Exhibit 99.2

CLNN (NASDAQ)

Forward Looking Statements 2 This presentation contains "forward - looking statements" within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995 . Clene's actual results may differ from its expectations, estimates, and projections and consequently, you should not rely on these forward - looking statements as predictions of future events . Words such as "expect," "estimate," "project," "budget," "forecast," "anticipate," "intend," "plan," "may," "will," "could," "should," "believes," "predicts," "potential," "might" and "continues," and similar expressions are intended to identify such forward - looking statements . These forward - looking statements involve significant known and unknown risks and uncertainties, many of which are beyond Clene’s control and could cause actual results to differ materially and adversely from expected results . Factors that may cause such differences include Clene’s ability to demonstrate the efficacy and safety of its drug candidates ; the clinical results for its drug candidates, which may not support further development or marketing approval ; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials and marketing approval ; Clene’s ability to achieve commercial success for its marketed products and drug candidates, if approved ; Clene’s ability to obtain and maintain protection of intellectual property for its technology and drugs ; Clene’s reliance on third parties to conduct drug development, manufacturing and other services ; Clene’s limited operating history and its ability to obtain additional funding for operations and to complete the licensing or development and commercialization of its drug candidates ; the impact of the COVID - 19 pandemic on Clene’s clinical development, commercial and other operations, as well as those risks more fully discussed in the section entitled “Risk Factors” in Clene’s recently filed registration statement on Form S - 4 /A as well as discussions of potential risks, uncertainties, and other important factors in Clene’s subsequent filings with the U . S . Securities and Exchange Commission . Clene undertakes no obligation to release publicly any updates or revisions to any forward - looking statements to reflect any change in its expectations or any change in events, conditions or circumstances on which any such statement is based, subject to applicable law . All information in this presentation is as of the date of presented or the date made publicly available . The information contained in any website referenced herein is not, and shall not be deemed to be, part of or incorporated into this presentation .

Lanxide Corporation Dupont Lanxide Composites Lanxide Armor Company Lanxide Performance Materials Lanxide Electronic Components CDO CLENE | Management Team Ted Jeong , DM 3
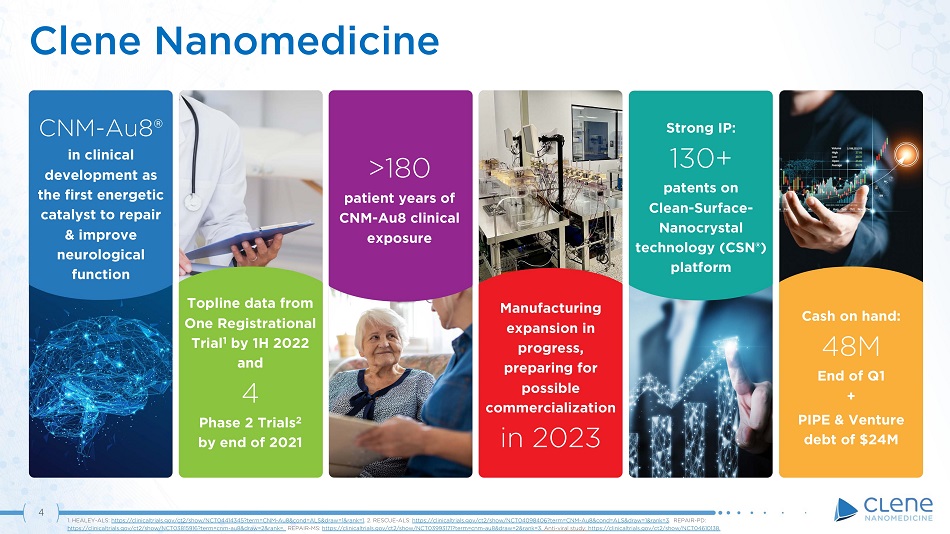
4 Clene Nanomedicine Cash on hand: 48M End of Q1 + PIPE & Venture debt of $24M Topline data from One Registrational Trial 1 by 1H 2022 and 4 Phase 2 Trials 2 by end of 2021 >180 patient years of CNM - Au8 clinical exposure CNM - Au8® in clinical development as the first energetic catalyst to repair & improve neurological function Manufacturing expansion in progress, preparing for possible commercialization in 2023 Strong IP: 130+ patents on Cle a n - S u r fac e - Nanocrystal technology (CSN®) platform 1. HEALEY - ALS: https://clinicaltrials.gov/ct2/show/NCT04414345?term=CNM - Au8&cond=ALS&draw=1&rank=1 . 2. RESCUE - ALS: https://clinicaltrials.gov/ct2/show/NCT04098406?term=CNM - Au8&cond=ALS&draw=1&rank=3 . REPAIR - PD: https://clinicaltrials.gov/ct2/show/NCT03815916?term=cnm - au8&draw=2&rank=. REPAIR - MS: https://clinicaltrials.gov/ct2/show/NCT03993171?term=cnm - au8&draw=2&rank=3 . Anti - viral study: https://clinicaltrials.gov/ct2/show/NCT04610138.
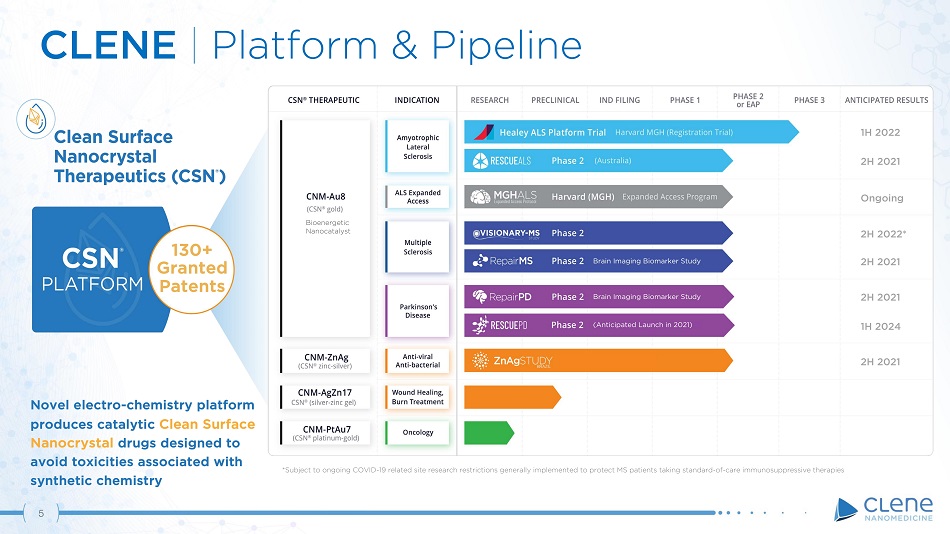
Novel electro - chemistry platform produces catalytic Clean Surface Nanocrystal drugs designed to avoid toxicities associated with synthetic chemistry 130+ Gr a n t ed Patents CLENE | Platform & Pipeline (Anticipated Launch in 2021) Brain Imaging Biomarker Study Brain Imaging Biomarker Study 1H 2022 2H 2021 O ngo i n g 5 2H 2022* 2H 2021 2H 2021 1H 2024 2H 2021 *Subject to ongoing COVID - 19 related site research restrictions generally implemented to protect MS patients taking standard - of - care immunosuppressive therapies Bioenergetic Na noc a t a lyst
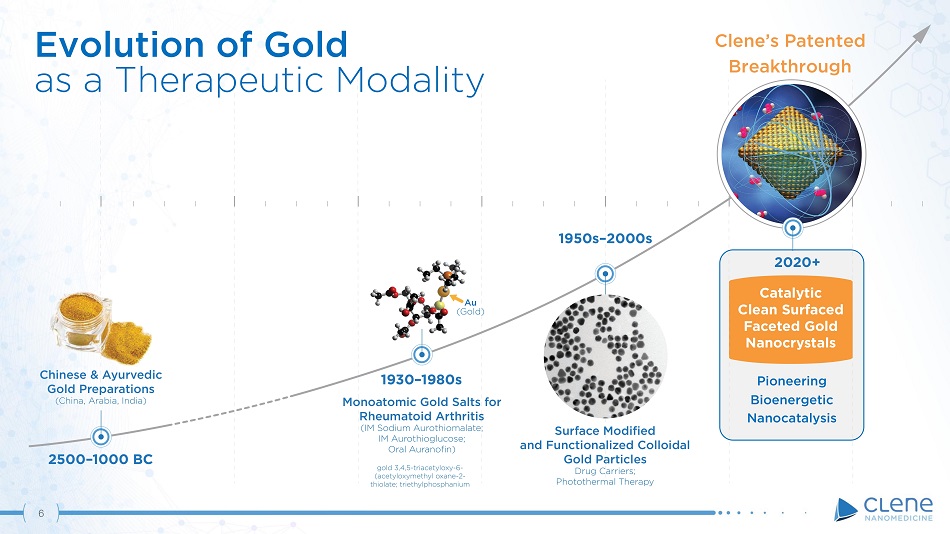
Chinese & Ayurvedic Gold Preparations (China, Arabia, India) Monoatomic Gold Salts for Rheumatoid Arthritis (IM Sodium Aurothiomalate; IM Aurothioglucose; Oral Auranofin) gold 3,4,5 - triacetyloxy - 6 - (acetyloxymethyl oxane - 2 - thiolate; triethylphosphanium Au ( Gold) 2020+ Catalytic Clean Surfaced Faceted Gold Nanocrystals 1950s – 2000s 2500 – 1000 BC 1 9 30 – 1980s Surface Modified and Functionalized Colloidal Gold Particles Drug Carriers; Photothermal Therapy 6 Evolution of Gold as a Therapeutic Modality Pioneering Bioenergetic Na noc a t a l y s i s Clene’s Patented Breakthrough

CNM - Au8® | Energy Enhancing Nanotherapeutic Improved Cellular Energy Production & Utilization E nergy > 100 Trillion Nanocrystals per 60 mL Dose (At 30mg) Oral Suspension; Once Daily 13 nm Median Diameter (Ribosome = 20 - 30 nm) Clean Surfaced Faceted Nanocrystal Novel mechanism of action to address a range of CNS diseases CNM - Au8 Nanocrystal Cellular 7

CNM - Au8 | Integrating Physics With Biology Electron Transfer Is Fundamental to Energy Production Surface Based Catalytic Activity Electrons (e - ) Move Freely Across Nanocrystal Surface Vertices, Edges, & Faces Key to Catalytic Activity AuNP Catalyzed Oxidation of Ascorbic Acid 1 a. Rayleigh scattering measured by dark field microscopy of surface plasmon resonance of scattering spectra of the AuNP decahedron before and at 1 , 2 , 3 and 60 min after electron injection by ascorbate ions . b. Spectral shift as a function of time for the catalysis reaction and for the control experiment .. 1 Novo et al. Nature Nanotech 3, 598 – 602 (2008). Clean - Surfaced Nanocrystals Up to 4,600 e - per second per nanocrystal 1 8

Treating Energetic Failure | Common Pathological Mechanism In Neurodegenerative Disorders (MS, ALS, PD) Respiratory In s u ff icie ncy D y sp h agia/ Dysarthria Cognitive Im p a i rm ent Muscle Weakness/Atrophy ALS Neuronal Metabolic Failure MS Oligodendrocyte Remyelination Failure and Neuronal Die - Off Vandoorne et al. Acta Neuropathologica (2018) 135:489 – 509. Rone et al. J Neurosci. 2016 Apr 27;36(17):4698 - 707. Cognitive Im p a i rm ent Spinal Cord (Move m ent) Dexterity & Coo r di n atio n Visual Im p a i rm ent Neu r o n Ac ti v at e d A str ocy te Demyelination Oligodendrocyte Myelin She ath Com pr om is e d Axon Dendritic R e tra c ti o n Neuromuscular Junction Impairment A c tivat e d Astrocyte 9
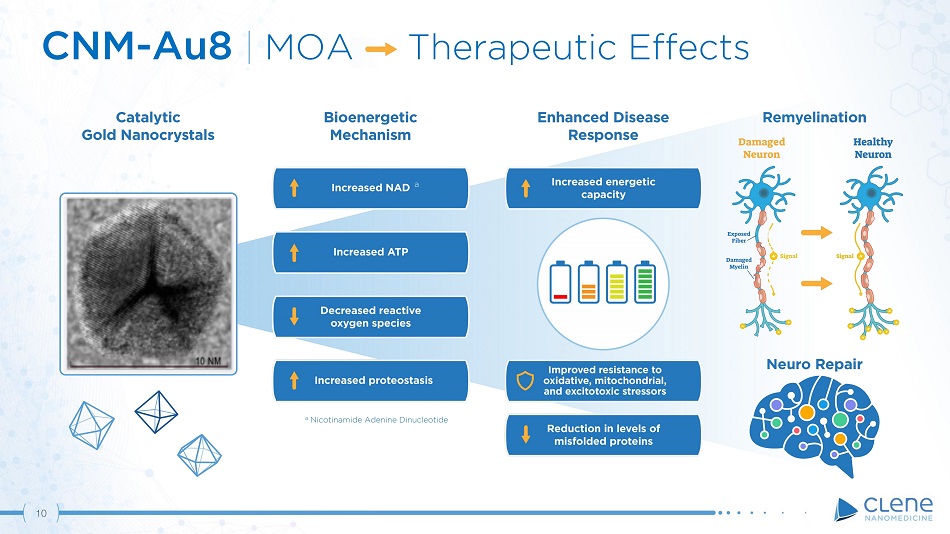
CNM - Au8 | MOA Therapeutic Effects a Nicotinamide Adenine Dinucleotide a 10
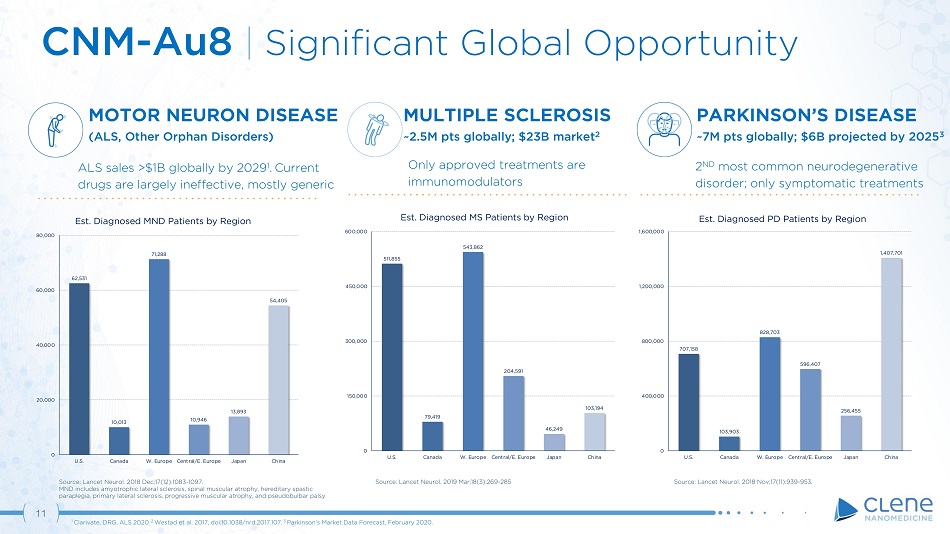
11 CNM - Au8 | Significant Global Opportunity 707,158 103,903 828,703 596,407 256,455 1,407,701 0 4 00 , 0 0 0 800,000 1 , 2 00 , 00 0 1 , 6 00 , 0 0 0 U . S . C a n a d a W. Europe Central/E. Europe J a p a n C h i n a Est. Diagnosed PD Patients by Region Source: Lancet Neurol. 2018 Nov;17(11):939 - 953. PARKINSON’S DISEASE ~7M pts globally; $6B projected by 2025 3 2 ND most common neurodegenerative disorder; only symptomatic treatments 62,531 10,013 71,288 10,946 13,893 54,405 0 2 0 , 00 0 40,000 6 0 , 00 0 8 0 , 00 0 U . S . C a n a d a W. Europe C e n t r a l / E . E ur o p e J a p a n C h i n a Est. Diagnosed MND Patients by Region Source: Lancet Neurol. 2018 Dec;17(12):1083 - 1097. MND includes amyotrophic lateral sclerosis, spinal muscular atrophy, hereditary spastic paraplegia, primary lateral sclerosis, progressive muscular atrophy, and pseudobulbar palsy MOTOR NEURON DISEASE (ALS, Other Orphan Disorders) ALS sales >$1B globally by 2029 1 . Current drugs are largely ineffective, mostly generic Source: Lancet Neurol. 2019 Mar;18(3):269 - 285 511,855 79,419 543,862 204,591 46,249 103,194 0 1 5 0 , 0 0 0 300 , 00 0 4 5 0 , 0 0 0 600,000 U . S . C a n a d a W. Europe C e n t r a l / E . E ur o p e J a p a n C h i n a Est. Diagnosed MS Patients by Region MULTIPLE SCLEROSIS ~2.5M pts globally; $23B market 2 Only approved treatments are immunomodulators 1 Clarivate, DRG, ALS 2020 . 2 Westad et al. 2017, doi:10.1038/nrd.2017.107. 3 Parkinson’s Market Data Forecast, February 2020.
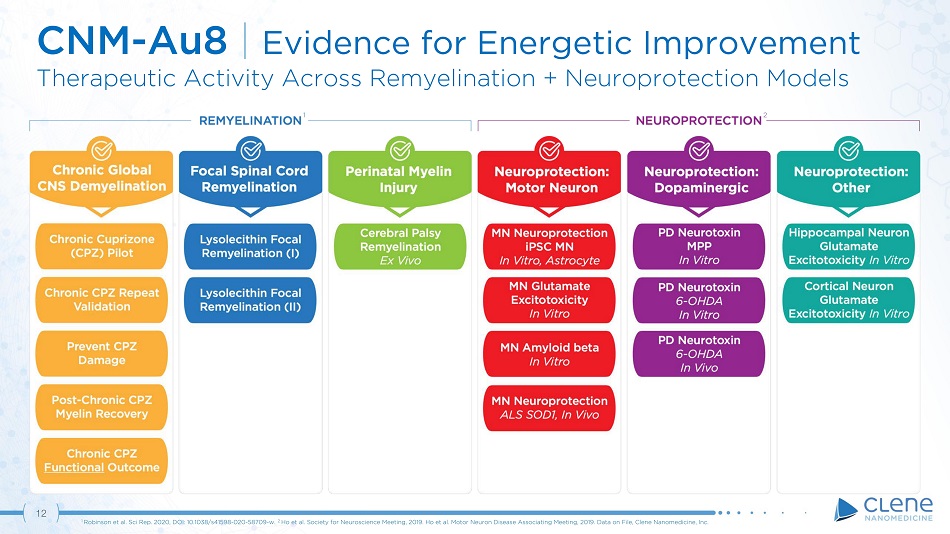
12 CNM - Au8 | Evidence for Energetic Improvement Therapeutic Activity Across Remyelination + Neuroprotection Models 1 Robinson et al. Sci Rep. 2020, DOI: 10.1038/s41598 - 020 - 58709 - w. . 2 Ho et al. Society for Neuroscience Meeting, 2019. Ho et al. Motor Neuron Disease Associating Meeting, 2019. Data on File, Clene Nanomedicine, Inc. 1 2
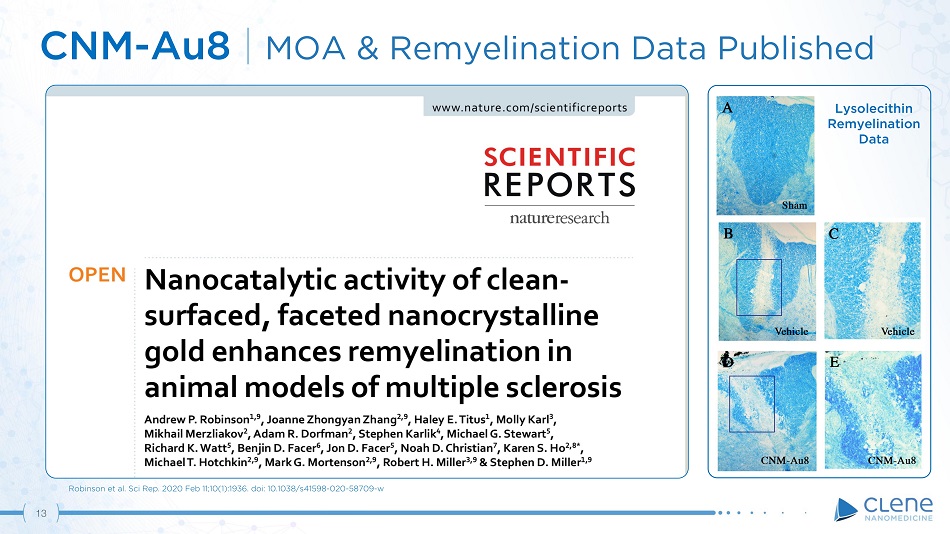
CNM - Au8 | MOA & Remyelination Data Published Robinson et al. Sci Rep. 2020 Feb 11;10(1):1936. doi: 10.1038/s41598 - 020 - 58709 - w Lysolecithin R emye li n ati on Data 13

Successful Phase 1 First - In Humans Safety Trial + Chronic Animal Toxicity Studies Phase 2 Brain Target Engagement 31 P - Magnetic Resonance Phase 2 MS Clinical Remyelination & Neurorepair CNM - Au8 | Clinical Program Overview Phase 3 Phase 2 & 3 ALS Clinical Neurorepair Phase 2 14

CNM - Au8 | Clean Toxicology Findings All Studies Resulted in No Adverse Effect Level (NOAEL) a Standard ICH M3(R2) Toxicology Program Genotoxicity In Vitro & In Vivo (Rodent) Safety Pharmacology CNS, CV, Renal Dose Range Finding Rodent, Minipig Single Dose Tox icokin e tic s Canine Multi - Dose Tox icokin e tic s Canine (7 - Day) MTD Toxicokinetics Canine (4 - Wk) Max Feasible Tox icokin e tic s Rodent (1 - Wk, SQ) Max Feasible Tox icokin e tic s Canine (3 - Wk) Chronic Toxicity Rodent Rodent (6 - Month) Chronic Toxicity Canine Canine (9 - Month) High Dose Toxicokinetics Rodent (3 - Wk) a NOAEL = No Dose Limiting Toxicities Observed Carcinogenicity Dose Range Finding rasH2 (1 - Month) 15
• Most frequent TEAEs by System Organ Class: Nervous/GI - Nearly all of the TEAEs were Grade 1 severity (mild) • No serious TEAEs, TEAEs leading to discontinuation of treatment, or TEAEs considered severe, life - threatening, or resulting in death • No dose responsive TEAEs observed in SAD or MAD • Single - ascending dose – 4 cohorts of 8 subjects plus one repeat (n=40) – 15, 30, 60, 90 mg – 3:1 randomized ( ac t ive:con t rol) – 1 dose; 17 - day follow - up • Multi - ascending dose – 4 cohorts of ~12 subjects (n=46) – 15, 30, 60, 90 mg – 3:1 randomized ( ac t ive:con t rol) – 21 days daily dosing + follow - up (Up to 50 days) Phase 1 First In Human Study Completed (n=86) CNM - Au8 | Well Tolerated; No Dose - Limiting Safety Issues + Long - Term Extension 16 + Long - Term Extension Phase 2 & 3 Clinical (>180 Years Exposure) Up to 89 Weeks Exposure in Clinical Trials; Up to 96 Weeks in ALS Expanded Access + Long - Term Extension
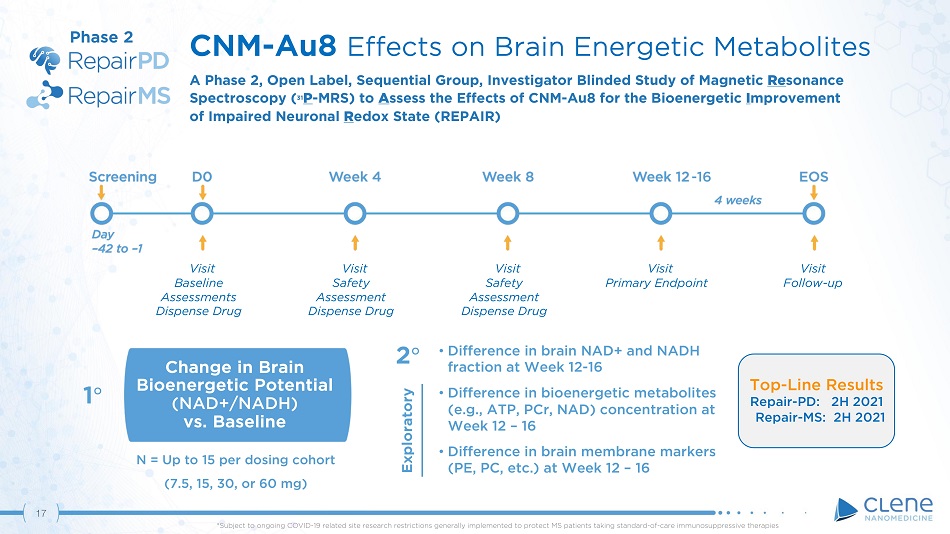
17 • Difference in brain NAD+ and NADH fraction at Week 12 - 16 • Difference in bioenergetic metabolites (e . g . , ATP, PCr, NAD) concentration at Week 12 – 16 • Difference in brain membrane markers (PE, PC, etc.) at Week 12 – 16 Change in Brain Bioenergetic Potential (NAD+/NADH) vs. Baseline 1 ι 2 ι CNM - Au8 Effects on Brain Energetic Metabolites A Phase 2 , Open Label, Sequential Group, Investigator Blinded Study of Magnetic Re sonance Spectro s co p y ( 31 P - M R S) to A sses s the Effects of C N M - A u 8 f or t h e Bioe n ergetic I m prove m ent of Impaired Neuronal R edox State (REPAIR) Visit Safety Assessment Dispense Drug Visit Safety Assessment Dispense Drug Visit Primary Endpoint Visit F ollo w - up Visit Ba seline Assessments Dispense Drug - 16 N = Up to 15 per dosing cohort (7.5, 15, 30, or 60 mg) Phase 2 Top - Line Results Repair - PD: 2H 2021 Repair - MS: 2H 2021 *Subject to ongoing COVID - 19 related site research restrictions generally implemented to protect MS patients taking standard - of - care immunosuppressive therapies Exploratory
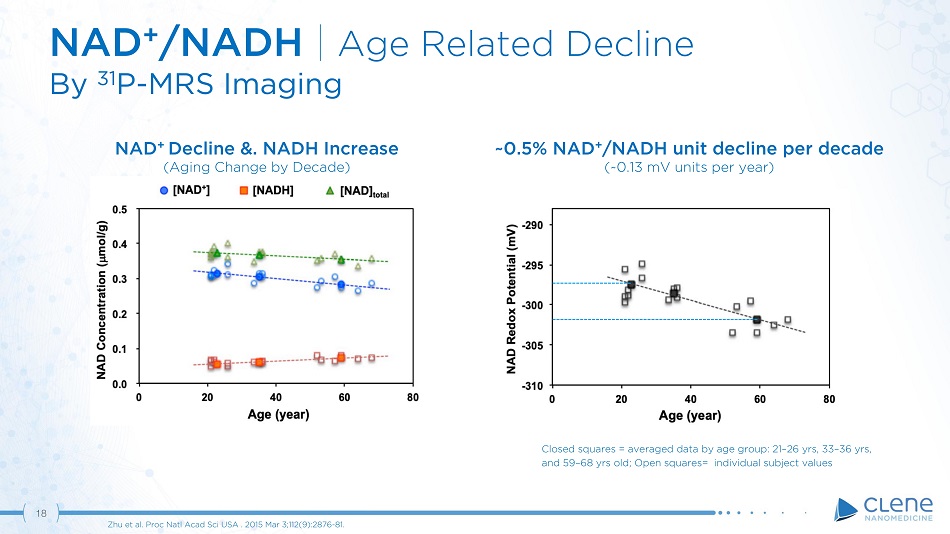
18 NAD + /NADH | Age Related Decline By 31 P - MRS Imaging Zhu et al. Proc Natl Acad Sci USA . 2015 Mar 3;112(9):2876 - 81. Closed squares = averaged data by age group: 21 – 26 yrs, 33 – 36 yrs, and 59 – 68 yrs old; Open squares= individual subject values ~0.5% NAD + /NADH unit decline per decade (~0.13 mV units per year) NAD + Decline &. NADH Increase (Aging Change by Decade)
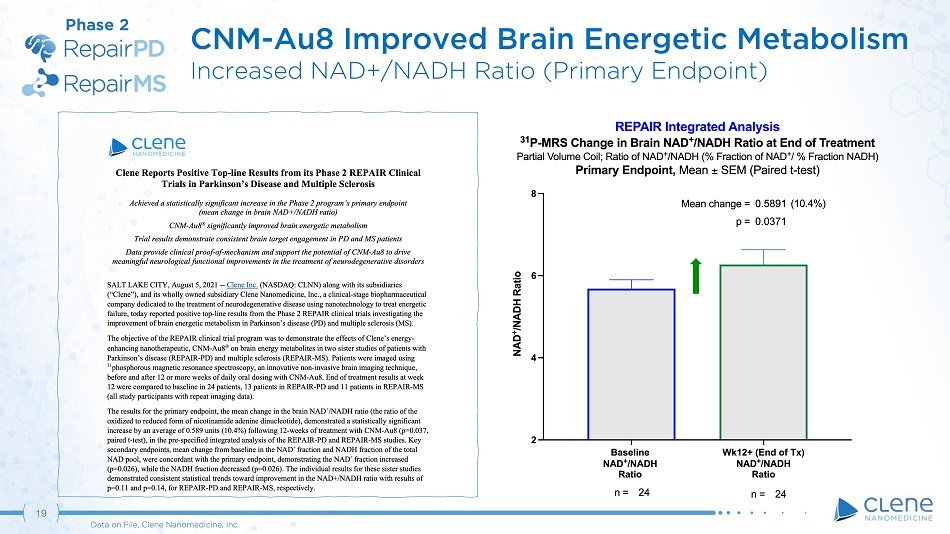
CNM - Au8 Improved Brain Energy Ratio Elevated NAD+/NADH Ratio 19 Data on File, Clene Nanomedicine, Inc. Phase 2
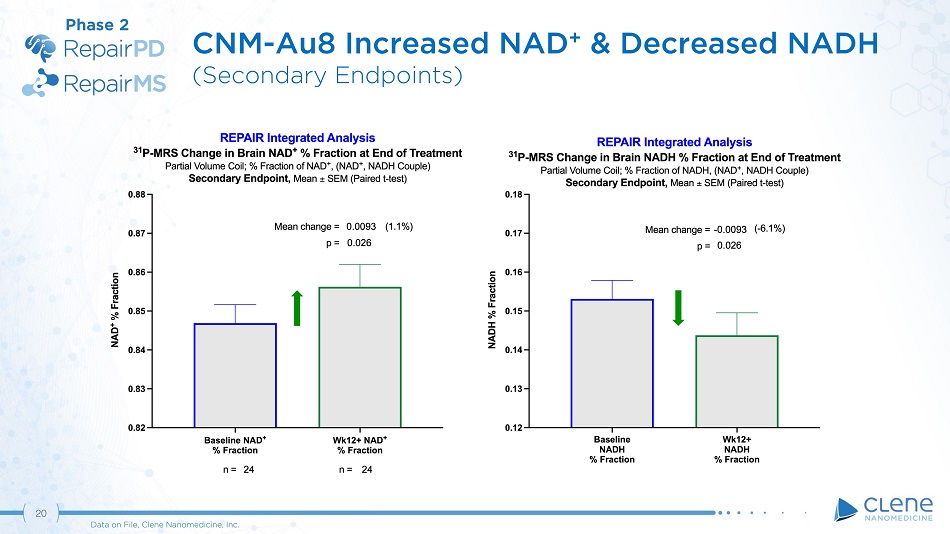
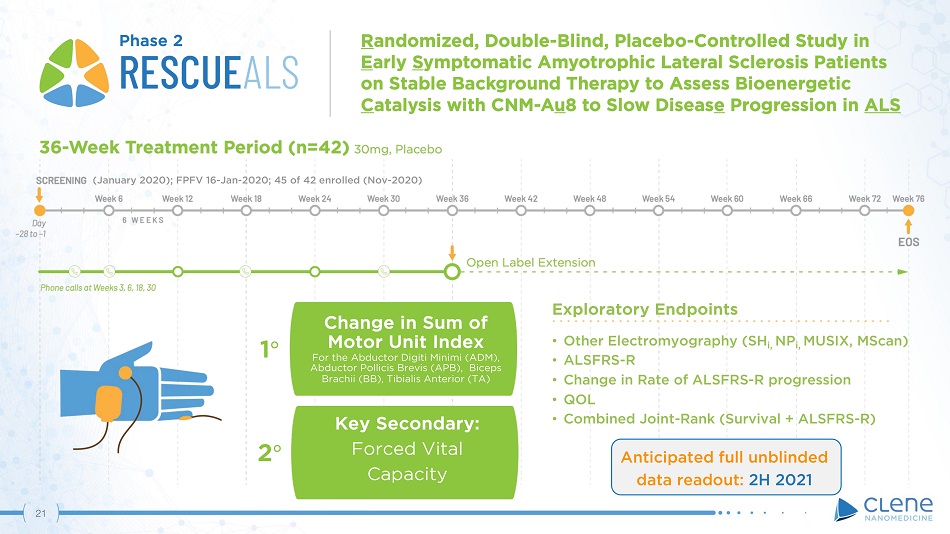
(January 2020); FPFV 16 - Jan - 2020; 45 of 42 enrolled (Nov - 2020) Phase 2 Anticipated full unblinded data readout: 2H 2021 Exploratory Endpoints • Other Electromyography (SH i, NP i, MUSIX, MScan) • ALSFRS - R • Change in Rate of ALSFRS - R progression • QOL • Combined Joint - Rank (Survival + ALSFRS - R) Change in Sum of Motor Unit Index For the Abductor Digiti Minimi (ADM), Abductor Pollicis Brevis (APB), Biceps Brachii (BB), Tibialis Anterior (TA) 1 ι Key Secondary: Forced Vital Capacity 2 ι 20
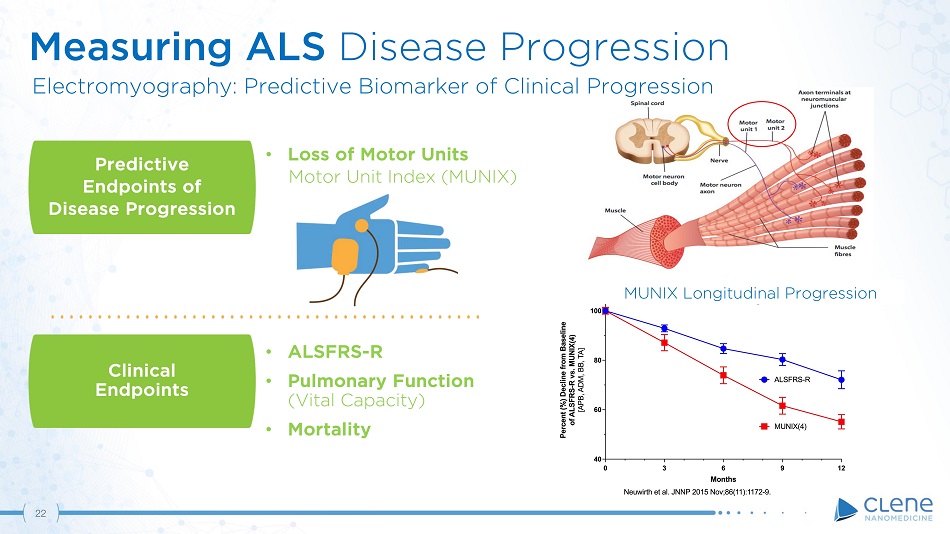
Predictive Endpoints of Disease Progression Clinical E ndpoint s • ALSFRS - R • Pulmonary Function (Vital Capacity) • Mortality • Loss of Motor Units Motor Unit Index (MUNIX) Measuring ALS Disease Progression Electromyography: Predictive Biomarker of Clinical Progression MUNIX Longitudinal Progression 21
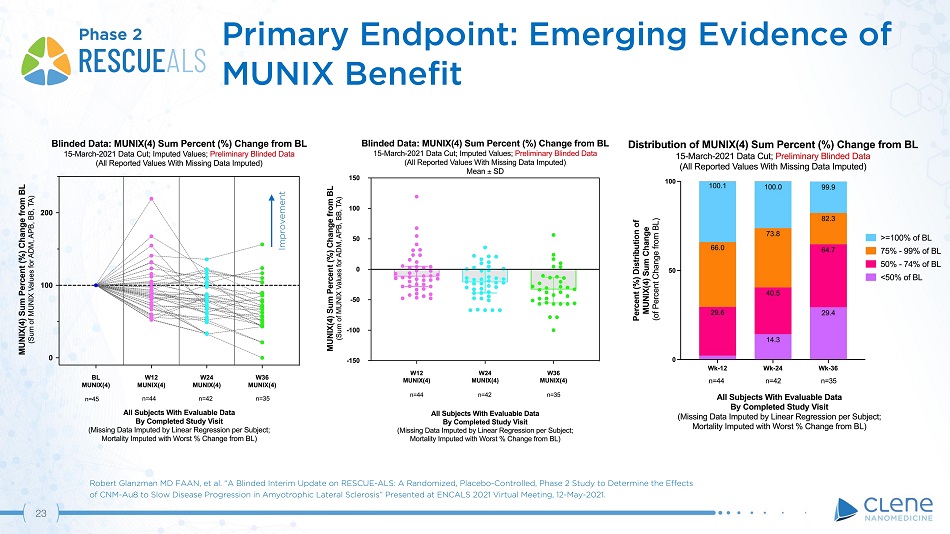
Primary Endpoint: Emerging Evidence of MUNIX Benefit Phase 2 Improvement Robert Glanzman MD FAAN, et al. “A Blinded Interim Update on RESCUE - ALS: A Randomized, Placebo - Controlled, Phase 2 Study to Determine the Effects of CNM - Au8 to Slow Disease Progression in Amyotrophic Lateral Sclerosis” Presented at ENCALS 2021 Virtual Meeting, 12 - May - 2021. 22
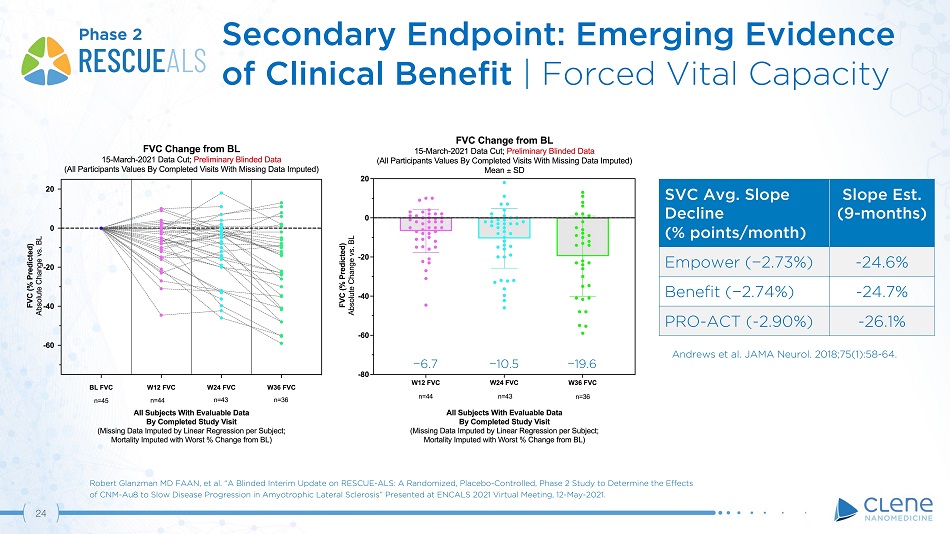
SVC Avg. Slope Decline (% points/month) Slope Est. (9 - mo n t h s) Empower ( − 2.73%) - 24.6% Benefit ( − 2.74%) - 24.7% PRO - ACT ( - 2.90%) - 26.1% − 10. 5 − 19. 6 − 6. 7 Andrews et al. JAMA Neurol. 2018;75(1):58 - 64. Phase 2 Robert Glanzman MD FAAN, et al. “A Blinded Interim Update on RESCUE - ALS: A Randomized, Placebo - Controlled, Phase 2 Study to Determine the Effects of CNM - Au8 to Slow Disease Progression in Amyotrophic Lateral Sclerosis” Presented at ENCALS 2021 Virtual Meeting, 12 - May - 2021. 23 Secondary Endpoint: Emerging Evidence of Clinical Benefit | Forced Vital Capacity
Exploratory Endpoints • Combined Joint Rank (Survival + ALSFRS - R) • Voice pathology • PRO (ALSAQ) • Pharmacodynamic markers Slow Vital Capacity Hand Held Dynamometry Change in ALSF RS - R Registration Study: 24 - Week Treatment Period (3:1 randomization, 120 active [30mg, 60mg]: 40 placebo) (July 2020) (n=160) (n=160) (n=160) 1 ι 2 ι Phase 3 Multiple Independent Regimens with Pooled Placebo Anticipated full unblinded data readout : 1 H 2022 24

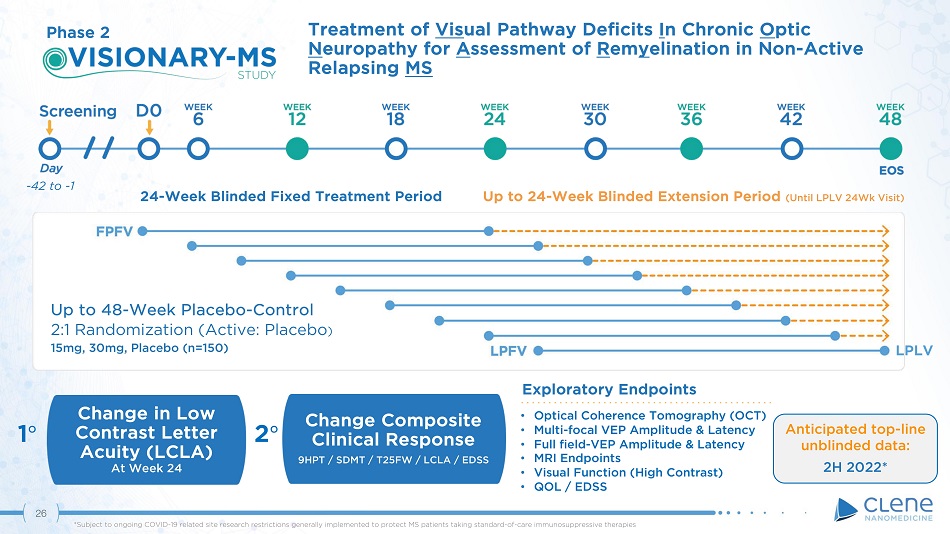
Exploratory Endpoints Treatment of Vis ual Pathway Deficits I n Chronic O ptic N europathy for A ssessment of R em y elination in Non - Active Relapsing MS Change in Low Contrast Letter Acuity (LCLA) At Week 24 Up to 48 - Week Placebo - Control 2:1 Randomization (Active: Placebo ) 15mg, 30mg, Placebo (n=150) • Optical Coherence Tomography (OCT) • Multi - focal VEP Amplitude & Latency • Full field - VEP Amplitude & Latency • MRI Endpoints • Visual Function (High Contrast) • QOL / EDSS 1 ι 2 ι Change Composite Clinical Response 9HPT / SDMT / T25FW / LCLA / EDSS Phase 2 - 42 to - 1 24 - Week Blinded Fixed Treatment Period Up to 24 - Week Blinded Extension Period (Until LPLV 24Wk Visit) LPLV Anticipated top - line unblinded data: 2H 2022* 25 *Subject to ongoing COVID - 19 related site research restrictions generally implemented to protect MS patients taking standard - of - care immunosuppressive therapies
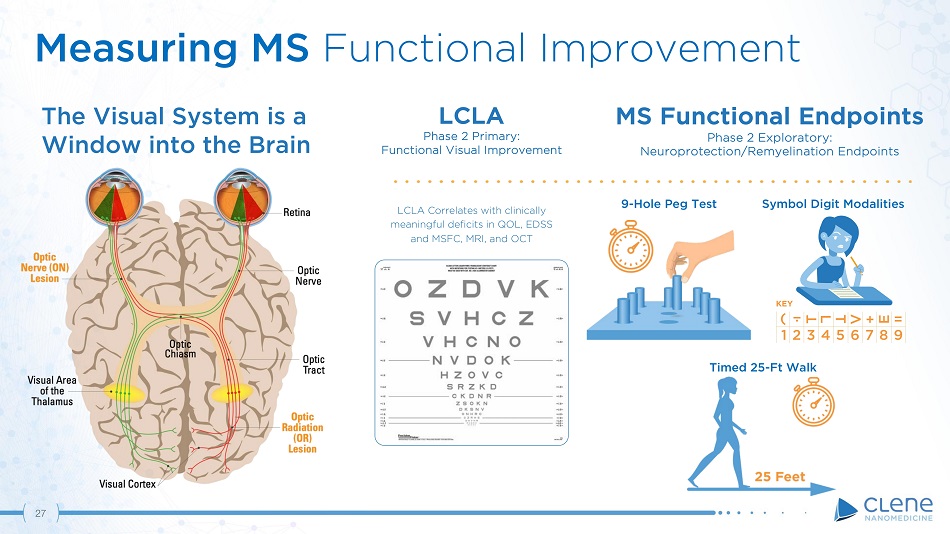
LCLA Phase 2 Primary: Functional Visual Improvement LCLA Correlates with clinically meaningful deficits in QOL, EDSS and MSFC, MRI, and OCT MS Functional Endpoints Phase 2 Exploratory: Neuroprotection/Remyelination Endpoints The Visual System is a Window into the Brain ( T L T V + E = 1 2 3 4 5 6 7 8 9 KEY 9 - Hole Peg Test 26 Symbol Digit Modalities Timed 25 - Ft Walk Measuring MS Functional Improvement
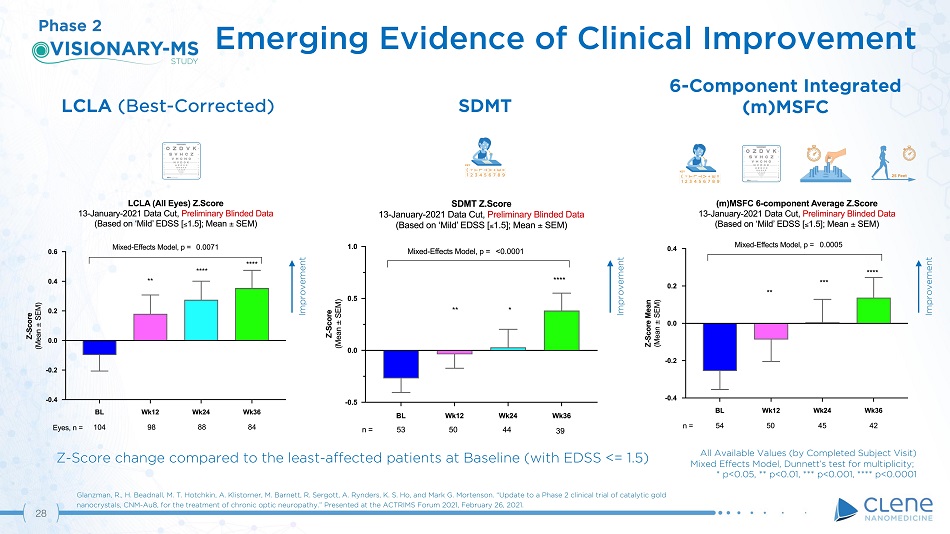
LCLA (Best - Corrected) Emerging Evidence of Clinical Improvement Glanzman, R., H. Beadnall, M. T. Hotchkin, A. Klistorner, M. Barnett, R. Sergott, A. Rynders, K. S. Ho, and Mark G. Mortenson. “Update to a Phase 2 clinical trial of catalytic gold nanocrystals, CNM - Au8, for the treatment of chronic optic neuropathy.” Presented at the ACTRIMS Forum 2021, February 26, 2021. SD M T 6 - Component Integrated (m)MSFC Phase 2 Improvement Improvement Improvement ( T L T V + E = 1 2 3 4 5 6 7 8 9 KEY ( T L T V + E = 1 2 3 4 5 6 7 8 9 KEY Z - Score change compared to the least - affected patients at Baseline (with EDSS <= 1.5) 27 All Available Values (by Completed Subject Visit) Mixed Effects Model, Dunnett’s test for multiplicity; * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001
C S N 28 ® Clean surface nanocrystal (CSN) therapeutics Trade Secrets Patent Status Issued & Allowed Patents 130+ Pending Applications >30 Total Patents/ Applications >160 Patent Description Process And Me thod/ D e vic e (Clean Surface; Gold CSN) State of Matter (CNM - Au8) Method of Use (Prevent Demyelination & MoA) Method of Use (Bi - Metallic Au/Pt; Antimicrobial) Plasma Conditioning Electrode Design & Cycling Trough Flow, Temp, Pressure Concentration & Filtration Strong Intellectual Property Extensive Patent Portfolio With Protection Through 2035 a & Proprietary Trade Secrets; Plus 7 - year Orphan Drug Designation a With Patent Restoration Term (assuming 5 - year extension).

Clene | Proprietary Nanocrystal Manufacturing In - House ISO8 Clean Room Clinical Production in North East, MD Validated CMC Processes 29 Designed to be Scalable to Commercialization Patented H ydro - electr o - Crystallization Proprietary Trade Secrets

Anticipated Timeline & Investor Catalysts 2020 - 2023 30

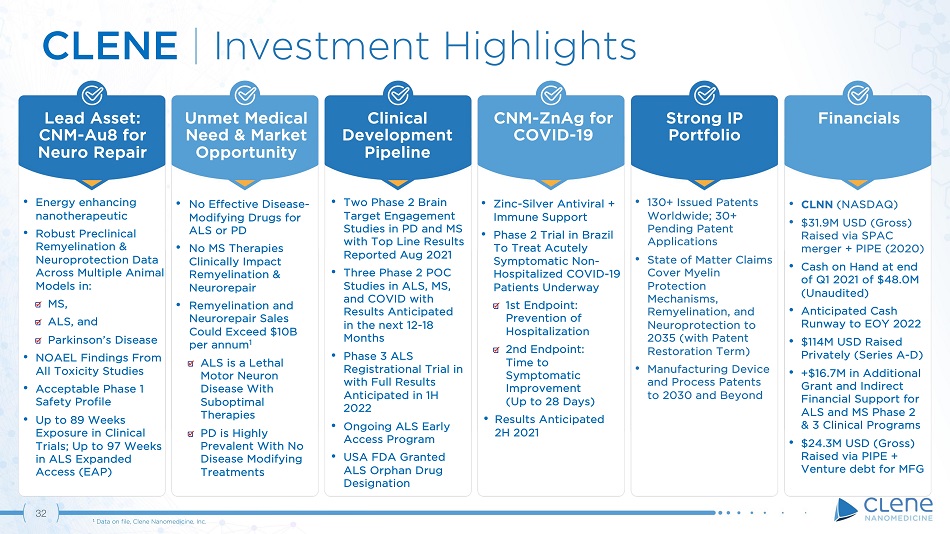
31 Unmet Medical Need & Market Opportunity Lead Asset: CNM - Au8 for Neuro Repair Clinical D e v e lo p m en t Pipeline Strong IP Portfolio Financials CNM - ZnAg for COVID - 19 CLENE | Investment Highlights • Energy enhancing nanotherapeutic • Robust Preclinical Remyelination & Neuroprotection Data Across Multiple Animal Models in: MS, ALS, and Parkinson’s Disease • NOAEL Findings From All Toxicity Studies • Acceptable Phase 1 Safety Profile • Up to 89 Weeks Exposure in Clinical Trials; Up to 97 Weeks in ALS Expanded Access (EAP) • Two Phase 2 Brain Target Engagement Studies in PD and MS with Top Line Results Reported Aug 2021 • Three Phase 2 POC Studies in ALS, MS, and COVID with Results Anticipated in the next 12 - 18 Months • Phase 3 ALS Registrational Trial in with Full Results Anticipated in 1H 2022 • Ongoing ALS Early Access Program • USA FDA Granted ALS Orphan Drug Designation • CLNN (NASDAQ) • $ 31 . 9 M USD (Gross) Raised via SPAC merger + PIPE ( 2020 ) • Cash on Hand at end of Q 1 2021 of $ 48 . 0 M (Unaudited) • Anticipated Cash Runway to EOY 2022 • $ 114 M USD Raised Privately (Series A - D) • +$16.7M in Additional Grant and Indirect Financial Support for ALS and MS Phase 2 & 3 Clinical Programs • $24.3M USD (Gross) Raised via PIPE + Venture debt for MFG • Zinc - Silver Antiviral + Immune Support • Phase 2 Trial in Brazil To Treat Acutely Symptomatic Non - Hospitalized COVID - 19 Patients Underway 1st Endpoint: Prevention of Hospitalization 2nd Endpoint: Time to S y m p t o m at i c Imp r o vemen t (Up to 28 Days) • Results Anticipated 2H 2021 • 130+ Issued Patents Worldwide; 30+ Pending Patent Applications • State of Matter Claims Cover Myelin Protection M ech a nisms , Remyelination, and Neuroprotection to 2035 (with Patent Restoration Term) • Manufacturing Device and Process Patents to 2030 and Beyond • No Effective Disease - Modifying Drugs for ALS or PD • No MS Therapies Clinically Impact Remyelination & Neurorepair • Remyelination and Neurorepair Sales Could Exceed $10B per annum 1 ALS is a Lethal Motor Neuron Disease With Suboptimal Therapies PD is Highly Prevalent With No Disease Modifying Treatments 1 Data on file, Clene Nanomedicine, Inc.
Clene Inc. HQ

& Clinical Development 6550 South Millrock Drive, Suite G50 Salt Lake City, UT 84121 R&D and Manufacturing 500 Principio Parkway, Suite 400 North East, MD 21901 © 2021 Clene Inc. Version: 4 - August - 2021
































