
Corporate Overview May 2021 Next Generation Targeted Therapy for Cancer Exhibit 99.2

Disclaimer 1 ABOUT THIS PRESENTATION This presentation is for informational purpose sonly to assist interested parties in making their own evaluation with respect to a proposed business combination (the Business Combination) between Locust Walk Acquisition Corp. (LWAC) and eFFECTOR Therapeutics, Inc. (eFFECTOR or the Company) and related transactions, and for no other purpose. The information contained herein does not purport to be all inclusive and no representation or warranty, express or implied, is or will be given by LWAC, eFFECTOR, or any of their respective affiliates, directors, officers, employees or advisers or any other person as to the accuracy, completeness or reliability of the information contained in this presentation. The distribution of this presentation may be restricted by law and persons into whose possession this presentation comes should inform themselves about and observe any such restrictions. FORWARD-LOOKING STATEMENTS This presentation contains forward-looking statements. All statements other than statements of historical facts contained in this presentation, including statements regarding LWAC or the Company’s future results of operations and financial position, the amount of cash expected to be available to eFFECTOR after giving effect to any redemptions by LWAC stockholders, eFFECTOR’s business strategy, prospective products, product approvals, research and development costs, timing and likelihood of success, plans and objectives of management for future operations, future results of current and anticipated products and expected use of proceeds by LWAC, are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as “may”, “believe”, “anticipate”, “could”,“ should”, “estimate”, “expect”, “intend”, “plan”, “project”, “will”, “forecast” and similar terms. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, but not limited to, the risks relating to the Company set forth in the Appendix and the following risks relating to the Business Combination: the risk that the Business Combination may not be completed in a timely manner or at all, which may adversely affect the price of LWAC’s securities; the failure to satisfy the conditions to closing the Business Combination, including the approval by the stockholders of LWAC and the receipt of certain governmental and regulatory approvals; the risk that some or all of LWAC’s stockholders may redeem their shares at the closing of the transaction; the effect of the announcement or pendency of the Business Combination on the Company’s business relationships and business generally; the outcome of any legal proceedings that may be instituted related to the Business Combination; and the ability to realize the anticipated benefits of the Business Combination. Moreover, the Company operates in a very competitive and rapidly changing environment. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond LWAC’s and the Company’s control, you should not rely on these forward-looking statements as predictions of future events. The foregoing list of factors is not exclusive, and readers should also refer to those risks that will be included under the header “Risk Factors” in the registration statement on Form S-4 to be filed by LWAC with the Securities and Exchange Commission (SEC) and those included under the header “Risk Factors” in LWAC’s Annual Report on Form 10-K for the year ended December 31, 2020 filed with the SEC on March 29, 2021. The events and circumstances reflected in these forward-looking statements may not be achieved or occur and actual results could differ materially from those Except as required by applicable law, neither LWAC nor the Company plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information ,future events, changed circumstances or otherwise. MARKET AND INDUSTRY DATA This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. CLINICAL INVESTIGATION/FDA This presentation concerns product candidates that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration (FDA). They are currently limited by Federal law to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated. ADDITIONAL INFORMATION In connection with the proposed Business Combination, LWAC will file a registration statement on Form S-4 with the SEC, which will include a proxy statement/prospectus, that will be both the proxy statement to be distributed to holders of LWAC common stock in connection with its solicitation of proxies for the vote by LWAC stockholders with respect to the proposed Business Combination and other matters as may be described in the registration statement, as well as the prospectus relating to the offer and sale of the securities to be issued in the Business Combination. After the registration statement is declared effective, LWAC will mail a definitive proxy statement/prospectus and other relevant documents to its stockholders. This presentation does not contain all the information that should be considered concerning the proposed Business Combination and is not intended to form the basis of any investment decision or any other decision in respect of the Business Combination. LWAC’s stockholders, the Company, and other interested persons are advised to read, when available, the definitive proxy statement/prospectus and other documents filed in connection with the proposed Business Combination, as these materials will contain important information about the Company, LWAC and the Business Combination. When available, the definitive proxy statement and other relevant materials for the proposed Business Combination will be sent to stockholders of LWAC as of a record date to be established for voting on the proposed Business Combination. Stockholders will also be able to obtain copies of the definitive proxy statement/prospectus and other documents filed with the SEC, without charge, once available, at the SEC’s website at www.sec.gov, or by directing a request to: LWAC c/o at eFFECTOR Therapeutics, Inc., 11120 Roselle Street, Suite A, San Diego, CA 92121. PARTICIPANTS IN THE SOLICITATION LWAC and its directors and executive officers may be deemed participants in the solicitation of proxies from LWAC’s stockholders with respect to the proposed Business Combination. A list of the names of those directors and executive officers and a description of their interests in LWAC is contained in LWAC’s final prospectus dated January 11, 2021 relating to its initial public offering, which was filed with the SEC and is available free of charge at the SEC’s web site at www.sec.gov. Additional information regarding the interests of such participants will be contained in the proxy statement/prospectus for the proposed Business Combination when available. The Company and its directors and executive officers may also be deemed to be participants in the solicitation of proxies from the stockholders of LWAC in connection with the proposed Business Combination. A list of the names of such directors and executive officers and information regarding their interests in the proposed Business Combination will be included in the proxy statement/prospectus for the proposed Business Combination when available. NO SOLICITATION OR OFFER This presentation and any oral statements made in connection with this presentation shall neither constitute an offer to sell nor the solicitation of an offer to buy any securities, or the solicitation of any proxy, vote, consent or approval in any jurisdiction in connection with the proposed Business Combination, nor shall there be any sale of securities in any jurisdiction in which the offer, solicitation or sale would be unlawful prior to any registration or qualification under the securities laws of any such jurisdictions. This communication is restricted by law; it is not intended for distribution to, or use by any person in, any jurisdiction where such distribution or use would be contrary to local law or regulation. TRADEMARKS This presentation contains trademarks, service marks, and trade names of the Company, LWAC and other companies, which are the property of their respective owners.
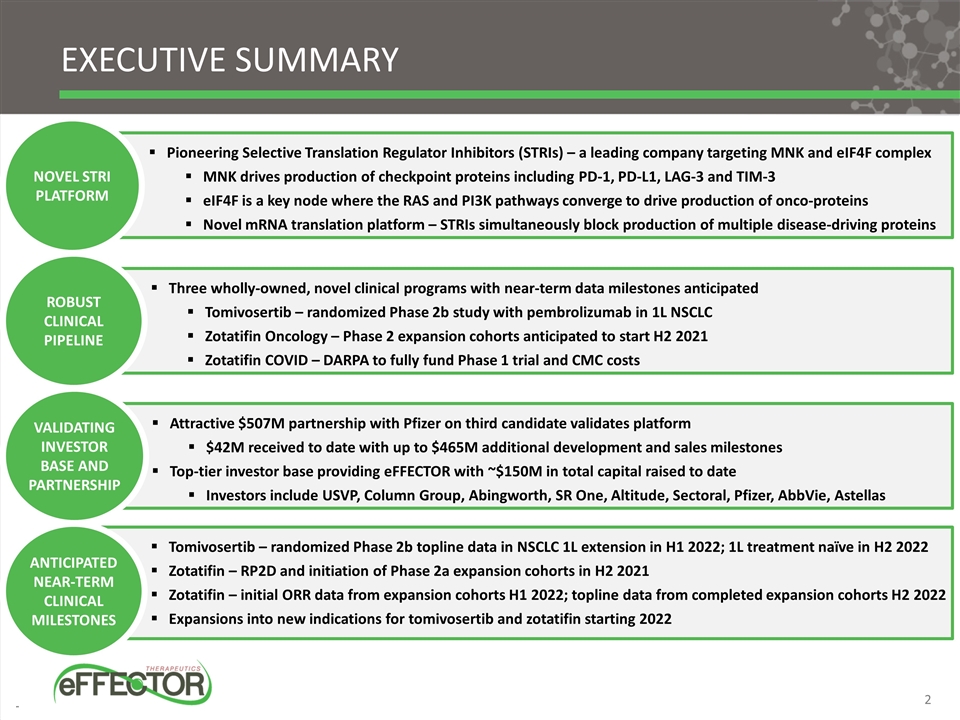
EXECUTIVE SUMMARY Pioneering Selective Translation Regulator Inhibitors (STRIs) – a leading company targeting MNK and eIF4F complex MNK drives production of checkpoint proteins including PD-1, PD-L1, LAG-3 and TIM-3 eIF4F is a key node where the RAS and PI3K pathways converge to drive production of onco-proteins Novel mRNA translation platform – STRIs simultaneously block production of multiple disease-driving proteins NOVEL STRI PLATFORM Three wholly-owned, novel clinical programs with near-term data milestones anticipated Tomivosertib – randomized Phase 2b study with pembrolizumab in 1L NSCLC Zotatifin Oncology – Phase 2 expansion cohorts anticipated to start H2 2021 Zotatifin COVID – DARPA to fully fund Phase 1 trial and CMC costs ROBUST CLINICAL PIPELINE Attractive $507M partnership with Pfizer on third candidate validates platform $42M received to date with up to $465M additional development and sales milestones Top-tier investor base providing eFFECTOR with ~$150M in total capital raised to date Investors include USVP, Column Group, Abingworth, SR One, Altitude, Sectoral, Pfizer, AbbVie, Astellas VALIDATING INVESTOR BASE AND PARTNERSHIP Tomivosertib – randomized Phase 2b topline data in NSCLC 1L extension in H1 2022; 1L treatment naïve in H2 2022 Zotatifin – RP2D and initiation of Phase 2a expansion cohorts in H2 2021 Zotatifin – initial ORR data from expansion cohorts H1 2022; topline data from completed expansion cohorts H2 2022 Expansions into new indications for tomivosertib and zotatifin starting 2022 ANTICIPATED NEAR-TERM CLINICAL MILESTONES

eFFECTOR MEETS DEMANDING CRITERIA OF LOCUST WALK ACQUISITION CORP Locust Walk Acquisition Corp (LWAC) is a $175M SPAC focused on biotechnology with a comprehensive investment thesis that has been used to evaluate over 90 potential targets SUPERLATIVE CRITERIA Validating Partnership Novel Lead Asset Experienced Management Team PRELIMINARY CRITERIA Platform Technology ≥ 2 Assets Last Valuation <$600M Strong Investor Base (>$50M Raised) eFFECTOR Therapeutics has experienced management, a promising lead asset, validating partnership and is ready to go public via LWAC 3
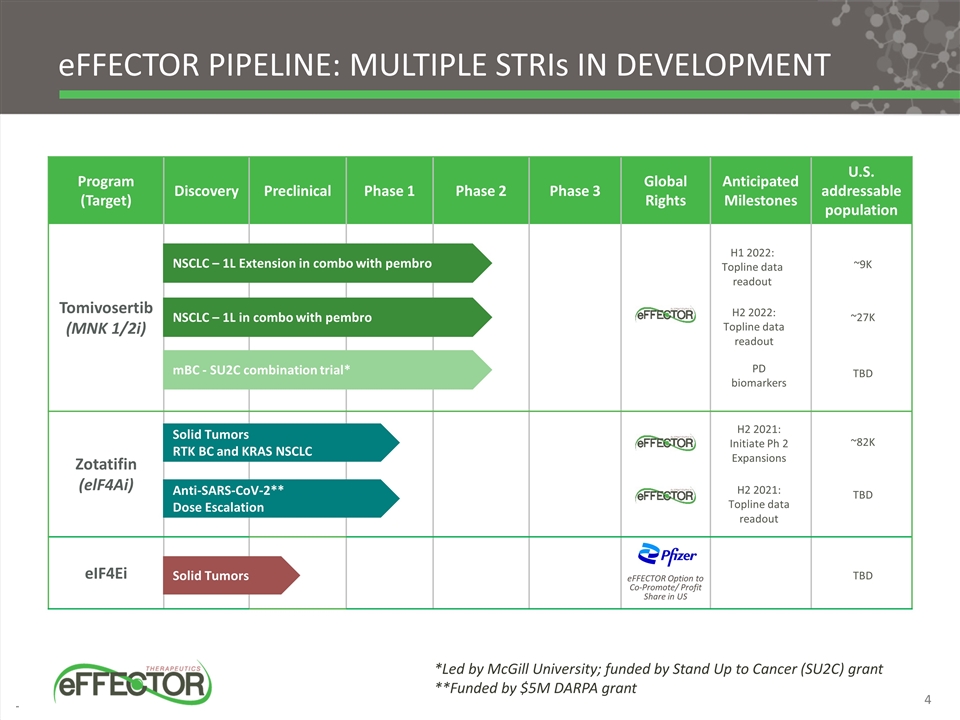
eFFECTOR Pipeline: Multiple STRIs in development Program (Target) Discovery Preclinical Phase 1 Phase 2 Phase 3 Global Rights Anticipated Milestones U.S. addressable population Tomivosertib (MNK 1/2i) Zotatifin (elF4Ai) eIF4Ei NSCLC – 1L Extension in combo with pembro Solid Tumors RTK BC and KRAS NSCLC Solid Tumors mBC - SU2C combination trial* *Led by McGill University; funded by Stand Up to Cancer (SU2C) grant **Funded by $5M DARPA grant eFFECTOR Option to Co-Promote/ Profit Share in US 4 Anti-SARS-CoV-2** Dose Escalation NSCLC – 1L in combo with pembro H1 2022: Topline data readout H2 2022: Topline data readout H2 2021: Topline data readout H2 2021: Initiate Ph 2 Expansions ~9K ~27K ~82K TBD TBD PD biomarkers TBD
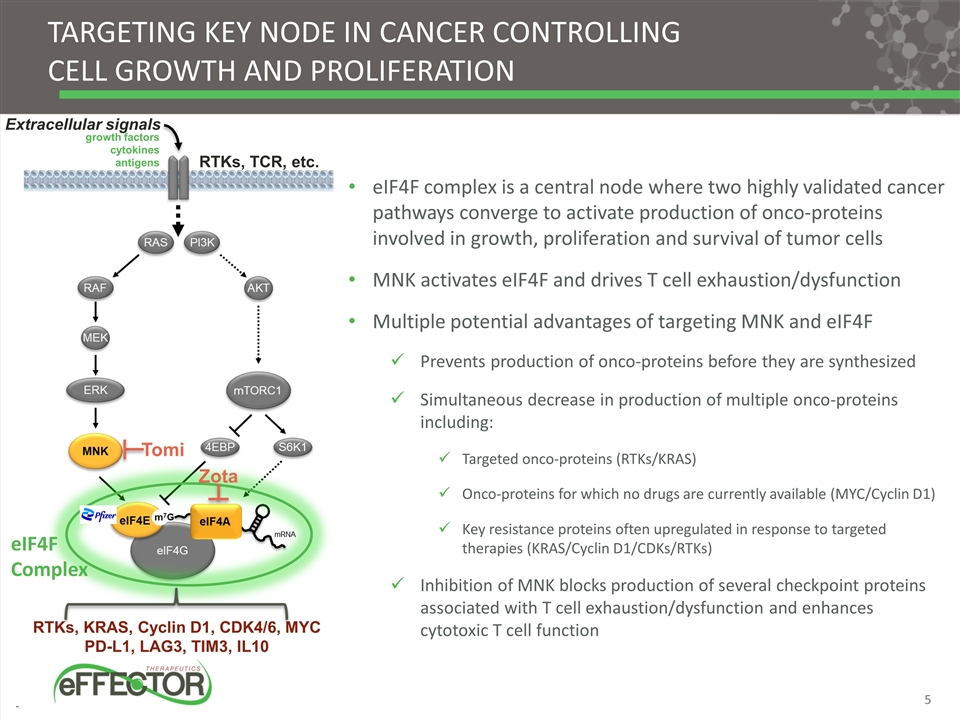
Targeting Key node in cancer controlling Cell Growth and proliferation eIF4F complex is a central node where two highly validated cancer pathways converge to activate production of onco-proteins involved in growth, proliferation and survival of tumor cells MNK activates eIF4F and drives T cell exhaustion/dysfunction Multiple potential advantages of targeting MNK and eIF4F Prevents production of onco-proteins before they are synthesized Simultaneous decrease in production of multiple onco-proteins including: Targeted onco-proteins (RTKs/KRAS) Onco-proteins for which no drugs are currently available (MYC/Cyclin D1) Key resistance proteins often upregulated in response to targeted therapies (KRAS/Cyclin D1/CDKs/RTKs) Inhibition of MNK blocks production of several checkpoint proteins associated with T cell exhaustion/dysfunction and enhances cytotoxic T cell function RTKs, KRAS, Cyclin D1, CDK4/6, MYC PD-L1, LAG3, TIM3, IL10 RTKs, TCR, etc. Extracellular signals growth factors cytokines antigens eIF4F Complex Tomi Zota
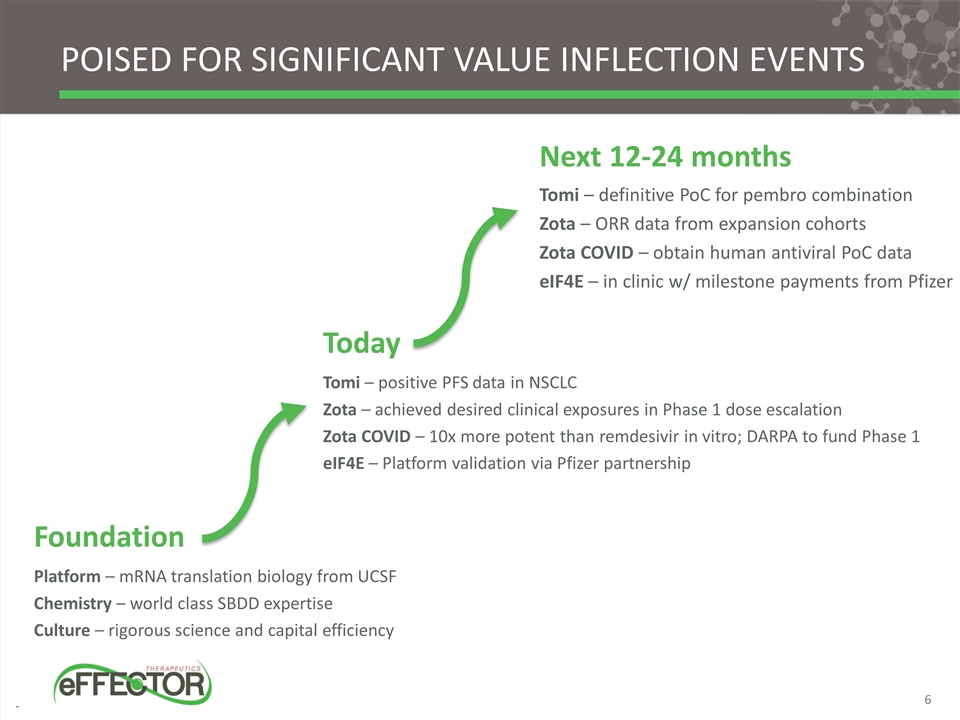
Foundation Platform – mRNA translation biology from UCSF Chemistry – world class SBDD expertise Culture – rigorous science and capital efficiency Next 12-24 months Tomi – definitive PoC for pembro combination Zota – ORR data from expansion cohorts Zota COVID – obtain human antiviral PoC data eIF4E – in clinic w/ milestone payments from Pfizer Poised for significant value inflection events Today Tomi – positive PFS data in NSCLC Zota – achieved desired clinical exposures in Phase 1 dose escalation Zota COVID – 10x more potent than remdesivir in vitro; DARPA to fund Phase 1 eIF4E – Platform validation via Pfizer partnership
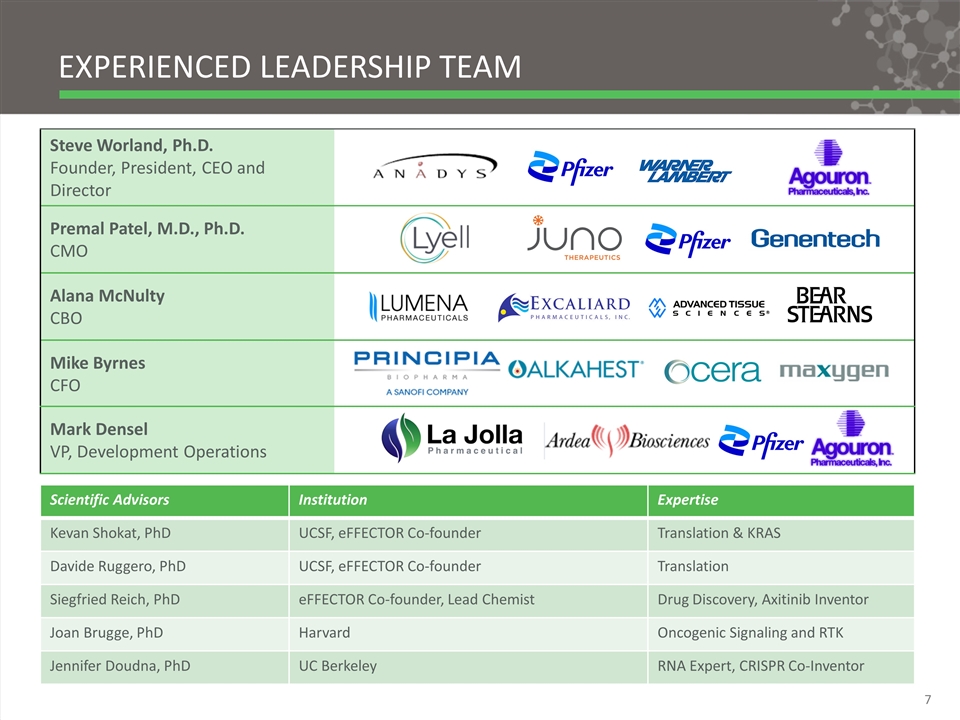
Steve Worland, Ph.D. Founder, President, CEO and Director Premal Patel, M.D., Ph.D. CMO Alana McNulty CBO Mike Byrnes CFO Experienced leadership team Scientific Advisors Institution Expertise Kevan Shokat, PhD UCSF, eFFECTOR Co-founder Translation & KRAS Davide Ruggero, PhD UCSF, eFFECTOR Co-founder Translation Siegfried Reich, PhD eFFECTOR Co-founder, Lead Chemist Drug Discovery, Axitinib Inventor Joan Brugge, PhD Harvard Oncogenic Signaling and RTK Jennifer Doudna, PhD UC Berkeley RNA Expert, CRISPR Co-Inventor Mark Densel VP, Development Operations
Tomivosertib (eFT508) Designed to stimulate activation, prevent exhaustion and prolong memory of T cells MNK 1/2 Inhibitor
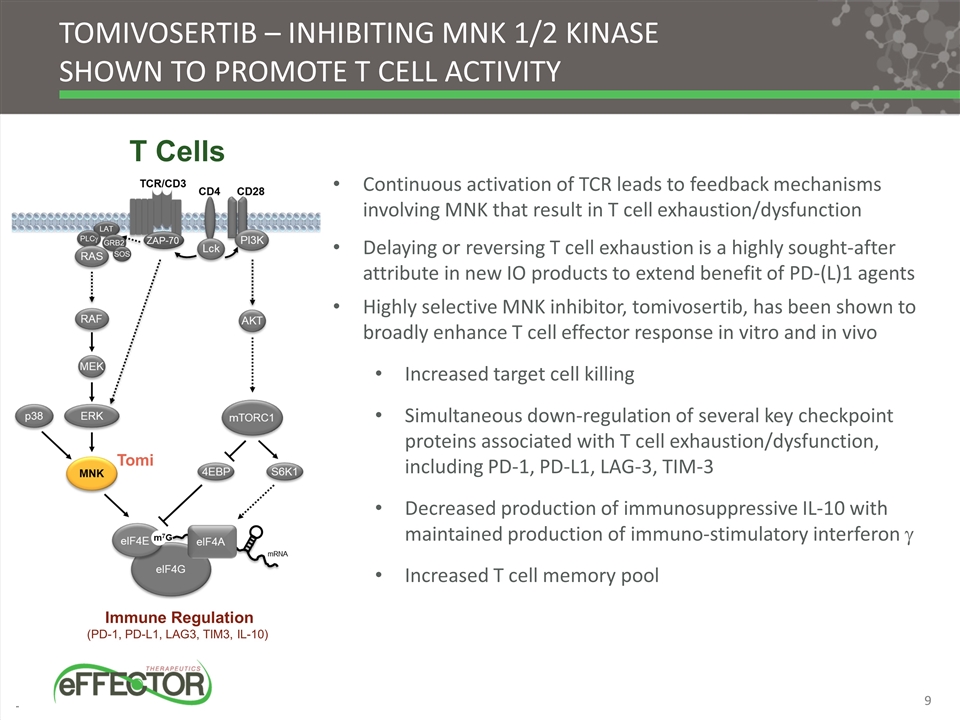
T Cells Continuous activation of TCR leads to feedback mechanisms involving MNK that result in T cell exhaustion/dysfunction Delaying or reversing T cell exhaustion is a highly sought-after attribute in new IO products to extend benefit of PD-(L)1 agents Highly selective MNK inhibitor, tomivosertib, has been shown to broadly enhance T cell effector response in vitro and in vivo Increased target cell killing Simultaneous down-regulation of several key checkpoint proteins associated with T cell exhaustion/dysfunction, including PD-1, PD-L1, LAG-3, TIM-3 Decreased production of immunosuppressive IL-10 with maintained production of immuno-stimulatory interferon g Increased T cell memory pool Immune Regulation (PD-1, PD-L1, LAG3, TIM3, IL-10) Tomivosertib – inhibiting MNK 1/2 kinase shown to promote T cell activity Tomi
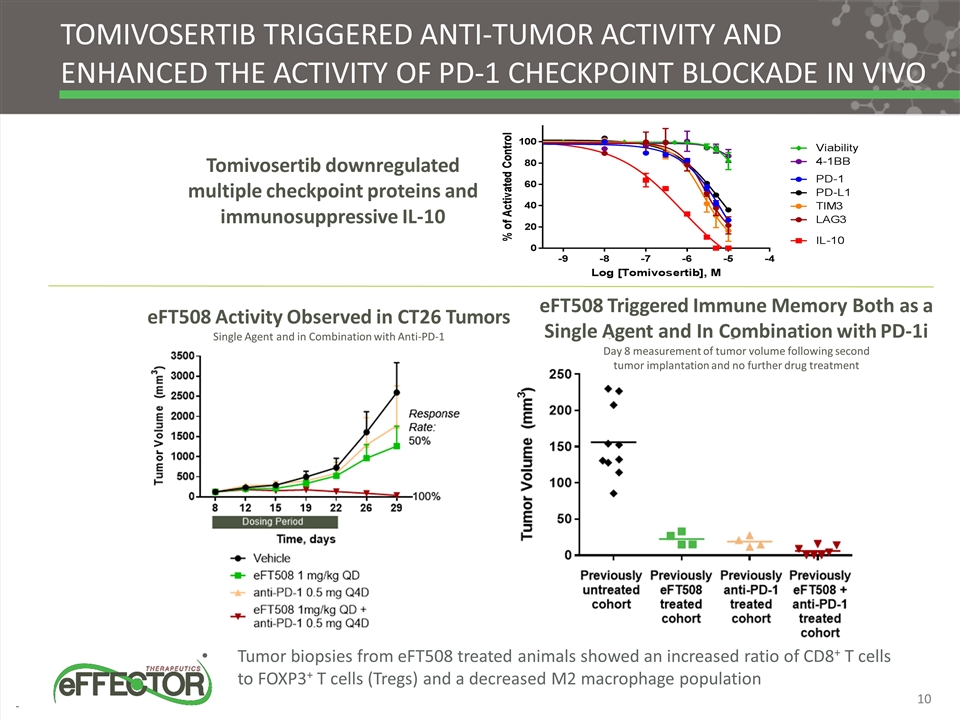
TOMIVOSERTIB TRIGGERED ANTI-TUMOR ACTIVITY AND ENHANCED THE ACTIVITY OF PD-1 CHECKPOINT BLOCKADE IN VIVO Tumor biopsies from eFT508 treated animals showed an increased ratio of CD8+ T cells to FOXP3+ T cells (Tregs) and a decreased M2 macrophage population Tomivosertib downregulated multiple checkpoint proteins and immunosuppressive IL-10 eFT508 Triggered Immune Memory Both as a Single Agent and In Combination with PD-1i Day 8 measurement of tumor volume following second tumor implantation and no further drug treatment eFT508 Activity Observed in CT26 Tumors Single Agent and in Combination with Anti-PD-1
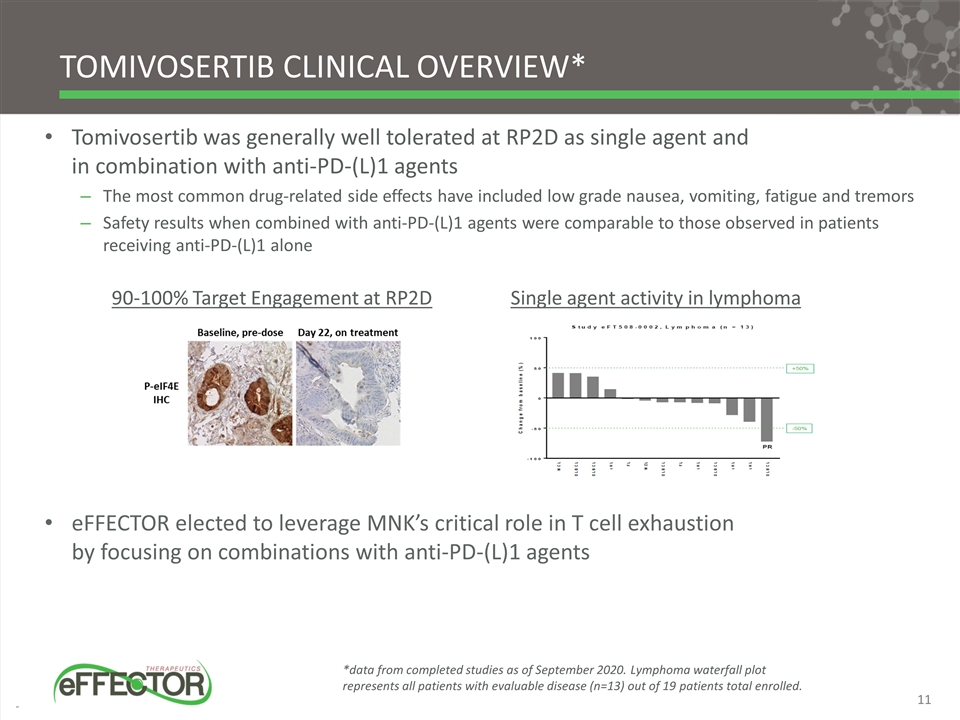
Tomivosertib clinical Overview* Tomivosertib was generally well tolerated at RP2D as single agent and in combination with anti-PD-(L)1 agents The most common drug-related side effects have included low grade nausea, vomiting, fatigue and tremors Safety results when combined with anti-PD-(L)1 agents were comparable to those observed in patients receiving anti-PD-(L)1 alone eFFECTOR elected to leverage MNK’s critical role in T cell exhaustion by focusing on combinations with anti-PD-(L)1 agents 90-100% Target Engagement at RP2D *data from completed studies as of September 2020. Lymphoma waterfall plot represents all patients with evaluable disease (n=13) out of 19 patients total enrolled. Single agent activity in lymphoma
Tomivosertib demonstrated prolonged pfs when combined with anti-PD-(L)1 agents Phase 2a study combined with anti-PD-(L)1 demonstrated encouraging activity* Tomivosertib was added to patients not responding to ongoing anti-PD-(L)1 therapy with no change or break in anti-PD-(L)1 regimen Confirmed PR observed in 1/4 RCC patients 66% reduction in target lesions observed in 1/1 gastric patients Strong signal with most experience of tomi + anti-PD-(L)1 in NSCLC All 17 NSCLC patients had worsening metastatic disease on anti-PD-(L)1 therapy prior to adding tomivosertib (16 of 17 had RECIST progression) Inflection in tumor growth and durable tumor control observed in many patients after adding tomivosertib Confirmed PRs in 2/17 patients (12%), third patient with 28% reduction Prolonged PFS (up to 18 months) after adding tomivosertib delayed need for chemotherapy in these responding patients *data through study completion in September 2020
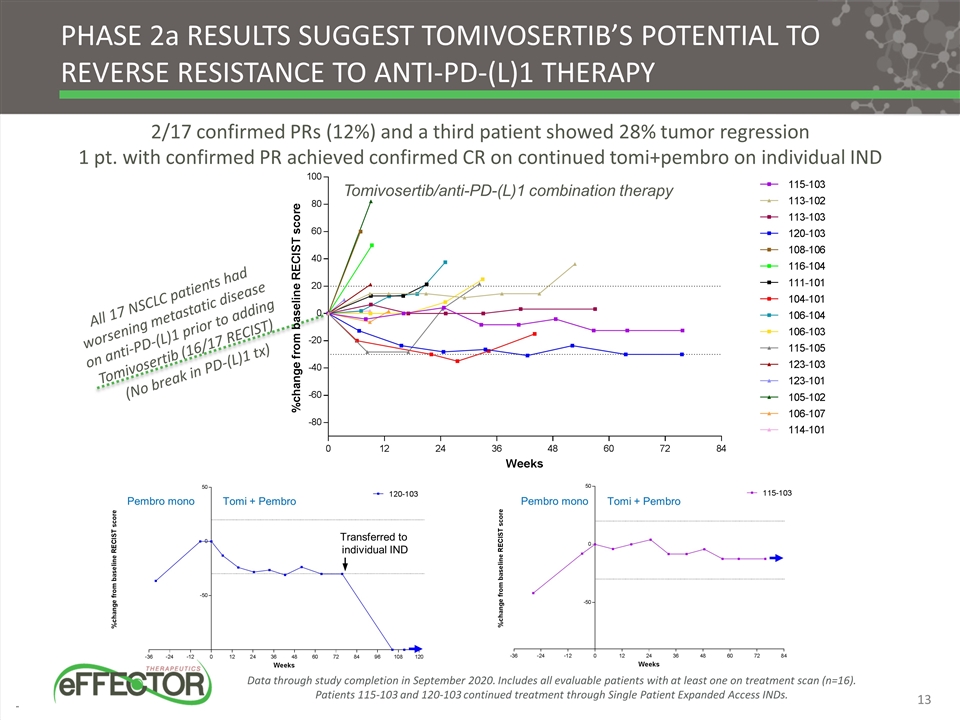
Tomi + Pembro Pembro mono Transferred to individual IND phase 2a results suggest tomivosertib’s potential to reverse resistance to anti-PD-(L)1 therapy Data through study completion in September 2020. Includes all evaluable patients with at least one on treatment scan (n=16). Patients 115-103 and 120-103 continued treatment through Single Patient Expanded Access INDs. All 17 NSCLC patients had worsening metastatic disease on anti-PD-(L)1 prior to adding Tomivosertib (16/17 RECIST) (No break in PD-(L)1 tx) 2/17 confirmed PRs (12%) and a third patient showed 28% tumor regression 1 pt. with confirmed PR achieved confirmed CR on continued tomi+pembro on individual IND Tomivosertib/anti-PD-(L)1 combination therapy Pembro mono Tomi + Pembro
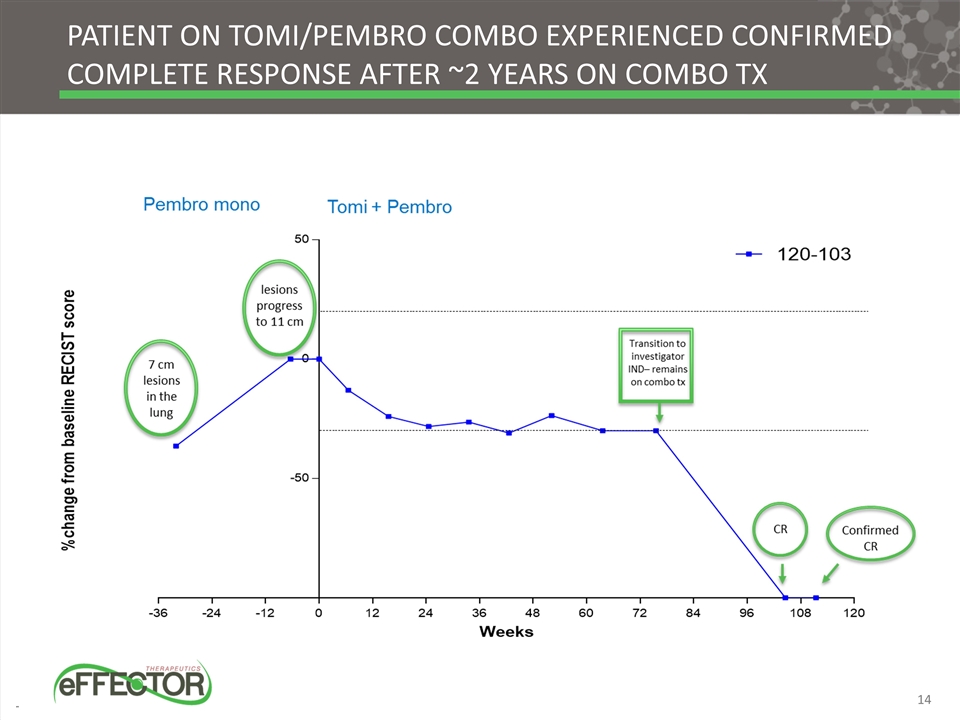
Patient on Tomi/Pembro Combo Experienced Confirmed Complete Response after ~2 Years on Combo Tx
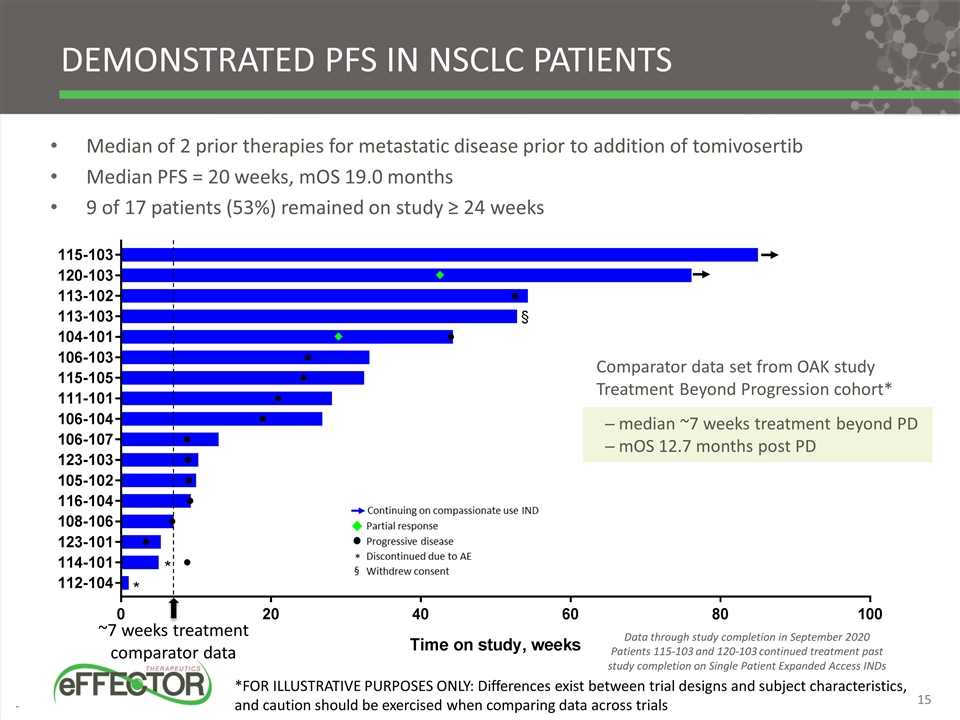
Demonstrated PFS in NSCLC patients Median of 2 prior therapies for metastatic disease prior to addition of tomivosertib Median PFS = 20 weeks, mOS 19.0 months 9 of 17 patients (53%) remained on study ≥ 24 weeks Comparator data set from OAK study Treatment Beyond Progression cohort* ─ median ~7 weeks treatment beyond PD ─ mOS 12.7 months post PD ~7 weeks treatment comparator data *FOR ILLUSTRATIVE PURPOSES ONLY: Differences exist between trial designs and subject characteristics, and caution should be exercised when comparing data across trials Data through study completion in September 2020 Patients 115-103 and 120-103 continued treatment past study completion on Single Patient Expanded Access INDs
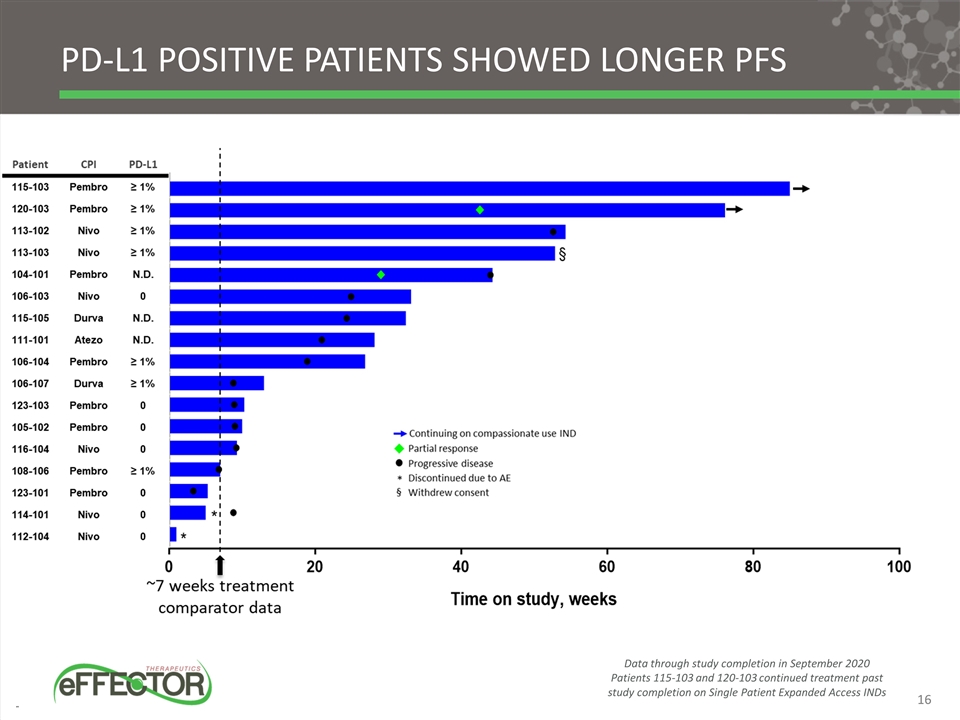
PD-L1 positive patients showed longer PFS Data through study completion in September 2020 Patients 115-103 and 120-103 continued treatment past study completion on Single Patient Expanded Access INDs
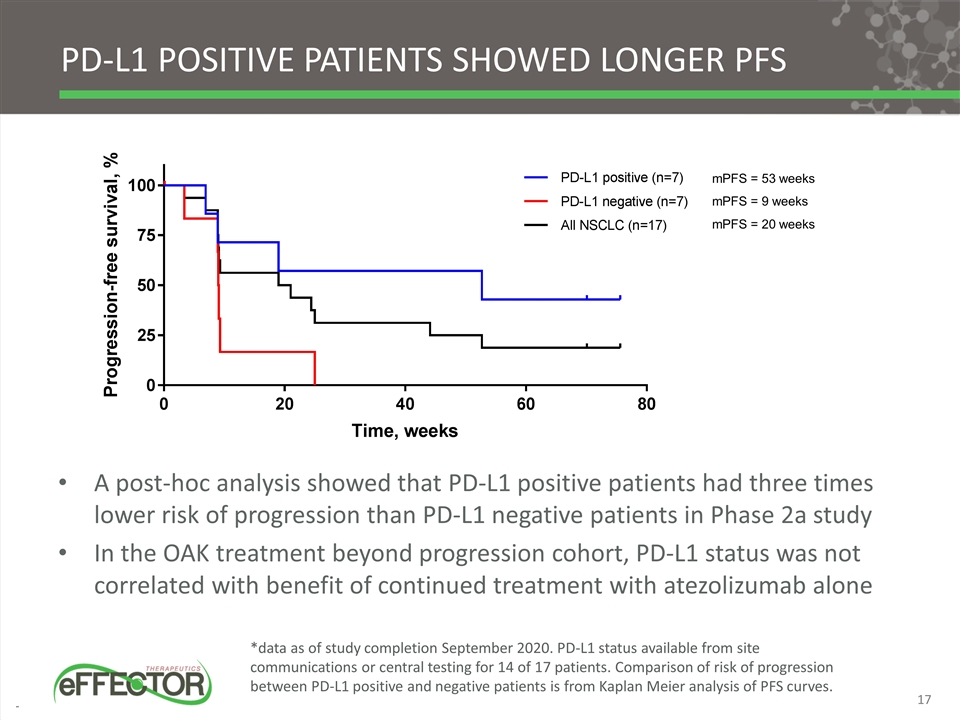
Pd-l1 positive patients showed longer pfs A post-hoc analysis showed that PD-L1 positive patients had three times lower risk of progression than PD-L1 negative patients in Phase 2a study In the OAK treatment beyond progression cohort, PD-L1 status was not correlated with benefit of continued treatment with atezolizumab alone *data as of study completion September 2020. PD-L1 status available from site communications or central testing for 14 of 17 patients. Comparison of risk of progression between PD-L1 positive and negative patients is from Kaplan Meier analysis of PFS curves. mPFS = 53 weeks mPFS = 9 weeks mPFS = 20 weeks
Plan to evaluate novel treatment setting – 1L Extension Nsclc Based on our 17 NSCLC patient data, we received FDA allowance to proceed with the following 1L extension study Tomivosertib added to pembrolizumab after initial progression In patients suitable to continue on pembrolizumab after PD Control arm of placebo added to pembrolizumab Aligned with FDA on PFS as the primary endpoint Data from trial, if positive, may support NDA submission This novel treatment setting is prior to any chemotherapy treatment Because delaying resistance is a lower biological hurdle than reversing resistance, also plan to include a frontline combination arm in next study
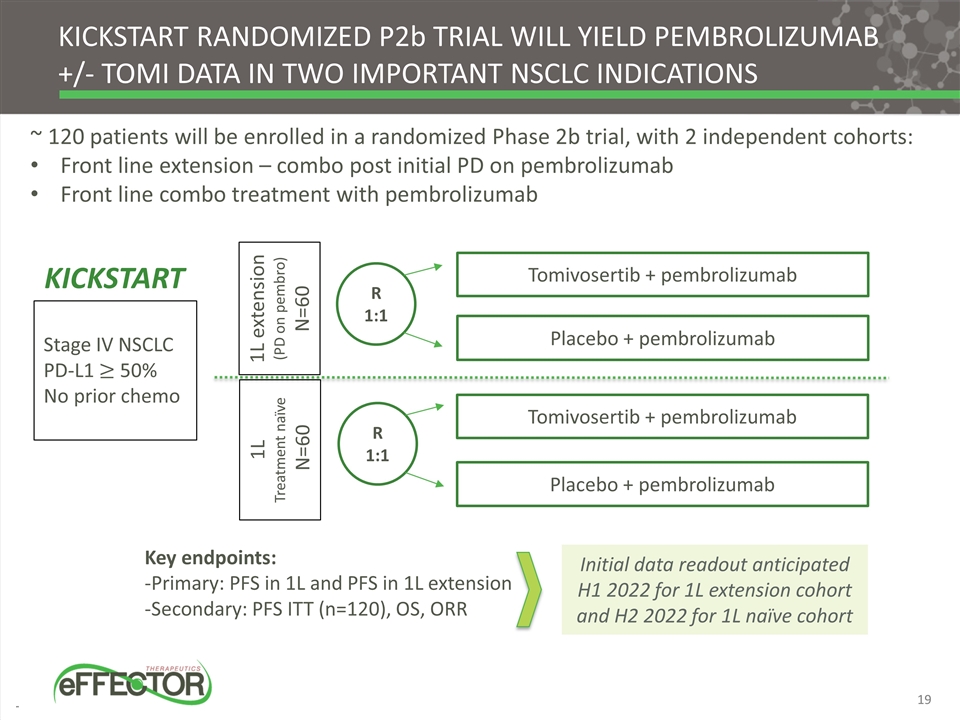
~ 120 patients will be enrolled in a randomized Phase 2b trial, with 2 independent cohorts: Front line extension – combo post initial PD on pembrolizumab Front line combo treatment with pembrolizumab Stage IV NSCLC PD-L1 50% No prior chemo Tomivosertib + pembrolizumab Placebo + pembrolizumab Key endpoints: -Primary: PFS in 1L and PFS in 1L extension -Secondary: PFS ITT (n=120), OS, ORR KICKSTART randomized p2b trial will yield Pembrolizumab +/- tomi data in two important Nsclc indications KICKSTART 1L extension (PD on pembro) N=60 1L Treatment naïve N=60 R 1:1 Tomivosertib + pembrolizumab Placebo + pembrolizumab R 1:1 Initial data readout anticipated H1 2022 for 1L extension cohort and H2 2022 for 1L naïve cohort
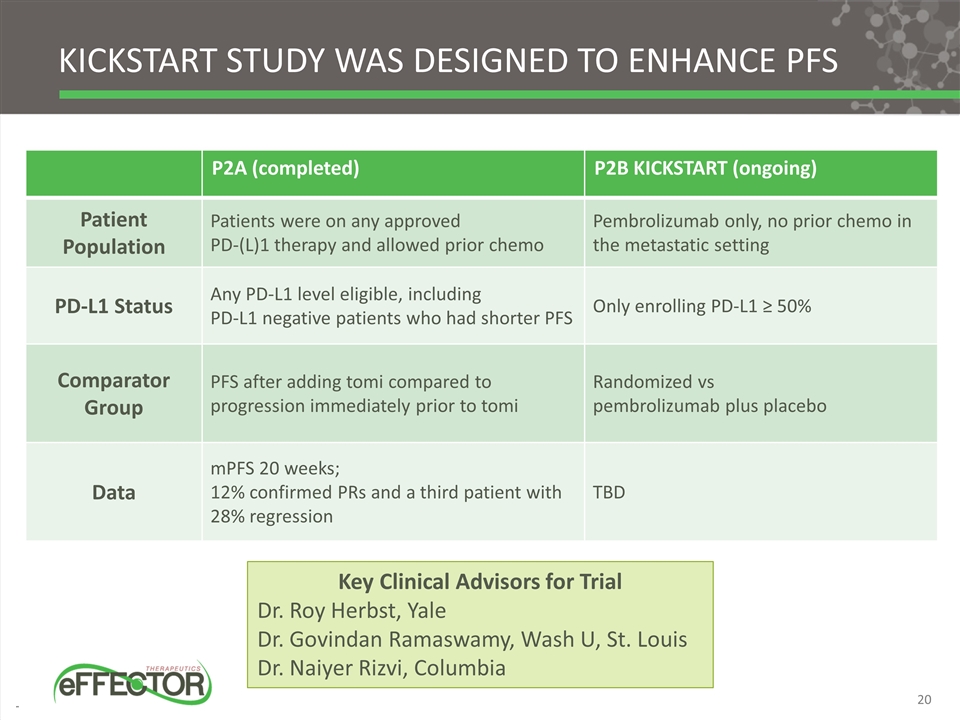
P2A (completed) P2B KICKSTART (ongoing) Patient Population Patients were on any approved PD-(L)1 therapy and allowed prior chemo Pembrolizumab only, no prior chemo in the metastatic setting PD-L1 Status Any PD-L1 level eligible, including PD-L1 negative patients who had shorter PFS Only enrolling PD-L1 ≥ 50% Comparator Group PFS after adding tomi compared to progression immediately prior to tomi Randomized vs pembrolizumab plus placebo Data mPFS 20 weeks; 12% confirmed PRs and a third patient with 28% regression TBD KICKSTART STUDY WAS DESIGNED TO ENHANCE PFS Key Clinical Advisors for Trial Dr. Roy Herbst, Yale Dr. Govindan Ramaswamy, Wash U, St. Louis Dr. Naiyer Rizvi, Columbia
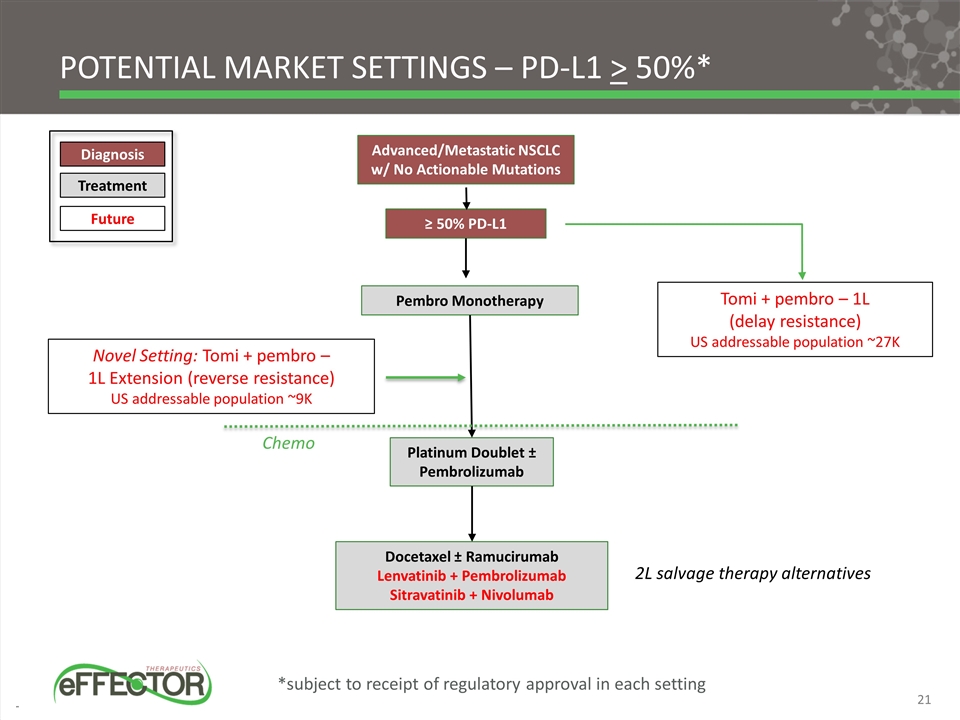
Pembro Monotherapy ≥ 50% PD-L1 Advanced/Metastatic NSCLC w/ No Actionable Mutations Platinum Doublet ± Pembrolizumab Docetaxel ± Ramucirumab Lenvatinib + Pembrolizumab Sitravatinib + Nivolumab 2L salvage therapy alternatives Novel Setting: Tomi + pembro – 1L Extension (reverse resistance) US addressable population ~9K Treatment Diagnosis Future Tomi + pembro – 1L (delay resistance) US addressable population ~27K Potential Market Settings – PD-L1 > 50%* Chemo *subject to receipt of regulatory approval in each setting
Zotatifin (eFT226) eIF4A Helicase Inhibitor Oncology: Designed to downregulate key oncoproteins and cell cycle proteins
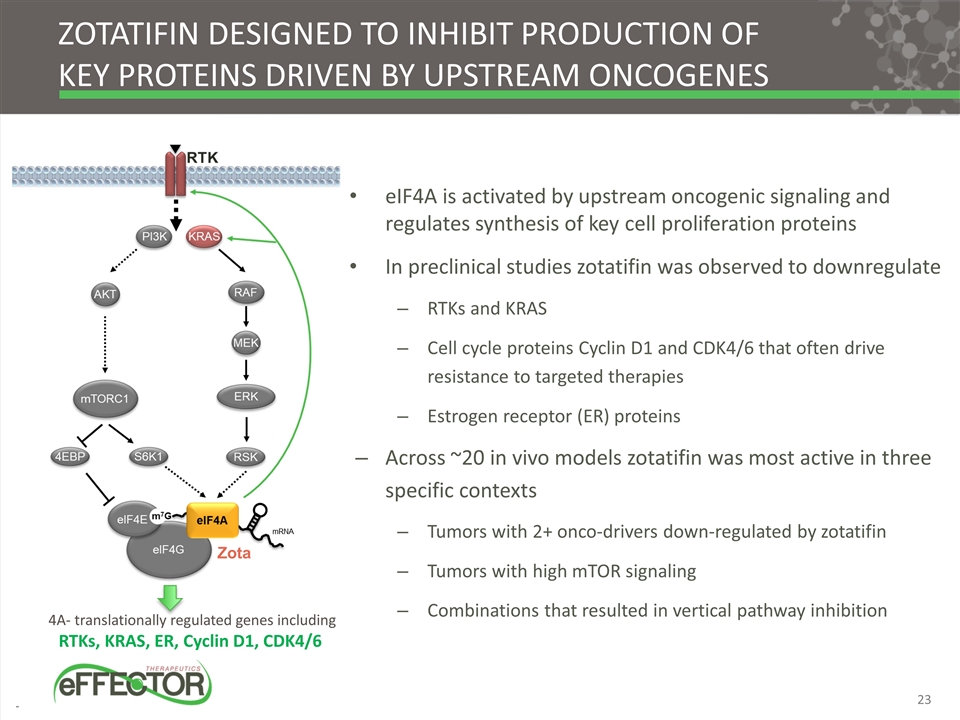
4A- translationally regulated genes including RTKs, KRAS, ER, Cyclin D1, CDK4/6 Zotatifin DESIGNED TO inhibit production of key proteins driven by upstream oncogenes eIF4A is activated by upstream oncogenic signaling and regulates synthesis of key cell proliferation proteins In preclinical studies zotatifin was observed to downregulate RTKs and KRAS Cell cycle proteins Cyclin D1 and CDK4/6 that often drive resistance to targeted therapies Estrogen receptor (ER) proteins Across ~20 in vivo models zotatifin was most active in three specific contexts Tumors with 2+ onco-drivers down-regulated by zotatifin Tumors with high mTOR signaling Combinations that resulted in vertical pathway inhibition Zota
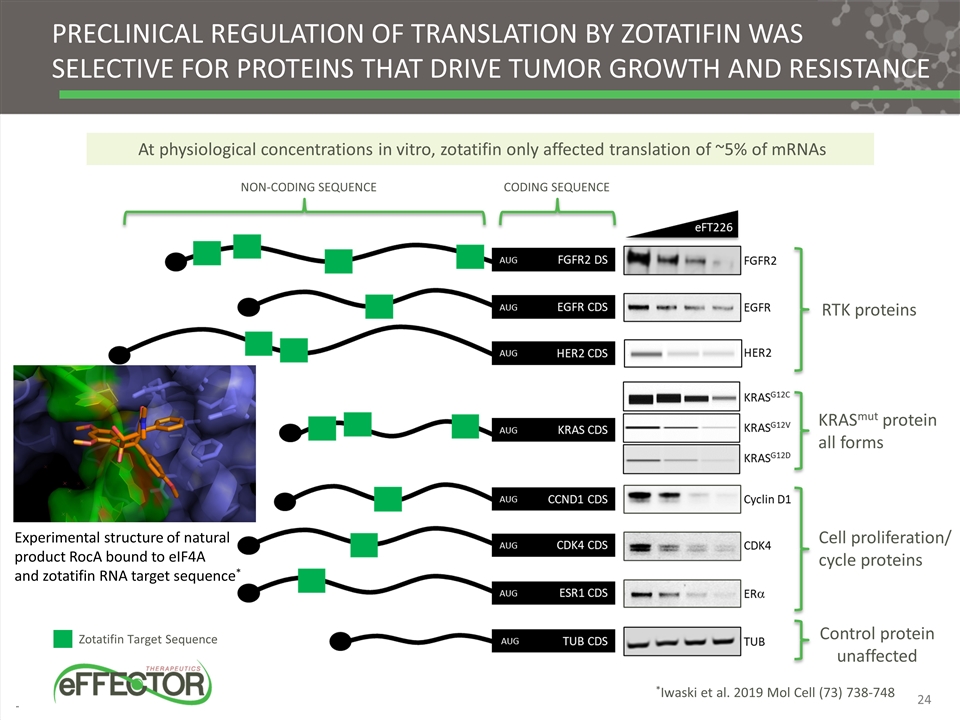
Preclinical regulation of translation by zotatifin was selective for proteins that drive tumor growth and resistance Zotatifin Target Sequence KRASmut protein all forms Cell proliferation/ cycle proteins RTK proteins Control protein unaffected Non-coding sequence coding sequence Experimental structure of natural product RocA bound to eIF4A and zotatifin RNA target sequence* *Iwaski et al. 2019 Mol Cell (73) 738-748 At physiological concentrations in vitro, zotatifin only affected translation of ~5% of mRNAs
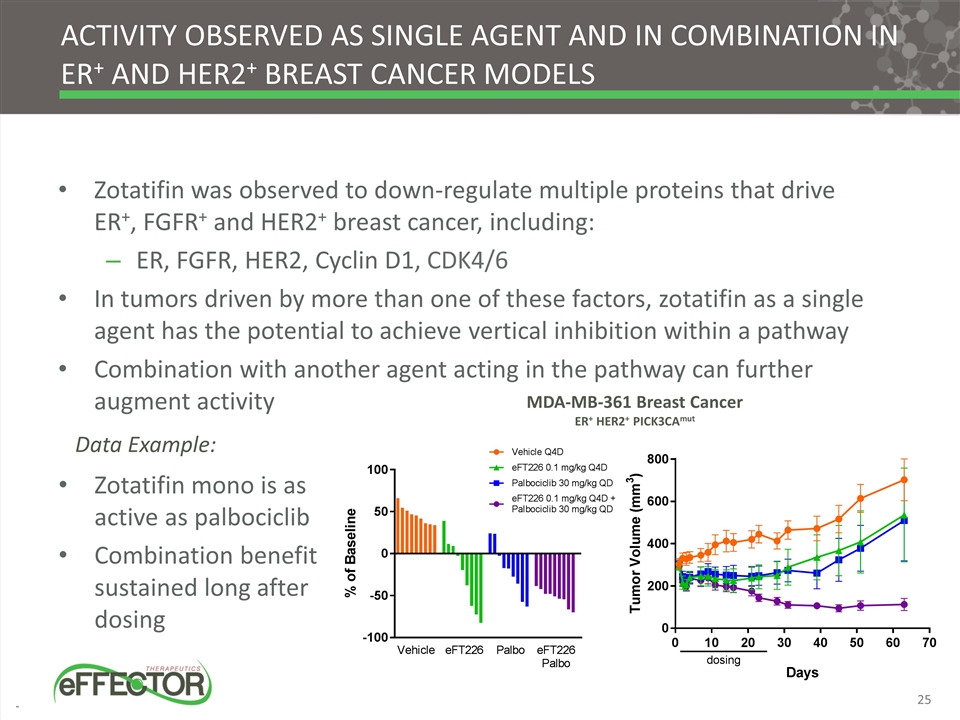
Activity observed as single agent and in combination in ER+ and HER2+ breast cancer models Zotatifin was observed to down-regulate multiple proteins that drive ER+, FGFR+ and HER2+ breast cancer, including: ER, FGFR, HER2, Cyclin D1, CDK4/6 In tumors driven by more than one of these factors, zotatifin as a single agent has the potential to achieve vertical inhibition within a pathway Combination with another agent acting in the pathway can further augment activity MDA-MB-361 Breast Cancer ER+ HER2+ PICK3CAmut Zotatifin mono is as active as palbociclib Combination benefit sustained long after dosing Data Example:
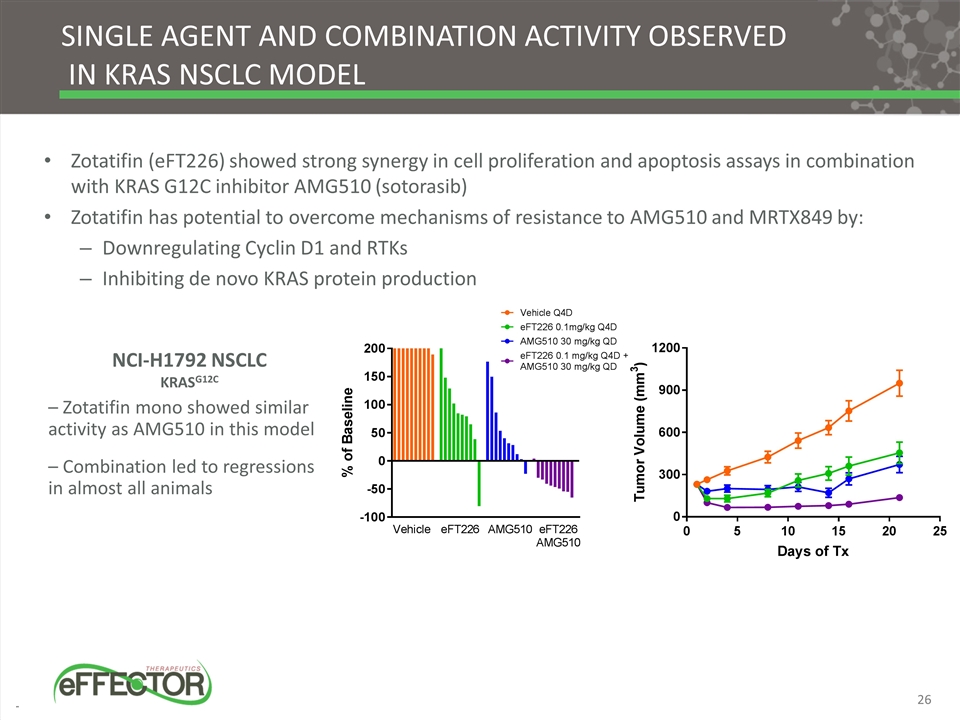
SINGLE AGENT AND COMBINATION ACTIVITY OBSERVED IN KRAS NSCLC MODEL NCI-H1792 NSCLC KRASG12C – Zotatifin mono showed similar activity as AMG510 in this model – Combination led to regressions in almost all animals Zotatifin (eFT226) showed strong synergy in cell proliferation and apoptosis assays in combination with KRAS G12C inhibitor AMG510 (sotorasib) Zotatifin has potential to overcome mechanisms of resistance to AMG510 and MRTX849 by: Downregulating Cyclin D1 and RTKs Inhibiting de novo KRAS protein production
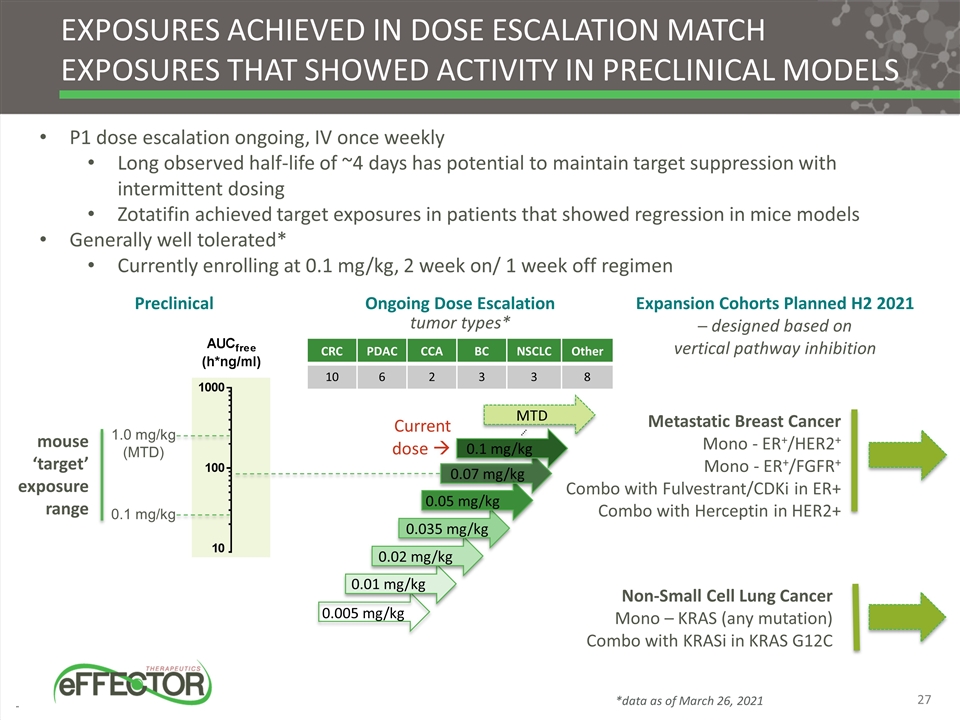
Exposures achieved in dose escalation match exposures that showed activity in preclinical models P1 dose escalation ongoing, IV once weekly Long observed half-life of ~4 days has potential to maintain target suppression with intermittent dosing Zotatifin achieved target exposures in patients that showed regression in mice models Generally well tolerated* Currently enrolling at 0.1 mg/kg, 2 week on/ 1 week off regimen 0.005 mg/kg 0.01 mg/kg 0.02 mg/kg 0.035 mg/kg 0.05 mg/kg MTD Current dose à Metastatic Breast Cancer Mono - ER+/HER2+ Mono - ER+/FGFR+ Combo with Fulvestrant/CDKi in ER+ Combo with Herceptin in HER2+ Non-Small Cell Lung Cancer Mono – KRAS (any mutation) Combo with KRASi in KRAS G12C Expansion Cohorts Planned H2 2021 ─ designed based on vertical pathway inhibition Ongoing Dose Escalation Preclinical CRC PDAC CCA BC NSCLC Other 10 6 2 3 3 8 0.07 mg/kg tumor types* 1.0 mg/kg (MTD) 0.1 mg/kg mouse ‘target’ exposure range 0.1 mg/kg *data as of March 26, 2021

financial OVERVIEW Top-tier Life Sciences Investors 2015 Series B 2013 Series A 2017 Series C Financial Highlights AbbVie Biotech Ventures $150M raised from top tier investors Up to $507M achievable under Pfizer agreement $42M received to date $465M potential additional development and sales milestones Retained US co-promote/profit share option
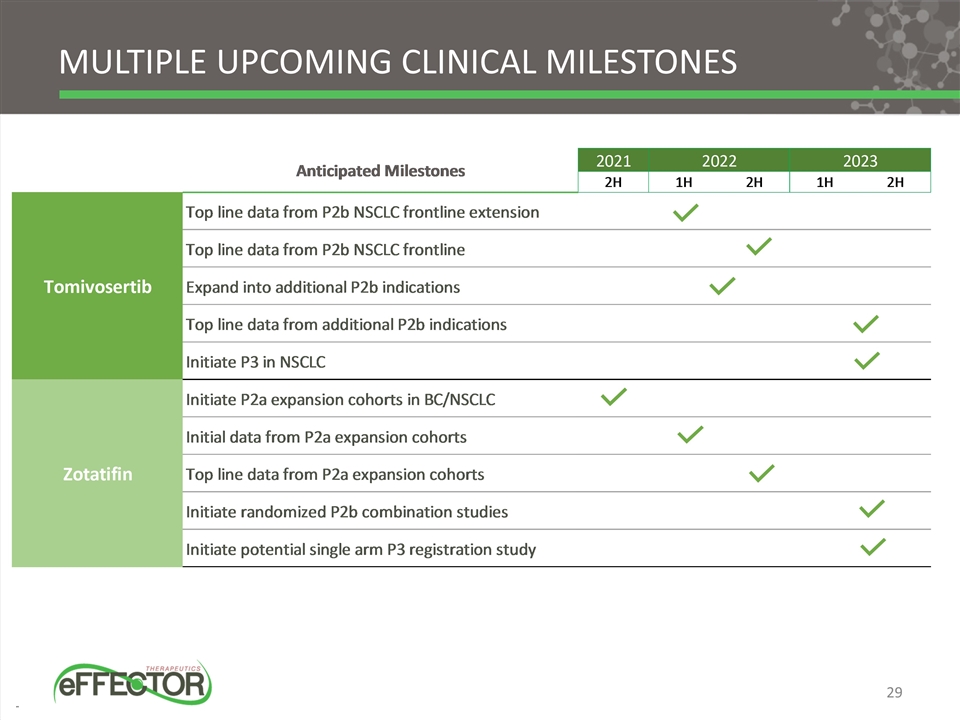
Multiple upcoming clinical milestones 29

Transaction summary
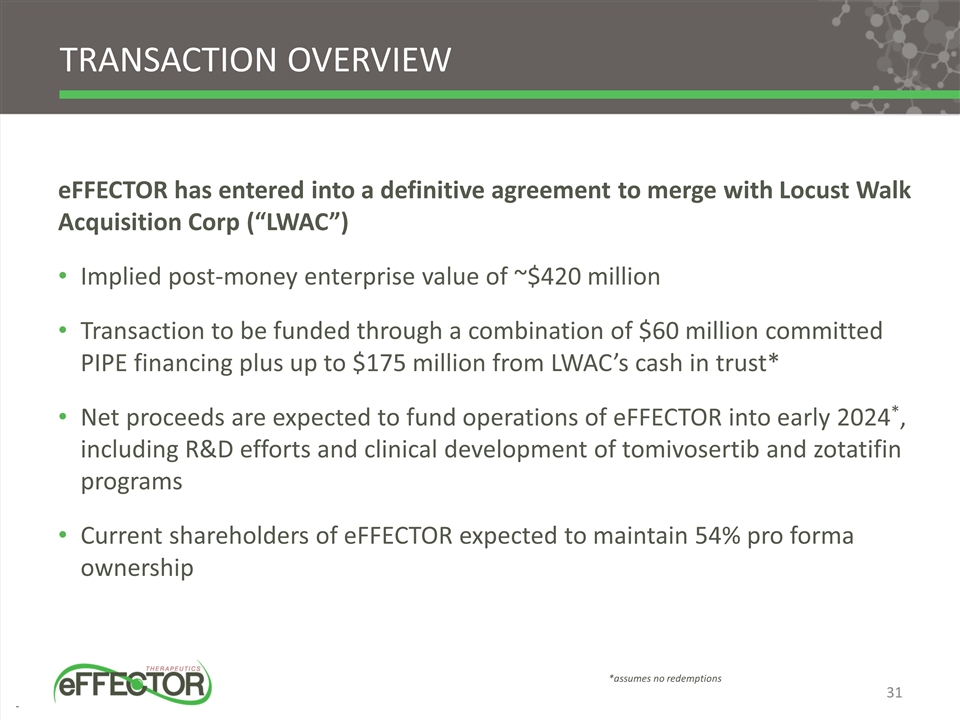
TRANSACTION OVERVIEW eFFECTOR has entered into a definitive agreement to merge with Locust Walk Acquisition Corp (“LWAC”) Implied post-money enterprise value of ~$420 million Transaction to be funded through a combination of $60 million committed PIPE financing plus up to $175 million from LWAC’s cash in trust* Net proceeds are expected to fund operations of eFFECTOR into early 2024*, including R&D efforts and clinical development of tomivosertib and zotatifin programs Current shareholders of eFFECTOR expected to maintain 54% pro forma ownership *assumes no redemptions 31
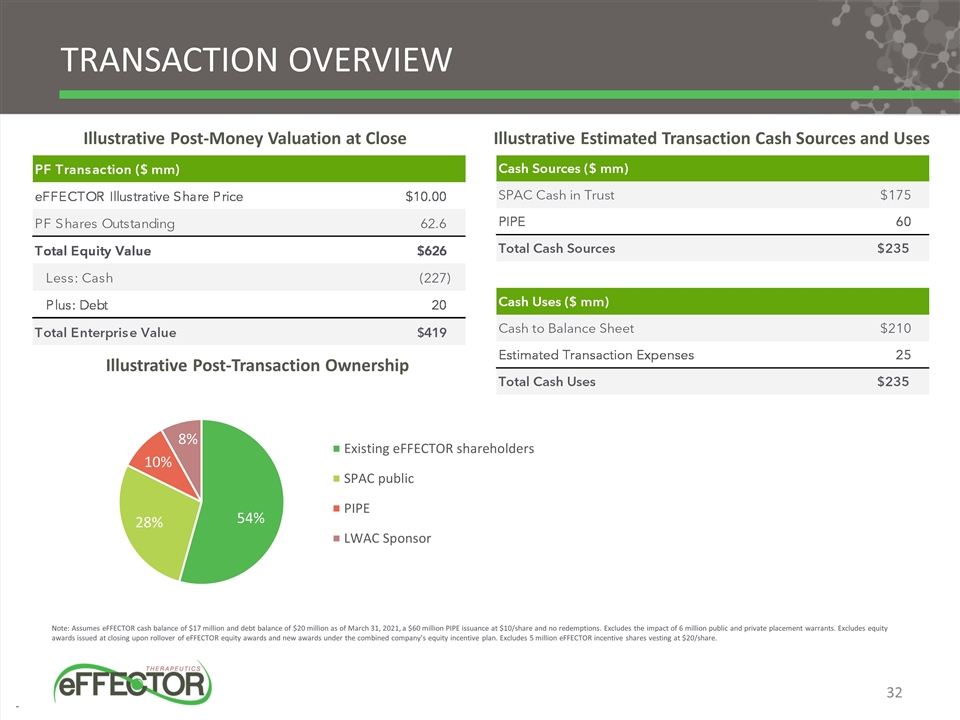
TRANSACTION OVERVIEW Illustrative Post-Money Valuation at Close Illustrative Estimated Transaction Cash Sources and Uses Illustrative Post-Transaction Ownership Note: Assumes eFFECTOR cash balance of $17 million and debt balance of $20 million as of March 31, 2021, a $60 million PIPE issuance at $10/share and no redemptions. Excludes the impact of 6 million public and private placement warrants. Excludes equity awards issued at closing upon rollover of eFFECTOR equity awards and new awards under the combined company’s equity incentive plan. Excludes 5 million eFFECTOR incentive shares vesting at $20/share. 32

Appendix
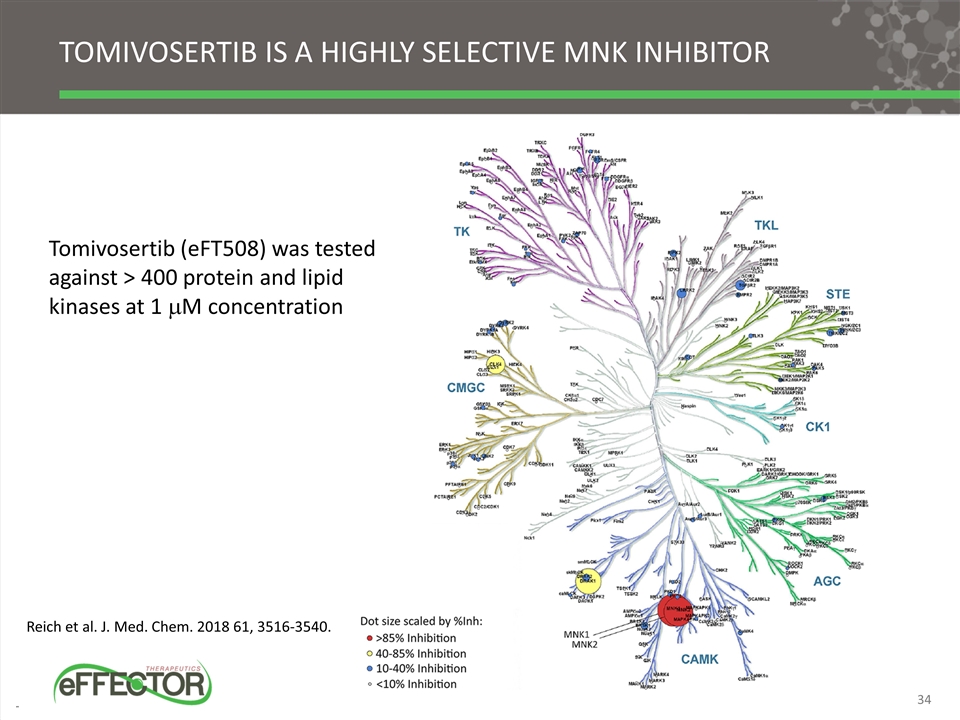
Tomivosertib is a highly selective mnk inhibitor Tomivosertib (eFT508) was tested against > 400 protein and lipid kinases at 1 mM concentration Reich et al. J. Med. Chem. 2018 61, 3516-3540. 34
tomivosertib background - Preclinical data summary 35 Tomivosertib is an immunologically active agent Downregulated expression of checkpoint proteins, including PD-1,PD-L1, LAG-3 and TIM-3 Inhibited production of immunosuppressive cytokine IL-10 without interfering with interferon-g production Functionally activates T cell effector function and expands T cell central memory pool Consistent with literature on role of MNK, p38 and MEK in T cell function Broad potential activity of tomivosertib on reprogramming T cell biology contrasts with agents acting at a single point (LAG3, TIM3, adenosine receptor) Clinically, tomivosertib has led to marked down-regulation of PD-1 and PD-L1 in on-treatment biopsies and has shown single agent activity in B cell diseases
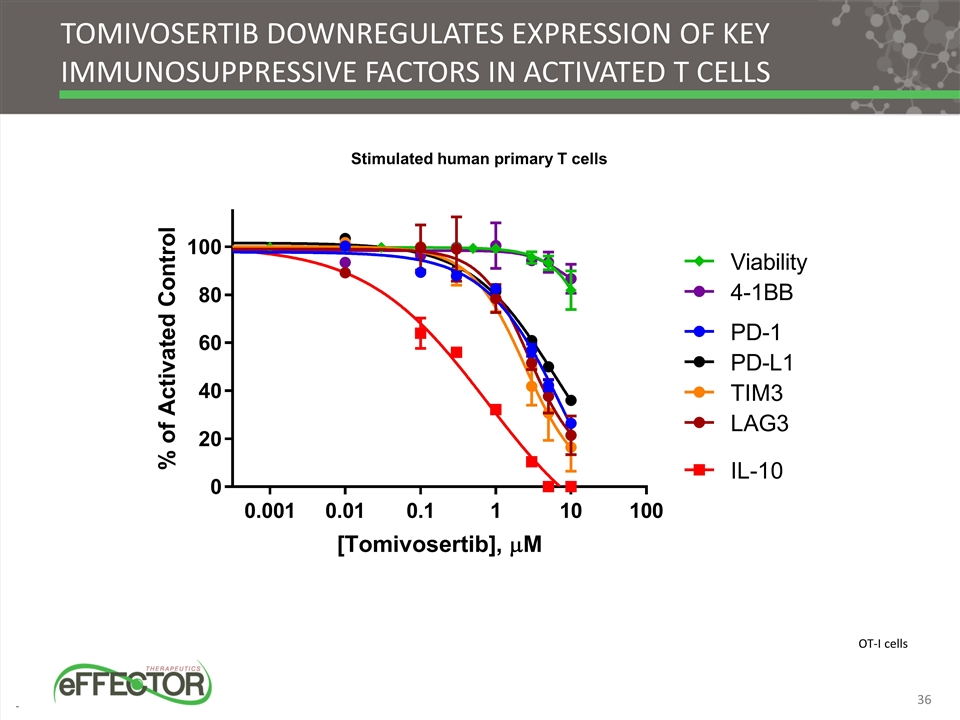
Tomivosertib downregulates expression of key immunosuppressive factors in activated T cells Stimulated human primary T cells OT-I cells 36
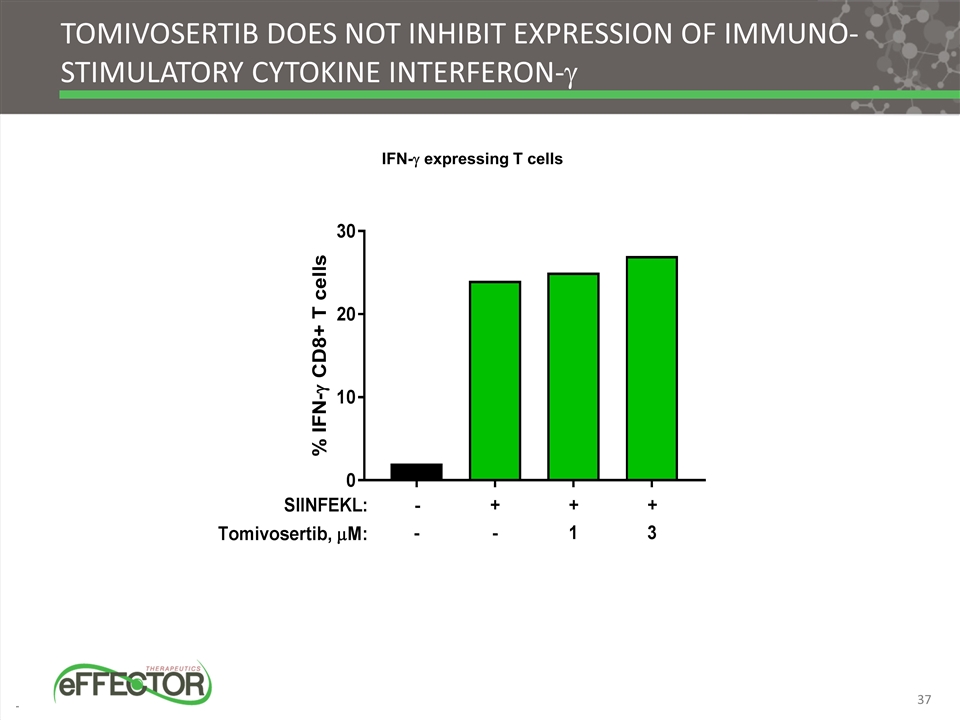
Tomivosertib does not inhibit expression of immuno-stimulatory cytokine interferon-g IFN-g expressing T cells 37
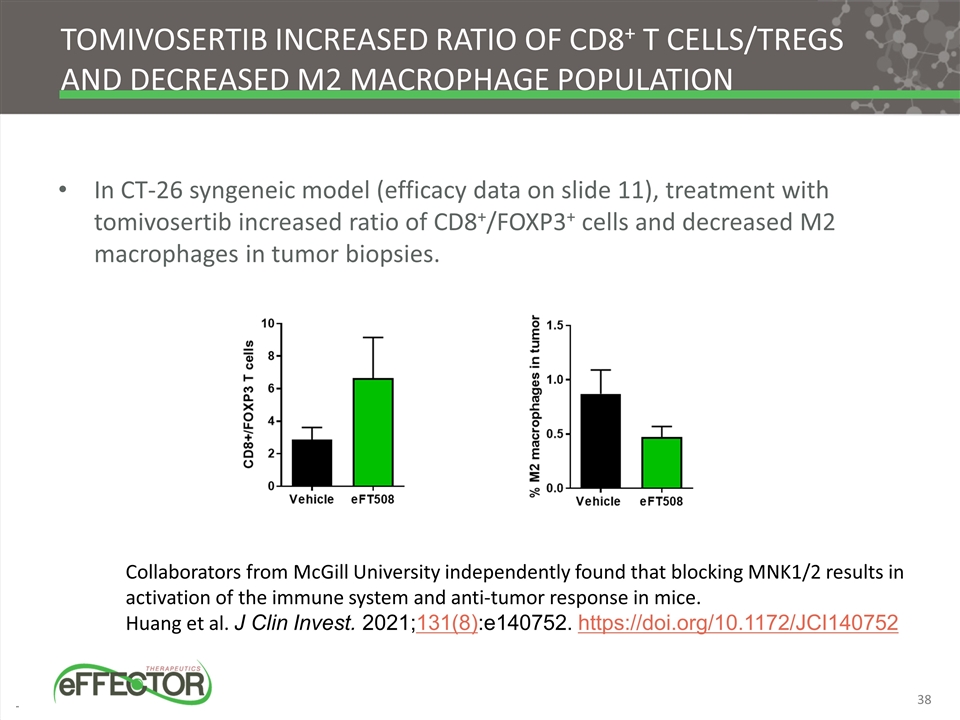
Tomivosertib increased ratio of CD8+ T cells/Tregs and decreased M2 macrophage population In CT-26 syngeneic model (efficacy data on slide 11), treatment with tomivosertib increased ratio of CD8+/FOXP3+ cells and decreased M2 macrophages in tumor biopsies. Collaborators from McGill University independently found that blocking MNK1/2 results in activation of the immune system and anti-tumor response in mice. Huang et al. J Clin Invest. 2021;131(8):e140752. https://doi.org/10.1172/JCI140752 38
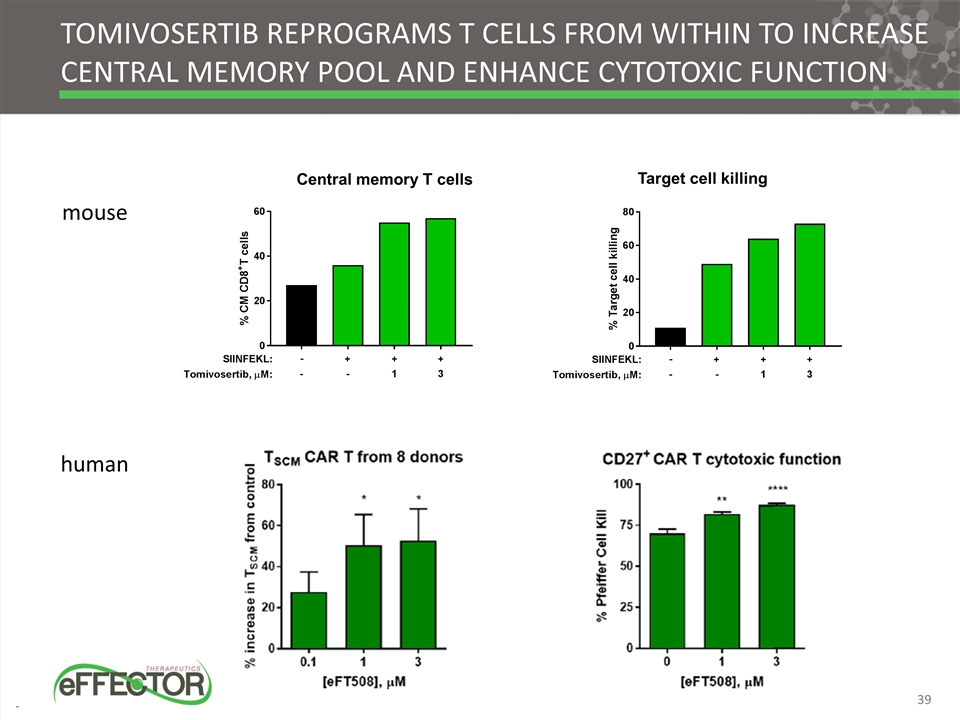
Target cell killing Tomivosertib Reprograms t cells from within to increase central memory pool and enhance cytotoxic function Central memory T cells mouse human 39
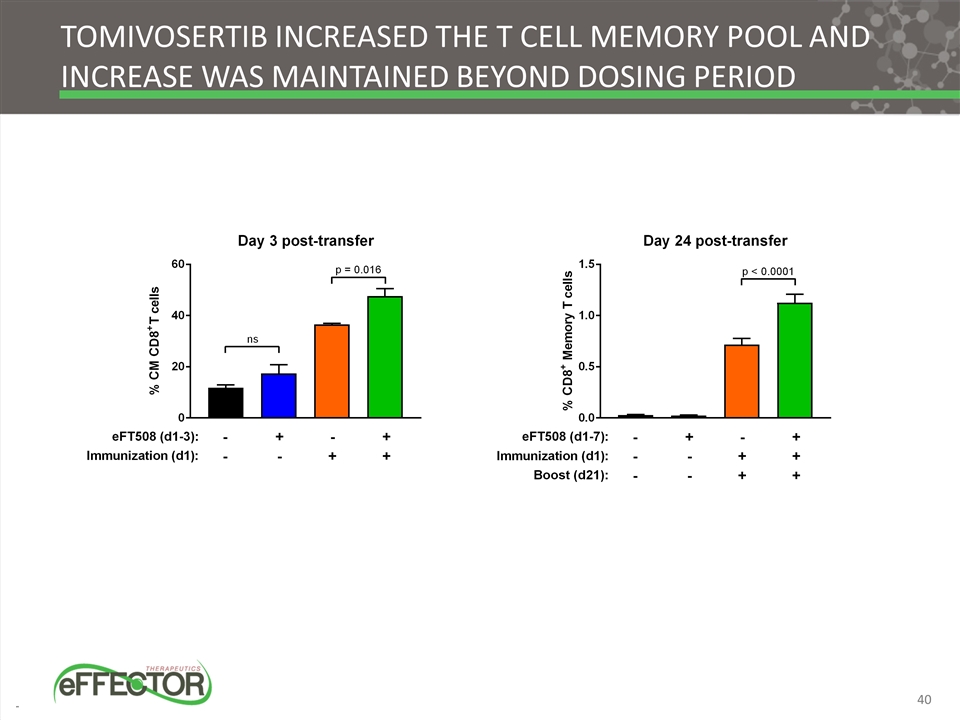
Tomivosertib increased the T cell memory pool and increase was maintained beyond dosing period 40
Zotatifin (eFT226) eIF4A Helicase Inhibitor COVID-19: Designed to inhibit unwinding of complex RNA structures necessary to translate SARS-CoV-2 proteins
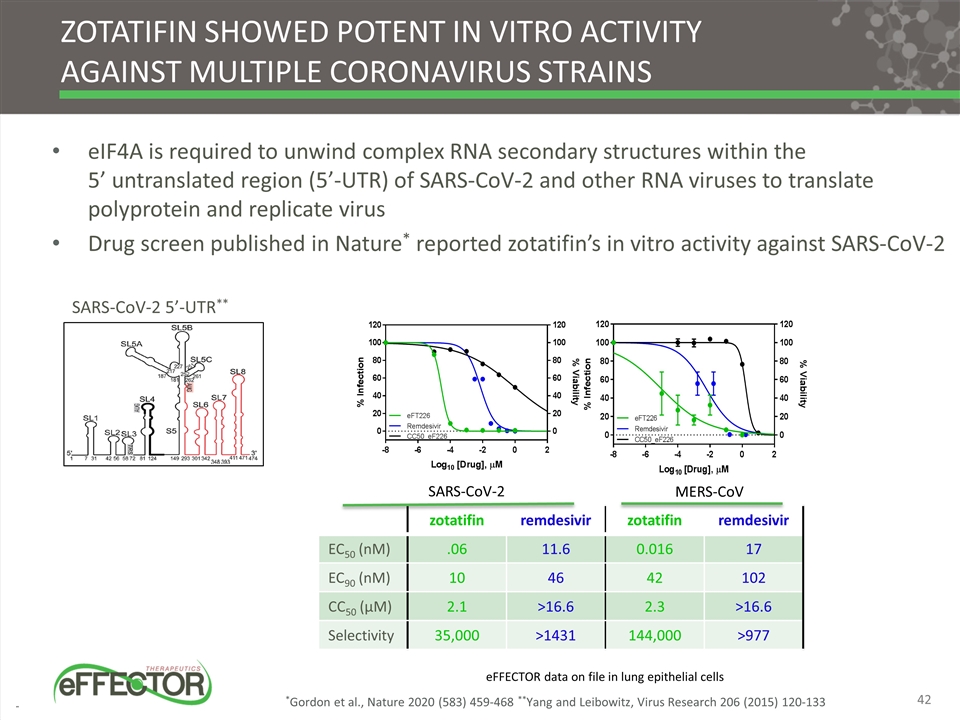
Zotatifin showed potent in vitro activity against multiple coronavirus strains eIF4A is required to unwind complex RNA secondary structures within the 5’ untranslated region (5’-UTR) of SARS-CoV-2 and other RNA viruses to translate polyprotein and replicate virus Drug screen published in Nature* reported zotatifin’s in vitro activity against SARS-CoV-2 *Gordon et al., Nature 2020 (583) 459-468 **Yang and Leibowitz, Virus Research 206 (2015) 120-133 SARS-CoV-2 5’-UTR** zotatifin remdesivir zotatifin remdesivir EC50 (nM) .06 11.6 0.016 17 EC90 (nM) 10 46 42 102 CC50 (µM) 2.1 >16.6 2.3 >16.6 Selectivity 35,000 >1431 144,000 >977 eFFECTOR data on file in lung epithelial cells SARS-CoV-2 MERS-CoV 42

RISK FACTORS Risks Related to Our Limited Operating History, Financial Position and Capital Requirements We have a limited operating history, have incurred significant operating losses since our inception and expect to incur significant losses for the foreseeable future. We may never generate any revenue or become profitable or, if we achieve profitability, we may not be able to sustain it. We will require substantial additional financing to achieve our goals, and a failure to obtain this necessary capital when needed on acceptable terms, or at all, could force us to delay, limit, reduce or terminate our development programs, commercialization efforts or other operations. Raising additional capital may cause dilution to our stockholders, including purchasers of common stock in this offering, restrict our operations or require us to relinquish rights to our technologies or product candidates. Risks Related to the Discovery, Development and Regulatory Approval of Our Product Candidates We depend heavily on the success of Tomivosertib and Zotatifin, which are in either Phase 1 or Phase 2 clinical development. If we are unable to successfully develop and commercialize our product candidates or experience significant delays in doing so, our business will be materially harmed. Our approach to the discovery and development of product candidates based on our technology platform is unproven, and we do not know whether we will be able to develop any products of commercial value. Clinical and preclinical drug development involves a lengthy and expensive process with an uncertain outcome, and the results of preclinical studies and early clinical trials are not necessarily predictive of future results. Any of our product candidates may not have favorable results in later clinical trials, if any, or receive regulatory approval on a timely basis, if at all. Any difficulties or delays in the commencement or completion, or termination or suspension, of our clinical trials could result in increased costs to us, delay or limit our ability to generate revenue and adversely affect our commercial prospects. We may find it difficult to enroll patients in our clinical trials. If we encounter difficulties enrolling subjects in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected. Use of our product candidates could be associated with side effects, adverse events or other properties or safety risks, which could delay or preclude approval, cause us to suspend or discontinue clinical trials, abandon a product candidate, limit the commercial profile of an approved label or result in other significant negative consequences that could severely harm our business, prospects, operating results and financial condition. We have never completed any later-stage clinical trials, and may be unable to do so for any of our product candidates. Our product candidates are subject to extensive regulation and compliance, which is costly and time consuming, and such regulation may cause unanticipated delays or prevent the receipt of the required approvals to commercialize our product candidates. We may expend our limited resources to pursue a particular product candidate and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success. We may seek Breakthrough Therapy designation or Fast Track designation by the FDA for one or more of our product candidates, but we may not receive such designation, and even if we do, such designation may not lead to a faster development or regulatory review or approval process and it does not increase the likelihood that our product candidates will receive marketing approval. We may conduct certain of our clinical trials for our product candidates outside of the United States. However, the FDA and other foreign equivalents may not accept data from such trials, in which case our development plans will be delayed, which could materially harm our business. Interim, topline and preliminary data from our clinical trials and preclinical studies that we announce or publish from time to time may change as more patient data become available and are subject to audit and verification procedures that could result in material changes in the final data. Disruptions at the FDA and other government agencies caused by funding shortages or global health concerns could hinder their ability to hire, retain or deploy key leadership and other personnel, or otherwise prevent new or modified products from being developed, approved or commercialized in a timely manner or at all, which could negatively impact our business. 43

RISK FACTORS (Cont’d) Risks Related to Our Reliance on Third Parties We rely on third parties to conduct our clinical trials and preclinical studies. If these third parties do not successfully carry out their contractual duties, comply with applicable regulatory requirements or meet expected deadlines, our development programs and our ability to seek or obtain regulatory approval for or commercialize our product candidates may be delayed. We rely on third parties for the manufacture of our product candidates for clinical and preclinical development. This reliance on third parties increases the risk that we will not have sufficient quantities of our product candidates or products or such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts. Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed. We are dependent on our collaboration agreement with Pfizer for the discovery, development and commercialization of small-molecule inhibitors of eIF4E. Under certain circumstances, Pfizer may unilaterally terminate the agreement for convenience, which could materially and adversely affect our business. We may seek to enter into additional collaborations, licenses and other similar arrangements and may not be successful in doing so, and even if we are, we may relinquish valuable rights and may not realize the benefits of such relationships. Risks Related to Commercialization of Our Product Candidates Even if we receive regulatory approval for any product candidate, we will be subject to ongoing regulatory obligations and continued regulatory review, which may result in significant additional expense. Additionally, our product candidates, if approved, could be subject to labeling and other restrictions on marketing or withdrawal from the market, and we may be subject to penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our product candidates, when and if any of them are approved. The commercial success of our product candidates will depend upon the degree of market acceptance of such product candidates by physicians, patients, healthcare payors and others in the medical community. The successful commercialization of our product candidates, if approved, will depend in part on the extent to which governmental authorities and health insurers establish coverage, adequate reimbursement levels and favorable pricing policies. Failure to obtain or maintain coverage and adequate reimbursement for our products could limit our ability to market those products and decrease our ability to generate revenue. We face significant competition from entities that have developed or may develop product candidates for cancer, including companies developing novel treatments and technology platforms. If our competitors develop technologies or product candidates more rapidly than we do or their technologies are more effective, our business and our ability to develop and successfully commercialize products may be adversely affected. The market opportunities for our product candidates may be limited to patients who are ineligible for or have failed prior treatments and may be small or different from our estimates. We currently have no marketing and sales organization and have no experience as a company in commercializing products, and we may have to invest significant resources to develop these capabilities. If we are unable to establish marketing and sales capabilities or enter into agreements with third parties to market and sell our products, we may not be able to generate product revenue. Our future growth may depend, in part, on our ability to operate in foreign markets, where we would be subject to additional regulatory burdens and other risks and uncertainties. 44

RISK FACTORS (Cont’d) Risks Related to Our Business Operations and Industry Our operating results may fluctuate significantly, which makes our future operating results difficult to predict and could cause our operating results to fall below expectations or any guidance we may provide. We are dependent on the services of our management and other clinical and scientific personnel, and if we are not able to retain these individuals or recruit additional management or clinical and scientific personnel, our business will suffer. We may encounter difficulties in managing our growth and expanding our operations successfully. We are subject to various federal, state and foreign healthcare and privacy laws and regulations, which could increase compliance costs, and our failure to comply with these laws and regulations could harm our results of operations and financial condition. Recently enacted legislation, future legislation and healthcare reform measures may increase the difficulty and cost for us to obtain marketing approval for and commercialize our product candidates and may affect the prices we may set. If product liability lawsuits are brought against us, we may incur substantial liabilities and may be required to limit commercialization of our products. Our insurance policies are expensive and only protect us from some business risks, which will leave us exposed to significant uninsured liabilities. We and any of our current and potential future collaborators will be required to report to regulatory authorities if any of our approved products cause or contribute to adverse medical events, and any failure to do so would result in sanctions that would materially harm our business Our internal computer systems, or those of any of our CROs, manufacturers, other contractors or consultants or current or potential future collaborators, may fail or suffer security breaches, which could result in a material disruption of our product development programs. Our business is subject to risks arising from COVID-19 and other epidemic diseases. Our business could be affected by litigation, government investigations and enforcement actions. Our employees and independent contractors, including principal investigators, CROs, consultants and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements. We may engage in strategic transactions that could impact our liquidity, increase our expenses and present significant distractions to our management. Our ability to use net operating loss carryforwards and other tax attributes may be limited in connection with this offering or other ownership changes. We prepare our consolidated financial statements in accordance with U.S. generally accepted accounting principles. These principles are subject to interpretation by the Securities and Exchange Commission and various bodies formed to create and interpret appropriate accounting principles and guidance. A change in these principles or guidance, or in their interpretations, may have a material effect on our reported results, as well as our processes and related controls, and may retroactively affect previously reported results. 45

RISK FACTORS (Cont’d) Risks Related to Our Intellectual Property If we are unable to obtain and maintain patent protection for our product candidates, or if the scope of the patent protection obtained is not sufficiently broad, our competitors could develop and commercialize products similar or identical to ours, and our ability to successfully commercialize our product candidates may be adversely affected. We may not be able to protect our intellectual property and proprietary rights throughout the world. Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements. Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products. Issued patents covering our product candidates could be found invalid or unenforceable if challenged in court or before administrative bodies in the United States or abroad. Patent terms may be inadequate to protect our competitive position on products and product candidates for an adequate amount of time. If we do not obtain patent term extension for our product candidates, our business may be materially harmed. We may be subject to claims challenging the inventorship of our patents and other intellectual property. If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed. We may not identify relevant third-party patents or may incorrectly interpret the relevance, scope or expiration of a third-party patent, which might adversely affect our ability to develop and market our products and product candidates. We may be subject to claims that our employees, consultants or advisors have wrongfully used or disclosed alleged trade secrets of their current or former employers or claims asserting ownership of what we regard as our own intellectual property. Third-party claims of intellectual property infringement, misappropriation or other violations against us or our collaborators may prevent or delay the development and commercialization of our product candidates. We may become involved in lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive, time consuming and unsuccessful. If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected. Intellectual property rights do not necessarily address all potential threats. If we fail to comply with any of our obligations under our existing license agreement or any future license agreements, or disputes arise with respect to those agreements, it could have a negative impact on our business and our intellectual property rights. We may not be successful in obtaining or maintaining necessary rights to product components and processes for our development pipeline through acquisitions and in-licenses. We, our collaborator and our service providers may be subject to a variety of privacy and data security laws and contractual obligations, which could increase compliance costs and our failure to comply with them could subject us to potentially significant fines or penalties and otherwise harm our business. 46

RISK FACTORS (Cont’d) Risks Related to Becoming a Public Company We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives. We will be an emerging growth company and a smaller reporting company, and the reduced disclosure requirements applicable to emerging growth companies and smaller reporting companies may make our common stock less attractive to investors. If securities or industry analysts do not publish research or reports or publish unfavorable research or reports about our business, our stock price and trading volume could decline. If we fail to maintain proper and effective internal control over financial reporting, our ability to produce accurate and timely financial statements could be impaired, investors may lose confidence in our financial reporting and the trading price of our common stock may decline. Provisions in our proposed charter documents and under Delaware law could discourage a takeover that stockholders may consider favorable and may lead to entrenchment of management. Our proposed certificate of incorporation will provide that the Court of Chancery of the State of Delaware will be the exclusive forum for substantially all disputes between us and our stockholders and that the federal district courts shall be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the Securities Act, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with us or our directors, officers or employees. We could be subject to securities class action litigation. Risks Related to the Private Placement The conditions to closing the Private Placement may not be met and LWAC may be otherwise unable to raise additional financing to complete the Business Combination or to fund the operations and growth of the combined company following the Business Combination (the “Combined Company”). The issuance of shares of the Combined Company’s securities in connection with the Private Placement will dilute substantially the voting power of purchasers in the Private Placement. LWAC may issue shares of its Class A common stock upon the conversion of its Class B common stock at a ratio greater than one-to-one at the closing of the Business Combination as a result of the anti-dilution provisions contained in its amended and restated certificate of incorporation. Any such issuance would dilute the interest of the Combined Company’s stockholders, including purchasers in the Private Placement. In addition to acting as placement agents, (i) Credit Suisse Securities (USA) LLC is acting as equity capital markets and financial advisor to the Company and (ii) an entity affiliated with certain members of LWAC's management, Locust Walk Partners, LLC, has provided financial advisory services to LWAC in connection with the proposed transaction, which may give rise to potential conflicts of interests or the appearance thereof. Risks Related to the Business Combination Each of LWAC and eFFECTOR will incur significant transaction costs in connection with the Business Combination. The consummation of the Business Combination is subject to a number of conditions and if those conditions are not satisfied or waived, the Business Combination agreement may be terminated in accordance with its terms and the Business Combination may not be completed. The ability to successfully effect the Business Combination and the Combined Company’s ability to successfully operate the business thereafter will be largely dependent upon the efforts of certain key personnel of eFFECTOR. The loss of such key personnel could negatively impact the operations and financial results of the combined business. 47

RISK FACTORS (Cont’d) There is no assurance that a stockholder’s decision whether to redeem its shares for a pro rata portion of LWAC’s trust account will put the stockholder in a better future economic position. If the Business Combination’s benefits do not meet the expectations of investors or securities analysts, the market price of LWAC’s securities or, following the consummation of the Business Combination, the Combined Company’s securities, may decline. A market for the Combined Company’s securities may not develop, which would adversely affect the liquidity and price of such securities. There can be no assurance that the Combined Company’s securities will be approved for listing on the Nasdaq Global Market (“Nasdaq”) or that the Combined Company will be able to comply with the continued listing standards of Nasdaq. Directors of LWAC have potential conflicts of interest in recommending that LWAC’s stockholders vote in favor of the adoption of the Business Combination. LWAC may redeem unexpired warrants prior to their exercise at a time that is disadvantageous to the holders of LWAC warrants, thereby making such warrants worthless. Further, even if the Business Combination is completed, there can be no assurance that LWAC’s warrants will be in the money during their exercise period, and they may expire worthless. If LWAC seeks stockholder approval of the Business Combination, its sponsor, directors, officers, advisors and their affiliates may elect to purchase shares or warrants from public stockholders, which may influence a vote on the Business Combination and reduce the public “float” of LWAC’s Class A common stock or warrants. If LWAC seeks stockholder approval of the Business Combination, its sponsor, officers and directors have agreed to vote in favor of such Business Combination, regardless of how its public stockholders vote. The ability of LWAC’s public stockholders to exercise redemption rights with respect to a large number of its shares could increase the probability that the Business Combination would be unsuccessful. The LWAC public shareholders can redeem some or all of the $175 million held in trust, and significant redemptions could materially impact our cash position and runway. LWAC is not required to obtain an opinion from an independent investment banking firm or from an independent accounting firm, and consequently, its stockholders may have no assurance from an independent source that the price it is paying for the business is fair to LWAC from a financial point of view. Legal proceedings in connection with the Business Combination, the outcomes of which are uncertain, could delay or prevent the completion of the Business Combination. The Business Combination or Combined Company may be materially adversely affected by the recent COVID-19 outbreak. Changes in laws or regulations, or a failure to comply with any laws and regulations, may adversely affect LWAC’s and the Combined Company’s business, including LWAC’s and the Combined Company’s ability to consummate the Business Combination, and results of operations. General Risk Factors We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We could face criminal liability and other serious consequences for violations, which could harm our business. We and any of our third-party manufacturers or suppliers may use potent chemical agents and hazardous materials, and any claims relating to improper handling, storage or disposal of these materials could be time consuming or costly. Business disruptions could seriously harm our future revenue and financial condition and increase our costs and expenses. Unstable market and economic conditions may have serious adverse consequences on our business, financial condition and stock price. Changes in U.S. tax law may materially adversely affect our financial condition, results of operations and cash flows. 48

Corporate Overview May 2021 Next Generation Targeted Therapy for Cancer

















































