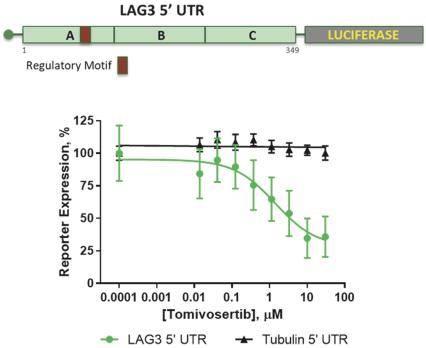
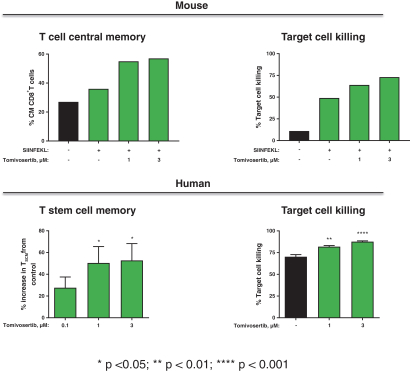
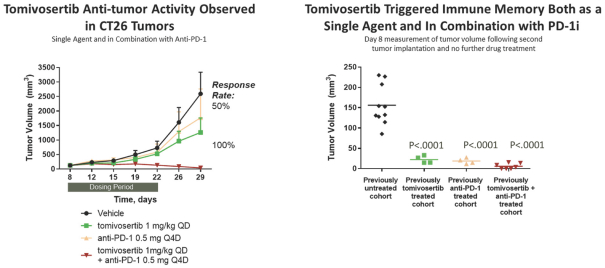
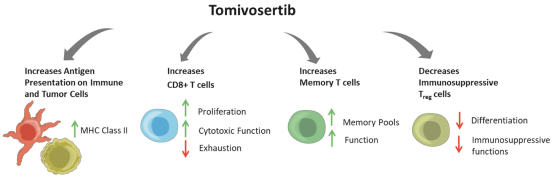
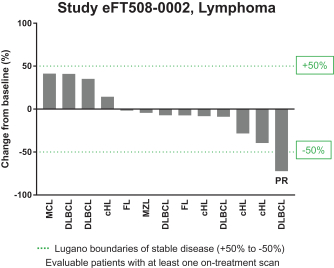
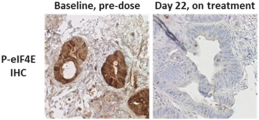
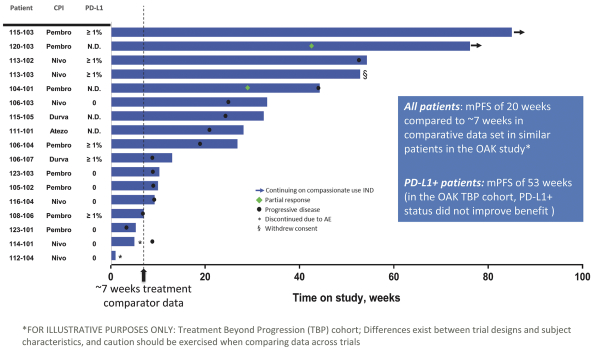
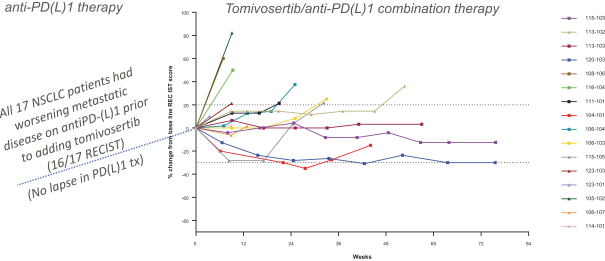
trial, the risk that enrolled patients will not complete a clinical trial, our ability to recruit clinical trial investigators with the appropriate competencies and experience, our ability to obtain and maintain patient consents, patient referral practices of physicians, ability to monitor patients adequately during and after treatment, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages and risks of the product candidate being studied in relation to other available therapies, including any new products that may be approved for the indications we are investigating as well as any product candidates under development.
We will be required to identify and enroll a sufficient number of subjects for each of our clinical trials. Potential subjects for any planned clinical trials may not be adequately diagnosed or identified with the diseases which we are targeting or may not meet the entry criteria for such trials. We also may encounter difficulties in identifying and enrolling patients with a stage of disease appropriate for our ongoing and planned clinical trials and monitoring such patients adequately during and after treatment. The large number of clinical trials concurrently seeking to enroll patients with NSCLC and breast cancers, as well as the other cancers we intend to evaluate, may result in delays or difficulties enrolling a sufficient number of patients, particularly patients that meet our specific enrollment criteria, and completing the trials on schedule, if at all. In addition, with respect to our planned Phase 1b clinical trial of zotatifin, we intend to assess zotatifin as a potential host-direct anti-viral therapy for
With declining
COVID-19
infection rates globally, enrollment has been slower than expected in this clinical trial because the enrollment criteria requires that patients enroll within 5 days of symptoms of the virus. This, together with the large number of clinical trials seeking to enroll
COVID-19
patients, may materially delay our expectations with respect to the clinical timeline for this and any future clinical trials. We may not be able to initiate or continue clinical trials if we are unable to locate a sufficient number of eligible subjects to participate in the clinical trials required by the FDA or comparable foreign regulatory authorities. In addition, the process of finding and diagnosing patients is and will likely continue to be costly. The timing of our clinical trials depends, in part, on the speed at which we can recruit patients to participate in our trials, as well as completion of required
follow-up
periods. The eligibility criteria of our clinical trials further limits the pool of available trial participants. If patients are unwilling to participate in our trials for any reason, including the existence of concurrent clinical trials for similar patient populations, the availability of approved therapies or as a result of the
COVID-19
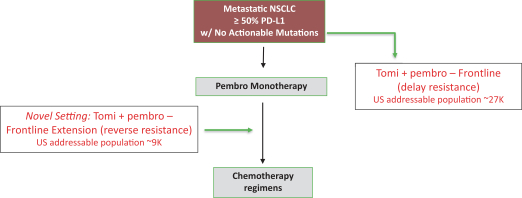
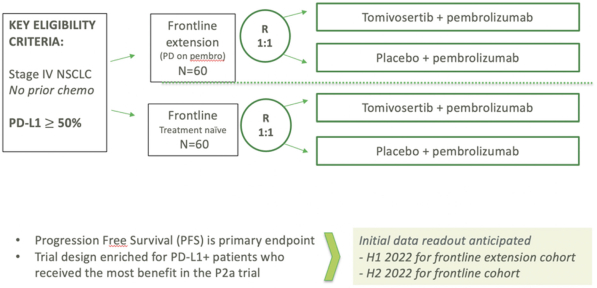
pandemic, or we otherwise have difficulty enrolling a sufficient number of patients, the timeline for recruiting subjects, conducting studies and obtaining regulatory approval of our product candidates may be delayed. In particular, the frontline extension portion of our KICKSTART trial requires patients to experience disease progression while taking pembrolizumab before receiving tomivosertib or proceeding to a second line of therapy, and patients or physicians may be unwilling to delay chemotherapy or other approved treatments to enroll in our trial. Furthermore, the FDA placed a partial clinical hold on our Phase 2b KICKSTART clinical trial of tomivosertib in combination with pembrolizumab treatment in frontline and frontline extension NSCLC patients which prevents us from enrolling more than 50 patients for experimental treatment until the results of
13-week
toxicology studies are submitted to and reviewed by the FDA. While we expect to file the results of the
13-week
animal toxicology studies with the FDA by the end of 2021, if we do not complete these toxicology studies on our expected timeline or if the results of the studies do not support a continued development, we will be unable to enroll a sufficient number of subjects which may cause the trial to be delayed or not completed at all. Additionally, because our clinical trials may enroll patients with advanced/metastatic cancers, the patients are typically in the late stages of their disease and may experience clinical disease progression independent from our product candidates, making them unevaluable for purposes of the clinical trial and requiring additional patient enrollment. Our inability to enroll a sufficient number of subjects for any of our future clinical trials would result in significant delays or may require us to abandon one or more clinical trials altogether. In addition, we expect to rely on CROs and clinical trial sites to ensure proper and timely conduct of our future clinical trials, and while we have entered into agreements governing their services, we have limited influence over their actual performance. We cannot assure you that our assumptions used in determining expected clinical trial timelines are correct or that we will not experience delays in enrollment, which would result in the delay of completion of such trials beyond our expected timelines.