Exhibit 99.1

Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved August 2022 Investor Presentation

Forward looking - Statements This presentation of Inspira TM Technologies Oxy B . H . N . Ltd . (“Inspira Technologies” or the “Company”) contains “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act and other securities law . Words such as “expects,” “intends,” “plans,” “believes,” “seeks,” “estimates,” and similar expressions or variations of such words are intended to identify forward - looking statements . For example, the Company is using forward - looking statements when it discusses intended uses of its products and technology, its business model, its strategy for gaining market penetration and market share, the potential amounts to be derived from its distribution agreements, its go - to market strategy, potential strategic collaborations with third parties, its reimbursement strategy, regulatory submissions, market potential for its products, commercialization of its products, the potential to use its products together with mechanical ventilation, the regulatory approval process of its product candidates and the potential submission of approvals with such regulators, the benefits and uses of its product candidates for intended patient populations, lines of therapy, targeted major milestones for 2022 - 2023 and market milestones for 2022 - 2023 and its future growth . The presentation also contains estimates with respect to the Company’s health economics model . Forward - looking statements are not historical facts, and are based upon management’s current expectations, beliefs and projections, many of which, by their nature, are inherently uncertain . Such expectations, beliefs and projections are expressed in good faith . However, there can be no assurance that management’s expectations, beliefs and projections will be achieved, and actual results may differ materially from what is expressed or indicated by the forward - looking statements . Forward - looking statements are subject to risks and uncertainties that could cause actual performance or results to differ materially from those expressed in the forward - looking statements . More detailed information about the risks and uncertainties affecting the Company is contained under the heading “Risk Factors” in the Company’s annual report on Form 20 - F for the fiscal year ended December 31 , 2021 filed with the U . S . Securities and Exchange Commission (the “SEC”), which is available on the SEC’s website, www . sec . govForward - looking statements speak only as of the date the statements are made . The Company assumes no obligation to update forward - looking statements to reflect actual results, subsequent events or circumstances, changes in assumptions or changes in other factors affecting forward - looking information except to the extent required by applicable securities laws as well as subsequent filings with the SEC . If the Company does update one or more forward - looking statements, no inference should be drawn that the Company will make additional updates with respect thereto or with respect to other forward - looking statements . 2 * The Inspira ART and the ART presented in this presentation are both referring to the Inspira ART Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

3 3 * The Inspira Ρ ART and the ART presented in this presentation are both referring to the Inspira Ρ ART Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

4 4 We need to unlock your dependency on invasive mechanical ventilation Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

The Respiratory Gap 5 Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

Respiratory Failure Multiple Trends Propelling the Market in the Coming Years Respiratory failure is a serious medical condition that develops when the lungs cannot get sufficient oxygen into the blood. Buildup of carbon dioxide can also damage the tissues and organs. Acute respiratory failure can occur quickly and without much warning and often requires immediate emergency treatment¹. 1. National Heart, Lung and Blood Institute website. https://www.nhlbi.nih.gov/health - topics/respiratory - failure 6 Global Market Expected to Grow (Based on invasive mechanical ventilation market) Increase in the prevalence of respiratory diseases Growing geriatric population Respiratory comorbidities following COVID - 19 Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

7 7 * The Inspira ART and the ART presented in this presentation are both referring to the Inspira ART. The Inspira ART has not been cleared or approved or cleared by the U.S. Food and Drug Administration (FDA) or any other regulatory authority. Play Video Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

1. Am J Respir Crit Care Med Vol. 196, P3 - 4, 2017. ATS Patient Education Series © 2017 American Thoracic Society 2. Diling Wu et al. Frontiers in pharmacology MINI REVIEW published: 09 May 2019. doi: 10.3389/fphar.2019.00482 3. Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital - acquired and Ventilator - associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61 - e111. MV requires intrusive intubation, coma induction, and can be very traumatic for patients: Potential Risks & Complications • Ventilator - induced lung injury (VILI) • Ventilator - associated pneumonia (VAP) • Ventilator - induced diaphragmatic dysfunction • Pneumothorax & tracheomalacia • Increased Delirium • Muscular atrophy • Drug withdrawal symptoms High Cost of Treatment • Prolonged duration of Ventilation • Prolonged ICU stay • Increased complication rate • Patient re - admissions • Requires weaning process • Extended rehabilitation period Mechanical Ventilation (MV) Puts Patients at Risk¹ˉ³ 8 Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

9 9 o Aims to reduce the need for invasive mechanical ventilation (IMV) with the potential to reduce risks, complications and high costs o Targets for patient to remain awake & spontaneously breathing o Will be designed to utilize direct blood oxygenation to boost patient saturation levels within minutes o Anticipates to allow for larger patient populations in and beyond ICU settings Augmented Respiratory Technology (Inspira ART Ρ ) Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved
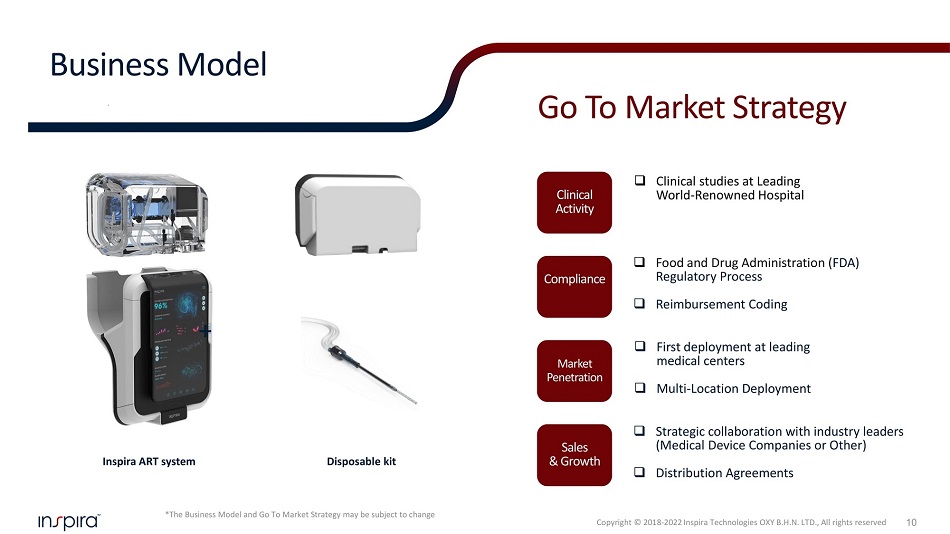
Inspira ART system Disposable kit + □ Clinical studies at Leading World - Renowned Hospital □ Food and Drug Administration ( FDA) Regulatory Process □ Reimbursement Coding □ Strategic collaboration with industry leaders (Medical Device Companies or Other) □ Distribution Agreements Clinical Activity *The Business Model and Go To Market Strategy may be subject to change 10 Business Model Go To Market Strategy □ First deployment at leading medical centers □ Multi - Location Deployment Compliance Market Penetration Sales & Growth Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

* To - date the Inspira Ρ ART System, ALICE Ρ Device and HYLA Ρ Blood Sensor have not been tested or used on humans and are subject to the regulator’s approvals or clearance. Timelines and milestones may be subject to change. 11 Market Opportunity By Product * ALICE TM Device Life Support - The Alice Ρ System (previously identified as the Liby Ρ or ECLS system) is designed as a cardiopulmonary bypass (CPB) or heart - lung bypass device designed to do work of providing the body blood & oxygen when heart is stopped for a surgical procedure up to 6hrs. Treatment setting: Operating Theaters FDA Submission H2 - 2023, Planning FDA Class II 510(k) Inspira ART TM System HYLA TM Blood Sensor This respiratory support technology targeted to perform direct blood oxygenation and CO2 removal to boost patient saturation levels within minutes. The designed treatment is intended for longer than 6 hours, providing assisted extracorporeal circulation and physiologic gas exchange of the patient's bloodin adult patients with acute respiratory failure. The system is targeted to allow for treatmentof patients while awake and spontaneously breathing. Treatment setting: ICUs & Hospital Units FDA Pre - Submission H2 - 2023 A non - invasive blood sensor to be designed to perform real - time and continuous blood measurements, potentially minimizing the need for blood samples from patients. The HYLA’s measurement shall be designed to assist physicians in the monitoring of a patient’s clinical condition. Treatment setting: Operating Theaters, Hospital Units FDA Submission date to be defined Targeting Millions of Patients in ICUs Targeting Millions of Patients Targeting Operating Theaters Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

The milestones and timelines are subject to change. There is no guarantee as to the success of any trial. In addition, there is inherent risk and variability in the overall regulatory process. Approval by the FDA may not be granted, or the FDA may require different study parameters from those that are intended to be included in the submission. While the Company intends to execute on strategic distribution agreements, there is no guarantee any sales will occur pursuant to those existing agreements, and agreements that may be executed in the future. * The Distribution agreements are over a 7 - year period and subject to completion of development, and the required regulatory approvals or clearances. Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved 12 Planned Milestones 2022 - 2023 FDA Submissions ALICE Ρ System Inspira Ρ ART System (2023/2024) Inspira DL Cannula (2023/2024) Inspira Disposable Kit Strategic Collabortions Leading World Renowned Hospital Manufacturing Agreement Licensing Agreement HYLA Ρ Blood Sensor Distribution Agreements Signed Agreementin U.S. for future deployment* of Inspira Ρ ART Systems Signed Agreementin IL for future deployment* of Inspira Ρ ART Systems Signed Agreementin U.S for future deployment* of HYLA Ρ Blood Sensors Clinical Studies Commencement of HYLA Ρ Study Results of HYLA Ρ Study Commencement of New Product Study Results of New Product Study New Technology & Product Reveals ALICE Ρ System HYLA Ρ Blood Sensor New Tech Device Inspira Ρ ART System New Region

Company $12 million $9.4 million $16 million incorporation Raised prior to IPO: Including funds from Israeli Innovation Authority Raised at initial public offering on NASDAQ Warrants exercised at $5.50 per share Feb.2018 2019 up to Q1 - 2021 Jul. 2021 Oct. 2021 $21.7 million, Cash on hand as of March 31, 2022 $0.8 million, H1 - 2022 average monthly burn - rate ~$37.4 million Total cash raised to - date 13 Inspira Cash Management Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

Prof. Benad Goldwasser, MD, MBA Chairman Multiple well - known industry exits Dagi Ben - Noon, BSc Co - Founder, Director & CEO Co - founder of Nano Dimension Nasdaq: NNMD 14 Daniella Yeheskely - Hayon, PhD CTO 13 yrs development experience Joe Hayon, MBA Co - Founder, Director, President & CFO M&A experience & track record • Elscint Technologies • Arazim Advanced Technologies Yafit Tehila, CPA VP Finance & Legal Experience with public companies Avi Shabtay, BSc COO 25 yrs of startup development experience Experienced Leadership Team Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

Dr. Yigal Kasif, MD ECMO Advisor Chairman of the Israel ECMO Society. Director of ECMO program at Sheba Medical Center. Surgeon, Specializing in Adult Cardiac Surgery, Heart Transplantation and Assist Devices. Dr. Stephan Ledot Intensivist & ECMO Advisor World - renowned expert in critical care, ECMO, anesthesia and echocardiography. Fellowships in ECMO at the NHS, UK and cardiothoracic anesthesia at Harefield Hospital` Prof. Eddy Fan Intensivist & ECMO Advisor Medical director of the extracorporeal life support program at the Toronto General Hospital. Intensivist at the University Health Network/Mount Sinai Hospital Prof. Daniel Brodie Intensivist & ECMO Advisor President - elect of the Extracorporeal Life Support Organization (ELSO). Chairman of the Executive Committee of the International ECMO Network (ECMONet) 15 Scientific Advisory Board - Several world - renowned KOLs Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

16 You Need to be Conscious of Your Potential Alternatives * The Inspira ART and the ART presented in this presentation are both referring to the Inspira ART. To - date the Inspira ART System has not been tested or used on humans and is subject to the regulator’s approvals or clearances. Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved

Copyright © 2018 - 2022 Inspira Technologies OXY B.H.N. LTD., All rights reserved THANK YOU
















