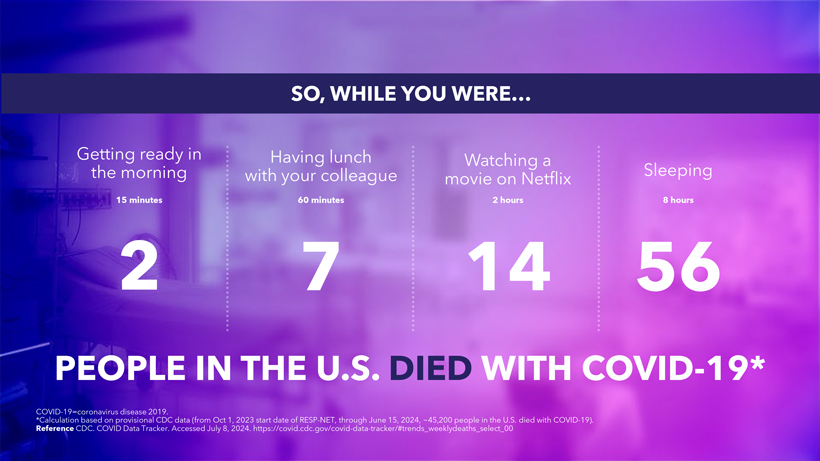
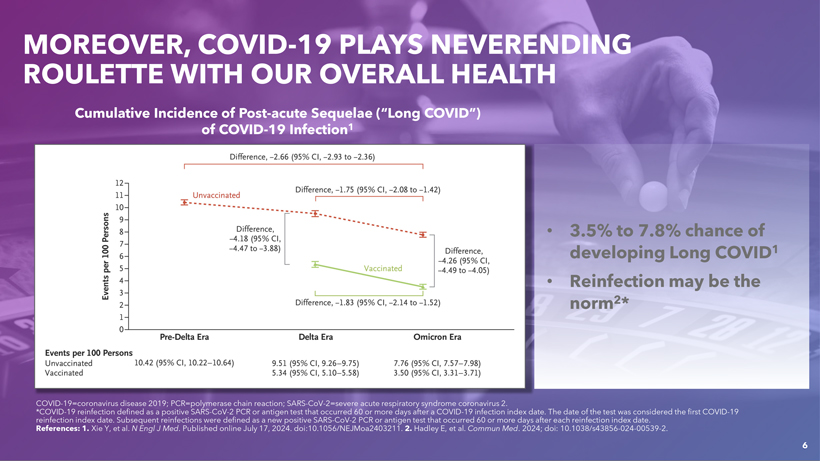
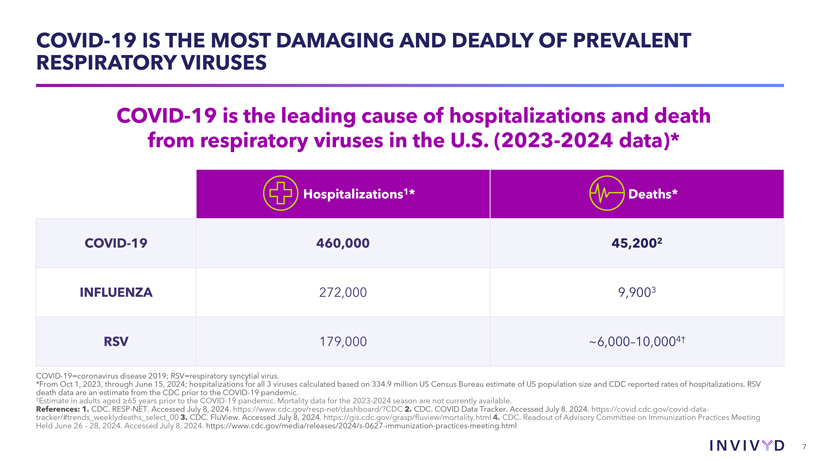
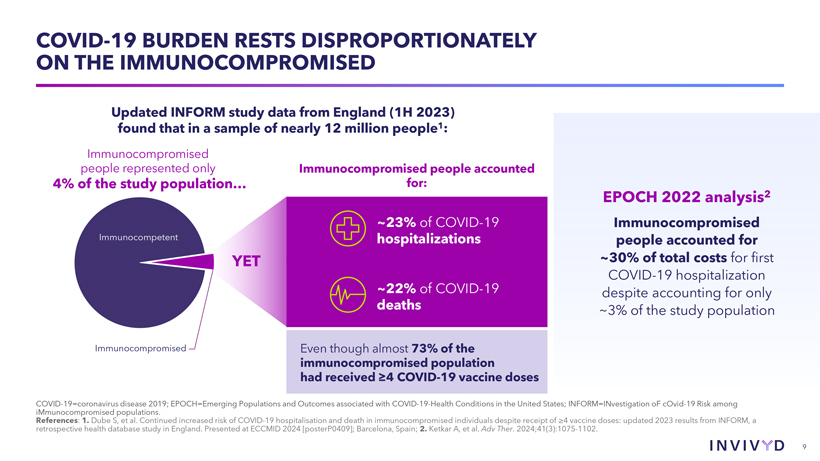
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS This presentation contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Statements in this presentation that are not statements of historical fact are forward-looking statements. Words such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “seek,” “could,” “intend,” “target,” “aim,” “project,” “designed to,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements include statements concerning, among other things, PEMGARD (pemivibart) as a monoclonal antibody (mAb) for pre-exposure prophylaxis (PrEP) of COVID-19 in certain adults and adolescents with moderate-to-severe immune compromise; our plans, strategy and expectations related to the launch and commercialization of PEMGARDA; the potential of our platform, including with respect to our mAb engineering capabilities, anticipated future immunobridging studies, and the potential commercial opportunity for our product candidates; the company’s general alignment with the U.S. Food and Drug Administration (FDA) on a repeatable immunobridging pathway to future potential Emergency Use Authorization (EUA) for serial, novel mAbs for the prevention and treatment of symptomatic COVID-19, including the company’s beliefs regarding the potential benefits, certain anticipated costs, and possible outcomes of utilizing such pathway; the company’s EUA amendment request to the FDA for PEMGARDA for COVID-19 treatment in certain immunocompromised patients; the company’s expectation that, if authorized, PEMGARDA would represent the first and only mAb authorized in PrEP and treatment of COVID-19 in certain immunocompromised patients; the potential of pemivibart for clinical protection from symptomatic COVID-19 based on the 180-day exploratory clinical efficacy data from the CANOPY clinical trial; the future of the COVID-19 landscape; our beliefs regarding the sufficiency of certain other COVID-19 therapies; our expectations about the potential market opportunity for mAbs, as well as our market position; our research and clinical development efforts, including statements regarding initiation or completion of studies or trials, the time-frame during which results may become available, and the potential utility of generated data; our expectations regarding advancement of our pipeline and anticipated improved drug profiles; our expectations regarding the biophysical properties and development of VYD2311; our beliefs regarding potential adjacent opportunities; our business strategies and objectives, and ability to execute on them; our future prospects; and other statements that are not historical fact. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements and you should not place undue reliance on our forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause our actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation: how long the EUA granted by the FDA for PEMGARDA for COVID-19 PrEP in certain adults and adolescents with moderate-to-severe immune compromise will remain in effect and whether such EUA is revoked or revised by the FDA; our ability to maintain and expand sales, marketing and distribution capabilities to successfully commercialize PEMGARDA; changes in expected or existing competition; the outcome of the company’s EUA amendment request for PEMGARDA for COVID-19 treatment in certain immunocompromised patients, and the timing thereof; our ability to effectively utilize an immunobridging pathway to potential EUA for serial, novel mAbs for the prevention and treatment of COVID-19; whether we are able to successfully submit any future EUA request to the FDA, and the timing, scope and outcome of any such EUA request; uncertainties related to the regulatory authorization or approval process, and available development and regulatory pathways for authorization or approval of the company’s product candidates; changes in the regulatory environment; the timing, progress and results of our discovery, preclinical and clinical development activities; unexpected safety or efficacy data observed during preclinical studies or clinical trials; the ability to maintain a continued acceptable safety, tolerability and efficacy profile of any product candidate following regulatory authorization or approval; the predictability of clinical success of our product candidates based on neutralizing activity in nonclinical studies; the risk that results of nonclinical studies or clinical trials may not be predictive of future results, and interim data are subject to further analysis; our reliance on third parties with respect to virus assay creation and product candidate testing and with respect to our clinical trials; variability of results in models used to predict activity against SARS-CoV-2 variants; potential variability in neutralizing activity of product candidates tested in different assays, such as pseudovirus assays and authentic assays; whether pemivibart, VYD2311, or any other product candidate is able to demonstrate and sustain neutralizing activity against major SARS-CoV-2 variants, particularly in the face of viral evolution; the complexities of manufacturing mAb therapies; our dependence on third parties to manufacture, label, package, store and distribute clinical and commercial supplies of our product candidates; whether we are able to provide sufficient commercial supply of PEMGARDA to meet market demand; whether we can obtain and maintain third-party coverage and adequate reimbursement for PEMGARDA or any other product candidate; whether we are able to achieve high potency and/or variation resistance with our future product pipeline; any legal proceedings or investigations relating to the company; our ability to continue as a going concern; and whether we have adequate funding to meet future operating expenses and capital expenditure requirements. Other factors that may cause our actual results to differ materially from those expressed or implied in the forward-looking statements in this presentation are described under the heading “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2023 and our Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, each filed with the Securities and Exchange Commission (SEC), and in our other filings with the SEC, and in our future reports to be filed with the SEC and available at www.sec.gov. Forward-looking statements contained in this presentation are made as of this date, and we undertake no duty to update such information whether as a result of new information, future events or otherwise, except as required under applicable law. 2