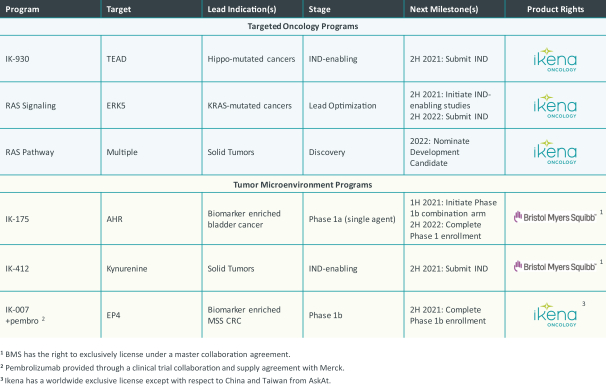
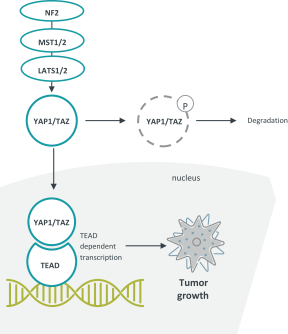
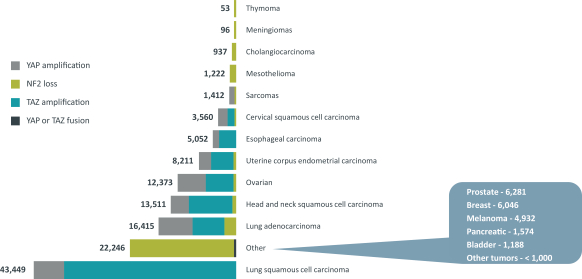
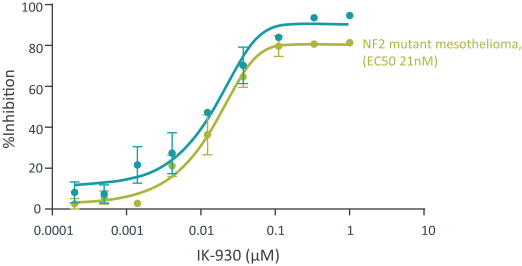
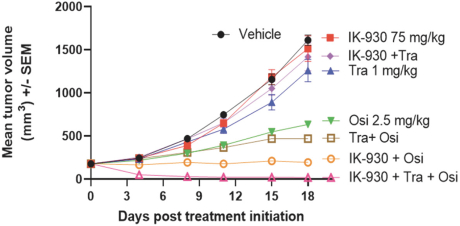
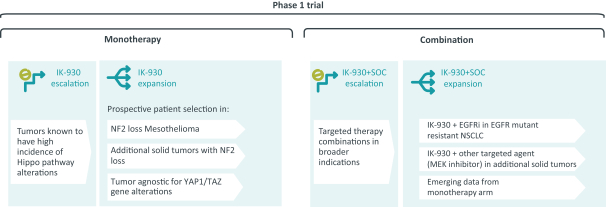
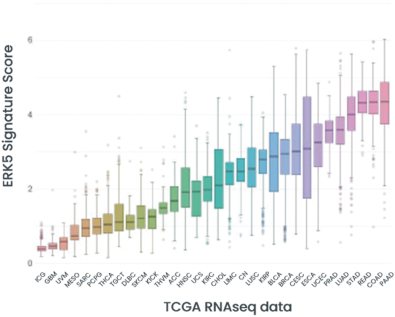
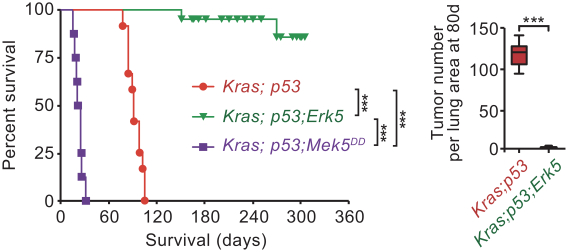
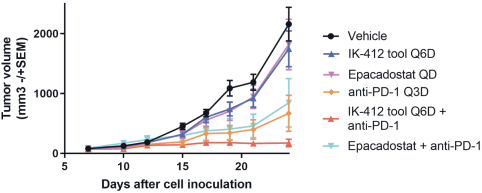
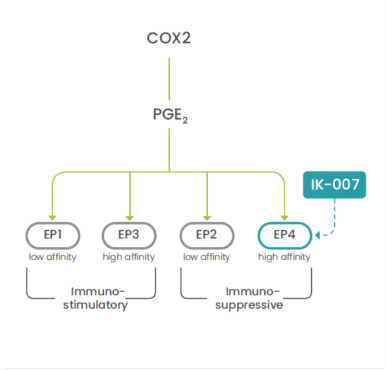
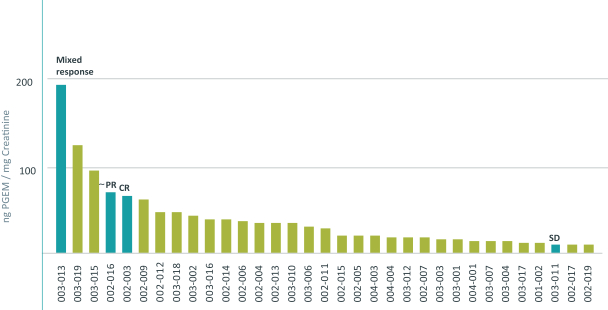
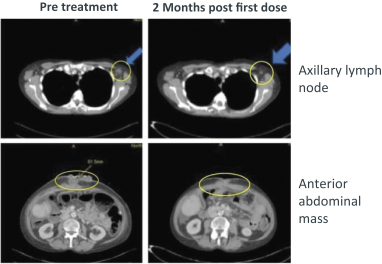
combination with other therapeutic agents. The three exclusively licensed patent families are licensed from the University of Texas at Austin.
The first in-licensed patent family includes two issued patents in the U.S., which are projected to expire in 2034 and 2035, respectively, excluding any patent term extensions, if applicable. The first in-licensed patent family also includes issued patents in Europe, Hong Kong, Australia, Israel, Japan, and South Africa, and such patents are expected to expire in 2034, excluding any patent term extensions, if applicable. Within this first in-licensed patent family, patent applications are pending in the U.S., Brazil, Canada, China, Europe, India, Japan, Korea, New Zealand, and South Africa.
The second in-licensed patent family includes one issued patent in the U.S., which is projected to expire in 2035, excluding any patent term extensions, if applicable. Within this second in-licensed patent family, patent applications are pending in the U.S., Canada, Europe, Israel, and Japan.
The third in-licensed patent family includes patent applications in the U.S., Argentina, Australia, Brazil, Canada, Chile, China, Colombia, Eurasian Patent Organization, Hong Kong, Europe, Israel, India, Japan, Korea, Mexico, New Zealand, Singapore, Thailand, Taiwan, and South Africa. Patents that issue from these applications are projected to expire in 2039, excluding any patent term adjustments or extensions, if applicable.
We also solely own patent applications related to our IK-412 program. The company-owned patent portfolio related to this program consists of one patent family that currently includes one U.S. patent application and one PCT international patent application covering our IK-412 biologic drug product as a composition of matter and methods of using the same, alone or in combination with other therapeutic agents. Patents issuing from the company-owned patent family are projected to expire in 2040, excluding any patent term adjustments or extensions, if applicable.
Prosecution is a lengthy process, during which the scope of the claims initially submitted for examination by the USPTO or other foreign jurisdiction are often significantly narrowed by the time they issue, if they issue at all. Any of our pending PCT patent applications are not eligible to become issued patents until, among other things, we file national stage patent applications within 30 months in the countries in which we seek patent protection. If we do not timely file any national stage patent applications, we may lose our priority date with respect to our PCT patent applications and any patent protection on the inventions disclosed in such PCT patent applications. Our provisional patent applications may never result in issued patents and are not eligible to become issued patents until, among other things, we file a non-provisional patent application and/or PCT patent application within 12 months of filing the related provisional patent application. If we do not timely file non-provisional patent applications, we may lose our priority date with respect to our provisional patent applications and any patent protection on the inventions disclosed in our provisional patent applications. While we intend to timely file non-provisional and national stage patent applications relating to our provisional and PCT patent applications, we cannot predict whether any of our current or future patent applications will issue as patents. If we do not successfully obtain patent protection, or, if the patent protection scope is not sufficiently broad, we may be unable to prevent others from using our proprietary technology or from developing or commercializing technology and products similar or identical to ours or other competing products and technologies.
Trade Secret Protection
In addition to patents, we rely on unpatented trade secrets, know-how and continuing technological innovation to develop and maintain our competitive position. However, trade secrets and confidential know-how are difficult to protect. In particular, we anticipate that with respect to the building of our compound library, our trade secrets and know-how will over time be disseminated within the industry through independent development and public presentations describing the methodology. We seek to protect our proprietary information, in part, by executing confidentiality agreements with our collaborators and scientific advisors and non-competition, non-solicitation, confidentiality and invention assignment agreements with our employees and consultants. We have also executed
143