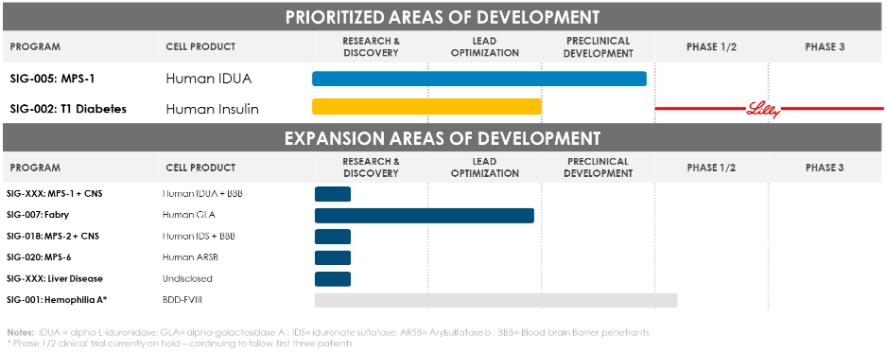
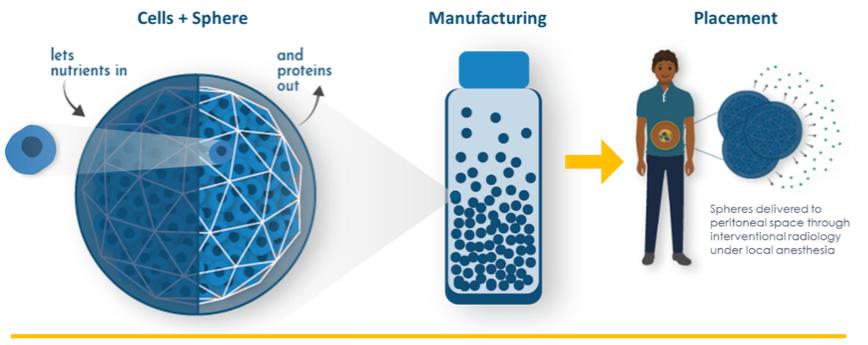
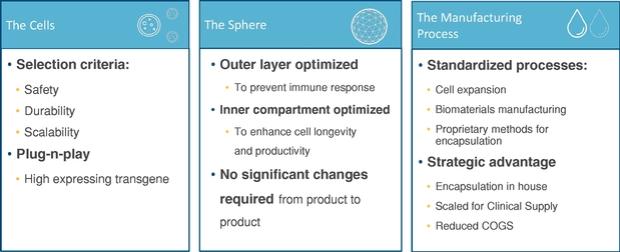
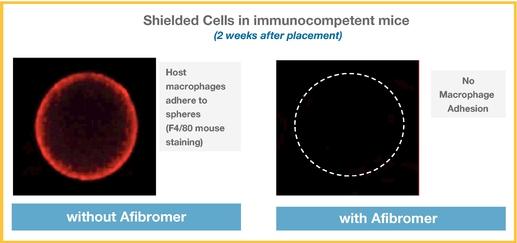
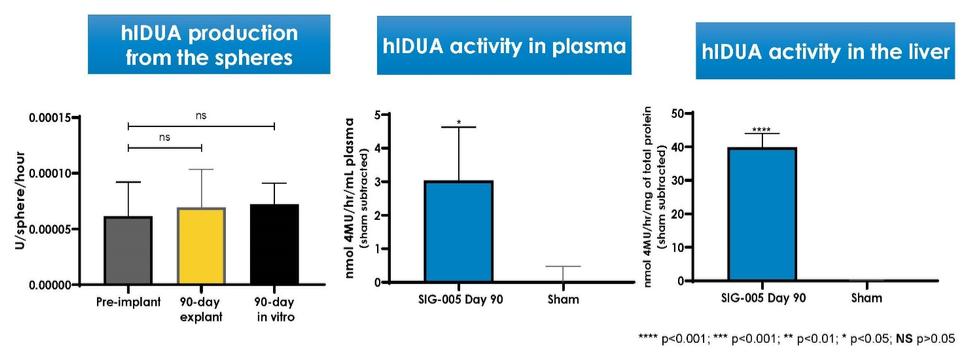
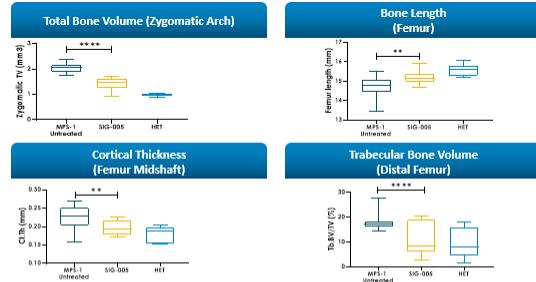
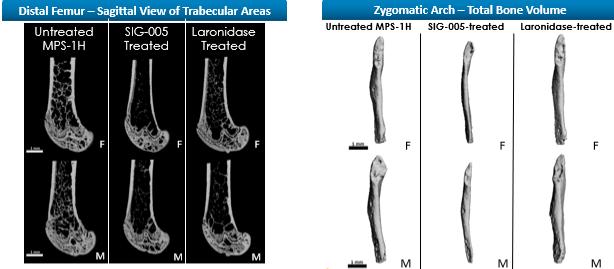
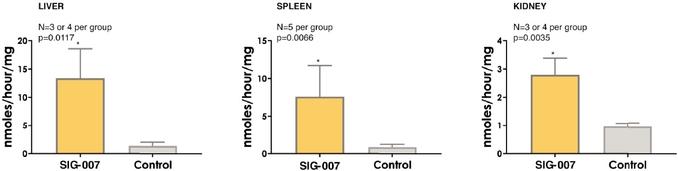
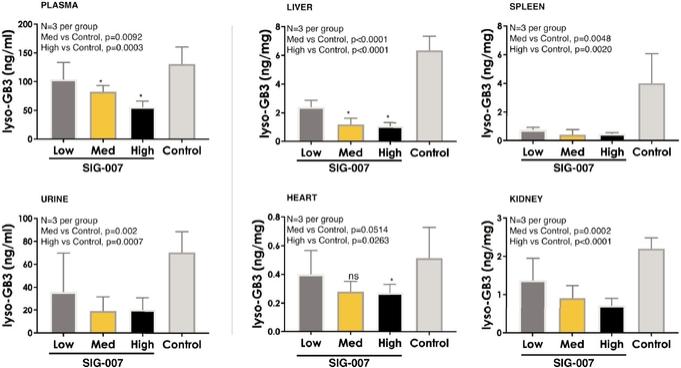
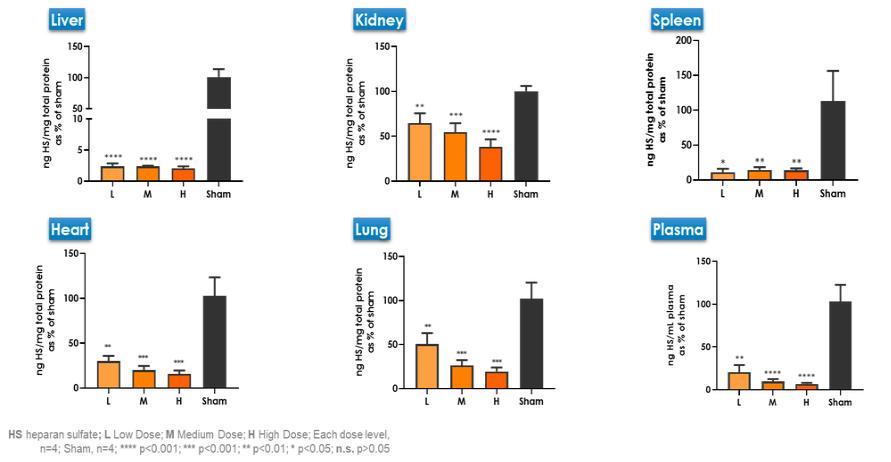
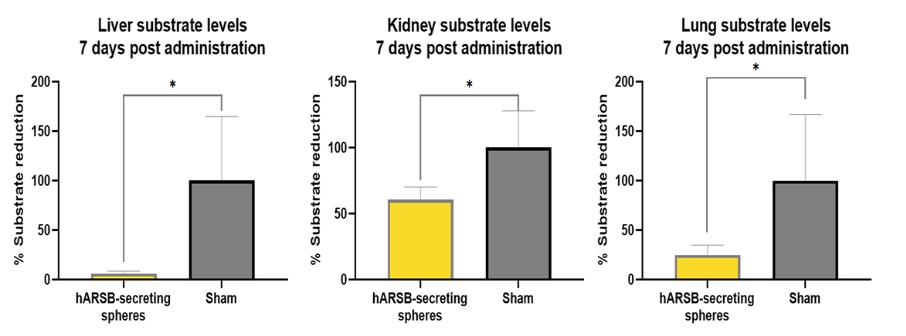
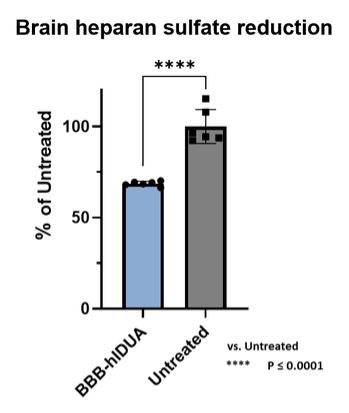
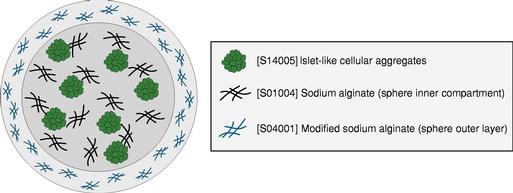
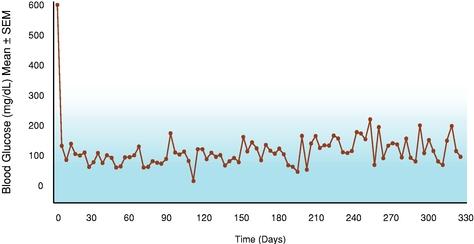
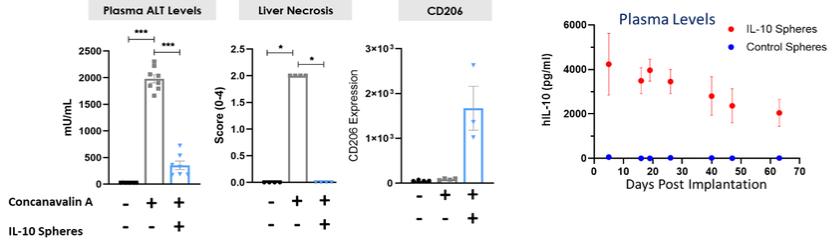
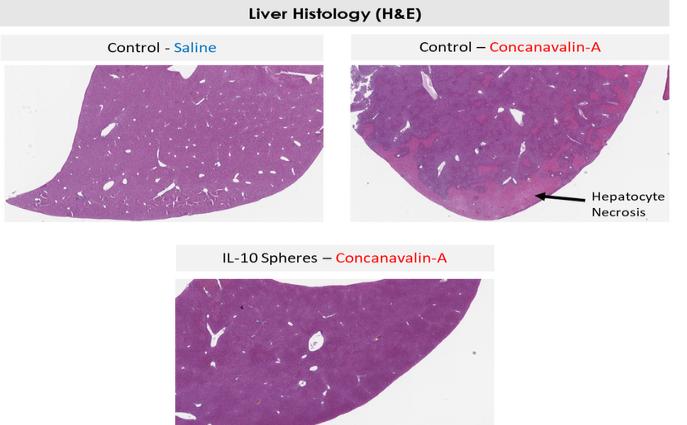
Even if we, or any collaborators we may have, obtain marketing approvals for any product candidates we develop, the terms of approvals and ongoing regulation of our product candidates could require the substantial expenditure of resources and may limit how we, or they, manufacture and market our product candidates, which could materially impair our ability to generate revenue.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, packaging, distribution, import, export, adverse event reporting, storage, recordkeeping, advertising, and promotional activities for such product candidate, will be subject to extensive and ongoing requirements imposed by the FDA, EMA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, facility registration and drug listing requirements, cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents, requirements regarding the distribution of samples to physicians and recordkeeping, and with respect to any medical device components of our product candidates, compliance with applicable provisions of the FDA’s Quality System regulation. Even if marketing approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the product may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the product.
Accordingly, assuming we, or any collaborators we may have, receive marketing approval for one or more product candidates we develop, we, and such collaborators, and our and their contract manufacturers will continue to expend time, money, and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance, and quality control. If we and such collaborators are not able to comply with post-approval regulatory requirements, we and such collaborators could have the marketing approvals for our products withdrawn by regulatory authorities and our, or such collaborators’, ability to market any future products could be limited, which could adversely affect our ability to achieve or sustain profitability. Further, the cost of compliance with post-approval regulations may have a negative effect on our business, operating results, financial condition, and prospects.
If there are changes in the application of legislation or regulatory policies, or if problems are discovered with a product or our manufacture of a product, or if we or one of our distributors, licensees or co-marketers fails to comply with regulatory requirements, the regulators could take various actions. These include issuing warning letters or untitled letters, imposing fines on us, imposing restrictions on the product or its manufacture and requiring us to recall or remove the product from the market. The regulators could also suspend or withdraw our marketing authorizations, requiring us to conduct additional clinical trials, change our product labeling or submit additional applications for marketing authorization. If any of these events occurs, our ability to sell such product may be impaired, and we may incur substantial additional expense to comply with regulatory requirements, which could materially adversely affect our business, financial condition and results of operations.
In addition, the FDA, the EMA, the MHRA and other regulatory agencies closely regulate the post-approval marketing and promotion of medicines to ensure that they are marketed only for the approved indications and in accordance with the provisions of the approved labeling. In particular, a product may not be promoted for uses that are not approved by the FDA, EMA, MHRA or other regulatory agencies as reflected in the product’s approved labeling. If we receive marketing approval for a product candidate, physicians may nevertheless prescribe it to their patients in a manner that is inconsistent with the approved label. If we are found to have promoted such off-label uses, we may become subject to significant liability. The FDA, the EMA, the MHRA and other regulatory agencies impose stringent restrictions on manufacturers’ communications regarding off-label use, and if we market our medicines for off-label use, we may be subject to enforcement action for off-label marketing by the FDA and other federal and state enforcement agencies, including the Department of Justice. Violation of the Federal Food, Drug, and Cosmetic Act and other statutes, including the False Claims Act, and equivalent legislation in other countries relating to the promotion and advertising of prescription products may also lead to investigations or allegations of violations of federal and state and other countries’ health care fraud and abuse laws and state consumer protection laws. Even if it is later determined we were not in violation of these laws, we may be faced with negative publicity, incur significant expenses defending our actions and have to divert significant management resources from other matters.
In addition, later discovery of previously unknown problems with our products, manufacturers, or manufacturing processes, or failure to comply with regulatory requirements, may yield various negative consequences, including: