Barinthus Biotherapeutics Corporate Presentation Guiding the Immune System to Cure Disease January 2025

Disclosure 2 This presentation includes express and implied “forward-looking statements,” including forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward looking statements include all statements that are not historical facts, and in some cases, can be identified by terms such as “may,” “will,” “could,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “potential,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. Forward- looking statements contained in this presentation include, but are not limited to, statements regarding: our product development activities and clinical trials, including timing for readouts of any interim data for any of our programs and initiation of clinical trials, our regulatory filings and approvals, our estimated cash runway and cash burn, our ability to develop and advance our current and future product candidates and programs, our ability to establish and maintain collaborations or strategic relationships or obtain additional funding, the rate and degree of market acceptance and clinical utility of our product candidates, and the ability and willingness of our third-party collaborators to continue research and development activities relating to our product candidates. By their nature, these statements are subject to numerous risks and uncertainties, including factors beyond our control, that could cause actual results, performance or achievement to differ materially and adversely from those anticipated or implied in the statements. Such risks and uncertainties, include, without limitation, risks and uncertainties related to: preclinical and clinical studies, the success, cost and timing of our product development activities and planned and ongoing preclinical studies and clinical trials, including the risks of the timing for preliminary, interim or final data or initiation of our clinical trials may be delayed, the risk that interim or topline data may not reflect final data or results, our ability to execute on our strategy, regulatory developments, the risk that we may not achieve the anticipated benefits of our pipeline prioritization and corporate restructuring, our ability to fund our operations, and access capital, our cash runway, including the risk that our estimate of our cash runway may be incorrect, global economic uncertainty, including disruptions in the banking industry, and other risks, uncertainties and other factors identified in our filings with the Securities and Exchange Commission (the “SEC”), including our Annual Report on Form 10-K for the year ended December 31, 2023, our Quarterly Report on Form 10-Q for the most recently ended fiscal quarter and subsequent filings with the SEC. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our statements are reasonable, we cannot guarantee that the future results, performance or events and circumstances described in the forward-looking statements will be achieved or occur and actual results may vary. Recipients are cautioned not to place undue reliance on these forward- looking statements, which speak only as of the date such statements are made and should not be construed as statements of fact. Except as required by law, we do not assume any intent to update any forward-looking statements after the date on which the statement is made, whether as a result of new information, future events or circumstances or otherwise. Certain information contained in this presentation and statements made orally during this presentation relate to or are based on studies, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, publications, surveys and other data to be reliable as of the date of this presentation, it has not independently verified, and makes no representations as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal estimates and research.

Our Mission To advance the next generation of immunotherapies for autoimmunity and inflammatory diseases.

Company Overview 4 • Barinthus Bio is developing immunotherapies for autoimmunity and other inflammatory diseases (“I&I” area) • Publicly traded on Nasdaq under ticker BRNS • Current focus leveraging SNAP-TI platform to restore immune tolerance • Barinthus Bio’s legacy portfolio based on viral vector platforms to be advanced with partner support About us • Differentiated platform for antigen-specific immune tolerance, potentially more effective & patient friendly • Aims to reduce inflammation & restores the natural state of immune non-responsiveness to healthy tissue • Lead candidate for Celiac disease (VTP-1000) in ongoing Phase 1 clinical trial with data readout expected in mid-2025 • Advancing undisclosed preclinical candidates based on SNAP-TI platform for other indications within I&I area SNAP-TI Platform • Strong balance sheet: • Cash of $112 million.1 • Outstanding ordinary shares: 40.2 million.1 • Estimated cash runway into 2027.1 • No debt or outstanding warrants. Financials 1 As of December 31, 2024; preliminary estimate based on management's current views and may change as a result of management’s review of results and other factors. The preliminary financial estimate of the Company's cash as of December 31, 2024, may not ultimately be indicative of the Company’s results for such periods and actual results may differ materially from those described above. No independent registered public accounting firm has audited, reviewed or compiled, examined or performed any procedures with respect to these preliminary results, nor have they expressed any opinion or any other form of assurance on these preliminary estimated results.
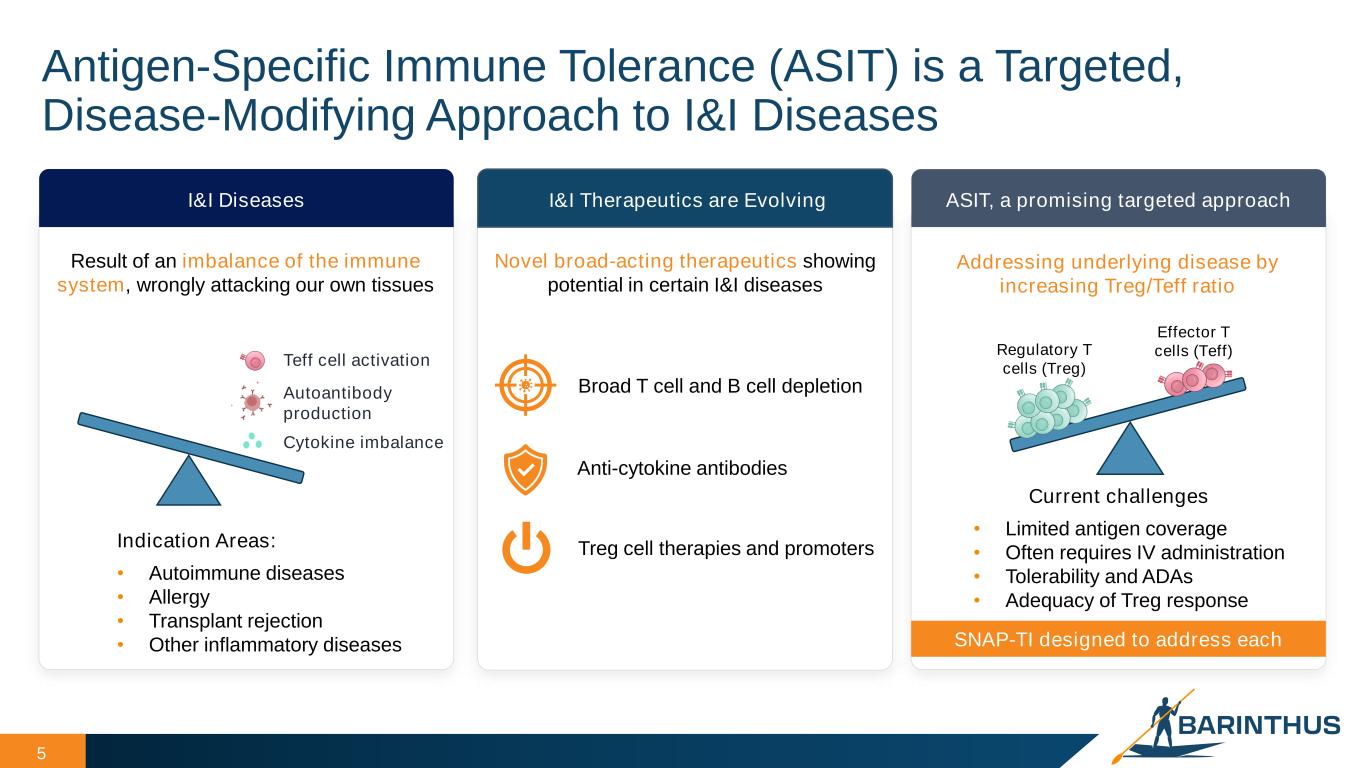
Antigen-Specific Immune Tolerance (ASIT) is a Targeted, Disease-Modifying Approach to I&I Diseases 5 I&I Diseases Teff cell activation Autoantibody production Cytokine imbalance Indication Areas: • Autoimmune diseases • Allergy • Transplant rejection • Other inflammatory diseases I&I Therapeutics are Evolving ASIT, a promising targeted approach Current challenges • Limited antigen coverage • Often requires IV administration • Tolerability and ADAs • Adequacy of Treg response Effector T cells (Teff)Regulatory T cells (Treg) Treg cell therapies and promoters Broad T cell and B cell depletion Anti-cytokine antibodies SNAP-TI designed to address each Result of an imbalance of the immune system, wrongly attacking our own tissues Novel broad-acting therapeutics showing potential in certain I&I diseases Addressing underlying disease by increasing Treg/Teff ratio

Teff (II) Anergy or clonal deletion (I) Induction or transdifferentiation SNAP-TI targets lymph node APCs, critical for T cell immunity Treg 6 SNAP-TI polarizes to Treg phenotype, restoring immune homeostasis SNAP-TI Designed to Promote Antigen-Specific Tolerance SNAP-TI Cytokines (e.g. IL-10) Enables • Broad antigen coverage • Patient friendly intramuscular/subcutaneous routes • Based on preclinical data, potentially improved tolerability and increased Treg/Teff ratio SOURCE: Based on unpublished preclinical data, Barinthus Bio, Data on File. APC: Antigen presenting cell Treg: Regulatory T cell Teff: Effector T cell APC Characteristics and Mechanism of Action Self-Assembling Nanoparticle • Co-delivery of multiple disease-associated antigens and an immunomodulator • Nanoparticles of precise composition for ease of manufacturing Target tissue cells
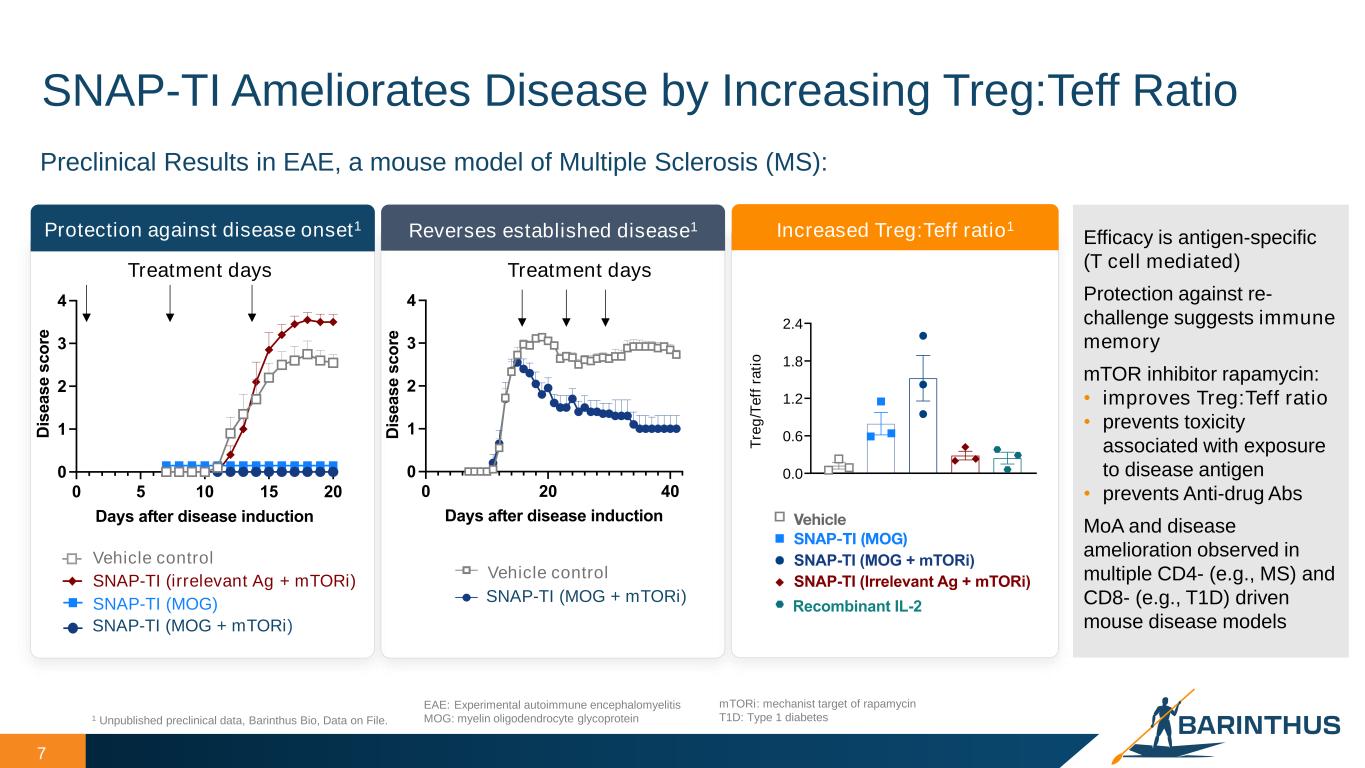
7 SNAP-TI Ameliorates Disease by Increasing Treg:Teff Ratio 1 Unpublished preclinical data, Barinthus Bio, Data on File. EAE: Experimental autoimmune encephalomyelitis MOG: myelin oligodendrocyte glycoprotein Preclinical Results in EAE, a mouse model of Multiple Sclerosis (MS): Protection against disease onset1 Reverses established disease1 Treatment days Vehicle control SNAP-TI (MOG + mTORi) SNAP-TI (MOG) SNAP-TI (irrelevant Ag + mTORi) 0 20 40 0 1 2 3 4 Days after disease induction D is e a s e s c o re Treatment days [1] Vehicle [6] SNAPvax TV MOG; Rapamycin; 40 nmol Vehicle control SNAP-TI (MOG + mTO i) 0 5 10 15 20 0 1 2 3 4 Days after disease induction D is e a s e s c o re Efficacy is antigen-specific (T cell mediated) Protection against re- challenge suggests immune memory mTOR inhibitor rapamycin: • improves Treg:Teff ratio • prevents toxicity associated with exposure to disease antigen • prevents Anti-drug Abs MoA and disease amelioration observed in multiple CD4- (e.g., MS) and CD8- (e.g., T1D) driven mouse disease models Increased Treg:Teff ratio1 mTORi: mechanist target of rapamycin T1D: Type 1 diabetes 0 5 10 15 20 0 1 2 3 4 Day D is e a s e s c o re Legend Legend Legend Legend 0 5 10 15 20 0 1 2 3 4 Day D is e a s e s c o re Legend Legend Legend Legend 0 5 10 15 20 0 1 2 3 4 Day D is e a s e s c o re Legend Legend Legend Legend 0.0 0.6 1.2 1.8 2.4 T re g /T e ff r a ti o
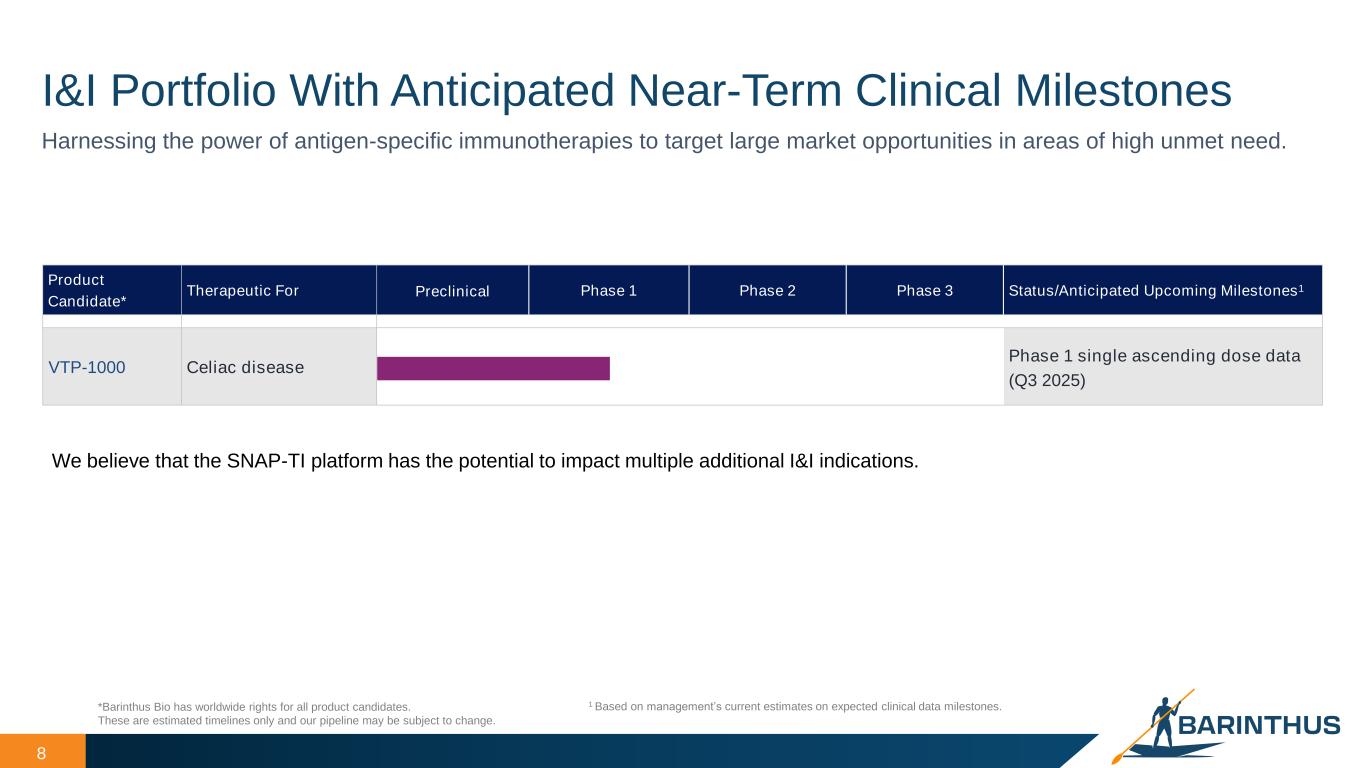
8 I&I Portfolio With Anticipated Near-Term Clinical Milestones *Barinthus Bio has worldwide rights for all product candidates. These are estimated timelines only and our pipeline may be subject to change. Harnessing the power of antigen-specific immunotherapies to target large market opportunities in areas of high unmet need. Product Candidate* Therapeutic For Preclinical Phase 1 Phase 2 Phase 3 Status/Anticipated Upcoming Milestones1 VTP-1000 Celiac disease Phase 1 single ascending dose data (Q3 2025) 1 Based on management’s current estimates on expected clinical data milestones. We believe that the SNAP-TI platform has the potential to impact multiple additional I&I indications.

VTP-1000 Celiac Disease Therapeutic Guiding the immune system to cure disease
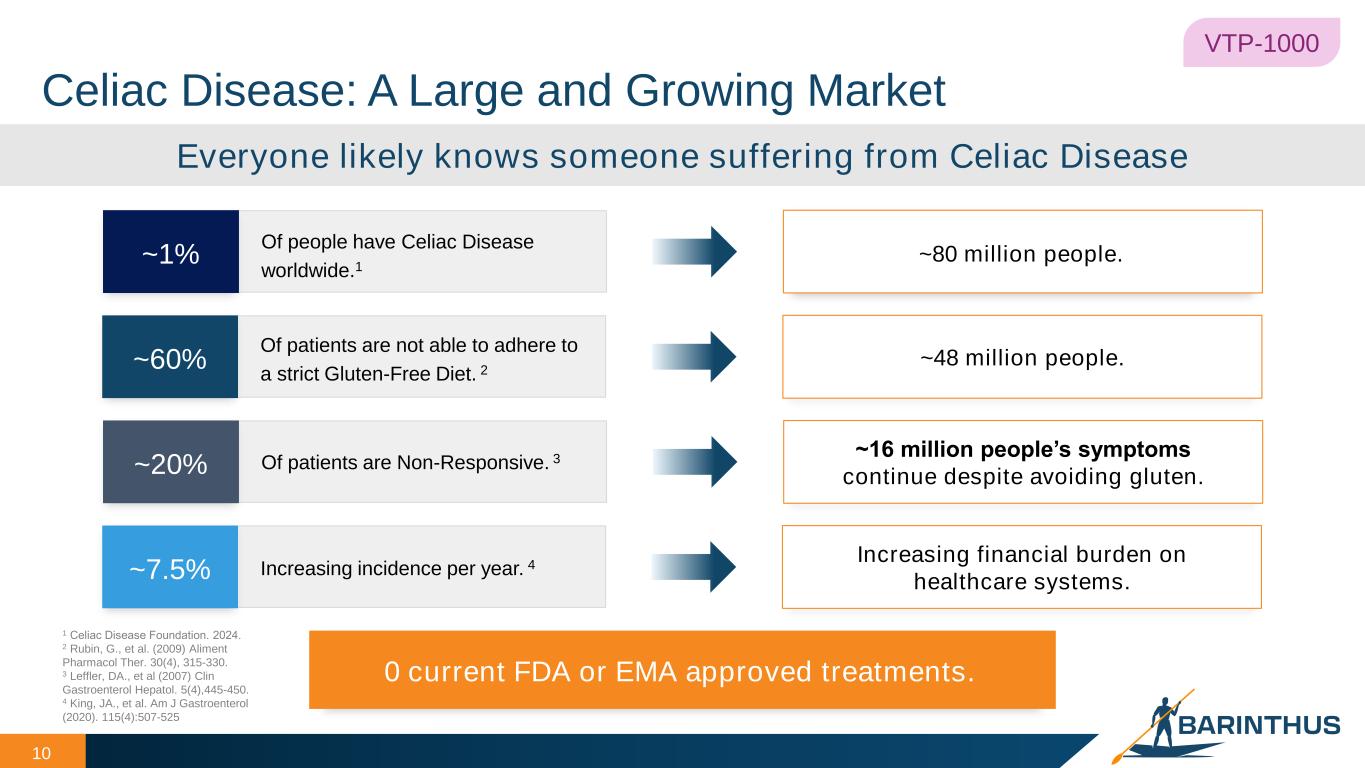
Celiac Disease: A Large and Growing Market 10 VTP-1000 0 current FDA or EMA approved treatments. Everyone likely knows someone suffering from Celiac Disease Of people have Celiac Disease worldwide.1 ~1% ~80 million people. Increasing incidence per year. 4~7.5% Increasing financial burden on healthcare systems. Of patients are Non-Responsive. 3~20% ~16 million people’s symptoms continue despite avoiding gluten. Of patients are not able to adhere to a strict Gluten-Free Diet. 2 ~60% ~48 million people. 1 Celiac Disease Foundation. 2024. 2 Rubin, G., et al. (2009) Aliment Pharmacol Ther. 30(4), 315-330. 3 Leffler, DA., et al (2007) Clin Gastroenterol Hepatol. 5(4),445-450. 4 King, JA., et al. Am J Gastroenterol (2020). 115(4):507-525
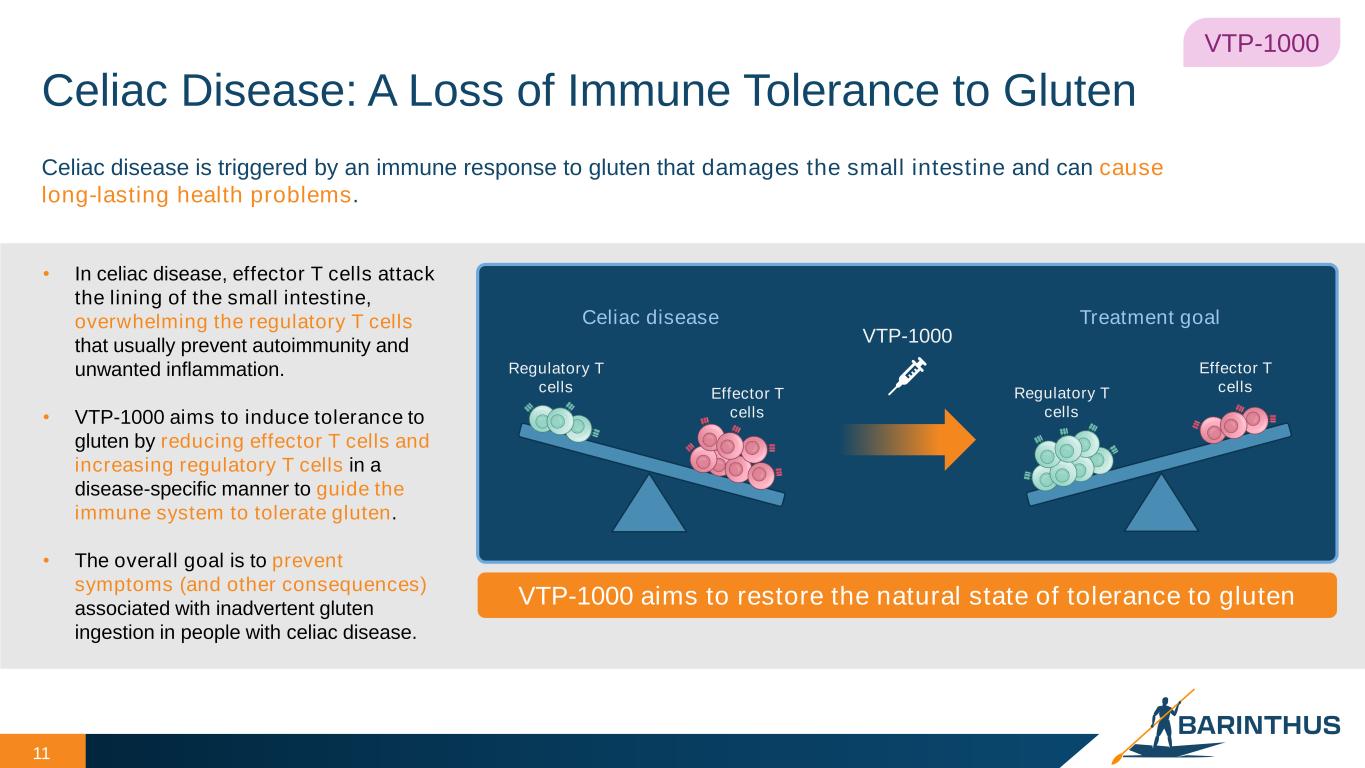
Celiac Disease: A Loss of Immune Tolerance to Gluten 11 VTP-1000 • In celiac disease, effector T cells attack the lining of the small intestine, overwhelming the regulatory T cells that usually prevent autoimmunity and unwanted inflammation. • VTP-1000 aims to induce tolerance to gluten by reducing effector T cells and increasing regulatory T cells in a disease-specific manner to guide the immune system to tolerate gluten. • The overall goal is to prevent symptoms (and other consequences) associated with inadvertent gluten ingestion in people with celiac disease. VTP-1000 aims to restore the natural state of tolerance to gluten Celiac disease is triggered by an immune response to gluten that damages the small intestine and can cause long-lasting health problems. Celiac disease Treatment goal Regulatory T cells Effector T cells VTP-1000 Regulatory T cells Effector T cells
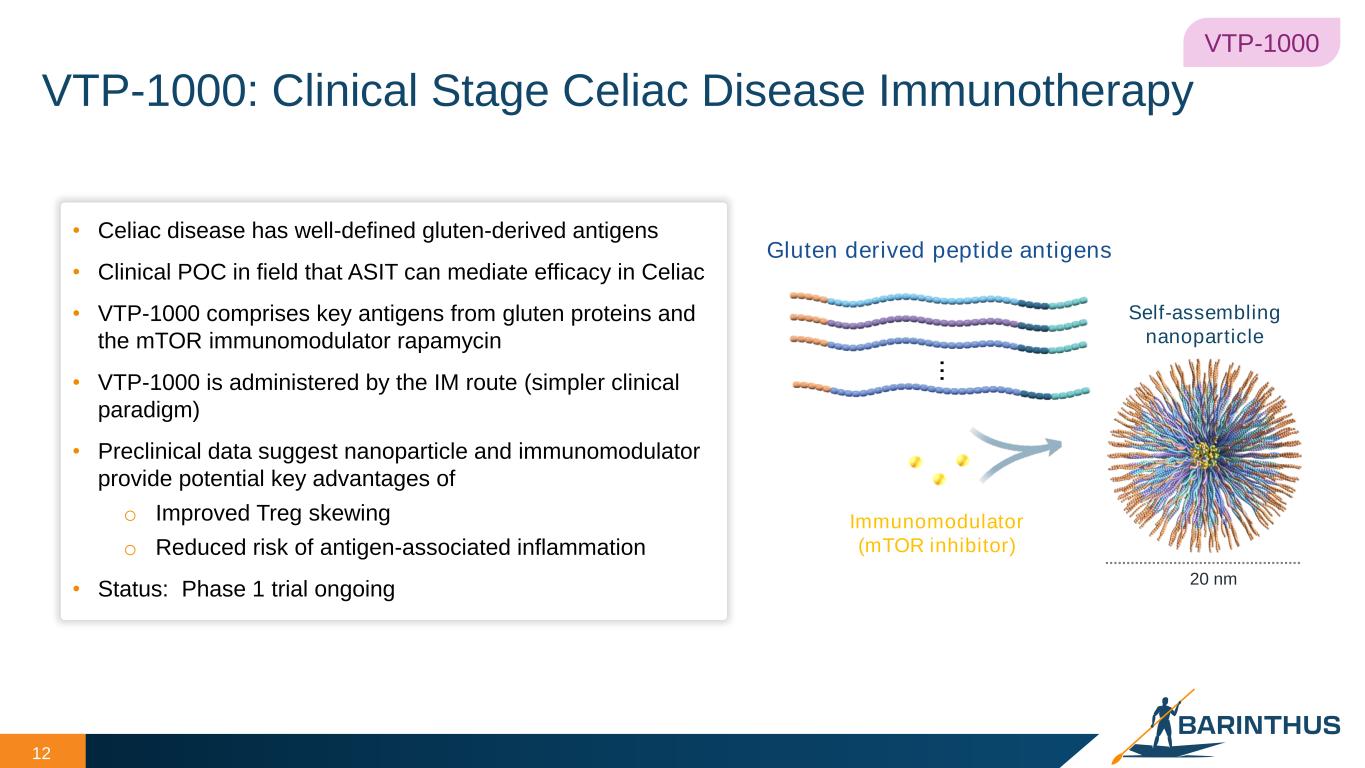
VTP-1000: Clinical Stage Celiac Disease Immunotherapy 12 • Celiac disease has well-defined gluten-derived antigens • Clinical POC in field that ASIT can mediate efficacy in Celiac • VTP-1000 comprises key antigens from gluten proteins and the mTOR immunomodulator rapamycin • VTP-1000 is administered by the IM route (simpler clinical paradigm) • Preclinical data suggest nanoparticle and immunomodulator provide potential key advantages of o Improved Treg skewing o Reduced risk of antigen-associated inflammation • Status: Phase 1 trial ongoing 20 nm Gluten derived peptide antigens Immunomodulator (mTOR inhibitor) .. . Self-assembling nanoparticle VTP-1000

13 VTP-1000 Preclinical Data Showed Potential Differentiated Profile VTP-1000 antigens recognized by Celiac disease subjects (n=6) VTP-1000 observed to reduce IL-2 and other inflammatory cytokines* *IL-1, IL-6, IL-8, TNF, IFNg, etc. VTP-1000 1 Unpublished preclinical data, Barinthus Bio, Data on File. Irrelevant antigens or VTP-1000 incubated with subject whole blood and assessed for recognition by T cells VTP-1000 accesses majority of APCs in lymphoid and disease tissue Other tissues ✓ Liver ✓ Spleen ✓ Intestines 0.0 0.1 0.2 5 10 15 1 10 100 Time (days) F lu o re s c e n c e in te n s it y (F o ld c h a n g e ) Intestines VTP-1000 Free peptide TP-1000 Gluten peptides Gluten peptides or VTP-1000 incubated with subject (n=6) blood VTP-1000 traffics to draining lymph nodes and remains detectable in APCs past day 15 0.0 0.2 5 10 15 0 50 100 Time (days) F re q u e n c y ( % ) a n ti g e n p o s it iv e 0.01 0.1 1 10 M a g n it u d e 0 10 20 30 IL -2 ( p g /m L ) ✱

AVALON: Phase 1 – Trial Design, Initiated Q3 2024 14 Key Inclusion Criteria Part A – Single Ascending Dose (N=18) Part B – Multiple Ascending Dose (N=24) Key Primary Endpoints VTP-1000 Dose Levels VTP-1000 (Part A/B) Placebo 1 N=4/6 N=2 2 N=4/6 N=2 3 N=4/6 N=2 Objective: Evaluating safety and tolerability of single and multiple doses of VTP-1000 in participants with Celiac disease • Safety: incidence of AEs and SAEs. • Changes from baseline in anti-tissue transglutaminase immunoglobulin A antibodies. Other Outcome Measures • Serum cytokine (IL-2) concentrations. • Diagnosis of celiac disease as confirmed by positive serology and intestinal histology. • Well-controlled, gluten restricted diet ≥12 months. Study Reference: NCT06310291 Dosing End of Follow up Day 1 Day 22 Day 1 Day 57Day 15 Day 29 Day 43 Dosing Dosing Dosing End of Follow up Gluten Challenge • Sequential dosing levels: 7-day gap from first 2 participants at each level and safety review before escalation to next dosing level. Next anticipated milestone: Single ascending dose data: Q3 2025
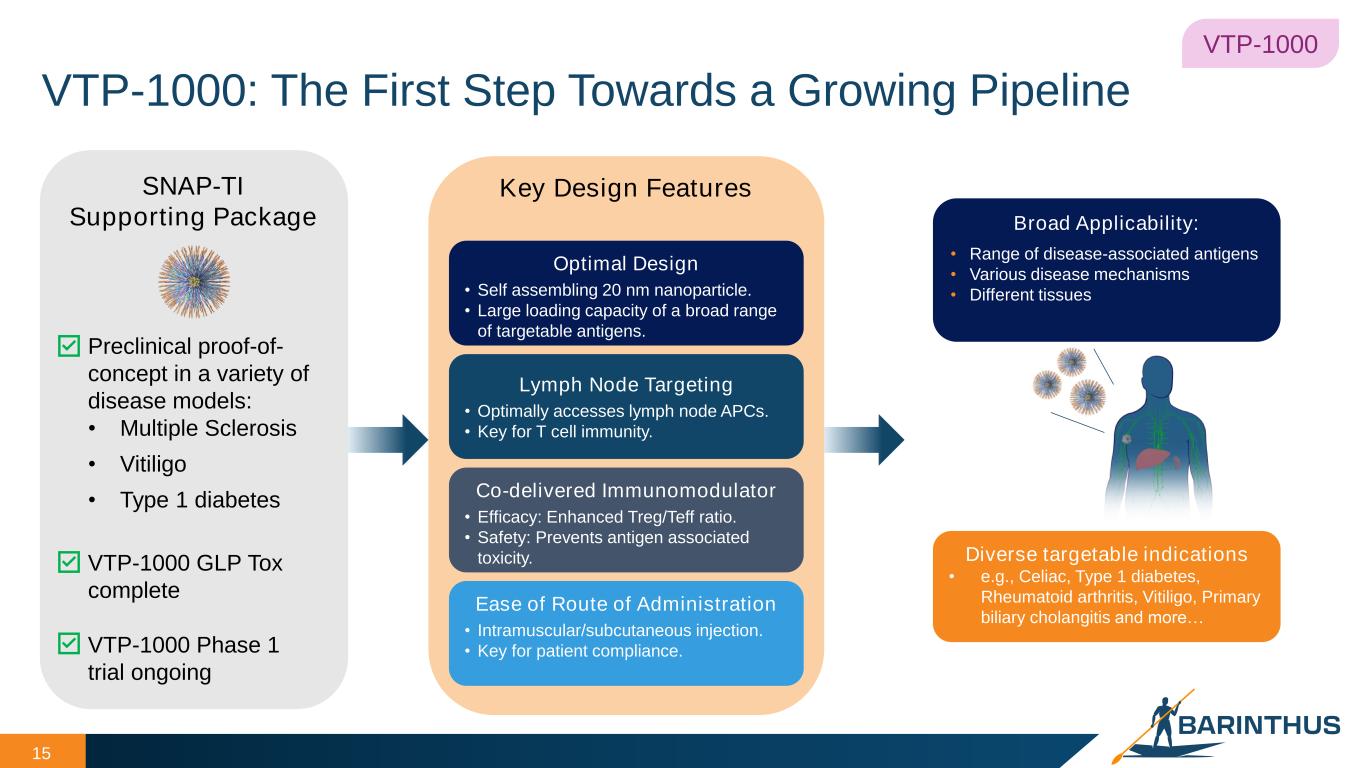
VTP-1000: The First Step Towards a Growing Pipeline 15 15 Key Design Features Optimal Design • Self assembling 20 nm nanoparticle. • Large loading capacity of a broad range of targetable antigens. Lymph Node Targeting • Optimally accesses lymph node APCs. • Key for T cell immunity. Co-delivered Immunomodulator • Efficacy: Enhanced Treg/Teff ratio. • Safety: Prevents antigen associated toxicity. Ease of Route of Administration • Intramuscular/subcutaneous injection. • Key for patient compliance. SNAP-TI Supporting Package Preclinical proof-of- concept in a variety of disease models: • Multiple Sclerosis • Vitiligo • Type 1 diabetes VTP-1000 GLP Tox complete VTP-1000 Phase 1 trial ongoing VTP-1000 Diverse targetable indications • e.g., Celiac, Type 1 diabetes, Rheumatoid arthritis, Vitiligo, Primary biliary cholangitis and more… Broad Applicability: • Range of disease-associated antigens • Various disease mechanisms • Different tissues

Viral Vector Platform Programs Program Looking for Partners to Advance Guiding the immune system to cure disease
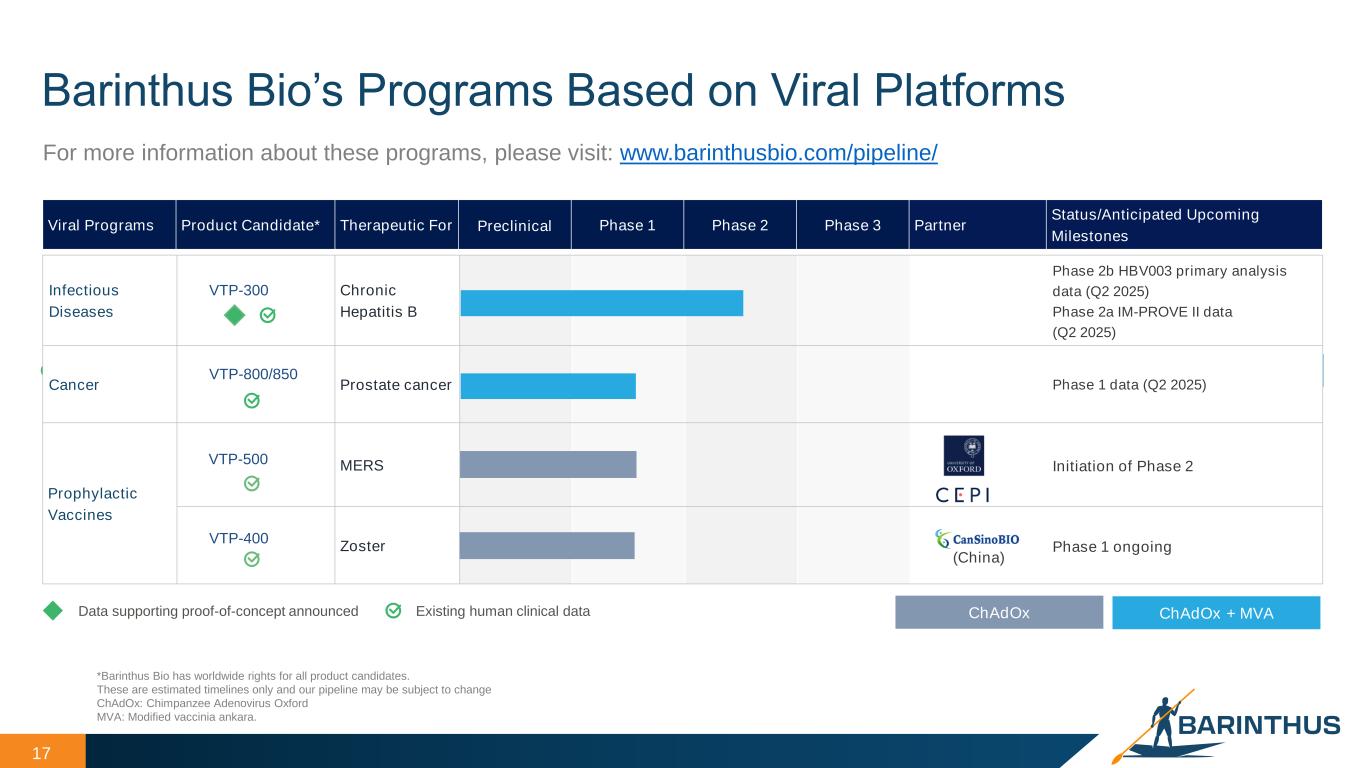
17 Barinthus Bio’s Programs Based on Viral Platforms *Barinthus Bio has worldwide rights for all product candidates. These are estimated timelines only and our pipeline may be subject to change ChAdOx: Chimpanzee Adenovirus Oxford MVA: Modified vaccinia ankara. Existing human clinical data ChAdOx + MVA Infectious Diseases VTP-300 Chronic Hepatitis B Phase 2b HBV003 primary analysis data (Q2 2025) Phase 2a IM-PROVE II data (Q2 2025) Cancer VTP-800/850 Prostate cancer Phase 1 data (Q2 2025) Prophylactic Vaccines VTP-500 MERS Initiation of Phase 2 VTP-400 Zoster (China) Phase 1 ongoing Viral Programs Product Candidate* Therapeutic For Preclinical Phase 1 Phase 2 Phase 3 Partner Status/Anticipated Upcoming Milestones For more information about these programs, please visit: www.barinthusbio.com/pipeline/ Existing human clinical dataData supporting proof-of-concept announced ChAdOx + MVAChAdOx

VTP-300 Hepatitis B Virus (HBV) Therapeutic Guiding the immune system to cure disease
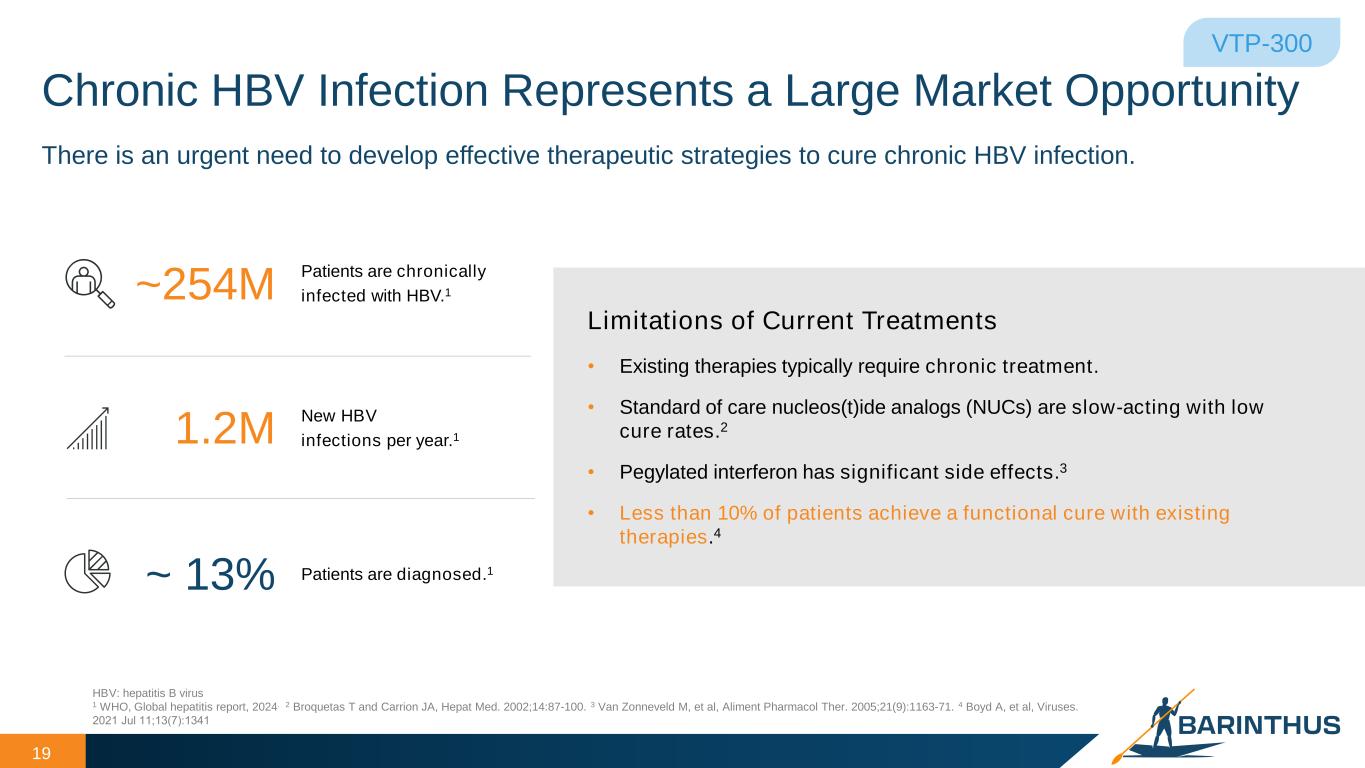
Chronic HBV Infection Represents a Large Market Opportunity 19 HBV: hepatitis B virus 1 WHO, Global hepatitis report, 2024. 2 Broquetas T and Carrion JA, Hepat Med. 2002;14:87-100. 3 Van Zonneveld M, et al, Aliment Pharmacol Ther. 2005;21(9):1163-71. 4 Boyd A, et al, Viruses. 2021 Jul 11;13(7):1341 • Existing therapies typically require chronic treatment. • Standard of care nucleos(t)ide analogs (NUCs) are slow-acting with low cure rates.2 • Pegylated interferon has significant side effects.3 • Less than 10% of patients achieve a functional cure with existing therapies.4 Limitations of Current Treatments Patients are chronically infected with HBV.1~254M New HBV infections per year.11.2M Patients are diagnosed.1~ 13% VTP-300 There is an urgent need to develop effective therapeutic strategies to cure chronic HBV infection.
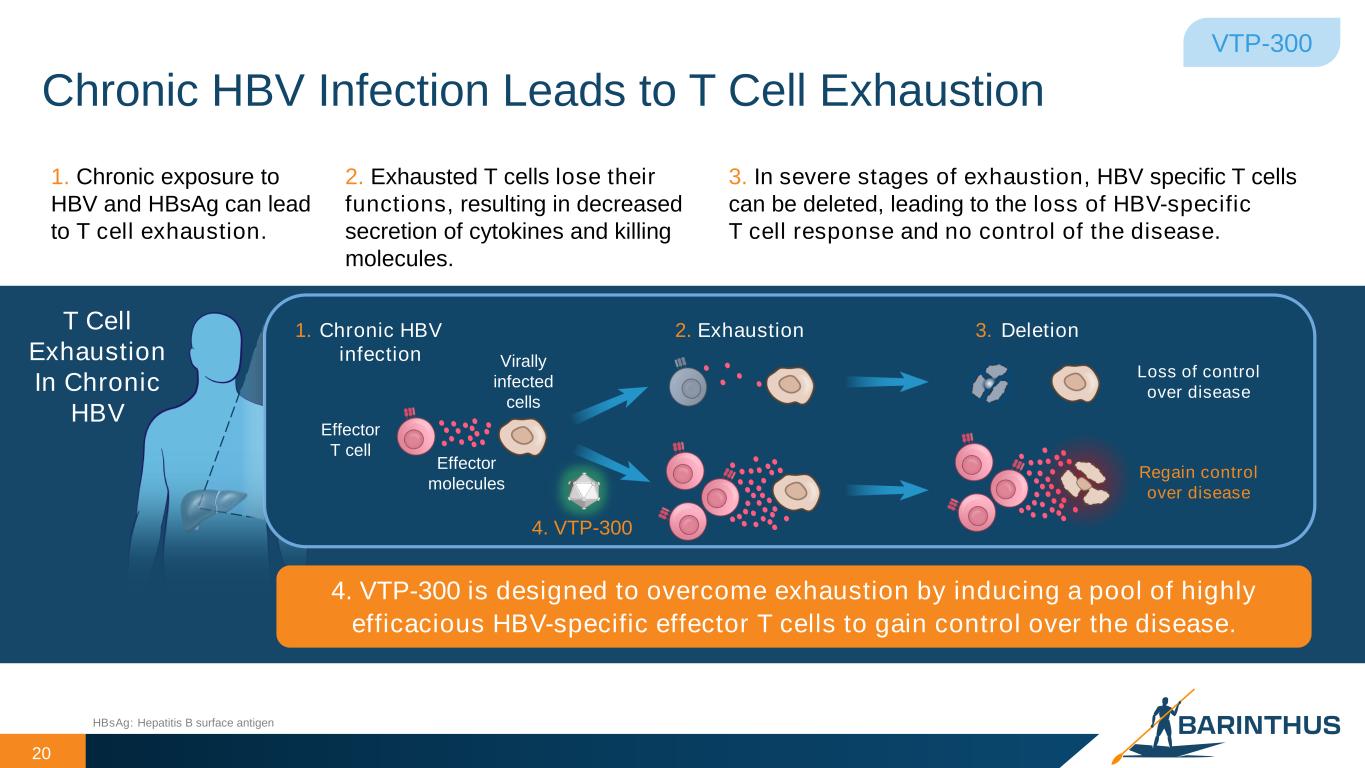
Chronic HBV Infection Leads to T Cell Exhaustion 20 VTP-300 Exhaustion DeletionChronic HBV infection HBsAg: Hepatitis B surface antigen 1. 2. 3.T Cell Exhaustion In Chronic HBV Virally infected cells Effector T cell 4. VTP-300 Loss of control over disease Regain control over disease Effector molecules 3. In severe stages of exhaustion, HBV specific T cells can be deleted, leading to the loss of HBV-specific T cell response and no control of the disease. 1. Chronic exposure to HBV and HBsAg can lead to T cell exhaustion. 2. Exhausted T cells lose their functions, resulting in decreased secretion of cytokines and killing molecules. 4. VTP-300 is designed to overcome exhaustion by inducing a pool of highly efficacious HBV-specific effector T cells to gain control over the disease.
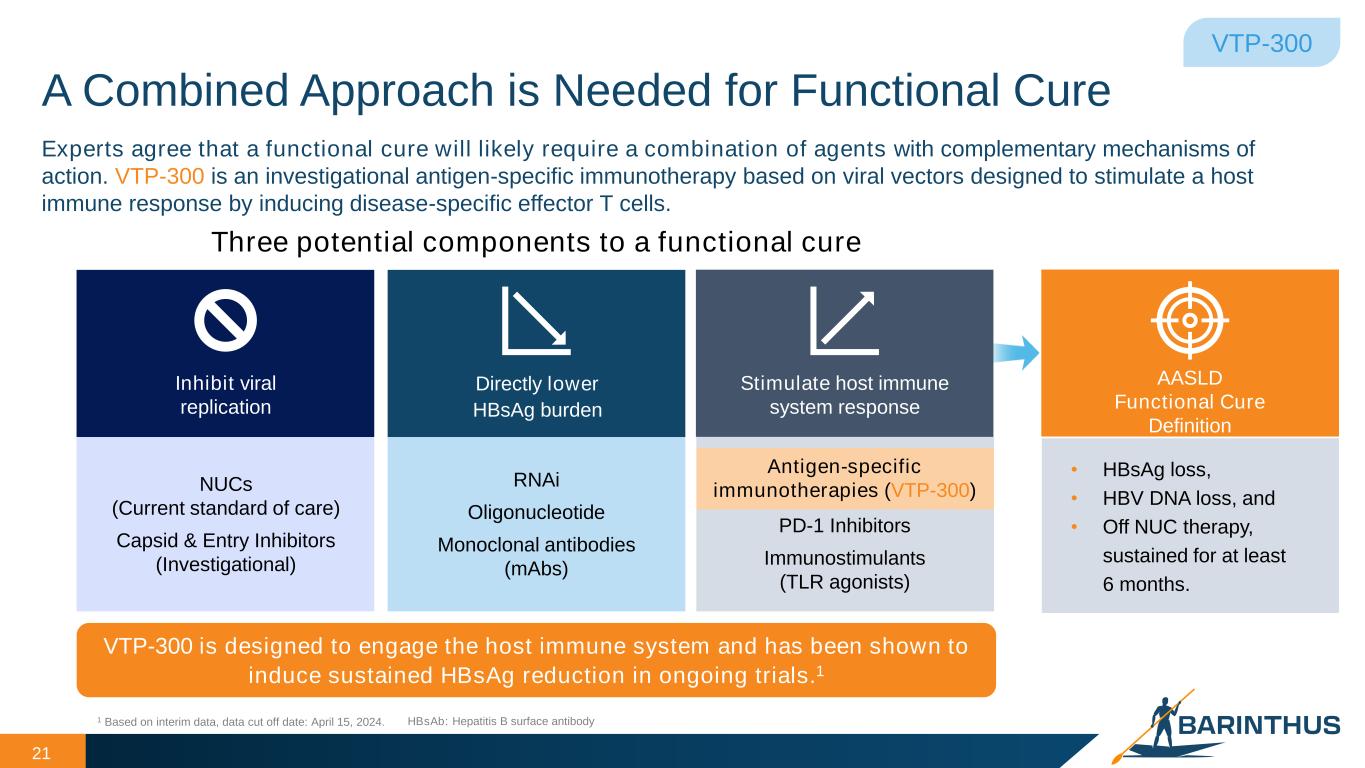
A Combined Approach is Needed for Functional Cure 21 VTP-300 Experts agree that a functional cure will likely require a combination of agents with complementary mechanisms of action. VTP-300 is an investigational antigen-specific immunotherapy based on viral vectors designed to stimulate a host immune response by inducing disease-specific effector T cells. Three potential components to a functional cure 1 Based on interim data, data cut off date: April 15, 2024. NUCs (Current standard of care) Capsid & Entry Inhibitors (Investigational) Inhibit viral replication RNAi Oligonucleotide Monoclonal antibodies (mAbs) Directly lower HBsAg burden Stimulate host immune system response Antigen-specific immunotherapies (VTP-300) PD-1 Inhibitors Immunostimulants (TLR agonists) HBsAb: Hepatitis B surface antibody VTP-300 is designed to engage the host immune system and has been shown to induce sustained HBsAg reduction in ongoing trials.1 • HBsAg loss, • HBV DNA loss, and • Off NUC therapy, sustained for at least 6 months. AASLD Functional Cure Definition

HBV003: Phase 2b Trial – Enrolment Complete Inclusion Criteria • HBV DNA ≤1,000 IU/mL. • HBsAg ≤200 IU/mL.* • On NUCs for ≥6 months. Primary Endpoint • % participants with a greater than 1 log HBsAg reduction at 6 months after initiation of therapy. Secondary Endpoints • Safety: incidence of AEs and SAEs. • T cell response. HBV003 results will inform treatment dosing regimen Group 1: Mirrors Group 3 in HBV002 to further support response effect observed. Group 2: Assesses if additional dose of MVA-HBV with LDN at Day 85 further reduces HBsAg. Group 3: Assesses if delaying LDN until after MVA-HBV is more optimal (plus adds option of 2nd MVA-HBV dose). VTP-300 + Low-dose nivolumab (LDN), N=69, with baseline HBsAg ≤200 IU/mL* Objective: Evaluating Additional Dosing and PD-1 Inhibition Timing MVA + LDN Day 1 Week 1 Group 1 (n=22) Group 2 (n=22) Group 3 (n=25) ChAdOx Day 29 Week 4 Day 36 Week 5 Day 85 Week 12 ChAdOx ChAdOx MVA + LDN MVA MVA + LDN LDN MVA Day 169 Week 24 Patients to discontinue NUCs if eligible • ALT <2 × ULN, and • HBV DNA <LLOQ, and • HBeAg negative, and • HBsAg <100 IU/mL, and/or • HBsAb positive D is c o n ti n u a ti o n c ri te ri a 22 Study Reference: NCT05343481 ALT: Alanine aminotransferase; LLOQ: lower limit of quantification; ULN: upper limit of normal; HBeAg: Hepatitis B e Antigen. *Inclusion criteria were amended in 2023 to focus on participants with HBsAg ≤200 IU/mL, as such data now focuses on this group. VTP-300 Next anticipated readout: Q2 2025
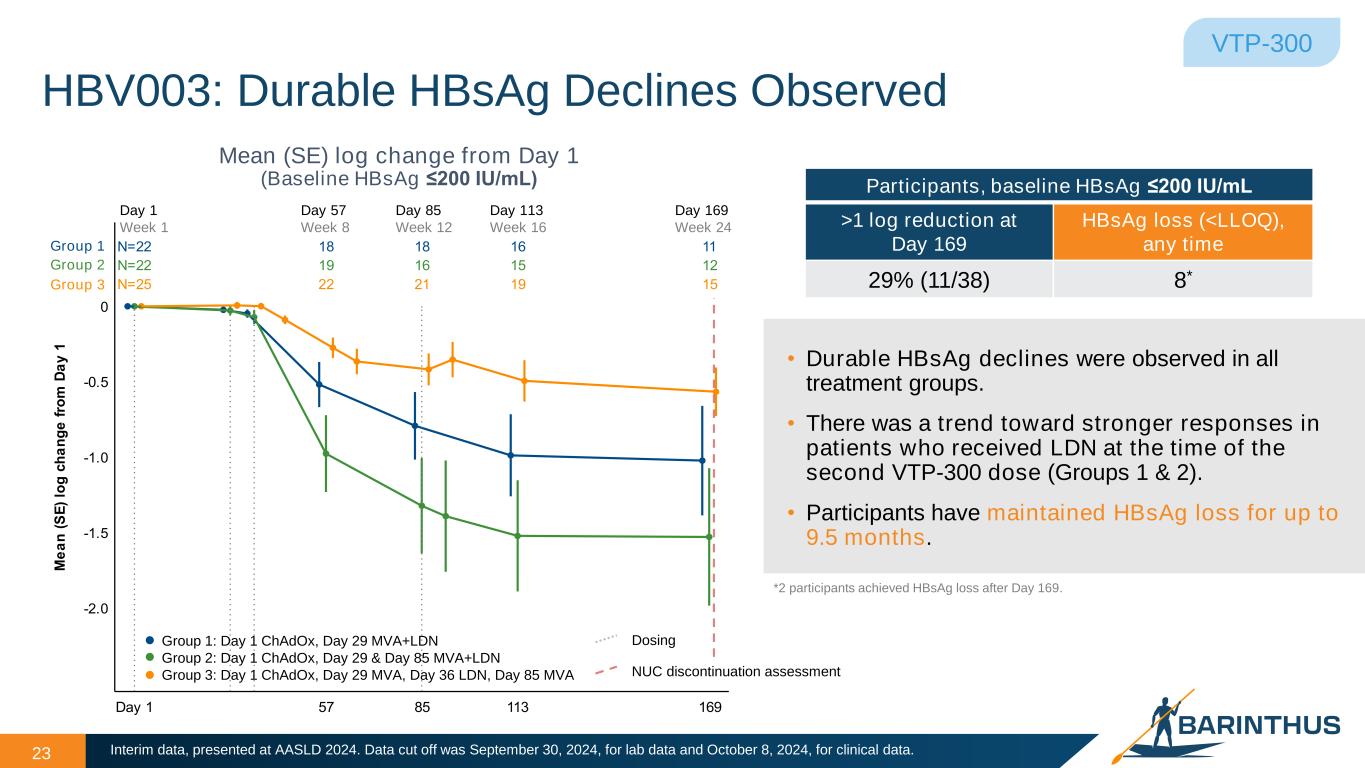
N 22 1 1 16 11 N 22 1 16 1 12 N 2 22 21 1 1 Day 1 7 113 16 2.0 1. 1.0 0. 0 e a n ( ) lo h a n e f ro m a y 1 23 Mean (SE) log change from Day 1 (Baseline HBsAg ≤200 IU/mL) HBV003: Durable HBsAg Declines Observed • Durable HBsAg declines were observed in all treatment groups. • There was a trend toward stronger responses in patients who received LDN at the time of the second VTP-300 dose (Groups 1 & 2). • Participants have maintained HBsAg loss for up to 9.5 months. Group 1 Group 2 Group 3 Day 1 Week 1 Day 57 Week 8 Day 85 Week 12 Day 113 Week 16 Day 169 Week 24 VTP-300 Group 1: Day 1 ChAdOx, Day 29 MVA+LDN Group 2: Day 1 ChAdOx, Day 29 & Day 85 MVA+LDN Group 3: Day 1 ChAdOx, Day 29 MVA, Day 36 LDN, Day 85 MVA Dosing NUC discontinuation assessment Interim data, presented at AASLD 2024. Data cut off was September 30, 2024, for lab data and October 8, 2024, for clinical data. *2 participants achieved HBsAg loss after Day 169. Participants, baseline HBsAg ≤200 IU/mL >1 log reduction at Day 169 HBsAg loss (<LLOQ), any time 29% (11/38) 8*
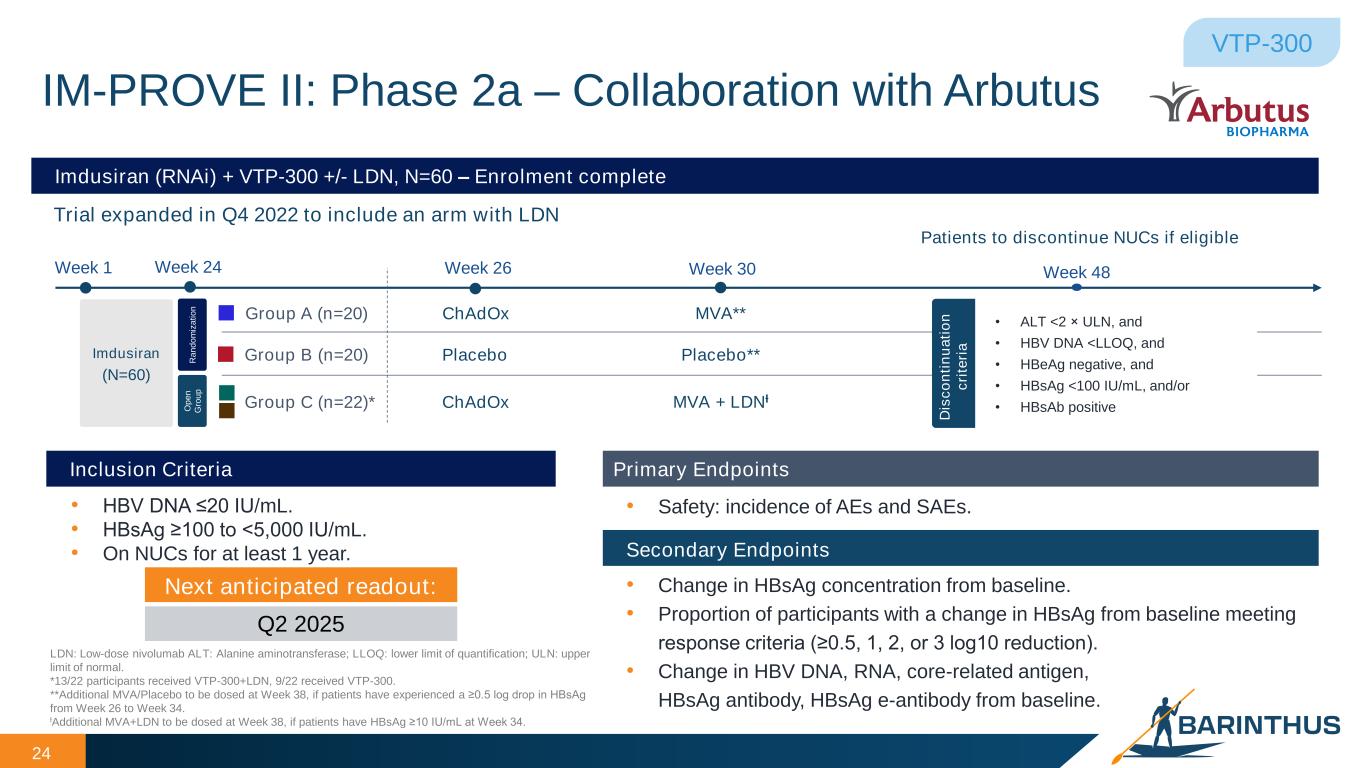
• HBV DNA ≤20 IU/mL. • HBsAg ≥100 to < ,000 IU/mL. • On NUCs for at least 1 year. Inclusion Criteria Primary Endpoints Secondary Endpoints • Safety: incidence of AEs and SAEs. • Change in HBsAg concentration from baseline. • Proportion of participants with a change in HBsAg from baseline meeting response criteria (≥0. , 1, 2, or 3 log10 reduction). • Change in HBV DNA, RNA, core-related antigen, HBsAg antibody, HBsAg e-antibody from baseline. Imdusiran (RNAi) + VTP-300 +/- LDN, N=60 – Enrolment complete Trial expanded in Q4 2022 to include an arm with LDN 24 LDN: Low-dose nivolumab ALT: Alanine aminotransferase; LLOQ: lower limit of quantification; ULN: upper limit of normal. *13/22 participants received VTP-300+LDN, 9/22 received VTP-300. **Additional MVA/Placebo to be dosed at Week 38, if patients have experienced a ≥0. log drop in HBsAg from Week 26 to Week 34. ƚAdditional MVA+LDN to be dosed at Week 38, if patients have HBsAg ≥10 IU/mL at Week 34. Imdusiran (N=60) R a n d o m iz a ti o n O p e n G ro u p IM-PROVE II: Phase 2a – Collaboration with Arbutus VTP-300 Week 1 Group A (n=20) Group B (n=20) Week 24 Week 26 Week 48 ChAdOx Placebo Group C (n=22)* ChAdOx Week 30 MVA + LDNƚ MVA** Placebo** Patients to discontinue NUCs if eligible • ALT <2 × ULN, and • HBV DNA <LLOQ, and • HBeAg negative, and • HBsAg <100 IU/mL, and/or • HBsAb positive D is c o n ti n u a ti o n c ri te ri a Next anticipated readout: Q2 2025
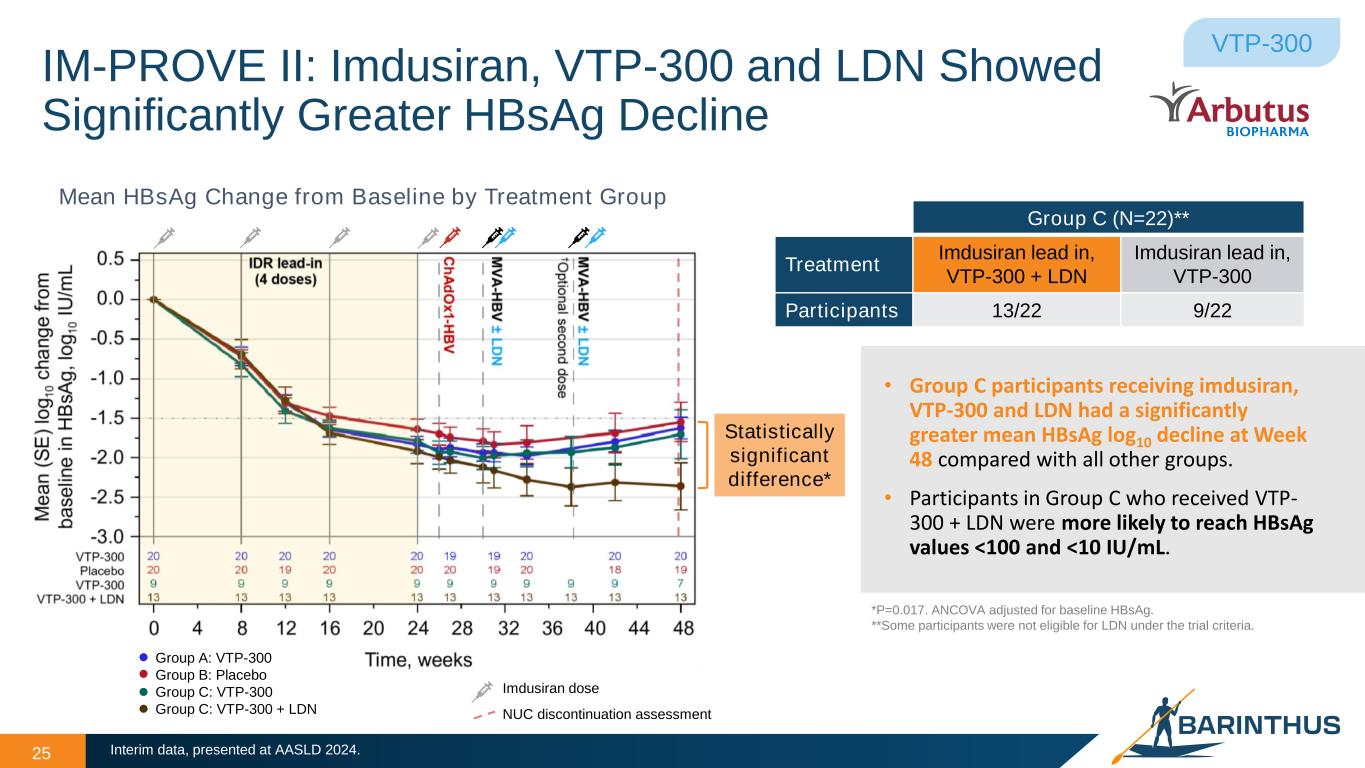
IM-PROVE II: Imdusiran, VTP-300 and LDN Showed Significantly Greater HBsAg Decline 25 VTP-300 Mean HBsAg Change from Baseline by Treatment Group • Group C participants receiving imdusiran, VTP-300 and LDN had a significantly greater mean HBsAg log10 decline at Week 48 compared with all other groups. • Participants in Group C who received VTP- 300 + LDN were more likely to reach HBsAg values <100 and <10 IU/mL. Interim data, presented at AASLD 2024. Imdusiran dose Group A: VTP-300 Group B: Placebo Group C: VTP-300 Group C: VTP-300 + LDN NUC discontinuation assessment Statistically significant difference* Group C (N=22)** Treatment Imdusiran lead in, VTP-300 + LDN Imdusiran lead in, VTP-300 Participants 13/22 9/22 *P=0.017. ANCOVA adjusted for baseline HBsAg. **Some participants were not eligible for LDN under the trial criteria.
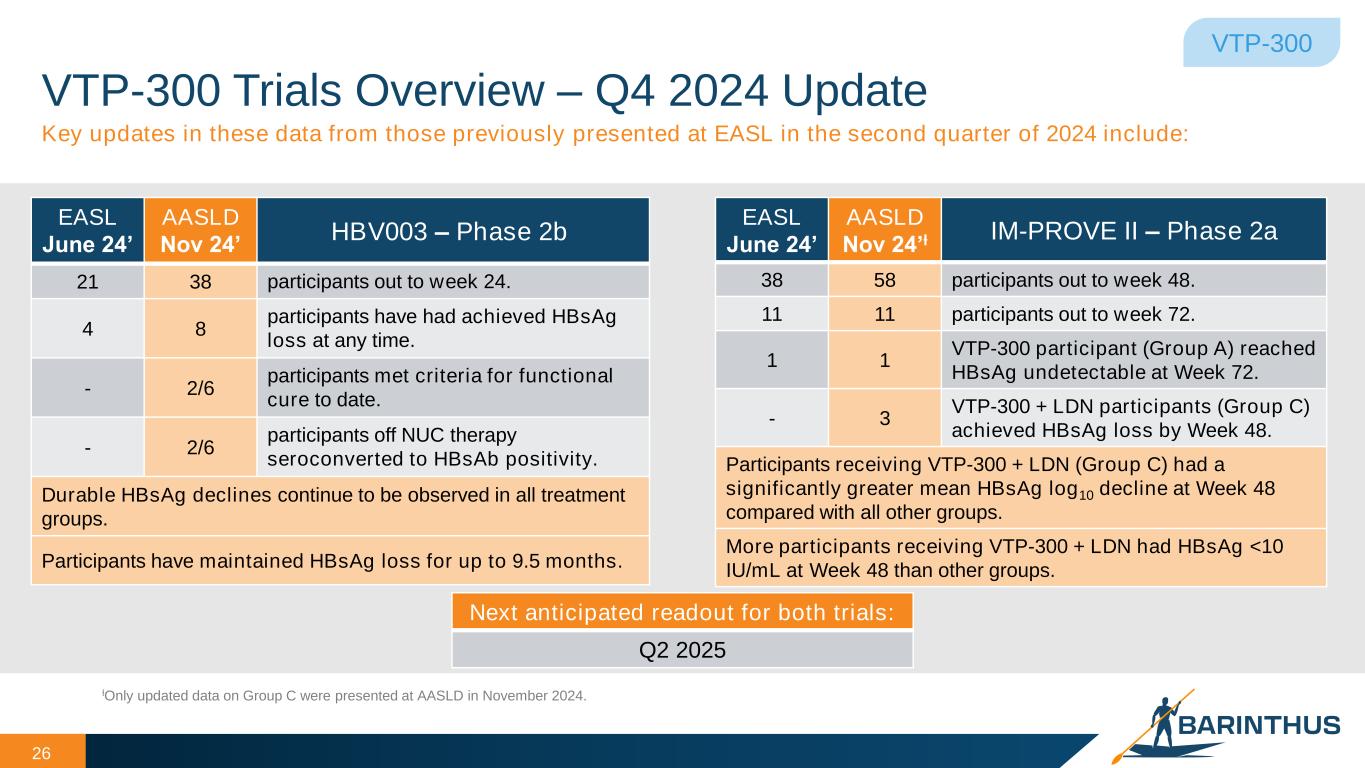
VTP-300 Trials Overview – Q4 2024 Update 26 VTP-300 Key updates in these data from those previously presented at EASL in the second quarter of 2024 include: EASL June 24’ AASLD Nov 24’ HBV003 – Phase 2b 21 38 participants out to week 24. 4 8 participants have had achieved HBsAg loss at any time. - 2/6 participants met criteria for functional cure to date. - 2/6 participants off NUC therapy seroconverted to HBsAb positivity. Durable HBsAg declines continue to be observed in all treatment groups. Participants have maintained HBsAg loss for up to 9.5 months. EASL June 24’ AASLD Nov 24’ƚ IM-PROVE II – Phase 2a 38 58 participants out to week 48. 11 11 participants out to week 72. 1 1 VTP-300 participant (Group A) reached HBsAg undetectable at Week 72. - 3 VTP-300 + LDN participants (Group C) achieved HBsAg loss by Week 48. Participants receiving VTP-300 + LDN (Group C) had a significantly greater mean HBsAg log10 decline at Week 48 compared with all other groups. More participants receiving VTP-300 + LDN had HBsAg <10 IU/mL at Week 48 than other groups. ƚOnly updated data on Group C were presented at AASLD in November 2024. Next anticipated readout for both trials: Q2 2025

Company Highlights Guiding the immune system to cure disease
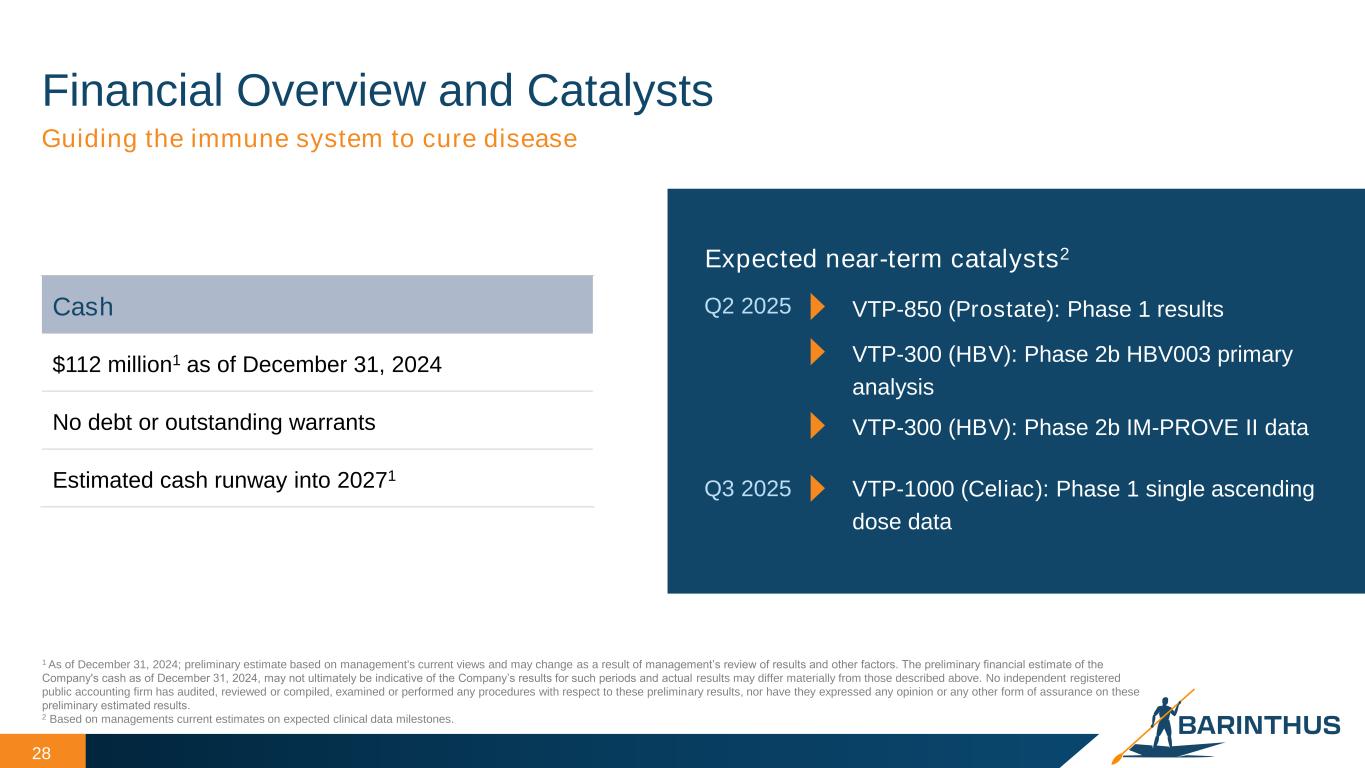
Financial Overview and Catalysts 28 Guiding the immune system to cure disease Cash $112 million1 as of December 31, 2024 No debt or outstanding warrants Estimated cash runway into 20271 Expected near-term catalysts2 Q2 2025 VTP-300 (HBV): Phase 2b HBV003 primary analysis VTP-850 (Prostate): Phase 1 results Q3 2025 VTP-1000 (Celiac): Phase 1 single ascending dose data VTP-300 (HBV): Phase 2b IM-PROVE II data 1 As of December 31, 2024; preliminary estimate based on management's current views and may change as a result of management’s review of results and other factors. The preliminary financial estimate of the Company's cash as of December 31, 2024, may not ultimately be indicative of the Company’s results for such periods and actual results may differ materially from those described above. No independent registered public accounting firm has audited, reviewed or compiled, examined or performed any procedures with respect to these preliminary results, nor have they expressed any opinion or any other form of assurance on these preliminary estimated results. 2 Based on managements current estimates on expected clinical data milestones.

Guiding the Immune System to Cure Disease Thank You




























