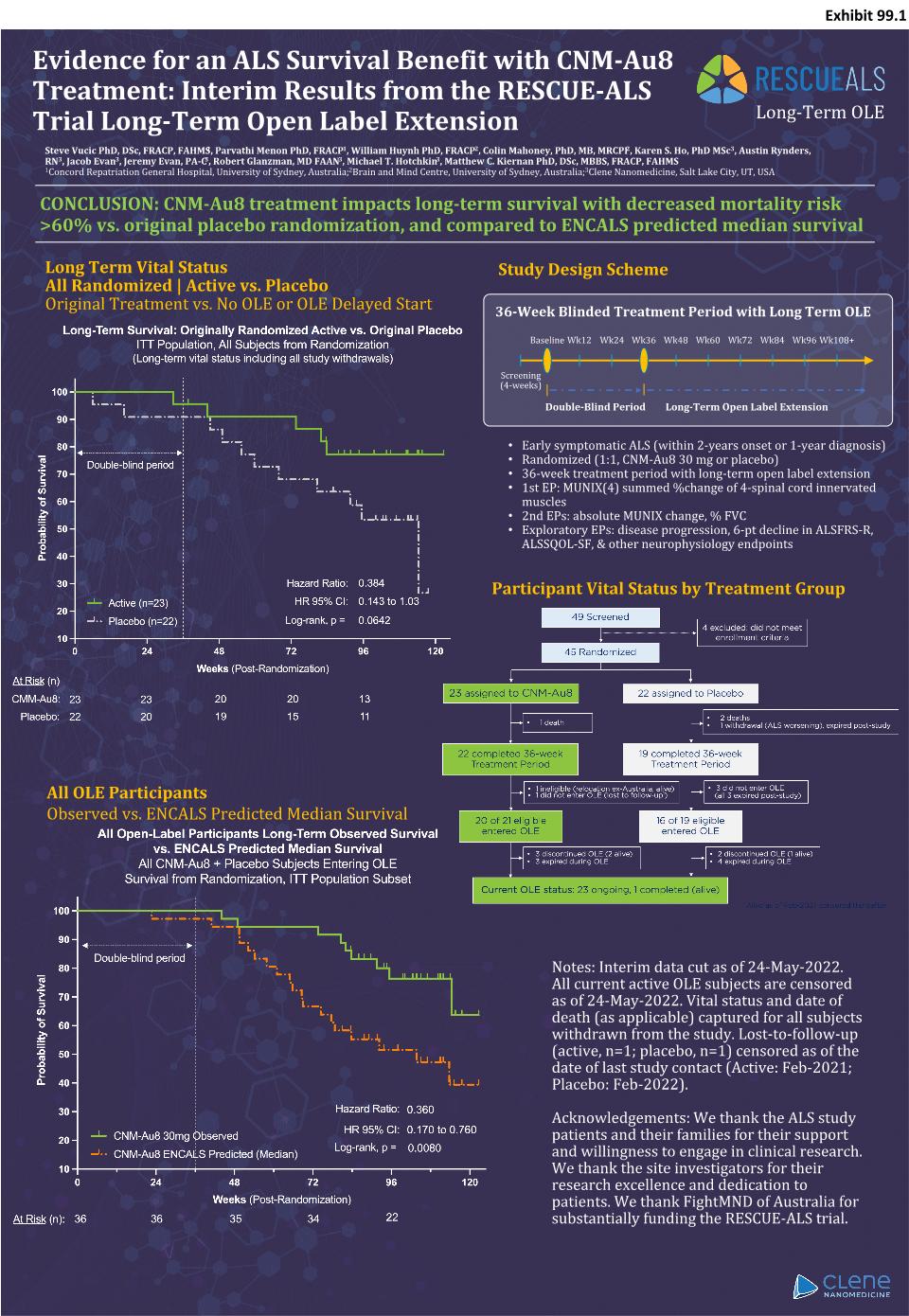
Evidence for an ALS Survival Benefit with CNM-Au8 Treatment: Interim Results from the RESCUE-ALS Trial Long-Term Open Label Extension Long Term Vital Status All Randomized | Active vs. Placebo Original Treatment vs. No OLE or OLE Delayed Start All OLE Participants Observed vs. ENCALS Predicted Median Survival Steve Vucic PhD, DSc, FRACP, FAHMS1, Parvathi Menon PhD, FRACP1, William Huynh PhD, FRACP2, Colin Mahoney, PhD, MB, MRCPI2, Karen S. Ho, PhD MSc3, Austin Rynders, RN3, Jacob Evan3, Jeremy Evan, PA-C3, Robert Glanzman, MD FAAN3, Michael T. Hotchkin3, Matthew C. Kiernan PhD, DSc, MBBS, FRACP, FAHMS 1Concord Repatriation General Hospital, University of Sydney, Australia; 2Brain and Mind Centre, University of Sydney, Australia; 3Clene Nanomedicine, Salt Lake City, UT, USA CONCLUSION: CNM-Au8 treatment impacts long-term survival with decreased mortality risk >60% vs. original placebo randomization, and compared to ENCALS predicted median survival Long-Term OLE Participant Vital Status by Treatment Group Notes: Interim data cut as of 24-May-2022. All current active OLE subjects are censored as of 24-May-2022. Vital status and date of death (as applicable) captured for all subjects withdrawn from the study. Lost-to-follow-up (active, n=1; placebo, n=1) censored as of the date of last study contact (Active: Feb-2021; Placebo: Feb-2022). Acknowledgements: We thank the ALS study patients and their families for their support and willingness to engage in clinical research. We thank the site investigators for their research excellence and dedication to patients. We thank FightMND of Australia for substantially funding the RESCUE-ALS trial. Study Design Scheme 36-Week Blinded Treatment Period with Long Term OLE Screening (4-weeks) Baseline Wk12 Wk36 Wk24 Wk48 Wk60 Wk72 Wk84 Wk96 Wk108+ Double-Blind Period Long-Term Open Label Extension Early symptomatic ALS (within 2-years onset or 1-year diagnosis) Randomized (1:1, CNM-Au8 30 mg or placebo) 36-week treatment period with long-term open label extension 1st EP: MUNIX(4) summed %change of 4-spinal cord innervated muscles 2nd EPs: absolute MUNIX change, % FVC Exploratory EPs: disease progression, 6-pt decline in ALSFRS-R, ALSSQOL-SF, & other neurophysiology endpoints Exhibit 99.1
