Exhibit 99.1

See Disclaimers on slides 1 - 16 . Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 1 See Disclaimers in deck on slides 3 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 1 Inspira Technologies WEBINAR NOVEMBER 2024

See Disclaimers on slides 2 - 3 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 2 This presentation contains express or implied forward - looking statements pursuant to U . S . Federal securities laws . These forward - looking statements and their implications are based on the current expectations of the management of the Company only and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward - looking statements . For example, the Company is using forward - looking statements when it discusses the prospective commercialization of its products, prospective U . S . Food and Drug Administration (FDA) regulatory submissions and clearances for its products the projected size of the mechanical ventilation market and perfusion systems market, the projected size of any other markets the Company may operate, the potential market sizes of its future products, the intended outcomes of the use of its products, including the HYLA Blood Sensor, INSPIRA ART 100 , INSPIRA ART, and INSPIRA Cardi - ART, and the intended uses and potential benefits of its products and technology . This presentation also contains estimates of the Company ’ s health economics model . These forward - looking statements and their implications are based solely on the current expectations of the Company ’ s management and are subject to a number of factors and uncertainties that could cause actual results to differ materially from those described in the forward - looking statements . Except as otherwise required by law, the Company undertakes no obligation to publicly release any revisions to these forward - looking statements to reflect events or circumstances after the date hereof or to reflect the occurrence of unanticipated events . More detailed information about the risks and uncertainties affecting the Company is contained under the heading “ Risk Factors ” in the Company ’ s annual report on Form 20 - F for the fiscal year ended December 31 , 2023 filed with the U . S . Securities and Exchange Commission (the “ SEC ” ), which is available on the SEC ’ s website, www . sec . gov . Forward Looking Statement Disclaimer

See Disclaimers on slides 2 - 3 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 3 The reference to INSPIRA TM ART should be interpreted as the fact that it is a platform. The use of the term INSPIRA TM ART can refer to the INSPIRA TM ART 500 or INSPIRA TM ART Gen 2 . Mechanical Ventilation refers to Invasive Mechanical Ventilation. Planned timelines, milestones, estimates or assumptions are subject to change. The INSPIRA ART 100 system has FDA 510 (k) clearance and Israeli AMAR certification for Cardiopulmonary Bypass and ECMO procedures. The INSPIRA ART 100 System is FDA 510 (k) cleared for use in an extracorporeal perfusion circuit to pump blood during short - duration cardiopulmonary bypass procedures lasting 6 hours or less. INSPIRA ART 100 and INSPIRA ART are different devices The Augmented Respiration Technology, Adaptive Blood Oxygenation Technology, INSPIRA TM AI, HYLA TM , INSPIRA TM ART 500 , INSPIRA TM ART, INSPIRA TM Cardi - ART, VORTX TM and any other Inspira products, devices, disposables, components, software or technologies are still in development and have not been tested or used on humans. Product intent of uses and regulatory pathways are yet to be defined or finalized. The products have not been cleared or approved by the FDA or any other regulatory or authorizing authority. The estimated date/time of regulatory clearance or approval may be subject to change. Approval or clearance by the FD A, CE or any other authorizing entity may not be granted or may require different study parameters or data or validation from those that are intended or were included in the submission. In addition, there is inherent risk and variability in the overall regulatory process and no guarantee as to the success of any trial or regulatory approval or clearance. Some or all the clinical studies may be conducted outside of the U.S. INSPIRA TM Cardi - ART, INSPIRA TM ART 500 , INSPIRA TM ART or HYLA TM may either have embedded or integrated INSPIRA TM AI or other software with selective levels or functionality, yet to be decided by the Company. Disclaimers Source: $19 Billion Predicted Global Mechanical Ventilation Market By 2030 Source: Perfusion S ystems M arket Source: $ 2.5 Billion ABG Market By 2030 Source: $ 39.4 Billion Cardiac Arrest Treatment Market By 2029 Source: Extracorporeal Membrane Oxygenation Machine Market Size by 2036 Source: After Out - of - Hospital Cardiac Arrest Source: inspira - technologies.com/news/ Source: High Mortality Rate Source : ± 20M Patients/Year on MV Source: > 50 % Mortality Rate Source: Washington Post The Dark Side of Ventilators Source: CNBC Why Some Doctors Are Moving Away From Ventilators Source: CNN When Life Support is Really Death Support Source: Forbes Millions May Soon Breath Better Source: WhitePaper_Oxygenators V 11 _ 5.1.2023

See Disclaimers on slides 2 - 3 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 4 “ Why some doctors are moving away from ventilators ” “ The dark side of ventilators ” Patients can often experience legacy complications: delirium, lung infections, lung injury or severe life - threatening conditions > 50 % Mortality rate Patients under heavy sedation ( COMA) with tubes pushed down their throat ± 20 Million People a Year on Mechanical Ventilation “ When ‘ life support ’ is really ‘ death support ’ ” High Mortality Rate > 50 % Mortality Rate ± 20 M Patients On MV Washington Post NBC News CNN

See Disclaimers on slides 2 - 3 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 5 “ A new and innovative direct blood oxygenation technology may provide a solution . Augmented Respiration Technology, (ART), developed by Inspira Technologies , tackles the increased global demand for long - term and short - term respiratory support and the surge in ICU admissions as a result of Covid - 19 . Even prior to Covid - 19 , approximately 20 million patients were treated annually with MV in ICUs around the world . ” Millions May Soon Breath Better (Article by Carie Rubenstein October 25 , 2021 ) INSPIRA ART Forbes Millions May Soon Breath Better
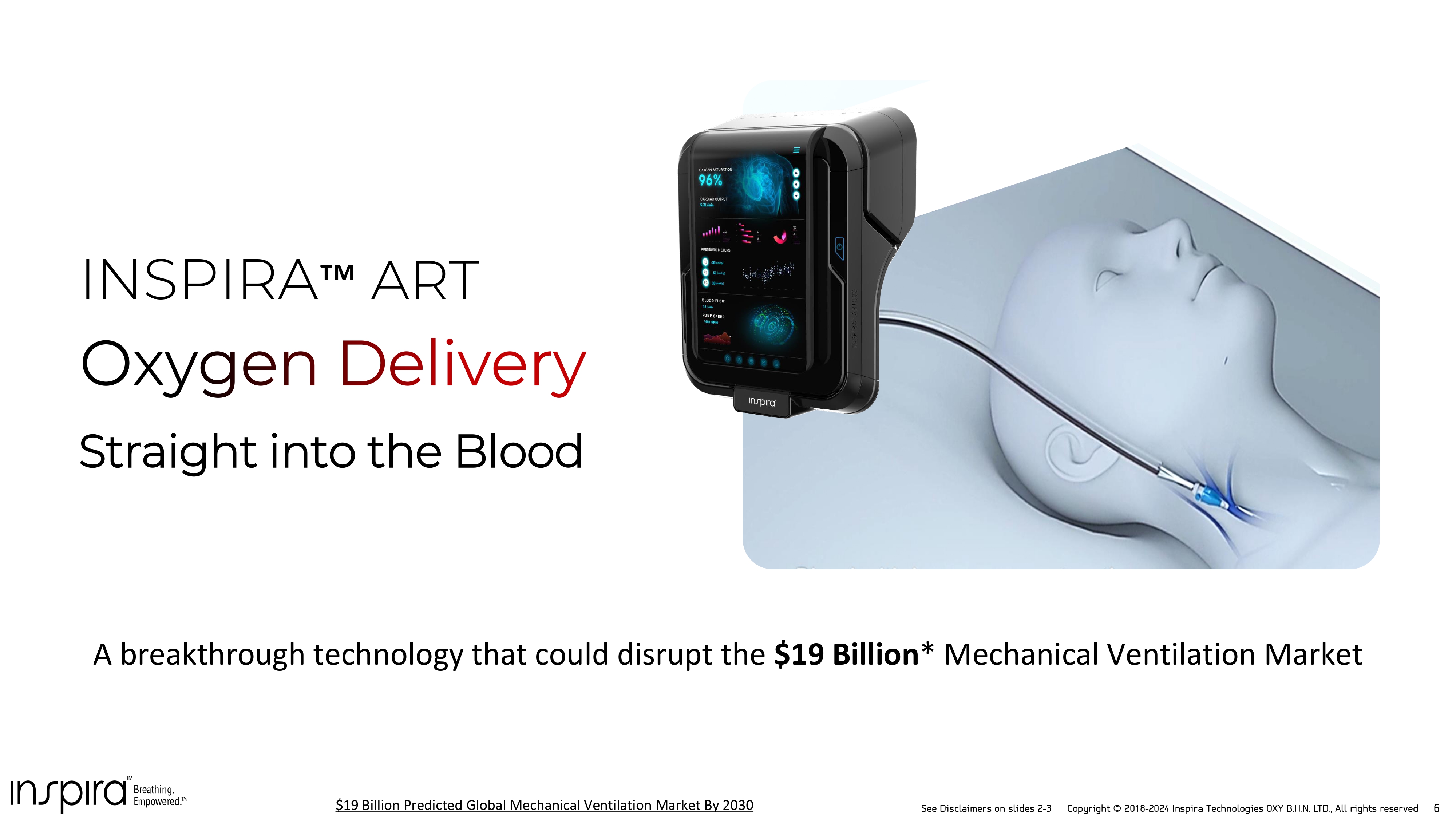
See Disclaimers on slides 2 - 3 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 6 Straight into the Blood INSPIRA ART A breakthrough technology that could disrupt the $ 19 Billion * Mechanical Ventilation Market $ 19 Billion Predicted Global Mechanical Ventilation Market By 2030
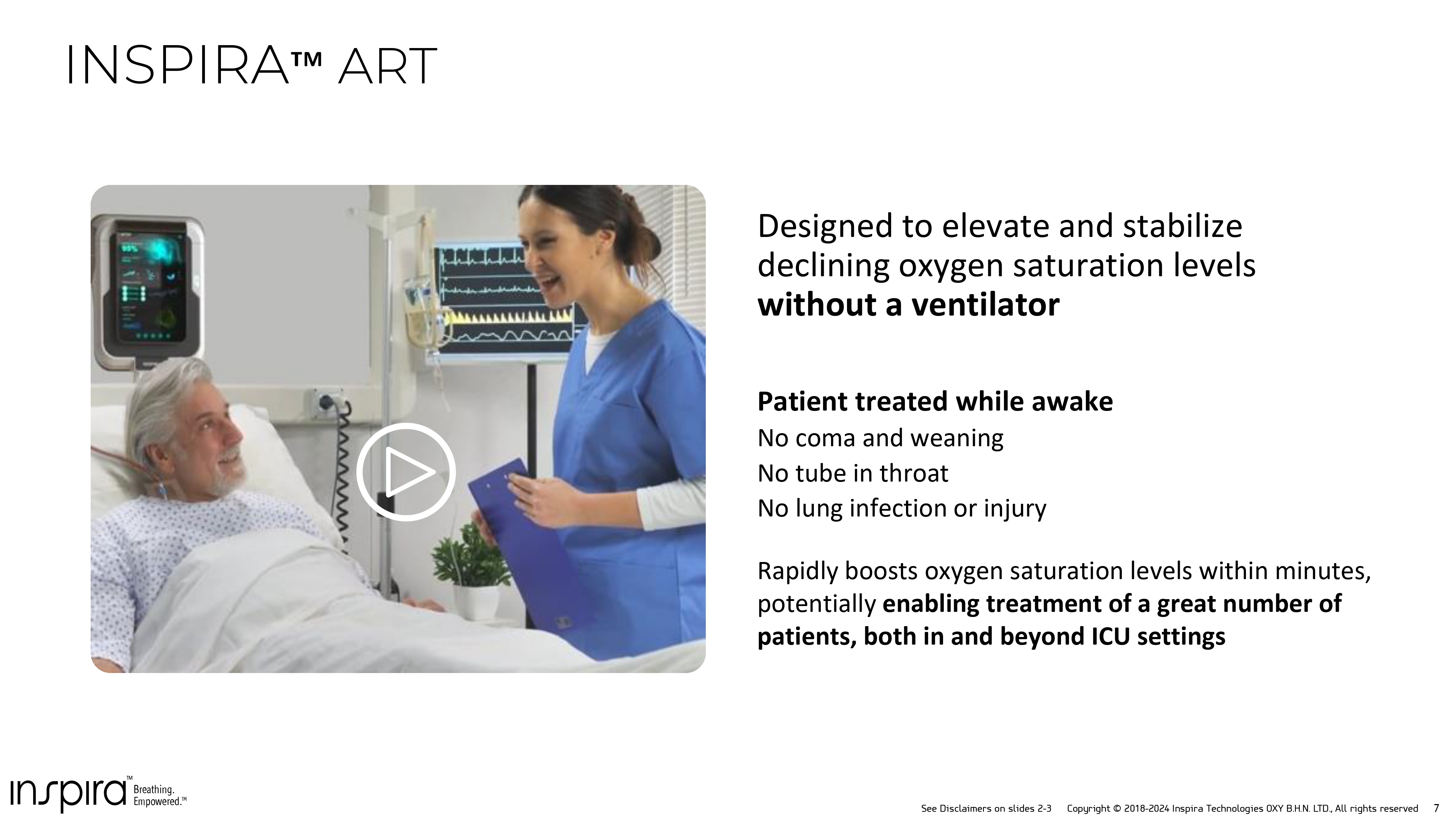
See Disclaimers on slides 2 - 3 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 7 Patient treated while awake No coma and weaning No tube in throat No lung infection or injury Rapidly boosts oxygen saturation levels within minutes, potentially enabling treatment of a great number of patients, both in and beyond ICU settings Designed to elevate and stabilize declining oxygen saturation levels without a ventilator INSPIRA ART

See Disclaimers on slides 2 - 3 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 8 Disruptive Core Technologies of INSPIRA ART VORTX A Direct Oxygenation Delivery Technology Being designed to defuse oxygen into the blood and remove carbon dioxide with minimal contact with foreign materials to avoid blood trauma • Targets to replace legacy Hollow Fiber Membrane technology associated with blood cells destruction and clotting HYLA A Clip - on Optic Blood Sensor Technology Being designed with state - of - the - art algorithms that utilize light to continuously monitor blood parameters in real - time • Without physical contact with the blood • Aiming to reduce intermittent actual blood samples • Provide early detection and decision supporting data WhitePaper Oxygenators V 11 _ 5.1.2023

See Disclaimers on slides 2 - 3 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 9 *The INSPIRA ART 100 System is FDA 510 (k) cleared for use in an extracorporeal perfusion circuit to pump blood during short - duration cardiopulmonary bypass procedures lasting 6 hours or less INSPIRA ART 100 Cardiopulmonary Bypass* FDA 510 (k) - cleared Inspira has a proven track record taking the INSPIRA ART 100 all the way from R&D through regulatory clearance to deployment ** **We expect to deploy the INSPIRA ART 100 by the end of 2024
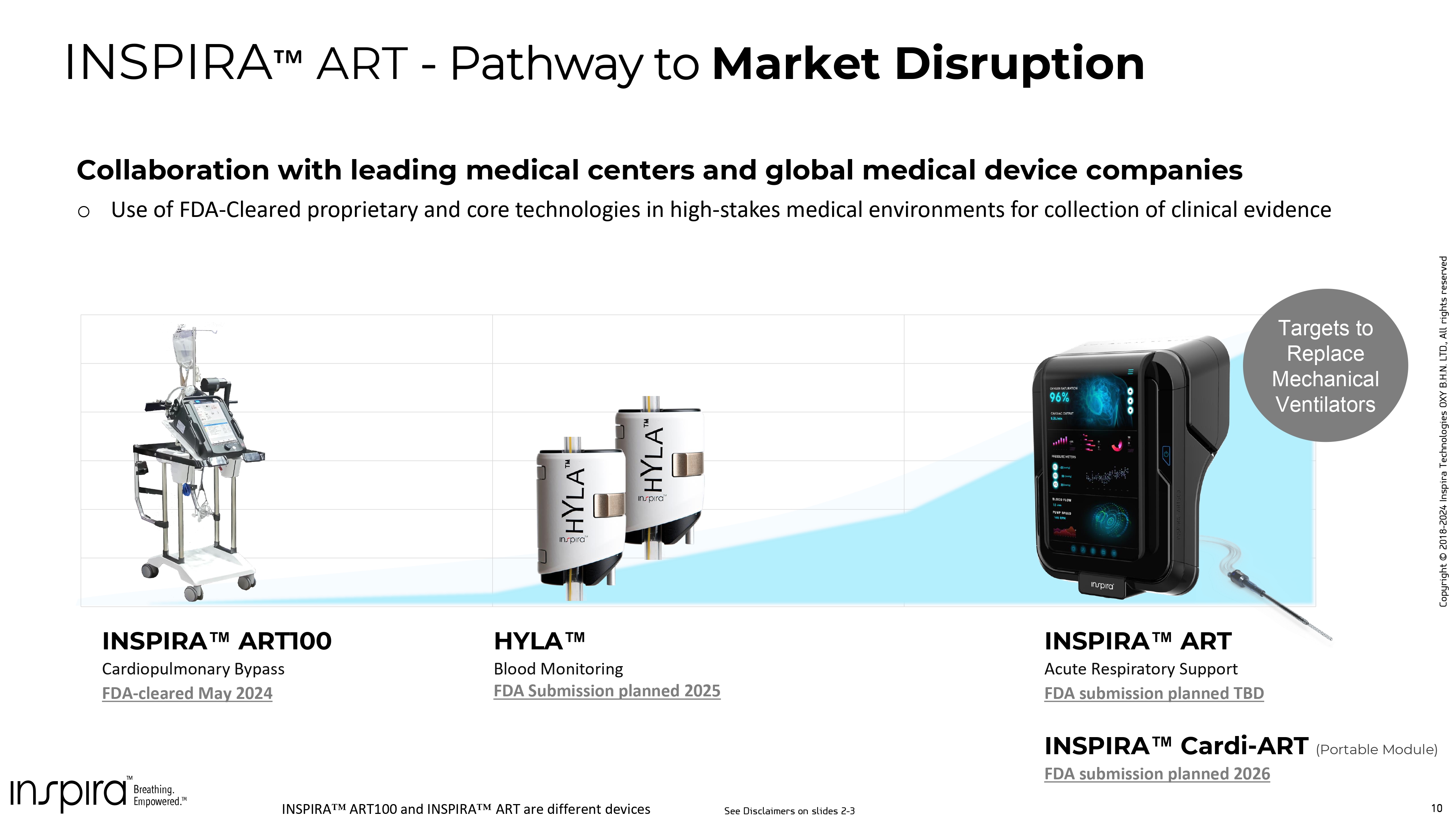
10 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved Collaboration with leading medical centers and global medical device companies o Use of FDA - Cleared proprietary and core technologies in high - stakes medical environments for collection of clinical evidence Category 1 Category 2 Category 3 Category 4 INSPIRA ART 100 Cardiopulmonary Bypass FDA - cleared May 2024 HYLA Blood Monitoring FDA Submission planned 2025 INSPIRA ART - Pathway to Market Disruption Targets to Replace Mechanical Ventilators INSPIRA ART Acute Respiratory Support FDA submission planned TBD INSPIRA Cardi - ART (Portable Module) FDA submission planned 2026 INSPIRA ART 100 and INSPIRA ART are different devices. The timelines assume certain approvals by regulatory and or other authorizing authorities and are subject to change. There i s i nherent risk and variability in the overall regulatory process and no guarantee as to the success of any device or the approval or clearance of such devices by any regulatory authorities and or other authorizing authorities. See Disclaimers on slides 2 - 3

See Disclaimers on slides 2 - 3 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 11 Dr. Daniella Yeheskely - Hayon, PhD CTO Renowned Expert in the field of Artificial Lung Development Yafit Tehila , CPA CFO & Legal Serial Financial management experience in public companies Dagi Ben - Noon, BSc Co - Founder & CEO Co - founded Nano Dimension Nasdaq: NNMD Joe Hayon , MBA Co - Founder & President M&A experience & track record Elscint Technologies, Arazim Avi Shabtay COO Serial New - tech development & delivery track record Prof. Benad Goldwasser, MD, MBA Executive Chairman Urologic surgeon, inventor & entrepreneur. Multiple well - known industry exits Dr. Dekel Stavi , MD Medical Director Senior Intensive Care physician at Tel Aviv Sourasky Medical Center. Chairman of the Israeli ECMO Society INSPIRED TEAM

See Disclaimers on slides 1 - 16 . Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 12 See Disclaimers in deck on slides 3 Copyright © 2018 - 2024 Inspira Technologies OXY B.H.N. LTD., All rights reserved 12 TARGETING TO DISRUPT THE $ 19 BILLION MECHANICAL VENTILATION MARKET $ 19 Billion Predicted Global Mechanical Ventilation Market By 2030











