
Corporate Presentation August 1, 2024 Exhibit 99.2

Forward-Looking Statements and Other Legal Notices This presentation contains forward-looking statements about Longboard Pharmaceuticals, Inc. (“we,” “Longboard” or the “Company”), including statements regarding: our vision; commercial opportunities and analogs; the potential of bexicaserin (LP352) and LP659, including to be best-in-class, to treat indications, to have differentiated or next-generation design, selectivity, specificity and other characteristics, and to meet an unmet need; anticipated milestones and timing; the prevalence of, unmet need associated with, and market opportunity for, DEEs; the bexicaserin phase 3 global program design, initiation timing and DEE path forward; Breakthrough Therapy designation for bexicaserin; LP659’s broad applicability, predictive data, commercial opportunities, next generation and selectivity characteristics, mechanism of action and preclinical data, potential to limit off-target effects, potential neurodegenerative disease therapeutic areas, indications and opportunities, topline SAD data and Phase 1 MAD initiation timing; our intellectual property; our ability to obtain regulatory approval and commercialize our drug candidates (in the manner we may propose or at all); and other statements that are not historical facts, including statements that may include words such as “will”, “may”, “can”, “would”, “plan”, “anticipate”, “expect”, “believe”, “potential”, “goal”, “opportunity” and similar words. For such statements, we claim the protection of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including, but not limited to: topline or interim data may not reflect the complete or final results of a particular study or trial, and are subject to change; nonclinical and clinical data are voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than us or others, request additional information, have additional recommendations or change their guidance or requirements before or after approval; we have a limited operating history, a history of incurring net losses and expectation that we will continue to incur net losses for the foreseeable future, and we may never be profitable; we will need additional capital to finance our operations; clinical and preclinical drug development involves lengthy and expensive processes with uncertain timing and outcomes; we have multiple product candidates with a variety of target indications, and we may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on a product candidate or indication that may be more profitable; the regulatory approval process in the U.S. and in other territories is lengthy, time consuming and inherently unpredictable, and we may not be able to obtain or maintain regulatory approval to conduct our clinical trials (in the manner we propose or at all) or, ultimately, to market our product candidates; receiving Breakthrough Therapy designation ma not lead to a faster development or regulator review or approval and does not mean bexicaserin will receive marketing approval for seizures associated with DEEs or for any other indication; risks relating to our ability to commercialize our product candidates and compete in the marketplace; risks regarding our license and dependencies on others; risks relating to our ability to obtain and maintain intellectual property protection and freedom to operate for our product candidates; risks relating to our ability to manage our growth; and other risks and factors disclosed in our filings with the U.S. Securities and Exchange Commission (the “SEC”). We operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. The forward-looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Except as required by law, we assume no responsibility for the accuracy and completeness of the forward-looking statements, and we undertake no obligation to update any forward-looking statements after the date of this presentation to conform these statements to actual results or to changes in our expectations. Certain information contained in or that may orally accompany this presentation relate to or are based on studies, research, publications, surveys and other data obtained from third-party sources and our own internal estimates and research. While we believe these third-party studies, research, publications, surveys and other data to be reliable as of the date of this presentation, they have not been independently verified, and we make no representations as to the adequacy, fairness, accuracy or completeness of any information obtained from third-party sources. This presentation discusses product candidates, bexicaserin and LP659, that have not yet been approved for marketing by the U.S. Food and Drug Administration (the “FDA”) or any other regulatory authority.

Our Vision is Backed by 20+ Years of World Class GPCR Research Longboard Thesis Vision A world where devastating neurological conditions are no longer devastating 3 Longboard Pharmaceuticals Differentiated & innovative clinical approaches Relevant M&A analogs JAZZ - GW $7.2B PFE - ARNA $6.7B UCB - ZGNX $1.9B Bold & experienced leadership with expertise in CNS and rare disorders Pipeline with differentiated PK / PD and target engagement CNS programs with significant commercial opportunities Well-understood targets
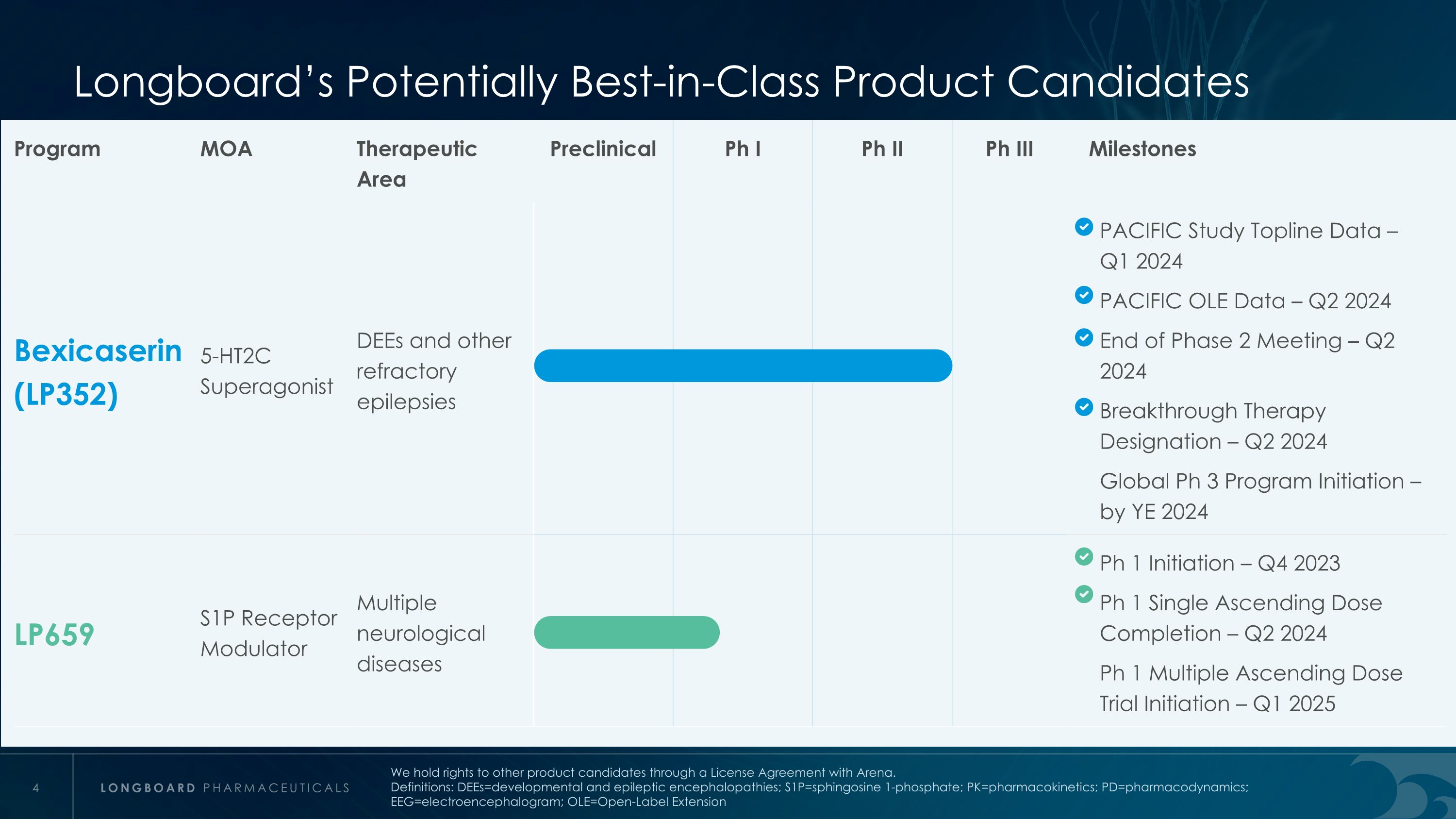
Longboard’s Potentially Best-in-Class Product Candidates Program MOA Therapeutic Area Preclinical Ph I Ph II Ph III Milestones Bexicaserin (LP352) 5-HT2C Superagonist DEEs and other refractory epilepsies PACIFIC Study Topline Data – Q1 2024 PACIFIC OLE Data – Q2 2024 End of Phase 2 Meeting – Q2 2024 Breakthrough Therapy Designation – Q2 2024 Global Ph 3 Program Initiation – by YE 2024 LP659 S1P Receptor Modulator Multiple neurological diseases Ph 1 Initiation – Q4 2023 Ph 1 Single Ascending Dose Completion – Q2 2024 Ph 1 Multiple Ascending Dose Trial Initiation – Q1 2025 We hold rights to other product candidates through a License Agreement with Arena. Definitions: DEEs=developmental and epileptic encephalopathies; S1P=sphingosine 1-phosphate; PK=pharmacokinetics; PD=pharmacodynamics; EEG=electroencephalogram; OLE=Open-Label Extension

Developmental & Epileptic Encephalopathies (DEE) Landscape
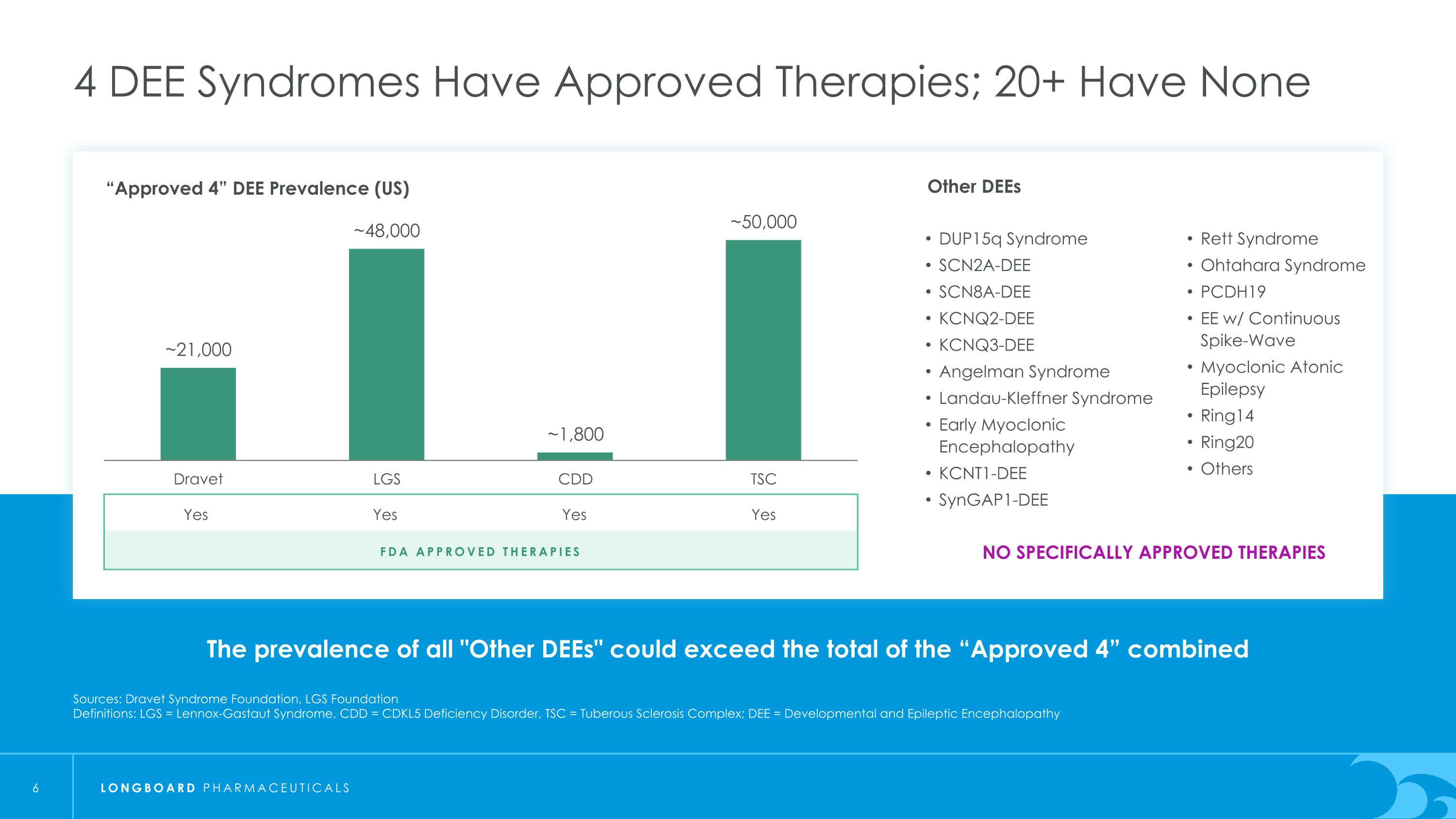
The prevalence of all "Other DEEs" could exceed the total of the “Approved 4” combined 4 DEE Syndromes Have Approved Therapies; 20+ Have None Sources: Dravet Syndrome Foundation, LGS Foundation Definitions: LGS = Lennox-Gastaut Syndrome, CDD = CDKL5 Deficiency Disorder, TSC = Tuberous Sclerosis Complex; DEE = Developmental and Epileptic Encephalopathy “Approved 4” DEE Prevalence (US) FDA Approved Therapies Yes Yes Yes Yes NO SPECIFICALLY APPROVED THERAPIES Other DEEs DUP15q Syndrome SCN2A-DEE SCN8A-DEE KCNQ2-DEE KCNQ3-DEE Angelman Syndrome Landau-Kleffner Syndrome Early Myoclonic Encephalopathy KCNT1-DEE SynGAP1-DEE Rett Syndrome Ohtahara Syndrome PCDH19 EE w/ Continuous Spike-Wave Myoclonic Atonic Epilepsy Ring14 Ring20 Others
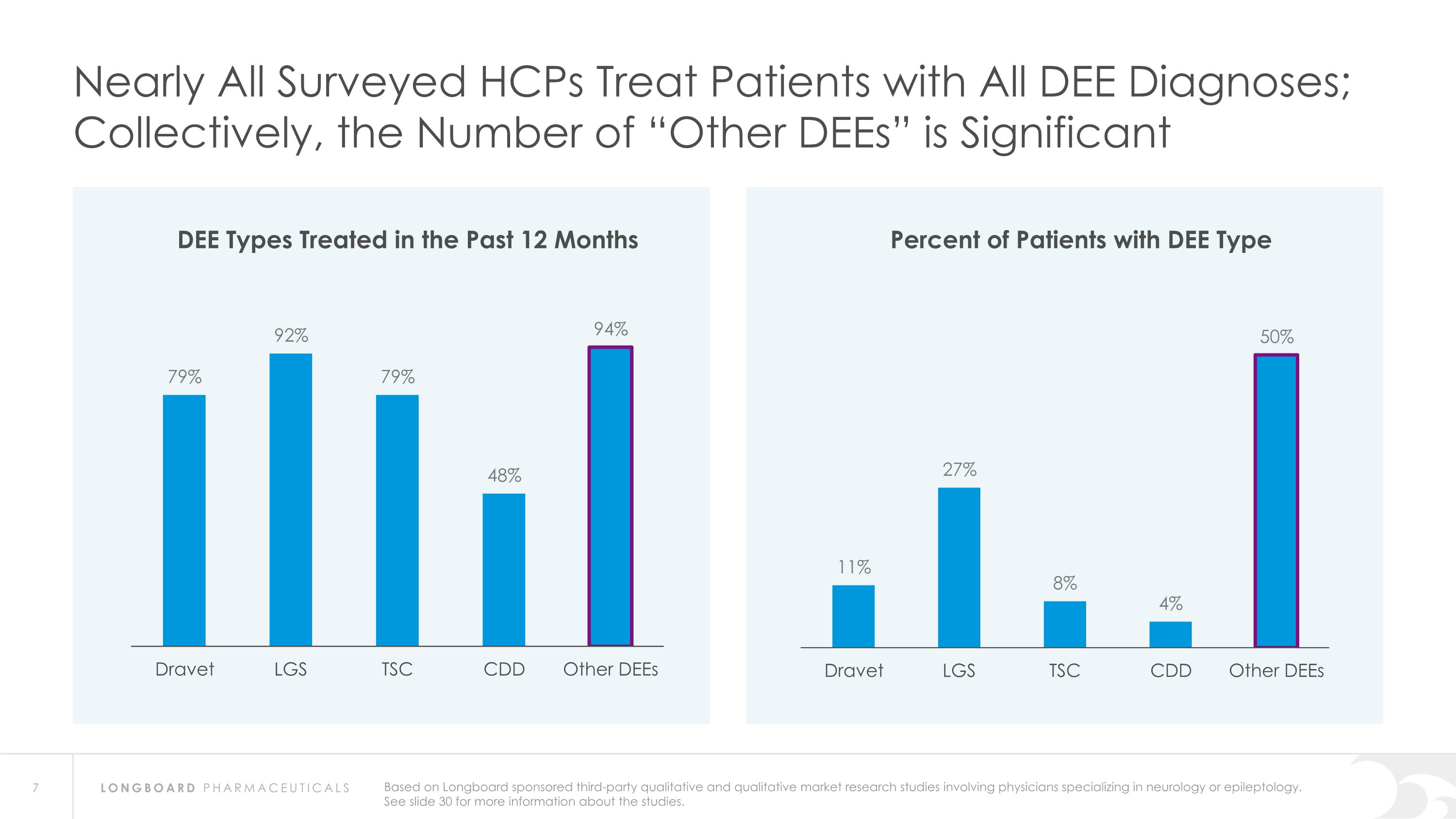
Nearly All Surveyed HCPs Treat Patients with All DEE Diagnoses; Collectively, the Number of “Other DEEs” is Significant DEE Types Treated in the Past 12 Months Percent of Patients with DEE Type Based on Longboard sponsored third-party qualitative and qualitative market research studies involving physicians specializing in neurology or epileptology. See slide 30 for more information about the studies.

Bexicaserin (LP352) Potential Best-in-Class 5-HT2C Superagonist - Entering a Ph 3 Program with the Goal of Treating a Broad Range of DEEs
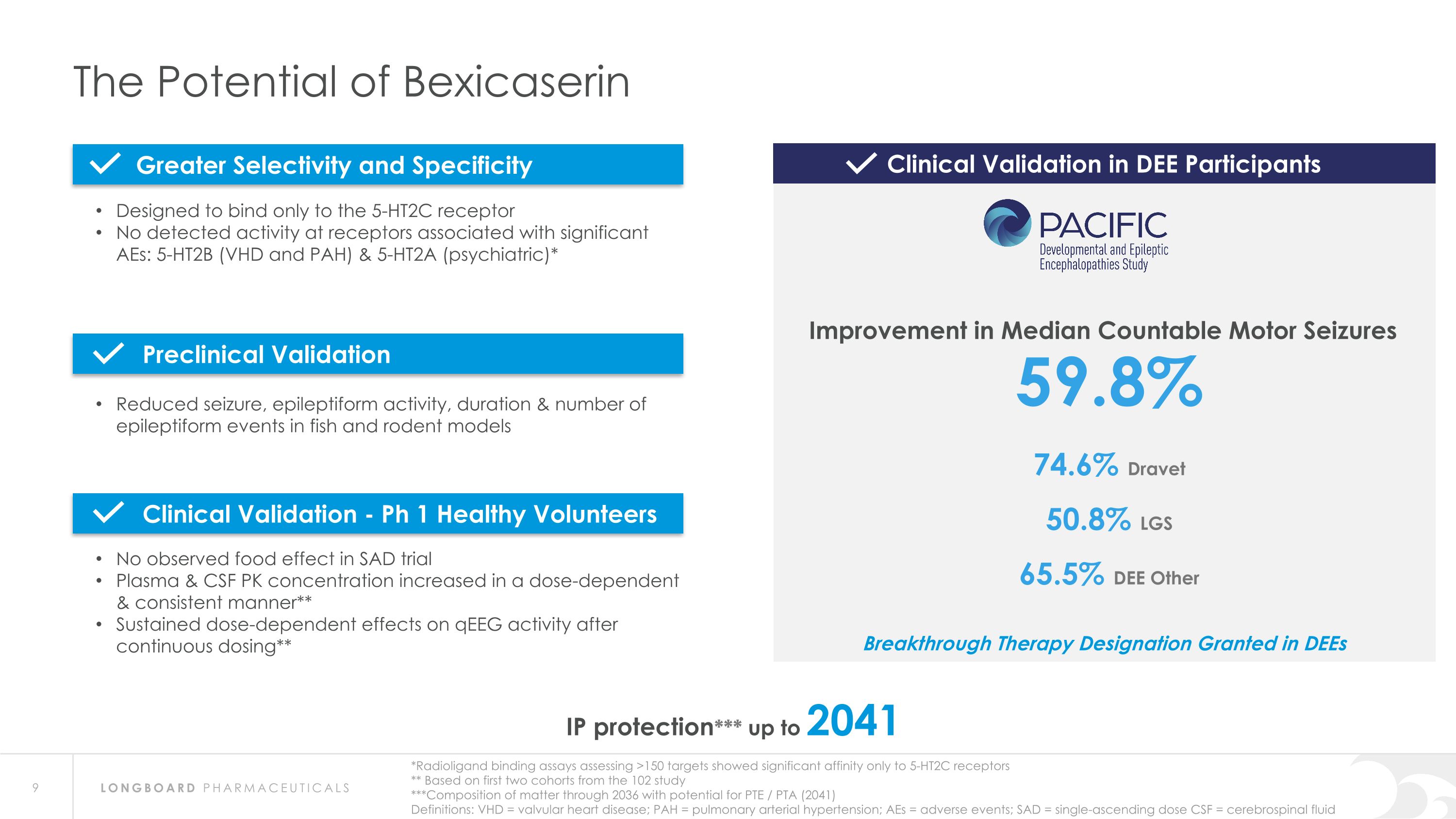
The Potential of Bexicaserin IP protection*** up to 2041 *Radioligand binding assays assessing >150 targets showed significant affinity only to 5-HT2C receptors ** Based on first two cohorts from the 102 study ***Composition of matter through 2036 with potential for PTE / PTA (2041) Definitions: VHD = valvular heart disease; PAH = pulmonary arterial hypertension; AEs = adverse events; SAD = single-ascending dose CSF = cerebrospinal fluid 59.8% 74.6% Dravet 50.8% LGS 65.5% DEE Other Designed to bind only to the 5-HT2C receptor No detected activity at receptors associated with significant AEs: 5-HT2B (VHD and PAH) & 5-HT2A (psychiatric)* Reduced seizure, epileptiform activity, duration & number of epileptiform events in fish and rodent models No observed food effect in SAD trial Plasma & CSF PK concentration increased in a dose-dependent & consistent manner** Sustained dose-dependent effects on qEEG activity after continuous dosing** Clinical Validation in DEE Participants Improvement in Median Countable Motor Seizures Greater Selectivity and Specificity Preclinical Validation Clinical Validation - Ph 1 Healthy Volunteers Breakthrough Therapy Designation Granted in DEEs
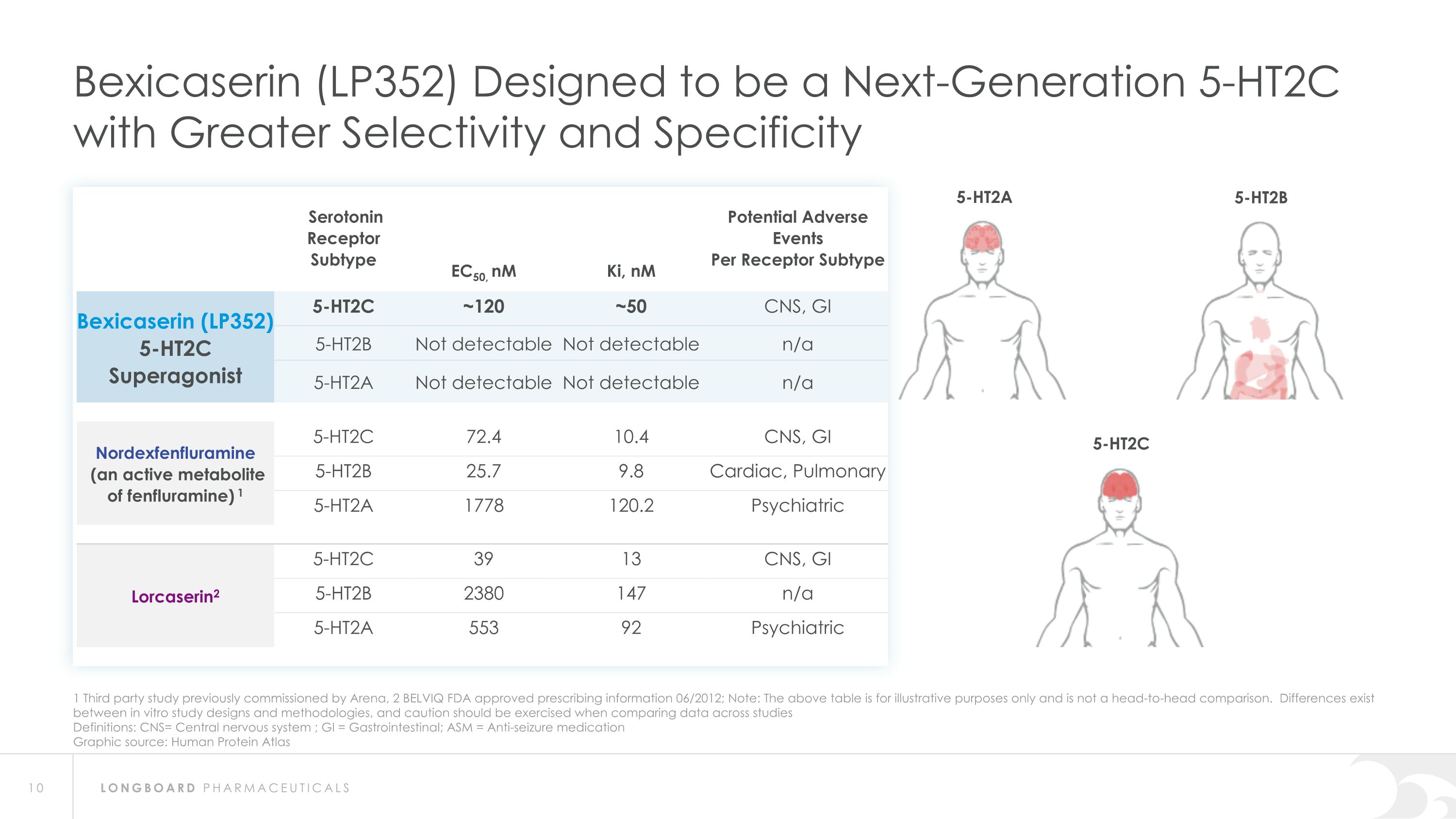
1 Third party study previously commissioned by Arena, 2 BELVIQ FDA approved prescribing information 06/2012; Note: The above table is for illustrative purposes only and is not a head-to-head comparison. Differences exist between in vitro study designs and methodologies, and caution should be exercised when comparing data across studies Definitions: CNS= Central nervous system ; GI = Gastrointestinal; ASM = Anti-seizure medication Graphic source: Human Protein Atlas Bexicaserin (LP352) Designed to be a Next-Generation 5-HT2C with Greater Selectivity and Specificity Serotonin Receptor Subtype EC50, nM Ki, nM Potential Adverse Events Per Receptor Subtype Bexicaserin (LP352) 5-HT2C Superagonist 5-HT2C ~120 ~50 CNS, GI 5-HT2B Not detectable Not detectable n/a 5-HT2A Not detectable Not detectable n/a Nordexfenfluramine (an active metabolite of fenfluramine) 1 5-HT2C 72.4 10.4 CNS, GI 5-HT2B 25.7 9.8 Cardiac, Pulmonary 5-HT2A 1778 120.2 Psychiatric Lorcaserin2 5-HT2C 39 13 CNS, GI 5-HT2B 2380 147 n/a 5-HT2A 553 92 Psychiatric 5-HT2A 5-HT2B 5-HT2C
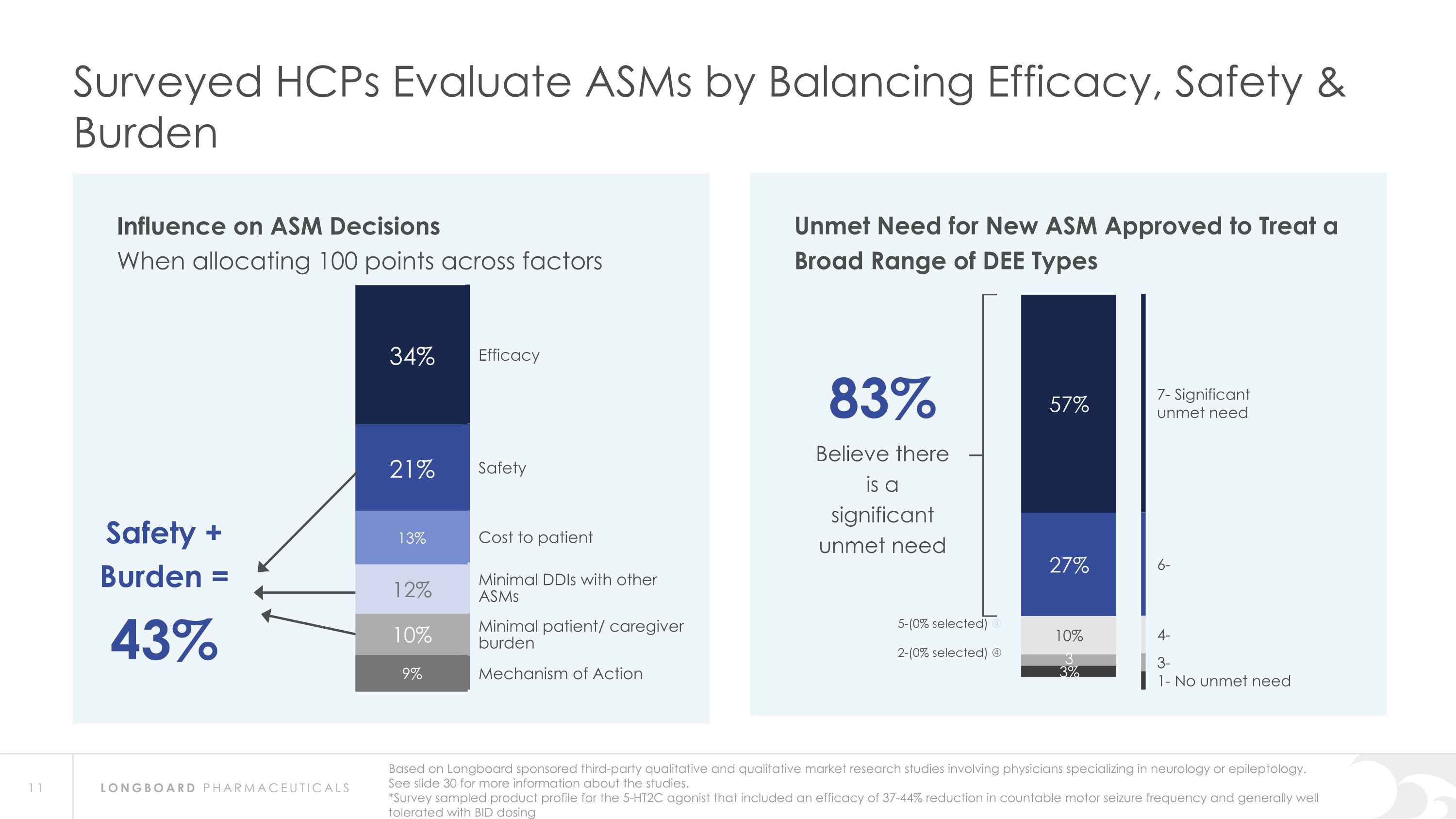
Influence on ASM Decisions When allocating 100 points across factors Surveyed HCPs Evaluate ASMs by Balancing Efficacy, Safety & Burden Efficacy Safety Cost to patient Minimal DDIs with other ASMs Minimal patient/ caregiver burden Mechanism of Action Safety + Burden = 43% Based on Longboard sponsored third-party qualitative and qualitative market research studies involving physicians specializing in neurology or epileptology. See slide 30 for more information about the studies. *Survey sampled product profile for the 5-HT2C agonist that included an efficacy of 37-44% reduction in countable motor seizure frequency and generally well tolerated with BID dosing Unmet Need for New ASM Approved to Treat a Broad Range of DEE Types 83% Believe there is a significant unmet need 7- Significant unmet need 6- 4- 3- 1- No unmet need 5-(0% selected) 2-(0% selected)
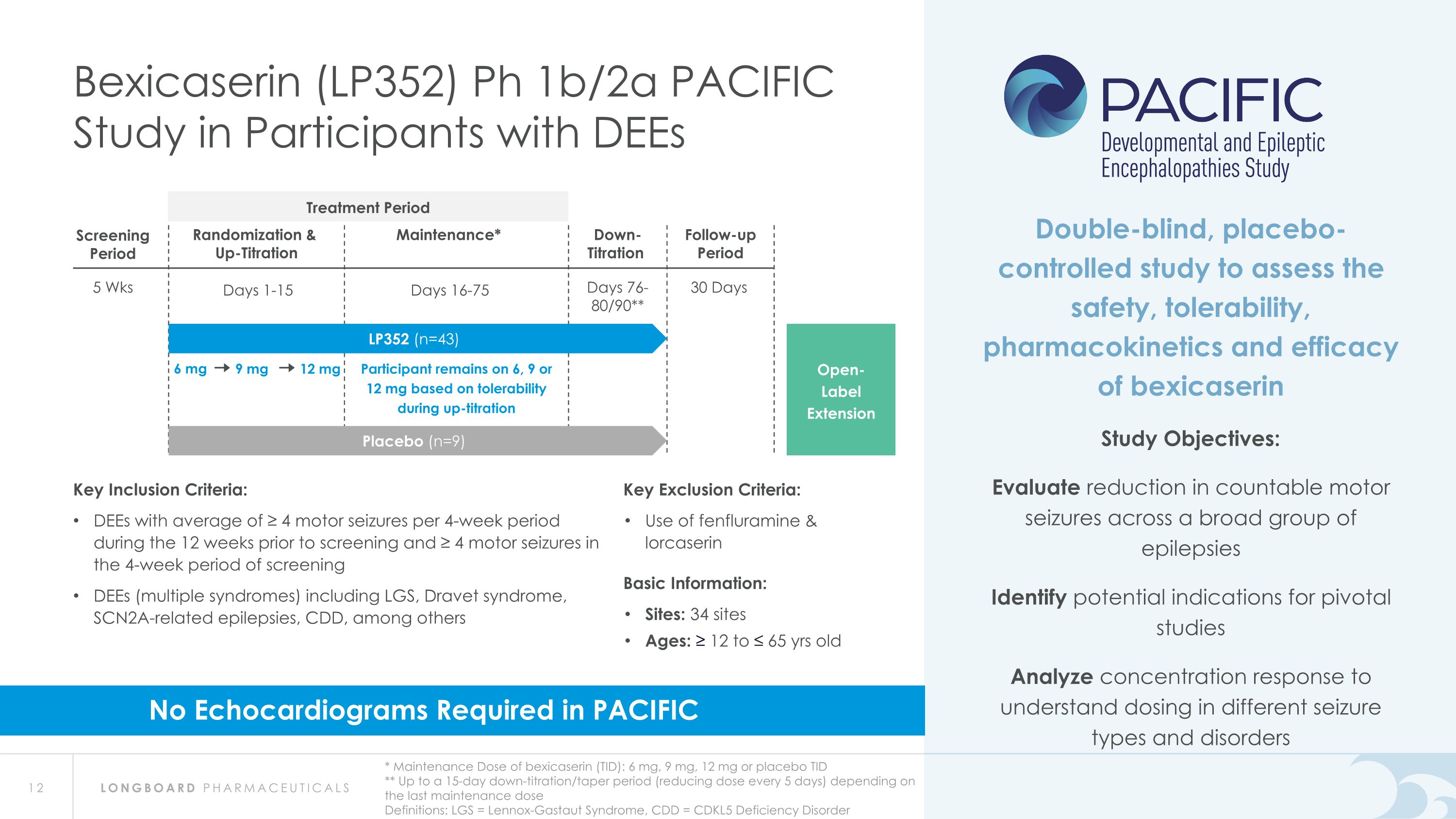
6 mg 9 mg 12 mg Participant remains on 6, 9 or 12 mg based on tolerability during up-titration 5 Wks Screening Period 30 Days Follow-up Period Randomization & Up-Titration Days 1-15 Maintenance* Days 16-75 Down- Titration Days 76- 80/90** Bexicaserin (LP352) Ph 1b/2a PACIFIC Study in Participants with DEEs * Maintenance Dose of bexicaserin (TID): 6 mg, 9 mg, 12 mg or placebo TID ** Up to a 15-day down-titration/taper period (reducing dose every 5 days) depending on the last maintenance dose Definitions: LGS = Lennox-Gastaut Syndrome, CDD = CDKL5 Deficiency Disorder Open- Label Extension Double-blind, placebo-controlled study to assess the safety, tolerability, pharmacokinetics and efficacy of bexicaserin Study Objectives: Evaluate reduction in countable motor seizures across a broad group of epilepsies Identify potential indications for pivotal studies Analyze concentration response to understand dosing in different seizure types and disorders Placebo (n=9) LP352 (n=43) Key Inclusion Criteria: DEEs with average of ≥ 4 motor seizures per 4-week period during the 12 weeks prior to screening and ≥ 4 motor seizures in the 4-week period of screening DEEs (multiple syndromes) including LGS, Dravet syndrome, SCN2A-related epilepsies, CDD, among others Key Exclusion Criteria: Use of fenfluramine & lorcaserin Basic Information: Sites: 34 sites Ages: ≥ 12 to ≤ 65 yrs old No Echocardiograms Required in PACIFIC Treatment Period
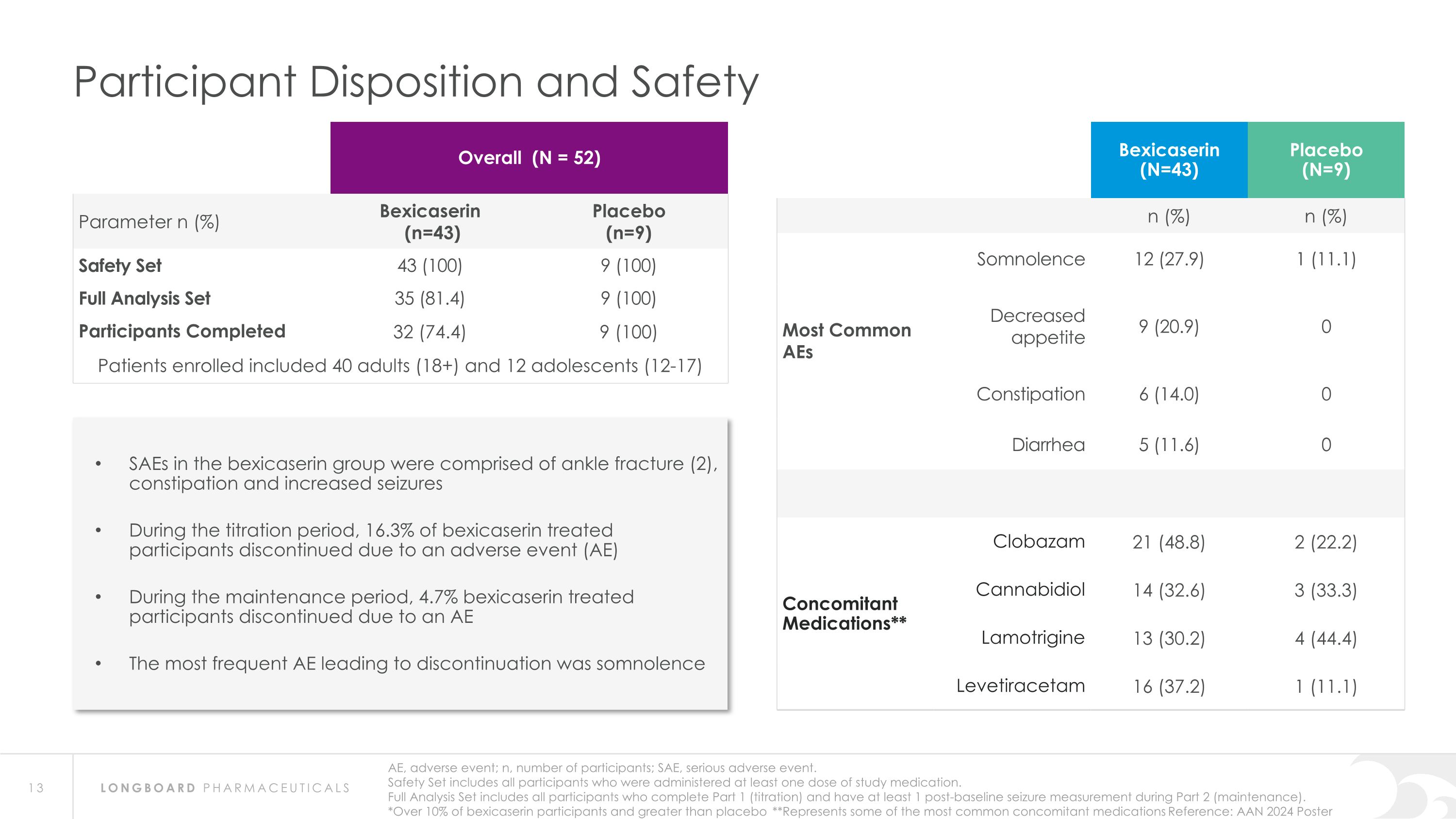
AE, adverse event; n, number of participants; SAE, serious adverse event. Safety Set includes all participants who were administered at least one dose of study medication. Full Analysis Set includes all participants who complete Part 1 (titration) and have at least 1 post-baseline seizure measurement during Part 2 (maintenance). *Over 10% of bexicaserin participants and greater than placebo **Represents some of the most common concomitant medications Reference: AAN 2024 Poster Participant Disposition and Safety Overall (N = 52) Parameter n (%) Bexicaserin (n=43) Placebo (n=9) Safety Set 43 (100) 9 (100) Full Analysis Set 35 (81.4) 9 (100) Participants Completed 32 (74.4) 9 (100) Patients enrolled included 40 adults (18+) and 12 adolescents (12-17) Bexicaserin (N=43) Placebo (N=9) n (%) n (%) Most Common AEs Somnolence 12 (27.9) 1 (11.1) Decreased appetite 9 (20.9) 0 Constipation 6 (14.0) 0 Diarrhea 5 (11.6) 0 Concomitant Medications** Clobazam 21 (48.8) 2 (22.2) Cannabidiol 14 (32.6) 3 (33.3) Lamotrigine 13 (30.2) 4 (44.4) Levetiracetam 16 (37.2) 1 (11.1) SAEs in the bexicaserin group were comprised of ankle fracture (2), constipation and increased seizures During the titration period, 16.3% of bexicaserin treated participants discontinued due to an adverse event (AE) During the maintenance period, 4.7% bexicaserin treated participants discontinued due to an AE The most frequent AE leading to discontinuation was somnolence
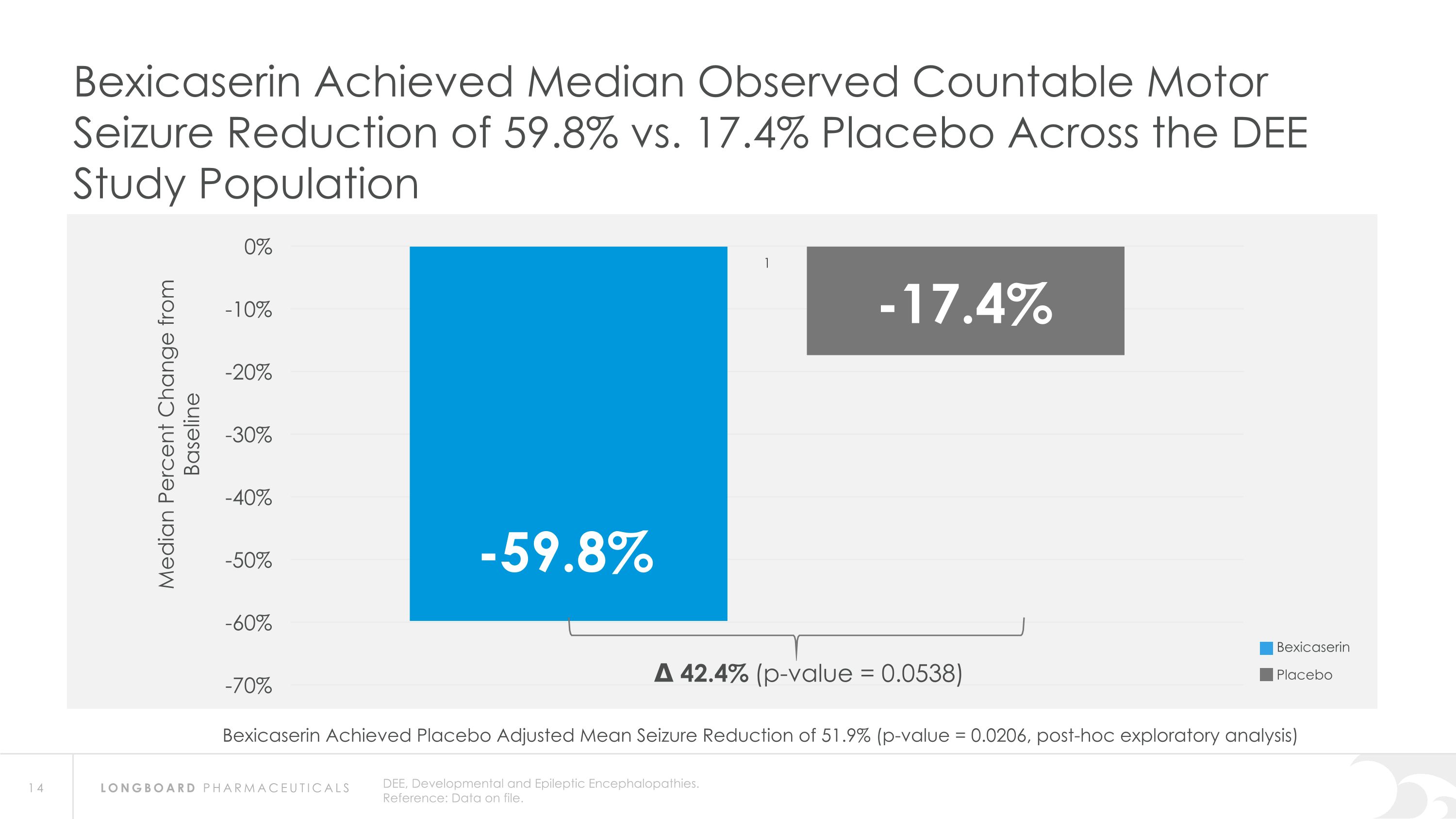
DEE, Developmental and Epileptic Encephalopathies. Reference: Data on file. Bexicaserin Achieved Median Observed Countable Motor Seizure Reduction of 59.8% vs. 17.4% Placebo Across the DEE Study Population Δ 42.4% (p-value = 0.0538) Bexicaserin Placebo Bexicaserin Achieved Placebo Adjusted Mean Seizure Reduction of 51.9% (p-value = 0.0206, post-hoc exploratory analysis)
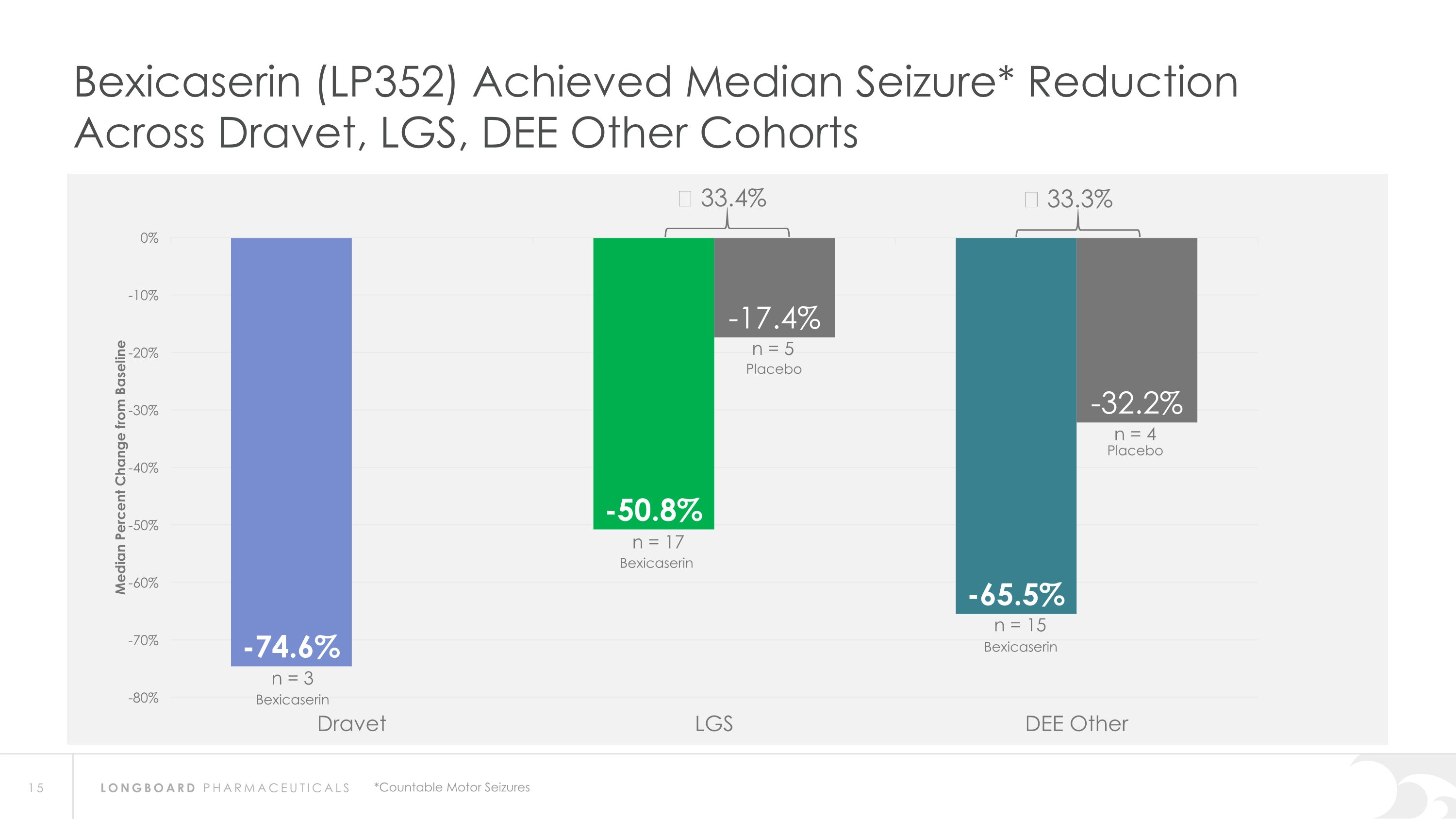
Bexicaserin (LP352) Achieved Median Seizure* Reduction Across Dravet, LGS, DEE Other Cohorts Δ 33.4% Δ 33.3% *Countable Motor Seizures n = 3 n = 5 n = 15 n = 4 Placebo Bexicaserin Placebo Bexicaserin Bexicaserin n = 17
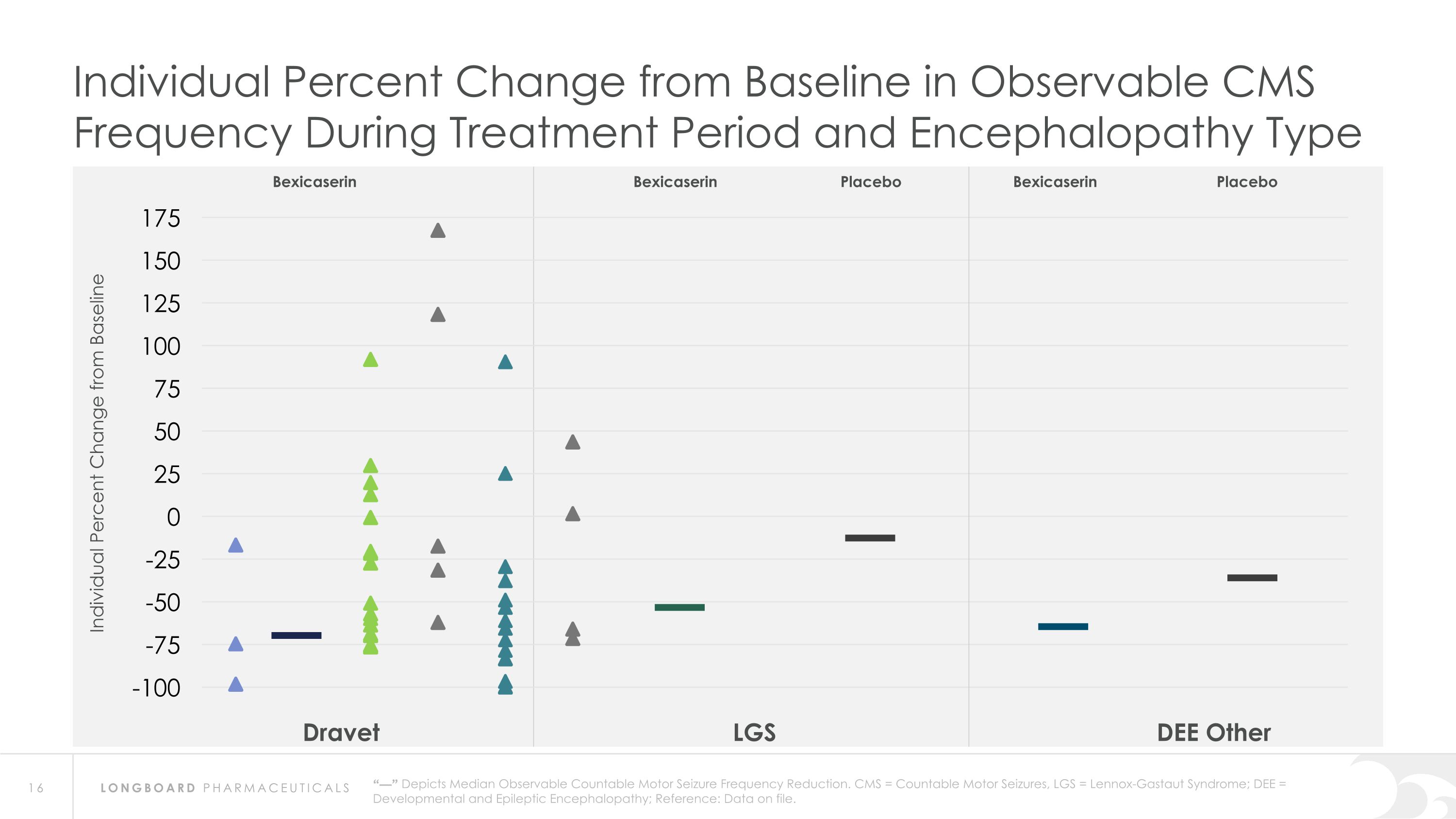
Dravet LGS DEE Other “—” Depicts Median Observable Countable Motor Seizure Frequency Reduction. CMS = Countable Motor Seizures, LGS = Lennox-Gastaut Syndrome; DEE = Developmental and Epileptic Encephalopathy; Reference: Data on file. Bexicaserin Placebo Bexicaserin Placebo Bexicaserin Individual Percent Change from Baseline in Observable CMS Frequency During Treatment Period and Encephalopathy Type
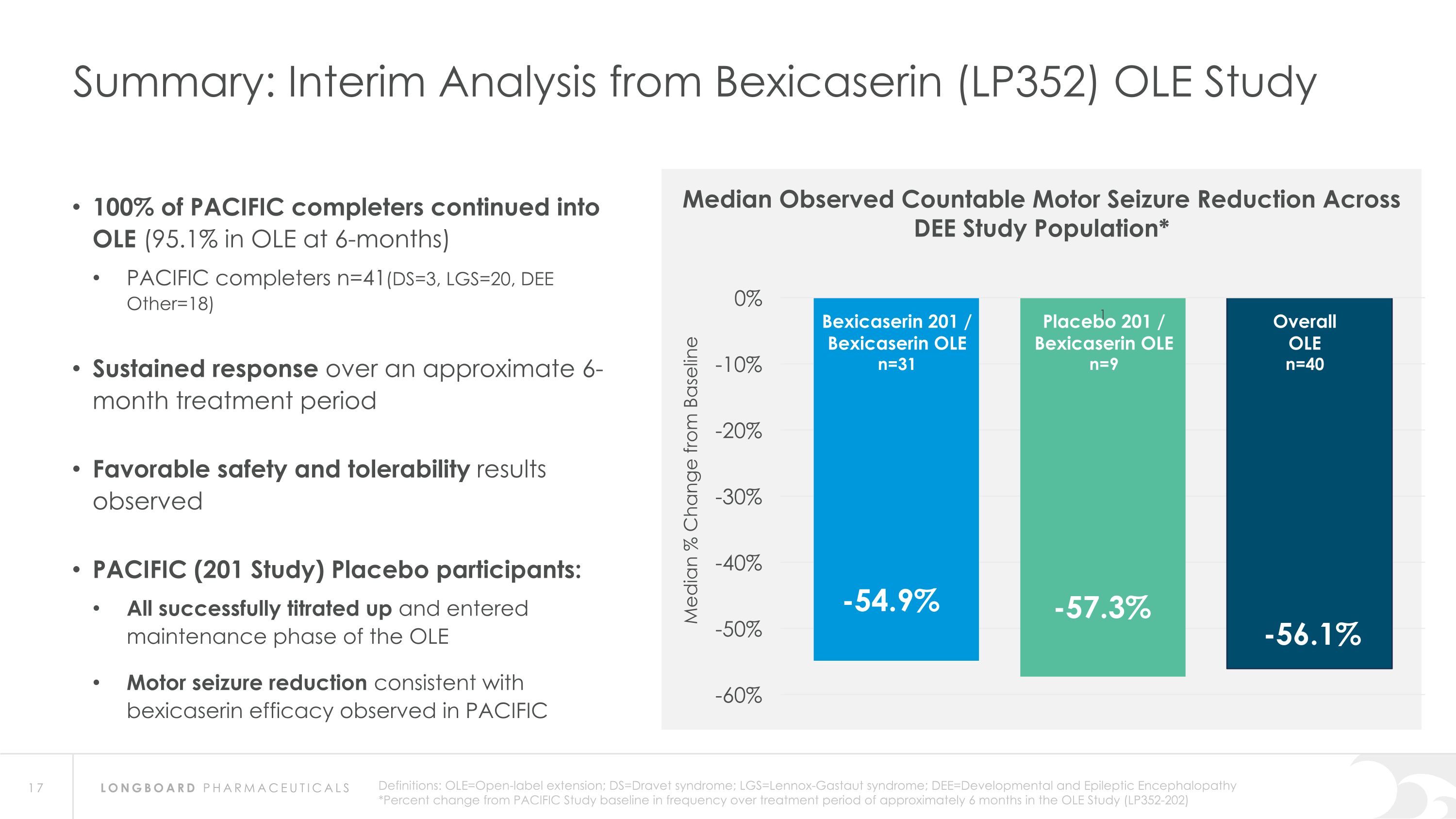
Summary: Interim Analysis from Bexicaserin (LP352) OLE Study Bexicaserin 201 / Bexicaserin OLE n=31 Placebo 201 / Bexicaserin OLE n=9 Overall OLE n=40 Median Observed Countable Motor Seizure Reduction Across DEE Study Population* 100% of PACIFIC completers continued into OLE (95.1% in OLE at 6-months) PACIFIC completers n=41(DS=3, LGS=20, DEE Other=18) Sustained response over an approximate 6-month treatment period Favorable safety and tolerability results observed PACIFIC (201 Study) Placebo participants: All successfully titrated up and entered maintenance phase of the OLE Motor seizure reduction consistent with bexicaserin efficacy observed in PACIFIC Definitions: OLE=Open-label extension; DS=Dravet syndrome; LGS=Lennox-Gastaut syndrome; DEE=Developmental and Epileptic Encephalopathy *Percent change from PACIFIC Study baseline in frequency over treatment period of approximately 6 months in the OLE Study (LP352-202)
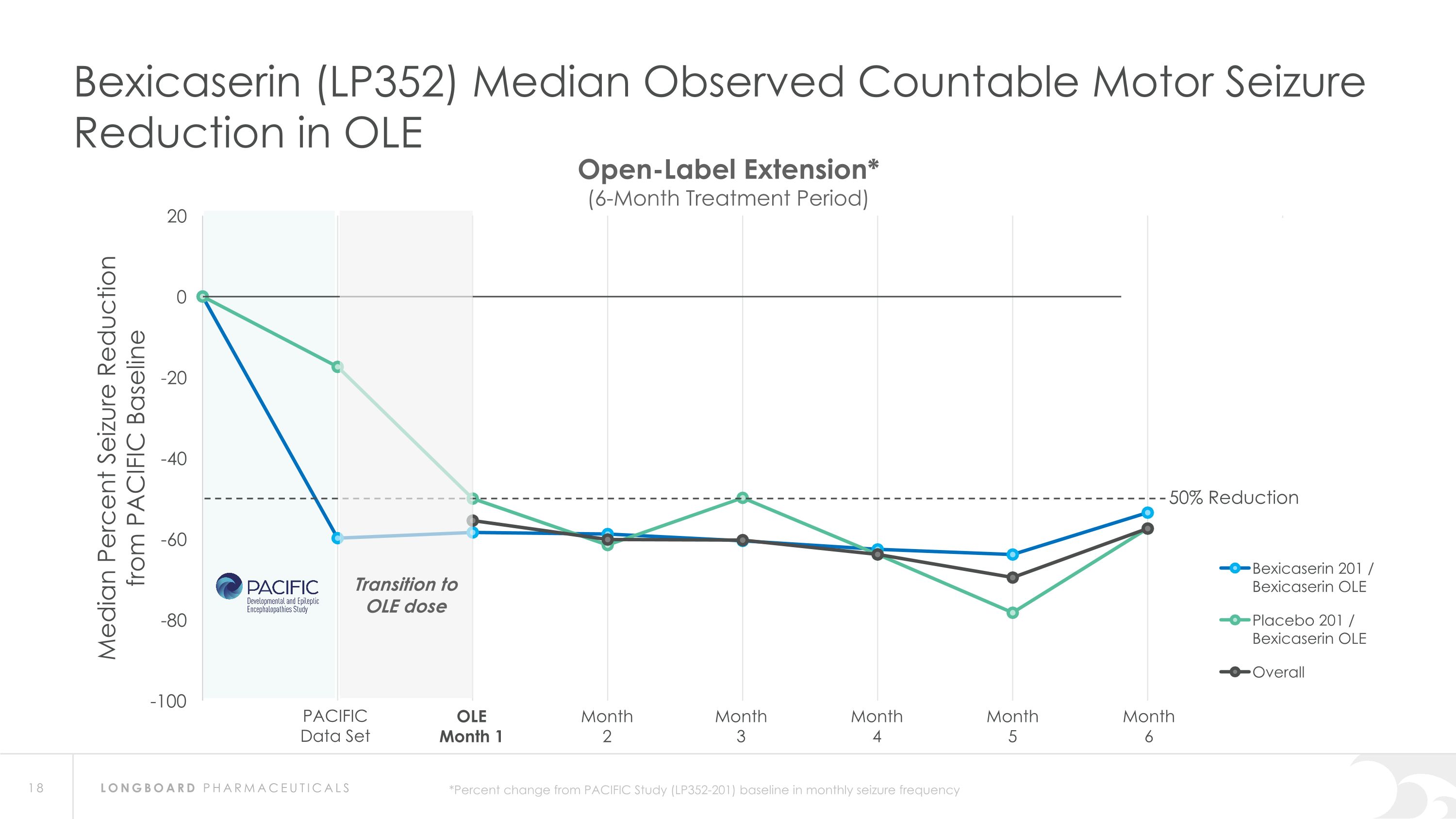
Bexicaserin (LP352) Median Observed Countable Motor Seizure Reduction in OLE PACIFIC Data Set OLE Month 1 Month 2 Month 3 Month 4 Month 5 Month 6 Open-Label Extension* (6-Month Treatment Period) 50% Reduction *Percent change from PACIFIC Study (LP352-201) baseline in monthly seizure frequency Transition to OLE dose

LGS = Lennox-Gastaut Syndrome Bexicaserin Phase 3 Global Program – DEE Path Forward Subject to Ongoing Discussions with Regulatory Agencies Planned Study Parameters: Primary Endpoint: Reduction in Countable Motor Seizures Ages: ≥ 2 to ≤ 65 yrs old (weight-based dosing for pts of lower weight/age) Sites: Sites across the US, AUS, EU, other potential regions Open-Label Extension (OLE): Participants who complete either of the Ph 3 studies are eligible to enter a 52-week OLE Study 302: Dravet Syndrome Study 301: DEEs (LGS + Other DEEs) Expected to be run in parallel Breakthrough Therapy designation granted for bexicaserin for the treatment of seizures associated with Developmental and Epileptic Encephalopathies (DEEs) for patients ≥ 2 years of age

Global Phase 3 Program Expected to Initiate in 2024 Bexicaserin (LP352) achieved a median percent reduction from baseline in seizure frequency during the treatment period of: 59.8% in broad DEE population (42.4% placebo-adjusted) 74.6% in Dravet cohort 50.8% in LGS cohort (33.4% placebo-adjusted) 65.5% in DEE Other cohort (33.3% placebo-adjusted) Results were shown on top of a contemporary polytherapy background with multiple ASMs including cannabidiol (32.7% of participants were receiving cannabidiol) *Definitions: ASMs = Anti-Seizure Medications; UGT = Uridine Diphosphate Glucuronosyltransferase Favorable safety and tolerability results No echocardiograms required in PACIFIC study Metabolized via UGT pathway – potentially reduces risk of Drug-Drug Interactions 86% of participants achieved the highest dose of 12 mg of bexicaserin in the maintenance period 100% of PACIFIC participants who completed the study entered the Open Label Extension (OLE) Study OLE Interim Analysis Sustained response over an approximate 6-month treatment period Favorable safety and tolerability results observed All PACIFIC Placebo participants successfully titrated up and entered maintenance phase of the OLE Motor seizure reduction consistent with bexicaserin efficacy observed in PACIFIC

LP659 Centrally Acting, Highly Selective Sphingosine-1-Phosphate (S1P) Receptor Modulator Targeting Multiple Neurological Diseases
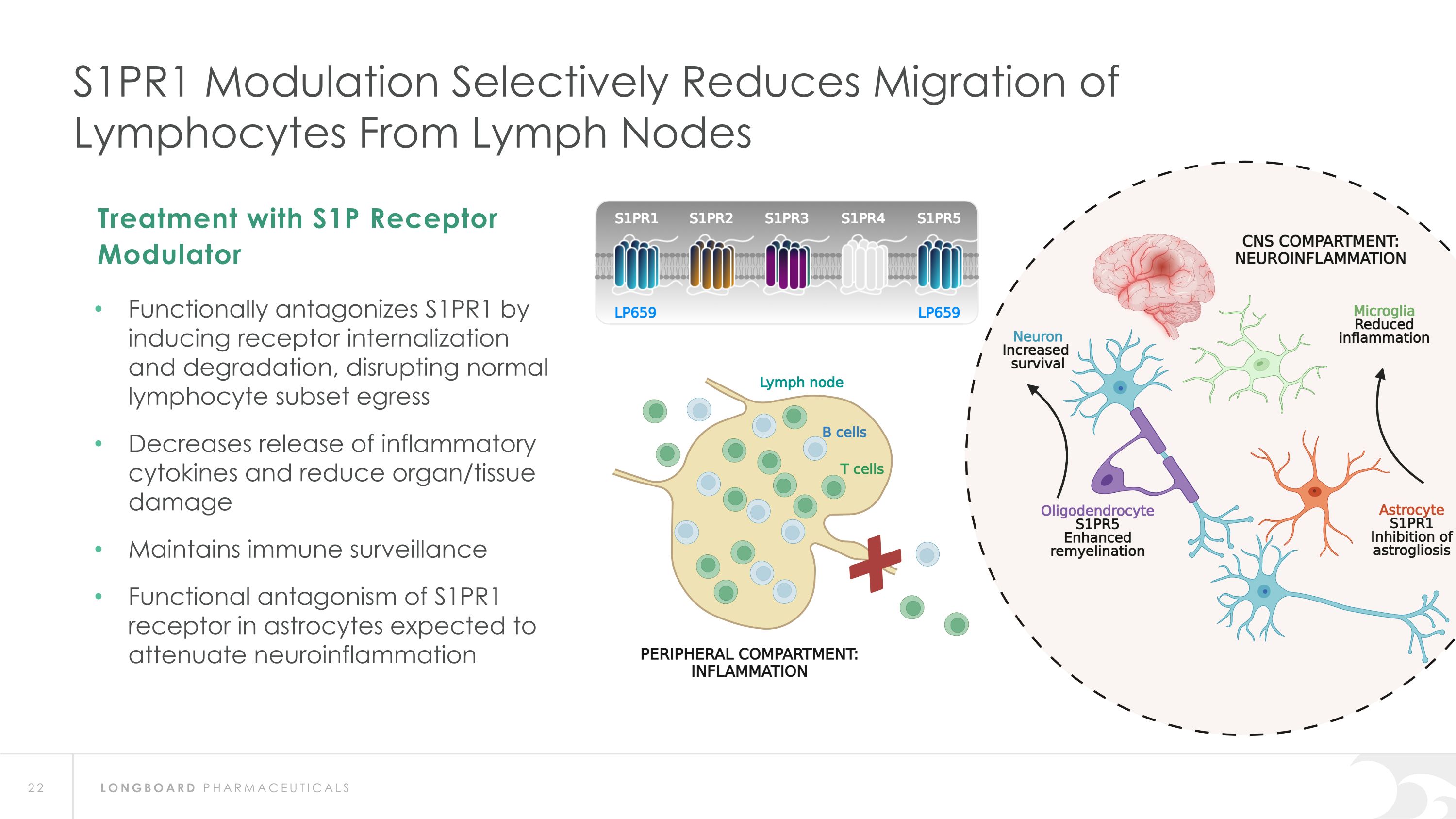
S1PR1 Modulation Selectively Reduces Migration of Lymphocytes From Lymph Nodes Functionally antagonizes S1PR1 by inducing receptor internalization and degradation, disrupting normal lymphocyte subset egress Decreases release of inflammatory cytokines and reduce organ/tissue damage Maintains immune surveillance Functional antagonism of S1PR1 receptor in astrocytes expected to attenuate neuroinflammation Treatment with S1P Receptor Modulator
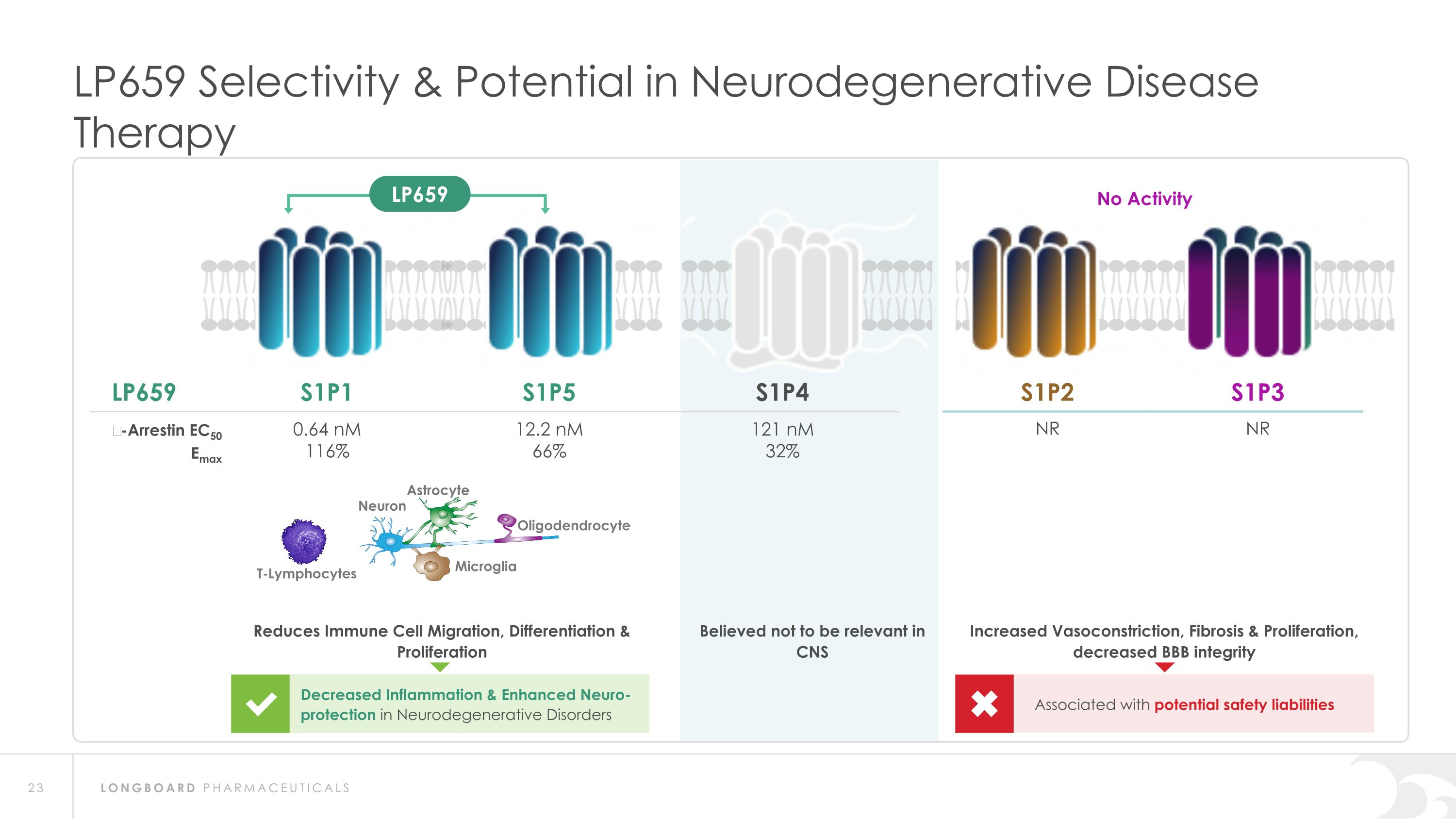
LP659 Selectivity & Potential in Neurodegenerative Disease Therapy LP659 S1P1 S1P5 S1P4 S1P2 S1P3 β-Arrestin EC50 Emax 0.64 nM 116% 12.2 nM 66% 121 nM 32% NR NR T-Lymphocytes Neuron Astrocyte Oligodendrocyte Microglia Decreased Inflammation & Enhanced Neuro-protection in Neurodegenerative Disorders Reduces Immune Cell Migration, Differentiation & Proliferation Associated with potential safety liabilities Increased Vasoconstriction, Fibrosis & Proliferation, decreased BBB integrity No Activity LP659 Believed not to be relevant in CNS
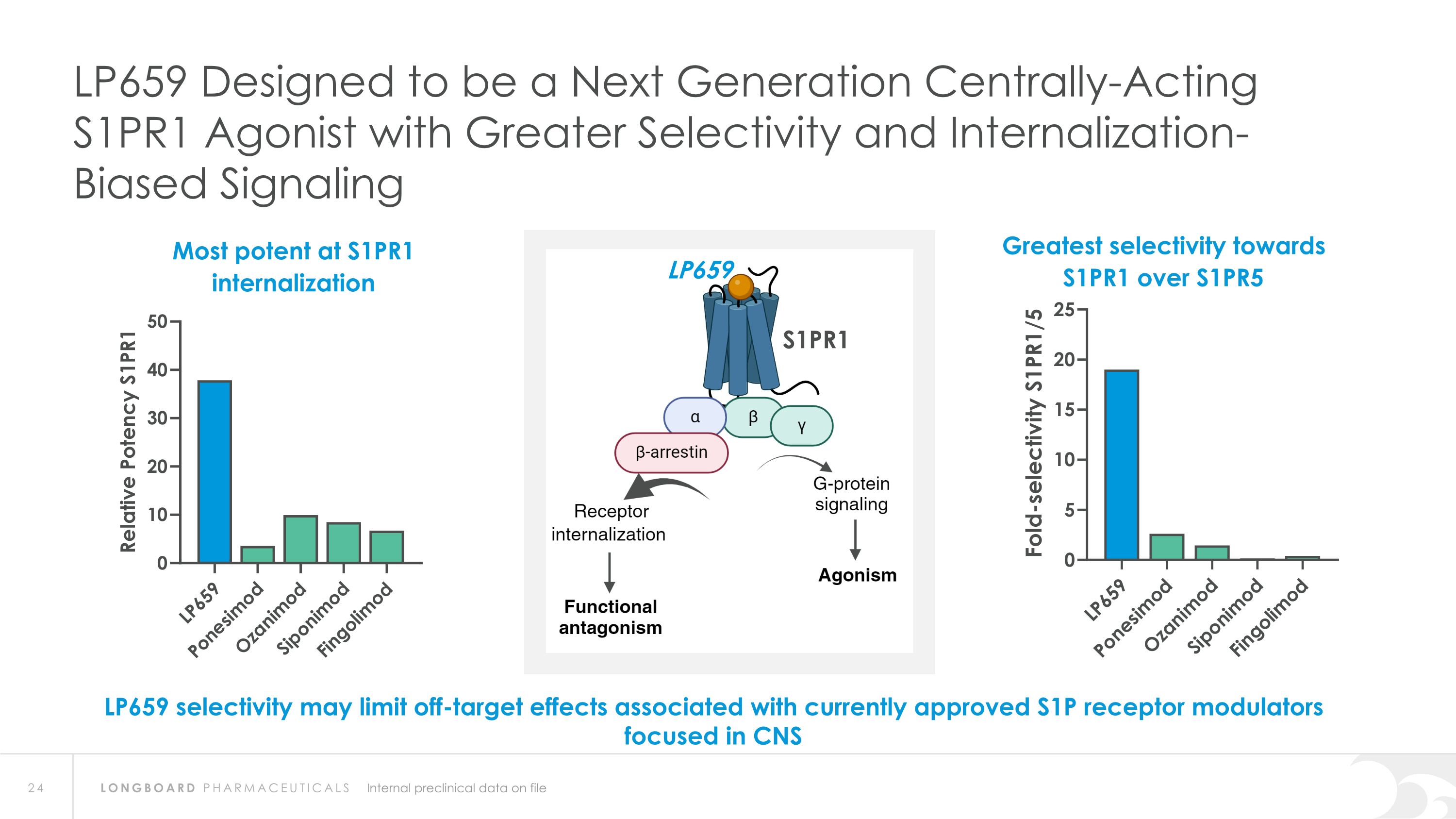
S1PR1 Internal preclinical data on file LP659 Designed to be a Next Generation Centrally-Acting S1PR1 Agonist with Greater Selectivity and Internalization-Biased Signaling LP659 selectivity may limit off-target effects associated with currently approved S1P receptor modulators focused in CNS Greatest selectivity towards S1PR1 over S1PR5 Most potent at S1PR1 internalization LP659
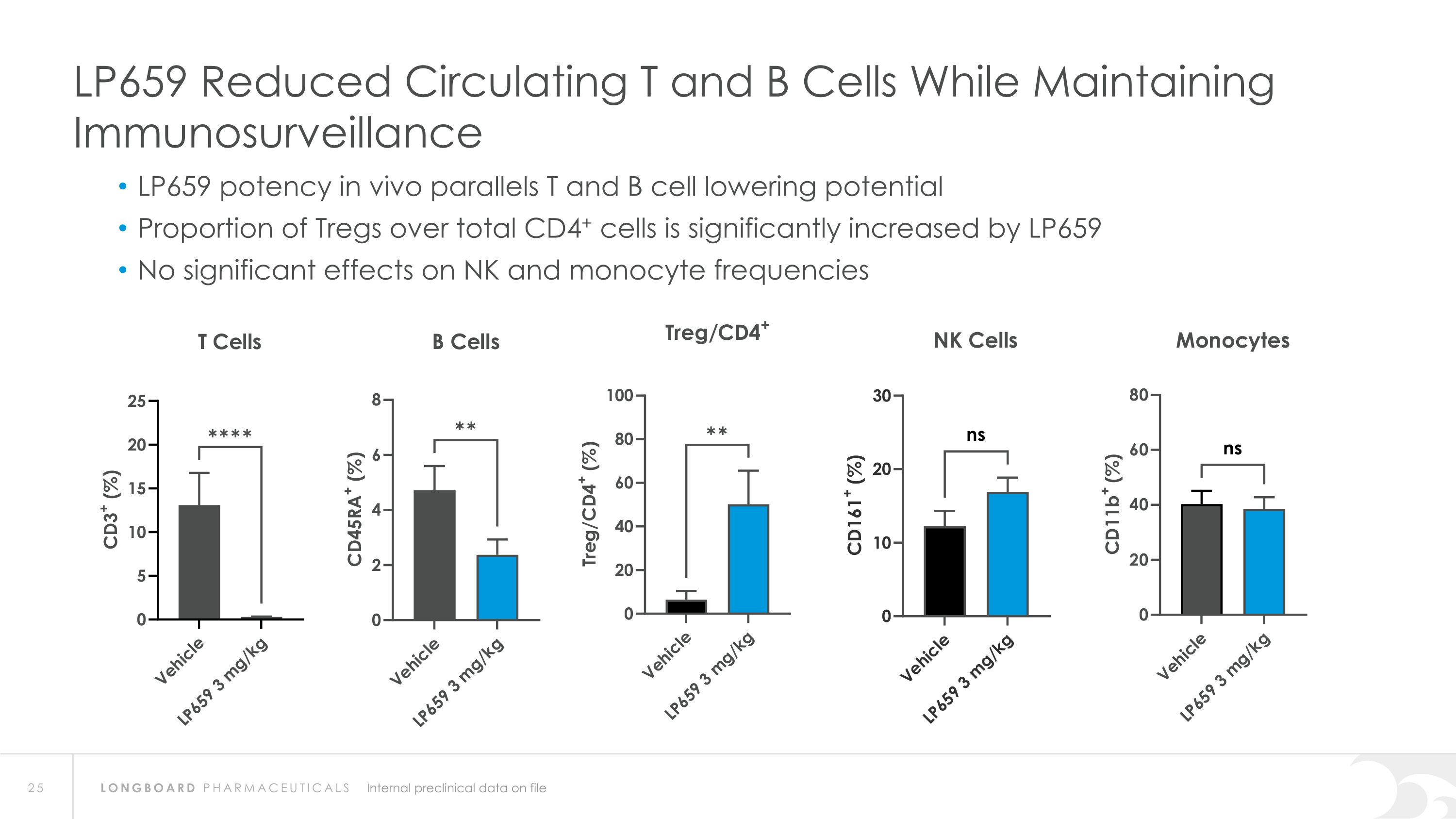
LP659 Reduced Circulating T and B Cells While Maintaining Immunosurveillance LP659 potency in vivo parallels T and B cell lowering potential Proportion of Tregs over total CD4+ cells is significantly increased by LP659 No significant effects on NK and monocyte frequencies Internal preclinical data on file

LP659 LP659 Phase 1 SAD Topline Data
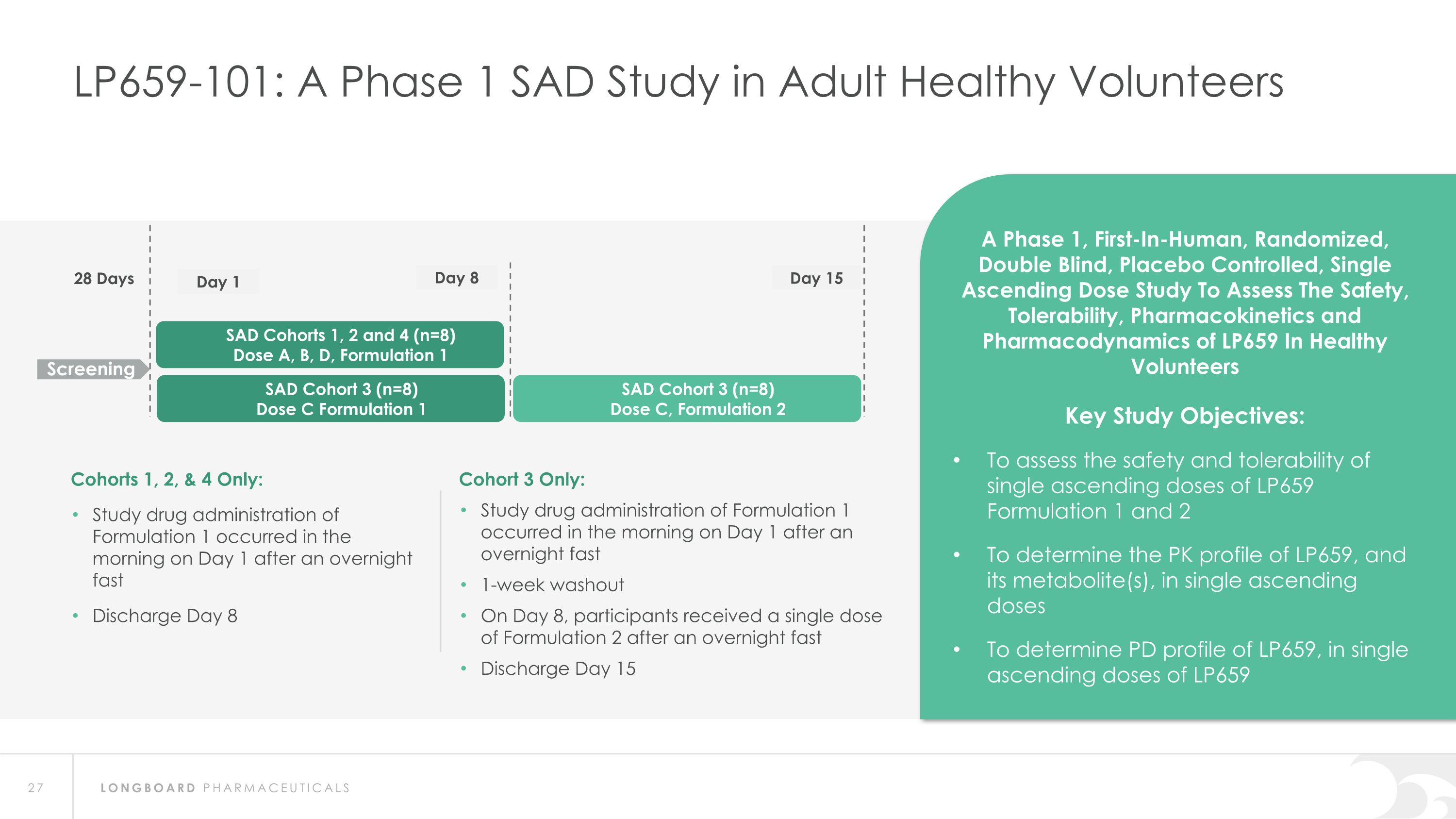
LP659-101: A Phase 1 SAD Study in Adult Healthy Volunteers SAD Cohorts 1, 2 and 4 (n=8) Dose A, B, D, Formulation 1 28 Days Screening A Phase 1, First-In-Human, Randomized, Double Blind, Placebo Controlled, Single Ascending Dose Study To Assess The Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of LP659 In Healthy Volunteers Key Study Objectives: To assess the safety and tolerability of single ascending doses of LP659 Formulation 1 and 2 To determine the PK profile of LP659, and its metabolite(s), in single ascending doses To determine PD profile of LP659, in single ascending doses of LP659 Day 1 Day 15 Cohorts 1, 2, & 4 Only: Study drug administration of Formulation 1 occurred in the morning on Day 1 after an overnight fast Discharge Day 8 Cohort 3 Only: Study drug administration of Formulation 1 occurred in the morning on Day 1 after an overnight fast 1-week washout On Day 8, participants received a single dose of Formulation 2 after an overnight fast Discharge Day 15 Day 8 SAD Cohort 3 (n=8) Dose C, Formulation 2 SAD Cohort 3 (n=8) Dose C Formulation 1

Adverse events were mild No TEAEs leading to discontinuation No SAEs observed Impact on HR was low throughout the study with no first dose bradycardia No abnormal ECGs (no AV block) or abnormal echocardiograms No abnormal pulmonary/spirometry and ophthalmologic assessments No infections Phase 1 Safety Results LP659 was generally safe and well tolerated ► Abbreviations: TEAEs = Treatment-Emergent Adverse Events; SAEs = serious adverse events, HR = Heart Rate, ECG = electrocardiogram, AV = atrioventricular
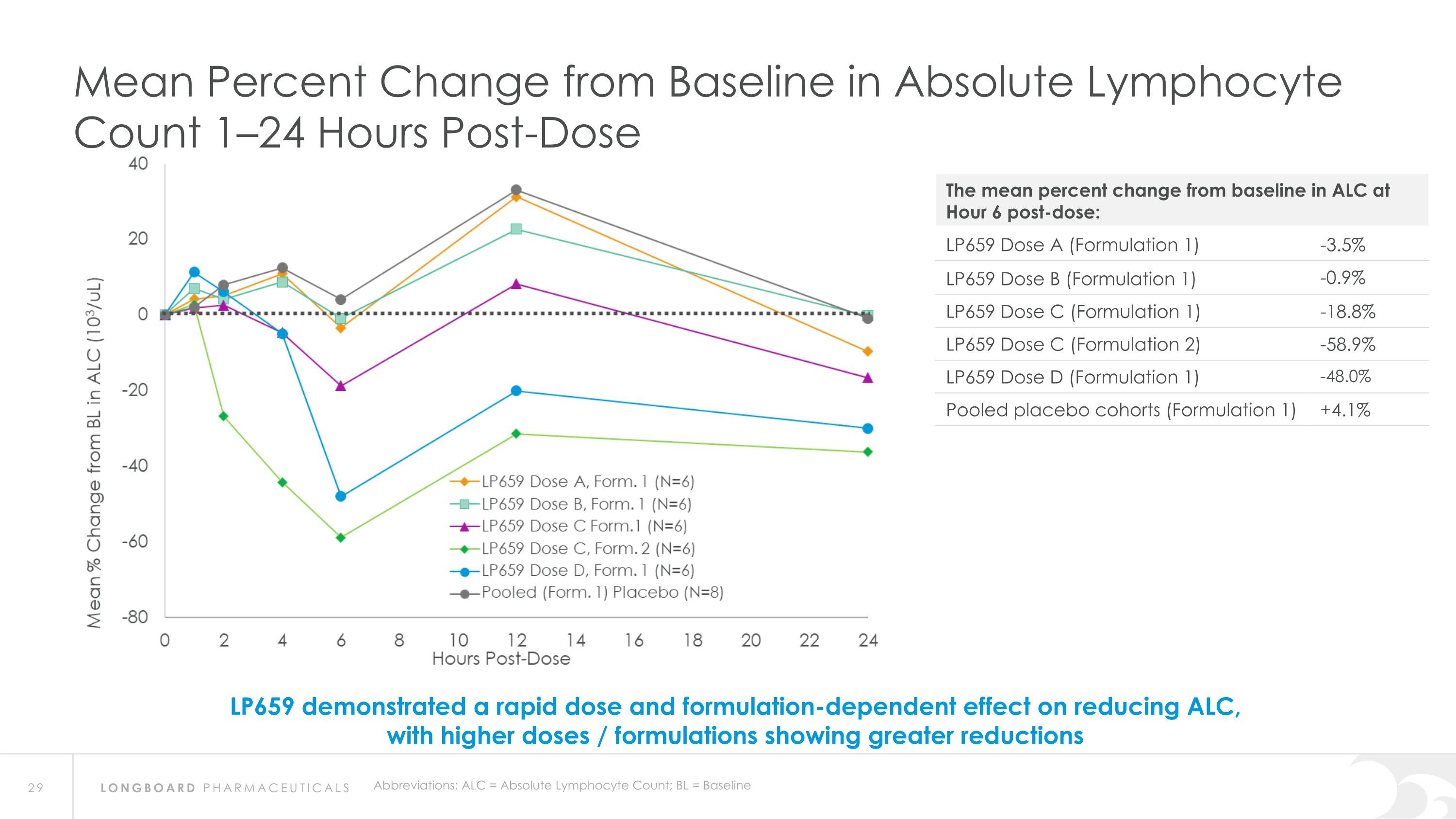
Abbreviations: ALC = Absolute Lymphocyte Count; BL = Baseline Mean Percent Change from Baseline in Absolute Lymphocyte Count 1–24 Hours Post-Dose LP659 demonstrated a rapid dose and formulation-dependent effect on reducing ALC, with higher doses / formulations showing greater reductions The mean percent change from baseline in ALC at Hour 6 post-dose: LP659 Dose A (Formulation 1) -3.5% LP659 Dose B (Formulation 1) -0.9% LP659 Dose C (Formulation 1) -18.8% LP659 Dose C (Formulation 2) -58.9% LP659 Dose D (Formulation 1) -48.0% Pooled placebo cohorts (Formulation 1) +4.1%
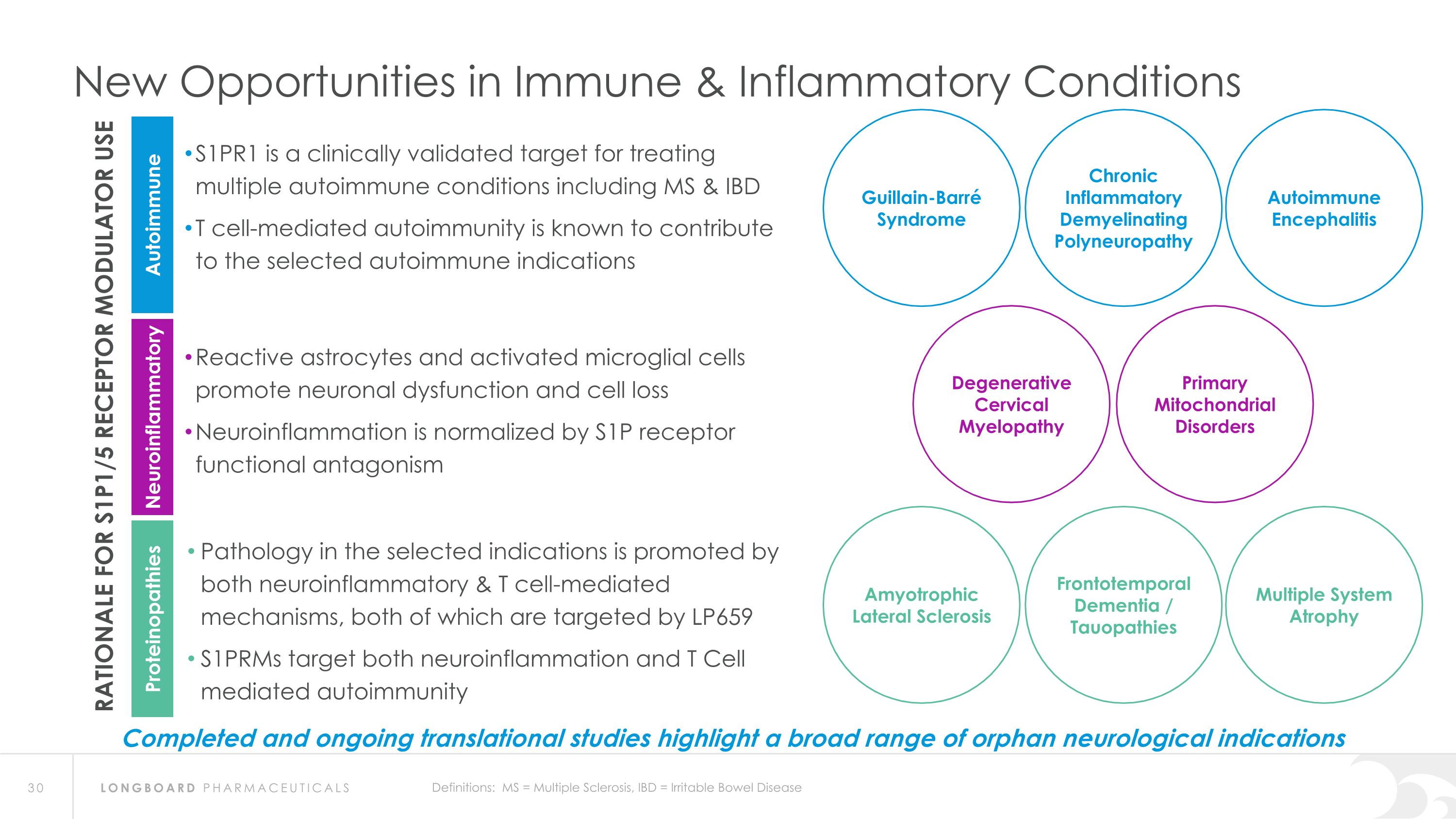
New Opportunities in Immune & Inflammatory Conditions S1PR1 is a clinically validated target for treating multiple autoimmune conditions including MS & IBD T cell-mediated autoimmunity is known to contribute to the selected autoimmune indications RATIONALE FOR S1P1/5 RECEPTOR MODULATOR USE Autoimmune Neuroinflammatory Proteinopathies Reactive astrocytes and activated microglial cells promote neuronal dysfunction and cell loss Neuroinflammation is normalized by S1P receptor functional antagonism Pathology in the selected indications is promoted by both neuroinflammatory & T cell-mediated mechanisms, both of which are targeted by LP659 S1PRMs target both neuroinflammation and T Cell mediated autoimmunity Degenerative Cervical Myelopathy Primary Mitochondrial Disorders Autoimmune Encephalitis Guillain-Barré Syndrome Chronic Inflammatory Demyelinating Polyneuropathy Definitions: MS = Multiple Sclerosis, IBD = Irritable Bowel Disease Multiple System Atrophy Amyotrophic Lateral Sclerosis Frontotemporal Dementia / Tauopathies Completed and ongoing translational studies highlight a broad range of orphan neurological indications
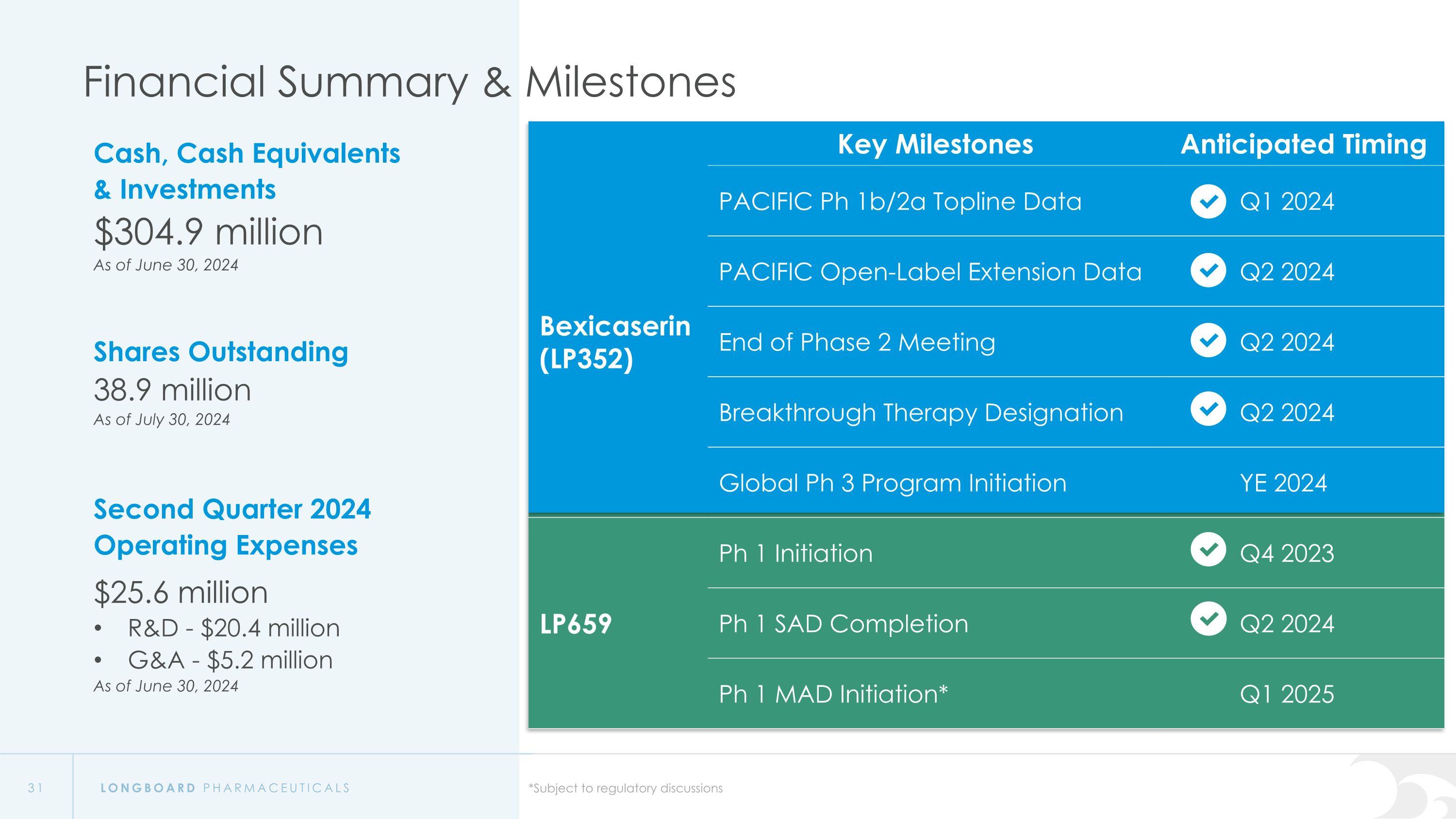
Financial Summary & Milestones Cash, Cash Equivalents & Investments $304.9 million As of June 30, 2024 Shares Outstanding 38.9 million As of July 30, 2024 Second Quarter 2024 Operating Expenses $25.6 million R&D - $20.4 million G&A - $5.2 million As of June 30, 2024 Key Milestones Anticipated Timing Bexicaserin (LP352) PACIFIC Ph 1b/2a Topline Data Q1 2024 PACIFIC Open-Label Extension Data Q2 2024 LP352 End of Phase 2 Meeting Q2 2024 Breakthrough Therapy Designation Q2 2024 Global Ph 3 Program Initiation YE 2024 LP659 Ph 1 Initiation Q4 2023 Ph 1 SAD Completion Q2 2024 Ph 1 MAD Initiation* Q1 2025 *Subject to regulatory discussions

Thank you Nasdaq: LBPH IR@longboardpharma.com