Exhibit 99.1

January 2025 www.SeaStarMedical.com Nasdaq: ICU Investor Presentation Bringing organ - restoring solutions to critically ill patients

Forward - looking statements This presentation contains certain forward - looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1955. These forward - looking statements include, without limitation, SeaStar Medical’s expectations with respect to the ability of the SCD to treat patients with AKI and other diseases; the total addressable market for adult SCD applications including the annual U.S. patient population, cartridge pricing and the number of cartridges used per patient; the ability of SeaStar Medical to gain market share and generate sales with respect to the total addressable market for adult SCD application; anticipated patient enrollment and the expansion of the clinical trial sites; the number of patients and annual sales for the addressable AKI market; the anticipated Medicare and Medicaid reimbursement by CMS for patients enrolled in clinical trials; planned and potential future clinical trials and associated costs; the expected regulatory approval process and timeline for commercialization of our clinical products; and the ability of SeaStar Medical to meet the expected timeline.. Words such as “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions are intended to identify such forward - looking statements. Forward - looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to significant risks and uncertainties that could cause the actual results to differ materially from the expected results. Most of these facto rs are outside SeaStar Medical’s control and are difficult to predict. Factors that may cause actual future events to differ materially from the expected results include, but are not limited to: (i) the risk that SeaStar may not be able to obtain regulatory approval of its SCD product candidates; (ii) the risk that SeaStar may not be able to raise sufficient capital to fund its operations, including clinical trials; (iii) the risk that SeaStar Medical and its current and future collaborators are unable to successfully develop and commercialize its products or services, or experience significant delays in doing so, including failure to achieve approval of its products by applicable federal and state regulators, (iv) the risk that SeaStar Medical may never achieve or sustain profitability; (v) the risk that SeaStar Medical may not be able to access funding under existing agreements; (vi) the risk that third - parties suppliers and manufacturers are not able to fully and timely meet their obligations, (vii) the risk of product liability or regulatory lawsuits or proceedings relating to SeaStar Medical’s products and services, (xiii) the risk that SeaStar Medical is unable to secure or protect its intellectual property, and (xi) other risks and uncertainties indicated from time to time in SeaStar Medical’s Annual Report on Form 10 - K, including those under the “Risk Factors” section therein and in SeaStar Medical’s other filings with the SEC. The foregoing list of factors is not exhaustive. Forward - looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward - looking statements, and SeaStar Medical assume no obligation and do not intend to update or revise these forward - looking statements, whether as a result of new information, future events, or otherwise.

SEASTAR MEDICAL Commercial - stage company with patented, c linically validated, organ - agnostic therapeutic device targeting life - threatening hyperinflammation The Selective Cytopheretic Device (SCD) extracorporeal platform stops the cytokine storm and safely restores organ function Changing the standard of care, one patient at a time

Seasoned, d ynamic leadership ERIC SCHLORFF CEO + Board Member TOM MULLEN SVP, Manufacturing and Product Development SAI IYER, PHD SVP, Medical Affairs and Clinical Development DAVID GREEN Chief Financial Officer KEVIN CHUNG, MD Chief Medical Officer TIM VARACEK SVP, Commercial Business Operations 4
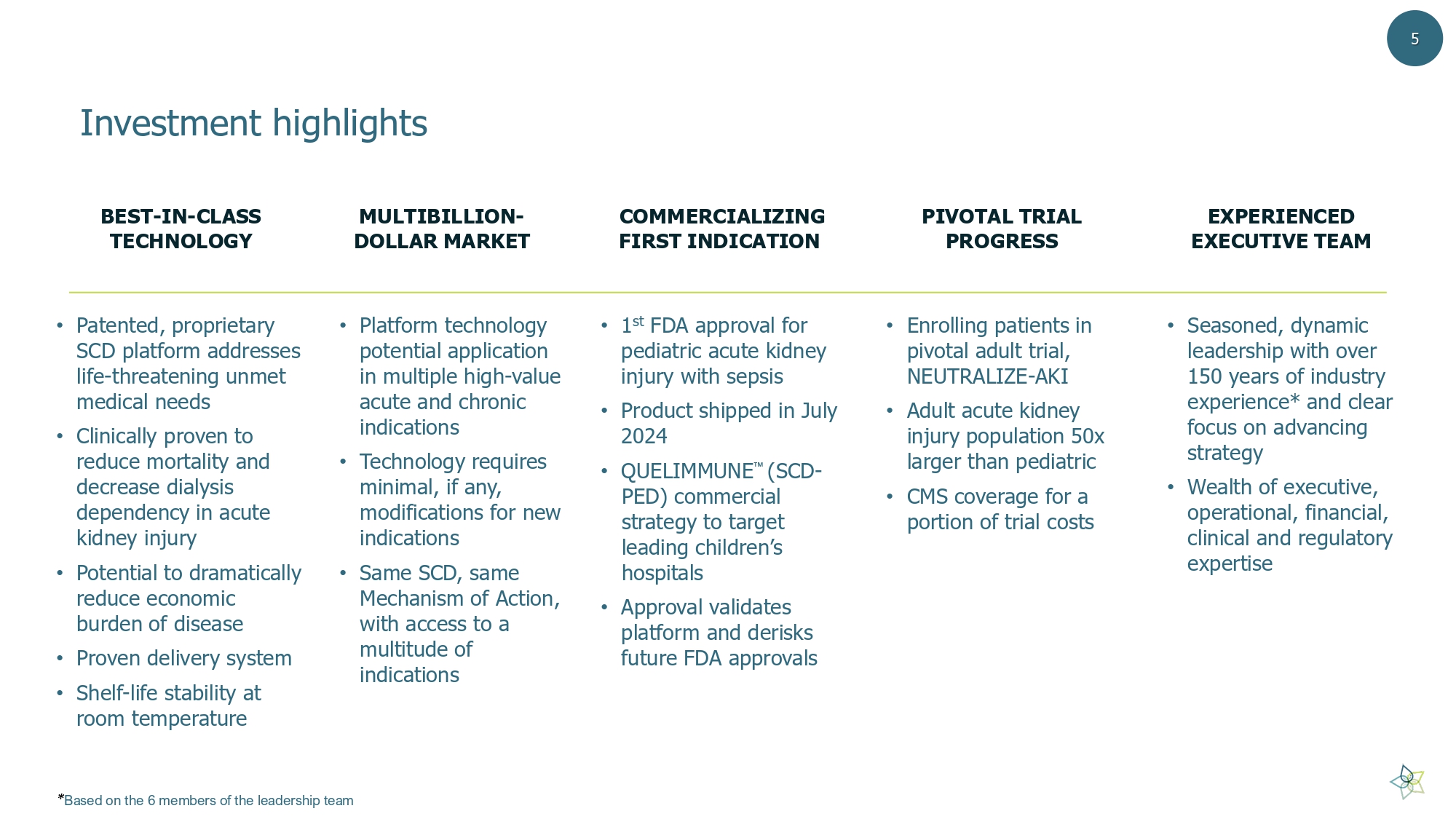
Investment highlights • Patented, proprietary SCD platform addresses life - threatening unmet medical needs • Clinically proven to reduce mortality and decrease dialysis dependency in acute kidney injury • Potential to dramatically reduce economic burden of disease • P roven delivery system • Shelf - life stability at room temperature • 1 st FDA approval for pediatric acute kidney injury with sepsis • Product shipped in July 2024 • QUELIMMUNE (SCD - PED) commercial strategy to target leading children’s hospitals • Approval validates platform and derisks future FDA approvals • Enrolling patients in pivotal adult trial, NEUTRALIZE - AKI • Adult acute kidney injury population 50x larger than pediatric • CMS coverage for a portion of trial costs • Platform technology potential application in multiple high - value acute and chronic indications • Technology requires minimal, if any, modifications for new indications • Same SCD, same Mechanism of Action, with access to a multitude of indications • Seasoned, dynamic leadership with over 150 years of industry experience* and clear focus on advancing strategy • Wealth of executive, operational, financial, clinical and regulatory expertise BEST - IN - CLASS TECHNOLOGY COMMERCIALIZING FIRST INDICATION PIVOTAL TRIAL PROGRESS MULTIBILLION - DOLLAR MARKET 5 EXPERIENCED EXECUTIVE TEAM * Based on the 6 members of the leadership team
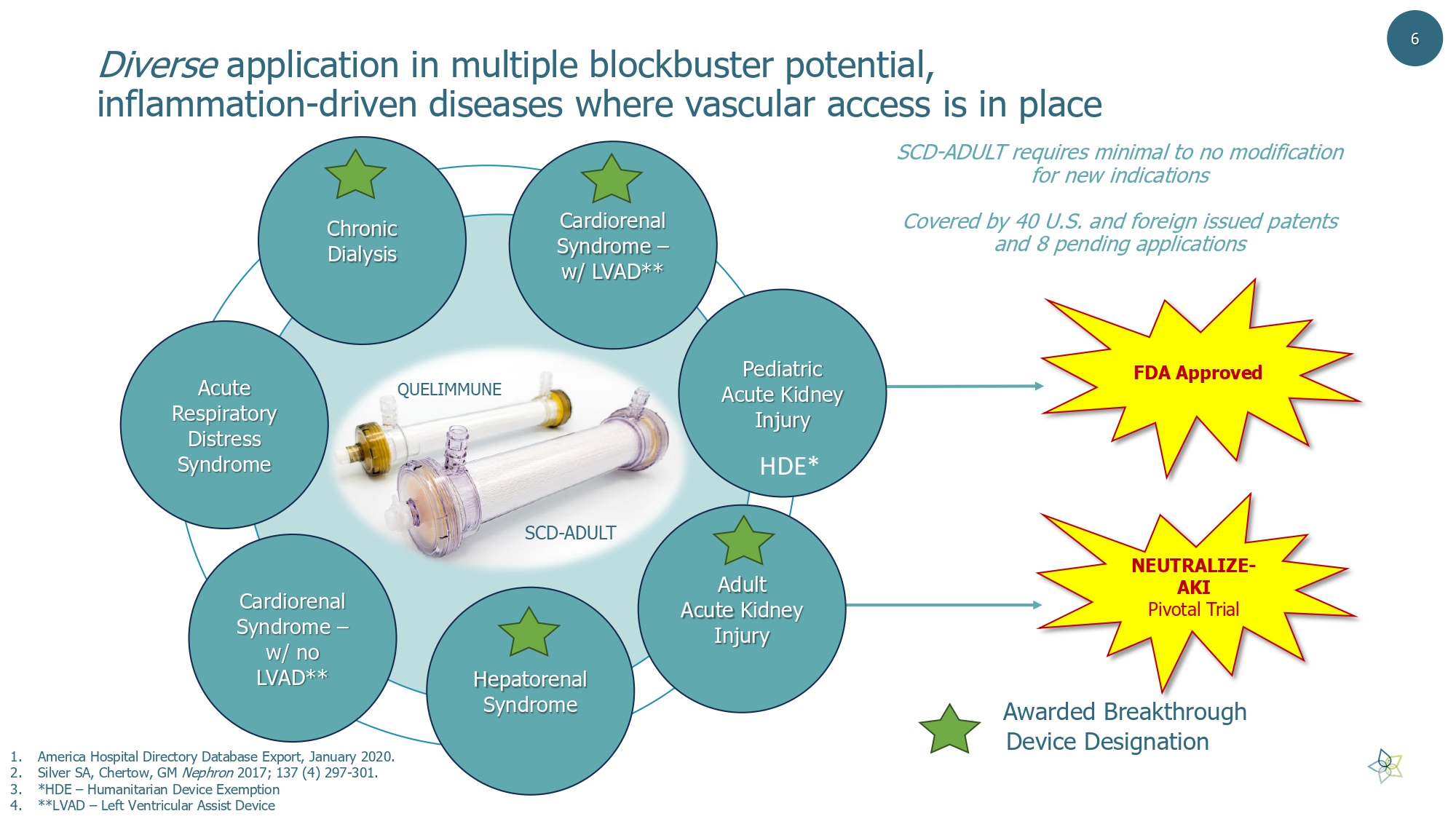
Diverse application in multiple blockbuster potential, inflammation - driven diseases where vascular access is in place 1. America Hospital Directory Database Export, January 2020. 2. Silver SA, Chertow , GM Nephron 2017; 137 (4) 297 - 301. 3. *HDE – Humanitarian Device Exemption 4. **LVAD – Left Ventricular Assist Device Cardiorenal Syndrome – w/ LVAD** Pediatric Acute Kidney Injury Adult Acute Kidney Injury Hepatorenal Syndrome Chronic Dialysis Acute Respiratory Distress Syndrome Cardiorenal Syndrome – w/ no LVAD** FDA Approved Awarded Breakthrough Device Designation NEUTRALIZE - AKI Pivotal Trial SCD - ADULT requires minimal to no modification for new indications Covered by 40 U.S. and foreign issued patents and 8 pending applications 6 HDE*
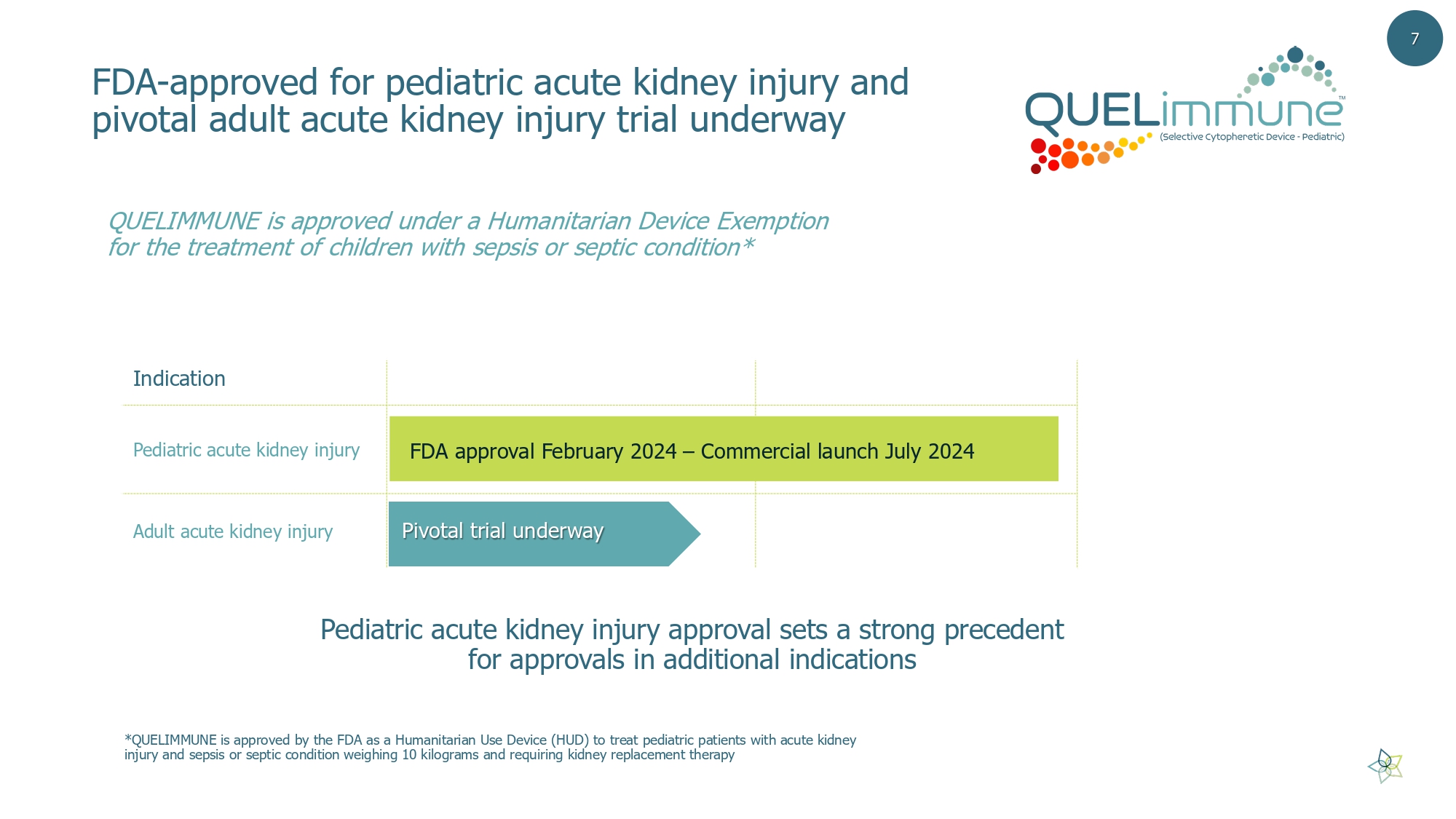
FDA - approved for pediatric acute k idney i njury and pivotal adult acute kidney injury trial underway Pediatric acute kidney injury approval sets a strong precedent for approvals in additional indications Indication Pediatric acute kidney injury Adult acute kidney injury Pivotal trial underway FDA approval February 2024 – Commercial launch July 2024 QUELIMMUNE is approved under a Humanitarian Device Exemption for the treatment of children with sepsis or septic condition* 7 *QUELIMMUNE is approved by the FDA as a Humanitarian Use Device (HUD) to treat pediatric patients with acute kidney injury and sepsis or septic condition weighing 10 kilograms and requiring kidney replacement therapy
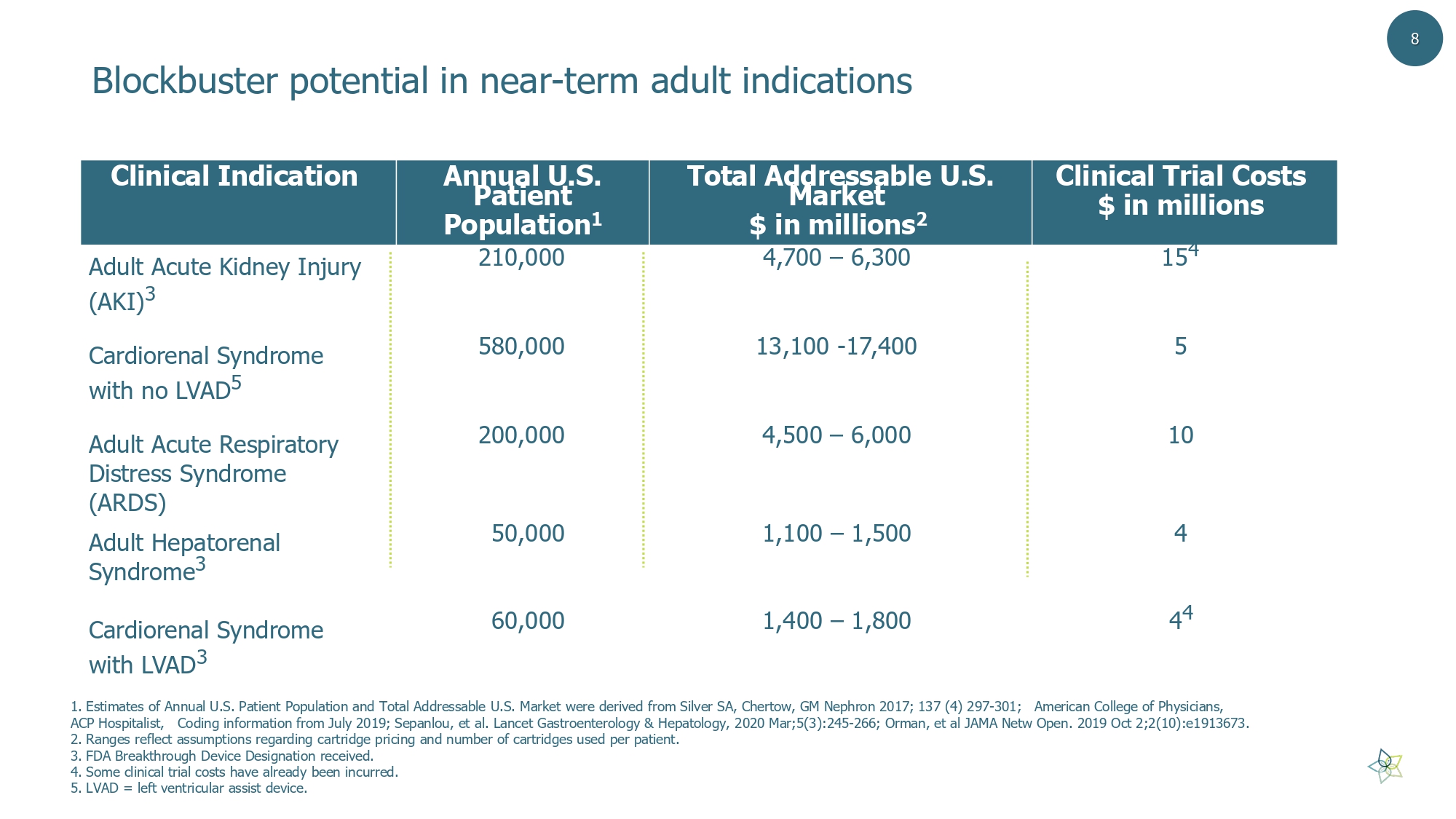
Blockbuster potential in near - term adult indications 8 Clinical Trial Costs $ in millions Total Addressable U.S. Market $ in millions 2 Annual U.S. Patient Population 1 Clinical Indication 15 4 4,700 – 6,300 210,000 Adult Acute Kidney Injury (AKI) 3 5 13,100 - 17,400 580,000 Cardiorenal Syndrome with no LVAD 5 10 4,500 – 6,000 200,000 Adult Acute Respiratory Distress Syndrome (ARDS) 4 1,100 – 1,500 50,000 Adult Hepatorenal Syndrome 3 4 4 1,400 – 1,800 60,000 Cardiorenal Syndrome with LVAD 3 1. Estimates of Annual U.S. Patient Population and Total Addressable U.S. Market were derived from Silver SA, Chertow , GM Nephron 2017; 137 (4) 297 - 301; American College of Physicians, ACP Hospitalist, Coding information from July 2019; Sepanlou , et al. Lancet Gastroenterology & Hepatology, 2020 Mar;5(3):245 - 266; Orman, et al JAMA Netw Open. 2019 Oct 2;2(10):e1913673. 2. Ranges reflect assumptions regarding cartridge pricing and number of cartridges used per patient. 3. FDA Breakthrough Device Designation received. 4. Some clinical trial costs have already been incurred. 5. LVAD = left ventricular assist device.

SCD TECHNOLOGY PLATFORM
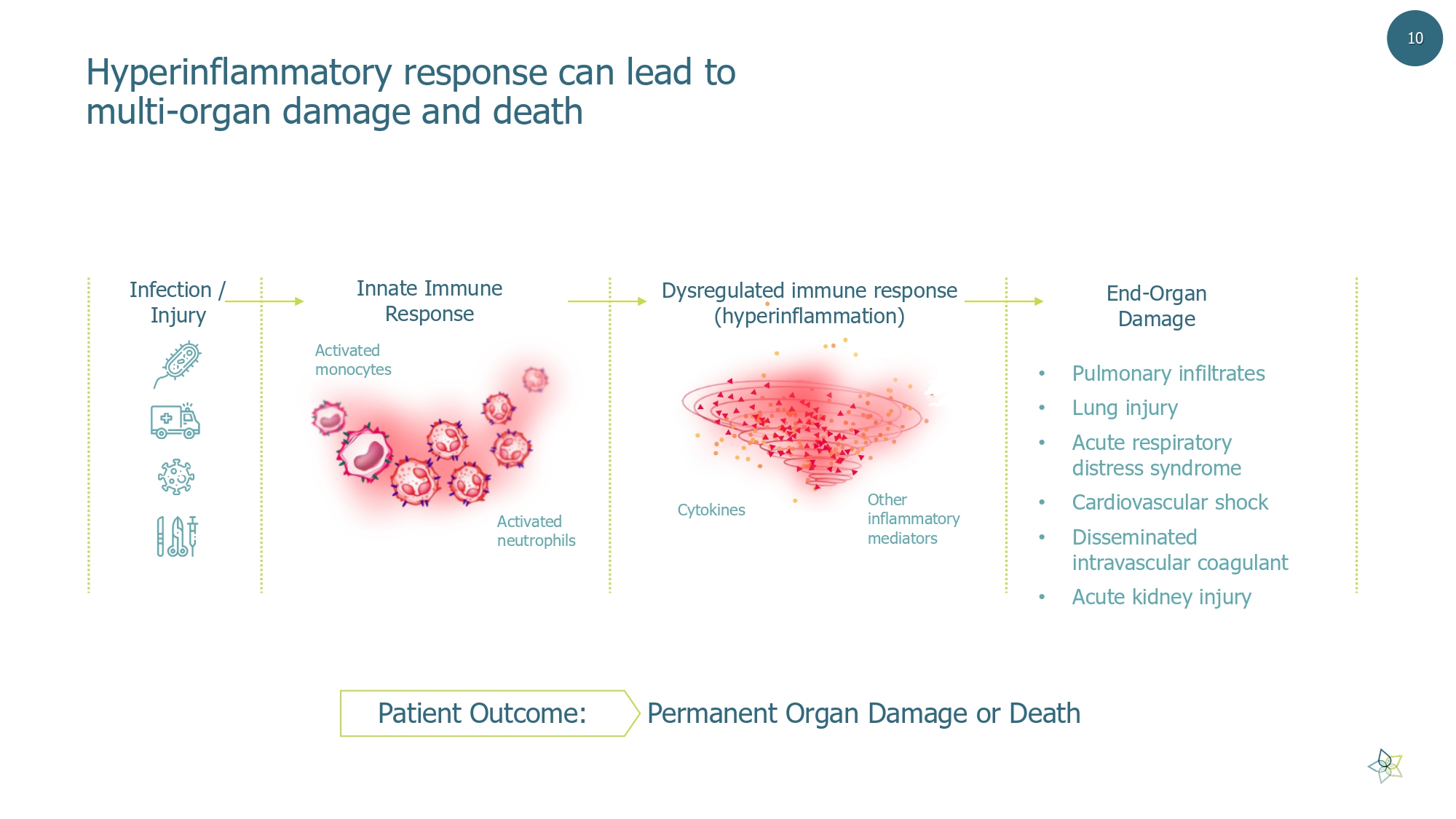
• Pulmonary infiltrates • Lung injury • Acute respiratory distress syndrome • Cardiovascular shock • Disseminated intravascular coagulant • Acute kidney injury End - Organ Damage Innate Immune Response Dysregulated immune response (hyperinflammation) H yperinflammatory response can lead to multi - organ damage and death Infection / Injury Activated monocytes Activated neutrophils Other inflammatory mediators Cytokines Patient Outcome: Permanent Organ Damage or Death 10
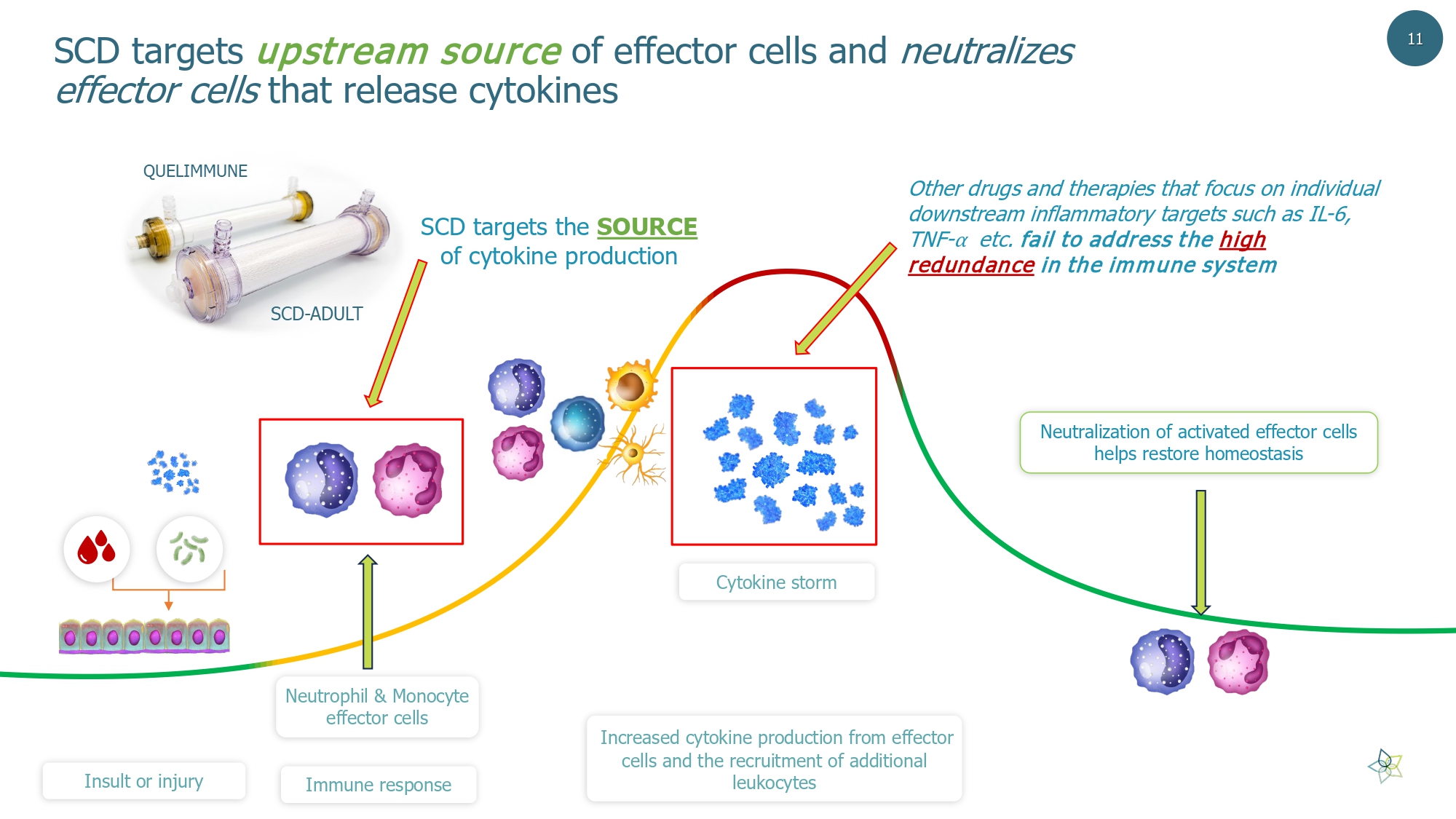
Neutralization of activated effector cells helps restore homeostasis Cyt okine storm Insult or injury Immune response Increased cytokine production from effector cells and the recruitment of additional leukocytes SCD targets the SOURCE of cytokine production SCD targets upstream source of effector cells and neutralizes effector cells that release cytokines Other drugs and therapies that focus on individual downstream inflammatory targets such as IL - 6, TNF - ⍺ etc. fail to address the high redundance in the immune system 11 Neutrophil & Monocyte effector cells
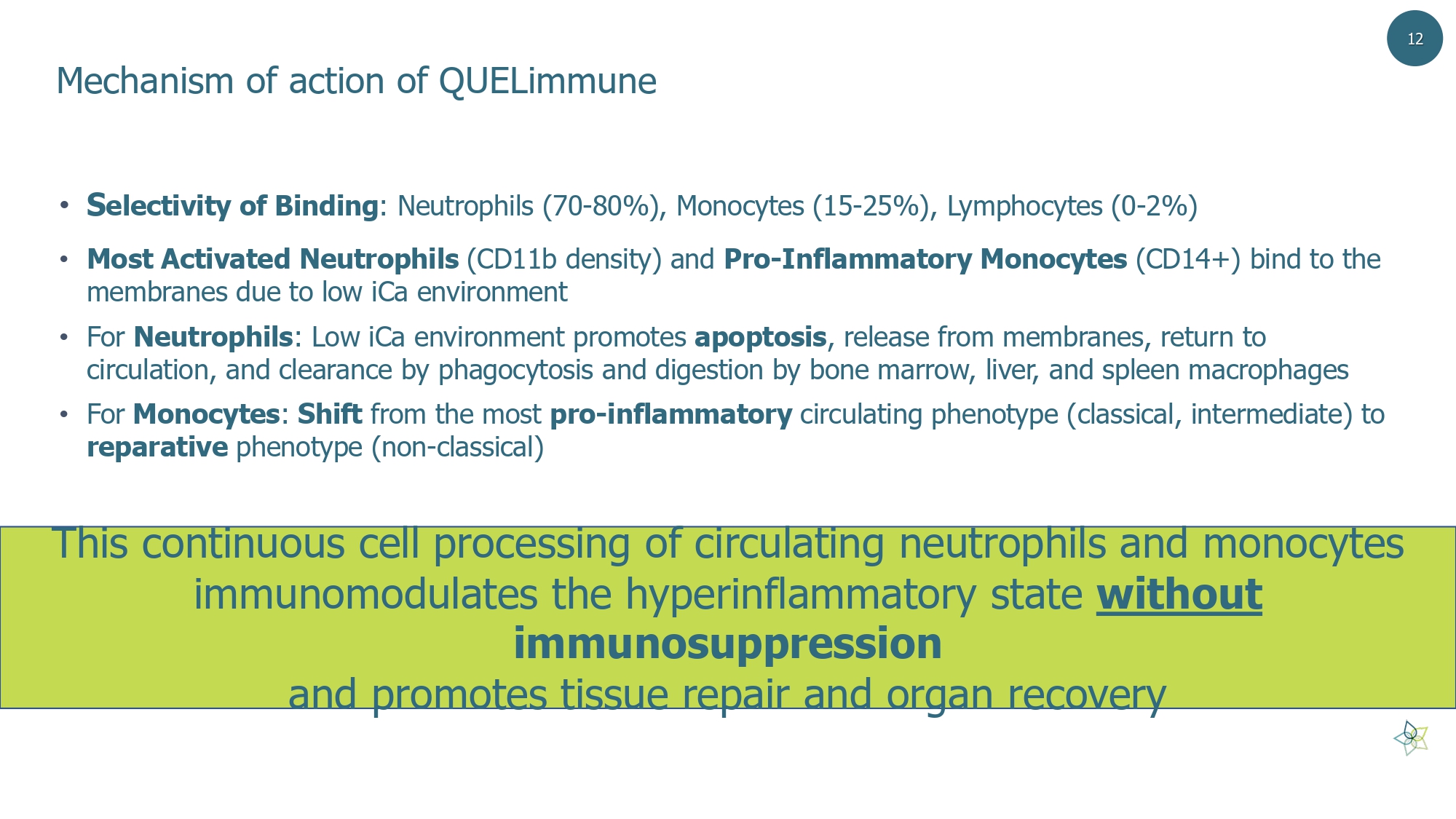
Mechanism of action of QUELimmune • S electivity of Binding : Neutrophils (70 - 80%), Monocytes (15 - 25%), Lymphocytes (0 - 2%) • Most Activated Neutrophils (CD11b density) and Pro - Inflammatory Monocytes (CD14+) bind to the membranes due to low iCa environment • For Neutrophils : Low iCa environment promotes apoptosis , release from membranes, return to circulation, and clearance by phagocytosis and digestion by bone marrow, liver, and spleen macrophages • For Monocytes : Shift from the most pro - inflammatory circulating phenotype (classical, intermediate) to reparative phenotype (non - classical) This continuous cell processing of circulating neutrophils and monocytes immunomodulates the hyperinflammatory state without immunosuppression and promotes tissue repair and organ recovery 12
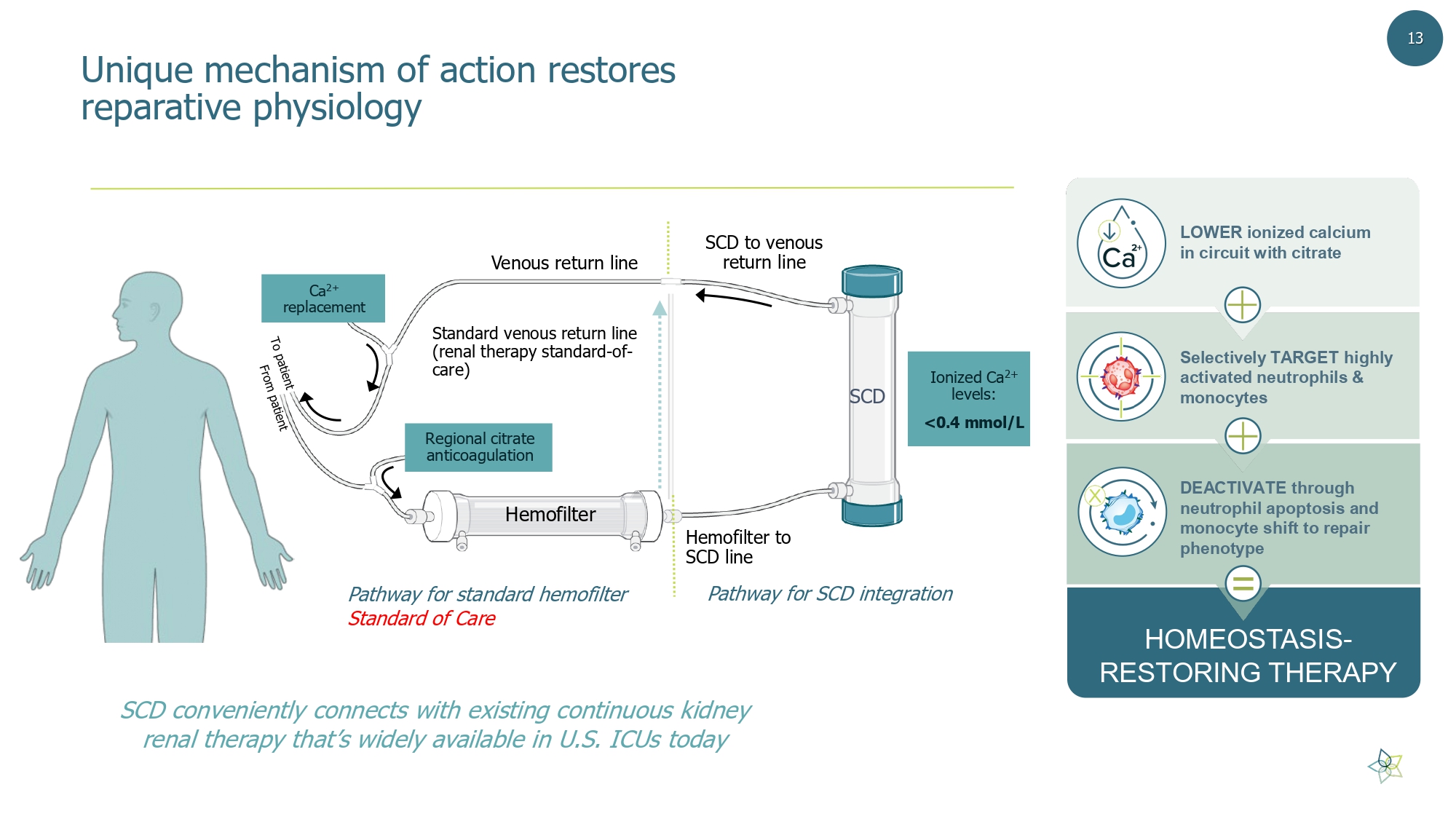
Standard venous return line (renal therapy standard - of - care) Hemofilter Hemofilter to SCD line SCD Venous return line SCD to venous return line Pathway for SCD integration SCD conveniently connects with existing continuous kidney renal therapy that’s widely available in U.S. ICUs today Pathway for standard hemofilter Standard of Care Ca 2+ replacement Regional citrate anticoagulation Ionized Ca 2+ levels: <0.4 mmol/L Unique mechanism of action restores reparative physiology HOMEOSTASIS - RESTORING THERAPY LOWER ionized calcium in circuit with citrate Selectively TARGET highly activated neutrophils & monocytes DEACTIVATE through neutrophil apoptosis and monocyte shift to repair phenotype = 13

SCD CLINICAL EVIDENCE
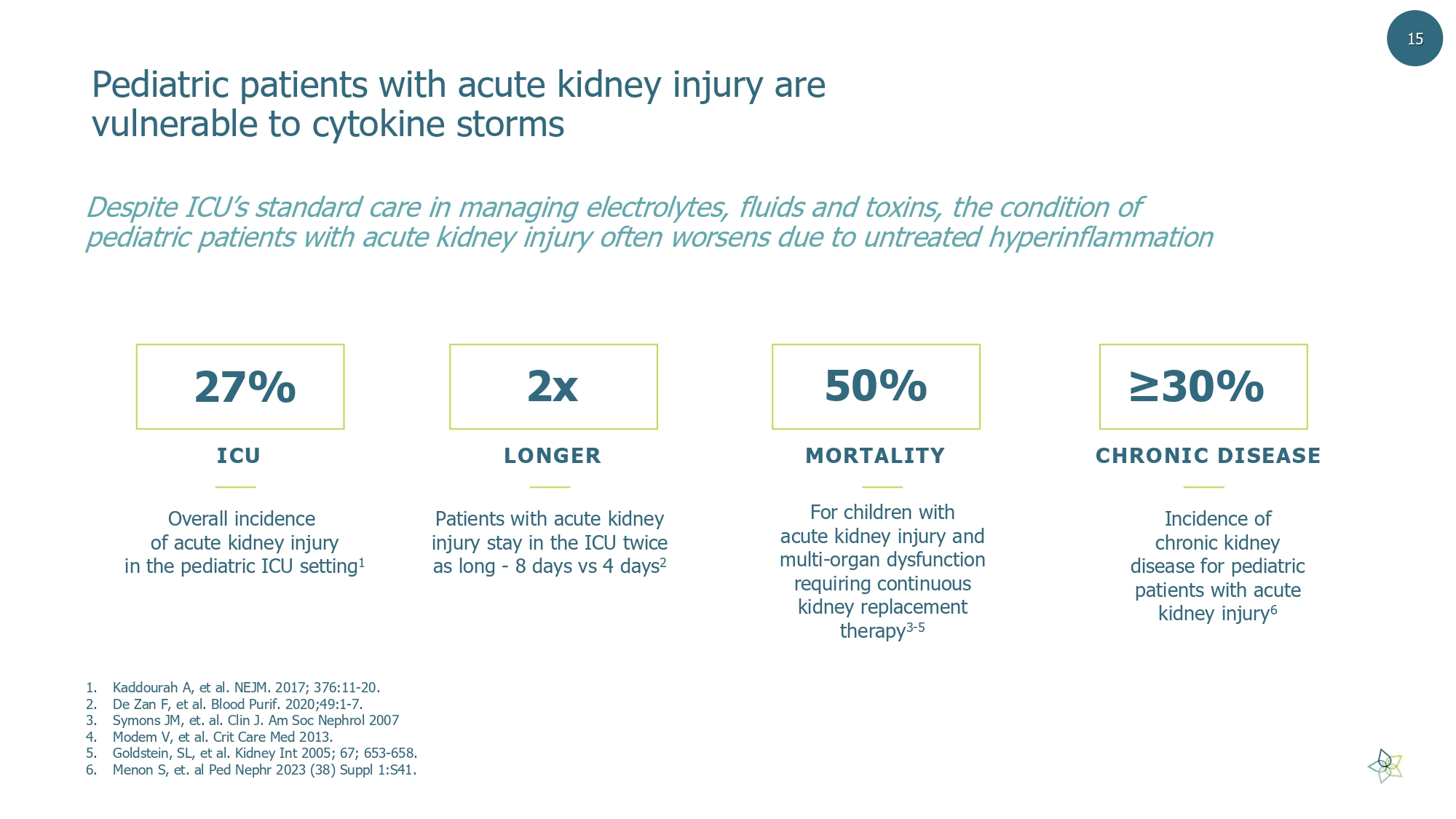
Pediatric patients with acute kidney injury are vulnerable to cytokine storms D espite ICU’s standard care in managing electrolytes, fluids and toxins, the condition of pediatric patients with acute kidney injury often worsens due to untreated hyperinflammation 27% Overall incidence of acute kidney injury in the pediatric ICU setting 1 2x Patients with acute kidney injury stay in the ICU twice as long - 8 days vs 4 days 2 50% For children with acute kidney injury and multi - organ dysfunction requiring continuous kidney replacement therapy 3 - 5 ≥30% Incidence of chronic kidney disease for pediatric patients with acute kidney injury 6 ICU LONGER MORTALITY CHRONIC DISEASE 15 1. Kaddourah A, et al. NEJM. 2017; 376:11 - 20. 2. De Zan F, et al. Blood Purif . 2020;49:1 - 7. 3. Symons JM, et. al. Clin J. Am Soc Nephrol 2007 4. Modem V, et al. Crit Care Med 2013. 5. Goldstein, SL, et al. Kidney Int 2005; 67; 653 - 658. 6. Menon S, et. al Ped Nephr 2023 (38) Suppl 1:S41.
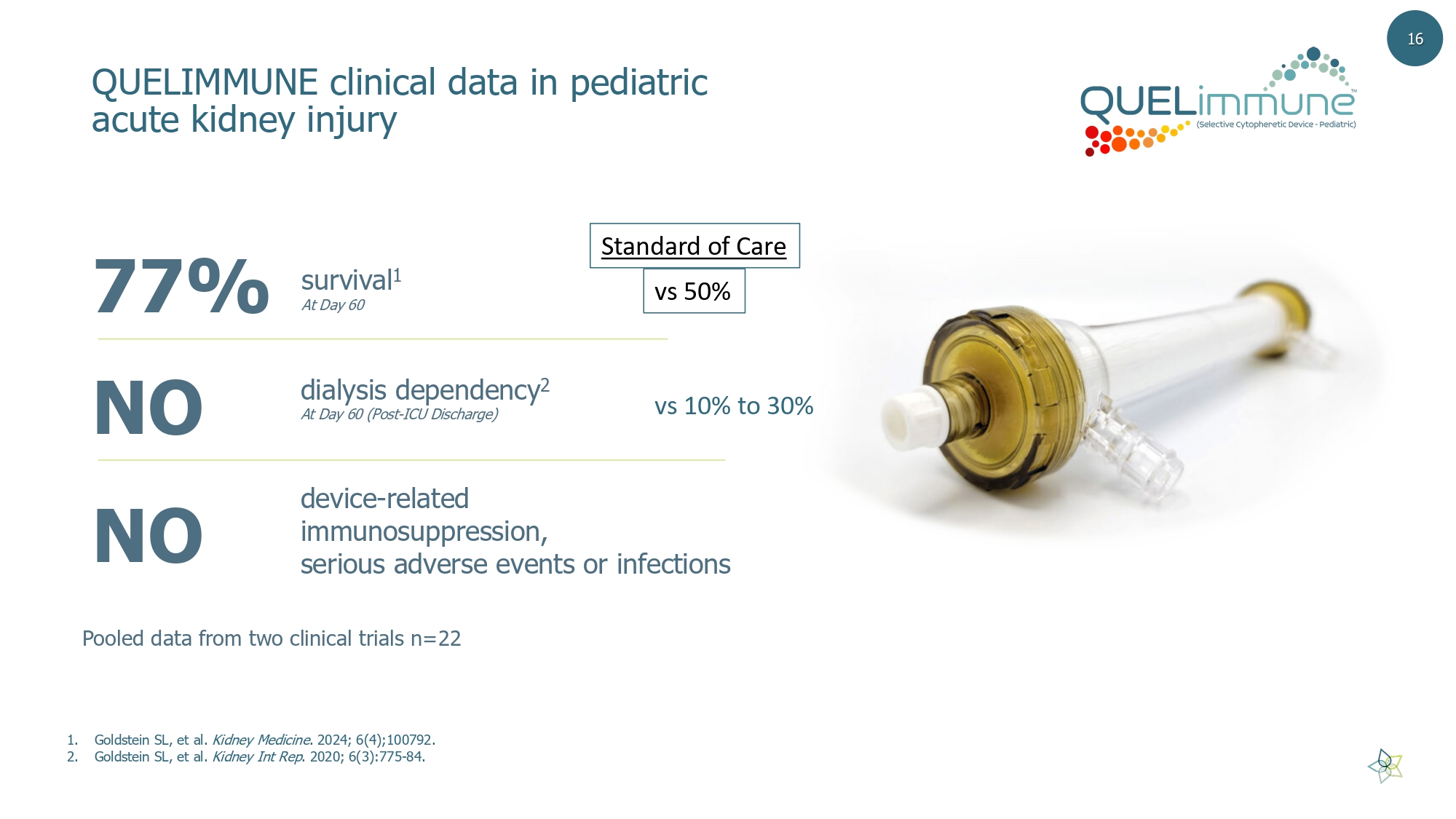
77% survival 1 At Day 60 dialysis dependency 2 At Day 60 (Post - ICU Discharge) device - related immunosuppression, serious adverse events or infections NO NO 1. Goldstein SL, et al. Kidney Medicine . 2024; 6(4);100792. 2. Goldstein SL, et al. Kidney Int Rep . 2020; 6(3):775 - 84. QUELIMMUNE clinical data in pediatric acute kidney injury Pooled data from two clinical trials n=22 16 vs 50% Standard of Care vs 10% to 30%
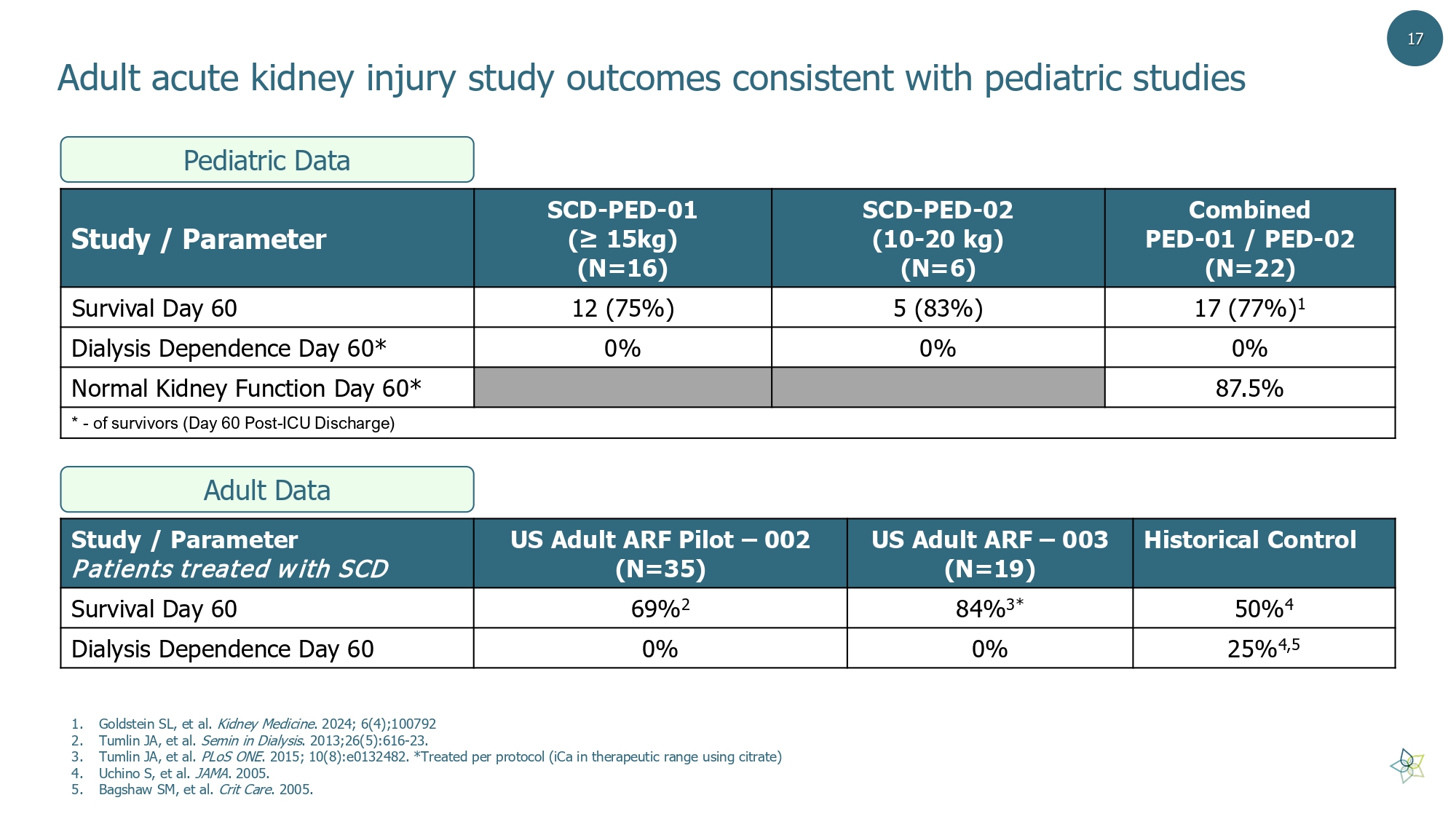
Adult acute kidney injury study outcomes consistent with pediatric studies Combined PED - 01 / PED - 02 (N=22) SCD - PED - 02 (10 - 20 kg) (N=6) SCD - PED - 01 (≥ 15kg) (N=16) Study / Parameter 17 (77%) 1 5 (83%) 12 (75%) Survival Day 60 0% 0% 0% Dialysis Dependence Day 60* 87.5% Normal Kidney Function Day 60* * - of survivors (Day 60 Post - ICU Discharge) Historical Control US Adult ARF – 003 (N=19) US Adult ARF Pilot – 002 (N=35) Study / Parameter Patients treated with SCD 50% 4 84% 3* 69% 2 Survival Day 60 25% 4,5 0% 0% Dialysis Dependence Day 60 1. Goldstein SL, et al. Kidney Medicine . 2024; 6(4);100792 2. Tumlin JA, et al. Semin in Dialysis . 2013;26(5):616 - 23. 3. Tumlin JA, et al. PLoS ONE . 2015; 10(8):e0132482. *Treated per protocol ( iCa in therapeutic range using citrate) 4. Uchino S, et al. JAMA . 2005. 5. Bagshaw SM, et al. Crit Care . 2005. Pediatric Data Adult Data 17
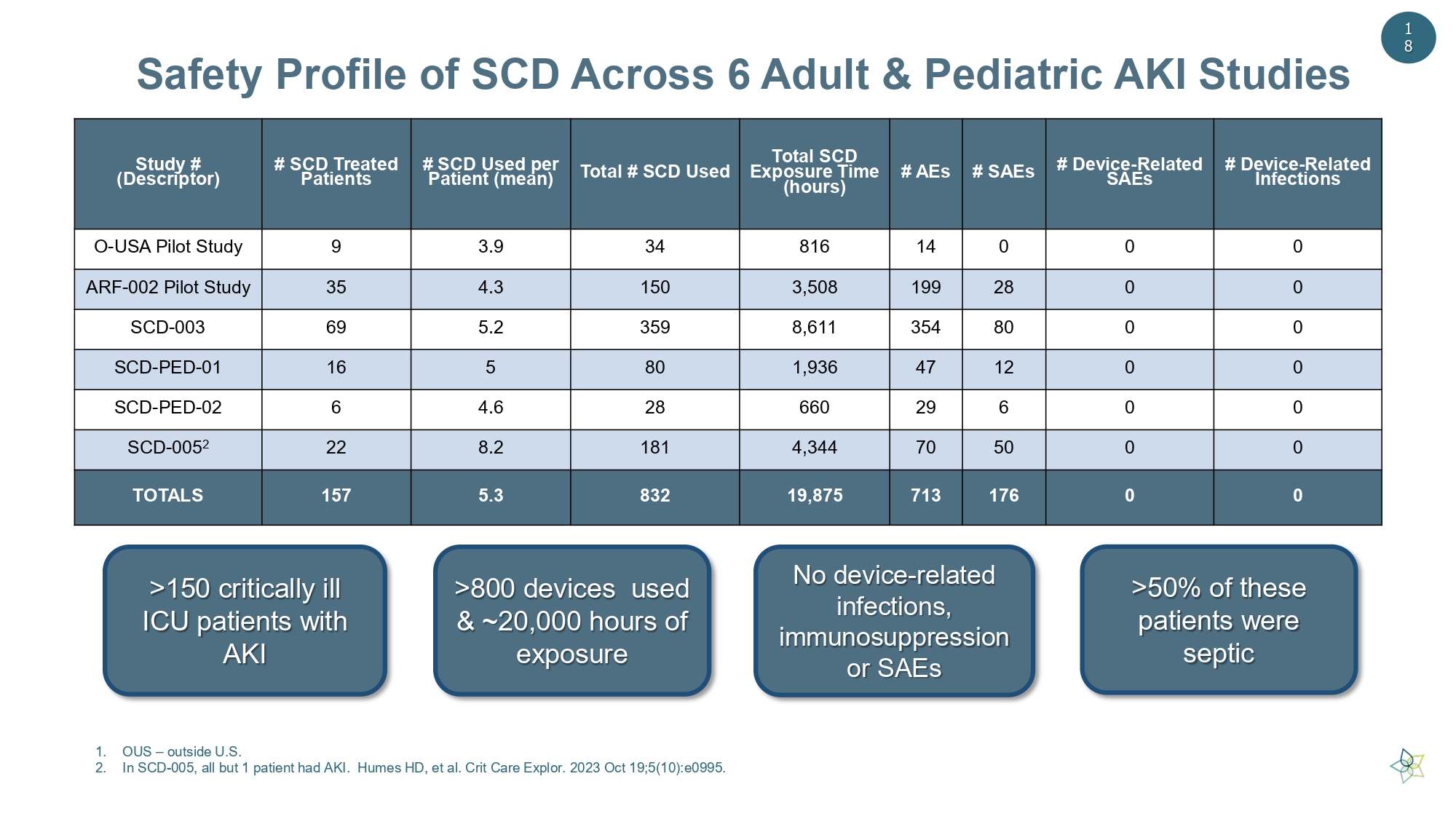
Safety Profile of SCD Across 6 Adult & Pediatric AKI Studies >150 critically ill ICU patients with AKI >800 devices used & ~20,000 hours of exposure No device - related infections, immunosuppression or SAEs >50% of these patients were septic # Device - Related Infections # Device - Related SAEs # SAEs # AEs Total SCD Exposure Time (hours) Total # SCD Used # SCD Used per Patient (mean) # SCD Treated Patients Study # (Descriptor) 0 0 0 14 816 34 3.9 9 O - USA Pilot Study 0 0 28 199 3,508 150 4.3 35 ARF - 002 Pilot Study 0 0 80 354 8,611 359 5.2 69 SCD - 003 0 0 12 47 1,936 80 5 16 SCD - PED - 01 0 0 6 29 660 28 4.6 6 SCD - PED - 02 0 0 50 70 4,344 181 8.2 22 SCD - 005 2 0 0 176 713 19,875 832 5.3 157 TOTALS 1 8 1. OUS – outside U.S. 2. In SCD - 005, all but 1 patient had AKI. Humes HD, et al. Crit Care Explor . 2023 Oct 19;5(10):e0995.

COMMERCIAL & REGULATORY STRATEGIES PEDIATRICS
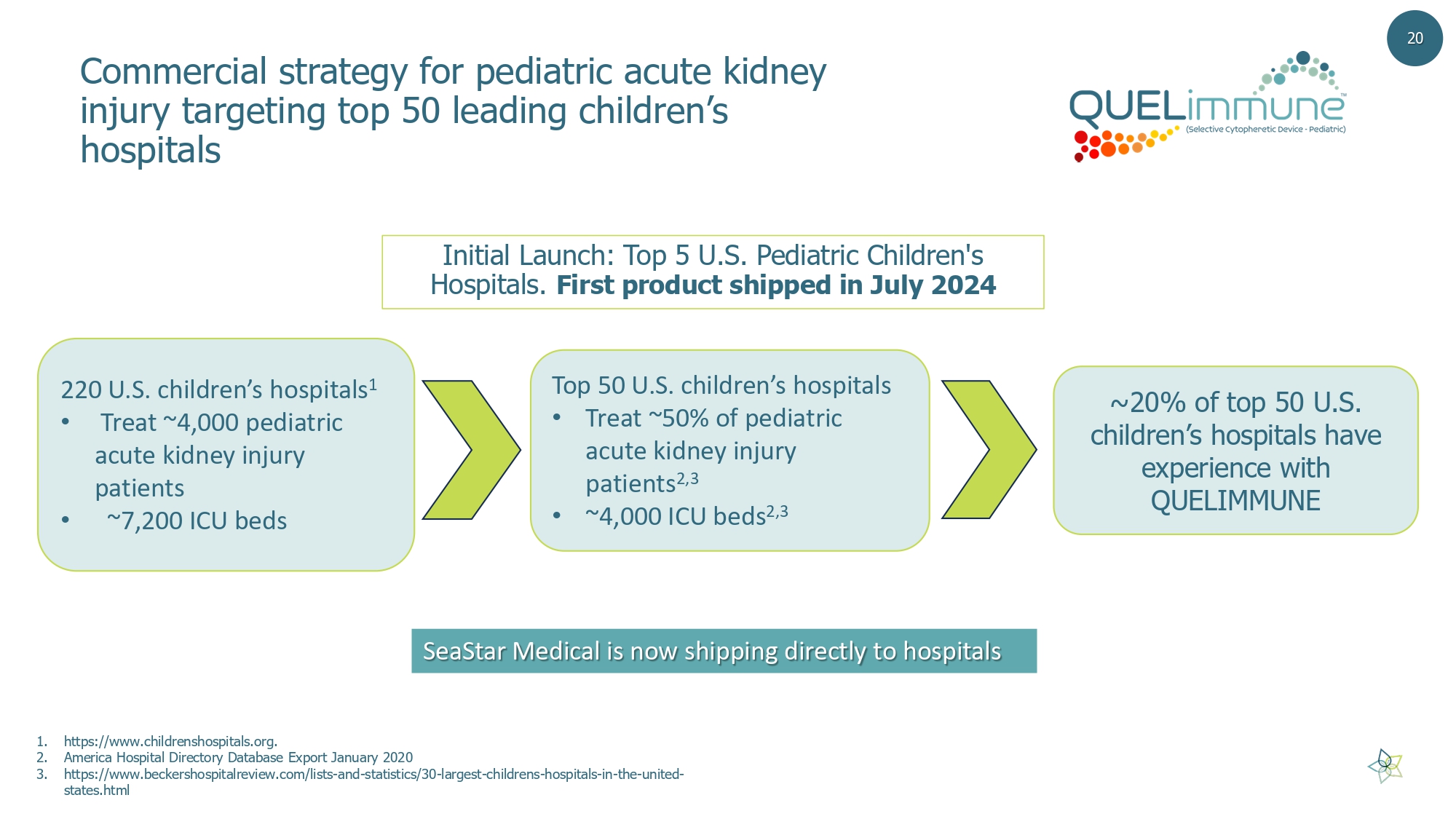
Commercial strategy for pediatric acute kidney injury targeting top 50 leading children’s hospitals 1. https://www.childrenshospitals.org. 2. America Hospital Directory Database Export January 2020 3. https://www.beckershospitalreview.com/lists - and - statistics/30 - largest - childrens - hospitals - in - the - united - states.html 20 Initial Launch: Top 5 U.S. Pediatric Children's Hospitals. First product shipped in July 2024 220 U.S. children’s hospitals 1 • Treat ~4,000 pediatric acute kidney injury patients • ~7,200 ICU beds Top 50 U.S. children’s hospitals • Treat ~50% of pediatric acute kidney injury patients 2,3 • ~4,000 ICU beds 2,3 ~20% of top 50 U.S. children’s hospitals have experience with QUELIMMUNE SeaStar Medical is now shipping directly to hospitals
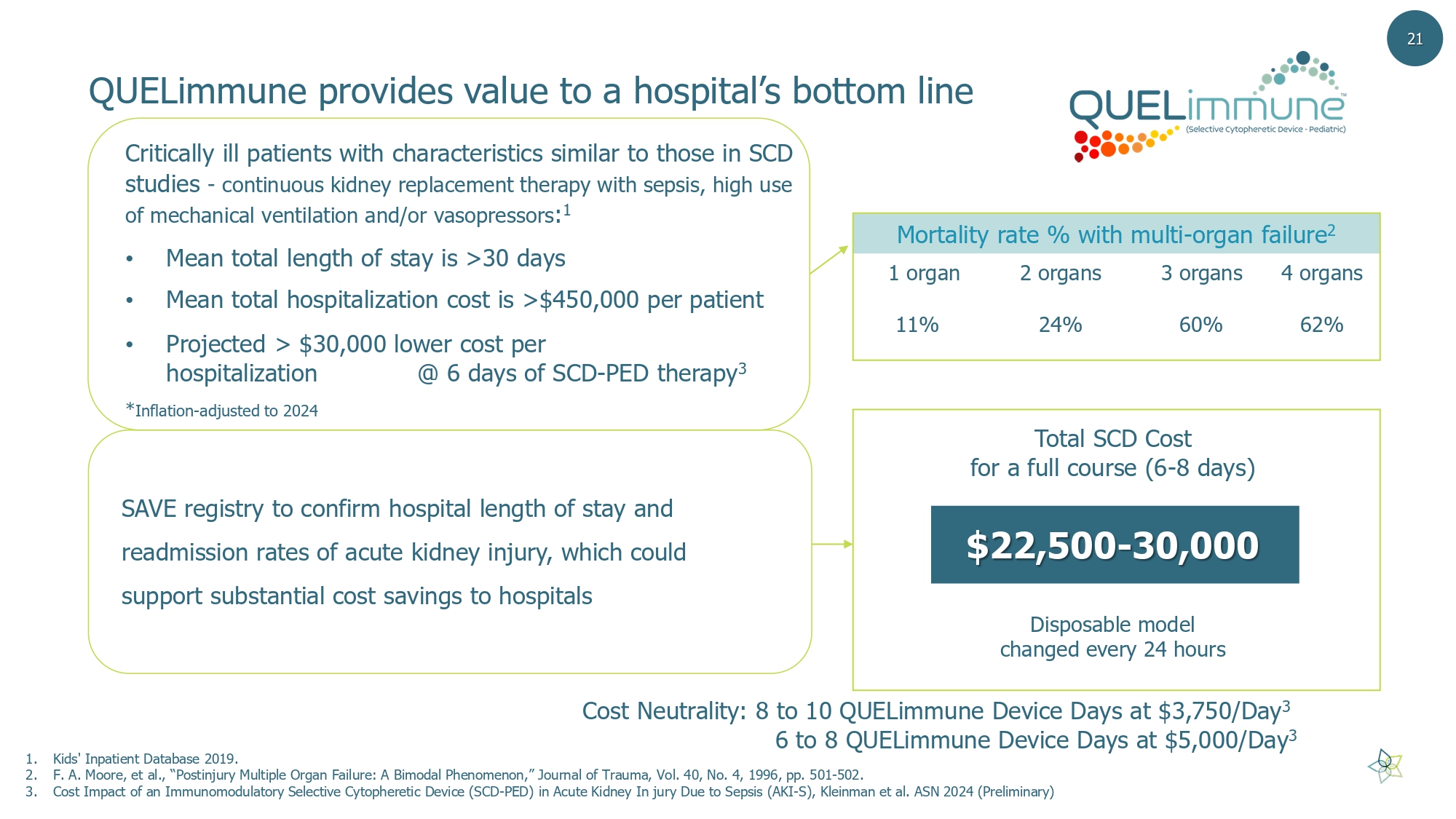
QUELimmune provides value to a hospital’s bottom line Critically ill patients with characteristics similar to those in SCD studies - continuous kidney replacement therapy with sepsis, high use of mechanical ventilation and/or vasopressors : 1 • Mean total length of stay is >30 days • Mean total hospitalization cost is >$450,000 per patient • Projected > $30,000 lower cost per hospitalization @ 6 days of SCD - PED therapy 3 * Inflation - adjusted to 2024 1. Kids' Inpatient Database 2019. 2. F. A. Moore, et al., “Postinjury Multiple Organ Failure: A Bimodal Phenomenon,” Journal of Trauma, Vol. 40, No. 4, 1996, pp. 501 - 502. 3. Cost Impact of an Immunomodulatory Selective Cytopheretic Device (SCD - PED) in Acute Kidney In jury Due to Sepsis (AKI - S), Kleinm an et al. ASN 2024 (Preliminary) Mortality rate % with multi - organ failure 2 4 organs 3 organs 2 organs 1 organ 62% 60% 24% 11% 21 $22,500 - 30,000 Disposable model changed every 24 hours Total SCD C ost for a full course (6 - 8 days ) SAVE registry to confirm hospital length of stay and readmission rates of acute kidney injury, which could support substantial cost savings to hospitals Cost Neutrality: 8 to 10 QUELimmune Device Days at $3,750/Day 3 6 to 8 QUELimmune Device Days at $5,000/Day 3

CLINICAL STUDY & REGULATORY STRATEGIES ADULT
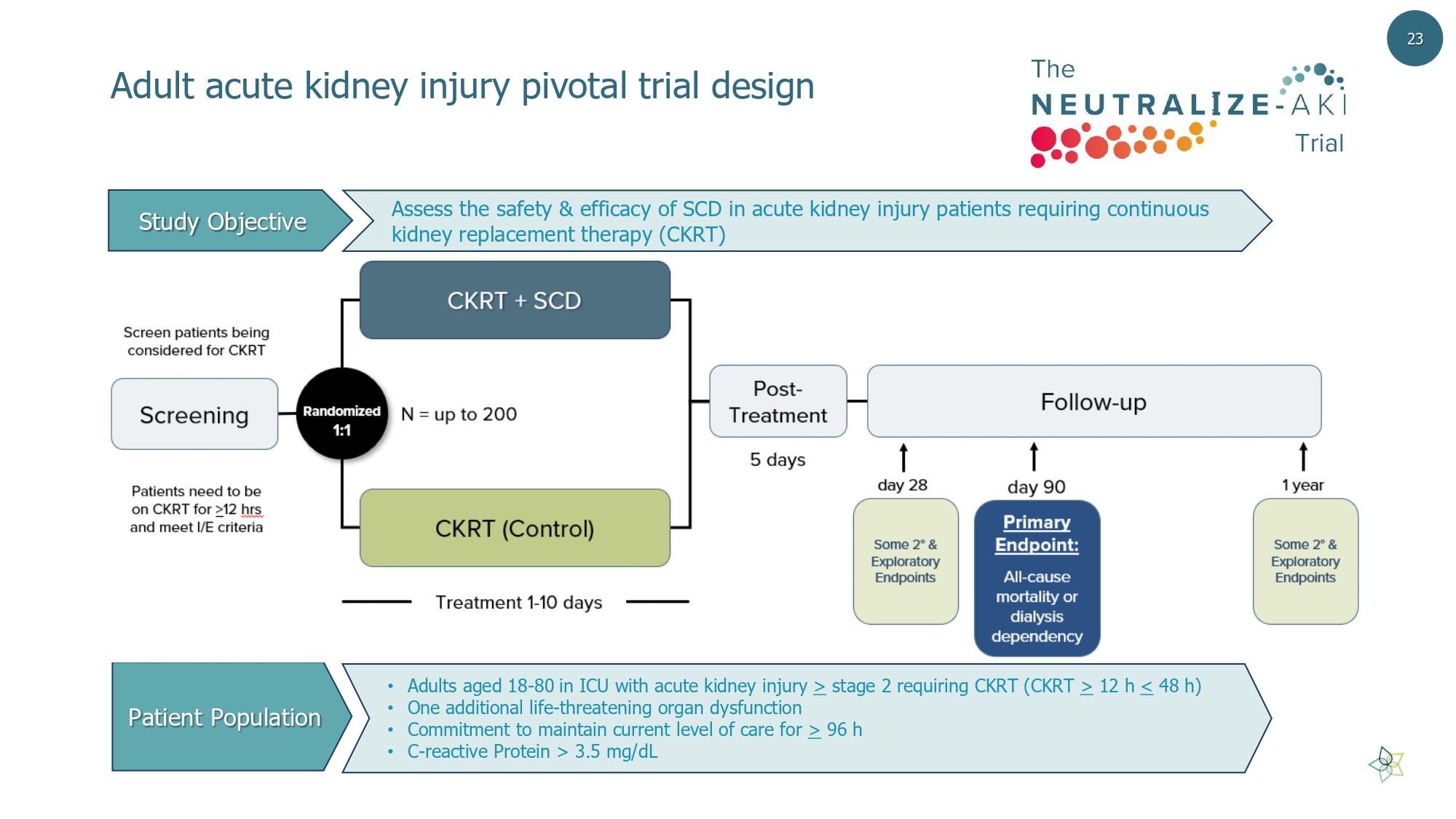
Adult acute kidney injury pivotal trial design • Adults aged 18 - 80 in ICU with acute kidney injury > stage 2 requiring CKRT (CKRT > 12 h < 48 h) • One additional life - threatening organ dysfunction • Commitment to maintain current level of care for > 96 h • C - reactive Protein > 3.5 mg/dL Patient Population Assess the safety & efficacy of SCD in acute kidney injury patients requiring continuous kidney replacement therapy (CKRT) Study Objective 23
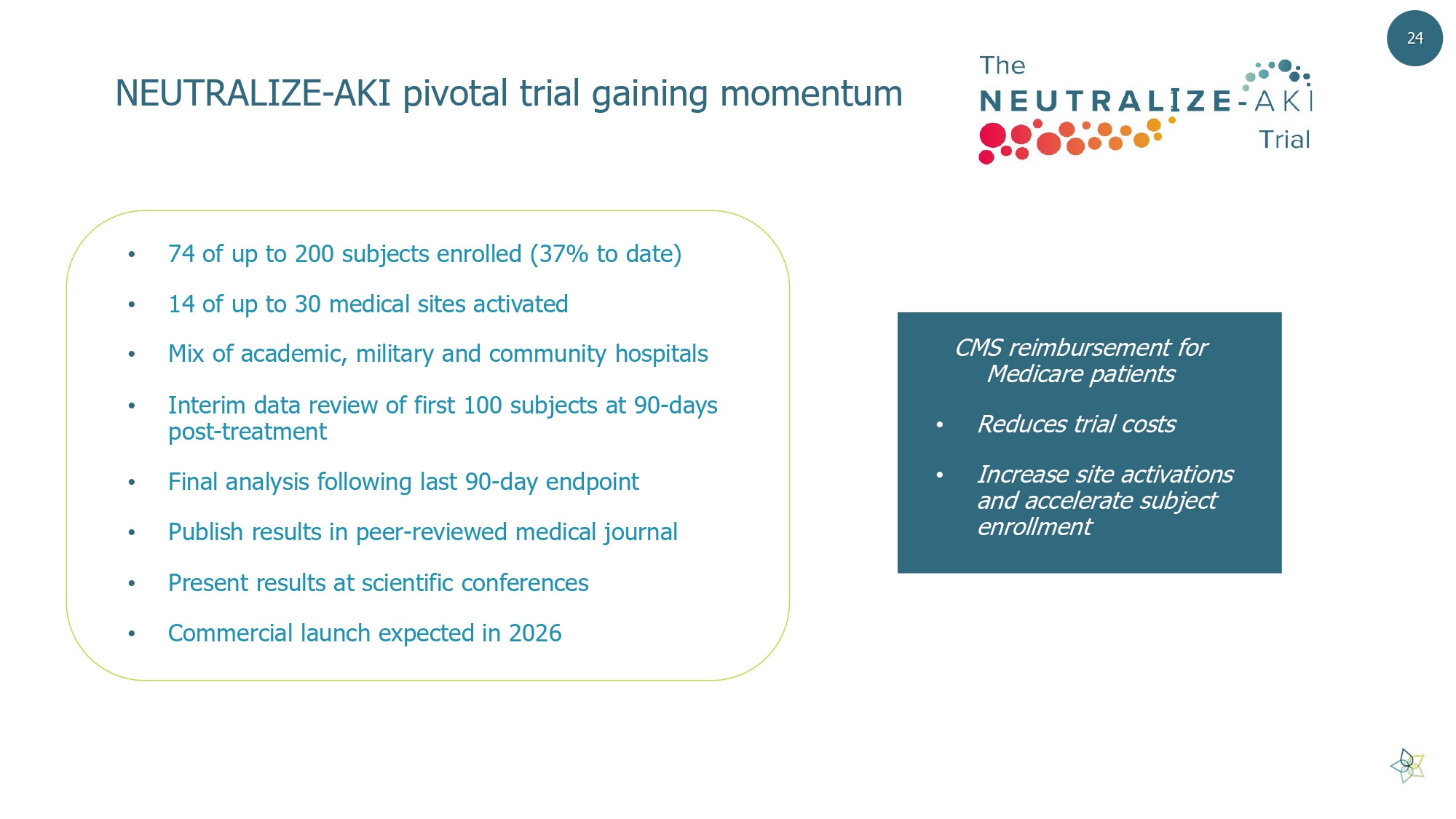
NEUTRALIZE - AKI pivotal trial gaining momentum • 74 of up to 200 subjects enrolled (37% to date) • 14 of up to 30 medical sites activated • Mix of academic, military and community hospitals • Interim data review of first 100 subjects at 90 - days post - treatment • Final analysis following last 90 - day endpoint • Publish results in peer - reviewed medical journal • Present results at scientific conferences • Commercial launch expected in 2026 24 CMS reimbursement for Medicare patients • Reduces trial costs • Increase site activations and accelerate subject enrollment

EXPANDING SCIENCE & FUTURE INDICATIONS
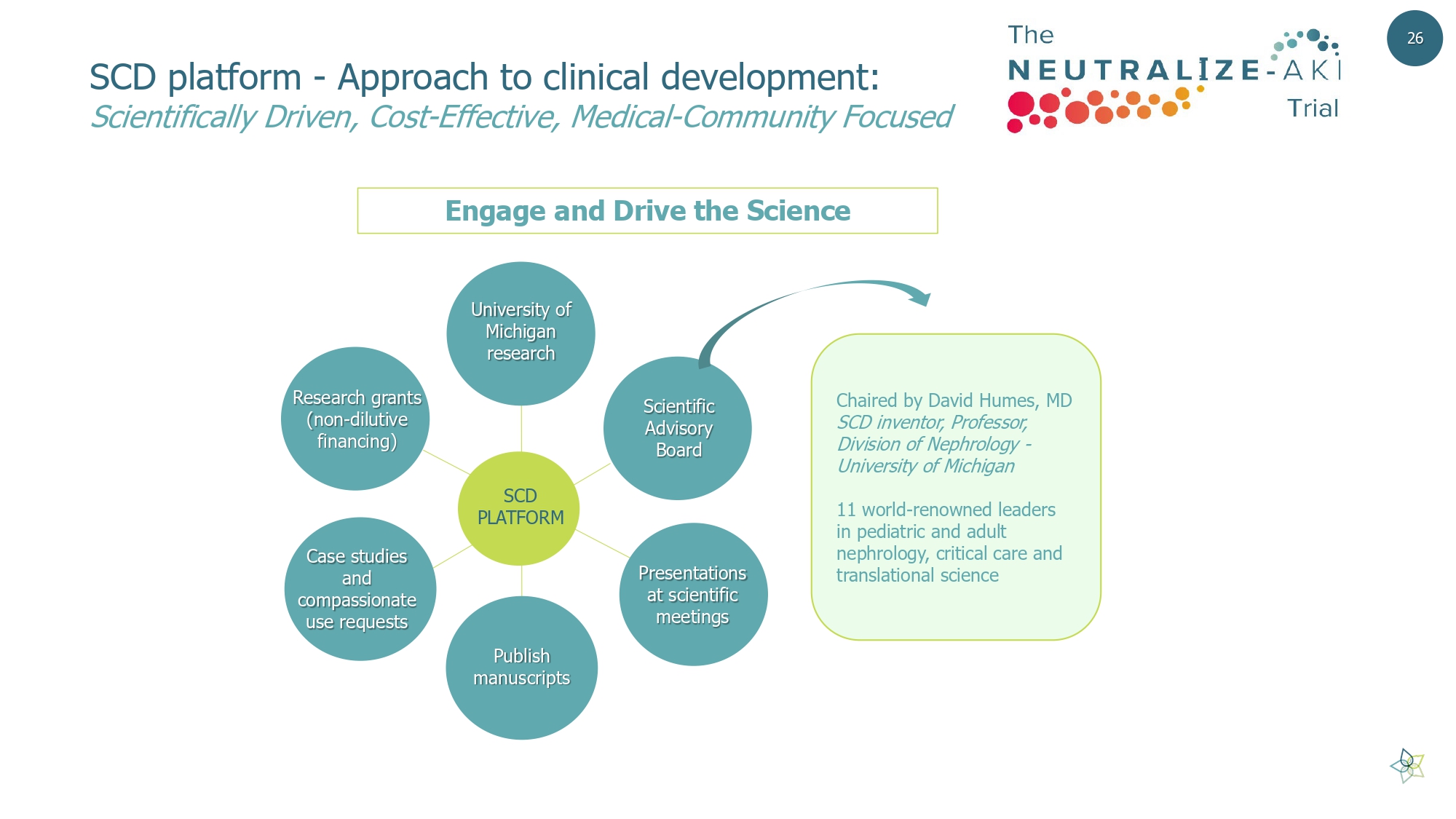
SCD platform - Approach to clinical development: Scientifically Driven, Cost - Effective, Medical - Community Focused 26 Engage and Drive the Science SCD PLATFORM Presentations at scientific meetings Publish manuscripts Research grants (non - dilutive financing) University of Michigan research Scientific Advisory Board Case studies and compassionate use requests Chaired by David Humes, MD SCD inventor, Professor, Division of Nephrology - University of Michigan 11 world - renowned leaders in pediatric and adult nephrology, critical care and translational science

Strategy is to expand indications with platform technology Evaluate inflammation - based conditions or diseases driven by activated neutrophils / monocytes High Burden of disease and unmet medical need in an operable patient setting Clear commercial opportunity based on population size and or lack of approved therapies Clear clinical value proposition that delivers clinical outcomes to the patient and economic value to the customer Clear reimbursement pathway that provides pricing power and flexibility Indication Evaluation Process Results – Where we are Investing Resources Adult Acute Kidney Injury – Awarded Breakthrough Status Cardiorenal Syndrome w/ LVAD – Awarded Breakthrough Status Hepatorenal Syndrome – Awarded Breakthrough Status 27 Chronic Dialysis – Awarded Breakthrough Status
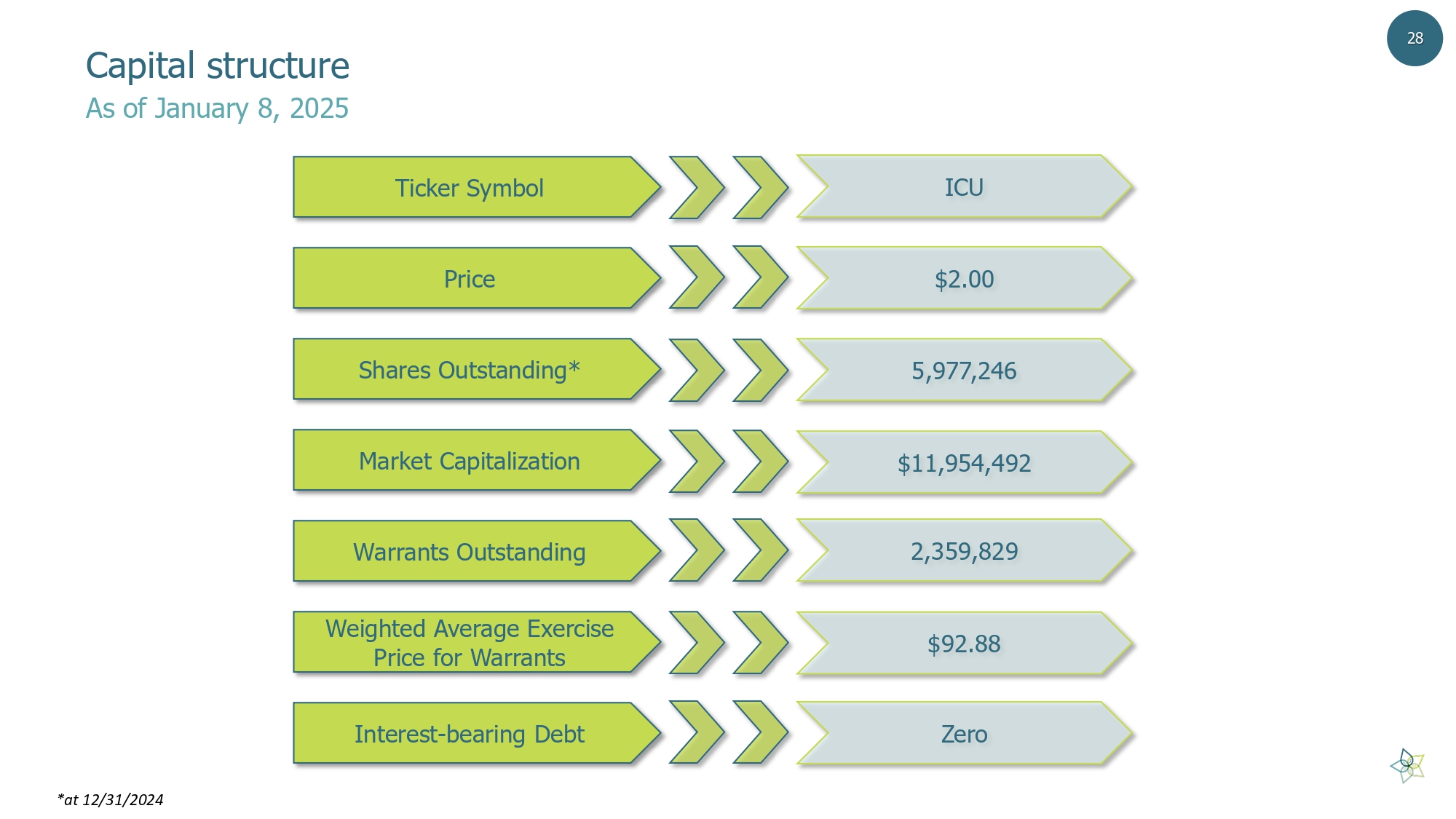
28 Capital structure As of January 8, 2025 Ticker Symbol Price Shares Outstanding* Market Capitalization Warrants Outstanding Weighted Average Exercise Price for Warrants Interest - bearing Debt ICU $2.00 5,977,246 $11,954,492 2,359,829 $92.88 Zero *at 12/31/2024
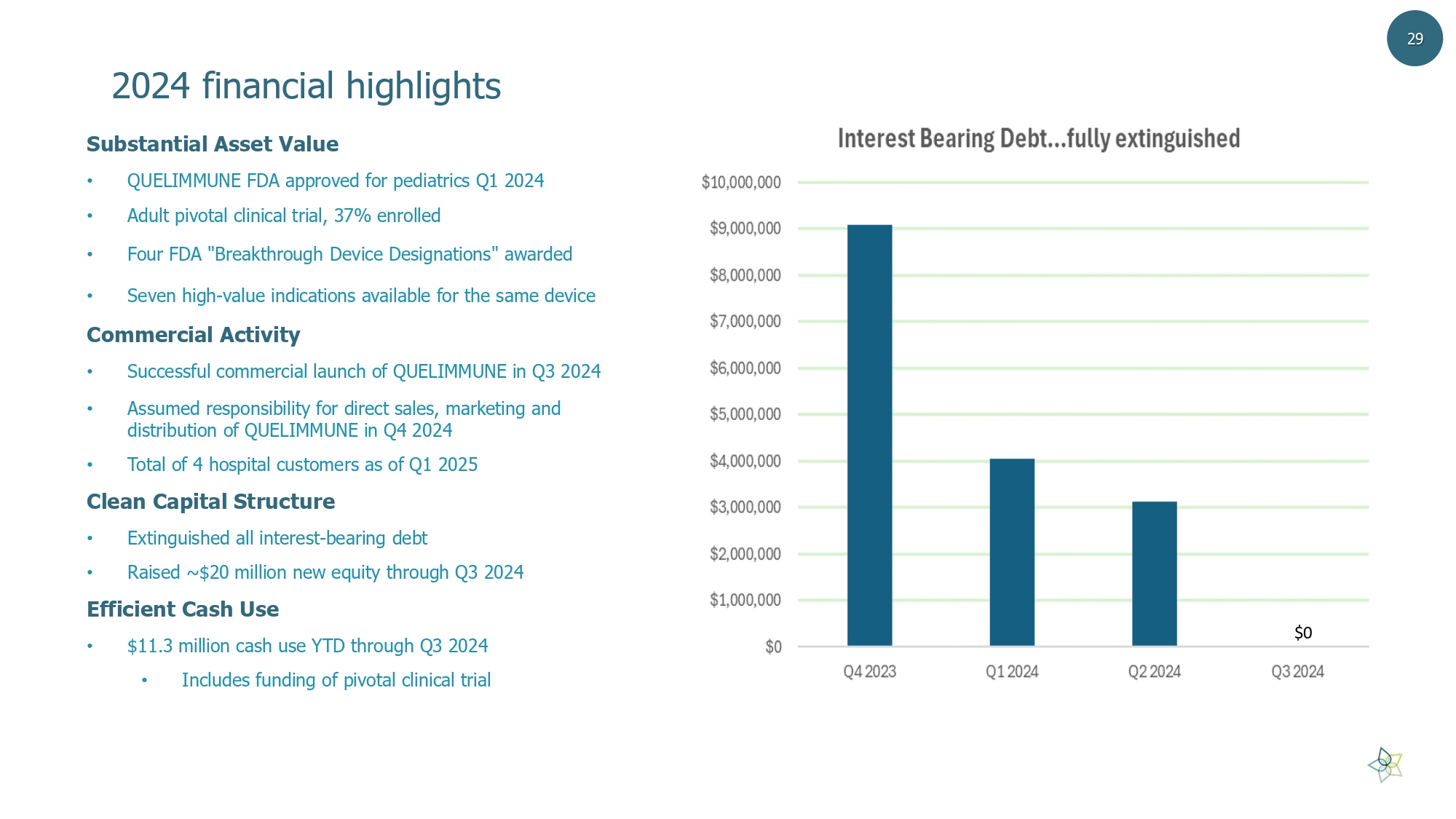
2024 financial highlights 29 $0 Substantial Asset Value • QUELIMMUNE FDA approved for pediatrics Q1 2024 • Adult pivotal clinical trial, 37% enrolled • Four FDA "Breakthrough Device Designations" awarded • Seven high - value indications available for the same device Commercial Activity • Successful commercial launch of QUELIMMUNE in Q3 2024 • Assumed responsibility for direct sales, marketing and distribution of QUELIMMUNE in Q4 2024 • Total of 4 hospital customers as of Q1 2025 Clean Capital Structure • Extinguished all interest - bearing debt • Raised ~$20 million new equity through Q3 2024 Efficient Cash Use • $ 11.3 million cash use YTD through Q3 2024 • Includes funding of pivotal clinical trial
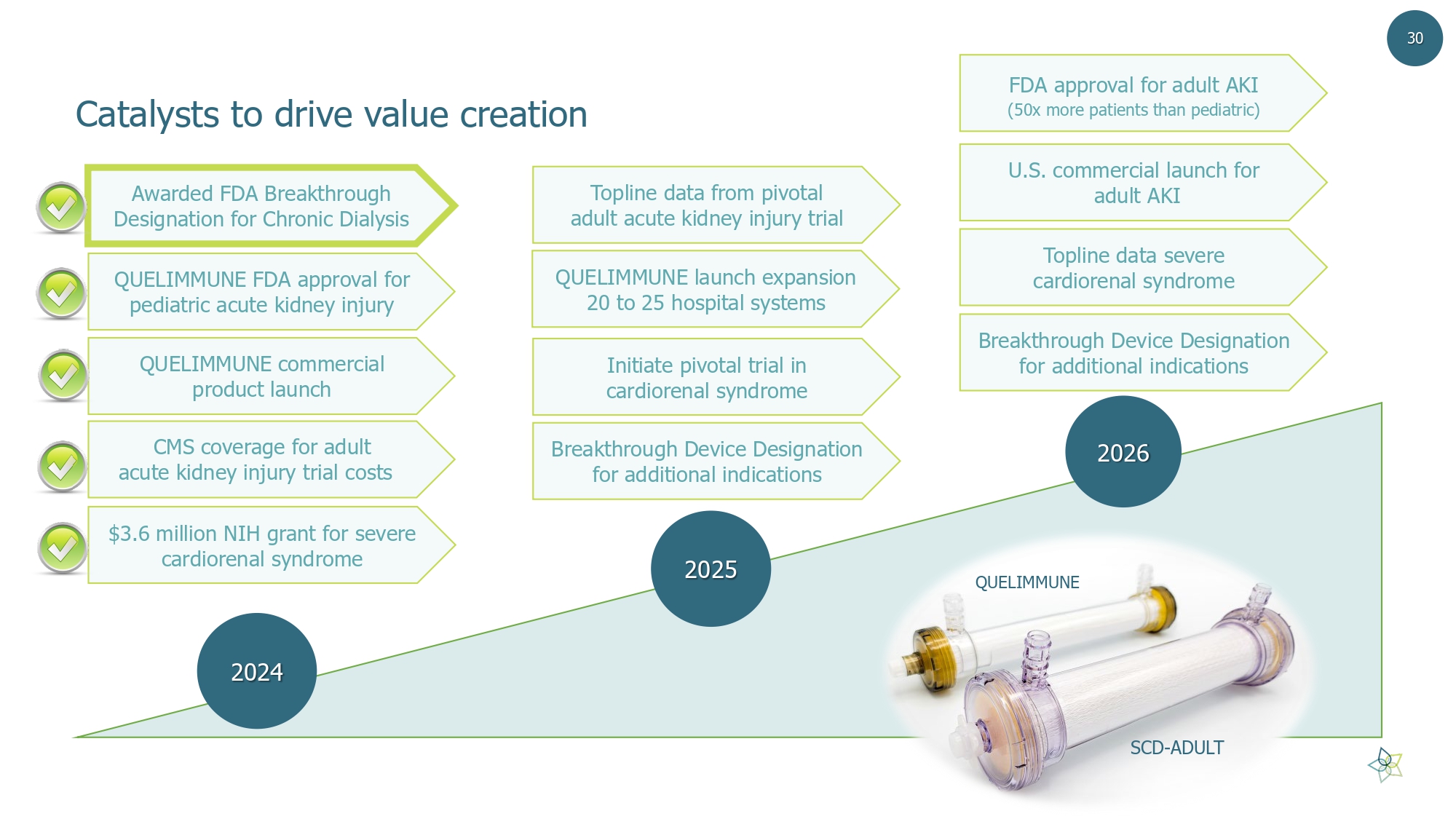
2024 2025 2026 QUELIMMUNE commercial product launch Breakthrough Device Designation for additional indications Initiate pivotal trial in cardiorenal syndrome QUELIMMUNE launch expansion 20 to 25 hospital systems $3.6 million NIH grant for severe cardiorenal syndrome Topline data severe cardiorenal syndrome U.S. commercial launch for adult AKI FDA approval for adult AKI (50x more patients than pediatric) Catalysts to drive value creation QUELIMMUNE FDA approval for pediatric acute kidney injury SCD - ADULT QUELIMMUNE CMS coverage for adult acute kidney injury trial costs 30 Topline data from pivotal adult acute kidney injury trial Breakthrough Device Designation for additional indications Awarded FDA Breakthrough Designation for Chronic Dialysis
Investment highlights • Patented, proprietary SCD platform addresses life - threatening unmet medical needs • Clinically proven to reduce mortality and decrease dialysis dependency in acute kidney injury • Potential to dramatically reduce economic burden of disease • Proven delivery system • Shelf - life stability at room temperature • 1 st FDA approval for pediatric acute kidney injury with sepsis • First commercial sale in Q3 2024 • QUELIMMUNE commercial strategy to target leading children’s hospitals • Approval validates platform and derisks future FDA approvals • Enrolling patients in pivotal adult trial, NEUTRALIZE - AKI • Adult AKI population 50x larger than pediatric • CMS coverage for a portion of trial costs • Platform technology potential application in multiple high - value acute and chronic indications • Technology requires minimal, if any, modifications for new indications • Same SCD, same Mechanism of Action, with access to a multitude of indications • Seasoned, dynamic leadership with over 150 years of industry experience* and clear focus on advancing strategy • Wealth of executive, operational, financial, clinical and regulatory expertise BEST - IN - CLASS TECHNOLOGY COMMERCIALIZING FIRST INDICATION PIVOTAL TRIAL PROGRESS MULTIBILLION - DOLLAR MARKET 31 EXPERIENCED EXECUTIVE TEAM * Based on the 6 members of the leadership team I nvestor Contact: Alliance Advisors IR Jody Cain Jcain@allianceadvisors.com 310 - 691 - 7107






























