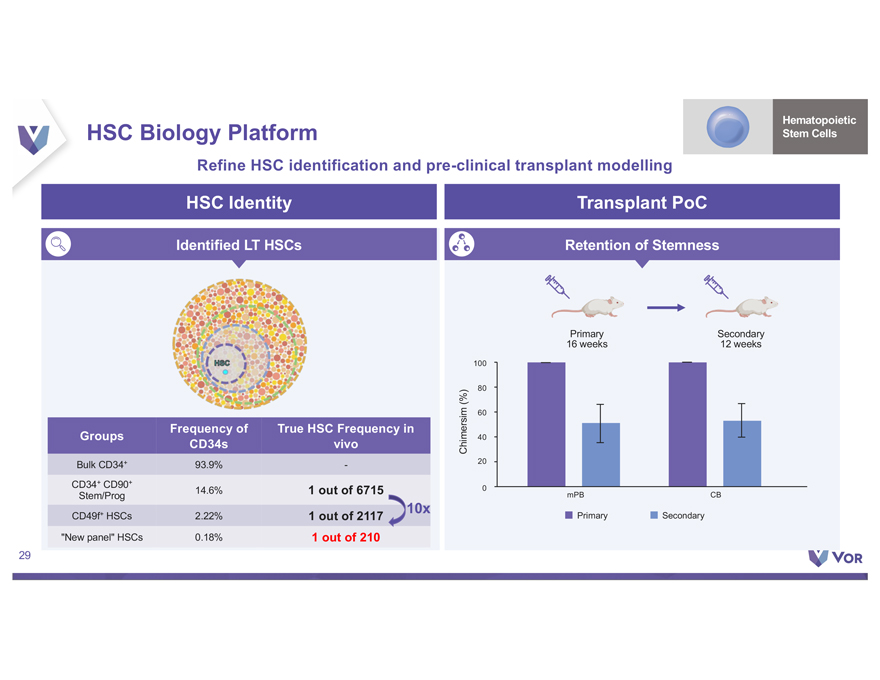
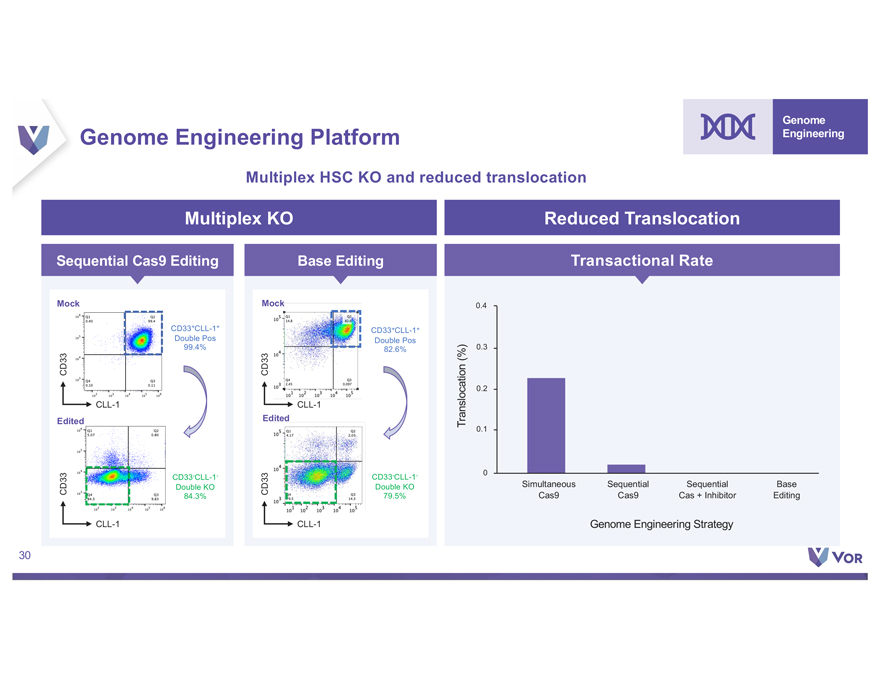
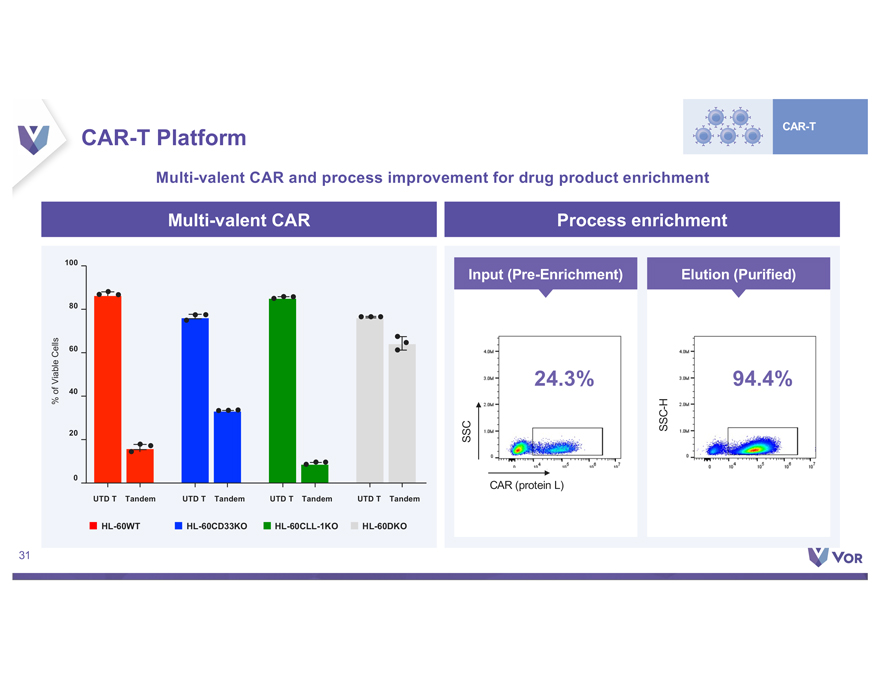
Disclaimer This presentation (the “Presentation”) contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 about Vor Biopharma Inc. (“Vor,” “Vor Bio” or the “Company”) that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this Presentation, “plan,” “potential,” are forward “project,” looking “should,” statements “target,” including, “vision,” “will,” but not “would,” limited or to, other terms similar such expressions. as “anticipate,” Such “believe,” forward-looking “continue,” statements “could,” “estimate,” in this Presentation “expect,” “intend,” include those “may,” regarding Vor Bio may Vor not Bio’s actually plans, achieve strategies the and plans, expectations intentions, for or expectations its preclinical disclosed and clinical in these programs, forward-looking including the statements. anticipated These milestones forward-looking and related statements catalysts of should such not programs. be relied upon to, Vor as Bio’s representing dependence Vor on Bio’s its views product as candidates of any date VOR33 subsequent (trem-cel) to the and date VCAR33 of this Presentation. ALLO, the risks Factors inherent that in biopharmaceutical could cause actual product results development to differ include, and but clinical are not trials, limited including clinical trials the will lengthy validate and the uncertain safety and regulatory efficacy approval of VOR33 process, (trem-cel) uncertainties and VCAR33 related programs to the timing in acute of initiation, myeloid leukemia enrollment or and other completion indications, of and clinical the trials, impact whether of the COVID-19 the pandemic change. These on Vor results Bio’s may business, not be operations, reproduced strategy in subsequent and anticipated patients. milestones, These and among other risks others. are described The first patient in greater data detail presented under herein the caption is preliminary “Risk Factors” and is in subject Vor Bio’s to reports plans, intentions filed with or the expectations Securities and disclosed Exchange in our Commission forward-looking (“SEC”), statements, and in other and filings you should that Vor not may place make undue with reliance the SEC on in our the forward-looking future. We may statements. not actually Actual achieve results the or included events could in this differ Presentation materially represent from the plans, our views intentions as of the and date expectations of this Presentation. disclosed in We the anticipate forward-looking that subsequent statements events we make. and developments In addition, the will forward-looking cause our views statements to change. otherwise. We undertake no obligation to update or revise any forward-looking statements, whether as a result of new information, the occurrence of certain events or Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third party sources and Vor Bio’s own internal representation estimates as to and the research. adequacy, While fairness, we believe accuracy these or third-party completeness sources of any to information be reliable as obtained of the date from of third this party Presentation, sources. In it has addition, not independently the third party verified, information and makes included no in we this believe Presentation our own may internal involve research a number is reliable, of assumptions such research and limitations, has not been and verified there can by any be no independent guarantee source. as to the accuracy or reliability of such assumptions. Finally, while VCAR33AUTO and NMDP-Sponsored Trial. A T cell therapy using the same chimeric antigen receptor construct as VCAR33ALLO is being studied in a Phase 1/2 clinical trial sponsor sponsored of this by the trial, National the NMDP Marrow has Donor permitted Program us to cross-reference (“NMDP”), and timing its IND of for data this release trial in is future dependent IND applications on the investigators that we may conducting submit with the the trial. FDA. Although For more we are information not the regarding of results that the NMDP may be trial, observed see “Risk in clinical Factors trials” – We in have our Annual not successfully Report on tested Form 10-K our product for the year candidates ended in December clinical trials 31, 2021 and any filed favorable with the SEC preclinical and such results other are filings not predictive that we may make with the SEC from time to time. 2