| | • | | Anti-PLA2R and anti-nephrin podocytopathies – The PIONEER study will expand to additional autoimmune glomerular diseases characterized by the presence of antibodies to glomerular antigens, including primary membranous nephropathy, focal segmental glomerulosclerosis, and minimal change disease. |
About the Phase 2b ORIGIN clinical trial
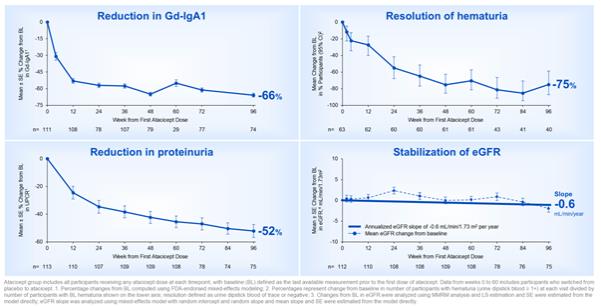
The Phase 2b ORIGIN clinical trial (NCT04716231) is a global, multicenter, randomized, double-blind, placebo-controlled trial evaluating the safety and efficacy of atacicept in 116 patients with IgAN who continue to have persistent proteinuria and remain at high risk of disease progression despite being on a stable prescribed regimen of a renin-angiotensin-aldosterone system inhibitor for at least 12 weeks that is the maximum labeled or tolerated dose. The Phase 2b ORIGIN clinical trial evaluated three dose strengths of atacicept versus placebo, administered weekly by prefilled syringe. Patients were randomized 2:2:1:2 to atacicept 150 mg, atacicept 75 mg, atacicept 25 mg or matching placebo. Upon completion of the 36-week blinded treatment period, all patients were offered open-label atacicept 150 mg for an additional 60 weeks.
The primary endpoint was the change in proteinuria as evaluated by urine protein to creatinine ratio (UPCR) at week 24, and the key secondary endpoint was the change in proteinuria as evaluated by UPCR at week 36. Additional exploratory endpoints include change in proteinuria as evaluated by UPCR at weeks 12, 48, and 96; change in eGFR; change in serum immunoglobulin levels, and change in serum Gd-IgA1 levels; safety and tolerability; and serum pharmacokinetics.
The trial met its primary and key secondary endpoints, with statistically significant and clinically meaningful proteinuria reductions and stabilization of eGFR versus placebo through week 36. The safety profile was comparable between atacicept and placebo.
For more information about the Phase 2b ORIGIN clinical trial, please visit www.clinicaltrials.gov.
About the Phase 3 ORIGIN 3 clinical trial
The ORIGIN 3 clinical trial (NCT04716231) is a global, multicenter, randomized, double-blind, placebo-controlled Phase 3 trial evaluating the safety and efficacy of atacicept in patients with IgAN who continue to have persistent proteinuria and remain at high risk of disease progression despite being on a stable prescribed regimen of renin-angiotensin system inhibitors for at least 12 weeks that is the maximum labeled or tolerated dose. The objectives of the trial are to determine the effect of atacicept on proteinuria and preservation of kidney function compared to placebo.
For more information about the ORIGIN 3 clinical trial, please visit http://www.clinicaltrials.gov.