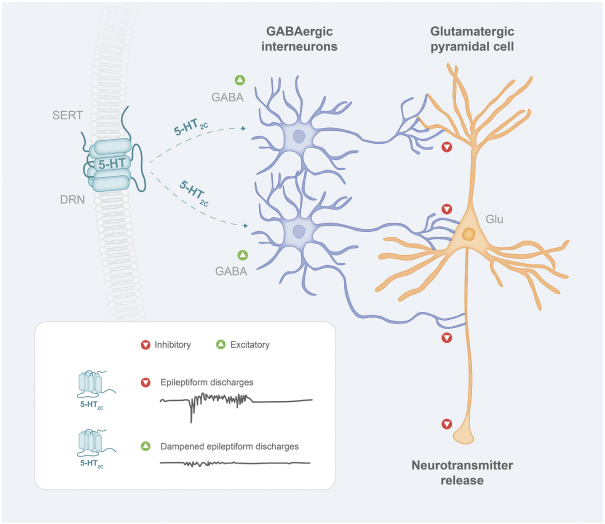
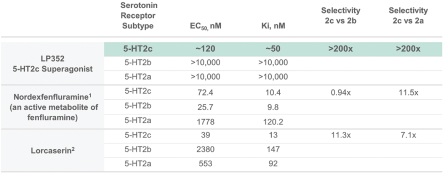
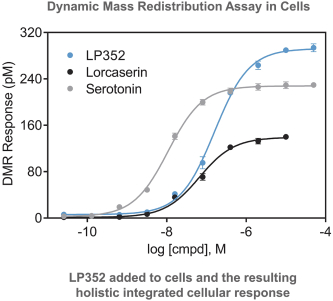
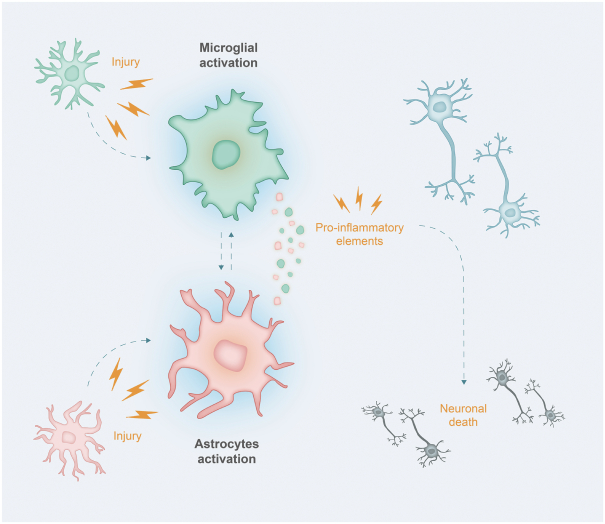
others, and in part, on our ability to prevent others from infringing our proprietary rights. A comprehensive discussion on risks relating to intellectual property is provided under the section of this prospectus entitled “Risk Factors—Risks Related to Our Intellectual Property.”
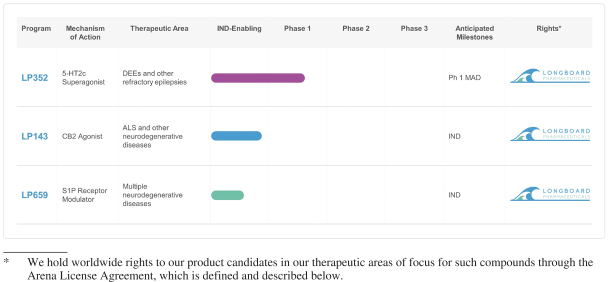
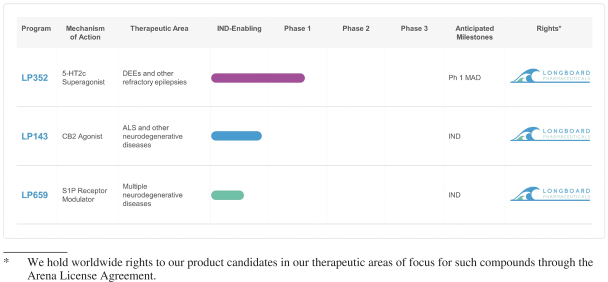
As of February 1, 2021, we held an exclusive, worldwide license to issued and pending patent claims for compositions of matter and certain methods of treatment using LP352 in several jurisdictions, including issued patents in the United States, Europe (17 countries), Japan, Mexico, Australia and Russia, and pending applications in China, Brazil, Canada, India, South Korea, New Zealand, and Israel. The terms of these patents (and applications, if issued) are capable of continuing into 2036, without taking into account any patent term adjustment or extension regimes of any country (e.g., up to five additional years in certain jurisdictions if maximum PTE or SPC applies) or any additional term of exclusivity we might obtain by virtue of later filed patent applications.
As of February 1, 2021, we held an exclusive, worldwide license to issued and pending patent claims for compositions of matter and certain methods of treatment using LP659 in several jurisdictions, including issued patents in the United States, Europe (39 countries), China, Japan, Canada, South Korea, Australia, Mexico, South Africa, New Zealand, Singapore, Israel, and Eurasia, and a pending application in Brazil. The terms of these patents (and applications, if issued) are capable of continuing into 2029, without taking into account any patent term adjustment or extension regimes of any country (e.g., up to five additional years in certain jurisdictions if maximum PTE or SPC applies) or any additional term of exclusivity we might obtain by virtue of later filed patent applications.
As of February 1, 2021, we held an exclusive, worldwide license to issued and pending patent claims for compositions of matter and certain methods of treatment using LP143 in several jurisdictions, including issued patents in China, Japan, Canada, India, Eurasia (9 countries), South Korea, Australia, Mexico, Taiwan, New Zealand, and Israel, and pending applications in the United States, Europe, Venezuela, Brazil, Argentina, South Africa, Bangladesh, Hong Kong, and the GCC. The terms of these patents (and applications, if issued) are capable of continuing into 2030, without taking into account any patent term adjustment or extension regimes of any country (e.g., up to five additional years in certain jurisdictions if maximum PTE or SPC applies) or any additional term of exclusivity we might obtain by virtue of later filed patent applications.
In addition to patent protection, we rely on trade secret protection, trademark protection and know-how to expand our proprietary position around our chemistry, technology and other discoveries and inventions that we consider important to our business. We are a party to a license agreement under which we are granted intellectual property rights to know-how that are important to our business. We have licensed know-how related to the LP352, LP143 and LP659 compounds in all countries around the world from Arena. The Arena License Agreement imposes various development, regulatory and/or commercial diligence obligations, payment of royalties, including a mid-single digit royalty on net sales of Licensed Products of LP352, and a low-single digit royalty on net sales of all other Licensed Products, by our company, its affiliates or its sublicensees, subject to standard reductions, and other obligations.
We also seek to protect our intellectual property by having confidentiality terms in our agreements with companies with whom we share proprietary and confidential information in the course of business discussions, and by having confidentiality terms in our agreements with our employees, consultants, scientific advisors, clinical investigators and other contractors and also by requiring our employees, commercial contractors, and certain consultants and investigators, to enter into invention assignment agreements that grant us ownership of any discoveries or inventions made by them while in our employ.
Sales and Marketing
Given our stage of development, we have not yet established a commercial organization or distribution capabilities. We intend to build a commercial infrastructure to support sales of any of our approved products. We expect to manage sales, marketing and distribution through internal resources and third-party relationships. While we may commit significant financial and management resources to commercial activities, we will also consider collaborating with one or more pharmaceutical companies to enhance our commercial capabilities.
107