
Exhibit 99.2 INVIVYD Q3 2024 FINANCIAL RESULTS & BUSINESS HIGHLIGHTS November 14, 2024 © 2024 Invivyd, Inc. Invivyd , Pemgarda , and the Ribbon logos are trademarks of Invivyd, Inc. All trademarks in this presentation are the property of their respective owners.

CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS This presentation contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. Statements in this presentation that are not statements of historical fact are forward-looking statements. Words such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “seek,” “could,” “intend,” “target,” “aim,” “project,” “designed to,” “estimate,” “believe,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. Forward-looking statements include statements concerning, among other things, PEMGARDA (pemivibart) as a monoclonal antibody (mAb) for pre-exposure prophylaxis (PrEP) of COVID-19 in certain immunocompromised patients; the company’s plans, strategies, goals and expectations related to the commercialization of PEMGARDA; the future of the COVID-19 landscape and impacts of COVID-19; the company’s expectation regarding its cash and cash equivalents balance at the end of 2024; the company’s aim for near-term profitability; the company’s belief that its existing cash and cash equivalents, anticipated growth of net product revenue, and various operational efficiency improvements will be sufficient to fund operations through profitability; the company’s belief that it is positioned for a return to growth; the company’s research and clinical development efforts, including statements regarding initiation or completion of studies or trials, the time-frame during which results may become available, and the potential utility of generated data; the company’s expectations regarding advancement of its pipeline and the expected profile and development program for VYD2311; the company’s expectations regarding the neutralization activity of pemivibart against SARS-CoV-2 variants, including XEC; the company’s business strategies and objectives, and ability to execute on them; the company’s future prospects; and other statements that are not historical fact. The company may not actually achieve the plans, intentions or expectations disclosed in the company’s forward-looking statements and you should not place undue reliance on the company’s forward-looking statements. These forward-looking statements involve risks and uncertainties that could cause the company’s actual results to differ materially from the results described in or implied by the forward-looking statements, including, without limitation: uncertainties regarding the company’s expectations, projections and estimates regarding future costs and expenses, future revenue, capital requirements, and the availability of and the need for additional financing; whether the company’s cash and cash equivalents are sufficient to support its operating plan for as long as anticipated; uncertainties regarding market acceptance, payor coverage or future sales and revenue generated by PEMGARDA; uncertainties regarding the potential advantages from the company’s planned operational efficiency improvements; how long the EUA granted by the U.S. Food & Drug Administration (FDA) for PEMGARDA for COVID-19 PrEP in certain immunocompromised patients will remain in effect and whether such EUA is revised or revoked by the FDA; the potential negative impacts on Invivyd’s business of any virologic activity data in the public domain that creates doubt regarding the neutralization activity of pemivibart or any other of Invivyd’s product candidates that is generated by academic or other third-party labs and not as part of Invivyd’s ongoing industrial-grade virology efforts; the company’s ability to maintain and expand sales, marketing and distribution capabilities to successfully commercialize PEMGARDA; changes in expected or existing competition; the outcome of the company’s EUA amendment request for PEMGARDA for treatment of mild-to-moderate COVID-19 in certain immunocompromised patients, and the timing thereof; uncertainties related to the regulatory authorization or approval process; changes in the regulatory environment; the timing, progress and results of the company’s discovery, preclinical and clinical development activities; unexpected safety or efficacy data observed during preclinical studies or clinical trials; the ability to maintain a continued acceptable safety, tolerability and efficacy profile of any product candidate following regulatory authorization or approval; the predictability of clinical success of the company’s product candidates based on neutralizing activity in nonclinical studies; the risk that results of nonclinical studies or clinical trials may not be predictive of future results, and interim data are subject to further analysis; the company’s reliance on third parties with respect to virus assay creation and product candidate testing and with respect to its clinical trials; potential variability in neutralizing activity of product candidates tested in different assays, such as pseudovirus assays and authentic assays; variability of results in models and methods used to predict activity against SARS-CoV-2 variants; formal assay assessment results in comparison to predictions made using Invivyd’s molecular panel approach with respect to neutralization activity of pemivibart; whether PEMGARDA, VYD2311, or any other product candidate is able to demonstrate and sustain neutralizing activity against major SARS-CoV-2 variants, particularly in the face of viral evolution; the complexities of manufacturing mAb therapies; the company’s dependence on third parties to manufacture, label, package, store and distribute clinical and commercial supplies of its product candidates; the company’s ability to leverage its INVYMAB platform approach to facilitate the rapid, serial generation of new mAbs to address evolving viral threats; any legal proceedings or investigations relating to the company; the company’s ability to continue as a going concern; and whether the company has adequate funding to meet future operating expenses and capital expenditure requirements. Other factors that may cause the company’s actual results to differ materially from those expressed or implied in the forward-looking statements in this presentation are described under the heading “Risk Factors” in the company’s Annual Report on Form 10-K for the year ended December 31, 2023 and the company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2024, each filed with the Securities and Exchange Commission (SEC), and in the company’s other filings with the SEC, and in its future reports to be filed with the SEC and available at www.sec.gov. Forward-looking statements contained in this press release are made as of this date, and Invivyd undertakes no duty to update such information whether as a result of new information, future events or otherwise, except as required under applicable law. 2

u Executive Summary u CANOPY Phase 3 Clinical Trial: 12 Month Data Update u Pipeline u Commercial Update Finance Q&A 3
A QUARTER OF HIGH ENGAGEMENT DESPITE UNEXPECTED FACT SHEET HEADWIND; POSITIONED FOR RETURN TO GROWTH • CANOPY exploratory clinical efficacy data, to date, reconfirm a high level of risk reduction from developing symptomatic COVID-19 in immunocompetent participants (84% RRR months 1-6, and 64% RRR months 7-12 with no additional drug) • Structural biology predicts continued neutralization activity for pemivibart against SARS- CoV-2 variant XEC, with formal assay assessment from Monogram pending • PEMGARDA uptake accelerated nicely prior to FDA inclusion of a link to inaccurate non- PEMGARDA data in August product Fact Sheet; flat September sales growth with return to growth after September Fact Sheet update. Impact of recent publication in the New England Journal of Medicine (Aaron Diamond AIDS Research Laboratory / Dr. David D. Ho) unclear • Commercial efforts have established breadth and now targeting pull-through • Pemivibart treatment EUA application pending; VYD2311 offering potential improved clinical & commercial profile advancing with anticipated preliminary data readout late Q4 2024 RRR = Relative Risk Reduction 4

u Executive Summary u CANOPY Phase 3 Clinical Trial: 12 Month Data Update Pipeline Commercial Update Finance Q&A 5
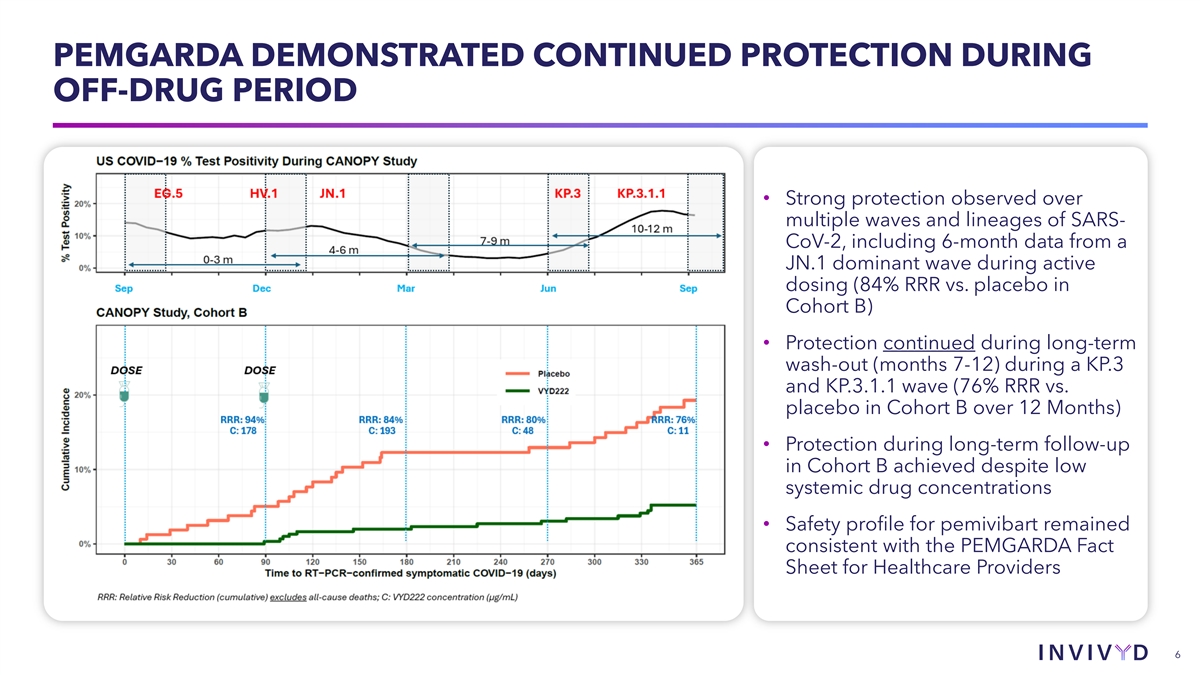
PEMGARDA DEMONSTRATED CONTINUED PROTECTION DURING OFF-DRUG PERIOD • Strong protection observed over multiple waves and lineages of SARS- CoV-2, including 6-month data from a JN.1 dominant wave during active dosing (84% RRR vs. placebo in Cohort B) • Protection continued during long-term wash-out (months 7-12) during a KP.3 and KP.3.1.1 wave (76% RRR vs. placebo in Cohort B over 12 Months) • Protection during long-term follow-up in Cohort B achieved despite low systemic drug concentrations • Safety profile for pemivibart remained consistent with the PEMGARDA Fact Sheet for Healthcare Providers 6 6

u Executive Summary u CANOPY Phase 3 Clinical Trial: 12 Month Data Update u Pipeline Commercial Update Finance Q&A 7
NEXT UP: VYD2311, A MAB WITH HIGH IN VITRO POTENCY SHOWN AGAINST POST-OMICRON COVID-19 VARIANTS TESTED TO DATE Our next-generation mAb, VYD2311, improves biophysical properties; shows continued in vitro neutralization activity in pseudovirus assays against KP1.1 FLiRT, KP.2 FLiRT, KP.3, KP.3.1.1 and LB.1 variants Development: • First-in-human clinical trial dosing began in August 2024 assessing PK and safety with anticipated preliminary data readout late Q4 2024 with additional clinical readouts throughout 2025 • Development program for VYD2311 designed to evaluate diverse routes of administration (e.g., IV, IM, SC) for Treatment and Prevention • Assessment of authorization pathways and titer thresholds with regulators ongoing COVID-19= COVID-19=coronavirus disease 2019; IM=intramuscular; IV=intravenous; mAb=monoclonal antibody; PrEP=pre-exposure prophylaxis; SC=subcutaneous; PK=Pharmacokinetics. Reference: Invivyd. Data on File. 8

u Executive Summary u CANOPY Phase 3 Clinical Trial: 12 Month Data Update u Pipeline u Commercial Update Finance Q&A 9
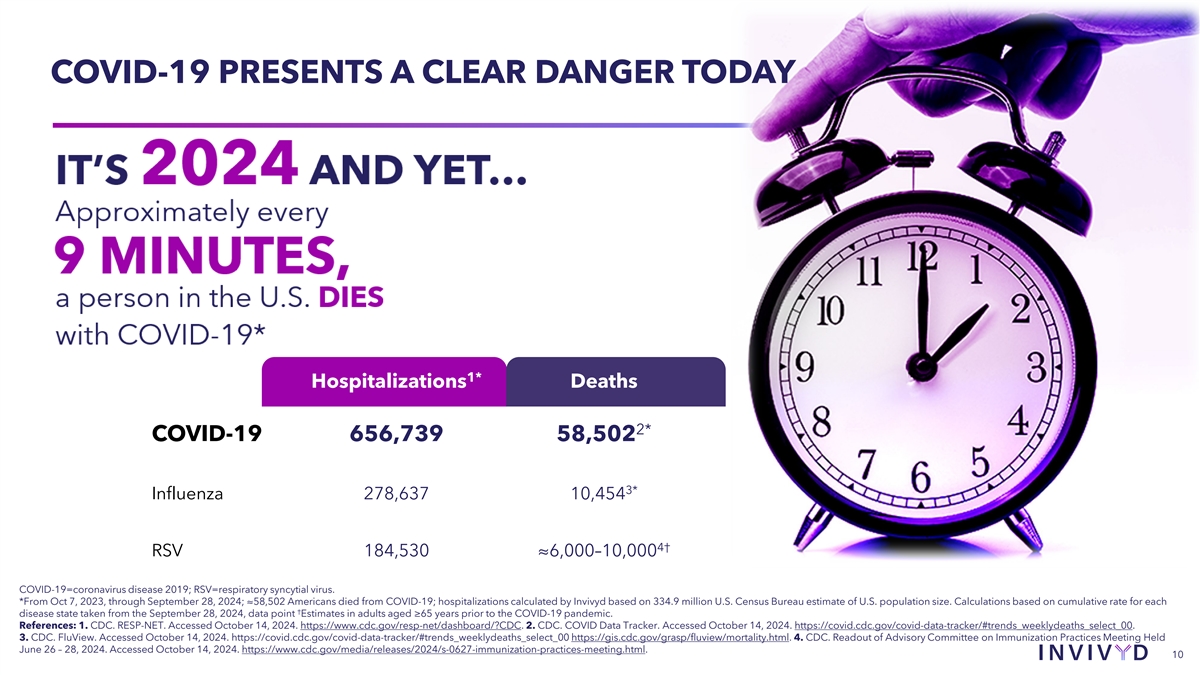
COVID-19 PRESENTS A CLEAR DANGER TODAY 1* Hospitalizations Deaths 2* COVID-19 656,739 58,502 3* Influenza 278,637 10,454 4† RSV 184,530 ≈6,000–10,000 COVID-19=coronavirus disease 2019; RSV=respiratory syncytial virus. *From Oct 7, 2023, through September 28, 2024; ≈58,502 Americans died from COVID-19; hospitalizations calculated by Invivyd based on 334.9 million U.S. Census Bureau estimate of U.S. population size. Calculations based on cumulative rate for each † disease state taken from the September 28, 2024, data point Estimates in adults aged ≥65 years prior to the COVID-19 pandemic. References: 1. CDC. RESP-NET. Accessed October 14, 2024. https://www.cdc.gov/resp-net/dashboard/?CDC. 2. CDC. COVID Data Tracker. Accessed October 14, 2024. https://covid.cdc.gov/covid-data-tracker/#trends_weeklydeaths_select_00. 3. CDC. FluView. Accessed October 14, 2024. https://covid.cdc.gov/covid-data-tracker/#trends_weeklydeaths_select_00 https://gis.cdc.gov/grasp/fluview/mortality.html. 4. CDC. Readout of Advisory Committee on Immunization Practices Meeting Held June 26 – 28, 2024. Accessed October 14, 2024. https://www.cdc.gov/media/releases/2024/s-0627-immunization-practices-meeting.html. 10
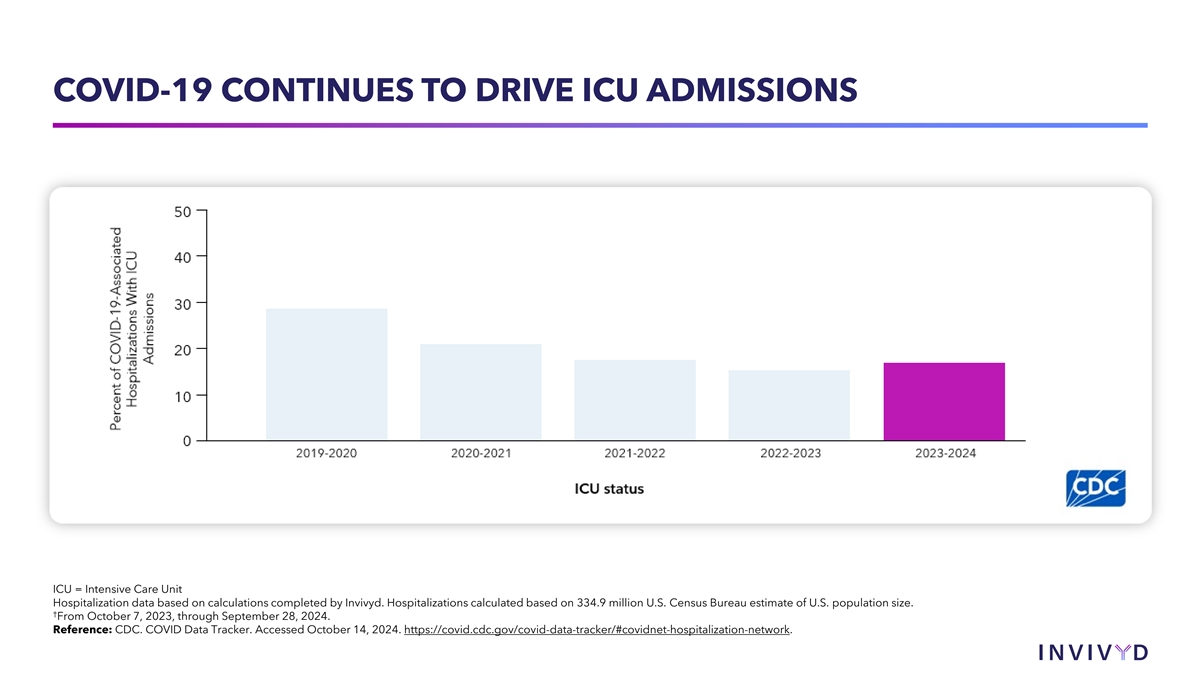
COVID-19 CONTINUES TO DRIVE ICU ADMISSIONS ICU = Intensive Care Unit Hospitalization data based on calculations completed by Invivyd. Hospitalizations calculated based on 334.9 million U.S. Census Bureau estimate of U.S. population size. † From October 7, 2023, through September 28, 2024. Reference: CDC. COVID Data Tracker. Accessed October 14, 2024. https://covid.cdc.gov/covid-data-tracker/#covidnet-hospitalization-network.
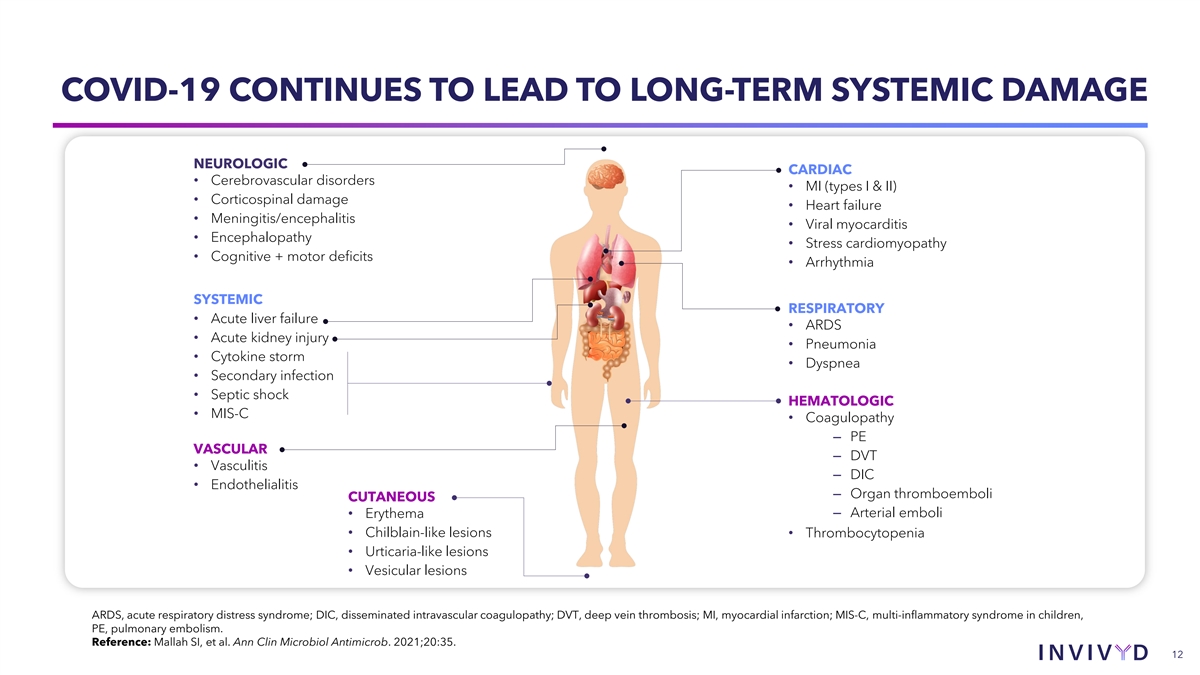
COVID-19 CONTINUES TO LEAD TO LONG-TERM SYSTEMIC DAMAGE NEUROLOGIC CARDIAC • Cerebrovascular disorders • MI (types I & II) • Corticospinal damage • Heart failure • Meningitis/encephalitis • Viral myocarditis • Encephalopathy • Stress cardiomyopathy • Cognitive + motor deficits • Arrhythmia SYSTEMIC RESPIRATORY • Acute liver failure • ARDS • Acute kidney injury • Pneumonia • Cytokine storm • Dyspnea • Secondary infection • Septic shock HEMATOLOGIC • MIS-C • Coagulopathy ‒ PE VASCULAR ‒ DVT • Vasculitis ‒ DIC • Endothelialitis ‒ Organ thromboemboli CUTANEOUS ‒ Arterial emboli • Erythema • Chilblain-like lesions • Thrombocytopenia • Urticaria-like lesions • Vesicular lesions ARDS, acute respiratory distress syndrome; DIC, disseminated intravascular coagulopathy; DVT, deep vein thrombosis; MI, myocardial infarction; MIS-C, multi-inflammatory syndrome in children, PE, pulmonary embolism. Reference: Mallah SI, et al. Ann Clin Microbiol Antimicrob. 2021;20:35. 12
13 POISED FOR ACCELERATION AND GROWTH Initiatives for Growth Q4 into 2025 • Anticipated Tail Winds from COVID-19 Seasonal • Headwinds created by August Fact Sheet Spike with the Holidays and Indoor Gatherings Update o Caused 30 days of confusion • Investing in Direct Hire Resources • Developing Infusion Networks at Scale o Strategic Account Management (SAM) team with deep o Partnering with Independent academic center expertise Infusion Networks and Integrated Delivery o Key Account Managers (KAMs) expand coverage to Networks to increase availability community academic centers o Deployed Inside Sales – Focus on Rheumatology • Developing a Digital Ecosystem • Expanding Access o Expanding healthcare provider and patient o Deployed Field Reimbursement Managers Reach o Standing Up Patient Support Program o Federal Account Managers • First Promotional Speaker Programs

KEY LAUNCH METRICS SHOWING EXPANDED COMMERCIAL COVERAGE As of July 31 As of Aug 31 As of Sept 30 As of Oct 31 HCP Interactions 2,032 2,698 3,198 3,722 Logged Unique Accounts 911 1,099 1,216 1,365 Called On Unique Accounts 208 274 347 426 Ordered • Breadth of clinician and patient experience with new team driving depth while expanding deeper into target universe • Commercial coverage across national and regional plans, including United Health Care, Aetna, Cigna, and Regional Blue Cross Plans Source: Invivyd data on file 14

u Executive Summary u CANOPY Phase 3 Clinical Trial: 12 Month Data Update u Pipeline u Commercial Update u Finance Q&A 15

FINANCIALS • Q3 2024 PEMGARDA (pemivibart) net product revenue of $9.3 million • Ended Q3 2024 with approximately $106.9 million in cash and cash equivalents • Targeting near-term (1H 2025) profitability with existing cash and cash equivalents, anticipated growth of net product revenue, and various operational efficiency improvements underway • VYD2311 clinical and launch material production included in YTD financial results; meaningful quantities expensed to R&D • Continuing to evaluate multiple sources of additional capital 16 Source: Invivyd Data on File

u Executive Summary u CANOPY Phase 3 Clinical Trial: 12 Month Data Update u Pipeline u Commercial Update u Finance u Q&A 17
















