As filed with the Securities and Exchange Commission on June 2, 2023
Registration No. 333-253037
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 10 to
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
IMPACT BIOMEDICAL INC.
(Exact name of registrant as specified in its charter)
| Nevada | | 8731 | | 85-3926944 |
(State or other jurisdiction of incorporation or organization) | | (Primary Standard Industrial Classification Code Number) | | (I.R.S. Employer Identification Number) |
275 Wiregrass Pkwy
West Henrietta, NY 14586
+1-585-325-3610
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Frank D. Heuszel
Chief Executive Officer
Impact BioMedical Inc.
275 Wiregrass Pkwy
West Henrietta, NY 14586
+1-585-325-3610
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With a copy to:
Darrin M. Ocasio, Esq.
Sichenzia Ross Ference LLP
1185 Avenue of the Americas
New York, NY 10036
Telephone: +1-212-930-9700
As soon as practicable after the effective date of this registration statement.
(Approximate date of commencement of proposed sale to the public)
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☐
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated filer | ☐ | | Accelerated filer | ☐ |
| Non-accelerated filer | ☒ | | Smaller reporting company | ☒ |
| | | | Emerging growth company | ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not issue these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
SUBJECT TO COMPLETION
PRELIMINARY PROSPECTUS DATED JUNE 2, 2023
IMPACT BIOMEDICAL INC.
486,706,712 shares of Common Stock
(par value $0.001 per share)
This prospectus is being furnished to you as a stockholder of DSS, Inc. (“DSS”), in connection with the planned distribution to its stockholders of shares of common stock, par value $0.001 per share (the “Common Stock”) of Impact BioMedical Inc. (the “Company,” “Impact,” “we,” “our” or “us” as applicable) beneficially held by DSS, through its wholly-owned subsidiary, DSS BioHealth Security, Inc. (the “Distribution”).
DSS’s long-term plans for the Company include taking the Company public through an initial public offering. In concert with this planned public offering, DSS, through its wholly-owned subsidiary DSS BioHealth Security, Inc. plans to distribute an aggregate of approximately 486,706,712 shares of our Common Stock (the “Impact Shares”) beneficially held by DSS BioHealth Security, Inc. in a distribution to holders of DSS common stock, par value $0.02 per share (“DSS Common Stock”) as of June 30, 2023 (the “Record Date”), except DSS will not cause to be distributed by DSS BioHealth Security, Inc. any Impact Shares to the Chairman of the Board of DSS, Mr. Chan Heng Fai Ambrose, or any of his affiliates that hold shares of DSS Common Stock. As of the Record Date, Mr. Chan, individually and through affiliates, beneficially held 18,587,572 shares of DSS Common Stock (the “Affiliate Shares”). Except for the Affiliate Shares, each share of DSS Common Stock outstanding as of 5:00 p.m., New York City time, held on a Record Date, will entitle the holder thereof to receive four (4) Impact Shares.
Immediately prior to the Distribution, DSS, through its wholly-owned subsidiary Impact BioMedical Inc., will beneficially hold 100%, or 3,877,282,251 of the issued and outstanding shares of our Common Stock. The Distribution will be effective as of 5:00 p.m., New York City time, on July 14, 2023 (“Distribution Date”).
The Distribution will not occur until this Registration Statement becomes effective under the Securities Act of 1933, as amended (the “Securities Act”). The Distribution will be made in book-entry form by a distribution agent. Each Impact Share distributed as part of the Distribution will be not be eligible for resale until 180 days from the date of the Company’s initial public offering becomes effective under the Securities Act, subject to the discretion of the Company to lift the restriction sooner.
All of our outstanding shares of Common Stock are currently beneficially owned by DSS, through its wholly-owned subsidiary DSS BioHealth Security, Inc. Accordingly, there currently is no public trading market for our Common Stock. Our Common Stock is currently not listed for trading on any stock exchange or market. The Impact Shares may be illiquid as we cannot predict whether any trading market will develop. We currently plan to apply for listing of our Common Stock on the over-the-counter board upon the effectiveness of the registration statement, of which this prospectus forms a part. However, we can provide no assurance that our shares will be traded on the bulletin board or, if traded, that a public market will materialize. If no market is ever developed for our shares, it will be difficult for shareholders to sell their stock.
DSS stockholders are not required to vote on or take any other action in connection with the Distribution. We are not asking you for a proxy, and we request that you do not send us a proxy. DSS stockholders will not be required to pay any consideration for the Impact Shares that they receive in the Distribution, and they will not be required to surrender or exchange their shares of DSS Common Stock or take any other action in connection with the Distribution.
We are an “emerging growth company,” as defined under the federal securities laws, and have elected to comply with certain reduced public company reporting requirements for this prospectus and for future filings.
Investing in our securities involve a high degree of risk. Before buying any securities, you should carefully consider the matters described in the section titled “Risk Factors” beginning on page 11 of this Prospectus.
You should rely only on the information contained in this prospectus or any prospectus supplement or amendment hereto. We have not authorized anyone to provide you with different information.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved these securities or determined if this Prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
This Prospectus is not an offer to sell, or a solicitation of an offer to buy, any securities.
The date of this Prospectus is ________ ___, 2023.
TABLE OF CONTENTS
QUESTIONS AND ANSWERS ABOUT THE DISTRIBUTION
The following questions and answers briefly address some commonly asked questions about the Distribution. They may not include all the information that is important to you. We encourage you to read carefully this entire Prospectus and the other documents to which we have referred you. We have included references in certain parts of this section to direct you to a more detailed discussion of each topic presented in this section.
| Q: | What is the Distribution? |
| | |
| A: | The Distribution is a method by which we will begin to separate from DSS. DSS currently beneficially owns through its wholly-owned subsidiary, DSS BioHealth Security, Inc., 3,877,282,251 shares of our Common Stock, representing 100% of our issued and outstanding capital stock. In connection with the Distribution, DSS, through its wholly-owned subsidiary DSS BioHealth Security, Inc., will distribute to holders of DSS Common Stock an aggregate of 486,706,712 Impact Shares. Following the Distribution DSS, through its wholly-owned subsidiary DSS BioHealth Security, Inc., will retain approximately an 87% ownership interest in the Company or otherwise possess control over us. |
| | |
| Q: | Will the number of shares of DSS Common Stock I own change as a result of a Distribution? |
| | |
| A: | No, the number of shares of DSS Common Stock you own will not change as a result of a Distribution. |
| | |
| Q: | What are the reasons for the Distribution? |
| | |
| A: | The Distribution is a method by which DSS will begin to carry out the intention of management and the board of directors of DSS (“DSS Board”) to take the Company public and to reward DSS stockholders via the issuance of the Impact Shares. |
| | |
| Q: | What will I receive in a Distribution? |
| | |
| A: | As a holder of DSS Common Stock, you will receive a dividend of four Impact BioMedical Shares for every share of DSS Common Stock you hold on the Record Date. The distribution agent will distribute only whole Impact Shares in the Distribution. Your proportionate interest in DSS will not change as a result of the Distribution. For a more detailed description, see “The Distribution.” |
| | |
| Q: | What is being distributed to holders of DSS Common Stock in the Distribution? |
| | |
| A: | DSS will cause its wholly-owned subsidiary DSS BioHealth Security, Inc. to distribute 486,706,712 Impact BioMedical Shares in the Distribution. Subsequent to the Distribution DSS will maintain through its wholly-owned subsidiary DSS BioHealth Security, Inc. approximately 87% of our issued and outstanding shares of Common Stock. For more information on the shares being distributed in the Distribution, see “Description of Our Capital Stock—Common Stock.” |
| Q: | What is the record date for the Distribution? |
| | |
| A: | The DSS Board has designated 5:00 p.m., New York City time, on June 30, 2023 as the record ownership date for the Distribution. |
| | |
| Q: | When will the Distribution to holders of DSS Common Stock occur? |
| | |
| A: | The Distribution will be effective as of 5:00 p.m., New York City time on July 14, 2023 (the “Distribution Date”). On or shortly after the Distribution Date, the Impact Shares will be credited in book-entry accounts for stockholders entitled to receive those shares in the Distribution. We expect that it may take the distribution agent up to two weeks after each Distribution Date to fully distribute the Impact Shares to DSS stockholders. See “Questions and Answers About the Distribution—How will DSS distribute the Impact Shares?” for more information on how to access your book-entry account or your bank, brokerage or other account holding Impact Shares that you will receive in the Distribution. |
| | |
| Q: | What do I have to do to participate in the Distribution? |
| | |
| A: | You are not required to take any action, but we urge you to read this Prospectus carefully. Holders of DSS Common Stock on the Record Date will not need to pay any cash or deliver any other consideration, including any shares of DSS Common Stock, in order to receive Impact Shares in the Distribution. No stockholder approval of the Distribution is required. We are not asking you for a vote, and we request that you do not send us a proxy card. |
| | |
| Q: | If I sell my shares of DSS Common Stock on or before the Distribution Date, will I still be entitled to receive shares of Common Stock in the Distribution? |
| | |
| A: | If you hold shares of DSS Common Stock as of the Record Date and decide to sell them on or before the relevant Distribution Date, you will not be entitled to receive Impact Shares. You should discuss these alternatives with your bank, broker or other nominee. Each Impact Share distributed as part of the Distribution will be not be eligible for resale until 180 days from the date of the Company’s initial public offering becomes effective under the Securities Act, subject to the discretion of the Company to lift the restriction sooner. |
| | |
| Q: | How will DSS distribute the Impact Shares? |
| | |
| A: | Registered stockholders: If you are a registered stockholder (meaning you own your shares of DSS Common Stock directly through DSS’s transfer agent, American Stock Transfer & Trust Company, LLC), V Stock Transfer LLC our transfer agent, which is serving as the distribution agent in connection with the Distribution, will credit the Impact Shares you receive in the Distribution to a new book-entry account on or shortly after the Distribution Date. Our distribution agent will mail you a book-entry account statement that reflects the number of Impact Shares you own. You will be able to access information regarding your book-entry account holding the Impact Shares at V Stock Transfer LLC. |
| | |
| | “Street name” or beneficial stockholders: If you own your shares of DSS Common Stock beneficially through a bank, broker or other nominee, your bank, broker or other nominee will credit your account with the Impact Shares you receive in the Distribution on or shortly after the Distribution Date. Please contact your bank, broker or other nominee for further information about your account. |
| | |
| | We will not issue any physical stock certificates to any stockholders, even if requested. See “The Distribution—When and How You Will Receive Company Shares” for a more detailed explanation. |
| Q: | What is the U.S. federal income tax consequences to me of the Distribution? |
| | |
| A: | DSS shareholders will be subject to being taxed on the distribution of the Impact Shares. |
| | |
| | See “Material U.S. Federal Income Tax Consequences of the Distribution” for more information regarding the potential tax consequences to you from the Distribution. |
| | |
| | You should consult your own tax advisors regarding the particular tax consequences of the Distribution to you, including the applicability and effect of any U.S. federal, state, local and non-U.S. tax laws. |
| | |
| Q: | Does the Company intend to pay cash dividends? |
| | |
| A: | Following the Distribution, we do not anticipate paying any dividends on Impact Shares in the foreseeable future. See “Dividend Policy” for more information. |
| | |
| Q: | How will our Common Stock trade? |
| | |
| A: | Our shares of Common Stock are not listed on any securities exchange and the Impact Shares may be illiquid as we cannot predict whether any trading market will develop. |
| | |
| Q: | Will my shares of DSS Common Stock continue to trade on the NYSE American LLC exchange (“NYSE Amex”) following the Distribution? |
| | |
| A: | Yes. Following the Distribution, DSS Common Stock will continue to trade on the NYSE Amex under the symbol “DSS” through and after the Distribution Date. |
| | |
| Q: | Will the Distribution affect the trading price of my DSS Common Stock? |
| | |
| A: | We do not expect the trading price of shares of DSS Common Stock immediately following the Distribution to be materially lower or higher than immediately prior to the Distribution. However, until the market has fully analyzed the value of DSS without its ownership of the Impact Shares, the trading price of shares of DSS Common Stock may fluctuate. We cannot assure you that, following the Distribution, the combined trading prices of the DSS Common Stock and Impact Shares will equal or exceed what the trading price of DSS Common Stock would have been in the absence of the Distribution. It is possible that after the Distribution, the combined equity value of DSS and the Impact Shares will be less than DSS’s equity value before the Distribution. |
| Q: | Do I have appraisal rights in connection with the Distribution? |
| | |
| A: | No. Holders of DSS Common Stock are not entitled to appraisal rights in connection with the Distribution. |
| Q: | What will the relationship be between DSS and the Company after the Distribution? |
| | |
| A: | Following the Distribution, DSS, through its wholly-owned subsidiary DSS BioHealth Security, Inc., will still have a continuing stock ownership interest in and is expected to possess control over the Company, until and if the proposed public offering of the Company is completed. |
| | |
| Q: | Who is the transfer agent and registrar for our Common Stock? Who is the distribution agent in connection with the Distribution? |
| | |
| A: | V Stock Transfer LLC is the transfer agent and registrar for our Common Stock and is serving as the distribution agent in connection with the Distribution. |
| | |
| Q: | Are there risks associated with owning shares of our Common Stock? |
| | |
| A: | Yes. Our business faces both general and specific risks and uncertainties. Our business also faces risks relating to the Distribution. Accordingly, you should read carefully the information set forth in the section titled “Risk Factors” in this Prospectus. |
| | |
| Q: | Are there any conditions to completing the Distribution? |
| | |
| A: | Yes. The Distribution is conditional upon a number of matters, including but not limited to the authorization and approval of the DSS Board (which has been obtained) and the declaration of effectiveness of our Registration Statement on Form S-1, of which this Prospectus is a part, by the Securities and Exchange Commission. See “Summary—Summary of the Distribution— Conditions to the Distribution” for a more detailed explanation of the conditions to completing the Distribution. |
| | |
| Q: | Can DSS decide to not proceed with the Distribution even if all the conditions to the Distribution have been met? |
| | |
| A: | Yes. Until the Distribution has occurred, the DSS Board has the right to not proceed with the Distribution, even if all the conditions are satisfied. |
| | |
| Q: | Could there be any other classes of capital stock of the Company outstanding after the Distribution? |
| | |
| A: | No. After giving effect to the Distribution, the only class of our capital stock then outstanding is expected to be our Common Stock. |
| | |
| Q: | Where can I get more information? |
| | |
| A: | If you have any questions relating to the mechanics of the Distribution, you should contact the distribution agent, V Stock Transfer LLC, at: |
V Stock Transfer LLC 18 Lafayette Place
Woodmere, NY 11598
+1 212-828-8435
info@vstocktransfer.com
www.vstocktransfer.com
SUMMARY
This summary highlights certain information contained elsewhere in this Prospectus and may not contain all the information that is important to you. To understand fully and for a more complete description of the terms and conditions of the Distribution, you should read this Prospectus in its entirety, including the information presented under the section titled “Risk Factors” and the consolidated financial statements and related notes, and the documents to which you are referred. See “Where You Can Find More Information.”
Except where the context otherwise requires, or where otherwise indicated, references to the “Company,” “we,” “us,” or “our” are to are to Impact BioMedical Inc. and its subsidiaries.
Introduction
Impact BioMedical is the developer of unique and differentiated technologies to address unmet healthcare and wellness needs. Our activities range from the discovery of technologies and leveraging those technologies to create and commercialize product candidates. Currently, our operations are conducted, and our assets are owned primarily through our principal subsidiaries: (i) Global BioLife, Inc. (“Global BioLife”), which was incorporated on April 14, 2017, (ii) Impact BioLife Science (“Impact BioLife”), which was incorporated on August 28, 2020, Global BioMedical, Inc. (“Global BioMedical”), which was incorporated on April 18, 2017, and (iii) Sweet Sense, Inc. (“Sweet Sense”), which was incorporated on April 30, 2018.
Risks Associated with Our Business
Our business is subject to numerous risks described in the section entitled “Risk Factors” and elsewhere in this prospectus. You should carefully consider these risks. Some of these risks include, but are not limited to that:
| | ● | we cannot guarantee that we will find third-parties or customers that are interested in purchasing, licensing, or co-developing our products; |
| | ● | even if we are able to establish licensing arrangements, we cannot guarantee that licensors will be successful in their development efforts of products; |
| | ● | we cannot guarantee that our products will ever be approved for clinical testing or commercialization by the FDA; |
| | ● | we have not yet generated any revenue from our operations; |
| | ● | we have a history of net losses, negative cash flows, and accumulated deficits over the last two years; and |
| | ● | an occurrence of an uncontrolled event such as the Covid-19 pandemic, is likely to negatively affect our operations. |
Implications of Being an Emerging Growth Company and a Smaller Reporting Company
As a company with less than $1.235 billion in revenue during our last fiscal year, we qualify as an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act (the “JOBS Act”). Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended (or the “Securities Act”), for complying with new or revised accounting standards. Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies.
An emerging growth company may also take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
| | ● | we may present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations; |
| | | |
| | ● | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act; |
| | | |
| | ● | reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements and registration statements; and |
| | | |
| | ● | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval |
We may take advantage of these provisions until the last day of our fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act, which such fifth anniversary will occur in 2026. However, if certain events occur prior to the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1.235 billion or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company prior to the end of such five-year period.
We have elected to take advantage of certain of the reduced disclosure obligations regarding executive compensation in this prospectus and, as long as we continue to qualify as an emerging growth company, we may elect to take advantage of this and other reduced burdens in future filings. As a result, the information that we provide to our stockholders may be different than you might receive from other public reporting companies in which you hold equity interests.
We are also a “smaller reporting company,” as defined under SEC Regulation S-K. As such, we also are exempt from the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and also are subject to less extensive disclosure requirements regarding executive compensation in our periodic reports and proxy statements. We will continue to be deemed a smaller reporting company until our public float exceeds $75 million on the last day of our second fiscal quarter in the preceding fiscal year.
Business Overview
Impact BioMedical Inc. (“Impact”) targets urgent medical needs and expands the borders of medical and pharmaceutical science. Impact drives mission-oriented research and development, and commercialization of solutions for medical advances in human wellness and healthcare. By leveraging technology and new science with strategic partnerships, Impact BioMedical provides advances in drug discovery for the prevention, inhibition, and treatment of neurological, oncology and immuno-related diseases. Other exciting technologies include a breakthrough alternative sugar aimed to combat diabetes and functional fragrance formulations aimed at the industrial and medical industry. The research and development efforts have been primarily conducted in collaboration with GRDG Sciences, LLC (“GRDG”) and with the expertise of Mr. Daryl Thompson as its Director of Scientific Initiatives. We have initiated research regarding universal therapeutics as part of an attempt to help address some of the world’s deadliest diseases.
The business model of Impact BioMedical revolves around two methodologies – Licensing and Sales Distribution. Impact BioMedical develops valuable and unique patented technologies which will be licensed to pharmaceutical, large consumer package goods companies and venture capitalists in exchange for usage licensing and royalties. Impact also utilizes the DSS ecosystem to leverage distribution networks on a global scale. Impact will engage in branded and private labelling of certain products for sales generation through these channels. This global distribution model will give direct access to end users of Impact’s nutraceutical and health related products.
Below is a list of our principal subsidiaries:
| | ● | Impact BioLife Science, Inc.; |
| | ● | Global BioMedical, Inc.; |
| | ● | Global BioLife, Inc.; and |
| | ● | Sweet Sense, Inc. |
Impact BioLife Science, Inc. We are the sole owner of the outstanding equity of Impact BioLife Science, Inc.
Global Biomedical, Inc. We own 90.91% of Global Biomedical, Inc. outstanding equity, and the balance minority equity owner is Peggy Tang.
Global BioLife, Inc. Through our majority owned subsidiary Global Biomedical, Inc., we own 90% of the outstanding equity of Global BioLife, Inc. The other equity owner is Holista CollTech Limited (“Holista”) (10%).
Sweet Sense, Inc. We are the owner of 50% of the outstanding equity of Sweet Sense. The other equity owner is BioLife Sugar, Inc. (“BioLife Sugar”).
Below is an organization chart showing our ownership structure and ownership interests.
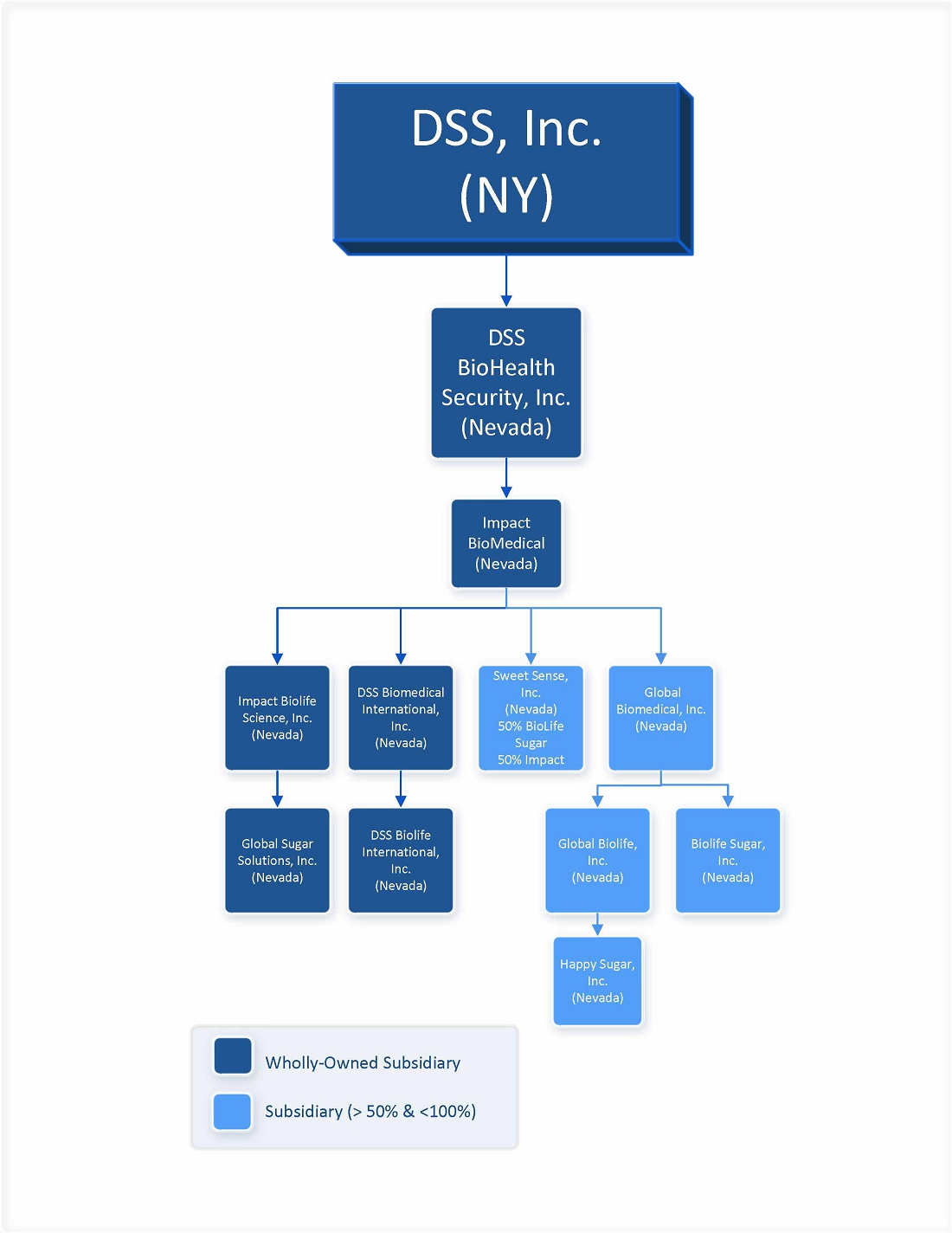
Impact BioMedical and our majority-owned subsidiaries own or have rights to a portfolio of biomedical intellectual property and leverages its scientific know-how and intellectual property rights to develop various emerging technologies, including biopharmaceuticals, antivirals, antimicrobials, sugar alternatives, insect repellents, fragrances, bioplastics and natural preservatives.
Impact BioMedical has had several important and valuable products, technology, or compounds that are in continuing development and/or licensing stages. The Company has not conducted and has no current plans to conduct any preclinical testing for these products, technology, or compounds:
| | ● | LineBacker: Multi-faceted therapeutic platform intended for metabolic, neurologic, cancer, and infectious diseases. |
| | | |
| | ● | Equivir: A compound intended for antiviral infection treatments. Equivir/Nemovir is a novel blend of FDA Generally Recognized as Safe (“GRAS”) eligible natural compounds. These compounds are generally sourced from fruits, vegetables, and other natural substances. Please note, the FDA has not approved this product, GRAS designation means that the FDA does not question the basis for a notifier’s GRAS determination, and GRAS determination does not increase the likelihood that product candidates will receive marketing approval. |
| | | |
| | ● | Laetose: Laetose technology is derived from a unique combination of sugar and other naturally occurring compounds which demonstrate the ability to inhibit the inflammatory and metabolic response of sugar alone. This compound is a sugar alternative aimed to combat diabetes. |
| | | |
| | ● | 3F: A botanical compound intended to serve as an insect repellent and anti-microbial agent. 3F is a unique formulation of specialized ingredients from naturally occurring botanical sources. |
| | | |
| | ● | 3F Mosquito Repellent: 3F repellent contains botanical ingredients that mosquitos avoid. These ingredients affect the mosquito’s receptors, essentially making the insect blind to a human’s presence. This can be utilized as a stand-alone repellent or as an additive in detergents, lotions, shampoo, and other substances to provide mosquito protection. |
| | | |
| | ● | 3F Antimicrobial: 3F antimicrobial contains botanical ingredients known to kill viruses. These ingredients are intended to inhibit viral replication. This can be utilized as a stand-alone antimicrobial or as an additive in detergents, lotions, shampoo, fabrics, and other substances. |
Commercialization Business Strategies
Our business model revolves mainly around two approaches – Licensing and Sales Distribution.
1. Licensing
The licensing strategy includes developing unique patented technologies which would then be licensed to external partners for developing, registration, and commercialization as appropriate. We believe that interest in licensing certain projects may rise over time as validating data becomes available.
In June of 2022, Impact BioMedical signed a License Agreement with ProPhase Labs to produce and distribute compound Equivir, which has shown potential as a treatment to limit the occurrence of or reduce the risk or severity of viral outbreaks.
In July of 2022, Global BioLife, Inc., a subsidiary of Impact, executed a license agreement with ProPhase BioPharma, Inc. (“PBIO”), a subsidiary of ProPhase Labs, Inc., a rapidly growing and diversified diagnostics, genomics and biotech company, for Global BioLife’s Linebacker portfolio (LB-1 and LB-2), two patented small molecule PIM kinase inhibitors with significant potential across multiple therapeutic indications.
2. Sales Distribution
We are intending to pursue 3rd party partnerships specifically with large manufactures in the pharmaceutical, food, health and beauty, and nutraceutical industries to commercialize our products, ideas and intellectual property. We will leverage their product development, commercialization and distribution capabilities to take products to market through a licensing and/or private label sales model.
3. Research and Development
We have secured arrangements with certain affiliates to undertake activities to improve existing product candidates and develop new ones. We believe the timely development of new, and the enhancement of our existing products, is essential to our success. Although we intend to invest a substantial amount of our resources in research and development, there is no guarantee that our investment will translate into improvements of existing products or the development of new products.
Stockholders Agreement between Global BioLife and the Global BioLife Stockholders
On April 26, 2017, Global BioLife entered into a stockholders’ agreement with its stockholders Global BioMedical, GRDG and Holista (the “Global BioLife Stockholders’ Agreement”). Pursuant to the Global BioLife Stockholder’s Agreement, GRDG has agreed to contribute to Global BioLife any and all right, title, interest and ownership held by GRDG in any patent related to the uses of the “Linebacker Patents” as defined in the Global BioLife Stockholders’ Agreement. Further, pursuant to the Global BioLife Stockholders Agreement, GRDG has agreed to contribute to Global BioLife the advice and services of Daryl Thompson as a scientist during the term of the agreement in connection with the development of the Linebacker Patents and all projects associated therewith, as well as such other projects as Global BioLife may from time to time pursue. In addition, Global BioLife agreed to contract with GRDG for the needed research in order to develop the Linebacker Patents as well as new intellectual property. Compensation paid by Global BioLife for this work, if any, shall be provided for in Global BioLife’ budget
Pursuant to the Global BioLife Stockholders’ Agreement, Global Biomedical has agreed to contribute to Global BioLife the funds set forth in the Global BioLife budget and such reasonable amounts as the Global BioLife board of directors shall in future annual periods authorize as Global BioLife’s business plan and budget. Such budget shall include (i) a payment of $20,994 per month to GRDG and (ii) such other amounts as shall be necessary to fund the scientific operations that the Global BioLife board shall agree to pursue. The monthly payments were adjusted for the increase in rent of GRDG office and general inflation. Current monthly payments approximate to $43,000. For the years ended December 31, 2022 and 2021, the Company incurred expenses of $546,000 and $509,176, respectively. On December 31, 2022 and 2021, the Company owed this related party $0, and had prepaid monthly fees approximating $43,000.
Under the Global BioLife Stockholders’ Agreement, Holista has agreed to (i) assist in the global commercialization of Global BioLife’s intellectual property, (ii) assist in the initiation and development of joint venture opportunities for Global BioLife, and (iii) provide the expertise of Dr. Rajen M. Dato to Global BioLife as strategic director.
The Global BioLife Stockholders’ Agreement shall terminate (a) upon the dissolution and winding up of Global BioLife or (b) on the date the parties terminate the agreement by unanimous written consent.
On May 22, 2018, the parties to the Global BioLife Stockholders’ Agreement entered into Amendment No. 1 to the agreement (“Amendment No. 1 to the Global BioLife Stockholders Agreement”), and in August of 2020 the parties entered into Amendment No. 2 to the agreement (“Amendment No. 2 to the Global BioLife Stockholders Agreement”). Pursuant to Amendment No. 2, the parties to the Global BioLife Stockholders Agreement agreed to waive the obligations of GRDG to contribute any further inventions, discoveries or other items of intellectual property developed by GRDG during the term of the Stockholders’ Agreement subsequent to the date of Amendment No. 2, except for any invention, discovery or other items of intellectual property which is directly related to, or necessary for the sale, licensing or further development of intellectual property owned by Global BioLife.
Licensing Proceeds Distribution Agreement
In May 2022, Impact entered into a new licensing proceeds distribution agreement whereas GRDG will continue to invent and develop Improvements and Discoveries for and on behalf Impact and agree to assign any such Discoveries/Improvements/advances to Impact generated by its efforts and through the resources funded by Impact, directly or indirectly. Any such Discoveries assigned to Impact would be considered Intellectual Property under this Agreement such that GRDG would receive 20% of the gross licensing or sale proceeds received by Impact from the licensing of such discoveries. Under the same agreement, GRDG transferred all of its right, title, and interest that GRDG has, if any, in Global as set forth in the Global Agreement back to Global, and all right, title and interest that GRDG has, if any, in Impact back to Impact, and hereinafter having no further ownership, control, or interest in either Global or Impact.
Stockholders Agreement between Impact BioLife and the Impact BioLife Stockholders
In December 2020, Impact BioLife entered into a stockholder’s agreement with its stockholders Impact BioMedical and GRDG (the “Impact BioLife Stockholders’ Agreement”). Pursuant to the Impact BioLife Stockholders’ Agreement, GRDG agreed to (i) transfer certain intellectual property as identified in the Stockholders’ Agreement to Impact BioLife, (ii) present all suitable technologies developed by GRDG to Impact BioLife, so as to provide Impact BioLife with the opportunity to fund, own and develop any intellectual property developed by GRDG and (iii) retain the advice and services of Mr. Daryl Thompson as a scientist during the term of the Impact BioLife Stockholders’ Agreement in connection with all projects as Impact BioLife may from time to time pursue. Further, pursuant to the Impact BioLife Stockholders’ Agreement, GRDG also agreed that Daryl Thompson will devote most of his professional time and efforts to the business of Impact BioLife, including but not limited to, the development of new intellectual property for Impact BioLife.
In addition, pursuant to the Impact BioLife Stockholder’s Agreement, the Company has agreed to contribute to Impact BioLife such reasonable amounts as the board of directors of Impact BioLife shall in future annual periods authorize as Impact BioLife’s business plan and budget (the “Impact BioLife Budget”). The Impact BioLife Budget shall include (i) a payment approximating $43,000 per month to GRDG and (ii) such other amounts as shall be necessary to fund the research, development and other scientific operations that the Impact BioLife board of directors shall agree to pursue. For the years ended December 31, 2022 and 2021, the Company incurred expenses of $546,000 and $509,176, respectively. On December 31, 2022 and 2021, the Company owed this related party $0, and had prepaid monthly fees approximating $43,000.
Pursuant to the Impact BioLife Stockholders’ Agreement, the board of directors of Impact BioLife shall never be less than one nor more than five directors. GRDG shall be entitled to nominate one director to the Impact BioLife board of directors so long as it shall remain a stockholder of Impact BioLife. The Company shall be entitled to nominate the remaining directors of the Impact BioLife. In addition, pursuant to the Impact BioLife Stockholders’ Agreement, so long as it is a stockholder of Impact BioLife, the Company is entitled to appoint Impact BioLife’s chief executive officer, who, at the discretion of the Company, may also serve as a director of the Impact BioLife board of directors. The parties to the Impact BioLife Stockholders’ Agreement have agreed that the initial directors of the Impact BioLife board of directors shall be Heng Fai Ambrose Chan, Frank D. Heuszel and Daryl Thompson. The Company shall appoint the chairman of the Impact BioLife board of directors.
The Impact BioLife Stockholders’ Agreement will terminate (a) upon the dissolution and winding up of Impact BioLife, (b) on the date the parties terminate the agreement by unanimous written, (c) on the fifth anniversary of the date of the Impact BioLife Stockholders’ Agreement, unless the parties mutually agree to an extension, or (d) upon three months’ written notice by either party.
Licensing Proceeds Distribution Agreement between GRDG Sciences, LLC, Global BioLife, Inc., and Impact BioLife Sciences, Inc., to include Impact Biomedical Inc.
On February 15, 2022, the Company and Impact BioLife Sciences, Inc. (together, “Impact”), entered into a Licensing Proceeds Distribution Agreement (the “LPDA”) with the Company’s subsidiary, Global BioLife, Inc. (“Global,” and together with Impact BioLife Sciences, Inc. and the Company, the “Impact Parties”), and GRDG Sciences, LLC (“GRDG”), pursuant to which the parties clarified the consideration due to GRDG under pre-existing agreements by and between GRDG and the Impact Parties. Pursuant to the LPDA, GRDG will receive 20% of the gross licensing or sale proceeds from any of its developed improvements for and on behalf of Global and the Impact Parties. Further, Impact agreed to provide periodic payments to GRDG for the purpose of paying or reimbursing certain salaries, overhead, office rent reimbursements, and other operating costs. As compensation, GRDG will receive 20% of the gross licensing or sale proceeds received by the Impact Parties for any intellectual property generated. Pursuant to the GRDG, Impact retroactively acknowledged that GDRG (1) is not a shareholder in any company or subsidiary of the Impact Parties, and (2) that GDRG’s full consideration for the improvement developments which it has or will develop is/was (a) monthly operating cash flow payments, and (2) 20% of the gross proceeds from any licensing or sale of such improvements and discoveries. In addition, GRDG agreed to transfer all of its right, title and interest in Global to Global, and all right, title and interest in Impact to Impact. Global has agreed to provide GRDG a financial interest in revenue received directed to the licensing of the Global intellectual property, and Impact has agreed to provide GRDG an interest in revenue received directed to the licensing of the impact intellectual property.
Share Exchange Transaction
On August 21, 2020, we closed on a Share Exchange Agreement by and among DSS, DSS BioHealth Security, Inc., Alset International Ltd. (formerly Singapore eDevelopment Ltd.), and Global Biomedical Pte Ltd. (“GBM”), pursuant to which we became a wholly-owned subsidiary of DSS BioHealth Security, Inc. Shortly after closing, Frank D. Heuszel, Jason Grady, and John Thatch were appointed directors, and Mr. Heng Fai Ambrose Chan remained on the Board of Directors and appointed Chief Executive Officer, Mr. Heuszel was appointed President, Mr. Grady was appointed Chief Operating Officer and Todd Macko was appointed Secretary and Treasurer, replacing the officers and directors who resigned in connection with the share exchange.
Mr. Chan is the Chief Executive Officer and largest shareholder of Alset International Ltd., as well as the Chairman of the Board and largest shareholder of the DSS.
Corporate Information
Impact BioMedical Inc. is a Nevada corporation and was incorporated in October 2018. Our principal executive offices are located at 275 Wiregrass Parkway, West Henrietta, NY 14586. Our telephone number is +1-585-325-3610. Our website address is https://www.impbio.com. Our website and the information contained thereon, or connected thereto, does not and will not constitute part of this Prospectus or the Registration Statement on Form S-1 of which this Prospectus is a part.
Summary of the Distribution
| Distributing Company | | |
| | | DSS, Inc., a New York corporation, which beneficially owns through its wholly-owned subsidiary DSS BioHealth Security, Inc. 3,877,282,251 shares of our Common Stock, representing 100% of our outstanding shares of our Common Stock prior to the Distribution. After the Distribution, DSS will own approximately 87% of our Common Stock. |
| | | |
| Distributed Company | | |
| | | Impact BioMedical Inc., a Nevada corporation and a subsidiary of DSS. At the time of the Distribution, we operate primarily through our principal subsidiaries, Impact BioLife, Global Biomedical, Global BioLife and Sweet Sense. Through our principal subsidiaries, we are committed to both funding research, developing and commercializing new offerings in pharmaceuticals, consumer and wellness products, including, but not limited to, a focus on: (i) the development of a universal therapeutic drug platform; (ii) a new sugar substitute; and (iii) a multi-use fragrance developed for industrial and medical applications, including an insect repellant, and (iv) an OTC medication with broad antiviral activity. Impact. |
| Distributed Securities in the Distribution | | |
| | | 486,706,712 shares of our Common Stock indirectly owned by DSS, which represent approximately 12% of our Common Stock issued and outstanding immediately prior to the Distribution. Based on 140,264,250 shares of DSS Common Stock outstanding as of the close of business on the Record Date, not including the Affiliate Shares, this reflects a distribution ratio of four (4) Impact Shares for every one (1) share of DSS Common Stock. |
| | | |
| Record Date | | The Record Date is 5:00 p.m., New York City time, on June 30, 2023. |
| | | |
| Distribution Date | | The Distribution Date is July 14, 2023, and we expect that the Distribution will be effective as of 5:00 p.m., New York City time. |
| | | |
| | | |
| Distribution Ratio | | Each holder of DSS Common Stock will receive four (4) shares of our Common Stock for every one (1) share of DSS Common Stock it holds on the Record Date. The distribution agent will distribute only whole shares of our Common Stock in the Distribution. |
| | | |
| | | Please note that if you sell your shares of DSS Common Stock on or before the Distribution Date, the buyer of those shares will in most circumstances be entitled to receive the shares of our Common Stock to be distributed in respect of the DSS shares that you sold. |
| | | |
| | | |
| The Distribution | | On the Distribution Date, DSS will release the shares of our Common Stock to the distribution agent to distribute to DSS stockholders. DSS will distribute our shares in book-entry form, and thus we will not issue any physical stock certificates. We expect that it will take the distribution agent up to two weeks to electronically issue shares of our Common Stock to you or your bank or brokerage firm on your behalf by way of direct registration in book-entry form. If you own your shares of DSS Common Stock through a bank, broker or other nominee, your bank, broker or other nominee will credit your account with the whole shares of our Common Stock that you receive in the Distribution on or shortly after the Distribution Date. You will not be required to make any payment, surrender or exchange your shares of DSS Common Stock or take any other action to receive your shares of our Common Stock. |
| | | |
| Conditions to the Distribution | | The Distribution is subject to the satisfaction, or the DSS Board’s waiver, of the following conditions: |
| | | ● | the DSS Board shall have authorized and approved the Distribution by its wholly-owned subsidiary DSS BioHealth Security, Inc. and not withdrawn such authorization and approval, and shall have declared the dividend of our Common Stock to DSS stockholders; |
| | | ● | the SEC shall have declared effective our Registration Statement on Form S-1, of which this Prospectus is a part, under the Securities Act of 1933, as amended (the “Securities Act”), and no stop order suspending the effectiveness of our Registration Statement shall be in effect and no proceedings for that purpose shall be pending before or threatened by the SEC; |
| | | ● | no order, injunction or decree issued by any governmental authority of competent jurisdiction or other legal restraint or prohibition preventing consummation of the Distribution shall be in effect, and no other event outside the control of DSS shall have occurred or failed to occur that prevents the consummation of the Distribution; and |
| | | | |
| | | ● | no other events or developments shall have occurred prior to the Distribution Date that, in the sole judgment of the DSS Board, would result in the Distribution having a material adverse effect on DSS or its stockholders; and |
| | | | |
| | | ● | Each Impact Share distributed as part of the Distribution will be not be eligible for resale until 180 days from the date of the Company’s initial public offering becomes effective under the Securities Act, subject to the discretion of the Company to lift the restriction sooner. |
| | | The fulfillment of the foregoing conditions will not create any obligation on the part of DSS to effect the Distribution. We are not aware of any material federal, foreign or state regulatory requirements with which we must comply, other than SEC rules and regulations, or any material approvals that we must obtain, other than the SEC’s declaration of the effectiveness of the Registration Statement, in connection with the Distribution. DSS has the right not to complete the Distribution if, at any time, the DSS Board determines, in its sole and absolute discretion, that the Distribution is not in the best interests of DSS or its stockholders or is otherwise not advisable. For a more detailed description, see “The Distribution—Conditions to the Distribution.” |
| | | |
| Trading Market | | All of our outstanding shares of Common Stock are currently beneficially owned by DSS through its wholly-owned subsidiary DSS BioHealth Security, Inc. Accordingly, there currently is no public trading market for the Common Stock. The Common Stock is currently not listed for trading on any stock exchange or market. The Common Stock may be illiquid as we cannot predict whether any trading market will develop. |
| Tax Consequences to DS Stockholders | | DSS shareholders will be subject to a taxable event on the distribution of the Impact Shares. See “Material U.S. Federal Income Tax Consequences of the Distribution” for more information regarding the potential tax consequences to you of the Distribution. |
| | | |
| | | You should consult your own tax advisors regarding the particular tax consequences of the Distribution to you, including the applicability and effect of any U.S. federal, state, local and non-U.S. tax laws. |
| | | |
| Dividend Policy | | Following the Distribution, we do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. See “Dividend Policy” for more information. |
| | | |
| Transfer Agent and Distribution Agent | | V Stock Transfer LLC, the transfer agent and registrar for our Common Stock, will serve as distribution agent in connection with the Distribution. |
| | | |
| Risk Factors | | Our business faces both general and specific risks and uncertainties. Our business also faces risks relating to the Distribution. Accordingly, you should read carefully the information set forth under the section titled “Risk Factors.” |
RISK FACTORS
You should carefully consider all the information in this Prospectus and each of the risks described below, which we believe are the principal risks that we face. Some of the risks relate to our business, others to the Distribution. Some risks relate principally to the securities markets and ownership of our Common Stock. The risks and uncertainties described below are not the only ones faced by us. Additional risks and uncertainties not presently known or that are currently deemed immaterial also may impair our business operations. If any of the following risks occur, our business, financial condition, operating results and cash flows and the trading price of our Common Stock could be materially adversely affected.
Risks Relating to the Distribution
The Distribution may not be completed on the terms or timeline currently contemplated, if at all.
While we are actively engaged in planning for the Distribution, unanticipated developments could delay or negatively affect the Distribution, including, but not limited to, those related to the filing and effectiveness of appropriate filings with the SEC and receiving any required regulatory approvals. In addition, until a Distribution has occurred, the DSS Board, in its sole discretion, has the right to not proceed with the Distribution, even if all the conditions are satisfied. Therefore, the Distribution may not be completed on the terms or timeline currently contemplated, if at all.
DSS expects the Distribution to be treated as a taxable non-liquidating distribution to its stockholders. As a result, a U.S. stockholder of DSS may have a U.S. Federal income tax liability in respect of the Distribution without the receipt of cash from DSS or Impact.
DSS expects the Distribution to be treated as a taxable, non-liquidating distribution to its stockholders. As such, for U.S. federal income tax purposes, each U.S. stockholder of DSS receiving shares of Impact Common Stock in the Distribution would be treated as if such stockholder had received a distribution in an amount equal to the fair market value of Impact Common Stock received, which would result in (1) a taxable dividend to the extent of such stockholder’s pro rata share of DSS’s current and accumulated earnings and profits, (2) a reduction in such stockholder’s basis (but not below zero) in DSS common stock to the extent the amount received exceeds such stockholder’s share of earnings and profits and (3) a taxable gain to the extent the amount received exceeds the sum of the amount treated as a dividend and the stockholder’s basis in the DSS Common Stock. Accordingly, such stockholder may have a U.S. federal income tax liability in respect of the Distribution without the receipt of cash from DSS or Impact.
For a more detailed discussion, see the section entitled “The Distribution – U.S. Federal Income Tax Consequences of the Distribution” below.
We have a limited operating history, and our historical financial information may not be a reliable indicator of our future results.
There is no historical financial information about us upon which to base an evaluation of our performance. As of the date of this report, we have not generated any revenues from operations. We cannot guarantee we will be successful in our business operations. Our business is subject to risks inherent in the establishment of a new business enterprise, including limited capital resources, possible delays our research, testing and marketing efforts or wider economic downturns. For additional information about our past financial performance and the basis of presentation of our financial statements, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our historical financial statements and the notes thereto included elsewhere in this Prospectus.
Risks Relating to Our Business
If we do not adequately protect our intellectual property rights, our operations may be materially harmed.
We rely on and expect to continue to rely on agreements with parties with whom we have relationships, as well as patent, trademark and trade secret protection laws, to protect our intellectual property and proprietary rights. We cannot assure you that we can adequately protect our intellectual property or successfully prosecute potential infringement of its intellectual property rights. Also, we cannot assure you that others will not assert rights in, or ownership of, trademarks and other proprietary rights of ours or that we will be able to successfully resolve these types of conflicts to our satisfaction. Our failure to protect our intellectual property rights may result in a loss in potential revenue and could materially harm our operations and financial condition
New legislation, regulations or rules related to obtaining patents or enforcing patents could significantly increase our operating costs and decrease any potential revenue we might otherwise make.
We spend a significant amount of resources on its patent assets. If new legislation, regulations or rules are implemented either by Congress, the U.S. Patent and Trademark Office (the “USPTO”) or the courts that impact the patent application process, the patent enforcement process or the rights of patent holders, these changes could negatively affect its expenses, potential revenue and could negatively impact the value of its assets.
Safety and effectiveness concerns can have significant negative impacts on sales and results of operations, lead to litigation and cause reputational damage.
Concerns about product safety, whether raised internally or by litigants, regulators or consumer advocates, and whether or not based on scientific evidence, can result in safety alerts, product recalls, governmental investigations, regulatory action on the part of the FDA (or its counterpart in other countries), private claims and lawsuits, payment of fines and settlements, declining sales and reputational damage. These circumstances can also result in damage to brand image, brand equity and consumer trust in products. Product recalls could in the future prompt government investigations and inspections, the shutdown of manufacturing facilities, continued product shortages and related sales declines, significant remediation costs, reputational damage, possible civil penalties and criminal prosecution.
Significant challenges or delays in our innovation and development of new products, technologies and indications could have an adverse impact on our long-term success.
Our continued growth and success depend on our ability to innovate and develop new and differentiated products and services that address the evolving health care needs of patients, providers and consumers. Development of successful products and technologies may also be necessary to offset revenue losses should our products lose market share due to various factors such as competition and loss of patent exclusivity. We cannot be certain when or whether we will be able to develop, license or otherwise acquire companies, products and technologies, whether particular product candidates will be granted regulatory approval, and, if approved, whether the products will be commercially successful. We pursue product development through internal research and development as well as through collaborations, acquisitions, joint ventures and licensing or other arrangements with third parties. In all of these contexts, developing new products, particularly biotechnology products, requires a significant commitment of resources over many years. Only a very few biopharmaceutical research and development programs result in commercially viable products. The process depends on many factors, including the ability to discern patients’ and healthcare providers’ future needs; develop new compounds, strategies and technologies; achieve successful clinical trial results; secure effective intellectual property protection; obtain regulatory approvals on a timely basis; and, if and when they reach the market, successfully differentiate its products from competing products and approaches to treatment. New products or enhancements to existing products may not be accepted quickly or significantly in the marketplace for healthcare providers, and there may be uncertainty over third-party reimbursement. Even following initial regulatory approval, the success of a product can be adversely impacted by safety and efficacy findings in larger patient populations, as well as market entry of competitive products.
We are subject to risks related to corporate social responsibility and reputational matters.
Our reputation and the reputation of our brands, including the perception held by our customers, end-users, business partners, investors, other key stakeholders and the communities in which we do business are influenced by various factors. There is an increased focus from our stakeholders on ESG practices and disclosure - and if we fail, or are perceived to have failed, in any number of ESG matters, such as environmental stewardship, inclusion and diversity, workplace conduct and support for local communities, or to effectively respond to changes in, or new, legal or regulatory requirements concerning climate change or other sustainability concerns, our reputation or the reputation of our brands may suffer. Such damage to our reputation and the reputation of our brands may negatively impact our business, financial condition and results of operations. In addition, negative or inaccurate postings or comments on social media or networking websites about the Company or our brands could generate adverse publicity that could damage our reputation or the reputation of our brands. If we are unable to effectively manage real or perceived issues, including concerns about product quality, safety, corporate social responsibility or other matters, sentiments toward the Company or our products could be negatively impacted, and our financial results could suffer.
We may not have adequate funds to implement our business plan.
Although we have received capital from our parent companies to meet our working capital and financing needs in the past, additional financing may be required in order to meet our current and projected cash requirements for operations. We cannot assure that we will secure all or any of the funding we anticipate. If our entire original capital is fully expended and additional costs cannot be funded from borrowings or capital from other sources, then our financial condition, results of operations and business performance would be materially adversely affected. We cannot assure that we will have adequate capital or financing to conduct our business or to grow.
Risks Related to Our Common Stock
Currently, there is no public market for our securities, and there can be no assurances that any public market will ever develop.
Currently, our Common Stock is not listed or quoted on any public market, exchange, or quotation system. Although we plan to take steps to have our Common Stock publicly traded, a market for our Common Stock may never develop. Even if we are successful in developing a public market, there may not be enough liquidity in such market to enable stockholders to sell their stock. If a public market for our Common Stock does not develop, DSS stockholders may not be able to re-sell the shares of our Common Stock that they have received, rendering their shares effectively worthless.
Future sales of our Common Stock in the public market could lower our stock price, and any additional capital raised by us through the sale of equity or convertible securities may dilute our stockholders’ ownership in us.
We may sell additional shares of our Common Stock in public or private offerings. We also may issue convertible debt or equity securities. No prediction can be made as to the effect, if any, that future sales or distributions of our Common Stock will have on the market price of our Common Stock from time to time. Sales or distributions of substantial amounts of our Common Stock, or the perception that such sales or distributions are likely to occur, could adversely affect the prevailing fair market price for our Common Stock.
We do not intend to pay any cash dividends in the foreseeable future and, therefore, any return on your investment in our Common Stock must come from increases in the fair market value of our Common Stock.
We do not intend to pay cash dividends on our Common Stock in the foreseeable future. We expect to retain future earnings, if any, for reinvestment in our business. Also, any credit agreements, which we may enter into, may restrict our ability to pay dividends. Whether we pay cash dividends in the future will be at the discretion of our Board and will be dependent upon our financial condition, results of operations, cash requirements, future prospects and any other factors our Board deems relevant. Therefore, any return on your investment in our Common Stock must come from increases in the fair market value of our Common Stock. For more information, see “Dividend Policy.”
Your percentage ownership in the Company may be diluted in the future.
Your percentage ownership in the Company may be diluted in the future because of equity awards that we may grant to our directors, officers and other employees. In addition, we may issue equity as all or part of the consideration paid for acquisitions and strategic investments that we may make in the future or as necessary to finance our ongoing operations.
CAUTIONARY STATEMENT CONCERNING FORWARD-LOOKING STATEMENTS
This Prospectus contains “forward-looking statements” within the meaning of the federal securities laws. Forward-looking statements give our current expectations or forecasts of future events. You can identify these statements because they do not relate strictly to historical or current facts. In some cases, these forward-looking statements can be identified by words or phrases such as “may,” “will,” “expect,” “anticipate,” “aim,” “estimate,” “intend,” “plan,” “believe,” “potential,” “continue,” “is/are likely to” or other similar expressions. They also appear in any discussion of future operating or financial performance. In particular, these include statements relating to future actions, future performance of our products, level of expenses and anticipated expense reductions, the outcome of any legal proceedings, and financial results. Although we believe that we are basing our expectations and beliefs on reasonable assumptions within the bounds of what we currently know about our business and operations, there can be no assurance that our actual results will not differ materially from what we expect or believe. The following factors could cause our actual results to differ from our expectations or beliefs:
| | ● | the potential adverse impact on our business resulting from the Distribution; |
| | ● | the adverse effect from a decline in the securities markets or from catastrophic, unpredictable events like a global health pandemic; |
| | ● | a decline in the performance of our products; |
| | ● | a general downturn in the economy; |
| | ● | changes in government policy or regulation; |
| | ● | changes in our ability to attract or retain key employees; |
| | ● | unforeseen costs and other effects related to legal proceedings or investigations of governmental and self-regulatory organizations; and |
| | ● | other factors (including the risks contained in the section titled “Risk Factors”) relating to our industry, our operations and results of operations. |
The preceding list is not intended to be an exhaustive list of all of our forward-looking statements. Forward-looking statements are not historical facts, and are based on current expectations, estimates and projections about our industry, management’s beliefs and certain assumptions made by management, many of which, by their nature, are inherently uncertain and beyond our control. Accordingly, you are cautioned that any forward-looking statements are not guarantees of future performance and are subject to certain risks, uncertainties and assumptions that are difficult to predict. Although we believe that the expectations reflected in our forward-looking statements are reasonable as of the date made, expectations may prove to have been materially different from the results expressed or implied by such forward-looking statements. Unless otherwise required by law, we also disclaim any obligation to update our view of any such risks or uncertainties or to announce publicly the result of any revisions to the forward-looking statements made in this Prospectus.
THE DISTRIBUTION
Background
DSS currently beneficially owns through its wholly owned subsidiary, DSS BioHealth Security, Inc., 3,877,282,251 shares of our Common Stock, which represents 100% of the shares outstanding. These shares were acquired on August 21, 2020, upon closing of the Share Exchange Agreement. DSS is deemed to be a beneficial owner of the shares of our Common Stock covered by this Prospectus because it has voting and dispositive power over such shares.
On August 27, 2020, the DSS Board approved the Distribution. DSS will distribute part of its equity interest that it holds in us, consisting of 486,706,712 shares of our Common Stock, to DSS stockholders, except for the holders of the Affiliate Shares. Following the Distribution, DSS will own an equity interest in us of approximately 87%, giving DSS effective control over us. No approval of DSS Common Stockholders is required in connection with the Distribution, and DSS stockholders will not have any appraisal rights in connection with the Distribution.
The Distribution is subject to the satisfaction, or the DSS Board’s waiver, of a number of conditions. In addition, DSS has the right not to complete the Distribution if, at any time, the DSS Board determines, in its sole and absolute discretion, that the Distribution is not in the best interests of DSS or its stockholders or is otherwise not advisable. For a more detailed description, see “The Distribution—Conditions to the Distribution.”
Reasons for the Distribution
The Distribution is the method by which DSS will begin to carry out the intention of management of DSS and the DSS Board to take the Company public and to reward DSS stockholders via the issuance of our shares of Common Stock.
When and How You Will Receive Company Shares
DSS will distribute to its stockholders, as a dividend, 4 shares of our Common Stock for every share of DSS Common Stock outstanding, except for the Affiliate Shares, as of 5:00 p.m., New York City time, June 30, 2023, the Record Date of the Distribution.
Prior to the Distribution, DSS will deliver the Common Stock to the distribution agent. V Stock Transfer LLC, the transfer agent and registrar for our Common Stock, will serve as distribution agent in connection with the Distribution.
Each Impact Share distributed as part of the Distribution will be not be eligible for resale until 180 days from the date of the Company’s initial public offering becomes effective under the Securities Act, subject to the discretion of the Company to lift the restriction sooner.
If you own DSS common stock on the Record Date, the shares of our Common Stock that you are entitled to receive in the Distribution will be issued to your account as follows:
| | ● | Registered stockholders. If you own your shares of DSS Common Stock directly through DSS’s transfer agent, American Stock Transfer & Trust Company, you are a registered stockholder. In this case, our transfer agent, V Stock Transfer LLC, which is serving as the distribution agent in connection with the Distribution, will credit the whole shares of our Common Stock you receive in the Distribution to a new book-entry account established on or shortly after the Distribution Date. Registration in book-entry form refers to a method of recording share ownership where no physical stock certificates are issued to stockholders, as is the case in the Distribution. You will be able to access information regarding your book-entry account holding our shares of Common Stock at V Stock Transfer LLC. Commencing on or shortly after the Distribution Date, the distribution agent will mail to you an account statement that indicates the number of whole shares of our Common Stock that have been registered in book-entry form in your name. We expect it will take the distribution agent up to two weeks after the Distribution Date to complete the distribution of the shares of our Common Stock and mail statements of holding to all registered stockholders. |
| | ● | “Street name” or beneficial stockholders. If you own your shares of DSS Common Stock beneficially through a bank, broker or other nominee, the bank, broker or other nominee holds the shares in “street name” and records your ownership on its books. If you own your shares of DSS Common Stock through a bank, broker or other nominee, your bank, broker or other nominee will credit your account with the whole Impact Shares that you receive in the Distribution on or shortly after the Distribution Date. We encourage you to contact your bank, broker or other nominee if you have any questions concerning the mechanics of having shares held in “street name.” |
We will not issue any physical stock certificates to any stockholders, even if requested.
If you sell any of your shares of DSS Common Stock on or before a Distribution Date, the buyer of those shares will in most circumstances be entitled to receive the Impact Shares to be distributed in respect of the shares of DSS Common Stock you sold.
We are not asking DSS stockholders to take any action in connection with the Distribution. No approval of the holders of DSS Common Stock is required for the Distribution. We are not asking you for a proxy and request that you not send us a proxy. We are also not asking you to make any payment or surrender or exchange any of your shares of DSS Common Stock for Impact Shares. The number of outstanding shares of DSS Common Stock will not change as a result of the Distribution.
Number of Shares You Will Receive
On the Distribution Date, you will receive four Impact Shares for every share of DSS Common Stock you held on the Record Date, unless you sell any of your shares of DSS Common Stock on or before a Distribution Date, in which case the buyer of those shares may be entitled to receive the Impact Shares.
Results of the Distribution
After the Distribution, we expect to have approximately [•] holders of shares of our Common Stock, based on the number of DSS stockholders and shares of DSS Common Stock outstanding. The actual number of holders of shares of our Common Stock will depend on the actual number of holders of shares of DSS Common Stock outstanding on the Record Date. The Distribution will not affect the number of outstanding shares of DSS Common Stock or any rights of DSS stockholders, although it is possible that the trading price of shares of DSS Common Stock immediately following the Distribution may be lower than immediately prior to the Distribution because the trading price of DSS Common Stock will no longer reflect the value of DSS ownership stake immediately before the Distribution. Nevertheless, we do not expect the trading price of shares of DSS Common Stock immediately following the Distribution to be materially lower. However, until the market has fully analyzed the value of DSS without the value represented by the Impact Shares distributed to DSS stockholders, the trading price of shares of DSS Common Stock may fluctuate.
Listing and Trading of our Common Stock
All of our outstanding shares of Common Stock are currently beneficially owned by DSS. Accordingly, there currently is no public trading market for the Impact Shares. Our Common Stock is currently not listed for trading on any stock exchange or market. The Impact Shares may be illiquid as we cannot predict whether any trading market will develop.
Conditions to the Distribution
We expect that each Distribution will be effective on the Distribution Date, provided that the following conditions shall have been satisfied or waived by DSS:
| | ● | the DSS Board shall have authorized and approved the Distribution and not withdrawn such authorization and approval, and shall have declared the dividend of our Common Stock to DSS stockholders; |
| | ● | the SEC shall have declared effective our Registration Statement on Form S-1, of which this Prospectus is a part, under the Securities Act, and no stop order suspending the effectiveness of our Registration Statement shall be in effect and no proceedings for that purpose shall be pending before or threatened by the SEC; |
| | ● | no order, injunction or decree issued by any governmental authority of competent jurisdiction or other legal restraint or prohibition preventing consummation of the Distribution shall be in effect, and no other event outside the control of DSS shall have occurred or failed to occur that prevents the consummation of the Distribution; and |
| | ● | no other events or developments shall have occurred prior to the Distribution Date that, in the judgment of the DSS Board, would result in the Distribution having a material adverse effect on DSS or its stockholders. |
DSS shall, in its sole and absolute discretion, determine the Record Date, the Distribution Date and all terms of the Distribution, including the form, structure and terms of any transactions and/or offerings to effect the Distribution and the timing of and conditions to the consummation thereof. In addition and notwithstanding anything to the contrary set forth in this Prospectus, DSS may at any time and from time to time until the Distribution decide to abandon the Distribution including by accelerating or delaying the timing of the consummation of all or part of the Distribution or modifying or changing the terms of the Distribution if, at any time, the DSS Board determines, in its sole and absolute discretion, that the Distribution is not in the best interests of DSS or its stockholders or is otherwise not advisable.
Reasons for Furnishing this Prospectus
We are furnishing this Prospectus solely to provide information to DSS’s stockholders who will receive Impact Shares in the Distribution. You should not construe this Prospectus as an inducement or encouragement to buy, hold or sell any of our securities or any securities of DSS. We believe that the information contained in this Prospectus is accurate as of the date set forth on the cover. Changes to the information contained in this Prospectus may occur after that date, and neither we nor DSS undertakes any obligation to update the information except in the normal course of our and DSS’s public disclosure obligations and practices and except as required by applicable law.
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE DISTRIBUTION
Consequences to U.S. Holders of DSS Common Stock
The following is a summary of the material U.S. federal income tax consequences to holders of DSS Common Stock in connection with the Distribution. This summary is based on the Code, the Treasury Regulations promulgated under the Code and judicial and administrative interpretations of those laws, in each case as in effect and available as of the date of this Prospectus and all of which are subject to change at any time, possibly with retroactive effect. Any such change could affect the tax consequences described below.
This summary is limited to holders of DSS Common Stock that are U.S. Holders, as defined immediately below, that hold their DSS Common Stock as a capital asset. A “U.S. Holder” is a beneficial owner of DSS Common Stock that is, for U.S. federal income tax purposes:
| | ● | an individual who is a citizen or a resident of the United States; |
| | ● | a corporation, or other entity taxable as a corporation for U.S. federal income tax purposes, created or organized under the laws of the United States or any state thereof or the District of Columbia; |
| | ● | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| | ● | a trust if a court within the United States is able to exercise primary jurisdiction over its administration and one or more U.S. persons have the authority to control all of its substantial decisions or, in the case of a trust that was treated as a domestic trust under law in effect before 1997, a valid election is in place under applicable Treasury Regulations. |
This summary does not discuss all tax considerations that may be relevant to stockholders in light of their particular circumstances, nor does it address the consequences to stockholders subject to special treatment under the U.S. federal income tax laws, such as:
| | ● | dealers or traders in securities or currencies; |
| | ● | tax-exempt entities; |
| | ● | banks, financial institutions or insurance companies; |
| | ● | real estate investment trusts, regulated investment companies or grantor trusts; |
| | ● | persons who acquired DSS Common Stock pursuant to the exercise of employee stock options or otherwise as compensation; |
| | ● | stockholders who own, or are deemed to own, 10% or more, by voting power or value, of DSS equity; |
| | ● | stockholders owning DSS Common Stock as part of a position in a straddle or as part of a hedging, conversion or other risk reduction transaction for U.S. federal income tax purposes; |
| | ● | certain former citizens or long-term residents of the United States or green card holders; |
| | ● | stockholders who are subject to the alternative minimum tax; or |
| | ● | persons who own DSS Common Stock through partnerships, certain trusts or other pass-through entities. |
This summary does not address any U.S. state, local or non-U.S. tax consequences or any estate, gift or other non-income tax consequences.
If a partnership, or any other entity treated as a partnership for U.S. federal income tax purposes, holds DSS Common Stock, the tax treatment of a partner in that partnership will generally depend on the status of the partner and the activities of the partnership. Such a partner or partnership should consult its own tax advisor as to its tax consequences.
YOU SHOULD CONSULT YOUR OWN TAX ADVISOR WITH RESPECT TO THE U.S. FEDERAL, STATE AND LOCAL AND NON-U.S. TAX CONSEQUENCES OF THE DISTRIBUTION.
General
The Distribution will be treated as a taxable non-liquidating distribution to DSS stockholders, As such, each U.S. stockholder receiving Impact Shares in the Distribution will be treated as if such stockholder had received a distribution in an amount equal to the fair market value of our Common Stock received, which would result in (1) a taxable dividend to the extent of such stockholder’s pro rata share of Impact’s current and accumulated earnings and profits, (2) a reduction in such stockholder’s basis (but not below zero) in DSS Common Stock to the extent the amount received exceeds such stockholder’s share of earnings and profits and (3) a taxable gain to the extent the amount received exceeds the sum of the amount treated as a dividend and the stockholder’s basis in the DSS Common Stock. Any such gain would generally be a capital gain if the DSS Common Stock is held by such stockholder as a capital asset on the Distribution Date and subject to long-term capital gain treatment if the DSS Common Stock has been held by such stockholder for more than one year on the Distribution Date. Distributions treated as dividends will be subject to tax at favorable long-term capital gains rates provided that the stockholder has held his DSS stock for the period required for preferential dividend tax treatment generally.
A U.S. stockholder also may be subject to information reporting with respect to the Distribution. In addition, such stockholder may be subject to “backup withholding” on the Distribution, unless such stockholder provides proof of any applicable exemption or a correct taxpayer identification number, and otherwise complies with the requirements of the backup withholding rules. Backup withholding is not an additional tax, and it may be refunded or credited against a U.S. stockholder’s U.S. federal income tax liability if the required information is timely supplied to the IRS.
USE OF PROCEEDS
We will not receive any proceeds from the Distribution.
DETERMINATION OF OFFERING PRICE
No consideration will be paid for the Impact Shares in the Distribution.
DIVIDEND POLICY
We do not intend, following the Distribution, to pay cash dividends on our Common Stock in the foreseeable future. We expect to retain future earnings, if any, for reinvestment in our business. Any credit agreements which we may enter into may restrict our ability to pay dividends. The payment of dividends in the future will be subject to the discretion of our Board and will depend, among other things, on our financial condition, results of operations, cash requirements, future prospects and any other factors our Board deems relevant. There can be no assurance that a payment of a dividend will occur in the future.
CAPITALIZATION
The following table sets forth our cash and capitalization as of December 31, 2022. The following table should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our historical consolidated financial statements and the notes thereto included elsewhere in this Prospectus.
| | | As of December 31, 2022 | |
| | | Actual | |
| | | | |
| Cash and cash equivalents | | $ | 2,000 | |
| Long-term debt, net | | $ | - | |
| Stockholders’ equity: | | | | |
| | | | | |
| Common stock, $.001 par value; 4,000,000,000 shares authorized, 3,877,282,251 shares issued and outstanding (3,877,282,251 on December 31, 2021) | | | 125,000 | |
| Additional paid-in capital | | | 38,058,000 | |
| Accumulated deficit | | | (8,625,000 | ) |
| Total stockholders’ equity of the company | | | 29,558,000 | |
| Non-controlling interest in subsidiary | | | 3,111,000 | |
| Total stockholder’s equity | | | 32,669,000 | |
| Total capitalization | | $ | 46,497,000 | |
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our audited and unaudited financial statements and related notes, respectively, beginning on page F-1 below of this Prospectus. Some of the information contained in this discussion and analysis constitutes forward-looking statements that involve risks and uncertainties. Actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to these differences include, but are not limited to, those discussed below and elsewhere in this Prospectus, particularly under the section titled “Cautionary Statement Concerning Forward-Looking Statements.”
Overview
Impact BioMedical Inc. targets urgent medical needs and expands the borders of medical and pharmaceutical science. Impact drives mission-oriented research and development, and commercialization of solutions for medical advances in human wellness and healthcare. By leveraging technology and new science with strategic partnerships, Impact Bio provides advances in drug discovery for the prevention, inhibition, and treatment of neurological, oncology and immuno-related diseases. Other exciting technologies include a breakthrough alternative sugar aimed to combat diabetes and functional fragrance formulations aimed at the industrial and medical industry. The research and development efforts are primarily conducted in collaboration with GRDG Sciences, LLC (“GRDG”) and with the expertise of Mr. Daryl Thompson as its Director of Scientific Initiatives. We have initiated research regarding universal therapeutics as part of an attempt to help address some of the world’s deadliest diseases.
The business model of Impact BioMedical revolves around a Licensing methodology for commercialization, sales and distribution. Impact develops valuable and unique patented technologies which will be licensed to pharmaceutical, food, large consumer package goods companies and venture capitalists in exchange for usage licensing and royalties.
Below is a list of our principal subsidiaries:
| | ● | Impact BioLife Science, Inc.; |
| | ● | Global Biomedical, Inc.; |
| | ● | Global BioLife, Inc.; and |
| | ● | Sweet Sense, Inc. |
Impact BioLife Science, Inc. We are the sole owner of the outstanding equity of Impact BioLife Science, Inc.
Global Biomedical, Inc. We own 90.91% of Global Biomedical, Inc. outstanding equity, and the balance minority equity owner is Peggy Tang.
Global BioLife, Inc. Through our majority owned subsidiary Global Biomedical, Inc., we own 90% of the outstanding equity of Global BioLife, Inc. The other equity owner is Holista CollTech Limited (“Holista”) (10%).
Sweet Sense, Inc. We are the owner of 50% of the outstanding equity of Sweet Sense. The other equity owner is BioLife Sugar, Inc. (“BioLife Sugar”).
Below is an organization chart showing our ownership structure and ownership interests.

Through our majority-owned subsidiary Global BioLife, we own or have rights to a portfolio of biomedical intellectual property, including intellectual property assigned to Global BioLife by GRDG. Global BioLife leverages its scientific know-how and intellectual property rights to develop various emerging technologies, including biopharmaceuticals, antivirals, antimicrobials, sugar alternatives, insect repellents, fragrances, bioplastics and natural preservatives.
Biotech and Impact BioMedical have several important and valuable products, technology or compounds that are in continuing development and/or licensing stages:
Linebacker
Linebacker is a multi-faceted therapeutic platform intended for metabolic, neurologic, cancer, and infectious diseases. Our process features a molecular tuning technique that is believed to modify a natural compound to induce potency, efficacy, bioavailability, and trans-membrane permeability while maintaining safety, toxicity, and tolerability. Natural compounds used in the Linebacker platform may have potential in treating and preventing a range of diseases by inhibiting TNF-alpha and indication specific causes (e.g. neurology, anti-inflammatory diseases, oncology). Linebacker’s intended use is subject to FDA regulation, and the FDA must approve any drug or biologic product before it can be marketed in the United States. In addition, prior to being sold outside the United States, our Linebacker technology must be approved by the regulatory agencies of foreign governments. Prior to filing a new drug application or biologics license application with the FDA, we would have to perform extensive clinical trials, and prior to beginning any clinical trial, we would need to perform extensive preclinical testing which could take several years and may require substantial expenditures. To date, we have not conducted no preclinical testing or clinical trials (human or otherwise) on Linebacker, nor have we received any FDA approval to conduct such any such preclinical testing or clinical trials. Further, because we have not conducted any testing or studies on Linebacker, we are not able to substantiate or demonstrate that Linebacker will function as planned, nor can we seek FDA approval to market and sell Linebacker. To date, we have not performed the extensive preclinical testing necessary to begin any clinical trials for Linebacker; and until we have conducted the necessary clinical trials, we will not be able to seek FDA approval for Linebacker. Currently, we have no plans to conduct preclinical testing or clinical trials (human or otherwise) involving Linebacker.
We intend to identify third parties or customers that are interested in purchasing, licensing or co-developing products that leverage our Linebacker technology. We have not entered into any such agreements to date, and there can be no guarantee that we will enter into any such agreements or that such agreements will be on terms that are favorable to the Company.
Laetose
Laetose technology is derived from a unique combination of sugar and other compounds, which is intended to inhibit the inflammatory response of sugar alone. Based on our intended use for Laetose, Laetose is intended to be an high-intensity sweetener, yet to be determined by the FDA. High-intensity sweeteners are commonly used as sugar substitutes or sugar alternatives because they are many times sweeter than sugar but contribute only a few to no calories when added to foods. High-intensity sweeteners, like all other ingredients added to food in the United States, must be safe for consumption. Further, a high intensity sweetener is regulated as a food additive, unless its use as a sweetener is generally recognized as safe (GRAS). The use of a food additive must undergo premarket review and approval by FDA before it can be used in food. In contrast, use of a GRAS substance does not require premarket approval. Rather, the basis for a GRAS determination based on scientific procedures is that experts qualified by scientific training and experience to evaluate its safety conclude, based on publicly available information, that the substance is safe under the conditions of its intended use. A company can make an independent GRAS determination for a substance with or without notifying FDA. Regardless of whether a substance is approved for use as a food additive or its use is determined to be GRAS, scientists must determine that it meets the safety standard of reasonable certainty of no harm under the intended conditions of its use. This standard of safety is defined in FDA’s regulations.
As of this date, we have not performed any scientific testing to determine the safety of Laetose for human consumption, and no qualified experts have evaluated the safety of Laetose. Therefore, we have not been able to make any determination that Laetose is safe for human consumption. Currently, we have no plans to conduct any scientific testing relating to the safety of Laetose, and any there can be no guarantee that any such testing will conclude that Laetose is safe for human consumption.
We intend to identify third parties or customers that are interested in purchasing, licensing or co-developing products that leverage Laetose. We have not entered into any such agreements to date, and there can be no guarantee that we will enter into any such agreements or that such agreements will be on terms that are favorable to the Company.
The Company is presently seeking to license Laetose by way of a business development agreement between Sweet Sense, Inc., a joint venture established by Global BioLife and Quality Ingredients, LLC (“Sweet Sense”), and BFS. Sweet Sense was established for the development, manufacture, and global distribution of the new sugar substitute. On November 8, 2019, the Company purchased 50% of Sweet Sense Inc. from Quality Ingredients, LLC for $91,000.
Functional Fragrance Formulation (“3F”)
3F is a unique formulation of specialized ingredients from naturally occurring botanical sources with intended application as an insect repellent and an antimicrobial. Currently, approximately 20 combination insect repellent-sunscreen drug products are available for consumers. These products consist of one of three insect repellents (N,N-diethyl-meta-toluamide (DEET), oil of citronella, or IR3535) and a sunscreen component (one or more sunscreen ingredients). Internal repellent tests results demonstrated the prevention and limitation of insect bites up to eight (8) hours. To date, we have not conducted studies to establish marketing claims.
We intend to identify third parties or customers that are interested in purchasing, licensing or co-developing products that leverage 3F. We have not entered into any such agreements to date, and there can be no guarantee that we will enter into any such agreements or that such agreements will be on terms that are favorable to the Company.
Equivir
Equivir/Nemoviris a novel blend of naturally occurring compounds that are sourced from fruits, vegetables, and other natural substances. Equivir is designed to work by impeding virulence while also blocking multiple methods used by viruses to infect and replicate in host cells, following deployment in a manner similar to a vitamin, and this intended use is subject to FDA regulation. The FDA must approve any drug or biologic product before it can be marketed in the United States. In addition, prior to being sold outside the United States, our Equivir technology must be approved by the regulatory agencies of foreign governments. Prior to filing a new drug application or biologics license application with the FDA, we would have to perform extensive clinical trials, and prior to beginning any clinical trial, we would need to perform extensive preclinical testing which could take several years and may require substantial expenditures. To date, we have conducted no preclinical testing or clinical trials (human or otherwise) on Equivir. Further, because we have not conducted any testing or studies on Equivir, we are not able to substantiate or demonstrate its benefits as a health supplement or seek FDA approval at this time. Currently, we have no plans to conduct any preclinical testing or clinical trials (human or otherwise) involving Equivir.
We intend to identify third parties or customers that are interested in purchasing, licensing or co-developing products that leverage Equivir. We have not entered into any such agreements to date, and there can be no guarantee that we will enter into any such agreements or that such agreements will be on terms that are favorable to the Company.
In addition, other Equivir analogues are under development and provisional patents have been filed.
The Company was incorporated in the State of Nevada as a for-profit company on October 16, 2018, and established a fiscal year end of December 31st. The Company issued 9,000 shares to Global BioMedical Pte. Ltd., which was wholly–owned by Alset International Limited (formally Singapore eDevelopment Limited), a multinational public company, listed on the Singapore Exchange Securities Trading Limited (“SGXST”). On March 31, 2020, the Company issued 125,064,621 shares of common stock to its sole shareholder Global BioMedical Pte. Ltd. On July 24, 2020, the Board approved the Stock Split, pursuant to which each share of the Company’s common stock issued and outstanding was split into nine shares of the Company’s common stock. The numbers of authorized common stock and issued and outstanding common stock in the reporting periods were retrospectively adjusted for the stock split.
On March 12, 2020 Alset International Limited (“Alset”), a related party, Global BioMedical Pte Ltd., a related party, DSS, Inc (“DSS”), a related party, and DSS BioHealth Security Inc. (“DSS BioHealth”), a related party, signed Term Sheets and subsequently on April 21, 2020, these four companies entered into Share Exchange Agreement (“Share Exchange”), based on which Global BioMedical Pte Ltd., agreed to sell all of the issued and outstanding shares of the Company to DSS BioHealth in exchange for the combination of common and preferred shares of DSS. Under the terms of the Share Exchange, DSS issued 483,334 shares of the DSS Common Stock nominally valued at $6.48 per share, and 46,868 newly issued shares of the DSS Series A Convertible Preferred Stock (“Series A Preferred Stock”), with a stated value of $46,868,000, or $1,000 per share, for a total consideration valued at $50 million. Due to several factors, including a discount for illiquidity, the value of the Series A Preferred Stock was discounted from $46,868,000 to $35,187,000, thus reducing the final consideration given to approximately $38,319,000. The Company’s Chairman, Heng Fai Ambrose Chan, a related party, who is also the largest shareholder of Alset, at the time of the signing of the Share Exchange Agreement was the beneficial owner of approximately 18.3% of the outstanding shares of DSS and is the Chairman of the Board of Directors of DSS. On August 21, 2020, the transaction was concluded, and the Company became a direct wholly owned subsidiary of DSS BioHealth. In connection with the acquisition, and the related accounting determination, DSS BioHealth has elected to apply push-down accounting and reflect in its financial statements of Impact BioMedical, the fair value of its assets and liabilities. Utilizing an income approach, the Company has completed its valuations of certain developed technology and pending patents assets acquired in the transaction as well the fair value of the non-controlling interests. More specifically, a Multi-Period Excess Earnings Method (“MPEEM”) estimates the value of an intangible asset by quantifying the amount of residual (or excess) estimated cash flows generated by the asset and discounting those cash flows to the present. These have been valued at approximately $22,260,000 and $3,910,000, respectively, and are included on the Consolidated Balance Sheet on December 31, 2020. Estimated useful life of these assets is twenty years, based on the remaining terms of the related patents, with annual amortization approximating $1,112,000. The Company has also completed its valuation of goodwill and deferred tax liabilities of Impact BioMedical, and has recorded goodwill of approximately $25,093,000, driven by other intangible assets that do not qualify for separate recognition, and a deferred tax liability of approximately $5,234,000. The goodwill is not deductible for tax purposes and has been allocated to Impact BioMedical in totality as a single reporting unit. The Company is committed to both funding research and developing intellectual property portfolio.
As of the date of this report, we have not generated any revenues from operations. We cannot guarantee we will be successful in our business operations. Our business is subject to risks inherent in the establishment of a new business enterprise, including possible delays our research, testing and marketing efforts or wider economic downturns.
Fiscal Year Ended December 31, 2022, compared to Year Ended December 31, 2021
Results of operations for the year ended December 31, 2022, as compared to year ended December 31, 2021.
Cost and expenses:
| | | 2022 | | | 2021 | | | % Change | |
| | | | | | | | | | |
| Sales, general and administrative compensation | | $ | 325,000 | | | $ | 398,000 | | | | -18 | % |
| Depreciation and amortization | | | 1,113,000 | | | | 1,112,941 | | | | 0 | % |
| Professional fees | | | 722,000 | | | | 551,796 | | | | 31 | % |
| Research and development | | | 1,226,000 | | | | 1,080,051 | | | | 14 | % |
| Other operating expenses | | | 68,000 | | | | 42,553 | | | | 62 | % |
| | | | | | | | | | | | | |
| Total costs and expenses | | $ | 3,454,000 | | | $ | 3,185,341 | | | | 8 | % |
Selling, general and administrative compensation costs decreased 18% for the year ended December 31, 2022, as compared to the year ended December 31, 2021 due to decreases in head count at the Company. Also, the Company is allocated certain administrative labor cost from DSS as shared resources which approximates $12,000 per month during the year ended December 31, 2022, as compared to $17,000 per month during the year ended December 31, 2021.
Depreciation and Amortization expense of $1,113,000 for the year ended December 31, 2022, and 2021 represents the amortization of the associated with the developed technology and patents acquired as part of the acquisition of Impact BioMedical by DSS. Amortization of these assets began on January 1, 2021, and will have a 20-year term.
Professional fees increased 31% for the year ended December 31, 2022, as compared to the year ended December 31, 2021 mostly due to increases in consulting and legal services associated with developing and implementing Impact BioMedical’s business plan.
Research and development costs represent costs consisted primarily of independent, third-party testing of the various properties of each technology the Company owns possesses as well as research on new technologies. Research and development increased 14% for the year ended December 31, 2022, as compared to the year ended December 31, 2021 primarily due to exploration and development of new technologies during 2022.
Other operating expenses consist primarily of office supplies, IT support, travel and insurance costs. These costs remained increased 62% for the year ended December 31, 2022, as compared to year ended December 31, 2021 due primarily to increased IT support and travel costs
Other income (expense):
| | | 2022 | | | 2021 | | | % Change | |
| | | | | | | | | | |
| Interest Income | | $ | 24,000 | | | $ | 165,000 | | | | -85 | % |
| Interest Expense | | | (462,000 | ) | | | (300,000 | ) | | | 54 | % |
| Other Income (expense) | | | 116,000 | | | | 77,000 | | | | 51 | % |
| Impairment of investment | | | (4,100,000 | ) | | | - | | | | N/A | |
| | | | | | | | | | | | | |
| Total other loss | | $ | (4,422,000 | ) | | $ | (58,000 | ) | | | 7524 | % |
Total other income and expense as of December 31, 2022, is driven by the impairment of the Company’s Vivacitas investment in the amount of $4,100,000. As of December 31, 2021, total other income and expense represents interest income and origination fee income associated with the Company’s notes receivable offset by interest expense on its note payable to DSS.
Net loss:
| | | 2022 | | | 2021 | | | % Change | |
| | | | | | | | | | | | | |
| Net loss | | $ | (7,255,000 | ) | | $ | (1,848,000 | ) | | | -293 | % |
The increase in net loss of 293% for the year ended December 31, 2022 as compared to the year ended December 31, 2021 is driven by the impairment of the Company’s Vivacitas investment in the amount of $4,100,000.
Other expense:
LIQUIDITY AND CAPITAL RESOURCES
The Company has historically met its liquidity and capital requirements primarily through the sale of its equity securities and debt financings. As of December 31, 2022, the Company had cash of approximately $2,000. As of December 31, 2022, the Company believes that it has sufficient availability to cash via its revolving promissory note with DSS to meet its cash requirements for at least the next 12 months from the filing date of this Prospectus.
Critical Accounting Policies
The preparation of financial statements and related disclosures in conformity with generally accepted accounting principles in the U.S. (“U.S. GAAP”) requires management to make judgments, assumptions and estimates that affect the amounts reported in our consolidated financial statements and accompanying notes. The Company’s consolidated financial statements for the year ended December 31, 2022 and 2021, describe the significant accounting policies and methods used in the preparation of the consolidated financial statements.
Fair Value of Financial Instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Fair Value Measurement Topic of the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
● Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets.
● Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and
● Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable.
The carrying amounts reported in the balance sheet of cash and cash equivalents, prepaids, accounts payable and accrued expenses approximate fair value because of the immediate or short-term maturity of these financial instruments. The fair value of notes receivable approximates their carrying value as the stated or discounted rates of the notes do reflect recent market conditions. The Company’s investments are record at cost as the fair value of these investment in are not readily available. The fair value of notes payable approximates its carrying value as the stated interest rate reflects recent market conditions.
Investments
Investments in equity securities with a readily determinable fair value, not accounted for under the equity method, are recorded at fair value with unrealized gains and losses included in earnings. For equity securities without a readily determinable fair value, the investment is recorded at cost, less any impairment, plus or minus adjustments related to observable transactions for the same or similar securities, with unrealized gains and losses included in earnings.
For equity method investments, the Company regularly reviews its investments to determine whether there is a decline in fair value below book value. If there is a decline that is other-than-temporary, the investment is written down to fair value.
Goodwill
Goodwill is the excess of cost of an acquired entity over the fair value of amounts assigned to assets acquired and liabilities assumed in a business combination. Goodwill is subject to impairment testing at least annually and will be tested for impairment between annual tests if an event occurs or circumstances change that would indicate the carrying amount may be impaired. FASB ASC Topic 350 provides an entity with the option to first assess qualitative factors to determine whether the existence of events or circumstances leads to a determination that it is more likely than not that the fair value of a reporting unit is less than its carrying amount. Some of the qualitative factors considered in applying this test include consideration of macroeconomic conditions, industry and market conditions, cost factors affecting the business, and overall financial performance of the business. If, after completing the assessment, it is determined that it is more likely than not that the fair value of a reporting unit is less than its carrying value, the Company will proceed to a quantitative test. If qualitative factors are not deemed sufficient to conclude that the fair value of the reporting unit more likely than not exceeds its carrying value, then a one-step approach is applied in making an evaluation. The evaluation utilizes multiple valuation methodologies, including a market approach (market price multiples of comparable companies) and an income approach (discounted cash flow analysis). The computations require management to make significant estimates and assumptions, including, among other things, selection of comparable publicly traded companies, the discount rate applied to future earnings reflecting a weighted average cost of capital, and earnings growth assumptions. The Company believes the estimates and assumptions used in our impairment assessments are reasonable and based on available market information, but variations in any of the assumptions could result in materially different calculations of fair value and determinations of whether or not an impairment is indicated. A discounted cash flow analysis requires management to make various assumptions about future sales, operating margins, capital expenditures, working capital, and growth rates. Cash flow projections are derived from one-year budgeted amounts plus an estimate of later period cash flows, all of which are determined by management. Subsequent period cash flows are developed for each reporting unit using growth rates that management believes are reasonably likely to occur. Impairment of goodwill is measured as the excess of the carrying amount of goodwill over the fair values of recognized and unrecognized assets and liabilities of the reporting unit.
On October 15, 2020, a third party engaged by the Company had completed a valuation of the acquisition price of ImpactBio by DSS BioHealth Security, Inc. It was determined that DSS BioHealth Security, Inc.’s parent company, DSS (NYSE: DSS) publicly traded stock price on the valuation date of August 21, 2020, reflected all relevant data regarding the transaction and was applied to both the common A shares and preferred A shares given as consideration for the acquisition of ImpactBio. The preferred A shares contained certain sell restrictions and a liquidity discount was applied. Further, a valuation of the assets acquired was also completed as of the valuation date on March 25, 2021. As no factors changed between, the completion of the valuation of the consideration given and the completion valuation of the assets received no impairment was recorded during the fiscal year end December 31, 2021. Subsequently, as no events or circumstances occurred as of December 31, 2022, and no impairment of goodwill was recorded for the year ended December 31, 2022.
Intangible Assets
The estimated fair values of acquired intangibles are generally determined based upon future economic benefits such as earnings and cash flows. Acquired identifiable intangible assets are recorded at fair value and are amortized over their estimated useful lives. Acquired intangible assets with an indefinite life are not amortized but are reviewed for impairment at least annually as of December 31st, or more frequently whenever events or changes in circumstances indicate that the carrying amounts of those assets are below their estimated fair values. Impairment is tested under ASC 350. No impairment was recognized during the years ended December 31, 2022, or 2021.
Continuing Operations and Going Concern
Due to incurred operating losses as well as negative cash flows from operating and investing activities over the past two years, the accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. This basis of accounting contemplates the recovery of our assets and the satisfaction of liabilities in the normal course of business. These consolidated financial statements do not include any adjustments to the specific amounts and classifications of assets and liabilities, which might be necessary should we be unable to continue as a going concern.
To continue as a going concern, the Company has entered into an updated revolving promissory note which extended the maturity through December 31, 2022, and DSS intends to continue to fund the operations of the Company through a year from the date these financial statements were available to be issued. The Company’s management intends to take actions necessary to continue as a going concern. Management’s plans concerning these matters includes, among other things, monetization of its intellectual properties, and tightly controlling operating costs. Based on this, the Company has concluded that substantial doubt of its ability to continue as a going concern has been alleviated.
Related Party Transactions
Research and Development Activities
Based on Shareholders Agreement entered into on April 26, 2017, the Company would fund the scientific operations of GRDG, a company involved in research and development of biomedical products which is a minority stockholder of two of the Company’s subsidiaries and is owned by Daryl Thompson, a director of many subsidiaries of the Company, to do the development and research works on the biomedical products for the Company. As of December 31, 2022, this funding approximates $43,000 per month, and incurred approximately $546,000 in expenses. On December 31, 2022 and December 31, 2021, the Company owed this related party $0 and had prepaid monthly fees approximating $43,000 and $43,000, respectively.
General and Administrative Costs
There are certain general and administrative costs incurred by DSS, a related party on behalf of the Company which are passed through to the Company on a monthly basis. These costs consist of primarily payroll costs for certain DSS employees and are allocated based on estimated time spent on behalf of the Company. These costs approximate $12,000 per month. As of December 31, 2022, the Company incurred $117,000 in related expenses. As of December 31, 2021, the Company incurred approximately $155,000 in related expenses.
Research and Development Activities
Based on Shareholders Agreement entered into on April 26, 2017, the Company would fund the scientific operations of GRDG, a company involved in research and development of biomedical products which is a minority stockholder of two of the Company’s subsidiaries and is owned by Daryl Thompson, a director of many subsidiaries of the Company, to do the development and research works on the biomedical products for the Company. As of December 31, 2022, this funding approximates $43,000 per month, and incurred approximately $546,000 in expenses. On December 31, 2022 and December 31, 2021, the Company owed this related party $0 and had prepaid monthly fees approximating $43,000 and $43,000, respectively.
General and Administrative Costs
There are certain general and administrative costs incurred by DSS, a related party on behalf of the Company which are passed through to the Company on a monthly basis. These costs consist of primarily payroll costs for certain DSS employees and are allocated based on estimated time spent on behalf of the Company. These costs approximate $12,000 per month. As of December 31, 2022, the Company incurred $117,000 in related expenses. As of December 31, 2021, the Company incurred approximately $155,000 in related expenses.
Off-Balance Sheet Arrangements
We do not have any material off-balance sheet arrangements that have, or are reasonably likely to have, an effect on our financial condition, financial statements, revenues or expenses.
BUSINESS
Introduction
Currently, we are a holding company operating mainly through our majority owned subsidiary, Global BioLife, Inc., which was incorporated on April 14, 2017. The Company is committed to both funding research and developing intellectual property portfolio. We currently focus on research in three main areas: (i) development of a universal therapeutic drug platform; (ii) a new sugar substitute; and (iii) a multi-use fragrance.
Our Company
Impact BioMedical Inc. targets urgent medical needs and expands the borders of medical and pharmaceutical science. Impact drives mission-oriented research and development, and commercialization of solutions for medical advances in human wellness and healthcare. By leveraging technology and new science with strategic partnerships, Impact Bio provides advances in drug discovery for the prevention, inhibition, and treatment of neurological, oncology and immuno-related diseases. Other exciting technologies include a breakthrough alternative sugar aimed to combat diabetes and functional fragrance formulations aimed at the industrial and medical industry. The research and development efforts are primarily conducted in collaboration with GRDG Sciences, LLC (“GRDG”) and with the expertise of Mr. Daryl Thompson as its Director of Scientific Initiatives. We have initiated research regarding universal therapeutics as part of an attempt to help address some of the world’s deadliest diseases.
Below is a list of our principal subsidiaries:
| ● | Impact BioLife Science, Inc.; |
| ● | Global Biomedical, Inc.; |
| ● | Global BioLife, Inc.; and |
| ● | Sweet Sense, Inc. |
Impact BioLife Science, Inc. We are the sole owner of the outstanding equity of Impact BioLife Science, Inc.
Global Biomedical, Inc. We own 90.91% of Global Biomedical, Inc. outstanding equity, and the balance minority equity owner is Peggy Tang.
Global BioLife, Inc. Through our majority owned subsidiary Global Biomedical, Inc., we own 90% of the outstanding equity of Global BioLife, Inc. The other equity owner is Holista CollTech Limited (“Holista”) (10%).
Sweet Sense, Inc. We are the owner of 50% of the outstanding equity of Sweet Sense. The other equity owner is BioLife Sugar, Inc. (“BioLife Sugar”).
Below is an organization chart showing our ownership structure and ownership interests.

Through our majority-owned subsidiaries, Global BioLife and Impact BioLife, we own or have rights to a portfolio of biomedical intellectual property, including intellectual property assigned to Global BioLife by GRDG. Global BioLife leverages its scientific know-how and intellectual property rights to develop various emerging technologies, including biopharmaceuticals, antivirals, antimicrobials, sugar alternatives, insect repellents, fragrances, bioplastics and natural preservatives.
Linebacker
Linebacker is a multi-faceted therapeutic platform intended for metabolic, neurologic, cancer, and infectious diseases. Our process features a molecular tuning technique that is intended to modify a natural compound to induce potency, efficacy, bioavailability, and trans-membrane permeability while maintaining safety, toxicity, and tolerability. Natural compounds used in the Linebacker platform is designed to treat and prevent a range of diseases by inhibiting TNF-alpha and indication specific causes (e.g., neurology, anti-inflammatory diseases, oncology). Linebacker’s intended use is subject to FDA regulation, and the FDA must approve any drug or biologic product before it can be marketed in the United States. In addition, prior to being sold outside the United States, our Linebacker technology must be approved by the regulatory agencies of foreign governments. Prior to filing a new drug application or biologics license application with the FDA, we would have to perform extensive clinical trials, and prior to beginning any clinical trial, we would need to perform extensive preclinical testing which could take several years and may require substantial expenditures. To date, we have not conducted preclinical testing or clinical trials (human or otherwise) on Linebacker nor have we received any FDA approval to conduct such any such preclinical testing or clinical trials. Further, because we have not conducted any testing or studies on Linebacker, we are not able to substantiate or demonstrate that Linebacker will function as planned, nor can we seek FDA approval to market and sell Linebacker. To date, we have not performed the extensive preclinical testing necessary to begin any clinical trials for Linebacker; and until we have conducted the necessary clinical trials, we will not be able to seek FDA approval for Linebacker. Currently, we have no plans to conduct preclinical testing or clinical trials (human or otherwise) involving Linebacker.
We intend to identify third parties or customers that are interested in purchasing, licensing or co-developing products that leverage our Linebacker technology. We have not entered into any such agreements to date, and there can be no guarantee that we will enter into any such agreements or that such agreements will be on terms that are favorable to the Company.
Laetose
Laetose technology is derived from a unique combination of sugar and other compounds, which demonstrates the ability to inhibit the inflammatory response of sugar alone. Based on our intended use for Laetose, Laetose may be classified as a high-intensity sweetener by the FDA. High-intensity sweeteners are commonly used as sugar substitutes or sugar alternatives because they are many times sweeter than sugar but contribute only a few to no calories when added to foods. High-intensity sweeteners, like all other ingredients added to food in the United States, must be safe for consumption. Further, a high intensity sweetener is regulated as a food additive, unless its use as a sweetener is generally recognized as safe (GRAS). The use of a food additive must undergo premarket review and approval by FDA before it can be used in food. In contrast, use of a GRAS substance does not require premarket approval. Rather, the basis for a GRAS determination based on scientific procedures is that experts qualified by scientific training and experience to evaluate its safety conclude, based on publicly available information, that the substance is safe under the conditions of its intended use. A company can make an independent GRAS determination for a substance with or without notifying FDA. Regardless of whether a substance is approved for use as a food additive or its use is determined to be GRAS, scientists must determine that it meets the safety standard of reasonable certainty of no harm under the intended conditions of its use. This standard of safety is defined in FDA’s regulations.
As of this date, we have not performed any scientific testing to determine the safety of Laetose for human consumption, and no qualified experts have evaluated the safety of Laetose. Therefore, we have not been able to make any determination that Laetose is safe for human consumption. Currently, we have no plans to conduct any scientific testing relating to the safety of Laetose, and any there can be no guarantee that any such testing will conclude that Laetose is safe for human consumption.
We intend to identify third parties or customers that are interested in purchasing, licensing or co-developing products that leverage Laetose. We have not entered into any such agreements to date, and there can be no guarantee that we will enter into any such agreements or that such agreements will be on terms that are favorable to the Company.
The Company is presently seeking to license Laetose by way of a business development agreement between Sweet Sense, Inc., a joint venture established by Global BioLife and Quality Ingredients, LLC (“Sweet Sense”), and BFS. Sweet Sense was established for the development, manufacture, and global distribution of the new sugar substitute. On November 8, 2019, the Company purchased 50% of Sweet Sense Inc. from Quality Ingredients, LLC for $91,000. Sweet Sense is now an 81.8% owned subsidiary of the Impact BioMedical.
Functional Fragrance Formulation (“3F”)
3F is a unique formulation of specialized ingredients (e.g., terpenes) from botanical sources with potential application as an insect repellent and an antimicrobial. Currently, approximately 20 combination insect repellent-sunscreen drug products are available for consumers. These products consist of one of three insect repellents (N,N-diethyl-meta-toluamide (DEET), oil of citronella, or IR3535) and a sunscreen component (one or more sunscreen ingredients). Internal repellent test results demonstrated the prevention and limitation of insect bites up to eight (8) hours. To date, we have not conducted studies to establish marketing claims.
We also intend to identify third parties or customers that are interested in purchasing, licensing or co-developing products that leverage 3F. We have not entered into any such agreements to date, and there can be no guarantee that we will enter into any such agreements or that such agreements will be on terms that are favorable to the Company.
Equivir
Equivir/Nemovir is a novel blend of naturally occurring compounds that are sourced from fruits, vegetables, and other natural substances. Equivir is designed to work by impeding virulence while also blocking multiple methods used by viruses to infect and replicate in host cells, following deployment in a manner similar to a vitamin, and this intended use is subject to FDA regulation. The FDA must approve any drug or biologic product before it can be marketed in the United States. In addition, prior to being sold outside the United States, our Equivir technology must be approved by the regulatory agencies of foreign governments. Prior to filing a new drug application or biologics license application with the FDA, we would have to perform extensive clinical trials, and prior to beginning any clinical trial, we would need to perform extensive preclinical testing which could take several years and may require substantial expenditures. To date, we have conducted no preclinical testing or clinical trials (human or otherwise) on Equivir. Further, because we have not conducted any testing or studies on Equivir, we are not able to substantiate or demonstrate its benefits as a health supplement or seek FDA approval at this time. Currently, we have no plans to conduct any preclinical testing or clinical trials (human or otherwise) involving Equivir.
We intend to identify third parties or customers that are interested in purchasing, licensing or co-developing products that leverage Equivir. We have not entered into any such agreements to date, and there can be no guarantee that we will enter into any such agreements or that such agreements will be on terms that are favorable to the Company.
In addition, other Equivir analogues are under development and provisional patents have been filed.
Additional Products
Though not of substantive material impact to our business currently, we have three additional products; VanXin, Quantum, and CRST1, which we believe to have high potentiality of viability and commercialization. VanXin is a natural food preservative booster made up of natural occurring compounds, that can extend the shelf life of various products. Quantum, our Patent Cliff solution, uses advanced methods to modify natural compounds and existing drugs, while maintaining the safety attributes of the original molecules. CRST 1 is our advanced adjuvant for next generation vaccine applications. We intend to commercialize these future portfolio products through partnerships with large manufactures in the pharmaceutical, food, health and beauty, and nutraceutical industries. We will leverage their product development, commercialization, and distribution capabilities to take products to market through a licensing and/or private label sales model.
Recent Developments
BioMed Technologies Asia Pacific Holdings Limited Investment
On December 19, 2020, the Company, entered into a subscription agreement (the “Subscription Agreement”) with BioMed Technologies Asia Pacific Holdings Limited (“BioMed”), a limited liability company incorporated in the British Virgin Islands, pursuant to which the Company agreed to purchase 525 ordinary shares or 4.99% of BioMed at a purchase price of approximately $630,000. The Subscription Agreement provides, among other things, the Company the right to appoint a new director to the board of BioMed. With respect to an issuance of shares to a third party by BioMed, the Company will have the right of first refusal to purchase such shares, as well as customary tag-along rights.
In connection with the Subscription Agreement, on December 9, 2020, the Company entered into an exclusive distribution agreement (the “Distribution Agreement”) with BioMed, to directly market, advertise, promote, distribute, and sell certain BioMed products, which focus on manufacturing natural probiotics, to resellers. The Distribution Agreement is for an initial term of ten years, and upon expiration, renews for additional successive one-year terms unless either party elects not to renew. The Distribution Agreement may be terminated by either party for cause as specified therein. Pursuant to the Distribution Agreement, BioMed provides its products to the Company at the prices set forth in the Distribution Agreement, such prices being firm and not subject to increase except for as provided in the Distribution Agreement. Further, pursuant to the Distribution Agreement, BioMed is solely responsible for all costs and expenses relating to the Company’s distribution of BioMed’s products. This investment is valued at cost as it does not have a readily determined fair value. As of December 31, 2021, the Company has generated approximately $83,000 through the sale of BioMed’s products under the Distribution Agreement.
BioMed focuses on manufacturing natural probiotics, pursuant to which the Company will directly market, advertise, promote, distribute and sell certain BioMed products to resellers. The products to be distributed by the Company include BioMed’s PGut Premium Probiotics®, PGut Allergy Probiotics®, PGut SupremeSlim Probiotics®, PGut Kids Probiotics®, and PGut Baby Probiotics®.
Vivacitas Investment
On March 15, 2021, the Company entered into a Stock Purchase Agreement (the “Vivacitas Agreement #1”) with Vivacitas Oncology Inc. (“Vivacitas”), to purchase 500,000 shares of its common stock at the per share price of $1.00, with an option to purchase 1,500,000 additional shares at the per share price of $1.00. This option will terminate upon one of the following events: (i) Vivacitas’ board of directors cancels this option because it is no longer in the best interest of the Company; (ii) December 31, 2021; or (iii) the date on which Vivacitas receives more than $1.00 per share of the Company’s common stock in a private placement with gross proceeds of $500,000. Under the terms of the Vivacitas Agreement #1, the Company will be allocated two seats on the board of Vivacitas. On March 18, 2021, the Company entered into an agreement with Alset EHome International, Inc. (“Seller”) to purchase from the Seller’s its wholly owned subsidiary Impact Oncology PTE Ltd. (“IOPL”) for a purchase price $2,480,000. The acquisition of IOPL has been treated as an asset acquisition as IOPL does not meet the definition of a business as defined in Topic 805. IOPL owns 2,480,000 shares of common stock of Vivacitas along with the option to purchase an additional 250,000 shares of common stock. The Sellers largest shareholder is Mr. Chan Heng Fai Ambrose, the Chairman of the Company’s board of directors and its largest shareholder.
On April 1, 2021, the Company entered into an additional stock purchase agreement with Vivacitas (“Vivacitas Agreement #2”), whereas Vivacitas wished to employ the service of the Chief Business Officer of Impact Biomedical, and in return for the services of this individual, Vivacitas shall issue to the Company, the aggregate purchase price for the Class A Common Shares of Vivacitas at the value of $1.00 per share shall be $120,000 to be paid in twelve (12) equal monthly installments for the period between April 1, 2021 and March 31, 2022.
On July 22, 2021, the Company exercised 1,000,000 of the available options under the Vivacitas Agreement #1 for $1,000,000. This, along with the shares received as part Vivacitas Agreement #2 increased the Company’s equity position in Vivacitas to approximately 120,000 shares or 16% as of September 30, 2022. As of September 30, 2022, and December 31, 2021, the fair value of the Company’s investment in Vivacitas is not readily available, and therefore is recorded at cost in the amount of $4,100,000 and $4,035,000, respectively. As of December 31, 2022, the Company determined to impair 100% of its investment in Vivacitas, in the amount of $4,100,000.
Promissory Note 1
On February 21, 2021, Impact BioMedical Inc. entered into a promissory note (“Promissory Note 1”) with an individual. The Company loaned the principal sum of $206,000, with interest at a rate of 6.5%, and maturity date of August 19, 2022. Monthly payments are due on the twenty-first day of each month and continuing each month thereafter until August 19, 2022, at which time all accrued interest and the entire remaining principal shall be due and payable in full. This note is secured by certain real property situated in Collier County, Florida.
The outstanding principal and interest approximated $206,000 and $197,000 as of December 31, 2022 and December 31, 2021, respectively. Of the $206,000 outstanding at December 31, 2022, $16,000 is classified in current notes receivable on the accompanying consolidated balance sheets, and the remaining $190,000 is classified as note receivable.
Promissory Note 2
On May 14, 2021, DSS PureAir, Inc. (“DSS PureAir”), a wholly owned subsidiary of the Company, entered into a convertible promissory note (“Promissory Note 2”) with a limited liability company registered in the state of Texas (“Borrower”). The Promissory Note 2 has an aggregate principal balance up to $5,000,000, to be funded at request of the borrower. The Promissory Note 2, which incurs interest at a rate of 6.5% due quarterly, has a maturity date of May 14, 2023. The Promissory Note 2 contains an options conversion clause that allows the Company to convert all, or a portion of all, into new issued member units of the borrower with the maximum principal amount equal to 18% of the total equity position at conversion. The outstanding principal and interest as of March 31, 2022, approximated $5,164,000, which is classified as Notes receivable on the consolidated balance sheet. Subsequent to the execution of the Promissory Note 2, DSS PureAir entered into distribution agreement with Borrower, who is in the business of manufacturing and supplying proactive air and surface purification systems, to sell its products within specified territories. In June of 2022, the Company transferred its 100% of its interest in DSS Pure Air, Inc. to DSS Biohealth Holdings, Inc., a related party and wholly owned subsidiary of DSS BioHealth Security, Inc. Associated with this transfer is Promissory Note 2 and the related Note payable, related party. The outstanding principal and interest on December 31, 2022, and December 31, 2021 is $0 and $5,081,000, respectively.
DSS Note
On December 31, 2020, and later amended on December 31, 2022, the Company executed a Revolving Promissory Note (“Note”) with DSS, a related party which accrues interest at a rate of 4.25% and is due in full at the maturity date of December 31, 2023. The revolving nature of this Note permits for principal amounts borrowed to be repaid and reborrowed. As of December 31, 2022 and December 31, 2021, this Note had an outstanding balance of $9,991,000 and $12,523,615, respectively.
Although there is no formal written agreement to fund the Company, DSS may continue to fund the operations of the Company on an as needed basis to be decided by its board of directors.
GRDG Licensing Proceeds Distribution Agreement
On February 15, 2022, the Company entered into a Licensing Proceeds Distribution Agreement (the “Licensing Agreement”) with GRDG Sciences, LLC (“GRDG”), Global BioLife, Inc., and Impact BioLife Sciences, Inc., pursuant to which GRDG will receive 20% of the gross licensing or sale proceeds received by the Company from the licensing of improvements with patent and patent applications (the “Improvements”), and all research and technology, developed, made, owned, conceived, by GRDG in exchange for funding from the Company for research and technology development activities. Pursuant to the Licensing Agreement, Impact continues to receive consulting payments (the “Consulting Payments”) approximating $43,000 per month for the purpose of paying or reimbursing certain salaries, overhead, office rent reimbursement, and other operating costs. As of December 1, 2022, the Company not made any payments under the Licensing Agreement.
The term of the Licensing Agreement is from February 15, 2022, through the later of (1) the date of the last to expire of a valid patent of intellectual property, or (2) the date of the last license or fee income generated from the Improvements. The Licensing Agreement provides for termination for cause, which includes failure to make payment when due, unauthorized disclosure of confidential information, and unauthorized assignment of the agreement. The parties may terminate the Licensing Agreement with or without cause by providing the other party with at least two months advance written of a notice of intention of termination.
ProPhase License Agreement
On March 17, 2022, the Company entered into a License Agreement (the “License Agreement”) with ProPhase Labs, Inc. (“ProPhase”) and Global BioLife, Inc., pursuant to which ProPhase obtained a license to intellectual property rights of Global BioLife, Inc. in exchange for a royalty fee of five- and one-half percent (5.5%) of net sales. Pursuant to the License Agreement, Global BioLife, Inc. shall reimburse ProPhase for fifty percent (50%) of the development costs up to one million two hundred fifty dollars ($1,250,000). The term (the “Term”) of the License Agreement is the later of (a) the expiration date of the last to expire a valid claim comprising the licensed patents, or (b) twelve (12) years from the date of first commercial sale. ProPhase may terminate this Agreement for any or no reason upon thirty (30) days prior written notice to Global BioLife, Inc. In addition, At any time prior to expiration of the Term, either party may terminate the License Agreement for cause by providing written notice.
On July 18, 2022, the Company entered into a License Agreement (“July License Agreement”) by and between ProPhase, and Global BioLife, Inc., pursuant to which Global BioLife, Inc. licensed compounds to ProPhase for research purposes for a one-time upfront license fee of fifty thousand dollars ($50,000). The July License Agreement contains milestones that may result in payment to Global BioLife, Inc. of (a) nine hundred thousand dollars ($900,000) upon successful completion of a first Phase 3 study, which may be required by the FDA, and (b) one million dollars ($1,000,000) for regulatory approval of an NDA for the first licensed product. In addition, ProPhase will pay to Global BioLife, Inc. 3% royalties on net revenue of each licensed product from the date of first commercial sale and for the term of the agreement. The term of the July License Agreement is automatically upon the last to occur of the expiration of the last-to-expire licensed patent. ProPhase has the right to terminate the July License Agreement for any reason or for convenience in its sole discretion. Global BioLife, Inc. has the right to terminate the July License Agreement only for uncured material breaches by ProPhase.
Property
Office space is provided to by DSS at no cost.
Commercialization Business Strategies
Our business model revolves mainly around two approaches – Licensing and Sales Distribution.
1. Licensing
The licensing strategy includes developing unique patented technologies which would then be licensed to external partners for developing, registration, and commercialization as appropriate. We believe that interest in licensing certain projects may rise over time as validating data becomes available.
2. Sales Distribution
Certain affiliates of ours have relationships with developing global distribution networks. We currently intend to pursue private labelling opportunities in these and other networks. In addition, we intend to utilize our affiliates newly launched retail e-commerce site to allow us to take orders and deliver products.
Distribution Agreement by and between BioMed Technologies Asia Pacific Holdings Limited and the Company
On December 9, 2020, the Company entered into a distribution agreement (the “BioMed Distribution Agreement”) with BioMed Technologies Asia Pacific Holdings Limited (“BioMed Technologies”), whereby BioMed Technologies agreed to sell to the Company, and the Company agreed to purchase and resell BioMed Technologies’ BioMed Gut Health Products (the “BioMed Products”) as identified in the BioMed Distribution Agreement. Pursuant to the BioMed Distribution Agreement, the Company will act as the exclusive distributor of the BioMed Products in the U.S., Canada, Singapore, Malaysia, and South Korea. The BioMed Distribution Agreement does not preclude the Company from selling competing products.
The initial term of the BioMed Distribution Agreement is ten (10) years from the December 9, 2020, effective date, unless earlier terminated. Upon its expiration, the BioMed Distribution Agreement shall automatically renew for additional successive one (1) year term unless and until either party provides notice of nonrenewal at least sixty (60) days before the end of the then-current term. The Company may terminate the BioMed Distribution Agreement if BioMed Technologies: (a) repudiates any of its obligations under the BioMed Distribution Agreement; (b) materially breaches any its representations, warranties or covenants; (c) fails to or threatens to fail to, timely deliver the BioMed Products; (d) becomes insolvent or is generally unable to pay, or fails to pay, its debts as they become due and payable, (ii) files or has filed against it, a petition for voluntary or involuntary bankruptcy, (iii) seeks reorganization, arrangement, adjustment, winding-up, liquidation or dissolution or other relief from its debts, (iv) makes or seeks to make a general assignment for the benefit of its creditors, (v) or applies for or has a receiver, trustee, custodian, or similar agent appointed by order of any court of competent jurisdiction or (e) is unable to perform its obligations for more than thirty (30) consecutive business days due to a force majeure event. In addition, the Company may terminate the BioMed Distribution Agreement at its option, at any time, and for any reason.
3. Research and Development
Stockholders Agreement between Global BioLife and the Global BioLife Stockholders
On April 26, 2017, Global BioLife entered into a stockholders’ agreement with its stockholders Global BioMedical, GRDG and Holista (the “Global BioLife Stockholders’ Agreement”). Pursuant to the Global BioLife Stockholder’s Agreement, GRDG has agreed to contribute to Global BioLife any and all right, title, interest and ownership held by GRDG in any patent related to the uses of the “Linebacker Patents” as defined in the Global BioLife Stockholders’ Agreement. Further, pursuant to the Global BioLife Stockholders Agreement, GRDG has agreed to contribute to Global BioLife the advice and services of Daryl Thompson as a scientist during the term of the agreement in connection with the development of the Linebacker Patents and all projects associated therewith, as well as such other projects as Global BioLife may from time to time pursue. In addition, Global BioLife has agreed to contract with GRDG for the needed research in order to develop the Linebacker Patents as well as new intellectual property. Compensation paid by Global BioLife for this work, if any, shall be provided for in Global BioLife’ budget
Pursuant to the Global BioLife Stockholders’ Agreement, Global Biomedical has agreed to contribute to Global BioLife the funds set forth in the Global BioLife budget and such reasonable amounts as the Global BioLife board of directors shall in future annual periods authorize as Global BioLife’s business plan and budget. Such budget shall include (i) a payment of $20,994 per month to GRDG and (ii) such other amounts as shall be necessary to fund the scientific operations that the Global BioLife board shall agree to pursue. The monthly payments were adjusted for the increase in rent of GRDG office and general inflation. Current monthly payments approximate to $43,000. For the years ended December 31, 2022 and 2021, the Company incurred expenses of $546,000 and $509,176, respectively. On December 31, 2022 and 2021, the Company owed this related party $0, and had prepaid monthly fees approximating $43,000.
Under the Global BioLife Stockholders’ Agreement, Holista has agreed to (i) assist in the global commercialization of Global BioLife’s intellectual property, (ii) assist in the initiation and development of joint venture opportunities for Global BioLife, and (iii) provide the expertise of Dr. Rajen M. Dato to Global BioLife, who shall be available to provide management service and assist in the strategic director of Global BioLife.
The Global BioLife Stockholders’ Agreement shall terminate (a) upon the dissolution and winding up of Global BioLife or (b) on the date the parties terminate the agreement by unanimous written consent.
On May 22, 2018, the parties to the Global BioLife Stockholders’ Agreement entered into Amendment No. 1 to the agreement (“Amendment No. 1 to the Global BioLife Stockholders Agreement”), and in August of 2020 the parties entered into Amendment No. 2 to the agreement (“Amendment No. 2 to the Global BioLife Stockholders Agreement”). Pursuant to Amendment No. 2, the parties to the Global BioLife Stockholders Agreement agreed to waive the obligations of GRDG to contribute any further inventions, discoveries or other items of intellectual property developed by GRDG during the term of the Stockholders’ Agreement subsequent to the date of Amendment No. 2, except for any invention, discovery or other items of intellectual property which is directly related to, or necessary for the sale, licensing or further development of intellectual property owned by Global BioLife.
Stockholders Agreement between Impact BioLife and the Impact BioLife Stockholders
In December 2020, Impact BioLife entered into a stockholders agreement with its stockholders Impact BioMedical and GRDG (the “Impact BioLife Stockholders’ Agreement”). Pursuant to the Impact BioLife Stockholders’ Agreement, GRDG agreed to (i) transfer certain intellectual property as identified in the Stockholders’ Agreement to Impact BioLife, (ii) present all suitable technologies developed by GRDG to Impact BioLife, so as to provide Impact BioLife with the opportunity to fund, own and develop any intellectual property developed by GRDG and (iii) retain the advice and services of Mr. Daryl Thompson as a scientist during the term of the Impact BioLife Stockholders’ Agreement in connection with all projects as Impact BioLife may from time to time pursue. Further, pursuant to the Impact BioLife Stockholders’ Agreement, GRDG also agreed that Daryl Thompson will devote most of his professional time per week to the business of Impact BioLife, including but not limited to, the development of new intellectual property for Impact BioLife.
In addition, pursuant to the Impact BioLife Stockholder’s Agreement, the Company has agreed to contribute to Impact BioLife such reasonable amounts as the board of directors of Impact BioLife shall in future annual periods authorize as Impact BioLife’s business plan and budget (the “Impact BioLife Budget”). The Impact BioLife Budget shall include (i) a payment approximating $43,000 per month to GRDG and (ii) such other amounts as shall be necessary to fund the research, development and other scientific operations that the Impact BioLife board of directors shall agree to pursue. For the years ended December 31, 2022 and 2021, the Company incurred expenses of $546,000 and $509,176, respectively. On December 31, 2022 and 2021, the Company owed this related party $0, and had prepaid monthly fees approximating $43,000.
Pursuant to the Impact BioLife Stockholders’ Agreement, the board of directors of Impact BioLife shall never be less than one nor more than five directors. GRDG shall be entitled to nominate one director to the Impact BioLife board of directors so long as it shall remain a stockholder of Impact BioLife. The Company shall be entitled to nominate the remaining directors of the Impact BioLife. In addition, pursuant to the Impact BioLife Stockholders’ Agreement, so long as it is a stockholder of Impact BioLife, the Company is entitled to appoint Impact BioLife’s chief executive officer, who, at the discretion of the Company, may also serve as a director of the Impact BioLife board of directors. The parties to the Impact BioLife Stockholders’ Agreement have agreed that the initial directors of the Impact BioLife board of directors shall be Heng Fai Ambrose Chan, Frank D. Heuszel and Daryl Thompson. The Company shall appoint the chairman of the Impact BioLife board of directors.
The Impact BioLife Stockholders’ Agreement will terminate (a) upon the dissolution and winding up of Impact BioLife, (b) on the date the parties terminate the agreement by unanimous written, (c) on the fifth anniversary of the date of the Impact BioLife Stockholders’ Agreement, unless the parties mutually agree to an extension, or (d) upon three months’ written notice by either party.
Employees
We have two employees focused primarily on the development and marketing of existing technologies as well as identifying new opportunities. Certain services are provided to us and our subsidiaries by DSS as well as by GRDG, a related party, pursuant to the Global BioLife Stockholders’ Agreement and the Impact BioLife Stockholders’ Agreement. At the present time, Global BioLife pays GRDG approximately $43,000 per month for services provided by GRDG.
Legal Proceedings
From time to time, we may be involved in various other claims and legal proceedings relating to claims arising out of our operations. We are not currently a party to any material legal proceedings.
Competition
The biohealth business is highly competitive. Existing and future competitors may introduce products and services in the same markets we serve, and competing products or services may have better performance, lower prices, better functionality and broader acceptance than our products. This competition could result in increased sales and marketing expenses, thereby materially reducing our operating margins, and could harm our ability to increase, or cause us to lose, market share.
Most, if not all, of our current and potential competitors may have significantly greater resources or better competitive positions in certain product segments, geographic regions or user demographics than we do. These factors may allow our competitors to respond more effectively than us to new or emerging technologies and changes in market conditions.
Our competitors may develop products, features or services that are similar to its own or that achieve greater acceptance, may undertake more far-reaching and successful product development efforts or marketing campaigns, or may adopt more aggressive pricing policies. Certain competitors could use strong or dominant positions in one or more markets to gain competitive advantage us in its target market or markets. As a result, our competitors may acquire and engage customers or generate revenue at the expense of our own efforts.
Future Research/Emerging Technologies
In addition to our current technologies, we continue our exploration and discovery efforts. Current areas of interest include natural entities for drug development/treatment, food preservatives, and other categories. These efforts may result in new intellectual property, drug treatments, and consumer and wellness offerings subject to study/data results. They may also never reach development milestones, prove effective, or result in material value to the company.
Intellectual Property
We strive to protect the intellectual property that we believe is important to our business, including seeking and maintaining patent protection intended to cover the composition of matter of our product candidates, their methods of use, their methods of production, related technologies and other inventions. In addition to patent protection, we also rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection, including certain aspects of technical know-how.
Our commercial success depends in part upon our ability to obtain and maintain patent and other proprietary protection for commercially important technologies, inventions and know-how related to our business, defend and enforce our intellectual property rights, particularly our patent rights, preserve the confidentiality of our trade secrets and operate without infringing valid and enforceable intellectual property rights of others.
The patent positions for companies like us are generally uncertain and can involve complex legal, scientific and factual issues. In addition, the coverage claimed in a patent application can be significantly reduced before a patent is issued, and its scope can be reinterpreted and even challenged after issuance. As a result, we cannot guarantee that any of our product candidates will be protectable or remain protected by enforceable patents. We cannot predict whether the patent applications we are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient proprietary protection from competitors. Any patents that we hold may be challenged, circumvented or invalidated by third parties.
The following table shows our material patents and the expiration date of each patent as of March 31, 2022:
| Product | | Patent No. | | Status | | Expiration Date | | Summary | | Ex US Status |
LineBacker | | U.S. 8,034,838 | | Granted | | 2029 | | Method/Composition for Treatment of Neurological Disorders with Modified Flavonoid Compound | | None |
| | U.S. 10,123,991 | | Granted | | 2036 | | Composition of Electrophilically Enhanced Phenolic Compounds (any chlorinated myricetin) for Treating Inflammatory Diseases and Disorders | | PCT/US17/32897 (Foreign Filed in AU, BR, CA, CN, EP, HK, IN, JP, KR, RU) |
| | U.S. 10,966,954 | | Granted | | 2036 | | Composition of Electrophilically Enhanced Phenolic Compounds (specific mono and di-chlorinated myricetin) for Treating Inflammatory Diseases and Disorders | | PCT/US2017/032897 (Foreign AU, BR, CN, EP, JP, KR, RU) (30-month date: Nov. 16, 2018) |
| | PCT/US21/22538 | | Pending | | N/A (1) (2) | | Method and Compositions for Treating, Preventing or Limiting the Occurrence of Viral Infection | | PCT/US21/22538 (30-month date: Sept. 16, 2022) |
Equivir Nemovir Equivir G | | U.S. 10,383,842; US 15/043,472 | | Granted | | 2036 | | Method/Composition for Preventing and Treating Viral Infections (Influenza) | | PCT/US2017/032897* (Foreign AU, BR, CN, EP, JP, KR, RU) (30-month date: Nov. 16, 2018) (* disclosure of both Linebacker and Equivir) |
| | US 11,033,528 | | Granted | | 2036 | | Method/Composition for Preventing and Treating Viral Infections (Ebola, Rhinovirus) | | PCT/US17/048892** (Foreign AU, CA, CN, JP, KR, EPO, RU) (** Equivir w/gallic acid) (30-month date: Feb. 28, 2020) |
| | US 17/346,569 | | Pending | | N/A (1) (3) | | Method/Composition for Preventing and Treating Viral Infections (potentially Covid, others). | | N/A |
| | | | | | | | | |
| Laetose | | 15/698,159 | | Pending | | N/A (1) (4) | | A composition including a select sugar source and myo-inositol that impacts the signaling of TNF-a and pro-inflammatory cytokines when metabolized, with ability to control blood glucose levels, treat diabetes and inflammatory diseases. | | PCT/US18/49965 Foreign Filed in AU, BR, CA, CN, EP, HK, IN, JP, KR and RU (30-month date: Mar. 7, 2020) |
| 3F (Anti-Viral) | | 16/212,966 | | Pending | | N/A (1) (5) | | Composition of natural elements for use as an antibacterial and antiviral agent | | PCT/US19/64254 Foreign Filed in AU, BR, CA, CN, EP, IN, JP, KR and RU (30- month Jun. 7, 2021 |
| 3F (DB Repellent) | | U.S. 16/593,693; U.S. 10,966,424 PCT/US520/54042 | | Granted | | 2039 | | Unique weight-based composition/method of natural elements with DEET for use as insect repellent | | PCT Filed October 2, 2020, PCT/US20/54042) – 30 Month Date April 4, 2022 (currently no foreign national stage countries pending |
| 3F (Anti-Insect) | | 16/270,857 | | Pending | | N/A (1) (6) | | Unique weight-based composition/method of natural elements without DEET for use as insect repellent | | PCT/US20/17183 (Foreign Filed in CA, JP, CN, KR, EPO, RU) (30 month: Jun. 7, 2021) |
1. The expiration date of pending patents are currently unknown but typically approximately 20 years from filing.
2. Patent application filed on September 23, 2021.
3. Patent application filed on June 14, 2021.
4. Patent application filed on September 7, 2017.
5. Patent application filed December 7, 2018.
6. Patent application filed February 8, 2019.
Regulation of the Biohealth Business
In the United States, the drug, device and cosmetic industries have long been subject to regulation by various federal and state agencies, primarily as to product safety, efficacy, manufacturing, advertising, labeling and safety reporting. The U.S. Food and Drug Administration, or FDA, has broad regulatory powers. Extensive testing and documentation is required for FDA approval of new drugs and devices, increasing the expense of product introduction. Significant expenses are also evident in major markets outside of the United States.
The costs of human health care have been and continue to be a subject of study, investigation and regulation by governmental agencies and legislative bodies around the world. In the United States, attention has been focused on drug prices and profits and programs that encourage doctors to write prescriptions for particular drugs, or to recommend, use or purchase particular medical devices. The regulatory agencies under whose purview Impact BioMedical may operate in the future have administrative powers that may subject partners to whom Impact BioMedical licenses products to actions such as product withdrawals, recalls, seizure of products and other civil and criminal sanctions.
In addition, business practices in the health care industry have come under increased scrutiny, particularly in the United States, by government agencies and state attorneys general, and resulting investigations and prosecutions carry the risk of significant civil and criminal penalties.
Partners to whom we license products in the future may need to rely on global supply chains, and production and distribution processes, that are complex, subject to increasing regulatory requirements, and may be faced with unexpected changes that may affect sourcing, supply and pricing of materials used in products which we have or will develop. These processes also are subject to lengthy regulatory approvals.
Share Exchange Transaction
On August 21, 2020, we closed on a Share Exchange Agreement by and among DSS, DSS BioHealth Security, Inc., Alset International Ltd. (formerly Singapore eDevelopment Ltd.), and Global Biomedical Pte Ltd. (“GBM”), pursuant to which we became a wholly-owned subsidiary of DSS BioHealth Security, Inc. Shortly after closing, Frank D. Heuszel, Jason Grady and John Thatch were appointed directors, and Mr. Heng Fai Ambrose Chan remained on the Board of Directors and appointed Chief Executive Officer, Mr. Heuszel was appointed President, Mr. Grady was appointed Chief Operating Officer and Todd Macko was appointed Secretary and Treasurer, replacing the officers and directors who resigned in connection with the share exchange.
Mr. Chan is the Chief Executive Officer and largest beneficial owner of outstanding shares of common stock of Alset International Ltd., as well as the Chairman of the Board and largest beneficial owner of outstanding shares of common stock of DSS.
Limited Operating History
There is no historical financial information about us upon which to base an evaluation of our performance. As of the date of this report, we have not generated any revenues from operations. We cannot guarantee we will be successful in our business operations. Our business is subject to risks inherent in the establishment of a new business enterprise, including limited capital resources, possible delays our research, testing and marketing efforts or wider economic downturns.
Corporate Information
Impact BioMedical Inc. is a Nevada corporation and was incorporated in October 2018. Our principal executive offices are located at 275 Wiregrass Parkway, West Henrietta, New York 14568. Our telephone number is +1-585-325-3610. Our website address is https://www.impbio.com. Our website and the information contained thereon, or connected thereto, does not and will not constitute part of this Prospectus or the Registration Statement on Form S-1 of which this Prospectus is a part.
MANAGEMENT
Officers and Board of Directors Following the Distribution
Under Nevada law, the business and affairs of the Company is managed under the direction of our Board. Our Amended and Restated Articles of Incorporation provide that the number of directors may be fixed by our Board from time to time. Members of the Board are elected and serve for one-year terms or until their successors are elected qualify. The following sets forth information regarding individuals who are currently officers and directors in office and are expected to continue to serve in their positions after the Distribution (ages as of June 24, 2022):
| Name | | Age | | Position |
| Heng Fai Ambrose Chan | | 77 | | Chairman of the Board |
| Frank D. Heuszel | | 65 | | President, Chief Executive Officer and Director |
| TJ Leonardo | | 44 | | Chief Operating Officer and Secretary |
| Todd D. Macko | | 50 | | Chief Financial Officer |
| Brian DeFrees | | 38 | | Controller |
| Dr. Elise Brownell | | 67 | | Director |
| Melissa Sims | | 54 | | Director |
Biographical and certain other information concerning the Company’s officers and directors is set forth below. There are no familial relationships among any of our directors. Except as indicated below, none of our directors is a director in any other reporting companies. None of our directors has been affiliated with any company that has filed for bankruptcy within the last ten years. We are not aware of any proceedings to which any of our directors, or any associate of any such director is a party adverse to us or any of our subsidiaries or has a material interest adverse to us or any of our subsidiaries. Each executive officer serves at the pleasure of the Board of Directors.
| Name | | Age | | Director/Officer Since | | Principal Occupation or Directorships |
| Heng Fai Ambrose Chan | | 77 | | 2017 | | Mr. Chan Heng Fai has served as an Executive director of DSS, Inc. (NYSE: DSS) (formerly known as Document Security Systems, Inc.) since January 2017 and as Executive Chairman of the Board since March 2019. Mr. Chan founded Alset EHome International, Inc. and has served as Chairman of the Board and Chief Executive Officer since inception in March 2018. Mr. Chan is an expert in banking and finance, with 45 years of experience in these industries. He has restructured numerous companies in various industries and countries during the past 40 years. Mr. Chan has served as the Chief Executive Officer of Alset EHome International Inc.’s subsidiary Alset International Limited (“Alset”) (SGX: 40V) since April 2014. Mr. Chan joined the Board of Directors of Alset in May 2013. Mr. Chan has served as a Director of Sharing Services Global Corporation since April 2020. Mr. Chan has served as a director of Alset’s 99.69%-owned subsidiary GigWorld Inc. since October 2014. He also served as a director of Alset’s indirect subsidiary LiquidValue Development Inc. since January 2017. Mr. Chan has also appointed as Chairman and Chief Executive Officer of Alset Capital Acquisition Corp, a New York Stock Exchange Listed company, since October 2021. In addition, Mr. Chan appointed as a board member of Value Exchange International, Inc. since December 2021. |
| Frank D. Heuszel | | 64 | | 2020 | | Frank Heuszel has served as President and Director of the Company since August 2020. Since April 2023, Mr. Heuszel has also served as Chief Executive Officer of the Company. Since April 11, 2019, Mr. Heuszel has served as the Chief Executive Officer of DSS since April 11, 2019, DSS’s Interim Chief Financial Officer from April 2019 to October 2020, and a director of DSS since July 30, 2018. Mr. Heuszel has extensive expertise in a wide array of strategic, business, turnaround, and regulatory matters across several industries as a result of his executive management, educational, and operational experience. Prior to joining DSS, Mr. Heuszel had a very successful career in commercial banking. For over 35 years, Mr. Heuszel served in many senior executive roles with major US and international banking organizations. As a banker Mr. Heuszel has served as General Counsel, Director of Special Assets, Credit Officer, Chief Financial Officer and Auditor. Mr. Heuszel also operated a successful law practice focused on the litigation, corporate restructures, and merger and acquisitions, and collections. In addition to being an attorney and executive manager, Mr. Heuszel is also a Certified Public Accountant (retired), and a Certified Internal Auditor. Mr. Heuszel graduated from The University of Texas at Austin and from The South Texas College of Law, Houston. |
| | | | | | | |
TJ Leonardo | | 44 | | 2023 | | Ms. Leonardo joined DSS, Inc. in 2021 as the company’s Director of Strategic Operations, and was named Impact BioMedical’s Chief Operating Officer and Secretary in May 2023. Through her diverse areas of expertise and problem-solving agility, she has become a trusted partner and advisor to the leadership team of DSS, Inc. Prior to joining DSS, Inc., Ms. Leonardo has held various operational positions at a number of NYC-based firms. Most recently, she led Operations, Onboarding, and Training and Development at Odgers Berndtson, Executive Recruiting Firm, from 2016 to 2020. During her time in NYC from 2001-2016, Ms. Leonardo worked in operations and administrative roles within the investment banking and wealth advisory services industries in fast-paced, demanding, and client-centric environments at Allen & Co., Mount Kellett Capital Management, and Wilmington Trust Company, while also working as a freelance Editor/Writer. Prior to that, TJ supported Managing Directors at DDB Worldwide Advertising Agency and a fellowship program within Columbia Law School. Ms. Leonardo is President of the Board of IC3 in Ithaca, NY, holds a BFA Degree from the State University of New York at Brockport, and obtained a Human Resource Management Certificate from NYU’s School for Continuing and Professional Studies. |
| | | | | | | |
| Todd D. Macko | | 50 | | 2021 | | Todd D. Macko has been Secretary and Treasurer of the Company since January 2021 and in May 2023 became Chief Financial Officer of the Company. Mr. Macko was promoted to Interim Chief Financial Officer of DSS effective October 28, 2020, and was appointed Chief Financial Officer on August 16, 2021. Mr. Macko previously served as the Vice President of Finance of DSS. As the Vice President of Finance, Mr. Macko’s responsibilities included assisting DSS’s Interim Chief Financial Officer in all aspects of financial and regulatory reporting. In addition, his responsibilities included the day-to-day management of the Company’s Accounting and Finance team and the financial leadership in the directing and improving of the accounting, reporting, audit, and tax activities. Prior to his role as Vice President of Finance for the Company, Mr. Macko joined the wholly owned subsidiary of DSS, Premier Packaging Corporation in January 2019, as its Vice President of Finance. Mr. Macko is a Certified Public Accountant with over 25 years of public and corporate financial management, business leadership and corporate strategy. Mr. Macko brings a wealth of experience with strengths in financial planning and analysis, business process re-engineering, budgeting, merger and acquisitions, financial reporting systems, project evaluation and treasury and capital management. Prior to joining the Company, Mr. Macko served as the Corporate Controller for Baldwin Richardson Foods, a leading custom ingredients manufacturer for the food and beverage industry from November 2015 until January 2019. Prior to that, Mr. Macko served as the Controller for The Outdoor Group, LLC., Genesis Vision, Inc., Complemar Partners, Inc., and Level 3 Communications, Inc. Mr. Macko obtained is Bachelor of Science in Accounting from Rochester Institute of Technology. |
Brian DeFrees | | 38 | | 2023 | | Brian DeFrees has served as Controller of the Company since May 2023. Mr. DeFrees joined DSS, Inc in the capacity of Controller in September 2022. In this role, he is responsible for the accounting operations of the company and its subsidiaries, production of periodic financial reports, maintenance of accounting records, and oversight of the accounting department. Mr. DeFrees brings a combination of Public and Corporate sector accounting experience that spans a wide range of industries. Most recently, he was the Financial Controller for Goodwill of the Finger Lakes from May 2015 to May 2021 and a Senior Manager of Accounting for Genesee & Wyoming Railroad from May 2021 to September 2022. |
| | | | | | | |
| Dr. Elise Brownell | | 67 | | 2021 | | Dr. Elise Brownell has served as a director of the Company since January 2021. Dr. Brownell has more than 20 years of biotechnology and pharmaceutical project management experience with a proven track record of advancing programs through clinical development. She serves as a Life Sciences entrepreneurial advisor for ASTIA, the nation’s premier entrepreneurial organization focused on women-led businesses. Dr. Brownell is also a member of the Editorial Advisory Board for Contract Pharma Magazine, and previous Chair of the Leaders Network program of Women in Consulting. She is the co-founder of ZephyrBiotech, LLC, a project management firm dedicated to advancing therapeutic candidates through development to key inflection points for clients. Earlier, Dr. Brownell was a founding member, head of project management and senior director of Aerovance, Inc., a venture-backed biotechnology company spun out from Bayer Healthcare, where she created and managed effective team processes to bring product candidates into full scale clinical Phase 1 and 2 developments. Prior to Aerovance, Dr. Brownell acted as head of project management for Bayer’s Biotechnology Unit, where she integrated project strategies to meet therapeutic and market needs. Other roles included building and negotiating partnerships with third parties to support development programs, leading research teams through early bench-to-clinic development phases, as well as entrepreneurial investment experience with Angel’s Forum. Dr. Brownell received her M.S., M.Phil. and Ph.D. in biology from Yale University and her B.S. in biology from Allegheny College. |
| | | | | | | |
| Melissa Sims | | 54 | | 2023 | | Since May 2023, Melissa Sims has served as a director of the Company. Ms. Sims is an Illinois licensed attorney having practiced law since 1995. Following graduation from Northern Illinois University College of Law, Ms. Sims started the general practice of law representing clients in banking, health care, real estate, criminal, dissolution, municipal and probate matters in state and appellate courts. In 2006, she represented the Village of DePue, Illinois regarding legacy pollution from a Superfund site and set national precedent before the Seventh Circuit Court of Appeals. In 2021, the United States Supreme Court cited the Village of DePue v. ExxonMobil as precedent in the Atlantic Richfield v. Christian case. For the past six years, Ms. Sims has been employed with the international law firm, Milberg Coleman Bryson Phillps Grossman, PLLC and recently represented clients in the National Opioid multidistrict litigation in the Northern District of Ohio. She also represents municipalities across the country in tort actions in state, federal and appellate courts. Ms. Sims will be an asset to the Board in that her decades of plaintiff litigation with offer keen insight into potential matters which may be of importance on behalf of the Company. The Board believes that her legal background, knowledge expertise, and litigation experience will add great value to the board slate. |
| | | | | | | |
Daryl Thompson | | 52 | | 2015 | | Since 2015, Daryl Thompson has served as the Director of Science Initiatives at GRDG Sciences, LLC. From 2006 to 2014, Mr. Thompson served as the President and Director of Research at ATM Metabolics LLC, providing oversight of research and identification of capital, business opportunities, and research partnerships for an entrepreneurial start-up company specializing in the development of food supplements and pharmaceuticals naturally derived from phyto-chemical platforms aimed at preventing and reversing metabolic diseases. Mr. Thompson obtain a degree in chemistry and biology at Florida Southern College. |
Corporate Governance
The Board of Directors of the Company plans to adopt corporate governance guidelines that serve as a flexible framework within which our Board of Directors and its committees operate. These guidelines will cover a number of areas including the size and composition of the board, board membership criteria and director qualifications, director responsibilities, board agenda, roles of the chairman of the board and Chief Executive Officer, meetings of independent directors, committee responsibilities and assignments, board member access to management and independent advisors, director communications with third parties, director compensation, director orientation and continuing education, evaluation of senior management and management succession planning.
Committees of Our Board
We presently do not have any committee of our Board. Currently, our full Board serves as the audit committee and approves, when applicable, the appointment of auditors and the inclusion of financial statements in our periodic reports.
Board Leadership Structure and Role in Risk Oversight
Our Board of Directors is primarily responsible for overseeing our risk management processes on behalf of our Company. The Board of Directors receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate regarding our company’s assessment of risks. The Board of Directors focuses on the most significant risks facing our company and our company’s general risk management strategy, and also ensures that risks undertaken by our Company are consistent with the board’s appetite for risk. While the board oversees our company’s risk management, management is responsible for day-to-day risk management processes. We believe this division of responsibilities is the most effective approach for addressing the risks facing our company and that our board leadership structure supports this approach.
Legal Proceedings
None of directors and executive officers has been involved in legal proceedings that would be material to an evaluation of our management.
Compensation of Directors
The Company has not paid any compensation to any directors since inception.
Stock Ownership of Directors and Executive Officers
See “Security Ownership of Certain Beneficial Owners and Management.”
EXECUTIVE COMPENSATION
The Company has not paid any compensation to any name executive officers or directors since inception.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
DSS currently beneficially owns 100% of all of our Common Stock. After the Distribution, DSS will own approximately 87% of the outstanding shares of our Common Stock. The following table provides information with respect to the expected beneficial ownership of our Common Stock after giving effect to the Distribution by:
| | (i) | each person or entity that we believe, based on the assumptions described below, will be a beneficial owner of more than 5% of our outstanding Common Stock following the Distribution; |
| | | |
| | (ii) | each person who we expect will serve as a director following the Distribution and each named executive officer; and |
| | | |
| | (iii) | all our expected directors and executive officers following the Distribution as a group. |
Except as otherwise noted in the footnotes below, we based the share amounts on each person’s or entity’s existing beneficial ownership of our Common Stock and the number to be distributed to holders of DSS Common Stock at the distribution ratio of 4-1 shares of our Common Stock for each share of DSS Common Stock.
To the extent our directors and officers own DSS Common Stock at the time of the Distribution, they will participate in the Distribution on the same terms as other holders of DSS Common Stock, except for holders of any Affiliate Shares.
Except as otherwise noted in the footnotes below, each person or entity identified below has sole voting and investment power with respect to such securities. The address of all of the officers and directors listed in the table below are in the care of Impact BioMedical Inc., 275 Wiregrass Parkway, West Henrietta, NY 14586, unless otherwise indicated.
As of December 31, 2022, there were 125,073,621 shares of our Common Stock issued and outstanding. The actual number of shares of our Common Stock outstanding following the Distribution will depend on several factors including then number of issued and outstanding shares of DSS at the record date and the number of shares eliminated as a result of the cash out of fractional shares.
| Name of Beneficial Owner | | Number of Shares
Beneficially Owned
Before Distribution | | | Percentage of Shares
Beneficially Owned
Before Distribution | | | Number of Shares
Beneficially Owned
After Distribution | | | Percentage of Shares
Beneficially Owned
After Distribution | |
| 5% or More Stockholders | | | | | | | | | | | | | | | | |
| DSS, Inc. | | | 125,073,621 | (1) | | | 100 | % | | | | | | | | |
| Directors and Executive Officers | | | - | | | | | | | | | | | | | |
| Heng Fai Ambrose Chan | | | - | | | | - | | | | | | | | | |
TJ Leonardo | | | - | | | | - | | | | | | | | | |
| Frank D. Heuszel | | | - | | | | - | | | | | | | | | |
| Dr. Elise Brownell | | | - | | | | - | | | | | | | | | |
| Todd D. Macko | | | - | | | | - | | | | | | | | | |
Brian DeFrees | | | - | | | | - | | | | | | | | | |
| Melissa Sims | | | - | | | | - | | | | | | | | | |
Daryl Thompson | | | - | | | | - | | | | | | | | | |
| All officers and directors as a group (6 persons) | | | - | | | | - | | | | | | | | | |
| (1) | DSS indirectly owns the shares through DSS BioHealth Security, Inc., its wholly-owned subsidiary. |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
On August 21, 2020, DSS completed its acquisition of the Company, pursuant to a Share Exchange Agreement by and among DSS, DSS BioHealth Security, Inc., Alset International Ltd. (formerly Singapore eDevelopment Ltd.), and Global Biomedical Pte Ltd. (“GBM”) (the “Share Exchange”). Under the terms of the Share Exchange, DSS issued 483,334 shares of the DSS Common Stock nominally valued at $6.48 per share, and 46,868 newly issued shares of the DSS Series A Convertible Preferred Stock (“Series A Preferred Stock”), with a stated value of $46,868,000, or $1,000 per share, for a total consideration valued at $50 million. Due to several factors, including a discount for illiquidity, the value of the Series A Preferred Stock was discounted from $46,868,000 to $35,187,000, thus reducing the final consideration given to approximately $38,319,000. As a result of the Share Exchange, Impact BioMedical became a wholly-owned subsidiary of DSS BioHealth Security, Inc., DSS’s wholly-owned subsidiary.
Mr. Heng Fai Ambrose Chan, the Chief Executive Officer and Chairman of the Board of the Company, is the Chief Executive Officer and largest shareholder of Alset International Ltd., as well as the Chairman of the Board and largest shareholder of DSS.
Prior to the execution of the Share Exchange Agreement, our ownership of a suite of antiviral and medical technologies was valued at $382 million. Because the valuation was higher than the previously agreed value, the purchase price was capped at a value of $50 million, pursuant to the terms of the Share Exchange Agreement.
The research and development efforts are currently headed by Mr. Daryl Thompson, Founder and Owner of GRDG. Pursuant to a stockholders’ agreement between Impact BioLife, a majority-owned subsidiary of the Company, and its stockholders (including GRDG) and a stockholders agreement between Global BioLife, a majority-owned subsidiary of the Company, and its stockholder (including GRDG), Mr. Thompson has agreed, through GRDG, to conduct the development and research work on the biomedical products for Impact BioLife and Global BioLife, respectively. Mr. Thompson also serves as the Director of Scientific Initiatives at Impact BioLife. The above-referenced stockholders’ agreements are described more fully below. For services provided by GRDG, Global BioLife pays GRDG approximately $43,000 per month.
On March 15, 2021, the Company, entered into a Stock Purchase Agreement (the “Agreement”) with Vivacitas Oncology Inc. (“Vivacitas”), to purchase 500,000 shares of its common stock at the per share price of $1.00, with an option to purchase 1,500,000 additional shares at the per share price of $1.00. This option will terminate upon one of the following events: (i) The Seller’s board of directors cancels this option because it is no longer in the best interest of the Company; (ii) December 31, 2021; or (iii) the date on which the Seller receives more than $1.00 per share of the Company’s common stock in a private placement with gross proceeds of $500,000. Under the terms of the Agreement, the Company will be allocated two seats on the board of Vivacitas. On July 22, 2021, the Company exercised its right to purchase 1,000,000 additional shares of Vivacitas at the per share price of $1.00. On March 18, 2021, the Company entered into an agreement with Alset EHome International, Inc. (“Seller”) to purchase from the Seller’s its wholly owned subsidiary Impact Oncology PTE Ltd. (“IOPL”) for a purchase price $2,480,000. The acquisition of IOPL has been treated as an asset acquisition as IOPL does not meet the definition of a business as defined in Topic 805. IOPL owns 2,480,000 shares of common stock of Vivacitas along with the option to purchase an additional 250,000 shares of common stock. As a result of these two transactions, the Company will have an approximate 15.7% (subsequent transactions increase the Company ownership to approximately 19%) equity position in Vivacitas. As of September 30, 2022, and December 31, 2021, the fair value of the Company’s investment in Vivacitas is not readily available, and therefore is recorded at cost in the amount of $4,100,000 and $4,035,000, respectively. As of December 31, 2022, the Company determined to impair 100% of its investment in Vivacitas, in the amount of $4,100,000. The Sellers largest shareholder is Mr. Heng Fai Ambrose Chan, the Chief Executive Officer and Chairman of DSS’s board of directors and the largest shareholder of DSS.
Stockholders Agreement between Global BioLife and the Global BioLife Stockholders
On April 26, 2017, Global BioLife entered into a stockholders’ agreement with its stockholders Global BioMedical, GRDG and Holista (the “Global BioLife Stockholders’ Agreement”). Pursuant to the Global BioLife Stockholder’s Agreement, GRDG has agreed to contribute to Global BioLife any and all right, title, interest and ownership held by GRDG in any patent related to the uses of the “Linebacker Patents” as defined in the Global BioLife Stockholders’ Agreement. Further, pursuant to the Global BioLife Stockholders Agreement, GRDG has agreed to contribute to Global BioLife the advice and services of Daryl Thompson as a scientist during the term of the agreement in connection with the development of the Linebacker Patents and all projects associated therewith, as well as such other projects as Global BioLife may from time to time pursue. In addition, Global BioLife has agreed to contract with GRDG for the needed research in order to develop the Linebacker Patents as well as new intellectual property. Compensation paid by Global BioLife for this work, if any, shall be provided for in Global BioLife’ budget
Pursuant to the Global BioLife Stockholders’ Agreement, Global Biomedical has agreed to contribute to Global BioLife the funds set forth in the Global BioLife budget and such reasonable amounts as the Global BioLife board of directors shall in future annual periods authorize as Global BioLife’s business plan and budget. Such budget shall include (i) a payment of $20,994 per month to GRDG and (ii) such other amounts as shall be necessary to fund the scientific operations that the Global BioLife board shall agree to pursue. The monthly payments were adjusted for the increase in rent of GRDG office and general inflation. The current agreement provides for monthly payments of approximately $43,000. As of December 31, 2022, this funding approximates $43,000 per month, and incurred approximately $546,000 in expenses. On December 31, 2022 and December 31, 2021, the Company owed this related party $0 and had prepaid monthly fees approximating $43,000 and $43,000, respectively.
Under the Global BioLife Stockholders’ Agreement, Holista has agreed to (i) assist in the global commercialization of Global BioLife’s intellectual property, (ii) assist in the initiation and development of joint venture opportunities for Global BioLife, and (iii) provide the expertise of Dr. Rajen M. Dato to Global BioLife, who shall be available to provide management service and assist in the strategic director of Global BioLife.
The Global BioLife Stockholders’ Agreement shall terminate (a) upon the dissolution and winding up of Global BioLife or (b) on the date the parties terminate the agreement by unanimous written consent.
On May 22, 2018, the parties to the Global BioLife Stockholders’ Agreement entered into Amendment No. 1 to the agreement (“Amendment No. 1 to the Global BioLife Stockholders Agreement”), and in August of 2020 the parties entered into Amendment No. 2 to the agreement (“Amendment No. 2 to the Global BioLife Stockholders Agreement”). Pursuant to Amendment No. 2, the parties to the Global BioLife Stockholders Agreement agreed to waive the obligations of GRDG to contribute any further inventions, discoveries or other items of intellectual property developed by GRDG during the term of the Stockholders’ Agreement subsequent to the date of Amendment No. 2, except for any invention, discovery or other items of intellectual property which is directly related to, or necessary for the sale, licensing or further development of intellectual property owned by Global BioLife.
Stockholders Agreement between Impact BioLife and the Impact BioLife Stockholders
In December 2020, Impact BioLife entered into a stockholders agreement with its stockholders Impact BioMedical and GRDG (the “Impact BioLife Stockholders’ Agreement”). Pursuant to the Impact BioLife Stockholders’ Agreement, GRDG agreed to (i) transfer certain intellectual property as identified in the Stockholders’ Agreement to Impact BioLife, (ii) present all suitable technologies developed by GRDG to Impact BioLife, so as to provide Impact BioLife with the opportunity to fund, own and develop any intellectual property developed by GRDG and (iii) retain the advice and services of Mr. Daryl Thompson as a scientist during the term of the Impact BioLife Stockholders’ Agreement in connection with all projects as Impact BioLife may from time to time pursue. Further, pursuant to the Impact BioLife Stockholders’ Agreement, GRDG also agreed that Daryl Thompson will devote most of his professional time and efforts each week to the business of Impact BioLife, including but not limited to, the development of new intellectual property for Impact BioLife.
In addition, pursuant to the Impact BioLife Stockholder’s Agreement, the Company has agreed to contribute to Impact BioLife such reasonable amounts as the board of directors of Impact BioLife shall in future annual periods authorize as Impact BioLife’s business plan and budget (the “Impact BioLife Budget”). The Impact BioLife Budget shall include (i) a payment approximating $43,000 per month to GRDG and (ii) such other amounts as shall be necessary to fund the research, development and other scientific operations that the Impact BioLife board of directors shall agree to pursue. For the years ended December 31, 2022 and 2021, the Company incurred expenses of $546,000 and $509,176, respectively. On December 31, 2022 and 2021, the Company owed this related party $0, and had prepaid monthly fees approximating $43,000.
Pursuant to the Impact BioLife Stockholders’ Agreement, the board of directors of Impact BioLife shall never be less than one nor more than five directors. GRDG shall be entitled to nominate one director to the Impact BioLife board of directors so long as it shall remain a stockholder of Impact BioLife. The Company shall be entitled to nominate the remaining directors of Impact BioLife. In addition, pursuant to the Impact BioLife Stockholders’ Agreement, so long as it is a stockholder of Impact BioLife, the Company is entitled to appoint Impact BioLife’s chief executive officer, who, at the discretion of the Company, may also serve as a director of the Impact BioLife board of directors. The parties to the Impact BioLife Stockholders’ Agreement have agreed that the initial directors of the Impact BioLife board of directors shall be Heng Fai Ambrose Chan, Frank D. Heuszel and Daryl Thompson. The Company shall appoint the chairman of the Impact BioLife board of directors.
The Impact BioLife Stockholders’ Agreement will terminate (a) upon the dissolution and winding up of Impact BioLife, (b) on the date the parties terminate the agreement by unanimous written, (c) on the fifth anniversary of the date of the Impact BioLife Stockholders’ Agreement, unless the parties mutually agree to an extension, or (d) upon three months’ written notice by either party.
On March 15, 2021, the Company entered into a Stock Purchase Agreement (the “Vivacitas Agreement #1”) with Vivacitas Oncology Inc. (“Vivacitas”), to purchase 500,000 shares of its common stock at the per share price of $1.00, with an option to purchase 1,500,000 additional shares at the per share price of $1.00. This option will terminate upon one of the following events: (i) Vivacitas’ board of directors cancels this option because it is no longer in the best interest of the Company; (ii) December 31, 2021; or (iii) the date on which Vivacitas receives more than $1.00 per share of the Company’s common stock in a private placement with gross proceeds of $500,000. Under the terms of the Vivacitas Agreement #1, the Company will be allocated two seats on the board of Vivacitas. On March 18, 2021, the Company entered into an agreement with Alset EHome International, Inc. (“Seller”) to purchase from the Seller’s its wholly owned subsidiary Impact Oncology PTE Ltd. (“IOPL”) for a purchase price $2,480,000. The acquisition of IOPL has been treated as an asset acquisition as IOPL does not meet the definition of a business as defined in Topic 805. IOPL owns 2,480,000 shares of common stock of Vivacitas along with the option to purchase an additional 250,000 shares of common stock. The Sellers largest shareholder is Mr. Chan Heng Fai Ambrose, the Chairman of the Company’s board of directors and its largest shareholder.
On April 1, 2021, the Company entered into an additional stock purchase agreement with Vivacitas (“Vivacitas Agreement #2”), whereas Vivacities wished to employ the service of the Chief Business Officer of Impact Biomedical, and in return for the services of this individual, Vivacitas shall issue to the Company, the aggregate purchase price for the Class A Common Shares of Vivacitas at the value of $1.00 per share shall be $120,000 to be paid in twelve (12) equal monthly installments for the period between April 1, 2021 and March 31, 2022.
On July 22, 2021, the Company exercised 1,000,000 of the available options under the Vivacitas Agreement #1 for $1,000,000. This, along with the shares received as part Vivacitas Agreement #2 increased the Company’s equity position in Vivacitas to approximately 16% as of December 31, 2022. As of December 31, 2021, the fair value of the Company’s investment in Vivacitas is not readily available, and therefore is recorded at cost in the amount of $4,035,000. For the fiscal year December 31, 2022, the company was unable to obtain viable documentation in regards to Vivacitas’s ability to continue as a business and therefore as of December 31, 2022, the Company determined to impair 100% of its investment in Vivacitas, in the amount of $4,100,000.
On December 31, 2020, and later amended on December 31, 2021, March 31, 2022, September 30, 2022, and December 31, 2022, the Company executed a Revolving Promissory Note (“Note”) with DSS which accrues interest at a rate of 4.25% and is due in full at the maturity date of December 31, 2023. The revolving nature of this Note permits for principal amounts borrowed to be repaid and reborrowed. DSS has not established a maximum borrowing amount and intends to continue to fund the operations of the Company through a year from the date these financial statements were available to be issued. As of December 31, 2022 and 2021, this Note had an outstanding balance of $9,991,000 and $12,524,000, respectively.
GRDG Licensing Proceeds Distribution Agreement
On February 15, 2022, the Company entered into a Licensing Proceeds Distribution Agreement (the “Licensing Agreement”) with GRDG Sciences, LLC (“GRDG”), Global BioLife, Inc., and Impact BioLife Sciences, Inc., pursuant to which GRDG will receive 20% of the gross licensing or sale proceeds received by the Company from the licensing of improvements with patent and patent applications (the “Improvements”), and all research and technology, developed, made, owned, conceived, by GRDG in exchange for funding from the Company for research and technology development activities. The term of the Licensing Agreement is from February 15, 2022, through the later of (1) the date of the last to expire of a valid patent of intellectual property, or (2) the date of the last license or fee income generated from the Improvements.
ProPhase License Agreement
On March 17, 2022, the Company entered into a License Agreement (the “License Agreement”) with ProPhase Labs, Inc. (“ProPhase”) and Global BioLife, Inc., pursuant to which ProPhase obtained a license to intellectual property rights of Global BioLife, Inc. in exchange for a royalty fee of five- and one-half percent (5.5%) of net sales. Pursuant to the License Agreement, Global BioLife, Inc. shall reimburse ProPhase for fifty percent (50%) of the development costs up to one million two hundred fifty dollars ($1,250,000). The term (the “Term”) of the License Agreement is the later of (a) the expiration date of the last to expire a valid claim comprising the licensed patents, or (b) twelve (12) years from the date of first commercial sale. ProPhase may terminate this Agreement for any or no reason upon thirty (30) days prior written notice to Global BioLife, Inc. In addition, at any time prior to expiration of the Term, either party may terminate the License Agreement for cause by providing written notice.
On July 18, 2022, the Company entered into a License Agreement (“July License Agreement”) by and between ProPhase, and Global BioLife, Inc., pursuant to which Global BioLife, Inc. licensed compounds to ProPhase for research purposes for a one-time upfront license fee of fifty thousand dollars ($50,000). The July License Agreement contains milestones that may result in payment to Global BioLife, Inc. of (a) nine hundred thousand dollars ($900,000) upon successful completion of a first Phase 3 study, which may be required by the FDA, and (b) one million dollars ($1,000,000) for regulatory approval of an NDA for the first licensed product. In addition, ProPhase will pay to Global BioLife, Inc. 3% royalties on net revenue of each licensed product from the date of first commercial sale and for the term of the agreement. The term of the July License Agreement is automatically upon the last to occur of the expiration of the last-to-expire licensed patent. ProPhase has the right to terminate the July License Agreement for any reason or for convenience in its sole discretion. Global BioLife, Inc. has the right to terminate the July License Agreement only for uncured material breaches by ProPhase.
DSS PureAir, Inc. Assignment Agreement
On June 1, 2022, Impact Biolife Science, Inc. assigned and transferred its entire interest of 100 shares of common stock of DSS PureAir, Inc. to DSS BioHealth Holdings, Inc. for consideration of $100.
DESCRIPTION OF OUR CAPITAL STOCK
General
Our Amended and Restated Articles of Incorporation authorizes us to issue up to 4,000,000,000 shares of Common Stock, $0.001 par value per share, and 100,000,000 shares of preferred stock, par value $0.001 per share. As of December 31, 2022, there were 125,073,621 shares of our Common Stock and no shares of preferred stock issued and outstanding. On May 11, 2023, the Company effected a forward split. As a result, there were 3,877,282,251 shares of our Common Stock and no shares of preferred stock issued and outstanding.
Common Stock
Voting Rights
Subject to the rights of holders of any then-outstanding preferred stock, each holder of our Common Stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, including the election of directors. Under our Amended and Restated Articles of Incorporation, our stockholders will not have cumulative voting rights. Because of this, the holders of a majority of the shares of Common Stock entitled to vote in any election of directors can elect all of the directors standing for election.
Dividend Rights
Subject to the rights of holders of any then-outstanding preferred stock, the holders of our Common Stock are entitled to receive ratably those dividends, if any, as may be declared from time to time by the board of directors out of legally available funds. We do not anticipate paying any cash dividends in the foreseeable future.
Liquidation Rights
In the event of our liquidation, dissolution or winding up, holders of common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then-outstanding shares of preferred stock.
Preemptive or Similar Rights
Holders of Common Stock have no preemptive, conversion or subscription rights and there is no redemption or sinking fund provisions applicable to the common stock. The rights, preferences and privileges of the holders of common stock are subject to, and may be adversely affected by, the rights of the holders of shares of any series of preferred stock that we may designate in the future.
Preferred Stock
No shares of preferred stock are currently outstanding. We have no present plans to issue any shares of preferred stock.
Limitations of Liability and Indemnification Matters
Our Amended and Restated Articles of Incorporation contains provisions that limit the liability of our current and former directors for monetary damages to the fullest extent permitted by Nevada law. Nevada law provides that directors of a corporation will not be personally liable for monetary damages for any breach of fiduciary duties as directors, except liability for intentional misconduct, fraud or a knowing violation of law.
Transfer Agent and Registrar
The transfer agent and registrar for our Common Stock is V Stock Transfer, LLC. The transfer agent’s address is 6201 15th Avenue, Brooklyn, New York 11219 and the telephone number is (800) 937-5449.
No Listing
Our Common Stock is not listed on any securities exchange.
LEGAL MATTERS
The validity of our Common Stock to be distributed in the Distribution will be passed upon for the Company by Sichenzia Ross Ference LLP, New York, New York.
EXPERTS
The consolidated financial statements of Impact BioMedical Inc. and subsidiaries as of and for the year ended December 31, 2021, included in this prospectus and elsewhere in the registration statement have been so included in reliance upon the report of Turner, Stone & Company. LLP., independent registered public accountants upon the authority of said firm as experts in accounting and auditing.
The consolidated financial statements of Impact BioMedical Inc. and subsidiaries as of and for the year ended December 31, 2022, have been included in reliance on the report of by Grassi & Co. CPAs, P.C., an independent registered public accounting firm, as stated in its report incorporated by reference herein, and have been so incorporated in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act to register the shares of our Common Stock to be distributed in the Distribution. The term registration statement means the original registration statement and any and all amendments thereto, including the exhibits and schedules to the original registration statement and any amendments. This Prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules filed therewith. For further information with respect to us, reference is made to the registration statement and the exhibits and schedules filed therewith. Statements contained in this Prospectus regarding the contents of any contract or any other document that is filed as an exhibit to the registration statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the registration statement. A copy of the registration statement, along with the exhibits and schedules filed therewith, may be inspected without charge at the SEC’s Internet website. The SEC maintains an Internet website at www.sec.gov that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC.
The information contained on or accessible through our website or the SEC’s website shall not be deemed to be a part of this Prospectus or the Registration Statement on Form S-1, of which this Prospectus is a part.
We have not authorized anyone to give any information or make any representation about the Distribution or of the Company that is different from, or in addition to, that contained in this Prospectus. Therefore, if anyone does give you information of this sort, you should not rely on it. If you are in a jurisdiction where offers to sell, or solicitations of offers to purchase, the securities offered by this Prospectus or the solicitation of proxies is unlawful, or if you are a person to whom it is unlawful to direct these types of activities, then the offer presented in this Prospectus does not extend to you. The information contained in this Prospectus speaks only as of the date of this Prospectus unless the information specifically indicates that another date applies
FINANCIAL STATEMENTS
Impact BioMedical Inc and Subsidiaries
December 31, 2022 and December 31, 2021
Table of Contents
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Impact BioMedical, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Impact BioMedical, Inc. and subsidiaries (collectively, the “Company”) as of December 31, 2021, and the related consolidated statements of operations, stockholders’ equity and cash flows for the period ended December 31, 2021, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position for the Company as of December 31, 2021, and the results of its operations and its cash flows for the period ended December 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatements, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Turner, Stone & Company, LLP
We have served as the Company’s auditor since 2022.
Dallas, Texas
June 24, 2022
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors of
Impact Biomedical, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheet of Impact Biomedical, Inc., and its subsidiaries (the “Company”) as of December 31, 2022, and the related consolidated statement of operations, stockholders’ equity, and cash flows for the year ended December 31, 2022, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022, and the results of its operations and its cash flows for the year then ended, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Emphasis of Matters
As discussed in Note 3 to the consolidated financial statements, the December 31, 2022 consolidated financial statements have been restated to correct certain errors. Our opinion is not modified with respect to this matter.
 | |
| GRASSI & CO., CPAs, P.C. | |
| | |
| We have served as the Company’s auditor since 2021. | |
| | |
| Jericho, New York | |
| May 15, 2023, except for Note 3, as to which the date is June 2, 2023 | |
Impact BioMedical Inc and Subsidiaries
Consolidated Balance Sheets
As of December 31,
| | | 2022 (restated) | | | 2021 | |
| ASSETS | | | | | | | | |
| Current assets: | | | | | | | | |
| Cash and cash equivalents | | $ | 2,000 | | | $ | 46,000 | |
| Current portion of notes receivable | | | 16,000 | | | | 197,000 | |
| Prepaid expenses and other current assets | | | 104,000 | | | | 44,000 | |
| Total current assets | | | 122,000 | | | | 287,000 | |
| | | | | | | | | |
| Property, plant and equipment, net | | | 276,000 | | | | - | |
| Other investments | | | 782,000 | | | | 4,817,000 | |
| Notes receivable | | | 190,000 | | | | 5,081,000 | |
| Goodwill | | | 25,093,000 | | | | 25,093,000 | |
| Other intangible assets, net | | | 20,034,000 | | | | 21,147,000 | |
| Total assets | | $ | 46,497,000 | | | $ | 56,425,000 | |
| | | | | | | | | |
| LIABILITIES AND STOCKHOLDER’S EQUITY | | | | | | | | |
| | | | | | | | | |
| Current liabilities: | | | | | | | | |
| Accounts payable | | $ | 539,000 | | | $ | 115,000 | |
| Accrued expenses | | | 63,000 | | | | 6,000 | |
| Note payable, related party | | | 9,991,000 | | | | 12,524,000 | |
| Total current liabilities | | | 10,593,000 | | | | 12,645,000 | |
| | | | | | | | | |
| Deferred tax liability, net | | | 3,235,000 | | | | 3,856,000 | |
| Total liabilities | | | 13,828,000 | | | | 16,501,000 | |
| | | | | | | | | |
| Commitments and contingencies (Note 12) | | | | | | | | |
| | | | | | | | | |
| Stockholder’s equity | | | | | | | | |
| Common stock, $.001 par value; 4,000,000,000 shares authorized, 3,877,282,251 shares issued and outstanding (3,877,282,251 on December 31, 2021) | | | 125,000 | | | | 125,000 | |
| Additional paid-in capital | | | 38,058,000 | | | | 38,058,000 | |
| Accumulated deficit | | | (8,625,000 | ) | | | (1,574,000 | ) |
| Total stockholder’s equity of the company | | | 29,558,000 | | | | 36,609,000 | |
| Non-controlling interest in subsidiary | | | 3,111,000 | | | | 3,315,000 | |
| Total stockholder’s equity | | | 32,669,000 | | | | 39,924,000 | |
| | | | | | | | | |
| Total liabilities and stockholder’s equity | | $ | 46,497,000 | | | $ | 56,425,000 | |
See accompanying notes to the consolidated financial statements
Impact BioMedical Inc and Subsidiaries
Consolidated Statements of Operations
| | | 2022 (restated) | | | 2021 | |
| | | | | | | |
| Costs and expenses: | | | | | | | | |
| Selling, general and administrative compensation | | | 325,000 | | | | 398,000 | |
| Research & Development | | | 1,226,000 | | | | 1,080,000 | |
| Professional Services | | | 722,000 | | | | 552,000 | |
| Amortization | | | 1,113,000 | | | | 1,113,000 | |
| Other General Expense | | | 68,000 | | | | 42,000 | |
| Total costs and expenses | | | 3,454,000 | | | | 3,185,000 | |
| | | | | | | | | |
| Operating loss | | | (3,454,000 | ) | | | (3,185,000 | ) |
| | | | | | | | | |
| Other income (expense): | | | | | | | | |
| Interest income | | | 24,000 | | | | 165,000 | |
| Other income (expense) | | | 116,000 | | | | 77,000 | |
| Interest expense | | | (462,000 | ) | | | (300,000 | ) |
| Impairment of investment | | | (4,100,000 | ) | | | - | |
| Loss from continuing operations before income taxes | | | (7,876,000 | ) | | | (3,243,000 | ) |
| | | | | | | | | |
| Income tax (loss)/benefit | | | 621,000 | | | | 1,395,000 | |
| | | | | | | | | |
| Net loss | | | (7,255,000 | ) | | | (1,848,000 | ) |
| | | | | | | | | |
| Loss from continuing operations attributed to noncontrolling interest | | | 204,000 | | | | 545,000 | |
| | | | | | | | | |
| Net loss attributable to common stockholders | | $ | (7,051,000 | ) | | $ | (1,303,000 | ) |
| | | | | | | | | |
| Loss per common share: | | | | | | | | |
| Basic | | $ | (0.00 | ) | | $ | (0.00 | ) |
| Diluted | | $ | - | | | $ | - | |
| | | | | | | | | |
| Shares used in computing loss per common share: | | | | | | | | |
| Basic | | | 3,877,282,251 | | | | 3,877,282,251 | |
| Diluted | | | 3,877,282,251 | | | | 3,877,282,251 | |
See accompanying notes to the consolidated financial statements.
Impact BioMedical Inc and Subsidiaries
Consolidated Statements of Stockholder’s Equity
| | | Common Stock | | | Additional Paid-in | | | Accumulated | | | Non- controlling Interest in | | | | |
| | | Shares | | | Amount | | | Capital | | | Deficit (restated) | | | Subsidiary | | | Total (restated) | |
| | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2021 | | | 3,877,282,251 | | | $ | 125,000 | | | $ | 38,058,000 | | | $ | (1,574,000 | ) | | $ | 3,315,000 | | | $ | 39,924,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Net loss | | | - | | | | - | | | | - | | | | (6,589,000 | ) | | | (204,000 | ) | | | (6,793,000 | ) |
| Balance December 31, 2022 | | | 3,877,282,251 | | | $ | 125,000 | | | $ | 38,058,000 | | | $ | (8,163,000 | ) | | $ | 3,111,000 | | | $ | 33,131,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Balance, December 31, 2020 | | | 3,877,282,251 | | | $ | 125,000 | | | $ | 38,058,000 | | | $ | (271,000 | ) | | $ | 3,860,000 | | | $ | 41,772,000 | |
| | | | | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock, net | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| Proceeds from shareholder | | | - | | | | - | | | | - | | | | - | | | | - | | | | - | |
| Net loss | | | - | | | | - | | | | - | | | | (1,303,000 | ) | | | (545,000 | ) | | | (1,848,000 | ) |
| Balance, December 31, 2021 | | | 3,877,282,251 | | | $ | 125,000 | | | $ | 38,058,000 | | | $ | (1,574,000 | ) | | $ | (3,315,000 | ) | | $ | 39,924,000 | |
See accompanying notes to the consolidated financial statements
Impact BioMedical Inc and Subsidiaries
Consolidated Statements of Cash Flows
| | | 2022 (restated) | | | 2021 | |
| Cash flows from operating activities: | | | | | | | | |
| Net loss | | $ | (7,255,000 | ) | | $ | (1,848,000 | ) |
| Adjustments to reconcile net loss to net cash used by operating activities: | | | | | | | | |
| Depreciation and amortization | | | 1,113,000 | | | | 1,113,000 | |
| Deferred tax benefit | | | (621,000 | ) | | | (1,395,000 | ) |
| Impairment of other investments | | | 4,100,000 | | | | - | |
| Decrease (increase) in assets: | | | | | | | | |
| Prepaid expenses and other current assets | | | (60,000 | ) | | | (8,000 | ) |
| Increase (decrease) in liabilities: | | | | | | | | |
| Accounts payable | | | 424,000 | | | | 106,000 | |
| Accrued expenses | | | 57,000 | | | | 5,000 | |
| Net cash used by operating activities | | | (2,242,000 | ) | | | (2,027,000 | ) |
| | | | | | | | | |
| Cash flows from investing activities: | | | | | | | | |
| Purchase of property, plant and equipment | | | (276,000 | ) | | | - | |
| Purchase of investment | | | (65,000 | ) | | | (4,185,000 | ) |
| Note receivable investment | | | (8,000 | ) | | | (5,278,000 | ) |
| Net cash used by investing activities | | | (349,000 | ) | | | (9,463,000 | ) |
| | | | | | | | | |
| Cash flows from financing activities: | | | | | | | | |
| Borrowings from parent | | | 2,547,000 | | | | 11,464,000 | |
| Net cash provided by financing activities | | | 2,547,000 | | | | 11,464,000 | |
| | | | | | | | | |
| Net decrease in cash | | | (44,000 | ) | | | (27,000 | ) |
| Cash and cash equivalents at beginning of year | | | 46,000 | | | | 72,000 | |
| Cash and cash equivalents at end of year | | $ | 2,000 | | | $ | 46,000 | |
See accompanying notes to the consolidated financial statements
IMPACT Biomedical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Note 1. Nature of Operations and Basis of Presentation
Nature of Operations
Impact BioMedical, Inc. (the “Company”, “Impact BioMedical”, “We”) through the utilization of its intellectual property rights, or through investment in, or through acquisition of companies in the biohealth and biomedical fields, focuses on the advancement of drug discovery and prevention, inhibition, and treatment of neurological, oncological, and immune related diseases. The Company is also developing open-air defense initiatives, which curb transmission of air-borne infectious diseases, such as tuberculosis and influenza.
Global BioLife, Inc. (“Global BioLife”), one of the Company’s subsidiaries and the main operating company of the group, focuses on research in four main areas: (i) the “Linebacker” project, which aims to develop a universal therapeutic drug platform; (ii) a new sugar substitute called “Laetose,”; (iii) a multi-use fragrance called “3F” (Functional Fragrance Formulation); and (iv) Equivir/Nemovir, a blend of natural polyphenols designed as an antimicrobial medication.
Linebacker
Unlike the traditional approach to treat individual diseases with specific drugs, the Linebacker platform seeks to offer a breakthrough therapeutic option for multiple diseases. Linebacker is designed to work by inhibiting a cascade of inflammatory responses responsible for many diseases. Its design is in direct contrast to the traditional approach of targeting individual diseases with specific drugs.
Laetose
We have also developed a low-calorie, low glycemic level, natural modified sugar through Global BioLife. The product, “Laetose,” is designed to possess low glycemic properties and mitigate inflammation. The Company is presently seeking to license Laetose. Global BioLife established a joint venture, Sweet Sense, Inc. (“Sweet Sense”), with Quality Ingredients, LLC for the development, manufacture, and global distribution of the new sugar substitute. On November 8, 2019, the Company purchased 50% of Sweet Sense Inc. from Quality Ingredients, LLC for $91,000. Sweet Sense is now an 81.8% owned subsidiary of Impact BioMedical.
Functional Fragrance Formulation (“3F”)
Global BioLife has established a collaboration with U.S.-based Chemia Corporation (“Chemia”) to develop specialized fragrances to counter mosquito-borne diseases such as Zika and Dengue, among other medical applications. The 3F mosquito fragrance product is made from specialized oils sourced from botanicals that mosquitos avoid. Global BioLife is seeking to commercialize this product. Together with Chemia, we are attempting to license 3F. Any potential profits from the 3F project will be split between Global BioLife and Chemia pursuant to the terms of the 20- year Royalty Agreement (Note 12).
Equivir
Equivir, is a polyphenol compound that is believed to be successful in antiviral infection treatments. Equivir is a patented medication, that has broad antiviral efficacy against multiple types of infectious disease.
The Company was incorporated in the State of Nevada as a for-profit company on October 16, 2018 and established a fiscal year end of December 31st. The Company issued 9,000 shares to its sole shareholder Global BioMedical Pte. Ltd., which was wholly owned by Alset International Limited (formally Singapore eDevelopment Limited), a multinational public company, listed on the Singapore Exchange Securities Trading Limited (“SGXST”). On March 31, 2020, the Company issued 125,064,621 shares of common stock to its sole shareholder Global BioMedical Pte. Ltd. On July 24, 2020, the Board approved the Stock Split, pursuant to which each share of the Company’s common stock issued and outstanding was split into nine shares of the Company’s common stock. The numbers of authorized common stock and issued and outstanding common stock in the reporting periods were retrospectively adjusted for the stock split.
As of the date of this report, we have not generated significant revenues from operations. We cannot guarantee we will be successful in our business operations. Our business is subject to risks inherent in the establishment of a new business enterprise, including possible delays in our research, testing and marketing efforts or wider economic downturns.
Basis of Presentation and Principles of Consolidation
The Company’s consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The consolidated financial statements include all accounts of the Company and its majority owned and controlled subsidiaries. The Company consolidates entities in which it owns more than 50% of the voting common stock and controls operations. All intercompany transactions and balances among consolidated subsidiaries have been eliminated. Non–controlling interest represents the minority equity investment in the Company’s subsidiaries, plus the minority investors’ share of the net operating results and other components of equity relating to the non–controlling interest.
IMPACT Biomedical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The consolidated financial statements include all accounts of the entities as of the reporting period ending dates and for the reporting periods as follows:
| Name of consolidated subsidiary | | State or other jurisdiction of incorporation or organization | | Date of incorporation or formation | | Attributable interest as of
December 31,
2022 | | | Attributable
interest as of
December 31,
2021 | |
| | | | | | | | | | | |
| Global BioMedical, Inc. | | Nevada | | April 18, 2017 | | | 90.9 | % | | | 90.9 | % |
| Global BioLife, Inc. | | Nevada | | April 14, 2017 | | | 81.8 | % | | | 63.6 | % |
| BioLife Sugar, Inc | | Nevada | | April 23, 2018 | | | 90.9 | % | | | 63.6 | % |
| Happy Sugar Inc | | Nevada | | August 17, 2018 | | | 81.8 | % | | | 63.6 | % |
| Sweet Sense Inc. | | Nevada | | April 30, 2018 | | | 95.5 | % | | | 81.8 | % |
| Global Sugar Solutions Inc. | | Nevada | | November 7, 2019 | | | 100 | % | | | 80 | % |
As of December 31, 2022, and 2021, the aggregate noncontrolling interest was equity of $3,111,000 and $3,315,000, respectively, which are separately disclosed on the Consolidated Balance Sheets.
Note 2. Summary of Significant Accounting and Reporting Policies
Use of estimates
The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the dates of the balance sheets and reported amounts of revenues and expenses during the reporting periods. Actual results could differ from these estimates.
Earnings (Loss) per Share
Basic earnings (loss) per share is computed by dividing the net income (loss) attributable to the common stockholders by weighted average number of shares of common stock outstanding during the period. Fully diluted earnings (loss) per share is computed like basic income (loss) per share except that the denominator is increased to include the number of additional common shares that would have been outstanding if the potential common shares had been issued and if the additional common shares were dilutive. There were no dilutive financial instruments issued or outstanding for the years ended December 31, 2022 or 2021.
Fair Value of Financial Instruments
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. The Fair Value Measurement Topic of the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) establishes a three-tier fair value hierarchy which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). These tiers include:
● Level 1, defined as observable inputs such as quoted prices for identical instruments in active markets.
● Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable such as quoted prices for similar instruments in active markets or quoted prices for identical or similar instruments in markets that are not active; and
● Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions, such as valuations derived from valuation techniques in which one or more significant inputs or significant value drivers are unobservable.
The carrying amounts reported in the balance sheet of cash and cash equivalents, prepaids, accounts payable and accrued expenses approximate fair value because of the immediate or short-term maturity of these financial instruments. The fair value of notes receivable approximates their carrying value as the stated or discounted rates of the notes do reflect recent market conditions. The Company’s investments are record at cost as the fair value of these investment in are not readily available. The fair value of notes payable approximates its carrying value as the stated interest rate reflects recent market conditions.
IMPACT Biomedical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Cash and cash equivalents
The Company considers all highly liquid investments with a maturity of three months or less at the date of acquisition to be cash equivalents. There were no cash equivalents as of December 31, 2022, and 2021.
Investments
Investments in equity securities with a readily determinable fair value, not accounted for under the equity method, are recorded at fair value with unrealized gains and losses included in earnings. For equity securities without a readily determinable fair value, the investment is recorded at cost, less any impairment, plus or minus adjustments related to observable transactions for the same or similar securities, with unrealized gains and losses included in earnings.
For equity method investments, the Company regularly reviews its investments to determine whether there is a decline in fair value below book value. If there is a decline that is other-than-temporary, the investment is written down to fair value. See Note 7 for further discussion on investments.
Goodwill
Goodwill is the excess of cost of an acquired entity over the fair value of amounts assigned to assets acquired and liabilities assumed in a business combination. Goodwill is subject to impairment testing at least annually and will be tested for impairment between annual tests, which takes place during the fourth quarter, if an event occurs or circumstances change that would indicate the carrying amount may be impaired. FASB ASC Topic 350 provides an entity with the option to first assess qualitative factors to determine whether the existence of events or circumstances leads to a determination that it is more likely than not that the fair value of a reporting unit is less than its carrying amount. Some of the qualitative factors considered in applying this test include consideration of macroeconomic conditions, industry and market conditions, cost factors affecting the business, and overall financial performance of the business. If, after completing the assessment, it is determined that it is more likely than not that the fair value of a reporting unit is less than its carrying value, the Company will proceed to a quantitative test. If qualitative factors are not deemed sufficient to conclude that the fair value of the reporting unit more likely than not exceeds its carrying value, then a one-step approach is applied in making an evaluation. The evaluation utilizes an income approach (discounted cash flow analysis). The computations require management to make significant estimates and assumptions, including, among other things, selection of comparable publicly traded companies, the discount rate applied to future earnings reflecting a weighted average cost of capital, and earnings growth assumptions. The Company believes the estimates and assumptions used in our impairment assessments are reasonable and based on available market information, but variations in any of the assumptions could result in materially different calculations of fair value and determinations of whether or not an impairment is indicated. A discounted cash flow analysis requires management to make various assumptions about future sales, operating margins, capital expenditures, working capital, and growth rates. Cash flow projections are derived from one year budgeted amounts plus an estimate of later period cash flows, all of which are determined by management. Subsequent period cash flows are developed for each reporting unit using growth rates that management believes are reasonably likely to occur. Impairment of goodwill is measured as the excess of the carrying amount of goodwill over the fair values of recognized and unrecognized assets and liabilities of the reporting unit. No impairment was recognized during the years ended December 31, 2022 or 2021 (see Note 8).
Intangible Assets
The estimated fair values of acquired intangibles are generally determined based upon future economic benefits such as earnings and cash flows. Acquired identifiable intangible assets are recorded at fair value and are amortized over their estimated useful lives. Acquired intangible assets with an indefinite life are not amortized but are reviewed for impairment at least annually as of December 31st, or more frequently whenever events or changes in circumstances indicate that the carrying amounts of those assets are below their estimated fair values. Impairment is tested under ASC 350. No impairment was recognized during the years ended December 31, 2022 or 2021.
Research and Development
Research and development costs are expensed as incurred. Total research and development costs were $1,226,000 for the year ended December 31, 2022, and $1,080,000 for year ended 2021.
IMPACT Biomedical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Recent Accounting Standards
The Financial Accounting Standards Board (FASB) issues various Accounting Standards Updates relating to the treatment and recording of certain accounting transactions. There are several new accounting pronouncements issued by FASB which are not yet effective. Each of these pronouncements, as applicable, has been or will be adopted by the Company. As of December 31, 2022, none of these pronouncements is expected to have a material effect on the financial position, results of operations or cash flows of the Company.
Continuing Operations and Going Concern
Due to incurred operating losses as well as negative cash flows from operating and investing activities over the past two years, the accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. This basis of accounting contemplates the recovery of our assets and the satisfaction of liabilities in the normal course of business. These consolidated financial statements do not include any adjustments to the specific amounts and classifications of assets and liabilities, which might be necessary should we be unable to continue as a going concern.
To continue as a going concern, the Company has entered into an updated revolving promissory note which extended the maturity through December 31, 2022, and DSS intends to continue to fund the operations of the Company through a year from the date these financial statements were available to be issued. The Company’s management intends to take actions necessary to continue as a going concern. Management’s plans concerning these matters include, among other things, monetization of its intellectual properties, and tightly controlling operating costs. Based on this, the Company has concluded that substantial doubt of its ability to continue as a going concern has been alleviated.
Note 3. Restatement
The Company has determined that a restatement of the December 31, 2022 consolidated financial statements is appropriate in order to correct certain errors in the reporting of interest expense on the related party note. In previously issued consolidated financial statements, there was no interest expense recorded. Interest expense has been corrected and restated from $0 to $462,000.
Note 4. Prepaid Expenses
Prepaid expenses for the year ended December 31, 2022, includes prepared inventory approximating $60,000 and research and development fees paid to GRDG Sciences, LLC. (“GRDG”), a related party, approximating $43,000. Prepaid expenses at December 31, 2021 consists primarily of research and development costs to GRDG approximating $43,000.
Note 5. Notes Receivable
On February 21, 2021, Impact BioMedical, Inc. entered into a promissory note (“Promissory Note 1”) with an individual. The Company loaned the principal sum of $206,000, with interest at a rate of 6.5%, and maturity date of August 19, 2022. Monthly payments are due on the twenty-first day of each month and continuing each month thereafter until August 19, 2022, at which time all accrued interest and the entire remaining principal shall be due and payable in full. In August 2022, this note was amended to extend the maturity date to August 19, 2023. This note is secured by certain real property situated in Collier County, Florida. The outstanding principal and interest approximated $206,000 and $197,000 as of December 31, 2022 and December 31, 2021, respectively. Of the $206,000 outstanding at December 31, 2022, $16,000 is classified in current notes receivable on the accompanying consolidated balance sheets, and the remaining $190,000 is classified as note receivable.
On May 14, 2021, DSS PureAir, Inc. (“DSS PureAir”), a wholly owned subsidiary of the Company, entered into a convertible promissory note (“Promissory Note 2”) with a limited liability company registered in the state of Texas (“Borrower”). The Promissory Note 2 has an aggregate principal balance up to $5,000,000, to be funded at the request of the borrower. Promissory Note 2, which incurs interest at a rate of 6.5% due quarterly, has a maturity date of May 14, 2023. Promissory Note 2 contains an options conversion clause that allows the Company to convert all, or a portion of all, into newly issued member units of the borrower with the maximum principal amount equal to 18% of the total equity position at conversion. On June 1, 2022, 100% of the Company’s interest in DSS PureAir was assigned to DSS BioHealth Holdings, Inc., a related party, and wholly owned subsidiary of DSS BioHealth Security, Inc. Included in this transaction was the outstanding principal and interest of Promissory Note 2 along with the related Note payable, related party. The outstanding principal and interest on December 31, 2022, and December 31, 2021 is $0 and $5,081,000, respectively.
Note 6. Investments
On December 19, 2020, Impact BioMedical entered into a subscription agreement (the “Subscription Agreement”) with BioMed Technologies Asia Pacific Holdings Limited (“BioMed”), a limited liability company incorporated in the British Virgin Islands, pursuant to which the Company agreed to purchase 525 ordinary shares or 4.99% of BioMed at a purchase price of approximately $632,000. The Subscription Agreement provides, among other things, the Company the right to appoint a new director to the board of BioMed. With respect to an issuance of shares to a third party by BioMed, the Company will have the right of first refusal to purchase such shares, as well as customary tag-along rights. In connection with the Subscription Agreement, the Company entered into an exclusive distribution agreement (the “Distribution Agreement”) with BioMed, to directly market, advertise, promote, distribute, and sell certain BioMed products, which focus on manufacturing natural probiotics, to resellers. This investment is valued at cost as it does not have a readily determined fair value.
BioMed focuses on manufacturing natural probiotics, pursuant to which the Company will directly market, advertise, promote, distribute and sell certain BioMed products to resellers. The products to be distributed by the Company include BioMed’s PGut Premium Probiotics®, PGut Allergy Probiotics®, PGut SupremeSlim Probiotics®, PGut Kids Probiotics®, and PGut Baby Probiotics®.
Effective January 1, 2021, the Company entered into a securities purchase agreement (“SPA”) with Nano9, LLC. (“Nano9”), a Utah limited partnership. For the consideration of $150,000 the Company obtained 1,000 membership units, or approximately 10% equitable ownership of Nano9. Nano9 is a scientifically driven company, specializing in the development and production of leading nano-sized health & wellness products utilizing their proprietary nano technology. As of December 31, 2022 and December 31, 2021, the fair value of the Company’s investment in Nano9 is not readily available, and therefore is recorded at cost of $150,000.
IMPACT Biomedical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
On March 15, 2021, the Company, through one of its subsidiaries, entered into a Stock Purchase Agreement (the “Vivacitas Agreement #1”) with Vivacitas Oncology Inc. (“Vivacitas”), to purchase 500,000 shares of its common stock at the per share price of $1.00, with an option to purchase 1,500,000 additional shares at the per share price of $1.00. This option will terminate upon one of the following events: (i) Vivacitas’ board of directors cancels this option because it is no longer in the best interest of the Company; (ii) December 31, 2022; or (iii) the date on which Vivacitas receives more than $1.00 per share of the Company’s common stock in a private placement with gross proceeds of $500,000. Under the terms of the Vivacitas Agreement #1, the Company will be allocated two seats on the board of Vivacitas. On March 18, 2021, the Company entered into an agreement with Alset EHome International, Inc. (“Seller”) to purchase from the Seller’s its wholly owned subsidiary Impact Oncology PTE Ltd. (“IOPL”) for a purchase price $2,480,000. The acquisition of IOPL has been treated as an asset acquisition as IOPL does not meet the definition of a business as defined in Topic 805. IOPL owns 2,480,000 shares of common stock of Vivacitas along with the option to purchase an additional 250,000 shares of common stock. The Sellers largest shareholder is Mr. Chan Heng Fai Ambrose, the Chairman of the Company’s board of directors and its largest shareholder.
On April 1, 2021, the Company entered into an additional stock purchase agreement with Vivacitas (“Vivacitas Agreement #2”), whereas Vivacities wished to employ the service of the Chief Business Officer of Impact Biomedical, and in return for the services of this individual, Vivacitas shall issue to the Company, the aggregate purchase price for the Class A Common Shares of Vivacitas at the value of $1.00 per share shall be $120,000 to be paid in twelve (12) equal monthly installments for the period between April 1, 2021 and March 31, 2022.
On July 22, 2021, the Company exercised 1,000,000 of the available options under the Vivacitas Agreement #1 for $1,000,000. This, along with the shares received as part Vivacitas Agreement #2 increased the Company’s equity position in Vivacitas to approximately 16% as of December 31, 2022. As of December 31, 2021, the fair value of the Company’s investment in Vivacitas is not readily available, and therefore is recorded at cost in the amount of $4,035,000. For the fiscal year December 31, 2022, the company was unable to obtain viable documentation in regards to Vivacitas’s ability to continue as a business and therefore as of December 31, 2022, the Company determined to impair 100% of its investment in Vivacitas, in the amount of $4,100,000.
Note 7. PROPERTY PLANT AND EQUIPMENT and INVESTMENT IN REAL ESTATE, NET
Property, plant and equipment consisted of the following as of December 31, 2022:
| | | Estimated | | | | | | |
| | | Useful Life | | 2022 | | | 2021 | |
| Machinery and equipment | | 5-10 years | | $ | 25,000 | | | $ | - | |
| Construction in progress | | | | | 251,000 | | | | - | |
| Total Cost | | | | | 276,000 | | | | - | |
| Less accumulated depreciation | | | | | - | | | | - | |
| Property, plant and equipment, net | | | | $ | 276,000 | | | $ | - | |
Depreciation expense for the years ended December 31, 2022 and 2021 was $- and $- respectively.
Note 8. Goodwill
Goodwill balances and activity for the year ended December 31, 2022 and year ended December 31, 2021 consisted of the following:
| Balance at December 31, 2021 | | $ | 25,093,000 | |
| Goodwill adjustment | | | - | |
| Balance at December 31, 2022 | | $ | 25,093,000 | |
During 2022 and 2021, management performed annual goodwill impairment testing. No goodwill impairment was identified as a result of these tests. During 2022 a quantitative analysis was prepared utilizing the market approach and income approach has the most world of method for valuing the Company. These approaches produce value indications through reference to either the recent historical financial performance or the projected future financial performance of the Company. During 2021, we used qualitative factors to determine whether it was more likely than not (likelihood of more than 50%) that the fair value of a reporting unit exceeded its carrying amount.
Note 9. Intangible Assets
The definite-lived intangible assets, to be amortized over 20 years, balances and activity for the years ended December 31, 2022, and 2021 consisted of the following:
| | | 12/31/2022 | | | | | | | | | 12/31/2021 | |
| | | Gross Carrying Amount | | | Accumulated Amortization | | | Net Carrying Amount | | | Gross Carrying Amount | | | Accumulated Amortization | | | Net Carrying Amount | |
| Definitive-lived: | | | | | | | | | | | | | | | | | | | | | | | | |
| Developed technology | | | 22,258,000 | | | $ | (2,226,000 | ) | | $ | 20,033,000 | | | $ | 22,260,000 | | | $ | (1,113,000 | ) | | $ | 21,147,000 | |
| Total | | | 22,258,000 | | | $ | (2,226,000 | ) | | $ | 20,033,000 | | | $ | 22,260,000 | | | $ | (1,113,000 | ) | | $ | 21,147,000 | |
The following table represents future amortization of developed technologies:
| 2022 | | $ | 1,113,000 | |
| 2023 | | $ | 1,113,000 | |
| 2024 | | $ | 1,113,000 | |
| 2025 | | $ | 1,113,000 | |
| 2026 | | $ | 1,113,000 | |
| Thereafter | | $ | 14,468,000 | |
IMPACT Biomedical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
In March 2021, the fair value of the assets acquired by DSS on August 21, 2020, was concluded. An impairment analysis was conducted for financial reporting purposes in connection with U.S. GAAP and ASC 360-10, Impairment and Disposal of Long-Lived Assets. Although the Company has a history of negative cash flows from operations, its financing arrangement with DSS (see Note 9) provides the opportunity to fund ongoing operations. This, as well as there being no other indicators present for impairment, has led management to conclude that no impairment is necessary for the years ended December 31, 2022, and 2021.
Note 10. Debt
On December 31, 2020, and later amended on December 31, 2022, the Company executed a Revolving Promissory Note (“Note”) with DSS, a related party, which accrues interest at a rate of 4.25% and is due in full at the maturity date of December 31, 2023. The revolving nature of this Note permits for principal amounts borrowed to be repaid and reborrowed. In the case of default, at DSS’s option, (i) eighteen percent (18%) per annum, or (ii) such lesser rate of interest as Lender in its sole discretion may choose to charge; but never more than the Maximum Lawful Rate. DSS has not established a maximum borrowing amount and intends to continue to fund the operations of the Company through a year from the date these financial statements were available to be issued. As of December 31, 2022 and 2021, this Note had an outstanding balance of $9,991,000 and $12,524,000, respectively.
Note 11. Stockholders’ Equity
On September 23, 2021, the Company, the Company’s Board of Directors approved the total number of shares of Common Stock to be 3,000,000,000 shares with a par value of $0.001. Each share of Common Stock when issued, shall have one (1) vote on all matters presented to the stockholders.
Our Amended and Restated Articles of Incorporation authorizes us to issue up to 4,000,000,000 shares of Common Stock, $0.001 par value per share, and 100,000,000 shares of preferred stock, par value $0.001 per share. On May 11, 2023, the Company effected a forward split. As a result, there were 3,877,282,251 shares of our Common Stock and no shares of preferred stock issued and outstanding. As of December 31, 2022 and 2021, there were 125,073,621 shares of our Common Stock and outstanding which was converted to 3,877,282,251 shares. As of December 31, 2022 and 2021, there were no preferred shares issued.
Note 12. Related Party Transactions
Research and Development Activities
Based on Shareholders Agreement entered into on April 26, 2017, the Company would fund the scientific operations of GRDG, a company involved in research and development of biomedical products which is a minority stockholder of two of the Company’s subsidiaries and is owned by Daryl Thompson, a director of many subsidiaries of the Company, to do the development and research works on the biomedical products for the Company. As of December 31, 2022, this funding approximates $43,000 per month, and incurred approximately $546,000 in expenses. On December 31, 2022 and December 31, 2021, the Company owed this related party $0 and had prepaid monthly fees approximating $43,000 and $43,000, respectively.
General and Administrative Costs
There are certain general and administrative costs incurred by DSS, a related party on behalf of the Company which are passed through to the Company on a monthly basis. These costs consist of primarily payroll costs for certain DSS employees and are allocated based on estimated time spent on behalf of the Company. These costs approximate $12,000 per month. As of December 31, 2022, the Company incurred $117,000 in related expenses. As of December 31, 2021, the Company incurred approximately $155,000 in related expenses.
IMPACT Biomedical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
Note 13. Commitments and Contingencies
On August 15, 2018, the Company entered into Royalty Agreement with Chemia Corporation (“Chemia”) pursuant to which Chemia transferred to the Company all of its right to 3F (Functional Fragrance Formulation). This agreement has a 20-year term and auto renews for a period of 1 year unless mutually agreed upon by both parties. 3F consists of 3F Mosquito Repellant and 3F Anti-Viral formulations. Based on the Royalty Agreement, the Company should cover all the costs to prepare and finalize necessary patent application and other intellectual property related to 3F. Chemia agreed to support the Company in efforts leading to development of 3F intellectual property and it’s licensing. Based on Royalty Agreement any payments received from development, sales, licensing or transfer of 3F technology will be paid 50% to the Company and 50% to Chemia. On November 27, 2018, Company and Chemia signed an Addendum to Royalty Agreement (“Addendum”), according to which the Company granted Chemia a royalty-based limited license for purposes of making and selling fragrances embodying the 3F technology. Based on the Addendum, Chemia should pay the Company 5% of net sales in royalty. On November 8, 2019, both companies entered into Amendment no.1 to Royalty Agreement, based on which certain expenses bore by the Company towards patent application and licensing should be reimbursed to the Company before any royalty payments are made. For the nine months ended December 31, 2022 and 2021, there were no reimbursements or royalties paid to the Company and the Company cannot be assured that Chemia’s efforts will end up in any future sales of the technology.
On February 15, 2022, the Company and its subsidiaries, Global BioLife, Inc. (“Global”), and Impact BioLife Sciences, Inc. (“BioLife Sciences”), and GRDG entered into a Licensing Proceeds Distribution Agreement (“GRDG Agreement”), whereas GRDG would transfer its 20% equity position in both Global and BioLife Sciences to the Company in exchange for 20% interest in Global and/or BioLife Science revenue received from the exclusive or non-exclusive licensing of and/or the sale of Global Intellectual Property to a Third Party, net of specific costs. As of the date of this report, no contingent liability has been recognized under the GRDG Agreement.
On March 19, 2022, Impact BioMedical entered into a License Agreement (“Equivir License”) with ProPhase Labs, Inc. (“ProPhase”) where the ProPhase is granted the right, amongst other things, to develop, commercialize, and sell the Company’s Equivir technology. In exchange, the Licensee shall pay the Company a royalty of 5.5% of net sales. Under the terms of the Equivir Agreement, the Company shall reimburse ProPhase for 50% of the development costs provided that the development costs shall not exceed $1,250,000. As of December 31, 2022, no liability has been recorded in relation to the Equivir License as development of the Equivir technology has not begun and no reasonable amount can be estimated.
Note 14. Income Taxes
The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the financial reporting and tax basis of assets and liabilities. Deferred tax assets are reduced, if deemed necessary, by a valuation allowance for the amount of tax benefits which are not expected to be realized.
IMPACT Biomedical, Inc. and Subsidiaries
Notes to Consolidated Financial Statements
The components of income tax benefit for the years ended December 31, 2022, and 2021 are as follows:
| Income Tax Expense (Benefit) | | Year Ended
December 31, 2022 | | | Year Ended December 31, 2021 | |
| Current tax payable | | | | | | | | |
| Federal | | $ | - | | | $ | - | |
| State | | | - | | | | - | |
| Total current tax payable | | | - | | | | - | |
| Deferred tax | | | | | | | | |
| Federal | | | (1,619,000 | ) | | | (456,000 | ) |
| State | | | (165,000 | ) | | | (1,068,000 | ) |
| Total deferred tax | | $ | (1,784,000 | ) | | $ | (1,524,000 | ) |
| Less increase in valuation allowance | | | 1,163,000 | | | | 129,000 | |
| Total income tax benefit | | $ | (621,000 | ) | | $ | (1,395,000 | ) |
Individual components of deferred tax assets and liabilities are approximately as follows:
| Deferred Tax Assets & Liabilities: | | | | | | |
| Deferred Tax assets: | | | | | | | | |
| Impairment of investment | | $ | 929,000 | | | $ | - | |
| Research & development cost | | | 250,000 | | | | - | |
| Net Operating loss | | | 1,611,000 | | | | 1,258,000 | |
| Gross deferred tax assets | | | 2,790,000 | | | | 1,258,000 | |
| | | | | | | | | |
| Deferred tax liability: | | | | | | | | |
| Intangible assets | | | (4,414,000 | ) | | | (4,667,000 | ) |
| Gross deferred tax liability | | | (4,414,000 | ) | | | (4,667,000 | ) |
| | | | | | | | | |
| Less valuation allowance | | | (1,611,000 | ) | | | (447,000 | ) |
| Net deferred tax liability | | $ | (3,235,000 | ) | | $ | (3,856,000 | ) |
| | | 2022 | | | 2021 | |
| Statutory United States federal rate | | | 21.0 | % | | | 21.0 | % |
| State income taxes net of federal benefit | | | 1.7 | % | | | 26.0 | % |
| Change in valuation allowance | | | (14.8 | )% | | | (4.0 | )% |
| | | | | | | | | |
| Effective rate | | | 7.9 | % | | | 43 | % |
As of December 31, 2022, and 2021, the Company has net operating loss carry forwards of approximately $7,109,000 and $5,551,000 respectively. The Company does not have other temporary differences associated with the amortization of intangible assets. As of December 31, 2022, and 2021, the total deferred tax assets carry-forward were $2,790,000 and $1,258,000, respectively. The deferred tax assets could be carried forward indefinitely. The full utilization of the deferred tax assets in the future is dependent upon the Company’s ability to generate taxable income. Considering the development stage of the Company, management believed that it was probable that the Company would not use tax assets in the near future. Accordingly, a valuation allowance of an equal amount has been established. During the years ended December 31, 2022 and December 31, 2021, the valuation allowance increased by $1,163,000 and decreased by $129,000, respectively.
The Company recognizes interest accrued and penalties related to unrecognized tax benefits in tax expense. During the years ended December 31, 2022 and 2021 the Company recognized no interest and penalties.
Note 15. Supplemental Cash Flow Information
The following table summarizes supplemental cash flows for the year ended December 31, 2022, and 2021:
| | | 2022 | | | 2021 | |
| | | | | | | |
| Transfer of promissory note 2 to related party | | $ | 5,081,000 | | | $ | - | |
| Transfer of note payable, related party | | $ | (5,081,000 | ) | | $ | - | |
Note 16. Subsequent Events
The Company has evaluated all subsequent events and transactions through June 2, 2023, the date that the consolidated financial statements were available to be issued, and noted no subsequent events requiring financial statement recognition or disclosure.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 11. | Change in Auditors |
On June 29, 2022, the Company’s board of directors approved replacing Turner Stone as our independent registered public accounting firm, with Grassi & Co. CPAs, P.C. (the “New Accountant”) as our independent registered public accounting firm, effective July 1, 2022.
For the year ended December 31, 2021, and through the interim period ended September 30, 2022, there were no “disagreements” (as such term is defined in Item 304 of Regulation S-K) with Turner Stone on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedures, which disagreements, if not resolved to the satisfaction of the Turner Stone, would have caused them to make reference thereto in their reports on the financial statements for such periods.
Turner Stone’s audit report on our financial statements for the year ended December 31, 2021 contained no adverse opinion or disclaimer of opinion, nor was it qualified or modified as to uncertainty, audit scope or accounting principles.
We authorized the former accountants to respond fully and without limitation to all requests of the New Accountant concerning all matters related to the audited periods by the former accountants, including with respect to the subject matter of each reportable event.
Prior to retaining the New Accountant, the Company did not consult with the New Accountant regarding either: (i) the application of accounting principles to a specified transaction, either contemplated or proposed, or the type of audit opinion that might be rendered on the Company’s financial statements; or (ii) any matter that was the subject of a “disagreement” or a “reportable event” (as those terms are defined in Item 304 of Regulation S-K).
| Item 13. | Other Expenses of Issuance and Distribution. |
The following table sets forth an itemization of all estimated expenses in connection with the issuance and distribution of the securities to be registered. All of the fees set forth below are estimates except for the SEC registration fee and transfer and distribution agent fees and expenses:
| Item | | Amount | |
| Registration Statement filing fee | | $ | 10.05 | |
| Accountants fees and expenses | | $ | 15,000 | |
| Legal fees and expenses | | $ | 75,000 | |
| Printing | | $ | 5,000 | |
| Transfer and distribution agent fees and expenses | | $ | 5,000 | |
| Miscellaneous | | $ | - | |
| Total | | $ | 100,010.05 | |
| Item 14. | Indemnification of Directors and Officers. |
Under the provisions of the Amended and Restated Articles of Incorporation of the Company, as amended, as of the date of this Registration Statement, the liability of directors and officers of the Company shall be eliminated or limited to the fullest extent permitted by the Nevada Revised Statutes (“NRS”). If the NRS is amended to further eliminate or limit or authorize corporate action to further eliminate or limit the liability of directors or officers, the liability of directors and officers of the Corporation shall be eliminated or limited to the fullest extent permitted by the NRS, as so amended from time to time.
| Item 15. | Recent Sales of Unregistered Securities. |
None.
| Item 16. | Exhibits and Financial Statement Schedules. |
See the Exhibit Index immediately preceding the signature page hereto, which is incorporated by reference as if fully set forth herein.
| (b) | Financial Statement Schedules |
No financial statement schedules are provided because the information called for is not required or is shown either in our consolidated financial statements or notes thereto.
| | (a) | (1) The undersigned registrant hereby undertakes to file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: (i) To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933; (ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and (iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| | | |
| | (2) | The undersigned registrant hereby undertakes that, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| | | |
| | (3) | The undersigned registrant hereby undertakes to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| | (4) | The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| | (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§ 230.424 of this chapter); |
| | | |
| | (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| | | |
| | (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| | | |
| | (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| | (b) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue. |
| | | |
| | (c) | The undersigned registrant hereby undertakes that: |
| | (1) | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| | | |
| | (2) | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
EXHIBIT INDEX
Exhibit
Number | | Exhibit Description |
| 3.1** | | Amended and Restated Articles of Incorporation of Impact BioMedical Inc. dated July 29, 2020 |
| | | |
| 3.2** | | Certificate of Amendment to the Amended and Restated Articles of Incorporation of Impact BioMedical Inc. |
| | | |
| 3.3** | | Certificate of Amendment to the Amended and Restated Articles of Incorporation of Impact BioMedical Inc. |
| | | |
| 3.4 | | Certificate of Amendment to the Amended and Restated Articles of Incorporation of Impact BioMedical Inc. |
| | | |
| 5.1 | | Opinion of Sichenzia Ross Ference LLP |
| | | |
| 10.1** | | Share Exchange Agreement dated as of April 27, 2020, among Document Security Systems, Inc., DSS BioHealth Security, Inc., Singapore eDevelopment Limited and Global BioMedical Pte Ltd. |
| | | |
| 10.2** | | Subscription Agreement dated December 19, 2020, between the Company and BioMed Technologies Asia Pacific Holdings Limited |
| | | |
| 10.3** | | Promissory Note with Dustin Michael Crum dated February 21, 2021 |
| | | |
| 10.4** | | Stock Purchase Agreement dated March 15, 2021 between the Company and Vivacitas Oncology Inc. |
| | | |
| 10.5** | | Convertible Promissory Note dated May 14, 2021 |
| | | |
| 10.6** | | Revolving Promissory Note dated December 31, 2020 |
| | | |
| 10.7** | | Royalty Agreement by and between Global BioLife Inc. and Chemia Corporation, dated August 15, 2018 |
| | | |
| 10.8** | | Addendum to Royalty Agreement by and between Global BioLife Inc. and Chemia Corporation, dated November 27, 2018 |
| | | |
| 10.9** | | Distribution Agreement by and between BioMed Technologies Asia Pacific Holdings Limited and Impact BioMedical Inc., dated December 9, 2020 |
| | | |
| 10.10** | | Global BioLife, Inc. Stockholders’ Agreement among Global BioLife, Inc., Global BioMedical, Inc., Holista Colltech Limited, and GRDG Sciences, LLC, dated April 26, 2017 |
| | | |
| 10.11** | | Amendment No. 1 to Global BioLife, Inc. Stockholders’ Agreement among Global BioLife, Inc., Global BioMedical, Inc., Holista Colltech Limited, and GRDG Sciences, LLC, dated May 22, 2018 |
| | | |
| 10.12** | | Amendment No. 2 to Global BioLife, Inc. Stockholders’ Agreement among Global BioLife, Inc., Global BioMedical, Inc., Holista Colltech Limited, and GRDG Sciences, LLC, dated August 2020 |
| | | |
| 10.13** | | Impact BioLife Science, Inc. Stockholders Agreement among Impact BioLife Science, Inc., Impact BioMedical Inc. and GRDG Sciences, LLC, dated December 11, 2020 |
| | | |
| 10.14** | | Licensing Proceeds Distribution Agreement with GRDG Sciences, LLC dated May 16, 2022 |
| | | |
| 10.15** | | Amendment No. 1 to Revolving Promissory Note dated December 31, 2021. |
| | | |
| 10.16** | | Amendment No. 2 to Revolving Promissory Note dated March 31, 2022. |
| | | |
| 10.17** | | License Agreement with ProPhase Labs, Inc. dated March 17, 2022. |
| | | |
| 10.18** | | License Agreement with ProPhase Labs, Inc. dated July 18, 2022. |
| | | |
| 10.19** | | Licensing Proceeds Distribution Agreement with GRDG Sciences, LLC dated February 15, 2022. |
| | | |
| 16.1** | | Letter from Freed Maxick CPAs, P.C. |
| | | |
| 16.2 | | Letter from Turner Stone & Company LLP |
| | | |
| 21.1** | | List of subsidiaries of Impact BioMedical Inc. |
| | | |
| 23.1 | | Consent of Turner, Stone & Company L.L.P. |
| | | |
| 23.2 | | Consent of Grassi & Co., CPAs, P.C. |
| | | |
| 23.3 | | Consent of Sichenzia Ross Ference LLP (included as part of Exhibit 5.1). |
| | | |
| 107** | | Filing Fee Table |
| | * | To be filed by amendment |
| | ** | Previously filed |
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the city of West Henrietta, on the 2nd day of June, 2023.
| | IMPACT BIOMEDICAL INC. |
| | | |
| | By: | /s/ Frank D. Heuszel |
| | Name: | Frank D. Heuszel |
| | Title: | President |
| Dated: June 2, 2023 | | |
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed below by the following persons in the capacities indicated.
| Signature | | Title | | Date |
| | | | | |
| /s/ Frank D. Heuszel | | | | |
| Frank D. Heuszel | | Chief Executive Officer (Principal Executive Officer), President and Director | | June 2, 2023 |
| | | | | |
| /s/ * | | | | |
| Heng Fai Ambrose Chan | | Chairman | | June 2, 2023 |
| | | | | |
| /s/ * | | | | |
| Todd D. Macko | | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer | | June 2, 2023 |
| | | | | |
| /s/ * | | | | |
TJ Leonardo | | Chief Operating Officer and Secretary | | June 2, 2023 |
| | | | | |
| /s/ * | | | | |
| Dr. Elise Brownell | | Director | | June 2, 2023 |
| | | | | |
| /s/ * | | | | |
| Melissa Sims | | Director | | June 2, 2023 |
| * By: | /s/ Frank D. Heuszel | |
| | Frank D. Heuszel | |
| | Attorney-in-fact | |