Exhibit 10.14
FIRST AMENDMENT TO LICENSE AGREEMENT
This First Amendment to License Agreement (this “First Amendment”) is entered into as of January 28, 2022 (the “First Amendment Date”) by and between Arena Pharmaceuticals, Inc., a Delaware corporation (“Licensor”), and Longboard Pharmaceuticals, Inc., a Delaware corporation (“Licensee”), each individually a “Party” and together, the “Parties”. For the purposes of Nelotanserin and Nelotanserin Product and provisions related thereto only, 125 Royalty Inc., a Delaware corporation (“125 Royalty”), is considered a Party to this First Amendment and to the Agreement as amended by this First Amendment as set forth above its signature herein.
Recitals
Whereas, Licensor and Licensee are parties to that certain License Agreement, dated October 27, 2020 (the “Agreement”); and
Whereas, Licensor and 125 Royalty have rights to the compound nelotanserin; and
Whereas, the Parties now mutually desire to amend, modify and restate certain terms and conditions of the Agreement to include nelotanserin as a Licensed Product under the Agreement.
Now, Therefore, for and in consideration of the mutual covenants contained herein, and for other good and valuable considerations, the receipt and sufficiency of which are hereby acknowledged, Licensor and Licensee hereby agree as follows:
Agreement
(a)Capitalized terms and section or article references used but not otherwise defined in this First Amendment shall have the meanings ascribed to them in the Agreement.
(b)The following definitions shall be added to Section 1 of the Agreement:
1.88 “Nelotanserin” shall mean (a) the compound designated on Exhibit 1.1A and referred to by Licensor as nelotanserin, and (b) any salt, solvate, hydrate, intermediate, pro-drug or metabolite thereof. Nelotanserin shall be a 2A Compound, in addition to the 2A Compounds originally listed in Exhibit 1.1 to the Agreement.
1.89 “Nelotanserin Product” means any pharmaceutical product, in any dosage strength or formulation, containing Nelotanserin as an active pharmaceutical ingredient. Nelotanserin Product shall be a 2A Product, in addition to the 2A Products originally provided in the Agreement.
1.90 “125 Royalty” shall mean 125 Royalty Inc., a Delaware corporation.
1
258997870 v5
(c)The definition of “Effective Date” contained in the Agreement is hereby amended and restated as follows:
“1.44 “Effective Date” shall mean (a) October 27, 2020, except as expressly provided in clause (b), and (b) with respect to Nelotanserin and Nelotanserin Product (and defined terms and other provisions of this Agreement (including 2A Know-How and 2A Patents) only as they relate to Nelotanserin and Nelotanserin Product, January 28, 2022.”
(d)The definition of “Licensor Product” set forth on Appendix A of the Agreement is hereby amended and restated to read in its entirety as follows:
““Licensor Product” shall mean any of etrasimod, lorcaserin, olorinab, or temanogrel, in any dosage strength or formulation.”
(a)Section 2.1(d) of the Agreement is hereby amended to add the following sentence at the end of such section:
“With respect to the license granted to the Nelotanserin Product under this Section 2.1(d), the 2A Technology includes Patents and Information licensed to 125 Royalty, an Affiliate of Licensor, pursuant to the Development, Marketing and Supply Agreement by and between Arena Pharmaceuticals GmbH and Roivant Sciences Ltd., dated May 18, 2015, which license survived termination of such agreement pursuant to the Termination Agreement by and between 125 Royalty (a successor-in-interest to API Development Ltd. and Arena Pharmaceuticals GmbH) and Axovant Sciences GmbH (a successor-in-interest to Axovant Sciences Ltd. and Roivant Sciences Ltd.), dated December 1, 2019.”
(b)Article 4 of the Agreement is hereby amended to add Section 4.5 as follows:
“4.5 Commercial Milestone Payments for Nelotanserin Product. When Aggregate Annual Net Sales of Nelotanserin Product in any calendar year first reach the applicable threshold set forth in the table below, Licensee shall pay to Licensor the corresponding non-refundable, non-creditable milestone payment set forth in the table below. Each milestone payment in this Section 4.5 is payable one time only upon the first achievement of the applicable milestone event, and the total amount of the milestone payments payable under this Section 4.5 if both milestone events are achieved is $50,000,000.
2
258997870 v5
| |
Aggregate Annual Net Sales of Nelotanserin Product | Milestone Payment |
First calendar year in which Aggregate Annual Net Sales of Nelotanserin Product equal or exceed $100,000,000 | $25,000,000 |
First calendar year after the above milestone is due in which Aggregate Annual Net Sales of Nelotanserin Product equal or exceed $500,000,000* | $25,000,000 |
*For clarity, Licensor is only eligible to receive one milestone payment in a calendar year. By way of example, and not limitation, should Aggregate Annual Net Sales of Nelotanserin Product equal or exceed $600,000,000 prior to achievement of the first milestone event, then the total $50,000,000 of milestone payments under Section 4.5 will be payable in two installments; the first $25,000,000 installment will be due pursuant to Section 5.1 and the second $25,000,000 installment will be due on the date that is 12 months afterwards.
(c)Section 5.1 of the Agreement is hereby amended and restated to read in its entirety as follows:
“5.1 Payment; Reports. Royalties and milestone payments due by Licensee to Licensor under Article 4 shall be calculated and reported for each calendar quarter. Licensee shall, within five Business Days after the end of each calendar quarter, provide Licensor (i) a good faith estimate of the royalties due for such calendar quarter, and (ii) a statement of any milestone payments due for such calendar quarter. Licensee shall pay Licensor the royalties and any milestone payments due within 45 days after the end of such calendar quarter. Each payment shall be accompanied by a report of Net Sales of each Licensed Product by Licensee and its Affiliates and Sublicensees in sufficient detail to permit confirmation of the accuracy of the payment made, including gross sales and Net Sales of each Licensed Product on a country-by-country basis, including deductions as applicable to calculate Net Sales the royalty payable, the method used to calculate the royalties, and the exchange rates used to calculate the royalties.”
(d)Section 7.2(b) of the Agreement is hereby amended and restated to read in its entirety as follows:
“(b) There are no agreements in effect as of the Effective Date between Licensor and a Third Party under which rights with respect to the Licensed Technology has been licensed to Licensor, except as described in Section 2.1(d) with respect to Nelotanserin Product;”
3
258997870 v5
(e)Exhibit 1.1 of the Agreement, listing the 2A Compounds, is hereby amended to add Nelotanserin as set forth on Exhibit 1.1A to this First Amendment.
(f)Exhibit 1.5 of the Agreement, listing the 2A Patents, is hereby amended to add Patents for Nelotanserin as set forth on Exhibit 1.5A to this First Amendment.
3.2A Patents for Nelotanserin. Notwithstanding Section 8.2(a)(ii) of the Agreement, upon written notice to Licensor, Licensee shall have the right to elect to control the preparation, filing, prosecution and maintenance of 2A Patents with respect to Nelotanserin from and after the Effective Date with respect to Nelotanserin.
4.Effectiveness of the Agreement. Except as expressly amended by this First Amendment, the Agreement shall remain in full force and effect in accordance with its terms.
5.Counterparts. This First Amendment may be executed in counterparts, each of which counterparts, when so executed and delivered, shall be deemed to be an original, and all of which counterparts, taken together, shall constitute one and the same instrument even if the Parties have not executed the same counterpart. Signatures provided by facsimile transmission or by email of a .pdf attachment shall be deemed to be original signatures.
[Remainder of this page intentionally blank.]
4
258997870 v5
In Witness Whereof, each Party has duly executed this First Amendment as of the First Amendment Date.
| |
Arena Pharmaceuticals, Inc. | Longboard Pharmaceuticals, Inc. |
By: Name: Title: | By: Name: Title: |
125 Royalty acknowledges and agrees to the terms of this First Amendment and to the terms of the Agreement that apply to Nelotanserin, Nelotanserin Product or provisions related thereto only, and is a Party to this First Amendment and the Agreement for those purposes only; and any amendment to the Agreement as applicable to Nelotanserin, Nelotanserin Product or provisions related thereto to which 125 Royalty is a Party will require written agreement of both Licensor and 125 Royalty. 125 Royalty Inc. | |
By: Name: Title: | |
5
258997870 v5
Exhibit 1.1A
2A Compound - Nelotanserin
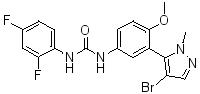
AR231142
258997870 v5
Exhibit 1.5A
2A Patents - Nelotanserin
| | | | | | |
Ref. No. | Application No. | Filing Date | Patent No. | Issue Date | Country | Status |
072.AR2.DIV | P140102303 | 7/21/2004 | | | ARGENTINA | PUBLISHED |
072.CL1.REG | 2005-0085 | 1/17/2005 | 46.245 | 1/12/2010 | CHILE | ISSUED |
072.DE1.EPO | 04758159.0 | 7/21/2004 | 1558582 | 12/21/2005 | GERMANY | ISSUED |
072.ES1.EPO | 04758159.0 | 7/21/2004 | 1558582 | 12/21/2005 | SPAIN | ISSUED |
072.FR1.EPO | 04758159.0 | 7/21/2004 | 1558582 | 12/21/2005 | FRANCE | ISSUED |
072.GB1.EPO | 04758159.0 | 7/21/2004 | 1558582 | 12/21/2005 | UNITED KINGDOM | ISSUED |
072.IL1.PCT | 172582 | 7/21/2004 | 172582 | 2/1/2011 | ISRAEL | ISSUED |
072.IT1.EPO | 04758159.0 | 7/21/2004 | 1558582 | 12/21/2005 | ITALY | ISSUED |
072.JP1.PCT | 2006-521215 | 7/21/2004 | 4198733 | 10/10/2008 | JAPAN | ISSUED |
072.MX1.PCT | PA/a/2006/000795 | 7/21/2004 | 258421 | 7/3/2008 | MEXICO | ISSUED |
072.US10.CON | 16/799,556 | 7/21/2004 | | | UNITED STATES | PUBLISHED |
072.US3.REG | 10/895,789 | 7/21/2004 | 8,754,238 | 6/17/2014 | UNITED STATES | ISSUED |
072.US6.DIV | 13/619,137 | 9/14/2012 | 8,871,797 | 10/28/2014 | UNITED STATES | ISSUED |
258997870 v5
| | | | | | |
072.US7.DIV | 14/332,207 | 7/15/2014 | 9,273,035 | 3/1/2016 | UNITED STATES | ISSUED |
072.US8.CON | 15/013,057 | 2/2/2016 | 9,775,829 | 10/3/2017 | UNITED STATES | ISSUED |
072.VE1.REG | 2005-000033 | 1/10/2005 | | | VENEZUELA | PUBLISHED |
083.US2.PCT | 10/593,847 | 9/21/2006 | 7,812,176 | 10/12/2010 | UNITED STATES | ISSUED |
142.CN4.DIV | 200980152821.4 | 10/27/2009 | | | CHINA | PUBLISHED |
142.HK2.CN | 19128214.4 | 10/27/2009 | | | HONG KONG | PUBLISHED |
142.US2.PCT | 13/126564 | 4/28/2011 | 9,034,911 | 5/19/2015 | UNITED STATES | ISSUED |
142.US3.CON | 14/679,487 | 4/6/2015 | 9,801,856 | 10/31/2017 | UNITED STATES | ISSUED |
142.US4.CON | 15/718,886 | 9/28/2017 | 10,117,851 | 11/6/2018 | UNITED STATES | ISSUED |
142.US5.CON | 16/144,330 | 9/27/2018 | 10,583,122 | 3/10/2020 | UNITED STATES | ISSUED |
142.US7.CON | 17/365,797 | 10/27/2009 | | | UNITED STATES | PENDING |
163.US2.PCT | 13/126,563 | 4/28/2011 | 9,126,946 | 9/8/2015 | UNITED STATES | ISSUED |
163.US3.CON | 14/679,421 | 4/6/2015 | 9,353,064 | 5/31/2016 | UNITED STATES | ISSUED |
163.US4.CON | 15/144,012 | 5/2/2016 | 9,745,270 | 8/29/2017 | UNITED STATES | ISSUED |
163.US5.CON | 15/681,766 | 8/21/2017 | 10,071,075 | 9/11/2018 | UNITED STATES | ISSUED |
258997870 v5
| | | | | | |
163.US6.CON | 16/057,587 | 8/7/2018 | 10,543,193 | 1/28/2020 | UNITED STATES | ISSUED |
275.AU2.DIV* | 2021269398 | 6/10/2016 | | | AUSTRALIA | PENDING |
275.CA1.PCT* | 2,989,343 | 6/10/2016 | | | CANADA | PUBLISHED |
275.EP1.PCT* | 16808478.8 | 6/10/2016 | | | EUROPEAN PATENT CONVENT | PUBLISHED |
275.HK1.EPO* | 18105276.4 | 6/10/2016 | | | HONG KONG | PUBLISHED |
275.JP2.DIV* | 2021-129480 | 6/10/2016 | | | JAPAN | PENDING |
275.KR1.PCT* | 10-2018-7000937 | 6/10/2016 | | | SOUTH KOREA | PENDING |
275.MX1.PCT* | MX/a/2017/016413 | 6/10/2016 | | | MEXICO | ALLOWED |
275.US6.REG* | 15/179,926 | 6/10/2016 | 10,022,355 | 7/17/2018 | UNITED STATES | ISSUED |
275.US8.CON* | 16/774,248 | 1/28/2020 | | | UNITED STATES | PUBLISHED |
275.ZA1.PCT* | 2018/00186 | 6/10/2016 | | | SOUTH AFRICA | PENDING |
277.CA1.PCT* | 2,992,518 | 7/15/2016 | | | CANADA | PUBLISHED |
277.CN1.PCT* | 201680050628X | 7/15/2016 | | | CHINA | PUBLISHED |
277.EP1.PCT* | 16825262.5 | 7/15/2016 | | | EUROPEAN PATENT CONVENT | PUBLISHED |
277.HK1.EPO* | 18106917.7 | 7/15/2016 | | | HONG KONG | PUBLISHED |
258997870 v5
| | | | | | |
277.JP2.DIV* | 2021-125769 | 7/15/2016 | | | JAPAN | PUBLISHED |
277.KR1.PCT* | 10-2018-7004357 | 7/15/2016 | | | SOUTH KOREA | PENDING |
277.MX1.PCT* | MX/a/2018/000465 | 7/15/2016 | | | MEXICO | PENDING |
277.US2.REG* | 15/211,638 | 7/15/2016 | 10,034,859 | 7/31/2018 | UNITED STATES | ISSUED |
277.US4.CON* | 17/100,528 | 11/20/2020 | | | UNITED STATES | ALLOWED |
280.US3.DIV* | 16/998,918 | 8/20/2020 | | | UNITED STATES | PUBLISHED |
* licensed from Axovant Sciences GmbH (a successor-in-interest to Axovant Sciences Ltd. and Roivant Sciences Ltd.) to 125 Royalty Inc.
258997870 v5
