Exhibit 99.2

is a clinical - stage biopharmaceutical company revolutionizing oncolytic viral therapies with stem cell - based platforms May 2022

This presentation contains forward - looking statements for purposes of the “safe harbor” provisions under the United States Private Securities Litigation Reform Act of 1995. Terms such as “anticipates,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predicts,” “project,” “should,” “would” as well as similar terms, are forward - looking in nature. The forward - looking statements contained in this discussion are based on the Calidi’s current expectations and beliefs concerning future developments and their potential effects. There can be no assurance that future developments affecting Calidi will be those that it has anticipated. These forward - looking statements involve a number of risks, uncertainties (some of which are beyond Calidi’s control) or other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward - looking statements. Factors that may cause actual results to differ materially from current expectations include, but are not limited to: the occurrence of any event, change or other circumstances that could give rise to the termination of negotiations and any subsequent definitive agreements with respect to the business combination (the “Business Combination”) with Edoc Acquisition Corp. (“Edoc”); the outcome of any legal proceedings that may be instituted against Edoc, Calidi, the combined company or others following the announcement of the Business Combination, the private placement financing proposed to be consummated concurrently with the Business Combination (the “PIPE”), and any definitive agreements with respect thereto; the inability to complete the Business Combination due to the failure to obtain approval of the shareholders of Edoc, the possibility that due diligence completed following execution of the principal definitive transaction documents for the Business Combination and PIPE will not be satisfactorily concluded, the inability to complete the PIPE or other financing needed to complete the Business Combination, or to satisfy other conditions to closing; changes to the proposed structure of the Business Combination that may be required or appropriate as a result of applicable laws or regulations or as a condition to obtaining regulatory approval of the Business Combination; the ability to meet stock exchange listing standards following the consummation of the Business Combination; the risk that the Business Combination disrupts current plans and operations of Calidi as a result of the announcement and consummation of the Business Combination; the ability to recognize the anticipated benefits of the Business Combination or to realize estimated pro forma results and underlying assumptions, including with respect to estimated shareholder redemptions; costs related to the Business Combination; changes in applicable laws or regulations; the evolution of the markets in which Calidi competes; the inability of Calidi to defend its intellectual property and satisfy regulatory requirements; the ability to implement business plans, forecasts, and other expectations after the completion of the proposed Business Combination, and identify and realize additional opportunities; the risk of downturns and a changing regulatory landscape in the highly competitive pharmaceutical industry; the impact of the COVID - 19 pandemic on Calidi’s business; and other risks and uncertainties set forth in the section entitled “Risk Factors” and “Cautionary Note Regarding Forward - Looking Statements” in Edoc’s preliminary prospectus dated March 16, 2022, in the Registration Statement on Form S - 4 filed with the Securities and Exchange Commission (“SEC”) on March 16, 2022. Page 2 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Forward - Looking Statements

This presentation relates to a proposed business combination between Edoc Acquisition Corp. a Cayman Islands exempted company, EDOC Merger Sub Inc., a Nevada corporation and Calidi Biotherapeutics, Inc., a Nevada corporation . A full description of the terms and conditions Agreement and Plan of Merger constituting the business combination is provided in the registration statement on Form S - 4 filed with the U.S.Securities and Exchange Commission (SEC) by Edoc Acquisition Corp., that includes a prospectus with respect to the securities to be issued in connection with the merger, and information with respect to an extraordinary meeting of Edoc Acquisition Corp. shareholders to vote on the merger and related transactions. Edoc Acquisition Corp. and Calidi Biotherapeutics, Inc. urgesits investors, shareholders and other interested persons to read the proxy statement andprospectus as well as other documents filed with the SEC because these documents will contain important information about Calidi Biotherapeutics, Inc., Edoc Acquisition Corp., and the business combination transaction. After the registration statement is declared effective, the definitive proxy statement and prospectus to be included in the registration statement will be distributed toshareholders of Edoc Acquisition Corp. and Calidi Biotherapeutics, Inc., as of a record date to be established for voting on the proposed merger and related transactions. Shareholders may obtain a copy of the Form S - 4 registration statement, including the proxy statement and prospectus, and other documents filedwith the SEC without charge, by directing a request to: Edoc Acquisition Corp. at 7612 Main Street Fishers, Suite 200, Victor, New York 14564. The preliminary and definitive proxy statement and prospectusincluded in the registration statement can also be obtained, without charge, at the SEC’s website ( www.sec.gov ). Page 3 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Important Information About the Business Combination Transaction and Where to Find It

Edoc Acquisition Corp., Calidi Biotherapeutics, Inc., and their respective directors and executive officers may be deemed to be participants in the solicitation of proxies or consents from Edoc Acquisition Corp. and Calidi Biotherapeutics, Inc. shareholders in connection with the proposed transaction. A list of the names of thedirectors and executive officers of Edoc Acquisition Corp. and Calidi Biotherapeutics, Inc. and information regarding their interests in the business combination transaction is contained in the proxy statement and prospectus. You may obtain free copies of these documents as described in the preceding paragraph. No Offer or Solicitation This presentation will not constitute a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the proposed business combination. This presentation will also not constitute an offer to sell or the solicitation of an offer to buy any securities of Calidi Biotherapeutics, Inc., nor will there be any sale of securities in any states or jurisdictions in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities will be made except by means of a prospectus meeting the requirements of section 10 of the Securities Act of 1933, as amended, or an exemption therefrom. Page 4 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Participation in the Solicitation

Calidi Financing I. Historical II. Planned DeSPAC with EDOC Acquisition Corp: Anticipated in July – August 2022 III. Use of Proceeds: Funding for 3 years of operations and 3 clinical trials IV. Crossover Funding Page 5 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

Company Highlights Two First - in - Class Novel Clinically Focused Platforms With Broad and Long - Lasting IP Protection NeuroNova ( NNV ): - Off - the - shelf Allogeneic neural stem cells (HB1.F3.CD21) loaded with oncolytic adenovirus (CRAD - s - pk7) to treat Glioblastoma (GBM) - Completed Phase 1 trial (NNV in newly diagnosed GBM). - Plan NNV Phase 1b/2a trial in newly diagnosed GBM and Phase 1 trial in recurrent GBM initiating in first half 2023 SuperNova ( SNV ): - Off - the - shelf Allogeneic adipose tissue - derived Mesenchymal Stem Cells (VP - 01) loaded with oncolytic vaccinia virus (CAL1) to treat advanced solid tumors. - Completed safety trial autologous adipose derived stromal cells loaded with oncolytic vaccinia virus (CAL1) - Allogeneic SNV1 phase 1/2 trial in TNBC, Head & Neck, and Metastatic/Unresectable Melanoma initiating in 2023 State - of - the - art Virus and Cell Manufacturing Processes - Early investment on process and assay development allowed to generate a scalable, and cost - efficient manufacturing platform. - Available, GMP manufactured allogeneic stem cells and oncolytic virus banks for SNV1 and NNV © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Page 6 Page 6 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

COMPANY Oncolytic Virus Platform Cell Protected : Vaccinia Virus Adenovirus Herpes Virus Herpes Virus Adenovirus Herpes Virus Adenovirus Vaccinia Virus Engineered Virus FDA Approved Virus Reduced viral elimination by the immune system Virally - encoded therapeutic expressed at administration Stem cell - derived immune modulators expressed at administration Calidi’s Platform Advantages Based on publicly available information © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Page 7
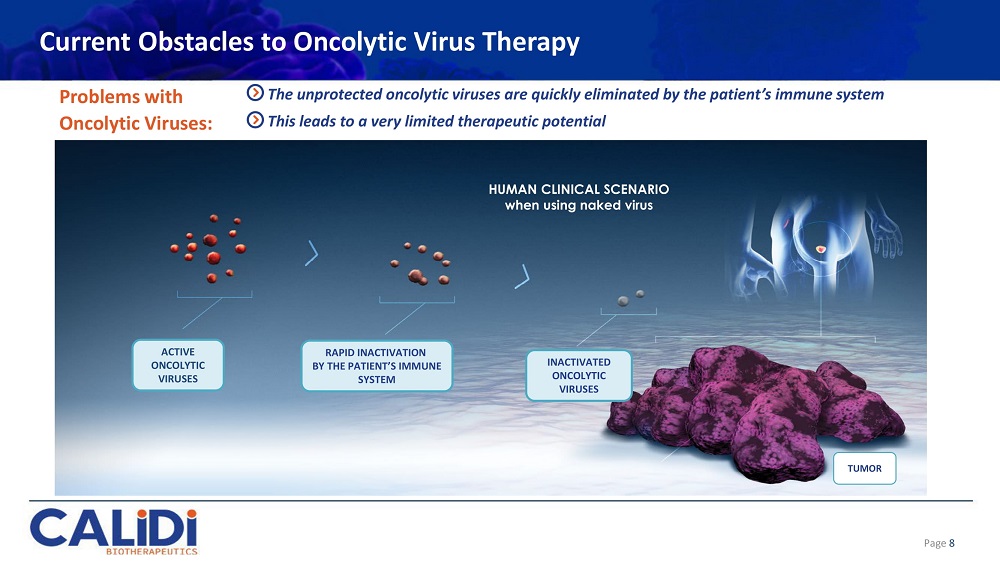
Current Obstacles to Oncolytic Virus Therapy Problems with Oncolytic Viruses: The unprotected oncolytic viruses are quickly eliminated by the patient’s immune system This leads to a very limited therapeutic potential HUMAN CLINICAL SCENARIO when using naked virus ACTIVE ONCOLYTIC VIRUSES RAPID INACTIVATION BY THE PATIENT’S IMMUNE SYSTEM INACTIVATED ONCOLYTIC VIRUSES TUMOR Page 8

CALIDI’S SOLUTION: Stem Cell - Based Platforms to Potentiate Oncolytic Viral Therapies Stem cells loaded with oncolytic viruses are manufactured in a single off - the - shelf vial 1. Stem cell loaded with Oncolytic Viruses (OV) 2. Stem cell protects and amplifies OV 3. Protected viruses delivered to tumor cells, altering the Tumor Micro - Environment and inducing an anti - tumor response to kill tumor cells TARGETED TUMOR Page 9 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending x Prevents viral elimination by patients' immune system x Supports oncolytic viral amplification in the stem cells x Leads to efficient induction of potent anti - tumor immune responses

Preclinical in vitro data shows that Vaccinia Virus (VACV) kills tumor cells PC3 (prostate cancer) + HUMAN SERUM Vaccinia virus (VACV) engineered to express red fluorescent protein. RED signal indicates virus amplification leading to killing of cancer cells Treatment: CAL1 – naked virus Treatment: CAL1 - naked virus Treatment: SNV1 (CAL1+MSC) Advantages of Calidi’s Technology Human serum can inhibit oncolytic virus activity by blocking its capacity to infect and kill tumor cells Treatment efficacy is restored when adipose derived stem cells (AD - MSC) are used to protect and potentiate the oncolytic vaccinia virus 4 PC3 (prostate cancer) 24h Page 10 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending 24h PC3 (prostate cancer) + HUMAN SERUM 24h

Differentiated Pipeline Targeting Multiple Cancer Indications * Calidi has observed data related to a physician sponsored 26 - patient safety trial (IRB) with autologous adipose - derived stromal cells delivering CAL1 oncolytic virus NeuroNova SuperNova Program Description Target Indications Discovery Non - clinical Studies Phase 1 Phase 2 Phase 3 Calidi Worldwide Rights Manufacturing Partner NNV1 Allogeneic NSC - delivering CRAd - S - pk7 oncolytic virus Newly diagnosed Glioblastoma FDA - cleared IND – Entering Phase 2 NNV2 Allogeneic NSC - delivering CRAd - S - pk7 oncolytic virus Recurring Glioblastoma FDA - cleared IND – Entering Phase 1 SNV1* Allogeneic AD - MSC - delivering CAL1 oncolytic virus Solid tumors (metastatic breast / melanoma / head and neck) FDA Pre - IND – Planned Phase 1 Page 11 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

© 2022 Calidi Biotherapeutics Ρ | Proprietary & Confidential, Patents issued and pending NeuroNova Platform 12

NeuroNova Platform Overview Image Source: Lancet Oncology Journal (June 29 th , 2021) » Possess inherent ability to distribute throughout tumor mass as well as migrate to distant tumor sites regardless of tumor size, anatomic location, or tissue type » Calidi’s platform, NeuroNova1 (NNV1), is comprised of an immortalized NSC line loaded with an engineered oncolytic adenovirus » Completed Phase 1 clinical trial documenting excellent safety and signals of efficacy in patients with newly diagnosed Glioblastoma (GBM) » Peer reviewed publication (Lancet Oncology Journal (June 29th, 2021) Neural Stem Cells (NSC): Adenovirus (CRAd - S - pk7) for NeuroNova Amplifies selectively in tumor cells Radiation treatment upregulates Survivin expression Page © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending 13 The dotted line emphasizes the engineered part of the virus

Neural Stem Cell Delivery of an Oncolytic Adenovirus in Newly Diagnosed Patients with Malignant Glioma: A First - in - Human, Phase 1 Clinical Trial (Lancet Oncology, 2021 Aug;22(8):1103 - 1114) NNV1 (NSC.CRAd - S - pk7) Phase 1 Trial Design Agent: NSC - CRAd - S - pk7: Neural stem cells loaded with CRAd - S - pk7 - a chimeric adeno viral vector containing a pk7 fiber modification and a survivin promoter driving E1A replication Study design: Open - label, phase 1, dose - escalation clinical trial in patients with newly diagnosed high - grade gliomas Methods: After neurosurgical resection, NSCCRAd - S - pk7 was injected into the walls of the resection cavity. Within 10 - 14 days, treatment with temozolomide and radiotherapy was initiated Endpoints: a) Primary endpoint: to determine the safety and toxicity profile and the maximum tolerated dose b) Secondary endpoint: to determine the best tumor response using the iRANO criteria, estimate survival outcomes in patients and evaluate quality of life while on treatment c) Exploratory endpoints: to evaluate blood immune responses and cytokine profiles and to determine whether survival rates correlated with extent of immune response. © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Page 14 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

NNV1 Clinical Trial Results Treatment with NNV1 (NSC - CRAd - S - pk7) was safe and tolerable Treatment achieved favorable therapeutic outcomes in patients with newly diagnosed malignant glioma: best overall response saw one patient responding partially to treatment, another progressing, and 10 with stable disease The median progression - free survival was 9.1 months, and the median overall survival was 18.4 months Importantly, in the subset of patients with glioma containing an unmethylated MGMT promoter, the median progression - free survival and overall survivals were 9 vs. 5 months and 18 vs. 10 months, respectively Image Source: Lancet Oncology Journal (June 29 th , 2021) © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Page 15 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

© 2022 Calidi Biotherapeutics Ρ | Proprietary & Confidential, Patents issued and pending SuperNova Platform 16

SuperNova Platform Overview » AD - MSC have significant advantages over other MSCs because of their ease of extraction, maintained potency with age of the donor, significant anti - inflammatory & immune - suppressive properties and documented tumor homing ability » Calidi’s product candidate, SuperNova1 (SNV1), is comprised of a GMP manufactured AD - MSC line loaded with an oncolytic vaccinia virus » Completed Phase 1 autologous cell clinical trial that was well tolerated and observed signals of anti - tumor activity in 24 patients with advanced solid tumors and 2 patients with AML » Peer reviewed publication (Journal Translational Medicine 2019 Aug 19;17(1):271) Adipose - derived Mesenchymal Stem Cells (AD - MSC) Vaccinia Virus (CAL1) for SuperNova Not a human pathogen, safely used as a vaccine for smallpox Highly cytolytic for most tumor types Key natural attenuations improve tumor selectivity Large insertion capacity allows cloning of therapeutic payloads Page 17 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

Autologous adipose derived stromal cells loaded with oncolytic vaccinia virus CAL1: Phase 1 Trial Design Agent: SNV0: Adipose - derived autologous cells loaded with CAL1 – a non - modified oncolytic vaccinia virus Study design: Open - label, phase 1, dose - escalation clinical trial in patients with advanced solid tumors and AML Methods: Patients were treated with a single Intravenous, Intratumoral or Intraperitoneal injection of autologous cells loaded with CAL1 virus. Total virus dose: 1.4x10 6 – 1.8x10 7 live viral particles Endpoints: a) Primary endpoint: to determine the safety and toxicity profile and the maximum tolerated dose b) Secondary endpoint: to determine the best tumor response, estimate survival outcomes in patients and evaluate quality of life while on treatment c) Exploratory endpoints: to evaluate presence of viral particles in patients' blood over time, blood immune responses and cytokine profiles and to determine whether survival rates correlated with viral presence in blood samples © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Page 18 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

SI - 001 Patient Case: Patient #SI01 - 047 • Age/Sex: 68/M • Diagnosis: Thyroid Papillary Carcinoma • Stage IV • Treatment Date / Location: 7/19/17 BH • Treatment Details: IT in right lesion 37x10 6 SC in 0.75ml - (3.75x10 6 pfu) IT in left lesion 12x10 6 SC in 0.25ml - (1.25x10 6 pfu) Day 85 post - treatment Day 30 post - treatment Day 65 post - treatment Tumor Regression and Survival Potential Synergy With Immunotherapy (CTLA - 4: Yervoy) © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Minev, et al. Journal of Translational Medicine (2019) 17:271 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Page 19

SI - 001 Patient Case: Patient #SI01 - 021 • Age/Sex: 70/M • Diagnosis: Metastatic Head & Neck SCC • Stage IV_B • Injected tumor was resistant to chemo - and radio - therapy • Treatment Date / Location: 12/17/2015 / BH • Treatment Details: IV 19.7x10 6 SC in 0.6ml - (1.9x10 6 PFU) IT 3.3x10 6 SC in 0.1ml / lesion in 3 lesions - (0.33x10 6 PFU / lesion) Day 17 post Day 45 post Day 52 post Day 194 post Tumor Regression and Survival Potential Synergy With Approved Immunotherapy (PD - 1: Opdivo) Primary objective - Safety: No treatment - related side effects Secondary objective, Response and Patient Survival: 43 days after treatment the patient received Opdivo (anti - PD - 1 treatment) and 76 days after treatment the patient received local radiation therapy 194 days post treatment the previously resistant tumor regressed Minev, et al. Journal of Translational Medicine (2019) 17:271 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Page 20 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

Clinical Observations and Development Pathway Autologous adipose derived stromal cells delivering CAL1 oncolytic virus - Clinical Observations Combined application of stem cells and vaccina virus was well tolerated in all patients Results of the plasma cytokine assays suggested inflammatory reaction starting approximately 1 week after treatment, no cytokine storm was observed in any patient Results of the flow cytometry assays show induction of immune response with memory T cells approximately 1 month after treatment In 38% of patients, viral DNA reappeared in patient’s peripheral blood 1 week after treatment, indicative of intratumoral virus amplification without uncontrolled viremia or systemic toxicity Next Steps SNV1 – ( allogeneic AD - MSC - delivering CAL1 oncolytic virus) Phase 1 study for the treatment of solid tumors (metastatic breast / melanoma / head and neck) Pre - IND meeting held 2Q 2021 cGMP Final Drug Product Manufacturing to be completed in 1H 2023, Phase 1 initiation in 2H 2023 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Page 21 Page 21 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

Experienced Team with Proven Track Record ALLAN CAMAISA Chairman & CEO GEORGE NG President & Chief Operations Officer STEPHEN THESING Chief Business Officer BORIS MINEV, M.D. President, Medical & Scientific Affairs, Acting CMO WENDY PIZARRO, ESQ. Chief Admin Officer & Chief Legal Officer ANTONIO F. SANTIDRIAN, PH.D. SVP, Head of R&D THOMAS HERRMANN, PH.D. AVP, R&D BARBARA HAERTL, PH.D. AVP, Manufacturing Operations TONY KALAJIAN Acting CFO / Page 22 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending /

Company Highlights Two First - in - Class Novel Clinically Focused Platforms With Broad and Long - Lasting IP Protection NeuroNova ( NNV ): - Off - the - shelf Allogeneic neural stem cells (HB1.F3.CD21) loaded with oncolytic adenovirus (CRAD - s - pk7) to treat Glioblastoma (GBM) - Completed Phase 1 trial (NNV in newly diagnosed GBM). - Plan NNV Phase 1b/2a trial in newly diagnosed GBM and Phase 1 trial in recurrent GBM initiating in first half 2023 SuperNova ( SNV ): - Off - the - shelf Allogeneic adipose tissue - derived Mesenchymal Stem Cells (VP - 01) loaded with oncolytic vaccinia virus (CAL1) to treat advanced solid tumors. - Completed safety trial autologous adipose derived stromal cells loaded with oncolytic vaccinia virus (CAL1) - Allogeneic SNV1 phase 1/2 trial in TNBC, Head & Neck, and Metastatic/Unresectable Melanoma initiating in 2023 State - of - the - art Virus and Cell Manufacturing Processes - Early investment on process and assay development allowed to generate a scalable, and cost - efficient manufacturing platform. - Available, GMP manufactured allogeneic stem cells and oncolytic virus banks for SNV1 and NNV © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Page 23 Page 23 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

Contacts Stephen Thesing – Chief Business Officer & Investor Relations sthesing@calidibio.com 1.858.386.3776 George Ng – President and COO gng@calidibio.com 1.858.705.8594 Page 24 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

© 2022 Calidi Biotherapeutics Ρ | Proprietary & Confidential, Patents issued and pending Appendix 25

Broad IP Protection Across All Clinical Programs in Development Allogeneic Adipose Tissue - derived and Neural Stem Cell Platforms for Delivery of Oncolytic Viruses Tropic Cell - Based Virotherapy for the Treatment of Cancer Smallpox Vaccine for Treatment of Cancer Combination Immunotherapy Approach for Treatment of Cancer Enhanced Systems for Cell Mediated Oncolytic Viral Therapy Cell - Based Vehicle for Potentiation of Viral Therapy Two US Issued Patents, One application pending Allowance in 6 territories, 6 applications pending Allowance in 15 territories, 4 applications pending Filed in 2018, 10 applications pending Filed in 2018, 10 applications pending Use of Neural Stem Cells to Deliver Oncolytic Adenovirus Use of Adipose Derived Stomal Cells (Autologous & Allogeneic) to Deliver Oncolytic Viruses Use of Stem Cells and Oncolytic Viruses, in Combination with Immunotherapies Methods to Potentiate and Deliver Naturally Occurring and Armed Viruses Using Stem Cells New Genetically Modified Cell Delivery Vehicles Improving Potency © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Page 26 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

Recent Calidi Developments February 2nd, 2022 : Calidi announces merger with EDOC Acquisition Corp February 7 th , 2022 : Alfonso Zulueta , former President at Eli Lilly International joins Calidi Board of Directors March 16 th , 2022 : Calidi/EDOC files S - 4 to the SEC DeSPAC : Anticipated July - August 2022 Page 27 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

Board of Directors Allan Camaisa Chairman & CEO . Led $ 20 M early - stage Series A round and oversaw collaboration agreement with National Institutes of Health . Led key account engagements, including Roche, Johnson & Johnson, the US Veterans Administration, and CMS . Ernst and Young Entrepreneur of the Year . Graduate of the USNA and Harvard Business School . Former CEO : Scott Leftwich Vice - Chairman . Former consultant for Hospital Corporation of America (HCA) . Former P - 3 pilot in the Navy . Holds an MBA from Harvard Business School & a BS from the USNA . George Ng Partner at PENG Life Science Ventures . Raised over $ 140 million in non - dilutive commercialization funding for co - founded pain medication company . Holds a JD degree from the University of Notre Dame School of Law & a BAS double degree in Biochemistry and Economics from UCD . Dr . Heehyoung Lee Co - founder and managing partner, LumeBio, Inc . Drug discovery and development veteran — advised Sanofi, Genentech, and Eli Lilly and Company . Recipient of American Heart Association Fellowship . Research published in scientific journals : Nature Medicine & Nature Reviews Cancer . Holds a Ph . D . in Pathology & Molecular Medicine . James A . Schoeneck Director & Chairman of the Board at Fibrogen, Inc . Oversaw the development and launch of Remicade® as VP and General Manager at Centocor, Inc . , now Janssen Biotech, Inc . Spent 13 years at Rhone - Poulenc Rorer, Inc . , now Sanofi . Holds a BS in Education from JSU . Alfonso Zulueta Mr . Zulueta was President of International for Eli Lilly and Company (“Lilly”), where he was responsible for all geographies outside of the United States and Canada, from 2017 until his retirement at the end of 2021 . He also served as an Executive Committee Member and Corporate Officer . His other key senior roles at Lilly included President of Global Oncology and Critical Care, Vice President of Global Marketing, and President of both Lilly Japan and Emerging Markets . Page 28 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

Principal Investigators and KOL’s Roger Stupp, M.D. » Professor, Division Chief, Neuro - Oncology , Northwestern University » Internationally recognized medical oncologist, expert on GBM treatment » Established the “ stupp protocol” for temozolomide (Temodar®) and radiation in the first line treatment of brain tumors Matt Lesniak, M.D. » Professor, Chair Department of Neurological Surgery, Northwestern University » Completed first - in - human Phase 1 trial with NSC - Adeno in patients with newly diagnosed GBM » NeuroNova co inventor with 13+ years of research on the platform Karen Aboody, M.D. » Professor, Department of Stem Cell Biology, City of Hope Cancer Center » Documented safety of administering therapeutic NSCs into the brain tumor resection cavity Successful proof of concept for stem cell - based tumor targeting » NeuroNova co inventor with 13+ years of research on the platform Page 29 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

Principal Investigators and KOL’s Santosh Kesari, M.D. » Director of Neuro - oncology and chair of the Department of Translational Neurosciences and Neurotherapeutics at John Wayne Cancer Institute at Providence Saint John’s Health Center » Focused on personalized medicine for patients with malignant brain tumors » Research efforts have focused on oncolytic viruses, signal transduction, immunotherapy and translational clinical trials. Ewa Carrier, M.D. » Executive Director of Clinical Development, Fibrogen, Inc. » Extensive experience in clinical trial design and execution, including Phase 3 products Yan Michael Li, M.D. » Medical team member at MD Anderson with prior experience in treating patients with Adenovirus for Glioblastoma » Interim CEO and Board Director, ExoNanoRNA, LLC -- developing new RNA nanotechnology - based therapeutics for cancer, and vaccines. » Founder and Director, Minimally Invasive Brain and Spine Institute Page 30 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending

Benefits of Using Allogeneic Stem Cells to Manufacture Calidi’s Products Scale and Patient Compliance : Billions of doses can be manufactured from a single mini liposuction of a healthy donor. These allogeneic products have a long shelf life and can be shipped frozen to any clinic and used immediately upon thawing. Importantly, the patients will not be subjected to a liposuction procedure, leading to an improved patient compliance. Reproducibility and Potency : Calidi’s allogeneic products are composed of cultured and expanded mesenchymal stem cells only, ensuring reproducibility with very minimal lot - to - lot variations. Dose Accuracy : The allogeneic products are frozen at a specific time point following virus loading. This is a very important element of allogeneic products manufacturing to ensure sufficient oncolytic virus load of the stem cells and the presence of immune modulators in these cells. Importantly, this time point ensures the specific timing of stem cell destruction by the oncolytic virus (24 - 48 hours) following the injection into the targeted tumors Therapeutic Benefits : Calidi’s allogeneic stem cell platforms ensure protection of the OV from immune inactivation, OV amplification inside the allogeneic cells and effective modification of the tumor microenvironment, leading to improved treatment efficacy © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending Page 31 © 2022 Calidi Biotherapeutics, Inc. І Company Proprietary; Patents issued and pending