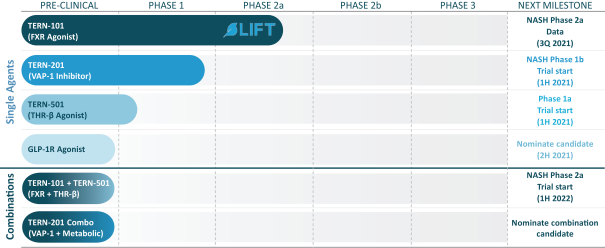
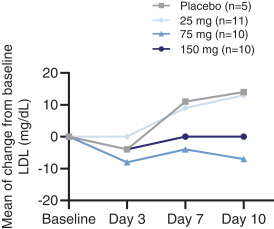
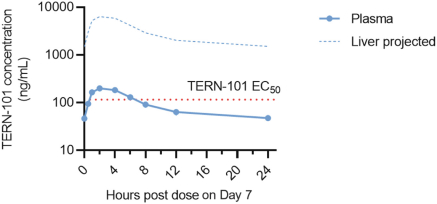
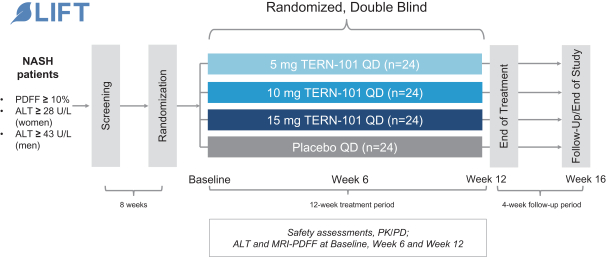
We are aware of both pharmaceutical and biotechnology companies with development programs in NASH. Large pharmaceutical companies participating in the development of NASH treatments include, but are not limited to, AbbVie, Inc., Amgen Inc., AstraZeneca PLC/MedImmune LLC, Bayer AG, Boehringer Ingelheim, Bristol-Myers Squibb Company, Eisai, Inc., Eli Lilly and Company, Gilead Sciences, Inc., GlaxoSmithKline plc, Johnson & Johnson, Merck & Co., Inc., Novartis Pharmaceuticals Corp., Novo Nordisk A/S, Pfizer Inc., Roche Holding AG, Sanofi, Sumitomo Dainippon Pharma Co., Ltd. and Takeda Pharmaceutical Co., Ltd.
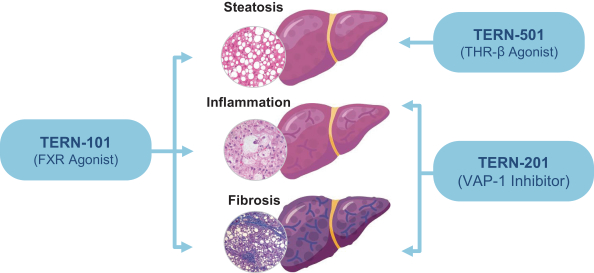
In relation to TERN-101, companies who are currently conducting clinical trials with FXR in the context of NASH include AbbVie, Inc., Enanta Pharmaceuticals, Inc., ENYO Pharma SA, Gilead Sciences, Inc., Intercept Pharmaceuticals, Inc., Metacrine, Inc. and Novartis Pharmaceuticals Corp.
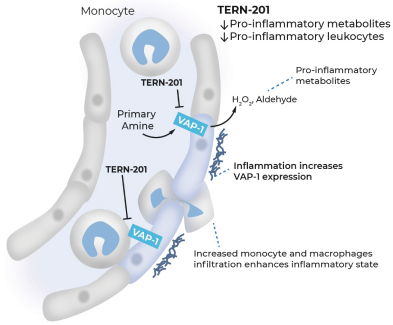
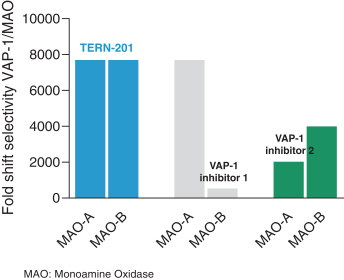
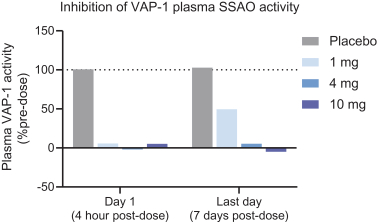
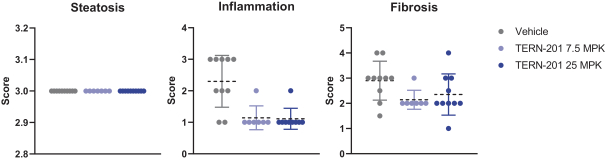
TERN-201, our VAP-1 inhibitor, is a relatively novel mechanism for the treatment of NASH, and thus has little competition we are aware of. The companies who are currently developing a SSAO/VAP-1 inhibitor with NASH as a lead indication are LG Chem Ltd. and Novo Nordisk A/S.
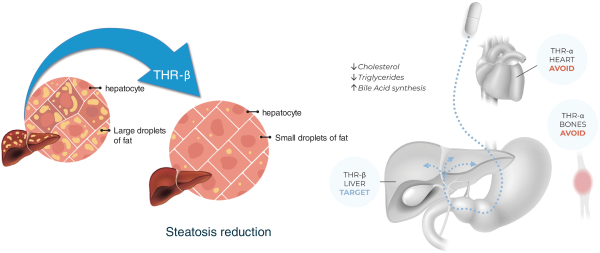
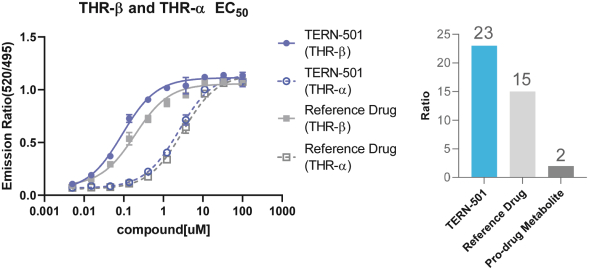
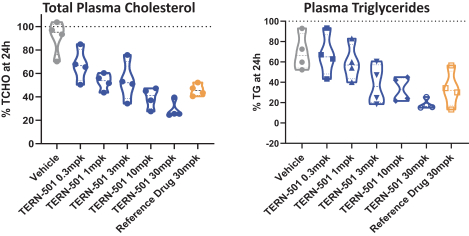
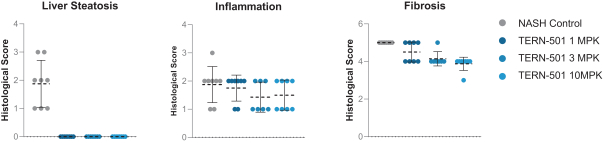
With regards to TERN-501, companies who are currently conducting clinical trials targeting THR-b in the context of NASH include Madrigal Pharmaceuticals, Inc. and Viking Therapeutics, Inc.
Furthermore, pharmaceutical and biotechnology companies who are developing clinical-stage drugs to treat NASH, using mechanisms not mentioned above, include 89Bio, Inc., Akero Therapeutics, Inc., Arrowhead Pharmaceuticals, Inc., Axcella Health, Inc., Carmot Therapeutics, Inc., Cirius Therapeutics, Inc., CohBar, Inc., Coherus Biosciences Inc., Corcept Therapeutics, Inc., CymaBay Therapeutics, Inc., Esperion Therapeutics, Inc., Galectin Therapeutics Inc., Galmed Pharmaceuticals Ltd., Hanmi Pharmaceutical Co., Ltd., Inventiva Pharma SA, Ionis Pharmaceuticals, Inc., MediciNova, Inc., NGM Biopharmaceuticals, Inc., NorthSea Therapeutics, Inc., Pliant Therapeutics, Inc., Poxel SA, Sagimet Biosciences, Inc., T3D Therapeutics, Inc. and Zydus Cadila Healthcare.
Many of our competitors have substantially greater financial, technical, human and other resources than we do and may be better equipped to develop, manufacture and market technologically superior products. In addition, many of these competitors have significantly greater experience than we have in undertaking preclinical studies and human clinical trials of new pharmaceutical products and in obtaining regulatory approvals of human therapeutic products. Accordingly, our competitors may succeed in obtaining FDA approval for superior products. Many of our competitors have established distribution channels for the commercialization of their products, whereas we have no such channel or capabilities. In addition, many competitors have greater name recognition and more extensive collaborative relationships. Smaller and earlier-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large, established companies.
Although we believe our drug and combination therapy candidate programs possess appealing attributes, we cannot guarantee that our products will achieve regulatory or market success. Our competitors may obtain regulatory approval of their products more rapidly than we do, or obtain patent protection or other intellectual property rights that limit our ability to develop or commercialize our drug candidate or any future drug candidates. Our competitors may also develop drugs that are more effective, more convenient, more widely used, and less costly, or have a better tolerability profile than our drugs. These competitors may also be more successful than we are in manufacturing and marketing their products. Should we not be able to compete with the aforementioned companies or others, it may hinder our ability to bring our product to market as planned.
Intellectual Property
The proprietary nature of, and protection for, our drug candidates and our discovery programs, processes and know-how are important to our business. For our patent portfolio for pipeline drug candidates, we seek to pursue patent protection covering compositions of matter and methods of use and manufacture. Our policy is to pursue, maintain, defend and enforce patent rights in strategic areas, whether developed internally or licensed from third parties, and to protect the technology, inventions and improvements that are commercially important
133