Exhibit 99.2

CLNN (NASDAQ) N o v e mb e r 8 , 2 0 2 1

Forward Looking Statements 2 This presentation contains "forward - looking statements" within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995 . Clene's actual results may differ from its expectations, estimates, and projections and consequently, you should not rely on these forward - looking statements as predictions of future events . Words such as "expect," "estimate," "project," "budget," "forecast," "anticipate," "intend," "plan," "may," "will," "could," "should," "believes," "predicts," "potential," "might" and "continues," and similar expressions are intended to identify such forward - looking statements . These forward - looking statements involve significant known and unknown risks and uncertainties, many of which are beyond Clene’s control and could cause actual results to differ materially and adversely from expected results . Factors that may cause such differences include Clene’s ability to demonstrate the efficacy and safety of its drug candidates ; the clinical results for its drug candidates, which may not support further development or marketing approval ; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials and marketing approval ; Clene’s ability to achieve commercial success for its marketed products and drug candidates, if approved ; Clene’s ability to obtain and maintain protection of intellectual property for its technology and drugs ; Clene’s reliance on third parties to conduct drug development, manufacturing and other services ; Clene’s limited operating history and its ability to obtain additional funding for operations and to complete the licensing or development and commercialization of its drug candidates ; the impact of the COVID - 19 pandemic on Clene’s clinical development, commercial and other operations, as well as those risks more fully discussed in the section entitled “Risk Factors” in Clene’s recently filed registration statement on Form S - 1 (filed July 22 , 2021 ), as well as discussions of potential risks, uncertainties, and other important factors in Clene’s subsequent filings with the U . S . Securities and Exchange Commission . Clene undertakes no obligation to release publicly any updates or revisions to any forward - looking statements to reflect any change in its expectations or any change in events, conditions or circumstances on which any such statement is based, subject to applicable law . All information in this presentation is as of the date of presented or the date made publicly available . The information contained in any website referenced herein is not, and shall not be deemed to be, part of or incorporated into this presentation .

Creating Elemental Solutions for Human Health Œ 3 CL E N E Ρ | M a n a g em e n t Te a m

CLENE | Overview September 30, 2021 Cash and restricted cash on hand (unaudited): $60.6M ALS R e g i s t r a t i o n Trial Topline data in 2H 2022 2 >230 patient years of CNM - Au8 clinical exposure CNM - Au8® a gold nanocrystal suspension, in development as the first energetic catalyst to remyelinate 1 & neuroprotect Manufacturing expansion in progress, preparing for possible commercialization in 2023 Strong IP: 130+ patents on Cle a n - S u r fac e - Nanocrystal technology (CSN®) platform 4 Data on File, Clene Nanomedicine, Inc. 1 Robinson et al. Sci Rep. 2020 Feb 11;10(1):1936. 2 https://clinicaltrials.gov/ct2/show/NCT04414345.

5 CLENE | Pipeline
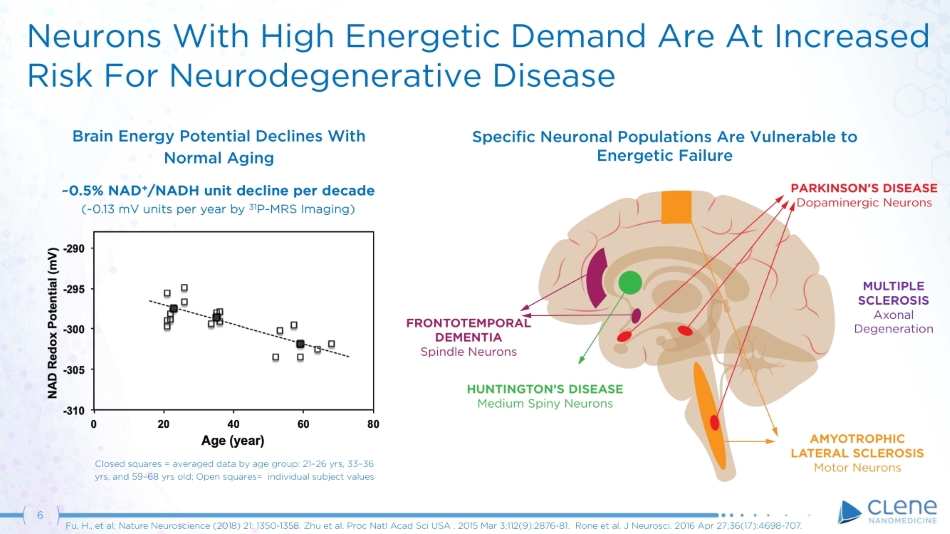
6 Fu, H., et al; Nature Neuroscience (2018) 21: 1350 - 1358. Zhu et al. Proc Natl Acad Sci USA . 2015 Mar 3;112(9):2876 - 81. Rone et al. J Neurosci. 2016 Apr 27;36(17):4698 - 707. Neurons With High Energetic Demand Are At Increased Risk For Neurodegenerative Disease Brain Energy Potential Declines With Normal Aging ~0.5% NAD + /NADH unit decline per decade (~0.13 mV units per year by 31 P - MRS Imaging) Closed squares = averaged data by age group: 21 – 26 yrs, 33 – 36 yrs, and 59 – 68 yrs old; Open squares= individual subject values AMYOTROPHIC LATERAL SCLEROSIS Motor Neurons HUNTINGTON’S DISEASE Medium Spiny Neurons FR O N T O T E M P O R A L DEMENTIA Spindle Neurons Specific Neuronal Populations Are Vulnerable to Energetic Failure PARKINSON’S DISEASE Dopaminergic Neurons MULTIPLE SC L E R O SIS Axonal De g e n e r a t i on
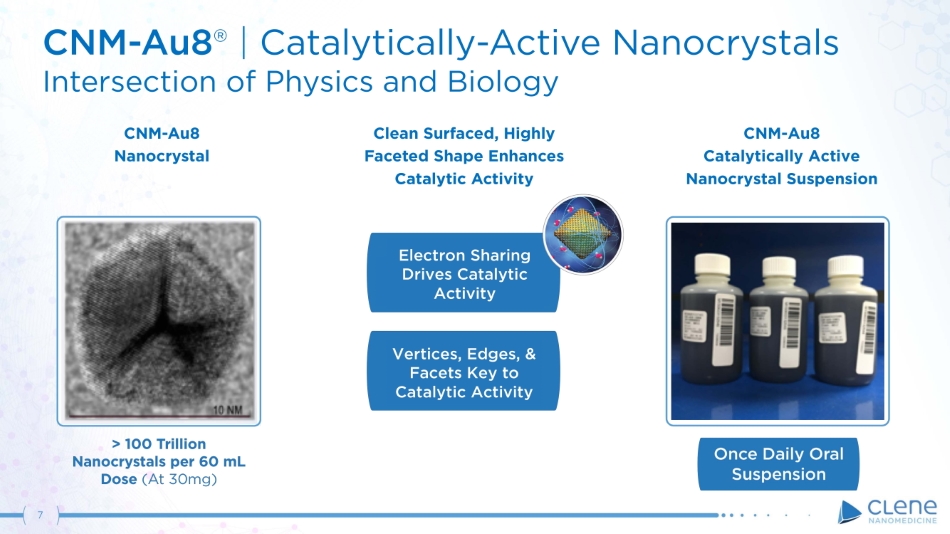
Vertices, Edges, & Facets Key to Catalytic Activity 7 CNM - Au8 Catalytically Active Nanocrystal Suspension Clean Surfaced, Highly Faceted Shape Enhances Catalytic Activity CNM - Au8 Nanocrystal CNM - A u 8 ® | C a ta l y t i c a l l y - A ct i v e Na noc r y s t a l s Intersection of Physics and Biology Electron Sharing Drives Catalytic Activity
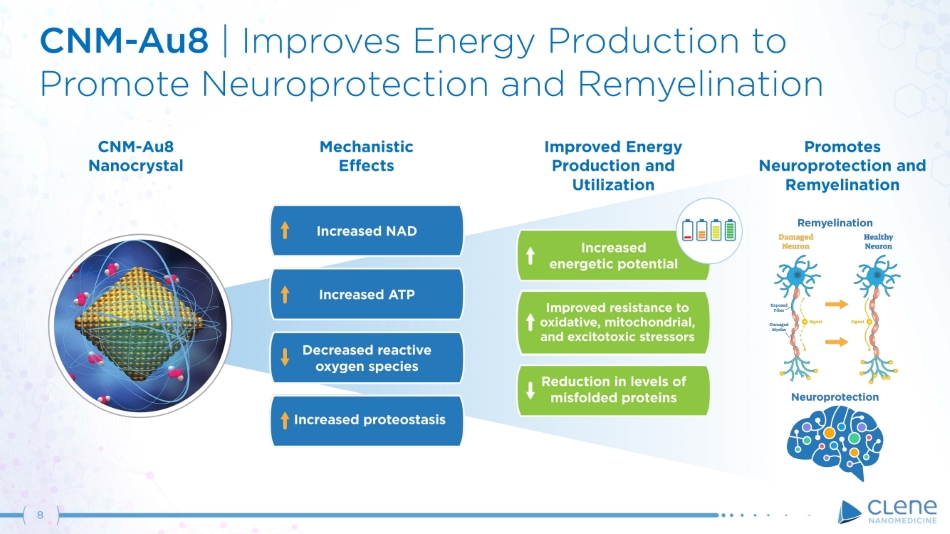
CNM - Au8 Nanocrystal M e ch a ni s tic Effects Improved Energy Production and Utilization Promotes Neuroprotection and Remyelination CNM - A u 8 | Im p r o v e s E n e r g y P r o d u c t i on t o Promote Neuroprotection and Remyelination 8

CNM - Au8 | Significant Global Opportunity 70 7 , 158 10 3,9 0 3 828,703 596,4 0 7 256,455 1,407,701 0 4 00 , 0 0 0 800,000 1 , 2 00 , 00 0 1 , 6 00 , 0 0 0 U . S . C a n a d a W. Europe Central/E. Europe J a p a n C h i n a Est. Diagnosed PD Patients by Region Source: Lancet Neurol. 2018 Nov;17(11):939 - 953; ~6.1M patients globally, data as of 2016.. PARKINSON’S DISEASE ~ 6 . 1 M p t s g l ob a ll y ; $ 6 B p r o j e c t e d b y 2 0 2 6 3 2 ND most common neurodegenerative disorder; only symptomatic treatments 62,531 10,013 71,288 10,946 13, 8 9 3 54,405 0 2 0 , 00 0 40,000 6 0 , 00 0 8 0 , 00 0 U . S . C a n a d a W. Europe C e n t r a l / E . E ur o p e J a p a n C h i n a Est. Diagnosed MND Patients by Region Source: Lancet Neurol. 2018 Dec;17(12):1083 - 1097. MND includes amyotrophic lateral sclerosis, spinal muscular atrophy, hereditary spastic paraplegia, primary lateral sclerosis, progressive muscular atrophy, and pseudobulbar palsy MOTOR NEURON DISEASE (ALS, Other Orphan Disorders) AL S s al e s > $ 1 B gl o b a ll y b y 2 0 2 9 1 . Cur r e nt drugs are largely ine ff ective, mostly generic Source: Lancet Neurol. 2019 Mar;18(3):269 - 285; ~2.2.M patients globally, data as of 2016 511,855 7 9, 4 19 543,862 204,591 46, 2 4 9 103,194 0 1 5 0 , 0 0 0 300 , 00 0 4 5 0 , 0 0 0 600,000 U . S . C a n a d a W. Europe C e n t r a l / E . E ur o p e J a p a n C h i n a Est. Diagnosed MS Patients by Region MULTIPLE SCLEROSIS ~ 2 . 2 M p t s g l ob a ll y ; $ 2 3 B m a r k et 2 Only approved treatments are immunomodulators 9 1 Clarivate, DRG, ALS 2020 . 2 Westad et al. 2017, doi:10.1038/nrd.2017.107;. 3 Parkinson’s Market Data Forecast, April 2021.

CNM - Au8 | Preclinical Evidence for Energetic Improvement Therapeutic Activity Across Remyelination + Neuroprotection Models 10 Data on File, Clene Nanomedicine, Inc. Robinson et al. Sci Rep. 2020 Feb 11;10(1):1936. MN Neuroprotection ALS SOD1, In Vitro Forebrain Neuroprotection C9ORF72 In Vitro Alzheimer’s Disease Induced Neurons In Vitro
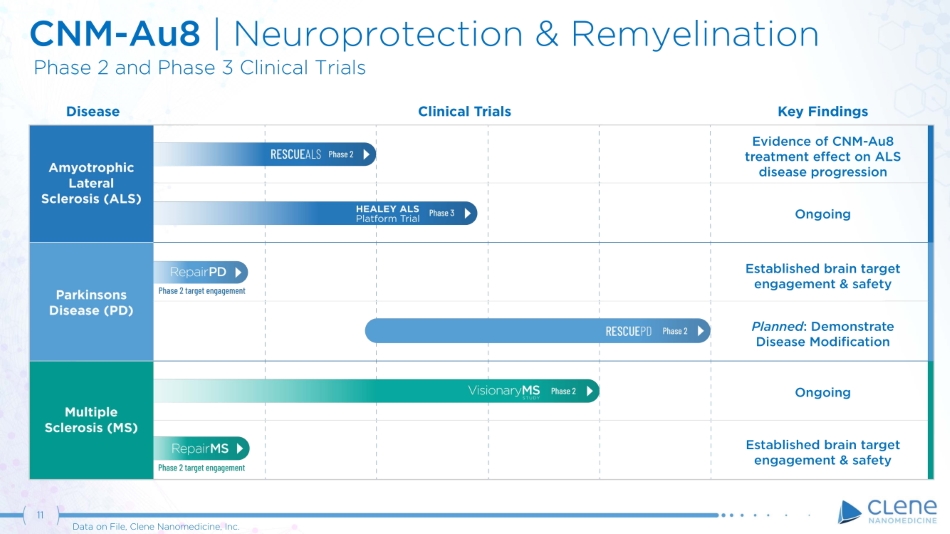
11 Data on File, Clene Nanomedicine, Inc. On g o i n g Established brain target e n g a geme n t & s a f e t y Planned : Demonstrate Disease Modification Ongoing Established brain target e n g a geme n t & s a fe t y CNM - Au8 | N e u r o p r o t e c t i on & R e my e li n a t i on P h a se 2 a n d P h a se 3 C li n i c a l T ria ls Key Findings Evidence of CNM - Au8 treatment e ff ect on ALS disease progression Disease Clinical Trials
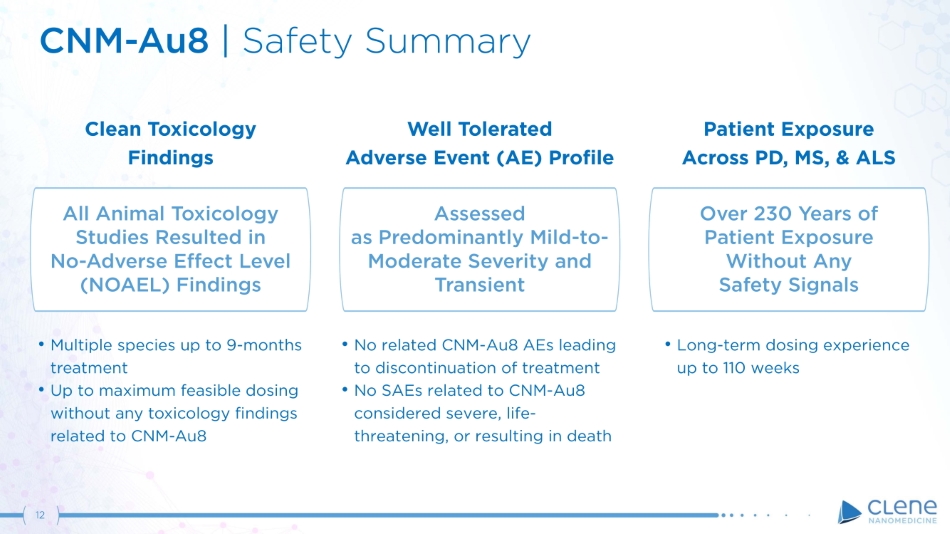
CNM - Au8 | Safety Summary Over 230 Years of Patient Exposure Without Any Safety Signals Patient Exposure Across PD, MS, & ALS • Long - term dosing experience up to 110 weeks All Animal Toxicology Studies Resulted in No - Adverse E ff ect Level (NOAEL) Findings Clean Toxicology Findings • Multiple species up to 9 - months treatment • Up to maximum feasible dosing without any toxicology findings related to CNM - Au 8 Assessed as Predominantly Mild - to - Moderate Severity and Transient 12 Well Tolerated Adverse Event (AE) Profile • No related CNM - Au8 AEs leading to discontinuation of treatment • No SAEs related to CNM - Au8 considered severe, life - threatening, or resulting in death
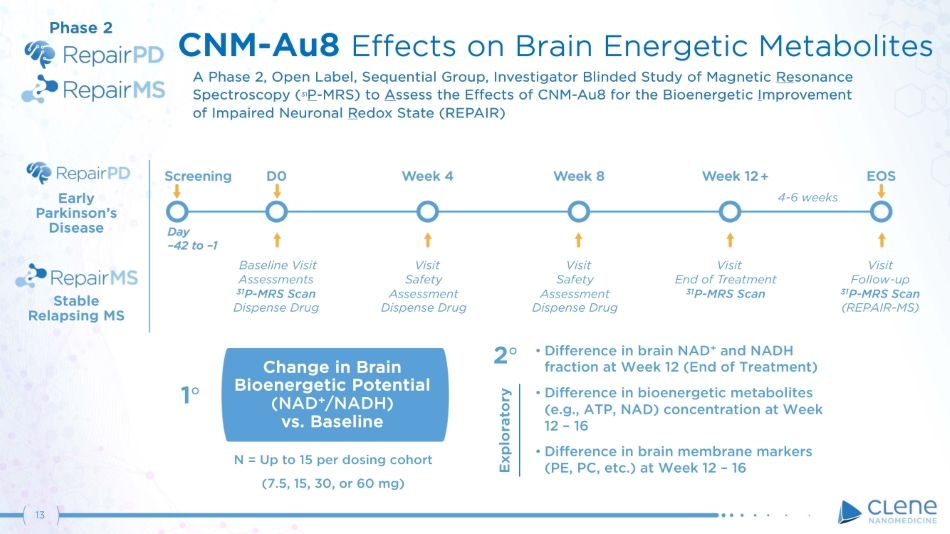
• Difference in brain NAD + and NADH fraction at Week 12 (End of Treatment) • Difference in bioenergetic metabolites (e.g., ATP, NAD) concentration at Week 12 – 16 • Difference in brain membrane markers (PE, PC, etc.) at Week 12 – 16 Change in Brain Bioenergetic Potential (NAD + /NADH) vs. Baseline 1 ι 2 ι CNM - Au8 Effects on Brain Energetic Metabolites A Phase 2 , Open Label, Sequential Group, Investigator Blinded Study of Magnetic Re sonance S pe c t r o s c o p y ( 3 1 P - MRS ) t o A s se s s t h e E ff e c t s o f CN M - A u 8 f o r t h e Bio ene r g et i c I m p r ov emen t of Impaired Neuronal R edox State (REPAIR) N = U p t o 1 5 p e r do s i n g co h o r t (7. 5 , 1 5 , 3 0 , or 6 0 m g ) Phase 2 Exploratory Stable Relapsing MS Early P a r k i n s o n ’ s Disease Visit S a f ety Assessment Dispense Drug Visit Safety Assessment Dispense Drug Visit End of Treatment 31 P - MRS Scan Visit F ollo w - up 31 P - MRS Scan (REPAIR - MS) Baseline Visit Assessments 31 P - MRS Scan Dispense Drug + 4 - 6 weeks 13
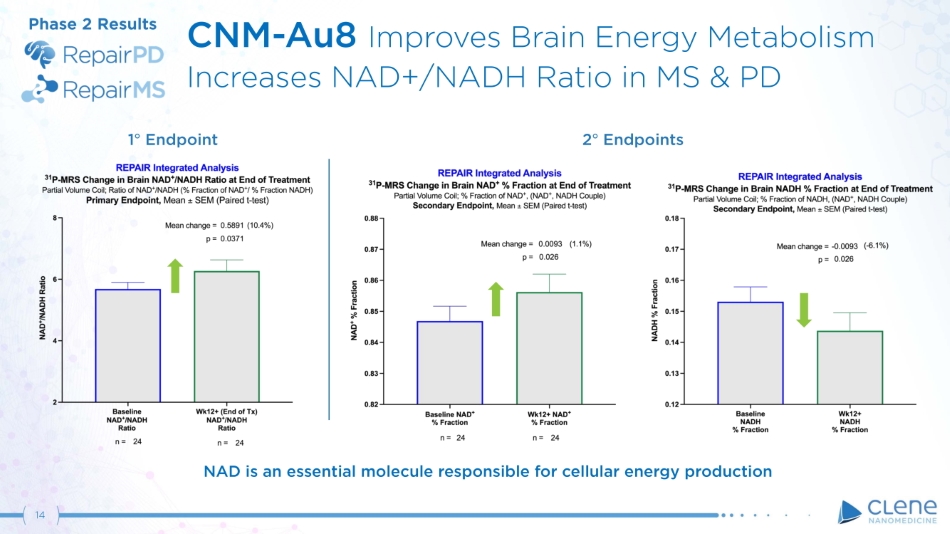
CNM - Au8 Improves Brain Energy Metabolism Increases NAD+/NADH Ratio in MS & PD Phase 2 Results 1 ƒ E n dpo i n t 2 ƒ Endpoints NAD is an essential molecule responsible for cellular energy production 14

(January 2020); FPFV 16 - Jan - 2020; 45 of 42 enrolled (Nov - 2020) Phase 2 15 1 Study was powered for MUNIX primary endpoint Neurophysiology MUNIX 1 Pulmonary Function Forced Vital Capacity F u n c t i o n & Q o L ALSFRS - R, ALSSQOL - SF Disease Progression & Survival
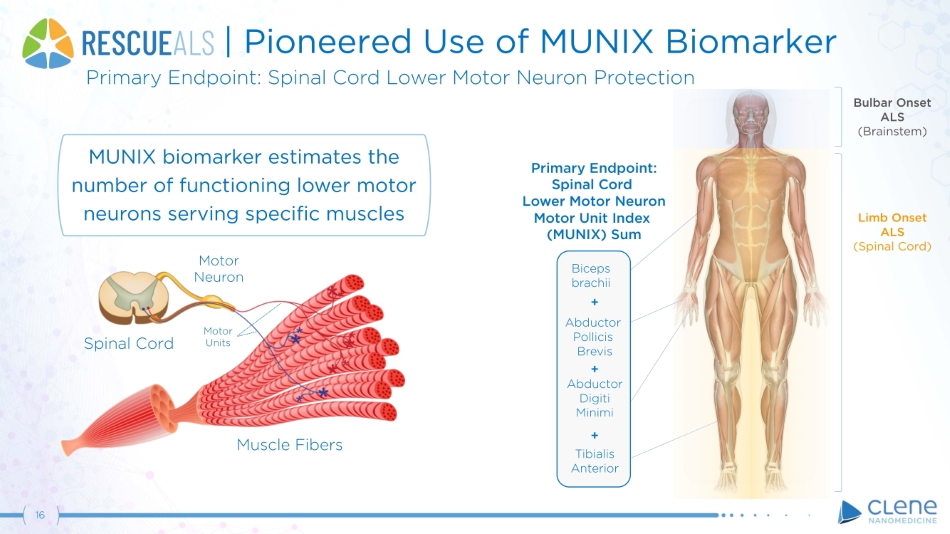
16 | Pioneered Use of MUNIX Biomarker Primary Endpoint: Spinal Cord Lower Motor Neuron Protection Muscle Fibers Spinal Cord Motor Ne u r o n Bulbar Onset ALS (Brainstem) Limb Onset ALS (Spinal Cord) Primary Endpoint: Spinal Cord Lower Motor Neuron Motor Unit Index (MUNIX) Sum Bi c e p s b r a chi i + Abduc t o r Pollicis Brevis + Abduc t o r Digiti Minimi + Tibialis An t e ri o r M o t o r Units MUNIX biomarker estimates the number of functioning lower motor neurons serving specific muscles
| Results Support Phase 3 Development 17 Data on File, Clene Nanomedicine, Inc.
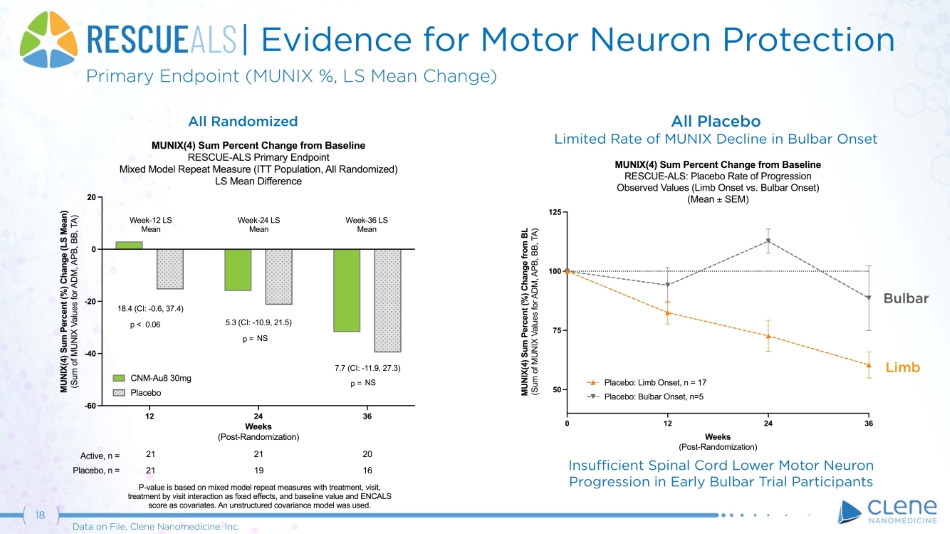
| Evidence for Motor Neuron Protection Primary Endpoint (MUNIX %, LS Mean Change) All Randomized All Placebo Limited Rate of MUNIX Decline in Bulbar Onset 18 Data on File, Clene Nanomedicine, Inc. L i m b Bu l b a r Insufficient Spinal Cord Lower Motor Neuron Progression in Early Bulbar Trial Participants
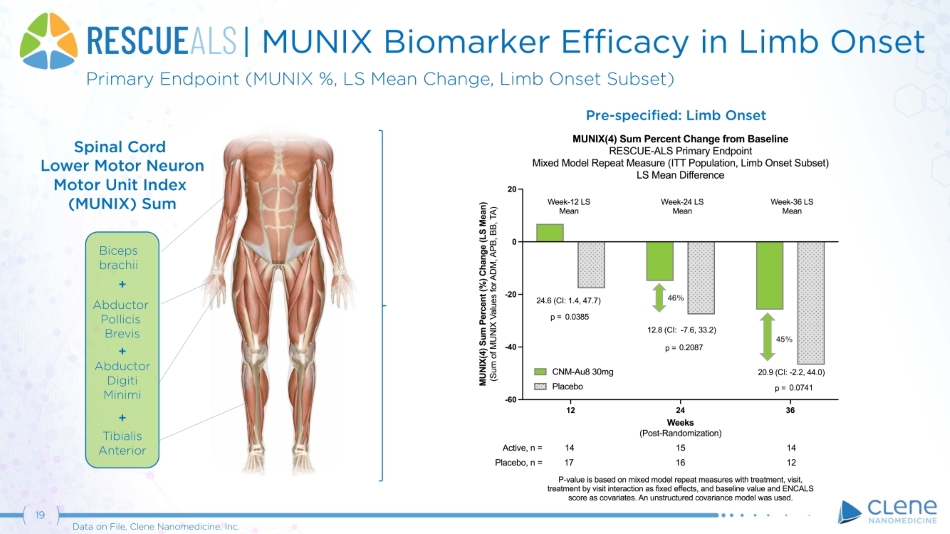
| MUNIX Biomarker Efficacy in Limb Onset Bi c e p s b r a chi i + Abduc t o r Pollicis Brevis + Abduc t o r Digiti Minimi + Tibialis An t e ri o r Primary Endpoint (MUNIX %, LS Mean Change, Limb Onset Subset) Pre - specified: Limb Onset Spinal Cord Lower Motor Neuron Motor Unit Index (MUNIX) Sum 45% 46% 19 Data on File, Clene Nanomedicine, Inc.
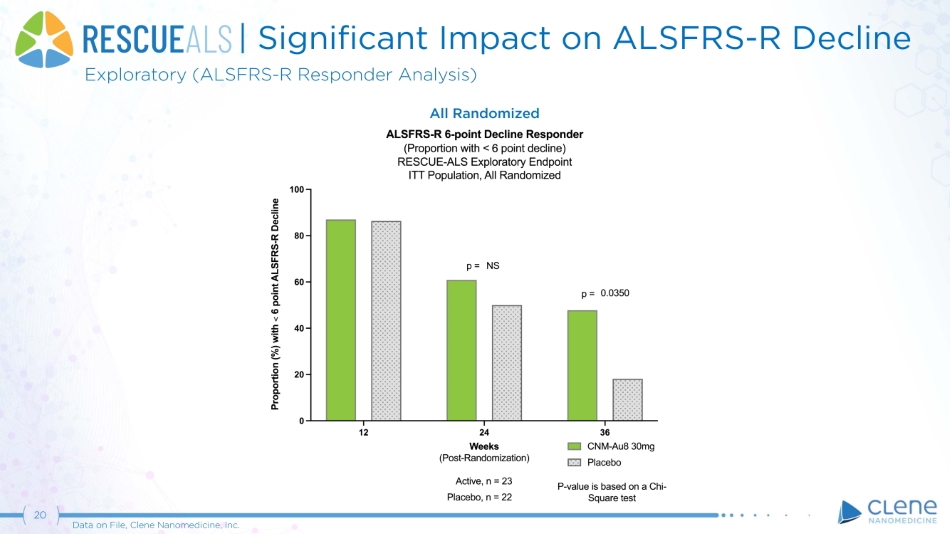
| Significant Impact on ALSFRS - R Decline Exploratory (ALSFRS - R Responder Analysis) All Randomized 20 Data on File, Clene Nanomedicine, Inc.
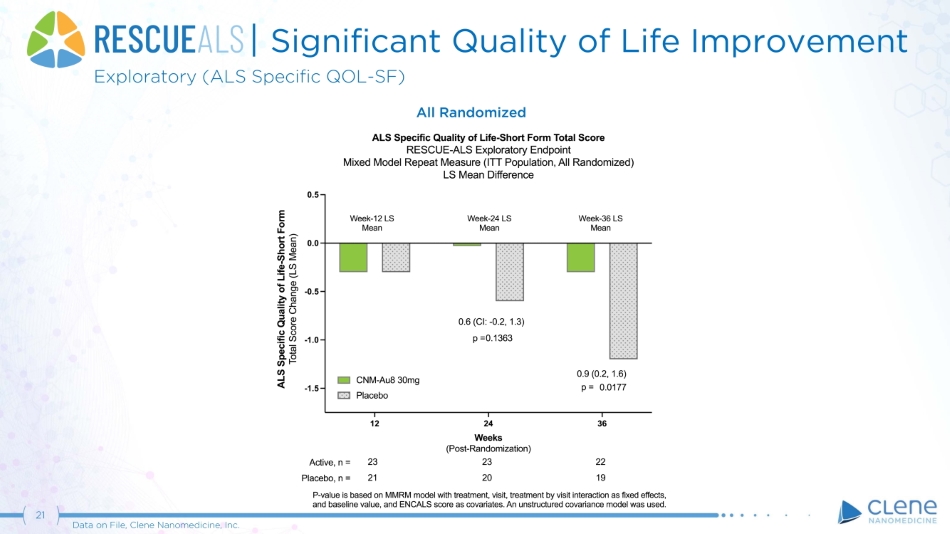
| Significant Quality of Life Improvement Exploratory (ALS Specific QOL - SF) All Randomized 21 Data on File, Clene Nanomedicine, Inc.

| Significant Impact on ALS Disease Progression Exploratory Endpoint (Disease Progression) ALS Disease Progression defined as: • D e a t h , o r • Tracheostomy, or • Non - invasive ventilation, or • Gastrostomy tube All B ul b ar All L i m b Sensitivity 22 Data on File, Clene Nanomedicine, Inc.
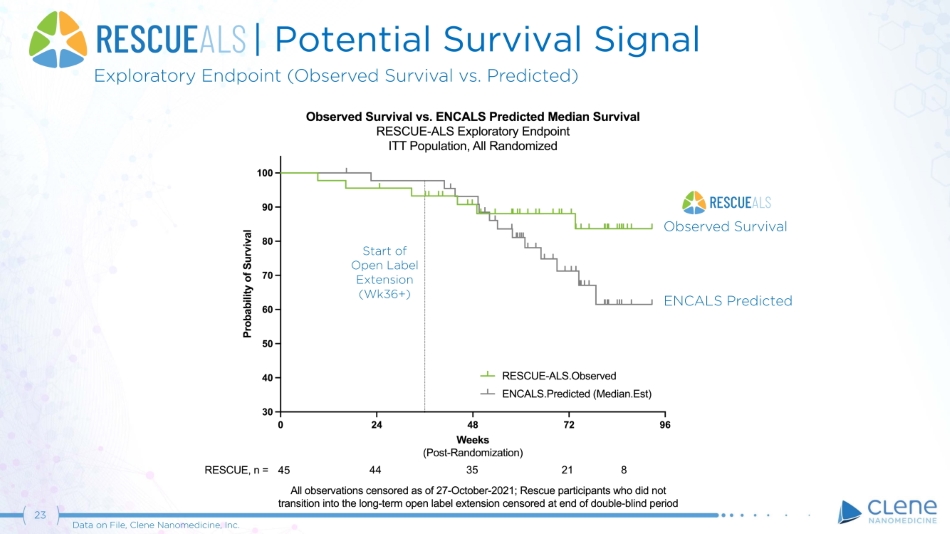
| Potential Survival Signal Exploratory Endpoint (Observed Survival vs. Predicted) ENCALS Predicted 23 Data on File, Clene Nanomedicine, Inc. Observed Survival Start of O p e n L a b el Extension (Wk36+)

| Well Tolerated & No Safety Signals Safety Summary • No CNM - Au8 related serious adverse events (SAEs) • No CNM - Au8 related drug discontinuations • No imbalances in treatment emergent adverse event (TEAEs) • TEAEs were predominantly mild - to - moderate and transient • Most common TEAEs associated with CNM - Au8 ( a s p i r a t i o n p n e u m o n i a , n =3 ; n a u s e a , n = 2 ; a bd o m i n a l d i s c o m f o r t , n = 2 ) 24 Data on File, Clene Nanomedicine, Inc.
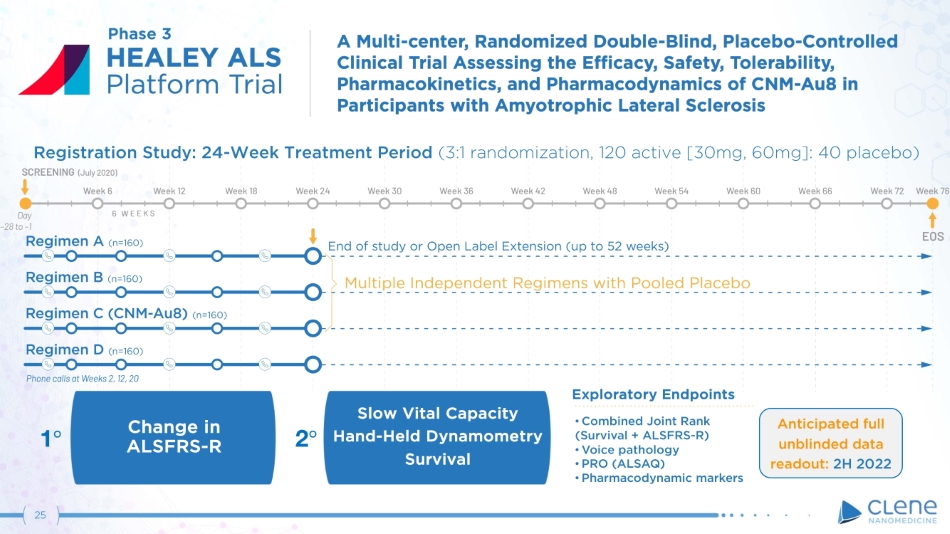
25 Exploratory Endpoints • Combined Joint Rank (S u r v i v al + AL S F R S - R) • Voice pathology • PRO (ALSAQ) • Pharmacodynamic markers Slow Vital Capacity Hand - Held Dynamometry Survival Change in AL S F R S - R Registration Study: 24 - Week Treatment Period (3:1 randomization, 120 active [30mg, 60mg]: 40 placebo) 1 ι 2 ι Phase 3 Anticipated full unblinded data readout : 2 H 2022
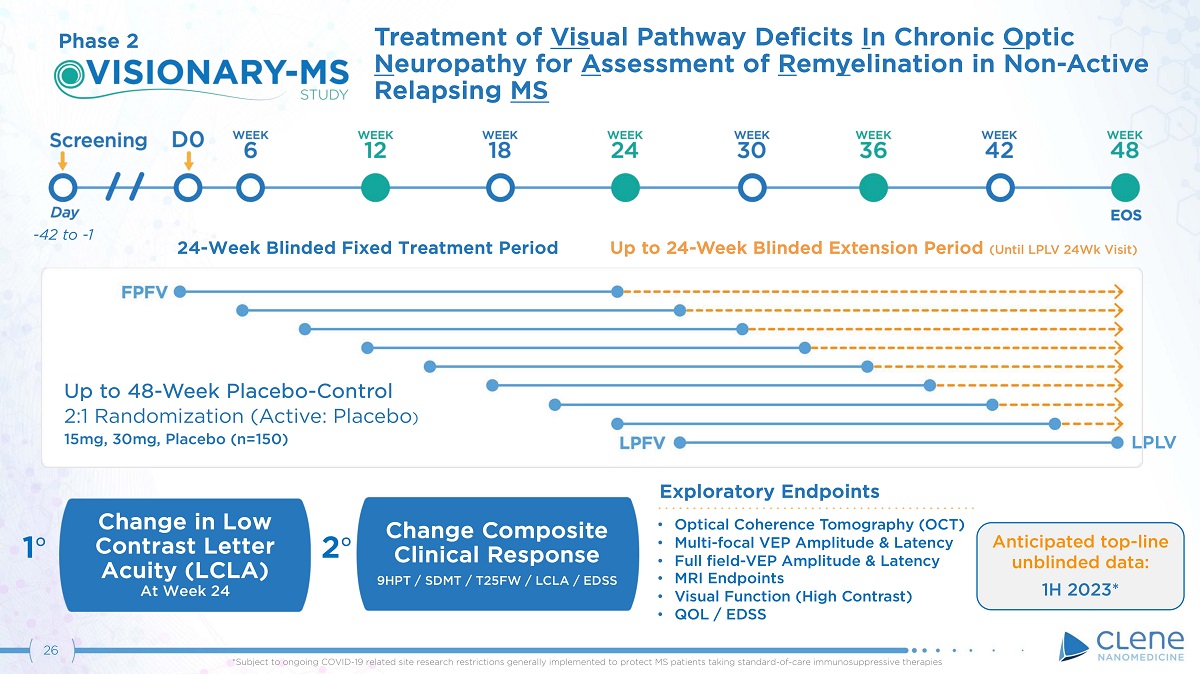
26 Exploratory Endpoints Treatment of Vis ual Pathway Deficits I n Chronic O ptic N eu r op a t h y f o r A s se s s m e n t o f R e m y el i n a t i o n i n N o n - A c t i v e Relapsing MS Change in Low Contrast Letter Acuity (LCLA) At Week 24 Up to 48 - Week Placebo - Control 2:1 Randomization (Active: Placebo ) 15 m g , 3 0 m g , P l a c e b o ( n= 15 0) • Optical Coherence Tomography (OCT) • Multi - focal VEP Amplitude & Latency • Full field - VEP Amplitude & Latency • MRI Endpoints • Visual Function (High Contrast) • QOL / EDSS 1 ι 2 ι Change Composite Clinical Response 9HPT / SDMT / T25FW / LCLA / EDSS Phase 2 - 42 to - 1 24 - Week Blinded Fixed Treatment Period Up to 24 - Week Blinded Extension Period (Until LPLV 24Wk Visit) LP L V Anticipated top - line unblinded data: 1H 2023* *Subject to ongoing COVID - 19 related site research restrictions generally implemented to protect MS patients taking standard - of - care immunosuppressive therapies
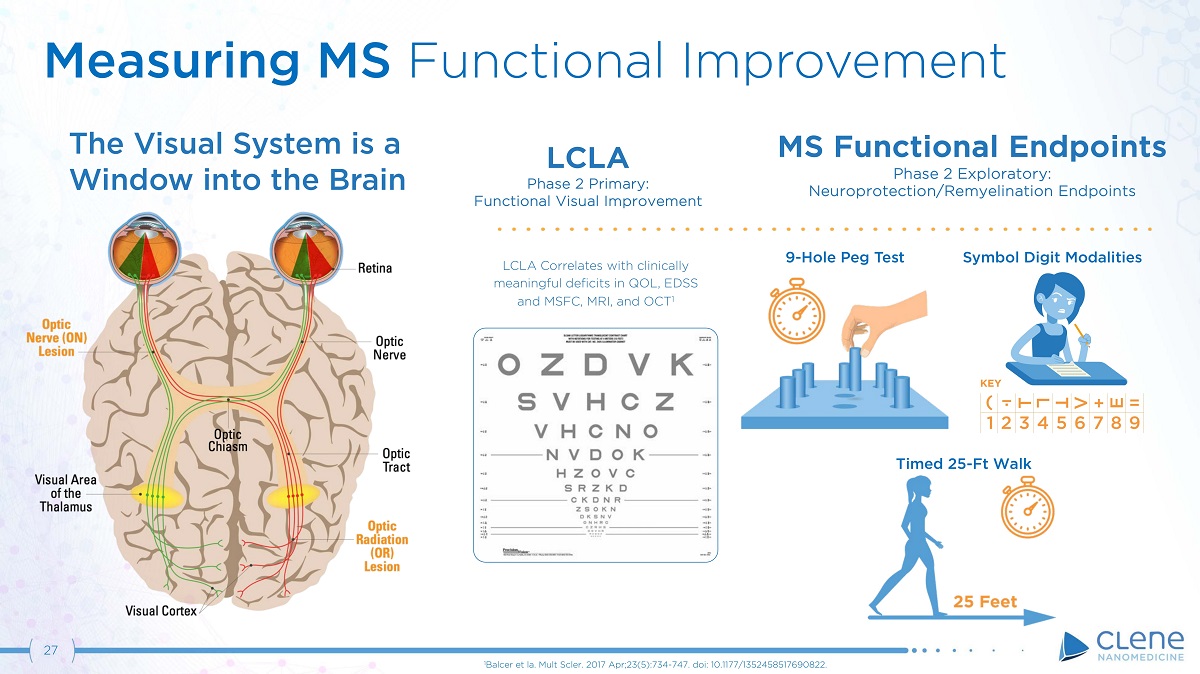
27 LCLA Phase 2 Primary: Functional Visual Improvement LCLA Correlates with clinically meaningful deficits in QOL, EDSS a n d M S F C , MR I , a n d O C T 1 MS Functional Endpoints Phase 2 Exploratory: Neuroprotection/Remyelination Endpoints The Visual System is a Window into the Brain ( T L T V + E = 1 2 3 4 5 6 7 8 9 KEY 9 - Hole Peg Test Symbol Digit Modalities Timed 25 - Ft Walk Measuring MS Functional Improvement 1 Balcer et la. Mult Scler. 2017 Apr;23(5):734 - 747. doi: 10.1177/1352458517690822.
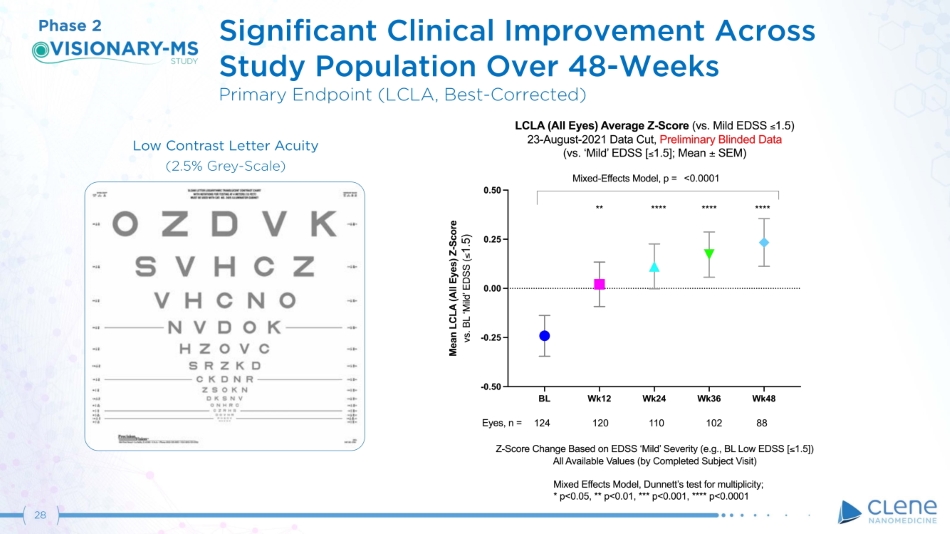
28 Significant Clinical Improvement Across Study Population Over 48 - Weeks Primary Endpoint (LCLA, Best - Corrected) Phase 2 Low Contrast Letter Acuity (2 . 5 % G r e y - S c a l e )
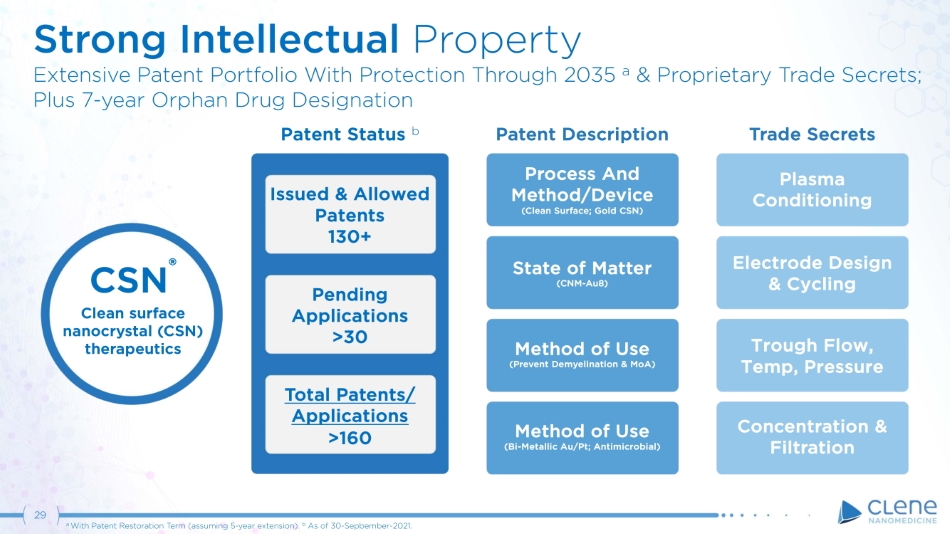
Trade Secrets Patent Status b Issued & Allowed Patents 130+ Pending Applications >30 Total Patents/ Applications >160 Patent Description Process And Me t h o d / D e v i c e (Clean Surface; Gold CSN) State of Matter (CNM - Au8) Method of Use (Prevent Demyelination & MoA) Method of Use (Bi - Metallic Au/Pt; Antimicrobial) Plasma C o n d i t i o n i n g Electrode Design & Cycling Trough Flow, Temp, Pressure Concentration & Filtration Strong Intellectual Property Extensive Patent Portfolio With Protection Through 2035 a & Proprietary Trade Secrets; P l us 7 - y e ar O r p han D r ug D e si g n a t i on 29 a With Patent Restoration Term (assuming 5 - year extension). b As of 30 - Sepbember - 2021. C S N ® Clean surface nanocrystal (CSN) therapeutics

Clene | Proprietary Nanocrystal Manufacturing In - House ISO8 Clean Room Clinical Production in Maryland Va l i d a t e d C M C Processes Designed to be Scalable to Commercialization Patented H y d r o - el ec t r o - Crystallization P r o p r i e t a r y T r ade Secrets 30
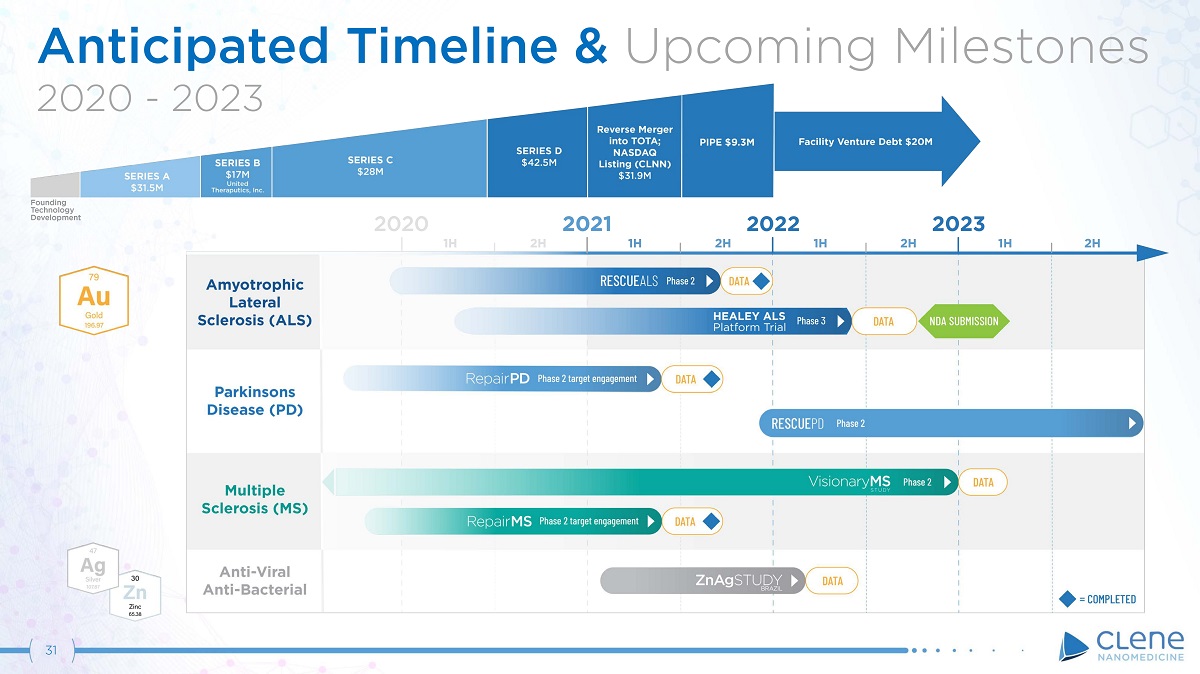
Anticipated Timeline & Upcoming Milestones 2020 - 2023 31
Na n o t h e r ap e u t i cs Platform • First - in - class nanotherapeutic with high catalytic activity to drive energy production and utilization in stressed CNS cells • Applications across neurology, infectious disease, and oncology Lead Asset: C N M - A u 8 f o r Neurorepair • CNM - Au8 improves cellular energy production and utilization to promote neuroprotection and remyelination • Phase 2 ALS proof - of - concept evidence of efficacy across clinical endpoints • Phase 3 Healey ALS platform trial results expected in 2H 2022 • Phase 2 VISIONARY - MS in multiple sclerosis underway S t r o n g E x e c u t i o n Capabilities 32 • Proprietary electrochemical manufacturing process produces nanotherapeutics, scalable to commercialization • Strong IP, including 130+ granted patents, and trade secrets CLENE | Company Highlights

Clene Inc. HQ & Clinical Development 6 55 0 S o u th Mill r o c k D ri v e , S u i te G 5 0 S al t La k e C i t y , U T 8 4 1 2 1 R&D and Manufacturing 5 00 P ri n c i p i o P ar k w a y , S u i te 4 00 North East, MD 21901 © 2 0 21 C l e n e In c . Version: 8 - November - 2021
































