- GRI Dashboard
- Financials
- Filings
-
Holdings
-
Transcripts
- ETFs
- Insider
- Institutional
- Shorts
-
FWP Filing
GRI Bio (GRI) FWPFree writing prospectus
Filed: 21 Jan 21, 4:36pm

Working to Reduce ADHD Medication Abuse Investor Presentation JANUARY 2021 Filed Pursuant to Rule 433 Issuer Free Writing Prospectus dated January 21, 2021 File No. 333 - 249636

Free Writing Prospectus Statement This presentation highlights basic information about us and the proposed offering . Because it is a summary, it does not contain all of the information that you should consider before investing . We have filed a registration statement (including a prospectus) with the SEC for the offering to which this presentation relates . The registration statement has not yet become effective . Before you invest, you should read the prospectus in the registration statement (including the risk factors described therein) and other documents we have filed with the SEC for more complete information about us and the offering . You may access these documents for free by visiting EDGAR on the SEC Web site at http : //www . sec . gov . Alternatively, we or any underwriter participating in the offering will arrange to send you the prospectus if you contact ThinkEquity , a division of Fordham Financial Management, Inc . , Prospectus Department, 17 State Street, 22 nd Floor, New York, New York 10004 , telephone : ( 877 ) 436 - 3673 or e - mail : prospectus@think - equity . com . This presentation shall not constitute an offer to sell, or the solicitation of an offer to buy, nor will there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to the registration or qualification under the securities laws of such state or jurisdiction . The offering will only be made by means of a prospectus pursuant to a registration statement that is filed with the SEC after such registration statement becomes effective . Market, Industry, and Other Data This presentation contains estimates, projections and other information concerning our industry, our business and the markets for our drug candidates, including data regarding the estimated size of such markets and the incidence of certain medical conditions . We obtained the industry, market and similar data set forth in this presentation from our internal estimates and research and from independent industry publications, governmental publications, reports by market research firms or other independent sources that we believe to be reliable sources . Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances that are assumed in this information . We are responsible for all of the disclosure contained in this presentation, and we believe these industry publications and third - party research, surveys and studies are reliable . While we are not aware of any misstatements regarding any third - party information presented in this presentation, their estimates, in particular, as they relate to projections, involve numerous assumptions, are subject to risks and uncertainties, and are subject to change . Please see the sections entitled “Risk Factors” and “Market, Industry, and Other Data” in the registration statement and the prospectus . 2

Forward Looking Statements This presentation contains projections of, and assumptions regarding, future events reflecting our beliefs and expectations . Such events may or may not occur . Consequently, these projections and assumptions must not be relied on to indicate, or guarantee, any actual results . Certain of the statements set forth in this presentation constitute “forward - looking statements” made pursuant to the safe harbor provisions of the private securities litigation reform act of 1995 . Forward - looking statements include, without limitation, any statement that may predict, forecast, indicate, or imply future results, performance or achievements . These forward - looking statements include, but are not limited to, statements concerning : estimates of our expenses, future revenue and capital requirements ; our ability to obtain additional financing on terms acceptable to us, or at all ; our ability to advance our product into, and successfully complete, clinical trials ; the timing or likelihood of regulatory filings and approvals ; our expectations regarding completion of required clinical trials and studies ; our compliance with federal and state laws and regulations ; and anticipated markets for our product . All such forward - looking statements involve risks and uncertainties . There can be no assurance that the forward - looking statements included in this presentation will prove to be accurate . In light of the significant uncertainties inherent to the forward - looking statements included herein, the inclusion of such information should not be regarded as a representation or warranty by Vallon or any other person that the objectives and plans of Vallon will be achieved in any specified timeframe, if at all . We do not undertake any obligation to update any such statements . You should not rely upon forward - looking statements as predictions of future events . Although we believe that the expectations reflected in the forward - looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance, events, circumstances or achievements reflected in the forward - looking statements will ever be achieved or occur . This presentation is being provided on a non - confidential basis solely for, and should be used only in connection with, the recipient’s consideration of a business transaction with Vallon . The use of this presentation for any other purpose is not authorized . The reproduction or distribution of this presentation in whole or in part, or the divulgence of any of its contents, without our prior written consent is prohibited . 3

Initial Public Offering Summary 4 Issuer Vallon Pharmaceuticals, Inc. Offering Size $15,000,000 Securities Offered 1,670,000 shares (100% primary) Overallotment Option 15% (100% primary) Expected Price Range $8.00 - $10.00 per share Proposed Symbol Nasdaq: VLON Use of Proceeds Planned clinical and non - clinical trials of our product candidate, ADAIR, manufacturing preparation, and development of other product candidates, such as ADMIR, and for working capital and general corporate purposes. Sole Book - Runner ThinkEquity , a division of Fordham Financial Management, Inc.
Adderall ® is the most abused medicine among both college and high s chool students 1,2 TO DATE, THERE ARE NO TECHNOLOGIES TO CREATE AN ABUSE - DETERRENT FORMULATION OF ADDERALL ® OR RITALIN ® 5 1: Monitoring the Future Study, University of Michigan, NIH, 2019 – Key Findings on Adolescent Drug Use 2 : Monitoring the Future Study, University of Michigan, NIH, 2019 Volume 2 – College Students & Adults Ages 19 - 60

Team with Extensive Development and Commercial Success in ADHD and CNS 6 David Baker, MBA Chief Executive Officer Timothy Whitaker, MD Acting Chief Medical Officer • Former Chief Medical Officer, Alder Biopharmaceuticals (NASDAQ: ALDR) • Former VP, Global Clinical Development, Neuroscience, Shire Plc • BA, Duke University; MD, Bowman Gray School of Medicine, Wake Forest University • Board certified in Psychiatry Led clinical development of Vyvanse ® , Intuniv ® , and novel Phase 3 - positive migraine medication • Former Chief Commercial Officer & Interim CEO of Alcobra (NASDAQ: ADHD) • Former VP, ADHD Marketing and Global General Manager for Vyvanse®, Shire Plc • Former Senior Director, Marketing, Merck & Co. - marketed three multi - billion $ drug • BA, Duke University; MBA, Fuqua School of Business, Duke University Led commercialization of the two most successful ADHD medications (Adderall XR ® , Vyvanse ® ) Penny Toren , MS SVP, Regulatory Affairs and Project Management • Former Director, Regulatory Affairs, Teva and Cephalon • BA, Florida Atlantic University; MS, Biostatistics & Epidemiology, New York Medical College Extensive experience with abuse - deterrent opioid development and FDA policy Different Billion - Dollar Products Commercialized Successful U.S. and Canadian NDA/NDS Approvals U.S. and International New Product Approvals 18 1 0 5
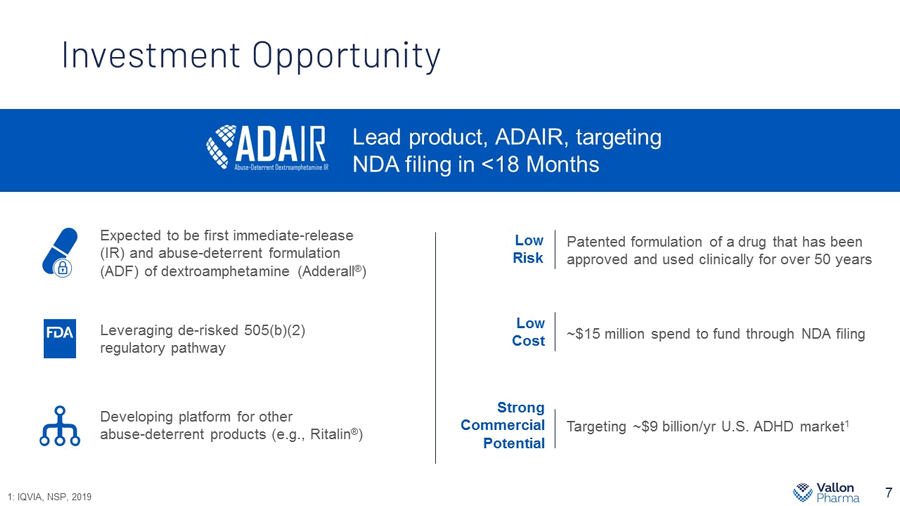
Investment Opportunity 7 Lead product, ADAIR, targeting NDA filing in <18 Months Developing platform for other abuse - deterrent products (e.g., Ritalin ® ) Low Cost Strong Commercial Potential Patented formulation of a drug that has been approved and used clinically for over 50 years Low Risk ~$15 million spend to fund through NDA filing Targeting ~$9 billion/ yr U.S. ADHD market 1 Expected to be first immediate - release (IR) and abuse - deterrent formulation (ADF) of dextroamphetamine (Adderall ® ) Leveraging de - risked 505(b)(2) regulatory pathway 1: IQVIA, NSP, 2019
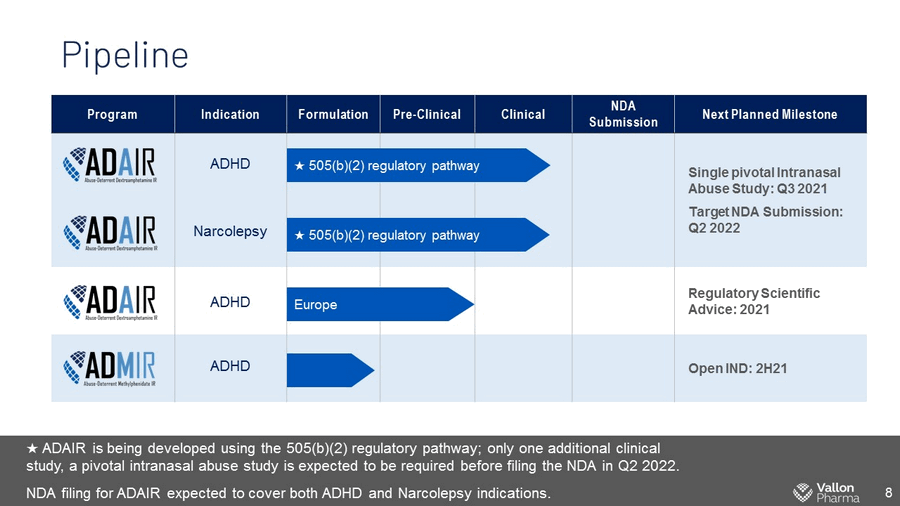
Pipeline 8 Program Indication Formulation Pre - Clinical Clinical NDA Submission Next Planned Milestone ADHD Single pivotal Intranasal Abuse Study: Q3 2021 Target NDA Submission: Q2 2022 Narcolepsy ADHD Regulatory Scientific Advice: 2021 ADHD Open IND: 2H21 Europe ★ 505(b)(2) regulatory pathway ★ 505(b)(2) regulatory pathway ★ ADAIR is being developed using the 505(b)(2) regulatory pathway; only one additional clinical study, a pivotal intranasal abuse study is expected to be required before filing the NDA in Q2 2022. NDA filing for ADAIR expected to cover both ADHD and Narcolepsy indications.
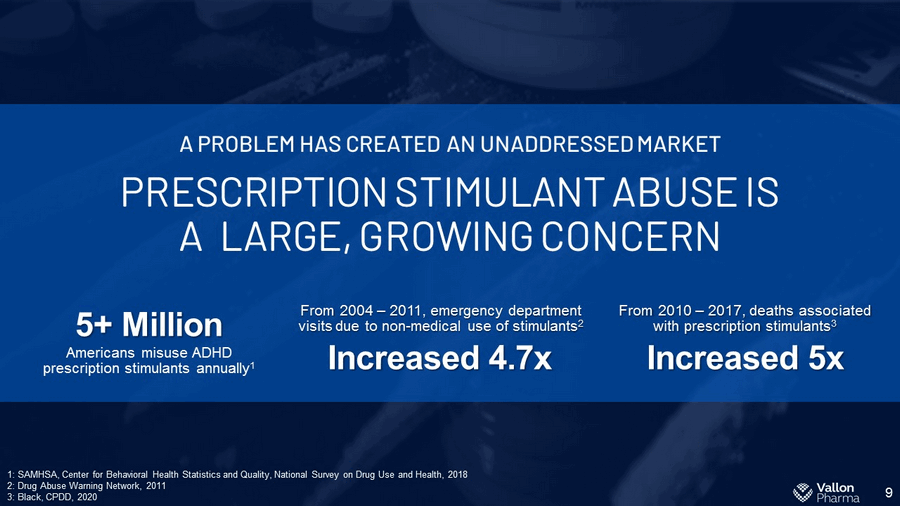
9 PRESCRIPTION STIMULANT ABUSE IS A LARGE, GROWING CONCERN A PROBLEM HAS CREATED AN UNADDRESSED MARKET Increased 5x From 2010 – 2017, deaths associated with prescription stimulants 3 5+ Million Americans misuse ADHD prescription stimulants annually 1 1: SAMHSA, Center for Behavioral Health Statistics and Quality, National Survey on Drug Use and Health, 2018 2: Drug Abuse Warning Network, 2011 3: Black, CPDD, 2020 Increased 4.7x From 2004 – 2011, emergency department visits due to non - medical use of stimulants 2

Rx Stimulant Abuse is a Problem of Youth Sources: NSDUH, 2015; McCabe, 2011; Garnier, 2012; Compton, 2018 10 Rates of Rx stimulant misuse/abuse grow from age 12 and peak at age 21, where an estimated 10% of the population reported misuse Rx stimulants are widely available in school and on campuses: • 24% of adolescents w/ADHD report being approached to divert their Rx • 62% of college students have been offered Rx stimulants Most common source is friends, not drug dealers Adolescents and young adults believe the risks are low because Rx stimulants are approved medicines

Snorting or Injecting Can Cause Serious Events 11 Chest Pain Rapid Irregular Heartbeat Heart Attack Seizures Stroke Hallucinations Hostile/Aggressive Behavior Psychosis Suicidal Thoughts and Behaviors Quick entry into the bloodstream increases risk 3 : Rapid delivery of CNS stimulants to the brain promotes addiction 4 1: Bright,G . Medscape Jrnl . of Medicine 2008; 10 (5): 111 & Cassidy, et al, Jrnl . Attention Disorders, 2015 19 (7) 630 - 640 2: White et al, Jrnl of Amer Coll Health 2006; Cassidy et al, Jrnl Attention Disorders, 2013 3: http://prescription - drug.addictionblog.org/snorting - adderall/ 4: Samaha, 2005; Volkow, 2003; Lile, 2011 of people who abuse or misuse prescription stimulants, report snorting or injecting them 2 likely to be abused or misused than extended release formulations 1 Immediate - Release Stimulants: “Will Adderall Be the New Opioid Crisis?” 2 - 4 Times More 40%

12 AN ABUSE - DETERRENT ORAL FORMULATION OF IMMEDIATE - RELEASE (IR) DEXTROAMPHETAMINE Our technologies are designed to resist manipulation for snorting, and provide barriers to injection US Patent #9,931,303 Issued Apr. 2018 – Expires 2037 US Patent #10,471,015 Issued Nov. 2019 – Expires 2037
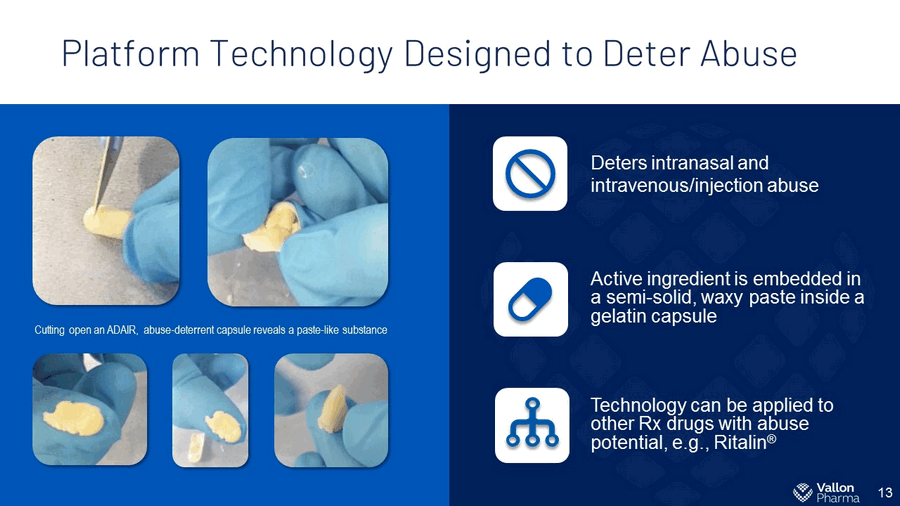
Platform Technology Designed to Deter Abuse 13 Cutting open an ADAIR, abuse - deterrent capsule reveals a paste - like substance Deters intranasal and intravenous/injection abuse Active ingredient is embedded in a semi - solid, waxy paste inside a gelatin capsule Technology can be applied to other Rx drugs with abuse potential, e.g., Ritalin ®
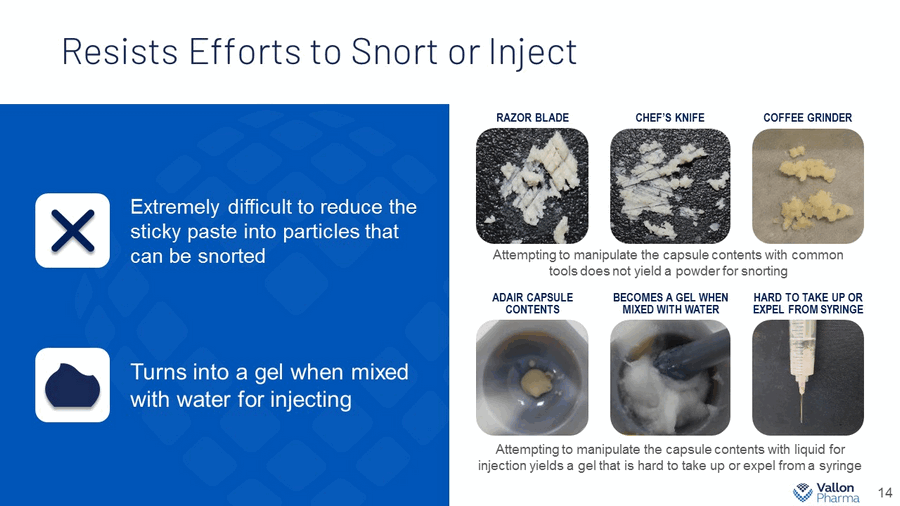
Resists Efforts to Snort or Inject 14 Extremely difficult to reduce the sticky paste into particles that can be snorted Turns into a gel when mixed with water for injecting RAZOR BLADE CHEF’S KNIFE ADAIR CAPSULE CONTENTS BECOMES A GEL WHEN MIXED WITH WATER HARD TO TAKE UP OR EXPEL FROM SYRINGE COFFEE GRINDER Attempting to manipulate the capsule contents with common tools does not yield a powder for snorting Attempting to manipulate the capsule contents with liquid for injection yields a gel that is hard to take up or expel from a syringe

Behaves Like Traditional ADHD Product if Taken Orally 15 Bioequivalence study showed ADAIR exhibited the same Pharmacokinetics as non - ADF Dexamphetamine 1 Single center, randomized, single - dose, laboratory - blinded, 2 - period, 2 - sequence, crossover design in healthy patients Treatment : 10 mg ADAIR; 10 mg Dexamphetamine sulfate IR Measures : Pk; Safety Number of Subjects : 24 Study Design 1: Presented at AAPS annual meeting, 2019 *ADAIR is an investigational drug and is not approved by the FDA
Well Established Approaches to Studying Abuse 16 FDA has established standard types of abuse evaluations and preferred endpoints 1 Manipulation Pharmacokinetics 2 Pharmacodynamics 3 Category 2/3 studies are conducted in recreational drug users in controlled settings Categories: Study subjects are provided with optimized, laboratory - manipulated study drug In real world settings, recreational drug users are typically willing to spend only 10 - 15 minutes to manipulate a product
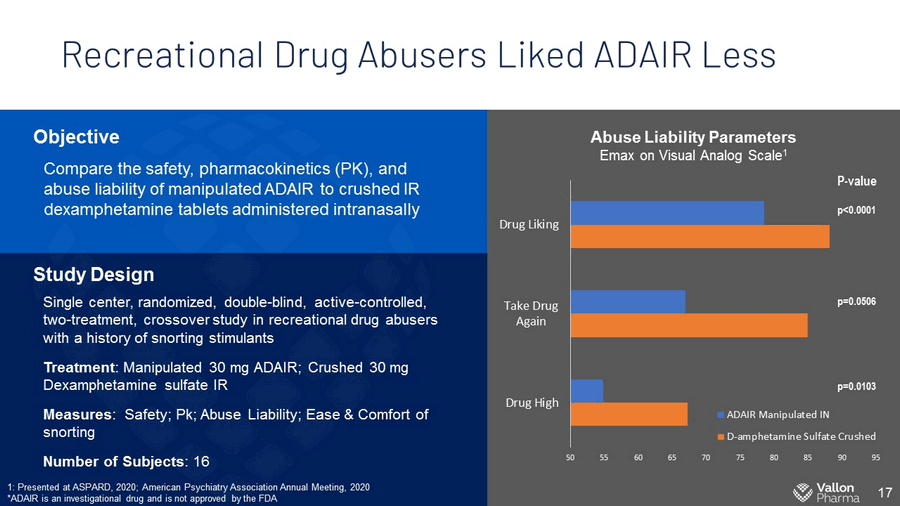
Recreational Drug Abusers Liked ADAIR Less 17 Objective Compare the safety, pharmacokinetics (PK), and abuse liability of manipulated ADAIR to crushed IR dexamphetamine tablets administered intranasally Study Design Single center, randomized , d ouble - blind, active - controlled, two - treatment, crossover s tudy in recreational drug abusers with a history of snorting stimulants Treatment : Manipulated 30 mg ADAIR; Crushed 30 mg Dexamphetamine sulfate IR Measures : Safety; Pk; Abuse Liability; Ease & Comfort of snorting Number of Subjects : 16 Abuse Liability Parameters Emax on Visual Analog Scale 1 50 55 60 65 70 75 80 85 90 95 Drug High Take Drug Again Drug Liking ADAIR Manipulated IN D-amphetamine Sulfate Crushed P - value p<0.0001 p=0.0506 p=0.0103 1: Presented at ASPARD, 2020; American Psychiatry Association Annual Meeting, 2020 *ADAIR is an investigational drug and is not approved by the FDA

Clinical Summary 18 Shown to be bioequivalent to reference dextroamphetamine Shown to have no food effect; similar Pk when taken with or without food Is difficult and time consuming to manipulate for snorting In a human abuse liability study in which subjects were given laboratory manipulated study drug to snort, ADAIR compared to dextroamphetamine: - demonstrated a blunted Pk profile - scored worse on nasal irritation and discomfort - was less desirable on standard abuse scales (likeability, high, take again)
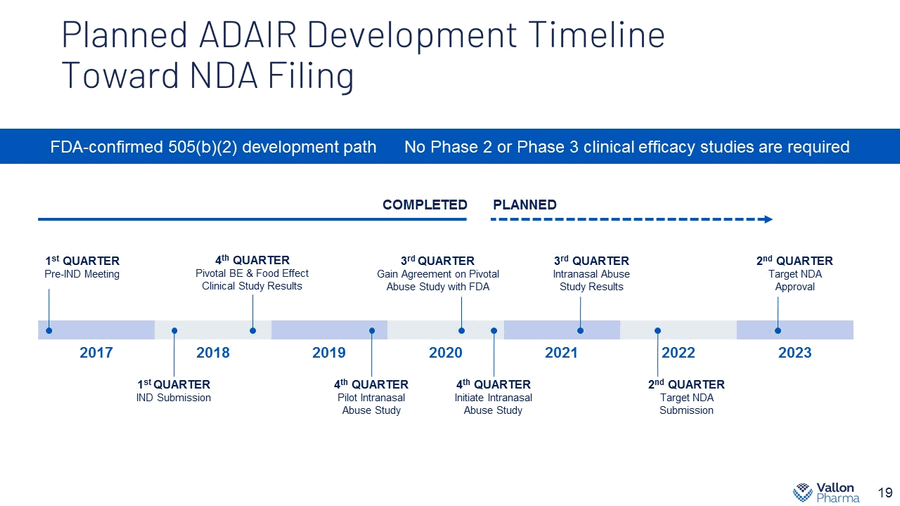
Planned ADAIR Development Timeline Toward NDA Filing 19 FDA - confirmed 505(b)(2) development path No Phase 2 or Phase 3 clinical efficacy studies are required 2017 2018 2019 2020 2021 2022 2023 1 st QUARTER Pre - IND Meeting 4 th QUARTER Pivotal BE & Food Effect Clinical Study Results 1 st QUARTER IND Submission 4 th QUARTER Pilot Intranasal Abuse Study 3 rd QUARTER Gain Agreement on Pivotal Abuse Study with FDA 4 t h QUARTER Initiate Intranasal Abuse Study 3 rd QUARTER Intranasal Abuse Study Results 2 nd QUARTER Target NDA Submission 2 nd QUARTER Target NDA Approval COMPLETED PLANNED
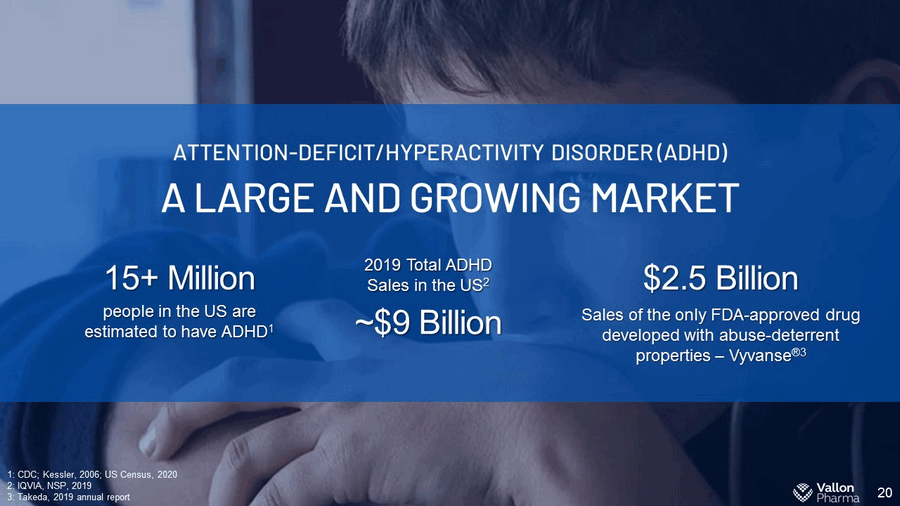
20 A LARGE AND GROWING MARKET ATTENTION - DEFICIT/HYPERACTIVITY DISORDER (ADHD) Sales of the only FDA - approved drug developed with abuse - deterrent properties – Vyvanse ® 3 $2.5 Billion 2019 Total ADHD Sales in the US 2 ~$9 Billion 15+ Million people in the US are estimated to have ADHD 1 1: CDC; Kessler, 2006; US Census, 2020 2: IQVIA, NSP, 2019 3: Takeda, 2019 annual report
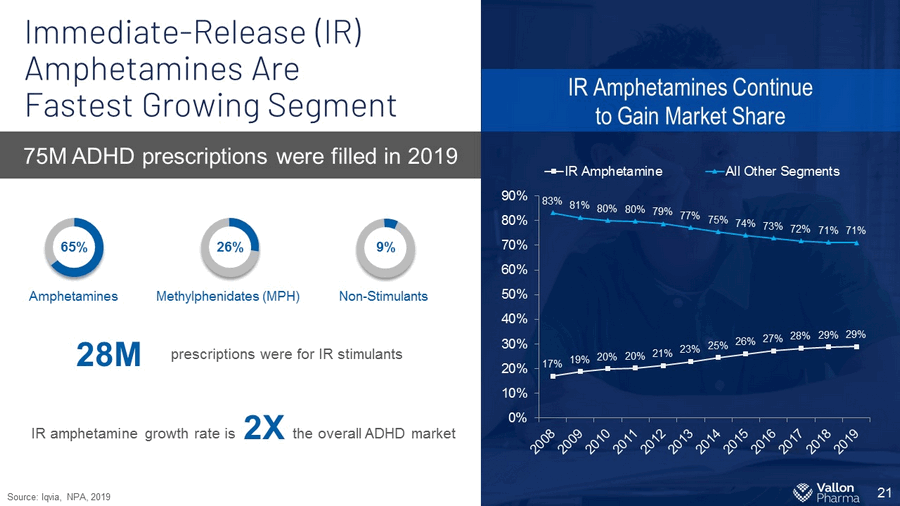
Immediate - Release (IR) Amphetamines Are Fastest Growing Segment 21 75M ADHD prescriptions were filled in 2019 IR Amphetamines Continue to Gain Market Share 17% 19% 20% 20% 21% 23% 25% 26% 27% 28% 29% 29% 83% 81% 80% 80% 79% 77% 75% 74% 73% 72% 71% 71% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% IR Amphetamine All Other Segments 65% Amphetamines prescriptions were for IR stimulants 28M IR amphetamine growth rate is 2X the overall ADHD market 26% Methylphenidates (MPH) 9% Non - Stimulants Source: Iqvia , NPA, 2019

Market Research Shows Strong Commercial Potential for ADAIR 22 1: HMS, 2017 2: Red Team Associates, 2017 High prescribing ADHD physicians demonstrated strong interest in ADAIR 1 Intention to prescribe for >50% of patients who otherwise would receive another IR amphetamine Parents of teenagers with ADHD and with young adult patients demonstrated strong interest in ADAIR 2 Willing to ask doctor about ADAIR Willing to pay a higher insurance co - pay for ADAIR Managed Care payers showed acceptability of product concept without intention to block access 2 19 out of 20 Plans said they would add ADAIR to their formulary

Competitive Landscape Favors Vallon 23 GENERIC Major brand players are focused on extended - release segments Includes other developers of abuse - deterrent stimulants, e.g., KemPharm , Durect Generic manufacturers have shown no interest in developing abuse - deterrent technologies Formulation challenges FDA advisory committee recently rejected competing IR stimulant due to safety issues with inactive ingredients not used in ADAIR Opportunity for abuse - deterrent IR stimulants is wide open with little active competition

Why We Believe ADAIR Can be Commercially Successful 24 Tailwind from growing public concern around addiction Clear, simple product differentiation Minimal competition Very appealing to physicians and parents of patients, based on quantitative market research Not expensive enough to be a major concern to managed care, based on payor research

Partnered with the #1 ADHD Company in Europe 1 Medice has well established development, manufacturing, sales, and marketing capabilities Large investor in Vallon , creating a multi - level alignment of incentives 25 Entered European licensing agreement in January 2020 Right to develop, manufacture, market and sell ADAIR in the European Union and United Kingdom Vallon will receive development and sales milestone payments and double - digit sales - based royalty payments 1: Based on ADHD prescription share
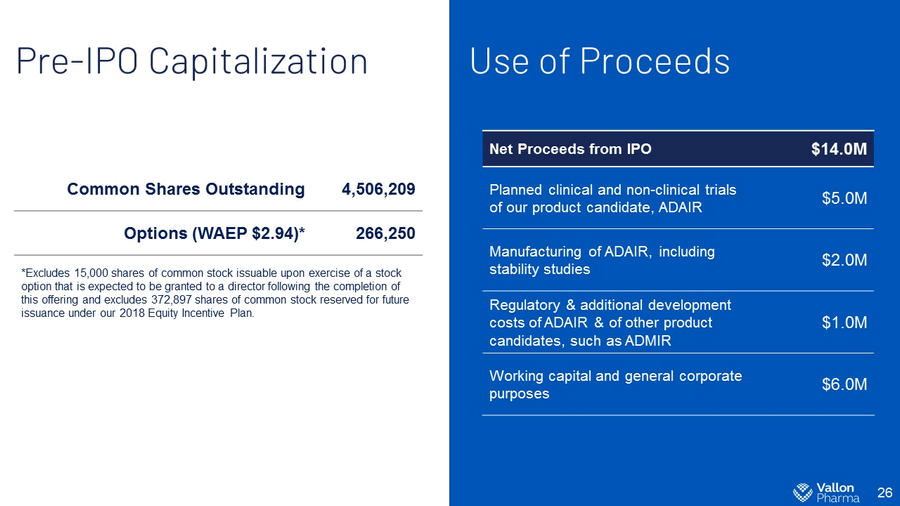
Use of Proceeds 26 Pre - IPO Capitalization Common Shares Outstanding 4,506,209 Options (WAEP $2.94)* 266,250 *Excludes 15,000 shares of common stock issuable upon exercise of a stock option that is expected to be granted to a director following the completion of this offering and excludes 372,897 shares of common stock reserved for future issuance under our 2018 Equity Incentive Plan. Net Proceeds from IPO $14.0M Planned clinical and non - clinical trials of our product candidate, ADAIR $5.0M Manufacturing of ADAIR, including stability studies $2.0M Regulatory & additional development costs of ADAIR & of other product candidates, such as ADMIR $1.0M Working capital and general corporate purposes $6.0M
Investment Summary 27 Pivotal data from lead program expected in 3Q21 with potential NDA filing in less than 18 months Patented novel formulation of a drug that has been used for over 50 years Leveraging lower cost and de - risked 505(b)(2) regulatory pathway Targeting large and growing ~$9B U.S. ADHD market Abuse - Deterrent Platform Technology Proven team with extensive experience in ADHD/CNS development and commercialization Pipeline expansion opportunities across multiple drugs and indications

Working to Reduce ADHD Medication Abuse Investor Relations JTC Team 833.475.8247 vallon@jtcir.com