regulations. Additionally, if third parties with which we work, such as vendors or service providers, violate applicable laws, rules or regulations or our policies, such violations may also put our or our clinical trial and employee data, including personal information, at risk, and our business, financial condition, results of operations, and prospects may be adversely affected.
We are subject to certain U.S. and foreign export and import controls, sanctions, embargoes, anti-corruption laws, and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations, which can harm our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Controls, the U.S. Foreign Corrupt Practices Act of 1977, as amended, or FCPA, the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other collaborators from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector.
We expect to have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations, and we expect our non-U.S. activities to increase in time. We may engage third parties to conduct clinical trials, obtain necessary permits, licenses, patent registrations and other regulatory approvals or sell our drugs outside the United States, to conduct clinical trials, and/or to obtain necessary permits, licenses, patent registrations, and other regulatory approvals. We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors, and other collaborators, even if we do not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm, and other consequences.
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes, including radioactive materials and gas. Our operations involve the use of hazardous and flammable materials, including chemicals and biological and radioactive materials. Our operations also produce hazardous waste products. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. We also could incur significant costs associated with civil or criminal fines and penalties.
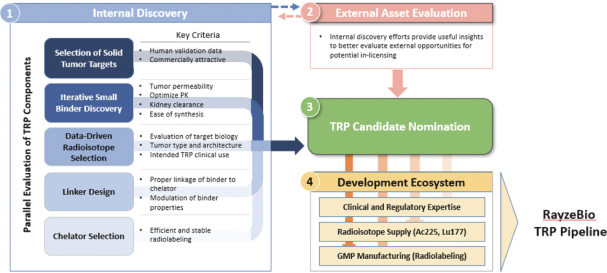
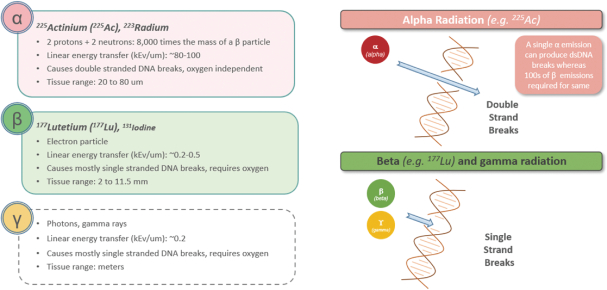
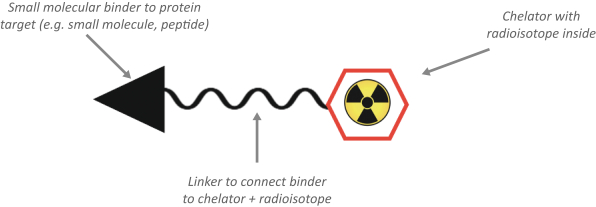
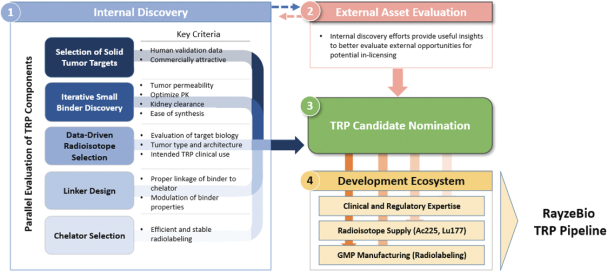
Our use of facilities that use and produce radioactive materials subjects us to compliance with decommissioning and decontamination, or D&D, requirements when we close those facilities, exposing us to potentially significant costs. Our drug candidates are manufactured using radioactive components, such as the radioisotopes Ac225 and Lu177. When one of such facilities or a cyclotron reaches the end of its useful life or if we need to abandon such facility for any other reason, we are obligated under the laws and regulatory rules of the various jurisdictions in which we operate to decommission and decontaminate such facility or cyclotron. We have no experience with D&D, and the costs of such D&D may be substantial. Estimating the amount and timing
47