Exhibit 10.31
Certain identified information has been omitted from this exhibit because it is not material and of the type that the registrant treats as private or confidential. [***] indicates that information has been omitted.
Contribution and License Agreement for Pharmaceutical FOU
This Contribution and License Agreement (“Agreement’) effective on the Closing Date (defined below) by The Invention Science Fund I, L.L.C. (“ISF1”), a Delaware limited liability company, and Pear Therapeutics, Inc. (“Licensee”), a Delaware corporation.
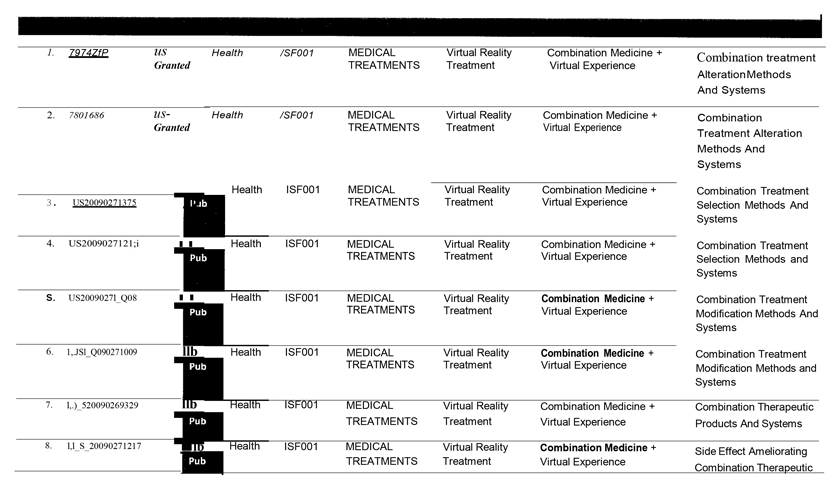
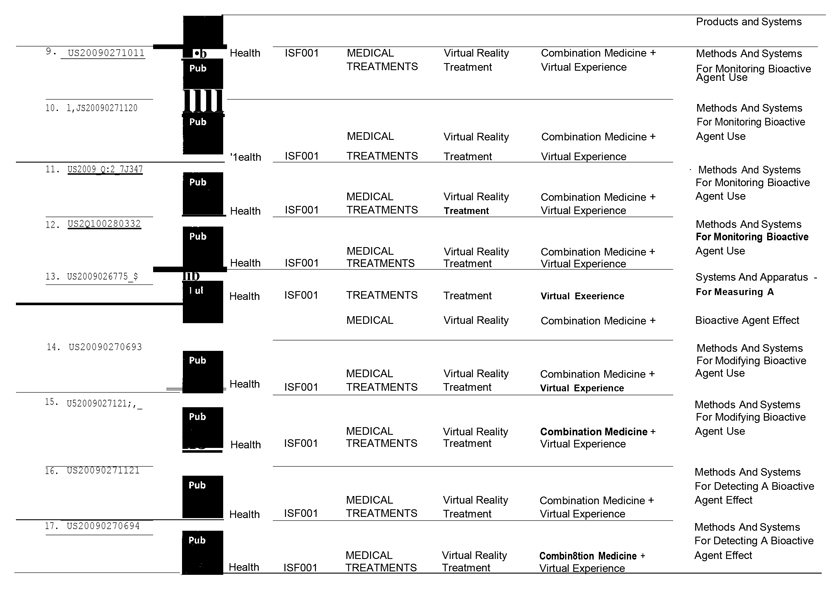
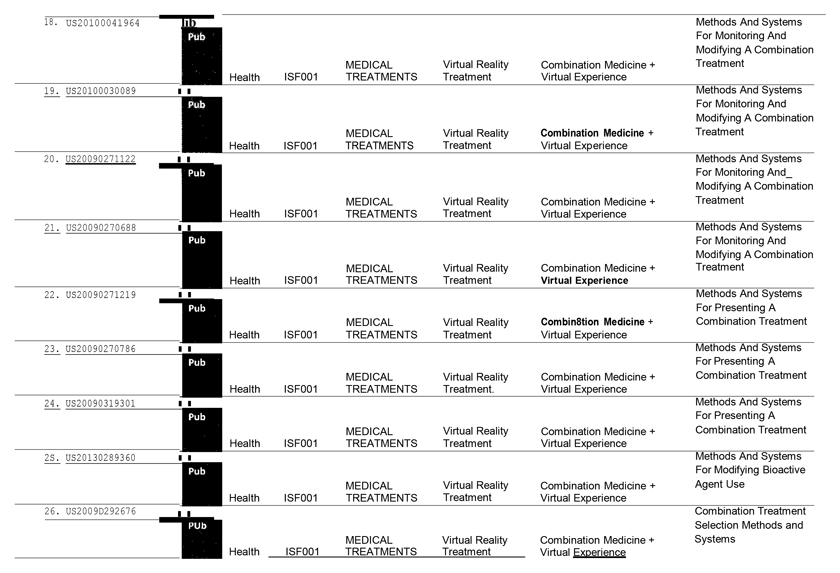
WHEREAS, ISF1 owns certain intellectual property and proprietary rights directed to combination medicine and virtual experience technology (“IPR”).
WHEREAS, ISF1 and Licensee are parties to that certain Contribution and License Agreement dated December 31, 2013 (the “Original Agreement”), pursuant to which ISF1 granted Licensee certain licenses to further develop the IPR for use with central nervous system indications with the goal of developing and commercializing drug and software combinations and services to address certain effects associated with central nervous system disorders.
WHEREAS, in order to facilitate an equity or debt financing of Licensee, ISF1 and Licensee now desire to terminate the Original Agreement upon closing of such financing and enter into new agreements under (i) the terms and conditions set forth under this Agreement, and (ii) the terms and conditions set forth under that certain Non-Exclusive Patent License Agreement entered into on the Agreement Date by and between ISF1 and Licensee (“Parallel Non-Exclusive License”).
NOW THEREFORE, in consideration of the foregoing and in further consideration of the covenants and promises set forth herein, the sufficiency of which is hereby acknowledged, and intending to be legally bound, the parties agree as follows:
For purposes of this Agreement, in addition to the capitalized terms defined elsewhere in this Agreement, the following terms shall have the meanings ascribed to them below:
“Abandoned Patent Rights” has the meaning set forth in Section 7.2.
“Affiliates” means with respect to any party, any entity that is Controlled by, under common Control with, or Controls such party. The term “Control” means possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of an entity, whether through the ownership of voting security, contract, or otherwise.
“Agreement Date” means the date entered below on which ISF1 signed this Agreement.
“Authorized Sublicensee” is defined in Section 2.2
“Claims” means claims, suits, and legal actions by any third party.
“Closing Date” has the meaning set forth in Section 8.l(a).
“Combination Product” means any Licensed Product for which Licensee, Sublicensee, any of their respective Affiliates or agents directly or indirectly uses, sells, licenses, distributes, or otherwise disposes of the Drug portion of the Licensed Product.