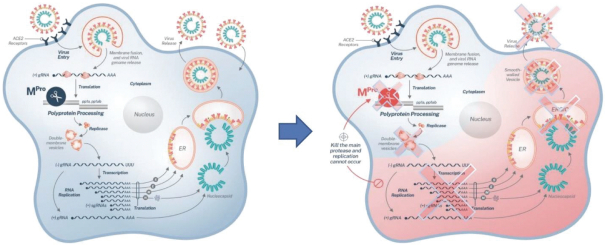
Many biotechnology and pharmaceutical companies are developing vaccines and treatments for
the virus that causes
COVID-19.
Many of these companies, which include large pharmaceutical companies, have greater resources for development and established commercialization capabilities and our competitors may obtain regulatory authorizations of their products more rapidly than Pardes. For example, in October 2020, the FDA approved the antiviral drug Veklury (remdesivir), a direct acting antiviral marketed by Gilead Sciences, Inc. for the treatment of
infections in certain patients requiring hospitalization and in November 2020 the FDA granted Regeneron Pharmaceuticals, Inc. an emergency use authorization for the use of casirivimab and imdevimab, administered together, for the treatment of mild to moderate
COVID-19
in adult and certain pediatric patients with positive results of direct
viral testing who are at high risk for progressing to severe
COVID-19
and/or hospitalization. In the same month, the FDA granted Eli Lily & Company emergency use authorization for Bamlanivimab for the treatment of mild to moderate
COVID-19
in adult and certain pediatric patients. There are other companies that are currently pursuing authorization for emergency use of their respective products. For example, in May 2021, the FDA granted VIR Biotechnology, Inc. and GlaxoSmithKline plc emergency use authorization for sotrovimab for the treatment of adults and pediatric patients 12 years of age and older with mild to moderate
COVID-19.
Brii Biosciences submitted an emergency use authorization to the FDA in October 2021 for its combination therapy,
which has been approved in China in December 2021, to treat
Covid-19
patients. In addition to therapeutics, vaccines indicated for active immunization to prevent
COVID-19
have been recently authorized for emergency use. In December 2020, the FDA granted authorization for emergency use for vaccines from Pfizer Inc. and BioNTech and Moderna, Inc., each of which announced clinical trial results showing that their respective vaccine candidate was found to be more than 90% effective in preventing
COVID-19
during such trials, and in February 2021, the FDA granted emergency use authorization to a vaccine developed by Janssen Pharmaceutical Company. On August 23, 2021, the FDA approved the Pfizer Inc. vaccine for the prevention of
COVID-19
disease in individuals 16 years of age or older, nine months after receiving authorization for emergency use. Additionally, in February 2021, the FDA granted EUA to a combination of Eli Lilly and AbCellera’s monocolonal antibody bamlanivimab and a second Eli Lilly antibody, etesevimab, for treatment of mild to moderate
COVID-19.
Additional vaccines and therapeutics are in development by other pharmaceutical and biopharmaceutical companies. For example, Molnupiravir, an orally administered direct-acting antiviral, which is being developed by Merck & Co. and Ridgeback Biotherapeutics LP, is currently in Phase 2/3 development in the outpatient setting for treatment and post-exposure prophylaxis. In June 2021, Merck entered into a procurement agreement with the U.S. government for Molnupiravir that was conditioned upon receiving emergency use authorization and in December 2021, Molnupiravir received emergency use authorization from the FDA for treatment of high-risk adults with mild to moderate
COVID-19.
Pfizer, Inc. initiated clinical development of its protease inhibitor, another form of direct acting antiviral in March 2021 and in September 2021 initiated its Phase 2/3 clinical trial to investigate its protease inhibitor direct acting antiviral in combination with ritonavir in
non-hospitalized
symptomatic participants who have a confirmed diagnosis of
In December 2021 Pfizer announced that it had received emergency use authorization for its antiviral candidate, PAXLOVID
™
(nirmatrelvir
(PF-07321332)
tablets and ritonavir tablets)) for the treatment of mild to moderate
COVID-19
in patients at high risk of hospitalizations or death. Previously Pfizer had entered into a government purchase agreement with the U.S. government for it to purchase certain quantities of
PF-07321331
upon receiving EUA. Given the products currently approved or authorized for use as well as those in development by others, any treatment we may develop could face significant competition that would negatively impact our commercial opportunity.