Exhibit 99.2

Acquisition of Avidea Technologies December 2021

2 This presentation includes express and implied “forward - looking statements,” including forward - looking statements within the mea ning of the Private Securities Litigation Reform Act of 1995. Forward looking statements include all statements that are not historical facts, and in some cases, can b e i dentified by terms such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “po tential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward - looking statements contai ned in this presentation include, but are not limited to, statements about our product development activities and clinical trials, our regulatory filings and approvals , o ur ability to develop and advance our current and future product candidates and programs, our ability to establish and maintain collaborations or strategic relationships or ob tai n additional funding, the rate and degree of market acceptance and clinical utility of our product candidates, the ability and willingness of our third - party collaborators t o continue research and development activities relating to our product candidates, our and our collaborators’ ability to protect our intellectual property for our products. By their nature, these statements are subject to numerous risks and uncertainties, including factors beyond our control, that could cause actual results, performance or achie vem ent to differ materially and adversely from those anticipated or implied in the statements. You should not rely upon forward - looking statements as predictions of future eve nts. Although our management believes that the expectations reflected in our statements are reasonable, we cannot guarantee that the future results, performance or even ts and circumstances described in the forward - looking statements will be achieved or occur. Recipients are cautioned not to place undue reliance on these forward - looking stat ements, which speak only as of the date such statements are made and should not be construed as statements of fact. Certain information contained in this presentation and statements made orally during this presentation relate to or are based on studies, publications, surveys and other data obtained from third - party sources and our own internal estimates and research. While we believe these third - party studies, publi cations, surveys and other data to be reliable as of the date of this presentation, it has not independently verified, and makes no representations as to the adequacy, fai rne ss, accuracy or completeness of, any information obtained from third - party sources. In addition, no independent source has evaluated the reasonableness or accuracy o f our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal esti mat es and research.
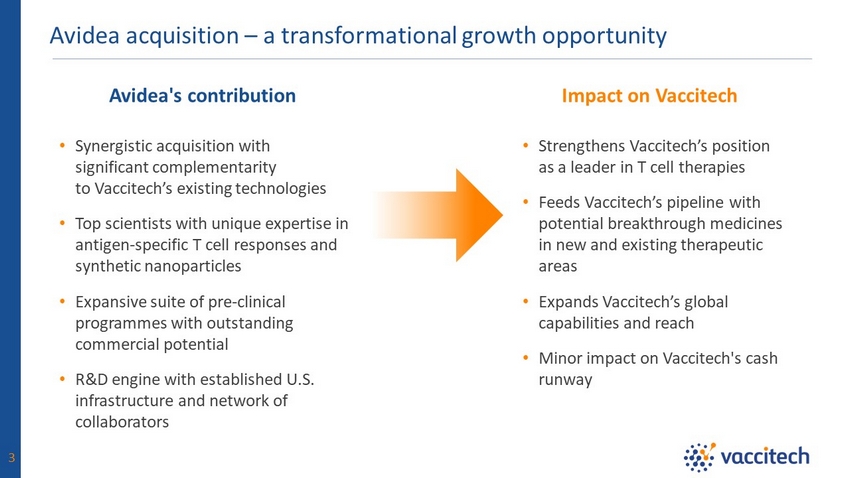
Avidea acquisition – a transformational growth opportunity 3 • Synergistic acquisition with significant complementarity to Vaccitech’s existing technologies • Top scientists with unique expertise in antigen - specific T cell responses and synthetic nanoparticles • Expansive suite of pre - clinical programmes with outstanding commercial potential • R&D engine with established U.S. infrastructure and network of collaborators • Strengthens Vaccitech’s position as a leader in T cell therapies • Feeds Vaccitech’s pipeline with potential breakthrough medicines in new and existing therapeutic areas • Expands Vaccitech’s global capabilities and reach • Minor impact on Vaccitech's cash runway Avidea's contribution Impact on Vaccitech
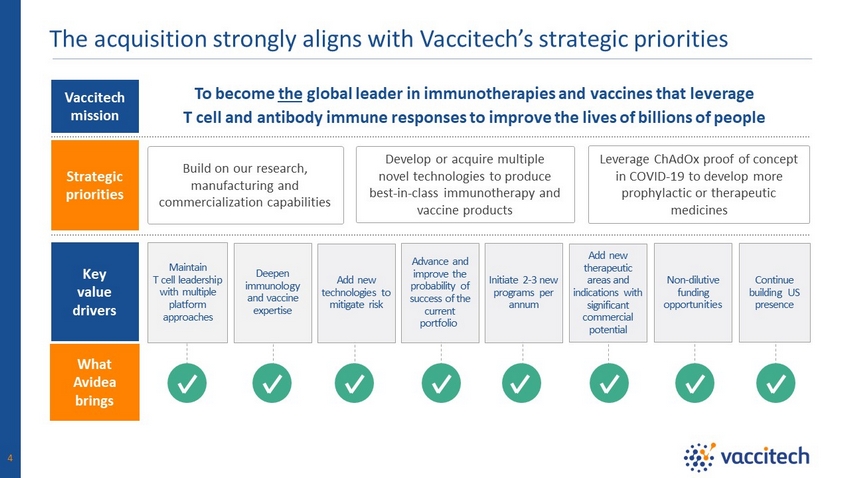
Strategic priorities Key value drivers Vaccitech mission What Avidea brings The acquisition strongly aligns with Vaccitech’s strategic priorities Build on our research, manufacturing and commercialization capabilities Leverage ChAdOx proof of concept in COVID - 19 to develop more prophylactic or therapeutic medicines Develop or acquire multiple novel technologies to produce best - in - class immunotherapy and vaccine products To become the global leader in immunotherapies and vaccines that leverage T cell and antibody immune responses to improve the lives of billions of people 4 Maintain T cell leadership with multiple platform approaches Deepen immunology and vaccine expertise Continue building US presence Add new therapeutic areas and indications with significant commercial potential Add new technologies to mitigate risk Initiate 2 - 3 new programs per annum Advance and improve the probability of success of the current portfolio Non - dilutive funding opportunities
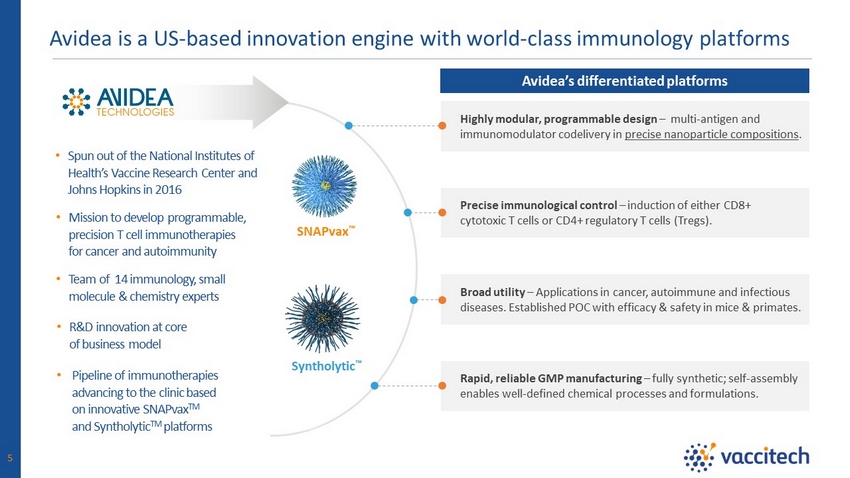
Avidea’s differentiated platforms Rapid, reliable GMP manufacturing – fully synthetic; self - assembly enables well - defined chemical processes and formulations. Broad utility – Applications in cancer, autoimmune and infectious diseases. Established POC with efficacy & safety in mice & primates. Highly modular, programmable design – multi - antigen and immunomodulator codelivery in precise nanoparticle compositions . Precise immunological control – induction of either CD8+ cytotoxic T cells or CD4+ regulatory T cells (Tregs). SNAPvax Ρ • Mission to develop programmable, precision T cell immunotherapies for cancer and autoimmunity • Team of 14 immunology, small molecule & chemistry experts • R&D innovation at core of business model • Pipeline of immunotherapies advancing to the clinic based on innovative SNAPvax TM and Syntholytic TM platforms • Spun out of the National Institutes of Health’s Vaccine Research Center and Johns Hopkins in 2016 Syntholytic Ρ 5 Avidea is a US - based innovation engine with world - class immunology platforms
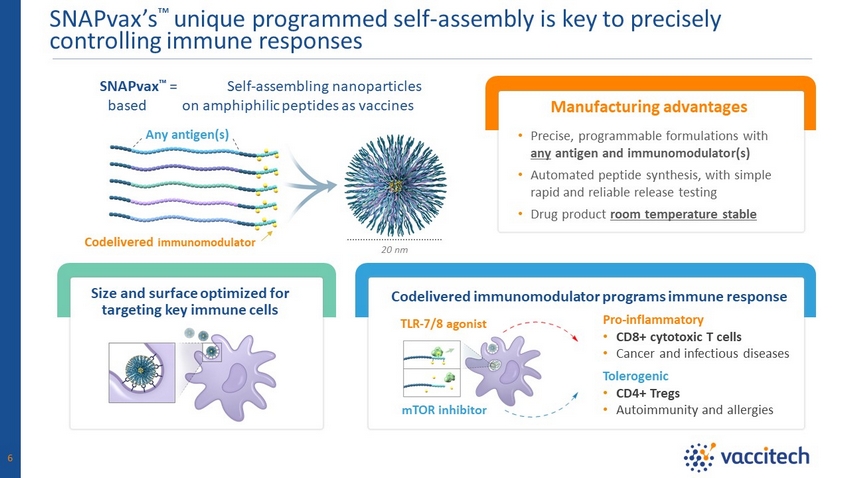
SNAPvax’s Ρ unique programmed self - assembly is key to precisely controlling immune responses Pro - inflammatory • CD8+ cytotoxic T cells • Cancer and infectious diseases 20 nm SNAPvax Ρ = Self - assembling nanoparticles based on amphiphilic peptides as vaccines Codelivered immunomodulator programs immune response TLR - 7/8 agonist mTOR inhibitor Tolerogenic • CD4+ Tregs • Autoimmunity and allergies Codelivered immunomodulator Any antigen(s) Size and surface optimized for targeting key immune cells Manufacturing advantages • Precise, programmable formulations with any antigen and immunomodulator(s) • Automated peptide synthesis, with simple rapid and reliable release testing • Drug product room temperature stable 6
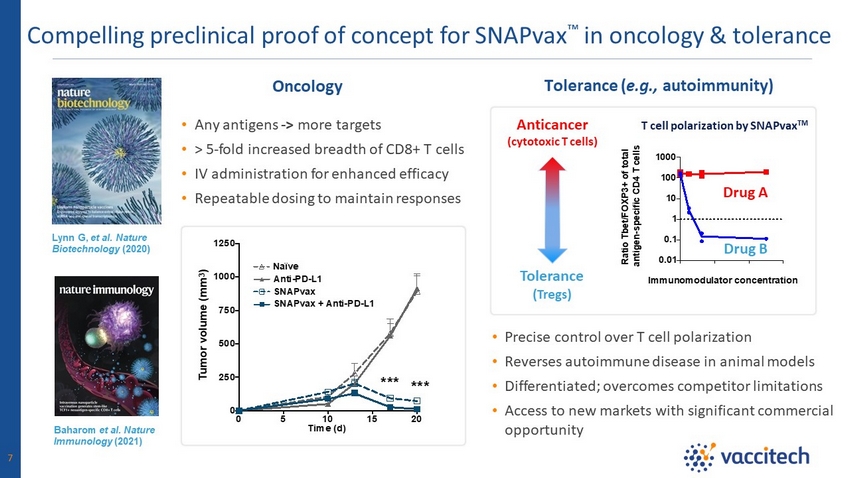
Compelling preclinical proof of concept for SNAPvax Ρ in oncology & tolerance 7 Lynn G, et al. Nature Biotechnology (2020) Oncology Baharom et al. Nature Immunology (2021) Tolerance ( e.g., autoimmunity) Anticancer (cytotoxic T cells) Tolerance (Tregs) • Any antigens - > more targets • > 5 - fold increased breadth of CD8+ T cells • IV administration for enhanced efficacy • Repeatable dosing to maintain responses • Precise control over T cell polarization • Reverses autoimmune disease in animal models • Differentiated; overcomes competitor limitations • Access to new markets with significant commercial opportunity T cell polarization by SNAPvax TM 0 5 10 15 20 0 250 500 750 1000 1250 *** *** Time (d) T u m o r v o l u m e ( m m 3 ) Naïve SNAPvax Anti-PD-L1 SNAPvax + Anti-PD-L1 Drug A Drug B
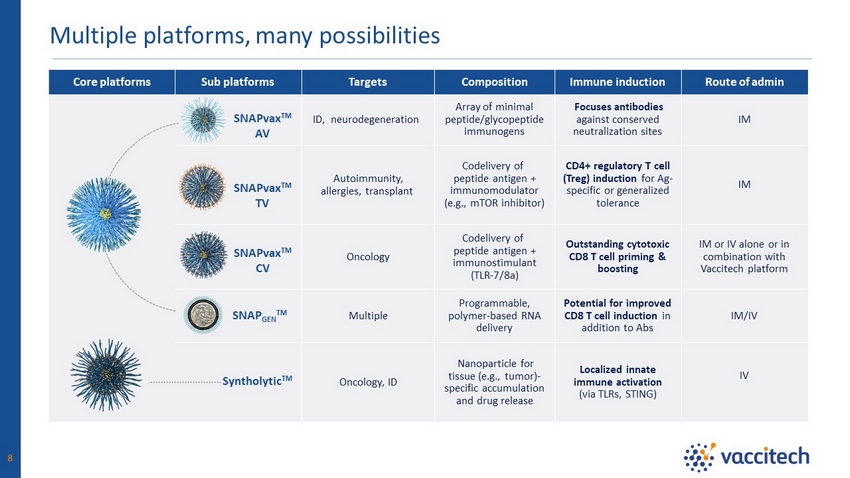
Core platforms Sub platforms Targets Composition Immune induction Route of admin ID, neurodegeneration Array of minimal peptide/glycopeptide immunogens Focuses antibodies against conserved neutralization sites IM Autoimmunity, allergies, transplant Codelivery of peptide antigen + immunomodulator (e.g., mTOR inhibitor) CD4+ regulatory T cell (Treg) induction for Ag - specific or generalized tolerance IM Oncology Codelivery of peptide antigen + immunostimulant (TLR - 7/8a) Outstanding cytotoxic CD8 T cell priming & boosting IM or IV alone or in combination with Vaccitech platform Multiple Programmable, polymer - based RNA delivery Potential for improved CD8 T cell induction in addition to Abs IM/IV Oncology, ID Nanoparticle for tissue (e.g., tumor) - specific accumulation and drug release Localized innate immune activation (via TLRs, STING) IV Multiple platforms, many possibilities 8 Syntholytic TM SNAPvax TM AV SNAPvax TM TV SNAPvax TM CV SNAP GEN TM
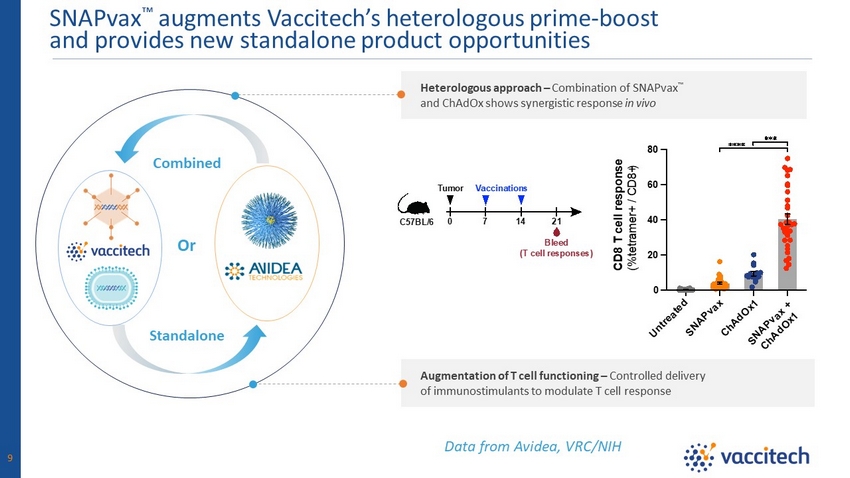
Augmentation of T cell functioning – Controlled delivery of immunostimulants to modulate T cell response Heterologous approach – Combination of SNAPvax Ρ and ChAdOx shows synergistic response in vivo SNAPvax Ρ augments Vaccitech’s heterologous prime - boost and provides new standalone product opportunities Data from Avidea, VRC/NIH 0 21 7 14 C57BL/6 Tumor Bleed (T cell responses) Vaccinations Or Combined Standalone 9
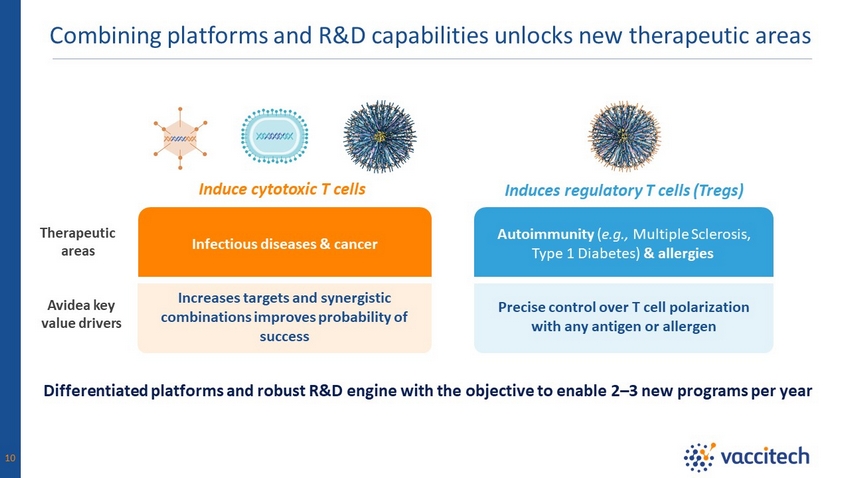
Combining platforms and R&D capabilities unlocks new therapeutic areas 10 Differentiated platforms and robust R&D engine with the objective to enable 2 – 3 new programs per year Avidea key value drivers Infectious diseases & cancer Autoimmunity ( e.g., Multiple Sclerosis, Type 1 Diabetes) & allergies Precise control over T cell polarization with any antigen or allergen Increases targets and synergistic combinations improves probability of success Therapeutic areas Induce cytotoxic T cells Induces regulatory T cells (Tregs)
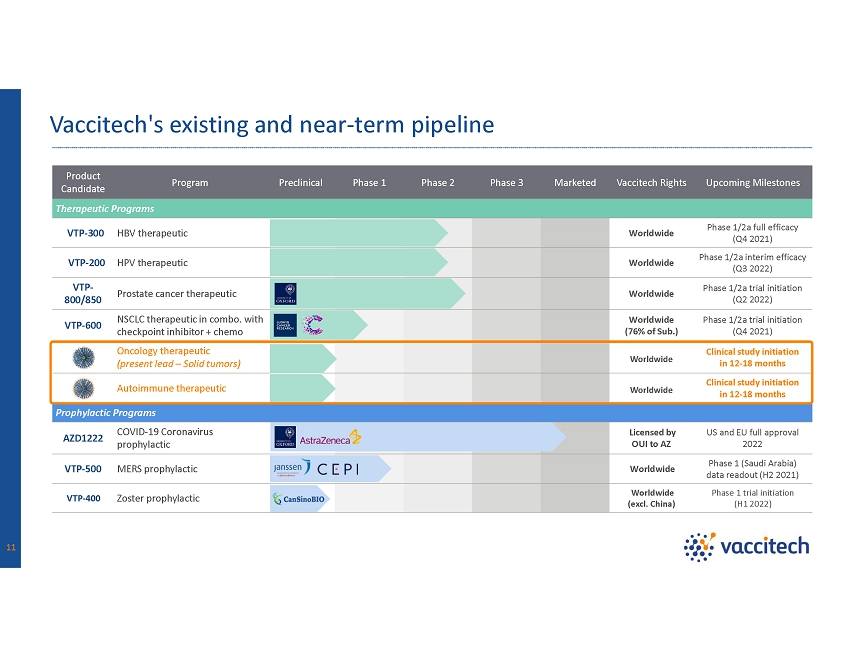
Product Candidate Program Preclinical Phase 1 Phase 2 Phase 3 Marketed Vaccitech Rights Upcoming Milestones Therapeutic Programs VTP - 300 HBV therapeutic Worldwide Phase 1/2a full efficacy (Q4 2021) VTP - 200 HPV therapeutic Worldwide Phase 1/2a interim efficacy (Q3 2022) VTP - 800/850 Prostate cancer therapeutic Worldwide Phase 1/2a trial initiation (Q2 2022) VTP - 600 NSCLC therapeutic in combo. with checkpoint inhibitor + chemo Worldwide (76% of Sub.) Phase 1/2a trial initiation (Q4 2021) Oncology therapeutic (present lead – Solid tumors) Worldwide Clinical study initiation in 12 - 18 months Autoimmune therapeutic Worldwide Clinical study initiation in 12 - 18 months Prophylactic Programs AZD1222 COVID - 19 Coronavirus prophylactic Worldwide (excl. China) US and EU full approval 2022 VTP - 500 MERS prophylactic Worldwide Phase 1 (Saudi Arabia) data readout (H2 2021) VTP - 400 Zoster prophylactic Licensed by OUI to AZ Phase 1 trial initiation (H1 2022) Vaccitech's existing and near - term pipeline 11
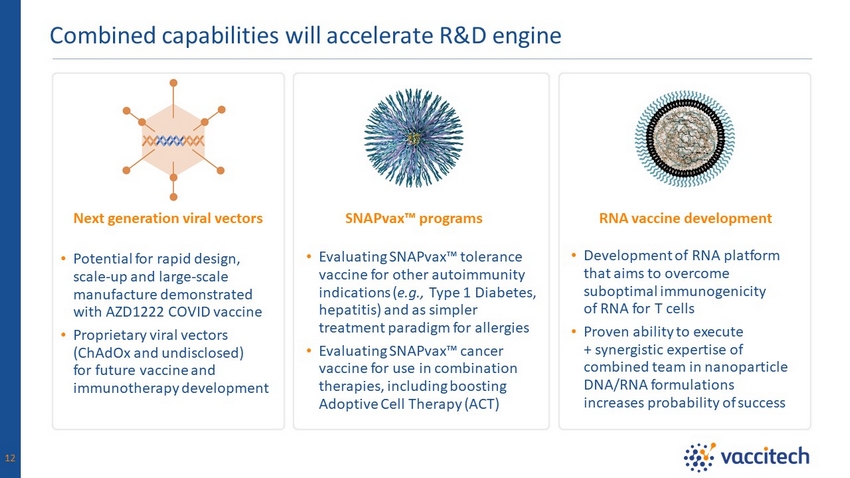
Combined capabilities will accelerate R&D engine 12 SNAPvax Ρ programs Next generation viral vectors RNA vaccine development • Evaluating SNAPvax Ρ tolerance vaccine for other autoimmunity indications ( e.g., Type 1 Diabetes, hepatitis) and as simpler treatment paradigm for allergies • Evaluating SNAPvax Ρ cancer vaccine for use in combination therapies, including boosting Adoptive Cell Therapy (ACT) • Development of RNA platform that aims to overcome suboptimal immunogenicity of RNA for T cells • Proven ability to execute + synergistic expertise of combined team in nanoparticle DNA/RNA formulations increases probability of success • Potential for rapid design, scale - up and large - scale manufacture demonstrated with AZD1222 COVID vaccine • Proprietary viral vectors (ChAdOx and undisclosed) for future vaccine and immunotherapy development

Transaction overview 13 • Cash post transaction is $217m. Cash runway at least into 2024 • Adds new programs to existing pipeline with two expected to go to the clinic in next 12 - 18 months. Expected combined R&D spend of $45m - $50m in 2022 • Transaction has been unanimously approved by both Vaccitech and Avidea board of directors • Closing took place on 10th December Funding Financial impact Purchase price Approvals and timing • No financing conditions. Financing through issuance of new ADSs and cash on balance sheet • Consideration: approx. $12.5 million cash + $27.5 million equity • Transaction value of approximately $40m (excluding net debt) • Certain milestones upon reaching clinical points or commercialisation
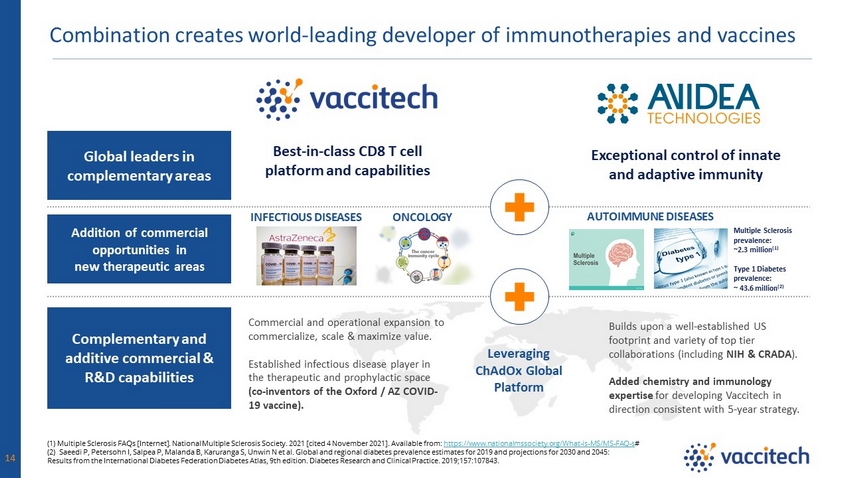
Combination creates world - leading developer of immunotherapies and vaccines 14 ` Leveraging ChAdOx Global Platform Best - in - class CD8 T cell platform and capabilities Exceptional control of innate and adaptive immunity ONCOLOGY INFECTIOUS DISEASES AUTOIMMUNE DISEASES Multiple Sclerosis prevalence: ~2.3 million (1) Type 1 Diabetes prevalence: ~ 43.6 million (2) Global leaders in complementary areas Addition of commercial opportunities in new therapeutic areas Complementary and additive commercial & R&D capabilities (1) Multiple Sclerosis FAQs [Internet]. National Multiple Sclerosis Society. 2021 [cited 4 November 2021]. Available from: https://www.nationalmssociety.org/What - is - MS/MS - FAQ - s # (2) Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N et al. Global and regional diabetes prevalence estimates for 20 19 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Research and Clinical Practice. 2019 ;15 7:107843. Commercial and operational expansion to commercialize, scale & maximize value. Established infectious disease player in the therapeutic and prophylactic space (co - inventors of the Oxford / AZ COVID - 19 vaccine). Builds upon a well - established US footprint and variety of top tier collaborations (including NIH & CRADA ). Added chemistry and immunology expertise for developing Vaccitech in direction consistent with 5 - year strategy.













