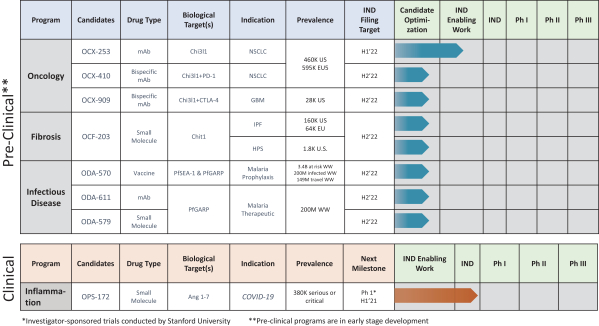
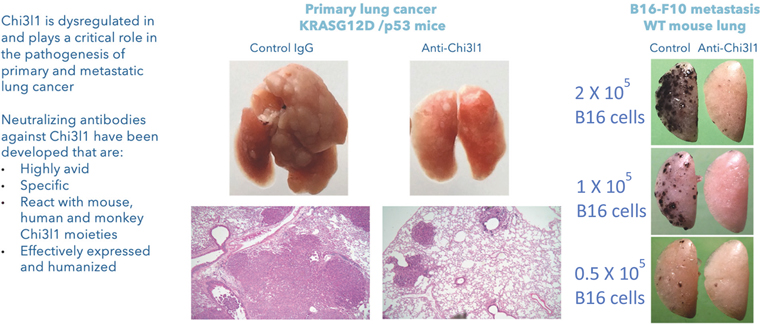
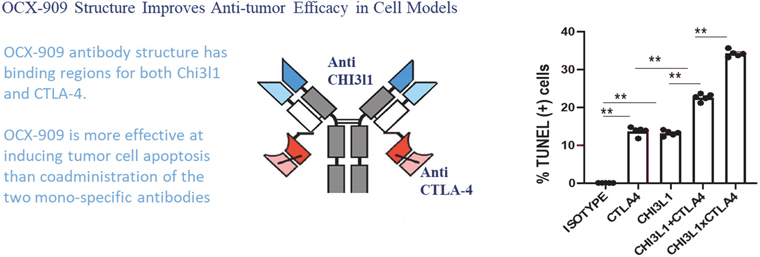
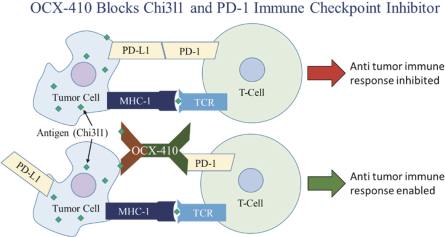
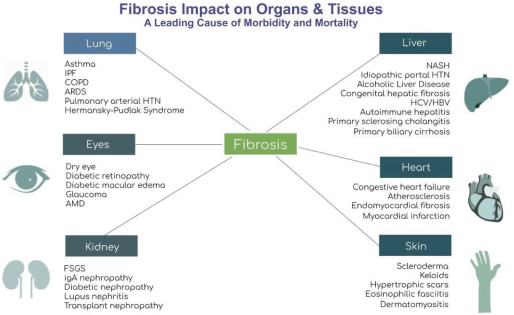
The Company will also pay the Licensor developmental and commercialization milestone payments for each of the Brown License Agreements ranging from $50,000 for the filing of an Investigational New Drug Application, or IND, or the equivalent outside of the United States, to $250,000 for enrollment of the first patient in a Phase 3 clinical trial in the United States or the equivalent outside of the United States. The Company is also responsible for reimbursement of patent costs. To date, the Company has reimbursed patent costs expenses to Brown University in the amount of $65,312.
The contract term for each of the Brown License Agreements continues until the later of the date on which the last valid claim expires or ten years. Either party may terminate each of the Brown License Agreements in certain situations, including the Licensor being able to terminate the Brown License Agreements at any time and for any reason after October 1, 2021 if the Company has not raised at least $10 million in equity financing by then. For the oncology programs, three of the license agreements have been sublicensed to the Company’s subsidiary, Ocean Chitorx, Inc., and for the Fibrosis program, one license agreement has been sublicensed to the Company’s subsidiary, Ocean Chitofibrorx, Inc.
Elkurt/Rhode Island Agreement
On January 25, 2021, the Company entered into an Exclusive License Agreement, or the Rhode Island License Agreement, with Licensor, a licensee of Rhode Island Hospital. In April 1, 2021, the Company and Licensor amended the Rhode Island License Agreement Under the Rhode Island License Agreement, the Licensor grants to the Company an exclusive, royalty-bearing license to patent rights and a non-exclusive, royalty-bearing license to know-how, solely to make, have made, market, offer for sale, use, and sell licensed products for use in a certain field.
For the Rhode Island License Agreement, the Company is required to pay the Licensor $110,000, due within 45 days of an equity financing of at least $10 million or October 15, 2021, whichever comes first, and an additional $3,000 annual maintenance fee thereafter, until January 1, 2028, at which point the annual maintenance fee will become $4,000 per year. The Company is also required to pay the Licensor 1.5% of net sales under the Rhode Island License Agreement. In addition, the Company must pay the Licensor 25% of all non-royalty sublicense income prior to the first commercial sale, and 10% of non-royalty sublicense income thereafter, in the event that the Company enters into sublicenses for the subject intellectual property. If net sales or non-royalty sublicense income are generated from know-how products, the amounts otherwise due (royalty or non-royalty sublicense income) shall be reduced by 50%.
The Company will also pay the Licensor developmental and commercialization milestone payments under the Rhode Island Agreement, ranging from $50,000 for the filing of an IND, or the equivalent outside of the United States, to $250,000 for enrollment of the first patient in a Phase 3 clinical trial in the United States or the equivalent outside of the United States.
The contract term for the Rhode Island License Agreement began February 1, 2020 and will continue until the later of the date on which the last valid claim expires or fifteen years. Either party may terminate the License Agreement in certain situations, including the Licensor being able to terminate the license agreement at any time and for any reason by October 1, 2021, if the Company has not raised at least $10 million in equity financing by then. Currently, the Rhode Island License Agreement is still in effect and the license agreement has been sublicensed to the Company’s subsidiary, Ocean Sihoma, Inc.
Teton Therapeutics, Inc.
On April 15, 2020, the Company entered into an Exclusive License Agreement, or the Teton License Agreement, with Teton Therapeutics, Inc., or Teton. We amended and restated this agreement on February 25, 2021 in order to assign the program to our subsidiary in the future. Pursuant to the Teton License Agreement, the Company obtained an exclusive license under certain patent rights, or the Teton Patents, and under certain data, expression and purification methods, information and other know-how, or the Teton Know-How, in each case relating to therapies for neurofibromatosis type 1 and 2 and schwannomatosis. The Company has the right to make, have made, market, offer for sale, use and sell in
99