Exhibit 99.2

CLNN (NASDAQ) November 30, 2021

2 Forward Looking Statements This presentation contains "forward - looking statements" within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995 . Clene's actual results may differ from its expectations, estimates, and projections and consequently, you should not rely on these forward - looking statements as predictions of future events . Words such as "expect," "estimate," "project," "budget," "forecast," "anticipate," "intend," "plan," "may," "will," "could," "should," "believes," "predicts," "potential," "might" and "continues," and similar expressions are intended to identify such forward - looking statements . These forward - looking statements involve significant known and unknown risks and uncertainties, many of which are beyond Clene’s control and could cause actual results to differ materially and adversely from expected results . Factors that may cause such differences include Clene’s ability to demonstrate the efficacy and safety of its drug candidates ; the clinical results for its drug candidates, which may not support further development or marketing approval ; actions of regulatory agencies, which may affect the initiation, timing and progress of clinical trials and marketing approval ; Clene’s ability to achieve commercial success for its marketed products and drug candidates, if approved ; Clene’s ability to obtain and maintain protection of intellectual property for its technology and drugs ; Clene’s reliance on third parties to conduct drug development, manufacturing and other services ; Clene’s limited operating history and its ability to obtain additional funding for operations and to complete the licensing or development and commercialization of its drug candidates ; the impact of the COVID - 19 pandemic on Clene’s clinical development, commercial and other operations, as well as those risks more fully discussed in the section entitled “Risk Factors” in Clene’s recently filed registration statement on Form S - 1 (filed July 22 , 2021 ), as well as discussions of potential risks, uncertainties, and other important factors in Clene’s subsequent filings with the U . S . Securities and Exchange Commission . Clene undertakes no obligation to release publicly any updates or revisions to any forward - looking statements to reflect any change in its expectations or any change in events, conditions or circumstances on which any such statement is based, subject to applicable law . All information in this presentation is as of the date of presented or the date made publicly available . The information contained in any website referenced herein is not, and shall not be deemed to be, part of or incorporated into this presentation .
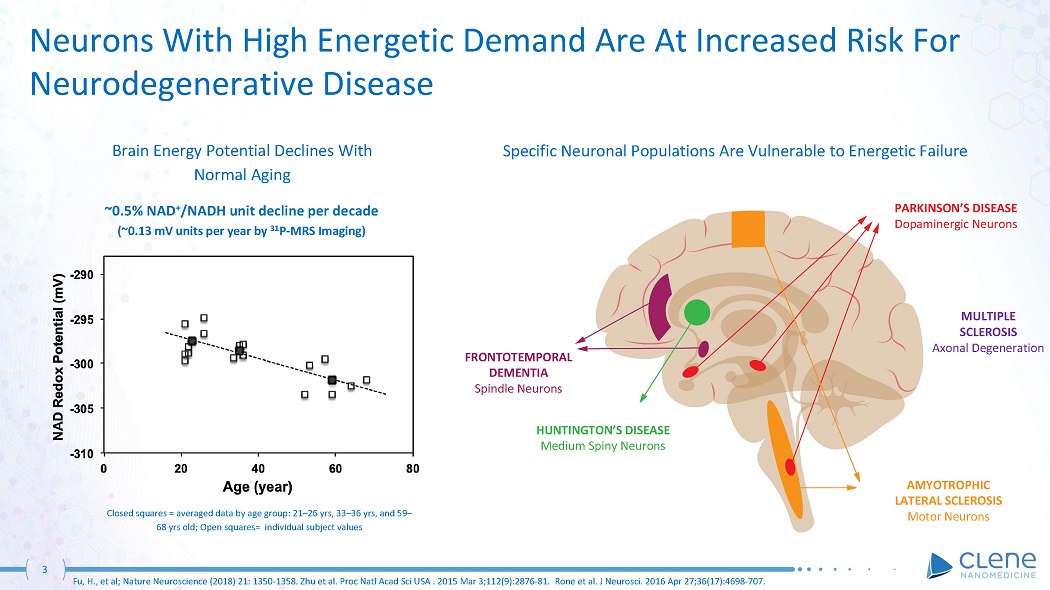
3 Fu, H., et al; Nature Neuroscience (2018) 21: 1350 - 1358. Zhu et al. Proc Natl Acad Sci USA . 2015 Mar 3;112(9):2876 - 81. Rone et al. J Neurosci. 2016 Apr 27;36(17):4698 - 707. Neurons With High Energetic Demand Are At Increased Risk For Neurodegenerative Disease ~0.5% NAD + /NADH unit decline per decade (~0.13 mV units per year by 31 P - MRS Imaging) Brain Energy Potential Declines With Normal Aging Closed squares = averaged data by age group: 21 – 26 yrs, 33 – 36 yrs, and 59 – 68 yrs old; Open squares= individual subject values PARKINSON’S DISEASE Dopaminergic Neurons AMYOTROPHIC LATERAL SCLEROSIS Motor Neurons HUNTINGTON’S DISEASE Medium Spiny Neurons FR O NT O TE M P O RA L DEMENTIA Spindle Neurons Specific Neuronal Populations Are Vulnerable to Energetic Failure MULTIPLE S C LER O S IS Axonal Degeneration

Vertices, Edges, & Facets Key to Catalytic Activity 4 CNM - Au8 Catalytically Active Nanocrystal Suspension Clean Surfaced, Highly Faceted Shape Enhances Catalytic Activity CNM - Au8 Nanocrystal CNM - Au8® | Catalytically - Active Nanocrystals Intersection of Physics and Biology Electron Sharing Drives Catalytic Activity

CNM - Au8 Nanocrystal Mecha ni s tic Effects Improved Energy Production and Utilization Promotes Neuroprotection and Remyelination CNM - Au8 | Improves Energy Production to Promote Neuroprotection and Remyelination 5
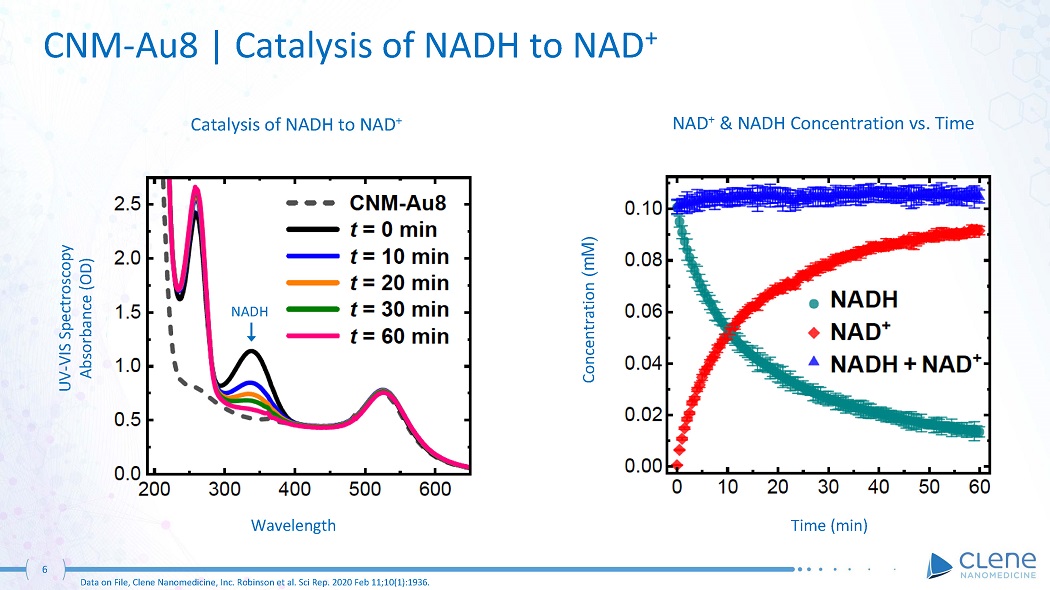
Catalysis of NADH to NAD + UV - VIS Spectroscopy Absorbance (OD) Wa v elen gth NAD + & NADH Concentration vs. Time Time (min) Concentration (mM) 6 Data on File, Clene Nanomedicine, Inc. Robinson et al. Sci Rep. 2020 Feb 11;10(1):1936. NA DH CNM - Au8 | Catalysis of NADH to NAD +
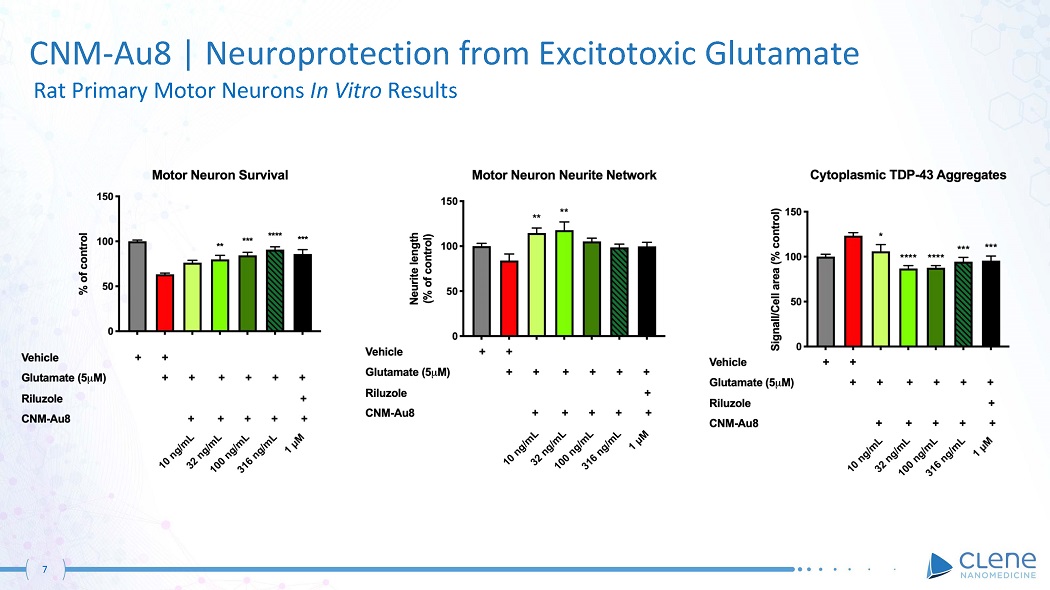
7 CNM - Au8 | Neuroprotection from Excitotoxic Glutamate Rat Primary Motor Neurons In Vitro Results

CNM - Au8 | ALS Neuroprotection in Human iPSCs Concentration (ng/mL) iPSC In Vitro Survival Results – SOD1 A4V Astrocytes with Human Motor Neuron 8 Ho et al.
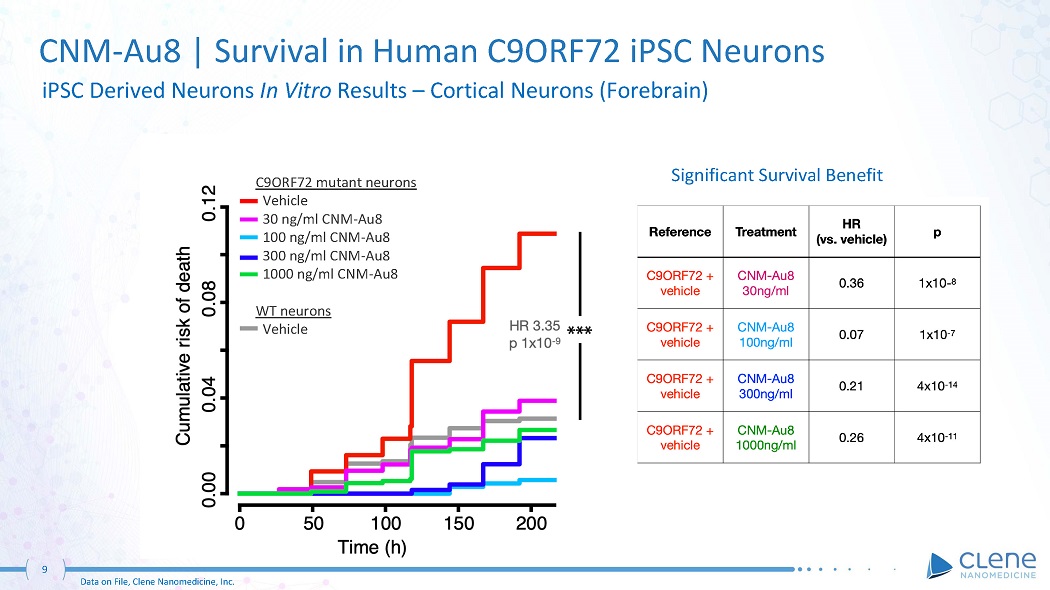
CNM - Au8 | Survival in Human C9ORF72 iPSC Neurons iPSC Derived Neurons In Vitro Results – Cortical Neurons (Forebrain) Significant Survival Benefit 9 Data on File, Clene Nanomedicine, Inc. C9ORF72 mutant neurons Vehicle 30 ng/ml CNM - Au8 100 ng/ml CNM - Au8 300 ng/ml CNM - Au8 1000 ng/ml CNM - Au8 WT neurons V e h ic le
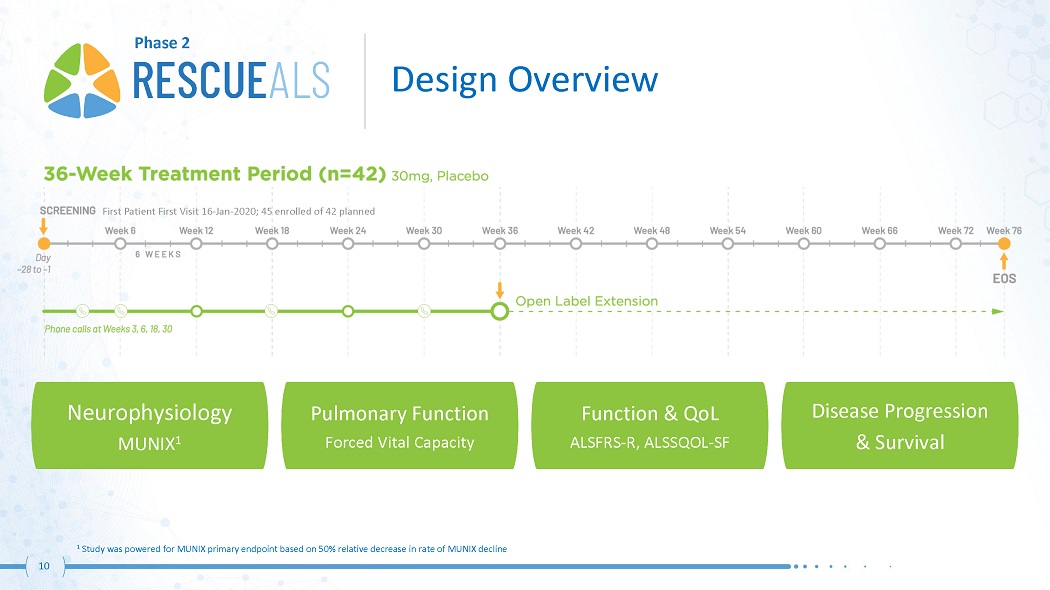
First Patient First Visit 16 - Jan - 2020; 45 enrolled of 42 planned Phase 2 1 Study was powered for MUNIX primary endpoint based on 50% relative decrease in rate of MUNIX decline Neurophysiology MUNIX 1 10 Pulmonary Function Forced Vital Capacity Function & QoL ALSFRS - R, ALSSQOL - SF Disease Progression & Survival Design Overview
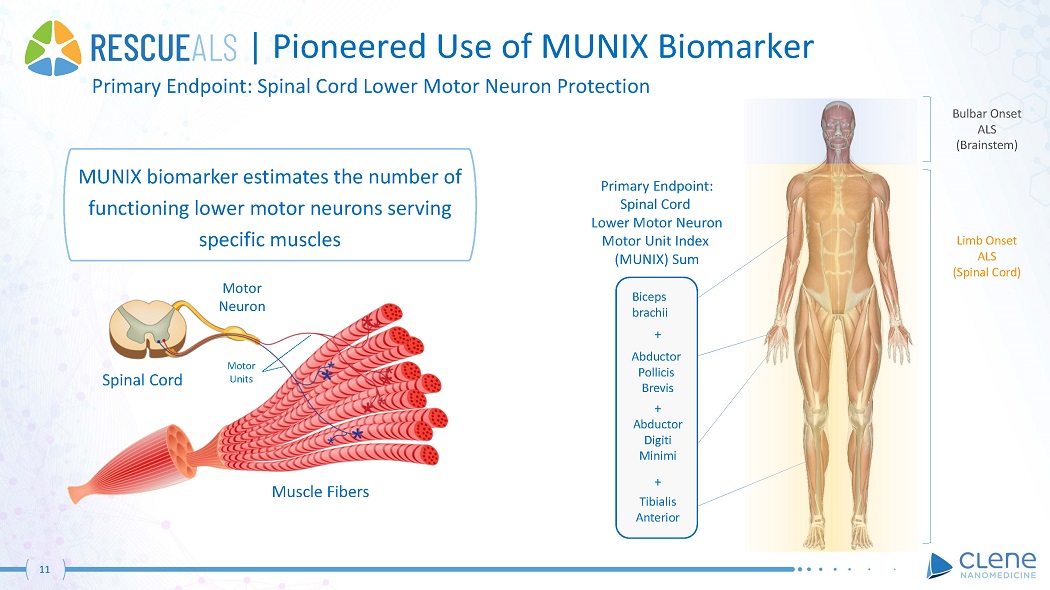
| Pioneered Use of MUNIX Biomarker Primary Endpoint: Spinal Cord Lower Motor Neuron Protection Muscle Fibers Spinal Cord Motor Neuro n Bulbar Onset ALS (Brainstem) Limb Onset ALS (Spinal Cord) Primary Endpoint: Spinal Cord Lower Motor Neuron Motor Unit Index (MUNIX) Sum Biceps b r a chii + Abduc t or Pollicis Brevis + Abduc t or Digiti Minimi + Tibialis A nt er ior Motor Units MUNIX biomarker estimates the number of functioning lower motor neurons serving specific muscles 11

| Evidence for Motor Neuron Protection Primary Endpoint (MUNIX %, LS Mean Change) All Randomized All Placebo Limited Rate of MUNIX Decline in Bulbar Onset 12 Data on File, Clene Nanomedicine, Inc. Limb Bulb ar Insufficient Spinal Cord Lower Motor Neuron Progression in Early Bulbar Trial Participants
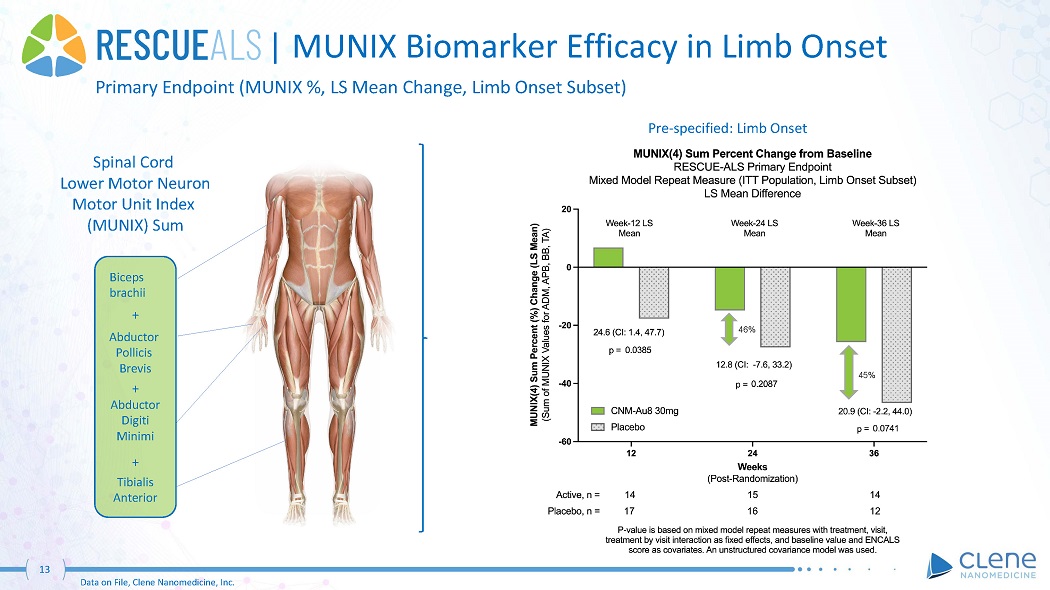
| MUNIX Biomarker Efficacy in Limb Onset 45% 46% Primary Endpoint (MUNIX %, LS Mean Change, Limb Onset Subset) Pre - specified: Limb Onset Spinal Cord Lower Motor Neuron Motor Unit Index (MUNIX) Sum Biceps b r a chii + Abduc t or Pollicis Brevis + Abduc t or Digiti Minimi + Tibialis A nt er ior 13 Data on File, Clene Nanomedicine, Inc.
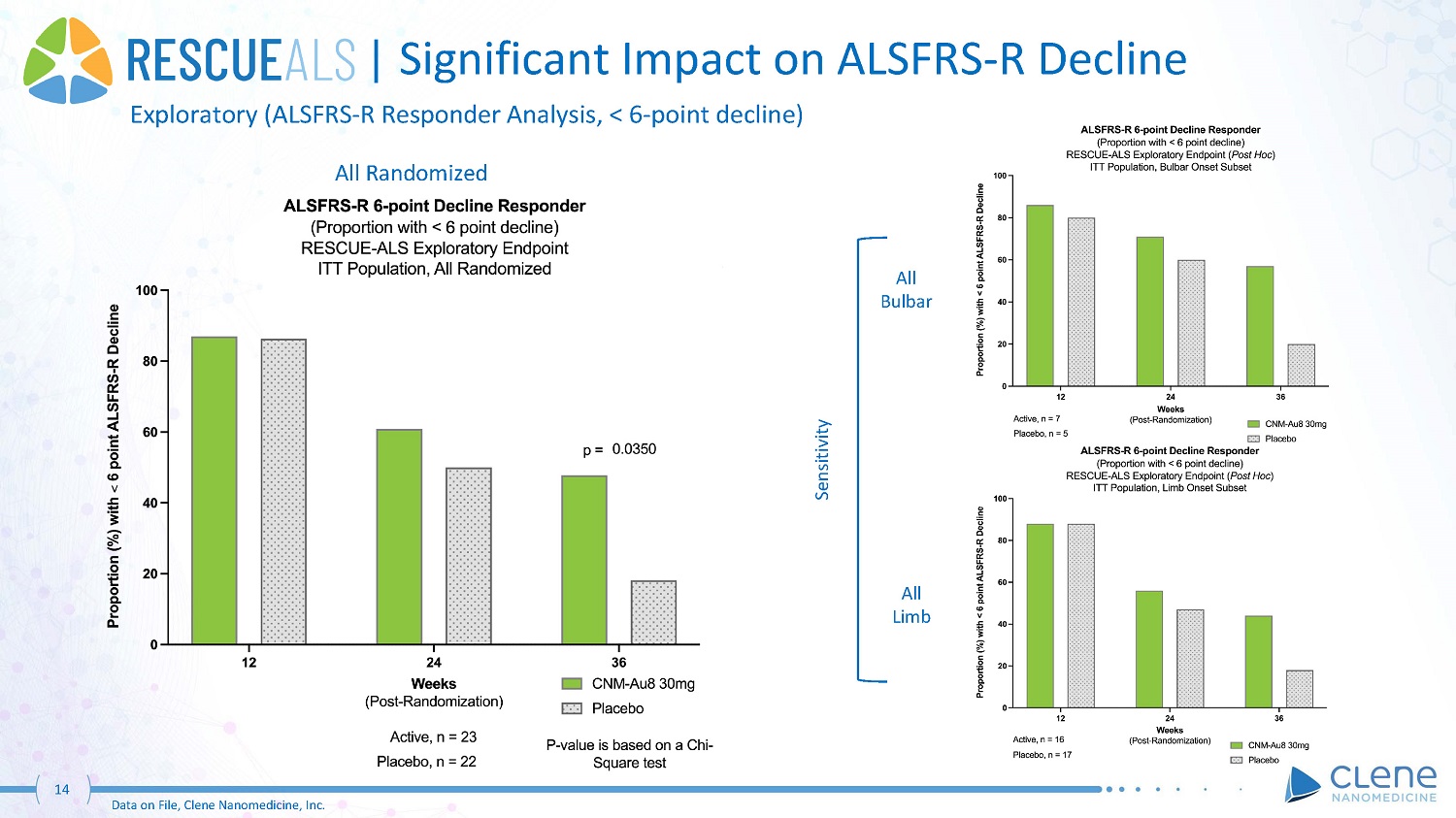
| Significant Impact on ALSFRS - R Decline Exploratory (ALSFRS - R Responder Analysis, < 6 - point decline) All Randomized All Bu l b a r All Limb Sensitivity 14 Data on File, Clene Nanomedicine, Inc.
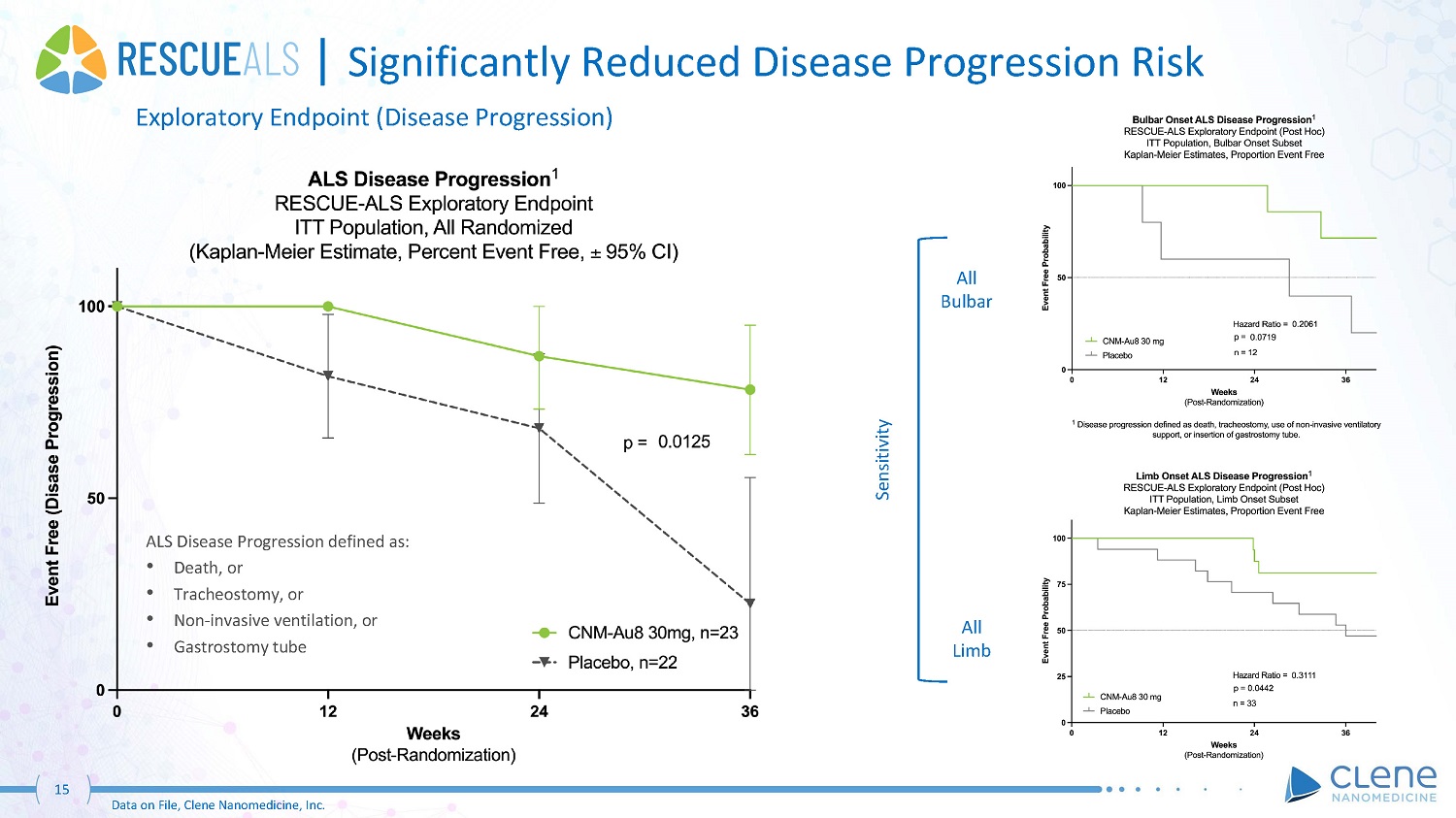
| Significantly Reduced Disease Progression Risk Exploratory Endpoint (Disease Progression) ALS Disease Progression defined as: • Death, or • Tracheostomy, or • Non - invasive ventilation, or • Gastrostomy tube All Bu l b a r All Limb Sensitivity 15 Data on File, Clene Nanomedicine, Inc.
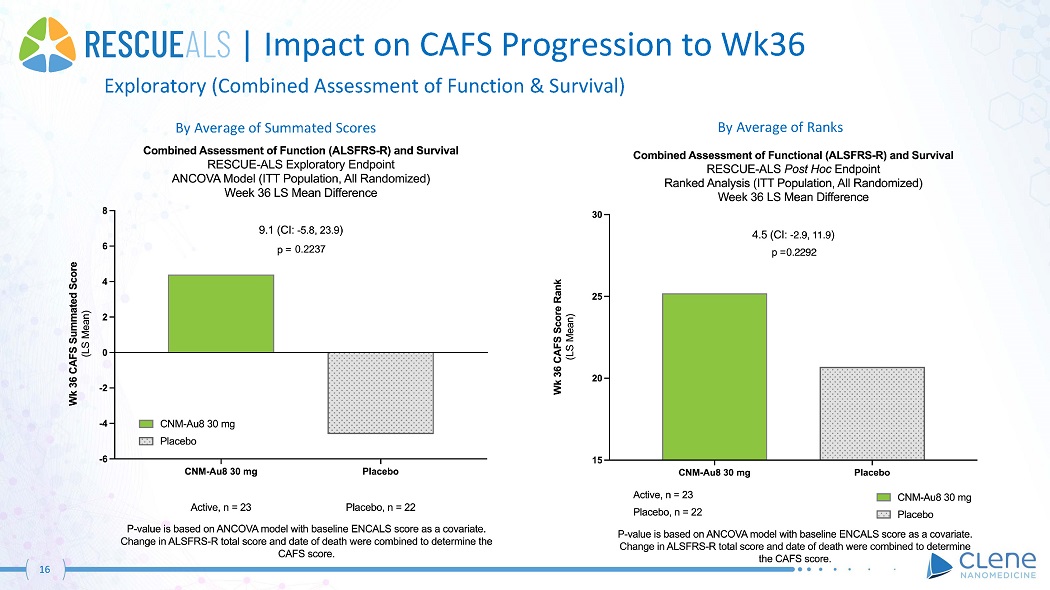
16 | Impact on CAFS Progression to Wk36 Exploratory (Combined Assessment of Function & Survival) By Average of Summated Scores By Average of Ranks
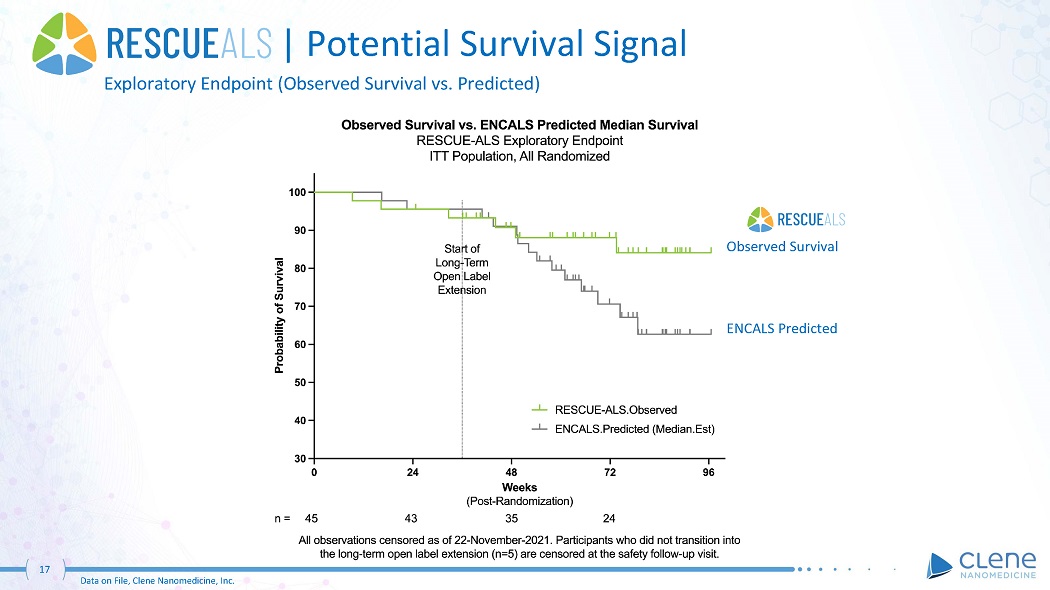
| Potential Survival Signal Exploratory Endpoint (Observed Survival vs. Predicted) ENCALS Predicted 17 Data on File, Clene Nanomedicine, Inc. Observed Survival

| Well Tolerated & No Safety Signals (aspiration 18 Data on File, Clene Nanomedicine, Inc. • Most common TEAEs associated with CNM - Au8 pneumonia, n=3; nausea, n=2; abdominal discomfort, n=2) Safety Summary • No CNM - Au8 related serious adverse events (SAEs) • No CNM - Au8 related drug discontinuations • No imbalances in treatment emergent adverse event (TEAEs) by system organ classification • TEAEs were predominantly mild - to - moderate and transient

Clene Inc. HQ & Clinical Development 6550 South Millrock Drive, Suite G50 Salt Lake City, UT 84121 R&D and Manufacturing 500 Principio Parkway, Suite 400 North East, MD 21901 © 2021 Clene Inc. Version: 30 - November - 2021


















