
Investor Presentation SeaStar Medical, Inc. LMF Acquisition Opportunities, Inc. 1 May 3, 2022

Important Disclosures SeaStar Medical 2 This investor presentation (the “presentation”) is for information proposes only to assist interested parties in making their own evaluation with respect to the business combination (the “Transaction”) between LMF Acquisition Opportunities, Inc. (“LMAO”) and SeaStar Medical, Inc. (“SeaStar”). The information contained herein does not purport to be all-inclusive and none of LMAO, SeaStar or their respective directors, officers, stockholders, affiliates or advisers or any other person makes any representation or warranty, express or implied, as to the accuracy, completeness or reliability of the information contained in this presentation or any other written or oral communication to the recipient in the course of the recipient’s evaluation of LMAO or SeaStar. The information contained herein is preliminary and is subject to change and such changes may be material. The information in this presentation assumes that the Transaction is consummated on the terms contemplated by the Agreement and Plan of Merger (“Merger Agreement”) entered into by LMAO and SeaStar. You should not construe the contents of this presentation as legal, tax, accounting or investment advice or a recommendation. You should consult your own counsel and tax and financial advisors as to legal and related matters concerning the matters described, and by accepting this presentation, you confirm that you are not relying upon the information contained herein to make any decision. No securities commission or securities regulatory authority in the United States or any other jurisdiction has in any way passed upon the merits of the Transaction or the accuracy or adequacy of this presentation.

Important Disclosures (cont.) SeaStar Medical 3 Forward Looking Statements This presentation contains certain forward-looking statements within the meaning of the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1955. These forward-looking statements include, without limitation, LMAO’s and SeaStar’s expectations with respect to the Transaction, including statements regarding the benefits of the Transaction, the anticipated timing of the Transaction, the implied valuation of SeaStar, the products offered by SeaStar and the markets in which it operates, and SeaStar’s projected future results. Words such as “believe,” “project,” “expect,” “anticipate,” “estimate,” “intend,” “strategy,” “future,” “opportunity,” “plan,” “may,” “should,” “will,” “would,” “will be,” “will continue,” “will likely result,” and similar expressions are intended to identify such forward-looking statements. Forward-looking statements are predictions, projections and other statements about future events that are based on current expectations and assumptions and, as a result, are subject to significant risks and uncertainties that could cause the actual results to differ materially from the expected results. Most of these factors are outside LMAO’s and SeaStar’s control and are difficult to predict. Factors that may cause actual future events to differ materially from the expected results, include, but are not limited to: (i) the risk that the Transaction may not be completed in a timely manner or at all, which may adversely affect the price of LMAO’s securities, (ii) the risk that the Transaction may not be completed by LMAO’s business combination deadline, even if extended by its sponsor, (iii) the failure to satisfy the conditions to the consummation of the Transaction, including the adoption of the Merger Agreement by the stockholders of LMAO and the satisfaction of the minimum trust account amount following redemptions by LMAO’s public stockholders, (iv) the occurrence of any event, change or other circumstance that could give rise to the termination of the Merger Agreement, (v) the receipt of an unsolicited offer from another party for an alternative transaction that could interfere with the Transaction, (vi) the effect of the announcement or pendency of the Transaction on SeaStar’s business relationships, performance, and business generally, (vii) the inability to recognize the anticipated benefits of the Transaction, which may be affected by, among other things, competition and the ability of the post-combination company to grow and manage growth profitability and retain its key employees, (viii) costs related to the Transaction, (ix) the outcome of any legal proceedings that may be instituted against SeaStar or LMAO following the announcement of the Transaction, (x) the ability to maintain the listing of LMAO’s securities on Nasdaq, (xi) the ability to implement business plans, forecasts, and other expectations after the completion of the Transaction, and identify and realize additional opportunities, (xii) the risk of downturns and the possibility of rapid change in the highly competitive industry in which SeaStar operates, (xiii) the risk that SeaStar and its current and future collaborators are unable to successfully develop and commercialize SeaStar’s products or services, or experience significant delays in doing so, including failure to achieve approval of its products by applicable federal and state regulators, (xiv) the risk that SeaStar may never achieve or sustain profitability; (xv) the risk that SeaStar may need to raise additional capital to execute its business plan, which many not be available on acceptable terms or at all; (xvi) the risk that third-parties suppliers and manufacturers are not able to fully and timely meet their obligations, (xvii) the risk of product liability or regulatory lawsuits or proceedings relating to SeaStar’s products and services, (xviii) the risk that SeaStar is unable to secure or protect its intellectual property, (xix) the risk that the post-combination company’s securities will not be approved for listing on Nasdaq or if approved, maintain the listing and (xx) other risks and uncertainties indicated from time to time in the proxy statement / prospectus to be filed relating to the Transaction, including those under the “Risk Factors” section therein and in LMAO’s other filings with the SEC. The foregoing list of factors is not exhaustive. Forward-looking statements speak only as of the date they are made. Readers are cautioned not to put undue reliance on forward-looking statements, and SeaStar and LMAO assume no obligation and do not intend to update or revise these forward-looking statements, whether as a result of new information, future events, or otherwise.

Important Disclosures (cont.) SeaStar Medical 4 Industry and Market Data In this presentation, we rely on and refer to information and statistics regarding market participants in the sectors in which SeaStar expects to compete and other industry data. We obtained this information and these statistics from a variety of publicly available sources, including reports by market research firms and other public company filings. No representation is made as to the reasonableness of the assumptions made within or the accuracy or completeness of any projections or modeling or any other information contained herein. Any data on past performance or modeling contained herein is not an indication as to future performance. Trademarks This presentation may contain trademarks, service marks, trade names and copyrights of other companies, which are the property of their respective owners. Solely for convenience, some of the trademarks, service marks, tradenames and copyrights referred to in this presentation may be listed without the TM, SM, © or ® symbols, but LMAO and SeaStar will assert, to the fullest extent under applicable law, the rights of the applicable owners, if any, to these trademarks, service marks, trade names and copyrights. Important Information and Where to Find It In connection with the Transaction, LMAO intends to file a registration statement on Form S-4 (the “Registration Statement”) with the SEC, which will include a preliminary proxy statement to be distributed to holders of LMAO’s common stock in connection with LMAO’s solicitation of proxies for the vote by LMAO’s stockholders with respect to the Transaction and other matters as described in the Registration Statement, as well as the prospectus relating to the offer of the securities to be issued to SeaStar’s stockholders in connection with the Transaction. After the Registration Statement has been filed and declared effective, LMAO will mail a definitive proxy statement, when available, to its stockholders. Investors and security holders and other interested parties are urged to read the proxy statement/prospectus, any amendments thereto and any other documents filed with the SEC carefully and in their entirety when they become available because they will contain important information about LMAO, SeaStar and the Transaction. Investors and security holders may obtain free copies of the preliminary proxy statement/prospectus and definitive proxy statement/prospectus (when available) and other documents filed with the U.S. Securities and Exchange Commission (the “SEC”) by LMAO through the website maintained by the SEC at http://www.sec.gov, or by directing a request to: LMF Acquisition Opportunities, Inc., 1200 Platt Street, Suite 1000 Tampa, FL 33602. Participants in Solicitation LMAO and SeaStar and their respective directors and certain of their respective executive officers and other members of management and employees may be considered participants in the solicitation of proxies with respect to the Transaction. Information about the directors and executive officers of LMAO is set forth in its Annual Report on Form 10-K for the fiscal year ended December 31, 2021. Additional information regarding the participants in the proxy solicitation and a description of their direct and indirect interests, by security holdings or otherwise, will be included in the proxy statement/prospectus and other relevant materials to be filed with the SEC regarding the transaction when they become available. Stockholders, potential investors and other interested persons should read the proxy statement/prospectus carefully when it becomes available before making any voting or investment decisions. When available, these documents can be obtained free of charge from the sources indicated above. No Offer or Solicitation This presentation shall not constitute a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the Transaction. This presentation shall also not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of securities in any states or jurisdictions in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction.

Overview of LMF Acquisition Opportunities SeaStar Medical 5 Bruce M. Rodgers, Esq. Chairman & CEO, President LMF’s team has significant public company experience to support SeaStar Medical’s growth plans 7+ years of public company experience plus over 20 years serving as outside counsel to public companies 2015 – Present: Chairman, CEO & President of LM Funding America, Inc. (Nasdaq:LMFA) Mr. Rodgers was instrumental in developing LMFA’s business model prior to its inception and in pivoting the business to Bitcoin mining As LMFA’s Chief Executive Officer, Mr. Rodgers has guided LMFA through its initial public offering and subsequent public offerings 1998 – 2003: Equity Partner at Foley & Lardner LLP Richard Russell Chief Financial Officer Craig Burson Director 100+ years combined investing & operating experience Growth oriented investors & operators Robust M&A and capital markets experience Long-term track record of value creation across sectors
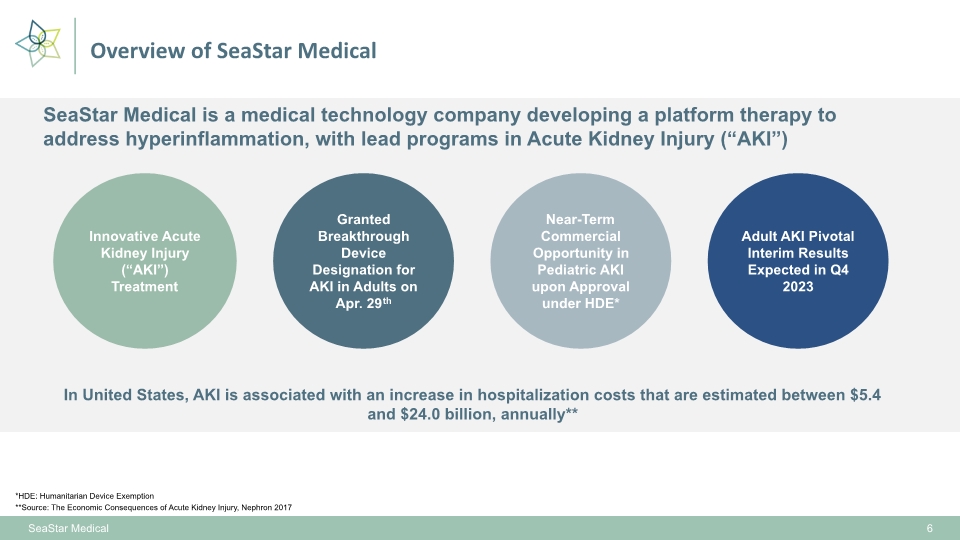
Overview of SeaStar Medical SeaStar Medical 6 Innovative Acute Kidney Injury (“AKI”) Treatment Near-Term Commercial Opportunity in Pediatric AKI upon Approval under HDE* SeaStar Medical is a medical technology company developing a platform therapy to address hyperinflammation, with lead programs in Acute Kidney Injury (“AKI”) In United States, AKI is associated with an increase in hospitalization costs that are estimated between $5.4 and $24.0 billion, annually** **Source: The Economic Consequences of Acute Kidney Injury, Nephron 2017 *HDE: Humanitarian Device Exemption Granted Breakthrough Device Designation for AKI in Adults on Apr. 29th Adult AKI Pivotal Interim Results Expected in Q4 2023
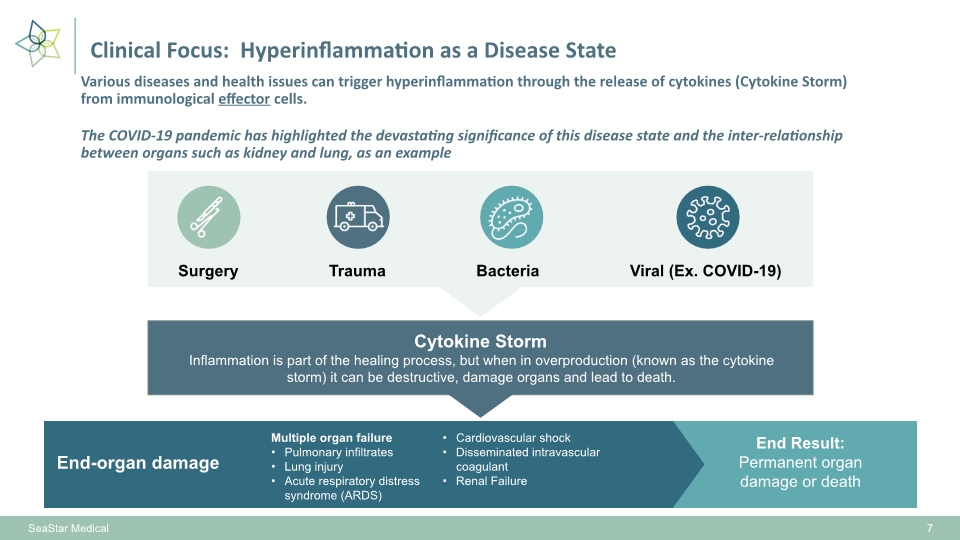
7 SeaStar Medical Various diseases and health issues can trigger hyperinflammation through the release of cytokines (Cytokine Storm) from immunological effector cells. The COVID-19 pandemic has highlighted the devastating significance of this disease state and the inter-relationship between organs such as kidney and lung, as an example Clinical Focus: Hyperinflammation as a Disease State
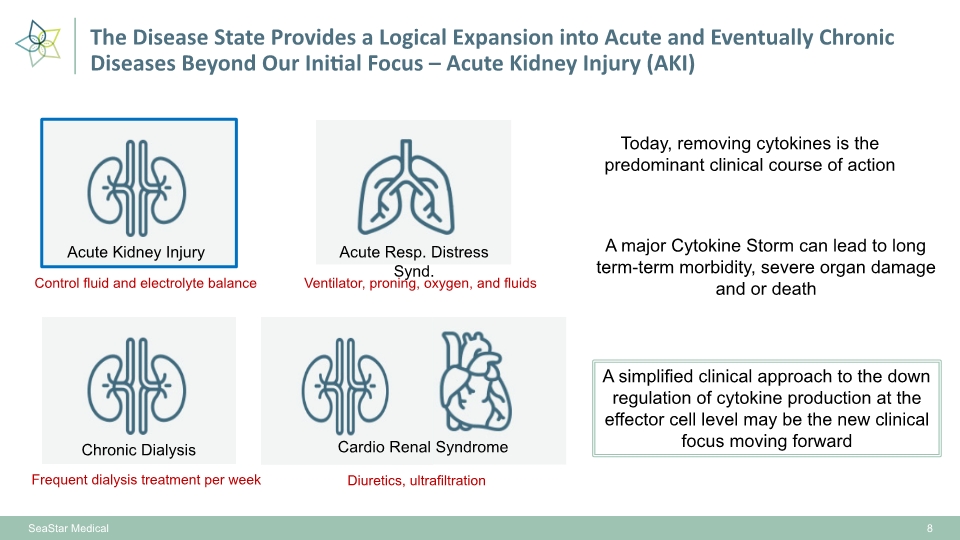
8 SeaStar Medical Control fluid and electrolyte balance Ventilator, proning, oxygen, and fluids Frequent dialysis treatment per week Diuretics, ultrafiltration Acute Kidney Injury Chronic Dialysis Acute Resp. Distress Synd. Cardio Renal Syndrome A major Cytokine Storm can lead to long term-term morbidity, severe organ damage and or death Today, removing cytokines is the predominant clinical course of action A simplified clinical approach to the down regulation of cytokine production at the effector cell level may be the new clinical focus moving forward The Disease State Provides a Logical Expansion into Acute and Eventually Chronic Diseases Beyond Our Initial Focus – Acute Kidney Injury (AKI)
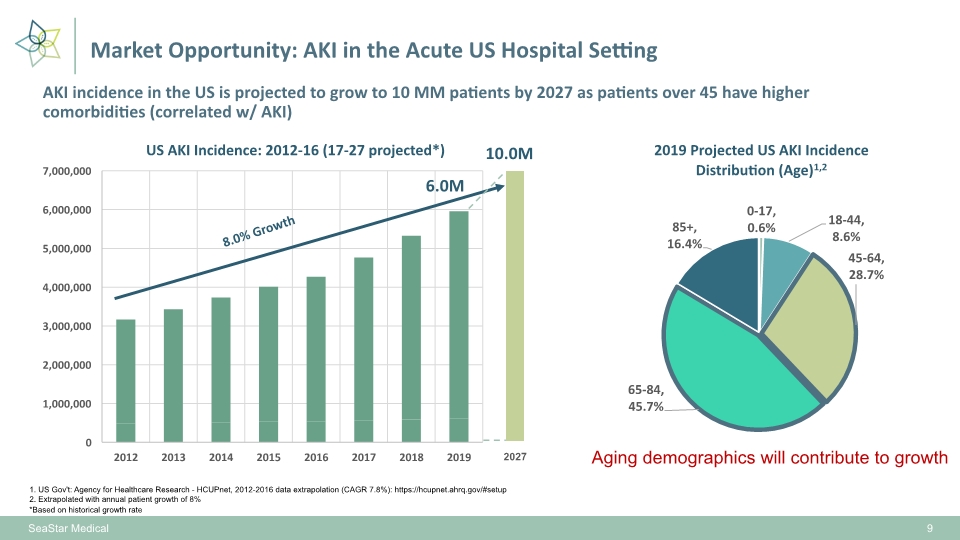
1. US Gov't: Agency for Healthcare Research - HCUPnet, 2012-2016 data extrapolation (CAGR 7.8%): https://hcupnet.ahrq.gov/#setup 2. Extrapolated with annual patient growth of 8% 8.0% Growth 6.0M Aging demographics will contribute to growth *Based on historical growth rate SeaStar Medical AKI incidence in the US is projected to grow to 10 MM patients by 2027 as patients over 45 have higher comorbidities (correlated w/ AKI) 9 Market Opportunity: AKI in the Acute US Hospital Setting 10.0M 2027
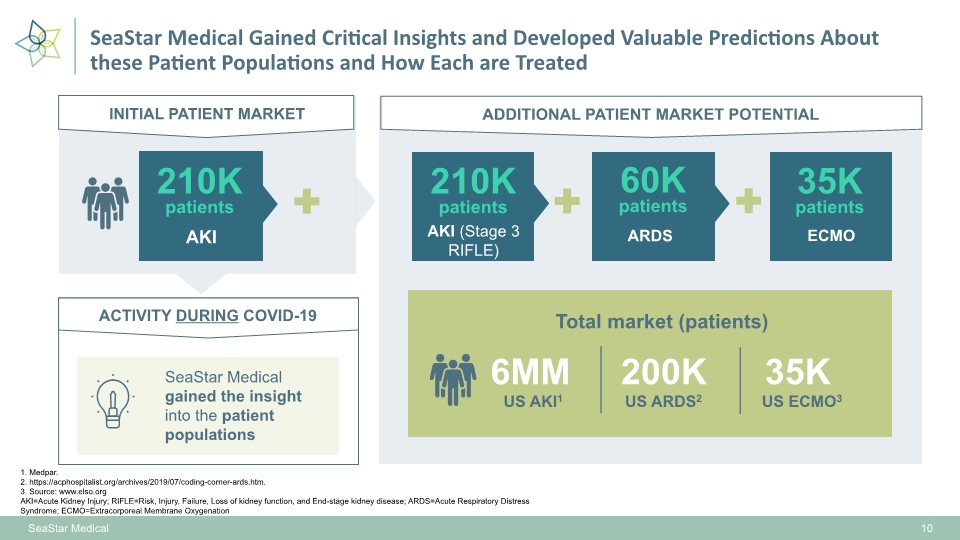
SeaStar Medical Gained Critical Insights and Developed Valuable Predictions About these Patient Populations and How Each are Treated ACTIVITY DURING COVID-19 ADDITIONAL PATIENT MARKET POTENTIAL SeaStar Medical gained the insight into the patient populations AKI (Stage 3 RIFLE) ARDS ECMO 1. Medpar. 2. https://acphospitalist.org/archives/2019/07/coding-corner-ards.htm. 3. Source: www.elso.org AKI=Acute Kidney Injury; RIFLE=Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease; ARDS=Acute Respiratory Distress Syndrome; ECMO=Extracorporeal Membrane Oxygenation INITIAL PATIENT MARKET AKI Total market (patients) 210K patients 6MM US AKI1 200K US ARDS2 US ECMO3 35K SeaStar Medical 10 60K patients 35K patients 210K patients
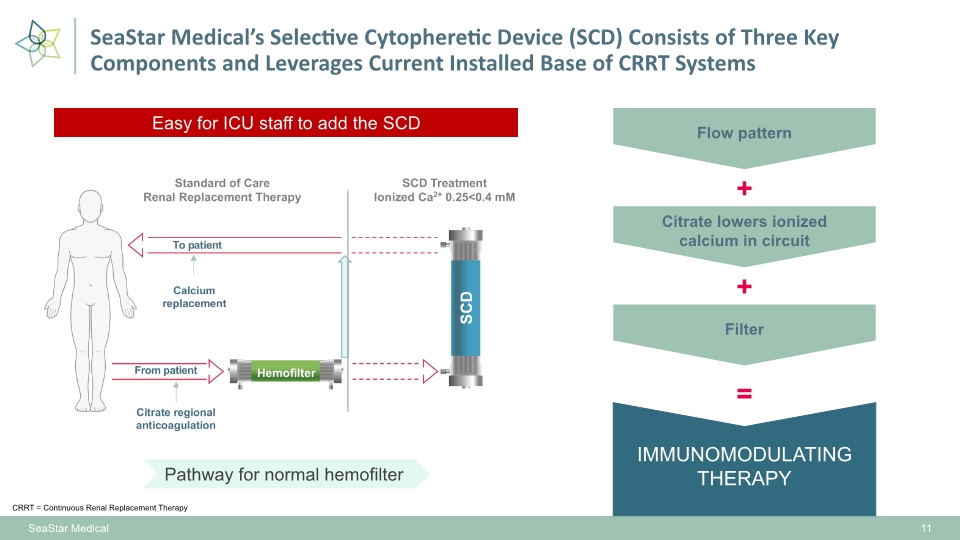
IMMUNOMODULATING THERAPY 11 SeaStar Medical SeaStar Medical’s Selective Cytopheretic Device (SCD) Consists of Three Key Components and Leverages Current Installed Base of CRRT Systems Easy for ICU staff to add the SCD Flow pattern Citrate lowers ionized calcium in circuit Filter + + = CRRT = Continuous Renal Replacement Therapy Pathway for normal hemofilter
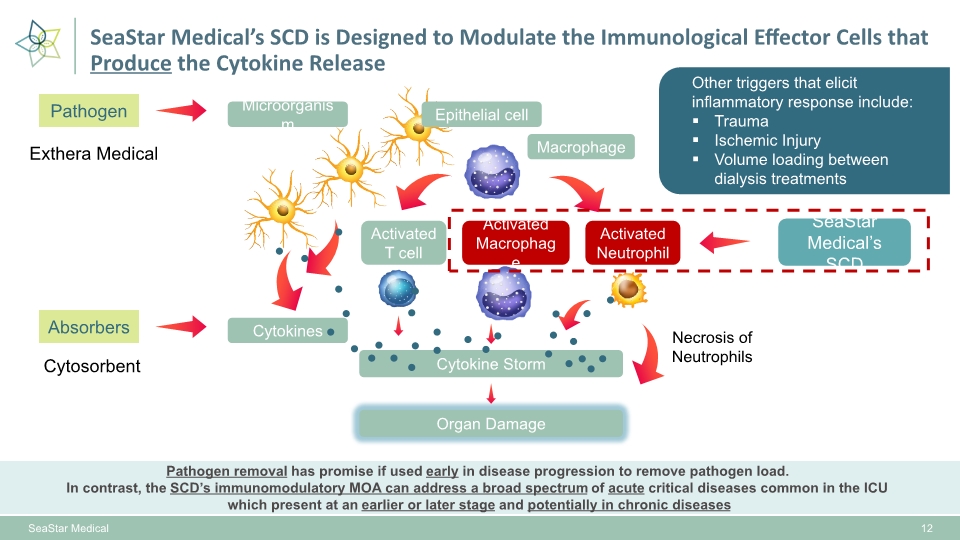
Absorbers SeaStar Medical’s SCD Pathogen SeaStar Medical Exthera Medical Cytosorbent 12 SeaStar Medical’s SCD is Designed to Modulate the Immunological Effector Cells that Produce the Cytokine Release Pathogen removal has promise if used early in disease progression to remove pathogen load. In contrast, the SCD’s immunomodulatory MOA can address a broad spectrum of acute critical diseases common in the ICU which present at an earlier or later stage and potentially in chronic diseases Cytokines Microorganism Epithelial cell Macrophage Activated T cell Activated Macrophage Activated Neutrophil Organ Damage Necrosis of Neutrophils Cytokine Storm Other triggers that elicit inflammatory response include: Trauma Ischemic Injury Volume loading between dialysis treatments
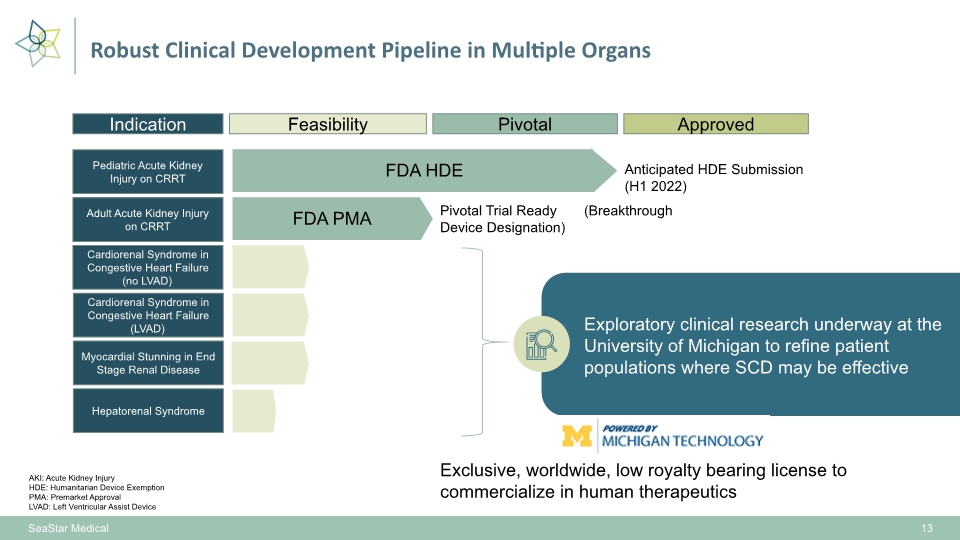
SeaStar Medical 13 Pivotal Approved Indication Pediatric Acute Kidney Injury on CRRT Feasibility FDA HDE Adult Acute Kidney Injury on CRRT FDA PMA Cardiorenal Syndrome in Congestive Heart Failure (no LVAD) Cardiorenal Syndrome in Congestive Heart Failure (LVAD) Myocardial Stunning in End Stage Renal Disease Hepatorenal Syndrome Exploratory clinical research underway at the University of Michigan to refine patient populations where SCD may be effective Pivotal Trial Ready (Breakthrough Device Designation) Anticipated HDE Submission (H1 2022) AKI: Acute Kidney Injury HDE: Humanitarian Device Exemption PMA: Premarket Approval LVAD: Left Ventricular Assist Device Robust Clinical Development Pipeline in Multiple Organs Exclusive, worldwide, low royalty bearing license to commercialize in human therapeutics
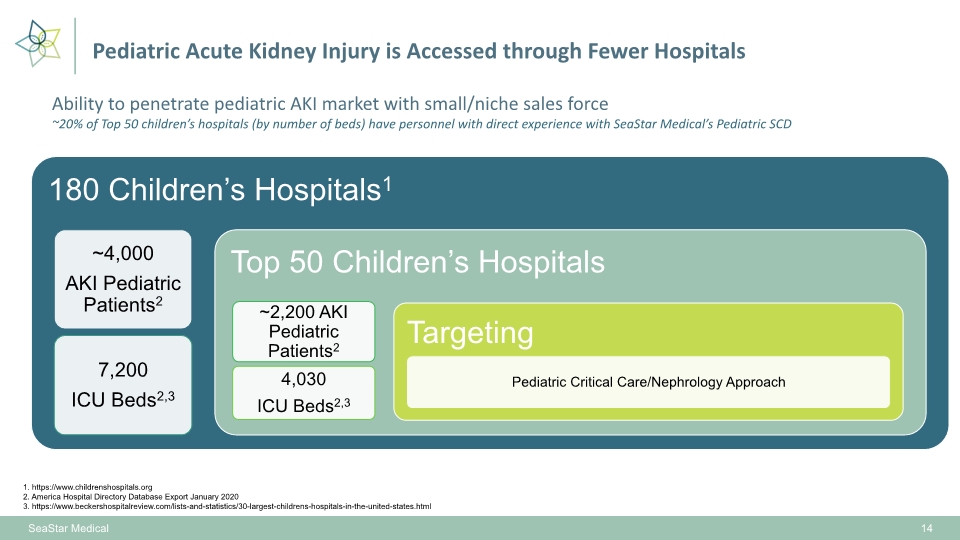
Pediatric Acute Kidney Injury is Accessed through Fewer Hospitals SeaStar Medical 14 1. https://www.childrenshospitals.org 2. America Hospital Directory Database Export January 2020 3. https://www.beckershospitalreview.com/lists-and-statistics/30-largest-childrens-hospitals-in-the-united-states.html Ability to penetrate pediatric AKI market with small/niche sales force ~20% of Top 50 children’s hospitals (by number of beds) have personnel with direct experience with SeaStar Medical’s Pediatric SCD
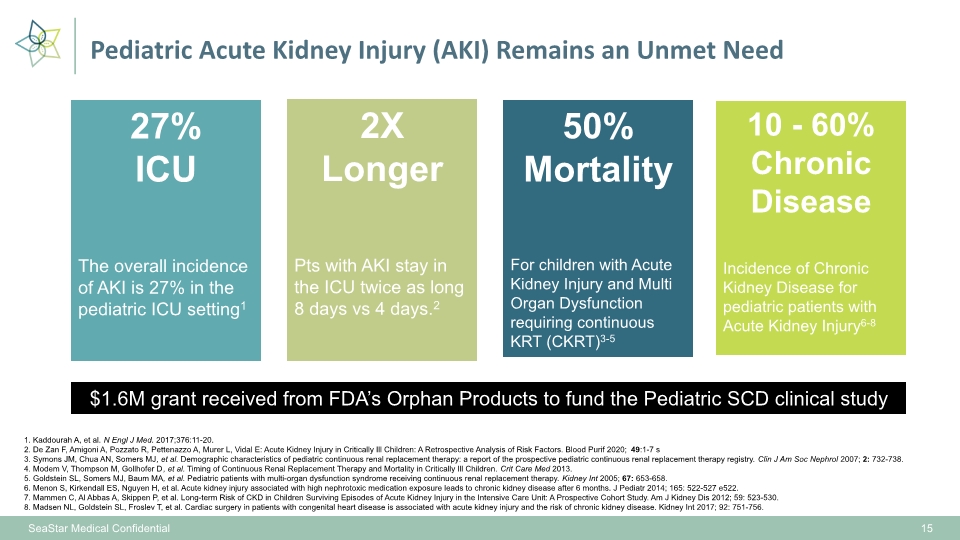
Pediatric Acute Kidney Injury (AKI) Remains an Unmet Need 1. Kaddourah A, et al. N Engl J Med. 2017;376:11-20. 2. De Zan F, Amigoni A, Pozzato R, Pettenazzo A, Murer L, Vidal E: Acute Kidney Injury in Critically Ill Children: A Retrospective Analysis of Risk Factors. Blood Purif 2020;49:1-7 s 3. Symons JM, Chua AN, Somers MJ, et al. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol 2007; 2: 732-738. 4. Modem V, Thompson M, Gollhofer D, et al. Timing of Continuous Renal Replacement Therapy and Mortality in Critically Ill Children. Crit Care Med 2013. 5. Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int 2005; 67: 653-658. 6. Menon S, Kirkendall ES, Nguyen H, et al. Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr 2014; 165: 522-527 e522. 7. Mammen C, Al Abbas A, Skippen P, et al. Long-term Risk of CKD in Children Surviving Episodes of Acute Kidney Injury in the Intensive Care Unit: A Prospective Cohort Study. Am J Kidney Dis 2012; 59: 523-530. 8. Madsen NL, Goldstein SL, Froslev T, et al. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int 2017; 92: 751-756. 10 - 60% Chronic Disease Incidence of Chronic Kidney Disease for pediatric patients with Acute Kidney Injury6-8 50% Mortality For children with Acute Kidney Injury and Multi Organ Dysfunction requiring continuous KRT (CKRT)3-5 27% ICU The overall incidence of AKI is 27% in the pediatric ICU setting1 SeaStar Medical Confidential 15 $1.6M grant received from FDA’s Orphan Products to fund the Pediatric SCD clinical study 2X Longer Pts with AKI stay in the ICU twice as long 8 days vs 4 days.2
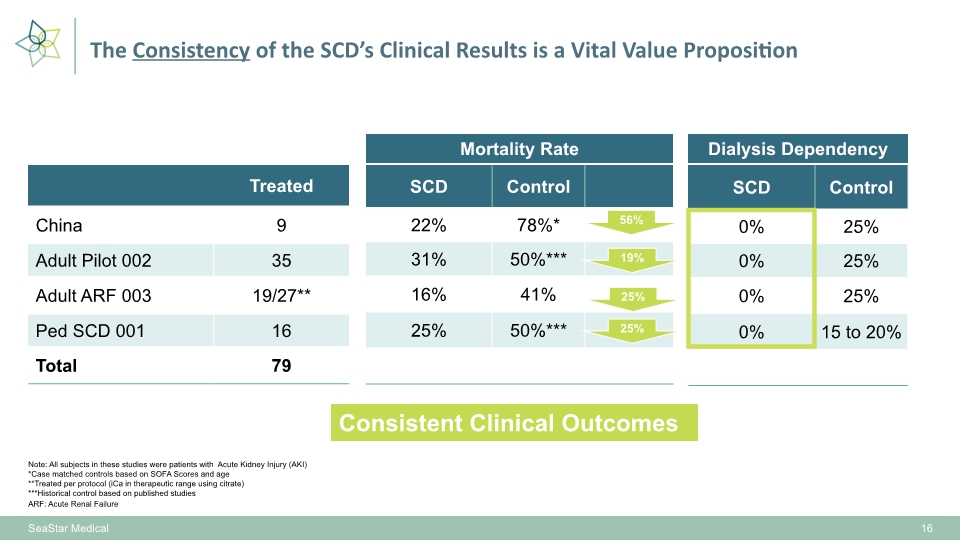
16 Note: All subjects in these studies were patients with Acute Kidney Injury (AKI) *Case matched controls based on SOFA Scores and age **Treated per protocol (iCa in therapeutic range using citrate) ***Historical control based on published studies ARF: Acute Renal Failure Dialysis Dependency Mortality Rate 25% Consistent Clinical Outcomes SeaStar Medical The Consistency of the SCD’s Clinical Results is a Vital Value Proposition
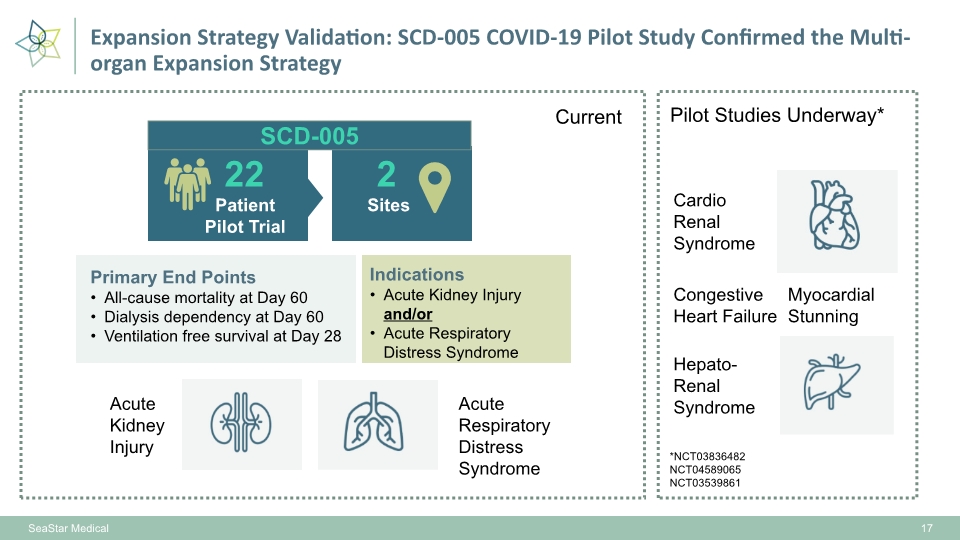
17 Primary End Points All-cause mortality at Day 60 Dialysis dependency at Day 60 Ventilation free survival at Day 28 Indications Acute Kidney Injury and/or Acute Respiratory Distress Syndrome Patient Pilot Trial 22 Sites 2 SCD-005 Current Pilot Studies Underway* Acute Kidney Injury Acute Respiratory Distress Syndrome Cardio Renal Syndrome Hepato-Renal Syndrome *NCT03836482 NCT04589065 NCT03539861 Congestive Heart Failure Myocardial Stunning SeaStar Medical Expansion Strategy Validation: SCD-005 COVID-19 Pilot Study Confirmed the Multi-organ Expansion Strategy
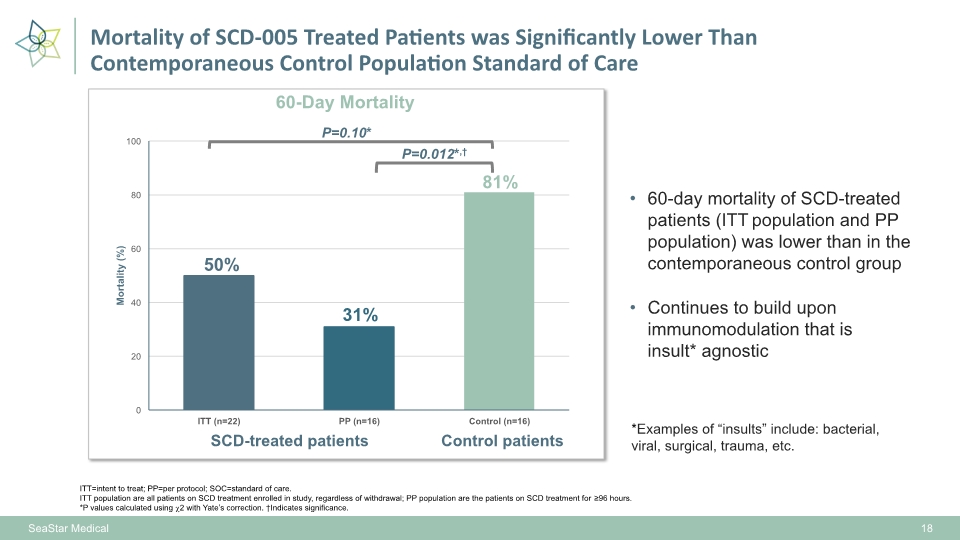
Mortality of SCD-005 Treated Patients was Significantly Lower Than Contemporaneous Control Population Standard of Care 60-day mortality of SCD-treated patients (ITT population and PP population) was lower than in the contemporaneous control group 18 ITT=intent to treat; PP=per protocol; SOC=standard of care. ITT population are all patients on SCD treatment enrolled in study, regardless of withdrawal; PP population are the patients on SCD treatment for ≥96 hours. *P values calculated using 2 with Yate’s correction. †Indicates significance. 50% 31% 81% P=0.012*,† SCD-treated patients Control patients P=0.10* SeaStar Medical Continues to build upon immunomodulation that is insult* agnostic *Examples of “insults” include: bacterial, viral, surgical, trauma, etc.
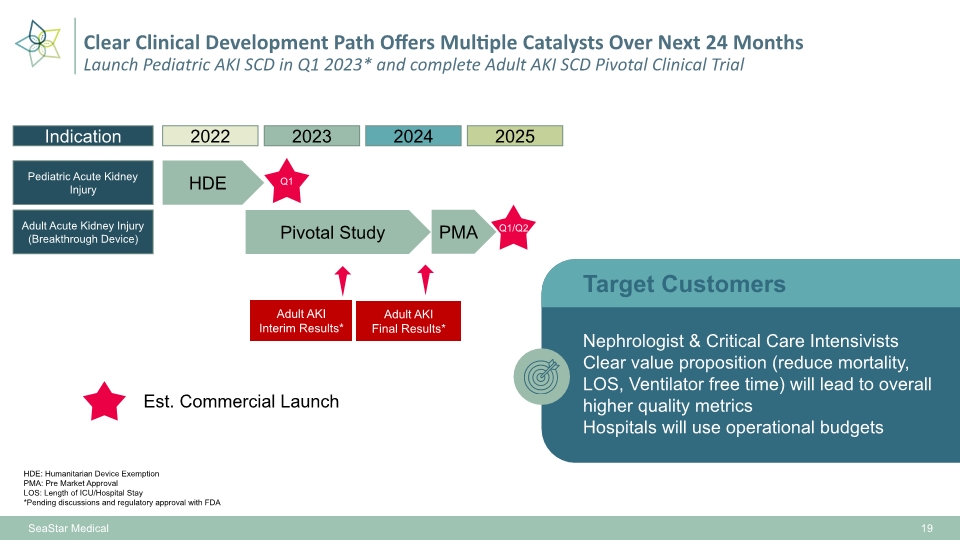
SeaStar Medical HDE: Humanitarian Device Exemption PMA: Pre Market Approval LOS: Length of ICU/Hospital Stay *Pending discussions and regulatory approval with FDA 19 Clear Clinical Development Path Offers Multiple Catalysts Over Next 24 Months Launch Pediatric AKI SCD in Q1 2023* and complete Adult AKI SCD Pivotal Clinical Trial Indication Pediatric Acute Kidney Injury 2022 HDE Adult Acute Kidney Injury (Breakthrough Device) Pivotal Study Nephrologist & Critical Care Intensivists Clear value proposition (reduce mortality, LOS, Ventilator free time) will lead to overall higher quality metrics Hospitals will use operational budgets 2023 2024 2025 Q1 PMA Q1/Q2 Adult AKI Final Results* Adult AKI Interim Results* Target Customers Est. Commercial Launch
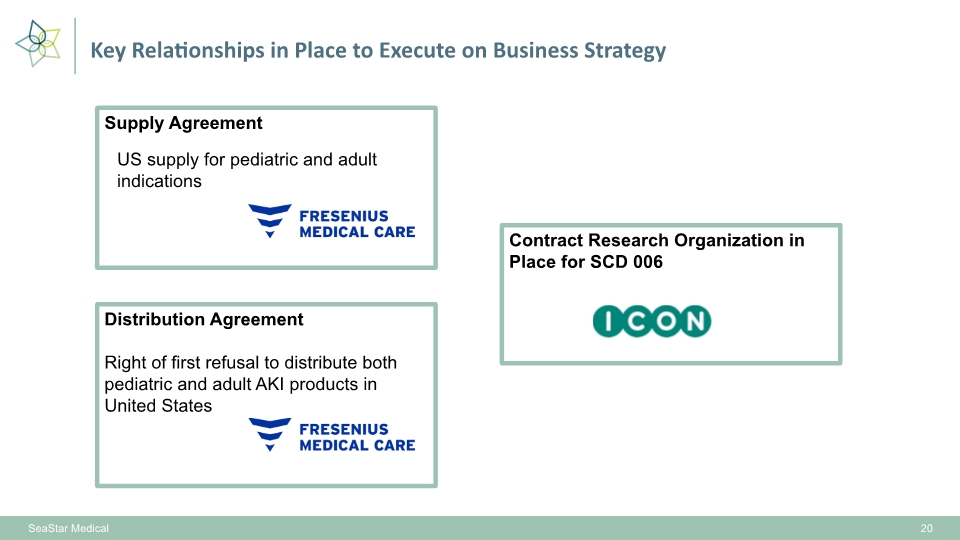
Key Relationships in Place to Execute on Business Strategy SeaStar Medical 20 Supply Agreement Distribution Agreement Right of first refusal to distribute both pediatric and adult AKI products in United States Contract Research Organization in Place for SCD 006 US supply for pediatric and adult indications
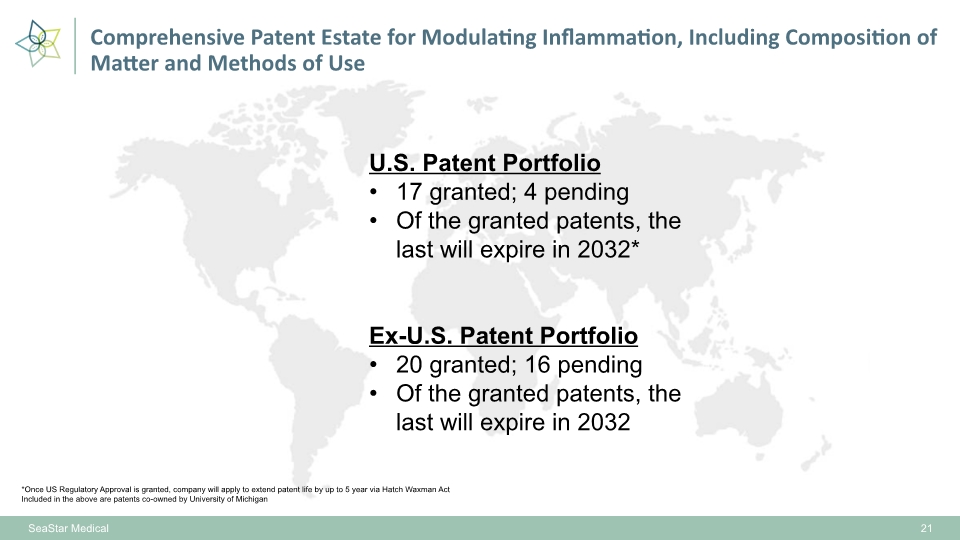
SeaStar Medical 21 U.S. Patent Portfolio 17 granted; 4 pending Of the granted patents, the last will expire in 2032* Ex-U.S. Patent Portfolio 20 granted; 16 pending Of the granted patents, the last will expire in 2032 *Once US Regulatory Approval is granted, company will apply to extend patent life by up to 5 year via Hatch Waxman Act Included in the above are patents co-owned by University of Michigan Comprehensive Patent Estate for Modulating Inflammation, Including Composition of Matter and Methods of Use

SeaStar Medical Management Team SeaStar Medical 22 Over 30 years of accounting and finance experience in private and public companies WPP, Tiffany & Co, CDM Group University of Maryland Caryl Baron Controller Over 20 years of business leadership at both large and growing companies Dow Chemical, Dow AgroSciences University of Illinois Eric Schlorff CEO & Chairman Over 10 years ICU nursing AdventHealth Hospital, Diabetes Institute University of Florida Matt Jacques Director of Clinical Nursing

Board of Directors – SeaStar Medical Holding Corporation SeaStar Medical 23
SeaStar Medical Corporate Highlights 24 SeaStar Medical 1. See slides 9-10 for market data 2. Pediatric Acute Kidney Injury product expected to launch in early 2023 under HDE; Pivotal trial ready program in Adult Acute Kidney Injury, with projected interim and final data readouts in 2023/2024 (PMA) HDE: Humanitarian Device Exemption PMA: Premarket Approval
25 SeaStar Medical Summary Transaction Overview The combined company will be known as SeaStar Medical Holding Corporation Will operate under the same management team as SeaStar Medical Anticipated closing in Q3 2022 Overview Ownership Financing Transaction Rationale Use of Proceeds The transaction contemplates an enterprise value of approximately $85 million for SeaStar Medical As part of the transaction, all SeaStar Medical shares owned by SeaStar Medical’s existing equity holders will be converted into Class A Common Stock of SeaStar Medical Holding Corporation Dow Pension Plans, as existing investors of SeaStar Medical, has committed to further participate in the transaction through a PIPE investment concurrent with the business combination Provides SeaStar Medical with access to the capital markets, allowing it to accelerate the development and advancement of its proprietary and innovative immunomodulatory platform for pediatric and adult AKI Multiple near-term value inflection points To submit SCD for FDA approval under HDE for pediatric AKI and initiate a pivotal trial of SCD in Adult AKI To launch commercialization of SCD in pediatric AKI upon HDE approval Working capital and general corporate purposes
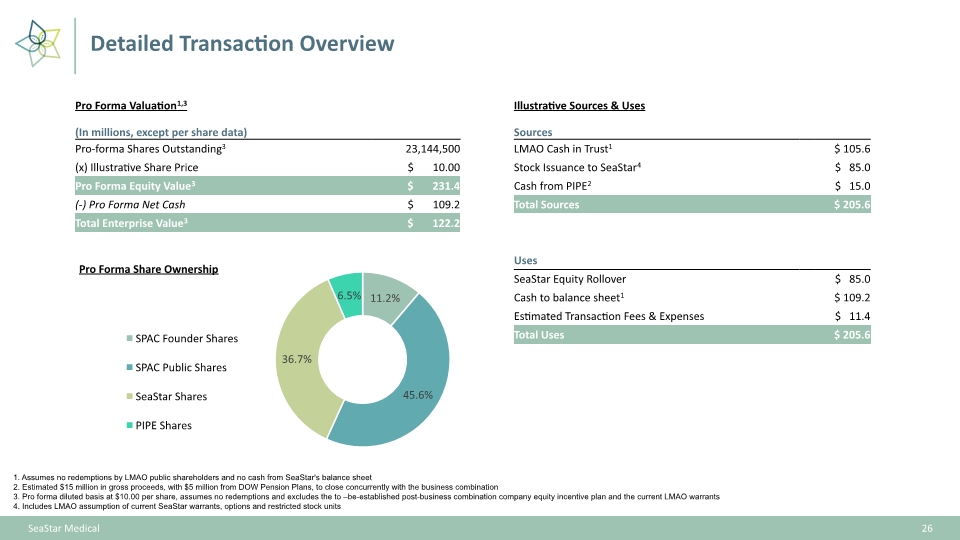
26 SeaStar Medical Detailed Transaction Overview

Thank You May 2022 27

Supplemental Medical Information SeaStar Medical 28
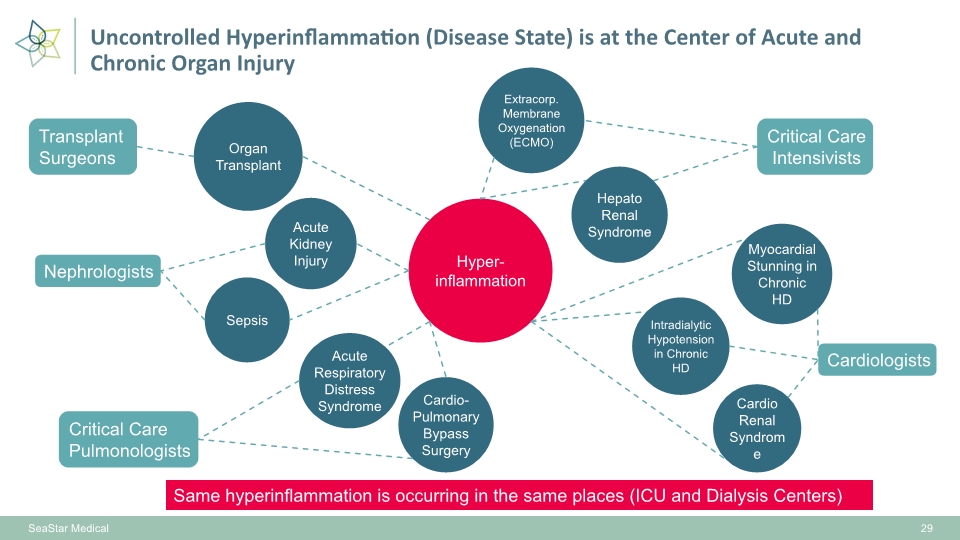
Hyper- inflammation Acute Kidney Injury Cardio Renal Syndrome Hepato Renal Syndrome Intradialytic Hypotension in Chronic HD Cardio-Pulmonary Bypass Surgery Acute Respiratory Distress Syndrome Extracorp. Membrane Oxygenation (ECMO) Sepsis Myocardial Stunning in Chronic HD Organ Transplant SeaStar Medical Nephrologists Critical Care Intensivists Critical Care Pulmonologists Cardiologists Transplant Surgeons Same hyperinflammation is occurring in the same places (ICU and Dialysis Centers) 29 Uncontrolled Hyperinflammation (Disease State) is at the Center of Acute and Chronic Organ Injury
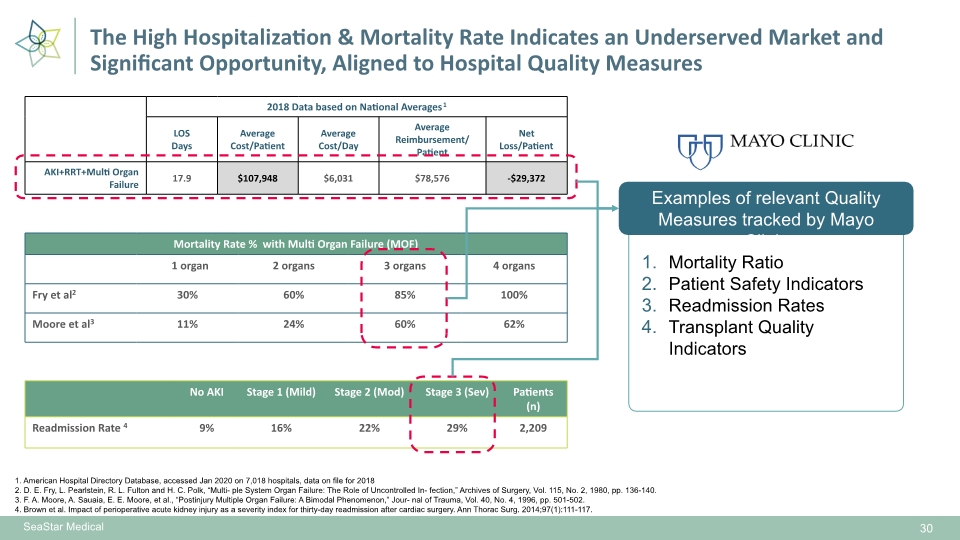
SeaStar Medical 30 1. American Hospital Directory Database, accessed Jan 2020 on 7,018 hospitals, data on file for 2018 2. D. E. Fry, L. Pearlstein, R. L. Fulton and H. C. Polk, “Multi- ple System Organ Failure: The Role of Uncontrolled In- fection,” Archives of Surgery, Vol. 115, No. 2, 1980, pp. 136-140. 3. F. A. Moore, A. Sauaia, E. E. Moore, et al., “Postinjury Multiple Organ Failure: A Bimodal Phenomenon,” Jour- nal of Trauma, Vol. 40, No. 4, 1996, pp. 501-502. 4. Brown et al. Impact of perioperative acute kidney injury as a severity index for thirty-day readmission after cardiac surgery. Ann Thorac Surg. 2014;97(1):111-117. The High Hospitalization & Mortality Rate Indicates an Underserved Market and Significant Opportunity, Aligned to Hospital Quality Measures Examples of relevant Quality Measures tracked by Mayo Clinic Mortality Ratio Patient Safety Indicators Readmission Rates Transplant Quality Indicators
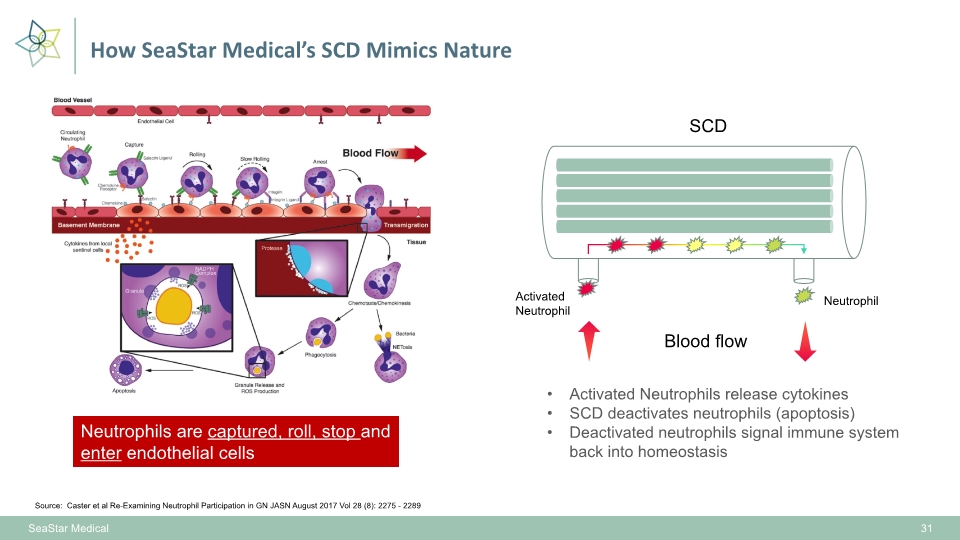
How SeaStar Medical’s SCD Mimics Nature 31 Source: Caster et al Re-Examining Neutrophil Participation in GN JASN August 2017 Vol 28 (8): 2275 - 2289 SeaStar Medical Neutrophils are captured, roll, stop and enter endothelial cells SCD Activated Neutrophil Neutrophil Activated Neutrophils release cytokines SCD deactivates neutrophils (apoptosis) Deactivated neutrophils signal immune system back into homeostasis Blood flow
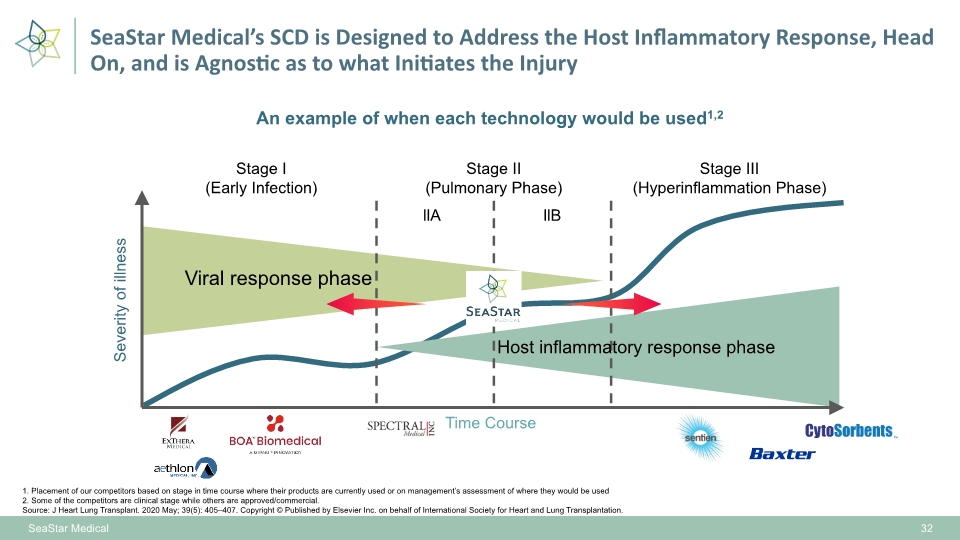
An example of when each technology would be used1,2 1. Placement of our competitors based on stage in time course where their products are currently used or on management’s assessment of where they would be used 2. Some of the competitors are clinical stage while others are approved/commercial. Source: J Heart Lung Transplant. 2020 May; 39(5): 405–407. Copyright © Published by Elsevier Inc. on behalf of International Society for Heart and Lung Transplantation. SeaStar Medical 32 SeaStar Medical’s SCD is Designed to Address the Host Inflammatory Response, Head On, and is Agnostic as to what Initiates the Injury Stage III (Hyperinflammation Phase) Stage II (Pulmonary Phase) Stage I (Early Infection) llA llB Time Course Severity of illness Viral response phase Host inflammatory response phase
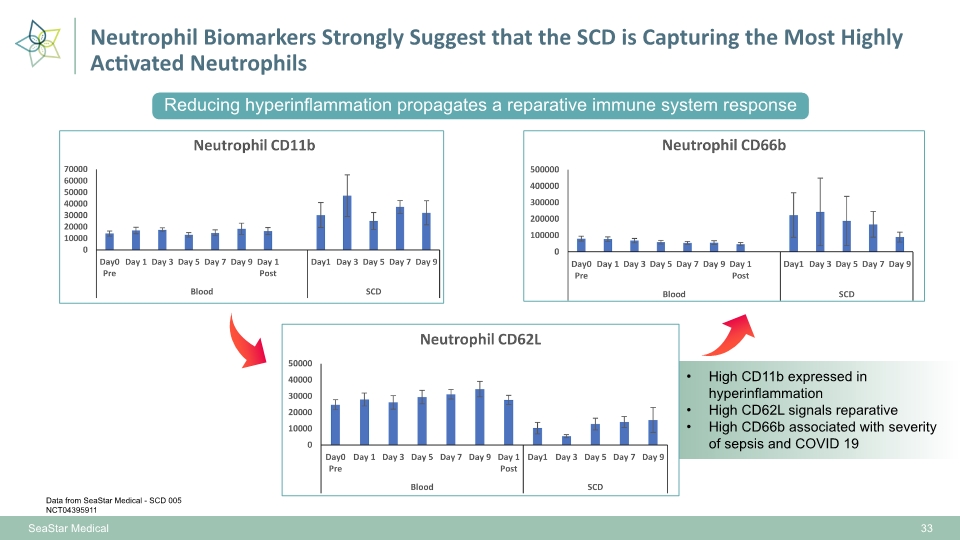
Neutrophil Biomarkers Strongly Suggest that the SCD is Capturing the Most Highly Activated Neutrophils 33 High CD11b expressed in hyperinflammation High CD62L signals reparative High CD66b associated with severity of sepsis and COVID 19 SeaStar Medical Data from SeaStar Medical - SCD 005 NCT04395911
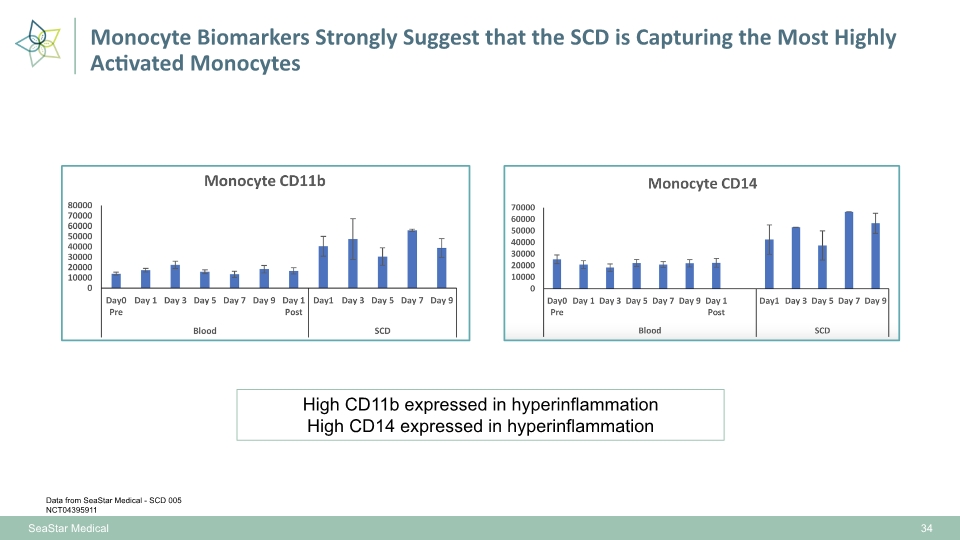
High CD11b expressed in hyperinflammation High CD14 expressed in hyperinflammation Monocyte Biomarkers Strongly Suggest that the SCD is Capturing the Most Highly Activated Monocytes 34 SeaStar Medical Data from SeaStar Medical - SCD 005 NCT04395911
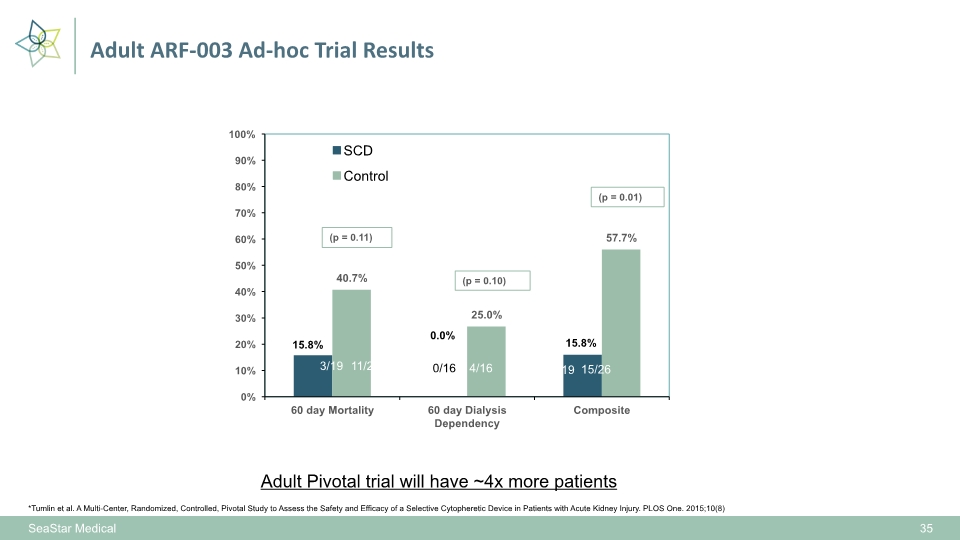
SeaStar Medical 35 *Tumlin et al. A Multi-Center, Randomized, Controlled, Pivotal Study to Assess the Safety and Efficacy of a Selective Cytopheretic Device in Patients with Acute Kidney Injury. PLOS One. 2015;10(8) Adult Pivotal trial will have ~4x more patients Adult ARF-003 Ad-hoc Trial Results
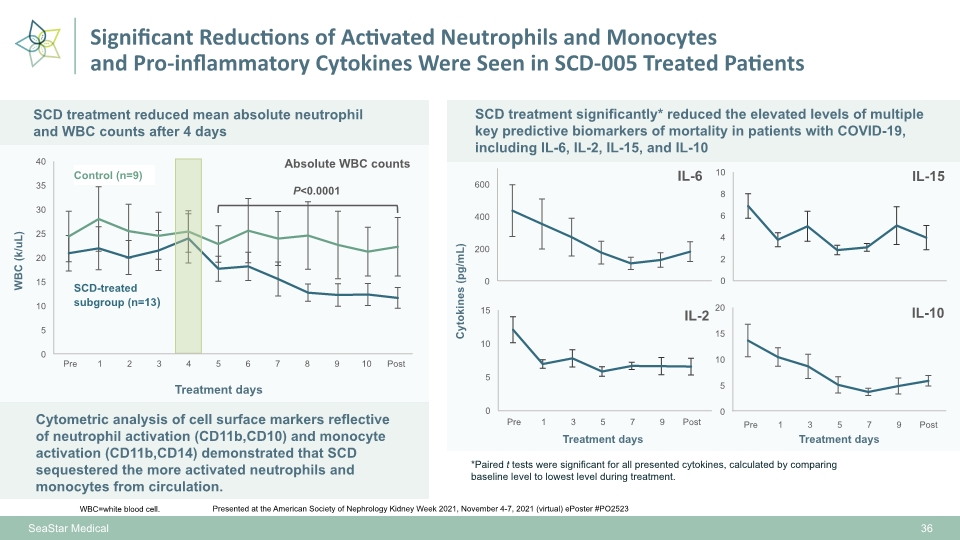
Significant Reductions of Activated Neutrophils and Monocytes and Pro-inflammatory Cytokines Were Seen in SCD-005 Treated Patients Cytometric analysis of cell surface markers reflective of neutrophil activation (CD11b,CD10) and monocyte activation (CD11b,CD14) demonstrated that SCD sequestered the more activated neutrophils and monocytes from circulation. 36 Presented at the American Society of Nephrology Kidney Week 2021, November 4-7, 2021 (virtual) ePoster #PO2523 WBC=white blood cell. SCD treatment reduced mean absolute neutrophil and WBC counts after 4 days Absolute WBC counts P<0.0001 SCD treatment significantly* reduced the elevated levels of multiple key predictive biomarkers of mortality in patients with COVID-19, including IL-6, IL-2, IL-15, and IL-10 Treatment days Cytokines (pg/mL) WBC (k/uL) Treatment days Treatment days *Paired t tests were significant for all presented cytokines, calculated by comparing baseline level to lowest level during treatment. SCD-treated subgroup (n=13) Control (n=9) SeaStar Medical
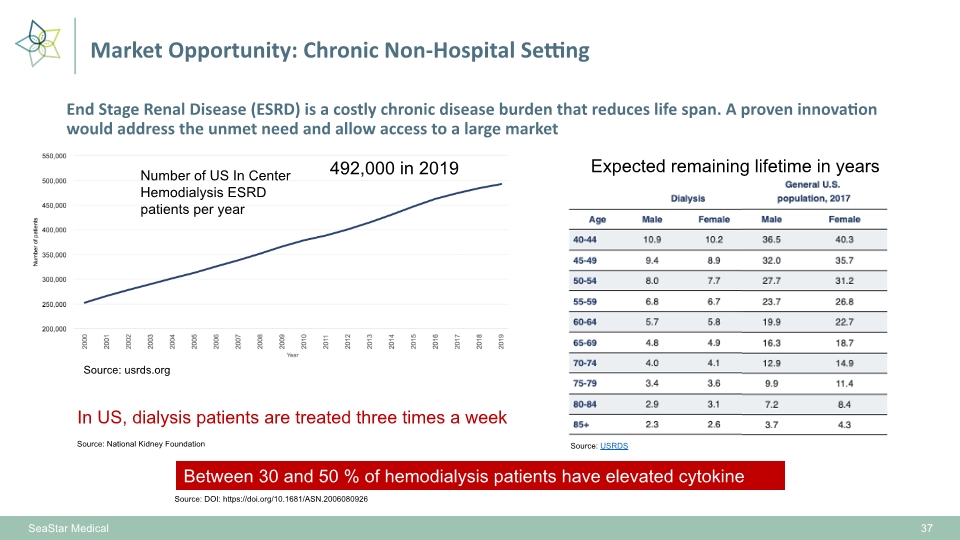
End Stage Renal Disease (ESRD) is a costly chronic disease burden that reduces life span. A proven innovation would address the unmet need and allow access to a large market 37 Number of US In Center Hemodialysis ESRD patients per year Source: usrds.org Between 30 and 50 % of hemodialysis patients have elevated cytokine markers 492,000 in 2019 Source: DOI: https://doi.org/10.1681/ASN.2006080926 In US, dialysis patients are treated three times a week SeaStar Medical Market Opportunity: Chronic Non-Hospital Setting Source: USRDS Source: National Kidney Foundation
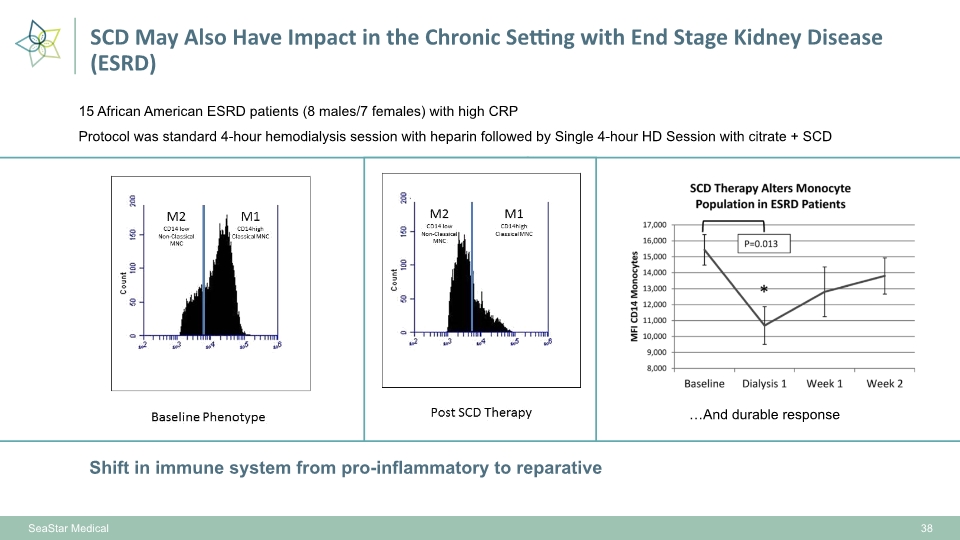
15 African American ESRD patients (8 males/7 females) with high CRP Protocol was standard 4-hour hemodialysis session with heparin followed by Single 4-hour HD Session with citrate + SCD Shift in immune system from pro-inflammatory to reparative …And durable response 38 SeaStar Medical SCD May Also Have Impact in the Chronic Setting with End Stage Kidney Disease (ESRD) …And durable response





































