| | transplantation involving non-myeloablative conditioning and with what was observed in the Phase 2 study. No events occurred to cause the Data Safety Monitoring Board (DSMB) to stop or modify the study protocol, nor was any stopping rule triggered. |
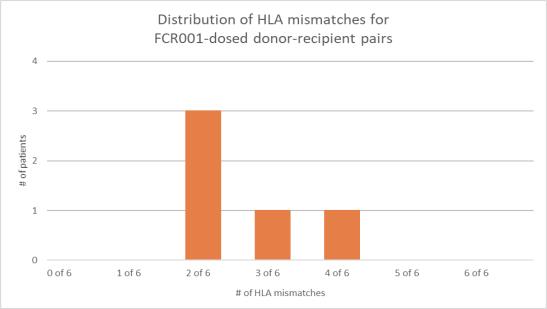
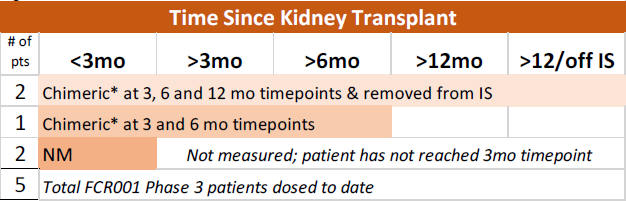
To date, no FCR001-dosed patients in the Phase 3 study have experienced BPAR. Moreover, to date, none of the 5 patients dosed with FCR001 in the Phase 3 study have developed donor-specific antibodies (DSA), the presence of which post-transplant predicts an increased risk for antibody-mediated rejection of the donated organ.
Phase 2 Long-Term Follow-Up Study Updates
| | • | | Consistent durability off IS and safety profile. In a poster presented at the 2021 ASN meeting, Talaris provided an update on the continued long-term follow-up of patients in its Phase 2 LDKT study. Talaris previously reported that 26 of 37 (70%) patients in its Phase 2 study achieved stable T-cell chimerism and were weaned off all chronic IS by approximately 12 months after their transplant. To date, 26 of 26 patients (100%) weaned off IS have continued to remain off chronic IS for the duration of their follow-up without rejecting their donated kidney. Talaris has followed these patients for a median >6 years and the longest >12 years. Six of these transplant recipients have now exceeded 10 years off all chronic IS without BPAR. Through June 11, 2021, the date of the most recent DSMB meeting for the Phase 2 study, there have been no additional AEs or SAEs reported since the prior Phase 2 data cut-off date that were determined to be related to FCR001. |
| | • | | The ability to discontinue chronic immunosuppression was observed across all levels of donor and recipient HLA matching, with 19 out of 26 recipients (73%) who were able to durably discontinue their chronic IS having an HLA match of three or less to their donor. In the Phase 2 study, Talaris did not observe any correlation between the degree of HLA mismatch and the safety or efficacy measures in the study. |
| | • | | As of October 1, 2021, Talaris has accumulated over 250 patient-years of exposure to FCR001 in LDKT, and the safety profile observed in the Phase 2 patients remains generally consistent with that expected of a patient who receives both a standard living donor kidney transplant and an allo-HSCT with nonmyeloablative conditioning. |
| | • | | Most adverse events in the Phase 2 study occurred during the first 12 months post-transplant when the patients were on conventional immunosuppression. |
New Poster Describes Potential Additional Signal of Immune Quiescence
| | • | | Potential urinary biomarker of immune quiescence. In a poster presented at the 2021 ASN meeting, Talaris reported findings of urinary mRNA profiling that it performed in a subgroup of Phase 2 LDKT patients who were tolerized to their donated kidney, as well as in a biopsy-matched cohort of standard of care LDKT recipients on chronic IS. In this analysis, Talaris identified potential signals of greater immune quiescence in the kidneys of tolerized patients, as compared to the cohort of biopsy-matched standard of care kidney transplant patients. These findings may provide further support that these patients have been tolerized to their donated kidney. |
Cautionary Note Regarding Forward-Looking Statements
This report contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, without limitation, implied and express statements regarding Talaris Therapeutics, Inc.’s (“Talaris,” the “Company,” “we,” or “our”) strategy, business plans and focus; the progress and timing of the preclinical and clinical development of Talaris’ programs, including FCR001; expectations regarding the dosing of additional patients in Talaris’ FREEDOM-1 study; and expectations regarding the identification of a potential signature of tolerance. The words “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “expect,” “estimate,” “seek,” “predict,” “future,” “project,” “potential,” “continue,” “target” or the negative of these terms and similar words or expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words.