Filed by Moringa Acquisition Corp pursuant
to Rule 425 under the Securities Act of 1933,
and deemed filed pursuant to Rule 14a-12
under the Securities Exchange Act of 1934
Subject Company: Biomotion Sciences
Commission File: 333-279281
Date: July 25, 2024

Sile ncing Oncogenes at the Level of Gene Expression Corporate Presentation July 2024 Filed by Moringa Acquisition Corp pursuant to Rule 425 under the Securities Act of 1933 , and deemed filed pursuant to Rule 14 a - 12 under the Securities Exchange Act of 1934 Subject Company: Biomotion Sciences Commission File : 333 - 279281 Date: July 25 , 2024

DISCLAIMER • ABOUT THIS PRESENTATION • This investor presentation (this “Presentation”) is for informational purposes only to assist interested parties in making th eir own evaluation with respect to the proposed business combination (the “Business Combination”) between Moringa Acquisition Cor p (“Moringa”) and Silexion Therapeutics Ltd. (together with its direct and indirect subsidiaries, collectively,“Silexion ”). The information contained herein does not purport to be all - inclusive or necessarily contain all the information that a pros pective investor may desire in investigating a prospective investment in the securities of Biomotion Sciences (“ PubCo ”), and none of Moringa, Silexion, PubCo or their respective representatives or affiliates makes any representation or warranty, express or implied, as to the accurac y, completeness or reliability of the information contained in this Presentation (and any other information, whether written or or al, that has been or will be provided to you). The information contained herein is preliminary and is subject to update, completion, revision, verification and amendment withou t n otice, and such changes may be material. The attached material is provided to you on the understanding that as a sophisticate d i nvestor, you will understand and accept its inherent limitation, will not rely on it in making any investment decision with respect to any securities that may be issued, and will use it only fo r purposes of discussing with your advisors your preliminary interest in investing in PubCo in connection with the proposed Business Combination. No statement contained herein should be considered binding on any party. Completion of the proposed Business Combination is subject to, among other matters, approval by Moringa ’s and Silexion’s shareholders and the satisfaction of the closing conditions to be set forth in the business combination agreem ent (“BCA”). No assurances can be given that the proposed Business Combination will be consummated on the terms or in the timeframe currently contemplated, if at all. • NO OFFER OR SOLICITATION • This Presentation does not constitute ( i ) a solicitation of a proxy, consent or authorization with respect to any securities or in respect of the proposed Business C omb ination or (ii) an offer to sell, a solicitation of an offer to buy or a recommendation to purchase any security of Moringa, Sil exion or PubCo or any of their respective affiliates. Any offer to sell securities will be made only pursuant to a definitive subscription or similar agreem ent and will be made in reliance on an exemption from registration under the Securities Act of 1933, as amended, for offers and s al es of securities that do not involve a public offering. You should not construe the contents of this Presentation as legal, tax, accounting or investment advice or a recommendation. You should con sul t your own counsel and tax and financial advisors as to legal and related matters concerning the matters described herein and , b y accepting this Presentation, you confirm that you are not relying upon the information contained herein to make any decision. No representations or warranties, express or implied are giv en in, or in respect of, this Presentation. No securities commission or securities regulatory authority in the United States or any other jurisdiction has in any way passed upon the merits of the proposed Business Combination or the accuracy or adequacy of this Presentation. The distribution of this Presentation may als o b e restricted by law and persons into whose possession this Presentation comes should inform themselves of and observe any suc h r estrictions. The recipient acknowledges that it is ( i ) aware that the United States securities law prohibits any person who has material, non - public information concerning a company from pu rchasing or selling securities of such company or from communicating such information to any other person under circumstances in which it is reasonably foreseeable that such person is likely to purchase or sell such securities, and (ii) familiar with the Securities Exchange Act of 1934, as amended, and the rules an d r egulation promulgated there under (collectively, the “Exchange Act”), and that the recipient will neither use, not cause any thi rd party to use, this Presentation or any information contained herein in contravention of the Exchange Act, including, without limitation, Rule 10b - 5 thereunder. • FORWARD - LOOKING STATEMENTS • The statements contained in this Presentation that are not purely historical are forward - looking statements.. The information in cluded in this Presentation in relation to Silexion has been provided by Silexion and its management team, and forward - looking s tatements include statements relating to the expectations of Silexion’s management team, hopes, beliefs, intentions or strategies regarding the future. In addition, any statements that r efe r to projections, forecasts or other characterizations of future events or circumstances, including any underlying assumption s, are forward - looking statements. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intends,” “may,” “might,” “plan,” “possible,” “potential,” “predict,” “project,” “sh ould,” “would” and similar expressions may identify forward - looking statements, but the absence of these words does not mean tha t a statement is not forward - looking. Forward - looking statements in this proxy statement may include, for example, statements about Moringa’s ability to complete the Business Combination, or , i f it does not consummate the Business Combination, any other initial business combination; the benefits of the Business Combi nat ion; the future performance of Silexion following the Business Combination, including Silexion’s projected timeline for regulatory submissions and approvals; and Silexion’s future plans an d o pportunities. The forward - looking statements contained in this Presentation are based on our current expectations and beliefs co ncerning future developments and their potential effects on us. There can be no assurance that future developments affecting Silexion will be those that it has anticipated. These forward - looki ng statements involve a number of risks, uncertainties (some of which are beyond the control of Silexion) or other assumption s t hat may cause actual results or performance to be materially different from those expressed or implied by these forward - looking statements. These risks and uncertainties include, but are no t limited to, the items in the following list, which summarize some of the principal risks relating to the Business Combinati on and Moringa’s and Silexion’s businesses: ( i ) the conditions to the closing of the Business Combination may not be fulfilled, or may be waived, (ii) the occurrence of any event, change or other ci rcumstances that could give rise to the termination of the BCA, (iii) Moringa may not succeed at having the Moringa ordinary sha res and Moringa warrants remain listed on Nasdaq until completion of the Business Combination, (iv) the ability to fulfill the Nasdaq listing conditions and/or maintain the listing of Pubco ordinary shares on the Nasdaq following the Business Combination, (v) Silexion is a development - stage company and has a limited operating history on which to assess its business; (vi) Silexion has never generated any revenue from product sales and may never be profitable, (vii) Pubco will need to raise substantial additional funding, which may not be available on acceptable terms, or at all, and which will ca use dilution to Pubco’s shareholders; (viii) the approach Silexion is taking to discover and develop novel RNAi therapeutics is unproven for oncology and may never lead to marketable products; (ix) Silexion does not have experience produ cin g its product candidates at commercial levels, currently has no marketing and sales organization, has an uncertain market rec ept iveness to its product candidates, and is uncertain as to whether there will be insurance coverage and reimbursement for its potential products; (x) Silexion may be unable to attract , d evelop and/or retain its key personnel or additional employees required for its development and future success; (xi) Moringa’ s s ponsor and other affiliates have potential conflicts of interest in recommending to vote in favor of the Business Combination, including economic incentives to complete a business combination, eve n if the target business is less favorable to Moringa’s public shareholders; (xii) Moringa’s shareholders who do not redeem the ir Moringa Class A ordinary shares will have a reduced, minority voting interest after the Business Combination and will exercise less influence over management; (xiii) Moringa does not have a specified maximum redemption limit, which will make it easier for Moringa to consummate the Business Combination even if a su bst antial majority of Moringa’s public shareholders do not support it; (xiv) The SPAC merger may be a taxable transaction for U.S. federal income tax purposes to U.S. Holders of Moring a C lass A ordinary shares and Moringa public warrants; (xv) Silexion may issue additional Pubco ordinary shares or other equity securities without your approval, including shares underlying warrants and note shares, which would dilute your ownership interest and may depress the market price of the Pubco ordinary shares; (xvi) additional factors relating to the business, operations and financial performance of Silexion; and (xv ii ) other factors detailed under the section entitled “Risk Factors” herein. Should one or more of these risks or uncertainties materialize, or should any of Moringa or Silexion’s assumptions prove incorrect, actual res ults may vary in material respects from those projected in these forward - looking statements. Moringa, Silexion and Pubco undertake no obligation to update or revise any forward - looking statements, whether as a result of new information, future events or otherwise, except as may be required under applicable se cur ities laws. Before a shareholder grants its proxy or instructs how its votes should be cast or vote on the proposals set fort h i n this Presentation, it should be aware that the occurrence of the events described in the “Risk Factors” section and elsewhere in the proxy statement/prospectus may adversely affect Moringa, Sil exion or Pubco . • IMPORTANT ADDITIONAL INFORMATION • In connection with the proposed Business Combination, PubCo filed a registration statement on Form S - 4 (Reg No. 333 - 279281) with the SEC that was declared effective on July 16, 2024 and i ncluded a proxy statement of Moringa and constituted a prospectus of PubCo . The proxy statement/prospectus contains important information about the proposed Business Combination and related matters. This Presentation does not contain all the informati on that should be considered concerning the proposed Business Combination and in not intended to form the basis of any investmen t d ecision or any other decision in respect of the proposed Business Combination. Moringa stockholders and other interested persons are advised to read the proxy statement/prospectus an d o ther documents filed in connection with the proposed Business Combination because these materials will contain important info rma tion about the parties to the business combination agreement, Pubco , Silexion, Moringa and the proposed Business Combination. Stockholders are able to obtain copies of the proxy statement/pros pec tus, without charge, once available, at the SEC’s website at www.sec.gov . • PARTICIPANTS IN SOLICITATION • Moringa, Silexion and Pubco and certain of their respective directors, executive officers, other members of management and employees, may, under SEC rule s, be deemed to be participants in the solicitation of proxies from the shareholders of Moringa in favor of the approval of the Bu siness Combinaregtion . Shareholders of Moringa and other interested persons may obtain more information regarding the names and interests in the Business Combina tio n of Moringa’s directors and officers in Moringa’s filings with the SEC. Additional information regarding the interests of th e p ersons who may be deemed participants in the solicitation of proxies from Moringa shareholders is available in the proxy statement/prospectus filed by PubCo , available on the SEC’s website at www.sec.gov . • INDUSTRY AND MARKET DATA • This Presentation has been prepared by Moringa and Silexion and includes market data and other statistical information from t hir d - party sources, including independent industry publications, governmental publications and other published independent sources . Some data is also based on the estimates of Silexion, which are derived from their review of internal sources as well as third - party sources described above. None of Moringa, Silexion or a ny of their respective representatives or affiliates has independently verified the information and cannot guarantee its accu rac y and completeness. • TRADEMARKS AND TRADE NAMES • This Presentation contains trademarks, service marks, trade names and copyrights of other companies, which are the property o f t heir respective owners. Solely for convenience, trademarks and trade names referred to in this Presentation may appear withou t t he ® or symbols, but such references are not intended to indicate, in any way, that the applicable licensor will not assert, to the fullest extent under applicable law, its rights to th ese trademarks and trade names. The use or display of third parties’ trademarks, service marks, trade manes or products in th is Presentation is not intended to, and does not imply, a relationship with Moringa or Silexion, or an endorsement or sponsorship by or of Moringa or Silexion. 2

Company Overview Silexion ’s siRNA platform technology is designed to silence oncogenes and prevent the production of the mutated KRAS proteins that drive cancer growth Loder siRNA with an extended - release PLGA delivery system • Completed Phase 2 trial • Results observed a 9.3 - month improvement in overall survival with Loder + chemo vs. chemo alone (SOC) in patients with KRAS G 12 D/V mutation Lead candidate SIL - 204 optimized upon Loder to enter Phase 2 / 3 trial Merger with Moringa Acquisition Corp, a special purpose acquisition company announced and expected to be completed in August 2024 To be listed on Nasdaq ( “ SLXN ” ) Clinical - stage company developing proprietary treatments for KRAS - driven cancers KRAS - Focused RNA Interference Platform with Targeted Delivery Promising Clinical Data in Locally Advanced Pancreatic Cancer Business Combination

Focused Pipeline to Address KRAS - driven Solid Tumor Localized Cancers 4 CTA=clinical trial application; SOC=standard of care. Status/ Anticipated Milestone Phase 3 Phase 2 Phase 1 Preclinical Discovery Setting Indication Program Phase 2 completed: observed 9.3 months improvement with LODER over SOC in KRAS G12D/V mutation patients. Continue development of SIL - 204. Combination with chemotherapy Locally advanced pancreatic cancer LODER siG12D/V + KRAS amplify with extended release PLGA delivery system 2025 : initiate toxicology studies Late 2025 : submission to initiate Phase 2 / 3 Combination with chemotherapy Locally advanced pancreatic cancer SIL - 204 (Intratumor) KRAS G12D/V + KRAS amplify formulation and extended - release delivery Phase 2 Completed O ptimized siRNA formulation and extended - release delivery

KRAS Oncogene is a Validated Target for Numerous Cancers 5 Prevalence of The Most Common Types of KRAS Mutations Across Cancers CRC=colorectal cancer; LAPC=locally advanced pancreatic cancer; NSCLC=non - small cell lung cancer. Lee, J.K. et al. NPJ Precis Oncol. 2022;6(1):91. PDAC CRC Non - sq NSCLC Small Bowel Adenocarcinoma Appendix KRAS is the most common oncogenic gene driver in human cancers with gastrointestinal cancers having high percentages of KRAS G12D/V mutations % KRAS mutations 92% 49% 35% 53% 61 %

Pancreatic Cancer Has One of the Highest Mortality Rates of All Major Cancers BRPC=borderline resectable pancreatic cancer; LAPC = locally advanced pancreatic cancer. 1. Bray F, et al. CA Cancer J Clin . 2024;74(3):229 - 263. 2. Hirshberg Foundation for Pancreatic Cancer Research. Pancreatic cancer Facts. https:// pancreatic.org /pancreatic - cancer/pancreatic - cancer - facts. 3. National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. https:// seer.cancer.gov / statfacts /html/ pancreas.html . 4. Gemenetzis G, et al. Ann Surg . 2019;270(2):340 - 347. 5. Kleeff J, et al. Nat Rev Dis Primers . 2016;2:16022. Local Metastatic At diagnosis: 10 - 20% resectable 30 - 40% LAPC + BRPC 50 - 60 % metastatic or systemic Types and Prevalence of Pancreatic Cancer 4,5 6 There are no effective treatment options for our intended indication LAPC 3 rd leading cause today in the U.S. 2 2 nd leading cause by 2030 2 12.8% 5 - year relative survival (2014 - 2020) is one of the poorest in the U.S. 3 Median overall survival for non - resectable PC populations is 14 - 17 months 4

Treating KRAS the Cancer - Driver at the Source and Site of Action Silexion siRNA technology prevents mutated KRAS from being produced while small molecule inhibitors target the functioning KRAS protein Silencing oncogene at the production stage is potentially more efficient and safe approach to treat cancer and overcome treatment - resistance Mutated KRAS gene Mutated KRAS mRNA Functional mutated KRAS protein Transcription Translation Silexion siRNA KRAS small molecule inhibitors ( Systemic delivery) Intratumor delivery Cell proliferation Migration Transformation Survival 7

Intratumor siRNA Administration is Advantageous to Systemic Delivery in Solid Tumors Especially Pancreatic x May overcome the limitations of systemic delivery, and allow obtaining higher intratumor concentrations, and better exposure throughout the primary tumor x Can potentially lead to lower systemic exposure due to limited escape from tumor after intratumor administration x EUS endoscopy provides access to different tumor locations within the affected organ EUS = endoscopic ultrasound; LAPC = locally advanced pancreatic cancer. Solid tumors, such as LAPC, are characterized by excessive fibrous tissue and low blood supply which limit the ability of systemically - administered drug to: 1) Reach to the tumor and within the tumor microenvironment 2) Obtain a sufficient concentration in the tumor for optimum activity Systemic exposure may lead to increased adverse events Limitations of Systemic Delivery Advantages of Intratumor Delivery

G12D G12V G12C G12A G12R Multiple Other KRAS Demographics KRAS mutations are present in ~92% pancreatic cancer cases 1 LAPC = localized advanced pancreatic cancer; ROW=rest of the world.*Number of KRAS G12D/V mutated LAPC were calculated based on KRAS mutations being present in 92% of pancreatic cancer patients, 70 - 75% with KRAS G12D and G12V mutations and 30 - 35% of cases being LAPC. 1. Lee, J.K. et al. NPJ Precis Oncol. 2022;6( 1):91. 2. Yousef, A. et al. NPJ Precis Oncol . 20024;8(1):27. 3. Global Cancer Observatory. Pancreatic Cancer. 2022. https://gco.iarc.who.int/media/globocan/factsheets/cancers/13 - pancreas - fact - sheet.pdf. 4. National Cancer Institute. Cancer Stat Facts: Pancreatic Cancer. 2023. https:// seer.cancer.gov / statfacts /html/ pancreas.html . SIL - 204 covers > 74% of KRAS mutations in PDAC 2 KRAS G12C treatment are treating ~1.5% E.U. U.S. 146,477 3 66,400 4 Annual PC cases ~35,000 ~16,000 KRAS - G12D/V mutated LAPC incidence* 9

LODER Phase 2 Trial Data
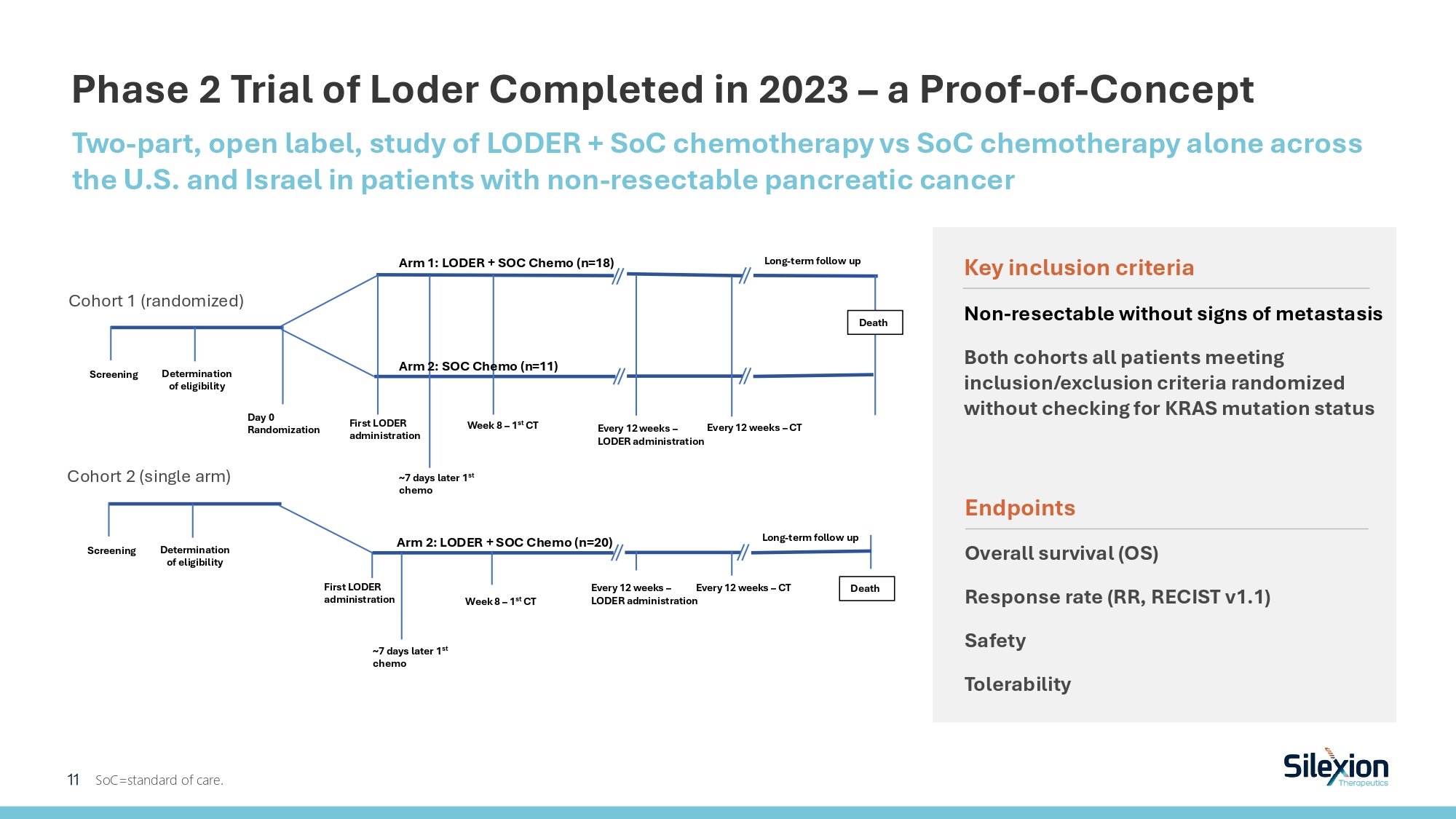
Phase 2 Trial of Loder Completed in 2023 – a Proof - of - Concept Two - part, open label, study of LODER + SoC chemotherapy vs SoC chemotherapy alone across the U.S. and Israel in patients with non - resectable pancreatic cancer SoC=standard of care. Arm 1: LODER + SOC Chemo (n=18) Day 0 Randomization Long - term follow up Screening Death Determination of eligibility First LODER administration ~7 days later 1 st chemo Week 8 – 1 st CT Arm 2: SOC Chemo (n=11) Every 12 weeks – LODER administration Every 12 weeks – CT Screening Death Determination of eligibility First LODER administration ~7 days later 1 st chemo Week 8 – 1 st CT Arm 2: LODER + SOC Chemo (n=20) Every 12 weeks – LODER administration Every 12 weeks – CT Long - term follow up Cohort 1 (randomized) Cohort 2 (single arm) 11 Key inclusion criteria Non - resectable without signs of metastasis Both cohorts all patients meeting inclusion/exclusion criteria randomized without checking for KRAS mutation status Endpoints Overall survival (OS) Response rate (RR, RECIST v1.1) Safety Tolerability

Baseline Characteristics and Cohorts Information Due to results of a clinical trial indicating FOLFIRINOX’s advantage over GnP as SoC chemotherapy, cohort 2’s SoC chemotherapy was changed from GnP (used in cohort 1) to FOLFIRINOX. *KRAS mutations were determined in 31 patients in total. In cohort 1 , 12 patients in the treatment arm and 10 patients in the control arm were tested; in cohort 1 , 9 patients were tested. BRPC=borderline resectable pancreatic cancer; GnP =gemcitabine/nab - paclitaxel; LAPC=locally advanced pancreatic cancer; SoC=standard - of - care. Cohort 2 (n=20) Cohort 1 (n=29) Single arm Randomized, controlled (SoC) Design/Arms Non - resectable (BRPC+ LAPC) Locally advanced PC (LAPC) Population 62% U .S. ( 4 sites) , 38% Israel (5 sites) Nationality 42% male; 58% female Male/ Female % 64.9 69.7 Median age (years) G12D/V*: Loder 7/9 G12R*: Loder: 2/9 G12D/V*: Loder 11/12, Control 5/10 G12R*: Loder: 1/12, Control 5/10 KRAS Mutations 2.1 2.8 Avg Loder cycles 370 Total number of Loder injections (modified) FOLFIRINOX ((m)FFX) gemcitabine/nab - paclitaxel ( GnP ) SoC chemotherapy 12
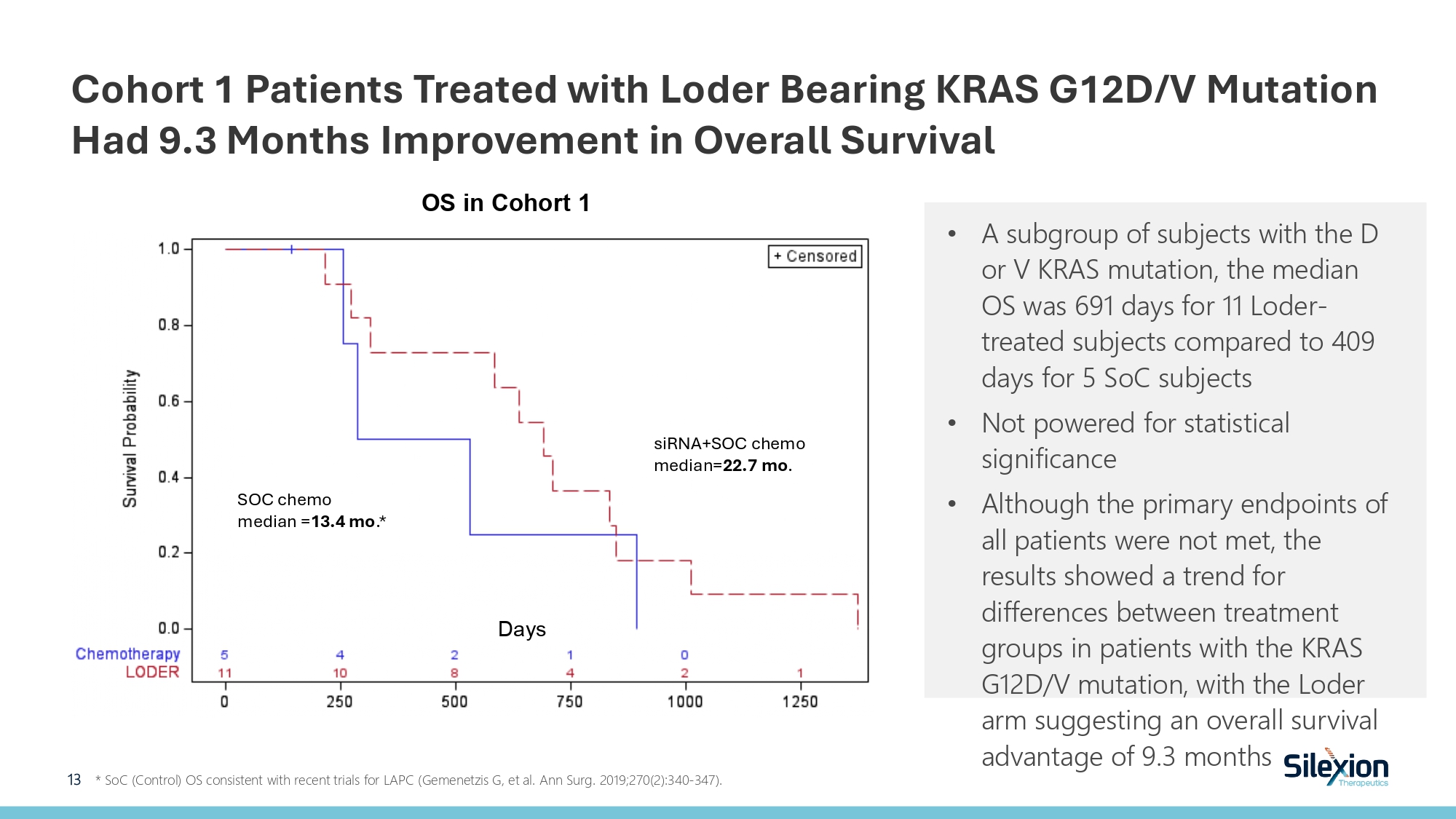
Cohort 1 Patients Treated with Loder Bearing KRAS G12D/V Mutation Had 9.3 Months Improvement in Overall Survival 13 * SoC (Control) OS consistent with recent trials for LAPC ( Gemenetzis G, et al. Ann Surg. 2019;270(2):340 - 347). • A subgroup of subjects with the D or V KRAS mutation, the median OS was 691 days for 11 Loder - treated subjects compared to 409 days for 5 SoC subjects • Not powered for statistical significance • Although the primary endpoints of all patients were not met, the results showed a trend for differences between treatment groups in patients with the KRAS G12D/V mutation, with the Loder arm suggesting an overall survival advantage of 9.3 months Days OS in Cohort 1 SOC chemo median = 13.4 mo .* siRNA+SOC chemo median= 22.7 mo .

Loder Was Overall Well Tolerated • The Phase 2 PoC c linical trial investigators reported that Loder treatment was well tolerated; Safety events were primarily related to procedure – Intratumor administration of extended - release siRNA via endoscopy (EUS) • N o Treatment Emergent Adverse Events (TEAEs) leading to study discontinuation nor deaths related to Loder treatment reported • No meaningful observations in any vital sign parameter nor any physical examination findings in the study reported • Independent Drug Safety Monitoring Board (DSMB) Reviews had no safety concerns nor safety restrictions • In a subset analysis, no measurable amount of Loder was detected (<BLQ) in any plasma samples suggesting low systemic levels 14

SIL - 204 KRAS G12D/V and KRAS amplification siRNA formulation

Leveraging Loder Clinical Data to Further Improve SIL - 204 Potential Efficacy and Safety *EUS endoscopy is a standard procedure used to obtain ultrasound guided biopsies once every 3 months . SIL - 204 LODER ` KRAS G12D/V+ KRAS amplify KRAS G12D/V siRNA target Added hydrophobic lead to increase siRNA access to - and within - the cell No hydrophobic lead Access to tumor cell site of action > 48 hrs <1 hr Stability in human serum PLGA microparticles suspension for better continuous 3 - month release PLGA depot rods Extended - release profile EUS Endoscopy* with smaller and more flexible needle; No loading device needed EUS - endoscopy* with larger needle; Required loading device Route and Ease of administration TBD in Phase 2/3 trial with the above improvement Trend for + 9.3 months OS with chemo vs. chemo alone 16
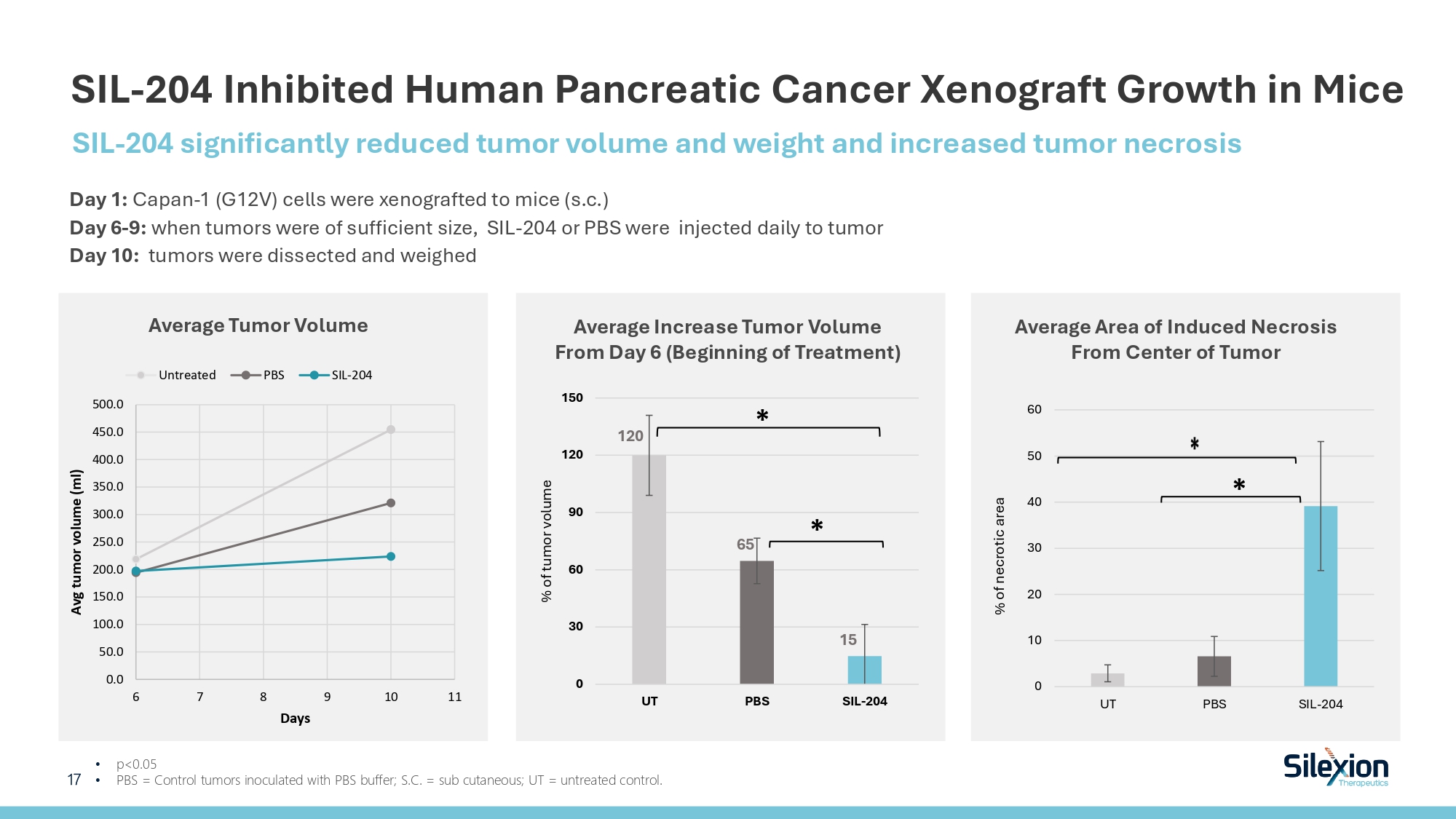
SIL - 204 Inhibited Human Pancreatic Cancer Xenograft Growth in Mice • p<0.05 • PBS = Control tumors inoculated with PBS buffer; S.C. = sub cutaneous; UT = untreated control. 0.0 50.0 100.0 150.0 200.0 250.0 300.0 350.0 400.0 450.0 500.0 6 7 8 9 10 11 Avg tumor volume (ml) Days Untreated PBS SIL-204 Average Tumor Volume Average Area of Induced Necrosis From Center of Tumor 0 10 20 30 40 50 60 UT PBS SIL-204 % of necrotic area * 120 65 15 0 30 60 90 120 150 UT PBS SIL-204 % of tumor volume * * SIL - 204 significantly reduced tumor volume and weight and increased tumor necrosis Day 1 : Capan - 1 (G 12 V) cells were xenografted to mice ( s.c. ) Day 6 - 9 : when tumors were of sufficient size, SIL - 204 or PBS were injected daily to tumor Day 10 : tumors were dissected and weighed 17 Average Increase Tumor Volume From Day 6 ( Beginning of Treatment )

SIL - 204 is Stable In Vitro for Over 48 Hours in Human Serum siRNA strand placed in human serum and tested for stability Stability of siRNA Strand in Human Serum Potential longer effectiveness of siRNA Greater ability to diffuse throughout the fibrous tumor environment Potential in other indications 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 0 1 2 4 6 14 24 48 % OF INTACT SIRNA GUIDE STRAND HOURS LODER SIL-204 18 Previous studies have shown siG12D (Loder) half - life to be 5 min in human serum.

SIL - 204 is Stable In Vivo in Rats for Five Hours 19 1. Givlaari ( givorisan ). EMA. 2. Alnylam. Givosiran NDA MULTI - DISCIPLINE REVIEW. 3. Lumasiran . Review ( fda.gov ). 4. Lumasiran . Leqvio , INN - inclisiran ( europa.eu ) . 5. Inclisiran . Leqvio , INN - inclisiran ( europa.eu ). 6. Inclisiran EMA Assessment Report. 7. Vutrisiran . FDA Review Summary. 8. EMA/FDA Approved siRNA Drugs: ADME Study Overview and Data Interpretation. Longer effectiveness of siRNA Greater ability to diffuse throughout the fibrous tumor environment Potential in other indications 0 1 2 3 4 5 6 7 8 9 10 SIL-204 Givosiran Lumasiran Inclisiran Vutrisiran Hour SIRNA siRNA Half - Life in Rats and Human Plasma (not a head - to - head comparison) Rat Human siRNA half - life in humans is 4 - 6x higher than in rats 1 - 8 potentially suggesting SIL - 204 may be the most stable siRNA

SIL - 204 Development Strategy in LAPC 2023 H1 2024 H 1 2025 H 2 2025 H1 2026 Clinical proof of concept for Loder in LAPC in an approvable endpoint for FDA Optimization of siRNA on various fronts; selection of SIL - 204 with new extended - release formulation Received guidance on trial design from the German Federal Institute for Drugs and Medical Devices ( BfArM ) Initiate toxicology studies SIL - 204 Initiate GMP production final formulation SIL - 204 Submit CTA in E.U. for Phase 2/3 Initiate Phase 2 / 3 of SIL - 204 in patients with locally advance pancreatic cancer in E.U. 20 Indicates completed activity. Unmarked activities to be performed.

Phase 2 / 3 Trial of SIL - 204 in LAPC: Proposed Study Design Received positive guidance from German regulatory agency on suggested trial design. Arm 1: SIL - 204B + SOC Chemo Arm 2 : SOC Chemo End of Study Day 1 , 2:1 Randomization DSMB ~ 15 subjects for safety following 1 mo. FU If pancreatectomy Post Surgery FU Treatment Chemo run - in Interim Analysis ~1/3 events P2→P3 Go/No - Go Screening 28d Death Each Patient Treatment Period 24 months or until death or the tumor is too small to treat Long Term Extension Trial for overall survival 5 yrs from randomization Chemo run - in Phase 2 Safety run - in Phase 2 expanded Phase 3 21

World - Renowned Expert Scientific Advisory Board Eileen M. O'Reilly, MD Memorial Sloan Kettering, NY, NY Winthrop Rockefeller Endowed Chair of Medical Oncology; Co - Director, Medical Initiatives, David M. Rubenstein Center for Pancreatic Cancer Research; Section Head, Hepatopancreatobi Hana Algul , MD Technical University of Munich, Germany chair for tumor metabolism; Director of the Comprehensive Cancer Center Munich, Germany at the Klinikum rechts der Isar, and Mildred - Scheel - professor and Milind Javle , MD The University of Texas & MD Anderson Cancer Center, Houston, TX Professor, Department of Gastrointestinal (GI) Medical Oncology, Division of Cancer Medicine Philip A. Philip, MD Henry Ford Health, Detroit, MI Director, Gastrointestinal Oncology; Co - Director, Pancreatic Cancer Center; Medical Director, Research and Clinical Care Integration, Henry Ford Cancer Institute Talia Golan, MD Sheba Tel Hashomer Hospital,, Israel Head, Sheba Pancreatic Cancer Center - SPCC Matthew Katz, MD The University of Texas & MD Anderson Cancer Center, Houston, TX Department Chair, Department of Surgical Oncology, Division of Surgery and Professor. Andrew M. Lowy, MD UC San Diego, San Diego, CA Chief, Division of Surgical Oncology; Professor of Surgery Mark A. Schattner , MD Memorial Sloan Kettering, NY, NY Chief, Gastroenterology, Hepatology and Nutrition Service 22

Highly Experienced Leadership Team Ilan Hadar , MBA Chairman and Chief Executive Officer > 25 years of multinational managerial and corporate experience with pharmaceutical and high - tech companies Mitchell Shirvan, PhD, MBA Chief Scientific and Development Officer > 25 years of experience in R&D, innovation and discovery in biotech companies Mirit Horenshtein Hadar, CPA Chief Financial Officer > 15 years of corporate finance experience in senior financial positions of public companies and privately held companies, in the pharmaceutical and high - tech industries Ilan Levin, Director Former Chairman & Chief Executive Officer of Moringa Acquisition Corp with 25 years of experience as an executive and venture capital/private equity investor in high - tech, Israel - related ventures 23

Investment Highlights CTA=clinical trial application; IND=investigational new drug. • Clinical - stage company with proprietary oncogene siRNA platform • Intratumor siRNA delivery for pancreatic cancer allow for better drug exposure compared with systemic KRAS inhibitors • Phase 2 clinical trial with Loder in LAPC showed 9.3 months improvement in the FDA approvable endpoint of overall survival • Lead Candidate SIL - 204 with enhanced siRNA stability, and a better extended - release profile Advanced RNA therapeutic candidate in oncology Late - Stage Ready Asset with Potential Regulatory Path Forward Strong Partnerships with Solid IP Portfolio • Guidance received from German Federal Institute for Drugs and Medical Devices ( BfArM ) on Phase 2 / 3 trial • Plans to submit CTA in E.U. late 2025 and initiate Phase 2 / 3 trial of SIL - 204 in 2026 • Established partnerships for GMP production of siRNA and delivery system • Strong IP portfolio for siRNA and microparticles with exclusivity through December 2043 with extension























