Filed Pursuant to Rule 424(b)(5)
Registration No. 333-267112
The information in this preliminary prospectus supplement is not complete and may be changed. This preliminary prospectus supplement and the accompanying prospectus are part of an effective registration statement filed with the Securities and Exchange Commission. This preliminary prospectus supplement and the accompanying prospectus are not an offer to sell these securities and are not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED JANUARY 26, 2023
PRELIMINARY PROSPECTUS SUPPLEMENT
(To prospectus dated September 7, 2022)

Shares of Class A Common Stock
We are offering shares of our Class A common stock (“Class A common stock”)
Our Class A common stock is traded on The Nasdaq Global Select Market (the “Nasdaq”) under the symbol “WGS.” On January 26, 2023, the last reported sales price of our Class A common stock was $0.37 per share. The actual offering price per share of Class A common stock will be as determined between us and the underwriter at the time of pricing. We are an “emerging growth company” and a “smaller reporting company” as those terms are defined under federal securities laws and, as such, we have elected to comply with certain reduced public company reporting requirements.
Investing in our securities involves a high degree of risk. You should review carefully the risks and uncertainties referenced under the heading “Risk Factors” on page S-8 of this prospectus supplement , as well as the sections entitled “Risk Factors” beginning on page 30 of our Annual Report on Form 10-K for the year ended December 31, 2021, beginning on page 46 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2022, beginning on page 60 of our Quarterly Report on Form 10-Q for the quarter ended June 30, 2022, and beginning on page 62 of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2022, which reports are incorporated by reference in this prospectus supplement and the accompanying prospectus, before investing in our securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share of Class A Common Stock | Total | ||||||||||
| Public offering price | $ | $ | |||||||||
Underwriting discounts and commissions(1) | $ | $ | |||||||||
| Proceeds to GeneDx Holdings Corp. (before expenses) | $ | $ | |||||||||
__________________
(1)See the section titled “Underwriting” for a description of the compensation payable to the underwriter.
We have granted the underwriter an option for a period of 30 days from the date of this prospectus supplement to purchase up to an additional ____ shares of Class A common stock. If the underwriter exercises its option in full, the total underwriting discounts and commissions payable by us will be $ and the total proceeds to us, before expenses, will be $ .
We are offering to sell directly to institutional investors affiliated with Keith Meister, a member of our board of directors, in a concurrent registered direct offering, shares of our Class A common stock at an offering price of $ per share, equal to the public offering price of this offering. We refer to this transaction as the concurrent registered direct offering. This offering is not contingent on the closing of the concurrent registered direct offering. See “Underwriting” for more information.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed on the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
Delivery of securities being offered pursuant to this prospectus supplement and accompanying prospectus will be made on or about , 2023, subject to the satisfaction of certain closing conditions.
| Jefferies | ||
The date of this prospectus supplement is , 2023.
TABLE OF CONTENTS
| Prospectus Supplement | PAGE | ||||
| Prospectus | |||||
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is part of the registration statement on Form S-3 that we filed with the Securities and Exchange Commission (the “SEC”) using a “shelf” registration process and consists of two parts. The first part is this prospectus supplement, including the documents incorporated by reference, which describes the specific terms of this offering. The second part, the accompanying prospectus, including the documents incorporated by reference, gives more general information, some of which may not apply to this offering. Generally, when we refer only to the “prospectus,” we are referring to both parts combined. This prospectus supplement may add to, update or change information in the accompanying prospectus and the documents incorporated by reference into this prospectus supplement or the accompanying prospectus.
This prospectus supplement describes the terms of this offering of Class A common stock and also adds to and updates information contained in the documents incorporated by reference into this prospectus supplement and the accompanying prospectus. To the extent there is a conflict between the information contained in this prospectus supplement, on the one hand, and the information contained in any document incorporated by reference into this prospectus supplement and the accompanying prospectus that was filed with the SEC before the date of this prospectus supplement, on the other hand, you should rely on the information in this prospectus supplement. If any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, a document incorporated by reference into this prospectus supplement and the accompanying prospectus—the statement in the document having the later date modifies or supersedes the earlier statement.
You should rely only on this prospectus supplement, the accompanying prospectus and the information incorporated or deemed to be incorporated by reference in this prospectus supplement and the accompanying prospectus. We have not authorized, and the underwriter has not authorized, anyone to provide you with information that is in addition to or different from that contained or incorporated by reference in this prospectus supplement and the accompanying prospectus. If anyone provides you with different or inconsistent information, you should not rely on it, and neither we nor the underwriter takes any responsibility for, and provide no assurance as to the reliability of, any other information that others may give you. You should not assume that the information contained or incorporated by reference in this prospectus supplement or the accompanying prospectus is accurate as of any date other than as of the date of this prospectus supplement or the accompanying prospectus, as the case may be, or in the case of the documents incorporated by reference, the date of such documents regardless of the time of delivery of this prospectus supplement and the accompanying prospectus or any sale of our Class A common stock. Our business, financial condition, liquidity, results of operations and prospects may have changed since those dates.
We are not offering to sell shares of our Class A common stock in any jurisdiction where the offer or sale is not permitted. The distribution of this prospectus supplement and the offering of the Class A common stock in certain jurisdictions may be restricted by law. Persons outside the United States who come into possession of this prospectus supplement must inform themselves about, and observe any restrictions relating to, the offering of the Class A common stock and the distribution of this prospectus supplement outside the United States. This prospectus supplement does not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy, any securities offered by this prospectus supplement by any person in any jurisdiction in which it is unlawful for such person to make such an offer or solicitation.
Unless the context otherwise requires, references in this prospectus supplement to:
•“GeneDx Holdings” refer to GeneDx Holdings Corp., a Delaware corporation (f/k/a Sema4 Holdings Corp. (“Sema4 Holdings”)), the issuer of the shares of Class A common stock in this offering and the concurrent registered direct offering;
•“Legacy GeneDx” refer to GeneDx, LLC, a Delaware limited liability company (formerly, GeneDx, Inc., a New Jersey corporation), which we acquired on April 29, 2022 (the “Acquisition”);
•“Legacy Sema4” refer to Mount Sinai Genomics, Inc. d/b/a as Sema4, a Delaware corporation, which consummated the business combination with CM Life Sciences, Inc. (“CMLS”) on July 22, 2021 (the “Business Combination”); and
S-1
•“we,” “us” and “our,” the “Company” and “GeneDx” refer, as the context requires, to:
◦Legacy Sema4 prior to the Business Combination, and GeneDx Holdings and its consolidated subsidiaries following the consummation of the Business Combination; and
◦Legacy GeneDx prior to the Acquisition, and GeneDx Holdings and its consolidated subsidiaries following the consummation of the Acquisition.
In addition, as described in more detail herein, we are pursuing a new strategic direction focused on our whole exome and genome sequencing business coupled with our Centrellis data platform. We are in the process of exiting our reproductive and women’s health testing business, which we expect to complete by the end of the first quarter of 2023, and we also completed the exit of our somatic tumor testing services during the fourth quarter of 2022. For more information, see “Prospectus Supplement Summary—Recent Developments—Legacy Sema4 Business Exits.” Unless the context otherwise requires, the description of our business and operations in this prospectus supplement assumes the completion of the exits from somatic tumor testing services and the reproductive and women’s health testing business
S-2
PROSPECTUS SUPPLEMENT SUMMARY
This summary may not contain all the information that you should consider before investing in our Class A common stock . You should read the entire prospectus supplement and the accompanying prospectus and the information incorporated by reference herein and therein carefully, including “Risk Factors” and the financial statements and related notes incorporated by reference herein, before making an investment decision.
Company Overview
GeneDx is focused on delivering personalized and actionable health insights to inform diagnosis, direct treatment and improve drug discovery. We sit at the intersection of diagnostics and data science, pairing decades of genomic expertise with an ability to interpret clinical data at scale. We are uniquely positioned to accelerate the use of genomics and leverage large-scale clinical data to enable precision medicine as the standard of care.
Legacy GeneDx was founded in 2000 by scientists from the National Institutes of Health whose mission was making genetic testing accessible for patients with rare diseases. The company quickly became a leader in genomics, creating the foundation for how to provide genomic information at scale and pioneering exome and genome sequencing for pediatric rare and ultra-rare genetic disorders. More than 20 years later, we have amassed one of the world’s largest rare disease data sets and remain a leader in genomics. In May 2022, Legacy GeneDx integrated with Sema4 Holdings, adding Centrellis®, a highly innovative health information platform to our portfolio of solutions. Centrellis® integrates digital tools and artificial intelligence and allows us to ingest and synthesize clinical and genomic data to deliver better, more comprehensive health insights.
Today, we are powered by our industry-leading genomic interpretation platform and Centrellis®, our innovative health information platform. We believe exome and genome testing will become the standard of care, transforming healthcare and improving patients’ quality of life for generations by sequencing once and analyzing for a lifetime.
Corporate Information
We were incorporated on July 10, 2020 as a special purpose acquisition company and a Delaware corporation under the name CM Life Sciences, Inc. On September 4, 2020, CMLS completed its initial public offering. On July 22, 2021, CMLS consummated the Business Combination with Legacy Sema4. In connection with the Business Combination, CMLS changed its name to Sema4 Holdings Corp. On January 9, 2023, Sema4 Holdings Corp. changed its name to GeneDx Holdings Corp.
Our address is 333 Ludlow Street, North Tower, 8th Floor, Stamford, Connecticut 06902. Our telephone number is +1 (800) 298-6470. Our website address is https://genedx.com. Information contained on our website or connected thereto does not constitute part of, and is not incorporated by reference into, this prospectus supplement or the registration statement of which it forms a part.
Recent Developments
Legacy Sema4 Business Exits
On August 11, 2022, our board of directors approved a restructuring plan that contemplated exiting our somatic tumor testing services and the closing of our laboratory in Branford, CT, which we completed as of December 31, 2022. In connection with the plan, we also eliminated approximately 250 positions, representing about 13% of our workforce, which, combined with our prior reductions in force during 2022, resulted in the elimination of approximately 30% of the roles from the Legacy Sema4 business.
On November 14, 2022, we announced our plan to pursue a new strategic direction focused on our exome and genome sequencing business coupled with our Centrellis data platform. As part of our strategic realignment, on November 11, 2022, our board of directors approved our exit from the reproductive and women’s health testing business, which includes carrier screening, noninvasive prenatal, and other ancillary reproductive testing offerings. We ceased accepting samples for reproductive and women’s health tests on December 14, 2022 and notified our customers impacted by this decision immediately. We expect to exit the operations of the reproductive and women’s health testing services by the end of the first quarter of 2023. As a result of this business exit, we eliminated
S-3
approximately 500 positions, representing approximately 32.5% of our workforce, and ceased operations at our Stamford, CT laboratory. Our go-forward testing services will be consolidated and performed out of our Gaithersburg, MD laboratory which was primarily used for our pediatric and rare disease testing services.
Nasdaq Compliance
On December 28, 2022, we received a written notice from the Listing Qualifications Department of The Nasdaq Stock Market, LLC that we were not in compliance with the minimum bid price requirements set forth in Nasdaq Listing Rule 5450(a)(1) (the “Minimum Bid Price Requirement”) for the last 30 consecutive trading days for continued listing on the Nasdaq Global Select Market (the “Nasdaq”). The Minimum Bid Price Requirement requires listed securities to maintain a minimum bid price of $1.00 per share, and Nasdaq Listing Rule 5810(c)(3)(A) provides that a failure to meet the Minimum Bid Price Requirement exists if the deficiency continues for a period of 30 consecutive trading days.
Pursuant to the Nasdaq Listing Rules, we have been provided an initial compliance period of 180 calendar days to regain compliance with the Minimum Bid Price Requirement. To regain compliance, the closing bid price of our Class A common stock must be at least $1.00 per share for a minimum of 10 consecutive trading days prior to June 26, 2023, and we must otherwise satisfy the Nasdaq’s requirements for listing.
We intend to monitor the closing bid price of our Class A common stock and consider our available options to resolve the noncompliance with the Minimum Bid Price Requirement. There can be no assurance that we will be able to regain compliance with the Nasdaq’s continued listing requirements or that Nasdaq will grant us a further extension of time to regain compliance, if applicable.
Please see “Risk Factors” below for additional disclosure regarding our compliance with Nasdaq listing qualifications.
Name Change
On January 9, 2023, we changed the name of the Company from “Sema4 Holdings Corp.” to “GeneDx Holdings Corp.” pursuant to a certificate of amendment to our certificate of incorporation. In conjunction with the name change, our Class A common stock and warrants also ceased trading under the ticker symbols “SMFR” and “SMFRW” and began trading under new ticker symbols, “WGS” and “WGSWW,” respectively, on the Nasdaq on January 10, 2023.
Implications of Being an Emerging Growth Company and a Smaller Reporting Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). An emerging growth company may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
•being permitted to present only two years of audited financial statements and only two years of related “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure in our periodic reports and registration statements
•not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, on the effectiveness of our internal controls over financial reporting
•reduced disclosure obligations regarding executive compensation arrangements in our periodic reports, proxy statements and registration statements; and
•exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved.
We will remain an emerging growth company until the earliest to occur of: (i) the last day of the fiscal year in which we have more than $1.07 billion in annual revenue; (ii) the date we qualify as a “large accelerated filer,” with
S-4
at least $700 million of equity securities held by non-affiliates; (iii) the date on which we have issued, in any three-year period, more than $1.0 billion in non-convertible debt securities; and (iv) December 31, 2025.
We have elected to take advantage of certain of the reduced disclosure obligations in the registration statement of which this prospectus is a part and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
We are also a “smaller reporting company,” meaning that the market value of our stock held by non-affiliates is less than $700.0 million and our annual revenue is less than $100.0 million during the most recently completed fiscal year. We may continue to be a smaller reporting company after this offering and the concurrent registered direct offering, if either (i) the market value of our stock held by non-affiliates is less than $250.0 million or (ii) our annual revenue is less than $100.0 million during the most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700.0 million. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
S-5
THE OFFERING
Securities Offered by Us | We are offering shares of our Class A common stock. | ||||
| Option to Purchase Additional Securities | We have granted the underwriter an option for a period of 30 days to purchase up to an additional shares of Class A common stock. | ||||
Concurrent Registered Direct Offering | We are offering to sell directly to institutional investors affiliated with Keith Meister, a member of our board of directors, in a concurrent registered direct offering, shares of our Class A common stock at an offering price of $ per share, equal to the public offering price of this offering. We refer to this transaction as the concurrent registered direct offering. This offering is not contingent on the closing of the concurrent registered direct offering. See “Underwriting” for more information | ||||
Class A Common Stock to be Outstanding Immediately After This Offering and the Concurrent Registered Direct Offering | shares (or shares if the underwriter exercises its option to purchase additional shares in full). | ||||
Use of Proceeds | We currently intend to use the net proceeds of this offering and the concurrent registered direct offering primarily for general corporate purposes, including additions to working capital, repayment or redemption of existing indebtedness, and strategic investment opportunities. See “Use of Proceeds.” | ||||
Risk Factors | Investing in our Class A common stock involves significant risks. See the disclosure under the heading “Risk Factors” in this prospectus supplement and under similar headings in other documents incorporated by reference into this prospectus supplement and the accompanying prospectus. | ||||
Listing | Our Class A common stock is listed on the Nasdaq under the symbol “WGS.” | ||||
The number of shares of our Class A common stock shown above to be outstanding after this offering and the concurrent registered direct offering is based on 381,428,905 shares of our Class A common stock outstanding as of September 30, 2022, and excludes:
•21,994,972 shares of Class A common stock issuable upon exercise of warrants outstanding as of September 30, 2022 with an exercise price of $11.50 per share;
•32,354,294 shares of Class A common stock issuable upon exercise of options outstanding as of September 30, 2022 with a weighted average exercise price of $1.47 per share;
•23,871,012 shares of common stock issuable upon vesting and settlement of restricted stock units (“RSUs”) (excluding the Earn-Out RSUs (as defined below)) outstanding as of September 30, 2022;
•up to 19,021,576 shares of Class A common stock that may be issuable (1) to certain former equity holders of Legacy Sema4 upon the achievement of certain vesting conditions and (2) upon the vesting and settlement of certain RSU awards (the “Earn-Out RSUs”) that were granted to certain former equity award holders of Legacy Sema4 and certain employees of the Company;
•up to 30,864,198 shares of our Class A common stock that may be issuable to OPKO Health, Inc. (“OPKO”) in connection with the achievement of certain revenue-based milestones for each of the fiscal years ending December 31, 2022 and December 31, 2023; and
•22,111,381 shares of Class A common stock reserved and available for future issuance as of September 30, 2022, under our equity incentive plan and employee stock purchase plan, consisting of (1) 14,881,582
S-6
shares of Class A common stock reserved and available for issuance under our 2021 Equity Incentive Plan as of September 30, 2022, and (2) 7,229,799 shares of Class A common stock reserved for issuance under our 2021 Employee Stock Purchase Plan as of September 30, 2022.
Except as otherwise indicated, the information in this prospectus supplement assumes no exercise of the underwriter’s option to purchase additional shares of our Class A common stock, no equity awards were issued under our 2021 Equity Incentive Plan and 2021 Employee Stock Purchase Plan after September 30, 2022, and that no outstanding warrants or options were exercised or terminated, and no outstanding RSUs vested and settled or terminated, after September 30, 2022.
S-7
RISK FACTORS
An investment in our securities involves a high degree of risk. You should consider the risk factors described in the “Risk Factors” sections of our Annual Report on Form 10-K for the fiscal year ended December 31, 2021, and our Quarterly Reports on Form 10-Q for the quarterly periods ended March 31, 2022, June 30, 2022, and September 30, 2022, respectively, which reports are incorporated herein by reference, in addition to the factors set forth below and other information contained in or incorporated by reference in this prospectus supplement and the accompanying prospectus before purchasing our securities. We may face additional risks and uncertainties that are not presently known to us, or that we currently deem immaterial, which may also impair our business or financial condition. See “Where You Can Find More Information; Incorporation by Reference” and “Cautionary Note Regarding Forward-Looking Statements.”
Risks Relating to this Offering and the Concurrent Registered Direct Offering
If you purchase securities sold in this offering, you will experience immediate and substantial dilution in your investment as a result of this offering and the concurrent registered direct offering. In addition, we may issue additional equity or equity-linked securities in the future, which may result in additional dilution to you.
The public offering price per share of Class A common stock being offered in this offering may be higher than the net tangible book value per share of our outstanding Class A common stock prior to this offering and the concurrent registered direct offering. Based on the public offering price of $ per share of Class A common stock and our net tangible book value as of September 30, 2022 of approximately $0.38 per share, if you purchase shares of Class A common stock in this offering, you will suffer immediate and substantial dilution of approximately $ per share, representing the difference between the public offering price per share of Class A common stock and the net tangible book value per share of our Class A common stock as of September 30, 2022 after giving effect to this offering and the concurrent registered direct offering. See the section titled “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase shares of Class A common stock in this offering.
To the extent outstanding stock options or warrants are exercised and RSUs become vested, there will be further dilution to new investors. In addition, to the extent we need to raise additional capital in the future and we issue additional shares of Class A common stock, preferred stock, warrants or other securities convertible or exchangeable for our Class A common stock, our then existing stockholders may experience dilution and the new securities may have rights senior to those of our Class A common stock offered in this offering.
We have broad discretion in the use of the net proceeds from this offering and the concurrent registered direct offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and the concurrent registered direct offering, including for any of the purposes described in the section entitled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering and the concurrent registered direct offering, their ultimate use may vary substantially from their currently intended use. Our management might not apply our net proceeds in ways that ultimately increase the value of your investment. We expect to use the net proceeds from this offering and the concurrent registered direct offering for general corporate purposes. The failure by our management to apply these funds effectively could harm our business. Pending their use, we plan to invest the net proceeds from this offering and the concurrent registered direct offering in short-term or long-term, investment-grade, interest-bearing securities. These investments may not yield a favorable return to our stockholders. If we do not invest or apply the net proceeds from this offering and the concurrent registered direct offering in ways that enhance stockholder value, we may fail to achieve expected financial results, which could cause our stock price to decline.
S-8
Future sales and issuances of our Class A common stock or rights to purchase our Class A common stock, including pursuant to our equity incentive plan and employee stock purchase plan, could result in additional dilution of the percentage ownership of our stockholders and may cause the price of our Class A common stock to decline.
We expect that significant additional capital may be needed in the future to continue our planned operations, including our commercialization efforts related to our Centrellis platform, expanded research and development activities and costs associated with operating as a public company. To raise capital, we may sell our Class A common stock, shares of preferred stock, other convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our Class A common stock.
After this offering and the concurrent registered direct offering, we will have shares of Class A common stock outstanding based on the number of shares of Class A common stock outstanding as of September 30, 2022. This includes the shares that we sell in this offering, which may be resold in the public market immediately without restriction, unless they were purchased in this offering by our affiliates, as that term is defined in Rule 144 under the Securities Act of 1933, as amended (the “Securities Act”), in which case they would only be able to be sold in compliance with the requirements of Rule 144.
In connection with this offering, subject to certain exceptions, we, all of our directors and executive officers and certain of our other stockholders, have agreed not to offer, sell, or agree to sell, directly or indirectly, any shares of Class A common stock without the permission of Jefferies LLC for a period of 60 days from the date of this prospectus supplement. We expect that investors in the concurrent registered direct offering will agree to a similar lock-up agreement with us that will apply for a period of six-months following such offering both to the shares that they purchase in such offering and the other shares that they own. However, all of the shares sold in this offering and the remaining shares of our Class A common stock outstanding prior to this will not be subject to lock-up agreements with the underwriter and, except to the extent such shares are held by our affiliates, will be freely tradable without restriction under the Securities Act.
Because we have no current plans to pay cash dividends on our Class A common stock for the foreseeable future, you may not receive any return on investment unless you sell your Class A common stock for a price greater than that which you paid for it.
We intend to retain future earnings, if any, for future operations and expansion of our business and have no current plans to pay any cash dividends for the foreseeable future. The declaration, amount and payment of any future dividends on shares of Class A common stock will be at the sole discretion of our board of directors. Our board of directors may take into account general and economic conditions, our financial condition and results of operations, our available cash and current and anticipated cash needs, capital requirements, contractual, legal, tax and regulatory restrictions, implications on the payment of dividends by us to our stockholders or by our subsidiaries to us and such other factors as our board of directors may deem relevant. In addition, our ability to pay dividends may be limited by covenants in connection with any indebtedness we or our subsidiaries may incur. As a result, you may not receive any return on an investment in our Class A common stock unless you sell our Class A common stock for a price greater than that which you paid for it.
If we fail to comply with the continued listing requirements of the Nasdaq, our Class A common stock may be delisted and the price of our Class A common stock and our ability to access the capital markets could be negatively impacted.
On December 28, 2022, we received a written notice from the Listing Qualifications Department of The Nasdaq Stock Market, LLC that we were not in compliance with the Minimum Bid Price Requirement set forth in Nasdaq Listing Rule 5450(a)(1) for the last 30 consecutive trading days for continued listing on the Nasdaq. The Minimum Bid Price Requirement requires listed securities to maintain a minimum bid price of $1.00 per share, and Nasdaq Listing Rule 5810(c)(3)(A) provides that a failure to meet the Minimum Bid Price Requirement exists if the deficiency continues for a period of 30 consecutive trading days. The notification provided that we had 180 calendar
S-9
days, or until June 26, 2023, to regain compliance with the Minimum Bid Price Requirement. To regain compliance, the closing bid price of our Class A common stock must be at least $1.00 per share for a minimum of 10 consecutive trading days prior to June 26, 2023, and we must otherwise satisfy The Nasdaq’s requirements for listing.
No assurance can be given that we will meet applicable Nasdaq continued listing standards or that future noncompliance will not occur. Failure to meet applicable Nasdaq continued listing standards could result in a delisting of our Class A common stock from the Nasdaq, which could materially reduce the liquidity of our Class A common stock and result in a corresponding material reduction in the price of our Class A common stock. Your ability to sell or purchase our Class A common stock when you wish to do so would also be impaired. In addition, delisting could harm our ability to raise capital through alternative financing sources on terms acceptable to us, or at all, and may result in the inability to expand our business, potential loss of confidence by investors and employees, and fewer business development and strategic investment opportunities.
The unaudited pro forma combined financial statements included in this prospectus supplement are presented for illustrative purposes only and may not be reflective of our operating results and financial condition following completion of the exits from the somatic tumor testing services and the reproductive and women’s health testing business.
The unaudited pro forma combined financial statements included in this prospectus supplement are presented for illustrative purposes only and are not necessarily indicative of what our actual financial condition or results of operations would have been had the exits from the COVID-19 and somatic tumor testing services and the reproductive and women’s health testing business or the Acquisition been completed on the dates indicated. The unaudited pro forma combined financial statements are subject to a number of assumptions. Our actual results and financial condition after the exits may differ materially and adversely from the unaudited pro forma combined financial statements. For further discussion, see “Unaudited Pro Forma Combined Financial Statements”.
We have a limited operating history of pursuing our new strategic direction, and the historical performance of our business may not be indicative of our future operating results and financial condition.
On November 14, 2022, we announced our plan to pursue a new strategic direction focused on our exome and whole genome testing business coupled with our Centrellis data platform. As a result, we have a limited operating history of pursuing this new strategic direction upon which to evaluate our business and forecast our future operating results and financial condition. Furthermore, when considering the historical performance of our business, investors should bear in mind that this information may not be indicative of the future results that investors should expect from us. In particular, our results could vary significantly from our historical results due to the fact that we are exiting our reproductive and women’s health testing business, which we expect to complete by the end of the first quarter of 2023, we completed the exit of our somatic tumor testing services during the fourth quarter of 2022 and we completed the exit of COVID-19 testing services during the first quarter of 2022. Further, prior to the April 2022 closing of the Acquisition, our historical results do not reflect the results of the Legacy GeneDx business that we acquired.
Risks Related to Our Business Model
We may need to raise additional capital to fund our existing operations, develop additional products and services, commercialize new products and services or expand our operations.
We have incurred net losses and negative cash flows from operations since our inception, including net losses of $245.4 million, $241.3 million and $29.7 million for the years ended December 31, 2021, 2020 and 2019, respectively. The net loss was $240.2 million for the nine months ended September 30, 2022. As of September 30, 2022, we had an accumulated deficit of $815.7 million. We expect to continue to generate significant operating losses for the foreseeable future.
Based on our current estimates of our cash and cash equivalents as of December 31, 2022, we do not believe these resources will be sufficient to fund our operations for the next twelve months. However, we believe that our cash and cash equivalents together with the anticipated net proceeds from this offering and the concurrent registered direct offering will be sufficient in the aggregate to fund our operations for at least the next twelve months, although
S-10
there can be no guarantee that we will successfully raise all the funding we require in this offering and the concurrent registered direct offering. We can offer no assurance that the concurrent registered direct offering will close, and if it does not close, the amount of our net proceeds will be limited to the net proceeds from this offering.
Depending on the amount of funding we receive in this offering and the concurrent registered direct offering as well as other factors, we may seek to sell common or preferred equity or convertible debt securities, enter into a credit facility or another form of third-party funding or seek other debt financing, whether in this offering, the concurrent registered direct offering or otherwise. We may also consider raising additional capital in the future to expand our business, to pursue strategic investments, to take advantage of financing opportunities or for other reasons, including to:
•increase our sales and marketing efforts to drive market adoption of our current and future products and services;
•fund development efforts for our current and future products and services;
•expand our products and services into other disease indications and clinical applications;
•acquire, license or invest in technologies;
•acquire or invest in complementary businesses or assets; and
•finance capital expenditures and general and administrative expenses.
Our present and future funding requirements will depend on many factors, including:
•our ability to achieve revenue growth;
•our rate of progress in establishing payor coverage and reimbursement arrangements with commercial third-party payors and government payors;
•the cost of expanding our laboratory operations and offerings, including our sales and marketing efforts;
•our rate of progress in, and cost of the sales and marketing activities associated with, establishing adoption of our Centrellis solution;
•our rate of progress in, and cost of research and development activities associated with, products and services in research and early development;
•the effect of competing technological, product and market developments;
•costs related to international expansion; and
•the potential cost of and delays in product development as a result of any regulatory oversight applicable to our products and services.
The various ways we could raise additional capital carry potential risks. If we raise funds by issuing equity securities, dilution to our stockholders could result. Any preferred equity securities issued also could provide for rights, preferences or privileges senior to those of holders of our Class A common stock. If we raise funds by issuing debt securities, those debt securities would have rights, preferences and privileges senior to those of holders of our Class A common stock. The terms of debt securities issued or borrowings pursuant to a credit agreement could impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our technologies or products and services or grant licenses on terms that are not favorable to us. If we are unable to raise additional capital, whether in this offering, the concurrent registered direct offering or otherwise, we may be required to undertake cost-cutting measures including delaying or discontinuing certain planned investments or programs.
S-11
We have identified material weaknesses, some of which have a pervasive effect across the organization, and may identify additional material weaknesses or significant deficiencies, in our internal controls over financial reporting. Our failure to remedy these matters could result in a material misstatement of our financial statements.
In the course of preparing Legacy Sema4’s financial statements for 2020, 2019 and 2018, we identified material weaknesses in our internal control over financial reporting as of December 31, 2020, which could, if not remediated, result in material misstatements in our financial statements. These material weaknesses had not been fully remediated as of September 30, 2022. In addition, during 2021, management identified a misclassification related to certain costs included within cost of services for the years ended December 31, 2021, 2020 and 2019. A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. The material weaknesses identified related to the fact that we did not design and maintain accounting policies, procedures and controls to ensure complete, accurate and timely financial reporting in accordance with U.S. GAAP. Specifically, the material weaknesses identified included the following:
•We did not design and maintain accounting policies, processes and controls to analyze, account for and report our revenue arrangements in accordance with ASC 606, Revenue from Contracts with Customers, and ASC 605, Revenue Recognition.
•We did not design and maintain formal accounting policies, procedures and controls to achieve complete, accurate and timely financial accounting, reporting and disclosures, including controls over the preparation and review of account reconciliations and journal entries; the accounting for cost capitalization policies in accordance with ASC 330, Inventory, and ASC 350-40, Intangibles – Goodwill and Other – Internal-Use Software; and the application of ASC 840, Leases.
•We had not developed and effectively communicated to our employees our accounting policies and procedures, which resulted in inconsistent practices. Since these entity level programs have a pervasive effect across the organization, management has determined that these circumstances constitute a material weakness.
•Our accounting and operating systems lacked controls over access, and program change management that are needed to ensure access to financial data is adequately restricted to appropriate personnel.
•We do not have sufficient, qualified finance and accounting staff with the appropriate U.S. GAAP technical accounting expertise to identify, evaluate and account for accounting and financial reporting, and effectively design and implement systems and processes that allow for the timely production of accurate financial information in accordance with internal financial reporting timelines, commensurate with our size and the nature and complexity of our operations. As a result, we did not design and maintain formal accounting policies, processes and controls related to complex transactions necessary for an effective financial reporting process.
Our management is in the process of implementing a remediation plan that is expected to include policies and procedures to support internal control over financial reporting for a public company as well as supplementing the accounting and finance function with robust technical accounting and financial reporting experience and training. However, we cannot guarantee that the steps we have taken or may subsequently take have been or will be sufficient to remediate the material weaknesses or ensure that our internal controls are effective. As noted above, as of September 30, 2022, the material weaknesses had not yet been fully remediated. Furthermore, we have not yet made any conclusions as to whether the actions we have taken have remediated the material weaknesses as of December 31, 2022 because our testing, evaluation and conclusion on the effectiveness of our internal control over financial reporting as of December 31, 2022 will not be completed until sometime in March 2023.
Furthermore, as a public company, we are required to comply with certain rules and requirements related to our disclosure controls and procedures and our internal control over financial reporting. Any failure to develop or maintain effective controls as a public company, any deficiencies found in the technology system we use to support our controls, or any difficulties encountered in their implementation or improvement, could harm our operating results or cause us to fail to meet our reporting obligations and may result in a restatement of our financial
S-12
statements for prior periods. For more information, see “Risk Factors—Risks Related to Being a Public Company—Our internal controls over financial reporting may not be effective and our independent registered public accounting firm may not be able to certify as to their effectiveness, which could have a significant and adverse effect on our business and reputation.” in our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2022, which is incorporated herein by reference.
Risks Related to Our Business, Industry and Operations
If third-party payors, including managed care organizations, private health insurers and government health plans, do not provide adequate reimbursement for our tests, or seek to amend or renegotiate their fee reimbursement schedules, or if we are unable to comply with their requirements for reimbursement, our commercial success could be negatively affected.
Our ability to increase the number of billable tests and our revenue therefrom will depend on our success in achieving reimbursement for our tests from third-party payors. Reimbursement by a payor may depend on a number of factors, including a payer’s determination that a test is appropriate, medically necessary, cost-effective, correctly billed, and has received prior authorization. The commercial success of our current and future products, if approved, will depend on the extent to which our customers receive coverage and adequate reimbursement from third-party payors, including managed care organizations and government payers (e.g., Medicare and Medicaid).
Since each payer makes its own decision as to whether to establish a policy or enter into a contract to cover our tests, as well as the amount it will reimburse for a test, seeking these approvals is a time-consuming and costly process. In addition, the determination by a payer to cover and the amount it will reimburse for our tests will likely be made on an indication-by-indication basis and may consider our billing practices and reimbursements from other payors and from our patient billing programs. To date, we have obtained policy-level reimbursement approval or contractual reimbursement for some indications for our tests from most of the large commercial third-party payors in the United States, and the Centers for Medicare & Medicaid Services, or CMS, provides reimbursement for our multi-gene tests for hereditary breast and ovarian cancer-related disorders as well as other tests. We believe that establishing adequate reimbursement from Medicare is an important factor in gaining adoption from healthcare providers. Our claims for reimbursement from third-party payors may be denied upon submission, and we must appeal the claims. The appeals process is time consuming and expensive and may not result in payment. In cases where there is not a contracted rate for reimbursement, there is typically a greater coinsurance or copayment requirement from the patient, which may result in further delay or decreased likelihood of collection.
A significant portion of the payments for our tests are paid or reimbursed under insurance programs with third-party payors. Billing and reimbursement for diagnostic tests is highly complex and closely scrutinized by payors. In particular, billing and reimbursement for multi-gene panel tests and other complex diagnostic tests continues to pose a particular risk of payor audit and potential overpayment obligations. Accurate billing requires sophisticated internal procedures and systems controls and ongoing oversight to ensure compliance with payor requirements
To contain reimbursement and utilization rates, third-party payors often attempt to, or do in fact, amend or renegotiate their fee reimbursement schedules. Loss of revenue caused by third-party payor cost containment efforts or an inability to negotiate satisfactory reimbursement rates could have a material adverse effect on our revenue and results of operations.
Furthermore, in cases where we or our partners have established reimbursement rates with third-party payors, we face additional challenges in complying with their procedural requirements for reimbursement. These requirements often vary from payer to payer and are reassessed by third party payors on a regular basis, and we have needed additional time and resources to comply with them. We have also experienced, and may continue to experience, delays in or denials of coverage if we do not adequately comply with these requirements.
Our third-party payors have also requested, and in the future may request, audits of the amounts paid to us. We have been required to repay certain amounts to payers as a result of such audits, including but not limited to the $42 million settlement disclosed in our Form 8-K of December 30, 2022 regarding certain overpayments to Legacy Sema4 allegedly received from a payor, and may be required to repay other payers for alleged overpayments due to lack of compliance with their reimbursement policies. In addition to potential repayment obligations, failure to
S-13
comply with payor reimbursement policies could result in government enforcement actions and, potentially, exclusion from certain payor programs, which could have a material adverse effect on our business.
We expect to continue to focus our resources on increasing adoption of, and expanding coverage and reimbursement for, our current tests and any future tests we may develop or acquire. If we fail to expand and maintain broad adoption of, and coverage and reimbursement for, our tests, our ability to generate revenue could be harmed and our future prospects and our business could suffer.
Risks Related to Legal, Regulatory and Compliance
We face uncertainty related to healthcare reform, pricing, coverage and reimbursement, which could reduce our revenue.
Healthcare reform laws, including the Patient Protection and Affordable Care Act or ACA, and the Protecting Access to Medicare Act of 2014, or PAMA, are significantly affecting the U.S. healthcare and medical services industry. Existing legislation, and possible future legal and regulatory changes, including potential repeal or modification of the ACA, elimination of penalties regarding the individual mandate for coverage, or approval of health plans that allow lower levels of coverage for preventive services, could materially change the structure and finances of the health insurance system and the methodology for reimbursing medical services, drugs and devices, including our current and future products and services. The ACA has also been the subject of various legal challenges if the plaintiffs in any case challenging the ACA are ultimately successful, insurance coverage for our tests could be materially and adversely affected. Any change in reimbursement policy could result in a change in patient cost-sharing, which could adversely affect a provider’s willingness to prescribe and patient’s willingness and ability to use our tests and any other product or service we may develop. Healthcare reforms, which may intend to reduce healthcare costs, may have the effect of discouraging third-party payors from covering certain kinds of medical products and services, particularly newly developed technologies, or other products or tests we may develop in the future. We cannot predict whether future healthcare reform initiatives will be implemented at the federal or state level or the effect any such future legislation or regulation will have on it. The taxes imposed by new legislation, cost reduction measures and the expansion in the government’s role in the U.S. healthcare industry may result in decreased profits to us, which may adversely affect our business, financial condition and results of operations.
PAMA presents significant uncertainty for future CMS reimbursement rates for our tests. Because Medicare currently covers a significant number of patients, any reduction in the CMS reimbursement rate for our tests would negatively affect our revenues and our business prospects. Under PAMA, unless delayed by an act of Congress, CMS reimbursement rates for clinical diagnostic laboratory tests are updated every three years, or annually for clinical laboratory tests that are considered "advanced diagnostic laboratory tests". The CMS reimbursement rates for clinical diagnostic laboratory tests are updated based on the volume-weighted median of private payer rates for each clinical diagnostic laboratory test based on data submitted by certain applicable laboratories. Further, laboratories that fail to report or erroneously report required payment information may be subject to substantial civil money penalties. There can be no assurance under PAMA that adequate CMS reimbursement rates will continue to be assigned to our tests. Congress could modify or repeal PAMA in the future or CMS could modify regulations under PAMA, and any such action could have the effect of reducing the CMS reimbursement rate for our tests. Further, it is possible that Medicare or other federal payers that provide reimbursement for our tests may suspend, revoke or discontinue coverage at any time, may require co-payments from patients, or may reduce the reimbursement rates payable to us. Any such action could have a negative impact on our revenues.
Compliance with the HIPAA security, privacy and breach notification regulations may increase our costs.
The HIPAA privacy, security and breach notification regulations, which include requirements implemented under the HITECH Act, establish federal standards with respect to the use and disclosure of protected health information, or PHI, by health plans, healthcare providers and healthcare clearinghouses. The HIPAA regulations generally prohibit the use and disclosure of PHI without patient authorization, unless the use or disclosure is for
S-14
payment, treatment or healthcare operations purposes. In setting standards to protect the confidentiality, integrity and security of PHI, the regulations establish a regulatory framework that addresses a variety of subjects, including:
•the circumstances under which uses and disclosures of PHI are permitted or required without a written authorization from the patient, including but not limited to treatment purposes, activities to obtain payments for our services, and our healthcare operations activities;
•a patient’s rights to access, amend and receive an accounting of certain disclosures of PHI;
•requirements to notify individuals if there is a breach of their PHI;
•the contents of notices of privacy practices related to the use and disclosure of PHI;
•administrative, technical and physical safeguards required of entities that use or receive PHI;
•criteria related to the deidentification and aggregation of PHI; and
•the use and protection of electronic PHI.
We are also required to comply with applicable state privacy, security and breach notification laws and regulations, which may be more stringent than federal HIPAA requirements. In addition, for healthcare data transfers from other countries relating to citizens and/or residents of those countries, we are also required to comply with the laws of those countries. Furthermore, on December 1, 2022, the U.S. Department of Health and Human Services, Office for Civil Rights (“OCR”) issued a Bulletin highlighting the obligations of HIPAA covered entities and business associates with respect to the use of online tracking technologies. To the extent that a covered entity or business associate permits a tracking technology vendor to collect PHI of its customers, the parties must enter into a business associate agreement. In addition, the PHI collected may only be used for treatment or health care operation purposes, in accordance with HIPAA. The PHI cannot be used for marketing purposes that are not connected with treatment or health care operations absent a HIPAA compliant authorization from each customer whose information is being shared.
Although HIPAA does not provide for private rights of action, HIPAA gives OCR and the Department of Justice the authority to assess significant fines and other penalties for wrongful use or disclosure of PHI, including potential civil and criminal fines and penalties. OCR may require an entity to enter into a settlement agreement which may include ongoing oversight and auditing of a company’s HIPAA compliance program.
In addition, computer networks are always vulnerable to breach and unauthorized persons may in the future be able to exploit weaknesses in the security systems of our computer networks and gain access to PHI. Additionally, we share PHI with third-parties who are legally obligated to safeguard and maintain the confidentiality of PHI. Despite such protections, unauthorized persons may also be able to gain access to PHI stored in such third-parties computer networks. Any wrongful use or disclosure of PHI by us or such third-parties, including disclosure due to data theft or unauthorized access to us or such third-parties’ computer networks, could subject us to fines or penalties that could adversely affect our business and results of operations. In addition, we distribute PHI to patients in physical form (e.g., test materials and/or test results), which introduces additional risk that human error will result in unauthorized disclosures of PHI. Although HIPAA does not expressly provide for a private right of action for damages, we could also be liable for damages under state privacy laws to private parties for the wrongful use or disclosure of confidential health information or other private personal information.
We have implemented practices intended to meet the requirements of the HIPAA privacy, security and breach notification regulations, as required by law, but cannot guarantee that such practices fully satisfy all applicable requirements under HIPAA. In addition, the Company has experienced a number of “security incidents” (as defined under HIPAA) that involved the unauthorized disclosure of PHI. A subset of these incidents were determined to be reportable breaches requiring disclosure to OCR, as well as to the affected patients. Moreover, we cannot confirm that we have identified all previous incidents that could constitute reportable breaches, or that the mitigation steps undertaken in response to known breaches are adequate to satisfy applicable regulatory requirements and prevent any future unauthorized disclosures.
S-15
As noted above, in addition to HIPAA, we are subject to myriad federal, state, and local requirements pertaining to the collection, retention, and disclosure of genetic material. While we endeavor to remain current with such requirements, we can provide no assurance that we are, or will remain, in compliance with all applicable requirements. Failure to comply with privacy and data security requirements could result in a variety of consequences, including significant fines and penalties as well as damage to our reputation, any of which could have a material adverse effect on our business.
Some of our activities may subject the Company to risks under federal and state healthcare fraud and abuse laws.
In addition to FDA marketing and promotion restrictions, several other types of state and federal healthcare fraud and abuse laws have been applied in recent years to restrict certain marketing practices in the healthcare product and service industry and to regulate billing practices and financial relationships with healthcare providers, hospitals and other healthcare providers. These laws include, among others, a federal law commonly known as the federal Anti-Kickback Statute, the federal False Claims Act, the federal physician self-referral law, known as the Stark Law, and corollary state laws, which prohibit payments intended to induce healthcare providers or others either to refer patients or to acquire or arrange for or recommend the acquisition of healthcare products or services. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices and providers of laboratory services by limiting the kinds of financial arrangements, including sales programs, free goods and services, consulting arrangements, speaker programs, compensated service arrangements (including specimen collection and processing), and other non-monetary compensation (e.g., meals, gifts and other business courtesies), that may be used with hospitals, healthcare providers, laboratories and other potential purchasers or prescribers of medical devices and laboratory services. The federal and state fraud and abuse laws prescribe civil and criminal penalties (including fines) for noncompliance that can be substantial. In addition, various states have enacted false claim laws analogous to the federal laws that apply where a claim is submitted to any third-party payor and not only a governmental payer program. Moreover, any claim for reimbursement that is predicated on a violation of the Anti-Kickback Statute may constitute a “false claim” under the False Claims Act (discussed in further detail below).
In 2018, Congress passed the Eliminating Kickbacks in Recovery Act, EKRA, as part of the Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities Act. Similar to the Anti-Kickback Statute, EKRA imposes criminal penalties for knowing or willful payment or offer, or solicitation or receipt, of any remuneration, whether directly or indirectly, overtly or covertly, in cash or in kind, in exchange for the referral or inducement of laboratory testing (among other healthcare services) unless a specific exception applies. However, unlike the Anti-Kickback Statute, EKRA is not limited to services covered by federal or state healthcare programs but applies more broadly to services covered by “healthcare benefit programs,” including commercial insurers. As currently drafted, EKRA potentially expands the universe of arrangements that could be subject to government enforcement under federal fraud and abuse laws. In addition, while the Anti-Kickback Statute includes certain exceptions that are widely relied upon in the healthcare industry, including safe harbors applicable to certain employment and personal services contracts, and not all of those same exceptions apply under EKRA. EKRA expressly does not protect employee compensation that varies by the number of individuals referred to a laboratory, the number of tests performed by a laboratory, or the amount billed to or received from a health benefit program from individuals referred to a laboratory. Because EKRA is a relatively new law, there is no agency guidance and only two courts have addressed the application of EKRA and those courts reached opposite conclusions. One Court ruled that the commission-based compensation provisions of a laboratory employee’s contract did not violate EKRA, while the other court expressly disagreed. Given the conflicting opinions, we cannot be assured that courts in our jurisdiction will reach the same conclusion or that the decision will not be overturned if there is an appeal. We cannot assure you that our relationships with healthcare providers, hospitals, customers, our own sales representatives, or any other party will not be subject to scrutiny or will survive regulatory challenge under EKRA or other anti-kickback laws.
The False Claims Act prohibits knowingly presenting (or causing the presentation of) a false claim for reimbursement by a federal health care program. Violation of the False Claims Act can result in substantial penalties, including treble damages. Moreover, the False Claims Act permits enforcement by qui tam relators (i.e., whistleblowers), such as competitors, customers, or current/former employees, who will receive a portion of any settlement. As discussed above, violations of the Anti-Kickback statute can serve as the basis for enforcement under
S-16
the False Claims Act. In addition, inaccurate or otherwise improper claims for reimbursement could constitute a false claim, meaning that we or our partners must carefully and accurately code claims for reimbursement, proactively monitor the accuracy and appropriateness of claims and payments received, diligently investigate any credible information indicating that we or our partners may have received an overpayment, and promptly return any overpayments. Medicare payments are subject to audit, including through the Comprehensive Error Rate Testing, (“CERT”), program, and payments may be recouped by CMS if it is determined that they were improperly made. Currently, a small percentage of our revenues are generated by payments from Medicare.
While we continually strive to comply with these complex requirements, interpretations of the applicability of these laws to marketing and billing practices are constantly evolving and even an unsuccessful challenge could cause adverse publicity and be costly to respond to, and thus could harm our business and prospects. In addition, while we have and will continue to enter into certain financial arrangements with referral sources, and we endeavor to ensure that such arrangements are designed to comply with applicable rules, laws and regulations, we can offer no assurance that such arrangements will not result in regulatory or enforcement scrutiny. Our failure to comply with applicable laws could result in various adverse consequences that could have a material adverse effect upon our business, including the exclusion of our products and services from government programs and the imposition of civil or criminal sanctions.
Complying with numerous statutes and regulations pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties.
Our operations are subject to other extensive federal, state, local and foreign laws and regulations, all of which are subject to change. These laws and regulations currently include, among others:
•HIPAA, which establishes comprehensive federal standards with respect to the privacy and security of protected health information and requirements for the use of certain standardized electronic transactions;
•amendments to HIPAA under HITECH, which strengthen and expand HIPAA privacy and security compliance requirements, increase penalties for violators and expand vicarious liability, extend enforcement authority to state attorneys general, and impose requirements for breach notification;
•the General Data Protection Regulation, or GDPR, which imposes strict privacy and security requirements on controllers and processors of European personal data, including enhanced protections for “special categories” of personal data, including sensitive information such as health and genetic information of data subjects;
•the CCPA, which, among other things, regulates how subject businesses may collect, use, disclose and/or sell the personal information of consumers who reside in California, affords rights to consumers that they may exercise against businesses that collect their information, and requires implementation of reasonable security measures to safeguard personal information of California consumers;
•Laws governing genetic counseling services, relating to, among other things, the adequacy of health care, the practice of medicine and other health professions (including the provision of remote care and cross-coverage practice), equipment, personnel, operating policies and procedures and the prerequisites for ordering laboratory tests. Some states have enacted regulations specific to providing services to patients via telehealth. Such regulations include, among other things, informed consent requirements that some states require providers to obtain from their patients before providing telehealth services. Health professionals who provide professional services using telehealth modalities must, in most instances, hold a valid license to practice the applicable health profession in the state in which the patient is located. In addition, certain states require a healthcare professional providing telehealth to be physically located in the same state as the patient. Any failure to comply with these laws and regulations could result in civil or criminal penalties against telehealth providers.
•Clinical and human subjects research regulations, including but not limited to the federal Policy for Protection of Human Subjects (45 C.F.R. Part 46), the FDCA and its applicable implementing regulations at 21 C.F.R. Parts 50, 54, 56, 58 and 812, the International Conference on Harmonization’s (ICH)
S-17
Guideline on Nonclinical Safety Studies for the Conduct of Human Clinical Trials for Pharmaceuticals and the ICH Guideline on Safety Pharmacology Studies for Human Pharmaceuticals, and all equivalent legal requirements in other jurisdictions.
•the federal Anti-Kickback Statute, which prohibits knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, overtly or covertly, in cash or in kind, to induce or in return for the referral of an individual, for the furnishing of or arrangement for the furnishing of any item or service for which payment may be made in whole or in part by a federal healthcare program, or the purchasing, leasing, ordering, arranging for, or recommend purchasing, leasing or ordering, any good, item or service for which payment may be made, in whole or in part, under a federal healthcare program;
•EKRA, which prohibits payments for referrals to recovery homes, clinical treatment facilities, and laboratories and reaches beyond federal health care programs, to include private insurance;
•the federal physician self-referral law, known as the Stark Law, which prohibits a physician from making a referral to an entity for certain designated health services covered by the Medicare program, including laboratory and pathology services, if the physician or an immediate family member has a financial relationship with the entity unless an exception applies, and prohibits an entity from billing for designated health services furnished pursuant to a prohibited referral;
•the federal False Claims Act, which imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment to the federal government;
•the federal Civil Monetary Penalties Law, which prohibits, among other things, the offering or transfer of remuneration to a Medicare or state healthcare program beneficiary if the person knows or should know it is likely to influence the beneficiary’s selection of a particular provider, practitioner or supplier of services reimbursable by Medicare or a state healthcare program, unless an exception applies;
•the HIPAA fraud and abuse provisions, which create new federal criminal statutes that prohibit, among other things, defrauding health care benefit programs, willfully obstructing a criminal investigation of a healthcare offense and falsifying or concealing a material fact or making any materially false statements in connection with the payment for healthcare benefits, items or services;
•other federal and state fraud and abuse laws, such as anti-kickback laws, prohibitions on self-referral, fee-splitting restrictions, insurance fraud laws, anti-markup laws, prohibitions on the provision of tests at no or discounted cost to induce physician or patient adoption, and false claims acts, which may extend to services reimbursable by any third-party payer, including private insurers;
•the 21st Century Cures Act information blocking prohibition, which prohibits covered actors from engaging in certain practices that are likely to interfere with the access, exchange, or use of electronic health information;
•the Physician Payments Sunshine Act and similar state laws that require reporting of certain payments and other transfers of value made by applicable manufacturers, directly or indirectly, to or on behalf of covered recipients including physicians (defined to include doctors, dentists, optometrists, podiatrists and chiropractors), physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists and certified nurse midwives and teaching hospitals as well as ownership and investment interests held by physicians and their immediate family members.
•state laws that limit or prohibit the provision of certain payments and other transfers of value to certain covered healthcare providers;
•the prohibition on reassignment of Medicare claims, which, subject to certain exceptions, precludes the reassignment of Medicare claims to any other party;
S-18
•state laws that prohibit other specified practices, such as billing clinicians for testing that they order; waiving coinsurance, copayments, deductibles and other amounts owed by patients; billing a state Medicaid program at a price that is higher than what is charged to one or more other payers;
•similar foreign laws and regulations that may apply to us in the countries in which we operate or may operate in the future; and
•laws that relate to maintaining accurate information and control over activities that may fall within the purview of the U.S. Foreign Corrupt Practices Act, its books and records provisions, or anti-bribery provisions.
We have adopted policies and procedures designed to comply with these laws and regulations. While the Company continues to develop and improve its compliance program, we acknowledge that further development will be necessary to help mitigate enforcement risk. Our compliance may also be subject to governmental review, and, in the event of a violation of certain legal requirements, any deficiencies in our policies, procedures, and controls may subject us to increased sanctions that could materially affect our business.
In addition the growth of our business and our expansion outside of the United States may increase the potential of violating these laws or our internal policies and procedures. The risk of us being found in violation of these or other laws and regulations is further increased by the fact that many have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of these laws and regulations, we may be subject to any applicable penalty associated with the violation, including significant administrative, civil and criminal penalties, damages, fines, imprisonment, exclusion from participation in Federal healthcare programs, refunding of payments received by us and curtailment or cessation of our operations, which may impact existing contracts with key payors, collaborators, health systems, and commercial partners. Any of the foregoing consequences could seriously harm our business and our financial results.
Risks Related to Cybersecurity, Privacy and Information Technology
Security breaches, privacy issues, loss of data and other incidents could continue to compromise sensitive, protected, or personal information related to our business, could prevent it from accessing critical information, and could expose it to regulatory liability, which could adversely affect our business.
In the ordinary course of our business, our collection and storing of PHI also includes more sensitive data such as genetic information, as well as personally identifiable information, genetic information, credit card information, financial information, intellectual property and proprietary business information owned or controlled by us or our customers, payers and other parties. We manage and maintain our applications and data utilizing a combination of on-site systems, managed data center systems and cloud based systems. We also communicate PHI and other sensitive patient data through our various customer tools and platforms, and in physical form. In addition to storing and transmitting sensitive data that is subject to multiple legal protections, these applications and data encompass a wide variety of business critical information including research and development information, commercial information, and business and financial information. We continue to face a number of risks relative to protecting this critical information, including loss of access risk, inappropriate disclosure, inappropriate modification, and the risk of our being unable to adequately monitor and modify our controls over our critical information. Any technical problems that may arise in connection with the data that we access and our systems, including those that are hosted by third-party providers, could result in interruptions to our business and operations or exposure to security vulnerabilities. These types of problems may be caused by a variety of factors, including infrastructure changes, intentional or accidental human actions or omissions, software errors, malware, viruses, security attacks, fraud, spikes in customer usage and denial of service issues. From time to time, large third-party web hosting providers have experienced outages or other problems that have resulted in their systems being offline and inaccessible. Such outages could materially impact our business and operations.
S-19
Although we take what we believe to be reasonable and appropriate measures, including a formal, dedicated enterprise security program, to protect sensitive information from various compromises (including unauthorized access, disclosure, or modification or lack of availability), our information technology and infrastructure may be vulnerable to attacks by hackers or viruses or breached due to employee error, malfeasance, lost or stolen technology, or other disruptions. Any such breach or interruption could compromise our networks and the information stored therein could be accessed by unauthorized parties, altered, publicly disclosed, lost or stolen.
Further, some of our customer tools and platforms are currently accessible through a portal, and there is no guarantee that we can protect our portal from a security breach. Unauthorized access, loss or dissemination could also disrupt our operations (including our ability to conduct our analyses, provide test results, bill payers or patients, process claims and appeals, provide customer assistance, conduct research and development activities, collect, process and prepare company financial information, provide information about our tests and other patient and physician education and outreach efforts through our website, and manage the administrative aspects of our business) and damage our reputation, any of which could adversely affect our business. In addition to data security risks, we also face privacy risks. For example, as noted above, pursuant to guidance recently issued by OCR, HIPAA covered entities and business associates who permit tracking technology vendors to collect PHI from their patients must enter into a HIPAA compliant business associate agreement with that vendor or obtain advance consent. We have utilized, and may continue to utilize, tracking technologies on one or more of our websites, and may not be able to do so in a manner that is consistent with what HIPAA requires. Should we actually violate, or be perceived to have violated, any privacy promises our business makes to patients or consumers, it could be subject to a complaint from an affected individual or interested privacy regulator, such as OCR, the FTC, a state Attorney General, an EU Member State Data Protection Authority, or a data protection authority in another international jurisdiction. This risk is heightened given the sensitivity of the data we collect.
Any security compromise that causes an apparent privacy violation could also result in legal claims or proceedings; liability under federal, state, foreign, or multinational laws that regulate the privacy, security, or breach of personal information, such as but not limited to the HIPAA, HITECH, state data security and data breach notification laws, the EU’s GDPR, the UK Data Protection Act of 2018; and related regulatory penalties. Penalties for failure to comply with a requirement of HIPAA or HITECH vary significantly, and, depending on the knowledge and culpability of the HIPAA-regulated entity, may include civil monetary penalties of up to $1.5 million per calendar year for each provision of HIPAA that is violated. A person who knowingly obtains or discloses individually identifiable health information in violation of HIPAA may face a criminal penalty of up to $50,000 and up to one-year imprisonment. The criminal penalties increase if the wrongful conduct involves false pretenses or the intent to sell, transfer or use identifiable health information for commercial advantage, personal gain or malicious harm. Penalties for unfair or deceptive acts or practices under the FTC Act or state Unfair and Deceptive Acts and Practices, or UDAP, statutes may also vary significantly.
There has been unprecedented activity in the development of data protection regulation around the world. As a result, the interpretation and application of consumer, health-related and data protection laws in the United States, Europe and elsewhere are often uncertain, contradictory and in flux. The GDPR took effect on May 25, 2018. The GDPR took effect on May 25, 2018. The GDPR applies to any entity established in the EU as well as extraterritorially to any entity outside the EU that offers goods or services to, or monitors the behavior of, individuals who are located in the EU. The GDPR imposes strict requirements on controllers and processors of personal data, including enhanced protections for “special categories” of personal data, which includes sensitive information such as health and genetic information of data subjects. The GDPR also grants individuals various rights in relation to their personal data, including the rights of access, rectification, objection to certain processing and deletion. The GDPR provides an individual with an express right to seek legal remedies if the individual believes his or her rights have been violated. Failure to comply with the requirements of the GDPR or the related national data protection laws of the member states of the EU, which may deviate from or be more restrictive than the GDPR, may result in significant administrative fines issued by EU regulators. Maximum penalties for violations of the GDPR are capped at 20 million euros or 4% of an organization’s annual global revenue, whichever is greater.
Further, the United Kingdom’s decision to leave the EU, often referred to as Brexit, has created uncertainty with regard to data protection regulation in the United Kingdom. In particular, it is still unclear whether the transfer of personal information from the EU to the United Kingdom will in the future remain lawful under the GDPR. The
S-20
United Kingdom-EU post-Brexit trade deal provides that transfers of personal information to the United Kingdom will not be treated as restricted transfers to a non-EU country for a period of up to six months from January 1, 2021. However, unless the EU Commission makes an “adequacy finding” with respect to the United Kingdom before the end of that transition period, from that date the United Kingdom will be a “third country” under the GDPR and transfers of personal information from the EU to the United Kingdom will require an “adequacy mechanism,” such as the SCCs.
Additionally, the implementation of GDPR has led other jurisdictions to either amend or propose legislation to amend their existing data privacy and cybersecurity laws to resemble the requirements of GDPR. For example, on June 28, 2018, California adopted the CCPA. The CCPA regulates how certain for-profit businesses that meet one or more CCPA applicability thresholds collect, use, and disclose the personal information of consumers who reside in California. Among other things, the CCPA confers to California consumers the right to receive notice of the categories of personal information that will be collected by a business, how the business will use and share the personal information, and the third parties who will receive the personal information; the CCPA also confers rights to access, delete, or transfer personal information; and the right to receive equal service and pricing from a business after exercising a consumer right granted by the CCPA. In addition, the CCPA allows California consumers the right to opt out of the “sale” of their personal information, which the CCPA defines broadly as any disclosure of personal information to a third party in exchange for monetary or other valuable consideration. The CCPA also requires a business to implement reasonable security procedures to safeguard personal information against unauthorized access, use, or disclosure. California amended the law in September 2018 to exempt all PHI collected by certain parties subject to HIPAA, and further amended the law in September 2020 to clarify that de-identified data as defined under HIPAA will also be exempt from the CCPA. The California Attorney General’s final regulations implementing the CCPA took effect on August 14, 2020. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches resulting from a business’s failure to implement and maintain reasonable data security procedures that is expected to increase data breach litigation. In addition, California voters recently approved the California Privacy Rights Act of 2020, or CPRA, that went into effect on January 1, 2023. The CPRA, among other things, amends the CCPA to give California residents the ability to limit the use of their sensitive information, provides for penalties for CPRA violations concerning California residents under the age of 16, and establishes a new California Privacy Protection Agency to implement and enforce the law. Other jurisdictions in the United States are beginning to propose laws similar to CCPA. Some observers have noted that the CCPA could mark the beginning of a trend toward more stringent privacy legislation, which could increase our potential liability and adversely affect our business, results of operations, and financial condition.
It is possible the GDPR, CCPA and other emerging United States and international data protection laws may be interpreted and applied in manner that is inconsistent with our practices. If so, this could result in government-imposed fines or orders requiring that we change our practices, which could adversely affect our business. In addition, these privacy laws and regulations may differ from country to country and state to state, and our obligations under these laws and regulations vary based on the nature of our activities in the particular jurisdiction, such as whether we collect samples from individuals in the local jurisdiction, perform testing in the local jurisdiction, or process personal information regarding employees or other individuals in the local jurisdiction. Complying with these various laws and regulations could cause us to incur substantial costs or require it to change our business practices and compliance procedures in a manner adverse to our business. We can provide no assurance that it is or will remain in compliance with diverse privacy and data security requirements in all of the jurisdictions in which we do business. Failure to comply with privacy and data security requirements could result in a variety of consequences, or damage to our reputation, any of which could have a material adverse effect on our business.
S-21
UNAUDITED PRO FORMA COMBINED FINANCIAL STATEMENTS
The following unaudited pro forma combined financial statements are based on the historical consolidated financial statements of the Company and historical combined financial statements of Legacy GeneDx and are adjusted to give effect to the completed Acquisition as well as the closure or abandonment of the COVID and somatic tumor testing services and the reproductive and women’s health business previously announced which have either occurred or are probable of occurring.
On December 16, 2021 and August 15, 2022, the Company announced its decision to exit COVID-19 and somatic tumor testing services, respectively. Similarly, on November 11, 2022, the board of directors of the Company unanimously approved the Company’s exit from the reproductive and women’s health testing business activities, which includes carrier screening, noninvasive prenatal, and other ancillary reproductive testing offerings. These are referred to as “Exited Activities” in the statements below. On November 14, 2022, the Company announced its plan to pursue a new strategic direction focused on the Company’s exome and whole genome testing business coupled with the Company’s Centrellis data platform.
The following unaudited pro forma combined statements of operations combine the historical consolidated statements of comprehensive loss of the Company, which includes the results of Legacy Sema4 and the results of Legacy GeneDx subsequent to the April 29, 2022 Acquisition, and the applicable pre-Acquisition periods of combined statements of comprehensive loss of Legacy GeneDx, giving effect to the Acquisition, as if the Acquisition had been consummated on January 1, 2021, in addition to the adjustments for the Exited Activities.
We have not presented a pro forma balance sheet as of September 30, 2022 since the assets and liabilities of Legacy GeneDx are already included in the consolidated balance sheet as of September 30, 2022. In addition, at the present time, the Exited Activities do not reflect selling or divesting such businesses, the individual assets and liabilities associated with the Exited Activities will remain as assets and liabilities of the Company. We continue to evaluate the ultimate disposition of the assets of Exited Activities, which may include the sale, return to suppliers, redirect the usage to ongoing business activities or abandonment, and will record the financial effects of the Exited Activities as our estimates evolve.
The unaudited pro forma combined financial statements should be read in conjunction with our financial statements and the related notes included in our Annual Report on Form 10-K for the year ended December 31, 2021 and our Quarterly Reports on Form 10-Q for the first, second and third quarters ended in 2022, which are incorporated by reference into this prospectus supplement. Subsequent to the unaudited pro forma combined financial statements presented, as part of its strategic realignment, the Company announced the elimination of approximately 500 positions, representing approximately 32.5% of its workforce. We have also identified indicators of impairment in the fourth quarter of 2022 related to the Exited Activities and are in the process of performing a detailed evaluation. Our evaluation will consider the fair value of the assets of the Exited Activities noted above. In addition, and in response to the significant declines in our share price through December 31, 2022, we will also evaluate the goodwill and long-lived intangible assets of the Company for impairment. Additionally, certain current and non-current assets previously utilized in the operation of reproductive and women’s health and somatic tumor testing services, primarily fixed assets and inventory, may be subject to fair market value adjustments in the future periods. As discussed above, we are evaluating the ultimate disposition of these assets, which may include a sale of any assets not expected to be used in our planned operations. Any impairments or fair market value adjustments to the carrying value of assets, if identified, will be recorded in the fourth quarter of 2022.
In addition, the unaudited pro forma combined financial statements update and supplement the unaudited pro forma combined financial information previously filed in the Company’s Current Reports on Form 8-K filed with the SEC on May 2, 2022 and August 26, 2022, which reports are incorporated by reference in this prospectus supplement. To the extent that information in the unaudited pro forma combined financial statements differs from or updates information contained in those Current Reports on Form 8-K, the information in the unaudited pro forma combined financial statements supersedes or supplements the information in those reports.
S-22
UNAUDITED PRO FORMA COMBINED STATEMENT OF OPERATIONS
FOR THE NINE MONTHS ENDED SEPTEMBER 30, 2022
(in thousands, except share and per share amounts)
| Historical | Pro forma | ||||||||||||||||||||||||||||
| Company | Legacy GeneDx (A) | Acquisition Adjustments (B) | Exited Activities Adjustments (H) | Pro Forma Statement of Operations | |||||||||||||||||||||||||
| Revenue: | |||||||||||||||||||||||||||||
| Diagnostic test revenue | $ | 167,989 | $ | 48,804 | $ | (544) | C | $ | (95,638) | $ | 120,611 | ||||||||||||||||||
| Other revenue | 5,355 | — | $ | 5,355 | |||||||||||||||||||||||||
| Total revenue | 173,344 | 48,804 | (544) | (95,638) | 125,966 | ||||||||||||||||||||||||
| Cost of services | 183,768 | 34,615 | 496 | D | (135,656) | $ | 83,223 | ||||||||||||||||||||||
| Total gross profit (loss) | (10,424) | 14,189 | (1,040) | 40,018 | 42,743 | ||||||||||||||||||||||||
| Operating expenses: | |||||||||||||||||||||||||||||
| Research and development | 61,837 | 5,487 | 464 | D | (11,143) | $ | 56,645 | ||||||||||||||||||||||
| Selling and marketing | 103,116 | 7,701 | 1,857 | D, E | (66,751) | $ | 45,923 | ||||||||||||||||||||||
| General and administrative | 162,681 | 22,040 | 4,938 | D, E | (24,290) | $ | 165,369 | ||||||||||||||||||||||
| Related party expenses | 4,712 | — | — | (805) | $ | 3,907 | |||||||||||||||||||||||
| Amortization of intangible assets | — | 5,604 | (5,604) | E | — | $ | — | ||||||||||||||||||||||
| Asset impairment charges | — | 5,121 | (5,121) | F | — | $ | — | ||||||||||||||||||||||
| Loss from operations | (342,770) | (31,764) | 2,426 | 143,007 | (229,101) | ||||||||||||||||||||||||
| Other income (expense): | |||||||||||||||||||||||||||||
| Change in fair market value of warrant and earn-out contingent liabilities | 54,350 | — | — | — | $ | 54,350 | |||||||||||||||||||||||
| Interest income | 1,405 | — | — | — | $ | 1,405 | |||||||||||||||||||||||
| Interest expense | (2,404) | — | �� | — | $ | (2,404) | |||||||||||||||||||||||
| Other income (expense), net | 58 | (3) | 3 | (3,653) | $ | (3,595) | |||||||||||||||||||||||
| Total other income (expense), net | 53,409 | (3) | 3 | (3,653) | 49,756 | ||||||||||||||||||||||||
| Net loss before income taxes | (289,361) | (31,767) | 2,429 | 139,354 | (179,345) | ||||||||||||||||||||||||
| Provision or benefit for income taxes | 49,142 | 11,806 | (60,948) | G | $ | — | |||||||||||||||||||||||
| Net loss | $ | (240,219) | $ | (19,961) | $ | (58,519) | $ | 139,354 | $ | (179,345) | |||||||||||||||||||
| Weighted average shares outstanding, basic and diluted | 321,461,266 | — | 56,190,476 | — | 377,651,742 | ||||||||||||||||||||||||
| Basic and diluted net loss per share | $ | (0.75) | $ | — | $ | — | $ | (0.47) | |||||||||||||||||||||
___________________
(A)Amounts represent four months operating results of Legacy GeneDx during the pre-Acquisition period.
(B)Amounts represent acquisition proforma adjustments to give effect to the completed Acquisition as if it had been consummated on January 1, 2021.
(C)Amount relates to intercompany revenue elimination for Legacy GeneDx revenue earned for services performed for BioReference Laboratories, Inc prior to the closing of the Acquisition.
(D)Adjustments relate to stock-based compensation expense for inducement awards granted to GeneDx employees. On May 2, 2022, the compensation committee of the Company’s board of directors granted newly-hired Legacy GeneDx employees inducement stock options to purchase an aggregate of 4,932,132 shares of the Company’s Class A common stock and 4,285,208 RSUs as inducements material to each employee entering into employment with the Company. The stock options have an exercise price of $2.20 per share, which was equal to the closing price of the Company’s Class A common stock on the grant date. The stock options and RSUs granted to the newly-hired employees other than the Company’s Chief Executive Officer, Chief Growth Officer, and Chief Financial Officer (formerly, the Senior Vice President, Operations) will vest with respect to 25% of the underlying shares on April 29, 2023, and will vest with respect to the remaining underlying shares in equal quarterly installments thereafter through April 29, 2026, in each case subject to the new employee’s continued service with the Company. The stock options and RSUs granted to the Company’s Chief Executive Officer, Chief Growth Officer, and Chief Financial Officer will vest with respect to 25% of the underlying shares on April 29, 2023 and 25% of the underlying shares on April 29, 2024, and will vest with respect to the remaining underlying shares in equal quarterly installments thereafter through April 29, 2026, in each case subject to his or her continued service with the Company. Each stock option has a 10-year term. The stock options and RSUs are subject to the terms and conditions identical to those of the Company’s 2021 Equity Incentive Plan and the form of stock option agreement or RSU agreement under such plan, as applicable, covering the grant. The adjustment includes elimination of $3.9 million in legacy stock-based compensation expense and recording of $7.0 million based on the inducement awards as discussed above.
(E)Pre-Acquisition period amortization expense related to the legacy intangible assets was eliminated and replaced with revised amortization expense based on the fair value of intangible assets recorded in connection with the Acquisition. A total of $196 million of intangible assets were recorded based on the Company’s preliminary purchase accounting assessments. This balance consists of tradenames and trademarks, developed technology and customer relationships of $50 million, $48 million and $98 million, respectively. Further details of the intangible assets are disclosed in the Company’s Quarterly Reports on Form 10-Q for the second and third quarters ended in 2022, which are incorporated by reference into this prospectus supplement. Related amortization expense for customer relationships of $1.6 million is recorded as an adjustment in selling and marketing and amortization expense for tradenames and trademarks and developed technology of
S-23
$3.1 million in total is recorded as an adjustment in general and administrative. The adjustment was offset by elimination of the legacy intangible asset amortization of $5.6 million.
(F)The Legacy GeneDx recorded a goodwill impairment charge of $5.1 million based on the difference between the carrying value of the business and its estimated fair value based on the fair value of consideration expected to be paid. The Company deemed the adjustment appropriate as the Company does not carry over the legacy goodwill balance recorded. Further details of the goodwill balance are disclosed in the Company’s Quarterly Reports on Form 10-Q for the second and third quarters ended in 2022, which are incorporated by reference into this prospectus supplement.
(G)Adjustment relates to elimination of the income tax benefit of $49.1 million for the Company because the acquisition is assumed to have occurred on January 1, 2021 and the tax benefit would be realized in the year of acquisition assumed. Legacy GeneDx tax benefit of $11.8 million was also eliminated as the benefit did not transfer over from OPKO.
(H)Adjustments in this column relate to revenue and expenses that are specifically attributable to operations of reproductive and women’s health testing, COVID-19 and somatic testing businesses that have been exited. Any corporate overhead is excluded from the adjustments as it is not specifically attributable to the exited activities.
S-24
UNAUDITED PRO FORMA COMBINED STATEMENT OF OPERATIONS
FOR THE YEAR ENDED DECEMBER 31, 2021
(in thousands, except share and per share amounts)
| Historical | Pro forma | ||||||||||||||||||||||||||||
| Company | Legacy GeneDx | Acquisition Adjustments (A) | Exited Activities Adjustments (F) | Pro Forma Statement of Operations | |||||||||||||||||||||||||
| Revenue: | |||||||||||||||||||||||||||||
| Diagnostic test revenue | $ | 205,100 | $ | 116,595 | $ | (2,070) | B | $ | (204,277) | $ | 115,348 | ||||||||||||||||||
| Other revenue | 7,095 | — | — | — | $ | 7,095 | |||||||||||||||||||||||
| Total revenue | 212,195 | 116,595 | (2,070) | (204,277) | 122,443 | ||||||||||||||||||||||||
| Cost of services | 228,797 | 84,361 | 1,204 | C | (219,685) | $ | 94,677 | ||||||||||||||||||||||
| Total gross profit (loss) | (16,602) | 32,234 | (3,274) | 15,408 | 27,766 | ||||||||||||||||||||||||
| Operating expenses: | |||||||||||||||||||||||||||||
| Research and development | 105,162 | 12,377 | 1,223 | C | (13,354) | $ | 105,408 | ||||||||||||||||||||||
| Selling and marketing | 112,738 | 12,145 | 5,443 | C, D | (92,733) | $ | 37,593 | ||||||||||||||||||||||
| General and administrative | 205,988 | 40,294 | 13,732 | C, D | (7,384) | $ | 252,630 | ||||||||||||||||||||||
| Related party expenses | 5,659 | — | — | (416) | $ | 5,243 | |||||||||||||||||||||||
| Amortization of intangible assets | — | 16,813 | (16,813) | D | — | $ | — | ||||||||||||||||||||||
| Loss from operations | (446,149) | (49,395) | (6,859) | 129,295 | (373,108) | ||||||||||||||||||||||||
| Other income (expense): | |||||||||||||||||||||||||||||
| Change in fair market value of warrant and earn-out contingent liabilities | 198,401 | — | — | — | $ | 198,401 | |||||||||||||||||||||||
| Interest income | 79 | — | — | — | $ | 79 | |||||||||||||||||||||||
| Interest expense | (2,835) | — | — | — | $ | (2,835) | |||||||||||||||||||||||
| Other income (expense), net | 5,114 | (44) | 40 | (1,833) | $ | 3,277 | |||||||||||||||||||||||
| Total other income (expense), net | 200,759 | (44) | 40 | (1,833) | 198,922 | ||||||||||||||||||||||||
| Net loss before income taxes | (245,390) | (49,439) | (6,819) | 127,462 | (174,186) | ||||||||||||||||||||||||
| Provision or benefit for income taxes | — | 12,547 | 36,595 | E | — | $ | 49,142 | ||||||||||||||||||||||
| Net loss | $ | (245,390) | $ | (36,892) | $ | 29,776 | $ | 127,462 | $ | (125,044) | |||||||||||||||||||
| Weighted average shares outstanding, basic and diluted | 108,077,439 | — | 130,000,000 | — | 238,077,439 | ||||||||||||||||||||||||
| Basic and diluted net loss per share | $ | (2.27) | $ | — | $ | — | $ | (0.53) | |||||||||||||||||||||
__________________
(A)Amounts represent acquisition proforma adjustments to give effect to the completed Acquisition as if it had been consummated on January 1, 2021.
(B)Amount relates to intercompany revenue elimination for Legacy GeneDx revenue earned for services performed for BioReference Laboratories, Inc prior to the closing of the Acquisition.
(C)Adjustments relate to stock-based compensation expense for inducement awards granted to GeneDx employees. On May 2, 2022, the compensation committee of the Company’s board of directors granted newly-hired Legacy GeneDx employees inducement stock options to purchase an aggregate of 4,932,132 shares of the Company’s Class A common stock and 4,285,208 RSUs as inducements material to each employee entering into employment with the Company. The stock options have an exercise price of $2.20 per share, which was equal to the closing price of the Company’s Class A common stock on the grant date. The stock options and RSUs granted to the newly-hired employees other than the Company’s Chief Executive Officer, Chief Growth Officer, and Chief Financial Officer (formerly, the Senior Vice President, Operations) will vest with respect to 25% of the underlying shares on April 29, 2023, and will vest with respect to the remaining underlying shares in equal quarterly installments thereafter through April 29, 2026, in each case subject to the new employee’s continued service with the Company. The stock options and RSUs granted to the Company’s Chief Executive Officer, Chief Growth Officer, and Chief Financial Officer will vest with respect to 25% of the underlying shares on April 29, 2023 and 25% of the underlying shares on April 29, 2024, and will vest with respect to the remaining underlying shares in equal quarterly installments thereafter through April 29, 2026, in each case subject to his or her continued service with the Company. Each stock option has a 10-year term. The stock options and RSUs are subject to the terms and conditions identical to those of the Company's 2021 Equity Incentive Plan and the form of stock option agreement or RSU agreement under such plan, as applicable, covering the grant. The adjustment includes elimination of $1.8 million in legacy stock-based compensation expense and recording of $9.4 million based on the inducement awards as discussed above.
(D)Pre-Acquisition period amortization expense related to the legacy intangible assets was eliminated and replaced with revised amortization expense based on the fair value of intangible assets recorded in connection with the Acquisition. A total of $196 million of intangible assets were recorded based on the Company’s preliminary purchase accounting assessments. This balance consists of tradenames and trademarks, developed technology and customer relationships of $50 million, $48 million and $98 million, respectively. Further details of the intangible assets are disclosed in the Company’s Quarterly Reports on Form 10-Q for the second and third quarters ended in 2022, which are incorporated by reference into this prospectus supplement. Related amortization expense for customer relationships of $4.9 million is recorded as an adjustment in selling and marketing and amortization expense for tradenames and trademarks and developed technology of $7.7 million in total is recorded as an adjustment in general and administrative. The adjustment was offset by elimination of the legacy intangible asset amortization of $16.8 million.
S-25
(E)Adjustment relates to addition of the income tax benefit of $49.1 million for the Company because the acquisition is assumed to have occurred on January 1, 2021. Legacy GeneDx tax benefit of $12.5 million was also eliminated as we note OPKO to absorb this benefit.
(F)Adjustments in this column relate to revenue and expenses that are specifically attributable to operations of reproductive and women’s health testing, COVID-19 and somatic testing businesses that have been exited. Any corporate overhead is excluded from the adjustments as it is not specifically attributable to the exited activities.
S-26
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain matters discussed in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein may constitute forward-looking statements for purposes of the Securities Act, and the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from the future results, performance or achievements expressed or implied by such forward-looking statements. The words “anticipate,” “believe,” “estimate,” “may,” “expect” and similar expressions are generally intended to identify forward-looking statements. Our actual results may differ materially from the results anticipated in these forward-looking statements due to a variety of factors, including, without limitation, those discussed in the section entitled “Risk Factors,” and elsewhere in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein, where such forward-looking statements appear. All written or oral forward-looking statements attributable to us are expressly qualified in their entirety by these cautionary statements. Such forward-looking statements include, but are not limited to, statements about:
•our expected application of the net proceeds from this offering and the concurrent registered direct offering;
•our estimates of the sufficiency of our existing capital resources combined with future anticipated cash flows to finance our operating requirements;
•our expected losses;
•our expectations for incurring capital expenditures;
•unforeseen circumstances or other disruptions to normal business operations, including supply chain interruptions and manufacturing constraints, arising from or related to the ongoing COVID-19 pandemic;
•our expectations regarding our plans to pursue a new strategic direction, exit our reproductive and women’s health testing business and our ability to scale to profitability, as well as our plans to exit our somatic tumor testing services and the associated cost savings and impact on our gross margins;
•our expectations for generating revenue or becoming profitable on a sustained basis;
•our expectations or ability to enter into service, collaboration and other partnership agreements;
•our expectations or ability to build our own commercial infrastructure to scale market and sell our products;
•actions or authorizations by the U.S. Food and Drug Administration or other regulatory authorities;
•risks related to governmental regulation and other legal obligations, including privacy, data protection, information security, consumer protection, and anti-corruption and anti-bribery;
•our ability to obtain and maintain intellectual property protection for our product candidates;
•our ability to compete against existing and emerging technologies;
•our stock price and its volatility;
•our ability to attract and retain key personnel;
•third-party payor reimbursement and coverage decisions, negotiations and settlements;
•our reliance on third-party laboratories and service providers for our test volume in connection with our diagnostic solutions and data programs;
•our ability to satisfy Nasdaq listing rules;
•our expectations for future capital requirements;
S-27
•our accounting estimates and judgments, including our expectations regarding our ability to continue as a going concern, the adequacy of our reserves for third party payor claims, our estimates of the fair value of the milestone payments related to the Acquisition and our conclusions regarding the appropriateness of the carrying value of intangible assets and goodwill;
•our ability to successfully implement our business strategy; and
•other factors detailed under the section entitled “Risk Factors.”
The forward-looking statements contained in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein and therein reflect our views and assumptions only as of the date of this prospectus supplement, the accompanying prospectus or such document, as applicable. Except as required by law, we assume no responsibility for updating any forward-looking statements.
We qualify all of our forward-looking statements by these cautionary statements. In addition, with respect to all of our forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
S-28
USE OF PROCEEDS
We estimate that our net proceeds from this offering and the concurrent registered direct offering will be approximately $ million after deducting the underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriter’s option to purchase additional shares of Class A common stock is exercised in full, we estimate that we will receive net proceeds of approximately $ .
We currently intend to use the net proceeds from this offering and the concurrent registered direct offering primarily for general corporate purposes, including additions to working capital, repayment or redemption of existing indebtedness, and strategic investment opportunities. We expect the net proceeds from this offering and the concurrent registered direct offering, together with our existing cash, and cash equivalents, to be sufficient to fund our business operations through at least the next 12 months.
The amounts and timing of our actual expenditures will depend on numerous factors, including the factors described under “Risk Factors” in this prospectus supplement, the accompanying prospectus and the documents incorporated by reference herein, as well as the amount of cash used in our operations. As a result, our management will have broad discretion over the uses of the net proceeds from this offering and the concurrent registered direct offering and investors will be relying on the judgment of our management regarding the application of the proceeds. Pending these uses, we intend to invest the net proceeds in short-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or guaranteed obligations of the U.S. government.
The expected use of the net proceeds to us from this offering and the concurrent registered direct offering represents our intentions based upon our current plans and business conditions. As of the date of this prospectus supplement, we cannot predict with any certainty all of the particular uses for the net proceeds or the amounts that we will actually spend on the uses set forth above.
S-29
DESCRIPTION OF THE SECURITIES WE ARE OFFERING
We are offering shares of our Class A common stock. The material terms and provisions of our Class A common stock and each other class of our securities which qualifies or limits our Class A common stock are described under the caption “Description of Our Capital Stock” starting on page 13 in the accompanying prospectus.
S-30
DIVIDEND POLICY
We have never declared or paid cash dividends on our capital stock. We intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to dividend policy will be made at the discretion of our board of directors, subject to applicable laws, and will depend on our financial condition, results of operations, capital requirements, restrictions in the agreements governing any indebtedness we may enter into, general business conditions and other factors that our board of directors considers relevant.
S-31
CAPITALIZATION
The following table sets forth our cash and cash equivalents and capitalization as of September 30, 2022:
•on an actual basis; and
•on an as adjusted basis giving effect to the sale and issuance by us of shares of Class A common stock in this offering and the concurrent registered direct offering, at the public offering price of $ per share, after deducting the underwriting discount and commissions, and estimated offering expenses payable by us.
We can offer no assurance that the concurrent registered direct offering will close, and if it does not close, the amount of our net proceeds will be limited to the net proceeds from this offering.
As of September 30, 2022
| (amounts in dollars and in thousands, except share and per share amounts) | Actual | As Adjusted | |||||||||
| Cash and cash equivalents | $ | 191,360 | |||||||||
Debt | |||||||||||
| Long-term debt, net of current portion | 10,651 | ||||||||||
| Stockholders’ equity (deficit): | |||||||||||
| Preferred stock, $0.0001 par value, 1,000,000 shares authorized: | — | ||||||||||
| Class A common stock, $0.0001 par value, 1,000,000,000 shares authorized; 381,428,905 shares issued, actual; shares issued, as adjusted | 38 | ||||||||||
| Additional paid-in capital | 1,376,916 | ||||||||||
| Accumulated deficit | (815,660) | ||||||||||
| Total stockholders’ equity | 561,294 | ||||||||||
Total capitalization | $ | 763,305 | |||||||||
The above discussion and table are based on 381,428,905 shares of Class A common stock outstanding as of September 30, 2022 and excludes the following securities:
•21,994,972 shares of Class A common stock issuable upon exercise of warrants outstanding as of September 30, 2022 with an exercise price of $11.50 per share;
•32,354,294 shares of Class A common stock issuable upon exercise of options outstanding as of September 30, 2022 with a weighted average exercise price of $1.47 per share;
•23,871,012 shares of common stock issuable upon vesting and settlement of RSUs (excluding the Earn-Out RSUs) outstanding as of September 30, 2022;
•up to 19,021,576 shares of Class A common stock that may be issuable (1) to certain former equity holders of Legacy Sema4 upon the achievement of certain vesting conditions and (2) upon the vesting and settlement of the Earn-Out RSUs that were granted to certain former equity award holders of Legacy Sema4 and certain employees of the Company;
•up to 30,864,198 shares of our Class A common stock that may be issuable to OPKO in connection with the achievement of certain revenue-based milestones for each of the fiscal years ending December 31, 2022 and December 31, 2023; and
•22,111,381 shares of Class A common stock reserved and available for future issuance as of September 30, 2022, under our equity incentive plan and employee stock purchase plan, consisting of (1) 14,881,582 shares of Class A common stock reserved and available for issuance under our 2021 Equity Incentive Plan as of September 30, 2022, and (2) 7,229,799 shares of Class A common stock reserved for issuance under our 2021 Employee Stock Purchase Plan as of September 30, 2022.
S-32
DILUTION
If you invest in our Class A common stock, your interest will be diluted to the extent of the difference between the price per share you pay in this offering and the net tangible book value per share of our Class A common stock immediately after this offering and the concurrent registered direct offering. Our net tangible book value of our Class A common stock as of September 30, 2022 was approximately $144.7 million, or approximately $0.38 per share of Class A common stock based upon 381,428,905 shares outstanding as of September 30, 2022. Net tangible book value per share is equal to our total tangible assets, less our total liabilities, divided by the total number of shares outstanding as of September 30, 2022.
After giving effect to the sale of our Class A common stock in this offering and the concurrent registered direct offering at the public offering price of $ per share and after deducting commissions and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2022 would have been approximately $ , or $ per share of Class A common stock. This represents an immediate increase in net tangible book value of $ per share of Class A common stock to our existing stockholders and an immediate dilution in net tangible book value of $ per share of Class A common stock to new investors purchasing Class A common stock in this offering at the public offering price.
The following table illustrates this calculation on a per share basis. The as-adjusted information is illustrative only and will adjust based on the actual price to the public, the actual number of shares sold and other terms of the offering and the concurrent registered direct offering determined at the time shares of our Class A common stock are sold pursuant to this prospectus supplement and the accompanying prospectus.
| Assumed public offering price per share | $ | ||||||||||
| Net tangible book value per share as of September 30, 2022 | $ | 0.38 | |||||||||
| Increase in net tangible book value per share attributable to the offering and the concurrent registered direct offering | |||||||||||
| As adjusted net tangible book value per share after giving effect to the offering and the concurrent registered direct offering | |||||||||||
| Dilution per share to new investors participating in the offering and the concurrent registered direct offering | $ | ||||||||||
If the underwriter exercises in full its option to purchase an additional shares of Class A common stock at the public offering price of $ per share, our as adjusted net tangible book value per share as of September 30, 2022 would have been $ per share, representing an immediate increase in net tangible book value of $ per share of Class A common stock to our existing stockholders and an immediate dilution in net tangible book value of $ per share of Class A common stock to new investors purchasing shares Class A common stock in this offering.
The number of shares of our Class A common stock shown above to be outstanding after this offering and the concurrent registered direct offering is based on 381,428,905 shares of our Class A common stock outstanding as of September 30, 2022, and excludes:
•21,994,972 shares of Class A common stock issuable upon exercise of warrants outstanding as of September 30, 2022 with an exercise price of $11.50 per share;
•32,354,294 shares of Class A common stock issuable upon exercise of options outstanding as of September 30, 2022 with a weighted average exercise price of $l.47 per share;
•23,871,012 shares of common stock issuable upon vesting and settlement of RSUs (excluding the Earn-Out RSUs) outstanding as of September 30;
•up to 19,021,576 shares of Class A common stock that may be issuable (1) to certain former equity holders of Legacy Sema4 upon the achievement of certain vesting conditions and (2) upon the vesting and settlement of the Earn-Out RSUs that were granted to certain former equity award holders of Legacy Sema4 and certain employees of the Company;
S-33
•up to 30,864,198 shares of our Class A common stock that may be issuable to OPKO in connection with the achievement of certain revenue-based milestones for each of the fiscal years ending December 31, 2022 and December 31, 2023; and
•22,111,381 shares of Class A common stock reserved and available for future issuance as of September 30, 2022, under our equity incentive plan and employee stock purchase plan, consisting of (1) 14,881,582 shares of Class A common stock reserved and available for issuance under our 2021 Equity Incentive Plan as of September 30, 2022, and (2) 7,229,799 shares of Class A common stock reserved for issuance under our 2021 Employee Stock Purchase Plan as of September 30, 2022.
S-34
BUSINESS
We are pursuing a new strategic direction focused on our exome and genome sequencing business coupled with our Centrellis® data platform. We are in the process of exiting the reproductive and women’s health testing business, which we expect to complete by the end of the first quarter of 2023, and we also completed the exit of the somatic tumor testing business during the fourth quarter of 2022. For more information, see “Prospectus Supplement Summary—Recent Developments—Legacy Sema4 Business Exits.” Unless the context otherwise requires, the description of our business and operations below assumes the completion of the exits from the somatic tumor testing and the reproductive and women’s health testing businesses.
Purpose
We operate with conviction that what is best for patients must be embedded in every aspect of our work. At GeneDx, we believe:
–genomic information has broad utility and every person should have access to their genome—delivered expertly, ethically and responsibly—to guide health decisions throughout life;
–exome and Whole Genome Sequencing (“WGS”) will facilitate a transition from hypothesis-based to genome-guided healthcare which will improve outcomes for patients and healthcare systems that benefit society as a whole;
–the ability to curate and combine genomic information with clinical and electronic medical record (“EMR”) data will transform therapeutic development, bringing better therapies to patients, faster; and;
–patients should control and have the ability to direct the use of their genomic information to both benefit themselves and advance scientific understanding that helps others.
In support of these beliefs, we value equitability, simplicity and transparency. Through this value system, we aim to deliver personalized and actionable health insights to inform diagnosis, direct treatment and improve drug discovery, bringing better health from genomics to patients around the world.
Overview
GeneDx is focused on delivering personalized and actionable health insights to inform diagnosis, direct treatment and improve drug discovery. We sit at the intersection of diagnostics and data science, pairing decades of genomic expertise with an ability to interpret clinical data at scale. We believe we are well-positioned to accelerate the use of genomics and leverage large-scale clinical data to enable precision medicine as the standard of care. Our initial focus is in pediatric and rare diseases, two areas in which we believe we have competitive advantage and can deliver on our vision today.
GeneDx was founded in 2000 by scientists from the National Institutes of Health whose mission was making genetic testing accessible for patients with rare diseases. The company quickly became a leader in genomics, creating the foundation for how to provide genomic information at scale and pioneering exome and genome sequencing for rare and ultra-rare genetic pediatric disorders. More than 20 years later, we have amassed one of the world’s largest rare disease data sets and remain a leader in genomics. In May 2022, GeneDx integrated with Sema4 Holdings, adding legacy Sema4’s Centrellis®, a highly innovative health information platform to our portfolio of solutions. Centrellis® integrates digital tools and artificial intelligence, allowing our scientists to ingest and synthesize clinical and genomic data to deliver better, more comprehensive health insights.
S-35
Today, we are powered by our industry-leading genomic interpretation platform and Centrellis®. We believe exome and genome testing will become the standard for diagnosis of genetic disease, with the potential to transform healthcare and improve patients’ quality of life for generations by sequencing once and analyzing for a lifetime.
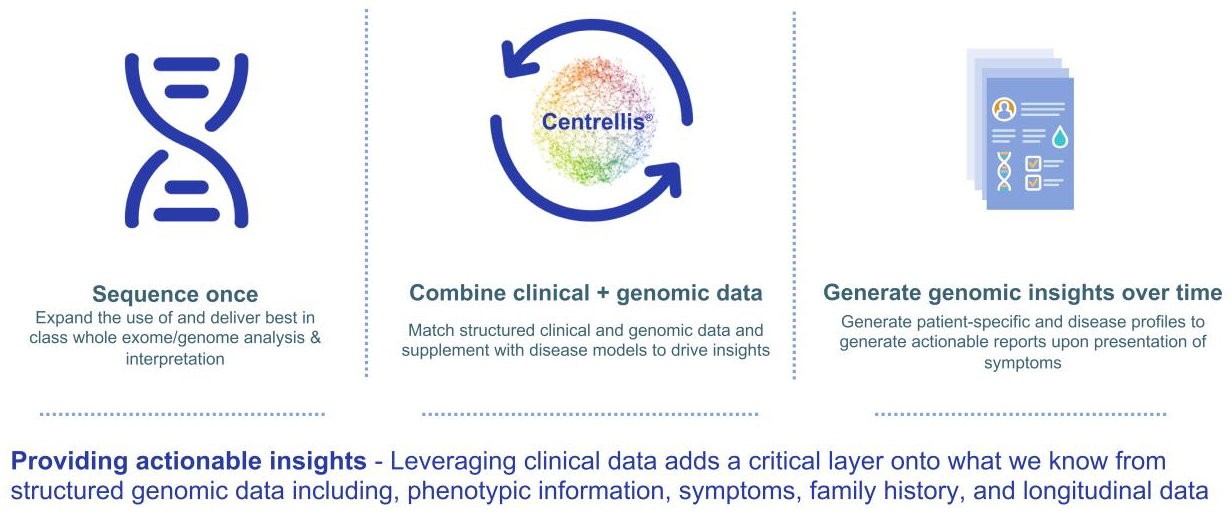
Industry Background
Targeted genetic tests and panel testing make up the vast majority of diagnostics tests ordered today. While panel testing can be immensely valuable, it has an increasing limitation as we move towards genetic-based healthcare. Panels only allow you to test for insights that physicians predefine based on symptoms, which can lead to inconclusive results and an inefficient process. It is hypothesis-based medicine based on symptoms that may overlap across diseases. We firmly believe that an affordable, scalable and actionable genome is the future of medicine. The barrier to having actionable information from a genomic sequence is significant—and not just due to costs, which are coming down. The less-discussed barrier to having actionable information lies in the ability to process a genome’s worth of information—quickly and scalably—and to deliver both a result that a clinician can easily act upon to help a patient, and a robust dataset that enables clinicians to drive precise diagnosis and researchers to develop and advance therapeutics.
Most companies in today’s genetics industry are taking a test-by-test approach to cross the chasm from genetics early adopters to genome guided healthcare in the mainstream market. We believe that driving clinician and patient awareness and influencing policy decisions may facilitate uptake within the industry. In addition, making genetics part of mainstream medicine requires advancing the technology to provide personalized and actionable health insights. It also will require having a robust, well-characterized dataset that can maximize answers and minimize unknowns and that can drive a new era of discovery.
Exome and whole genome sequencing provide the broadest view into the genomic variant—we are looking comprehensively into over 20,000 genes, while panels look at anywhere from two to a few hundred genes. While most companies in the industry have grown through a focus on panels, we have focused on exome and whole genome developing structured gene-disease knowledge curated by our team of experts to power automated interpretation and reporting.
One Test
The genome is composed of 3 billion “letters”, or base pairs, of DNA. The exome is a portion of the genome that encodes proteins, which are involved in many different types of cellular functions. Changes in the genome and exome can change the way proteins are formed or are utilized by the cell, potentially causing disease.
When patients present with complex issues, a genetic diagnosis may be available, but a traditional genetic panel test may be too narrow to identify the cause. Some genetic disorders present with very specific symptoms, so tests
S-36
that read the “letters” of a single gene or a small panel of genes, may make sense for physicians to use in diagnosis. But for many other genetic diseases, patients can present with overlapping symptoms and so finding the correct diagnosis is not always straightforward and may require multiple tests, costly evaluations, invasive procedures, and long hospital stays. Exome and genome sequencing can find different genetic alterations, or variants, that more targeted tests miss and are especially useful when the timing is critical to directing or altering medical management.
With over 20 years of operation, GeneDx has a proven track record of expertise in genetic testing. We launched the industry’s first commercially available next generation sequencing panels in 2008, pioneered exome sequencing in 2012 and have sequenced over 400,000 exomes to date. We have performed over a million genetic tests and worked tirelessly to develop the following:
•A curated database of disease-associated genomic variants.
•Proprietary bioinformatics and variant interpretation pipelines.
•Rapid exome and whole genome sequencing testing options.
The status quo of genetic testing requires repeated and fragmented testing, which in many cases, is conducted too late for physicians to use in treatment of patients. Targeted genetic tests and panels have been largely commoditized leaving physicians, healthcare partners and patients searching for deeper answers and enhanced utility. The scalable exome and whole genome interpretation that we can deliver at speed do not require a long, complex, expensive, expert-guided search and may make most other genetic tests obsolete. In addition, using whole genome testing is incredibly simple: it’s designed to be Just One Test.
Advanced Technology with a Human Touch
Our team includes approximately 250 genetic counselors, physicians, scientists, and clinical and molecular genomics specialists. We believe we are one of the industry’s leading genetic testing experts. We share the same goal as healthcare providers, patients, and families: to provide personalized and actionable health insights.
Our years of exome and genome sequencing experience have provided us with a substantial dataset, including over 2.7 million structured phenotypes with nearly 60% of all exomes to date processed as parent-child trios. We have invested resources over time to annotate the phenotypes and sequence the parents of patients, because their genetic sequences can often provide additional diagnostic information, potentially improving the precision of genetic analysis. In addition, the data from more families allows us to continually improve interpretation of genetic code and variants that may cause disease. We believe we have more expertly annotated disease-causing variants than the largest public archive.
Internally developed with over one million sequenced specimens, our database is designed to lead to increasingly reliable diagnostic test results. The structured gene-disease knowledge curated by our team of experts is powering automated interpretation and reporting built to handle genomic data at scale. Combined with our proprietary, state-of-the-art variant identification software, our ability to deliver highly accurate test results makes finding definitive diagnoses, even in complex cases, possible. Implemented with expert oversight, our advanced interpretation methods incorporate automation, bioinformatics, and cloud-based machine learning, enabling efficient discovery of genetic differences at previously undetectable levels.
As the number of new patients we test grows, so does our database as new data increases the potential for greater insights. Comparing new cases against the data from previous cases helps to confirm whether a genetic variant is significant. Once new findings are identified, we aim to proactively reach out to healthcare providers and offer to reanalyze their patients’ previous results. Over time, our objective is to fully automate this reanalysis process in a convenient, easy to understand, efficient method.
In this new world of “one test,” people may be able to carry their genomic data—their DNA blueprints—with them throughout their life. GeneDx intends to assist in providing new answers from within, decoding more insights over time. As we capture more genomic and phenotypic data, we hope to fuel a positive feedback cycle of discovery that continuously delivers more value for patients, providers and healthcare partners.
S-37
Delivering Health Insights
Centrellis®, our health information platform, is supported and fueled by genomic information from our diagnostic business and combined with an ever growing population of clinical health records and data. We engage with patients, physicians, health systems and other partners based on principles of transparency, choice, and consent. Driven by our direct engagement with patients and strategic relationships with multiple health systems, the database we have built contains extensive Electronic Medical Record (EMR) data, totaling approximately 3.1 million patient health records and has been designed to enable Centrellis® to draw from its extensive data assets in a way that enables physicians to proactively diagnose and manage disease. Our datasets include over 20 years of records abstracted from approximately 56 million clinical documents, 47 million phenotypes and 8 million disease diagnoses. We expect our current and targeted strategic relationships will provide us with access to additional active patient cohorts and datasets to continue to build our information base and enable our iterative, data-driven business model, including our genomic test solutions franchise.
Market Opportunity
Our primary growth engine in the short term will be expanding our current market-leading exome sequencing capabilities in the Neonatal Intensive Care Units (“NICU”) and Pediatric Developmental Disorder setting, as well as providing interpretation and information services for customers that sequence locally but look to GeneDx for analysis and interpretation, and providing our Centrellis® platform services to biopharmaceutical partners.
We believe we are particularly well-suited for helping rare disease and pediatric developmental disorder patients, their care teams and biopharma companies today. This is a large market with immense unmet medical need. There are nearly 7,000 individual diseases affecting nearly 10% of the total population in the United States, of which 50% are children. As a result, there are over 700 medicines in development for these diseases, with a regulatory pathway facilitated by the Orphan Drug Act of 1983. By providing the precise genetic diagnosis of patients with rare disease, our expertise and technology may provide researchers and biopharma companies with the information needed to develop and commercialize a new treatment for the disease.
Our longer-term growth strategy is the expansion into whole genome testing for Adult Disorders and Newborn Screening, supported with the launch of a new customer experience platform for non-geneticists, patients and caregivers, and evidence generation to establish the clinical and economic benefits of screening.
By unlocking the value of the products, our knowledge base, network of relationships, and expertise, our team is well positioned to lead what we believe is a nearly $30 billion global market opportunity.
S-38
Our Strategy
We believe that the span and depth of our experience and dataset allows us to return more positive findings and thus clinical utility both immediately and over time through reanalysis. Importantly, we believe that we return fewer uncertain findings compared to public data sets, which makes our analysis easier to interpret outside of the medical genetics community.
At the same time, we have improved quality and speed to delivery of exome and genome tests and have significantly lowered exome sequencing costs since 2013. Much of this decline was driven by reduced sequencing costs shared across the industry; however, we have reduced costs in the interpretation layer through accumulating data and experience, and we expect further decline in costs going forward.
Leveraging these capabilities, we aim to be the global market leader in the development and delivery of reliable, actionable, scalable exome and genome sequencing and interpretation and information services. Our strategy focuses on the following objectives:
•Expand the utilization of exome and genome sequencing as the first- or second-tier test over most other genetically targeted tests by leveraging decades of earned trust amongst expert geneticists; and
•Expand the utilization of industry-leading exome and genome sequencing beyond the genetic experts into the non-expert setting, potentially creating a new standard of care which enables faster diagnoses, reduces suffering, and helps healthcare systems save money. In the near term, our principal target markets will be settings with the most vulnerable patients who can benefit the most including, but not limited to, NICU and patients with Pediatric Developmental Disorders.
To achieve these objectives, we plan to:
•Complete the build out of our commercial footprint to nearly 60 field-based sales representatives in 2023, and construct an industry-leading brand, product, marketing, communications and market access platform by leveraging decades of earned trust across the genetics community.
•Partner with leaders across health systems, manufacturers, commercial and governmental payers and advocacy groups. We aim to collaborate on programs to establish definitive clinical and economic case for broad use of genomic-guided medicine. Such programs will focus on:
◦support for rapid whole genome sequencing in the NICU and Pediatric Developmental Disorder settings;
◦diagnosis of disease and prevention of chronic conditions in adults; and
◦use of rapid whole genome sequencing for broad newborn screening.
•Open new markets and geographies and unlock the value of our dataset with independently scalable cloud-based interpretation and information service offerings. This will enable healthcare partners to incorporate genetics into clinical care by accessing our analysis and interpretation capabilities remotely while sequencing locally to reduce complexity, logistics cost and wait times, and align to local restrictions where applicable;
•Launch a new provider and patient experience with the eventual goal of providing lifelong access and portability of genomic information. At initial sequence, rapid results provide clinicians simple, actionable, easy to understand results for non-geneticists and tailored resources for patients and caregivers. On an ongoing basis, reanalysis unlocks a renewable source of insight, replacing any future germline screening. We will sequence once, and analyze for life.
S-39
•Optimize Centrellis® to become a solutions provider of choice for biopharma. Such solutions will focus on three value-added services:
◦FIND: Finding rare disease patients for clinical trial recruitment and/or delivery of targeted therapeutics, eventually moving into other disease areas such as cardiology and oncology.
◦UNDERSTAND: Supporting research and development for targeted therapies with analytic reports leveraging clinicogenomics data across multiple therapeutic areas with an initial emphasis in rare disease and oncology.
◦PLATFORM: In the long term, providing a therapeutic area agnostic platform to access to data, patients and insights for real world evidence and data to support end-to-end drug discovery pipeline.
Research and Development
Our research and development activities include information technology, product development, customer experience, medical affairs, collaborations and research. These activities are principally focused on our efforts to develop and improve the software we use to analyze data, process genomic test orders, deliver reports, and improve customer experience.
We are also participating in several collaborative studies aimed to provide evidence of the clinical and economic benefit for exome and whole genome sequencing. Two such studies currently underway include the SeqFirst study—in collaboration with Seattle Children’s Hospital and University of Washington—which is designed to demonstrate the broad utility of rapid whole genome sequencing for critically ill newborns and, the Genomic Uniform-Screening Against Rare Diseases In All Newborns (“GUARDIAN”) study—in collaboration with New York-Presbyterian, Columbia University, New York State Department of Health and Illumina—which is designed to assess whole genome sequencing to screen newborns for more conditions than those currently included in standard newborn screening in the United States. The goals of these studies are to drive earlier diagnosis and treatment to improve the health of the newborns who participate in such studies, generate evidence to support the expansion of newborn screening through genomic sequencing, and characterize the prevalence and natural history of rare genetic conditions.
Competition
Our competitors include companies that offer molecular genetic testing and consulting services, including specialty and reference laboratories that offer traditional single- and multi-gene tests and biopharmaceutical companies. In addition, there are a large number of new entrants into the market for genetic information ranging from informatics and analysis pipeline developers to focused, integrated providers of genetic tools and services for health and wellness, including Illumina, Inc., which is also one of our suppliers. In addition to the companies that currently offer traditional genetic testing services and research centers, other established and emerging healthcare, information technology and service companies may commercialize competitive products including informatics, analysis, integrated genetic tools and services for health and wellness. Principal competitors include companies such Baylor, Centogene, Exact Sciences, Invitae, Neogenomics, Personalis, Caris and Tempus as well as other commercial and academic labs.
Customers and Seasonality
We receive payment for our products and services from third-party payers, patients, business-to-business clients, and from other healthcare partners. Substantially all of our revenue for the nine-month period ending September 30, 2022 has been primarily derived from diagnostic test reports and we expect this trend to continue in the near-term. In the past 12 months, 94% of pediatric specialists in the United States who order exome testing have ordered from GeneDx. We expect over time to achieve a mix of revenue from diagnostic tests, data and information solutions, newborn screening products and information and interpretation services.
S-40
Less than 5% of our revenues today are derived from referral sources outside of the United States. We expect over time to increase rest of world revenue as knowledge and understanding of the benefits of exome and whole genome sequencing continue to expand.
We have historically experienced higher revenue in our fourth quarter compared to other quarters in our fiscal year due in part to seasonal demand of our tests from patients who have met their annual insurance deductible. However, changes in our product and payer mix might cause these historical seasonal patterns to be different than future patterns of revenue or financial performance.
For information regarding our customer concentration in relation to certain of the Company’s third-party payors, see Note 2, “Summary of Significant Accounting Policies” in the Notes to our Unaudited Condensed Consolidated Financial Statements included in Part I, Item 1 of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2022, which is incorporated by reference in this prospectus supplement and the accompanying prospectus. We expect incrementally less concentration among third-party payors following the exits from reproductive health and somatic tumor testing. There are no single customers representing greater than 10% of our revenues or accounts receivable.
Raw Materials and Suppliers
We rely on a limited number of suppliers, including Illumina, Inc., Integrated DNA Technologies Incorporated, Agilent Technologies, Roche Holdings Ltd., QIAGEN, Inc. and Twist Biosciences, for certain laboratory reagents, as well as sequencers and other equipment and materials, which we use in our laboratory operations. Our operations could be interrupted if we encounter delays or difficulties in securing reagents, sequencers or other equipment or materials, and if we cannot obtain an acceptable substitute. Any such interruption could significantly affect our business, financial condition, results of operations and reputation. We believe that there are only a few other manufacturers that are currently capable of supplying and servicing the equipment necessary for our operations, including sequencers and various associated reagents and enzymes. The use of equipment or materials provided by these replacement suppliers would require us to alter our operations. Transitioning to a new supplier would be time consuming and expensive, may result in interruptions in operations, could affect the performance specifications of our laboratory operations or could require that we revalidate our tests. We cannot be certain that we will be able to secure alternative equipment, reagents and other materials, or bring such equipment, reagents and materials on line and revalidate them without experiencing interruptions in our workflow. If we encounter delays or difficulties in securing, reconfiguring or revalidating equipment and materials, our business and reputation could be adversely affected.
Intellectual Property
We rely on a combination of intellectual property rights, including trade secrets, copyrights, trademarks, customary contractual protections to protect our core technology and intellectual property.
Patents
The fields of genomic and health information analysis present limited opportunities for patent protection, based on current legal precedents. Our patent protection strategy has focused on seeking protection for certain of our non-gene specific technology and our specific biomarkers. In this regard, as of January 23, 2023, we have six pending non-provisional utility patent applications and one provisional patent application. The utility patent applications include a U.S. patent application related to a genome annotation software platform for annotating genomic intervals that are clinically relevant for analysis, a U.S. patent application related to a genetic carrier screening process, and U.S. and European patent applications related to therapeutic treatment for subjects having certain polymorphic markers associated with specific human leukocyte antigen alleles. If patents are issued from the currently pending applications, the earliest patents will begin expiring in 2040, subject to potential extensions of the patent term that will be calculated based on the length of the patent examination process. The claim scope of any potentially issued
S-41
patents stemming from the present applications may be narrowed from initial filings due to any amendments that may arise throughout their prosecution.
We do not presently have any patents or patent applications directed to the sequences of specific genes or variants of such genes, nor do we currently rely on any in-licensed gene patent rights of any third party. We may in time seek additional patent protection to protect technology that is not gene-specific and that provides us with a potential competitive advantage as we focus on making comprehensive genetic information less expensive and more broadly available to our customers.
Trade secrets
We rely on trade secrets, including unpatented know-how, technology and other proprietary information, to maintain and develop our competitive position. We have a trade secrecy program to prevent disclosure of our trade secrets to others, except under stringent conditions of confidentiality when disclosure is critical to our business. We attempt to protect trade secrets and know-how by establishing confidentiality agreements and invention assignment agreements with our employees, consultants, scientific advisors, contractors, and collaborators. These agreements also provide that all inventions resulting from work performed for us or relating to our business and conceived or completed during the period of employment or assignment, as applicable, will be our exclusive property. In addition, we take other appropriate precautions, such as physical and technological security measures, to guard against misappropriation of our proprietary information by third parties.
Our valuable trade secrets relate to proprietary bioinformatic tools such as:
•Custom data processing methods and analytical pipelines for NGS, aCGH, MLPA, Sanger, and other genomic data, optimized and validated to the highest performance standards;
•a novel detection method to uncover notoriously difficult to detect sequence variants called mobile element insertions and partial-exon deletions; and
•Custom variant analysis platforms built from the ground up for exome and genome-scale data interpretation.
Although we take steps to protect our proprietary information and trade secrets, including through contractual means with our employees and consultants, these steps may be circumvented or third parties may independently develop substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets or disclose our technology. Accordingly, we may not be able to meaningfully protect our trade secrets.
Trademarks
We own or are applying for various trademarks, service marks, trade names, and product service names in the U.S and other commercially important markets. We intend to invest significant resources in the growth and protection of our reputation and trademarks.
Human Capital Resources
Our mission
We have and will continue to attract and retain a team that recognizes top talent, values diverse experiences and demographics, shares an appreciation for servant-based leadership and, enables the ability for all individuals to make a difference for patients and our organization. We are building a team obsessed with the belief that GeneDx will change the world - advancing our mission to bring broad access and utility of genomics to every day medicine to help patients across the globe. We had approximately 1,100 employees as of January 20, 2023, of which approximately 66% are women and 33% men.
S-42
Our employee engagement and culture
We have established a Justice, Equity, Diversity and Inclusion, or JEDI roadmap, designed to engage, develop and retain talent from diverse backgrounds by providing education and support. We are committed to an environment of inclusiveness internally and a pursuit to close gaps in health disparities.
We are committed to maintaining and improving the health and safety of our employees at our Connecticut headquarters and our recently renovated laboratory and office space in Maryland, as well providing support for our teammates working remotely across and outside of the United States.
We empower our employees to own their career path and seek out training programs to take them to the next level. We are currently in the process of developing a structure of growth opportunities and ways to understand and communicate pathways and are investing in our training and development programs and infrastructure for our employees.
We have organized and measure progress around shared company-wide objectives and key results to enable prioritization, crisper decision-making and data-driven evaluation of our progress. Team level objectives and key results tied to our company goals allow us each to see how our individual contributions add to the overall success of the Company and ensure that we are collectively all pulling in one direction and measuring our progress beyond simply revenue or volume.
Properties
Properties for our core operations include our corporate office and headquarters located at 333 Ludlow Street, Stamford, Connecticut 06902, our primary operating laboratory located at 207 Perry Parkway, Gaithersburg, Maryland 20877, and a satellite meeting space located at 200 Park Avenue South, New York, NY 10002; each are leased spaces. Our laboratory in Stamford, CT will fully cease operations in March 2023 and our laboratory in Branford, CT has already ceased operations as part of the Company’s recently announced exits from reproductive health and somatic tumor testing. These facilities are actively being marketed for sublet; however, the outstanding lease obligations remain obligations of the Company.
Government Regulation
Our business and the services we provide are subject to and impacted by extensive and frequently changing laws and regulations in the United States (at both the federal and state levels) and internationally. Failure to comply with the applicable laws and regulations can subject us to repayment of amounts previously paid to us, significant civil and criminal penalties, loss of licensure, certification, or accreditation, or exclusion from state and federal health care programs. The significant areas of regulation are summarized below:
Clinical Laboratory Improvement Amendments of 1988 and State Regulation
Our clinical laboratories must hold certain federal, state and local licenses, certifications and permits to conduct our business. Laboratories in the United States that perform testing on human specimens for the purpose of providing information for the diagnosis, prevention, or treatment of disease or impairment, or the assessment of health are subject to the Clinical Laboratory Improvement Amendments of 1988, as amended, and its implementing regulations (“CLIA”). CLIA requires such laboratories to be certified by the federal government and mandates compliance with various operational, personnel, facilities administration, inspections, quality control, quality assessment and proficiency testing requirements intended to ensure that testing services are accurate, reliable and timely. CLIA certification also is a prerequisite to be eligible to bill state and federal health care programs, as well as many commercial third-party payers, for laboratory testing services. Our laboratory located in Gaithersburg, Maryland is CLIA certified to perform high complexity tests. Our laboratory located in Stamford, Connecticut is also CLIA certified to perform high complexity tests. Laboratories performing high complexity testing are required to meet more stringent requirements than laboratories performing less complex tests. The regulatory and compliance standards applicable to the testing we perform may change over time, and any such changes could have a material effect on our business.
S-43
As a condition of CLIA certification, our laboratory is subject to survey and inspection every two years to assess compliance with program standards, in addition to being subject to additional random inspections. The biennial survey is conducted by the Centers for Medicare & Medicaid Services (“CMS”), a CMS agent (typically a state agency), or, a CMS-approved accreditation organization. Our Gaithersburg and Stamford laboratories have been accredited by the College of American Pathologists (“CAP”), which means that our laboratories have been certified as following CAP guidelines in operating the laboratory and in performing tests that ensure the quality of our results. Because our laboratories are accredited by CAP, which is a CMS-approved accreditation organization, CMS does not perform these biennial surveys and inspections and relies on our CAP surveys and inspections. We may also be subject to additional unannounced inspections.
CLIA provides that a state may adopt laboratory regulations that are not inconsistent with those under federal law, and a number of states have implemented their own (sometimes more stringent) laboratory regulatory requirements. CLIA does not preempt state laws that have established laboratory quality standards that are at least as stringent as the federal law requirements under CLIA. State laws may require that nonresident laboratories, or out-of-state laboratories, maintain a laboratory license to perform tests on samples from patients who reside in that state. As a condition of state licensure, these state laws may require that laboratory personnel meet certain qualifications, specify certain quality control procedures or facility requirements, or prescribe record maintenance requirements. We maintain state laboratory licenses for our Gaithersburg and Stamford facilities in Maryland, New York, California, Pennsylvania and Rhode Island; our Stamford laboratory also maintains a Connecticut clinical laboratory permit. In addition to having laboratory licenses in New York, our laboratories are also required to obtain approval on a test-specific basis for the tests they run as laboratory developed tests (“LDTs”) by the New York Department of Health before specific testing is performed on samples from New York. If any states currently have or adopt similar licensure requirements in the future, we may be required to modify, delay or stop our operations in those states.
If a laboratory is out of compliance with state laws or regulations governing licensed laboratories or with CLIA, penalties may include suspension, limitation or revocation of the license or CLIA certificate, assessment of civil monetary penalties or fines, civil injunctive suit or criminal penalties. Failure to comply with CLIA could also result in a directed plan of correction and state on-site monitoring. Loss of a laboratory’s CLIA certificate or state license may also result in the inability to receive payments from state and federal health care programs as well as private third-party payers. We believe that we are in material compliance with CLIA and all applicable licensing laws and regulations.
CLIA and state laws and regulations, operating together, sometimes limit the ability of laboratories to offer consumer-initiated testing (also known as “direct access testing”). CLIA certified laboratories are permitted to perform testing only upon the order of an “authorized person,” defined as an individual authorized under state law to order tests or receive test results, or both. Many states do not permit persons other than licensed healthcare providers to order tests. We currently do not offer direct access testing and our CLIA tests may only be ordered by authorized healthcare providers.
Diagnostic Products and FDA Oversight of Laboratory Developed Tests
We provide our tests as LDTs. Under FDA’s regulatory framework, in vitro diagnostic devices (IVDs) are a type of medical device, including tests that can be used in the diagnosis or detection of diseases, such as cancer, or other conditions. FDA considers LDTs to be a subset of IVDs that are intended for clinical use and are designed, manufactured, and used within a single laboratory that is certified under CLIA. Such LDT testing is primarily under the purview of CMS and state agencies that provide oversight over clinical laboratory operations. Although the FDA has taken the position that it has statutory authority to assure that medical devices, including certain LDTs, are safe and effective for their intended use, FDA has historically exercised enforcement discretion with respect to most LDTs and has not required laboratories that furnish LDTs to comply with the agency’s requirements for medical devices (e.g., establishment registration, device listing, premarket clearance or approval, quality systems regulations, and post-market controls). In recent years, FDA has stated it intends to end its policy of general enforcement discretion and regulate certain LDTs as medical devices. For example, in 2014, the FDA issued two draft guidance documents that set forth a proposed risk-based regulatory framework that would apply varying levels of FDA oversight to LDTs. These documents have not been finalized to date. Subsequently, in August 2020, the U.S. Department of Health and Human Services – the parent agency for FDA – announced that FDA will not require
S-44
premarket review of LDTs absent notice-and-comment rulemaking, as opposed to through guidance documents and other informal issuances. In November 2021, the Biden Administration rescinded this policy. At this time, it is unclear when, or if, the FDA will finalize its plans to end enforcement discretion, and even then, the new regulatory requirements are expected to be phased-in over time. Nevertheless, the FDA may decide to regulate certain LDTs on a case-by-case basis at any time. Legislative proposals addressing the FDA’s oversight of LDTs have also been introduced in previous Congresses, and we expect that new legislative proposals will be introduced from time to time. For example, versions of the Verifying Accurate Leading-edge IVCT Development (“VALID”) Act have been introduced in Congress several times in recent years, but the VALID Act has not been enacted. The VALID Act, as most recently proposed, would create a new category of medical products separate from medical devices called “in vitro clinical tests,” or IVCTs. As most recently proposed, the VALID Act would modify the Federal Food, Drug, and Cosmetic Act (“FDCA”) and establish a risk-based approach to imposing requirements related to premarket review, quality systems, and labeling requirements on all IVCTs, including LDTs, but a grandfathering provision would create exemptions from certain requirements for certain LDTs offered for clinical use within 45 days of enactment of the bill. The likelihood that Congress will pass such legislation in the future and the extent to which such legislation may affect the FDA’s plans to regulate certain LDTs as medical devices is difficult to predict at this time. If the FDA ultimately regulates certain LDTs as medical devices, whether via final guidance, final regulation, or as instructed by Congress, our tests may be subject to certain additional regulatory requirements. Complying with the FDA’s requirements for medical devices can be expensive, time-consuming, and subject us to significant or unanticipated delays. Insofar as we may be required to obtain premarket clearance or approval to perform or continue performing an LDT, we cannot be sure that we will be able to obtain such authorization. Even if we obtain regulatory clearance or approval where required, such authorization may not be for the intended uses that we believe are commercially attractive or are critical to the commercial success of our tests. As a result, the application of the FDA’s oversight to our tests could materially and adversely affect our business, financial condition, and results of operations.
We will continue to monitor changes to all LDT regulatory policy so as to ensure compliance with the current regulatory scheme. The FDA in the course of enforcing the FDCA may subject a company to various sanctions for violating FDA regulations or provisions of the FDCA, including requiring recalls, issuing Warning Letters, seeking to impose civil money penalties, seizing devices that the agency believes are non-compliant, seeking to enjoin distribution of a specific device, seeking to revoke a clearance or approval, seeking disgorgement of profits and/or seeking to criminally prosecute a company and its officers and other responsible parties.
Additionally, certain of our diagnostic products in development may be subject to regulation by the FDA and similar international health authorities. For these products, we would have an obligation to adhere to the FDA’s current Good Manufacturing Practices (“cGMP”) and diagnostic product regulations, including providing for an establishment and product listing with the FDA. Additionally, we would be subject to periodic FDA inspections, quality control procedures, and other detailed validation procedures. If the FDA finds deficiencies in the validation of our manufacturing and quality control practices, it may impose restrictions on marketing specific products until corrected. Regulation by governmental authorities in the U.S. and other countries may be a significant factor in how we develop, test, produce and market our diagnostic test products.
Companion Diagnostics
For certain of our tests, we may pursue development as in vitro companion diagnostics for use in selecting the patients that may respond to our partners’ pharmaceutical products. Companion diagnostics (including those offered as LDTs) are regulated by the FDA as medical devices. The FDA issued a final guidance document in August 2014 addressing agency policy in relation to in vitro companion diagnostic tests. The guidance explains that for some drugs and therapeutic biologics, the use of a companion diagnostic test is essential for the safe and effective use of the product, such as when the use of a product is limited to a specific patient subpopulation that can be identified by using the test. According to the guidance, the FDA generally requires the therapeutic product and the companion diagnostic to be developed and approved or cleared contemporaneously. In July 2016, the FDA issued a draft guidance intended to assist sponsors of the drug or biological and in vitro companion diagnostic device on issues related to co-development of the products, and in April 2020, the FDA issued final guidance describing considerations for the development and labeling of in vitro companion diagnostic devices to support the indicated uses of multiple drug or biological oncology products.
S-45
Corporate Practice of Medicine
Numerous states prohibit business organizations from practicing medicine or employing or engaging physicians to practice medicine, which prohibitions are generally referred to as the prohibition against the corporate practice of medicine. These laws are intended to prevent interference in the medical decision-making process by anyone who is not a licensed physician. For example, California’s Medical Board has indicated that determining what diagnostic tests are appropriate for a particular condition and taking responsibility for the ultimate overall care of the patient, including providing treatment options available to the patient, would constitute the unlicensed practice of medicine if performed by an unlicensed person. Violation of these corporate practice of medicine prohibitions may result in civil or criminal fines, as well as sanctions imposed against us and/or the professional through licensure proceedings.
Other Regulatory Requirements
We are subject to laws and regulations related to the protection of the environment, the health and safety of employees and the handling, transportation and disposal of regulated medical waste, hazardous waste and biohazardous waste, including chemical, biological agents and compounds, blood and bone marrow samples and other human tissue, and radioactive materials. For example, the U.S. Occupational Safety and Health Administration (“OSHA”) has established extensive requirements relating specifically to workplace safety for healthcare employers in the U.S. For purposes of transportation, some biological materials and laboratory supplies are classified as hazardous materials and are subject to regulation by one or more of the following: the U.S. Department of Transportation, the U.S. Public Health Service, the United States Postal Service, the Office of Foreign Assets Control, and the International Air Transport Association. We generally use third-party vendors to dispose of regulated medical waste, hazardous waste and radioactive materials and contractually require them to comply with applicable laws and regulations. These vendors are licensed or otherwise qualified to handle and dispose of such wastes.
Federal and State Healthcare Fraud & Abuse Laws
Federal and State Physician Self-Referral Prohibitions
We are subject to the federal physician self-referral prohibitions, commonly known as the Stark Law. These restrictions generally prohibit a physician who has (or whose immediate family member has) a financial relationship, such as an ownership or investment interest in or compensation arrangement with us, from making referrals for “designated health services”, including clinical laboratory services, if payment for the services may be made under Medicare. If such a financial relationship exists, referrals are prohibited unless a statutory or regulatory exception applies. The Stark Law also prohibits us from billing for any such prohibited referral. These prohibitions apply regardless of any intent by the parties to induce or reward referrals or the reasons for the financial relationship and the referral. Several Stark Law exceptions are relevant to many common financial relationships involving clinical laboratories and referring physicians and may be relied upon if all of the elements of the applicable exception are satisfied. Penalties for violating the Stark Law include the return of funds received for all prohibited referrals, fines, civil monetary penalties and possible exclusion from federal health care programs. In addition, violations of the Stark Law may also serve as the basis for liability under the federal False Claims Act (“FCA”), which can result in additional civil and criminal penalties. Several states have enacted comparable self-referral laws which may be broader in scope and apply regardless of payer.
Federal and State Anti-Kickback Laws
The federal Anti-Kickback Statute (“AKS”), makes it a felony for a person or entity, including a clinical laboratory, to, among other things, knowingly and willfully offer, pay, solicit or receive any remuneration, directly or indirectly, overtly or covertly, in cash or in kind, in order to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal health care programs. The government may also assert that a claim that includes items or services resulting from a violation of the AKS constitutes a false or fraudulent claim under the FCA, which is discussed in greater detail below. Additionally, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. Although the AKS applies only to items and services reimbursable under any federal health care program, a number of states have passed statutes substantially similar to
S-46
the AKS that apply to all payers or to state program payers. Penalties for violations of such laws include imprisonment and significant monetary fines and, in the case of the AKS, exclusion from federal health care programs. Federal and state law enforcement authorities scrutinize arrangements between health care providers and potential referral sources to ensure that the arrangements are not designed as a mechanism to induce patient care referrals or induce the purchase or prescribing of particular products or services. Generally, courts have taken a broad interpretation of the scope of the AKS, holding that the statute may be violated if merely one purpose of a payment arrangement is to induce referrals or purchases. In addition to statutory exceptions to the AKS, regulations provide for a number of safe harbors. If an arrangement meets the conditions of an applicable exception or safe harbor, it is deemed not to violate the AKS. An arrangement must fully meet each condition of an applicable exception or safe harbor in order to qualify for protection. Failure to meet the conditions of a safe harbor, however, does not render an arrangement illegal. Rather, the government may evaluate such arrangements on a case-by-case basis, taking into account all facts and circumstances.
In addition, the federal Eliminating Kickbacks in Recovery Act (“EKRA”), prohibits knowingly and willfully soliciting or receiving any remuneration (including any kickback, bribe or rebate) directly or indirectly, overtly or covertly, in cash or in kind, in return for referring a patient or patronage to a laboratory; or paying or offering any remuneration (including any kickback, bribe or rebate) directly or indirectly, overtly or covertly, in cash or in kind, to induce a referral of an individual to a laboratory and certain other entities or in exchange for an individual using the services of such entities. The EKRA applies to all payers including commercial payers and government payers, and EKRA violations result in significant fines and/or up to 10 years in jail, separate and apart from existing AKS liability. Several EKRA exceptions are relevant to many common financial relationships involving clinical laboratories and may be relied upon if all of the elements of the applicable exception are satisfied. Failure to meet the requirements of an exception, however, does not render an arrangement illegal. Rather, the government may evaluate such arrangements on a case-by-case basis, taking into account all facts and circumstances.
Other Federal and State Fraud & Abuse Healthcare Laws
In addition to the requirements discussed above, several other health care fraud and abuse laws could have an effect on our business.
The FCA prohibits, among other things, a person from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment or approval and from, making, using, or causing to be made or used, a false record or statement material to a false or fraudulent claim in order to secure payment or retaining an overpayment by the federal government. Under the FCA, a person acts knowingly if he or she has actual knowledge of the information or acts in deliberate ignorance or in reckless disregard of the truth or falsity of the information. Specific intent to defraud is not required. FCA violations can result in penalties of up to three times the actual damages sustained by the government, plus civil penalties for each false claim. In addition to actions initiated by the government itself, the statute authorizes actions to be brought on behalf of the federal government by a private party having knowledge of the alleged fraud. Because the complaint is initially filed under seal, the action may be pending for some time before the defendant is even aware of the action. If the government intervenes and is ultimately successful in obtaining redress in the matter or if the plaintiff succeeds in obtaining redress without the government’s involvement, then the plaintiff will receive a percentage of the recovery. Several states have enacted comparable false claims laws which may be broader in scope and apply regardless of payer.
The Social Security Act includes civil monetary penalty provisions that impose penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent. Several states have enacted comparable laws which may be broader in scope and apply regardless of payer. In addition, a person who offers or provides to a Medicare or Medicaid beneficiary any remuneration, including waivers of co-payments and deductible amounts (or any part thereof), that the person knows or should know is likely to influence the beneficiary’s selection of a particular provider, practitioner or supplier of Medicare or Medicaid payable items or services may be liable under the civil monetary penalties law. Moreover, in certain cases, providers who routinely waive copayments and deductibles for Medicare and Medicaid beneficiaries, can also be held liable under the civil monetary penalty provisions and certain other laws, such as the AKS and FCA. One of the statutory exceptions to the civil monetary penalty prohibition is non-routine, unadvertised waivers of
S-47
copayments or deductible amounts based on individualized determinations of financial need or exhaustion of reasonable collection efforts. The Office of Inspector General of the U.S. Department of Health and Human Services (“HHS”), emphasizes, however, that this exception should only be used occasionally to address special financial needs of a particular patient. States may have similar prohibitions.
Other Federal and State Healthcare Laws
In addition to the fraud and abuse laws discussed above, our business potentially is subject to the following additional healthcare regulatory laws:
Laws Governing Genetic Counseling Services
Our genetic counseling partner may provide services via electronic means that could subject it to various federal, state and local certification and licensing laws, regulations and approvals, relating to, among other things, the adequacy of health care, the practice of medicine and other health professions (including the provision of remote care and cross-coverage practice), equipment, personnel, operating policies and procedures and the prerequisites for ordering laboratory tests. Some states have enacted regulations specific to providing services to patients via telehealth. Such regulations include, among other things, informed consent requirements that some states require providers to obtain from their patients before providing telehealth services. Health professionals who provide professional services using telehealth modalities must, in most instances, hold a valid license to practice the applicable health profession in the state in which the patient is located. In addition, certain states require a physician providing telehealth to be physically located in the same state as the patient. Any failure to comply with these laws and regulations could result in civil or criminal penalties against telehealth providers.
Clinical and Human Subjects Research Regulations
We may collaborate or support ongoing clinical or other human subjects research that could subject us to a number of laws and regulations pertaining to such research, including but not limited to the Federal Policy for Protection of Human Subjects (as set forth in the implementing regulations of any signatory federal department or agency), the FDCA and its applicable implementing regulations at 21 C.F.R. Parts 11, 50, 54, 56, 58 and 812 and all equivalent legal requirements in other jurisdictions.
Privacy and Security Laws
Health Insurance Portability and Accountability Act
Under the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), as amended by the Health Information Technology for Economic and Clinical Health Act (“HITECH”), HHS has issued regulations to protect the privacy and provide for the security of protected health information (“PHI”) used or disclosed by covered entities, including most health care providers and their respective business associates, as well as the business associates’ subcontractors. HIPAA also regulates standardization of data content, codes, and formats used in certain health care transactions and standardization of identifiers for health plans and providers. Four principal regulations with which we are required to comply have been issued in final form under HIPAA and HITECH: privacy regulations, security regulations, breach notification regulations, and standards for electronic transactions, which establish standards for common healthcare transactions.
The privacy regulations cover the use and disclosure of PHI by covered entities as well as business associates, which are persons or entities that perform certain functions for or on behalf of a covered entity that involve the creation, receipt, maintenance, or transmission of PHI. Business associates are defined to include a subcontractor to whom a business associate delegates a function, activity, or service, other than in the capacity of the business associate’s workforce. As a general rule, a covered entity or business associate may not use or disclose PHI except as permitted or required under the privacy regulations. The privacy regulations also set forth certain rights that an individual has with respect to his or her PHI maintained by a covered entity or business associate, including the right to access or amend certain records containing his or her PHI, request restrictions on the use or disclosure of his or her PHI, or request an accounting of disclosures of his or her PHI.
S-48
Covered entities and business associates also must comply with the security regulations, which establish requirements for safeguarding the confidentiality, integrity, and availability of PHI that is electronically transmitted or electronically stored. In addition, HITECH, among other things, established certain PHI breach notification requirements with which covered entities and business associates must comply. In particular, a covered entity must notify any individual whose unsecured PHI is breached according to the specifications set forth in the breach notification rule. A covered entity must also notify the Secretary of HHS and, under certain circumstances, the media of a breach of unsecured PHI.
The HIPAA privacy, security, and breach notification regulations establish a uniform federal “floor” and do not preempt state laws that are more stringent or provide individuals with greater rights with respect to the privacy or security of, and access to, their records containing PHI or insofar as such state laws apply to personal information that is broader in scope than PHI. In addition, individuals (or their personal representatives, as applicable) generally have the right to access test reports directly from laboratories and to direct that copies of those reports be transmitted to persons or entities designated by the individual.
HIPAA authorizes state attorneys general to file suit on behalf of their residents for violations. Courts are able to award damages, costs, and attorneys’ fees related to violations of HIPAA in such cases. While HIPAA does not create a private right of action allowing individuals to file suit against us in civil court for violations of HIPAA, its standards have been used as the basis for duty of care cases in state civil suits such as those for negligence or recklessness in the misuse or breach of PHI. In addition, violations of HIPAA could result in significant penalties imposed by the HHS’s Office for Civil Rights. HIPAA also mandates that the Secretary of HHS conduct periodic compliance audits of HIPAA covered entities, such as us, and their business associates for compliance with the HIPAA privacy and security standards. It also tasks HHS with establishing a methodology whereby harmed individuals who were the victims of breaches of unsecured PHI may receive a percentage of the civil monetary penalty paid by the violator.
Further, there are a number of state laws regarding the privacy and security of health information and personal data that are applicable to our clinical laboratories. We believe that we have taken the steps required of us to comply with health information privacy and security statutes and regulations in all jurisdictions, both state and federal, and we intend to continue to comprehensively protect all personal information and to comply with all applicable laws regarding the protection of such information. However, these laws constantly change, and we may not be able to maintain compliance in all jurisdictions where we do business. Failure to maintain compliance, or changes in state or federal laws regarding privacy or security, could result in civil and/or criminal penalties as well as significant reputational damage and could also have a material adverse effect on our business.
California Consumer Privacy Act
The California Consumer Privacy Act, as amended by the California Privacy Rights Act (“CPRA,” and together with the California Consumer Privacy Act, the “CCPA”), confers to California consumers, among other things, the right to receive notice of the categories of personal information that will be collected by a business, how the business will use and share the personal information, and the categories of third parties who will receive the personal information. The CCPA also confers rights to access, delete, correct, or request a portable data set, the right to limit processing of “sensitive personal information,” and the right to receive equal service and pricing from a business after exercising a consumer right granted by the CCPA. In addition, the CCPA allows California consumers the right to opt out of the “sale” of their personal information, which the CCPA defines broadly as any disclosure of personal information to a third party in exchange for monetary or other valuable consideration. The CCPA also allows California consumers to opt out of the “sharing” of information, which restricts a company’s use of personal information for cross-context behavioral advertising. The CCPA also requires a business to implement reasonable security procedures to safeguard personal information against unauthorized access, use, or disclosure and imposes purpose limitation, data minimization, data retention, and other security compliance obligations on regulated businesses. The CCPA requires businesses to include specific provisions in contracts with third parties that process data on a business’s behalf regarding the third party’s processing and management of such data.
The CCPA does not apply to personal information that is PHI under HIPAA and that is collected by a business associate or covered entity under HIPAA. The CCPA also exempts patient information that is processed by a
S-49
covered entity and maintained in the same manner as PHI. Accordingly, the CCPA will not apply to much of the genetic testing and patient information we collect and process. However, we are required to comply with the CCPA insofar as we collect other categories of California consumers’ personal information, such as information about California-based employees, contractors, business contacts and website visitors.
The CCPA is enforceable through administrative fines of up to $2,500 for each violation or $7,500 for intentional violations or where we have actual knowledge that the personal information relates to an individual under 16 years of age.
Further, five new state privacy laws have gone or will go into effect in 2023, including the CPRA, the Virginia Consumer Data Protection Act, the Utah Consumer Privacy Act, the Colorado Privacy Act, and the Connecticut Data Privacy Act, and a number of other states are considering similar laws. The new state privacy laws and any potential federal law will and would impose additional data protection obligations on covered businesses, including additional consumer rights, limitations on data uses, new audit requirements for higher risk data, and opt outs for certain uses of sensitive data. The new and proposed privacy laws may result in further uncertainty and may require us to incur additional expenditures to comply. These regulations and legislative developments have potentially far-reaching consequences and may require us to modify our data management and data use practices and incur substantial compliance expense. Our failure to comply with applicable laws and regulations or other obligations to which we may be subject relating to personal data, or to protect personal data from unauthorized access, use, or other processing, could result in enforcement actions and regulatory investigations against us, claims for damages by customers and other affected individuals, fines, damage to our reputation, and loss of goodwill, any of which could have a material adverse effect on our operations, financial performance, and business.
Genetic Privacy and Testing Laws
We are subject to myriad laws designed to establish safeguards regarding the conduct of genomic testing and analysis and to protect against the misuse of genetic information and human biological specimens (“samples”) from which genetic information can be derived. These laws vary in their scope and in the nature of their requirements and restrictions. For example, certain genetic privacy laws prohibit the retention of samples after performing a genomic analysis and prohibit the use or disclosure of genetic information or samples for certain purposes, such as research, without appropriate informed consent from the individual or unless the genetic information or samples are appropriately de-identified. Other laws may impose additional requirements, including requirements regarding institutional review board review and approval for certain research uses of genetic information or samples or requirements to implement certain security controls in connection with the transfer of genetic information. We must comply with such genetic privacy and testing laws in our collection, use, disclosure, and retention of genetic information and samples.
Other Data Protection Laws
There are a growing number of jurisdictions around the globe that have privacy and data protection laws that may apply to us as we enter or expand our business in jurisdictions outside of the United States. These laws are typically triggered by a company’s establishment or physical location in the jurisdiction, data processing activities that take place in the jurisdiction, and/or the processing of personal information about individuals located in that jurisdiction that are targeted, for example, by an offer of goods or services. Certain data protection laws, such as those in the European Union (“EU”) and United Kingdom, are comprehensive in nature and include significant requirements around the processing of personal information, while other jurisdictions may have laws less restrictive or prescriptive than those in the U.S. Enforcement of these laws varies from jurisdiction to jurisdiction, with a variety of consequences, including civil or criminal penalties, litigation, private rights of action, or damage to our reputation.
For example, the EU’s General Data Protection Regulation (“GDPR”) including as implemented and amended through the UK Data Protection Act 2018 and implementing laws and regulations (“UK GDPR”) applies to any data collection, use and sharing in the context of an establishment the EU or UK as well as extraterritorially to any entity outside the EU and UK when they process personal information related to an offer of goods or services to, or monitoring the behavior of, individuals who are located in the EU or UK. The GDPR and UK GDPR impose strict
S-50
requirements on controllers and processors of personal data, including enhanced protections for “special categories” of personal data, which include sensitive information such as health and genetic information of data subjects. The GDPR and UK GDPR also grant individuals various rights in relation to their personal data, including the rights of access, rectification, objection to certain processing, and deletion. The GDPR and UK GDPR provide an individual with an express right to seek legal remedies if the individual believes his or her rights have been violated. Failure to comply with the requirements of the GDPR or the related national data protection laws of the member states of the EU, which may deviate from or be more restrictive than the GDPR, or a failure to comply with the UK GDPR, may result in significant administrative fines issued by EU or UK regulators.
Information Blocking Prohibition
On May 1, 2020, the Office of the National Coordinator for Health Information Technology promulgated final regulations under the authority of the 21st Century Cures Act to impose new conditions to obtain and maintain certification of certified health information technology and prohibit certain covered actors, including developers of certified health information technology, health information networks/health information exchanges, and health care providers, from engaging in activities that are likely to interfere with the access, exchange, or use of electronic health information (information blocking). The final regulations further defined exceptions for activities that are permissible, even though they may have the effect of interfering with the access, exchange, or use of electronic health information. The information blocking regulations became effective on April 5, 2021. Under the 21st Century Cures Act, health care providers that violate the information blocking prohibition will be subject to appropriate disincentives, which the HHS has yet to establish through required rulemaking. Developers of certified information technology and health information networks/health information exchanges, however, may be subject to civil monetary penalties of up to $1 million per violation. The HHS Office of Inspector General has the authority to impose such penalties and on April 24, 2020 published a proposed rule to codify new authority in regulation, which the agency proposed would be effective 60 days after it issues a final rule but in no event before November 2, 2020. The HHS Office of Inspector General has not yet issued a final rule.
Federal and State Consumer Protection Laws
The Federal Trade Commission (“FTC”), is an independent U.S. law enforcement agency charged with protecting consumers and enhancing competition across broad sectors of the economy. The FTC’s primary legal authority with respect to data privacy and security comes from Section 5 of the FTC Act, which prohibits unfair or deceptive acts or practices in the marketplace. The FTC has increasingly used this broad authority to police data privacy and security, using its powers to investigate and bring lawsuits. Where appropriate, the FTC can seek a variety of remedies, such as but not limited to requiring the implementation of comprehensive privacy and security programs, biennial assessments by independent experts, monetary redress to consumers, and provision of robust notice and choice mechanisms to consumers. In addition to its enforcement mechanisms, the FTC uses a variety of tools to protect consumers’ privacy and personal information, including pursuing enforcement actions to stop violations of law, conducting studies and issuing reports, hosting public workshops, developing educational materials, and testifying before the U.S. Congress on issues that affect consumer privacy. Recently, the FTC has issued guidance emphasizing that their authority to prevent unfair or deceptive acts or practices extends to advertising and marketing claims for health care and health-related products.
The majority of data privacy cases brought by the FTC fall under the “deceptive” acts prong of Section 5. These cases often involve a failure on the part of a company to adhere to its own privacy and data protection principles set forth in its policies or other statements made to consumers. To avoid Section 5 violations, the FTC encourages companies to build privacy protections and safeguards into relevant portions of their business, and to consider privacy and data protection as the company grows and evolves. In addition, privacy notices should clearly and accurately disclose the type(s) of personal information the company collects, how the company uses and shares that information, and the security measures used by the company to protect that information.
In recent years, the FTC’s enforcement under Section 5 related to data security has included alleged violations of the “unfairness” prong. Many of these cases have alleged that companies were unfair to consumers because they failed to take reasonable and necessary measures to protect consumer data. The FTC has not provided bright line rules defining what constitutes “reasonable and necessary measures” for implementing a cybersecurity program, but
S-51
it has provided guidance, tips and advice for companies. The FTC has also published past complaints and consent orders, which it urges companies use as guidance to help avoid an FTC enforcement action, even if a data breach or loss occurs.
In addition to the FTC Act, most U.S. states have unfair and deceptive acts and practices statutes, known as UDAP statutes, that substantially mirror the FTC Act and have been applied in the privacy and data security context. These vary in substance and strength from state to state. Many have broad prohibitions against unfair and deceptive acts and practices. These statutes generally allow for private rights of action and are enforced by the states’ Attorneys General.
Reimbursement and Billing
In April 2014, Congress passed the Protecting Access to Medicare Act of 2014 (“PAMA”), which included substantial changes to the way in which clinical laboratory services are paid under Medicare. Under PAMA (as amended) and its implementing regulations, laboratories that realize at least $12,500 in Medicare Clinical Laboratory Fee Schedule (“CLFS”) revenues during the six month reporting period and that receive the majority of their Medicare revenue from payments made under the CLFS or the Physician Fee Schedule must report, beginning in 2017, and then in 2024 and every three years thereafter (or annually for “advanced diagnostic laboratory tests”), private payer payment rates and volumes for their tests. None of our tests meet the current definition of advanced diagnostic laboratory tests, and therefore we believe we are required to report private payer rates for our tests on an every three years basis starting next in 2024. CMS uses the rates and volumes reported by laboratories to develop Medicare payment rates for the tests equal to the volume-weighted median of the private payer payment rates for the tests. Laboratories that fail to report the required payment information may be subject to substantial civil money penalties.
As set forth under the regulations implementing PAMA, for tests furnished on or after January 1, 2018, Medicare payments for clinical diagnostic laboratory tests are paid based upon these reported private payer rates. For clinical diagnostic laboratory tests that are assigned a new or substantially revised code, initial payment rates for clinical diagnostic laboratory tests that are not advanced diagnostic laboratory tests will be assigned by the cross-walk or gap-fill methodology, as under prior law. Initial payment rates for new advanced diagnostic laboratory tests will be based on the actual list charge for the laboratory test.
The payment rates calculated under PAMA went into effect starting January 1, 2018. Where applicable, reductions to payment rates resulting from the new methodology were limited to 10% per test per year in each of the years 2018 through 2020. Rates were held at 2020 levels during 2021 and 2022 and will continue to be held at such levels in 2023. Then, where applicable based upon median private payer rates reported in 2017 or 2024, reduced by up to 15% per test per year in each of 2024 through 2026 (with a second round of private payer rate reporting in 2024 to establish rates for 2025 through 2027).
PAMA codified Medicare coverage rules for laboratory tests by requiring any local coverage determination to be made following the local coverage determination process. PAMA also authorizes CMS to consolidate coverage policies for clinical laboratory tests among one to four laboratory-specific Medicare Administrative Contractors (“MACs”). These same contractors may also be designated to process claims if CMS determines that such a model is appropriate. It is unclear whether CMS will proceed with contractor consolidation under this authorization.
PAMA also authorized the adoption of new, temporary billing codes and/or unique test identifiers for FDA-cleared or approved tests as well as advanced diagnostic laboratory tests. The American Medical Association has created a section of billing codes, Proprietary Laboratory Analyses (“PLA”), to facilitate implementation of this section of PAMA. These codes may apply to one or more of our tests if we apply for PLA coding.
Reimbursement and billing for diagnostic services is highly complex, and errors in billing potentially can result denied claims and/or in substantial obligations to repay overpayments to payors. Laboratories must bill various payers, such as private third-party payers, including managed care organizations (“MCO”), and state and federal health care programs, such as Medicare and Medicaid, and each may have different billing requirements. Additionally, the audit requirements we must meet to ensure compliance with applicable laws and regulations, as
S-52
well as our internal compliance policies and procedures, add further complexity to the billing process. Other factors that complicate billing include:
•variability in coverage and information requirements among various payers;
•patient financial assistance programs;
•missing, incomplete or inaccurate billing information provided by ordering physicians;
•billings to payers with whom we do not have contracts;
•disputes with payers as to which party is responsible for payment; and
•disputes with payers as to the appropriate level of reimbursement.
Depending on the reimbursement arrangement and applicable law, the party that reimburses us for our services may be:
•a third party who provides coverage to the patient, such as an insurance company or MCO;
•a state or federal healthcare program; or
•the patient.
Legal Proceedings
Except as described below, we, and our subsidiaries, are currently not a party to, and our property is not currently the subject of, any material pending legal proceedings; however, we may become involved in various claims and legal actions arising in the ordinary course of business.
On September 7, 2022, a shareholder class action lawsuit was filed in the United States District Court for the District of Connecticut against the Company and certain of our current and former officers. The complaint purports to bring suit on behalf of stockholders who purchased our publicly traded securities between March 14, 2022 and August 15, 2022. The complaint purports to allege that defendants made false and misleading statements about our business, operations and prospects in violation of Sections 10(b) and 20(a) of the Exchange Act, and seeks unspecified compensatory damages, fees and costs. We believe the allegations and claims made in the complaint are without merit.
S-53
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES
The following discussion is a summary of the material U.S. federal income tax consequences to U.S. holders and non-U.S. holders (each as defined below) of the purchase, ownership, conversion and disposition, as applicable, of the shares of our Class A common stock issued pursuant to this offering, but does not purport to be a complete analysis of all potential tax effects. The effects of other U.S. federal tax laws, such as estate and gift tax laws, and any applicable state, local or non-U.S. tax laws are not discussed. This discussion is based on the U.S. Internal Revenue Code of 1986, as amended (the “Code”), Treasury Regulations promulgated thereunder, judicial decisions, and published rulings and administrative pronouncements of the U.S. Internal Revenue Service (the “IRS”), in each case in effect as of the date hereof. These authorities may change or be subject to differing interpretations. Any such change or differing interpretation may be applied retroactively in a manner that could adversely affect a holder. We have not sought and will not seek any rulings from the IRS regarding the matters discussed below. There can be no assurance the IRS or a court will not take a contrary position to that discussed below regarding the tax consequences of the purchase, ownership, conversion and disposition, as applicable, of the shares of our Class A common stock .
This discussion is limited to holders that hold our Class A common stock as a “capital asset” within the meaning of Section 1221 of the Code (generally, property held for investment). This discussion does not address all U.S. federal income tax consequences relevant to a holder’s particular circumstances, including, without limitation, the impact of the Medicare contribution tax on net investment income and the alternative minimum tax. In addition, it does not address consequences relevant to holders subject to special rules, including, without limitation:
•U.S. expatriates and former citizens or long-term residents of the United States;
•persons holding our stock as part of a hedge, straddle or other risk reduction strategy or as part of a conversion transaction or other integrated investment;
•banks, insurance companies, and other financial institutions, regulated investment companies or real estate investment trusts;
•brokers or dealers in securities or currencies;
•traders in securities or other persons that elect to use a mark-to-market method of accounting for their holdings in our stock;
•“controlled foreign corporations (as defined in Section 957 of the Code),” “passive foreign investment companies (as defined in Section 1297 of the Code),” and corporations that accumulate earnings to avoid U.S. federal income tax;
•partnerships or other entities or arrangements treated as partnerships for U.S. federal income tax purposes (and investors in such entities);
•tax-exempt organizations or governmental organizations;
•persons deemed to sell our stock under the constructive sale provisions of the Code;
•persons who hold or receive our stock pursuant to the exercise of any employee stock option or otherwise as compensation;
•persons subject to special tax accounting rules as a result of any item of gross income with respect to our stock being taken into account in an applicable financial statement (as defined in Section 451(b) of the Code);
•pension plans or tax-qualified retirement plans; and
•“qualified foreign pension funds” as defined in Section 897(l)(2) of the Code and entities all of the interests of which are held by qualified foreign pension funds.
S-54
If an entity treated as a partnership for U.S. federal income tax purposes holds our Class A common stock, the tax treatment of a partner in the partnership will depend on the status of the partner, the activities of the partnership and certain determinations made at the partner level. Accordingly, partnerships holding our Class A common stock and the partners in such partnerships should consult their tax advisors regarding the U.S. federal income tax consequences to them.
THIS DISCUSSION IS FOR INFORMATIONAL PURPOSES ONLY AND IS NOT TAX ADVICE. INVESTORS SHOULD CONSULT THEIR TAX ADVISORS WITH RESPECT TO THE APPLICATION OF THE U.S. FEDERAL INCOME TAX LAWS TO THEIR PARTICULAR SITUATIONS AS WELL AS ANY TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP, AND DISPOSITION OF OUR CLASS A COMMON STOCK ARISING UNDER THE U.S. FEDERAL ESTATE OR GIFT TAX LAWS OR UNDER THE LAWS OF ANY STATE, LOCAL OR NON-U.S. TAXING JURISDICTION OR UNDER ANY APPLICABLE INCOME TAX TREATY.
Definition of a U.S. Holder and Non-U.S. Holder
For purposes of this discussion, a “U.S. holder” is any beneficial owner of our shares of Class A common stock (other than an entity treated as a partnership for U.S. federal income tax purposes) that, for U.S. federal income tax purposes, is or is treated as any of the following:
•an individual who is a citizen or resident of the United States;
•a corporation created or organized under the laws of the United States, any state thereof, or the District of Columbia;
•an estate, the income of which is subject to U.S. federal income tax regardless of its source; or
•a trust that (1) is subject to the primary supervision of a U.S. court and which has one or more “United States persons” (within the meaning of Section 7701(a)(30) of the Code) that have the authority to control all substantive decisions of the trust, or (2) has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes.
For purposes of this discussion, a “non-U.S. holder” is any beneficial owner of shares of our Class A common stock that is neither a “U.S. holder” nor an entity treated as a partnership for U.S. federal income tax purposes.
Tax Consequences Applicable to U.S. Holders
Distributions
As described in the section titled “Dividend Policy,” we do not anticipate declaring or paying dividends to holders of our Class A common stock in the foreseeable future. However, if we do make distributions of cash or property on our stock, such distributions will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Dividends paid to non-corporate U.S. holders generally will qualify for taxation at reduced rates if such U.S. holders meet certain holding period and other applicable requirements. Amounts not treated as dividends for U.S. federal income tax purposes will constitute a return of capital and first be applied against and reduce a U.S. holder’s adjusted tax basis in its stock, but not below zero. Any excess will be treated as capital gain and will be treated as described below under “—Sale or Other Taxable Disposition.” U.S. holders should consult their tax advisors regarding the application of reduced tax rates in their particular circumstances.
Sale or Other Taxable Disposition
A U.S. holder will recognize capital gain or loss on a sale or other taxable disposition of our stock equal to the difference between the amount of cash and the fair market value of any property received upon the sale or other taxable disposition and the U.S. holder’s adjusted tax basis in the shares sold or disposed of. Such capital gain or loss will be long-term capital gain or loss if the U.S. holder’s holding period for the shares sold or exchanged is
S-55
more than one year. Long-term capital gains recognized by certain non-corporate U.S. holders, including individuals, will be taxable at reduced rates. The deductibility of capital losses is subject to limitations.
Information Reporting and Backup Withholding
Distributions with respect to our stock to a U.S. holder and proceeds from the sale or other taxable disposition of our stock by the U.S. holder generally are subject to information reporting to the IRS, unless the U.S. holder is an exempt recipient. Backup withholding may apply to such payments if a U.S. holder is not otherwise exempt and:
•such holder fails to furnish its taxpayer identification number (“TIN”), which for an individual, is ordinarily his or her social security number;
•such holder furnishes an incorrect TIN;
•the applicable withholding agent is notified by the IRS that such holder has failed to properly report payments of dividends or interest; or
•such holder fails to certify, under penalties of perjury, that such holder has furnished a correct TIN and that the IRS has not notified such holder that such holder is subject to backup withholding.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a U.S. holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
Tax Consequences Applicable to Non-U.S. Holders
Distributions
Any distributions of cash or property on our stock will constitute dividends for U.S. federal income tax purposes to the extent paid from our current and accumulated earnings and profits, as determined under U.S. federal income tax principles. Amounts not treated as dividends for U.S. federal income tax purposes will first constitute a return of capital and be applied against and reduce a non-U.S. Holder’s adjusted tax basis in its stock, but not below zero. Any excess will be treated as capital gain and will be treated as described below under “—Sale or Other Taxable Disposition.”
Subject to the discussions below on effectively connected income, backup withholding and FATCA (as defined below), dividends paid to a non-U.S. holder on our stock will be subject to U.S. federal withholding tax at a rate of 30% of the gross amount of the dividends (or such lower rate specified by an applicable income tax treaty, provided the non-U.S. holder furnishes a valid IRS Form W-8BEN or W-8BEN-E (or other applicable documentation) certifying qualification for the lower treaty rate). A non-U.S. holder that does not timely furnish the required documentation, but that qualifies for a reduced treaty rate, may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS. Non-U.S. holders should consult their tax advisors regarding their entitlement to benefits under any applicable income tax treaty.
If dividends paid to a non-U.S. holder are effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the non-U.S. holder maintains a permanent establishment in the United States to which such dividends are attributable), the non-U.S. holder will be exempt from the U.S. federal withholding tax described above. To claim the exemption, the non-U.S. holder must furnish to the applicable withholding agent a valid IRS Form W-8ECI, certifying that the dividends are effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States.
Any such effectively connected dividends will be subject to U.S. federal income tax on a net income basis at the regular graduated rates. A non-U.S. holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on such effectively connected dividends, as adjusted for certain items. Non-U.S. holders should consult their tax advisors regarding any applicable tax treaties that may provide for different rules.
S-56
Sale or Other Taxable Disposition
Subject to the discussions below regarding backup withholding and FATCA, a non-U.S. holder will not be subject to U.S. federal income tax on any gain realized upon the sale or other taxable disposition of our stock unless:
•the gain is effectively connected with the non-U.S. holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, the non-U.S. holder maintains a permanent establishment in the United States to which such gain is attributable);
•the non-U.S. holder is a nonresident alien individual present in the United States for 183 days or more during the taxable year of the disposition and certain other requirements are met; or
•our stock constitutes a U.S. real property interest (“USRPI”) by reason of our status as a U.S. real property holding corporation (“USRPHC”) for U.S. federal income tax purposes.
Gain described in the first bullet point above generally will be subject to U.S. federal income tax on a net income basis at the regular rates. A non-U.S. holder that is a corporation also may be subject to a branch profits tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on such effectively connected gain, as adjusted for certain items.
A non-U.S. holder described in the second bullet point above will be subject to U.S. federal income tax at a rate of 30% (or such lower rate specified by an applicable income tax treaty) on gain realized upon the sale or other taxable disposition of our stock, which may be offset by U.S. source capital losses of the non-U.S. holder (even though the individual is not considered a resident of the United States), provided the non-U.S. holder has timely filed U.S. federal income tax returns with respect to such losses.
With respect to the third bullet point above, we believe we currently are not, and do not anticipate becoming, a USRPHC. Because the determination of whether we are a USRPHC depends, however, on the fair market value of our USRPIs relative to the fair market value of our non-U.S. real property interests and our other business assets, there can be no assurance we currently are not a USRPHC or will not become one in the future. Even if we are or were to become a USRPHC, gain arising from the sale or other taxable disposition of our Class A common stock by a non-U.S. holder will not be subject to U.S. federal income tax if our Class A common stock is “regularly traded,” as defined by applicable Treasury Regulations, on an established securities market, and such non-U.S. holder owned, actually and constructively, 5% or less of our Class A common stock throughout the shorter of the five-year period ending on the date of the sale or other taxable disposition or the non-U.S. holder’s holding period.
Non-U.S. holders should consult their tax advisors regarding potentially applicable income tax treaties that may provide for different rules.
Information Reporting and Backup Withholding
Payments of dividends on our stock will not be subject to backup withholding, provided the applicable withholding agent does not have actual knowledge or reason to know the holder is a United States person and the holder either certifies its non-U.S. status, such as by furnishing a valid IRS Form W-8BEN, W-8BEN-E or W-8ECI, or otherwise establishes an exemption. However, information returns are required to be filed with the IRS in connection with any distributions on our stock paid to the non-U.S. holder, regardless of whether such distributions constitute dividends or whether any tax was actually withheld. In addition, proceeds of the sale or other taxable disposition of our stock within the United States or conducted through certain U.S. related brokers generally will not be subject to backup withholding or information reporting if the applicable withholding agent receives the certification described above and does not have actual knowledge or reason to know that such holder is a United States person or the holder otherwise establishes an exemption. Proceeds of a disposition of our stock conducted through a non-U.S. office of a non-U.S. broker generally will not be subject to backup withholding or information reporting.
S-57
Copies of information returns that are filed with the IRS may also be made available under the provisions of an applicable treaty or agreement to the tax authorities of the country in which the non-U.S. holder resides or is established.
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules may be allowed as a refund or a credit against a non-U.S. holder’s U.S. federal income tax liability, provided the required information is timely furnished to the IRS.
Additional Withholding Tax on Payments Made to Foreign Accounts
Withholding taxes may be imposed under Sections 1471 to 1474 of the Code (such Sections commonly referred to as the Foreign Account Tax Compliance Act, or “FATCA”) on certain types of payments made to non-U.S. financial institutions and certain other non-U.S. entities. Specifically, a 30% withholding tax may be imposed on dividends on, or (subject to the proposed Treasury Regulations discussed below) gross proceeds from the sale or other disposition of, our stock paid to a “foreign financial institution” or a “non-financial foreign entity” (each as defined in the Code), unless (1) the foreign financial institution undertakes certain diligence and reporting obligations, (2) the non-financial foreign entity either certifies it does not have any “substantial United States owners” (as defined in the Code) or furnishes identifying information regarding each substantial United States owner, or (3) the foreign financial institution or non-financial foreign entity otherwise qualifies for an exemption from these rules. If the payee is a foreign financial institution and is subject to the diligence and reporting requirements in (1) above, it must enter into an agreement with the U.S. Department of the Treasury requiring, among other things, that it undertake to identify accounts held by certain “specified United States persons” or “United States owned foreign entities” (each as defined in the Code), annually report certain information about such accounts, and withhold 30% on certain payments to non-compliant foreign financial institutions and certain other account holders. Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules.
Under the applicable Treasury Regulations and administrative guidance, withholding under FATCA generally applies to payments of dividends on our stock. The U.S. Department of Treasury has issued proposed Treasury Regulations providing that the withholding provisions under FATCA do not apply with respect to the gross proceeds from a sale or other disposition of our common stock. In its preamble to such proposed regulations, the U.S. Department of the Treasury stated that taxpayers generally may rely on these proposed Treasury Regulations until final Treasury Regulations are issued.
Prospective investors should consult their tax advisors regarding the potential application of withholding under FATCA to their investment in our stock.
S-58
UNDERWRITING
Subject to the terms and conditions set forth in the underwriting agreement, dated January 26, 2022, between us and Jefferies LLC, as underwriter, we have agreed to sell to the underwriter, and the underwriter has agreed to purchase from us, the entire number of shares of Class A common stock offered by this prospectus supplement.
The underwriting agreement provides that the obligations of the underwriter are subject to certain conditions precedent such as the receipt by the underwriter of officers’ certificates and legal opinions and approval of certain legal matters by their counsel. The underwriting agreement provides that the underwriter will purchase all of the shares of Class A common stock if any of them are purchased. We have agreed to indemnify the underwriter and certain of its controlling persons against certain liabilities, including liabilities under the Securities Act, and to contribute to payments that the underwriter may be required to make in respect of those liabilities.
The underwriter has advised us that, following the completion of this offering, its currently intends to make a market in the Class A common stock as permitted by applicable laws and regulations. However, the underwriter is not obligated to do so, and the underwriter may discontinue any market-making activities at any time without notice, in their sole discretion. Accordingly, no assurance can be given as to the liquidity of the trading market for the Class A common stock, your ability to sell any of the Class A common stock held by you at a particular time or that the prices that you receive when you sell will be favorable.
The underwriter is offering the shares of Class A common stock subject to its acceptance of the shares of Class A common stock from us and subject to prior sale. The underwriter reserves the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
The closing of the concurrent registered direct offering is expected to occur subsequent to the closing of this offering. This offering is not contingent on the closing of the concurrent registered direct offering.
Commission and Expenses
The underwriter has advised us that it proposes to offer the shares of Class A common stock to the public at the public offering price set forth on the cover page of this prospectus supplement and to certain dealers, which may include the underwriter, at a price less a concession not in excess of $ per share of Class A common stock. After the offering, the public offering price, concession and reallowance to dealers may be reduced by the underwriter. No such reduction will change the amount of proceeds to be received by us as set forth on the cover page of this prospectus supplement.
The following table shows the public offering price, the underwriting discounts and commissions that we are to pay the underwriter and the proceeds to us, before expenses, in connection with this offering.
| Per Share | Total | ||||||||||
| Public offering price | $ | $ | |||||||||
| Underwriting discounts and commissions paid by us | $ | $ | |||||||||
| Proceeds to us, before expenses | $ | $ | |||||||||
We estimate expenses payable by us in connection with this offering and the concurrent registered direct offering, other than the underwriting discounts and commissions referred to above, will be approximately $ . We have also agreed to reimburse the underwriter for certain of its expenses in an amount not to exceed $ .
Listing
Our Class A common stock is listed on The Nasdaq Global Select Market under the trading symbol “WGS”.
No Sales of Similar Securities
We have agreed that, subject to certain exceptions, we will not (i) offer, sell, contract to sell, pledge, grant any option to purchase, make any short sale or otherwise transfer or dispose of, directly or indirectly, or file with the
S-59
SEC a registration statement under the Securities Act relating to, any securities of the Company that are substantially similar to the Class A common stock, including but not limited to any options or warrants to purchase shares of Class A common stock or any securities that are convertible into or exchangeable for, or that represent the right to receive, Class A common stock or any such substantially similar securities, or publicly disclose the intention to make any offer, sale, pledge, disposition or filing or (ii) enter into any swap or other agreement that transfers, in whole or in part, any of the economic consequences of ownership of the Class A common stock or any such other securities, whether any such transaction described in clause (i) or (ii) above is to be settled by delivery of Class A common stock or such other securities, in cash or otherwise, in each case, without the prior written consent of Jefferies LLC for a period of 60 days after the date of this prospectus supplement.
The restrictions described in the immediately preceding paragraph do not apply to us with respect to:
A.shares of Class A common stock to be sold pursuant to the underwriting agreement;
B.shares sold pursuant to the concurrent registered direct offering;
C.shares of Class A common stock issued upon the exercise of options granted under our stock incentive plans as described elsewhere in this prospectus supplement;
D.any shares of Class A common stock or any securities or other awards or rights (including without limitation options, restricted stock or restricted stock units) convertible into, exercisable or exchangeable for, or that represent the right to receive, shares of Class A common stock pursuant to any stock option plan, incentive plan or stock purchase plan of the Company or otherwise in equity compensation arrangements described elsewhere in this prospectus supplement;
E.any shares of Class A common stock issuable pursuant to (i) the Agreement and Plan of Merger, dated as of February 9, 2021, as amended, by and among CMLS, S-IV Sub, Inc. and Legacy Sema4 executed in connection with the Business Combination or (ii) the Agreement and Plan of Merger and Reorganization, dated as of January 14, 2022, as amended, by and among Sema4 Holdings Corp., Orion Merger Sub I, Inc., Orion Merger Sub II, LLC, GeneDx, Inc., OPKO Health, Inc., and GeneDx Holding 2, Inc. executed in connection with the Acquisition;
F.any shares of Class A common stock issued upon the exercise or redemption of warrants or the conversion or redemption of Company securities as described elsewhere in this prospectus supplement;
G.the filing by the Company of any registration statement on Form S-8 or a successor form thereto relating to any stock option plan, incentive plan or stock purchase plan described elsewhere in this prospectus supplement or otherwise in equity compensation arrangements; and
H.up to 5.0% worth of shares of Class A common stock or any securities convertible into or exercisable or exchangeable for, or that represent the right to receive, shares of Class A common stock issued in connection with any joint venture, commercial or collaborative relationship or the acquisition or license by the Company of the securities, businesses, property or other assets of another person or entity or pursuant to any employee benefit plan assumed by the Company in connection with any such acquisition).
Our officers and directors and certain of our shareholders have entered into lock-up agreements with the underwriter prior to the commencement of this offering pursuant to which each of these persons or entities, with limited exceptions, for a period of 60 days after the date of this prospectus (the “Lock-Up Period”), may not, without the prior written consent of Jefferies LLC (i) offer, sell, contract to sell, pledge, grant any option, right or warrant to purchase, purchase any option or contract to sell, lend or otherwise transfer or dispose of any shares of Class A common stock, or any options or warrants to purchase any shares of Class A common stock, or any securities convertible into, exchangeable for or that represent the right to receive shares of Class A common stock (such shares of Class A common stock, options, rights, warrants or other securities, collectively, “Lock-Up Securities”), including without limitation any such Lock-Up Securities now owned or hereafter acquired by the undersigned, (ii)
S-60
engage in any hedging or other transaction or arrangement (including, without limitation, any short sale or the purchase or sale of, or entry into, any put or call option, or combination thereof, forward, swap or any other derivative transaction or instrument, however described or defined) which is designed to or which reasonably could be expected to lead to or result in a sale, loan, pledge or other disposition (whether by the undersigned or someone other than the undersigned), or transfer of any of the economic consequences of ownership, in whole or in part, directly or indirectly, of any Lock-Up Securities, whether any such transaction or arrangement (or instrument provided for thereunder) would be settled by delivery of Common Stock or other securities, in cash or otherwise, (iii) make any demand for or exercise any right with respect to the registration of any Lock-Up Securities or (iv) otherwise publicly announce any intention to engage in or cause any action, activity, transaction or arrangement described in clause (i), (ii) or (iii) above.
The restrictions described in the immediately preceding paragraph do not apply with respect to:
A.transfers of Lock-Up Securities:
(i)as one or more bona fide gifts or charitable contributions, or for bona fide estate planning purposes;
(ii) upon death by will, testamentary document or intestate succession;
(iii)if the transferor is a natural person, to members of immediate family or to any trust for the direct or indirect benefit of an immediate family or to a trustor or beneficiary of a trust or the estate of a beneficiary of a trust;
(iv) to a partnership, limited liability company or other entity of which the transferor and the immediate family of such transferor are the legal and beneficial owner of all of the outstanding equity securities or similar interests;
(v)to a nominee or custodian of a person or entity to whom a disposition or transfer would be permissible under clauses (i) through (iv) above;
(vi)if the transferor is a corporation, partnership, limited liability company or other business entity, (a) to another corporation, partnership, limited liability company or other business entity that is an affiliate (as defined in Rule 405 under the Securities Act) of the transferor, or to any investment fund or other entity which fund or entity is controlled or managed by the transferor or affiliates of the transferor, or (b) as part of a distribution by the undersigned to its stockholders, partners, members or other equityholders or to the estate of any such stockholders, partners, members or other equityholders;
(vii)by operation of law, such as pursuant to a qualified domestic order, divorce settlement, divorce decree or separation agreement;
(viii)to the Company from an employee of the Company upon death, disability or termination of employment, in each case, of such employee;
(ix)in connection with a sale of the transferor’s Lock-Up Securities acquired (A) from the Underwriter in the offering contemplated by this prospectus supplement or (B) in open market transactions after the closing date of the offering contemplated by this prospectus supplement;
(x)in connection with the vesting, conversion, settlement, exchange or exercise of restricted stock units, options, warrants or other rights to purchase or receive shares of Class A common stock (including, in each case, a transfer to the Company by way of “net” or “cashless” exercise or a sale in the market to cover the payment of tax withholdings or remittance payments due in connection with such vesting, conversion, settlement, exchange or exercise), or in connection with the exercise or redemption of warrants or the conversion or redemption of convertible securities, in all such cases pursuant to equity
S-61
awards or rights granted under a stock incentive plan, other equity award plan or stock purchase plan or inducement awards, or pursuant to the terms of warrants or convertible securities, each as described elsewhere in this prospectus supplement, provided that any Lock-Up Securities received upon such vesting, conversion, settlement, exchange, exercise or redemption, and after giving effect to such transfers to the Company or sale to cover transactions, shall be subject to the terms of the lock-up agreements;
(xi)pursuant to a written plan for trading securities that is designed to satisfy the requirements of Rule 10b5-1 under the Exchange Act (a “10b5-1 Plan”) in effect as of the date of this prospectus supplement and disclosed to the Jefferies, provided that any filing under Section 16 of the Exchange Act made in connection with such sales shall clearly indicate in the footnotes thereto that such disposition of Lock-Up Securities was pursuant to a 10b5-1 Plan;
(xii)with the prior written consent of Jefferies LLC;
provided that (1) in the case of clauses (i), (ii), (iii), (iv), (v) and (vi) above, such transfer or distribution shall not involve a disposition for value, (2) in the case of clauses (i), (ii), (iii), (iv), (v), (vi) and (vii) above, it shall be a condition to the transfer or distribution that the donee, devisee, transferee or distributee, as the case may be, shall sign and deliver a lock up agreement, (3) in the case of clauses (i), (ii), (iii), (iv), (v) and (vi) above, no filing by any party (including, without limitation, any donor, donee, devisee, transferor, transferee, distributor or distributee) under the Exchange Act, or other public filing, report or announcement reporting a reduction in beneficial ownership of Lock-Up Securities shall be required or shall be voluntarily made in connection with such transfer or distribution, and (4) in the case of clauses (a)(vii), (viii), (ix) and (x) above, no filing under the Exchange Act or other public filing, report or announcement shall be voluntarily made, and if any such filing, report or announcement shall be legally required during the Lock-Up Period, such filing, report or announcement shall clearly indicate in the footnotes thereto (x) the circumstances of such transfer or distribution and (y) in the case of a transfer or distribution pursuant to clause (a)(vii) above, that the donee, devisee, transferee or distributee has agreed to be bound by a lock-up agreement;
B.in connection with shares of Class A common stock held by investment funds and accounts advised by the transferor, pledging and/or lending or hypothecating such shares pursuant to standard margin and pledge agreements entered into by such investment funds or accounts with its brokers in the ordinary course of their business;
C.entering into a 10b5-1 Plan under the Exchange Act relating to the transfer, sale or other disposition of the undersigned’s Lock-Up Securities, if then permitted by the Company, provided that none of the securities subject to such plan may be transferred, sold or otherwise disposed of until after the expiration of the Lock-Up Period and no public announcement, report or filing under the Exchange Act, or any other public filing, report or announcement, shall be required or shall be voluntarily made regarding the establishment of such plan during the Lock-Up Period; and
D.transfers of the Lock-Up Securities pursuant to a bona fide third-party tender offer, merger, consolidation or other similar transaction that is approved by the board of directors of the Company and made to all holders of the Company’s capital stock involving a Change of Control of the Company (for purposes hereof, “Change of Control” shall mean the transfer (whether by tender offer, merger, consolidation or other similar transaction), in one transaction or a series of related transactions, to a person or group of affiliated persons, of shares of capital stock if, after such transfer, such person or group of affiliated persons would hold at least a majority of the outstanding voting securities of the Company (or the surviving entity)); provided that in the event that such tender offer, merger, consolidation or other similar transaction is not completed, the Lock-Up Securities shall remain subject to the provisions of the lock-up agreements.
Jefferies LLC may, in its sole discretion, and at any time or from time to time before the termination of the 60-day period, release all or any portion of the securities subject to lock-up agreements. There are no existing
S-62
agreements providing consent to the sale of shares prior to the expiration of the lock-up period between the underwriter and any of our stockholders who will execute a lock-up agreement.
Stabilization
The underwriter has advised us that it, pursuant to Regulation M under the Securities Exchange Act of 1934, as amended, may engage in short sale transactions, stabilizing transactions, syndicate covering transactions or the imposition of penalty bids in connection with this offering. These activities may have the effect of stabilizing or maintaining the market price of the Class A common stock at a level above that which might otherwise prevail in the open market. Establishing short sales positions may involve either “covered” short sales or “naked” short sales.
“Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional shares of our Class A common stock in this offering. The underwriters may close out any covered short position by either exercising their option to purchase additional shares of Class A common stock or purchasing shares of our Class A common stock in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the option to purchase additional shares.
“Naked” short sales are sales in excess of the option to purchase additional shares of our Class A common stock. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of shares of our Class A common stock in the open market after pricing that could adversely affect investors who purchase in this offering.
A stabilizing bid is a bid for the purchase of shares of Class A common stock on behalf of the underwriter for the purpose of fixing or maintaining the price of the Class A common stock. A syndicate covering transaction is the bid for or the purchase of shares of Class A common stock on behalf of the underwriter to reduce a short position incurred by the underwriter in connection with the offering. Similar to other purchase transactions, the underwriter’s purchases to cover the syndicate short sales may have the effect of raising or maintaining the market price of our Class A common stock or preventing or retarding a decline in the market price of our Class A common stock. As a result, the price of our Class A common stock may be higher than the price that might otherwise exist in the open market. A penalty bid is an arrangement permitting the underwriter to reclaim the selling concession otherwise accruing to a syndicate member in connection with the offering if the Class A common stock originally sold by such syndicate member are purchased in a syndicate covering transaction and therefore have not been effectively placed by such syndicate member.
Neither we nor the underwriter make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our Class A common stock. The underwriter is not obligated to engage in these activities and, if commenced, any of the activities may be discontinued at any time.
The underwriter may also engage in passive market making transactions in our Class A common stock on The NASDAQ Stock Market, LLC in accordance with Rule 103 of Regulation M during a period before the commencement of offers or sales of shares of our Class A common stock in this offering and extending through the completion of distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, that bid must then be lowered when specified purchase limits are exceeded.
Electronic Distribution
A prospectus supplement in electronic format may be made available by e-mail or on the web sites or through online services maintained by the underwriter or its affiliates. In those cases, prospective investors may view offering terms online and may be allowed to place orders online. The underwriter may agree with us to allocate a specific number of shares of Class A common stock for sale to online brokerage account holders. Any such allocation for online distributions will be made by the underwriter on the same basis as other allocations. Other than the prospectus supplement in electronic format, the information on the underwriter’s web site and any information
S-63
contained in any other web site maintained by the underwriter is not part of this prospectus supplement, has not been approved and/or endorsed by us or the underwriter and should not be relied upon by investors.
Other Activities and Relationships
The underwriter and certain of its affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, investment research, principal investment, hedging, financing and brokerage activities. The underwriter and certain of its affiliates have, from time to time, performed, and may in the future perform, various commercial and investment banking and financial advisory services for us and our affiliates, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the underwriter and certain of its affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers, and such investment and securities activities may involve securities and/or instruments issued by us and our affiliates. If the underwriter or its affiliates have a lending relationship with us, they routinely hedge their credit exposure to us consistent with their customary risk management policies. The underwriter and its affiliates may hedge such exposure by entering into transactions which consist of either the purchase of credit default swaps or the creation of short positions in our securities or the securities of our affiliates, including potentially the Class A common stock offered hereby. Any such short positions could adversely affect future trading prices of the Class A common stock offered hereby. The underwriter and certain of its affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments.
Disclaimers About Non-U.S. Jurisdictions
Australia
This prospectus supplement is not a disclosure document for the purposes of Australia’s Corporations Act 2001 (Cth) of Australia, or Corporations Act, is not lodged with the Australian Securities & Investments Commission and is only directed to the categories of exempt persons set out below. Accordingly, if you receive this prospectus supplement in Australia:
You confirm and warrant that you are either:
•a “sophisticated investor” under section 708(8)(a) or (b) of the Corporations Act;
•a “sophisticated investor” under section 708(8)(c) or (d) of the Corporations Act and that you have provided an accountant’s certificate to the company which complies with the requirements of section 708(8)(c)(i) or (ii) of the Corporations Act and related regulations before the offer has been made;
•a person associated with the company under Section 708(12) of the Corporations Act; or
•a “professional investor” within the meaning of section 708(11)(a) or (b) of the Corporations Act.
To the extent that you are unable to confirm or warrant that you are an exempt sophisticated investor, associated person or professional investor under the Corporations Act, any offer made to you under this prospectus supplement is void and incapable of acceptance.
You warrant and agree that you will not offer any of the securities issued to you pursuant to this prospectus supplement for resale in Australia within 12 months of those securities being issued unless any such resale offer is exempt from the requirement to issue a disclosure document under section 708 of the Corporations Act.
S-64
Canada
(A)Resale Restrictions
The distribution of shares of Class A common stock in Canada is being made only in the provinces of Ontario, Quebec, Alberta, British Columbia, Manitoba, New Brunswick and Nova Scotia on a private placement basis exempt from the requirement that we prepare and file a prospectus with the securities regulatory authorities in each province where trades of these securities are made. Any resale of the shares of our Class A common stock in Canada must be made under applicable securities laws which may vary depending on the relevant jurisdiction, and which may require resales to be made under available statutory exemptions or under a discretionary exemption granted by the applicable Canadian securities regulatory authority. Purchasers are advised to seek legal advice prior to any resale of the shares of Class A common stock.
(B)Representations of Canadian Purchasers
By purchasing shares of our Class A common stock in Canada and accepting delivery of a purchase confirmation, a purchaser is representing to us and the dealer from whom the purchase confirmation is received that:
•the purchaser is entitled under applicable provincial securities laws to purchase the shares of our Class A common stock without the benefit of a prospectus qualified under those securities laws as it is an “accredited investor” as defined under National Instrument 45-106-Prospectus Exemptions or Section 73.3(1) of the Securities Act (Ontario), as applicable,
•the purchaser is a “permitted client” as defined in National Instrument 31-103-Registration Requirements, Exemptions and Ongoing Registrant Obligations,
•where required by law, the purchaser is purchasing as principal and not as agent, and
•the purchaser has reviewed the text above under Resale Restrictions.
(C) Conflicts of Interest
Canadian purchasers are hereby notified that the underwriter is relying on the exemption set out in section 3A.3 or 3A.4, if applicable, of National Instrument 33-105-Underwriting Conflicts from having to provide certain conflict of interest disclosure in this document.
(D) Statutory Rights of Action
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if the prospectus (including any amendment thereto) such as this document contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser of these securities in Canada should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
(E) Enforcement of Legal Rights
All of our directors and officers as well as the experts named herein may be located outside of Canada and, as a result, it may not be possible for Canadian purchasers to effect service of process within Canada upon us or those persons. All or a substantial portion of our assets and the assets of those persons may be located outside of Canada and, as a result, it may not be possible to satisfy a judgment against us or those persons in Canada or to enforce a judgment obtained in Canadian courts against us or those persons outside of Canada.
(F) Taxation and Eligibility for Investment
Canadian purchasers of shares of Class A common stock should consult their own legal and tax advisors with respect to the tax consequences of an investment in the shares of our Class A common stock in their particular
S-65
circumstances and about the eligibility of the shares of our Class A common stock for investment by the purchaser under relevant Canadian legislation.
European Economic Area
In relation to each Member State of the European Economic Area (each a “Relevant State”), no shares have been offered or will be offered pursuant to the offering to the public in that Relevant State prior to the publication of a prospectus in relation to the shares which has been approved by the competent authority in that Relevant State or, where appropriate, approved in another Relevant State and notified to the competent authority in that Relevant State, all in accordance with the Prospectus Regulation, except that the shares may be offered to the public in that Relevant State at any time:
•to any legal entity which is a qualified investor as defined under Article 2 of the Prospectus Regulation;
•to fewer than 150 natural or legal persons (other than qualified investors as defined under Article 2 of the Prospectus Regulation), subject to obtaining the prior consent of underwriter for any such offer; or
•in any other circumstances falling within Article 1(4) of the Prospectus Regulation,
provided that no such offer of the shares shall require us or the underwriter to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation.
For the purposes of this provision, the expression an “offer to the public” in relation to the shares in any Relevant State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for any shares, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
Hong Kong
No shares of our Class A common stock have been offered or sold, and no shares of our Class A common stock may be offered or sold, in Hong Kong, by means of any document, other than to persons whose ordinary business is to buy or sell shares or debentures, whether as principal or agent; or to “professional investors” as defined in the Securities and Futures Ordinance (Cap. 571) of Hong Kong, or the SFO, and any rules made under that Ordinance; or in other circumstances which do not result in the document being a “prospectus” as defined in the Companies Ordinance (Cap. 32) of Hong Kong, or the CO, or which do not constitute an offer or invitation to the public for the purpose of the CO or the SFO. No document, invitation or advertisement relating to the shares of our Class A common stock has been issued or may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is directed at, or the contents of which are likely to be accessed or read by, the public of Hong Kong (except if permitted under the securities laws of Hong Kong) other than with respect to shares of our Class A common stock which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” as defined in the SFO and any rules made under that Ordinance.
This prospectus supplement has not been registered with the Registrar of Companies in Hong Kong. Accordingly, this prospectus supplement may not be issued, circulated or distributed in Hong Kong, and the shares of our Class A common stock may not be offered for subscription to members of the public in Hong Kong. Each person acquiring the shares of our Class A common stock will be required, and is deemed by the acquisition of the shares of our Class A common stock, to confirm that he is aware of the restriction on offers of the shares of our Class A common stock described in this prospectus supplement and the relevant offering documents and that he is not acquiring, and has not been offered any shares of our Class A common stock in circumstances that contravene any such restrictions.
Israel
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968, or the Securities Law, and has not been filed with or approved by the Israel Securities Authority. In Israel, this prospectus supplement
S-66
is being distributed only to, and is directed only at, and any offer of shares is directed only at, (i) a limited number of persons in accordance with the Israeli Securities Law and (ii) investors listed in the first addendum, or the Addendum, to the Israeli Securities Law, consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters, venture capital funds, entities with equity in excess of NIS 50 million and “qualified individuals,” each as defined in the Addendum (as it may be amended from time to time), collectively referred to as qualified investors (in each case, purchasing for their own account or, where permitted under the Addendum, for the accounts of their clients who are investors listed in the Addendum). Qualified investors are required to submit written confirmation that they fall within the scope of the Addendum, are aware of the meaning of same and agree to it.
Japan
The offering has not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948 of Japan, as amended), or FIEL, and the underwriter will not offer or sell any securities, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan (which term as used herein means any person resident in Japan, including any corporation or other entity organized under the laws of Japan), or to others for re-offering or resale, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan, except pursuant to an exemption from the registration requirements of, and otherwise in compliance with, the FIEL and any other applicable laws, regulations and ministerial guidelines of Japan.
Singapore
This prospectus supplement has not been and will not be lodged or registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus supplement and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the Class A common stock may not be circulated or distributed, nor may the Class A common stock be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor under Section 274 of the Securities and Futures Act, Chapter 289 of Singapore, or the SFA, (ii) to a relevant person pursuant to Section 275(1), or any person pursuant to Section 275(1A), and in accordance with the conditions specified in Section 275, of the SFA, or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA.
Where the shares of our Class A common stock are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
•a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or
•a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor,
securities (as defined in Section 239(1) of the SFA) of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust shall not be transferred within six months after that corporation or that trust has acquired the shares of our Class A common stock pursuant to an offer made under Section 275 of the SFA except:
•to an institutional investor or to a relevant person defined in Section 275(2) of the SFA, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA;
•where no consideration is or will be given for the transfer;
•where the transfer is by operation of law;
•as specified in Section 276(7) of the SFA; or
•as specified in Regulation 32 of the Securities and Futures (Offers of Investments) (Shares and Debentures) Regulations 2005 of Singapore.
S-67
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange, or the SIX, or on any other stock exchange or regulated trading facility in Switzerland. This prospectus supplement has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this prospectus supplement nor any other offering or marketing material relating to the securities or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this prospectus supplement nor any other offering or marketing material relating to the offering, us or the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this prospectus supplement will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority FINMA, and the offer of securities has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes, or the CISA. The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of securities.
United Kingdom
No shares have been offered or will be offered pursuant to the offering to the public in the United Kingdom prior to the publication of a prospectus in relation to the shares which has been approved by the Financial Conduct Authority, except that the shares may be offered to the public in the United Kingdom at any time:
•to any legal entity which is a qualified investor as defined under Article 2 of the UK Prospectus Regulation;
•to fewer than 150 natural or legal persons (other than qualified investors as defined under Article 2 of the UK Prospectus Regulation), subject to obtaining the prior consent of the underwriter for any such offer; or
•in any other circumstances falling within Section 86 of the FSMA.
provided that no such offer of the shares shall require the Issuer or any Manager to publish a prospectus pursuant to Section 85 of the FSMA or supplement a prospectus pursuant to Article 23 of the UK Prospectus Regulation. For the purposes of this provision, the expression an “offer to the public” in relation to the shares in the United Kingdom means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for any shares and the expression “UK Prospectus Regulation” means Regulation (EU) 2017/1129 as it forms part of domestic law by virtue of the European Union (Withdrawal) Act 2018.
S-68
LEGAL MATTERS
The validity of the securities offered hereby and certain other legal matters in connection with this offering will be passed upon by Fenwick & West LLP, New York, New York. Certain legal matters relating to this offering will be passed upon for the underwriter by Ropes & Gray LLP, Boston, Massachusetts.
EXPERTS
The consolidated financial statements of GeneDx Holdings Corp. (f/k/a Sema4 Holdings Corp.) appearing in GeneDx Holdings Corp.’s Annual Report (Form 10-K) for the year ended December 31, 2021, have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon included therein, and incorporated herein by reference. Such financial statements are, and audited financial statements to be included in subsequently filed documents will be, incorporated herein in reliance upon the report of Ernst & Young LLP pertaining to such financial statements (to the extent covered by consents filed with the Securities and Exchange Commission) given on the authority of such firm as experts in accounting and auditing.
The combined carve out financial statements of GeneDx, Inc. and subsidiary as of December 31, 2021 and 2020, and for each of the two years in the period ended December 31, 2021, included in the Proxy Statement of GeneDx Holdings Corp. dated March 31, 2022, incorporated by reference in this Prospectus Supplement and Registration Statement, have been audited by Ernst & Young LLP, independent auditors, as set forth in their report thereon, and are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION; INCORPORATION BY REFERENCE
Available Information
We file reports, proxy statements and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements and other information about issuers, such as us, who file electronically with the SEC. The address of that website is http://www.sec.gov.
Our website address is www.sema4.com. The information on our website, however, is not, and should not be deemed to be, a part of this prospectus supplement.
This prospectus supplement and the accompanying prospectus are part of a registration statement that we filed with the SEC and do not contain all of the information in the registration statement. The full registration statement may be obtained from the SEC or us, as provided below. Statements in this prospectus supplement and the accompanying prospectus about these documents are summaries, and each statement is qualified in all respects by reference to the document to which it refers. You should refer to the actual documents for a more complete description of the relevant matters. You may inspect a copy of the registration statement through the SEC’s website, as provided above.
Incorporation by Reference
The SEC’s rules allow us to “incorporate by reference” information into this prospectus supplement and the accompanying prospectus, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is deemed to be part of this prospectus supplement and the accompanying prospectus, and subsequent information that we file with the SEC will automatically update and supersede that information. Any statement contained in this prospectus supplement and the accompanying prospectus or a previously filed document incorporated by reference will be deemed to be modified or superseded for purposes of this prospectus supplement and the accompanying prospectus to the extent that a statement contained in this prospectus supplement and the accompanying prospectus or a subsequently filed document incorporated by reference modifies or replaces that statement.
S-69
This prospectus supplement and the accompanying prospectus incorporate by reference the documents set forth below that have previously been filed with the SEC:
•our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 14, 2022;
•our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2022 filed with the SEC on May 12, 2022, our Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2022 filed with the SEC on August 15, 2022, and our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2022 filed with the SEC on November 14, 2022;
•our Current Reports on Form 8-K filed with the Commission on January 18, 2022 (but only with respect to Items 1.01, 3.02 and 5.02, and Exhibits 2.1, 10.1, 10.2, 10.3 and 10.4 thereto), January 31, 2022, March 14, 2022 (but only with respect to Items 4.02 and 9.01 thereto), April 27, 2022, May 2, 2022 (but only with respect to Items 1.01, 2.01, 3.02, 5.02, 5.03, 8.01, 9.01(a) and 9.01(b), and Exhibits 2.1, 3.1, 10.1, 10.2, 23.1, 99.2 and 99.3 thereto), June 14, 2022 (but only with respect to Items 5.02 and 9.01), July 1, 2022, August 26, 2022 (but only with respect to Items 5.02, 8.01, 9.01(a) and 9.01(b), and Exhibits 10.1, 99.2 and 99.3 thereto), November 14, 2022 (but only with respect to Item 2.05 thereto), December 30, 2022, and January 9, 2023 (but only with respect to Item 5.03 thereto and Exhibits 3.1 and 3.2 thereto);
•the audited combined carve-out balance sheets of GeneDx, Inc. and subsidiary as of December 31, 2021 and 2020, the related audited combined carve-out statements of comprehensive loss, equity and cash flows for each of the two years in the period ended December 31, 2021, and the related notes are included in our definitive proxy statement filed with the SEC on March 31, 2022 beginning on page F-41; and
•the description of our Class A common stock contained in our Registration Statement on Form 8-A filed with the Commission on August 31, 2020, as updated by the description of our Class A common stock contained in Exhibit 4.4 to our Annual Report on Form 10-K, including any subsequent amendments or reports filed for the purpose of updating such description.
All reports and other documents we subsequently file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act pursuant to this prospectus supplement and the accompanying prospectus, prior to the termination of this offering, including all such documents we may file with the SEC after the date of the initial registration statement and prior to the effectiveness of the registration statement, but excluding any information furnished to, rather than filed with, the SEC, will also be incorporated by reference into this prospectus supplement and the accompanying prospectus and deemed to be part of this prospectus supplement and the accompanying prospectus from the date of the filing of such reports and documents.
We will provide, without charge, to each person, including any beneficial owner, to whom a copy of this prospectus supplement is delivered, upon written or oral request of such person, a copy of any or all of the documents incorporated by reference in this prospectus supplement, other than exhibits to such documents unless such exhibits are specifically incorporated by reference into such documents. Requests may be made by telephone at 1(800) 298-6470, or by sending a written request to GeneDx Holdings Corp., 333 Ludlow Street, North Tower, 8th Floor, Stamford, Connecticut 06902, Attention: Investor Relations.
S-70
PROSPECTUS
$300,000,000

Sema4 Holdings Corp.
Class A Common Stock, Preferred Stock,
Debt Securities, Warrants, Subscription Rights and Units
From time to time, we may offer up to $300,000,000 aggregate dollar amount of shares of our Class A common stock (the “Class A common stock”) or preferred stock, debt securities, warrants to purchase our Class A common stock, preferred stock or debt securities, subscription rights to purchase our Class A common stock, preferred stock or debt securities and/or units consisting of some or all of these securities, in any combination, together or separately, in one or more offerings, in amounts, at prices and on the terms that we will determine at the time of the offering and which will be set forth in a prospectus supplement and, if permitted, any related free writing prospectus. The prospectus supplement and, if permitted, any related free writing prospectus may also add, update or change information contained in this prospectus. The total amount of these securities will have an initial aggregate offering price of up to $300,000,000.
You should read this prospectus, the information incorporated, or deemed to be incorporated, by reference in this prospectus, and any applicable prospectus supplement and, if permitted, related free writing prospectus carefully before you invest.
Our Class A common stock and public warrants are traded on The Nasdaq Global Select Market (the “Nasdaq”) under the symbols “SMFR” and “SMFRW,” respectively. On August 25, 2022, the last reported sales price of our Class A common stock was $1.22 per share and the last reported sales price of our public warrants was $0.31 per warrant. The applicable prospectus supplement and, if permitted, any related free writing prospectus will contain information, where applicable, as to any other listing on The Nasdaq Global Select Market or any securities market or exchange of the securities covered by the prospectus supplement and, if permitted, any related free writing prospectus.
We are an “emerging growth company” as defined in Section 2(a) of the Securities Act of 1933, as amended, and, as such, have elected to comply with certain reduced disclosure and regulatory requirements. An investment in our securities involves a high degree of risk. You should carefully consider the information under the heading “Risk Factors” beginning on page 5 of this prospectus, as well as the sections entitled “Risk Factors” beginning on page 30 of our Annual Report on Form 10-K for the year ended December 31, 2021, beginning on page 46 of our Quarterly Report on Form 10-Q for the quarter ended March 31, 2022 and beginning on page 60 of our Quarterly Report on Form 10-Q for the quarter ended June 30, 2022, which reports are incorporated by reference in this prospectus, before investing in our securities.
Class A common stock, preferred stock, debt securities, warrants, subscription rights and/or units may be sold by us to or through underwriters or dealers, directly to purchasers or through agents designated from time to time. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus. If any underwriters, dealers or agents are involved in the sale of any securities with respect to which this prospectus is being delivered, the names of such underwriters or agents and any applicable fees, discounts or commissions, details regarding over-allotment options, if any, and the net proceeds to us will be set forth in a prospectus supplement. The price to the public of such securities and the net proceeds we expect to receive from such sale will also be set forth in a prospectus supplement.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is September 7, 2022
TABLE OF CONTENTS
| PAGE | |||||
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission (the “SEC”) using a “shelf” registration process. Under this shelf registration process, from time to time, we may sell any combination of the securities described in this prospectus in one or more offerings, up to a total dollar amount of $300,000,000. We have provided to you in this prospectus a general description of the securities we may offer. Each time we sell securities under this shelf registration process, we will provide a prospectus supplement that will contain specific information about the terms of the offering. We may also add, update or change in the prospectus supplement any of the information contained in this prospectus. To the extent there is a conflict between the information contained in this prospectus and the prospectus supplement, you should rely on the information in the prospectus supplement; provided that, if any statement in one of these documents is inconsistent with a statement in another document having a later date—for example, a document incorporated by reference in this prospectus or any prospectus supplement—the statement in the document having the later date modifies or supersedes the earlier statement. You should read both this prospectus and any prospectus supplement together with additional information described under the heading “Where You Can Find More Information” and “Incorporation of Information by Reference.”
Neither we, nor any agent, underwriter or dealer have authorized anyone to give you any information or to make any representation other than the information and representations contained in or incorporated by reference into this prospectus or any applicable prospectus supplement. We and any agent, underwriter or dealer take no responsibility for, and can provide no assurance as to the reliability of, any other information others may give you. You may not imply from the delivery of this prospectus and any applicable prospectus supplement, nor from a sale made under this prospectus and any applicable prospectus supplement, that our affairs are unchanged since the date of this prospectus and any applicable prospectus supplement or that the information contained in any document incorporated by reference is accurate as of any date other than the date of the document incorporated by reference, regardless of the time of delivery of this prospectus and any applicable prospectus supplement or any sale of a security. This prospectus and any applicable prospectus supplement may only be used where it is legal to sell the securities.
THIS PROSPECTUS MAY NOT BE USED TO OFFER AND SELL SECURITIES UNLESS IT IS ACCOMPANIED BY AN ADDITIONAL PROSPECTUS OR A PROSPECTUS SUPPLEMENT.
Unless the context otherwise requires, references in this prospectus to the “Company,” “Sema4” and “we,” “us” and “our” refer to (i) Mount Sinai Genomics, Inc. d/b/a as Sema4 (“Legacy Sema4”) prior to the consummation of our business combination with CM Life Sciences, Inc. (“CMLS”) on July 22, 2021 (the “Business Combination”) and (ii) Sema4 Holdings Corp. (“Sema4 Holdings”) and its consolidated subsidiaries following the consummation of the Business Combination.
1
SELECTED DEFINITIONS
Unless otherwise stated in this prospectus or the context otherwise requires, references to:
“Amended and Restated Certificate of Incorporation” mean our Third Amended and Restated Certificate of Incorporation, dated as of July 22, 2021, as amended by the Amendment to the Amended and Restated Certificate of Incorporation, dated as of April 29, 2022.
“Board” or “Board of Directors” mean the board of directors of the Company.
“Business Combination” mean the transactions contemplated by the Business Combination Merger Agreement pursuant to which Legacy Sema4 consummated its business combination with CMLS on July 22, 2021.
“Business Combination Merger Agreement” mean that certain Agreement and Plan of Merger, dated as of February 9, 2021, as amended, by and among CMLS, S-IV Sub, Inc. and Legacy Sema4.
“CMLS” mean CM Life Sciences, Inc. prior to the closing of the Business Combination.
“Former Sponsor” mean CMLS Holdings LLC, a Delaware limited liability company.
“GeneDx” mean GeneDx, LLC, a Delaware limited liability company (formerly, GeneDx, Inc., a New Jersey corporation), which we acquired on April 29, 2022.
“IPO” mean the Company’s initial public offering, consummated on September 4, 2020, of 44,275,000 units (including 5,775,000 units that were subsequently issued to the underwriters in connection with the partial exercise of their over-allotment option) at $10.00 per unit.
“private placement warrants” mean the 7,236,667 warrants originally issued to the Former Sponsor and certain of the other initial stockholders of CMLS in a private placement in connection with our IPO.
“public warrants” mean the 14,758,305 warrants included in the units issued in our IPO.
“stockholders” mean holders of shares of our Class A common stock.
“warrant agreement” mean the warrant agreement between us and Continental Stock Transfer & Trust Company, as warrant agent, governing the terms of the private placement warrants and the public warrants.
2
PROSPECTUS SUMMARY
This summary may not contain all the information that you should consider before investing in our securities. You should read the entire prospectus and the information incorporated by reference in this prospectus carefully, including “Risk Factors” and the financial statements and related notes incorporated by reference herein, before making an investment decision.
Company Overview
We are a health insights company dedicated to accelerating the use of genomics and leveraging large-scale clinical data to enhance the standard of care through the more extensive use of precision medicine. Our mission is to unlock insights from data, leading to healthier lives and a healthier society. We are one of the largest and most advanced providers of genomic testing in the U.S. and have an industry-leading health information database to transform patient care and improve therapeutic development.
We are focused on effectively delivering our portfolio of genomic and data solutions to guide patients through their family health journey. That includes family planning, delivery, pediatrics, hereditary cancer screening, and rare disorders for children and adults. We are committed to helping families make better health decisions with our panels, exome, and genome – fueled by our interpretation platform designed for a genome’s worth of information and a data engine built to combine genomic and clinical data to deliver better insights.
Our integrated information platform leverages longitudinal patient data, artificial intelligence (“AI”)-driven predictive modeling, and genomics in combination with other molecular and high-dimensional data in our efforts both to deliver better outcomes for patients and to transform the practice of medicine, including how disease is diagnosed, treated, and prevented. We now maintain a database that includes patient data available for research on approximately 12 million patients from a number of public and proprietary sources, including more than five million patients available with clinical data through our partner health systems and genomic testing solutions. Our data asset also includes one of the world’s largest datasets of approximately 400,000 clinical exomes, the vast majority of which are associated with rare disease, providing us an unparalleled platform for pharmaceutical and biotech (“Biopharma”) companies to leverage.
Today, by providing differentiated insights through diagnostic testing solutions to physicians and patients across the United States (the “U.S.”) in areas such as reproductive and women’s health, pediatric and rare disease health, and population health, , we are reimbursed by payors, providers, and patients for providing these services. In collaboration with Biopharma companies, we receive payments for a broad range of services relating to the aggregated data on our information platform, such as consenting and recontacting patients, the development and implementation of a wide range of predictive models, including drug discovery programs, conducting real-world evidence studies, and aiding in the identification and recruitment of patients into clinical trials. Over the next several years, we expect to focus on expanding the revenue from our health system and Biopharma partners, while also working to continue to grow the volumes and revenues from our diagnostics test solutions.
While there are many companies seeking to harness the potential of “big data” to address the challenges within the healthcare ecosystem, we believe that few have the scale of our company combined with our revenue-generating diagnostics testing business and origins as a company conceived and nurtured within a world-class health system. These characteristics have enabled us to build a significant and highly differentiated technological and informational asset positioned to drive precision medicine solutions into the standard of care in an unparalleled way.
The Securities We May Offer
With this prospectus, we may offer shares of our Class A common stock or preferred stock, debt securities, warrants to purchase our Class A common stock, preferred stock or debt securities, subscription rights to purchase our Class A common stock, preferred stock or debt securities, and/or units consisting of some or all of these securities in any combination. The aggregate offering price of securities that we offer with this prospectus will not exceed $300,000,000. Each time we offer securities with this prospectus, we will provide offerees with a prospectus supplement that will contain the specific terms of the securities being offered. The following is a summary of the securities we may offer with this prospectus.
3
Class A Common Stock
We may offer shares of our Class A common stock, par value $0.0001 per share.
Preferred Stock
We may offer shares of our preferred stock, par value $0.0001 per share, in one or more series. Our board of directors (the “Board”) or a committee designated by the Board will determine the dividend, voting, conversion and other rights of the series of shares of preferred stock being offered. Each series of preferred stock will be more fully described in the particular prospectus supplement that will accompany this prospectus, including redemption provisions, rights in the event of our liquidation, dissolution or the winding up, voting rights and rights to convert into Class A common stock.
Debt Securities
We may offer general obligations, which may be secured or unsecured, senior or subordinated and convertible into shares of our Class A common stock or preferred stock. In this prospectus, we refer to the senior debt securities and the subordinated debt securities together as the “debt securities.” Our Board will determine the terms of each series of debt securities being offered.
We will issue the debt securities under an indenture between us and a trustee. In this prospectus, we have summarized general features of the debt securities from the indenture. We encourage you to read the indenture, which is an exhibit to the registration statement of which this prospectus is a part. The actual indenture we enter into in connection with an offering of debt securities may differ significantly from the form of indenture we have filed.
Warrants
We may offer warrants for the purchase of debt securities, shares of preferred stock or shares of Class A common stock. We may issue warrants independently or together with other securities. Our Board will determine the terms of the warrants.
Subscription Rights
We may offer subscription rights for the purchase of Class A common stock, preferred stock or debt securities. We may issue subscription rights independently or together with other securities. Our Board will determine the terms of the subscription rights.
Units
We may offer units consisting of some or all of the securities described above, in any combination, including Class A common stock, preferred stock, warrants and/or debt securities. The terms of these units will be set forth in a prospectus supplement. The description of the terms of these units in the related prospectus supplement will not be complete. You should refer to the applicable form of unit and unit agreement for complete information with respect to these units.
Corporate Information
We were incorporated on July 10, 2020 as a special purpose acquisition company and a Delaware corporation under the name CM Life Sciences, Inc. (“CMLS”). On September 4, 2020, CMLS completed its initial public offering. On July 22, 2021, CMLS consummated the Business Combination with Legacy Sema4 pursuant to the Business Combination Merger Agreement. In connection with the Business Combination, CMLS changed its name to Sema4 Holdings Corp. (“Sema4 Holdings”).
Our address is 333 Ludlow Street, North Tower, 8th Floor, Stamford, Connecticut 06902. Our telephone number is 1(800) 298-6470. Our website address is https://sema4.com. Information contained on our website or connected thereto does not constitute part of, and is not incorporated by reference into, this prospectus or the registration statement of which it forms a part.
4
RISK FACTORS
An investment in our securities involves a high degree of risk. You should consider the risk factors described in the “Risk Factors” sections of our Annual Report on Form 10-K for the fiscal year ended December 31, 2021, our Quarterly Report on Form 10-Q for the quarter ended March 31, 2022 and our Quarterly Report on Form 10-Q for the quarter ended June 30, 2022, which reports are incorporated herein by reference, in addition to the factors set forth below and other information contained in or incorporated by reference in this prospectus or in any prospectus supplement or post-effective amendment, if required, before purchasing any of our securities. We may face additional risks and uncertainties that are not presently known to us, or that we currently deem immaterial, which may also impair our business or financial condition. See “Where You Can Find More Information,” “Incorporation of Information by Reference” and “Cautionary Note Regarding Forward-Looking Statements.”
5
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain matters discussed in this prospectus and the documents incorporated by reference in this prospectus may constitute forward-looking statements for purposes of the Securities Act of 1933, as amended (the “Securities Act”), and the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from the future results, performance or achievements expressed or implied by such forward-looking statements. The words “anticipate,” “believe,” “estimate,” “may,” “expect” and similar expressions are generally intended to identify forward-looking statements. Our actual results may differ materially from the results anticipated in these forward-looking statements due to a variety of factors, including, without limitation, those discussed in the section entitled “Risk Factors,” and elsewhere in this prospectus and the documents incorporated by reference herein, where such forward-looking statements appear. All written or oral forward-looking statements attributable to us are expressly qualified in their entirety by these cautionary statements. Such forward-looking statements include, but are not limited to, statements about:
•our ability to realize the benefits expected from our April 2022 acquisition of GeneDx;
•our estimates of the sufficiency of our existing capital resources combined with future anticipated cash flows to finance our operating requirements;
•our expected losses;
•our expectations for incurring capital expenditures;
•unforeseen circumstances or other disruptions to normal business operations, including supply chain interruptions and manufacturing constraints, arising from or related to the ongoing COVID-19 pandemic;
•our expectations regarding our plans to exit out somatic tumor testing business and the associated cost savings and impact on our gross margins;
•our expectations for generating revenue or becoming profitable on a sustained basis;
•our expectations or ability to enter into service, collaboration and other partnership agreements;
•our expectations or ability to build our own commercial infrastructure to scale market and sell our products;
•actions or authorizations by the U.S. Food and Drug Administration (the “FDA”) or other regulatory authorities;
•risks related to governmental regulation and other legal obligations, including privacy, data protection, information security, consumer protection, and anti-corruption and anti-bribery;
•our ability to obtain and maintain intellectual property protection for our product candidates;
•our ability to compete against existing and emerging technologies;
•our stock price and its volatility;
•our ability to attract and retain key personnel;
•third-party payor reimbursement and coverage decisions, negotiations and settlements;
•our reliance on third-party laboratories and service providers for our test volume in connection with our diagnostic solutions and data programs;
•our ability to satisfy Nasdaq listing rules;
•our expectations for future capital requirements;
6
•our accounting estimates, including the adequacy of our reserves for third party payor claims and our estimates of the fair value of milestone payments related to the April 2022 acquisition of GeneDx;
•our ability to successfully implement our business strategy; and
•other factors detailed under the section entitled “Risk Factors.”
The forward-looking statements contained in this prospectus and the documents incorporated by reference herein reflect our views and assumptions only as of the date of this prospectus or such document, as applicable. Except as required by law, we assume no responsibility for updating any forward-looking statements.
We qualify all of our forward-looking statements by these cautionary statements. In addition, with respect to all of our forward-looking statements, we claim the protection of the safe harbor for forward-looking statements contained in the Private Securities Litigation Reform Act of 1995.
7
WHERE YOU CAN FIND MORE INFORMATION
We file reports, proxy statements and other information with the SEC. The SEC maintains a website that contains reports, proxy and information statements and other information about issuers, such as us, who file electronically with the SEC. The address of that website is http://www.sec.gov.
Our website address is www.sema4.com. The information on our website, however, is not, and should not be deemed to be, a part of this prospectus.
This prospectus and any applicable prospectus supplement are part of a registration statement that we filed with the SEC and do not contain all of the information in the registration statement. The full registration statement may be obtained from the SEC or us, as provided below. Statements in this prospectus or any prospectus supplement about these documents are summaries, and each statement is qualified in all respects by reference to the document to which it refers. You should refer to the actual documents for a more complete description of the relevant matters. You may inspect a copy of the registration statement through the SEC’s website, as provided above.
8
INCORPORATION OF INFORMATION BY REFERENCE
The SEC’s rules allow us to “incorporate by reference” information into this prospectus, which means that we can disclose important information to you by referring you to another document filed separately with the SEC. The information incorporated by reference is deemed to be part of this prospectus, and subsequent information that we file with the SEC will automatically update and supersede that information. Any statement contained in this prospectus or a previously filed document incorporated by reference will be deemed to be modified or superseded for purposes of this prospectus to the extent that a statement contained in this prospectus or a subsequently filed document incorporated by reference modifies or replaces that statement.
This prospectus incorporates by reference the documents set forth below that have previously been filed with the SEC:
•our Annual Report on Form 10-K for the year ended December 31, 2021, filed with the SEC on March 14, 2022;
•our Quarterly Report on Form 10-Q for the quarterly period ended March 31, 2022, filed with the SEC on May 12, 2022, and our Quarterly Report on Form 10-Q for the quarterly period ended June 30, 2022, filed with the SEC on August 15, 2022;
•our Current Reports on Form 8-K filed with the SEC on January 18, 2022 (but only with respect to Items 1.01, 3.02, 5.02 and Exhibits 2.1, 10.1, 10.2, 10.3 and 10.4 thereto), January 31, 2022, March 14, 2022 (but only with respect to Item 4.02 and 9.01 thereto), April 27, 2022, May 2, 2022 (but only with respect to Items 1.01, 2.01, 3.02, 5.02, 5.03, 8.01, 9.01(a) and 9.01(b), and Exhibits 2.1, 3.1, 10.1, 10.2, 23.1, 99.2 and 99.3 thereto), June 14, 2022 (but only with respect to Items 5.02 and 9.01), July 1, 2022, and August 26, 2022 (but only with respect to Items 5.02, 8.01, 9.01(a) and 9.01(b), and Exhibits 10.1, 99.2 and 99.3);
•the audited combined carve-out balance sheets of GeneDx and subsidiary as of December 31, 2021 and 2020, the related audited combined carve out statements of comprehensive loss, equity and cash flows for each of the two years in the period ended December 31, 2021, and the related notes are included in our definitive proxy statement filed with the SEC on March 31, 2022 beginning on page F-41; and
•the description of our Class A common stock contained in our Registration Statement on Form 8-A filed with the SEC on August 31, 2020, as updated by the description of our Class A common stock contained in Exhibit 4.4 to our Annual Report on Form 10-K for the year ended December 31, 2021, including any subsequent amendments or reports filed for the purpose of updating such description.
All reports and other documents we subsequently file pursuant to Section 13(a), 13(c), 14 or 15(d) of the Exchange Act in this prospectus, prior to the termination of this offering, including all such documents we may file with the SEC after the date of the initial registration statement and prior to the effectiveness of the registration statement, but excluding any information furnished to, rather than filed with, the SEC, will also be incorporated by reference into this prospectus and deemed to be part of this prospectus from the date of the filing of such reports and documents.
We will provide, without charge, to each person, including any beneficial owner, to whom a copy of this prospectus is delivered, upon written or oral request of such person, a copy of any or all of the documents incorporated by reference in this prospectus, other than exhibits to such documents unless such exhibits are specifically incorporated by reference into such documents. Requests may be made by telephone at 1(800) 298-6470, or by sending a written request to Sema4 Holdings Corp., 333 Ludlow Street, North Tower, 8th Floor, Stamford, Connecticut 06902, Attention: Investor Relations.
9
USE OF PROCEEDS
We will retain broad discretion over the use of the net proceeds to us from the sale of our securities under this prospectus. General corporate purposes may include additions to working capital, repayment or redemption of existing indebtedness, and strategic investment opportunities. Unless we state otherwise in the applicable prospectus supplement, pending the application of net proceeds, we expect to invest the net proceeds in investment grade, interest-bearing securities.
10
PLAN OF DISTRIBUTION
We may sell the securities covered by this prospectus to one or more underwriters for public offering and sale by them, and may also sell the securities to investors directly or through agents. We will name any underwriter or agent involved in the offer and sale of securities in the applicable prospectus supplement. We have reserved the right to sell or exchange securities directly to investors on our own behalf in jurisdictions where we are authorized to do so. We may distribute the securities from time to time in one or more transactions:
•at a fixed price or prices, which may be changed;
•at market prices prevailing at the time of sale;
•at prices related to such prevailing market prices; or
•at negotiated prices.
We may directly solicit offers to purchase the securities being offered by this prospectus. We may also designate agents to solicit offers to purchase the securities from time to time. We will name in a prospectus supplement any agent involved in the offer or sale of our securities. Unless otherwise indicated in a prospectus supplement, an agent will be acting on a best efforts basis, and a dealer will purchase securities as a principal for resale at varying prices to be determined by the dealer.
If we utilize an underwriter in the sale of the securities being offered by this prospectus, we will execute an underwriting agreement with the underwriter at the time of sale and we will provide the name of any underwriter in the prospectus supplement that the underwriter will use to make resales of the securities to the public. In connection with the sale of the securities, we, or the purchasers of securities for whom the underwriter may act as agent, may compensate the underwriter in the form of underwriting discounts or commissions. The underwriter may sell the securities to or through dealers, and those dealers may receive compensation in the form of discounts, concessions or commissions from the underwriters or commissions from the purchasers for whom they may act as agent.
We will provide in the applicable prospectus supplement any compensation we pay to underwriters, dealers, or agents in connection with the offering of the securities, and any discounts, concessions or commissions allowed by underwriters to participating dealers. Underwriters, dealers and agents participating in the distribution of the securities may be deemed to be underwriters within the meaning of the Securities Act, and any discounts and commissions received by them and any profit realized by them on resale of the securities may be deemed to be underwriting discounts and commissions. We may enter into agreements to indemnify underwriters, dealers and agents against civil liabilities, including liabilities under the Securities Act, and to reimburse them for certain expenses. We may grant underwriters who participate in the distribution of our securities under this prospectus an option to purchase additional securities to cover any over-allotments in connection with the distribution.
The securities we offer under this prospectus may or may not be listed through The Nasdaq Global Select Market or any other securities exchange. To facilitate the offering of securities, certain persons participating in the offering may engage in transactions that stabilize, maintain or otherwise affect the price of the securities. This may include short sales of the securities, which involves the sale by persons participating in the offering of more securities than we sold to them. In these circumstances, these persons would cover such short positions by making purchases in the open market or by exercising their option to purchase additional securities. In addition, these persons may stabilize or maintain the price of the securities by bidding for or purchasing securities in the open market or by imposing penalty bids, whereby selling concessions allowed to dealers participating in the offering may be reclaimed if securities sold by them are repurchased in connection with stabilization transactions. The effect of these transactions may be to stabilize or maintain the market price of the securities at a level above that which might otherwise prevail in the open market. These transactions may be discontinued at any time.
We may issue to our existing security holders, through a dividend or similar distribution, subscription rights to purchase our securities, which may or may not be transferable. In any distribution of subscription rights to our existing security holders, if all of the underlying securities are not subscribed for, we may then sell the unsubscribed securities directly to third parties or may engage the services of one or more underwriters, dealers or agents,
11
including standby underwriters, to sell the unsubscribed securities to third parties. The applicable prospectus supplement will describe the specific terms of any offering of our securities through the issuance of subscription rights, including, if applicable, the material terms of any standby underwriting or purchase arrangement.
We may engage in at the market offerings into an existing trading market in accordance with Rule 415(a)(4) under the Securities Act. In addition, we may enter into derivative transactions with third parties, or sell securities not covered by this prospectus to third parties in privately negotiated transactions. If the applicable prospectus supplement indicates, in connection with those derivatives, the third parties may sell securities covered by this prospectus and the applicable prospectus supplement, including short sale transactions. If so, the third party may use securities pledged by us or borrowed from us or others to settle those sales or to close out any related open borrowings of securities, and they may use securities received from us in settlement of those derivatives to close out any related open borrowings of securities. The third party in these sale transactions will be an underwriter and will be identified in the applicable prospectus supplement. In addition, we may otherwise loan or pledge securities to a financial institution or other third party that in turn may sell the securities short using this prospectus. The financial institution or other third party may transfer its economic short position to investors in our securities or in connection with a concurrent offering of other securities.
We will file a prospectus supplement to describe the terms of any offering of our securities covered by this prospectus. The prospectus supplement will disclose:
•the terms of the offer;
•the names of any underwriters, including any managing underwriters, as well as any dealers or agents;
•the purchase price of the securities from us;
•the net proceeds to us from the sale of the securities;
•any delayed delivery arrangements;
•any over-allotment or other options under which underwriters, if any, may purchase additional securities from us;
•any underwriting discounts, commissions or other items constituting underwriters’ compensation, and any commissions paid to agents;
•in a subscription rights offering, whether we have engaged dealer-managers to facilitate the offering or subscription, including their name or names and compensation;
•any public offering price; and
•other facts material to the transaction.
We will bear all or substantially all of the costs, expenses and fees in connection with the registration of our securities under this prospectus. The underwriters, dealers and agents may engage in transactions with us, or perform services for us, in the ordinary course of business.
12
DESCRIPTION OF CAPITAL STOCK
The following summary sets forth certain material terms and provisions of our capital stock. This description also summarizes relevant provisions of the General Corporation Law of Delaware (the “DGCL”). The following description is a summary and does not purport to be a complete description of the rights and preferences of our capital stock. It is subject to, and qualified in its entirety by reference to, the applicable provisions of the DGCL and our third amended and restated certificate of incorporation, as amended (our “Amended and Restated Certificate of Incorporation”) and our restated bylaws (our “Bylaws”), each of which is incorporated by reference as an exhibit to the registration statement of which this prospectus forms a part. We encourage you to read our Amended and Certificate of Incorporation, our Bylaws and the applicable provisions of the DGCL for additional information.
General
Our authorized capital stock consists of 1,000,000,000 shares of Class A common stock, $0.0001 par value per share, and 1,000,000 shares of preferred stock, $0.0001 par value per share.
As of June 30, 2022, there were 379,896,799 shares of our Class A common stock outstanding, no shares of preferred stock outstanding and 21,994,972 warrants outstanding. The outstanding shares of our Class A common stock are duly authorized, validly issued, fully paid and non-assessable.
Common Stock
Our Amended and Restated Certificate of Incorporation provides that each share of our Class A common stock has the same relative rights and is identical in all respects to each other share of our Class A common stock. The rights, preferences and privileges of holders of our Class A common stock are subject to the rights, preferences and privileges of the holders of shares of any series of preferred stock that we have issued or may issue in the future.
Voting Power
Except as otherwise required by law or as otherwise provided in any certificate of designation for any series of preferred stock, or under our Amended and Restated Certificate of Incorporation, the holders of Class A common stock possess all voting power for the election of our directors and all other matters requiring stockholder action and are entitled to one vote per share on matters to be voted on by stockholders. The holders of Class A common stock shall at all times vote together as one class on all matters submitted to a vote of the holders of Class A common stock under our Amended and Restated Certificate of Incorporation.
Dividends
Subject to the rights, if any of the holders of any outstanding shares of preferred stock, under our Amended and Restated Certificate of Incorporation, holders of Class A common stock are entitled to receive such dividends and other distributions, if any, as may be declared from time to time by our Board in its discretion out of funds legally available therefor and shall share equally on a per share basis in such dividends and distributions.
Liquidation, Dissolution and Winding Up
In the event of the voluntary or involuntary liquidation, dissolution or winding-up of the Company, under our Amended and Restated Certificate of Incorporation, holders of Class A common stock will be entitled to receive all the remaining assets of the Company available for distribution to stockholders, ratably in proportion to the number of shares of Class A common stock held by them, after the rights of the holders of the preferred stock have been satisfied.
Preemptive or Other Rights
Under our Amended and Restated Certificate of Incorporation, our stockholders have no preemptive or other subscription rights and there are no sinking fund or redemption provisions applicable to our Class A common stock.
13
Election of Directors
Our Amended and Restated Certificate of Incorporation provides for a classified board of directors with staggered three-year terms, consisting of the three classes: Class I, Class II and Class III. The term of the Class I Directors will expire at our 2025 annual meeting of the stockholders, the term of the Class II Directors will expire at our 2023 annual meeting of the stockholders and the term of the Class III Directors will expire at our 2024 annual meeting of the stockholders.
Preferred Stock
Our Amended and Restated Certificate of Incorporation provides that shares of preferred stock may be issued from time to time in one or more series. Our Board is authorized to fix the voting rights, if any, designations, powers, preferences and relative, participating, optional, special and other rights, if any, and any qualifications, limitations and restrictions thereof, applicable to the shares of each series. Our Board is able, without stockholder approval, to issue preferred stock with voting and other rights that could adversely affect the voting power and other rights of the holders of the Class A common stock and could have anti-takeover effects. The ability of our Board to issue preferred stock without stockholder approval could have the effect of delaying, deferring or preventing a change of control of us or the removal of existing management. We have no preferred stock outstanding at the date hereof. Although we do not currently intend to issue any shares of preferred stock, we cannot assure you that we will not do so in the future.
Certain Anti-Takeover Provisions of Delaware Law and Our Amended and Restated Certificate of Incorporation and Bylaws
Provisions of the DGCL and our Amended and Restated Certificate of Incorporation could make it more difficult to acquire us by means of a tender offer, a proxy contest or otherwise, or to remove incumbent officers and directors. These provisions, summarized below, are intended to discourage coercive takeover practices and inadequate takeover bids and to encourage persons seeking to acquire control of us to first negotiate with the board of directors. We believe that the benefits of these provisions outweigh the disadvantages of discouraging certain takeover or acquisition proposals because, among other things, negotiation of these proposals could result in an improvement of their terms and enhance the ability of our Board to maximize stockholder value. However, these provisions may delay, deter or prevent a merger or acquisition of us that a stockholder might consider is in its best interest, including those attempts that might result in a premium over the prevailing market price of the Class A common stock.
In addition, our Amended and Restated Certificate of Incorporation provide for certain other provisions that may have an anti-takeover effect:
•There is no cumulative voting with respect to the election of directors.
•Our Board is empowered to elect a director to fill a vacancy created by the expansion of the Board or the resignation, death, or removal of a director in certain circumstances.
•Directors may only be removed from the Board for cause.
•Our Board will be classified into three classes of directors. As a result, in most circumstances, a person can gain control of our Board by successfully engaging in a proxy contest at two or more annual meetings.
•A prohibition on stockholder action by written consent, which forces stockholder action to be taken at an annual or special meeting of our stockholders.
•A prohibition on stockholders calling a special meeting and the requirement that a meeting of stockholders may only be called by members of our Board, which may delay the ability of our stockholders to force consideration of a proposal or to take action, including the removal of directors.
•Our authorized but unissued Class A common stock and preferred stock are available for future issuances without stockholder approval and could be utilized for a variety of corporate purposes, including future
14
offerings to raise additional capital, acquisitions and employee benefit plans. Our Board is entitled, without further stockholder approval, to designate one or more series of preferred stock and the associated voting rights, preferences and privileges of such series of preferred stock. The existence of authorized but unissued and unreserved Class A common stock and preferred stock could render more difficult or discourage an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Forum Selection Clause
Our Amended and Restated Certificate of Incorporation includes a forum selection clause. Our Amended and Restated Certificate of Incorporation provides that, subject to limited exceptions, the Court of Chancery of the State of Delaware and federal court within the State of Delaware will be exclusive forums for any:
•derivative action or proceeding brought on our behalf;
•action asserting a claim of breach of a fiduciary duty owed by, or other wrongdoing by, any of our directors, officers, stockholders, employees or agents to us or our stockholders;
•action asserting a claim against us or any of our directors, officers, stockholders, employees or agents arising pursuant to any provision of the DGCL, our Amended and Restated Certificate of Incorporation or Bylaws or as to which the DGCL confers jurisdiction on the Court of Chancery of the State of Delaware;
•action to interpret, apply, enforce or determine the validity of our Amended and Restated Certificate of Incorporation or the Bylaws; or
•other action asserting a claim against us or any of our directors, officers, stockholders, employees or agents that is governed by the internal affairs doctrine.
This choice of forum provision does not apply to actions brought to enforce a duty or liability created by the Exchange Act or any other claim for which federal courts have exclusive jurisdiction. Furthermore, in accordance with our Bylaws, unless we consent in writing to the selection of an alternative forum, the federal district courts of the United States will be, to the fullest extent permitted by law, the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act. We intend for this provision to apply to any complaints asserting a cause of action under the Securities Act despite the fact that Section 22 of the Securities Act creates concurrent jurisdiction for the federal and state courts over all actions brought to enforce any duty or liability created by the Securities Act or the rules and regulations promulgated thereunder.
Transfer Agent and Registrar
The transfer agent for our Class A common stock is Continental Stock Transfer & Trust Company. We have agreed to indemnify Continental Stock Transfer & Trust Company in its roles as transfer agent, its agents and each of its stockholders, directors, officers and employees against all liabilities, including judgments, costs and reasonable counsel fees that may arise out of acts performed or omitted for its activities in that capacity, except for any liability due to any gross negligence, willful misconduct or bad faith of the indemnified person or entity.
Nasdaq Global Select Market listing
Our Class A common stock is traded on The Nasdaq Global Select Market under the symbol “SMFR.”
15
DESCRIPTION OF DEBT SECURITIES
General
We will issue the debt securities offered by this prospectus and any accompanying prospectus supplement under an indenture to be entered into between us and the trustee identified in the applicable prospectus supplement. The terms of the debt securities will include those stated in the indenture and those made part of the indenture by reference to the Trust Indenture Act of 1939, as in effect on the date of the indenture. We have filed a copy of the form of indenture as an exhibit to the registration statement in which this prospectus is included, which we refer to as the “base indenture,” and supplemental indentures and forms of debt securities containing the terms of the debt securities being offered and sold will be filed as exhibits to the registration statement and/or will be incorporated by reference from reports that we file with the SEC. The actual base indenture we enter into in connection with an offering of debt securities may differ significantly from the form of base indenture we have filed. The base indenture, as amended or supplemented from time to time by one or more supplemental indentures, is referred to below collectively as the “indenture.” The indenture will be subject to and governed by the terms of the Trust Indenture Act of 1939.
We may offer under this prospectus up to an aggregate principal amount of $300,000,000 in debt securities, or if debt securities are issued at a discount, or in a foreign currency, foreign currency units or composite currency, the principal amount as may be sold for an aggregate public offering price of up to $300,000,000. Unless otherwise specified in the applicable prospectus supplement, the debt securities will represent our direct, unsecured obligations and will rank equally with all of our other unsecured indebtedness.
We may issue the debt securities in one or more series with the same or various maturities, at par, at a premium, or at a discount. We will describe the particular terms of each series of debt securities in a prospectus supplement relating to that series, which we will file with the SEC. The prospectus supplement relating to the particular series of debt securities being offered will specify the particular amounts, prices and terms of those debt securities. These terms may include:
•the title of the series;
•the aggregate principal amount, and, if a series, the total amount authorized and the total amount outstanding;
•the issue price or prices, expressed as a percentage of the aggregate principal amount of the debt securities;
•any limit on the aggregate principal amount;
•the date or dates on which principal is payable;
•the interest rate or rates (which may be fixed or variable) or, if applicable, the method used to determine such rate or rates;
•the date or dates from which interest, if any, will be payable and any regular record date for the interest payable;
•the place or places where principal and, if applicable, premium and interest, is payable;
•the terms and conditions upon which we may, or the holders may require us to, redeem or repurchase the debt securities;
•the denominations in which such debt securities may be issuable, if other than denominations of $1,000 or any integral multiple of that number;
•whether the debt securities are to be issuable in the form of certificated securities (as described below) or global securities (as described below);
16
•the portion of principal amount that will be payable upon declaration of acceleration of the maturity date if other than the principal amount of the debt securities;
•the currency of denomination;
•the designation of the currency, currencies or currency units in which payment of principal and, if applicable, premium and interest, will be made;
•if payments of principal and, if applicable, premium or interest, on the debt securities are to be made in one or more currencies or currency units other than the currency of denomination, the manner in which the exchange rate with respect to such payments will be determined;
•if amounts of principal and, if applicable, premium and interest may be determined by reference to an index based on a currency or currencies or by reference to a commodity, commodity index, stock exchange index or financial index, then the manner in which such amounts will be determined;
•the provisions, if any, relating to any collateral provided for such debt securities;
•any addition to or change in the covenants and/or the acceleration provisions described in this prospectus or in the base indenture;
•any events of default, if not otherwise described below under “Events of Default”;
•the terms and conditions, if any, for conversion into or exchange for shares of our Class A common stock or preferred stock;
•any depositaries, interest rate calculation agents, exchange rate calculation agents or other agents; and
•the terms and conditions, if any, upon which the debt securities shall be subordinated in right of payment to our other indebtedness.
We may issue discount debt securities that provide for an amount less than the stated principal amount to be due and payable upon acceleration of the maturity of such debt securities in accordance with the terms of the indenture. We may also issue debt securities in bearer form, with or without coupons. If we issue discount debt securities or debt securities in bearer form, we will describe material U.S. federal income tax considerations and other material special considerations which apply to these debt securities in the applicable prospectus supplement.
We may issue debt securities denominated in or payable in a foreign currency or currencies or a foreign currency unit or units. If we do, we will describe the restrictions, elections, and general tax considerations relating to the debt securities and the foreign currency or currencies or foreign currency unit or units in the applicable prospectus supplement.
Debt securities offered under this prospectus and any prospectus supplement may be subordinated in right of payment to certain of our outstanding senior indebtedness. In addition, we will seek the consent of the holders of any such senior indebtedness prior to issuing any debt securities under this prospectus to the extent required by the agreements evidencing such senior indebtedness.
Registrar and Paying Agent
The debt securities may be presented for registration of transfer or for exchange at the corporate trust office of the security registrar or at any other office or agency that we maintain for those purposes. In addition, the debt securities may be presented for payment of principal, interest and any premium at the office of the paying agent or at any office or agency that we maintain for those purposes.
17
Conversion or Exchange Rights
Debt securities may be convertible into or exchangeable for shares of our Class A common stock. The terms and conditions of conversion or exchange will be stated in the applicable prospectus supplement. The terms will include, among others, the following:
•the conversion or exchange price;
•the conversion or exchange period;
•provisions regarding the convertibility or exchangeability of the debt securities, including who may convert or exchange;
•events requiring adjustment to the conversion or exchange price;
•provisions affecting conversion or exchange in the event of our redemption of the debt securities; and
•any anti-dilution provisions, if applicable.
Registered Global Securities
If we decide to issue debt securities in the form of one or more global securities, then we will register the global securities in the name of the depositary for the global securities or the nominee of the depositary, and the global securities will be delivered by the trustee to the depositary for credit to the accounts of the holders of beneficial interests in the debt securities.
The prospectus supplement will describe the specific terms of the depositary arrangement for debt securities of a series that are issued in global form. None of us, the trustee, any payment agent or the security registrar will have any responsibility or liability for any aspect of the records relating to or payments made on account of beneficial ownership interests in a global debt security or for maintaining, supervising or reviewing any records relating to these beneficial ownership interests.
No Protection in the Event of Change of Control
The base indenture does not have any covenants or other provisions providing for a put or increased interest or otherwise that would afford holders of our debt securities additional protection in the event of a recapitalization transaction, a change of control or a highly leveraged transaction. If we offer any covenants or provisions of this type with respect to any debt securities covered by this prospectus, we will describe them in the applicable prospectus supplement.
Covenants
Unless otherwise indicated in this prospectus or the applicable prospectus supplement, our debt securities will not have the benefit of any covenants that limit or restrict our business or operations, the pledging of our assets or the incurrence by us of indebtedness. We will describe in the applicable prospectus supplement any material covenants in respect of a series of debt securities.
Merger, Consolidation or Sale of Assets
The form of base indenture provides that we will not consolidate with or merge into any other person or convey, transfer, sell or lease our properties and assets substantially as an entirety to any person, unless:
•we are the surviving person of such merger or consolidation, or if we are not the surviving person, the person formed by the consolidation or into or with which we are merged or the person to which our properties and assets are conveyed, transferred, sold or leased, is a corporation organized and existing under the laws of the U.S., any state or the District of Columbia or a corporation or comparable legal entity organized under the laws of a foreign jurisdiction and has expressly assumed all of our obligations,
18
including the payment of the principal of and, premium, if any, and interest on the debt securities and the performance of the other covenants under the indenture; and
•immediately before and immediately after giving effect to the transaction on a pro forma basis, no event of default, and no event which, after notice or lapse of time or both, would become an event of default, has occurred and is continuing under the indenture.
Events of Default
Unless otherwise specified in the applicable prospectus supplement, the following events will be events of default under the indenture with respect to debt securities of any series:
•we fail to pay any principal or premium, if any, when it becomes due;
•we fail to pay any interest within 30 days after it becomes due;
•we fail to observe or perform any other covenant in the debt securities or the indenture for 90 days after written notice specifying the failure from the trustee or the holders of not less than 25% in aggregate principal amount of the outstanding debt securities of that series; and
•certain events involving bankruptcy, insolvency or reorganization of us or any of our significant subsidiaries
The trustee may withhold notice to the holders of the debt securities of any series of any default, except in payment of principal of or premium, if any, or interest on the debt securities of a series, if the trustee considers it to be in the best interest of the holders of the debt securities of that series to do so.
If an event of default (other than an event of default resulting from certain events of bankruptcy, insolvency or reorganization) occurs, and is continuing, then the trustee or the holders of not less than 25% in aggregate principal amount of the outstanding debt securities of any series may accelerate the maturity of the debt securities. If this happens, the entire principal amount, plus the premium, if any, of all the outstanding debt securities of the affected series plus accrued interest to the date of acceleration will be immediately due and payable. At any time after the acceleration, but before a judgment or decree based on such acceleration is obtained by the trustee, the holders of a majority in aggregate principal amount of outstanding debt securities of such series may rescind and annul such acceleration if:
•all events of default (other than nonpayment of accelerated principal, premium or interest) have been cured or waived;
•all lawful interest on overdue interest and overdue principal has been paid; and
•the rescission would not conflict with any judgment or decree.
In addition, if the acceleration occurs at any time when we have outstanding indebtedness that is senior to the debt securities, the payment of the principal amount of outstanding debt securities may be subordinated in right of payment to the prior payment of any amounts due under the senior indebtedness, in which case the holders of debt securities will be entitled to payment under the terms prescribed in the instruments evidencing the senior indebtedness and the indenture.
If an event of default resulting from certain events of bankruptcy, insolvency or reorganization occurs, the principal, premium and interest amount with respect to all of the debt securities of any series will be due and payable immediately without any declaration or other act on the part of the trustee or the holders of the debt securities of that series.
The holders of a majority in principal amount of the outstanding debt securities of a series will have the right to waive any existing default or compliance with any provision of the indenture or the debt securities of that series and
19
to direct the time, method and place of conducting any proceeding for any remedy available to the trustee, subject to certain limitations specified in the indenture.
No holder of any debt security of a series will have any right to institute any proceeding with respect to the indenture or for any remedy under the indenture, unless:
•the holder gives to the trustee written notice of a continuing event of default;
•the holders of at least 25% in aggregate principal amount of the outstanding debt securities of the affected series make a written request and offer reasonable indemnity to the trustee to institute a proceeding as trustee;
•the trustee fails to institute a proceeding within 60 days after such request; and
•the holders of a majority in aggregate principal amount of the outstanding debt securities of the affected series do not give the trustee a direction inconsistent with such request during such 60-day period.
These limitations do not, however, apply to a suit instituted for payment on debt securities of any series on or after the due dates expressed in the debt securities.
We will periodically deliver certificates to the trustee regarding our compliance with our obligations under the indenture.
Modification and Waiver
From time to time, we and the trustee may, without the consent of holders of the debt securities of one or more series, amend the indenture or the debt securities of one or more series, or supplement the indenture, for certain specified purposes, including:
•to provide that the surviving entity following a change of control permitted under the indenture will assume all of our obligations under the indenture and debt securities;
•to provide for certificated debt securities in addition to uncertificated debt securities;
•to comply with any requirements of the SEC under the Trust Indenture Act of 1939;
•to provide for the issuance of and establish the form and terms and conditions of debt securities of any series as permitted by the indenture;
•to cure any ambiguity, defect or inconsistency, or make any other change that does not materially and adversely affect the rights of any holder; and
•to appoint a successor trustee under the indenture with respect to one or more series.
From time to time we and the trustee may, with the consent of holders of at least a majority in principal amount of an outstanding series of debt securities, amend or supplement the indenture or the debt securities series, or waive compliance in a particular instance by us with any provision of the indenture or the debt securities. We may not, however, without the consent of each holder affected by such action, modify or supplement the indenture or the debt securities or waive compliance with any provision of the indenture or the debt securities in order to:
•reduce the amount of debt securities whose holders must consent to an amendment, supplement, or waiver to the indenture or such debt security;
•reduce the rate of or change the time for payment of interest or reduce the amount of or postpone the date for payment of sinking fund or analogous obligations;
•reduce the principal of or change the stated maturity of the debt securities;
•make any debt security payable in money other than that stated in the debt security;
20
•change the amount or time of any payment required or reduce the premium payable upon any redemption, or change the time before which no such redemption may be made;
•waive a default in the payment of the principal of, premium, if any, or interest on the debt securities or a redemption payment;
•waive a redemption payment with respect to any debt securities or change any provision with respect to redemption of debt securities; or
•take any other action otherwise prohibited by the indenture to be taken without the consent of each holder affected by the action.
Defeasance of Debt Securities and Certain Covenants in Certain Circumstances
The indenture permits us, at any time, to elect to discharge our obligations with respect to one or more series of debt securities by following certain procedures described in the indenture. These procedures will allow us either:
•to defease and be discharged from any and all of our obligations with respect to any debt securities except for the following obligations (which discharge is referred to as “legal defeasance”):
1.to register the transfer or exchange of such debt securities;
2.to replace temporary or mutilated, destroyed, lost or stolen debt securities;
3.to compensate and indemnify the trustee; or
4.to maintain an office or agency in respect of the debt securities and to hold monies for payment in trust; or
•to be released from our obligations with respect to the debt securities under certain covenants contained in the base indenture, as well as any additional covenants which may be contained in the applicable supplemental indenture (which release is referred to as “covenant defeasance”).
In order to exercise either defeasance option, we must irrevocably deposit with the trustee or other qualifying trustee, in trust for that purpose:
•money;
•U.S. Government Obligations (as described below) or Foreign Government Obligations (as described below) that through the scheduled payment of principal and interest in accordance with their terms will provide money; or
•a combination of money and/or U.S. Government Obligations and/or Foreign Government Obligations sufficient in the written opinion of a nationally-recognized firm of independent accountants to provide money;
that, in each case specified above, provides a sufficient amount to pay the principal of, premium, if any, and interest, if any, on the debt securities of the series, on the scheduled due dates or on a selected date of redemption in accordance with the terms of the indenture.
In addition, defeasance may be effected only if, among other things:
•in the case of either legal or covenant defeasance, we deliver to the trustee an opinion of counsel, as specified in the indenture, stating that as a result of the defeasance neither the trust nor the trustee will be required to register as an investment company under the Investment Company Act of 1940;
•in the case of legal defeasance, we deliver to the trustee an opinion of counsel stating that we have received from, or there has been published by, the Internal Revenue Service a ruling to the effect that, or there has
21
been a change in any applicable federal income tax law with the effect that (and the opinion shall confirm that), the holders of outstanding debt securities will not recognize income, gain or loss for U.S. federal income tax purposes solely as a result of such legal defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner, including as a result of prepayment, and at the same times as would have been the case if legal defeasance had not occurred;
•in the case of covenant defeasance, we deliver to the trustee an opinion of counsel to the effect that the holders of the outstanding debt securities will not recognize income, gain or loss for U.S. federal income tax purposes as a result of covenant defeasance and will be subject to U.S. federal income tax on the same amounts, in the same manner and at the same times as would have been the case if covenant defeasance had not occurred; and
•certain other conditions described in the indenture are satisfied.
If we fail to comply with our remaining obligations under the base indenture and applicable supplemental indenture after a covenant defeasance of the base indenture and applicable supplemental indenture, and the debt securities are declared due and payable because of the occurrence of any undefeased event of default, the amount of money and/or U.S. Government Obligations and/or Foreign Government Obligations on deposit with the trustee could be insufficient to pay amounts due under the debt securities of the affected series at the time of acceleration. We will, however, remain liable in respect of these payments.
The term “U.S. Government Obligations” as used in the above discussion means securities that are direct obligations of or non-callable obligations guaranteed by the United States of America for the payment of which obligation or guarantee the full faith and credit of the United States of America is pledged.
The term “Foreign Government Obligations” as used in the above discussion means, with respect to debt securities of any series that are denominated in a currency other than U.S. dollars, (1) direct obligations of the government that issued or caused to be issued such currency for the payment of which obligations its full faith and credit is pledged or (2) obligations of a person controlled or supervised by or acting as an agent or instrumentality of such government the timely payment of which is unconditionally guaranteed as a full faith and credit obligation by that government, which in either case under clauses (1) or (2), are not callable or redeemable at the option of the issuer.
Regarding the Trustee
We will identify the trustee with respect to any series of debt securities in the prospectus supplement relating to the applicable debt securities. You should note that if the trustee becomes a creditor of ours, the indenture and the Trust Indenture Act of 1939 limit the rights of the trustee to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim, as security or otherwise. The trustee and its affiliates may engage in, and will be permitted to continue to engage in, other transactions with us and our affiliates. If, however, the trustee acquires any “conflicting interest” within the meaning of the Trust Indenture Act of 1939, it must eliminate such conflict or resign.
The holders of a majority in principal amount of the then outstanding debt securities of any series may direct the time, method and place of conducting any proceeding for exercising any remedy available to the trustee. If an event of default occurs and is continuing, the trustee, in the exercise of its rights and powers, must use the degree of care and skill of a prudent person in the conduct of his or her own affairs. Subject to that provision, the trustee will be under no obligation to exercise any of its rights or powers under the indenture at the request of any of the holders of the debt securities, unless they have offered to the trustee reasonable indemnity or security.
No Individual Liability of Incorporators, Stockholders, Officers or Directors
The indenture provides that no incorporator and no past, present or future stockholder, officer or director of our company or any successor corporation in those capacities will have any individual liability for any of our obligations, covenants or agreements under the debt securities or the indenture.
22
Governing Law
The indenture and the debt securities will be governed by, and construed in accordance with, the laws of the State of New York.
23
DESCRIPTION OF WARRANTS
The following summary sets forth certain material terms and provisions of our outstanding warrants to purchase 14,758,305 shares of Class A common stock that we issued in our IPO (the “public warrants”), our outstanding warrants to purchase 7,236,667 shares of Class A common stock that we issued in a private placement in connection with our IPO (the “private placement warrants”) and the warrant agreement governing our public warrants and our private placement warrants (the “warrant agreement”), which is incorporated by reference as an exhibit to the registration statement of which this prospectus forms a part. In addition, the following summary sets forth certain terms and provisions of the additional warrants we may offer pursuant to this prospectus.
Existing Warrants
Public Warrants
Each whole public warrant entitles the registered holder to purchase one share of our Class A common stock at a price of $11.50 per whole share, subject to adjustment as discussed below, at any time commencing on September 4, 2021. Pursuant to the warrant agreement, a warrant holder may exercise its public warrants only for a whole number of shares of Class A common stock. This means that only a whole public warrant may be exercised at any given time by a warrant holder. No fractional public warrants will be issued upon separation of the units and only whole public warrants will trade. The public warrants will expire September 4, 2026, at 5:00 p.m., New York City time, or earlier upon redemption or liquidation.
We are not obligated to deliver any shares of Class A common stock pursuant to the exercise of a public warrant and will have no obligation to settle such public warrant exercise unless a registration statement under the Securities Act, with respect to the shares of Class A common stock underlying the public warrants is then effective and a prospectus relating thereto is current, subject to our satisfying our obligations described below with respect to registration. No public warrant will be exercisable for cash or on a cashless basis, and we will not be obligated to issue any shares to holders seeking to exercise their public warrants, unless the issuance of the shares upon such exercise is registered or qualified under the securities laws of the state of the exercising holder, or an exemption is available. In the event that the conditions in the two immediately preceding sentences are not satisfied with respect to a public warrant, the holder of such public warrant will not be entitled to exercise such public warrant and such public warrant may have no value and expire worthless.
Redemption of Warrants When the Price per Share of Class A Common Stock Equals or Exceeds $18.00 - We may redeem the outstanding public warrants:
•in whole and not in part;
•at a price of $0.01 per public warrant;
•upon not less than 30 days’ prior written notice of redemption to each warrant holder; and
•if, and only if, the closing price of the Class A common stock equals or exceeds $18.00 per share (as adjusted) for any 20 trading days within a 30-trading day period ending three trading days before sending the notice of redemption to warrant holders (the “Reference Value”).
If and when the warrants become redeemable by us, we may exercise its redemption right even if we are unable to register or qualify the underlying securities for sale under all applicable state securities laws.
Redemption of Warrants When the Price per Share of Class A Common Stock Equals or Exceeds $10.00 – We may redeem the outstanding warrants:
•in whole and not in part;
•at $0.10 per warrant upon a minimum of 30 days’ prior written notice of redemption provided that holders will be able to exercise their warrants on a cashless basis prior to redemption and receive that number of shares based on the redemption date and the fair market value of the Class A common stock;
24
•if, and only if, the closing price of the Class A common stock equals or exceeds $10.00 per share (as adjusted) for any 20 trading days within the 30-trading day period ending three trading days before we send the notice of redemption to the warrant holders; and
•if the closing price of the Class A common stock for any 20 trading days within a 30-trading day period ending three trading days before we send notice of redemption to the warrant holders is less than $18.00 per share (as adjusted), the private placement warrants must also be concurrently called for redemption on the same terms as the outstanding public warrants, as described above.
We have established the last of the redemption criterion discussed above to prevent a redemption call unless there is at the time of the call a significant premium to the warrant exercise price. If the foregoing conditions are satisfied and we issue a notice of redemption of the public warrants, each warrant holder will be entitled to exercise his, her or its public warrant prior to the scheduled redemption date. However, the price of the Class A common stock may fall below the $18.00 redemption trigger price as well as the $11.50 warrant exercise price after the redemption notice is issued.
Redemption procedures and cashless exercise.
If we call the public warrants for redemption as described above, our management will have the option to require any holder that wishes to exercise his, her or its public warrant to do so on a “cashless basis.” In determining whether to require all holders to exercise their public warrants on a “cashless basis,” our management will consider, among other factors, our cash position, the number of public warrants that are outstanding and the dilutive effect on our stockholders of issuing the maximum number of shares of Class A common stock issuable upon the exercise of our public warrants. If our management takes advantage of this option, all holders of public warrants would pay the exercise price by surrendering their public warrants for that number of shares of Class A common stock equal to the quotient obtained by dividing (i) the product of the number of shares of Class A common stock underlying the public warrants, multiplied by the difference between the exercise price of the public warrants and the “fair market value” (defined below) by (ii) the fair market value. The “fair market value” shall mean the average reported last sale price of the Class A common stock for the 10 trading days ending on the third trading day prior to the date on which the notice of redemption is sent to the holders of public warrants. If our management takes advantage of this option, the notice of redemption will contain the information necessary to calculate the number of shares of Class A common stock to be received upon exercise of the public warrants, including the “fair market value” in such case. Requiring a cashless exercise in this manner will reduce the number of shares to be issued and thereby lessen the dilutive effect of a warrant redemption. We believe this feature is an attractive option to us if we do not need the cash from the exercise of the public warrants. If we call our public warrants for redemption and our management does not take advantage of this option, the Former Sponsor and its permitted transferees would still be entitled to exercise their private placement warrants for cash or on a cashless basis using the same formula described above that other warrant holders would have been required to use had all warrant holders been required to exercise their public warrants on a cashless basis, as described in more detail below.
A holder of a public warrant may notify us in writing in the event it elects to be subject to a requirement that such holder will not have the right to exercise such public warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates), to the warrant agent’s actual knowledge, would beneficially own in excess of 9.8% (or such other amount as a holder may specify) of the shares of Class A common stock outstanding immediately after giving effect to such exercise.
Anti-dilution Adjustments. If the number of outstanding shares of Class A common stock is increased by a stock dividend payable in shares of Class A common stock, or by a split-up of shares of Class A common stock or other similar event, then, on the effective date of such stock dividend, split-up or similar event, the number of shares of Class A common stock issuable on exercise of each public warrant will be increased in proportion to such increase in the outstanding shares of Class A common stock. A rights offering to holders of Class A common stock entitling holders to purchase shares of Class A common stock at a price less than the fair market value will be deemed a stock dividend of a number of shares of Class A common stock equal to the product of (i) the number of shares of Class A common stock actually sold in such rights offering (or issuable under any other equity securities sold in such rights offering that are convertible into or exercisable for Class A common stock) multiplied by (ii) one minus the quotient
25
of (a) the price per share of Class A common stock paid in such rights offering divided by (b) the fair market value. For these purposes (1) if the rights offering is for securities convertible into or exercisable for Class A common stock, in determining the price payable for Class A common stock, there will be taken into account any consideration received for such rights, as well as any additional amount payable upon exercise or conversion and (2) fair market value means the volume weighted average price of Class A common stock as reported during the 10 trading day period ending on the trading day prior to the first date on which the shares of Class A common stock trade on the applicable exchange or in the applicable market, regular way, without the right to receive such rights.
In addition, if we, at any time while the public warrants are outstanding and unexpired, pay a dividend or make a distribution in cash, securities or other assets to the holders of Class A common stock on account of such shares of Class A common stock (or other shares of our capital stock into which the public warrants are convertible), other than (i) as described above; or (ii) certain ordinary cash dividends, then the warrant exercise price will be decreased, effective immediately after the effective date of such event, by the amount of cash and/or the fair market value of any securities or other assets paid on each share of Class A common stock in respect of such event.
If the number of outstanding shares of our Class A common stock is decreased by a consolidation, combination, reverse stock split or reclassification of shares of Class A common stock or other similar event, then, on the effective date of such consolidation, combination, reverse stock split, reclassification or similar event, the number of shares of Class A common stock issuable on exercise of each public warrant will be decreased in proportion to such decrease in outstanding shares of Class A common stock.
Whenever the number of shares of Class A common stock purchasable upon the exercise of the public warrants is adjusted, as described above, the warrant exercise price will be adjusted by multiplying the warrant exercise price immediately prior to such adjustment by a fraction (x) the numerator of which will be the number of shares of Class A common stock purchasable upon the exercise of the public warrants immediately prior to such adjustment, and (y) the denominator of which will be the number of shares of Class A common stock so purchasable immediately thereafter.
In case of any reclassification or reorganization of the outstanding shares of Class A common stock (other than those described above or that solely affects the par value of such shares of Class A common stock), or in the case of any merger or consolidation of us with or into another corporation (other than a consolidation or merger in which we are the continuing corporation and that does not result in any reclassification or reorganization of our outstanding shares of Class A common stock), or in the case of any sale or conveyance to another corporation or entity of the assets or other property of us as an entirety or substantially as an entirety in connection with which we are dissolved, the holders of the public warrants will thereafter have the right to purchase and receive, upon the basis and upon the terms and conditions specified in the public warrants and in lieu of the shares of our Class A common stock immediately theretofore purchasable and receivable upon the exercise of the rights represented thereby, the kind and amount of shares of stock or other securities or property (including cash) receivable upon such reclassification, reorganization, merger or consolidation, or upon a dissolution following any such sale or transfer, that the holder of the public warrants would have received if such holder had exercised their public warrants immediately prior to such event. Additionally, if less than 70% of the consideration receivable by the holders of Class A common stock in such a transaction is payable in the form of common stock in the successor entity that is listed for trading on a national securities exchange or is quoted in an established over-the-counter market, or is to be so listed for trading or quoted immediately following such event, and if the registered holder of the public warrant properly exercises the public warrant within 30 days following public disclosure of such transaction, the warrant exercise price will be reduced as specified in the warrant agreement based on the per share consideration minus Black-Scholes Warrant Value (as defined in the warrant agreement) of the public warrant.
The public warrants have been issued in registered form under a warrant agreement between Continental Stock Transfer & Trust Company, as warrant agent, and us. You should review a copy of the warrant agreement, which is filed as an exhibit to the registration statement of which this prospectus forms a part, for a complete description of the terms and conditions applicable to the public warrants. The warrant agreement provides that the terms of the public warrants may be amended without the consent of any holder to cure any ambiguity or correct any defective provision, but requires the approval by the holders of at least 50% of the then outstanding public warrants to make any change that adversely affects the interests of the registered holders of public warrants.
26
The public warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the warrant agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price (or on a cashless basis, if applicable), by certified or official bank check payable to us, for the number of public warrants being exercised. The warrant holders do not have the rights or privileges of holders of Class A common stock and any voting rights until they exercise their public warrants and receive shares of Class A common stock. After the issuance of shares of Class A common stock upon exercise of the public warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
Warrants may be exercised only for a whole number of shares of Class A common stock. No fractional shares will be issued upon exercise of the public warrants. If, upon exercise of the public warrants, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, round down to the nearest whole number the number of shares of Class A common stock to be issued to the warrant holder. As a result, warrant holders not purchasing public warrants in multiples of three warrants will not obtain value from the fractional interest that will not be issued.
Private Placement Warrants
The private placement warrants are identical to the public warrants, except that (1) the private placement warrants are exercisable on a cashless basis, (2) the private placement warrants are non-redeemable (except as described above in “Redemption of Warrants When the Price per Share of Class A Common Stock Equals or Exceeds $10.00”) so long as they are held by the initial purchasers or their permitted transferees, and (3) the holders of the private placement warrants and the Class A common stock issuable upon the exercise of the private placement warrants have certain registration rights. If the private placement warrants are held by someone other than the initial purchasers or their permitted transferees, the private placement warrants are redeemable by us and exercisable by such holders on the same basis as the public warrants.
Warrant Agent
The warrant agent for our public warrants and private placement warrants is Continental Stock Transfer & Trust Company.
Nasdaq Global Select Market listing
Our public warrants are traded on The Nasdaq Global Select Market under the symbol “SMFRW.”
Additional Warrants
In addition, we may issue additional warrants for the purchase of our debt securities, preferred stock, Class A common stock, or any combination thereof. Warrants may be issued independently or together with our debt securities, preferred stock or Class A common stock and may be attached to or separate from any offered securities. Each series of warrants will be issued under a separate warrant agreement to be entered into between us and a bank or trust company, as warrant agent. The warrant agent will act solely as our agent in connection with the warrants. The warrant agent will not have any obligation or relationship of agency or trust for or with any holders or beneficial owners of warrants. This summary of certain provisions of the warrants is not complete. For the terms of a particular series of warrants, you should refer to the prospectus supplement for that series of warrants and the warrant agreement for that particular series.
Debt Warrants
The prospectus supplement relating to a particular issue of warrants to purchase debt securities will describe the terms of the debt warrants, including the following:
•the title of the debt warrants;
•the offering price for the debt warrants, if any;
27
•the aggregate number of the debt warrants;
•the designation and terms of the debt securities, including any conversion rights, purchasable upon exercise of the debt warrants;
•if applicable, the date from and after which the debt warrants and any debt securities issued with them will be separately transferable;
•the principal amount of debt securities that may be purchased upon exercise of a debt warrant and the exercise price for the warrants, which may be payable in cash, securities or other property;
•the dates on which the right to exercise the debt warrants will commence and expire;
•if applicable, the minimum or maximum amount of the debt warrants that may be exercised at any one time;
•whether the debt warrants represented by the debt warrant certificates or debt securities that may be issued upon exercise of the debt warrants will be issued in registered or bearer form;
•information with respect to book-entry procedures, if any;
•the currency or currency units in which the offering price, if any, and the exercise price are payable;
•if applicable, a discussion of material U.S. federal income tax considerations;
•the antidilution provisions of the debt warrants, if any;
•the redemption or call provisions, if any, applicable to the debt warrants;
•any provisions with respect to the holder’s right to require us to repurchase the debt warrants upon a change in control or similar event; and
•any additional terms of the debt warrants, including procedures and limitations relating to the exchange, exercise, and settlement of the debt warrants.
Debt warrant certificates will be exchangeable for new debt warrant certificates of different denominations. Debt warrants may be exercised at the corporate trust office of the warrant agent or any other office indicated in the prospectus supplement. Prior to the exercise of their debt warrants, holders of debt warrants will not have any of the rights of holders of the debt securities purchasable upon exercise and will not be entitled to payment of principal or any premium, if any, or interest on the debt securities purchasable upon exercise.
Equity Warrants
The prospectus supplement relating to a particular series of warrants to purchase our Class A common stock or preferred stock will describe the terms of the warrants, including the following:
•the title of the warrants;
•the offering price for the warrants, if any;
•the aggregate number of warrants;
•the designation and terms of the Class A common stock or preferred stock that may be purchased upon exercise of the warrants;
•if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each security;
28
•if applicable, the date from and after which the warrants and any securities issued with the warrants will be separately transferable;
•the number of shares of Class A common stock or preferred stock that may be purchased upon exercise of a warrant and the exercise price for the warrants;
•the dates on which the right to exercise the warrants shall commence and expire;
•if applicable, the minimum or maximum amount of the warrants that may be exercised at any one time;
•the currency or currency units in which the offering price, if any, and the exercise price are payable;
•if applicable, a discussion of material U.S. federal income tax considerations;
•the antidilution provisions of the warrants, if any;
•the redemption or call provisions, if any, applicable to the warrants;
•any provisions with respect to a holder’s right to require us to repurchase the warrants upon a change in control or similar event; and
•any additional terms of the warrants, including procedures and limitations relating to the exchange, exercise and settlement of the warrants.
Holders of equity warrants will not be entitled to:
•vote, consent, or receive dividends;
•receive notice as stockholders with respect to any meeting of stockholders for the election of our directors or any other matter; or
•exercise any rights as stockholders.
29
DESCRIPTION OF SUBSCRIPTION RIGHTS
We may issue subscription rights to purchase our Class A common stock, preferred stock or debt securities. These subscription rights may be offered independently or together with any other security offered hereby and may or may not be transferable by the stockholder receiving the subscription rights in such offering. In connection with any offering of subscription rights, we may enter into a standby arrangement with one or more underwriters or other purchasers pursuant to which the underwriters or other purchasers may be required to purchase any securities remaining unsubscribed for after such offering.
The prospectus supplement relating to any subscription rights we offer, if any, will, to the extent applicable, include specific terms relating to the offering, including some or all of the following:
•the price, if any, for the subscription rights;
•the exercise price payable for our Class A common stock, preferred stock or debt securities upon the exercise of the subscription rights;
•the number of subscription rights to be issued to each stockholder;
•the number and terms of our Class A common stock, preferred stock or debt securities which may be purchased per each subscription right;
•the extent to which the subscription rights are transferable;
•any other terms of the subscription rights, including the terms, procedures and limitations relating to the exchange and exercise of the subscription rights;
•the date on which the right to exercise the subscription rights shall commence, and the date on which the subscription rights shall expire;
•the extent to which the subscription rights may include an over-subscription privilege with respect to unsubscribed securities or an over-allotment privilege to the extent the securities are fully subscribed; and
•if applicable, the material terms of any standby underwriting or purchase arrangement which may be entered into by us in connection with the offering of subscription rights.
The description in the applicable prospectus supplement of any subscription rights we offer will not necessarily be complete and will be qualified in its entirety by reference to the applicable subscription rights certificate, which will be filed with the SEC if we offer subscription rights. We urge you to read the applicable subscription rights certificate and any applicable prospectus supplement in their entirety.
30
DESCRIPTION OF UNITS
We may issue units consisting of some or all of the securities described above, in any combination, including Class A common stock, preferred stock, warrants and/or debt securities. The terms of these units will be set forth in a prospectus supplement. The description of the terms of these units in the applicable prospectus supplement will not be complete. You should refer to the applicable form of unit and unit agreement for complete information with respect to these units.
31
LEGAL MATTERS
Fenwick & West LLP, New York, New York, will issue an opinion about certain legal matters with respect to the securities. Any underwriters or agents will be advised about legal matters relating to any offering by their own counsel.
EXPERTS
The consolidated financial statements of Sema4 Holdings Corp. appearing in Sema4 Holdings Corp.’s Annual Report (Form 10-K) for the year ended December 31, 2021, have been audited by Ernst & Young LLP, independent registered public accounting firm, as set forth in their report thereon included therein, and incorporated herein by reference. Such financial statements are, and audited financial statements to be included in subsequently filed documents will be, incorporated herein in reliance upon the report of Ernst & Young LLP pertaining to such financial statements (to the extent covered by consents filed with the Securities and Exchange Commission) given on the authority of such firm as experts in accounting and auditing.
The combined carve out financial statements of GeneDx, Inc. and subsidiary at December 31, 2021 and 2020, and for each of the two years in the period ended December 31, 2021, included in the Proxy Statement of Sema4 Holdings Corp. dated March 31, 2022, incorporated by reference in this Prospectus and Registration Statement have been audited by Ernst & Young LLP, independent auditors, as set forth in their report thereon, and are incorporated herein by reference in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
32

Shares of Class A Common Stock
Jefferies
PROSPECTUS SUPPLEMENT
, 2023