Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with our condensed consolidated financial statements and the related notes appearing elsewhere in this unaudited Quarterly Report on Form 10-Q and our audited financial statements and related notes thereto for the year ended December 31, 2022 included in our Annual Report on Form 10-K for the year ended December 31, 2022, which was filed with the SEC on March 24, 2023. Some of the information contained in this discussion and analysis or set forth elsewhere in this report, including information with respect to our plans and strategy for our business, includes forward-looking statements that involve risks, uncertainties, and assumptions. Factors that might cause future results to differ materially from those projected in the forward-looking statements include, but are not limited to, those set forth in our Annual Report on Form 10-K and in other filings with the SEC.
Overview
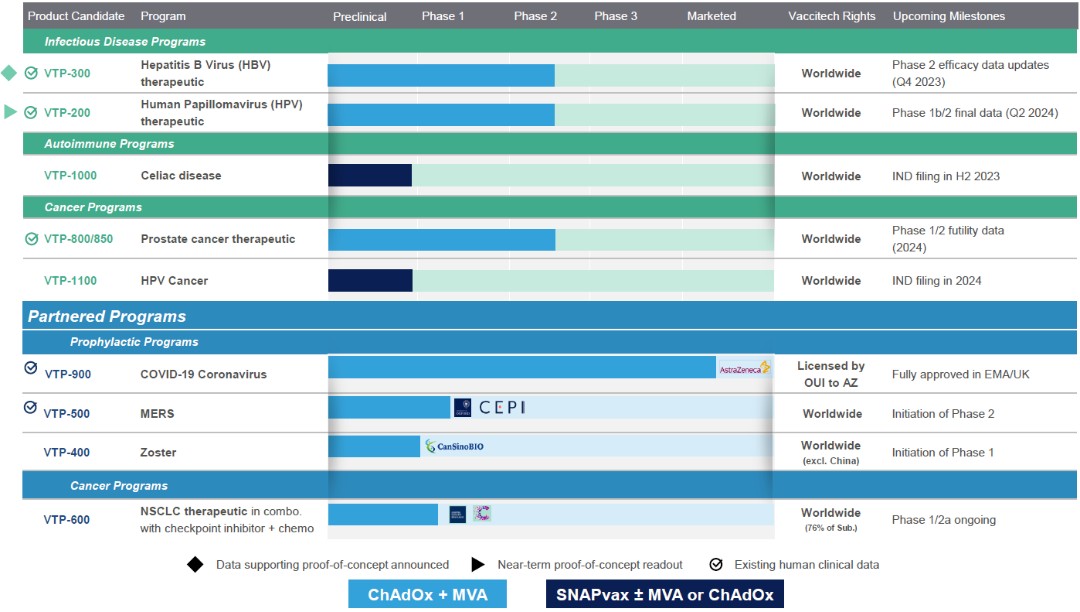
We are a clinical-stage biopharmaceutical company engaged in the discovery and development of novel T cell immunotherapeutics designed to harness the power of the immune system to treat chronic infectious diseases, cancer and autoimmunity. We aim to treat and prevent infectious diseases and cancer by using our proprietary platforms to develop product candidates that stimulate powerful, targeted immune responses against pathogens, infected cells, and tumor cells. We design these product candidates to stimulate immune responses that are robust, highly specific, and are differentiated by the magnitude of the T cell populations induced, which exhibit critical functionality and durability. In the field of autoimmunity, we use our proprietary platform to develop product candidates that are designed to induce regulatory T cells to suppress specific immune responses and prevent/reverse autoimmunity. We are focused on applying our platform capabilities and the expertise of our team to address significant unmet medical needs in two settings - the therapeutic setting, for the treatment of chronic infectious diseases, cancer, and autoimmunity and the prophylactic setting, for the prevention of infectious diseases, based on our platform’s ability to respond rapidly to epidemic and pandemic threats.
We have a broad pipeline of both clinical and preclinical stage therapeutic and prophylactic programs. Our current therapeutic programs include VTP-300 for the treatment of chronic hepatitis B infection, or CHB, VTP-200 for the treatment of HPV, VTP-850 for the treatment of prostate cancer, VTP-600 for the treatment of non-small cell lung cancer, or NSCLC, VTP-1000 for treatment of celiac disease, and VTP-1100 for treatment of HPV-associated cancers. The latter two programs are designed to utilize our SNAPvax platform. Our current prophylactic programs include VTP-400 for the prevention of herpes zoster, or shingles, and VTP-500 for the prevention of MERS. In addition, we co-invented a COVID-19 vaccine with the University of Oxford, the rights to which we assigned to Oxford University Innovation, or OUI, to facilitate the license of those rights by OUI to AstraZeneca UK Limited, or AstraZeneca. The vaccine, formerly referred to as AZD1222, is now authorized for use under the marketing name Vaxzevria in a number of countries. AstraZeneca has exclusive worldwide rights to develop and commercialize Vaxzevria.
On May 4, 2021, we completed our initial public offering, or IPO, pursuant to which we issued and sold 6,500,000 American Depository Shares, or ADSs, at a public offering price of $17.00 per ADS, resulting in net proceeds of $102.8 million, after deducting underwriting discounts and commissions and offering expenses. Prior to our IPO, we funded our operations primarily from private placements of our ordinary and preferred shares, private placements of loan notes convertible into ordinary shares, as well as from grants and licensing agreements, research tax credit payments, investments from non-controlling interest, and a $2.4 million upfront payment from OUI in July 2020 in connection with the Amendment, Assignment and Revenue Share Agreement, or the OUI License Agreement Amendment, related to the licensing of the COVID-19 vaccine, Vaxzevria. We do not expect to generate revenue from any of our own product candidates, excluding Vaxzevria, until we obtain regulatory authorization for one or more of such product candidates, if at all, and commercialize our products, or we enter into out-licensing agreements with third parties.
On March 28, 2022, pursuant to the OUI License Agreement Amendment, we were notified of the commencement of payments, arising from AstraZeneca’s commercial sales of Vaxzevria. Under the terms of an exclusive worldwide license agreement between OUI and AstraZeneca, OUI is entitled to milestone payments and royalties on commercial sales of Vaxzevria that began after the pandemic period. As part of the assignment from us to OUI, we are entitled to receive approximately 24% of payments received by OUI from AstraZeneca. For the three and six months ended June 30, 2023, we recognized $0.3 million and $0.8 million respectively as revenue (three and six months ended June 30, 2022: $17.1 million and $32.1 million). There is, however, no guarantee that such payments will continue in the future and, if they do, that we will be notified of such payments in a timely manner.