Non-compliance with environmental, social, and governance, or ESG, practices could harm our reputation, or otherwise adversely impact our business, while increased attention to ESG initiatives could increase our costs.
Expectations around a company's management of ESG matters continues to evolve rapidly, in many instances due to factors that are out of our control. To the extent ESG matters negatively impact our reputation, it may also impede our ability to compete as effectively to attract and retain employees or customers, which may adversely impact our operations.
The Israeli government is currently pursuing extensive changes to Israel’s judicial system. This has sparked extensive political debate. In response to the foregoing developments, many individuals, organizations and institutions, both within and outside of Israel, have voiced concerns that the proposed changes may negatively impact the business environment in Israel including due to reluctance of foreign investors to invest or transact business in Israel as well as to increased currency fluctuations, downgrades in credit rating, increased interest rates, increased volatility in security markets, and other changes in macroeconomic conditions. To the extent that any of these negative developments do occur, they may have an adverse effect on our business, our results of operations and our ability to raise additional funds, if deemed necessary by our management and board of directors.
| • | Israeli corporate law does not provide for shareholder action by written consent for public companies, thereby requiring all shareholder actions to be taken at a general meeting of shareholders; |
| • | our articles of association divide our directors into three classes, each of which is elected once every three years; |
| • | our articles of association generally require a vote of the holders of a majority of our outstanding ordinary shares entitled to vote present and voting on the matter at a general meeting of shareholders (referred to as simple majority), and solely the amendment of the provision relating to the removal of members of our board of directors, require a vote of the holders of 65% of our outstanding ordinary shares entitled to vote at a general meeting; |
| • | our articles of association provide that director vacancies may be filled by our board of directors. |
RISKS RELATED TO OWNERSHIP OF THE ADSs
If we are unable to maintain compliance with Nasdaq listing standards, our ADSs may be delisted from the Nasdaq Stock Market and you may face significant restrictions on the resale of your shares.
There can be no assurances that we will be able to maintain our Nasdaq listing in the future. On March 27, 2023, we received a notification from Nasdaq that the trading price of our ADSs did not meet the minimum bid price requirement of $1.00 per share for a period of 30 consecutive trading days. We were granted a 180-day compliance period, or September 18, 2023, to regain compliance by achieving a closing bid price of at least $1.00 per share for a minimum of 10 consecutive trading days. In the event we are unable to regain compliance with the Nasdaq Capital Market listing standards and our ADSs are delisted from the exchange, it would, among other things, likely lead to a number of negative implications, including an adverse effect on the price of our ADSs, reduced liquidity in our ADSs and greater difficulty in obtaining financing. In the event of a de-listing, we would take actions to restore our compliance with Nasdaq’s listing requirements, but we can provide no assurance that any such action taken by us would allow our ADSs to become listed again, stabilize the market price or improve the liquidity of our ADSs, prevent our ADSs from dropping below the Nasdaq minimum bid price requirement in the future, or prevent future non-compliance with Nasdaq’s listing requirements. If we cannot restore our compliance with Nasdaq’s listing requirements, we would pursue eligibility for trading of these securities on other markets or exchanges, such as the OTCQB or OTCQX, which are unorganized, inter-dealer, over-the-counter markets which provides significantly less liquidity than the Nasdaq Capital Market or other national securities exchanges.
The ADS price may be volatile, and you may lose all or part of your investment.
The market price of the ADSs could be highly volatile and may fluctuate substantially, including downward, as a result of many factors, including:
| • | changes in the prices of our raw materials or the products manufactured in factories using our technologies; |
| • | the trading volume of the ADSs; |
| • | general economic, market and political conditions, including negative effects on consumer confidence and spending levels that could indirectly affect our results of operations; |
| • | actual or anticipated fluctuations in our financial condition and operating results, including fluctuations in our quarterly and annual results; |
| • | announcements by us or our competitors of innovations, other significant business developments, changes in distributor relationships, acquisitions or expansion plans; |
| • | announcement by competitors or new market entrants of their entry into or exit from the alternative protein market; |
| • | overall conditions in our industry and the markets in which we intend to operate; |
| • | market conditions or trends in the packaged food sales industry that could indirectly affect our results of operations; |
| • | addition or loss of significant customers or other developments with respect to significant customers; |
| • | adverse developments concerning our manufacturers and suppliers; |
| • | changes in laws or regulations applicable to our products or business; |
| • | our ability to effectively manage our growth and market expectations with respect to our growth, including relative to our competitors; |
| • | changes in the estimation of the future size and growth rate of our markets; |
| • | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| • | additions or departures of key personnel; |
| • | competition from existing products or new products that may emerge; |
| • | issuance of new or updated research or reports about us or our industry, or positive or negative recommendations or withdrawal of research coverage by securities analysts; |
| • | variance in our financial performance from the expectations of market analysts; |
| • | our failure to meet or exceed the estimates and projections of the investment community or that we may otherwise provide to the public; |
| • | fluctuations in the valuation of companies perceived by investors to be comparable to us; |
| • | disputes or other developments related to proprietary rights, including patents, and our ability to obtain intellectual property protection for our products; |
| • | litigation or regulatory matters; |
| • | announcement or expectation of additional financing efforts; |
| • | sales and short-selling of the ADSs; |
| • | our issuance of equity or debt; |
| • | changes in accounting practices; |
| • | ineffectiveness of our internal controls; |
| • | negative media or marketing campaigns undertaken by our competitors or lobbyists supporting the conventional meat industry; |
| • | the public’s response to publicity relating to the health aspects or nutritional value of products to be manufactured in factories using our technologies; and |
| • | other events or factors, many of which are beyond our control. |
In addition, the stock markets have experienced extreme price and volume fluctuations. Broad market and industry factors may materially harm the market price of the ADSs, regardless of our operating performance. These fluctuations often have been unrelated or disproportionate to the operating performance of those companies. These fluctuations, as well as general economic, political and market conditions such as recessions, interest rate changes, tariffs, international currency fluctuations, or the effects of disease outbreaks or pandemics (such as the COVID-19 pandemic), may negatively impact the market price of the ADSs. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted against that company. If we were involved in any similar litigation, we could incur substantial costs and our management’s attention and resources could be diverted.
We have never paid dividends on our share capital and we do not intend to pay dividends for the foreseeable future.
We have never declared or paid any dividends on our share capital and do not intend to pay any dividends in the foreseeable future. We anticipate that we will retain all of our future earnings for use in the development and growth of our business and for general corporate purposes. Accordingly, any gains from an investment in the ADSs will depend on price appreciation of the ADSs, which may never occur. In addition, Israeli law limits our ability to declare and pay dividends, and may subject our dividends to certain Israeli withholding taxes.
ADS holders may not receive the same distributions or dividends as those we make to the holders of our ordinary shares, and, in some limited circumstances, they may not receive dividends or other distributions on our ordinary shares and may not receive any value for them, if it is illegal or impractical to make them available.
The depositary for the ADSs has agreed to pay to ADS holders the cash dividends or other distributions it or the custodian receives on ordinary shares or other deposited securities underlying the ADSs, after deducting its fees and expenses. ADS holders will receive these distributions in proportion to the number of ordinary shares their ADSs represent. However, the depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any holders of ADSs. For example, it would be unlawful to make a distribution to a holder of ADSs if it consists of securities that require registration under the Securities Act of 1933, as amended, or the Securities Act, but that are not properly registered or distributed under an applicable exemption from registration. In addition, conversion into U.S. dollars from foreign currency that was part of a dividend made in respect of deposited ordinary shares may require the approval or license of, or a filing with, any government or agency thereof, which may be unobtainable. In these cases, the depositary may determine not to distribute such property and hold it as “deposited securities” or may seek to affect a substitute dividend or distribution, including net cash proceeds from the sale of the dividends that the depositary deems an equitable and practicable substitute. We have no obligation to register under U.S. securities laws any ADSs, ordinary shares, rights or other securities received through such distributions. We also have no obligation to take any other action to permit the distribution of ADSs, ordinary shares, rights or anything else to holders of ADSs. In addition, the depositary may deduct from such dividends or distributions its fees and may withhold an amount on account of taxes or other governmental charges to the extent the depositary believes it is required to make such withholding. These restrictions may cause a material decline in the value of the ADSs.
ADS holders do not have the same rights as our shareholders.
ADS holders do not have the same rights as our shareholders. For example, ADS holders may not attend shareholders’ meetings or directly exercise the voting rights attaching to the ordinary shares underlying their ADSs. ADS holders may vote only by instructing the depositary to vote on their behalf. If we request the depositary to solicit voting instructions from ADS holders (which we are not required to do), the depositary will notify ADS holders of a shareholders’ meeting and send or make voting materials available to them. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote. For instructions to be valid, they must reach the depositary by a date set by the depositary. The depositary will try, as far as practical, subject to the laws of Israel and the provisions of our articles of association or similar documents, to vote or to have its agents vote the deposited ordinary shares as instructed by ADS holders. If we do not request the depositary to solicit voting instructions from ADS holders, they can still send voting instructions, and, in that case, the depositary may try to vote as they instruct, but it is not required to do so. Except by instructing the depositary as described above, ADS holders won’t be able to exercise voting rights unless they surrender their ADSs and withdraw the ordinary shares. However, they may not know about the meeting enough in advance to withdraw the ordinary shares. We cannot assure ADS holders that they will receive the voting materials in time to ensure that they can instruct the depositary to vote their ordinary shares. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that ADS holders may not be able to exercise voting rights and there may be nothing they can do if their ordinary shares are not voted as they requested. In addition, ADS holders have no right to call a shareholders’ meeting.
ADS holders may be subject to limitations on transfer of their ADSs.
ADSs will be transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deem it advisable to do so because of any requirement of law or of any government or governmental body, under any provision of the deposit agreement, or for any other reason in accordance with the terms of the deposit agreement.
As a foreign private issuer whose ADSs are listed on Nasdaq, we follow certain home country corporate governance practices instead of certain Nasdaq requirements, we are not subject to U.S. proxy rules and are exempt from certain Exchange Act reporting requirements. If we were to lose our foreign private issuer status, our costs to modify our practices and maintain compliance under U.S. securities laws and Nasdaq rules would be significantly higher.
We are a foreign private issuer and are not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. We are permitted to follow certain home country corporate governance practices instead of certain requirements of the rules of Nasdaq. As permitted under the Israeli Companies Law 1999, or Companies Law, pursuant to our articles of association, the quorum for an ordinary meeting of shareholders shall be the presence of at least two shareholders present in person, by proxy or by a voting instrument, who hold at least 25% of the voting power of our shares (and in an adjourned meeting, with some exceptions, a minimum of one shareholder) instead of 33 1⁄3% of our issued share capital as otherwise required under the Nasdaq corporate governance rules. We may also adopt and approve material changes to equity incentive plans in accordance with the Companies Law, which does not impose a requirement of shareholder approval for such actions. In addition, we follow Israeli corporate governance practice instead of the Nasdaq requirements to obtain shareholder approval for certain dilutive events (such as issuances that will result in a change of control, certain transactions other than a public offering involving issuances of a 20% or greater interest in us and certain acquisitions of the stock or assets of another company). Additionally, we follow Israeli corporate governance practices instead of Nasdaq requirements with regard to, among other things, the composition of our board of directors. For example, our board of directors currently comprises four directors, three of whom we have determined are independent, however during 2021, the majority of our board of directors was not deemed to be independent, in compliance with our home-country requirements. Accordingly, our shareholders may be afforded less protection that what is provided under the Nasdaq corporate governance rules to investors in U.S. domestic issuers. See “Item 16G. —Corporate Governance—Nasdaq Listing Rules and Home Country Practices.”
Additionally, we are exempt from the rules and regulations under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors, and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. Furthermore, although under regulations promulgated under the Companies Law, as an Israeli public company listed on Nasdaq, we are required to disclose the compensation of our five most highly compensated officers on an individual basis, this disclosure may not be as extensive as that required of U.S. domestic reporting companies. In addition, we are not required under the Exchange Act to file current reports and quarterly reports, including financial statements, with the SEC as frequently or as promptly as U.S. domestic reporting companies whose securities are registered under the Exchange Act. Moreover, we are not required to comply with Regulation FD, which restricts the selective disclosure of material information. These exemptions and leniencies reduce the frequency and scope of information and protections available to ADS holders in comparison to those applicable to U.S. domestic reporting companies.
If we cease to qualify as a foreign private issuer, we would be required to comply fully with the reporting requirements of the Exchange Act applicable to U.S. domestic issuers. We would lose our foreign private issuer status if a majority of our shares are owned by U.S. residents and a majority of our directors or executive officers are U.S. citizens or residents or we fail to meet additional requirements necessary to avoid loss of foreign private issuer status. If we are not a foreign private issuer, we will be required to file periodic reports and registration statements on U.S. domestic issuer forms with the SEC, which are more detailed and extensive than the forms available to a foreign private issuer. We may also be required to modify certain of our policies to comply with accepted governance practices associated with U.S. domestic issuers and we would lose our ability to rely upon exemptions from certain corporate governance requirements on U.S. stock exchanges that are available to foreign private issuers. Such modifications and subsequent compliance would cause us to incur significant legal, accounting and other expenses that we would not incur as a foreign private issuer.
If we are a “passive foreign investment company” for U.S. federal income tax purposes, there may be adverse U.S. federal income tax consequences to U.S. investors
Based on our income and assets, we believe that we may be treated as a PFIC for the preceding taxable year. However, the determination of our PFIC status is made annually based on the factual tests described below. Consequently, while we may be a PFIC in future years, we cannot estimate with certainty at this stage whether or not we are likely to be treated as a PFIC in the current taxable year or any future taxable years. Generally, if, for any taxable year, at least 75 percent of our gross income is “passive income” or at least 50 percent of the average percentage of our assets during the taxable year (based on the average of the fair market values of the assets determined at the end of each quarterly period) are assets that produce or are held for the production of passive income, we will be characterized as a PFIC for U.S. federal income tax purposes. Passive income for this purpose generally includes, among other things, dividends, interest, rents, royalties, gains from commodities and securities transactions, and gains from assets that produce passive income. However, rents and royalties received from unrelated parties in connection with the active conduct of a trade or business should not be considered passive income for purposes of the PFIC test. For example, if we were to be characterized as a PFIC for U.S. federal income tax purposes in any taxable year during which a U.S. Holder (as defined in “Item 10.—Additional Information—Taxation — Material United States federal income tax considerations”) holds ordinary shares or ADSs, such U.S. Holder could be subject to additional taxes and interest charges upon certain distributions by us and any gain recognized on a sale, exchange or other disposition of our shares, whether or not we continue to be characterized as a PFIC. Certain adverse consequences of PFIC status can be mitigated if a U.S. Holder makes a “mark to market” election or an election to treat us as a qualified electing fund, or QEF. See “Item 10.—Additional Information—Taxation—Passive foreign investment company considerations.”
Whether we are a PFIC for any taxable year will depend on the composition of our income and the composition and value of our assets from time to time. Each U.S. Holder is strongly urged to consult its tax advisor regarding these issues and any available elections to mitigate such tax consequences.
If we are a controlled foreign corporation, there could be adverse U.S. federal income tax consequences to certain U.S. Holders.
Each “Ten Percent Shareholder” (as defined below) in a non-U.S. corporation that is classified as a “controlled foreign corporation,” or a CFC, for U.S. federal income tax purposes generally is required to include in income for U.S. federal tax purposes such Ten Percent Shareholder’s pro rata share of the CFC’s “Subpart F income,” “tested income,” “global intangible low-taxed income” and investment of earnings in U.S. property, even if the CFC has made no distributions to its shareholders. Subpart F income generally includes dividends, interest, rents, royalties, gains from the sale of securities and income from certain transactions with related parties. A non-U.S. corporation generally will be classified as a CFC for U.S. federal income tax purposes if Ten Percent Shareholders own, directly or indirectly, more than 50% of either the total combined voting power of all classes of stock of such corporation entitled to vote or of the total value of the stock of such corporation. A “Ten Percent Shareholder” is a United States person (as defined by the Internal Revenue Code of 1986, as amended, or the Code) who owns or is considered to own, directly, indirectly, or constructively, 10% or more of the value or total combined voting power of all classes of stock entitled to vote of such corporation.
The determination of CFC status is complex and includes complex attribution rules. A non-corporate Ten Percent Shareholder with respect to a CFC generally will not be allowed certain tax deductions or foreign tax credits generally available to a corporate Ten Percent Shareholder. Failure to comply with CFC reporting obligations may subject a Ten Percent Shareholder to significant monetary penalties. We cannot provide any assurances that we will furnish to any Ten Percent Shareholder information that may be necessary to comply with the reporting and tax paying obligations applicable under the CFC rules of the Code. U.S. Holders should consult their own tax advisors with respect to the potential adverse U.S. tax consequences of becoming a Ten Percent Shareholder in a CFC.
| | ITEM 4. | INFORMATION ON THE COMPANY |
A. History and Development of the Company
We were incorporated in May 2018 in Israel as DocoMed Ltd., and originally provided digital health services. In July 2019, we changed our name to MeaTech Ltd. and later Steakholder Innovation Ltd., or Steakholder Innovation, and commenced our cultured meat technology development operations. In January 2020, Steakholder Innovation completed a merger with Ophectra Real Estate and Investment Ltd., or Ophectra, a company incorporated in Israel whose shares were traded on the TASE, whereupon the name of Ophectra was changed to Meat-Tech 3D Ltd., then MeaTech 3D Ltd. and finally Steakholder Foods Ltd., or Steakholder Foods.
According to the terms of the merger, Steakholder Foods acquired all outstanding shares of Steakholder Innovation from the shareholders of Steakholder Innovation, in return for the issuance of ordinary shares to the shareholders of Steakholder Innovation, as well as non-tradable merger warrants to receive ordinary shares upon the achievement of pre-defined milestones, which were achieved in 2020 and 2021. Steakholder Innovation become Steakholder Foods’ wholly-owned subsidiary.
In connection with the merger, the Tel Aviv District Court for Economic Affairs approved an arrangement whereby all of Ophectra’s assets and liabilities, whether certain or contingent, at the time of the merger were irrevocably assigned to a settlement fund, or Settlement Fund, for the purpose of settling Ophectra’s pre-merger liabilities (except for Ophectra’s ownership of 14.74% of the outstanding shares of Therapin Ltd., as described below). This includes all future liabilities arising from Ophectra’s activities prior to the merger (including tax liabilities, if any), and any commitments made by Ophectra prior to the merger. We also provided the Settlement Fund approximately NIS 1.3 million (approximately $0.4 million), which we included in our public listing expenses, for the purpose of settling any of Ophectra’s debts, and bear no additional liabilities to the Settlement Fund. Anyone who believed they had a claim to Ophectra’s assets were invited to lodge their claims to the trustees. Due to the fact that two years have passed since the merger, and the fact that the Settlement Fund no longer contains any assets, its trustees are expected to instigate proceedings to wind up the Settlement Fund.
Although Steakholder Foods was the legal acquirer of Steakholder Innovation’s shares as described above, the merger was not considered a business acquisition as defined in IFRS 3. As a result, it was determined that Steakholder Innovation is the acquirer of the business for accounting purposes and the transaction was treated as a reverse acquisition that does not constitute a business combination.
Therefore, the consolidated financial statements and financial data included herein for all periods through and including December 31, 2019 were adjusted retroactively to reflect the financial statements of Steakholder Innovation, other than the information concerning earnings per share, which is presented according to the equity information of Steakholder Foods (then called Ophectra Real Estate and Investments Ltd.), and our consolidated financial statements and financial data included herein from January 1, 2020 onward relate to Steakholder Foods.
We temporarily maintained ownership of 14.74% of the outstanding shares of Therapin Ltd., or Therapin, a company incorporated in Israel, while considering a possible collaboration, however, in May 2020, we returned these holdings to Therapin, and agreed to convert our investment of NIS 7.25 million in Therapin into an interest-free loan, to be repaid by the latter at a rate of NIS 0.48 million per annum for ten years (NIS 4.8 million in total) or in full upon an exit event, plus NIS 2.45 million to be paid upon an exit event, including a public offering, or repayment of 14.74% of any distributable surplus or dividend distributed by Therapin, up to the amount of the outstanding balance, as detailed in our separation agreement with Therapin. As part of the agreement, Therapin gave us an option to convert the cash payment to equity of Therapin.
B. Business Overview
Overview
We are an international deep-tech food company, headquartered in Rehovot, Israel, that initiated activities in 2019 and are listed on the Nasdaq Capital Market under the ticker “STKH”. We believe that cultivated meat technologies hold significant potential to improve meat production, develop a sustainable livestock system, simplify the meat supply chain, and offer consumers a range of new product offerings.
We are on a mission to make meat sustainable, delicious, and clean. We aim to provide an alternative to industrialized animal farming that reduces carbon footprint, minimizes water and land usage, and prevents the slaughtering of animals. By adopting a modular factory design, we expect to be able to offer a sustainable solution for producing a variety of beef, chicken, pork and seafood products, both as raw materials and whole cuts.
We are developing cultivated meat technologies, including three-dimensional printing technology, together with biotechnology processes and customizable manufacturing processes in order to manufacture cultivated meat that does not require animal slaughter. We are developing a novel, proprietary three-dimensional bioprinter to deposit layers of customized bio-ink in a three-dimensional geometry to form structured cultivated meat. We believe that the cultivated meat production processes we are developing, which are designed to offer our eventual customers an alternative to industrial slaughter, have the potential to improve the quality of the environment, shorten global food supply chains, and reduce the likelihood of health hazards such as zoonotic diseases transferred from animals to humans (including viruses, such as virulent avian influenza and COVID-19, and drug-resistant bacterial pathogens, such as some strains of salmonella).
In August 2020, we announced the completion of Project Carpaccio, whereby we printed a thin slice of meat consisting of muscle and fat tissue developed from stem cells, having developed the entire growth process of the tissue components, followed by three-dimensional printing using our dedicated, in-house printer.
In December 2021, we announced that we had successfully three-dimensionally printed a 3.67 oz cultivated steak, primarily composed of cultivated fat and muscle tissues. While cultivated meat companies have made some progress developing unstructured, or even undifferentiated, alternative meat products, such as minced meat and sausage, to the best of our knowledge, the industry has struggled in developing high-margin, high-value structured and cultivated meat products such as steak. Unlike minced meat, a cultivated meat steak product has to grow in fibers and contain connective tissues and fat. To be adopted by diners, we believe that cultivated steaks will need to be meticulously engineered to look and smell like conventional meat, both before and after cooking, and to taste and feel like meat to the diner. We believe that we are the first company to be developing both a proprietary bioprinter and the related processes for growing cultivated meat to focus on what we believe is a high value sector of the alternative protein market.
In May 2022, we joined the UN Global Compact initiative, committing to ten universally accepted principles in the areas of human rights, labor, environment, and anti-corruption and to act in support of the issues embodied in the UN’s Sustainable Development Goals.
We are led by our Chief Executive Officer, Arik Kaufman, who has founded various Nasdaq- and Tel Aviv Stock Exchange, or TASE, -traded foodtech companies, and currently serves as director of Wilk Technologies Ltd. He is also a founding partner of BlueOcean Sustainability Fund, LLC, or BlueSoundWaves, led by Ashton Kutcher, Guy Oseary and Effie Epstein, which has partnered with Steakholder to assist in attempting to accelerate the Company’s growth. Mr. Kaufman holds extensive personal experience in the fields of food-tech and bio-tech law, and has led and managed numerous complex commercial negotiations, as part of local and international fundraising, M&A transactions and licensing agreements. We have carefully selected personnel for the rest of our executive management team who possess substantial industry experience and share our core values.
Cultivated Meat Industry and Market Opportunity
Protein is a necessary staple for healthy nutrition. The growth in recent years of both the human population and global wealth is driving a decades-long trend of accelerating demand for meat. The demand for protein products has consistently risen in recent decades and is expected to continue to do so. The rising growth of demand for farm animals for the food industry has created significant environmental, health, financial and ethical challenges.
According to Statista, the value of the global meat sector was estimated at $838 billion in 2020, and was forecast to increase to $1.157 billion by 2025. According to market research firm Fortune Business Insights, the global meat substitute market was estimated at $5.4 billion in 2021 and is expected to grow to $10.8 billion by 2028. McKinsey & Company estimates between $20 and $25 billion in sales by 2030, and with regard to the longer term, Barclays predicted in November 2021 that by 2040, 20% of the demand for meat globally will be provided by cultivated meat – a $450 billion market opportunity. Jefferies likewise forecasts a $240-470 billion meat market, with 9%-18% of global meat demand provided by cultivated meat by 2040.
The meat industry is showing strong interest in the alternative protein space, both in plant-based and cell-based proteins. There are several drivers underlying the strong engagement with alternative proteins. We believe consumers are looking for less harmful protein sources, with approaches such as flexitarianism already an established middle path between vegetarian diets and those heavy in animal proteins, such as the paleo diet. Many meat processors have experienced the worst of the COVID-19 pandemic outbreaks and are seeking to minimize human involvement in the manufacturing process. To that end, retailers such as Costco and Walmart are increasingly opening their own meat processing facilities on which they can rely exclusively without the involvement of third party manufacturers.
Limitations of Conventional Meat Production
In addition to questions about whether conventional meat production can adequately provide for the growing global population, conventional meat production raises serious environmental issues. According to the United Nations, 8% of the world’s freshwater is used for raising livestock for meat and leather. At least 18% of the greenhouse gases entering the atmosphere are from the livestock industry. 26% of the planet’s ice-free land is used for livestock grazing and 33% of croplands are used for animal feed. With regard to treatment of animals in conventional meat production, more than 70 billion animals are slaughtered annually with steady increases to be expected in line with increased demand for meat.
Another common consumer concern with industrial-scale animal rearing is the reliance on the intensive use of antibiotics. Antibiotics are used in livestock, especially pigs and poultry, to manage animal health, and to treat or prophylactically prevent diseases such as avian flu and swine flu. Their effects on human health have not been fully resolved, with concerns including the potential growth of antibiotic-resistant diseases in meat for human consumption.
Existing Alternative Proteins and their Limitations
Negative consumer sentiment towards the perceived ethical, health and environmental effects of the global meat industry help explain the strong focus that has developed on creating methods of protein production that are more sustainable, nutritious and conscious of animal welfare. Recent years have seen a combination of increasing consumer awareness and advanced technological development that has led to substantially increased demand for proteins that do not involve animal slaughter besides traditional plant-based proteins, such as soy, peas and chickpeas. Some of the alternative proteins being developed for human consumption for this purpose include:
Mycoproteins: Some of the most commercially successful novel alternative protein products are currently mycoproteins, which are derived from fungi. They are high in protein and fiber, low in saturated fat, and contain no cholesterol. However, they have been associated with allergic and gastrointestinal reactions. They are fermented to become a dough, which can develop a texture similar to that of meat.
Jackfruit: Jackfruit is a tropical fruit native to India, which has a similar taste to fruits such as apples and mangoes. While it contains substantially greater protein than these fruits, its protein content is lower than that of meat. Therefore, while its texture is somewhat similar to that of shredded meat, it is not generally viewed as an alternative to meat for consumers used to animal proteins, due to the difference in taste from traditional meat products, and its lower protein content.
Insects: Insects are an environmentally-friendly source of protein that requires significantly less land and water, and emits significantly less greenhouse gases than large mammals raised for slaughter. In addition, they can be fed food unsuitable for livestock that would otherwise be wasted. While crickets are the most common source of edible insects, research is currently taking place on new insect species of value for food production, as well as methods to produce them economically at scale. Insects can be consumed in their natural state; however many cultures consider insect consumption to be taboo and many people are disgusted by the idea. As a result, research is taking place into developing insect-based products in different forms not easily discernable as insect-based, including flour.
The Cultivated Meat Solution
We believe that cultivated meat grown through cellular agriculture, which aims to produce cultivated animal proteins without the need for large-scale slaughter, has the potential to satisfy consumer desire for meat while also avoiding the negative impacts of conventional meat production. Cellular agriculture is an efficient, closely-controlled indoor agricultural process that utilizes advanced technologies with conceptual similarities to hydroponics, which are used for growing meat cells rather than fruit. Cultivated meat is grown in cell culture rather than inside animals and applies tissue engineering practices for fat and muscle production for the purpose of human consumption. Instead of animal slaughter, stem cells are isolated from animal tissue, such as from an umbilical cord (following birth), an adipose or a muscle tissue, and then cultivated in vitro to form protein biomass, muscle fibers and fat cells. While also known as “cultured meat”, “clean meat”, “in vitro meat” or “lab-grown meat”, the term “cultivated meat” has gained the most traction as of late and is the term believed to best appeal to consumers.
Cultivated meat production is an advanced technology that operates as part of the wider field of cellular agriculture, which entails growing animal cells in bioreactors and is an emerging solution to the growing demand for alternative proteins. We are aware of a few dozen companies and institutions actively working to develop technologies and other products to meet this demand, some of whom are focused on producing red and white meats, while others are focused on fish and crustaceans. Some of these companies are working on culturing various types of cells, such as chicken, pork, kangaroo and foie gras. We believe this push of scaling-up cellular agriculture has the potential to offer a solution to the scale and environmental challenges confronting conventional meat production. Other alternative protein companies are already selling plant-based meat substitutes, but to our knowledge, these companies are not focused on the production of real meat products produced with animal cells.
We are engaged with experimentation to develop optimal and cost-effective cell culture media composition. In so doing, we are also exploring a range of types of and sources for growth factors suited to cell culture. These sources are expected to be sustainable and ethical, providing a route to enabling efficient and cost-effective processes. While many challenges remain, surveys are consistently showing consumer openness toward, and enthusiasm for, cultivated meat. According to “Consumer Acceptance of Cultured Meat: An Updated Review (2018–2020)” published by researchers at the University of Bath, “the evidence suggests that, while most people see more societal benefits than personal benefits of eating cultivated meat, there is a large potential market for cultivated meat products in many countries around the world. Cultivated meat is generally seen as more acceptable than other food technologies, and more appealing than other alternative proteins like insects. Although it is not as broadly appealing as plant-based proteins, evidence suggests it may be more uniquely positioned to appeal to meat-lovers who are resistant to other alternative proteins, and it is more appealing to certain demographic groups”.
We believe that cultivated meat could have several potential advantages over conventionally-harvested meat:
| • | Environmental: At least 18% of the greenhouse gases entering the atmosphere today are from the livestock industry. Research shows that the expected environmental footprint of cultivated meat includes approximately 78% to 96% fewer greenhouse gas emissions, 63%-95% less land use, 51% to 78% less water use, and 7% to 45% less energy use than conventionally-produced beef, lamb, pork and poultry. This suggests that the environmental consequences of switching from large-scale, factory farming to lab-grown cultivated meat could have a long-term positive impact on the environment. |
| • | Mitigating and reducing of health risks: Another potential benefit of cultivated meat is that its growth environment is designed to be less susceptible to biological risk and disease, through standardized, tailored production methods consistent with controlled manufacturing practices that are designed to contribute to improved nutrition, health and wellbeing. Therefore, cultivated meat reduces the risk of new diseases and future pandemics. Plant-based and cultivated meats are expected to be insusceptible to animal diseases and should therefore not contribute to pandemic risk because they do not require the use of live animals. Moreover, cultivated meat does not require antibiotics during its production and therefore will not contribute to antibiotic resistance. |
| • | Cost: While the precise economic value of harvested cells has yet to be determined, the potential to harvest large numbers of cells from a small number of live donor animals gives rise to the possibility of considerably higher returns than traditional agriculture, with production cycles potentially measured in months rather than years. By comparison, raising a cow for slaughter generally takes an average of 18 months, over which period 15,400 liters of water and 7 kilograms of feed will be consumed for every kilogram of beef produced. While the original cultivated burger is thought to have cost around $330 thousand, consulting firm CE Delft estimates that economies of scale combined with technological improvements will bring the cost of cultivated meat down to less than $8 per kilogram by 2035. |
| • | Animal Suffering: More and more people are grappling with the ethical question of whether humanity should continue to slaughter animals for food. There is a growing trend of opposition to the way animals are raised for slaughter, often in small, confined spaces with unnatural feeding patterns. In many cases, such animals suffer terribly throughout their lives. This consideration is likely a factor in many consumers choosing to incorporate more flexitarian, vegetarian and vegan approaches to their diets in recent years. |
| • | Alternate Use of Natural Resources: Eight percent of the world’s freshwater supply and one third of croplands are currently used to provide for livestock. The development of cultivated meat is expected to free up many of these natural resources, especially in developing economies where they are most needed. |
| • | Food Waste: The conventional meat industry’s largest waste management problem relates to the disposal of partially-used carcasses, which are usually buried, incinerated, rendered or composted, with attendant problems such as land, water or air pollution. Cultivated meat offers a potential solution for this problem, with only the desired cuts of meat being produced for consumption and only minimal waste product generated with no leftover carcass. |
Our Strategy
Our vision is to be a global leader in the production of meat through advanced biotechnology and engineering solutions for a more sustainable world, enabling the production of the food of the future. We are committed to making the right choice of meat for end consumers simple by developing high-quality meat that is slaughter-free, delicious, nutritious, and safer than farm-raised meat, We accomplish this by adopting a factory design intended to offer a sustainable solution for producing a variety of beef, chicken, sea-food and pork products, whether as raw materials or final consumer products.
Our technologies and processes have the potential to be sustainable. We are developing a meat production process that is designed to provide sustainability in an industry that, due to inefficiencies inherent in conventional meat farming, is not otherwise expected to be able to meet the growing demand for protein caused by rising population numbers and global affluence. These include the large amounts of land and water use that are needed for raising livestock, which causes precious natural resources to be squandered and the release of methane and other greenhouse gases by livestock.
We are designing our cellular agriculture and bioprinting processes to be modular so that customers can initiate their cultivated meat activities at scales suitable for their specific needs and to grow their activities as their needs evolve. Whether a customer wishes to manufacture a hybrid product that includes cultivated and plant-based ingredients, cultivated fat as a raw material, or even 3D-printed steak, each facility can be adapted to scale-out product capabilities and production volumes.
We are developing a fully automated, clean and proprietary process for cultivated meat manufacturing in a controlled, sterile environment, which is expected to significantly increase food safety. Our production facilities will not house a single animal and will contain robust integrated monitoring systems and minimal human interaction, which will greatly reduce the risk of pathogen contamination of the type claimed to have caused the COVID-19 pandemic and numerous other human health crises.
We have carefully selected personnel for our management team who possess substantial industry experience, from diverse fields including the food industry, business development bioprinting, tissue engineering, industrial stem cell growth, software engineering, electronic and mechanical engineering and print materials development. We believe that this blend of talent and experience in managers who share our core values gives us the requisite insights and capabilities to execute our plan to develop technologies designed to meet demand in a scalable, profitable and sustainable way.
To achieve our mission, we intend to:
| • | Commercialize our technologies for use in consumer and business markets. We intend to commercialize our three-dimensional bio-printing capabilities, while also customizing bio-inks to enable the production of products based on a wide range of species in accordance with the needs of our partners and customers. We also intend to provide ingredients to business customers for use in consumer products in order to help meet the growing demand for sustainable, slaughter-free cultivated meat products. For example, manufacturers of meat alternatives, such as vegetarian sausages, may choose to include our cultivated fat biomass in their products in order to deliver the signature meaty flavors, aromas and textures of the meat that is otherwise provided by the conventional meat of species such as chicken, beef and pork. We believe that this combination has the potential to unlock a new level of meat experience. |
| • | Perfect the development of our cultivated meat manufacturing technology and processes. We intend to continue developing and refining our processes, procedures and equipment until we are in a position to commercialize our technologies, whether by manufacturing final products for consumers (B2B2C models) or ingredients for industrial use, as well as in outlicensing (B2B models). We are continuing to tackle the technological challenges involved in scaling up both our biological and printing processes to industrial-scale levels. |
In addition, we intend to license our production technology as well as provide associated products, such as cell lines, printheads, bioreactors and incubators, and services, such as technology implementation, training, and engineering support, whether directly or through contractors, to companies in fields including food processing, food retail and cultivated meat. We intend to charge our customers a production license fee, based upon the amount of meat printed. We expect that each production facility will periodically require us to provide them with our proprietary materials, such as fresh sets of starter cells, for a fee. In addition, other materials used in the production process, such as cell-culture media and additives in our bio-inks may be sourced from third parties. Whether these materials are customized for the specifics of our production processes, “white-labelled” generic materials or proprietary materials that we have developed, we may charge a fee for restocking such materials; however, we have not yet reached the stage where it would be possible to estimate to what extent this would contribute to any future revenue stream. Finally, we intend to provide paid product implementation and guidance services to our customers looking to establish cultivated meat manufacturing facilities. We expect that each facility licensing our technologies will need to deal with novel challenges and, as a result, will require the assistance of our expert knowledge in order to set up and implement our licensed technologies.
In December 2022, we announced that we will focus on commercialization of our 3D bio-printer in 2023 to accelerate our go-to-market strategy through business collaborations and partnerships. To facilitate an accelerated go-to-market plan, we will focus resources on dedicating business personnel to create and develop partnerships during 2023.
| • | Develop additional alternative proteins to meet growing industry demand. There are substantial technological challenges inherent in expanding our offering beyond our current cultivated beef technologies to additional alternative proteins and cell lines. However, we believe that our experience, know-how and intellectual property portfolio form an excellent basis from which to surmount such challenges. In January 2023, we announced a collaboration with Singaporean cultivated seafood developer, Umami Meats, to develop 3D-printed structured eel and grouper products pursuant to a grant from the Singapore-Israel R&D Foundation. The initiative is being funded by a grant from the Singapore-Israel Industrial R&D Foundation (SIIRD), a cooperation between Enterprise Singapore (ESG) and the Israel Innovation Authority (IIA). The collaboration aims to develop a scalable process for producing structured cultivated fish products and will involve the use of our newly-developed technology for mimicking the flaky texture of cooked fish which was the subject of a recent patent application. |
By the end of the first quarter of 2023, we intend to complete the project’s first prototype, a structured hybrid grouper product printed using our proprietary three-dimensional bio-printing technology and bio-inks, customized for cells provided by Umami Meats.
| • | Acquire synergistic and complementary technologies and assets. We intend to optimize our processes and diversify our product range to expand the cultivated meat technologies upon which marketable products can be based. We intend to accomplish this through a combination of internal development, acquisitions and collaborations, with a view to complementing our own processes and diversifying our product range along the cultivated meat production value chain in order to introduce cultivated products to the global market as quickly as possible. See also “- Additional Technologies” below. |
The Commercialization Roadmap
The following table sets forth a road map for the expected commercialization of substitutes for conventionally-farmed meat, which include:
| • | Fully-plant-based meat-like offerings that are already commercially available but lack the organoleptic properties of meat, primarily flavor, aroma, texture and color; |
| • | Hybrid meat products of the type that we are developing, which combines real cultivated fat with plant-based protein to offer meatier products with enhanced organoleptic properties; |
| • | Unstructured meat products, such as hamburgers and minced meat; |
| • | Thee-dimensional, printed, hybrid, structured products such as hybrid steaks, chicken breast and fish fillets (“ready to cook”); and |
| • | Fully-cultivated structured meat products, such as 3D-printed steaks. |
We are focusing on developing complex structured products, starting with ready-to-cook structured hybrid products, followed by fully cultivated and structured, maturated whole cuts of meat.
In September 2022, we announced the development of Omakase Beef Morsels, a richly-marbled, structured meat product developed using our proprietary 3D-printing process. Inspired by the marbling standard of Wagyu beef, we believe that Omakase Beef Morsels are an innovative culinary achievement elegantly designed as a meat lover’s delicacy for premium dining experiences.
The product is made up of multiple layers of muscle and fat tissue, which have been differentiated from bovine stem cells, and showcases the control, flexibility and consistency inherent in our bioprinting technology. Each layer is printed separately using two different bio-inks – one for muscle and one for fat. The layers can be printed in a variety of muscle/fat sequences to obtain differing results of juiciness and marbling of the cut.
We expect to reach industrial-speed printing capabilities in the second half of 2023 and generate initial revenues from our hybrid product technologies commencing in 2024, followed by whole cuts of meat commencing in 2025.
Omakase Beef Morsels (Photo credit: Shlomo Arbiv)
Meat Ingredients for Hybrid and Unstructured Cultivated Products
We continue to develop novel, proprietary, stem-cell-based technologies to produce fat, muscle and connective tissue biomass from multiple species, such as beef and fish, without harming any animals. We are leveraging this technology, including through novel hybrid food products, to expedite market entry while we develop an industrial process for cultivating and producing real meat, including through the use of three-dimensional bioprinting technology. The first expected application of the technology is in hybrid food products, which combines plant-based protein with cultivated animal biomass and is designed to provide meat analogues with qualities of “meatiness”, such as taste and texture, closer to that of conventional meat products than are currently available in the market today. To this end, we have conducted a number of taste tests where we demonstrated the potential that our cultivated fat biomass has to enhance the taste of plant-based protein products. We believe that a product comprised of as little as 10-25% of our cultivated fat biomass combined with plant-based protein has the potential to enhance meatiness. Our cultivated fat biomass is designed to be free of antibiotics and can be tailored to provide personalized nutritional profiles.
Our fat biomass production technology relies on the use of cells derived from proprietary cell lines. These cells grow naturally in suspension and in high densities. They also proliferate continuously, are relatively large and tend to easily accumulate lipid. This quality of the cells makes them an excellent candidate for producing cultivated fat, so we have used them to build a robust cell line that is free of genetic modifications, which we are now attempting to upscale towards industrial production volumes. Our most advanced cell line is being built with GMO pluripotent stem cells that can differentiate into muscle cells and fat cells and form connective tissue, which need fewer high-cost media components, such as growth factors, for their development. As a result, these cells may have higher growth potential with lower costs than alternative technologies. We have likewise developed the process for isolating, growing and differentiating bovine stem cells into muscle fibers, fat biomass and connective tissue.
Some of the steps which we are taking in order to keep the growth media cost low include:
| • | Replacing expensive, animal-derived components in cell growth media with chemical replacements, including through in-house production, with a view to completing animal-free growth media and bio-ink by the first half of 2023; |
| • | Cell line optimizations, such as through high-throughput analyses of evolved isolates; |
| • | Bioprocess optimization and media recycling; |
| • | Upscaled growth factor production, such as through hollow fiber bioreactors; and |
| • | Long-term market optimization as a result of expected increased demand. |
Structured Fully-Cultivated Meat
In addition to meat ingredients for use in hybrid meats and unstructured, cultivated meat, we are developing the technology and processes to produce cultivated meat steak at an industrial scale. We are working to achieve this by creating an end-to-end technology that combines cellular agriculture with bioprinting to produce complex meat structures. We are developing cellular agriculture technology, such as cell lines, and approaches to working with growth media to support the growth of cells such as fat and muscle cells in a scalable process, and have demonstrated an ability to differentiate stem cells into fat and muscle cells. We expect the media to be composed of food-grade ingredients, with growth factors similar to those produced naturally in the bodies of cattle, albeit free of fetal bovine serum, traditionally a significant component of cellular growth media that is harvested from animals. We are engaged with experimentation to develop optimal and cost-effective antibiotic-free cell culture media, and are exploring a range of types of, and sources for, growth factors suited to cell culture. These sources are expected to be sustainable and ethical, providing a route to enabling effective and cost-effective processes. The processes we are developing are designed to allow cells of interest, following humane tissue extraction from the umbilical cord or biopsy, to be isolated, replicated, grown and maintained in vitro under controlled, laboratory conditions.
In February 2023, we announced that we had analyzed our muscle cells and found that they offer the same amino acid profile as that of the native tissue. The amino acid profile has two important roles in our cultivated beef products – taste and nutritional value. Our biology team tested 17 amino acids and compared them to native tissue, with the results showing that the team was able to create the same amino acid profile in the lab as in animals, This demonstrates that cultivated meat has the same biochemical composition as conventional meat, and we believe that it has the potential to provide similar nutritional value.
Amino acids are the building blocks of proteins, playing a crucial role in human nutrition. Meat is a rich source of amino acids, particularly those that are considered "essential" because the human body cannot produce them on its own. These essential amino acids include leucine, isoleucine, valine, lysine, methionine, phenylalanine, threonine, tryptophan, and histidine. These amino acids are important for a variety of bodily functions, including muscle growth and repair, immune function, and hormone production. The specific amino acid profile of meat, as well as its taste, varies depending on the animal species and how it is raised, as well as the cut of meat.
We are also developing proprietary bioprinting and tissue engineering technologies to enable the design and bioprinting of three-dimensional tissues. Our goal is for the meat produced using these technologies to have an authentic texture, flavor, appearance and aroma without being limited to the precise combinations of existing meat tissue, so that fat content of the meat, for example, can be adjusted to amounts other than those occurring naturally in animals in order to meet varied consumer preferences for fattier or leaner cuts of meat. We believe that the novel processes that we are developing have the potential to eventually be competitive with conventional manufacturing technologies for premium products as large-scale production of meat tissues will create new lines of meat without any unnecessary animal use.
In the course of developing our technologies, we intend to develop a large-scale technology demonstration model. We have set forth below an illustration of the process that we are developing that we believe will, upon completion, allow us and our customers to develop and manufacture cultivated steaks at industrial scale.
We are working on slaughter-free meat development processes, including cell proliferation and differentiation and experiments with stem cell growth media to grow high-density stem cells based solely on compounds produced in laboratory processes.
In these experiments, we have developed stem cells able to differentiate into fat or muscle cells which allows for the maturation of fat tissue and muscle fibers following an isolation process of specific stem cells from sources, such as bovine umbilical cords or muscle tissue. These cells are nourished with nutritional compounds that we develop as a growth medium to direct their differentiation into fat tissue or muscle tissue as needed.
In February 2022, we announced the successful development of a novel technology process in which muscle cells are fused into significant muscle fibers that better resemble those in whole cuts of meat. Bovine stem cells were isolated, proliferated in the lab and differentiated into matured muscle cells with improved muscle fiber density, thickness and length.
Cell source for cultivated meat products
The process of industrial scale meat printing necessitates the isolation and development of cells able to produce both animal muscle and fat tissues. Our proprietary cell lines are isolated from various sources that harbor these properties. For example, adult stem cells can be isolated from various adult tissues and give rise to more cells of the same type, such as either fat or muscle tissues, while stem cells isolated from the umbilical cord immediately following birth can give rise to multiple types of cells, including both fat and muscle cells. Each of these cells has advantages and disadvantages and their adaptation to our robust meat production process is currently being evaluated.
Bioreactors
We are using software-controlled bioreactors to foster cell proliferation. The initial growth phase leverages exponential growth of stem cells to achieve sufficient cell volumes for food production. These stem cells initiate differentiation processes into multiple cell types, such as muscle and fat.
We are in the process of developing cell-culturing processes and protocols for use in bioreactor systems. Such bioreactor systems will enable monitoring and control of growth parameters, as well as testing and development of efficient and economical cell-growth processes in industrial breeding containers. Separate from the bioreactor development process, we have commenced development of a cell-suspension growth process. This growth process is different from cell growth on laboratory plates. We expect that the newly-developed processes may allow cell growth on a scale needed for industrial-scale meat development. We have already developed a cell-suspension growth process using chicken and beef cells in the course of developing both structured and unstructured products.
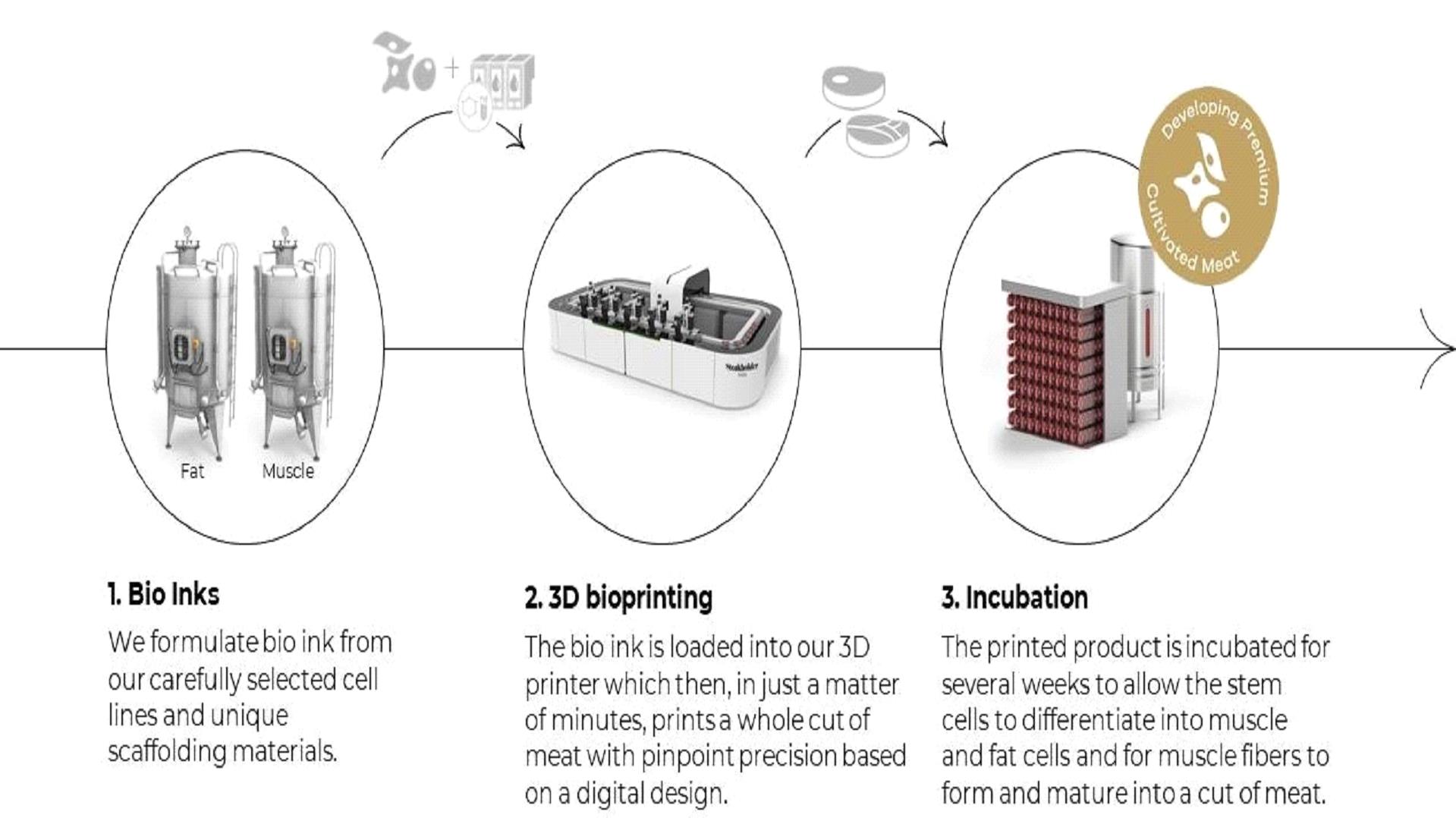
Our Bio-Inks
Structured, three-dimensional printed products require the use of edible bio-inks, which are printable biological materials produced from the biomass produced in our bioreactors, as well as scaffolding materials. Bioinks produced differ in their differentiation potential into muscle, fat and connective tissue. In this step, our bio-inks are printed in thin layers in the desired combination, which provides creative control over the steak design, in a process that maintains the ongoing viability of the bio-ink cells. Since the printed layers are composed of viable cells, they are then able to coalesce and mature in an incubator with the help of bonding agents that serve as a scaffold that forms three-dimensional tissues. We are in the process of optimizing the characteristics of our proprietary bio-inks, including composition, motility, viscosity, temperature, structural stability, density and jettability, or the ability to be dispersed by a printer, as well as the factors helping the cells to connect in three-dimensional tissues.
We also have the capability to customize bio-inks for businesses that we work with, offering the opportunity to 3D print with their cell lines. This is currently being proven in the collaboration with Umami Meat as we are customizing the bio-ink to the Umami Meats Grouper cells.
Proprietary Bioprinting
Bioprinting is a process of fashioning a specific type or types of native or manipulated cells configured to form the edible tissue analog by depositing scaffolding material mixed with cells and other bio-inks. This is done through the use of an inkjet-style printer with drop-on-demand capabilities where inks are printed precisely into a three-dimensional design.
The image below depicts a potential laboratory model that we could use for the development and production of cultivated meat steaks.
After the completion of the bioprinting process, the tissue is transferred into a special incubator, where, in addition to providing nutrients and other chemical and biological agents, the system may physically manipulate the tissue. This “training” process increases the muscle cells differentiation, a process in which a cell changes its function and phenotype, and produces a stronger, more fibrous tissue.
To date, we have printed several cell types, which coalesced into fat and muscle tissue grown in our laboratory. In December 2021, we announced that we had successfully three-dimensionally printed a 3.67 ounce cultivated steak that was primarily composed of cultivated fat and muscle tissues without using soy or pea protein. The cells used to make the steak were produced with an advanced proprietary process that started by isolating bovine stem cells from tissue samples and multiplying them. Upon reaching sufficient cellular mass, stem cells were formulated into bio-inks compatible with our proprietary 3D bioprinter. The bio-inks were printed from a digital design file of a steak structure. The printed product was placed in an incubator to mature, allowing the stem cells to differentiate into fat and muscle cells and develop into fat and muscle tissue to form our steak.
In May 2022, we announced the development of a novel, multi-nozzle 3D bioprinting system for industrial scale production of complex cultivated meat products without impacting cell viability. We plan to offer the technology to third parties via our wholly-owned private subsidiary, Steakholder Innovation Ltd. as a potential additional revenue stream and to accelerate commercialization. We aim to conclude our first strategic engagement to this end in the second half of 2023.
In addition, in December 2022, we announced the development of a temperature-controlled print bed for our industrial-scale printer, which is a step forward on our path toward mass production of cultivated meat using 3D printing technology. Temperature control is a critical requirement when printing a cultivated product containing live cells. Maintaining optimal temperature poses a challenge in the architecture of our industrial printers, so the development of temperature-controlled print beds is a major milestone on the path to production at scale, whereby contactless electromagnetic power is delivered to the print bed which is connected to a wireless communication module that monitors and controls its temperature.
Cultivated Steak Scaffolding
Growing three-dimensional meat presents a unique challenge. Typically, animal cells must remain within 200 microns of a nutrient supply in order to survive. This is little more than the width of a human hair and is known as the diffusion limit. It is the reason that cells grow along the surface of a petri dish rather than forming vertical piles.
In the next step of the process that we are developing, we intend to build a scaffold to support the growth of three-dimensional meat. A “scaffold”, or “biocompatible scaffolding”, refers to an engineered platform having a predetermined three-dimensional structure that mimics the environment of the natural extracellular material, or ECM. The ECM is a three-dimensional network of large molecules that provide structural and biochemical support to surrounding cells. Collagen is the most abundant component in the ECM that supports the development and growth of complex tissues, and specifically, also muscle tissues. Engineering of bovine muscle tissues in vitro while avoiding the use of animal derived collagen requires the development of plant based scaffolds that would imitate the properties of the ECM. Plants are an obvious candidate for scaffolding as they are sustainable, cost worthy and could be processed to have similar properties of collagen fibers. We are developing technology to allow for the formation of a composite scaffold.
Modularity
We are focused on developing a process that will allow our food technology customers to operate a high-throughput manufacturing process for high-quality, healthy meat. Our cellular agriculture and bioprinting processes are being designed to be modular, meaning that they can work using different factory sizes. We believe we could license our technology to customers with industrial plants close to urban areas seeking to provide “just in time”, logistically-efficient, local and premium cellular agriculture. In addition, we believe a licensee of our technology could build a plant in a locality that does not have the resources needed for industrial animal husbandry, which would allow places like the United Arab Emirates, Hong Kong or Singapore to potentially become more agriculturally independent by increasing food security. As costs continue to decrease, we believe licensees of our technology could also build production facilities in localities where there is high agricultural seasonality or desertification risk.

Illustration of a contemplated cultivated meat manufacturing plant.
Clean Energy
We are developing processes intended to achieve high-volume manufacturing capabilities in line with the needs of today’s value-added food processors and other meat and food industry players. To this end, we are working on processes to scale up production, beginning with different cell types, including induced pluripotent stem cells and embryonic stem cells. We expect high-volume stem cell production to feed into differentiation bioreactors that are dedicated to producing fat and muscle cells. These cells are the key input for our downstream productization stages.
The processes we are developing are advanced biotechnological processes that are intended to produce cultivated meat in a clean environment with minimal environmental impact. We envision that factories utilizing our technologies will exist in greater harmony with their environment than typical current factories by supporting sustainability, utilizing renewable energy sources and recycling or treating their own waste.
Additional Technologies
We may incorporate novel bioreactor technologies that benefit cellular agriculture and the development of low-cost cell culture media not based on fetal bovine serum.
We also plan to add cell line types to expand the development of cultivated meat to other types of animals, as well as achieving market penetration in the shortest timeframe possible, which would allow us to realize the great potential in the market. We are developing cultivated meat, both unstructured hybrid products and structured, three-dimensional printed products, with an initial emphasis on bovine cells. Beyond hybrid products, cultivated fat is expected to be a component in other fat-based products, whether edible or otherwise, and an integrated component in our printing technology. We are working to create synergy and added value to the cultivated meat market, while also sustaining animal welfare and meeting the growing global demand for meat.
International Expansion
United States
In March 2022, we announced that we intended to open a U.S. office. We expect the new space will include activities in research and development, investor relations and business development. In September 2022, we commenced development of a bovine cell line in the United States, by isolating cells sourced from cattle raised on a farm approved by the United States Department of Agriculture, or USDA. We plan to make a regulatory submission in the United States for approval of our cultivated meat in the second half of 2023.
Europe
Peace of Meat Acquisition
In February 2021, we finalized our acquisition of Peace of Meat, a Belgian producer of cultivated avian products, for up to $19.9 million in cash and equity, depending on milestone achievements. Peace of Meat was established in Belgium in 2019 and developed cultured avian fat directly from animal cells without the need to grow or kill animals.
In April 2021, we commenced food technology development activities through our European subsidiary, MeaTech Europe BV, with an initial focus on hybrid foods using Peace of Meat’s cultivated fat. Hybrid foods are products composed of both plant and cultivated meat ingredients that have the potential to offer a meatier experience than purely plant-based meat alternatives. We currently expect food technology development activities to continue at our Israeli headquarters.
On March 7, 2023, we announced a restructuring plan for Peace of Meat, to which end Peace of Meat began implementing a series of changes designed to streamline its operations. On April 4, 2023, we announced that Peace of Meat would close, in the context of optimizing our funds and investment strategy, alongside enabling a greater focus on recently-announced core goals such as accelerating the commercialization of our 3D printing technology.
As part of our purchase of the shares of Peace of Meat at the end of 2020, Peace of Meat’s management had been granted full contractual autonomy throughout 2021 and 2022, and was provided with the funding required to develop its technologies in accordance with the terms of the purchase. Following the conclusion of the autonomous period and following our previously announced plans to restructure Peace of Meat, we further evaluated the expected return on our investment, and decided not to provide additional funds to Peace of Meat, in order to focus our efforts in the advancement of its core technology 3D printing of cultured products, and potential collaborations. As a result, Peace of Meat has ceased operations and is expected to be liquidated. As part of this process, we expect Peace of Meat’s assets to be realized, following which we shall consider how and when to continue development of cultivated avian products. The closure of Peace of Meat is expected to reduce our expenses by about $4.5 million annually, relative to 2022.
Asia
In November 2022, we received a registered trademark for our name in Japan, which we view as an important next step in our plans to penetrate the Japanese market and other markets in Asia. This follows on our collaboration with Umami Meats for the joint development of 3D-printed cultivated structured seafood. In addition, we plan to make a regulatory submission for approval of our cultivated meat in Singapore in the first half of 2023.
On April 3, 2023, we announced our participation in a strategic investment round in Wilk Technologies Ltd. (TASE: WILK), alongside leading players in the food industry, such as Danone and the Central Bottling Co. Ltd. (owner of Tara, Coca Cola Israel and more). The transaction was approved by our audit committee (due to related party considerations) and board of directors. As part of the investment, we purchased ordinary shares of Wilk in the amount of $450,000 at a 15% discount below their 45-day average closing price, giving us a 2.5% stake in Wilk. In parallel with this investment, we aim to identify synergies with Wilk, including various types of strategic cooperation with the company surrounding our proprietary biology and printing technologies.
Sales and Distribution
We are working to develop and establish sales and distribution capabilities. In the event that we complete the development of our technologies and secure adequate funding, we intend to consider commercialization collaborations where appropriate.
Apart from end consumers in B2B2C models of branded products, we believe that our ideal business customers will be value-added food processors and retailers that wish to benefit from cultivated meat manufacturing capabilities. We intend to provide our corporate customers with a solution to these needs in the form of highly-automated, cleaner and ‘just-in-time’ manufacturing of cultivated meat products using a repeatable, consistent manufacturing process. Our goal is for our customers to be able to streamline their meat supply chain, introduce greater manufacturing flexibility and locate their cultivated meat production facilities closer to the point of retail or consumption.
We intend to provide our business licensees with assistance in constructing facilities to employ our proprietary technology and processes. We expect that we will need to collaborate with third parties to obtain and make available to our customers the expertise necessary to provide this assistance. In addition, we intend to procure the equipment our licensees need to deploy our proprietary technology and processes from third-party providers. Some equipment, such as piping, clean rooms and packing and freezing equipment are standard industry equipment and can be sourced on open markets. Other equipment, such as bioreactors and our proprietary bioprinters, will need to be produced by contract manufacturers.
Intellectual Property
We have sought and continue to seek patent protection as well as other intellectual property rights for our products, processes and technologies in the United States and internationally. Our policy is to pursue, maintain, expand, protect and defend our patent rights and trade secrets, which we believe enable us to deliver long-term protection for the proprietary technologies, inventions and improvements that are commercially important to the development of our business.
Since the beginning of 2022, we have received notices of grant or allowance for patent applications in the USA, Canada, Australia and New Zealand relating to our development of systems and methods to apply external forces to muscle tissue that result in the development of high-quality complex structured meat.
We have a growing portfolio of 15 provisional and non-provisional patent applications pending with the USPTO, WIPO (filed through the Paris Convention Treaty, or PCT, and in various countries worldwide. A provisional patent application is a preliminary application, and establishes a priority date for the patenting process of inventions disclosed therein.
Our existing patent portfolio can currently be divided into three main areas:
Mechanical: covering printer components and peripherals used in the fabrication of the tissue cultures with two applications filed at the national stage of prosecution. The first; directed to print heads operable in a bioprinting systems for the fabrication of edible biostructures using drop-on-demand, the print heads specifically designed to accommodate bio fluids of suspended systems without causing demixing, while still delivering bio fluids with high accuracy and precision.
Following a favorable patentability opinion, the Application was filed in the USPTO and has received a first office action indicating an intent to grant upon correction of some minor procedural deficiencies. The second application currently undergoing examination in 7 countries, is directed to systems and methods of physically manipulating a resilient container (bladder) of bioprinted tissue culture having non-random three dimensional cell structure over 4 dimensions, namely elongation, compression, torsion and shear, to modulate the tissue and achieve the desired texture for each meat type. The Application was already granted in the United States, Canada, Australia and New Zealand.
Current development work in the mechanical area will most likely result in the development of at least two additional intellectual property registrations.
Biological: covering initial materials used in the process with several provisional, and PCT applications filed and currently pending.
These include an application directed to methods for harvesting ICM from bovine blastocysts; methods and compositions for the xeno-free propagation of bESC on bovine umbilical stem cells (bUCSC), derived from a bovine umbilical cord; the use of plant-based lecithins and/or their components in a composition as a differentiation drivers for use in selectively promoting adipocytes differentiation; and methods and compositions for accelerated myotube formation.
Applications: covering the final consumable formed using mechanical and biological inputs, with a couple of applications currently pending.
These include a provisional application for a beef-emulating consumable formed of stacked 3D-pronted layers of muscle and fat tissues; and an application for a method and composition for achieving the flaky characteristics associated with fish.
In addition to patent applications, we maintain trade secrets covering know-how and proprietary information relating to our core technologies and make practicable efforts to protect our confidential trade secrets. To this end, we require our employees engaged in the development of intellectual property to enter into confidentiality agreements prohibiting the disclosure of confidential information and further, require disclosure and assignment of any inventions and associated intellectual property rights that are important to our business. Additionally, we require all entering employees to represent they are not bringing in, or are using any third party’s Trade Secrets.
We have also registered our new name, Steakholder Foods, and brand name as registered trademarks in various countries, and maintain ongoing rights to our domain name. Steakholder Foods® was registered in Japan and the European Community and is currently undergoing examination in several other countries, including the United States.
While our policy is to obtain patents by application, license or otherwise, to maintain trade secrets and to seek to operate without infringing on the intellectual property rights of third parties, technologies related to our business have been rapidly developing in recent years. Additionally, patent applications that we may file or license from third parties may not result in the issuance of patents, and our current or future issued patents may be challenged, invalidated or circumvented. Therefore, we cannot predict the extent of claims that may be allowed or enforced against our patents, nor be certain of the priority of inventions covered by pending third-party patent applications. If third parties prepare and file patent applications that also claim technology or therapeutics to which we have rights, we may have to engage in proceedings to determine priority of invention, which could result in substantial costs to us, even if the eventual outcome is favorable. Moreover, because of the extensive time required for clinical development and regulatory review of products we may develop, it is possible that the patent or patents on which we rely to protect such products could expire or be close to expiration by the commencement of commercialization, thereby reducing the value of such patent. Loss or invalidation of certain of our patents, or a finding of unenforceability or limited scope of certain of our intellectual property, could also have a material adverse effect on us. See “Risk Factors — Risks Related to our Intellectual Property and Potential Litigation.”.
Competition
We expect that demand for our cultivated meat manufacturing technologies will be driven by consumer demand for alternative proteins and, more specifically, consumer acceptance of cultivated meat as the alternative protein of choice. We believe that we will compete with other cultivated meat manufacturers, alternative protein manufacturers and the conventional meat industry as a whole. We expect to directly compete with companies licensing know-how or otherwise enabling the establishment of cultivated meat manufacturing plants. We are aware of certain companies that have announced plans to provide their cultivated meat technology on a B2B basis; however, we are not currently aware of a potential competitor focusing on complex, industrial-scale, bioprinted, high-value real structured meats, such as steak.
Companies such as Upside Foods, Inc. and Mosa Meat BV are focused on producing red meats, while BluNalu, Inc. is focusing on fish and Shiok Meats Pte. Ltd. is focusing on crustaceans. There are different companies working on culturing varying cell types, such as chicken, pork, kangaroo and foie gras. This push on scaling-up cellular agriculture can serve as a solution to the scale and environmental challenges confronting traditional meat production. Other alternative protein competitors such as Beyond Meat, Inc. and Impossible Foods, Inc. are already selling plant-based meat substitutes, but to the best of our knowledge, these companies are not focused on the production of real meat products produced with animal cells.
Companies Developing Vegetable and Insect Protein Alternatives
There are numerous companies focused on developing meat substitutes. In order for a product to achieve commercial acceptance as an alternative to meat, it must have an appearance, taste, smell and nutritional values that are similar enough to the type of meat that it seeks to replace or with which it seeks to compete. These meat substitute companies generally employ proprietary formulae for manufacturing that are based wholly on ingredients of plant origin. In addition, we are aware of several companies developing insect-protein production capabilities, employing among other insects, flies, larvae and grasshoppers.
Companies Developing Cultivated Meat
The cellular agriculture meat sector is in early stages of development. The sector is currently primarily comprised of companies developing a full technology stack from developing cell lines to scaling up cellular cultivation, developing media and researching the food technology aspects of the final product. Market dynamics have led to a large number of companies operating in this manner. We do not believe that any companies in this space have already developed the capability to produce industrial quantities at prices low enough to compete on a dollar-per-pound basis against conventionally-harvested meat.
A number of larger companies have begun engaging in this sector. For example, companies such as Cargill, Inc., JBS Foods, Nestlé S.A., Tyson Foods, Inc., Merck & Co., Inc. and Lonza Group AG are currently investing in capabilities to accommodate the market’s desire for change in the cell culture media market. Additionally, a number of bioreactor companies are rumored to be interested in the cellular agriculture market opportunity. Over time, we expect that larger players will continue to increase their exposure to cellular meat production either by selling to, or collaborating with, the many start-ups in the space.
Currently, cellular agriculture companies are for the most part paving their own path, with a goal of producing meat cells suitable as a replacement for ground meat. The ground meat type of cellular product may also be suitable as an ingredient in a hybrid plant-based food product. The cell-types relevant to this effort are primarily muscle and fat cells. What exactly these cell-based companies will offer is likely to be affected by consumer expectations and underlying cost structures. We believe that these companies may have to mix their cellular meat product with plant-based ingredients in the interests of cost or appearance.
Companies Developing Structured Cultivated Meat Products
To our knowledge, there is currently no other company that is advanced as Steakholder Foods who focused on the scaling up of three-dimensional bioprinting for the cultivated meat industry. As far as we know we are the only company to publicly demonstrate our printing technology in a few of the main food tech events in 2023. However, there are companies attempting to produce steaks by means of other approaches, such as growing bovine cells, including fat, muscle and connective tissue on a pre-prepared scaffold, in order to create a contiguous piece of meat, which has so far yielded steaks.
Government Regulation
Regulators around the world are in the process of developing a regulatory approval process for cultivated meat. Cultivated meat is not yet generally commercially available, but technologies like ours are anticipated to facilitate the imminent scaling up of cultivated meat production. In general, cultivated meat production is expected to be subject to extensive regulatory laws and regulations in the United States and in other jurisdictions such as Canada, Japan, the European Union and the United Kingdom. In the United States, existing food safety requirements are expected to apply. Additional details are being developed at the U.S. Food and Drug Administration, or FDA, and the U.S. Department of Agriculture, or USDA, pursuant to a Memorandum of Understanding, or MOU, published by the FDA and USDA on March 7, 2019 entitled the “Formal Agreement to Regulate Cell-Cultured Food Products from Cell Lines of Livestock and Poultry.” For example, the FDA anticipates publishing Draft Guidance on premarket safety oversight by December 31, 2022, and in September 2021, the USDA published an Advance Notice of Proposed Rulemaking (ANPR), indicating that the USDA will be developing new labeling requirements for foods under its jurisdiction produced through cell culture technology.
Under the MOU, which is expected to affect our customers producing cultivated meat, the two agencies will operate under a joint regulatory framework wherein the FDA will oversee cell collection, cell banks and cell growth and differentiation. A transition from FDA to USDA oversight will then occur during the cell harvest stage, at which point the USDA will oversee the production and labeling of cultivated meat. The USDA will be advancing new labeling requirements. To the best of our knowledge, the regulatory approval details under development, including the Draft Guidance on FDA premarket oversight, are not expected to apply to our business directly, but they are instructive as to the regulatory requirements that our cultivated meat production customers are expected to face and their expectations of us, in the form of customer assurances, regarding our products.
At this time, our business is limited to developing cultivated meat production technology, such as bioprinters, that will be marketed to cultivated meat producers. Production equipment manufacturers must ensure that their products do not contribute to the production of adulterated food. The regulatory obligation falls on the food manufacturer to ensure that all food produced, including cultivated meat, is wholesome and not adulterated. Therefore, when sourcing food processing equipment, such as the three-dimensional bioprinter that we are developing, our customers will request assurances that the bioprinter is safe for its intended use and will not result in the production of adulterated food. We intend to monitor developments at the FDA and USDA in connection with the MOU to determine whether any specific requirements or recommendations are published with specific regard to cultivated meat equipment manufacturers.
In the United States, we expect companies manufacturing cultivated meat products to be subject to regulation by various government agencies, including the FDA, USDA, and the FTC. Equivalent foreign regulatory authorities include the Canadian Food Inspection Agency, the Japanese Food Safety Commission, the European Food Safety Authority and authorities of the EU member states, the State Food and Drug Administration of China and the SFA. These agencies, among other things, prescribe the requirements and establish the standards for food quality and safety, and regulate various food technologies, including alternative meat product composition, ingredients, manufacturing, labeling and other marketing and advertising to consumers.
In June 2022, Singapore was the first country to approve cultivated meat for sale. The SFA has published comprehensive guidance explaining all of the requirements necessary for the safety assessment of novel foods, covering all of the specifications required for the approval of cultivated meat in Singapore.
In November 2022, the FDA announced that it completed its first pre-market consultation of human food made from cultured animal cells. Through a process with a U.S.-based cultivated meat technology company, which involved evaluating the company’s production process and the cultured cell material made by the production process, including the establishment of cell lines and cell banks, manufacturing controls, and all components and inputs, the FDA determined that it had no further questions about the company’s safety conclusion. As this was the first instance of the FDA giving the greenlight to a cultivated meat product, the FDA further announced that the world is experiencing a food revolution and the FDA is committed to supporting innovation in the food supply. In March 2023, the FDA completed a second such consultation.
We expect that federal, state and foreign regulators will have the authority to inspect our customers’ facilities to evaluate compliance with applicable food safety requirements. Federal, state and foreign regulatory authorities also require that certain nutrition and product information appear on the product labels of our customers’ food products and, more generally, that such labels, marketing and advertising be truthful, non-misleading and not deceptive to consumers.
As the cell-based agriculture industry is young and its regulatory framework is emerging and evolving, legislation and regulation may evolve to raise barriers to our go-to-market strategies.
In addition to federal regulatory requirements in the United States, certain states impose their own manufacturing and labeling requirements. For example, states typically require facility registration with the relevant state food safety agency, and those facilities are subject to state inspections as well as federal inspections. Further, states can impose state-specific labeling requirements. In the United States, the USDA will be developing new labeling requirements for foods under its jurisdiction produced through cell culture technology as noted in an Advance Notice of Proposed Rulemaking (ANPR) published in September 2021.
We are subject to labor and employment laws, laws governing advertising, privacy laws, safety regulations and other laws, including consumer protection regulations that regulate retailers or govern the promotion and sale of merchandise. Our operations are subject to various laws and regulations relating to environmental protection and worker health and safety matters. We monitor changes in these laws and believe that we are in material compliance with applicable laws.
Environmental, Health and Safety Matters
We, our agents and our service providers, including our manufacturers, may be subject to various environmental, health and safety laws and regulations, including those governing air emissions, water and wastewater discharges, noise emissions, the use, management and disposal of hazardous, radioactive and biological materials and wastes and the cleanup of contaminated sites. We believe that our business, operations and facilities, including, to our knowledge, those of our agents and service providers, are being operated in compliance in all material respects with applicable environmental and health and safety laws and regulations. Based on information currently available to us, we do not expect environmental costs and contingencies to have a material adverse effect on us. However, significant expenditures could be required in the future if we, our agents or our service providers are required to comply with new or more stringent environmental or health and safety laws, regulations or requirements.
Except as stated above, we are not aware of any environmental risks related to our operations, and therefore, we do not believe that environmental regulations will have a significant effect on us. However, in the future, we may be required to meet environmental protection standards or regulations which could have a material impact on our activities, activities, profitability and ability to remain competitive.
Organizational Structure
Our subsidiaries and the countries of their incorporation are as follows:
| Name | | Jurisdiction of
Incorporation | | Parent | | % Ownership
(direct or
otherwise) | |
| Steakholder Foods USA, Inc. | | Delaware, U.S. | | Steakholder Foods Ltd. | | | 100 | % |
| Steakholder Innovation Ltd. | | Israel | | Steakholder Foods Ltd. | | | 100 | % |
| Steakholder Foods Europe BV | | Belgium | | Steakholder Foods Ltd. | | | 100 | % |
| Peace of Meat BV | | Belgium | | Steakholder Foods Europe BV | | | 100 | % |
Property and Infrastructure
Our principal executive offices and laboratory are located at 5 David Fikes St., Rehovot, Israel. The laboratory and office space total approximately 18,300 square feet. The lease for this facility will expire in January 2026, although we have an option to renew it for four years, and the annual rent (including parking fees) is approximately $0.7 million, linked to the Israeli CPI.
Employees
As of December 31, 2022, we employed 49 employees based at our office and laboratory in Rehovot, Israel and Peace of Meat employed 32 employees based at its office and laboratory in Antwerp, Belgium. On March 7, 2023, we announced a strategic restructuring for Peace of Meat, including targeted layoffs in areas of the business that were unrelated to its updated focus, and on April 4, 2023, we announced the expected liquidation of Peace of Meat, as a result of which it will no longer employ employees.
Local labor laws govern the length of the workday and workweek, minimum wages for employees, procedures for hiring and dismissing employees, determination of severance pay, annual leave, sick days, advance notice of termination, Social Security payments or regional equivalents, and other conditions of employment, including equal opportunity and anti-discrimination laws. None of our employees is party to any collective bargaining agreements. We generally provide our employees with benefits and working conditions beyond the required minimums. We believe we have a good relationship with our employees, and have never experienced any employment-related work stoppages.
Legal Proceedings
From time to time, we may be party to litigation or other legal proceedings that we consider to be a part of the ordinary course of our business. We are not currently involved in any legal proceedings that could reasonably be expected to have a material adverse effect on our business, prospects, financial condition or results of operations.
| | ITEM 4A. | UNRESOLVED STAFF COMMENTS |
None.
| | ITEM 5. | OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
The following “Operating and Financial Review and Prospects” should be read together with the information in our financial statements and related notes included elsewhere in this Annual Report. The following discussion is based on our financial information prepared in accordance with the International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB, which may differ in material respects from generally accepted accounting principles in other jurisdictions, including U.S. GAAP. The following discussion includes forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those described in “Risk Factors” and elsewhere in this Annual Report. Please also see “Forward-Looking Statements.”
For a discussion of our results of operations for the year ended December 31, 2021, including a comparison between 2021 and 2020, refer to “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Results of Operations—Year Ended December 31, 2021 Compared to Year Ended December 31, 2020” in our annual report on Form 20-F filed on March 24, 2022.
A. Operating Results
To date, we have not generated any revenue since we commenced our cultured meat operations. We do not expect to receive any revenue unless and until we complete development of and successfully commence out-licensing our technologies, or until we receive revenue from a collaboration or other partnership such as a co-development agreement, or the acquisition of a company that generates revenues. There can be no assurance that we will be successful in developing or ultimately commercializing our technologies, in establishing revenue-generating collaborations or acquiring revenue-generating companies.
Research and Development Expenses
Research and development activities are our primary focus. We do not believe that it is possible at this time to accurately project total expenses required for us to reach the point at which we will be ready to out-license our technologies. Development timelines, the probability of success and development costs can differ materially from expectations. In addition, we cannot forecast whether and when collaboration arrangements will be entered into, if at all, and to what degree such arrangements would affect our development plans and capital requirements. We expect our research and development expenses to increase over the next several years as our development program progresses. We would also expect to incur increased research and development expenses if we were to identify and develop additional technologies.
Research and development expenses include the following:
| • | employee-related expenses, such as salaries and share-based compensation; |
| • | expenses relating to outsourced and contracted services, such as external laboratories and consulting, research and advisory services; |
| • | supply and development costs; |
| • | expenses, such as materials, incurred in operating our laboratories and equipment; and |
| • | costs associated with regulatory compliance. |
We recognize research and development expenses as we incur them.
Marketing expenses consist primarily of professional services, personnel costs, including share-based compensation related to employees, and business development, public relations and investor relations services.
General and Administrative Expenses
General and administrative expenses consist primarily of personnel costs, including share-based compensation related to directors and employees, corporate costs (such as insurance), facility costs, patent application and maintenance expenses, and professional service costs, including legal, accounting, audit, finance and human resource services, and other consulting fees.
Finance Expenses (income), Net
Finance expenses (income), net, consisted primarily of a change in the fair value of financial instruments mandatorily measured at fair value through profit or loss, and exchange rate fluctuations.
We have yet to generate taxable income. As of December 31, 2022, our operating tax loss carryforwards were approximately $23.6 million.
Our results of operations have varied in the past and can be expected to vary in the future due to numerous factors. We believe that period-to-period comparisons of our operating results are not necessarily meaningful and should not be relied upon as indications of future performance.
Below is a summary of our results of operations for the periods indicated (in thousands):
| | | Year Ended December 31, | |
| | | 2022 | | | 2021 | |
| | | | | | | |
| Operating expenses: | | | | | | |
| Research and development expenses | | $ | 9,801 | | | $ | 7,594 | |
| Marketing expenses | | | 3,044 | | | | 1,628 | |
| General and administrative expenses | | | 6,937 | | | | 8,010 | |
Impairment loss
| | | 15,577 | | | | - | |
| Loss from operations | | $ | 35,359 | | | $ | 17,232 | |
| Finance income | | | 4,878 | | | | 509 | |
| Finance expense | | | 286 | | | | 1,299 | |
| Finance expense (income), net | | | (4,592 | ) | | | 790 | |
| Net loss | | $ | 30,767 | | | $ | 18,022 | |
Year Ended December 31, 2022 Compared to Year Ended December 31, 2021
Research and development expenses
Research and development expenses increased by approximately $2.2 million, or 29%, to approximately $9.8 million for the year ended December 31, 2022, compared to $7.6 million for year ended December 31, 2021. The increase resulted mainly from payroll expenses, materials and professional services expenditures related to our cultured meat research and development operations. The increase reflects Steakholder Foods’ growing investment in research and development as it achieves its milestones and expands its cultured meat technology capabilities.
Marketing expenses increased by approximately $1.4 million, or 87%, to approximately $3.0 million for the year ended December 31, 2022, compared to $1.6 million for year ended December 31, 2021. The increase resulted mainly from professional services, personnel costs, including share-based compensation related to employees, and business development, public relations and investor relations services.
General and administrative expenses
General and administrative expenses decreased by approximately $1.1 million, or 13%, to approximately $6.9 million for the year ended December 31, 2022, compared to approximately $8.0 million for the year ended December 31, 2021. The decrease resulted mainly from corporate costs reduction.
Impairment from re-measurement of cash-generating unit
As part of an annual impairment test, we tested the Peace of Meat Cash-Generating Unit (“CGU”) for impairment as of December 31, 2022. We determined that the value in use of the operation is negative, and therefore assessed the fair value less costs of disposal of the CGU. As of the date of publication of these financial statements, we have not identified a potential market participant that may purchase the CGU in an arm’s-length transaction. Thus, we have concluded that the fair value less costs of disposal of the CGU is immaterial. The fair value less costs of disposal of the CGU’s IPR&D asset was determined to be zero, as the Company did not identify any potential buyer for this asset. We have allocated the impairment loss to the CGU’s fixed assets based on their fair value. The fair value of fixed assets that are available for sale to a market participant is based on their estimated selling value as of the valuation date, net of selling costs. We estimated the selling value net of selling costs of the fixed assets based on actual offers received, previous purchases, and our knowledge of the second-hand industry market. In allocating the impairment loss, we have not reduced the carrying amount of each asset below its fair value net of disposal costs on an individual basis, if determinable. As a result, we recognized an impairment loss of USD 14,367 thousand for our IPR&D asset and USD 1,210 thousand for our fixed assets. The total impairment loss recorded was USD 15,577 thousand.
Net loss increased by approximately $12.8 million to approximately $30.8 million for the year ended December 31, 2022, compared to $18.0 million for the year ended December 31, 2021. Net of the $15.6 million non-cash impairment and $3.8 million net financial income in 2021 and 2022, the net loss increased by approximately $2.6 million, or 15%, driven mainly by increased research and development and marketing expenses.
Critical Accounting Policies
We describe our significant accounting policies and estimates in Note 3, Summary of Significant Accounting Policies, to the consolidated financial statements contained elsewhere in this annual report. We believe that these accounting policies and estimates are critical in order to fully understand and evaluate our financial condition and results of operations.
We prepare our financial statements in accordance with IFRS as issued by the IASB.
In preparing these financial statements, management has made judgments, estimates and assumptions that affect the application of our accounting policies and the reported amounts recognized in the financial statements. On a periodic basis, we evaluate our estimates, including those related to share-based compensation and derivatives. We base our estimates on historical experience, authoritative pronouncements and various other assumptions that we believe to be reasonable under the circumstances. Actual results may differ from these estimates.
Recently-Issued Accounting Pronouncements
Certain recently-issued accounting pronouncements, if applicable, are discussed in Note 3 to our consolidated financial statements, regarding the impact of the IFRS standards as issued by the IASB that we will adopt in future periods in our consolidated financial statements.
Emerging Growth Company Status
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include:
| • | to the extent that we no longer qualify as a foreign private issuer, (i) reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and (ii) exemptions from the requirement to hold a non-binding advisory vote on executive compensation, including golden parachute compensation; |
| • | an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002; and |
| • | an exemption from compliance with the Critical Audit Matters requirement that the Public Company Accounting Oversight Board has adopted regarding a supplement to the auditor’s report providing additional information about the audit and the financial statements. |
We may take advantage of these exemptions for up to five years or until such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company upon the earliest to occur of: (i) the last day of the fiscal year in which we have total annual gross revenues of $1.235 billion or more; (ii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; (iii) the date on which we are deemed to be a large accelerated filer under the rules of the SEC; or (iv) the last day of the fiscal year following the fifth anniversary of our initial Nasdaq offering of March 2021. We may choose to take advantage of some but not all of these exemptions. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. This means that an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. Given that we currently report and expect to continue to report our financial results under IFRS as issued by the IASB, we will not be able to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required by the IASB.
B. Liquidity and Capital Resources
Since the commencement of our cultured meat operations, we have not generated any revenue and have incurred operating losses and negative cash flows from our operations. We have funded our operations primarily through the sale of equity securities. From the inception of Steakholder Innovation through December 31, 2022, we raised an aggregate of $53.0 million in four rounds of private placements of our securities, our initial public offering of securities on the Nasdaq and a registered direct offering, and $6.1 million in proceeds from option exercises. As of December 31, 2022, we had $6.3 million in cash and cash equivalents.
The table below shows a summary of our cash flows for the periods indicated:
| | | Year Ended December 31, | |
| | | 2022 | | | 2021 | |
| | | | | | | |
| Net cash used in operating activities | | $ | (14,253 | ) | | $ | (13,951 | ) |
| Net cash used in investing activities | | | (3,533 | ) | | | (9,191 | ) |
| Net cash provided by financing activities | | | 5,572 | | | | 28,865 | |
| Net increase (decrease) in cash and cash equivalents | | $ | (12,214 | ) | | $ | 5,723 | |
Year Ended December 31, 2022 Compared to Year Ended December 31, 2021
Net cash used in operating activities
Net cash used in operating activities increased by $0.3 million, or 2%, to approximately $14.3 million for the year ended December 31, 2022 compared to approximately $14.0 million for the year ended December 31, 2021. This increase was due to the increase in its research and development and marketing expenses growing activity with reduction in corporate costs.
Net cash used in investing activities
Net cash used in investing activities decreased by $5.7 million, or 62%, to approximately $3.5 million for the year ended December 31, 2022 compared to $9.2 million for the year ended December 31, 2021. This decrease was driven mainly by our investment in Peace of Meat in year ended December 31, 2021.
Net cash provided by financing activities
Net cash provided by financing activities decreased by $23.3 million, or 81%, to approximately $5.6 million for the year ended December 31, 2022 compared to $28.9 million for the year ended December 31, 2021. This decrease was driven mainly from the Company’s initial Nasdaq public offering and issuance of shares and warrants taking place in year ended December 31, 2021.
We have incurred losses and cash flow deficits from operations since the inception of Steakholder innovation, resulting in an accumulated deficit as of December 31, 2022 of approximately $67.7 million. We anticipate that we will continue to incur net losses for the foreseeable future. We believe that our existing cash and cash equivalents will be sufficient to fund our projected cash needs through the third quarter of 2023 (including January 2023 funding round). We do not currently have any specific commitments or plans for acquisitions; to the extent we do engage in acquisitions, we will do so after ensuring that we will have sufficient funds available to meet our capital requirements, and such acquisitions are likely to affect our projected cash needs. To meet future capital needs, we would need to raise additional capital through equity or debt financing or other strategic transactions. However, any such financing may not be on favorable terms or even available to us. Our failure to obtain sufficient funds on commercially acceptable terms when needed would have a material adverse effect on our business, results of operations and financial condition. Our forecast of the period of time through which our financial resources will be adequate to support our operations is a forward-looking statement that involves risks and uncertainties, and the actual amount of our expenses could vary materially and adversely as a result of a number of factors. We have based our estimates on assumptions that may prove to be wrong, and our expenses could prove to be significantly higher than we currently anticipate.
Our future capital requirements will depend on many factors, including, but not limited to:
| • | the progress and costs of our research and development activities; |
| • | the costs of development and expansion of our operational infrastructure; |
| • | the costs and timing of developing technologies sufficient to allow food production equipment manufacturers and food manufacturers to product products compliant with applicable regulations; |
| • | our ability, or that of our collaborators, to achieve development milestones and other events or developments under potential future licensing agreements; |
| • | the amount of revenues and contributions we receive under future licensing, collaboration, development and commercialization arrangements with respect to our technologies; |
| • | the costs of filing, prosecuting, enforcing and defending patent claims and other intellectual property rights; |
| • | the costs of contracting with third parties to provide sales and marketing capabilities for us or establishing such capabilities ourselves, once our technologies are developed and ready for commercialization; |
| • | the costs of acquiring or undertaking development and commercialization efforts for any future products or technology; |
| • | the magnitude of our general and administrative expenses; and |
| • | any additional costs that we may incur under future in- and out-licensing arrangements relating to our technologies and futures products. |
Until we can generate significant recurring revenues, we expect to satisfy our future cash needs through capital raising or by out-licensing and/or co-developing applications of one or more of our product candidates. We cannot be certain that additional funding will be available to us on acceptable terms, if at all. If funds are not available on favorable terms, or at all, we may be required to delay, reduce the scope of or eliminate research or development efforts or plans for commercialization with respect to our technologies and make necessary change to our operations to reduce the level of our expenditures in line with available resources.
We are a development-stage technology company and it is not possible for us to predict with any degree of accuracy the outcome of our research and development efforts. As such, it is not possible for us to predict with any degree of accuracy any significant trends, uncertainties, demands, commitments or events that are reasonably likely to have a material effect on our net loss, liquidity or capital resources, or that would cause financial information to not necessarily be indicative of future operating results or financial condition. However, to the extent possible, certain trends, uncertainties, demands, commitments and events are described herein.
Since inception, we have incurred significant losses and negative cash flows from operations and have an accumulated deficit of USD 67.7 million. We have financed our operations mainly through fundraising from various investors.
Our management expects that we will continue to generate losses and negative cash flows from operations for the foreseeable future. On January 9, 2023, we consummated a securities purchase agreement with gross proceeds of approximately USD 6.5 million. Consequently, our management is of the opinion that our existing cash will be sufficient to fund operations until the third quarter of 2023. As a result, there is substantial doubt about our ability to continue as a going concern.
Management’s plans include continuing to secure sufficient financing through the sale of additional equity securities or capital inflows from strategic partnerships. Additional funds may not be available when we need them on terms that are acceptable to us, or at all. If we are unsuccessful in securing sufficient financing, we may need to cease operations.
Our financial statements include no adjustments for measurement or presentation of assets and liabilities, which may be required should we fail to operate as a going concern.
Quantitative and Qualitative Disclosures About Market Risk
Liquidity risk is the risk that we will encounter difficulty in meeting the obligations associated with our financial liabilities that are settled in cash. Cash flow forecasting is performed in our operating entities and aggregated at a consolidated level. We monitor forecasts of our liquidity requirements to ensure we have sufficient cash to meet operational needs. We may be reliant on our ability to raise additional investment capital from the issuance of both debt and equity securities to fund our business operating plans and future obligations.
Credit risk is the risk of financial loss to us if a debtor or counterparty to a financial instrument fails to meet its contractual obligations, and arises mainly from our receivables.
As part of an agreement with Therapin from May 2020, we agreed to convert an NIS 7.25 million investment in Therapin made by Ophectra and assumed by us at the Merger, into an interest-free loan, to be repaid by the latter at a rate of NIS 0.48 million per annum for ten years (NIS 4.8 million in total) plus NIS 2.45 million to be paid upon an exit event, including a public offering, or repayment of 14.74% of any distributable surplus or dividend distributed by Therapin, up to the amount of the outstanding balance, as detailed in our separation agreement with Therapin. As part of the agreement, Therapin gave us an option to convert the cash payment to equity of Therapin. Therapin has not provided any guarantees in connection with its repayment of our loan.
We restrict exposure to credit risk in the course of our operations by investing only in bank deposits.
As we have not invested in securities riskier than short-term bank deposits, we do not believe that changes in equity prices pose a material risk to our holdings. However, decreases in the market price of our ordinary shares or ADSs could make it more difficult for us to raise additional funds in the future or require us to raise funds at terms unfavorable to us.
Foreign Currency Exchange Risk
Currency fluctuations could affect us primarily through increased or decreased foreign currency-denominated expenses. Currency fluctuations had a material effect on our results of operations during the year ended December 31, 2022, although not in the year ended December 31, 2021.
C. Research and development, patents and licenses, etc.
For a description of our research and development policies for the last three years, see “Item 4.—Information on the Company—Business Overview—Intellectual Property.”
D. Trend Information
Not applicable.
E. Critical Accounting Estimates
Critical accounting estimates are those estimates made in accordance with IFRS that involve a significant level of estimation uncertainty and have had or are reasonably likely to have a material impact on the financial condition or results of operations of the registrant. For further information, see Note 2E to our annual consolidated financial statements included in this Annual Report on Form 20-F.
| | ITEM 6. | DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES |
A. Directors and Senior Management
The following table sets forth the name, age and position of each of our executive officers and directors as of the date of this Annual Report on Form 20-F. Unless otherwise stated, the address of our executive officers and directors is Steakholder Foods Ltd., 5 David Fikes St., Rehovot 7638205, Israel.
| Name | | Age | | Position |
| | | | | |
| Executive Officers: | | | | |
| Arik Kaufman | | 42 | | Chief Executive Officer |
| Guy Hefer | | 41 | | Chief Financial Officer* |
| Dan Kozlovski | | 38 | | Chief Technologies Officer |
Non-Employee Directors:
| | | | |
| Yaron Kaiser | | 45 | | Chairman of the Board of Directors |
| David Gerbi(1)(2)(3) | | 43 | | Director |
| Eli Arad(1)(2)(3) | | 50 | | Director |
| Sari Singer(1)(2)(3) | | 43 | | Director |
| (1) | Member of the Audit Committee |
| (2) | Member of the Compensation Committee |
| (3) | Independent director as defined under Nasdaq Marketplace Rule 5605(a)(2) and SEC Rule 10A-3(b)(1). |
*As previously announced, effective April 5, 2023, Eitan Noah has been appointed as the Company’s Chief Financial Officer, replacing Mr. Hefer in this role. For more information, see Item 8 – Significant Changes.
Executive Officers
Arik Kaufman, Chief Executive Officer
Arik Kaufman has served as our Chief Executive Officer since January 2022. He has founded various Nasdaq- and TASE-traded foodtech companies, and currently serves as director of Wilk Technologies Ltd. He is also a founding partner of the BlueSoundWaves collective, led by Ashton Kutcher, Guy Oseary and Effie Epstein, which recently partnered with Steakholder Foods to assist in attempting to accelerate the Company’s growth. Mr. Kaufman holds extensive personal experience in the fields of food-tech and bio-tech law, and has led and managed numerous complex commercial negotiations, as part of local and international fundraising, M&A transactions and licensing agreements. He holds a B.A. degree in Law from Reichman University (formerly the Interdisciplinary Center Hertzliya).
Guy Hefer, Chief Financial Officer
Guy Hefer has served as our Chief Financial Officer since October 2020. Mr. Hefer will step down from his position as our Chief Financial Officer on April 5, 2023, at which time Mr. Eitan Noah, our current Vice President of Finance will assume such role. Mr. Hefer has over ten years of experience in investment banking and corporate finance roles. Between 2019 and 2020, he was the chief financial officer of Prytek Holdings Pte Ltd., a private holding group investing in technology companies globally. Prior to that, Mr. Hefer was an investment banker at Leumi Partners Ltd. between 2018 and 2019 and GCA Altium Israel Ltd. between 2017 and 2018 in Israel and at Barclays investment banking division between 2011 and 2016 in the UK and in Israel. Prior to that Guy worked at Fahn Kanne Grant Thornton Israel, an accounting firm in Israel between 2009 and 2011. Mr. Hefer holds a B.A. degree in Accounting and Economics from the Tel Aviv University, Israel.
Dan Kozlovski, Chief Technologies Officer
Dan Kozlovski has served as our Chief Technologies Officer since February 2022, having previously served as our Vice President of Research & Development from August 2020 after joining us in December 2019. He specializes in R&D and product development, with expertise in three-dimensional computer-aided design. Mr. Kozlovski has more than ten years of experience working in high-technology companies in the printing market. Previously, he served as Future Platform R&D Mechanical Engineer at HP Indigo Division from June 2018 to December 2019. Mr. Kozlovski has also worked as Mechanical Team Leader at Nano Dimension Ltd. from August 2015 to June 2018. Mr. Kozlovski holds a B.Sc. degree in Mechanical Engineering from Ben Gurion University of the Negev and an Executive MBA in Technology, Innovation & Entrepreneurship Management from Tel Aviv University.
Directors
Yaron Kaiser, Chairman of the Board of Directors
Yaron Kaiser has founded various Nasdaq- or TASE-traded foodtech companies, and has served as Chairperson of Wilk Technologies Ltd. since January 2021. Mr. Kaiser is a founding partner of the BlueSoundWaves collective since 2021, and practices law in the fields of securities, commercial and corporate law, representing numerous public companies on fundraising, IPOs, M&A, the Israel Securities Authority and corporate governance, most recently at JST & Co., Law Office, between 2010 and May 2021, and since then as a founding partner of Kaufman Kaiser Raz, Law Firm. He holds an LL.B. degree from the College of Management Academic Studies, Israel.
Eli Arad, Director
Eli Arad has served as a director since February 2018. Mr. Arad has been chief executive officer of the real-estate and life science investment company Merchavia Holdings and Investments Ltd (TASE:MRHL) since 2011. Mr. Arad has served as a director of Cleveland Diagnostics, Inc., a clinical-stage biotechnology company developing technology to improve cancer diagnostics since 2016, E.N. Shoham Business Ltd. (TASE:SHOM) since 2019, and a number of privately-held companies (Veoli Ltd., Train Pain Ltd., EFA Ltd., Nervio Ltd. and Cardiosert Ltd.). He has had leadership roles in many biomedical startup companies, and has extensive experience in all areas of financial management. Mr. Arad is a certified practicing accountant who holds a diploma in Accounting from Ramat Gan College and an Executive B.A. (Hons.) in Business Administration from the Ruppin Academic Center.
David Gerbi, Director
David Gerbi has served as a director since August 2019. Mr. Gerbi is managing partner of accounting firm Gerbi & Co., and serves as Chief Financial Officer of Israir Group Ltd. (TASE:ISRG) since 2017, Erech Finance Cahalacha Ltd. (TASE:EFNC) since 2019, Nur Ink Innovations Ltd. (TASE:NURI) since June 2021 and Bee-io Honey Ltd. (TASE:BHNY) since November 2021. Mr. Gerbi holds a B.A. in Business Administration and Accounting from the Israeli College of Management Academic Studies and an M.B.A. in Finance from Tel Aviv University.
Sari Singer, Director
Sari Singer has served as a director since March 2021. Ms. Singer has served as General Counsel and Executive Vice President at NewMed Energy LP (formerly Delek Drilling LP), the oil and gas arm of the Delek Group in Israel, and a partner in the Leviathan offshore gas field, as well as other petroleum assets offshore Israel and Cyprus, since 2012, where she has led significant strategic processes, including restructurings and complex financing rounds totaling some $7 billion in various transactions in the international and domestic markets. Ms. Singer holds an LL.B. (cum laude) from Tel Aviv University and has been a member of the Israel Bar since 2007.
Family Relationships
There are no family relationships among any of our directors or officers.
B. Compensation
Aggregate Compensation of Office Holders
The aggregate compensation we paid to our executive officers and directors for the year ended December 31, 2022, was approximately $1.3 million. This amount includes approximately $0.2 million paid, set aside or accrued to provide pension, severance, retirement or similar benefits or expenses, but does not include share-based compensation expenses, or business travel, professional and business association dues and expenses reimbursed to office holders, and other benefits commonly reimbursed or paid by companies in our industry. As of the date of this prospectus, options to purchase 2,472,540 Ordinary Shares granted to our officers and directors were outstanding under our share option plans at a weighted average exercise price of $0.62 per share, in addition to 157,790 restricted share units with no exercise price.
Individual Compensation of Office Holders
The table and summary below outlines the compensation granted to our Chief Executive Officer, Chief Financial Officer, Chief Technologies Officer, and the previous and current Chairmen of our board of directors, with respect to the year ended December 31, 2022. For purposes of the table and the summary below, “compensation” includes base salary, bonuses, equity-based compensation, retirement or termination payments, benefits and perquisites such as car, phone and social benefits and any undertaking to provide such compensation.
| Name and Principal Position | | Salary(1) | | | Bonus(2) | | | Equity-Based
Compensation(3) | | | Other
Compensation(4) | | | Total | |
| | | (USD in thousands) | |
| Mr. Arik Kaufman | | | | | | | | | | | | | | | |
| Chief Executive Officer | | $ | 235 | | | $ | - | | | $ | 88 | | | $
| 8 | | | $ | 331 | |
Mr. Guy Hefer(5) | | | | | | | | | | | | | | | | | | | | |
| Chief Financial Officer | | | 210 | | | | 34 | | | | 83 | | | | - | | | | 327 | |
| Mr. Dan Kozlovski | | | | | | | | | | | | | | | | | | | | |
| Chief Technologies Officer | | | 201 | | | | 42 | | | | 51 | | | | - | | | | 294 | |
| Mr. Yaron Kaiser | | | | | | | | | | | | | | | | | | | | |
| Chairman of the Board of Directors | | | 161 | | | | - | | | | 62 | | | | 8 | | | | 231 | |
Mr. Steven H. Levin (6) | | | | | | | | | | | | | | | | | | | | |
Former Chairman of the Board of Directors(5) | | $ | 57 | | | $ | - | | | $ | 108 | | | $
| - | | | $ | 165 | |
| (1) | Salary includes the officer’s gross salary plus payment by us of social benefits on behalf of the officer. Such benefits may include payments, contributions and/or allocations for savings funds (e.g., Managers’ Life Insurance Policy), pension, severance, risk insurance (e.g., life, or work disability insurance), payments for social security and tax gross-up payments, vacation, medical insurance and benefits, convalescence or recreation pay and other benefits and perquisites consistent with our policies. |
| (2) | Represents annual bonuses paid in 2022 with respect to 2021. |
| (3) | Represents the equity-based compensation expenses, based on the options’ fair value on the grant date, calculated in accordance with applicable accounting guidance for equity-based compensation. For a discussion of the assumptions used in reaching this valuation, see Note 10(B) to our annual consolidated financial statements included elsewhere in this prospectus. |
| (4) | Represents consulting services provided prior to commencement of the aforementioned current position. |
| (5) | Mr. Hefer will step down from his position as Chief Financial Officer on March 23, 2023, at which time Mr. Eitan Noah, our current Vice President of Finance, will assume the role of Chief Financial Officer. |
| (6) | Mr. Levin resigned his position as Chairman on January 24, 2022. |
Employment Agreements and Director Fees
We have entered into written employment agreements with each of our executive officers, which provide for notice periods of varying duration for termination of the agreement by us or by the relevant executive officer, during which time the executive officer will continue to receive base salary and benefits. These agreements also contain customary provisions regarding noncompetition, confidentiality of information and assignment of inventions. However, the enforceability of the noncompetition provisions may be limited under applicable law. See “Risk Factors — Risks relating to our operations — Under applicable employment laws, we may not be able to enforce covenants not to compete” for a further description of the enforceability of non-competition clauses.
The material employment terms for Mr. Kaufman, our Chief Executive Officer, are as follows: (1) a gross annual salary of NIS 564,000 ($160,000); (2) reimbursement of annual travel expenses of up to NIS 60,000 ($17,000); (3) options to purchase 500,000 Ordinary Shares (currently equivalent to 50,000 ADSs), vesting over three years from the date of his appointment as Chief Executive Officer, pursuant to which 1/12 will vest every quarter until fully vested, expiring one year following Mr. Kaufman’s cessation of service in all then-applicable capacities, but in any case after four years, with an exercise price of $0.519 per ordinary share (currently equivalent to $5.19 per ADS) and subject to acceleration upon termination pursuant to our sale or change in control; (4) an annual performance bonus in the aggregate amount of NIS 282,000 ($80,000), subject to his meeting certain performance milestones as determined by our board of directors on an annual basis; (5) termination of the employment relationship upon provision of six months’ advance notice by either party; (6) severance pay equal to 25% of the gross annual salary upon termination of Mr. Kaufman’s employment by us, not for cause, following three to twelve months of service, or 50% following twelve or more months of service (or 50% of these amounts upon Mr. Kaufman’s resignation); and (7) social benefits that we pay on behalf of officers, such as payments, contributions and/or allocations for savings funds (e.g., Managers’ Life Insurance Policy), pension, severance, risk insurance (e.g., life, or work disability insurance), payments for social security and tax gross-up payments, vacation, medical insurance and benefits, convalescence or recreation pay and other benefits and perquisites consistent with our policies, such as inclusion in our directors’ and officers’ liability insurance policy, and provision of indemnification, exculpation and exemption undertakings to the fullest extent permitted by the Companies Law.
The material terms for Mr. Kaiser, the Chairman of our board of directors, were as follows from his appointment in January 2022, until January 24, 2023: (1) an annual fee of $150,000, to be paid in four equal quarterly installments in USD or in NIS at the then-current exchange rate, which will automatically increase by an amount equal to seven percent at the end of each year of service; (2) reimbursement of annual travel expenses of up to $18,000; (3) options to purchase 350,000 Ordinary Shares (currently equivalent to 35,000 ADSs), vesting over three years from the date of his appointment as Chairman, pursuant to which 1/12 will vest every quarter until fully vested, expiring one year following Mr. Kaiser’s cessation of service in all then-applicable capacities, but in any case after four years, with an exercise price of $0.519 per ordinary share (currently equivalent to $5.19 per ADS) and subject to acceleration upon termination pursuant to our sale or change in control; (4) an annual bonus equal to 50% of the bonus awarded to the Chief Executive Officer in the applicable year; (5) severance pay equal to 12.5% of Mr. Kaiser’s annual fee upon the involuntary termination of his directorship, not for cause, following three to twelve months of service, or 25% following twelve or more months of service (or 50% of these amounts upon Mr. Kaiser’s resignation); and (6) other benefits and perquisites consistent with our policies, such as inclusion in our directors’ and officers’ liability insurance policy, and provision of indemnification, exculpation and exemption undertakings to the fullest extent permitted by the Companies Law.
Effective January 24, 2023, pursuant to approvals by our Compensation Committee, Board of Directors and a general meeting of our shareholders, the following amendments to the materials terms for Mr. Kaiser were adopted: his annual fee was adjusted to $290,000, automatically increased by an amount equal to seven percent at the end of each year of service commencing from the date of adjustment; his annual bonus shall be equal to 70% of the bonus awarded to the Chief Executive Officer in the applicable year; he received an allocation of restricted share units (RSUs) vesting into 1,340,000 ordinary shares (currently equivalent to 134,000 ADSs), vesting over three years from the date of adjustment, pursuant to which 1/12 will vest every quarter until fully vested, with no exercise price, issued under the Company’s 2022 Share Incentive Plan and the Capital Gains tax track pursuant to Section 102 of the Israeli Income Tax Ordinance (New Version), 1961 and subject to acceleration upon termination pursuant to either our sale or change in control; and he received an allocation of performance share units (PSUs) vesting into 1,340,000 ordinary shares (currently equivalent to 134,000 ADSs), vesting upon achievement of any one of the following milestones: (i) engagement with a strategic partner / investor (a corporation operating in the field of food, healthcare, pharmaceuticals or printing) for an investment in the company or its subsidiaries, in cash in an amount of not less than five hundred thousand dollars; (ii) submission of a regulatory approval to the U.S. FDA, Singapore Food Agency or European Food Safety Authority, for the commercial sale or distribution of our products; or (iii) engagement with a strategic partner (as defined above) in a joint development agreement to collaborate to develop technology or products for the purpose of later commercialization.
In addition, we pay fees to our non-executive directors in return for their service on our board of directors, in accordance with our compensation policy.
Our other employees are employed under the terms prescribed in their respective employment contracts. The employees are entitled to the social benefits prescribed by law and as otherwise provided in their agreements. These agreements each contain provisions standard for a company in our industry regarding non-competition, confidentiality of information and assignment of inventions. Under currently applicable labor laws, we may not be able to enforce covenants not to compete and therefore may be unable to prevent our competitors from benefiting from the expertise of some of our former employees. See “Risk Factors—Risks Related to Our Operations” for a further description of the enforceability of non-competition clauses.
Executive officers are also employed on the terms and conditions prescribed in employment agreements. These agreements provide for notice periods of varying duration for termination of the agreement by us or by the relevant executive officer, during which time the executive officer will continue to receive base salary and benefits. See “Risk Factors—Risks Related to Our Operations—If we are unable to attract and retain qualified employees, our ability to implement our business plan may be adversely affected.”
2018 Option and RSU Allocation Plan
In June 2018, the board of directors of Ophectra adopted our Option and RSU Allocation Plan, as amended, or the share option plan, to issue options to purchase our Ordinary Shares and restricted stock units to our directors, officers, employees and consultants, and those of our affiliated companies (as such term is defined under share option plan), or the Grantees. The share option plan is administered by our board of directors or a committee that was designated by the board of directors for such purpose, or the Administrator.
Under the share option plan, we may grant options to purchase Ordinary Shares and/or RSUs, or options, under four tracks: (i) Approved 102 capital gains options through a trustee, which was approved by the Israeli Tax Authority in accordance with Section 102(a) of the Israeli Income Tax Ordinance (New Version), 1961, or ITO, and granted under the tax track set forth in Section 102(b)(2) of the ITO. The holding period under this tax track is 24 months from the date of issuance of options to the trustee or such period as may be determined in any amendment of Section 102 of the ITO, or any applicable tax ruling or guidelines; (ii) Approved 102 earned income options through a trustee, granted under the tax track set forth is Section 102(b)(1) of the ITO. The holding period under this tax track is 12 months from the date of issuance of options to the trustee or such period as may be determined in any amendment of Section 102 of the ITO; (iii) Unapproved 102 options (the options will not be issued through a trustee and will not be subject to a holding period); and (iv) 3(i) options (the options will not be subject to a holding period). These options shall be subject to taxation pursuant to Section 3(i) of the ITO, or Section 3(i).
Options pursuant to the first three tax tracks (under Section 102 of the ITO) can be granted to our employees and directors and the grant of options under Section 3(i) can be granted to our consultants and controlling shareholders (a controlling shareholder is defined under the Section 102 of the ITO is a person who holds, directly or indirectly, alone or together with a “relative,” (i) the right to at least 10% of the company’s issued capital or 10% of the voting power; (ii) the right to hold at least 10% of the company’s issued capital or 10% of the voting power, or the right to purchase such rights; (iii) the right to receive at least 10% of the company’s profits; or (iv) the right to appoint a company’s director). Grantees who are not Israeli residents may be granted options that are subject to the applicable tax laws in their respective jurisdictions.
We determine, in our sole discretion, under which of the first three tax tracks above the options are granted and we notify the Grantee in a grant letter, as to the elected tax track. As mentioned above, consultants and controlling shareholders can only be granted Section 3(i) options.
The number of Ordinary Shares authorized to be issued under the share option plan will be proportionately adjusted for any increase or decrease in the number of Ordinary Shares issued as a result of a distribution of bonus shares, change in our capitalization (split, combination, reclassification of the shares or other capital change), or issuance of rights to purchase Ordinary Shares or payment of a dividend. We will not issue fractions of Ordinary Shares and the number of Ordinary Shares shall be rounded up to the closest number of ordinary shares.
In the event of a (i) merger or consolidation in which we (in this context, specifically Steakholder Foods Ltd.) are not the surviving entity or pursuant to which the other company becomes our parent company or that pursuant to which we are the surviving company but another entity holds 50% or more of our voting rights, (ii) an acquisition of all or substantially all of our Ordinary Shares, (iii) the sale of all or substantially all of our assets, or (iv) any other event with a similar impact, we may exchange all of our outstanding options granted under the share option plan that remain unexercised prior to any such transaction for options to purchase shares of the successor corporation (or those of an affiliated company) following the consummation of such transaction.
The exercise price of an option granted under the share option plan will be specified in the grant letter every Grantee received from us in which the Grantee notifies of the decision to grant him/her options under the share option plan, and will be denominated in our functional currency at the time of grant or the currency in which the Grantee is paid, at our discretion.
The Administrator may, in its absolute discretion, accelerate the time at which options granted under the share option plan or any portion of which will vest.
Unless otherwise determined by the Administrator, in the event that the Grantee’s employment was terminated, not for Cause (as defined in the share option plan), the Grantee may exercise that portion of the options that had vested as of the date of such termination until the end of the specified term in the grant letter or the share option plan. The portion of the options that had not vested at such date, will be forfeited and can be re-granted to other Grantees, in accordance with the terms of the share option plan.
At the discretion of our board of directors, and subject to receipt of taxation authority approvals, we may allow Grantees to exercise their options on a cashless basis.
2022 Share Incentive Plan
We adopted the 2022 Share Incentive Plan, or the 2022 Plan, on June 10, 2022, and a general meeting of our shareholders approved the 2022 Plan on March 30, 2023. The 2022 Plan provides for the grant of equity-based incentive awards to our employees, directors, office holders, service providers and consultants in order to incentivize them to increase their efforts on behalf of the Company and to promote the success of the Company’s business.
Shares Available for Grants. The maximum number Shares (which means ordinary shares, of no par value, (including ordinary shares resulting or issued as a result of share split, reverse share split, bonus shares, combination or other recapitalization events, and including in the form of ADSs), or shares of such other class of shares as shall be designated by the board of directors of the Company in respect of the relevant award) available for issuance under the 2022 Plan is equal to the sum of (i) 8,500,000 Shares, (ii) 1,127,850 Shares, which represents the number of Shares available for issuance under the Option and RSU Allocation Plan, or the Prior Plan, on the effective date of the 2022 Plan, and (iii) an annual increase on the first day of each year beginning in 2023 and on January 1st of each calendar year thereafter and ending on January 1, 2032, equal to the lesser of (A) 5% of the outstanding ordinary shares of the Company on the last day of the immediately preceding calendar year, on a fully diluted basis; and (B) such amount as determined by our board of directors if so determined prior to January 1 of a calendar year. Shares issued under the 2022 Plan may be, in whole or in part, authorized but unissued Shares, (and, subject to obtaining a ruling as it applies to 102 awards) treasury shares (dormant shares) or otherwise Shares that shall have been or may be repurchased by the Company (to the extent permitted pursuant to the Companies Law).
Any Shares (a) underlying an award granted under the 2022 Plan or an award granted under the Prior Plan (in an amount not to exceed 8,498,490 Shares under the Prior Plan) that has expired, or was cancelled, terminated, forfeited, or settled in cash in lieu of issuance of Shares, for any reason, without resulting in the issuance of Shares; (b) if permitted by the Company, subject to an award that are tendered to pay the exercise price of an award; or withholding tax obligations with respect to an award; or if permitted by the Company, subject to an award that are not delivered to a Grantee because such Shares are withheld to pay the exercise price of such award; or withholding tax obligations with respect to such award may again be available for issuance under the 2022 Plan and for issuance upon exercise or (if applicable) vesting thereof for the purposes of the 2022 Plan, unless determined otherwise by the Board. Our board of directors may also reduce the number of ordinary shares reserved and available for issuance under the 2022 Plan in its discretion.
The maximum aggregate number of Shares that may be issued pursuant to the exercise of incentive stock options granted under the 2022 Plan, or the ISO Limit, shall be the sum of (a) the aggregate number of Shares set forth in clauses (a) and (b) in the above paragraph; and (b) any Shares underlying awards granted under the Prior Plan that are returned to the 2022 Plan (not to exceed 8,498,490 Shares). To the extent permitted under Section 422 of the United States Internal Revenue Code of 1986, and any applicable regulations promulgated thereunder, all as amended (the “Code”), any Shares covered by an award that has expired, or was cancelled, terminated, forfeited, or settled in cash without the issuance of Shares shall not count against the ISO Limit. Shares that actually have been issued under the 2022 Plan shall not become available for future issuance hereunder pursuant to incentive stock options.
Administration. Our board of directors, or a duly authorized committee of our board of directors, or the Administrator, or the Administrator, will administer the 2022 Plan. Under the 2022 Plan, the Administrator has the authority, subject to applicable law, to interpret the terms of the 2022 Plan and any award agreements or awards granted thereunder, designate recipients of awards, determine and amend the terms of awards, including the exercise price of an option award, the fair market value of an ordinary share, the time and vesting schedule applicable to an award or the method of payment for an award, accelerate or amend the vesting schedule applicable to an award, prescribe the forms of agreement for use under the 2022 Plan and take all other actions and make all other determinations necessary for the administration of the 2022 Plan.
The Administrator also has the authority to approve the conversion, substitution, cancellation or suspension under and in accordance with the 2022 Plan of any or all option awards or ordinary shares, and the authority to modify option awards to eligible individuals who are foreign nationals or are individuals who are employed outside Israel to recognize differences in local law, tax policy or custom, in order to effectuate the purposes of the 2022 Plan but without amending the 2022 Plan; provided, that if the Administrator takes such action with respect to an award held by a U.S. service provider, it shall do so in accordance with the requirements of Section 409A of the Code, if applicable.
The Administrator also has the authority to amend and rescind rules and regulations relating to the 2022 Plan or terminate the 2022 Plan at any time before the date of expiration of its ten year term.
Eligibility. The 2022 Plan provides for granting awards under various tax regimes, including, without limitation, in compliance with Section 102 of the Israeli Income Tax Ordinance (New Version) 5271-1961, and the regulations and rules promulgated thereunder, all as amended from time to time (the “Ordinance”), and Section 3(i) of the Ordinance and in compliance with Section 422 of the Code and Section 409A of the Code as they relate to U.S. service providers when granted Nonqualified Stock Options, and to U.S. service providers who are Employees when granted Incentive Stock Options.
Grants. All awards granted pursuant to the 2022 Plan will be evidenced by an award agreement, in a form approved, from time to time, by the Administrator in its sole discretion. The award agreement will set forth the terms and conditions of the award, including the type of award, number of shares subject to such award, vesting schedule and conditions (including performance goals or measures) and the exercise price, if applicable. Certain awards under the 2022 Plan may constitute or provide for a deferral of compensation, subject to Section 409A of the Code, which may impose additional requirements on the terms and conditions of such awards.
Unless otherwise determined by the Administrator and stated in the award agreement, and subject to the conditions of the 2022 Plan, awards vest and become exercisable under the following schedule: 25% of the shares covered by the award on the first anniversary of the vesting commencement date determined by the Administrator (and in the absence of such determination, the date on which such award was granted) and 6.25% of the Shares covered by the award at the end of each subsequent three-month period thereafter over the course of the following three years; provided that the grantee remains continuously as an employee or provides services to the company throughout such vesting dates.
Each award will expire ten years from the date of the grant thereof, unless such shorter term of expiration is otherwise designated by the Administrator.
Awards. The 2022 Plan provides for the grant of stock options (including incentive stock options and nonqualified stock options), ordinary shares, restricted shares, RSUs, stock appreciation rights and other share-based awards.
Options granted under the 2022 Plan to the Company employees who are U.S. residents may qualify as “incentive stock options” within the meaning of Section 422 of the Code, or may be non-qualified stock options. The exercise price of an option may not be less than the par value of the Shares (if the Shares bear a par value) for which such option is exercisable. The exercise price of an Incentive Stock Option may not be less than 100% of the fair market value of the underlying share on the date immediately preceding the day of the grant or such other amount as may be required pursuant to the Code, and in the case of Incentive Stock Options granted to ten percent stockholders, not less than 110%.
Nonqualified stock options may not be granted to a U.S. service provider unless (i) the Shares underlying such options constitute “service recipient stock” under Section 409A of the Code and such options meet the other requirements to be exempt from Section 409A of the Code or (ii) such options comply with the requirements of Section 409A of the Code. A nonqualified stock option may be granted with an exercise price lower than the minimum exercise price set forth above if (i) such option is granted pursuant to an assumption or substitution for another option in accordance with and pursuant to Section 409A of the Code or (ii) the Administrator expressly determined that the option will have a lower exercise price and the Option complies with Section 409A of the Code or meets another exemption under Section 409A of the Code.
Incentive stock options may be granted only to U.S. service providers who are employees of the Company. However, if for any reason an option (or portion thereof) does not qualify as an incentive stock option, then, to the extent of such non-qualification, such option (or portion thereof) shall be treated as a nonqualified stock option granted under the 2022 Plan.
An RSU may be awarded to any service provider, including under Section 102 of the Ordinance. Subject to Applicable Law, RSUs may be granted in consideration of a reduction in the recipient’s other compensation. No payment of exercise price shall be required as consideration for RSUs, unless included in the award agreement or as required by applicable law. The grantee shall not possess or own any ownership rights in the Shares underlying the RSUs. Settlement of vested RSUs shall be made in the form of Shares. Distribution to a grantee of an amount (or amounts) from settlement of vested RSUs can be deferred to a date after vesting as determined by the Administrator; provided, that no such deferral shall be made with respect to RSUs held by a U.S. service provider if such deferral would cause such RSUs to fail to qualify for an exemption under Section 409A of the Code and become subject to the requirements of Section 409A of the Code, unless expressly determined by the Administrator, or would violate the requirements of Section 409A. In no event shall any dividends or dividend equivalent rights be paid before the vesting of the portion of the RSUs to which such dividends or dividend equivalent rights relate, unless otherwise provided for in an award agreement or determined by the Committee. Any RSUs granted under the 2022 Plan that are not exempt from the requirements of Section 409A of the Code shall contain such restrictions or other provisions so that such RSUs will comply with the requirements of Section 409A of the Code.
Exercise. An award under the 2022 Plan may be exercised by providing the Company with a written or electronic notice of exercise and full payment of the exercise price for such shares underlying the award, if applicable, in such form and method as may be determined by the Administrator and permitted by applicable law. An award may not be exercised for a fraction of a share. With regard to tax withholding, exercise price and purchase price obligations arising in connection with awards under the 2022 Plan, the Administrator may, in its discretion, accept cash, provide for net withholding of shares in a cashless exercise mechanism or direct a securities broker to sell shares and deliver all or a part of the proceeds to the Company or the trustee. The exercise period of an award will be determined by the Administrator and stated in the award agreement, but will in no event be longer than ten (10) years from the date of grant of the award. Notwithstanding anything to the contrary, the Administrator may extend the periods for which awards held by any grantee may continue to vest and/or be exercisable; it being clarified that such awards may lose their entitlement to certain tax benefits under applicable law; if done so with respect to a U.S service provider, the Administrator shall act in accordance with Section 409A of the Code, as applicable.
Transferability. Other than by will, the laws of descent and distribution or as otherwise provided under the 2022 Plan, neither the options nor any right in connection with such options are assignable or transferable.
Termination of Employment. In the event of termination of a grantee’s employment or service with the Company or any of its affiliates, all vested and exercisable awards held by such grantee as of the date of termination may be exercised within three months after such date of termination, unless otherwise determined by the Administrator, but in no event later than the date of expiration of the award as set forth in the award agreement. After such three-month period, all such unexercised awards will terminate and the shares covered by such awards shall again be available for issuance under the 2022 Plan.
In the event of termination of a grantee’s employment or service with the Company or any of its affiliates due to such grantee’s death or permanent disability, or in the event of the grantee’s death within the three month period (or such longer period as determined by the Administrator) following his or her termination of service, all vested and exercisable awards held by such grantee as of the date of termination may be exercised by the grantee or the grantee’s legal guardian, estate or by a person who acquired the right to exercise the award by bequest or inheritance, as applicable, within one year after such date of termination, unless otherwise provided by the Administrator, but in no event later than the date of expiration of the award as set forth in the award agreement. Any awards which are unvested as of the date of such termination or which are vested but not then exercised within the one year period following such date, will terminate and the shares covered by such awards shall again be available for issuance under the 2022 Plan.
Notwithstanding any of the foregoing, if a grantee’s employment or services with the Company or any of its affiliates is terminated for “cause” (as defined in the 2022 Plan), all outstanding awards held by such grantee (whether vested or unvested) will terminate on the date of such termination and the shares covered by such awards shall again be available for issuance under the 2022 Plan.
Any Option that is intended to be an incentive stock option and is exercised later than three (3) months after the grantee ceases to be employed by the Company (or any parent or subsidiary), except in the case of death or “Disability” (as defined in Section 22(e)(3) of the Code), will be deemed a nonqualified stock option. If the grantee ceases to be employed by the Company (or any parent or subsidiary) due to disability, any option that is intended to be an incentive stock option and is exercised later than twelve (12) months after such termination date will be deemed a nonqualified stock option.
Voting Rights. Except with respect to restricted share awards, grantees will not have the rights as a shareholder of the Company with respect to any shares covered by an award until the award has vested and/or the grantee has exercised such award, paid any exercise price for such award and becomes the record holder of the shares. With respect to restricted share awards, grantees will possess all incidents of ownership of the restricted shares, including the right to vote and receive dividends on such shares.
Dividends. Grantees holding restricted share awards will be entitled to receive dividends and other distributions with respect to the shares underlying the restricted share award. Any stock split, stock dividend, combination of shares or similar transaction will be subject to the restrictions of the original restricted share award. Grantees holding RSUs will not be eligible to receive dividend but may be eligible to receive dividend equivalents.
Transactions. In the event of a share split, reverse share split, share dividend, recapitalization, combination or reclassification of the Company’s shares, the Administrator in its sole discretion may, and where required by applicable law shall, without the need for a consent of any holder, make an appropriate adjustment in order to adjust (i) the number and class of shares reserved and available for the outstanding awards, (ii) the number and class of shares covered by outstanding awards, (iii) the exercise price per share covered by any award, (iv) the terms and conditions concerning vesting and exercisability and the term and duration of the outstanding awards, (v) the type or class of security, asset or right underlying the award (which need not be only that of the Company, and may be that of the surviving corporation or any affiliate thereof or such other entity party to any of the above transactions), and (vi) any other terms of the award that in the opinion of the Administrator should be adjusted; provided that any fractional shares resulting from such adjustment shall be rounded to the nearest whole share unless otherwise determined by the Administrator. In the event of a distribution of a cash dividend to all shareholders, the Administrator may determine, without the consent of any holder of an award, that the exercise price of an outstanding and unexercised award shall be reduced by an amount equal to the per share gross dividend amount distributed by the Company, subject to applicable law.
In the event of a merger or consolidation of the Company or a sale of all, or substantially all, of the Company’s shares or assets or other transaction having a similar effect on the Company, or change in the composition of the board of directors, or liquidation or dissolution, or such other transaction or circumstances that our board of directors determines to be a relevant transaction, then without the consent of the grantee, (i) unless otherwise determined by the Administrator, any outstanding award will be assumed or substituted by such successor corporation, or (ii) regardless of whether or not the successor corporation assumes or substitutes the award (a) provide the grantee with the option to exercise the award as to all or part of the shares, and may provide for an acceleration of vesting of unvested awards, (b) cancel the award and pay in cash, shares of the Company, the acquirer or other corporation which is a party to such transaction or other property as determined by the Administrator as fair in the circumstances, or (c) provide that the terms of any award shall be otherwise amended, modified or terminated, as determined by the Administrator to be fair in the circumstances. Changes with respect to awards held by U.S. service providers shall be made in accordance with the requirements of Section 409A of the Code or Section 424 of the Code, as applicable and to the extent necessary to avoid adverse tax consequences under Section 409A of the Code, a transaction or other event will not be deemed a Merger/Sale for purposes of awards granted to U.S. service providers unless the transaction or other event qualifies as a change in control event within the meaning of Section 409A of the Code.
C. Board Practices
Board of Directors
Our board of directors consists of four directors, three of whom are deemed independent directors under the corporate governance standards of the Nasdaq Marketplace Rules and the independence requirements of Rule 10A-3 of the Exchange Act, as well as the standards of the Companies Law.
Under our articles of association, our board of directors must consist of no less than three and no more than seven directors (including the external directors, if any), divided into three classes with staggered three-year terms. Each class of directors consists, as nearly as possible, of one-third of the total number of directors constituting the entire board of directors. At each annual general meeting of our shareholders, the election or re-election of directors following the expiration of the term of office of the directors of that class of directors will be for a term of office that expires on the third annual general meeting following such election or re-election.
Our directors are divided among the three classes as follows:
| • | the Class I directors are Messrs. Eli Arad and David Gerbi and their respective terms will expire at the Company’s annual general meeting of shareholders to be held in 2026; |
| • | the Class II director is Ms. Sari Singer and her term will expire at the Company’s annual general meeting of shareholders to be held in 2024; and |
| • | the Class III director is Mr. Yaron Kaiser his term will expire at the Company’s annual general meeting of shareholders to be held in 2025. |
Pursuant to our articles of association, the vote general required to appoint a director is a simple majority vote of holders of our voting shares participating and voting at the relevant meeting, provided that (i) in the event of a contested election, the method of calculation of the votes and the manner in which the resolutions will be presented to our shareholders at the general meeting shall be determined by our board of directors in its discretion, and (ii) in the event that our board of directors does not or is unable to make a determination on such matter, then the directors will be elected by a plurality of the voting power represented at the general meeting in person or by proxy and voting on the election of directors.
Each director will hold office until the annual general meeting of our shareholders for the year in which such director’s term expires, unless the tenure of such director expires earlier pursuant to the Companies Law or unless such director is removed from office. Under our articles of association, the approval of the holders of at least 65% of the total voting power of our shareholders is generally required to remove any of our directors from office.
In addition, our articles of association allow our board of directors to appoint new directors to fill vacancies which can occur for any reason or as additional directors, provided that the number of board members shall not exceed the maximum number of directors mentioned above. A director so appointed will hold office until the next annual general meeting of our shareholders for the election of the class of directors in respect of which the vacancy was created, or in the case of a vacancy due to the number of directors being less than the maximum number of directors stated in our articles of association, until the next annual general meeting of our shareholders for the election of the class of directors to which such director was assigned by our board of directors. Our board of directors may continue to operate for as long as the number of directors is no less than the minimum number of directors mentioned above.
In addition, under the Companies Law, our board of directors must determine the minimum number of directors who are required to have financial and accounting expertise. Under applicable regulations, a director with financial and accounting expertise is a director who, by reason of his or her education, professional experience and skill, has a high level of proficiency in and understanding of business accounting matters and financial statements. He or she must be able to thoroughly comprehend the financial statements of the company and initiate discussion regarding the manner in which financial information is presented. In determining the number of directors required to have such expertise, the board of directors must consider, among other things, the type and size of the company and the scope and complexity of its operations. Our board of directors has determined that we require at least one director with the requisite financial and accounting expertise and that Eli Arad and David Gerbi have such expertise.
External Directors
The Companies Law requires a public Israeli company to have at least two external directors who meet certain independence criteria to ensure that they are unaffiliated with the company and its controlling shareholder. An external director must have either financial and accounting expertise or professional qualifications, as defined in the regulations promulgated under the Companies Law, and at least one of the external directors is required to have financial and accounting expertise. An external director is entitled to reimbursement of expenses and compensation as provided in the regulations promulgated under the Companies Law, but is otherwise prohibited from receiving any other compensation from the company, directly or indirectly, during his or her term and for two years thereafter.
Pursuant to regulations promulgated under the Companies Law, as a company with shares traded on Nasdaq, we have elected no to comply with the requirements to appoint external directors and related rules concerning the composition of the audit committee and compensation committee of the board of directors. We are still subject to the gender diversity rule under the Companies Law, which requires that if, at the time a director is to be elected or appointed, all members of the board of directors are of the same gender, the director to be appointed must be of the other gender. The conditions to the exemptions from the Companies Law requirements are that: (i) the company does not have a “controlling shareholder,” as such term is defined under the Companies Law, (ii) its shares are traded on certain U.S. stock exchanges, including Nasdaq, and (iii) it comply with the director independence requirements and the audit committee and compensation committee composition requirements under U.S. laws, including the rules of the applicable exchange, that are applicable to U.S. domestic issuers.
Committees of the Board of Directors
Our board of directors has established the following committees. Each committee operates in accordance with a written charter that sets forth the committee’s structure, operations, membership requirements, responsibilities and authority to engage advisors.
Audit Committee
Under the Companies Law, the Exchange Act and Nasdaq Marketplace Rules, we are required to maintain an audit committee.
The responsibilities of an audit committee under the Companies Law include identifying and addressing flaws in the business management of the company, reviewing and approving related party transactions, establishing whistleblower procedures, overseeing the company’s internal audit system and the performance of its internal auditor, and assessing the scope of the work and recommending the fees of the company’s independent accounting firm. In addition, the audit committee is required to determine whether certain related party actions and transactions are “material” or “extraordinary” for the purpose of the requisite approval procedures under the Companies Law and to establish procedures for considering proposed transactions with a controlling shareholder.
In accordance with U.S. law and Nasdaq Marketplace Rules, our audit committee is also responsible for the appointment, compensation and oversight of the work of our independent auditors and for assisting our board of directors in monitoring our financial statements, the effectiveness of our internal controls and our compliance with legal and regulatory requirements.
Under the Companies Law, the audit committee must consist of at least three directors who meet certain independence criteria. Under the Nasdaq Marketplace Rules, we are required to maintain an audit committee consisting of at least three independent directors, all of whom are financially literate and one of whom has accounting or related financial management expertise. Each of the members of the audit committee is required to be “independent” as such term is defined in Rule 10A-3(b)(1) under the Exchange Act.
Our audit committee currently consists of Eli Arad, Sari Singer and David Gerbi. All members are independent directors as defined in the Companies Law, SEC rules and Nasdaq listing requirements. Our board of directors has determined that all members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and Nasdaq Marketplace Rules. Our board of directors has determined that Eli Arad and David Gerbi are audit committee financial experts as defined by the SEC rules and have the requisite financial experience as defined by the Nasdaq Marketplace Rules.
Compensation Committee
Under both the Companies Law and Nasdaq Marketplace Rules, we are required to establish a compensation committee.
The responsibilities of a compensation committee under the Companies Law include recommending to the board of directors, for ultimate shareholder approval by a special majority, a policy governing the compensation of directors and officers based on specified criteria, reviewing modifications to and implementing such compensation policy from time to time, and approving the actual compensation terms of directors and officers prior to approval by the board of directors.
The Companies Law stipulates that the compensation committee must consist of at least three directors who meet certain independence criteria. Under Nasdaq Marketplace Rules, we are required to maintain a compensation committee consisting of at least two independent directors; each of the members of the compensation committee is required to be independent under Nasdaq Marketplace Rules relating to compensation committee members, which are different from the general test for independence of board and committee members.
Our compensation committee currently consists of Eli Arad, Sari Singer and David Gerbi. All members are independent directors as defined in the Companies Law, SEC rules and regulations, and Nasdaq Marketplace Rules.
Director Nominations
We do not have a standing nominating committee. In accordance with Rule 5605(e)(2) of the Nasdaq Rules, a majority of the independent directors may recommend a director nominee for selection by the board of directors. Our board of directors believes that the independent directors can satisfactorily carry out the responsibility of properly selecting or approving director nominees without the formation of a standing nominating committee. As we do not have a standing nominating committee, we will not have a nominating committee charter in place.
Our board of directors will consider candidates for nomination who have a high level of personal and professional integrity, strong ethics and values and the ability to make mature business judgments. In general, in identifying and evaluating nominees for director, our board of directors will also consider experience in corporate management such as serving as an officer or former officer of a publicly held company, experience as a board member of another publicly held company, professional and academic experience relevant to our business, leadership skills, experience in finance and accounting or executive compensation practices, whether candidate has the time required for preparation, participation and attendance at board meetings and committee meetings, if applicable, independence and the ability to represent the best interests of our stockholders.
Internal Auditor
Under the Companies Law, the board of directors is required to appoint an internal auditor recommended by the audit committee. The role of the internal auditor is to examine, among other things, whether the company’s actions comply with applicable law and proper business procedures. The internal auditor may not be an interested party, a director or an officer of the company, or a relative of any of the foregoing, nor may the internal auditor be our independent accountant or a representative thereof. Our current internal auditor is Mr. Daniel Spira, CPA, who is a member of the board of directors of the Institute of Internal Auditors in Israel and Chairman of its Auditing and Knesset Relations Committee.
Fiduciary Duties and Approval of Related Party Transactions
Fiduciary duties of directors and officers
Israeli law imposes a duty of care and a duty of loyalty on all directors and officers of a company. The duty of care requires a director or officer to act with the level of care with which a reasonable director or officer in the same position would have acted under the same circumstances. The duty of care includes, among other things, a duty to use reasonable means, under the circumstances, to obtain information on the advisability of a given action brought for his approval or performed by virtue of his position and other important information pertaining to such action. The duty of loyalty requires the director or officer to act in good faith and for the benefit of the company.
Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions
Under the Companies Law, a company may approve an act specified above which would otherwise constitute a breach of the office holder’s fiduciary duty, provided that the office holder acted in good faith, the act or its approval does not harm the company, and the office holder discloses to the company his or her personal interest in the transaction (including any significant fact or document) a reasonable time before the approval of such act. Any such approval is subject to the terms of the Companies Law, setting forth, among other things, the appropriate bodies of the company required to provide such approval, and the methods of obtaining such approval.
The Companies Law requires that an office holder promptly disclose to the company any direct or indirect personal interest that he or she may have and all related material information or documents known to him or her relating to any existing or proposed transaction by the company. An interested office holder’s disclosure must be made promptly and, in any event, no later than the first meeting of the board of directors at which the transaction is considered. An office holder is not obliged to disclose such information if the personal interest of the office holder derives solely from the personal interest of his or her relative in a transaction that is not considered an extraordinary transaction.
If the transaction is an extraordinary transaction, the office holder must also disclose any personal interest held by:
| | • | the office holder’s relatives (spouse, siblings, parents, grandparents, descendants, spouse’s descendants and the spouses of any of these people); or |
| | • | any company in which the office holder or his or her relatives holds 5% or more of the shares or voting rights, serves as a director or general manager or has the right to appoint at least one director or the general manager. |
Under the Companies Law, unless the articles of association of a company provide otherwise, a transaction with an office holder or with a third party in which the office holder has a personal interest, which is not an extraordinary transaction, requires approval by the board of directors or a committee authorized by the board of directors. If the transaction considered is an extraordinary transaction with an office holder or third party in which the office holder has a personal interest, then audit committee approval is required prior to approval by the board of directors. Under specific circumstances, shareholder approval may also be required. For the approval of compensation arrangements with directors and executive officers, see “Item 6.B. Compensation—Compensation of Directors and Executive Officers.”
Any persons who have a personal interest in the approval of a transaction that is brought before a meeting of the board of directors or the audit committee may not be present at the meeting or vote on the matter. However, if the chairman of the board of directors or the chairman of the audit committee, as applicable, has determined that the presence of an office holder with a personal interest is required, such office holder may be present at the meeting for the purpose of presenting the matter. Notwithstanding the foregoing, a director who has a personal interest may be present at the meeting of the board of directors or the audit committee (as applicable) and vote on the matter if a majority of the members of the board of directors or the audit committee (as applicable) have a personal interest in the approval of such transaction. If a majority of the directors at a board of directors meeting have a personal interest in the transaction, such transaction also generally requires approval of the shareholders of the company.
A “personal interest” is defined under the Companies Law as the personal interest of a person in an action or in a transaction of the company, including the personal interest of such person’s relative or the interest of any other corporate body in which the person and/or such person’s relative is a director or general manager, a 5% shareholder or holds 5% or more of the issued and outstanding share capital of the company or of its voting rights, or has the right to appoint at least one director or the general manager, but excluding a personal interest stemming solely from the fact of holding shares in the company. A personal interest also includes (i) a personal interest of a person who votes according to a proxy of another person, including in the event that the other person has no personal interest, and (ii) a personal interest of a person who gave a proxy to another person to vote on his or her behalf regardless of whether the discretion of how to vote lies with the person voting.
An “extraordinary transaction” is defined under the Companies Law as any of the following:
| | • | a transaction other than in the ordinary course of business; |
| | • | a transaction that is not on market terms; or |
| | • | a transaction that may have a material impact on the company’s profitability, assets or liabilities. |
Disclosure of Personal Interests of a Controlling Shareholder and Approval of Transactions
Pursuant to the Companies Law, the disclosure requirements that apply to an office holder also apply to a controlling shareholder of a public company. Extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, including a private placement in which a controlling shareholder has a personal interest, and the terms of engagement of the company, directly or indirectly, with a controlling shareholder or a controlling shareholder’s relative (including through a corporation controlled by a controlling shareholder), regarding the company’s receipt of services from the controlling shareholder, and if such controlling shareholder is also an office holder or employee of the company, regarding his or her terms of employment, require the approval of each of (i) the audit committee (or the compensation committee with respect to the terms of the engagement as an office holder or employee, including insurance, indemnification and compensation), (ii) the board of directors and (iii) the shareholders, in that order. In addition, the shareholder approval must fulfill one of the following requirements:
| | • | a majority of the shares held by shareholders who have no personal interest in the transaction and are voting at the meeting must be voted in favor of approving the transaction, excluding abstentions; or |
| | • | the shares voted by shareholders who have no personal interest in the transaction who vote against the transaction represent no more than 2% of the voting rights in the company. |
Such majority determined in accordance with the majority requirement described above is hereinafter referred to as the Compensation Special Majority Requirement.
Any such transaction for which the term is more than three years must be approved in the same manner every three years, unless with respect to certain transactions as permitted by the Companies Law, the audit committee has determined that a longer term is reasonable under the circumstances. In addition, transactions with a controlling shareholder or a controlling shareholder’s relative who serves as an executive officer in a company, directly or indirectly (including through a corporation under his control), involving the receipt of services by a company or their compensation can have a term of five years from the company’s initial public offering under certain circumstances.
The Companies Law requires that every shareholder that participates, in person or by proxy, in a vote regarding a transaction with a controlling shareholder, must indicate in advance or in the ballot whether or not that shareholder has a personal interest in the vote in question. Failure to so indicate generally results in the invalidation of that shareholder’s vote.
Disclosure of Compensation of Executive Officers
For so long as we qualify as a foreign private issuer, we are not required to comply with the proxy rules applicable to U.S. domestic filers, including the requirement applicable to emerging growth companies to disclose the compensation of our chief executive officer and other two most highly compensated executive officers on an individual, rather than an aggregate, basis. Nevertheless, regulations promulgated under the Companies Law require us to disclose in the proxy statement for the annual general meeting of our shareholders (or to include a reference therein to other previously furnished public disclosure) the annual compensation of our five most highly compensated executive officers on an individual, rather than an aggregate, basis. This disclosure will not be as extensive as that required of a U.S. domestic issuer.
Compensation of Directors and Executive Officers
Directors. Under the Companies Law, the compensation of our directors requires the approval of our compensation committee, the subsequent approval of the board of directors and, unless exempted under regulations promulgated under the Companies Law, the approval of the shareholders at a general meeting. If the compensation of our directors is inconsistent with our compensation policy, then, provided that those provisions that must be included in the compensation policy according to the Companies Law have been considered by the compensation committee and board of directors, and provided that shareholder approval is obtained by the Compensation Special Majority Requirement.
Executive Officers (other than the Chief Executive Officer). The Companies Law requires the approval of the compensation of a public company’s executive officers (other than the chief executive officer) in the following order: (i) the compensation committee, (ii) the company’s board of directors and (iii) if such compensation arrangement is inconsistent with the company’s compensation policy, the company’s shareholders (the Compensation Special Majority Requirement). However, if the shareholders of the company do not approve a compensation arrangement with an executive officer that is inconsistent with the company’s compensation policy, the compensation committee and board of directors may override the shareholders’ decision if each of the compensation committee and the board of directors provide detailed reasons for their decision.
Chief Executive Officer. The Companies Law requires the approval of the compensation of a public company’s chief executive officer in the following order: (i) the company’s compensation committee, (ii) the company’s board of directors and (iii) the company’s shareholders (the Compensation Special Majority Requirement). However, if the shareholders of the company do not approve the compensation arrangement with the chief executive officer, the compensation committee and board of directors may override the shareholders’ decision if each of the compensation committee and the board of directors provide a detailed report for their decision. The approval of each of the compensation committee and the board of directors should be in accordance with the company’s compensation policy; however, in special circumstances, they may approve compensation terms of a chief executive officer that are inconsistent with such policy provided that they have considered those provisions that must be included in the compensation policy according to the Companies Law and that shareholder approval was obtained (by a special majority vote as discussed above with respect to the approval of director compensation). In addition, the compensation committee may waive the shareholder approval requirement with regards to the approval of the engagement terms of a candidate for the chief executive officer position, if the compensation committee determines that the compensation arrangement is consistent with the company’s compensation policy, and that the chief executive officer did not have a prior business relationship with the company or a controlling shareholder of the company and that subjecting the approval of the engagement to a shareholder vote would impede the company’s ability to employ the chief executive officer candidate.
Compensation Policy
Under the Companies Law, we are required to approve, at least once every three years, a compensation policy with respect to our directors and officers. Following the recommendation of our compensation committee, the compensation policy must be approved by our board of directors and our shareholders. The shareholder approval must be by a simple majority of all votes cast, provided that (i) such majority includes a simple majority of the votes cast by non-controlling shareholders having no personal interest in the matter or (ii) the total number of votes of shareholders mentioned in clause (i) above who voted against such transaction does not exceed 2% of the total voting rights in the company.
Directors’ Service Contracts
There are no arrangements or understandings between us and any of our subsidiaries, on the one hand, and any of our directors, on the other hand, providing for benefits upon termination of their employment or service as directors of our company or any of our subsidiaries.
Under the Companies Law, a shareholder has a duty to refrain from abusing its power in the company and to act in good faith and in an acceptable manner in exercising its rights and performing its obligations to the company and other shareholders, including, among other things, when voting at meetings of shareholders on the following matters:
| | • | an amendment to the articles of association; |
| | • | an increase in the company’s authorized share capital; |
| | • | the approval of related party transactions and acts of office holders that require shareholder approval. |
A shareholder also has a general duty to refrain from discriminating against other shareholders.
The remedies generally available upon a breach of contract also apply to a breach of the shareholder duties mentioned above, and in the event of discrimination against other shareholders, additional remedies may be available to the injured shareholder.
In addition, any controlling shareholder, any shareholder that knows that its vote can determine the outcome of a shareholder vote and any shareholder that, under a company’s articles of association, has the power to appoint or prevent the appointment of an office holder, or any other power with respect to a company, is under a duty to act with fairness towards the company. The Companies Law does not describe the substance of this duty except to state that the remedies generally available upon a breach of contract also apply in the event of a breach of the duty to act with fairness, taking the shareholder’s position in the company into account.
Exculpation, Insurance and Indemnification of Directors and Officers
Under the Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from liability to the company, in whole or in part, for damages caused to the company as a result of a breach of duty of care but only if a provision authorizing such exculpation is included in its articles of association. Our articles of association include such a provision. The company may not exculpate in advance a director from liability arising from a breach of his or her duty of care in connection with a prohibited dividend or distribution to shareholders.
As permitted under the Companies Law, our articles of association provide that we may indemnify an office holder in respect of the following liabilities, payments and expenses incurred for acts performed by him or her as an office holder, either in advance of an event or following an event:
| | • | financial liability that was imposed upon him in favor of another person pursuant to a judgment, including a compromise judgment or an arbitrator’s award approved by a court; |
| | • | reasonable litigation expenses, including attorneys’ fees paid by an officeholder following an investigation or proceeding conducted against him by an authority authorized to conduct such investigation or proceeding, and which ended without the filing of an indictment against him and without any financial obligation being imposed on him as an alternative to a criminal proceeding, or which ended without the filing of an indictment against him but with the imposition of a financial obligation as an alternative to a criminal proceeding for an offense which does not require proof of mens rea or in connection with a financial sanction; |
| | • | reasonable litigation expenses, including attorneys’ fees paid by the officeholder or which he was required to pay by a court, in a proceeding filed against him by the Company or on its behalf or by another person, or in criminal charges from which he was acquitted, or in criminal charges in which he was convicted of an offense which does not require proof of mens rea; |
| | • | a financial obligation imposed on the officeholder for the benefit of all of the parties damaged by the violation of an administrative proceeding; |
| | • | expenses incurred by an officeholder in connection with an Administrative Proceeding conducted in his regard, including reasonable litigation expenses, and including attorneys’ fees; |
| | • | expenses incurred by an officeholder in connection with a proceeding under the Antitrust Law, 5748-1988 and/or in connection with it (a “Proceeding Under the Antitrust Law”), conducted regarding him, including reasonable litigation expenses, and attorneys' fees; and |
| | • | any other liability or expense in respect of which it is permitted or shall be permitted by Law to indemnify an officeholder. |
As permitted under the Israeli Companies Law, our articles of association provide that we may insure an office holder against the following liabilities incurred for acts performed by him or her as an office holder:
| | • | Breach of the duty of care to the Company or to any other person; |
| | • | Breach of the fiduciary duty to the Company, provided that the officeholder acted in good faith and had reasonable grounds to assume that his act would not adversely affect the Company’s best interests; |
| | • | financial liability imposed upon him in favor of another person; |
| | • | financial liability imposed on the officeholder for the benefit of all of the parties damaged by the violation of an administrative proceeding; |
| | • | expenses incurred or to be incurred by an officer in connection with an Administrative Proceeding, including reasonable litigation expenses, and including attorneys’ fees; |
| | • | Expenses incurred or to be incurred in connection with a proceeding under the Antitrust Law, including reasonable litigation expenses, and including attorneys’ fees; and |
| | • | any other event in respect of which it is permitted and/or shall be permitted by Law to insure the liability of an officeholder. |
Under the Companies Law, a company may not indemnify, exculpate or insure an office holder against any of the following:
| | • | a breach of the duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty to the company to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company; |
| | • | a breach of duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder; |
| | • | an act or omission committed with intent to derive illegal personal benefit; or |
| | • | a fine, monetary sanction or forfeit levied against the office holder. |
Under the Companies Law, exculpation, indemnification and insurance of office holders must be approved by the compensation committee and the board of directors and, with respect to directors or controlling shareholders, their relatives and third parties in which controlling shareholders have a personal interest, also by the shareholders.
Our articles of association permit us to exculpate, indemnify and insure our office holders to the fullest extent permitted or to be permitted by law. Our office holders are currently covered by a directors’ and officers’ liability insurance policy. As of the date of this annual report, no claims for directors’ and officers’ liability insurance have been filed under this policy and we are not aware of any pending or threatened litigation or proceeding involving any of our office holders, including our directors, in which indemnification is sought.
D. Employees
As of December 31, 2022, we had 49 employees based at our office and laboratory in Rehovot, Israel and Peace of Meat employed 32 employees based at its office and laboratory in Antwerp, Belgium. On March 7, 2023, we announced a strategic restructuring for Peace of Meat, including targeted layoffs in areas of the business that were unrelated to its updated focus, and on April 4, 2023, we announced the expected liquidation of Peace of Meat, as a result of which it will no longer employ employees.
Local labor laws govern the length of the workday and workweek, minimum wages for employees, procedures for hiring and dismissing employees, determination of severance pay, annual leave, sick days, advance notice of termination, Social Security payments or regional equivalents, and other conditions of employment and include equal opportunity and anti-discrimination laws. None of our employees is party to any collective bargaining agreements. We generally provide our employees with benefits and working conditions beyond the required minimums. We believe we have a good relationship with our employees, and have never experienced any employment-related work stoppages.
E. Beneficial Ownership of Executive Officers and Directors
The beneficial ownership of our ordinary shares (including ordinary shares represented by ADSs) is determined in accordance with the rules of the SEC. Under these rules, a person is deemed to be a beneficial owner of a security if that person has or shares voting power, which includes the power to vote or to direct the voting of the security, or investment power, which includes the power to dispose of or to direct the disposition of the security. For purposes of the table below, we deem ordinary shares issuable pursuant to options or warrants that are currently exercisable or exercisable within 60 days of the date of this Annual Report on Form 20-F, if any, to be outstanding and to be beneficially owned by the person holding the options or warrants for the purposes of computing the percentage ownership of that person, but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person.
Unless otherwise noted below, each shareholder’s address is c/o Steakholder Foods Ltd., 5 David Fikes St., Rehovot 7638205, Israel.
| | | Shares Beneficially Owned | |
| Name of Beneficial Owner | | Number | | | Percentage(1) | |
| Directors and executive officers | | | | | | |
Arik Kaufman(2) | | | 491,600 | | | | * | |
Guy Hefer(3) | | | 333,330 | | | | * | |
Dan Kozlovski(4) | | | 133,340 | | | | * | |
Yaron Kaiser(5) | | | 3,003,610 | | | | 1.7 | % |
David Gerbi(6) | | | 218,510 | | | | * | |
Eli Arad(7) | | | 167,010 | | | | * | |
Sari Singer(8) | | | 168,460 | | | | * | |
| All directors and executive officers as a group (7 persons) | | | 4,515,860 | | | | 2.6 | % |
| * | Less than one percent (1%). |
| (1) | Based on 172,071,117 Ordinary Shares outstanding as of March 22, 2023. |
| (2) | Consists of 283,270 Ordinary Shares and options to purchase 208,330 Ordinary Shares exercisable within 60 days of the date of this annual report, with an exercise price of $0.519. These options expire on March 16, 2026. |
| (3) | Consists of options to purchase 187,500 Ordinary Shares exercisable within 60 days of the date of this annual report, with an exercise price of NIS 3.49 ($0.96), expiring on March 24, 2025, and options to purchase 145,830 Ordinary Shares exercisable within 60 days of the date of this annual report, with an exercise price of $0.716, expiring on July 20, 2025. |
| (4) | Consists of options to purchase 133,340 Ordinary Shares exercisable within 60 days of the date of this annual report, with an exercise price of NIS 1.90 ($0.52). These options expire on August 5, 2024. |
| (5) | Consists of 1,435,280 Ordinary Shares based on information provided to us by Mr. Kaiser, options to purchase 116,660 Ordinary Shares exercisable within 60 days of the date of this annual report, with an exercise price of $0.519, expiring on March 16, 2026, restricted share units vesting into 111,670 Ordinary Shares within 60 days of the date of this annual report, and performance share units that may vest into 1,340,000 Ordinary Shares within 60 days of this annual report if their associated performance targets are met during such period. |
| (6) | Consists of 96,450 Ordinary Shares, RSUs vesting into 7,490 Ordinary Shares within 60 days of the date of this annual report and options to purchase 114,570 Ordinary Shares within 60 days of the date of this annual report with an exercise price of $0.716. These options expire on July 20, 2025. |
| (7) | Consists of 44,950 Ordinary Shares, RSUs vesting into 7,490 Ordinary Shares within 60 days of the date of this annual report and options to purchase 114,570 Ordinary Shares within 60 days of the date of this annual report with an exercise price of $0.716. These options expire on July 20, 2025. |
| (8) | Consists of 44,910 Ordinary Shares, RSUs vesting into 8,980 Ordinary Shares within 60 days of the date of this annual report and options to purchase 114,570 Ordinary Shares within 60 days of the date of this annual report with an exercise price of $0.716. These options expire on July 20, 2025. |
F. Action to Recover Erroneously Awarded Compensation
Not applicable.
| | ITEM 7. | MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS |
A. Major Shareholders
The following table sets forth certain information regarding the beneficial ownership of our outstanding ordinary shares, including ordinary shares represented by ADSs, as of the date of this Annual Report on Form 20-F, by each person or entity who we know beneficially owns 5% or more of the outstanding ordinary shares. For purposes of the table below, we deem ordinary shares issuable pursuant to options or warrants that are currently exercisable or exercisable within 60 days of the date of this Annual Report on Form 20-F, if any, to be outstanding and to be beneficially owned by the person holding the options or warrants for the purposes of computing the percentage ownership of that person, but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person.
None of our shareholders have different voting rights from other shareholders. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
As of the date of this Annual Report on Form 20-F, there are five shareholders of record of our ordinary shares, of whom two are in the United States. The number of record holders is not representative of the number of beneficial holders of our ordinary shares, as most of the shares we have issued, including those represented by ADSs are currently recorded in the name of our ADS registrar, The Bank of New York Mellon. Based upon a review of the information provided to us by The Bank of New York Mellon, as of March 1, 2023, there were 25 holders of record of the ADSs on record with the Depository Trust Company. These numbers are not representative of the number of beneficial holders of our ADSs nor is it representative of where such beneficial holders reside, since many of these ADSs were held of record by brokers or other nominees.
| | | Ordinary Shares Beneficially Owned | |
| Name of Beneficial Owner | | Number | | | Percentage | |
| 5% or greater shareholders | | | | | | |
| Shimon Cohen | | | 12,175,320 | | | | 7.1 | % |
(1) Based on 172,071,117 Ordinary Shares outstanding as of March 22, 2023.
(2) This information is based solely on a Schedule 13D filed with the SEC on September 22, 2022, pursuant to which Shimon Cohen reported that he is the direct and beneficial owner of 12,175,320 Ordinary Shares, which represents (i) 305,616 ADSs held by Mr. Cohen in his individual capacity and (ii) 437,245 ADSs, 222,068 ADSs and 252,603 ADSs held indirectly by Mr. Cohen through S.C. Ma’agarei Enosh Ltd., Reshet Bitachon Ltd. and Ma’agarim Proyektim Ltd., respectively, each of which Mr. Cohen is the sole owner, manager and shareholder. The address for Shimon Cohen is 20 Derech HaShalom, Tel Aviv, 61250 Israel.
B. Related Party Transactions
The following is a description of the material transactions we entered into with related parties since the beginning of 2020. We believe that we have executed all of our transactions with related parties on terms no less favorable to us than those we could have obtained from unaffiliated third parties.
Our Board of Directors, acting through our Audit Committee, is responsible for the review, approval, or ratification of related party transactions between us and related persons. Under Israeli law, related party transactions are subject to special approval requirements, see “Management — Fiduciary duties and approval of specified related party transactions and compensation under Israeli law.”
Employment Agreements and Director Fees
We have entered into written employment agreements with each of our executive officers, which provide for notice periods of varying duration for termination of the agreement by us or by the relevant executive officer, during which time the executive officer will continue to receive base salary and benefits. These agreements also contain customary provisions regarding noncompetition, confidentiality of information and assignment of inventions. However, the enforceability of the noncompetition provisions may be limited under applicable law. See “Risk factors - Risks relating to our operations - Under applicable employment laws, we may not be able to enforce covenants not to compete” for a further description of the enforceability of non-competition clauses. For further information, see “Management - Employment and Consulting Agreements.”
Directors and Officers Insurance Policy and Indemnification and Exculpation Agreements
In accordance with our articles of association, we have obtained Directors and Officers insurance for our executive officers and directors, and provide indemnification, exculpation and exemption undertakings to each of our directors and officers to the fullest extent permitted by the Companies Law.
Private Issuances of Securities
In January 2020, following the closing of the merger between MeaTech and Ophectra, we issued former shareholders of Steakholder Innovations warrants to receive ordinary shares, including to the following then-related parties: (1) warrants to receive 1,036,098 ordinary shares each to Sharon Fima, then our Chief Executive Officer and Chief Technical Officer, and Omri Schanin, then our Deputy Chief Executive Officer; and (2) warrants to receive 1,291,158 ordinary shares to Liran Damati, then a substantial shareholder. The warrants had no exercise price and vested upon the achievement of pre-defined milestones in 2020 and 2021.
In May 2020, pursuant to approvals of our audit committee, board of directors and a general meeting of our shareholders: (1) we issued 1,043,846 ordinary shares and options to purchase 6,030,286 ordinary shares at an exercise price of NIS 3.36 (approximately $0.92) per share in return for a private investment of $750,000 by EL Capital Investments LLC, a company controlled by Mr. Steven Lavin, who was concurrently appointed to our board of directors as its chairman; and (2) we issued options to purchase 1,967,327 ordinary shares at an exercise price of NIS 2.49 (approximately $0.68) per share and options to purchase 1,967,328 ordinary shares at an exercise price of NIS 3.486 (approximately $0.96) to Silver Road Capital Ltd., the majority of whose shares were owned by directors at the time, Mr. Steven Lavin and Mr. Daniel Ayalon.
Engagement with BlueSoundWaves
On October 6, 2021, we entered into a services and collaboration agreement, or the Services and Collaboration Agreement, with BlueOcean Sustainability Fund, LLC, or BlueSoundWaves, pursuant to which BlueSoundWaves provides us with marketing and promotional services, strategic consulting advice, and partner and investor engagement services in the United States. As consideration for such services, BlueSoundWaves received (i) an option to purchase 6,215,770 ordinary shares, currently equal to 621,577 ADSs, and (ii) 1,243,150 of our ordinary restricted shares, currently equal to 124,315 ADSs.
BlueOcean Sustainability Management Fund LP, a Cayman partnership, and the managing partner of BlueSoundWaves holds all of the outstanding share capital of BlueOcean Kayomot Ltd., an Israeli company. Messrs. Kaufman and Kaiser are directors of BlueOcean Kayomot Ltd. and founding partners of BlueSoundWaves. Mr. Kaufman also serves as the chief executive officer of BlueOcean Kayomot Ltd. See Item 10.C for additional discussion of the Services and Collaboration Agreement.
C. Interests of Experts and Counsel
Not applicable.
| | ITEM 8. | FINANCIAL INFORMATION |
A. Consolidated Statements and other Financial Information
See “Item 18.—Financial Statements” in this Annual Report on Form 20-F.
Legal Proceedings
From time to time, we may be party to litigation or other legal proceedings that we consider to be a part of the ordinary course of our business. We are not currently involved in any legal proceedings that could reasonably be expected to have a material adverse effect on our business, prospects, financial condition or results of operations.
Dividend Distributions
We have never declared or paid cash dividends to our shareholders. Currently we do not intend to pay cash dividends. We currently intend to reinvest any future earnings in developing and expanding our business. Any future determination relating to our dividend policy will be at the discretion of our Board of Directors and will depend on a number of factors, including future earnings, our financial condition, operating results, contractual restrictions, capital requirements, business prospects, applicable Israeli law and other factors our Board of Directors may deem relevant.
B. Significant Changes
Since December 31, 2022, the following significant changes have occurred:
Fundraising Round
On January 9, 2023, we consummated an underwritten public offering of 1,550,000 American Depositary Shares (“ADSs”) at a price of $1.00 per ADS and pre-funded warrants to purchase 4,950,000 ADSs at a purchase price of $0.9999 per warrant and an exercise price of $0.0001 per warrant, for total immediate gross proceeds of approximately $6.5 million. As part of the offering, we issued warrants to purchase 6,500,000 ADSs, exercisable immediately for a period of five years, with an exercise price of $1.00 per ADS. Underwriting discounts and other offering expenses totaled approximately $0.7 million. In addition, we granted the underwriters a 45-day option to purchase up to an additional 975,000 ADSs and/or warrants at the public offering price, less discounts and commissions, solely to cover over-allotments, if any, which was not exercised.
In connection with the offering, we entered into an agreement with an existing investor to reduce the exercise price of outstanding warrants to purchase up to 1,857,143 ADS which were issued in our July 2022 registered direct offering (the “Prior Warrants”) from $3.50 per ADS to $1.00 per ADS, and to extend the term of the Prior Warrants until January 10, 2028.
Appointment of Chief Financial Officer
On March 2, 2023, we announced that our Vice President of Finance, Mr. Eitan Noah, will be assuming the position of Chief Financial Officer, as our current Chief Financial Officer, Guy Hefer, has decided to step down from his role for personal reasons, all effective April 5, 2023.
Eitan Noah, B.A., CPA, has been our Vice President of Finance since January 2021. He is an integral member of our finance team, demonstrating exceptional leadership and financial acumen during his tenure, with a deep understanding of our operations, financials, and strategic priorities. Before joining us, Mr. Noah was Director of Finance at Inception XR, Inc., and Controller and FP&A Manager at Fluence Corp. Ltd. Prior to that, he was a senior associate at PwC Israel. He holds a Bachelor’s degree in Economics from the Ben-Gurion University of the Negev, Israel, and is a certified practicing accountant in Israel.
| | ITEM 9. | THE OFFER AND LISTING |
A. Offer and Listing Details
ADSs
The ADSs, representing our ordinary shares, traded on Nasdaq under the symbol “MITC” between March 12, 2021 and August 2, 2022, and have traded under the symbol “STKH” since then.
Ordinary Shares
Our ordinary shares were traded on the TASE between January 26, 2020 and August 3, 2021, when we voluntarily de-listed them from trade on the TASE. Our ordinary shares were traded under the symbol “MEAT” until March 2021, and thereafter under the symbol “MITC.” Some of our ordinary shares are traded over the counter under the symbol MTTCF.
B. Plan of Distribution
Not applicable.
C. Markets
For a description of our publicly-traded ADSs, see “Item 9.— Offer and Listing Details —ADSs.” For a description of our ordinary shares, see “Item 9.— Offer and Listing Details —Ordinary Shares.”
D. Selling Shareholders
Not applicable.
E. Dilution
Not applicable.
F. Expenses of the Issue
Not applicable.
| | ITEM 10. | ADDITIONAL INFORMATION |
A. Share Capital
Not applicable.
B. Articles of Association
The information set forth in our prospectus dated January 9, 2023, filed with the SEC pursuant to Rule 424(b), under the headings “Description of Share Capital” is incorporated herein by reference.
C. Material Contracts
On October 6, 2021, we entered into the Services and Collaboration Agreement with BlueSoundWaves, a sustainability focused fund led by led by Ashton Kutcher, Guy Oseary and Effie Epstein. Pursuant to the Services and Collaboration Agreement, BlueSoundWaves provides us marketing and promotional services, strategic consulting advice, and partner and investor engagement services in the United States.
As consideration for such services, BlueSoundWaves received (i) an option to purchase 6,215,770 ordinary shares, currently equal to 621,577 ADSs, and (ii) 1,243,150 of our ordinary restricted shares, currently equal to 124,315 ADSs. The exercise price per ordinary share of the ordinary share option and restricted ordinary share option is the greater of (a) the closing price per ADS on October 5, 2021 ($6.65) divided by the number of ordinary shares represented by the ADS and (b) the closing price per ADS on the day prior to the exercise of the options less a discount ranging from 25% to 75% depending on how much higher the exercise price is compared to the price determined under subsection (a) of this paragraph. The options granted pursuant to this agreement will vest over a three-year period, with one-third vesting on the first anniversary of the Services and Collaboration Agreement with the remaining amount vesting in equal quarterly installments for the remaining period. If either party provides notice to terminate the agreement, the quarterly vesting will be cancelled. A percentage of the options described above will immediately vest and be exercisable upon the occurrence of certain trading milestones, investment milestones or change of control event. We have also agreed to reimburse BlueSoundWaves its reasonable out of pocket expenses which have been previously approved.
The Services and Collaboration Agreement will remain in effect until terminated in accordance with its terms. Each party has the right to terminate upon 60 days prior written notice after the 12-month anniversary of the effective date. Either party may also terminate the Services and Collaboration Agreement upon the occurrence of a material breach which remains uncured 30 days after the breaching party has received notice of the breach. Any portion of the vested options to purchase ordinary shares or restricted ordinary shares will expire on the second anniversary of the termination of the Services and Collaboration Agreement, or otherwise expire on the 10th anniversary.
For other agreements with related parties, see “Item 7.—Major Shareholders and Related Party Transactions—Related Party Transactions.”
D. Exchange Controls
Non-residents of Israel who purchase our ordinary shares outside of Israel with U.S. dollars or other foreign currency will be able to convert dividends (if any) thereon, and any amounts payable upon the dissolution, liquidation or winding up of the affairs of the Company, as well as the proceeds of any sale in Israel of the ordinary shares to an Israeli resident, into freely repatriable dollars, at a rate of exchange prevailing at the time of conversion, pursuant to regulations issued under the Currency Control Law, 1978, provided that Israeli income tax has been withheld by the Company with respect to such amounts.
E. Taxation
The following description is not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership and disposition of our ordinary shares or ADSs. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
Israeli tax considerations and government programs
The following is a summary of the current tax regime in the State of Israel, which applies to us and to persons who hold our ordinary shares or ADSs.
This summary does not discuss all the aspects of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment circumstances or to some types of investors subject to special treatment under Israeli law. Examples of this kind of investor include traders in securities or persons who do not hold our ordinary shares or ADSs as a capital asset. Some parts of this discussion are based on a new tax legislation which has not been subject to judicial or administrative interpretation. The discussion should not be construed as legal or professional tax advice and does not cover all possible tax considerations.
HOLDERS AND POTENTIAL INVESTORS ARE URGED TO CONSULT THEIR OWN TAX ADVISORS AS TO THE ISRAELI OR OTHER TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR ORDINARY SHARES OR ADSs, INCLUDING, IN PARTICULAR, THE EFFECT OF ANY FOREIGN, STATE OR LOCAL TAXES.
General corporate tax structure in Israel
Israeli resident companies are generally subject to corporate tax on both ordinary income and capital gains, currently at the rate of 23% of a company’s taxable income. Capital gains derived by an Israeli resident company are subject to tax at the prevailing corporate tax rate.
Taxation of our shareholders
Capital gains
Capital gains tax is generally imposed on the disposal of capital assets by an Israeli resident, and on the disposal of capital assets by a non-Israeli resident if those assets (i) are located in Israel, (ii) are shares or a right to shares in an Israeli resident corporation, (iii) represent, directly or indirectly, rights to assets located in Israel, or (iv) a right in a foreign resident corporation, which in its essence is the owner of a direct or indirect right to property located in Israel (with respect to the portion of the gain attributed to the property located in Israel), unless a specific exemption is available or unless a tax treaty between Israel and the shareholder’s country of residence provides otherwise. The ITO distinguishes between “Real Capital Gain” and “Inflationary Surplus.” Real Capital Gain is the excess of the total capital gain over Inflationary Surplus. The Inflationary Surplus is a portion of the total capital gain which is equivalent to the increase of the relevant asset’s price that is attributable to the increase in the Israeli consumer price index or, in certain circumstances, a foreign currency exchange rate, between the date of purchase and the date of sale. Inflationary Surplus is not currently subject to tax in Israel.
Real Capital Gain accrued by individuals on the sale of our ordinary shares or ADSs will be taxed at the rate of 25%. However, if the individual shareholder is a “Substantial Shareholder” (i.e., a person who holds, directly or indirectly, alone or together with another, 10% or more of one of the Israeli resident company’s means of control) at the time of sale or at any time during the preceding 12-month period, such gain will be taxed at the rate of 30%.
Individual and corporate shareholders dealing in securities in Israel are taxed at the tax rates applicable to business income in 2022, a tax rate of 23% for corporations and a marginal tax rate of up to 47% for individuals, unless contrary provisions in a relevant tax treaty applies. In addition, a 3% excess tax (as discussed below) is levied on individuals whose total taxable income in Israel in 2022 exceeded NIS 663,240.
Notwithstanding the foregoing, generally, capital gain derived from the sale of our ordinary shares or ADSs by a shareholder who is a non-Israeli resident (whether an individual or a corporation) should be exempt from Israeli capital gain tax, provided, among others, that:(i) the ordinary shares or ADSs were purchased upon or after the listing of the securities on the stock exchange, and (ii) the seller does not have a permanent establishment in Israel to which the derived capital gain is attributable. However, non-Israeli entities (including corporations) will not be entitled to the foregoing exemption if Israeli residents, whether directly or indirectly: (i) have a controlling interest of more than 25% in such non-Israeli entity or (ii) are the beneficiaries of, or are entitled to, 25% or more of the revenues or profits of such non-Israeli entity. In addition, such exemption is not applicable to a person whose gains from selling or otherwise disposing of the shares or ADSs are deemed to be business income. In addition, the sale of ordinary shares or ADSs may be exempt from Israeli capital gains tax under the provisions of an applicable tax treaty. For example, the U.S.-Israel Tax Treaty, or the Treaty, generally exempts U.S. residents (for purposes of the U.S.-Israel Treaty) holding the shares as a capital asset from Israeli capital gains tax in connection with such sale, exchange or disposition unless either (i) the U.S. treaty resident owns, directly or indirectly, 10% or more of the Israeli resident company’s voting rights at any time within the 12-month period preceding such sale, exchange or disposition; (ii) the seller, if an individual, has been present in Israel for a period or periods aggregating to 183 days or more during the relevant taxable year; (iii) the capital gain from the sale, exchange or disposition was derived through a permanent establishment of the U.S. resident in Israel; (iv) the capital gain arising from such sale, exchange or disposition is attributed to real estate located in Israel, or (v) the capital gains arising from such sale, exchange or disposition is attributed to royalties. In any such case, the sale, exchange or disposition of such shares or ADSs would be subject to Israeli tax, to the extent applicable; however, under the U.S.-Israel Treaty, a U.S. resident would be permitted to claim a credit for the Israeli tax against the U.S. federal income tax imposed with respect to the sale, exchange or disposition, subject to the limitations in U.S. laws applicable to foreign tax credits. The U.S.-Israel Treaty does not provide such credit against any U.S. state or local taxes.
In some instances where our shareholders may be liable for Israeli tax on the sale of their shares or ADSs, the payment of the consideration may be subject to withholding tax at source in Israel. Shareholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding tax at source at the time of sale. Specifically, in transactions involving a sale of all of the shares of an Israeli resident company, in the form of a merger or otherwise, the Israel Tax Authority may require from shareholders who are not liable for Israeli tax to sign declarations in forms specified by this authority or obtain a specific exemption from the Israel Tax Authority to confirm their status as non-Israeli residents, and, in the absence of such declarations or exemptions, may require the purchaser of the shares to withhold tax at source.
Upon the sale of securities traded on a stock exchange, a detailed return, including a computation of the tax due, must be filed and an advance payment must be made on January 31 and July 31 of every tax year, in respect of sales of securities made within the previous six months by Israeli residents for whom tax has not already been deducted. However, if all tax due was withheld at source according to applicable provisions of the ITO and the regulations promulgated thereunder, there is no need to file a return and no advance payment must be paid, provided that (i) such income was not generated from business conducted in Israel by the taxpayer, (ii) the taxpayer has no other taxable sources of income in Israel with respect to which a tax return is required to be filed and an advance payment does not need to be made, and (iii) the taxpayer is not obligated to pay excess tax (as further explained below). Capital gains are also reportable on the annual income tax return.
Dividends
Israeli residents who are individuals are generally subject to Israeli income tax for dividends paid (other than bonus shares or share dividends) at 25%, or 30% if the recipient of such dividend is a Substantial Shareholder, as defined above, at the time of distribution or at any time during the preceding 12-month period. Israeli resident corporations are generally exempt from Israeli corporate tax on the receipt of dividends paid on shares of Israeli resident corporations.
Non-Israeli residents (whether individuals or corporations) are generally subject to Israeli income tax on the receipt of dividends paid on ordinary shares at the rate of 25% or 30% (if the dividend recipient is a Substantial Shareholder at the time of distribution or at any time during the preceding 12-month period). Such dividends are generally subject to Israeli withholding tax at a rate of 25% so long as the shares are registered with a nominee company (whether the recipient is a Substantial Shareholder or not), unless a reduced rate is provided under an applicable tax treaty (subject to the receipt in advance of a valid certificate from the Israel Tax Authority allowing for a reduced tax rate). Under the U.S.- Israel Treaty and subject to the eligibility to the benefits under such treaty, the maximum rate of tax withheld at source in Israel on dividends paid to a holder of our ordinary shares who is a U.S. resident (for purposes of the U.S.-Israel Treaty) is 25%. However, for dividends not generated by an Approved Enterprise, Benefited Enterprise or Preferred Enterprises (which are benefitted tax regimes under the Law for Encouragement of Capital Investments-1959) and paid to a U.S. corporation holding 10% or more of the outstanding voting capital throughout the tax year in which the dividend is distributed as well as during the previous tax year, the maximum rate of withholding tax is generally 12.5%, provided that not more than 25% of the gross income of the Israeli resident paying corporation for such preceding year consists of certain types of dividends and interest. Notwithstanding the foregoing, dividends distributed from income attributed to an Approved Enterprise, Benefited Enterprise or Preferred Enterprise are not entitled to such reduction under such tax treaty but are subject to withholding tax at the rate of 15% or 20% for such a United States corporate shareholder, provided that the conditions related to the holding of 10% of our voting capital and to our gross income for the previous year (as set forth in the previous sentence) are met. The aforementioned rates under the U.S.-Israel Treaty would not apply if the dividend income is derived through a permanent establishment of the U.S. resident in Israel.
If the dividend is attributable partly to income derived from an Approved Enterprise, Benefited Enterprise or Preferred Enterprise, and partly to other sources of income, the withholding rate will be a blended rate reflecting the relative portions of the two types of income. U.S. residents (for purposes of the U.S.-Israel Treaty) who are subject to Israeli withholding tax on a dividend may be entitled to a credit or deduction for United States federal income tax purposes up to the amount of the taxes withheld, subject to detailed rules contained in U.S. tax law.
A non-Israeli resident who receives dividends from which tax was withheld is generally exempt from the obligation to file tax returns in Israel in respect of such income, provided, inter alia, that (i) such income was not derived from a business conducted in Israel by the taxpayer, (ii) the taxpayer has no other taxable sources of income in Israel with respect to which a tax return is required to be filed and (iii) the taxpayer is not obliged to pay excess tax (as further explained below).
Excess Tax
Individuals who are subject to tax in Israel (whether any such individual is an Israeli resident or non-Israeli resident) are also subject to an additional tax at a rate of 3% on annual income exceeding NIS 663,240 for 2022 (which amount is linked to the annual change in the Israeli consumer price index), including, but not limited to, dividends, interest and capital gain.
Estate and gift tax
Israeli law presently does not impose estate or gift taxes.
Material United States federal income tax considerations
The following discussion describes material United States federal income tax considerations relating to the acquisition, ownership, and disposition of shares or ADSs by a U.S. Holder (as defined below) that acquires our shares or ADSs and holds them as a capital asset. This discussion is based on the tax laws of the United States, including the Code, Treasury regulations promulgated or proposed thereunder, and administrative and judicial interpretations thereof, all as in effect on the date hereof. These tax laws are subject to change, possibly with retroactive effect, and subject to differing interpretations that could affect the tax consequences described herein. In addition, this section is based in part upon the representations of the depositary and the assumption that each obligation in the deposit agreement and any related agreements will be performed in accordance with its terms. This discussion does not address the tax consequences to a U.S. Holder under the laws of any state, local or foreign taxing jurisdiction.
For purposes of this discussion, a “U.S. Holder” is a beneficial owner of our shares or ADSs that, for United States federal income tax purposes, is:
| • | an individual who is a citizen or resident of the United States, |
| • | a domestic corporation (or other entity taxable as a corporation); |
| • | an estate the income of which is subject to United States federal income taxation regardless of its source; or |
| • | a trust if (1) a court within the United States is able to exercise primary supervision over the trust’s administration and one or more United States persons have the authority to control all substantial decisions of the trust or (2) a valid election under the Treasury regulations is in effect for the trust to be treated as a United States person. |
A “Non-U.S. Holder” is a beneficial owner of our ordinary shares that is neither a U.S. Holder nor a partnership (or other entity treated as a partnership for United States federal income tax purposes).
This discussion does not address all aspects of United States federal income taxation that may be applicable to U.S. Holders in light of their particular circumstances or status (including, for example, banks and other financial institutions, insurance companies, broker and dealers in securities or currencies, traders that have elected to mark securities to market, regulated investment companies, real estate investment trusts, partnerships or other pass-through entities, corporations that accumulate earnings to avoid U.S. federal income tax, tax-exempt organizations, pension plans, persons that hold our shares as part of a straddle, hedge or other integrated investment, persons subject to alternative minimum tax or whose “functional currency” is not the U.S. dollar).
If a partnership (including any entity or arrangement treated as a partnership for United States federal income tax purposes) holds our shares or ADSs, the tax treatment of a person treated as a partner in the partnership for United States federal income tax purposes generally will depend on the status of the partner and the activities of the partnership. Partnerships (and other entities or arrangements so treated for United States federal income tax purposes) and their partners should consult their own tax advisors.
In general, and taking into account the earlier assumptions, for United States federal income and Israeli tax purposes, a holder that holds ADRs evidencing ADSs will be treated as the owner of the shares represented by those ADRs. Exchanges of shares for ADRs, and ADRs for shares, generally will not be subject to United States federal income or Israeli tax.
This discussion addresses only U.S. Holders and does not discuss any tax considerations other than United States federal income tax considerations. Prospective investors are urged to consult their own tax advisors regarding the United States federal, state, and local, and non-United States tax consequences of the purchase, ownership, and disposition of our shares or ADSs.
We do not expect to make any distribution with respect to our shares or ADSs. However, if we make any such distribution, under the United States federal income tax laws, and subject to the passive foreign investment company, or PFIC, rules discussed below, the gross amount of any dividend we pay out of our current or accumulated earnings and profits (as determined for United States federal income tax purposes) will be includible in income for a U.S. Holder and subject to United States federal income taxation. Dividends paid to a noncorporate U.S. Holder that constitute qualified dividend income will be taxable at a preferential tax rate applicable to long-term capital gains of, currently, 20 percent, provided that the U.S. Holder holds the shares or ADSs for more than 60 days during the 121-day period beginning 60 days before the ex-dividend date and meets other holding period requirements. If we are treated as a PFIC, dividends paid to a U.S. Holder will not be treated as qualified dividend income. If we are not treated as a PFIC, dividends we pay with respect to the shares or ADSs generally may be qualified dividend income, provided that the holding period requirements are satisfied by the U.S. Holder.
A U.S. Holder must include any Israeli tax withheld from the dividend payment in the gross amount of the dividend even though the holder does not in fact receive it. The dividend is taxable to the holder when the holder, in the case of shares, or the Depositary, in the case of ADSs, receives the dividend, actually or constructively. The amount of the dividend distribution includible in a U.S. Holder’s income will be the U.S. dollar value of the NIS payments made, determined at the spot NIS/U.S. dollar rate on the date the dividend distribution is includible in income, regardless of whether the payment is in fact converted into U.S. dollars. Generally, any gain or loss resulting from currency exchange fluctuations during the period from the date the dividend payment is included in income to the date the payment is converted into U.S. dollars will be treated as ordinary income or loss and will not be eligible for the special tax rate applicable to qualified dividend income. The gain or loss generally will be income or loss from sources within the United States for foreign tax credit limitation purposes.
Dividends paid with respect to our ordinary shares or ADSs will be treated as foreign source income, which may be relevant in calculating the holder’s foreign tax credit limitation. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends that we distribute generally should constitute “passive category income,” or, in the case of certain U.S. Holders, “general category income.” A foreign tax credit for foreign taxes imposed on distributions may be denied if holders do not satisfy certain minimum holding period requirements. The rules relating to the determination of the foreign tax credit are complex, and you should consult your tax advisor to determine whether and to what extent you will be entitled to this credit.
To the extent a distribution with respect to our shares or ADSs exceeds our current or accumulated earnings and profits, as determined under United States federal income tax principles, the distribution will be treated, first, as a tax-free return of the U.S. Holder’s investment, up to the holder’s adjusted tax basis in its shares or ADSs, and, thereafter, as capital gain, which is subject to the tax treatment described below in “—Gain on Sale, Exchange or Other Taxable Disposition.”
Subject to certain limitations, the Israeli tax withheld in accordance with the Treaty and paid over to Israel will be creditable or deductible against a U.S. Holder’s United States federal income tax liability.
Subject to the discussion below under “Information reporting and backup withholding,” if you are a Non-U.S. Holder, you generally will not be subject to United States federal income (or withholding) tax on dividends received by you on your ordinary shares, unless you conduct a trade or business in the United States and such income is effectively connected with that trade or business (or, if required by an applicable income tax treaty, the dividends are attributable to a permanent establishment or fixed base that such holder maintains in the United States).
Gain on sale, exchange or other taxable disposition
Subject to the PFIC rules described below under “—Passive Foreign Investment Company Considerations,” a U.S. Holder that sells, exchanges or otherwise disposes of shares or ADSs in a taxable disposition generally will recognize capital gain or loss for United States federal income tax purposes equal to the difference between the U.S. Dollar value of the amount realized and the holder’s tax basis, determined in U.S. Dollars, in the shares or ADSs. Gain or loss recognized on such a sale, exchange or other disposition of shares or ADSs generally will be long-term capital gain if the U.S. Holder’s holding period in the shares or ADSs exceeds one year. Long-term capital gains of non-corporate U.S. Holders are generally taxed at preferential rates. The gain or loss generally will be income or loss from sources within the United States for foreign tax credit limitation purposes. A U.S. Holder’s ability to deduct capital losses is subject to limitations.
Subject to the discussion below under “Information reporting and backup withholding,” if you are a Non-U.S. Holder, you generally will not be subject to United States federal income or withholding tax on any gain realized on the sale or exchange of such ordinary shares unless:
| • | such gain is effectively connected with your conduct of a trade or business in the United States (or, if required by an applicable income tax treaty, the gain is attributable to a permanent establishment or fixed base that such holder maintains in the United States); or |
| • | you are an individual and have been present in the United States for 183 days or more in the taxable year of such sale or exchange and certain other conditions are met. |
For a cash basis taxpayer, units of foreign currency paid or received are translated into U.S. dollars at the spot rate on the settlement date of the purchase or sale. In that case, no foreign currency exchange gain or loss will result from currency fluctuations between the trade date and the settlement date of such a purchase or sale. An accrual basis taxpayer, however, may elect the same treatment required of cash basis taxpayers with respect to purchases and sales of our ordinary shares that are traded on an established securities market, provided the election is applied consistently from year to year. Such election may not be changed without the consent of the IRS. An accrual basis taxpayer who does not make such election may recognize exchange gain or loss based on currency fluctuations between the trade date and the settlement date. Any foreign currency gain or loss a U.S. Holder realizes will be U.S. source ordinary income or loss.
The determination of whether the ADSs or ordinary shares are traded on an established securities market is not entirely clear under current U.S. federal income tax law. Please consult your tax advisor regarding the proper treatment of foreign currency gains or losses with respect to a sale or other disposition of our ordinary shares.
Passive foreign investment company considerations
Based on our income and assets, we believe that we may be treated as a PFIC for the preceding taxable year. However, the determination of our status is made annually based on the factual tests described below. Consequently, while we may be treated as a PFIC in future years, we cannot estimate with certainty at this stage whether or not we are likely to be treated as a PFIC for the current or future taxable years. If we were classified as a PFIC in any taxable year, a U.S. Holder would be subject to special rules with respect to distributions on and sales, exchanges and other dispositions of the shares or ADSs. We will be treated as a PFIC for any taxable year in which at least 75 percent of our gross income is “passive income” or at least 50 percent of the average percentage of our assets during the taxable year, assuming we were not a controlled foreign corporation, or CFC, for the year being tested, based on the average of the fair market values of the assets determined at the end of each quarterly period, are assets that produce or are held for the production of passive income. Passive income for this purpose generally includes, among other things, dividends, interest, rents, royalties, gains from commodities and securities transactions, and gains from assets that produce passive income. However, rents and royalties received from unrelated parties in connection with the active conduct of a trade or business are not considered passive income for purposes of the PFIC test. In determining whether we are a PFIC, a pro rata portion of the income and assets of each corporation in which we own, directly or indirectly, at least a 25% interest (by value) is taken into account.
Excess distribution rules
If we were a PFIC with respect to a U.S. Holder, then unless the holder makes one of the elections described below, a special tax regime would apply to the U.S. Holder with respect to (a) any “excess distribution” (generally, aggregate distributions in any year that are greater than 125% of the average annual distribution received by the holder in the shorter of the three preceding years or the holder’s holding period for the shares or ADSs) and (b) any gain realized on the sale or other disposition of the shares or ADSs. Under this regime, any excess distribution and realized gain will be treated as ordinary income and will be subject to tax as if (a) the excess distribution or gain had been realized ratably over the U.S. Holder’s holding period, (b) the amount deemed realized in each year had been subject to tax in each year of that holding period at the highest marginal rate for such year (other than income allocated to the current period or any taxable period before we became a PFIC, which would be subject to tax at the U.S. Holder’s regular ordinary income rate for the current year and would not be subject to the interest charge discussed below), and (c) the interest charge generally applicable to underpayments of tax had been imposed on the taxes deemed to have been payable in those years. If we were determined to be a PFIC, this tax treatment for U.S. Holders would apply also to indirect distributions and gains deemed realized by U.S. Holders in respect of stock of any of our subsidiaries determined to be PFICs. In addition, dividend distributions would not qualify for the lower rates of taxation applicable to long-term capital gains discussed above under “Dividends”.
A U.S. Holder that holds the shares or ADSs at any time during a taxable year in which we are classified as a PFIC generally will continue to treat such shares or ADSs as shares or ADSs in a PFIC, even if we no longer satisfy the income and asset tests described above, unless the U.S. Holder elects to recognize gain, which will be taxed under the excess distribution rules as if such shares or ADSs had been sold on the last day of the last taxable year for which we were a PFIC.
Certain elections by a U.S. Holder would alleviate some of the adverse consequences of PFIC status and would result in an alternative treatment of the shares or ADSs, as described below. However, we do not currently intend to provide the information necessary for U.S. Holders to make “QEF elections,” as described below, and the availability of a “mark-to-market election” with respect to the shares or ADSs is a factual determination that will depend on the manner and quantity of trading of our shares or ADSs, as described below. A mark-to-market election cannot be made with respect to the stock of any of our subsidiaries.
QEF election
If we were a PFIC, the rules above would not apply to a U.S. Holder that makes an election to treat our shares or ADSs as stock of a “qualified electing fund” or QEF. A U.S. Holder that makes a QEF election is required to include in income its pro rata share of our ordinary earnings and net capital gain as ordinary income and long-term capital gain, respectively, subject to a separate election to defer payment of taxes, which deferral is subject to an interest charge. A U.S. Holder makes a QEF election generally by attaching a completed IRS Form 8621 to a timely filed United States federal income tax return for the year beginning with which the QEF election is to be effective (taking into account any extensions). A QEF election can be revoked only with the consent of the IRS. In order for a U.S. Holder to make a valid QEF election, we must annually provide or make available to the holder certain information. We cannot provide any assurances that we will provide to U.S. Holders the information required to make a valid QEF election.
Mark-to-market election
If we were a PFIC, the rules above also would not apply to a U.S. Holder that makes a “mark-to-market” election with respect to the shares or ADSs, but this election will be available with respect to the shares or ADSs only if they meet certain minimum trading requirements to be considered “marketable stock” for purposes of the PFIC rules. In addition, a mark-to-market election generally could not be made with respect to the stock of any of our subsidiaries unless that stock were itself marketable stock, and the election may therefore be of limited benefit to a U.S. Holder that wants to avoid the excess distribution rules described above. Shares or ADSs will be marketable stock if they are regularly traded on a national securities exchange that is registered with the Securities and Exchange Commission or on a non-U.S. exchange or market that meets certain requirements under the Treasury regulations. Shares or ADSs generally will be considered regularly traded during any calendar year during which they are traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. Any trades that have as their principal purpose meeting this requirement will be disregarded.
A U.S. Holder that makes a valid mark-to-market election for the first tax year in which the holder holds (or is deemed to hold) our shares or ADSs and for which we are a PFIC will be required to include each year an amount equal to the excess, if any, of the fair market value of such shares or ADSs the holder owns as of the close of the taxable year over the holder’s adjusted tax basis in such shares or ADSs. The U.S. Holder will be entitled to a deduction for the excess, if any, of the holder’s adjusted tax basis in the shares or ADSs over the fair market value of such shares or ADSs as of the close of the taxable year, but only to the extent of any net mark-to-market gains with respect to such shares or ADSs included by the U.S. Holder under the election for prior taxable years. The U.S. Holder’s basis in such shares or ADSs will be adjusted to reflect the amounts included or deducted pursuant to the election. Amounts included in income pursuant to a mark-to-market election, as well as gain on the sale, exchange or other taxable disposition of such shares or ADSs, will be treated as ordinary income. The deductible portion of any mark-to-market loss, as well as loss on a sale, exchange or other disposition of our shares or ADSs to the extent that the amount of such loss does not exceed net mark-to-market gains previously included in income, will be treated as ordinary loss.
The mark-to-market election applies to the taxable year for which the election is made and all subsequent taxable years, unless the shares cease to be treated as marketable stock for purposes of the PFIC rules or the IRS consents to its revocation. The excess distribution rules described above generally will not apply to a U.S. Holder for tax years for which a mark-to-market election is in effect. However, if we were a PFIC for any year in which the U.S. Holder owns the shares or ADSs but before a mark-to-market election is made, the interest charge rules described above would apply to any mark-to-market gain recognized in the year the election is made.
PFIC reporting obligations
A U.S. Holder of PFIC shares must generally file an annual information return on IRS Form 8621 (Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund) containing such information as the U.S. Treasury Department may require. The failure to file IRS Form 8621 could result in the imposition of penalties and the extension of the statute of limitations with respect to U.S. federal income tax.
U.S. Holders are urged to consult their tax advisors as to our status as a PFIC, and the tax consequences to them if we were a PFIC, including the reporting requirements and the desirability of making, and the availability of, a QEF election or a mark-to-market election with respect to the shares or ADSs.
Non-corporate U.S. Holders that are individuals, estates or trusts and whose income exceeds certain thresholds generally are subject to a 3.8% tax on all or a portion of their net investment income, which may include their gross dividend income and net gains from the disposition of shares or ADSs. A United States person that is an individual, estate or trust is encouraged to consult its tax advisors regarding the applicability of this Medicare tax to its income and gains in respect of any investment in our shares or ADSs.
Information reporting with respect to foreign financial assets
Individual U.S. Holders may be subject to certain reporting obligations on IRS Form 8938 (Statement of Specified Foreign Financial Asset) with respect to the shares or ADSs for any taxable year during which the U.S. Holder’s aggregate value of these and certain other “specified foreign financial assets” exceed a threshold amount that varies with the filing status of the individual. This reporting obligation also applies to domestic entities formed or availed of to hold, directly or indirectly, specified foreign financial assets, including the shares or ADSs. Significant penalties can apply if U.S. Holders are required to make this disclosure and fail to do so.
Information reporting and backup withholding
In general, information reporting, on IRS Form 1099, will apply to dividends in respect of shares or ADSs and the proceeds from the sale, exchange or redemption of shares of ADSs that are paid to a holder of shares or ADSs within the United States (and in certain cases, outside the United States), unless such holder is an exempt recipient such as a corporation. Backup withholding (currently at a 24% rate) may apply to such payments if a holder of shares or ADSs fails to provide a taxpayer identification number (generally on an IRS Form W-9) or certification of other exempt status or fails to report in full dividend and interest income.
Backup withholding is not an additional tax. A U.S. Holder generally may obtain a refund of any amounts withheld under the backup withholding rules that exceed the U.S. Holder’s income tax liability by filing a refund claim with the Internal Revenue Service.
F. Dividends and Paying Agents
Not applicable
G. Statement by Experts
Not applicable.
H. Documents on Display
We are subject to the information reporting requirements of the Exchange Act applicable to foreign private issuers and under those requirements will file reports with the SEC. Those reports may be inspected without charge at the locations described below. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act.
We maintain a corporate website at www.steakholderfoods.com. We intend to post our Annual Report on Form 20-F on our website promptly following it being filed with the SEC. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report on Form 20-F. We have included our website address in this Annual Report on Form 20-F solely as an inactive textual reference.
Our SEC filings are available to you on the SEC’s website at http://www.sec.gov. This site contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC. The information on that website is not part of this Annual Report on Form 20-F.
With respect to references made in this Annual Report on Form 20-F to any contract or other document of Steakholder Foods, such references are not necessarily complete and you should refer to the exhibits attached or incorporated by reference to this Annual Report on Form 20-F for copies of the actual contract or document.
I. Subsidiary Information
Not applicable.
J. Annual Report to Security Holders
Not applicable.
| | ITEM 11. | QUANTITATIVE AND QUALITATIVE DISCLOSURE ON MARKET RISK |
For quantitative and qualitative information regarding our market risk, see “Item 5 — Operating and Financial Review and Prospects — Liquidity and Capital Resources — Quantitative and Qualitative Disclosures About Market Risk” and Note 23 to our financial statements.
| | ITEM 12. | DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
A. Debt Securities
Not applicable.
B. Warrants and Rights
Not applicable.
C. Other Securities
Not applicable.
D. American Depositary Shares
The Bank of New York Mellon is the depositary of the ADSs. Each ADS represents ten ordinary shares (or a right to receive ten ordinary shares). Each ADS will also represent any other securities, cash or other property which may be held by the depositary. The depositary’s office at which the ADSs are administered and its principal executive office are located at 240 Greenwich Street, New York, New York 10286.
A deposit agreement among us, the depositary, ADS holders and all other persons indirectly or beneficially holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs. A copy of the deposit agreement was filed as Exhibit 4.1 to our amended registration statement on Form F-1 filed with the SEC on March 5, 2021 and is incorporated by reference herein.
Fees and Expenses
Pursuant to the terms of the deposit agreement, the holders of the ADSs will be required to pay the following fees:
Persons depositing or withdrawing ordinary
shares or ADS holders must pay | | For: |
| $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) | | Issuance of ADSs, including issuances resulting from a distribution of ordinary shares or rights or other property Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
| | | |
| $.05 (or less) per ADS | | Any cash distribution to ADS holders |
| | | |
| A fee equivalent to the fee that would be payable if securities distributed to you had been ordinary shares and the ordinary shares had been deposited for issuance of ADSs | | Distribution of securities distributed to holders of deposited securities (including rights) that are distributed by the depositary to ADS holders |
| | | |
| $.05 (or less) per ADS per calendar year | | Depositary services |
| | | |
| Registration or transfer fees | | Transfer and registration of ordinary shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw ordinary shares |
| | | |
| Expenses of the depositary | | Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement)
Converting foreign currency to U.S. dollars |
| | | |
| Taxes and other governmental charges the depositary or the custodian have to pay on any ADSs or ordinary shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes | | As necessary |
| | | |
| Any charges incurred by the depositary or its agents for servicing the deposited securities | | As necessary |
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing ordinary shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may collect any of its fees by deduction from any cash distribution payable (or by selling a portion of securities or other property distributable) to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
From time to time, the depositary may make payments to us to reimburse us for costs and expenses generally arising out of establishment and maintenance of the ADS program, waive fees and expenses for services provided to us by the depositary or share revenue from the fees collected from ADS holders. In performing its duties under the deposit agreement, the depositary may use brokers, dealers, foreign currency or other service providers that are owned by or affiliated with the depositary and that may earn or share fees, spreads or commissions.
The depositary may convert foreign currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and not as an agent, fiduciary or broker on behalf of any other person and earns revenue, including, without limitation, fees and spreads that it will retain for its own account. The revenue is based on, among other things, the difference between the exchange rate assigned to the currency conversion made under the deposit agreement and the rate that the depositary or its affiliate receives when buying or selling foreign currency for its own account. The depositary makes no representation that the exchange rate used or obtained in any currency conversion under the deposit agreement will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be most favorable to ADS holders, subject to its obligations under the deposit agreement. The methodology used to determine exchange rates used in currency conversions is available upon request.
PART II
| | ITEM 13. | DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES |
Not applicable.
| | ITEM 14. | MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
Initial Public Offering
In March 2021, we sold 2,721,271 ADSs, each representing ten ordinary shares, no par value, in our U.S. initial public offering at a public offering price of $10.30 per ADS. Gross proceeds, including proceeds generated from the partial exercise of the underwriter’s option to purchase additional ADSs, were $28 million. Net proceeds to us, after deducting underwriting discounts and commissions and estimated offering expenses payable by us were approximately $24.7 million. The effective date of the registration statement on Form F-1 (File No. 333-253257) for our U.S. initial public offering of ADSs was March 11, 2021. The offering closed on March 17, 2021. H.C. Wainwright, Inc. acted as the sole underwriter in the offering.
The net proceeds from our initial public offering are held in cash and cash equivalents and we expect they will meet our capital requirements until the fourth quarter of 2022. We do not currently have any specific commitments or plans for acquisitions; to the extent we do engage in acquisitions, we will do so after ensuring that we will have sufficient funds available to meet our capital requirements, and such acquisitions are likely to affect our projected cash needs. None of the net proceeds of our initial public offering were paid directly or indirectly to any director, officer, general partner of ours or to their associates, persons owning ten percent or more of any class of our equity securities, or to any of our affiliates.
| | ITEM 15. | CONTROLS AND PROCEDURES |
| A. | Disclosure Controls and Procedures |
We have performed an evaluation of the effectiveness of our disclosure controls and procedures that are designed to ensure that the material financial and non-financial information required to be disclosed to the SEC is recorded, processed, summarized and reported timely. Based on our evaluation, our management, including our chief executive officer and chief financial officer, has concluded that our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act) as of the end of the period covered by this report are effective. Notwithstanding the foregoing, there can be no assurance that our disclosure controls and procedures will detect or uncover all failures of persons within the Company to disclose material information otherwise required to be set forth in our reports.
| B. | Management’s Annual Report on Internal Control over Financial Reporting |
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) promulgated under the Exchange Act. Our internal control system was designed to provide reasonable assurance to our management and board of directors regarding the reliability of financial reporting and the preparation and fair presentation of published financial statements for external purposes in accordance with generally accepted accounting principles. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation and may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with policies or procedures may deteriorate.
Our management assessed our internal control over financial reporting as of December 31, 2022, the end of our fiscal year. Management based its assessment on criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The assessment included evaluation of elements such as the design and operating effectiveness of key financial reporting controls, process documentation, accounting policies and our overall control environment.
Based on its assessment, our management has concluded that our internal control over financial reporting was effective as of the end of the fiscal year to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with International Financial Reporting Standards. We reviewed the results of our management’s assessment with the Audit Committee of our Board of Directors.
| C. | Attestation Report of the Registered Public Accounting Firm |
This Annual Report on Form 20-F does not include an attestation report of the company’s registered public accounting firm as the company is considered an emerging growth company.
| D. | Changes in Internal Control over Financing Reporting |
There were no changes in our internal control over financial reporting that occurred during the period covered by this Annual Report on Form 20-F that have materially affected, or that are reasonably likely to materially affect, our internal control over financial reporting.
| | ITEM 16A. | AUDIT COMMITTEE FINANCIAL EXPERT |
Our board of directors has determined that all members of our audit committee are financially literate as determined in accordance with Nasdaq rules and that Messrs. Eli Arad and David Gerbi are qualified to serve as “audit committee financial experts” as defined by SEC rules. The audit committee financial experts are independent directors.
We have adopted a Code of Business Conduct and Ethics (the “Code of Ethics”) that is applicable to all of our directors, executives and other employees, and those of our affiliates. A copy of the Code of Conduct is available on our website at www.steakholderfoods.com. Any waivers of the Code of Ethics for directors and officers require the approval of the audit committee of our board of directors. We expect that any amendments to the Code of Conduct will be disclosed on our website.
| | ITEM 16C. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Fees Paid to Independent Registered Public Accounting Firm
Somekh Chaikin, a member firm of KPMG International, located in Tel Aviv, Israel (PCAOB ID 1057), has served as our independent registered public accounting firm for 2022 and 2021. Following are Somekh Chaikin’s fees for professional services in each of the respective fiscal years:
| | | Year ended December 31, | |
| | | 2022 | | | 2021 | |
| | | USD, in thousands | |
| | | | |
Audit fees(1) | | | 295 | | | | 336 | |
Tax fees(2) | | | 25 | | | | 3 | |
| Total | | | 320 | | | | 339 | |
| (1) | Audit fees consist of fees billed or expected to be billed for the annual audit services engagement and other audit services, which are those services that only the external auditor can reasonably provide, and include the Company audit; statutory audits; comfort letters and consents; attest services; and assistance with and review of documents filed with the TASE and SEC. |
| | |
| (2) | Tax fees include fees billed for tax compliance services that were rendered during the most recent fiscal year, including the preparation of original and amended tax returns and claims for refund; tax consultations, such as assistance and representation in connection with tax audits and appeals, tax advice related to mergers and acquisitions, transfer pricing, and requests for rulings or technical advice from taxing authority; tax planning services; and expatriate tax planning and services. |
Policy on Audit Committee Pre-Approval of Audit and Permissible Non-Audit Services of Independent Auditors
Our Audit Committee has the sole authority to approve the scope of the audit and any audit-related services, as well as all audit fees and terms. The Audit Committee must pre-approve any audit and non-audit services provided by our independent registered public accounting firm. The Audit Committee will not approve the engagement of the independent registered public accounting firm to perform any services that the independent registered public accounting firm would be prohibited from providing under applicable laws, rules and regulations, including those of self-regulating organizations. The Audit Committee reviews and pre-approves the statutory audit fees that can be provided by the independent registered public accounting firm on an annual basis.
| | ITEM 16D.
| EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES |
Not applicable.
| | ITEM 16E.
| PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS
|
Not applicable.
| | ITEM 16F.
| CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT |
Not applicable.
| | ITEM 16G.
| CORPORATE GOVERNANCE |
Nasdaq Listing Rules and Home Country Practices
The Sarbanes-Oxley Act, as well as related rules subsequently implemented by the SEC, requires foreign private issuers, such as us, to comply with various corporate governance practices. As a foreign private issuer, we are permitted to follow certain Israeli corporate governance practices instead of Nasdaq rules, provided that we disclose which requirements we are not following and the equivalent Israeli requirements. Below is a concise summary of the significant ways in which our corporate governance practices differ from the corporate governance requirements of Nasdaq applicable to domestic U.S. listed companies:
| • | Quorum. As permitted under the Companies Law, pursuant to our articles of association, the quorum required for an ordinary meeting of shareholders consists of at least two shareholders present in person or by proxy who hold or represent between them at least 25% of the voting power of our shares (and, with respect to an adjourned meeting, generally one or more shareholders who hold or represent any number of shares), instead of 33 1/3% of the issued share capital provided under Nasdaq Listing Rule 5260(c). |
| • | Shareholder Approval. Although the Nasdaq Listing Rules generally require shareholder approval of equity compensation plans and material amendments thereto, we follow Israeli practice, which is to have such plans and amendments approved only by the board of directors, unless such arrangements are for the compensation of chief executive officer or directors, in which case they also require the approval of the compensation committee and the shareholders. In addition, rather than follow the Nasdaq Listing Rules requiring shareholder approval for the issuance of securities in certain circumstances, we follow Israeli law, under which a private placement of securities requires approval by our board of directors and shareholders if it will cause a person to become a controlling shareholder (generally presumed at 25% ownership) or if: (a) the securities issued amount to 20% or more of our outstanding voting rights before the issuance; (b) some or all of the consideration is other than cash or listed securities or the transaction is not on market terms; and (c) transaction will increase the relative holdings of a shareholder that holds 5% or more of our outstanding share capital or voting rights or will cause any person to become, as a result of the issuance, a holder of more than 5% of our outstanding share capital or voting rights. |
| • | Executive Sessions. While the Nasdaq Listing Rules require that “independent directors,” as defined in the Nasdaq Listing Rules, must have regularly scheduled meetings at which only “independent directors” are present. Israeli law does not require, nor do our independent directors necessarily conduct, regularly scheduled meetings at which only they are present. |
| | ITEM 16H.
| MINE SAFETY DISCLOSURE |
Not applicable.
| | ITEM 16I. | DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
Not applicable.
PART III
| | ITEM 17. | FINANCIAL STATEMENTS |
The Registrant has responded to Item 18 in lieu of responding to this Item.
| | ITEM 18.
| FINANCIAL STATEMENTS |
See the financial statements beginning on page F-1. The following financial statements and financial statement schedules are filed as part of this Annual Report on Form 20-F together with the report of the independent registered public accounting firm.