
Exhibit 99.2 TD Cowen 43rd Annual Health Care Conference March 6, 2023

FORWARD LOOKING STATEMENTS This presentation contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Statements in this presentation that are not statements of historical fact are forward-looking statements. Words such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward- looking statements include statements concerning, among other things, our belief that our existing cash resources will be sufficient to fund our operations into the second quarter of 2024; the future of the COVID-19 landscape including the expectation of continued evolution and emergence of new variants and subvariants; our ongoing research and clinical development plans; the timing, progress and results of our preclinical studies and clinical trials of our product candidates; the initiation, modification and completion of studies or trials and related preparatory work; the period during which the results of our clinical trials and other studies and research activities will become available, and our research and development programs; our ability to identify novel antibodies designed to address the evolving SARS-CoV-2 threat; our ability to obtain and maintain regulatory approvals for our product candidates; our expectations regarding the size of the patient populations, market acceptance and opportunity for and clinical utility of our product candidates, if approved for commercial use; our expectations regarding the scope of any approved indication for our product candidates; our ability to successfully commercialize our product candidates, including for a new drug category; our ability to leverage our platform to identify and develop future product candidates in additional areas of need; our ability to identify patients with the diseases treated by our product candidates and to enroll these patients in our clinical trials; our manufacturing capabilities and strategy; our ability to successfully execute on the components of our vision to create the “perpetual machine”; the anticipation of ongoing discussions with health authorities; the potential for an emergency use authorization in the U.S. or other regulatory approval; our preclinical activity, plans, technology and resources to develop therapeutic or preventative options for other infectious diseases, such as additional coronaviruses and influenza, in the U.S. and globally; our belief in the potential to discover and develop pipeline candidates as potent and durable antibodies or combination of antibodies for COVID- 19; and other statements that are not historical fact. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause our actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation, the impacts of the COVID-19 pandemic on our business and those of our collaborators, our clinical trials and our financial position; unexpected safety or efficacy data observed during preclinical studies or clinical trials; the predictability of clinical success of our product candidates or combination of candidates based on neutralizing activity in pre-clinical studies; variability of results in models used to predict activity against SARS-CoV-2 variants of concern; clinical trial site activation or enrollment rates that are lower than expected; changes in expected or existing competition; changes in the regulatory environment; the uncertainties and timing of the regulatory approval process, including the outcome of our discussions with regulatory authorities concerning our clinical trials; whether we are able to successfully monitor, analyze, engineer and optimize new product candidates; whether we are able to create a flow of product candidates that address virus evolution; whether our product candidates or combination of candidates are able to demonstrate activity against predominant SARS-CoV-2 variant(s) in the U.S. and globally; whether we are able to successfully submit an emergency use authorization in the future, and the outcome of any such emergency use authorization submission; whether research and development efforts will improve efficacy of our product candidates against predominant variants or identify additional monoclonal antibodies or combination of antibodies for the prevention and treatment of COVID-19 and other infectious diseases; whether research and development efforts will identify and result in safe and effective therapeutic or preventative options for other infectious diseases in the U.S. or globally and whether we have adequate funding to meet future operating expenses and capital expenditure requirements. Other factors that may cause our actual results to differ materially from those expressed or implied in the forward-looking statements in this presentation are described under the heading “Risk Factors” in our most recent Annual Report on Form 10-K and our most recent Quarterly Report on Form 10-Q, each filed with the Securities and Exchange Commission (the “SEC”), and in our other filings with the SEC, and in our future reports to be filed with the SEC and available at www.sec.gov. Such risks may be amplified by the impacts of the COVID-19 pandemic. Forward-looking statements contained in this presentation are made as of this date, and we undertake no duty to update such information whether as a result of new information, future events or otherwise, except as required under applicable law. © 2023 Invivyd, Inc. Invivyd and the Invivyd logo are trademarks of Invivyd, Inc. 2 All trademarks in this presentation are the property of their respective owners. 2

OUR VISION & PURPOSE Providing Hope for Vulnerable People Against Viral Diseases Our purpose is to rapidly and perpetually deliver antibody-based therapies that protect vulnerable people from the devastating consequences of circulating viral threats, beginning with SARS-CoV-2 3
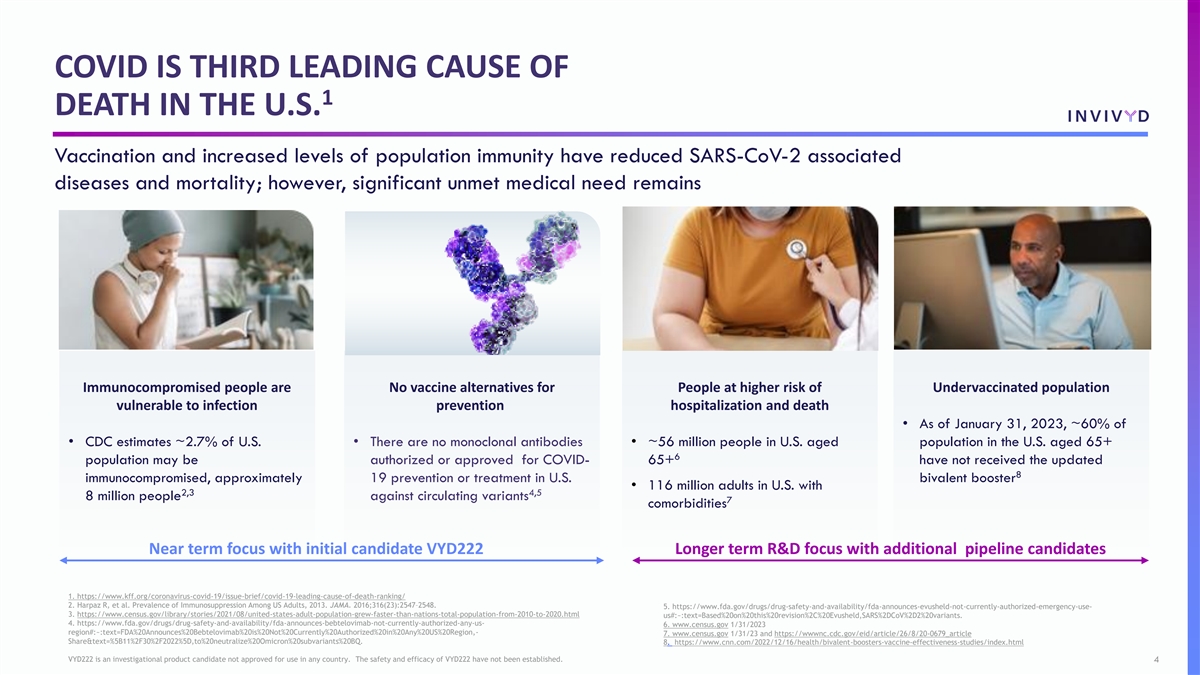
COVID IS THIRD LEADING CAUSE OF 1 DEATH IN THE U.S. Vaccination and increased levels of population immunity have reduced SARS-CoV-2 associated diseases and mortality; however, significant unmet medical need remains Immunocompromised people are No vaccine alternatives for People at higher risk of Undervaccinated population vulnerable to infection prevention hospitalization and death • As of January 31, 2023, ~60% of • CDC estimates ~2.7% of U.S. • There are no monoclonal antibodies • ~56 million people in U.S. aged population in the U.S. aged 65+ 6 population may be authorized or approved for COVID- 65+ have not received the updated 8 immunocompromised, approximately 19 prevention or treatment in U.S. bivalent booster • 116 million adults in U.S. with 2,3 4,5 8 million people against circulating variants 7 comorbidities Near term focus with initial candidate VYD222 Longer term R&D focus with additional pipeline candidates 1. https://www.kff.org/coronavirus-covid-19/issue-brief/covid-19-leading-cause-of-death-ranking/ 2. Harpaz R, et al. Prevalence of Immunosuppression Among US Adults, 2013. JAMA. 2016;316(23):2547–2548. 5. https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-evusheld-not-currently-authorized-emergency-use- 3. https://www.census.gov/library/stories/2021/08/united-states-adult-population-grew-faster-than-nations-total-population-from-2010-to-2020.html us#:~:text=Based%20on%20this%20revision%2C%20Evusheld,SARS%2DCoV%2D2%20variants. 4. https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-bebtelovimab-not-currently-authorized-any-us- 6. www.census.gov 1/31/2023 region#:~:text=FDA%20Announces%20Bebtelovimab%20is%20Not%20Currently%20Authorized%20in%20Any%20US%20Region,- 7. www.census.gov 1/31/23 and https://wwwnc.cdc.gov/eid/article/26/8/20-0679_article Share&text=%5B11%2F30%2F2022%5D,to%20neutralize%20Omicron%20subvariants%20BQ. 8. https://www.cnn.com/2022/12/16/health/bivalent-boosters-vaccine-effectiveness-studies/index.html VYD222 is an investigational product candidate not approved for use in any country. The safety and efficacy of VYD222 have not been established. 4 VYD
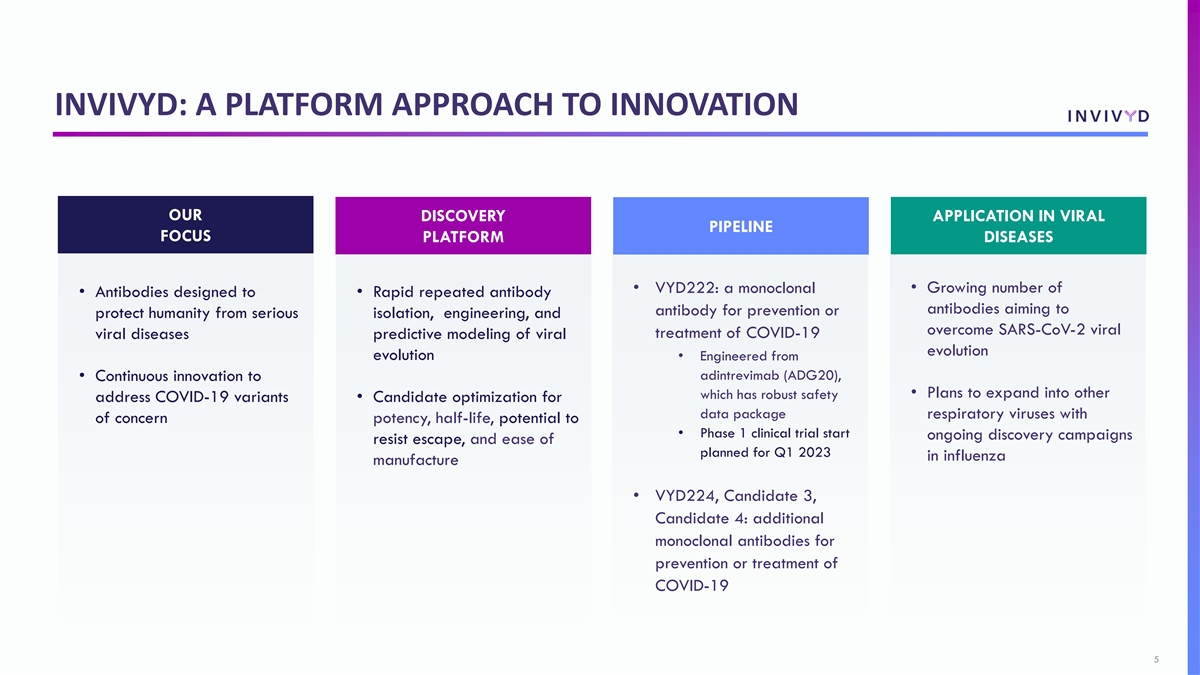
INVIVYD: A PLATFORM APPROACH TO INNOVATION OUR DISCOVERY APPLICATION IN VIRAL PIPELINE FOCUS PLATFORM DISEASES • Growing number of • VYD222: a monoclonal • Antibodies designed to • Rapid repeated antibody antibodies aiming to antibody for prevention or protect humanity from serious isolation, engineering, and overcome SARS-CoV-2 viral treatment of COVID-19 viral diseases predictive modeling of viral evolution evolution • Engineered from adintrevimab (ADG20), • Continuous innovation to • Plans to expand into other which has robust safety address COVID-19 variants • Candidate optimization for data package respiratory viruses with of concern potency, half-life, potential to • Phase 1 clinical trial start ongoing discovery campaigns resist escape, and ease of planned for Q1 2023 in influenza manufacture • VYD224, Candidate 3, Candidate 4: additional monoclonal antibodies for prevention or treatment of COVID-19 5
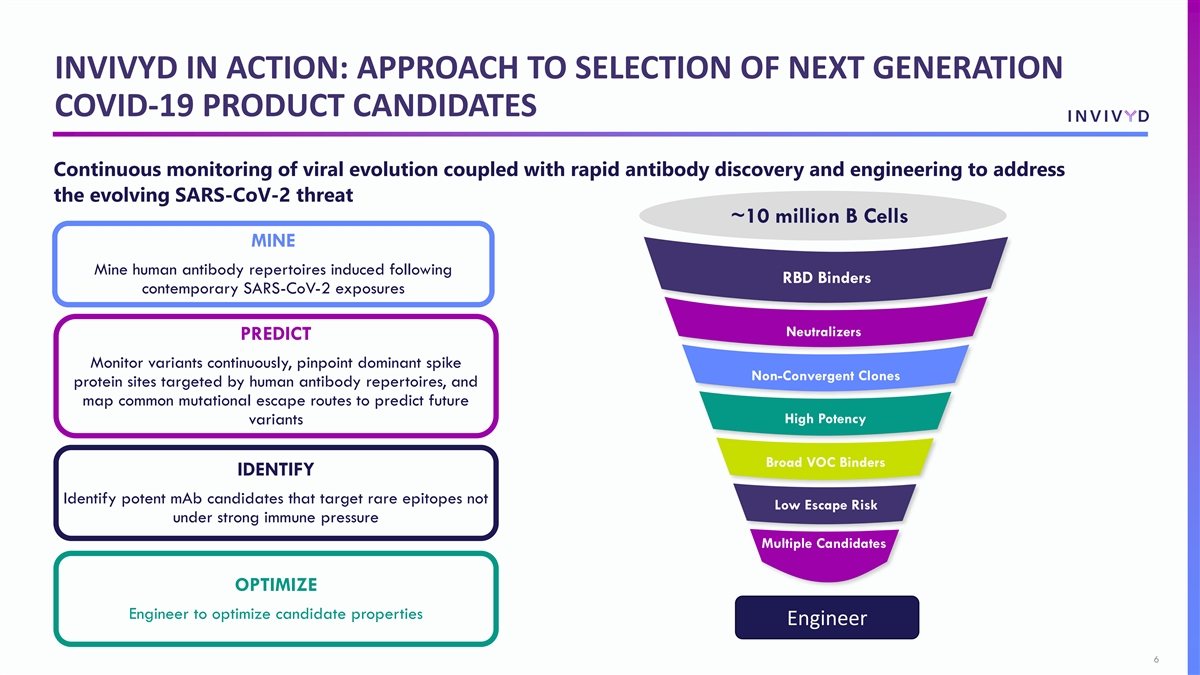
INVIVYD IN ACTION: APPROACH TO SELECTION OF NEXT GENERATION COVID-19 PRODUCT CANDIDATES Continuous monitoring of viral evolution coupled with rapid antibody discovery and engineering to address the evolving SARS-CoV-2 threat ~10 million B Cells MINE Mine human antibody repertoires induced following RBD Binders contemporary SARS-CoV-2 exposures Neutralizers PREDICT Monitor variants continuously, pinpoint dominant spike Non-Convergent Clones protein sites targeted by human antibody repertoires, and map common mutational escape routes to predict future High Potency variants Broad VOC Binders IDENTIFY Identify potent mAb candidates that target rare epitopes not Low Escape Risk under strong immune pressure Multiple Candidates OPTIMIZE Engineer to optimize candidate properties Engineer 6
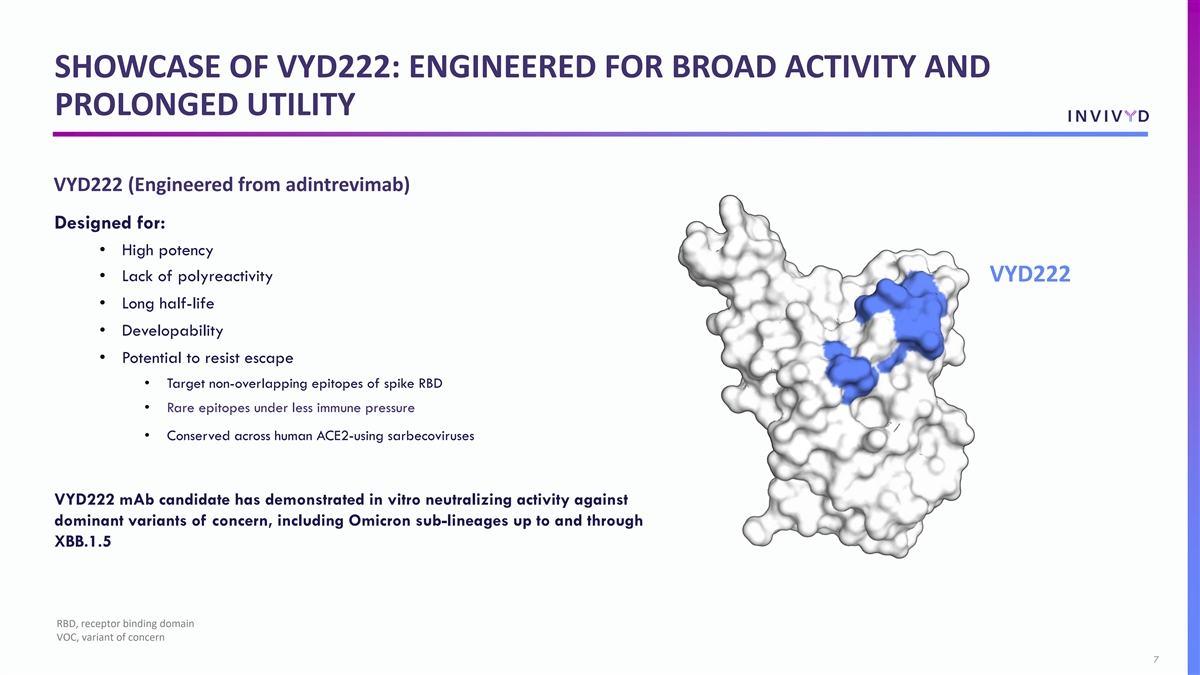
SHOWCASE OF VYD222: ENGINEERED FOR BROAD ACTIVITY AND PROLONGED UTILITY VYD222 (Engineered from adintrevimab) Designed for: • High potency • Lack of polyreactivity VYD222 • Long half-life • Developability • Potential to resist escape • Target non-overlapping epitopes of spike RBD • Rare epitopes under less immune pressure • Conserved across human ACE2-using sarbecoviruses VYD222 mAb candidate has demonstrated in vitro neutralizing activity against dominant variants of concern, including Omicron sub-lineages up to and through XBB.1.5 RBD, receptor binding domain VOC, variant of concern 7
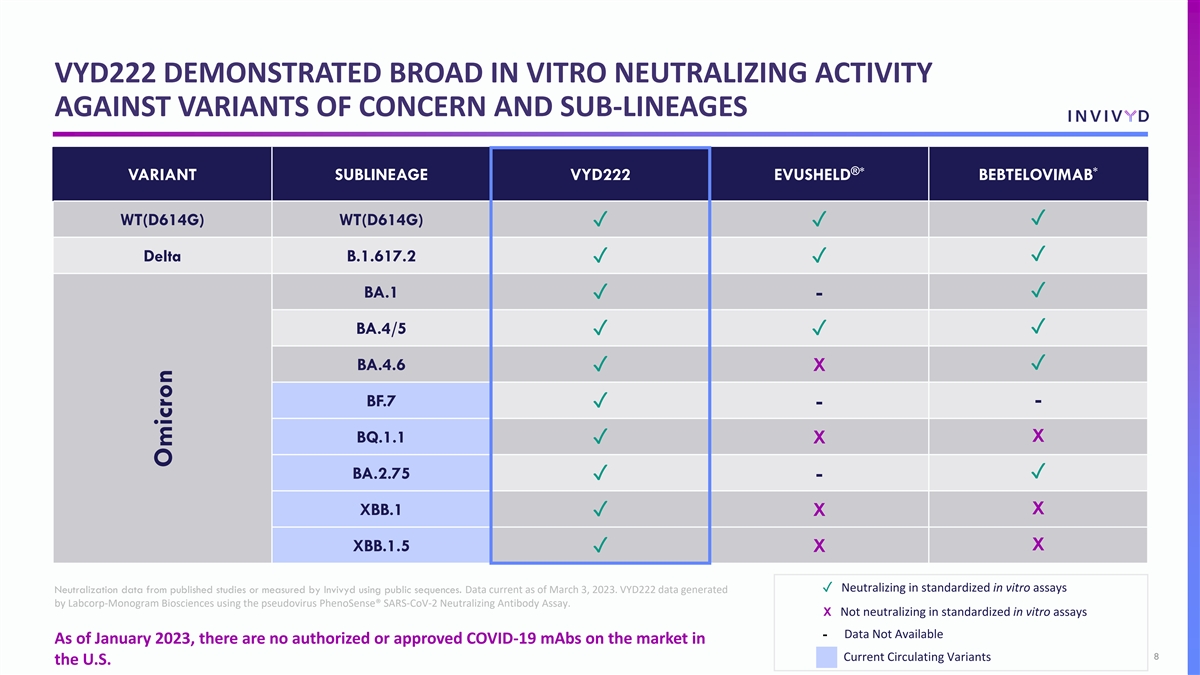
VYD222 DEMONSTRATED BROAD IN VITRO NEUTRALIZING ACTIVITY AGAINST VARIANTS OF CONCERN AND SUB-LINEAGES ®* * VARIANT SUBLINEAGE VYD222 EVUSHELD BEBTELOVIMAB WT(D614G) WT(D614G)✓ ✓✓ Delta B.1.617.2✓ ✓✓ BA.1✓ ✓ - BA.4/5✓ ✓✓ BA.4.6✓ ✓ X BF.7 - ✓ - BQ.1.1 X ✓ X BA.2.75✓ ✓ - XBB.1 X ✓ X X XBB.1.5 ✓ X ✓ Neutralizing in standardized in vitro assays Neutralization data from published studies or measured by Invivyd using public sequences. Data current as of March 3, 2023. VYD222 data generated by Labcorp-Monogram Biosciences using the pseudovirus PhenoSense® SARS-CoV-2 Neutralizing Antibody Assay. X Not neutralizing in standardized in vitro assays - Data Not Available As of January 2023, there are no authorized or approved COVID-19 mAbs on the market in 8 Current Circulating Variants the U.S. Omicron

DEMONSTRATED DEVELOPMENT SUCCESS AND SPEED WITH ADINTREVIMAB: FROM IND TO TOPLINE DATA IN 16 MONTHS Late Q4 2020 1H 2021 2H 2021 Q1 2022 Adintrevimab IND Phase 1 First in EVADE STAMP (treatment) Adintrevimab st Enabling & Human 1 Subject (prevention) Ph2/3 Enrollment Topline Data Submission Dosed Ph2/3 Enrollment Initiated Initiated (US) (ex-US) Adintrevimab is an investigational product candidate that is not approved for use in any country. The safety and efficacy of adintrevimab have not been established. 9
REGULATORS CONSIDERING STRATEGIES TO ACCELERTATE MONOCLONAL ANTIBODY DEVELOPMENT TIMELINES • Regulators are seeking strategies to streamline development of monoclonal antibodies and vaccines • The necessity of streamlined development for new mAb products is supported by the science • Understanding of SARS-CoV-2 biology has grown exponentially • Accumulating data enables scientists to predict mAb effectiveness based on in vitro data which can then be confirmed in patients • This is especially true for products closely related to those previously studied Possible Implication: Advance medicines to patients faster 10
BUILDING ON OUR VISION TO CREATE THE “PERPETUAL MACHINE” PREDICTION of viral evolution and rational selection of privileged epitopes Candidate antibody DISCOVERY and ENGINEERING COMMERCIAL design for a large drug category, not solely a pandemic emergency Efficient clinical DEVELOPMENT for multiple use cases and populations Flexible and highly efficient MANUFACTURING 11

VYD222 IS ONE OF MANY ANTIBODIES IN INVIVYD’S ROBUST PIPELINE DEVELOPMENT STATUS PROGRAMS PLATFORM INDICATION(S) STATUS DISCOVERY IND-ENABLING PHASE 1 PHASE 2 PHASE 3 CORONAVIRUSES Ph 1 trial planned Prevention or VYD222 mAb for Q1 2023 Treatment Engineering Prevention or VYD224 mAb variant matching Treatment COVID Prevention or Engineering mAb Candidate #3 Treatment variant matching COVID Prevention or Engineering mAb Candidate #4 Treatment variant matching Trials concluded, Adintrevimab mAb Prevention EUA filing dependent on variant Adintrevimab mAb Treatment susceptibility OTHER VIRUSES Investigational therapies are not approved for use by regulatory authorities. The safety and efficacy of pipeline candidates have not been established. mAb Influenza Prevention Early discovery Combination Investigational therapies are not approved for use by regulatory authorities. The safety and efficacy of pipeline candidates have not been established. 12

COMPANY WELL CAPITALIZED TO DEVELOP LEAD CANDIDATE & ADDITIONAL PIPELINE ASSETS Total fully diluted shares of Cash Position: common stock Cash, cash equivalents and Planned cash runway into outstanding* as of marketable securities were Q2 2024 $419 million as of September September 30, 2022: 30, 2022 130.4 million *Includes vested and unvested outstanding options as of September 30, 2022; excludes treasury stock 13

THANK YOU













