Next Generation Immuno-Oncology Medicines John K. Celebi, MBA President & Chief Executive Officer AUGUST 2022 | Nasdaq: SNSE Exhibit 99.2

Disclaimer This presentation has been prepared by Sensei Biotherapeutics, Inc. (the “Company,” "we," "us") and is made for informational purposes only. The information set forth herein does not purport to be complete or to contain all of the information you may desire. Statements contained herein are made as of the date of this presentation unless stated otherwise, and neither the delivery of this presentation at any time, nor any sale of securities, shall under any circumstances create an implication that the information contained herein is correct as of any time after such date or that information will be updated or revised to reflect information that subsequently becomes available or changes occurring after the date hereof. This presentation contains estimates and other statistical data made by independent parties and by us relating to market shares and other data about our industry. This presentation also contains "forward-looking" statements as that term is defined in the Private Securities Litigation Reform Act of 1995 that are based on our management's beliefs and assumptions and on information currently available to management. These forward-looking statements include, without limitation, expectations regarding the development of our product candidates and platforms, the availability of data from our preclinical studies, the timing of selection of product candidates, the timing of IND submissions to the FDA, and our belief that our existing cash and cash equivalents will be sufficient to fund our operations at least into the first quarter of 2025. When used in this presentation, the words and phrases "designed to," "may," "believes," "intends," "seeks," "anticipates," "plans," "estimates," "expects," "should," "assumes," "continues," "could," "will," "future" and the negative of these or similar terms and phrases are intended to identify forward-looking statements. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Risks and uncertainties that may cause actual results to differ materially include uncertainties inherent in the development of therapeutic product candidates, such as preclinical discovery and development, conduct of clinical trials and related regulatory requirements, our reliance on third parties over which we may not always have full control, and other risk and uncertainties that are described in our Annual Report on Form 10-K filed with the SEC on March 15, 2022 and our other Periodic Reports filed with the SEC. Forward-looking statements represent our management's beliefs and assumptions only as of the date of this presentation and include all matters that are not historical facts. Our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. Certain information contained in this presentation relates to, or is based on, studies, publications, surveys and other data obtained from third-party sources and the Company's own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.
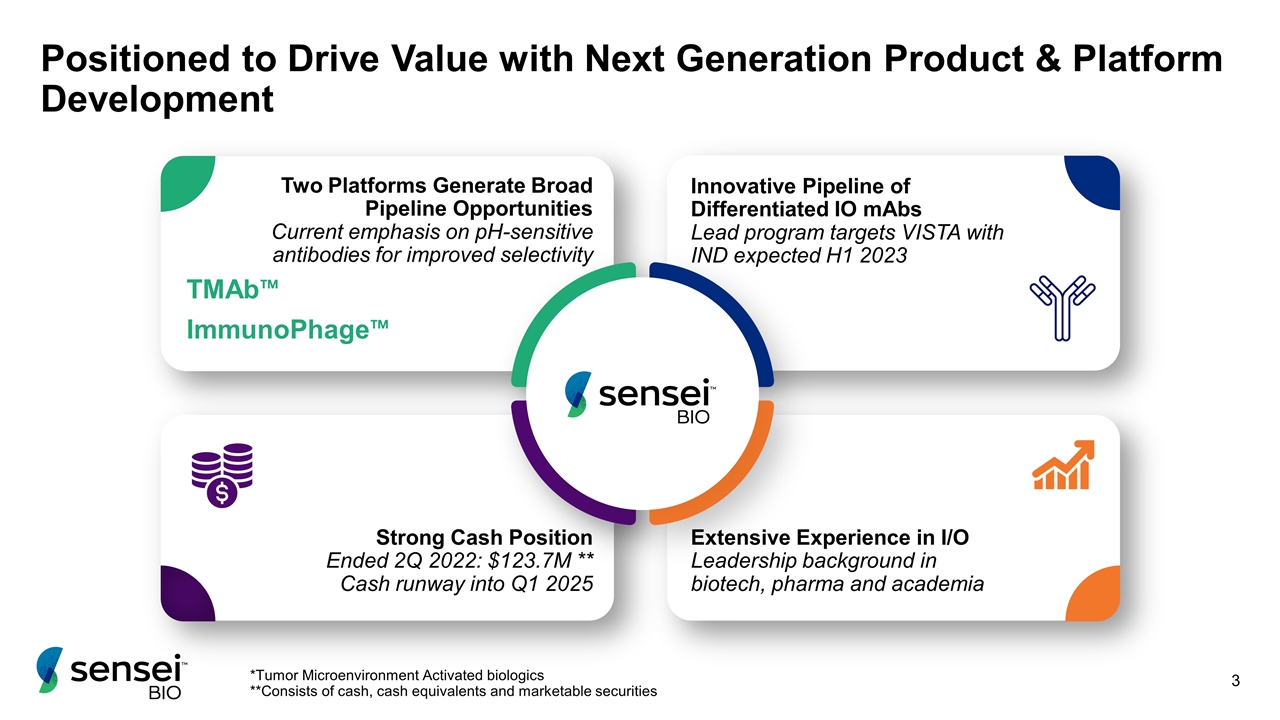
Positioned to Drive Value with Next Generation Product & Platform Development Two Platforms Generate Broad Pipeline Opportunities Current emphasis on pH-sensitive antibodies for improved selectivity TMAb™ ImmunoPhage™ Innovative Pipeline of Differentiated IO mAbs Lead program targets VISTA with IND expected H1 2023 Extensive Experience in I/O Leadership background in biotech, pharma and academia Strong Cash Position Ended 2Q 2022: $123.7M ** Cash runway into Q1 2025 *Tumor Microenvironment Activated biologics **Consists of cash, cash equivalents and marketable securities
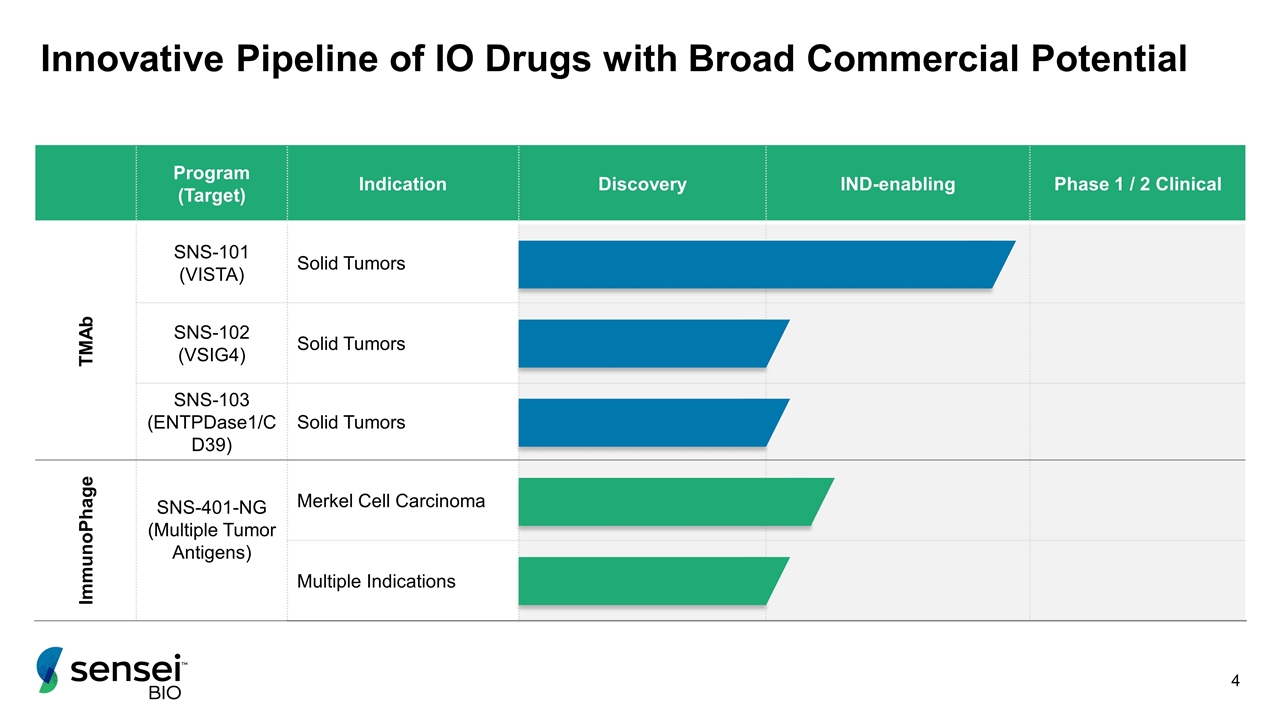
Innovative Pipeline of IO Drugs with Broad Commercial Potential Program (Target) Indication Discovery IND-enabling Phase 1 / 2 Clinical TMAb SNS-101 (VISTA) Solid Tumors SNS-102 (VSIG4) Solid Tumors SNS-103 (ENTPDase1/CD39) Solid Tumors ImmunoPhage SNS-401-NG (Multiple Tumor Antigens) Merkel Cell Carcinoma Multiple Indications
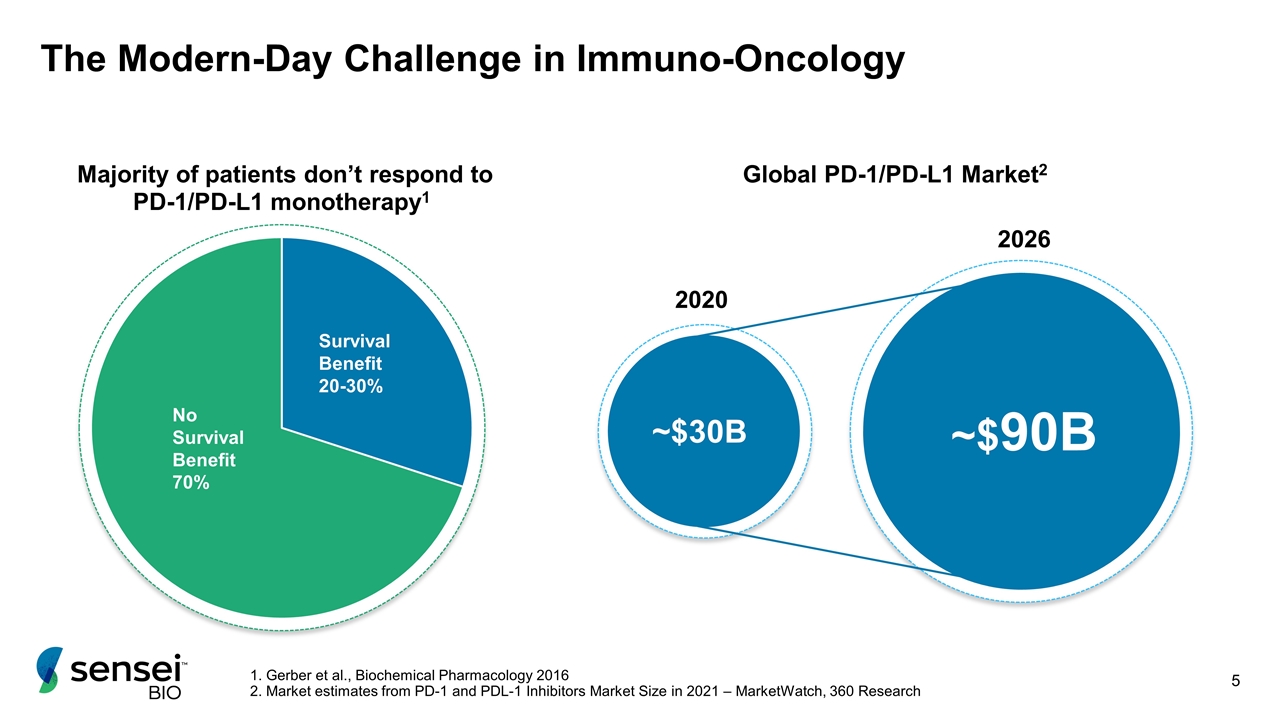
The Modern-Day Challenge in Immuno-Oncology No Survival Benefit 70% Survival Benefit 20-30% ~$30B ~$90B 2020 2026 Global PD-1/PD-L1 Market2 1. Gerber et al., Biochemical Pharmacology 2016 2. Market estimates from PD-1 and PDL-1 Inhibitors Market Size in 2021 – MarketWatch, 360 Research
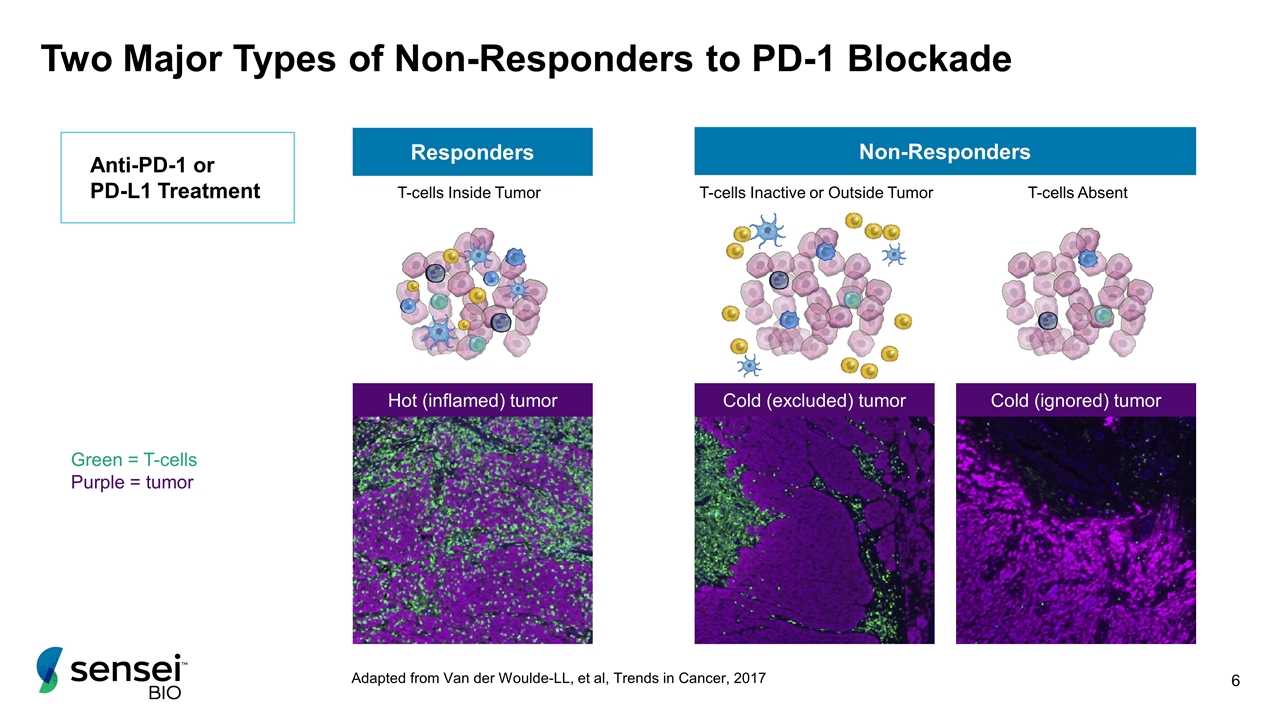
Two Major Types of Non-Responders to PD-1 Blockade Green = T-cells Purple = tumor Non-Responders T-cells Absent T-cells Inactive or Outside Tumor Responders T-cells Inside Tumor Anti-PD-1 or PD-L1 Treatment Adapted from Van der Woulde-LL, et al, Trends in Cancer, 2017 Hot (inflamed) tumor Cold (excluded) tumor Cold (ignored) tumor
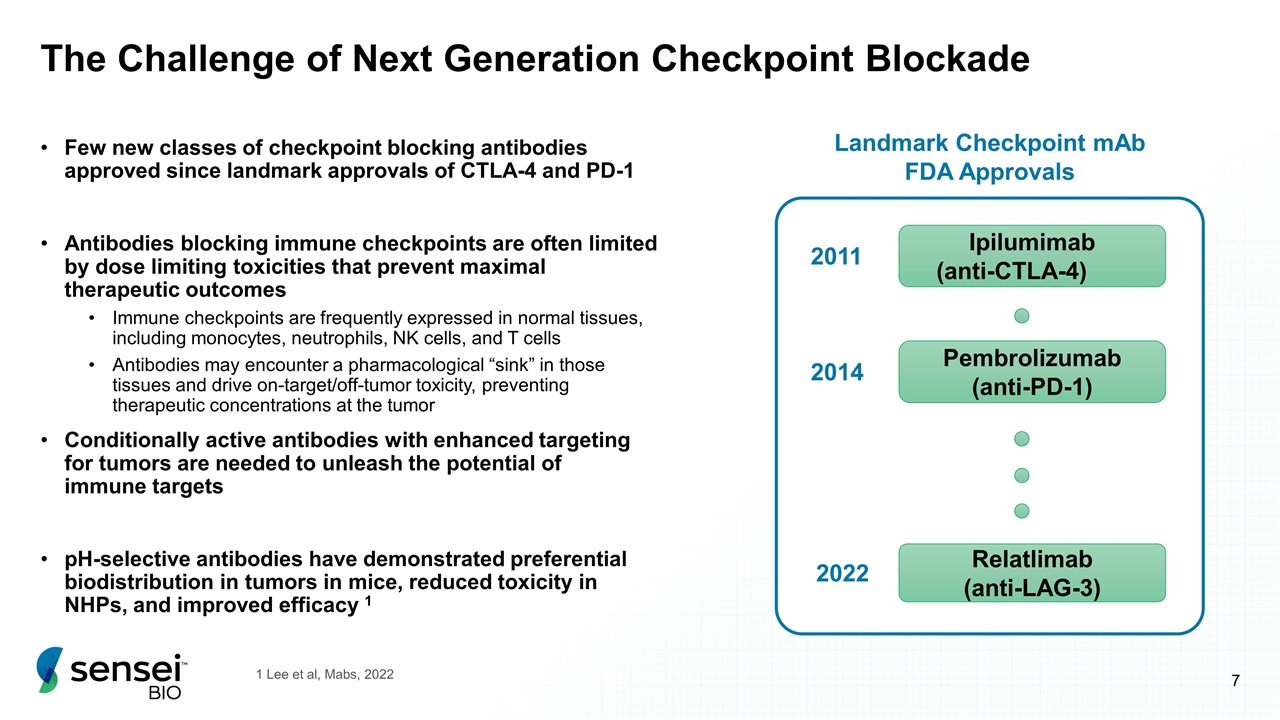
The Challenge of Next Generation Checkpoint Blockade Few new classes of checkpoint blocking antibodies approved since landmark approvals of CTLA-4 and PD-1 Antibodies blocking immune checkpoints are often limited by dose limiting toxicities that prevent maximal therapeutic outcomes Immune checkpoints are frequently expressed in normal tissues, including monocytes, neutrophils, NK cells, and T cells Antibodies may encounter a pharmacological “sink” in those tissues and drive on-target/off-tumor toxicity, preventing therapeutic concentrations at the tumor Conditionally active antibodies with enhanced targeting for tumors are needed to unleash the potential of immune targets pH-selective antibodies have demonstrated preferential biodistribution in tumors in mice, reduced toxicity in NHPs, and improved efficacy 1 1 Lee et al, Mabs, 2022 Ipilumimab (anti-CTLA-4) Pembrolizumab (anti-PD-1) 2011 2014 2022 Relatlimab (anti-LAG-3) Landmark Checkpoint mAb FDA Approvals
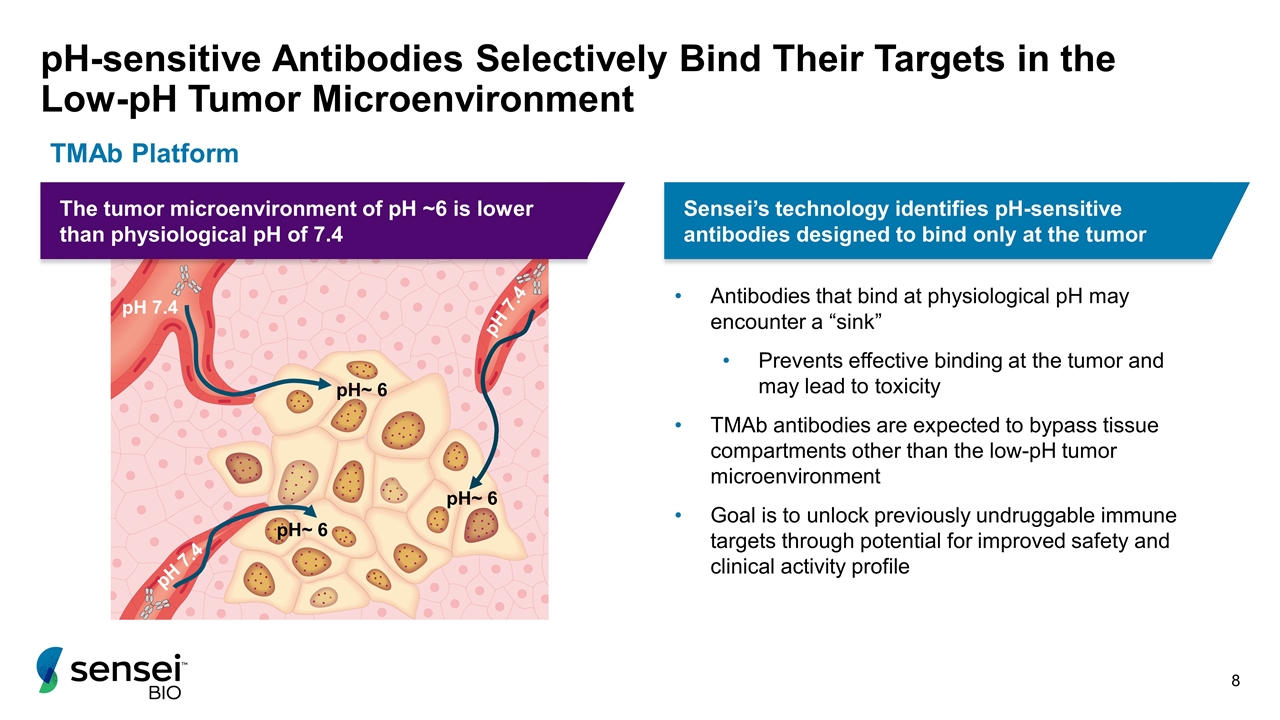
pH-sensitive Antibodies Selectively Bind Their Targets in the Low-pH Tumor Microenvironment Antibodies that bind at physiological pH may encounter a “sink” Prevents effective binding at the tumor and may lead to toxicity TMAb antibodies are expected to bypass tissue compartments other than the low-pH tumor microenvironment Goal is to unlock previously undruggable immune targets through potential for improved safety and clinical activity profile pH 7.4 pH 7.4 pH 7.4 pH~ 6 pH~ 6 pH~ 6 TMAb Platform Sensei’s technology identifies pH-sensitive antibodies designed to bind only at the tumor The tumor microenvironment of pH ~6 is lower than physiological pH of 7.4
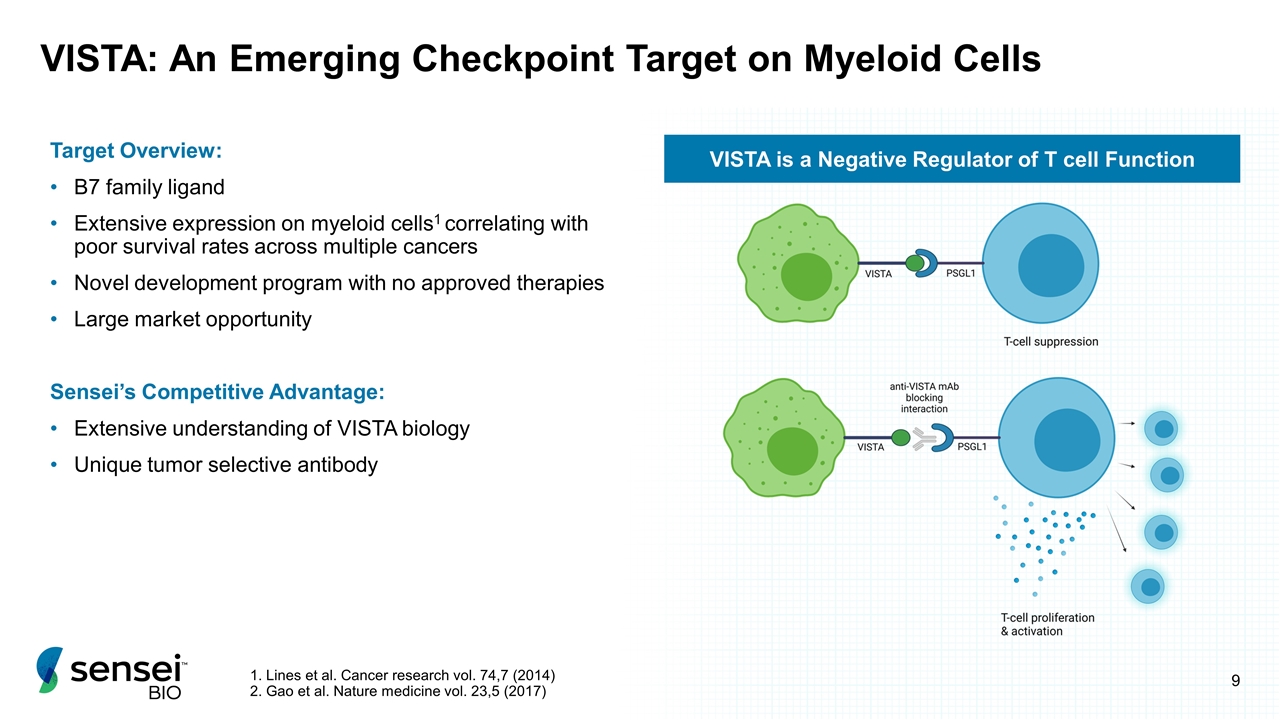
VISTA: An Emerging Checkpoint Target on Myeloid Cells Target Overview: B7 family ligand Extensive expression on myeloid cells1 correlating with poor survival rates across multiple cancers Novel development program with no approved therapies Large market opportunity Sensei’s Competitive Advantage: Extensive understanding of VISTA biology Unique tumor selective antibody 1. Lines et al. Cancer research vol. 74,7 (2014) 2. Gao et al. Nature medicine vol. 23,5 (2017) VISTA is a Negative Regulator of T cell Function

Increased Understanding of VISTA as a Promising Target to Address the Needs of Patients with Cancer
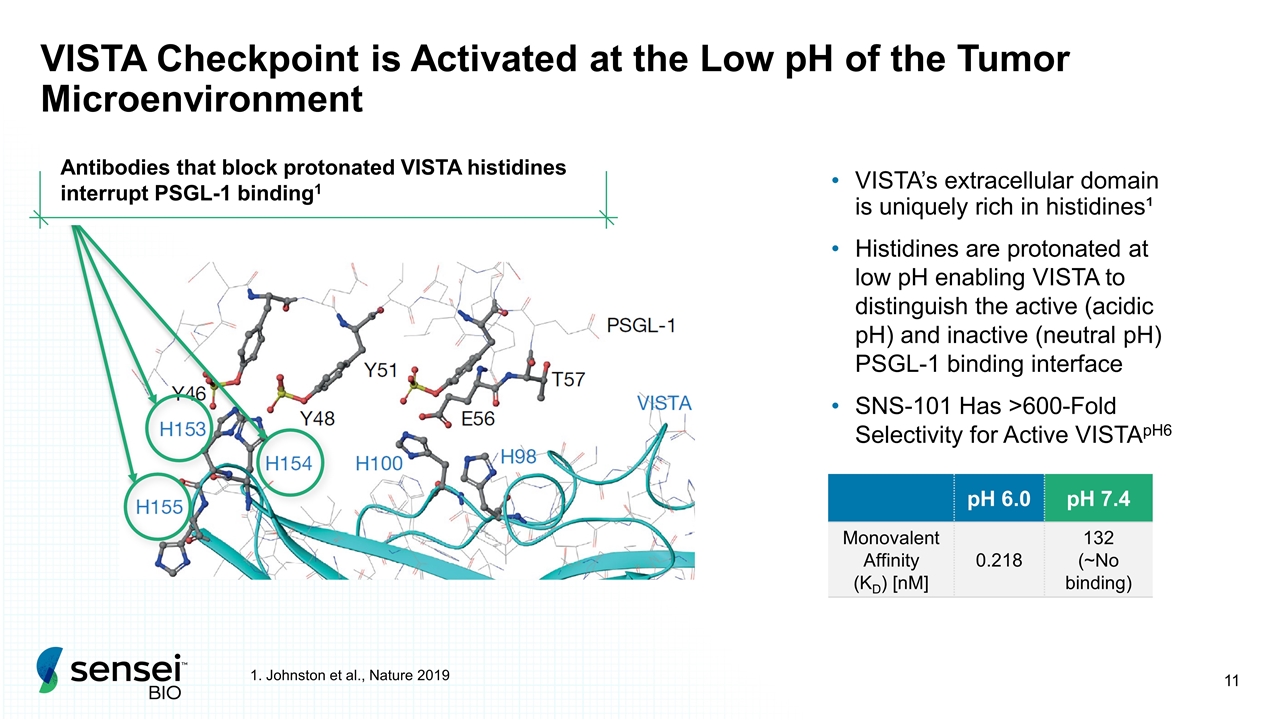
VISTA Checkpoint is Activated at the Low pH of the Tumor Microenvironment VISTA’s extracellular domain is uniquely rich in histidines¹ Histidines are protonated at low pH enabling VISTA to distinguish the active (acidic pH) and inactive (neutral pH) PSGL-1 binding interface SNS-101 Has >600-Fold Selectivity for Active VISTApH6 1. Johnston et al., Nature 2019 Antibodies that block protonated VISTA histidines interrupt PSGL-1 binding1 pH 6.0 pH 7.4 Monovalent Affinity (KD) [nM] 0.218 132 (~No binding)
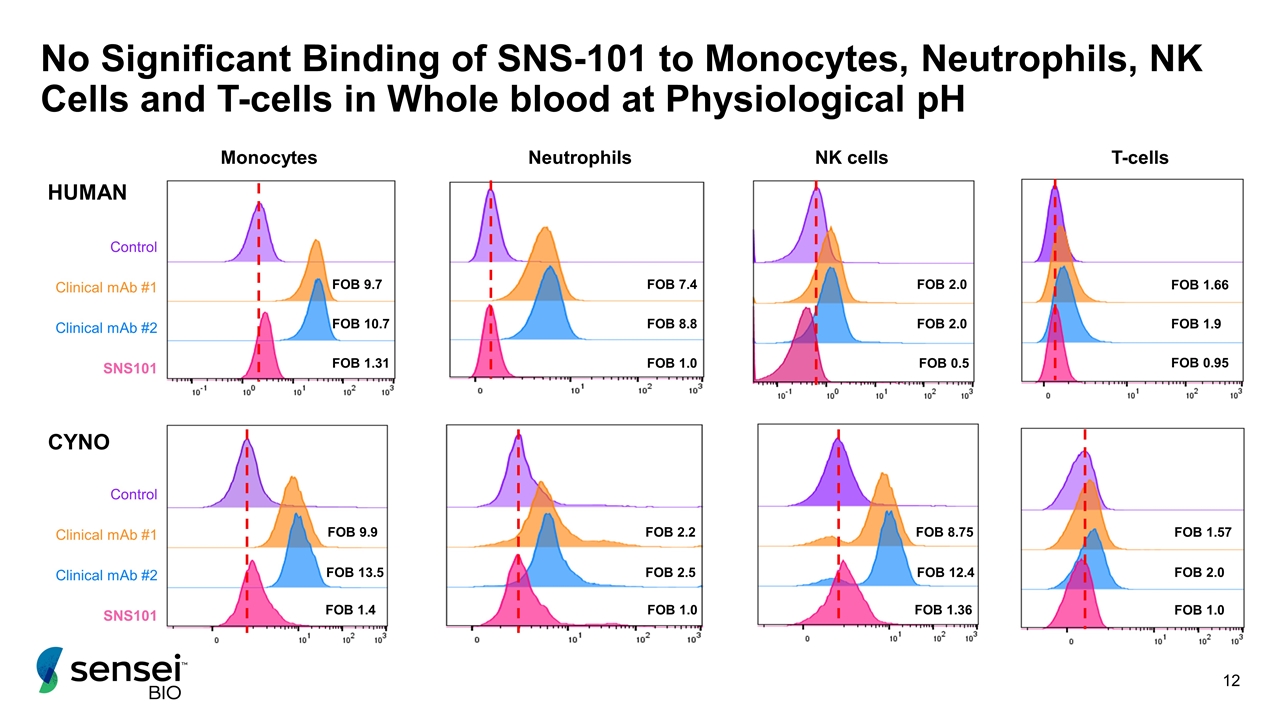
No Significant Binding of SNS-101 to Monocytes, Neutrophils, NK Cells and T-cells in Whole blood at Physiological pH T-cells NK cells FOB 1.57 FOB 2.0 FOB 1.0 FOB 8.75 FOB 12.4 FOB 1.36 Monocytes FOB 9.9 FOB 13.5 FOB 1.4 FOB 2.2 FOB 2.5 FOB 1.0 Neutrophils CYNO HUMAN FOB 7.4 FOB 8.8 FOB 1.0 Clinical mAb #1 Clinical mAb #2 SNS101 Control FOB 2.0 FOB 2.0 FOB 0.5 Clinical mAb #1 Clinical mAb #2 SNS101 Control FOB 1.66 FOB 1.9 FOB 0.95 FOB 9.7 FOB 10.7 FOB 1.31
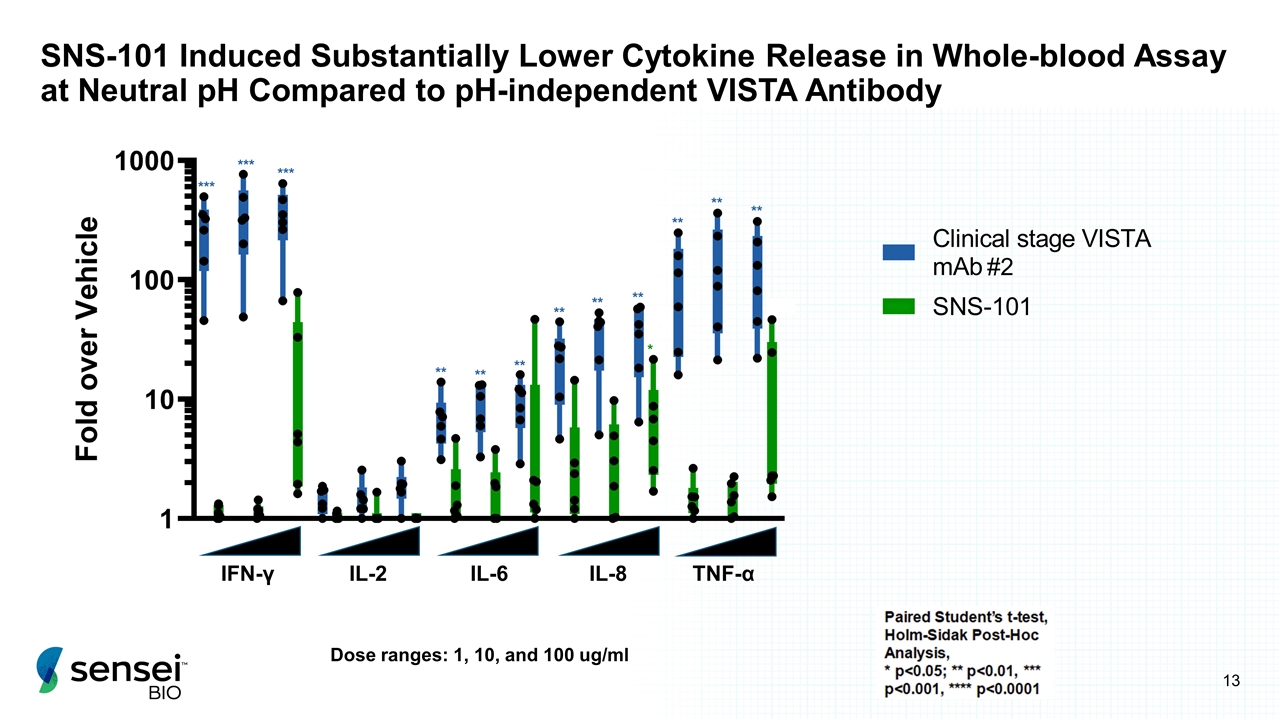
SNS-101 Induced Substantially Lower Cytokine Release in Whole-blood Assay at Neutral pH Compared to pH-independent VISTA Antibody IFN-γ IL-8 TNF-α IL-6 IL-2 *** *** *** ** * ** ** ** ** ** ** ** ** ** ** Dose ranges: 1, 10, and 100 ug/ml #2
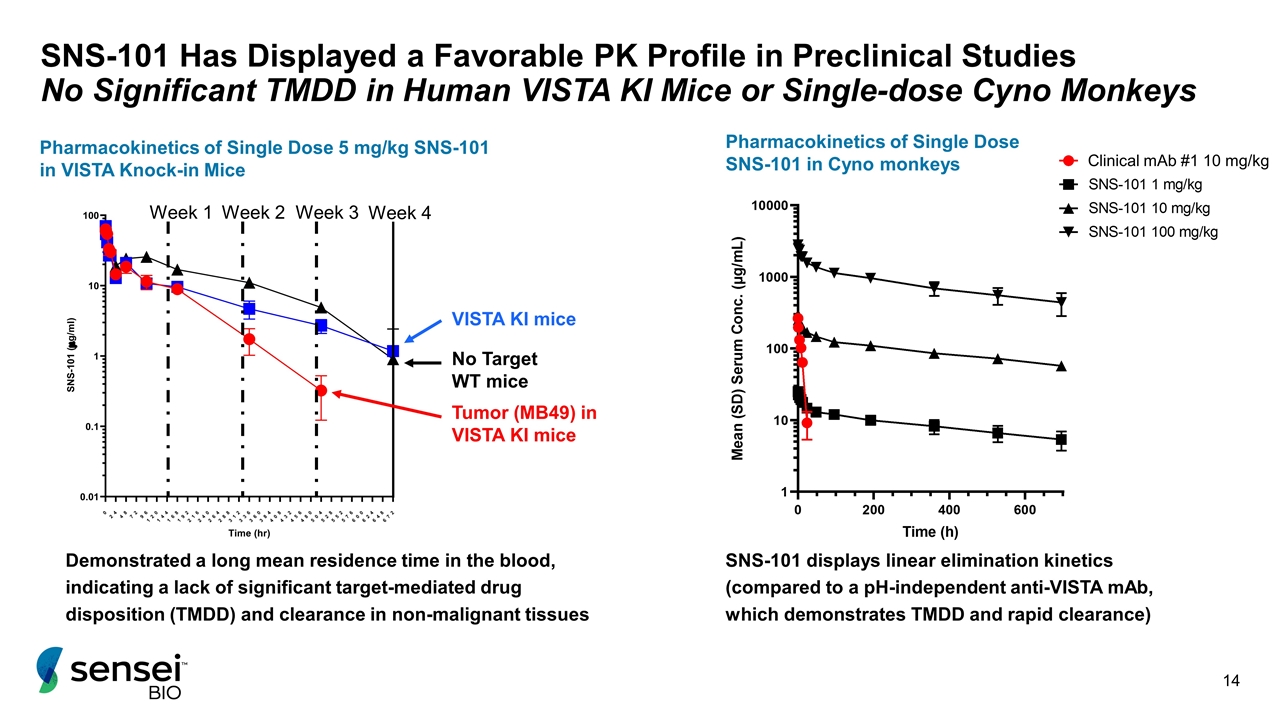
SNS-101 Has Displayed a Favorable PK Profile in Preclinical Studies No Significant TMDD in Human VISTA KI Mice or Single-dose Cyno Monkeys Pharmacokinetics of Single Dose 5 mg/kg SNS-101 in VISTA Knock-in Mice Demonstrated a long mean residence time in the blood, indicating a lack of significant target-mediated drug disposition (TMDD) and clearance in non-malignant tissues Week 1 Week 2 Week 3 Week 4 Tumor (MB49) in VISTA KI mice VISTA KI mice No Target WT mice SNS-101 displays linear elimination kinetics (compared to a pH-independent anti-VISTA mAb, which demonstrates TMDD and rapid clearance) Pharmacokinetics of Single Dose SNS-101 in Cyno monkeys Clinical mAb #1 10 mg/kg
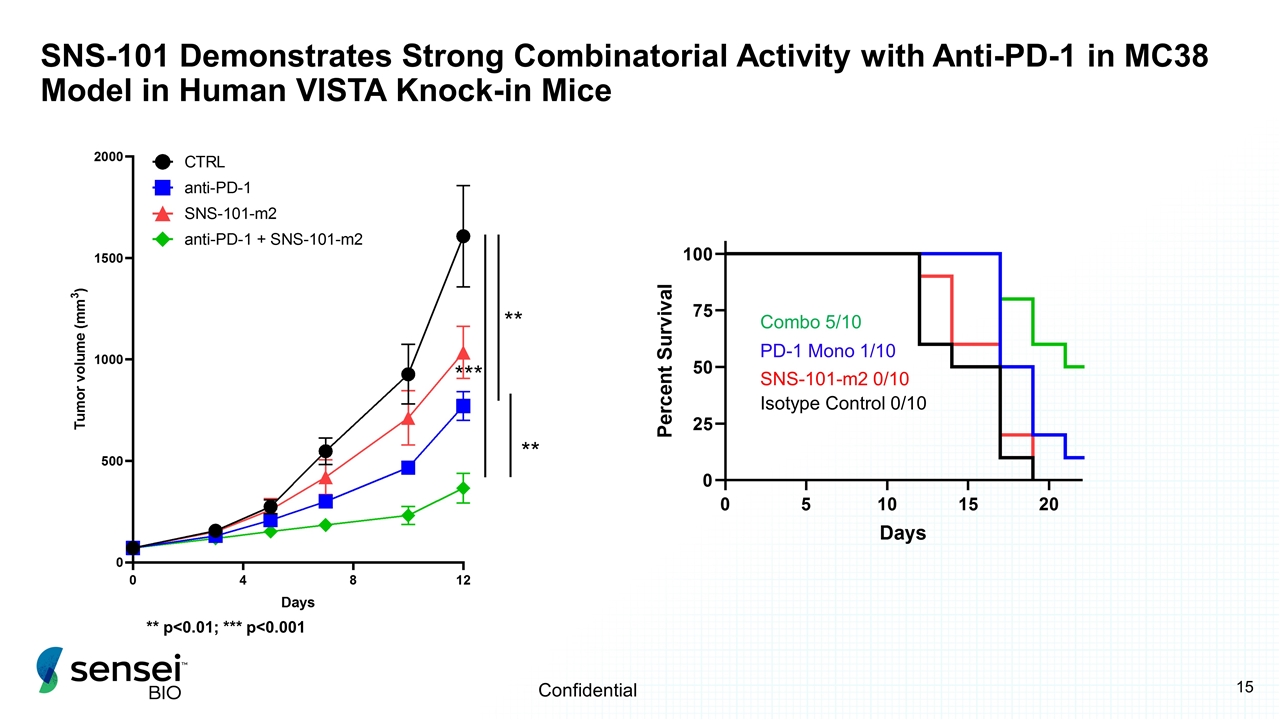
SNS-101 Demonstrates Strong Combinatorial Activity with Anti-PD-1 in MC38 Model in Human VISTA Knock-in Mice SNS-101-m2 0/10 PD-1 Mono 1/10 Combo 5/10 Isotype Control 0/10 *** ** ** ** p<0.01; *** p<0.001 Confidential
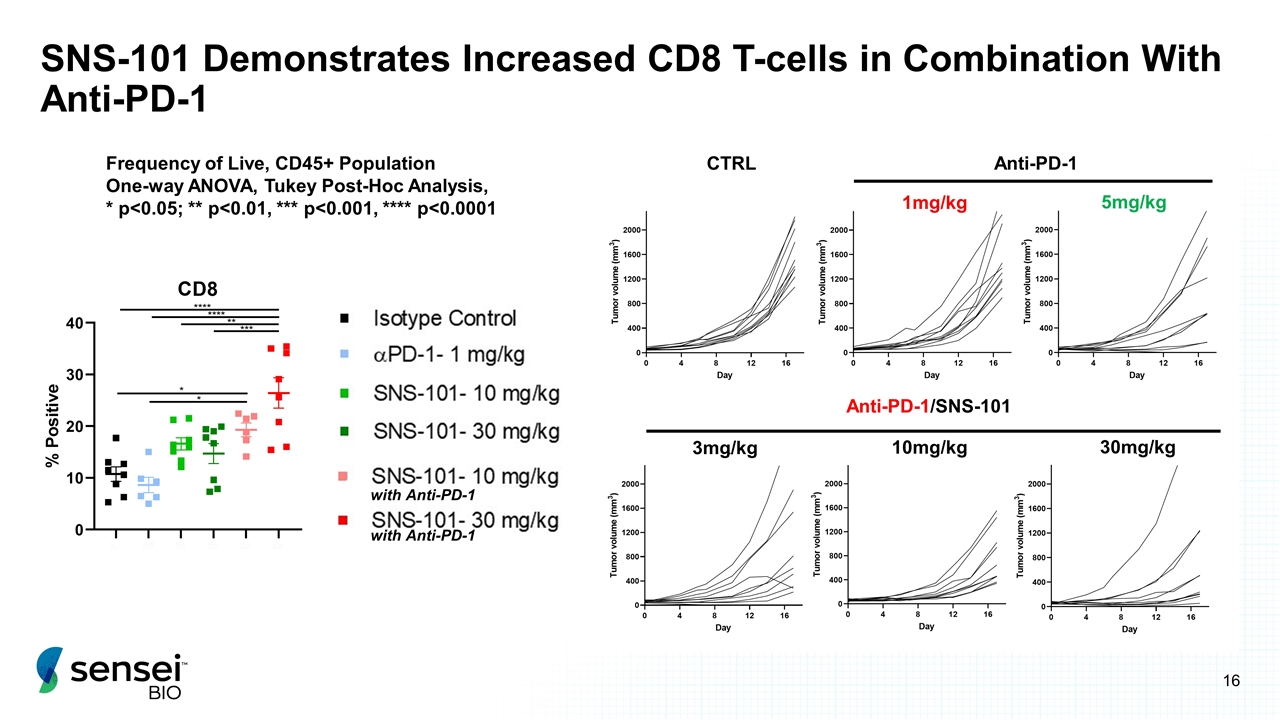
SNS-101 Demonstrates Increased CD8 T-cells in Combination With Anti-PD-1 CTRL Anti-PD-1 1mg/kg 5mg/kg Anti-PD-1/SNS-101 3mg/kg 10mg/kg 30mg/kg Frequency of Live, CD45+ Population One-way ANOVA, Tukey Post-Hoc Analysis, * p<0.05; ** p<0.01, *** p<0.001, **** p<0.0001 with Anti-PD-1 with Anti-PD-1
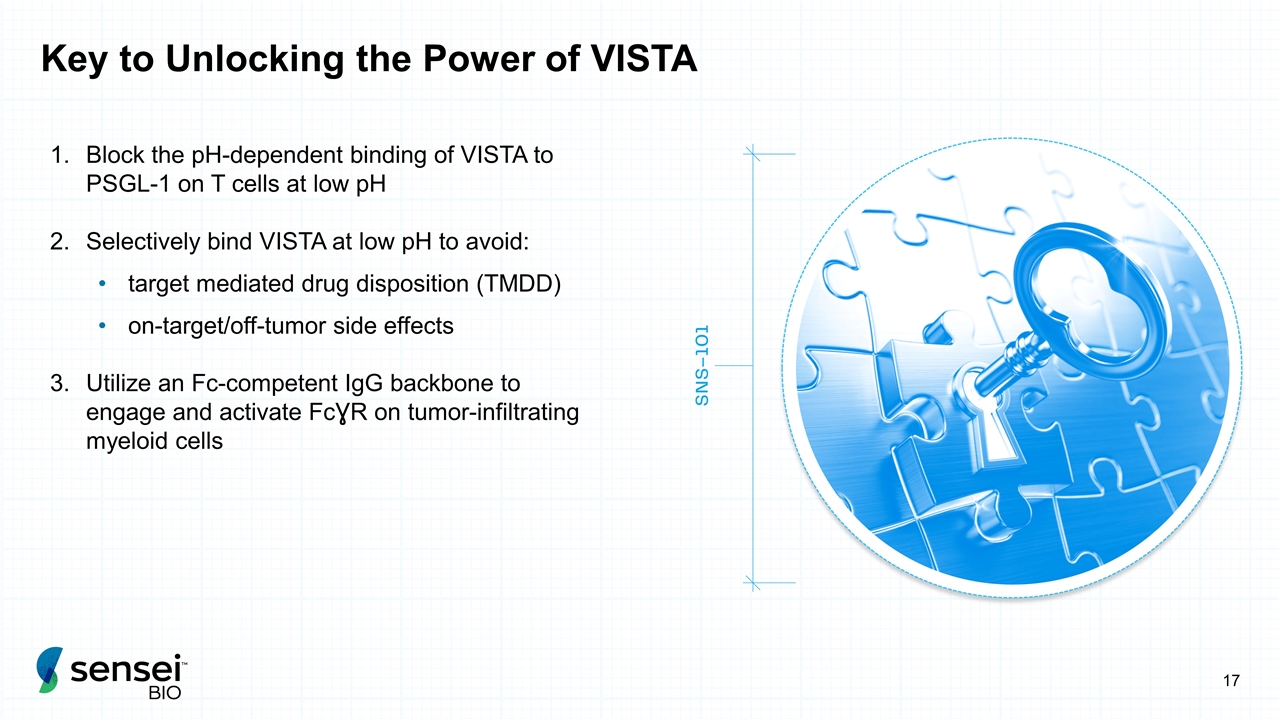
Key to Unlocking the Power of VISTA Block the pH-dependent binding of VISTA to PSGL-1 on T cells at low pH Selectively bind VISTA at low pH to avoid: target mediated drug disposition (TMDD) on-target/off-tumor side effects Utilize an Fc-competent IgG backbone to engage and activate FcƔR on tumor-infiltrating myeloid cells
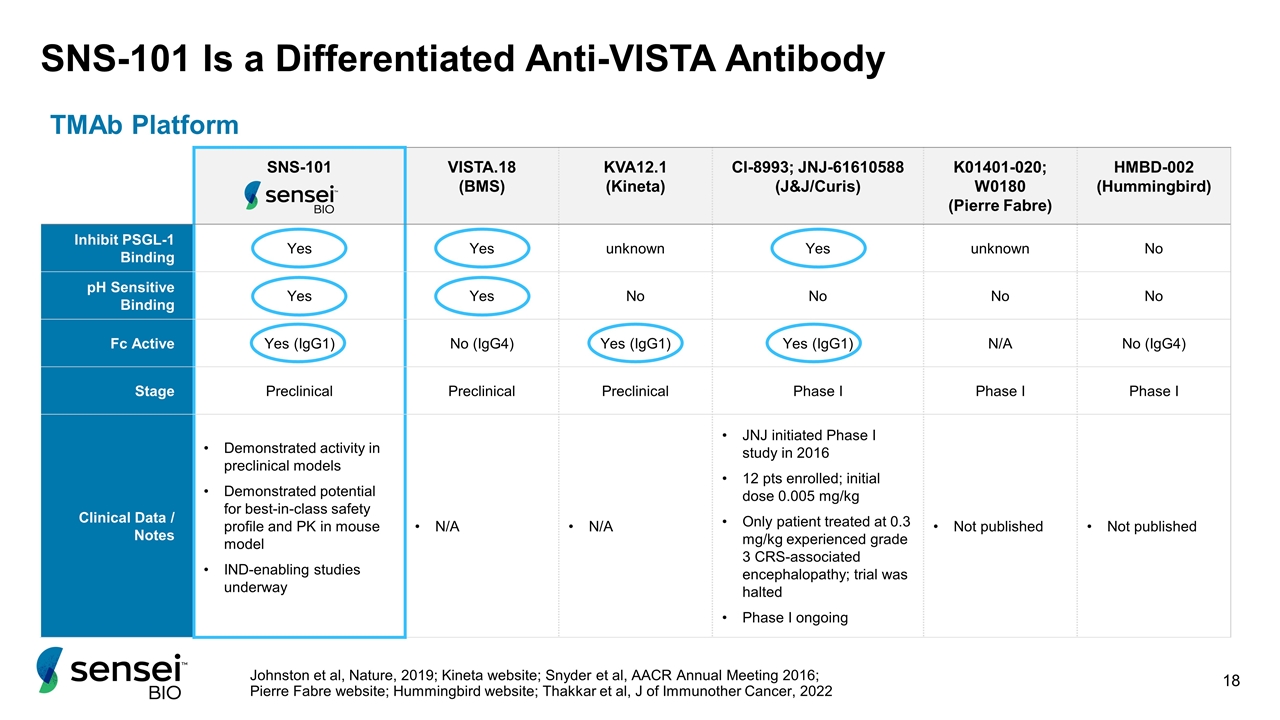
SNS-101 VISTA.18 (BMS) KVA12.1 (Kineta) CI-8993; JNJ-61610588 (J&J/Curis) K01401-020; W0180 (Pierre Fabre) HMBD-002 (Hummingbird) Inhibit PSGL-1 Binding Yes Yes unknown Yes unknown No pH Sensitive Binding Yes Yes No No No No Fc Active Yes (IgG1) No (IgG4) Yes (IgG1) Yes (IgG1) N/A No (IgG4) Stage Preclinical Preclinical Preclinical Phase I Phase I Phase I Clinical Data / Notes Demonstrated activity in preclinical models Demonstrated potential for best-in-class safety profile and PK in mouse model IND-enabling studies underway N/A N/A JNJ initiated Phase I study in 2016 12 pts enrolled; initial dose 0.005 mg/kg Only patient treated at 0.3 mg/kg experienced grade 3 CRS-associated encephalopathy; trial was halted Phase I ongoing Not published Not published SNS-101 Is a Differentiated Anti-VISTA Antibody TMAb Platform Johnston et al, Nature, 2019; Kineta website; Snyder et al, AACR Annual Meeting 2016; Pierre Fabre website; Hummingbird website; Thakkar et al, J of Immunother Cancer, 2022
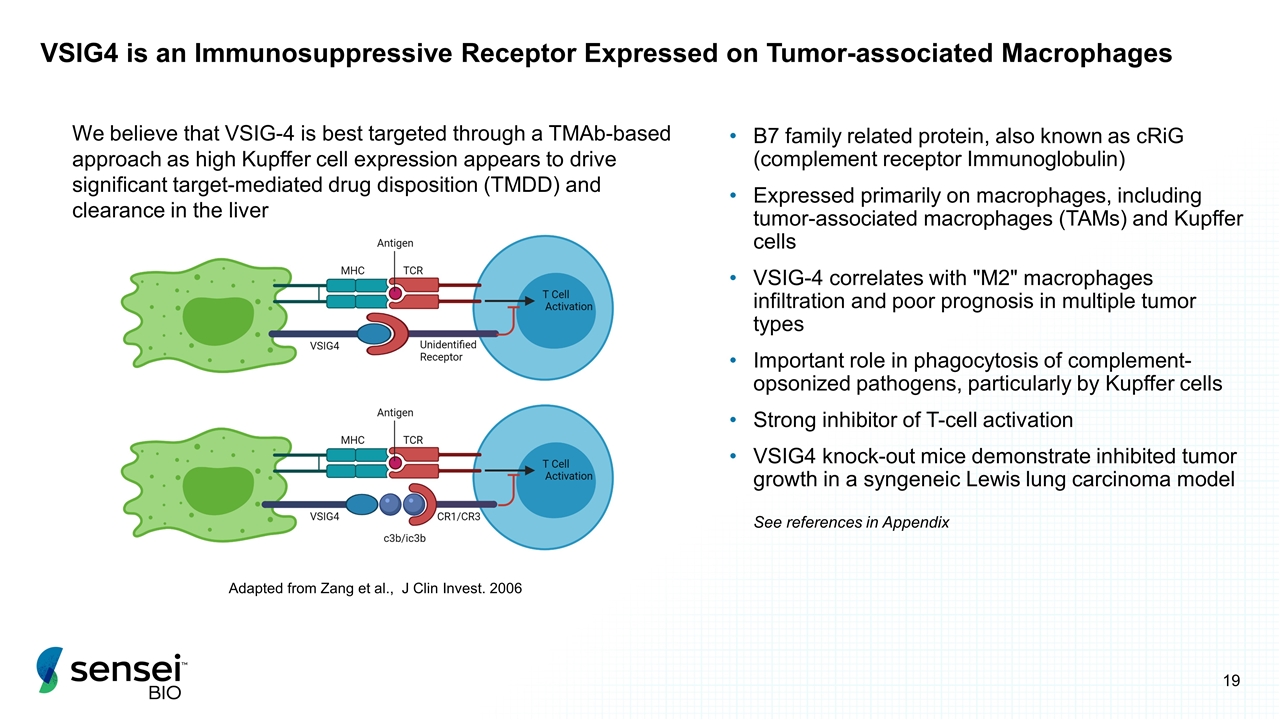
VSIG4 is an Immunosuppressive Receptor Expressed on Tumor-associated Macrophages B7 family related protein, also known as cRiG (complement receptor Immunoglobulin) Expressed primarily on macrophages, including tumor-associated macrophages (TAMs) and Kupffer cells VSIG-4 correlates with "M2" macrophages infiltration and poor prognosis in multiple tumor types Important role in phagocytosis of complement-opsonized pathogens, particularly by Kupffer cells Strong inhibitor of T-cell activation VSIG4 knock-out mice demonstrate inhibited tumor growth in a syngeneic Lewis lung carcinoma model Adapted from Zang et al., J Clin Invest. 2006 We believe that VSIG-4 is best targeted through a TMAb-based approach as high Kupffer cell expression appears to drive significant target-mediated drug disposition (TMDD) and clearance in the liver See references in Appendix
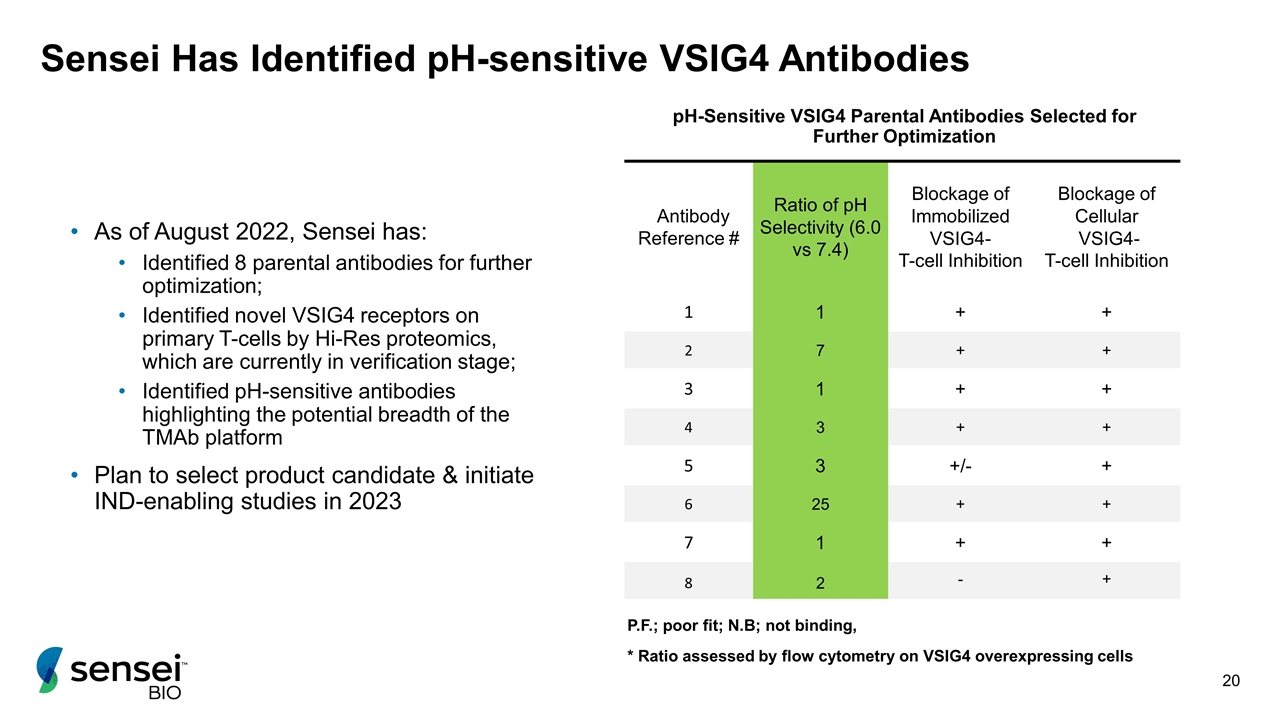
Sensei Has Identified pH-sensitive VSIG4 Antibodies pH-Sensitive VSIG4 Parental Antibodies Selected for Further Optimization Antibody Reference # Ratio of pH Selectivity (6.0 vs 7.4) Blockage of Immobilized VSIG4- T-cell Inhibition Blockage of Cellular VSIG4- T-cell Inhibition 1 1 + + 2 7 + + 3 1 + + 4 3 + + 5 3 +/- + 6 25 + + 7 1 + + 8 2 - + P.F.; poor fit; N.B; not binding, * Ratio assessed by flow cytometry on VSIG4 overexpressing cells As of August 2022, Sensei has: Identified 8 parental antibodies for further optimization; Identified novel VSIG4 receptors on primary T-cells by Hi-Res proteomics, which are currently in verification stage; Identified pH-sensitive antibodies highlighting the potential breadth of the TMAb platform Plan to select product candidate & initiate IND-enabling studies in 2023
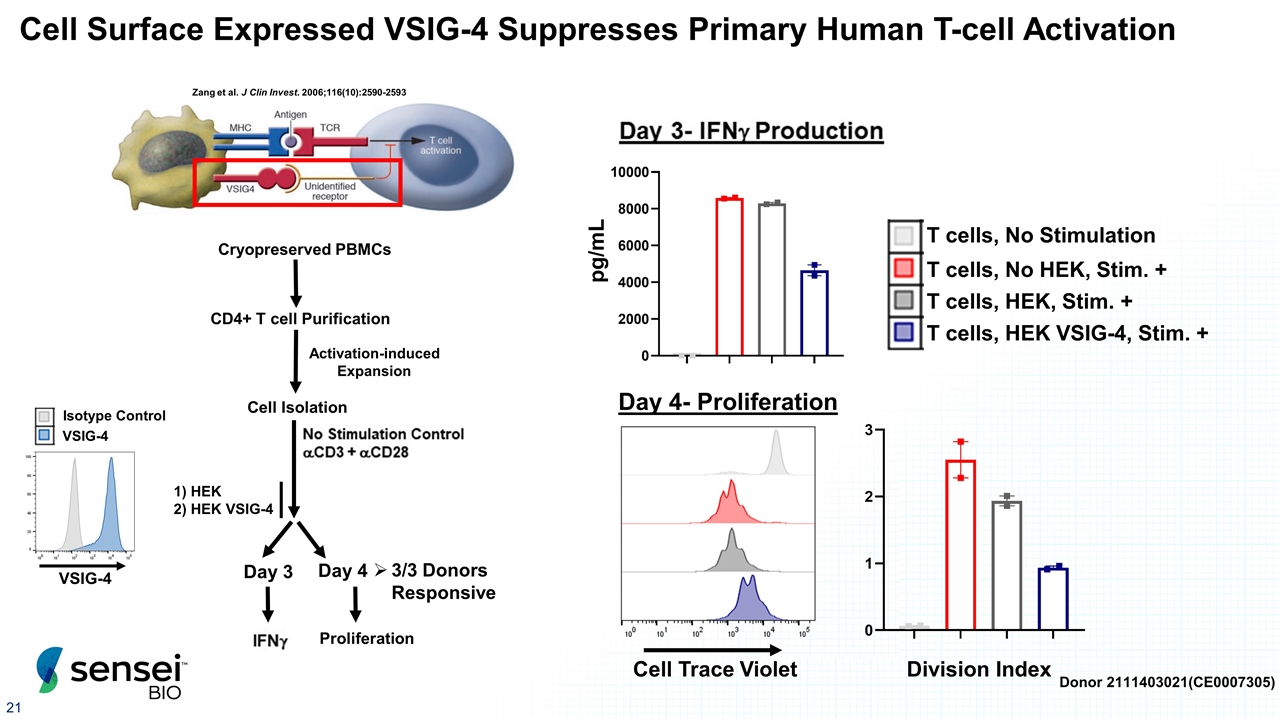
Cell Surface Expressed VSIG-4 Suppresses Primary Human T-cell Activation Zang et al. J Clin Invest. 2006;116(10):2590-2593 Day 4- Proliferation Cell Trace Violet Day 4 Cryopreserved PBMCs CD4+ T cell Purification Proliferation Cell Isolation Day 3 Activation-induced Expansion 3/3 Donors Responsive 1) HEK 2) HEK VSIG-4 Division Index VSIG-4 Isotype Control VSIG-4 Donor 2111403021(CE0007305) pg/mL T cells, HEK, Stim. + T cells, No HEK, Stim. + T cells, No Stimulation T cells, HEK VSIG-4, Stim. +
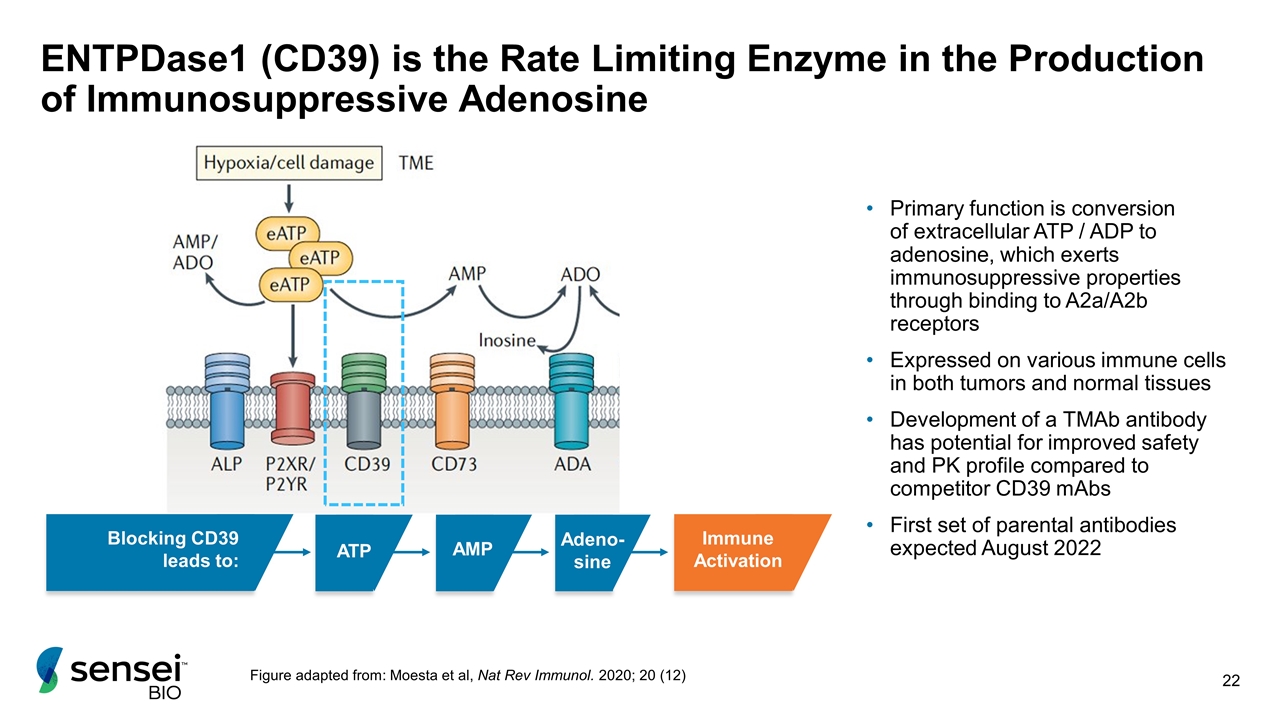
ENTPDase1 (CD39) is the Rate Limiting Enzyme in the Production of Immunosuppressive Adenosine ATP AMP Adeno-sine Figure adapted from: Moesta et al, Nat Rev Immunol. 2020; 20 (12) Primary function is conversion of extracellular ATP / ADP to adenosine, which exerts immunosuppressive properties through binding to A2a/A2b receptors Expressed on various immune cells in both tumors and normal tissues Development of a TMAb antibody has potential for improved safety and PK profile compared to competitor CD39 mAbs First set of parental antibodies expected August 2022 Blocking CD39 leads to: Immune Activation

SNS-101 (anti-VISTA) 1H 2023: Multi-dose Non-Human Primate (NHP) PK & Toxicology data 1H 2023: IND filing SNS-102 (anti-VSIG4) 2023: Select product candidate / initiate IND-enabling studies SNS-103 (anti-ENTPDase1/CD39) 2023: Select product candidate Expected Program Milestones

Proven Team With Deep Experience Patrick Gallagher Chief Business Officer Elisabeth Colunio VP, Human Resources Edward van der Horst, Ph.D. SVP, TMAb Antibodies John Celebi, MBA President and CEO Robert Pierce, M.D. Chief R&D Officer Erin Colgan Chief Financial Officer HansPeter Waldner,Ph.D. SVP, Cancer Immunology Christopher Gerry, J.D. VP, General Counsel

HQ: 451 D St, Unit 710 , Boston, MA 02210 / MD: 1405 Research Blvd, Suite 125, Rockville, MD 20850 senseibio.com

Appendix References for Slide 19 Zheng F, Devoogdt N, Sparkes A, Morias Y, Abels C, Stijlemans B, Lahoutte T, Muyldermans S, De Baetselier P, Schoonooghe S, Beschin A, Raes G. Monitoring liver macrophages using nanobodies targeting Vsig4: concanavalin A induced acute hepatitis as paradigm. Immunobiology. 2015 Feb;220(2):200-9. doi: 10.1016/j.imbio.2014.09.018. Epub 2014 Oct 2. PMID: 25440182. Reviewed in Zang X, Allison JP. To be or not to be B7. J Clin Invest. 2006 Oct;116(10):2590-3. doi: 10.1172/JCI30103. PMID: 17016555; PMCID: PMC1578606. Helmy KY, Katschke KJ Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006 Mar 10;124(5):915-27. doi: 10.1016/j.cell.2005.12.039. PMID: 16530040. Voillet V, Berger TR, McKenna KM, Paulson KG, Tan WH, Smythe KS, Hunter DS, Valente WJ, Weaver S, Campbell JS, Kim TS, Byrd DR, Bielas JH, Pierce RH, Chapuis AG, Gottardo R, Rongvaux A. An In Vivo Model of Human Macrophages in Metastatic Melanoma. J Immunol. 2022 Aug 1;209(3):606-620. doi: 10.4049/jimmunol.2101109. Epub 2022 Jul 11. PMID: 35817516; PMCID: PMC9377377. Reviewed in Small AG, Al-Baghdadi M, Quach A, Hii C, Ferrante A. Complement receptor immunoglobulin: a control point in infection and immunity, inflammation and cancer. Swiss Med Wkly. 2016 Apr 5;146:w14301. doi: 10.4414/smw.2016.14301. PMID: 27045607. Liu G, Fu Y, Yosri M, Chen Y, Sun P, Xu J, Zhang M, Sun D, Strickland AB, Mackey ZB, Shi M. CRIg plays an essential role in intravascular clearance of bloodborne parasites by interacting with complement. Proc Natl Acad Sci U S A. 2019 Nov 26;116(48):24214-24220. doi: 10.1073/pnas.1913443116. Epub 2019 Nov 13. PMID: 31723045; PMCID: PMC6883839. Vogt L, Schmitz N, Kurrer MO, Bauer M, Hinton HI, Behnke S, Gatto D, Sebbel P, Beerli RR, Sonderegger I, Kopf M, Saudan P, Bachmann MF. VSIG4, a B7 family-related protein, is a negative regulator of T cell activation. J Clin Invest. 2006 Oct;116(10):2817-26. doi: 10.1172/JCI25673. PMID: 17016562; PMCID: PMC1578631. Liao Y, Guo S, Chen Y, Cao D, Xu H, Yang C, Fei L, Ni B, Ruan Z. VSIG4 expression on macrophages facilitates lung cancer development. Lab Invest. 2014 Jul;94(7):706-15. doi: 10.1038/labinvest.2014.73. Epub 2014 May 26. PMID: 24862966.

























