Saturn-1 Phase 2b/3 Trial Design
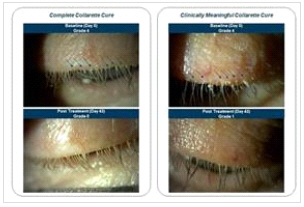
Saturn-1 was a randomized, controlled, multicenter, double-masked trial evaluating the safety and efficacy of TP-03 in adults with Demodex blepharitis. The trial enrolled 421 adults aged 18 and over having more than 10 collarettes on the upper lid and at least mild erythema of the upper eyelid margin. Each patient had at least 1.5 mites per lash on the upper and lower eyelids combined. One drop of TP-03 was self-administered twice per day in each eye for six weeks and patients were instructed not to touch or rub their lid margin. Enrolled patients received no treatment for blepharitis symptoms (i.e., lid hygiene) during the trial or 14 days prior to enrollment.
“Demodex blepharitis is a widespread, yet frequently overlooked condition that can negatively impact the quality of life for many patients and lead to more serious health outcomes if left untreated,” said Elizabeth Yeu, M.D., Chief Medical Advisor for Tarsus. “I am highly encouraged by the results seen in the Saturn-1 trial and I’m hopeful that there may be a treatment option on the horizon that targets the underlying cause of this disease to help patients finally find relief.”
Tarsus is also evaluating TP-03 in its pivotal Saturn-2 (Phase 3) trial, which has the same endpoints as Saturn-1, and commenced patient enrollment in May of 2021. Tarsus expects topline results for the Saturn-2 trial in Q1 2022, and, if the results are similarly positive, Tarsus expects data from both the Saturn-1 and Saturn-2 trials to support submission of a New Drug Application (NDA) to the FDA for TP-03 for the treatment of Demodex blepharitis. TP-03 has the potential to help millions of patients and eye care professionals struggling to manage Demodex blepharitis.
Conference Call and Webcast Information
A detailed summary of the Saturn-1 findings will be presented on a conference call and live, listen-only webcast today at 8:00 a.m. ET. The dial-in numbers are (833) 540-1160 for domestic callers and (929) 517-0351 for international callers. The Conference ID is 3766845. The webcast of the conference call can be accessed at https://edge.media-server.com/mmc/p/uh6zebmu. After the live webcast, the event will remain archived on the Tarsus Pharmaceuticals website at https://ir.tarsusrx.com/ for 90 days.
About TP-03
TP-03 (lotilaner ophthalmic solution, 0.25%) is a novel, investigational therapeutic designed to target and eradicate Demodex mites. TP-03 is a topical ophthalmic formulation of lotilaner, which is a well-characterized anti-parasitic agent that paralyzes and eradicates Demodex mites by selectively inhibiting parasite-specific GABA-Cl channels. It is a potent, non-competitive antagonist of insect and arachnid GABA-Cl channels and a highly lipophilic molecule, which may promote its uptake in the oily sebum of the hair follicle where the mites reside. Tarsus has completed four Phase 2 clinical trials of TP-03 in Demodex blepharitis, all of which met their respective endpoints with no significant adverse events nor any events leading to treatment discontinuation. TP-03 was also evaluated in the pivotal Saturn-1 (Phase 2b/3) trial and met all primary and secondary endpoints with no serious treatment-related adverse events and no treatment-related discontinuations. It is currently being evaluated in the Saturn-2 (Phase 3) pivotal trial. If approved, TP-03 may offer treatment for millions of patients around the world with Demodex blepharitis.
About Demodex Blepharitis
Blepharitis is a common ocular condition that is characterized by inflammation of the eyelid margin, redness and ocular irritation. Demodex blepharitis is caused by infestation of Demodex mites, the most common ectoparasite found on humans. Demodex mites cause approximately 45% of blepharitis, or about 9 million cases in the U.S. and the number may be as high as approximately 25 million based on Tarsus’ internal research indicating about 58% of patients presenting to eye care clinics have collarettes, a pathognomonic sign of Demodex infestation, and a published study estimating that at least 45 million people annually visit an eye care clinic. Currently, there are no FDA-approved treatments for Demodex blepharitis.
About Tarsus Pharmaceuticals, Inc.
Tarsus Pharmaceuticals, Inc. is a late clinical-stage biopharmaceutical company that applies proven science and new technology to revolutionize treatment for patients, starting with eye care. It is advancing its pipeline to address several diseases with high unmet need across a range of therapeutic categories, including eye care, dermatology, and infectious disease prevention. The company is studying two investigational medicines in clinical trials. Its lead product candidate, TP-03, is a novel therapeutic being studied in a second Phase 3 pivotal trial for the treatment of Demodex blepharitis. TP-03 is also being developed for the treatment of Meibomian Gland Disease. Tarsus is developing TP-05, an oral, non-vaccine therapeutic for the prevention of Lyme disease, which is currently being studied in a Phase 1 clinical trial.