
Innovative Solutions for the Consequences of Hyperinflammation February 2023 Investor Presentation Exhibit 99.1

Forward-Looking Statements This presentation contains certain forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1955. These forward-looking statements include, without limitation, SeaStar Medical’s expectations with respect to the timing of regulatory approval of its products, the expected timing on enrollment, generation of study results, submission of PMA and other corporate milestones, the ability of SCD to treat patients with AKI, and the potential benefits of SCD to treat other diseases. Words such as “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions are intended to identify such forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to significant risks and uncertainties that could cause the actual results to differ materially from the expected results. Most of these factors are outside SeaStar Medical’s control and are difficult to predict. Factors that may cause actual future events to differ materially from the expected results, include, but are not limited to: (i) the inability to recognize the anticipated benefits of the business combination with LMAO, which may be affected by, among other things, competition and the ability of the post-combination company to grow and manage growth profitability and retain its key employees, (ii) costs related to the business combination, (iii) the outcome of any legal proceedings that may be instituted against SeaStar Medical, (iv) the ability to maintain the listing of its securities on Nasdaq, (v) the ability to implement business plans, forecasts, and other expectations after the completion of the business combination, and identify and realize additional opportunities, (vi) the risk of downturns and the possibility of rapid change in the highly competitive industry in which SeaStar Medical operates, (vii) the risk that SeaStar Medical and its current and future collaborators are unable to successfully develop and commercialize its products or services, or experience significant delays in doing so, including failure to achieve approval of its products by applicable federal and state regulators, (viii) the risk that SeaStar Medical may never achieve or sustain profitability; (ix) the risk that SeaStar Medical may need to raise additional capital to execute its business plan, which may not be available on acceptable terms or at all; (x) the risk that third-parties suppliers and manufacturers are not able to fully and timely meet their obligations, (xi) the risk of product liability or regulatory lawsuits or proceedings relating to SeaStar Medical’s products and services, (xii) the risk that SeaStar Medical is unable to secure or protect its intellectual property, and (xiii) other risks and uncertainties indicated from time to time in SeaStar Medical’s registration statement on Form S-4, as amended (File No. 333-264993), including those under the “Risk Factors” section therein and in SeaStar Medical’s other filings with the SEC. The foregoing list of factors is not exhaustive. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and SeaStar Medical assume no obligation and do not intend to update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise.

About SeaStar Medical We are advancing an innovative platform therapy to address the life-threatening consequences of hyperinflammation The overproduction of inflammatory cells (i.e., cytokine storm) can damage vital organs, resulting in multi-organ failure and death Our SCD cell-directed extracorporeal therapy can bring activated cells back to a reparative state and homeostasis SeaStar Medical came public in October 2022 through a de-SPAC process Selective Cytopheretic Device (SCD) quells the hyperinflammatory process and cytokine storm by targeting and neutralizing activated effector cells

Investment Highlights SCD addresses hyperinflammation in multiple acute and chronic large, high-value indications SCD’s unique mechanism of action provides cell-directed extracorporeal therapy at the patient point of care under an FDA medical device regulatory pathway Initial indications in pediatric and adult acute kidney injury (AKI) are supported by clinical results demonstrating significantly reduced mortality and the elimination of dependency on hemodialysis Multiple near-term regulatory and clinical milestones provide a cadence of company news and the potential to create significant value for shareholders Targeting FDA approval of pediatric SCD for pediatric AKI in the first half of 2023 under the humanitarian device exemption (HDE) process Pivotal clinical trial with SCD for adult AKI to begin in the first quarter of 2023, with interim results expected in the fourth quarter of 2023 Seasoned leadership with highly relevant business experience and a proven track record of execution
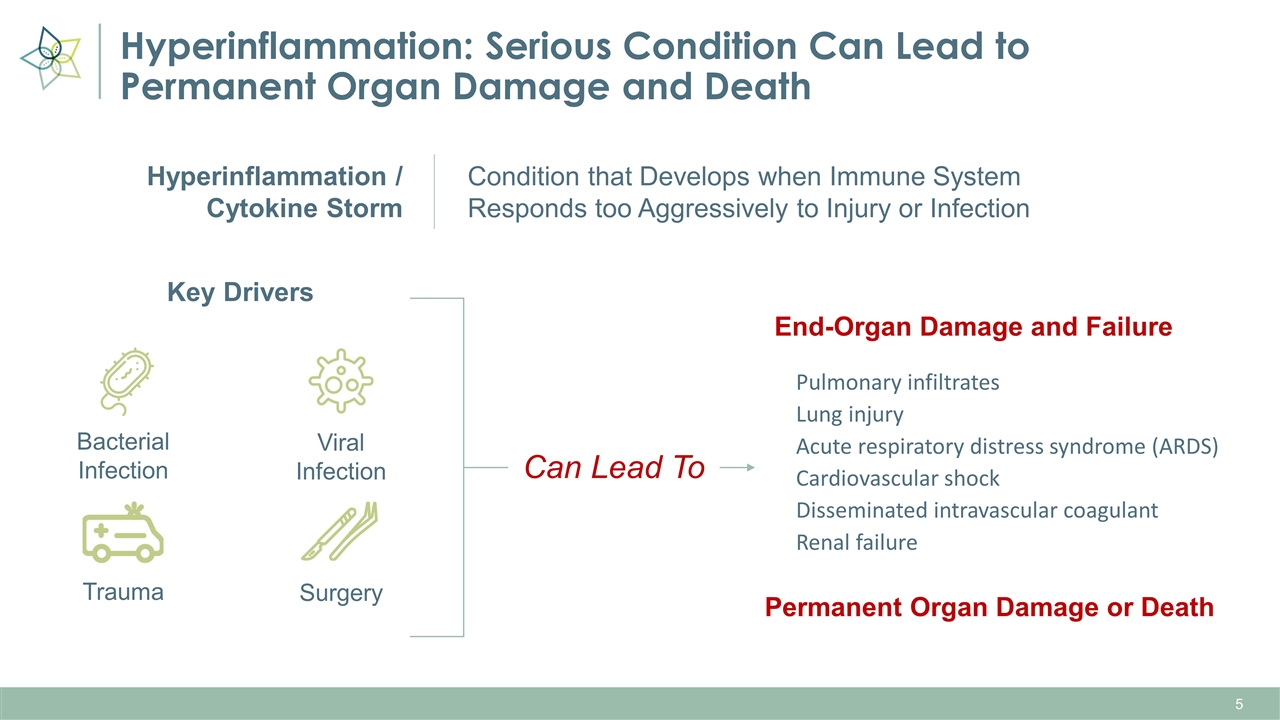
Hyperinflammation: Serious Condition Can Lead to Permanent Organ Damage and Death Key Drivers Pulmonary infiltrates Lung injury Acute respiratory distress syndrome (ARDS) Cardiovascular shock Disseminated intravascular coagulant Renal failure End-Organ Damage and Failure Permanent Organ Damage or Death Bacterial Infection Viral Infection Trauma Surgery Condition that Develops when Immune System Responds too Aggressively to Injury or Infection Hyperinflammation / Cytokine Storm Can Lead To
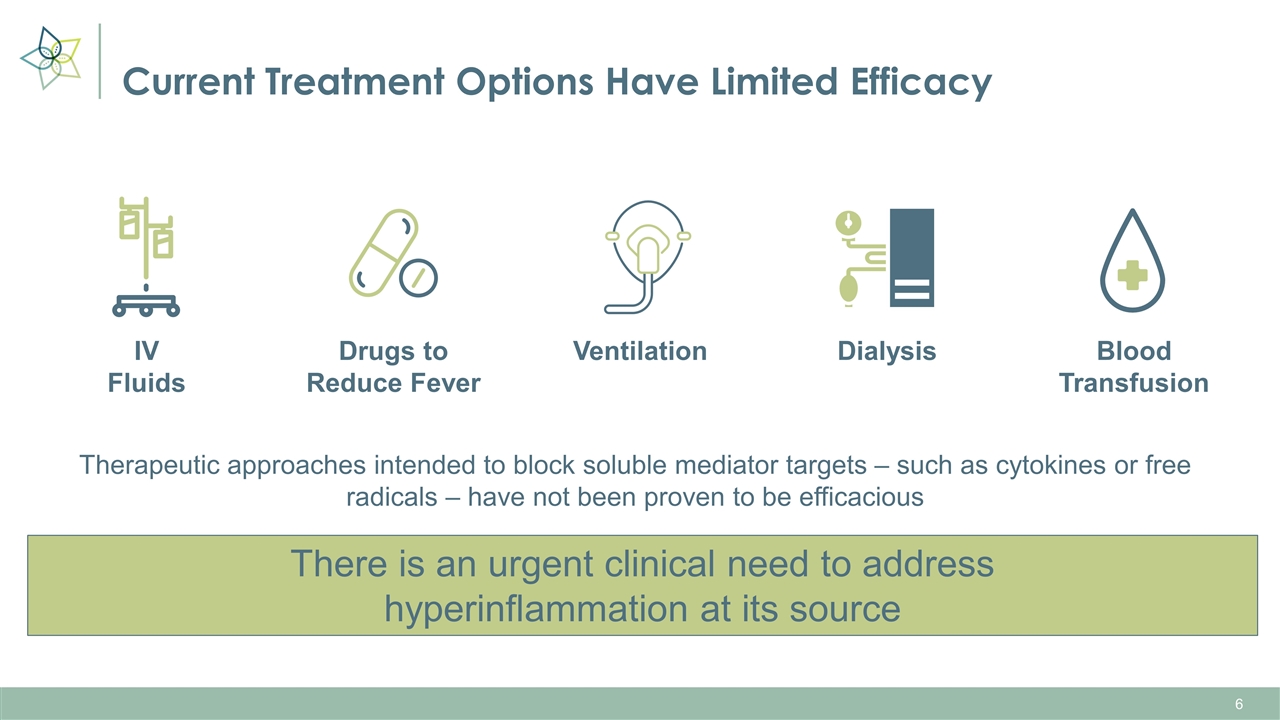
Therapeutic approaches intended to block soluble mediator targets – such as cytokines or free radicals – have not been proven to be efficacious IV Fluids Drugs to Reduce Fever Ventilation Dialysis Blood Transfusion Current Treatment Options Have Limited Efficacy There is an urgent clinical need to address hyperinflammation at its source

SCD Modulates the Immunological Effector Cells that Promote the Release of Cytokines and Other Harmful Mediators SeaStar Medical Confidential Innate immune response Activated monocytes Activated neutrophils IMPROVED CLINICAL OUTCOMES Cytokines Hyperinflammation/ Cytokine storm Effector cell immunomodulation and deactivation SCD* Infection (Endotoxins/ pathogens) Injury Deactivated neutrophils Deactivated monocytes Other inflammatory mediators *SCD is currently under investigation and has not been approved. There is no guarantee that the product will receive authority approval and become commercially available for the uses being investigated.
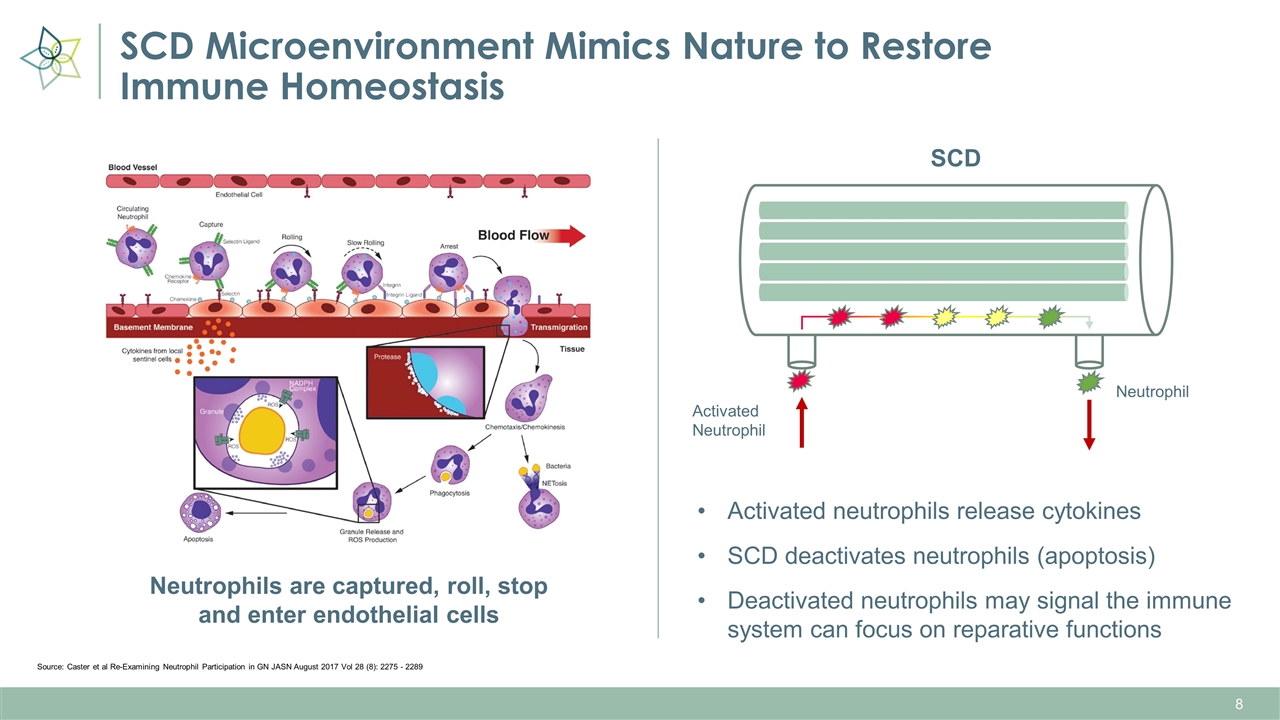
SCD Microenvironment Mimics Nature to Restore Immune Homeostasis Source: Caster et al Re-Examining Neutrophil Participation in GN JASN August 2017 Vol 28 (8): 2275 - 2289 SCD Activated Neutrophil Neutrophil Neutrophils are captured, roll, stop and enter endothelial cells Activated neutrophils release cytokines SCD deactivates neutrophils (apoptosis) Deactivated neutrophils may signal the immune system can focus on reparative functions
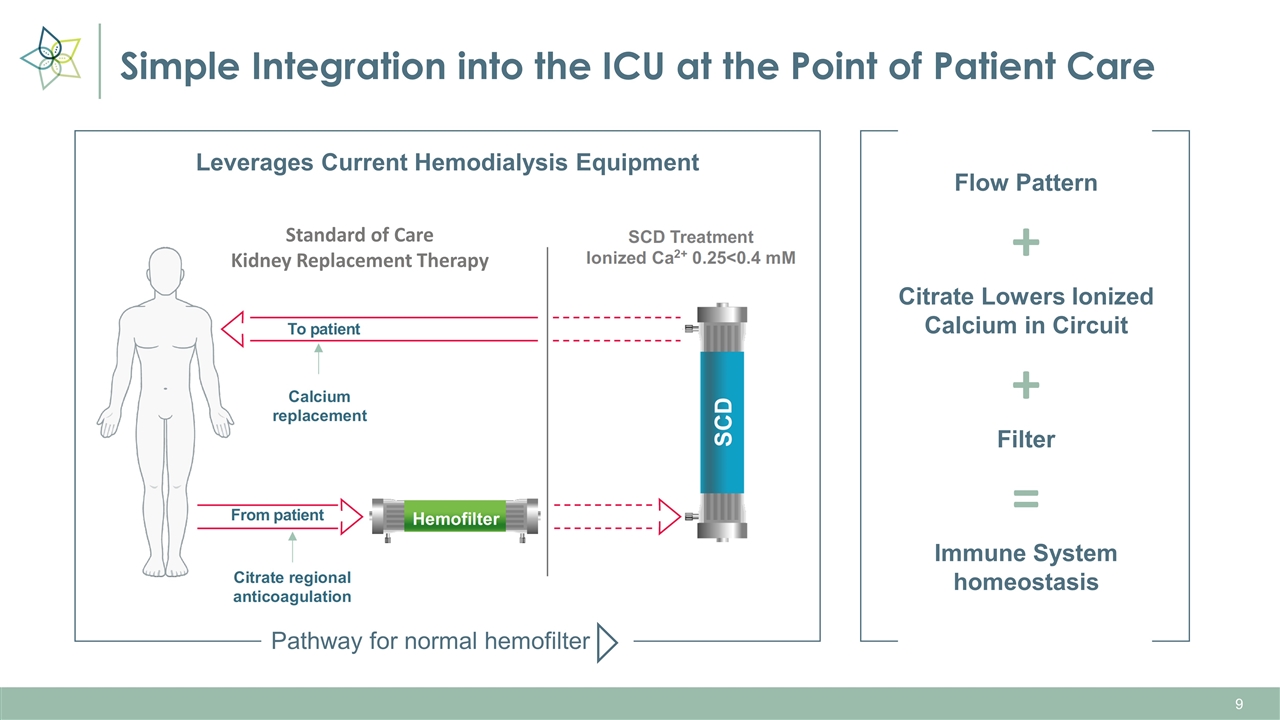
Simple Integration into the ICU at the Point of Patient Care + + = Leverages Current Hemodialysis Equipment Flow Pattern Citrate Lowers Ionized Calcium in Circuit Filter Immune System homeostasis Pathway for normal hemofilter Standard of Care Kidney Replacement Therapy
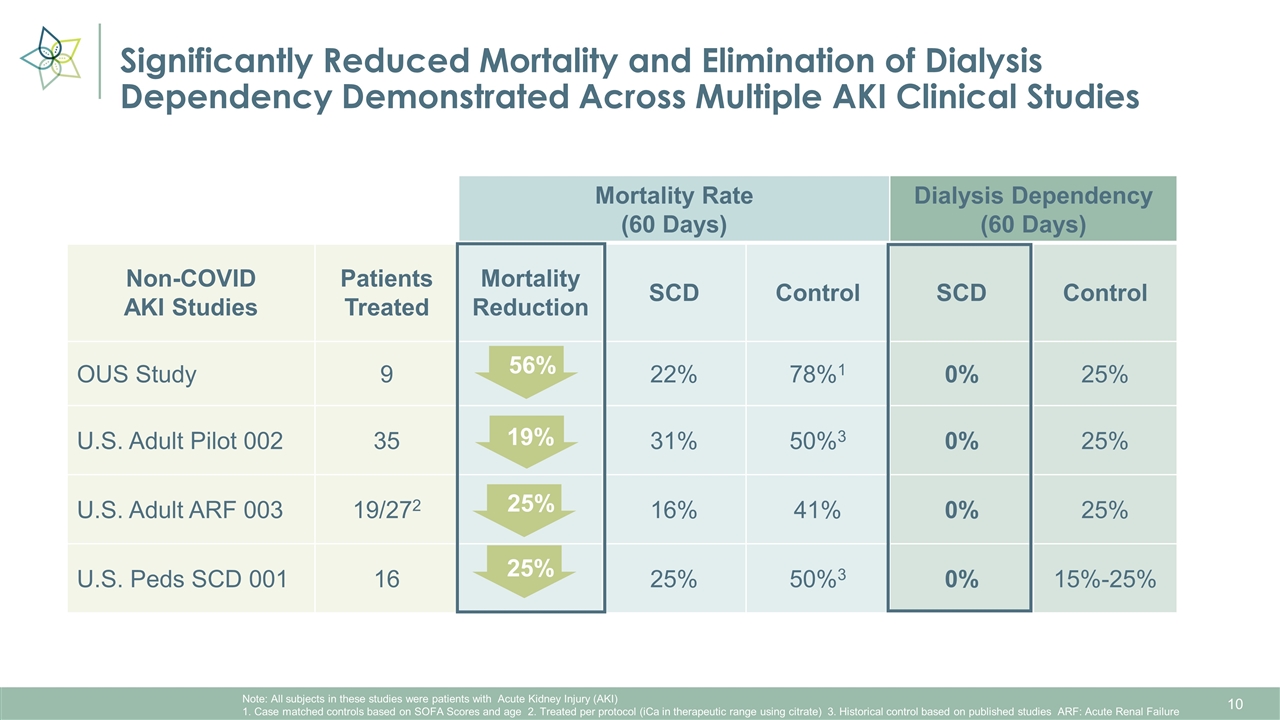
Significantly Reduced Mortality and Elimination of Dialysis Dependency Demonstrated Across Multiple AKI Clinical Studies Mortality Rate (60 Days) Control Dialysis Dependency (60 Days) Control Non-COVID AKI Studies Patients Treated Mortality Reduction SCD Control SCD Control OUS Study 9 22% 78%1 0% 25% U.S. Adult Pilot 002 35 31% 50%3 0% 25% U.S. Adult ARF 003 19/272 16% 41% 0% 25% U.S. Peds SCD 001 16 25% 50%3 0% 15%-25% 56% Note: All subjects in these studies were patients with Acute Kidney Injury (AKI) 1. Case matched controls based on SOFA Scores and age 2. Treated per protocol (iCa in therapeutic range using citrate) 3. Historical control based on published studies ARF: Acute Renal Failure 19% 25% 25%
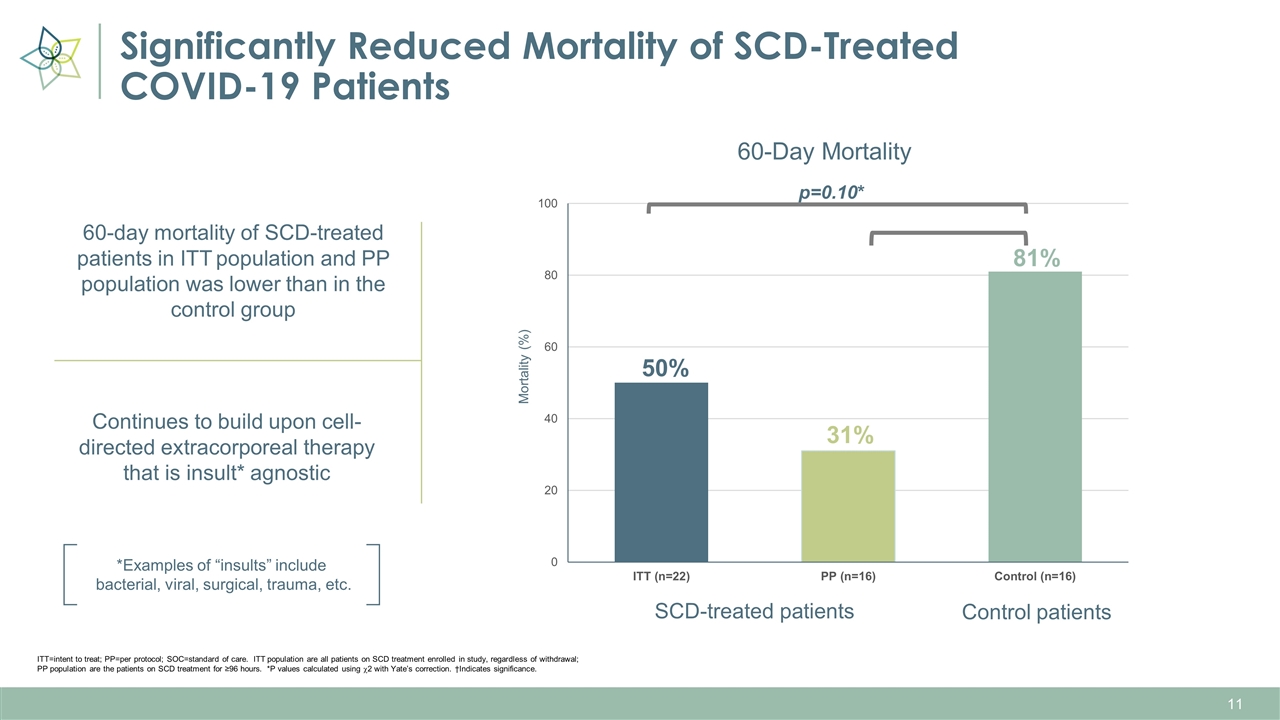
Significantly Reduced Mortality of SCD-Treated COVID-19 Patients Control patients 50% 31% 81% SCD-treated patients p=0.10* ITT=intent to treat; PP=per protocol; SOC=standard of care. ITT population are all patients on SCD treatment enrolled in study, regardless of withdrawal; PP population are the patients on SCD treatment for ≥96 hours. *P values calculated using c2 with Yate’s correction. †Indicates significance. 60-day mortality of SCD-treated patients in ITT population and PP population was lower than in the control group Continues to build upon cell-directed extracorporeal therapy that is insult* agnostic *Examples of “insults” include bacterial, viral, surgical, trauma, etc.
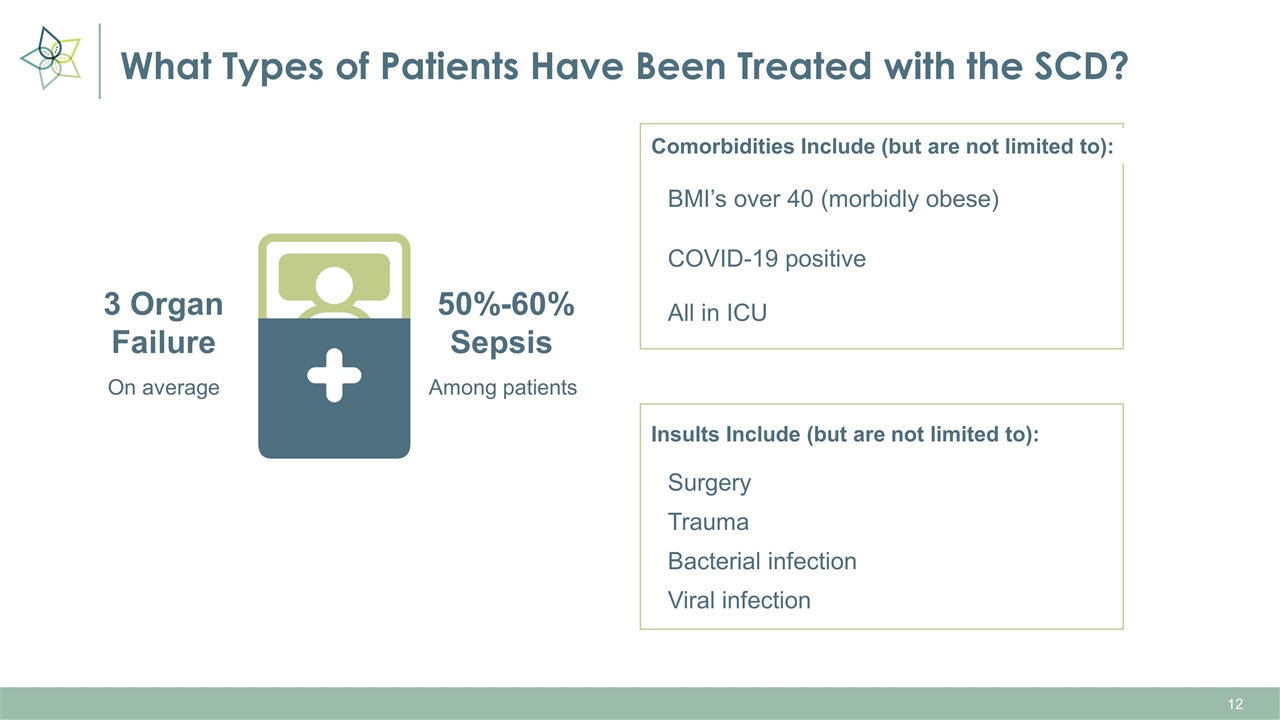
What Types of Patients Have Been Treated with the SCD? On average 3 Organ Failure Among patients 50%-60% Sepsis Comorbidities Include (but are not limited to): All in ICU BMI’s over 40 (morbidly obese) COVID-19 positive Insults Include (but are not limited to): Surgery Trauma Bacterial infection Viral infection
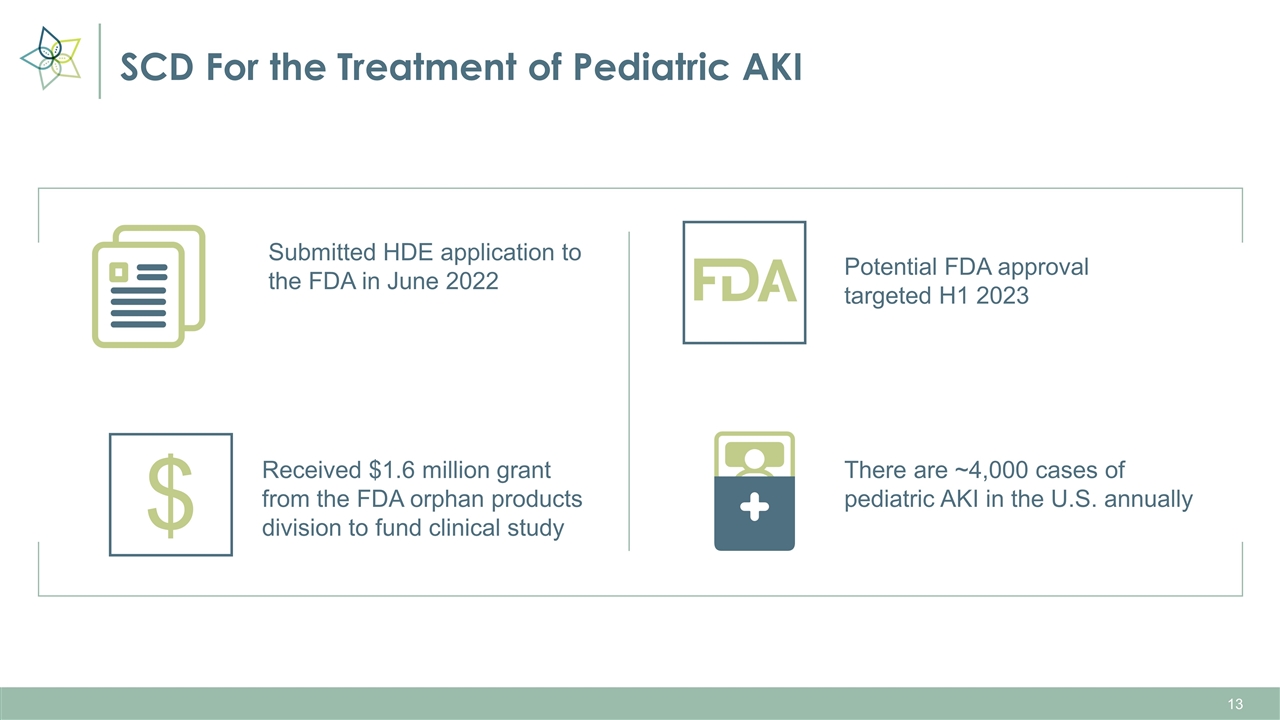
SCD For the Treatment of Pediatric AKI Potential FDA approval targeted H1 2023 Submitted HDE application to the FDA in June 2022 Received $1.6 million grant from the FDA orphan products division to fund clinical study There are ~4,000 cases of pediatric AKI in the U.S. annually $
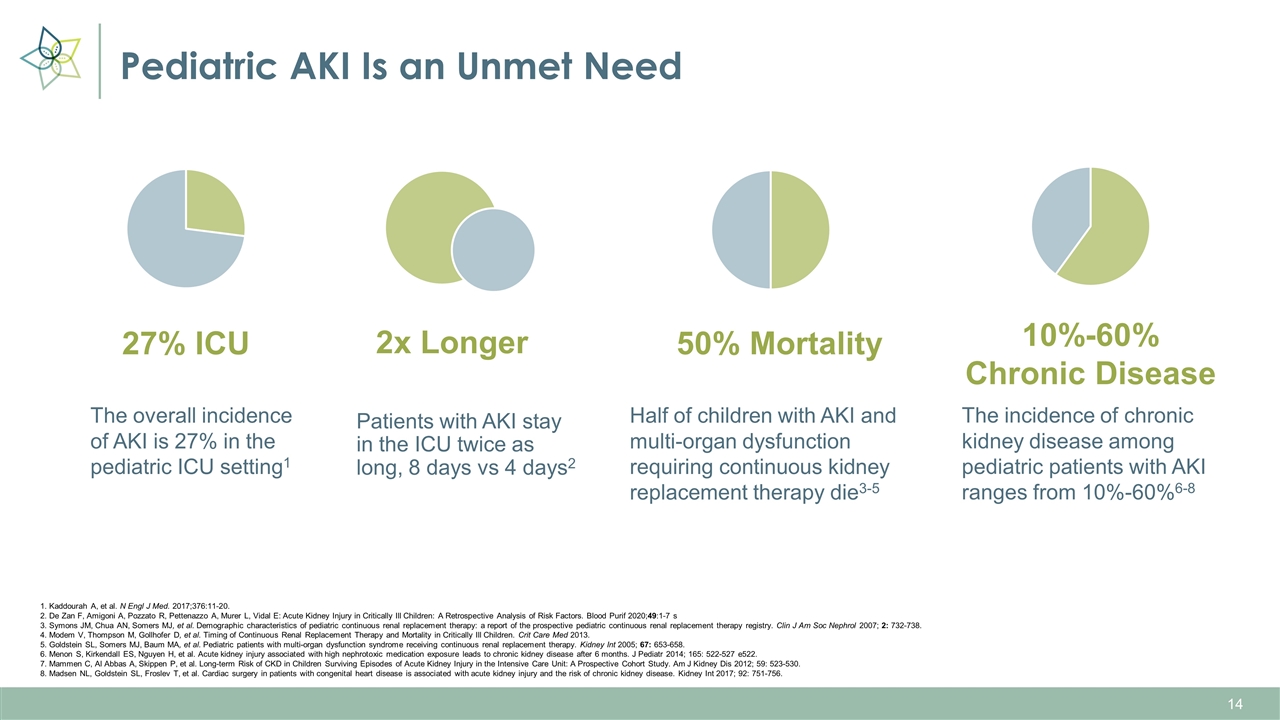
Pediatric AKI Is an Unmet Need The overall incidence of AKI is 27% in the pediatric ICU setting1 Patients with AKI stay in the ICU twice as long, 8 days vs 4 days2 Half of children with AKI and multi-organ dysfunction requiring continuous kidney replacement therapy die3-5 The incidence of chronic kidney disease among pediatric patients with AKI ranges from 10%-60%6-8 27% ICU 2x Longer 50% Mortality 10%-60% Chronic Disease 1. Kaddourah A, et al. N Engl J Med. 2017;376:11-20. 2. De Zan F, Amigoni A, Pozzato R, Pettenazzo A, Murer L, Vidal E: Acute Kidney Injury in Critically Ill Children: A Retrospective Analysis of Risk Factors. Blood Purif 2020;49:1-7 s 3. Symons JM, Chua AN, Somers MJ, et al. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol 2007; 2: 732-738. 4. Modem V, Thompson M, Gollhofer D, et al. Timing of Continuous Renal Replacement Therapy and Mortality in Critically Ill Children. Crit Care Med 2013. 5. Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int 2005; 67: 653-658. 6. Menon S, Kirkendall ES, Nguyen H, et al. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr 2014; 165: 522-527 e522. 7. Mammen C, Al Abbas A, Skippen P, et al. Long-term Risk of CKD in Children Surviving Episodes of Acute Kidney Injury in the Intensive Care Unit: A Prospective Cohort Study. Am J Kidney Dis 2012; 59: 523-530. 8. Madsen NL, Goldstein SL, Froslev T, et al. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int 2017; 92: 751-756.
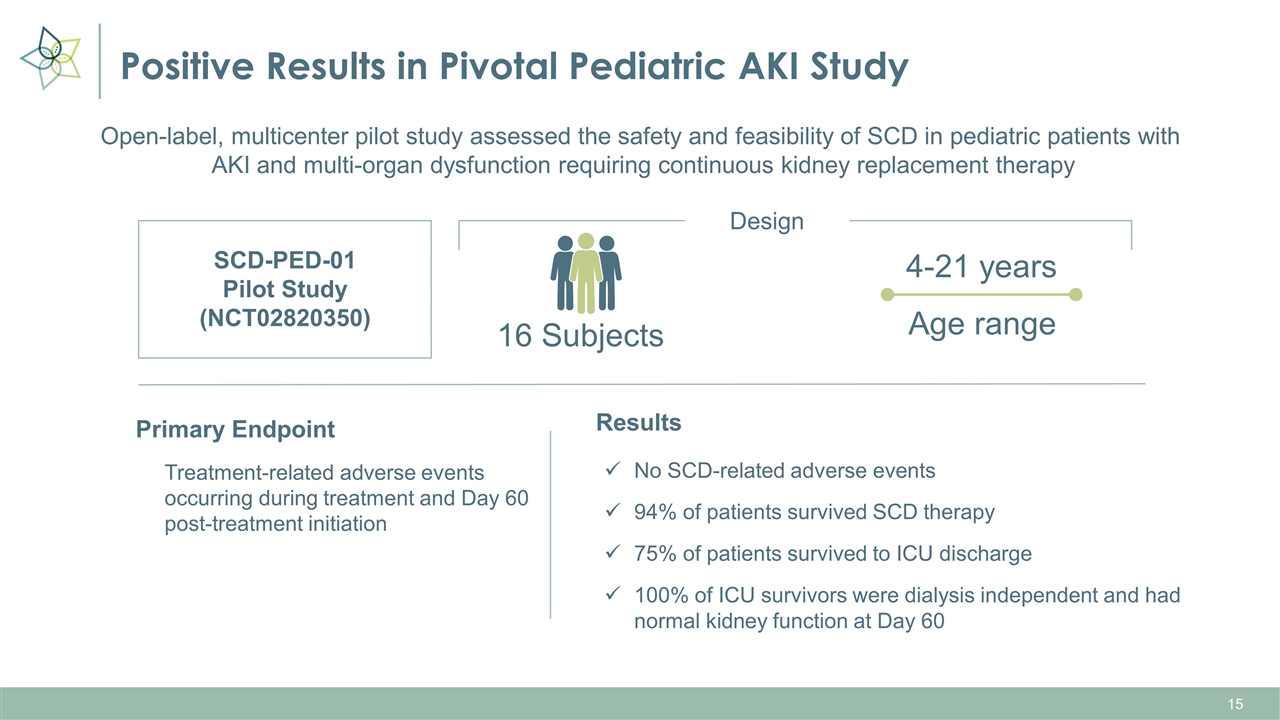
Positive Results in Pivotal Pediatric AKI Study SCD-PED-01 Pilot Study (NCT02820350) 16 Subjects 4-21 years Primary Endpoint Treatment-related adverse events occurring during treatment and Day 60 post-treatment initiation Design Open-label, multicenter pilot study assessed the safety and feasibility of SCD in pediatric patients with AKI and multi-organ dysfunction requiring continuous kidney replacement therapy Age range Results No SCD-related adverse events 94% of patients survived SCD therapy 75% of patients survived to ICU discharge 100% of ICU survivors were dialysis independent and had normal kidney function at Day 60
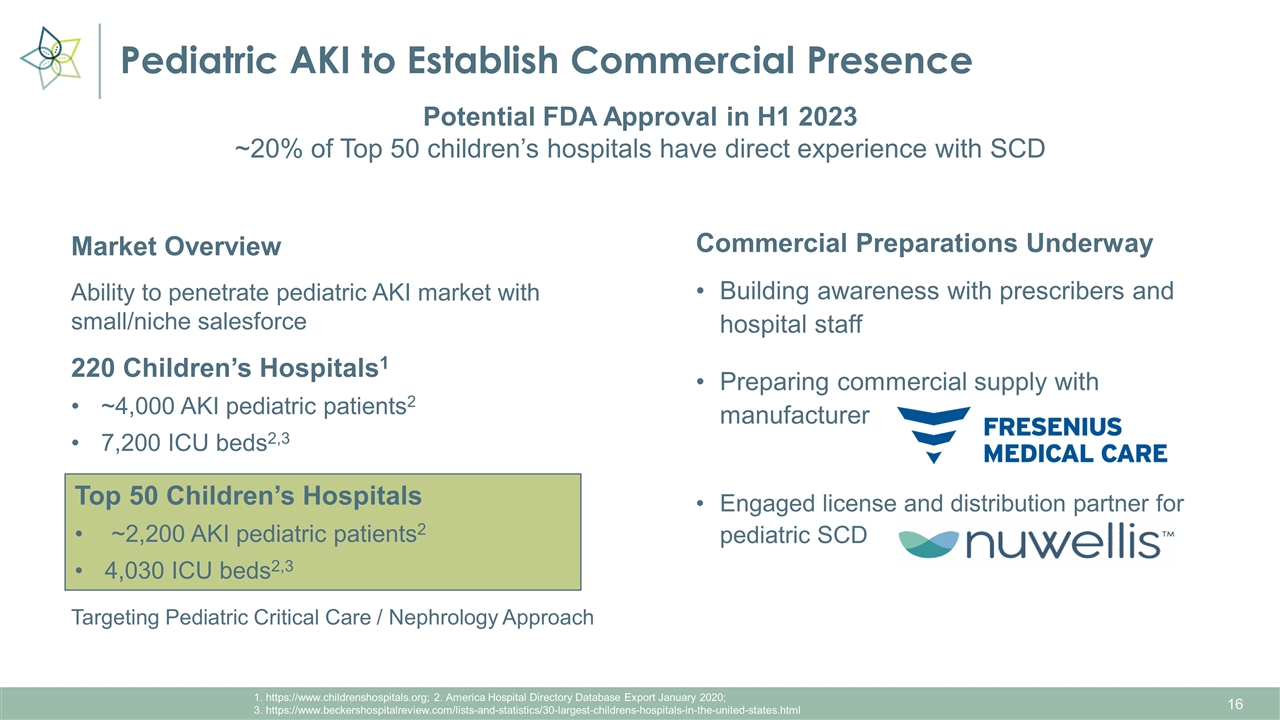
Pediatric AKI to Establish Commercial Presence 1. https://www.childrenshospitals.org; 2. America Hospital Directory Database Export January 2020; 3. https://www.beckershospitalreview.com/lists-and-statistics/30-largest-childrens-hospitals-in-the-united-states.html Market Overview Ability to penetrate pediatric AKI market with small/niche salesforce 220 Children’s Hospitals1 ~4,000 AKI pediatric patients2 7,200 ICU beds2,3 Targeting Pediatric Critical Care / Nephrology Approach Potential FDA Approval in H1 2023 ~20% of Top 50 children’s hospitals have direct experience with SCD Commercial Preparations Underway Building awareness with prescribers and hospital staff Preparing commercial supply with manufacturer Engaged license and distribution partner for pediatric SCD Top 50 Children’s Hospitals ~2,200 AKI pediatric patients2 4,030 ICU beds2,3
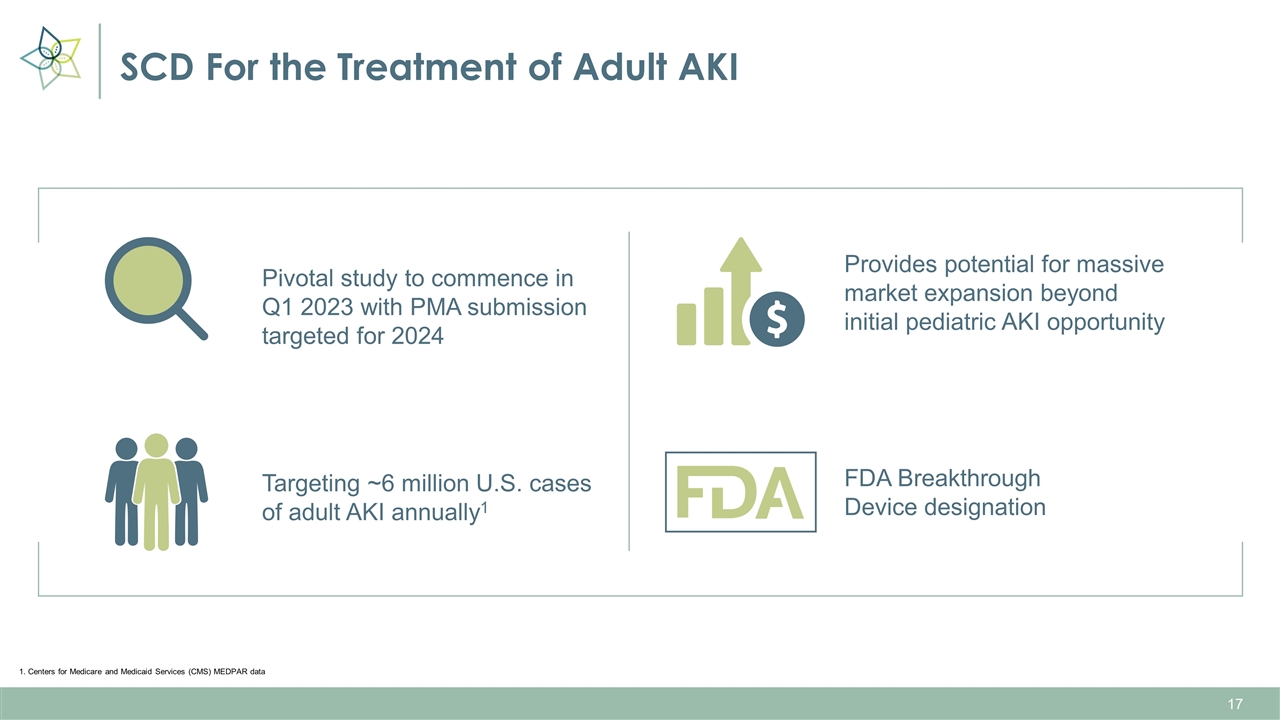
SCD For the Treatment of Adult AKI Pivotal study to commence in Q1 2023 with PMA submission targeted for 2024 Provides potential for massive market expansion beyond initial pediatric AKI opportunity Targeting ~6 million U.S. cases of adult AKI annually1 FDA Breakthrough Device designation 1. Centers for Medicare and Medicaid Services (CMS) MEDPAR data
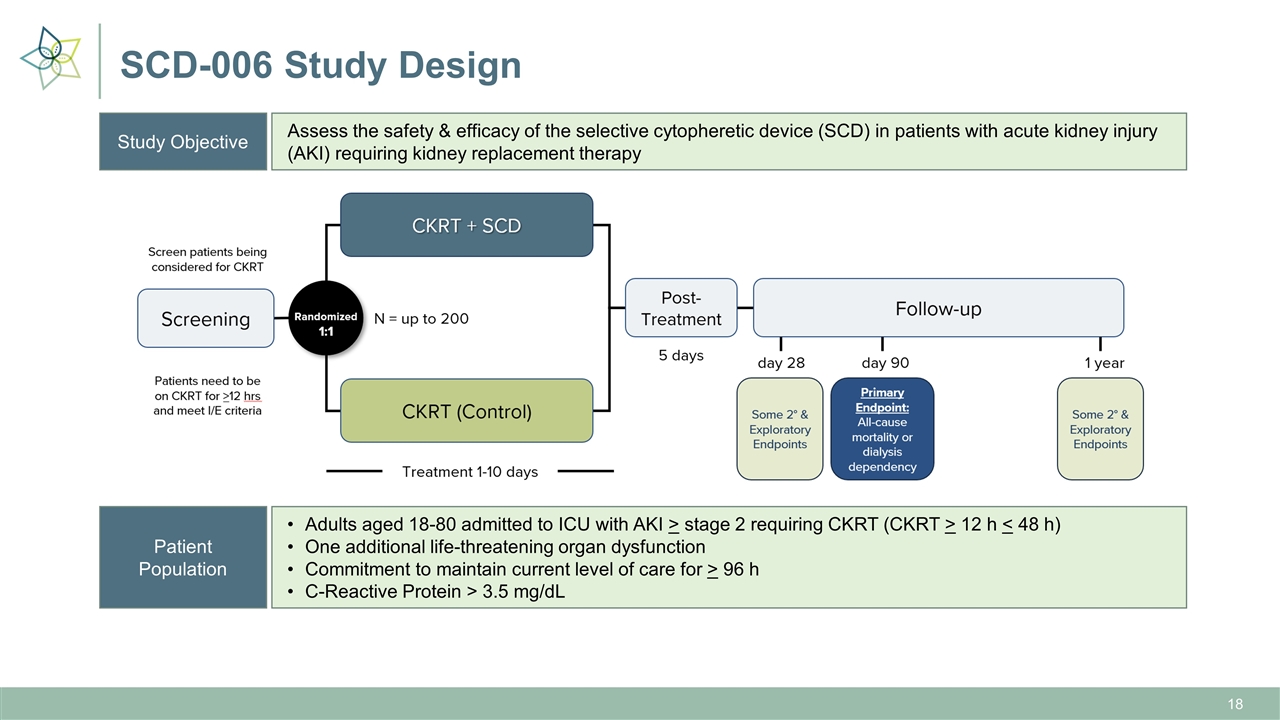
SCD-006 Study Design Study Objective Assess the safety & efficacy of the selective cytopheretic device (SCD) in patients with acute kidney injury (AKI) requiring kidney replacement therapy Patient Population Adults aged 18-80 admitted to ICU with AKI > stage 2 requiring CKRT (CKRT > 12 h < 48 h) One additional life-threatening organ dysfunction Commitment to maintain current level of care for > 96 h C-Reactive Protein > 3.5 mg/dL
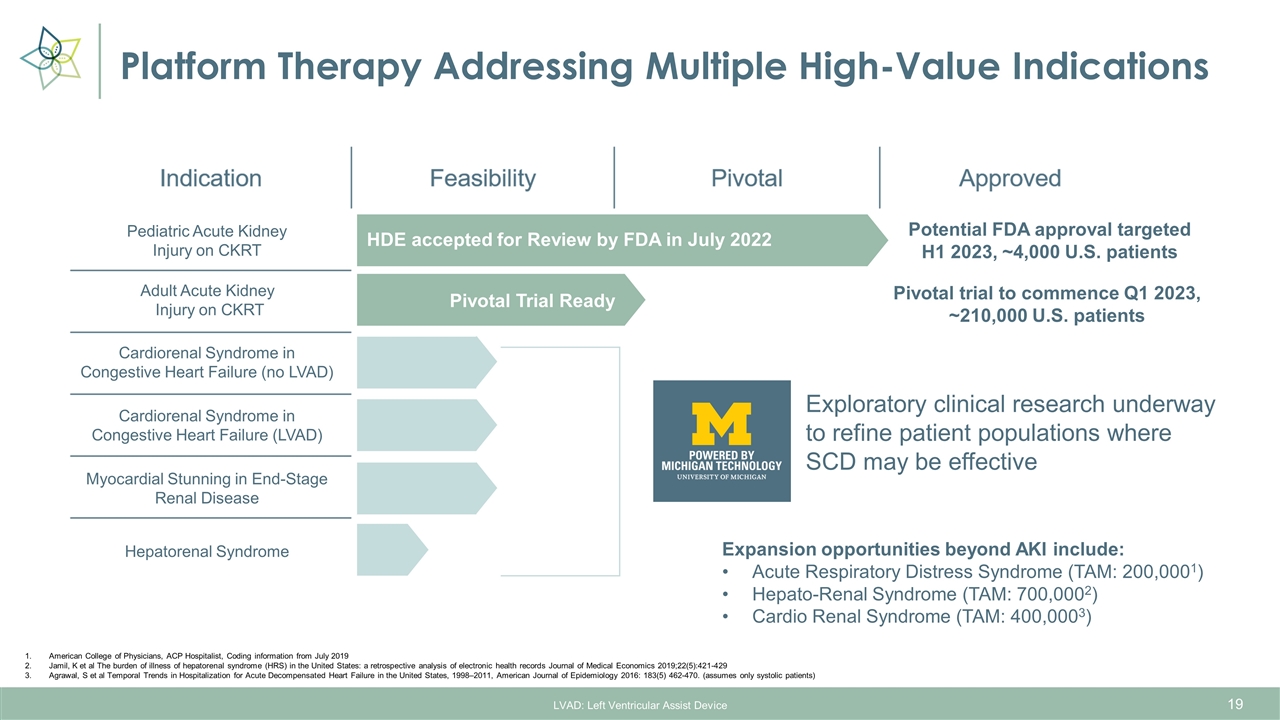
Platform Therapy Addressing Multiple High-Value Indications LVAD: Left Ventricular Assist Device American College of Physicians, ACP Hospitalist, Coding information from July 2019 Jamil, K et al The burden of illness of hepatorenal syndrome (HRS) in the United States: a retrospective analysis of electronic health records Journal of Medical Economics 2019;22(5):421-429 Agrawal, S et al Temporal Trends in Hospitalization for Acute Decompensated Heart Failure in the United States, 1998–2011, American Journal of Epidemiology 2016: 183(5) 462-470. (assumes only systolic patients) Expansion opportunities beyond AKI include: Acute Respiratory Distress Syndrome (TAM: 200,0001) Hepato-Renal Syndrome (TAM: 700,0002) Cardio Renal Syndrome (TAM: 400,0003) Pediatric Acute Kidney Injury on CKRT Adult Acute Kidney Injury on CKRT Cardiorenal Syndrome in Congestive Heart Failure (no LVAD) Cardiorenal Syndrome in Congestive Heart Failure (LVAD) Myocardial Stunning in End-Stage Renal Disease Hepatorenal Syndrome Exploratory clinical research underway to refine patient populations where SCD may be effective HDE accepted for Review by FDA in July 2022 Potential FDA approval targeted H1 2023, ~4,000 U.S. patients Pivotal Trial Ready Pivotal trial to commence Q1 2023, ~210,000 U.S. patients

Corporate Overview
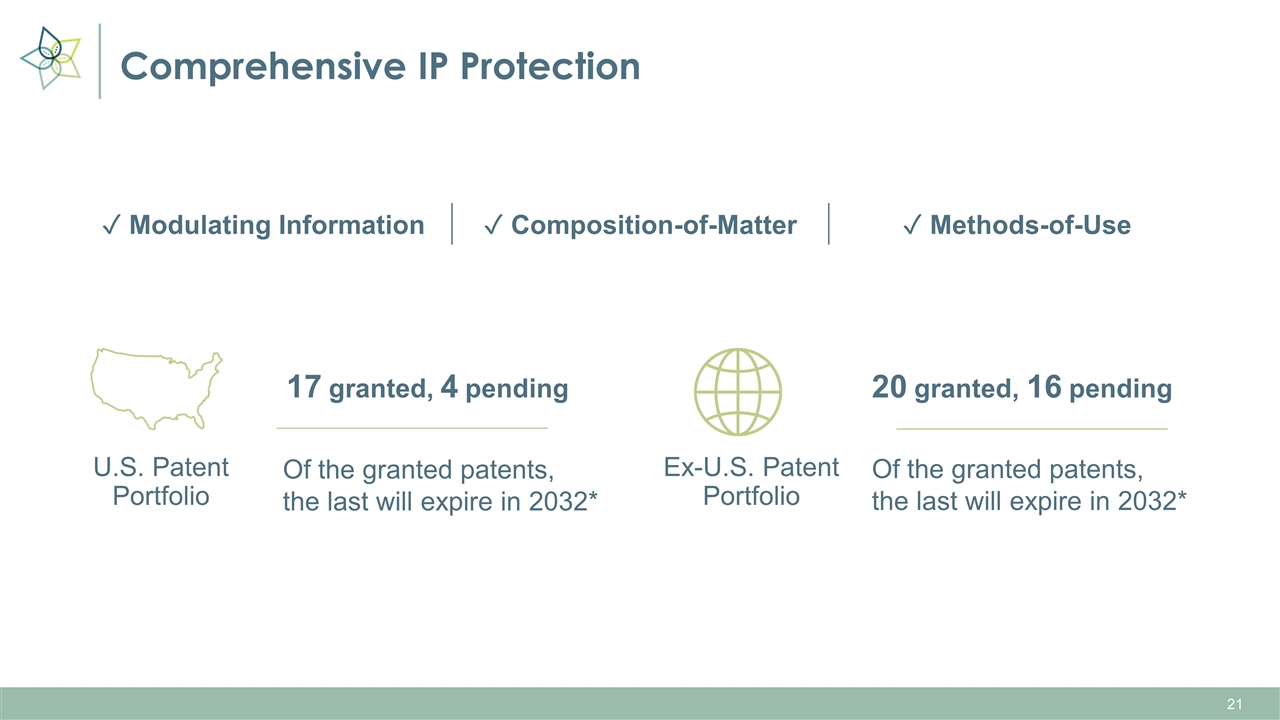
Comprehensive IP Protection U.S. Patent Portfolio 17 granted, 4 pending Of the granted patents, the last will expire in 2032* Ex-U.S. Patent Portfolio 20 granted, 16 pending Of the granted patents, the last will expire in 2032* ✓ Modulating Information ✓ Composition-of-Matter ✓ Methods-of-Use

Experienced Management Team Eric Schlorff CEO Caryl Baron Interim Chief Financial Officer Tom Mullen VP, Manufacturing and Product Development Kevin Chung, MD Chief Medical Officer Sai Iyer, PhD VP, Medical Affairs and Clinical Development Scientific Advisor: H. David Humes, MD Professor, Division of Nephrology, Internal Medicine University of Michigan Center for Integrative Research in Critical Care

Board of Directors Rick Barnett – Chairman Past President & CEO of Satellite Healthcare Board Chair National Kidney Foundation, Northern California Andres Lobo Dow Chemical Company Manages ~$16 billion NAV Allan Collins, MD Former Chief Medical Officer of NxStage, Fresenius Medical Care Past President of National Kidney Foundation Kenneth Van Heel Dow Chemical Company Former Board Member of Pfenex, Inc. Bruce Rodgers CEO – LM Funding America, Inc. and LMF Acquisition Opportunities Experienced M&A lawyer Richard Russell CFO – LMF Acquisition Opportunities Experienced public CFO Eric Schlorff CEO – SeaStar Medical Dow Chemical Company MS Pharmacology

Financial Update Merged into SPAC (NASDAQ: LMAO) on October 28, 2022 Completed simultaneous: PIPE of $7 MM Secured forward agreements for approximately 1.15 MM shares $100 MM Equity Line of Credit Warrants at $11.50 (NASDAQ: ICUCW) As of February 14, 2023 Proceeds of $1.9 MM were received from 378,000 shares under the forward purchase agreement Equity line of credit is available to company

Key Upcoming Potential Value-Driving Milestones H1 2023 FDA Approval H1 2023 Commercial Launch Pediatric Acute Kidney Injury Q1 2023 Commence Pivotal Study Q4 2023 Pivotal Study Interim Results Q3 2024 Pivotal Study Topline Results Q3 2024 PMA Submission H1 2025 PMA Approval H2 2025 Commercial Launch Adult Acute Kidney Injury

In Summary SCD addresses hyperinflammation in multiple acute and chronic large, high-value indications SCD’s unique mechanism of action provides cell-directed extracorporeal therapy at the patient point of care under an FDA medical device regulatory pathway Initial indications in pediatric and adult acute kidney injury (AKI) are supported by clinical results demonstrating significantly reduced mortality and the elimination of dependency on hemodialysis Multiple near-term regulatory and clinical milestones provide a cadence of company news and the potential to create significant value for shareholders Targeting FDA approval of pediatric SCD for pediatric AKI in the first half of 2023 under the humanitarian device exemption (HDE) process Pivotal clinical trial with SCD for adult AKI to begin in the first quarter of 2023, with interim results expected in the fourth quarter of 2023 Seasoned leadership with highly relevant business experience and a proven track record of execution
Appendix
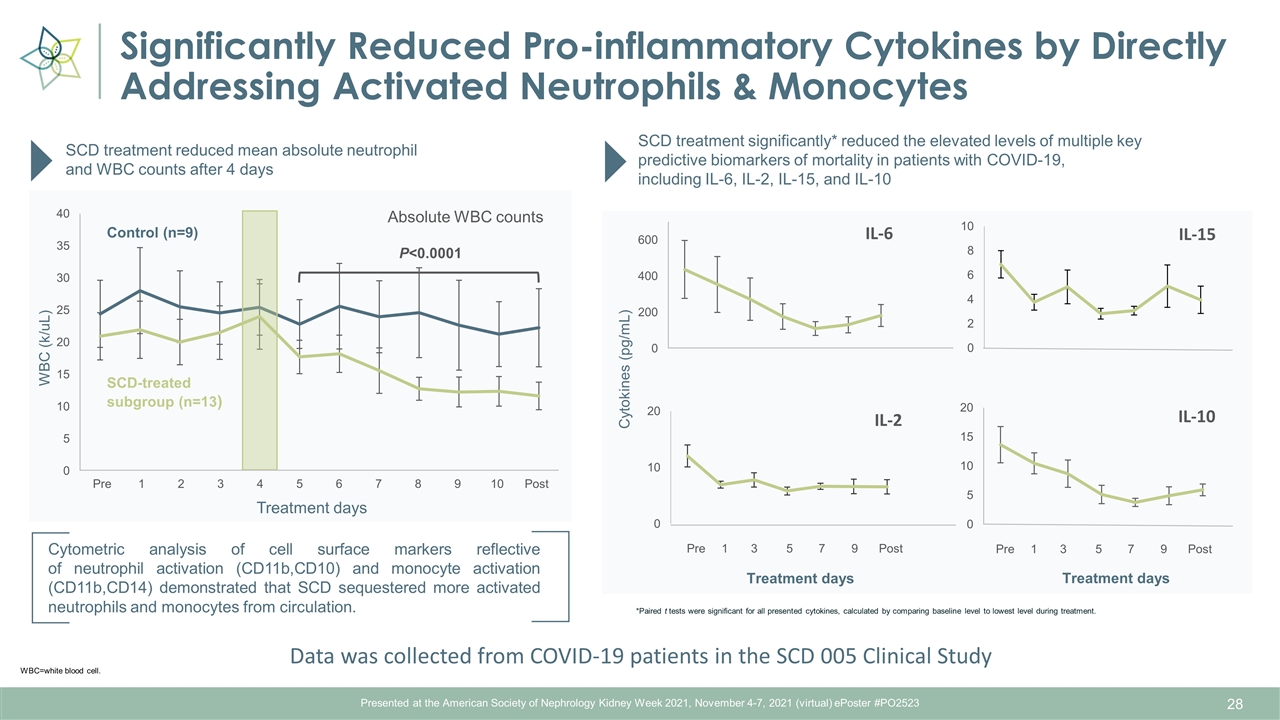
Presented at the American Society of Nephrology Kidney Week 2021, November 4-7, 2021 (virtual) ePoster #PO2523 Significantly Reduced Pro-inflammatory Cytokines by Directly Addressing Activated Neutrophils & Monocytes Cytometric analysis of cell surface markers reflective of neutrophil activation (CD11b,CD10) and monocyte activation (CD11b,CD14) demonstrated that SCD sequestered more activated neutrophils and monocytes from circulation. WBC=white blood cell. SCD treatment reduced mean absolute neutrophil and WBC counts after 4 days Absolute WBC counts P<0.0001 SCD treatment significantly* reduced the elevated levels of multiple key predictive biomarkers of mortality in patients with COVID-19, including IL-6, IL-2, IL-15, and IL-10 Treatment days Cytokines (pg/mL) WBC (k/uL) Treatment days Treatment days *Paired t tests were significant for all presented cytokines, calculated by comparing baseline level to lowest level during treatment. Pre Post 1 2 3 4 5 6 7 8 9 10 Pre 1 3 5 7 9 Post SCD-treated subgroup (n=13) Control (n=9) Pre 1 3 5 7 9 Post Data was collected from COVID-19 patients in the SCD 005 Clinical Study