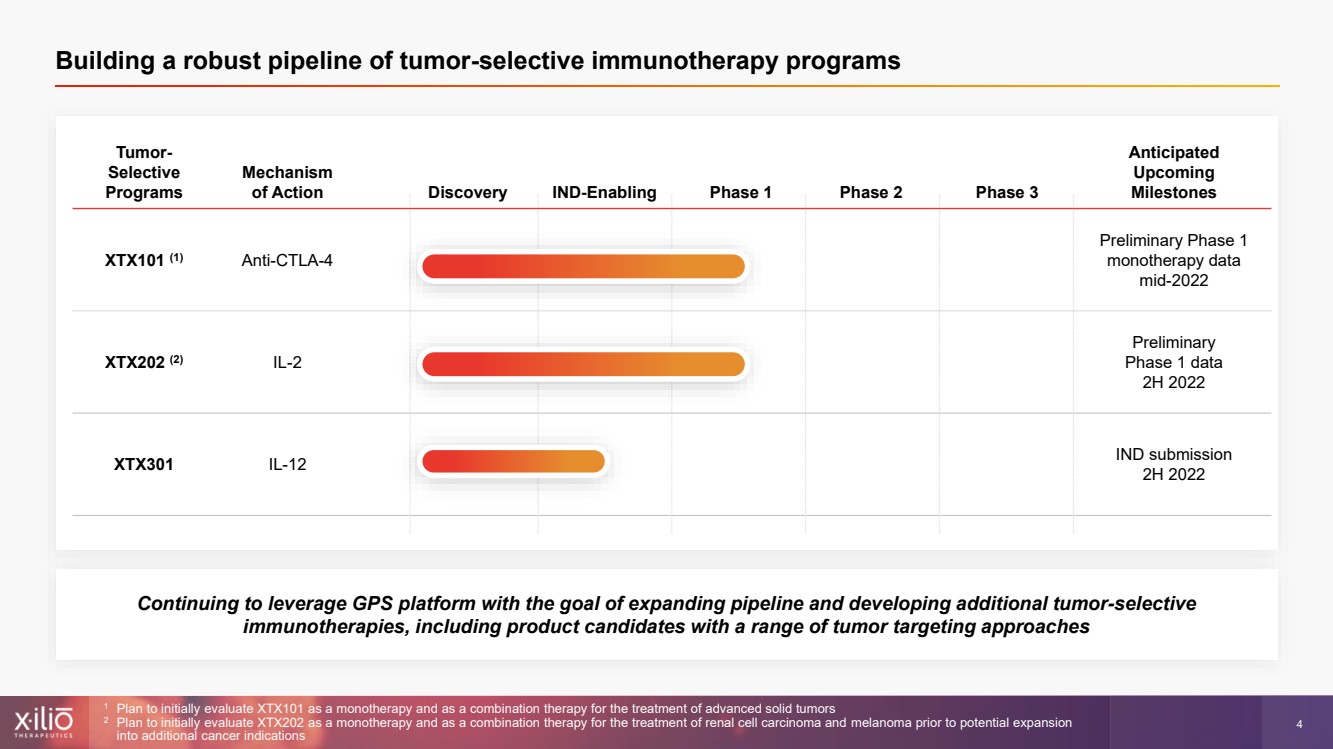
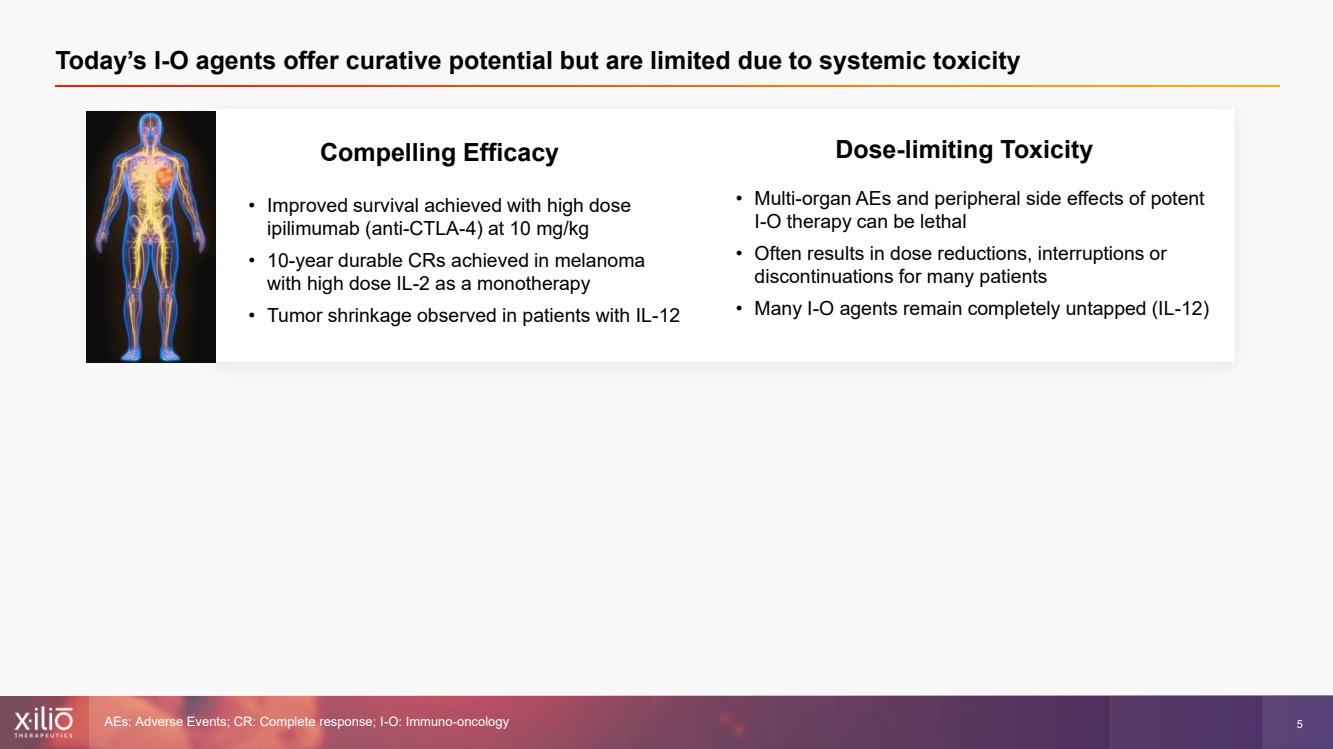
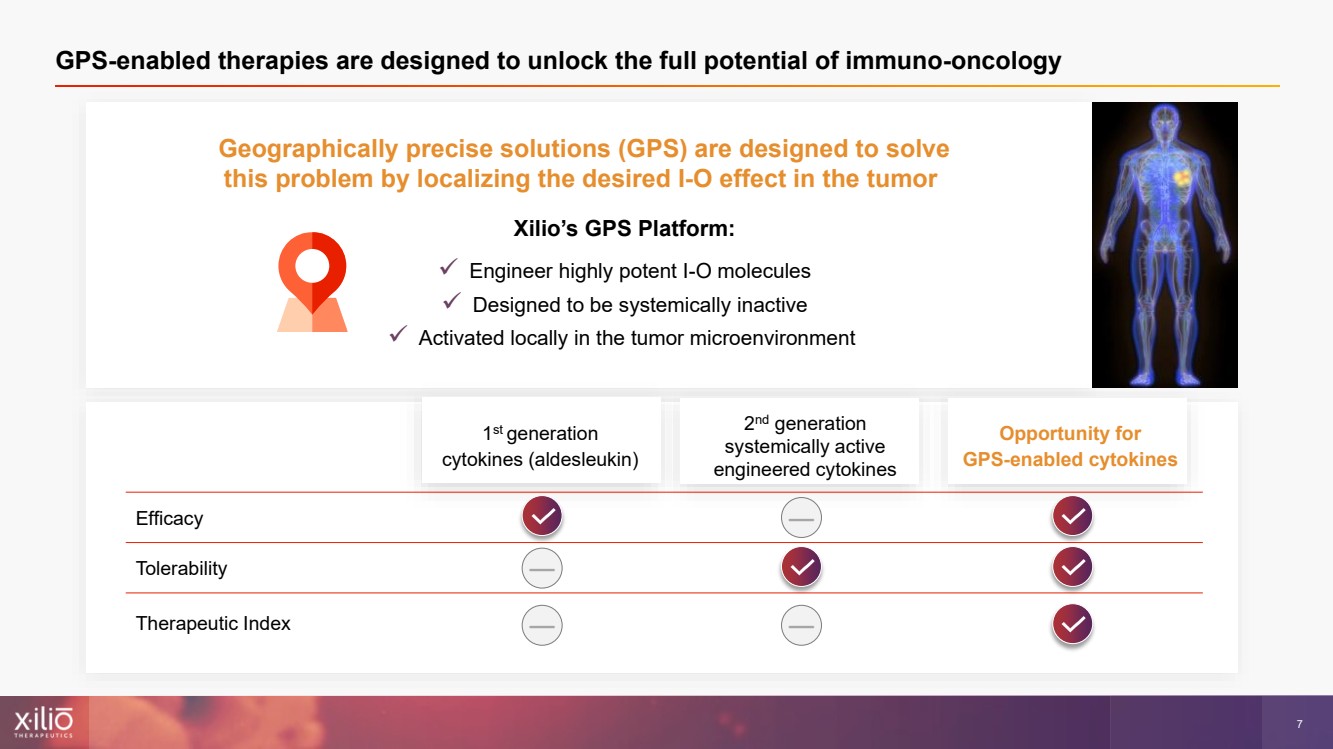
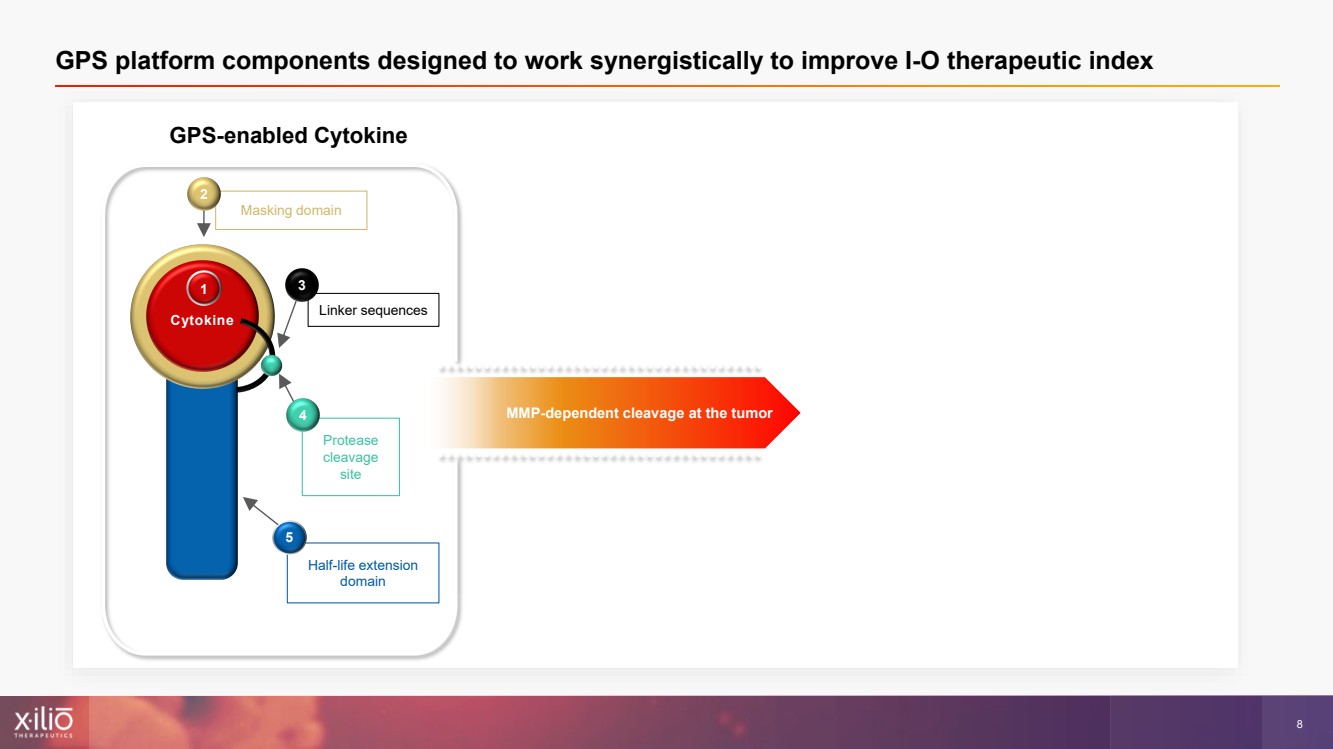
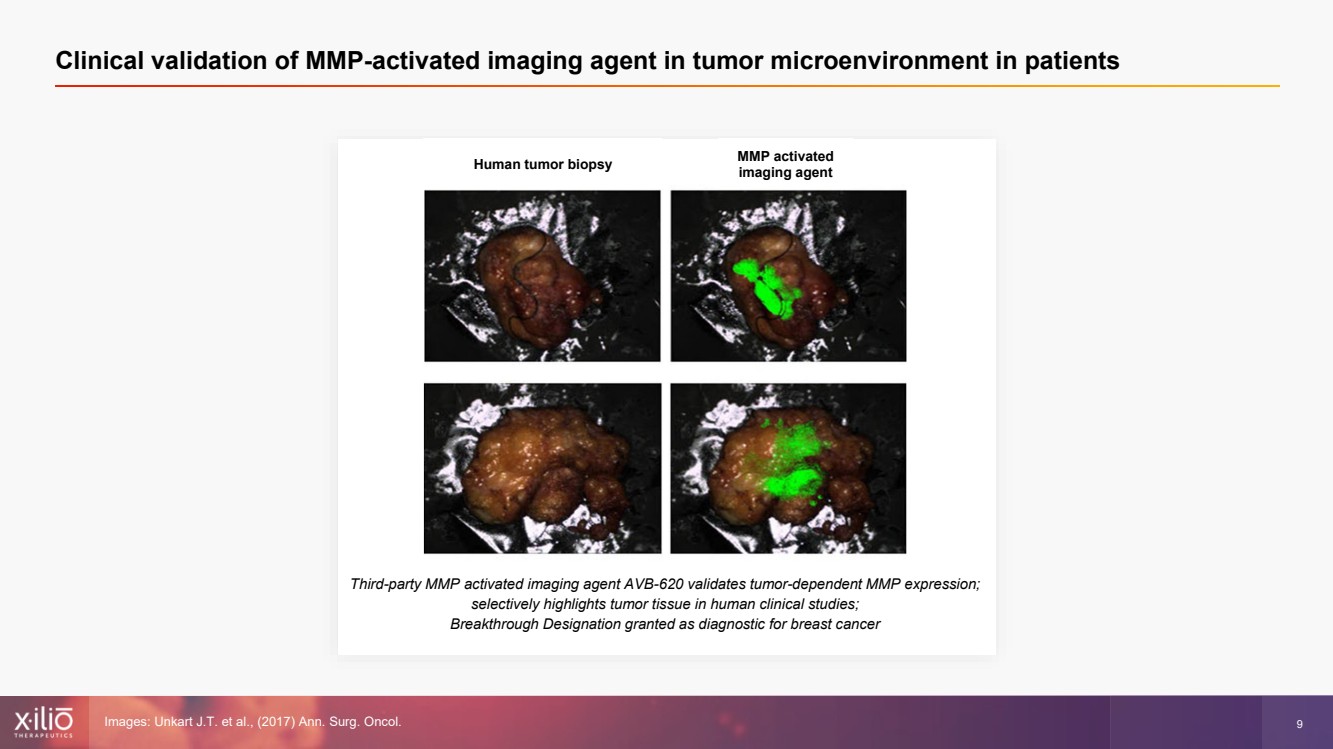
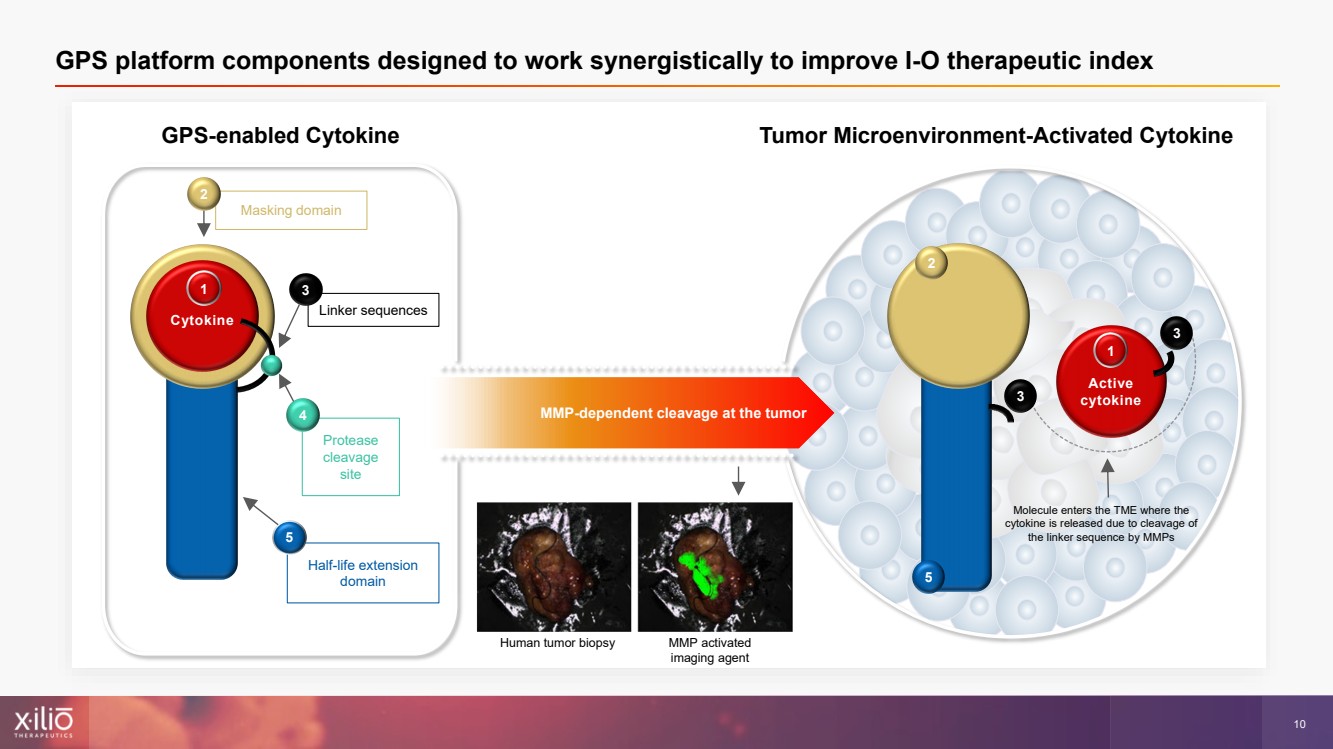
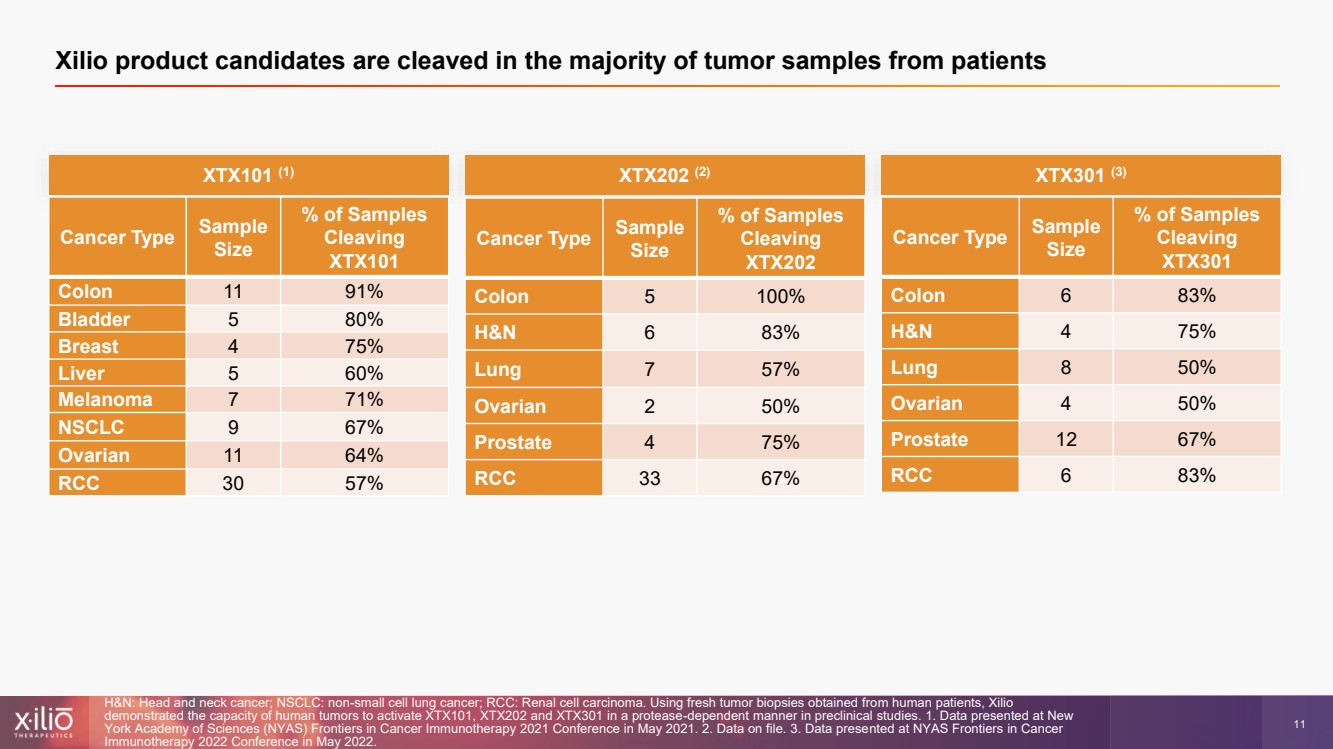
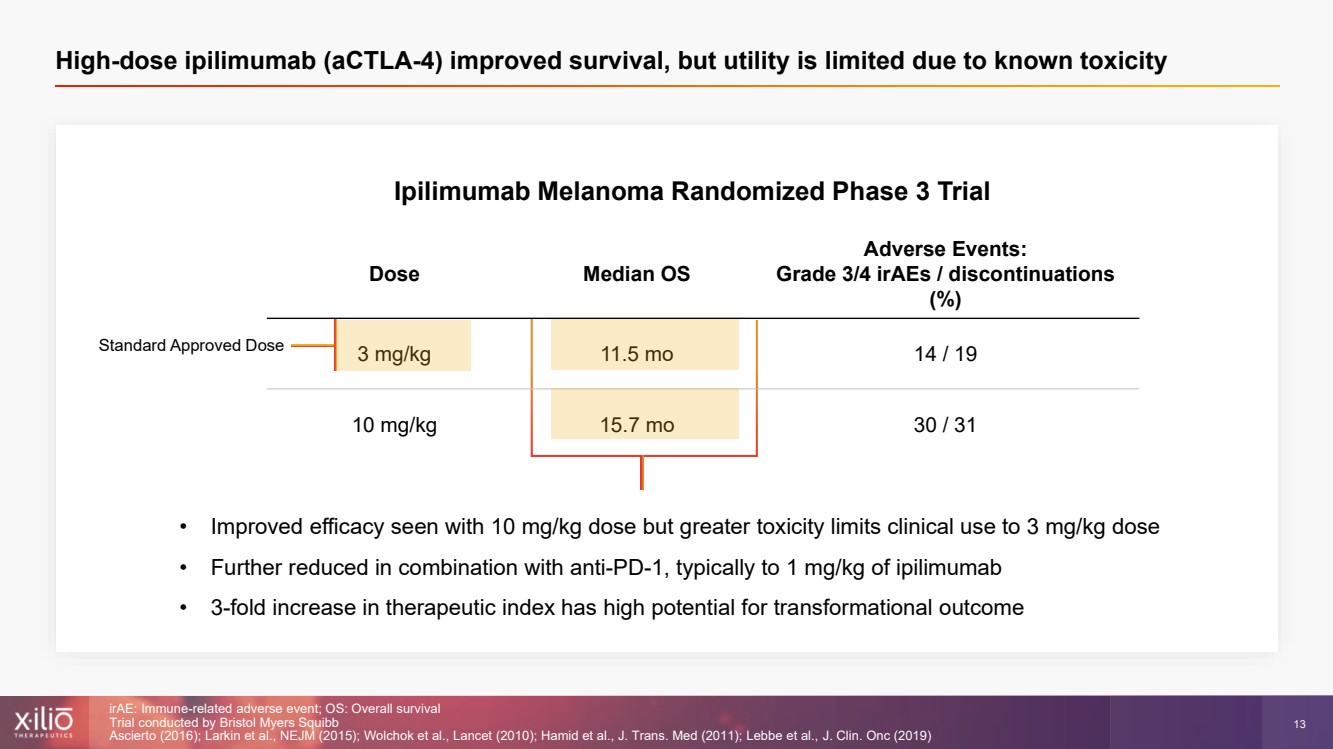
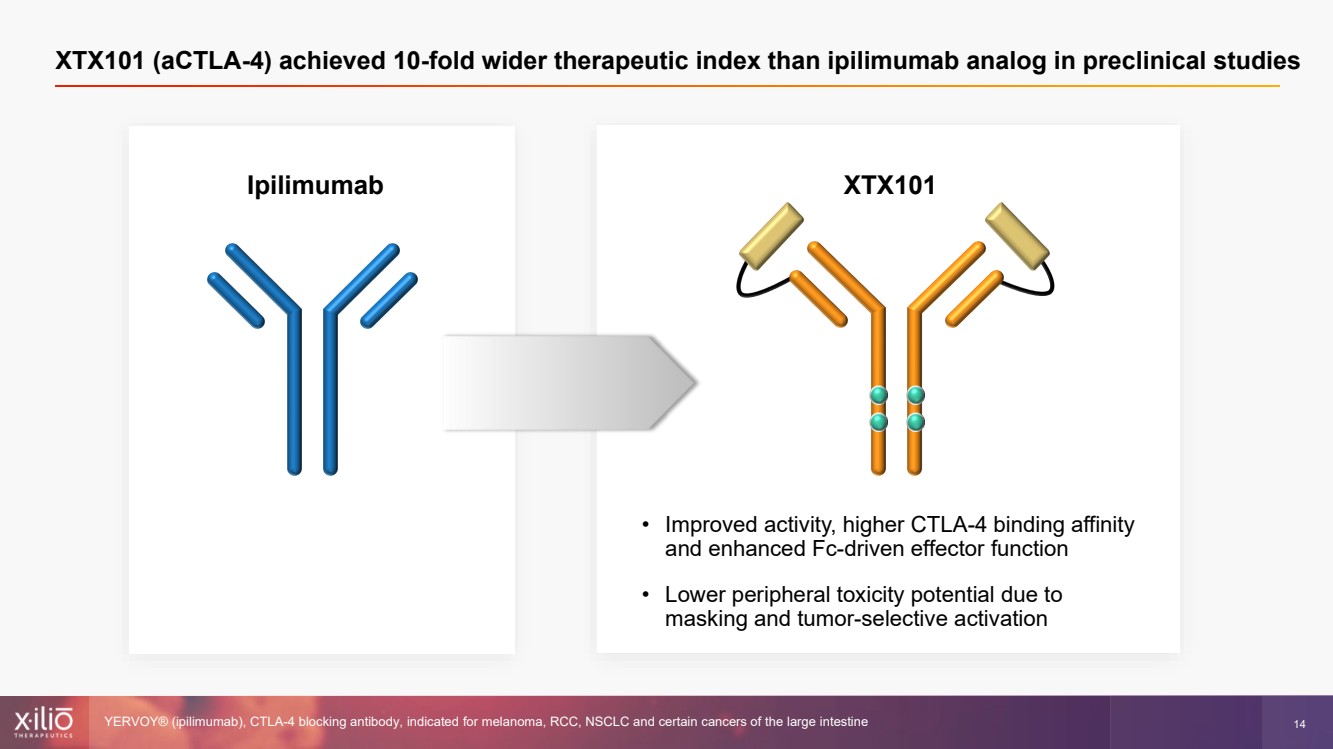
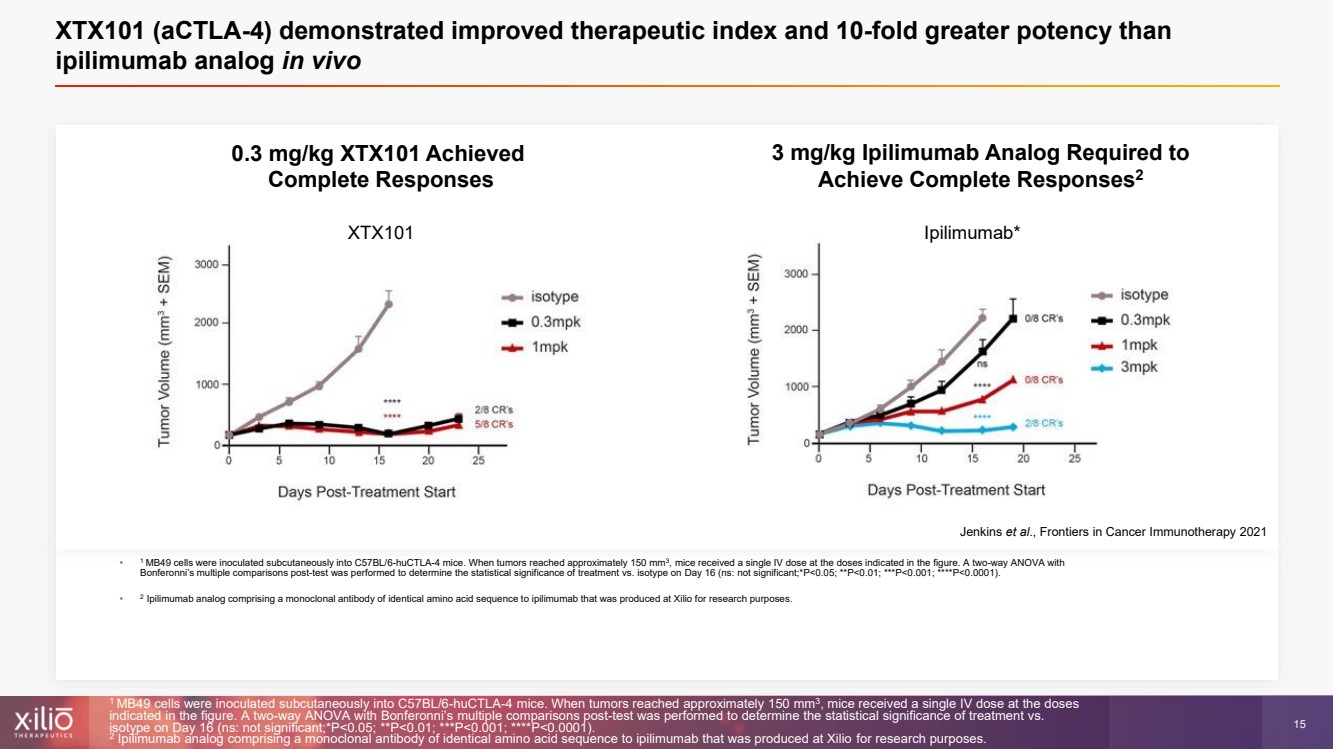
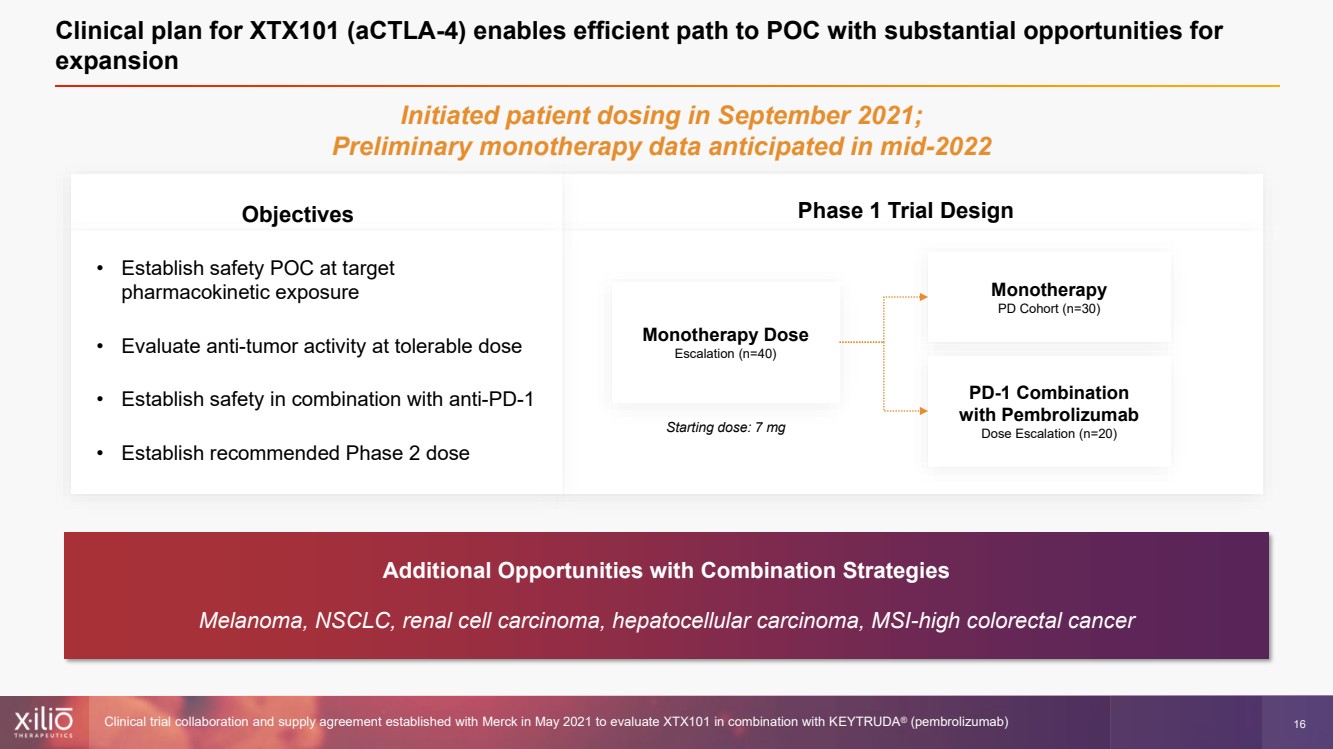
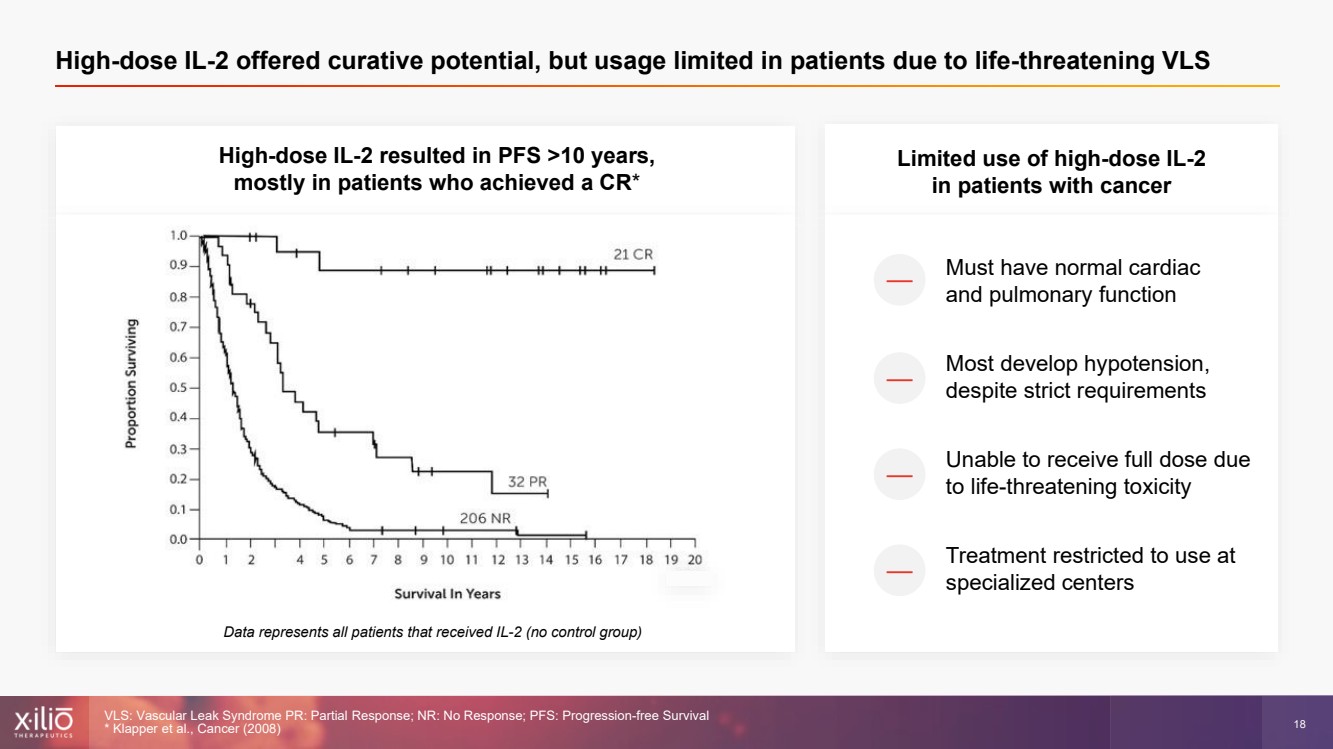
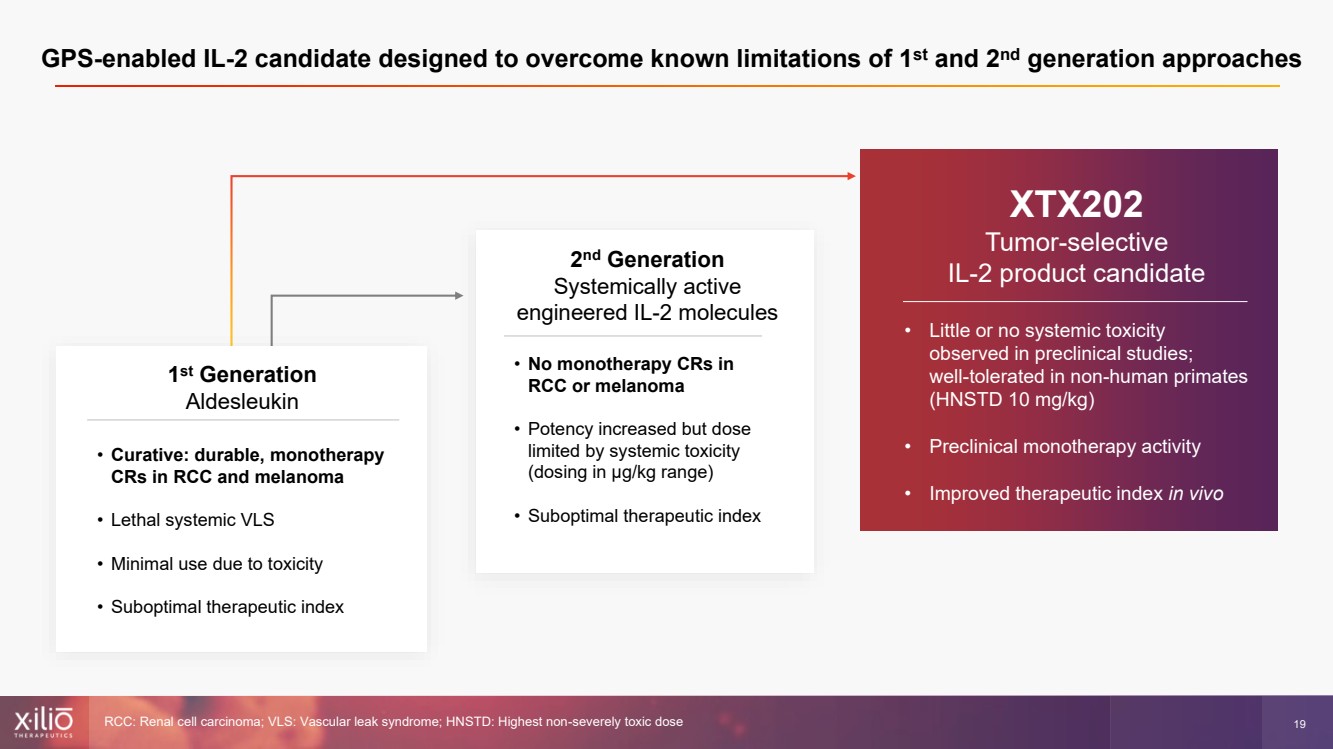
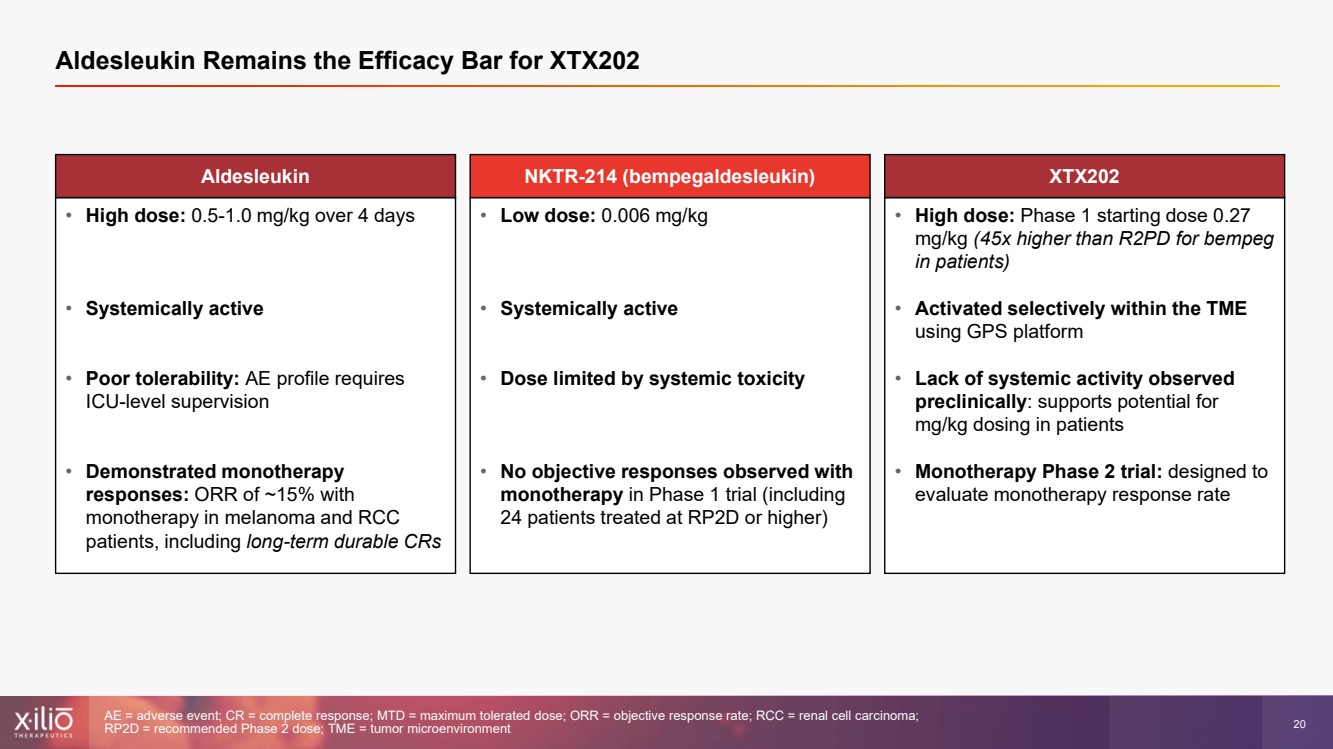
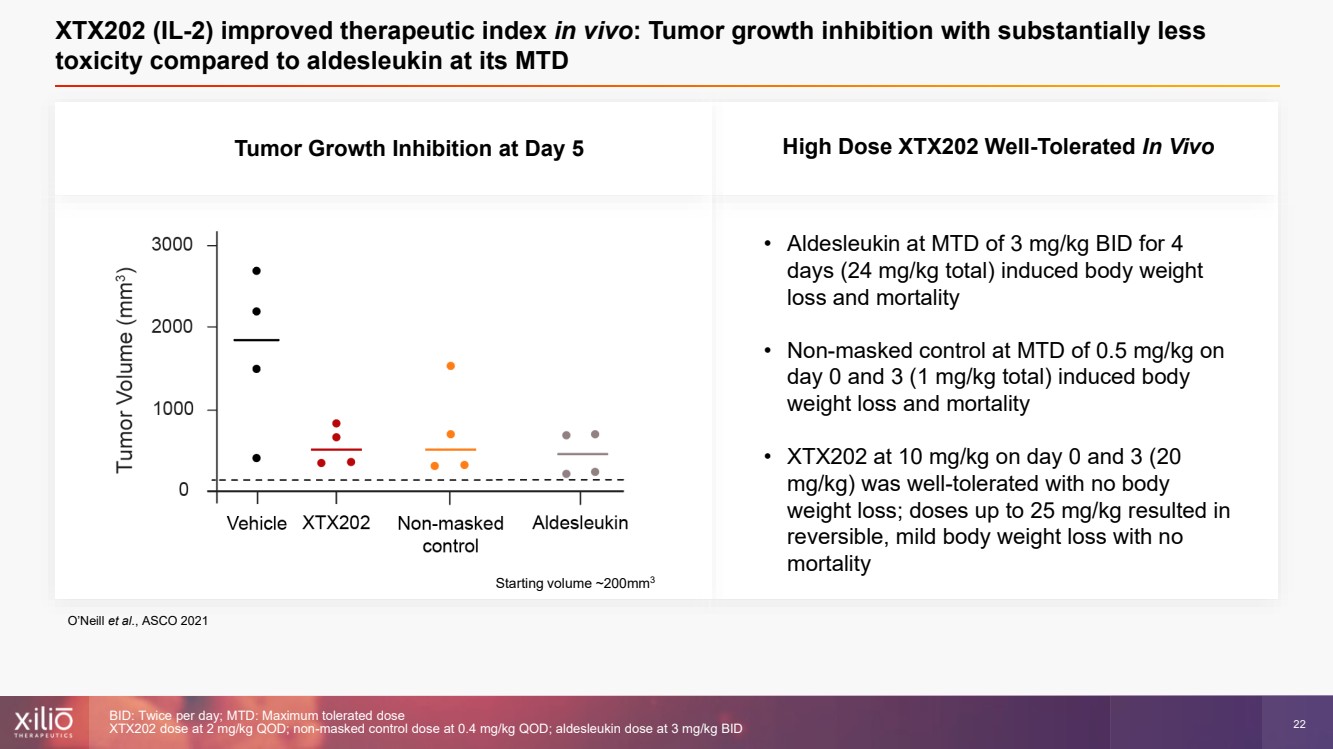
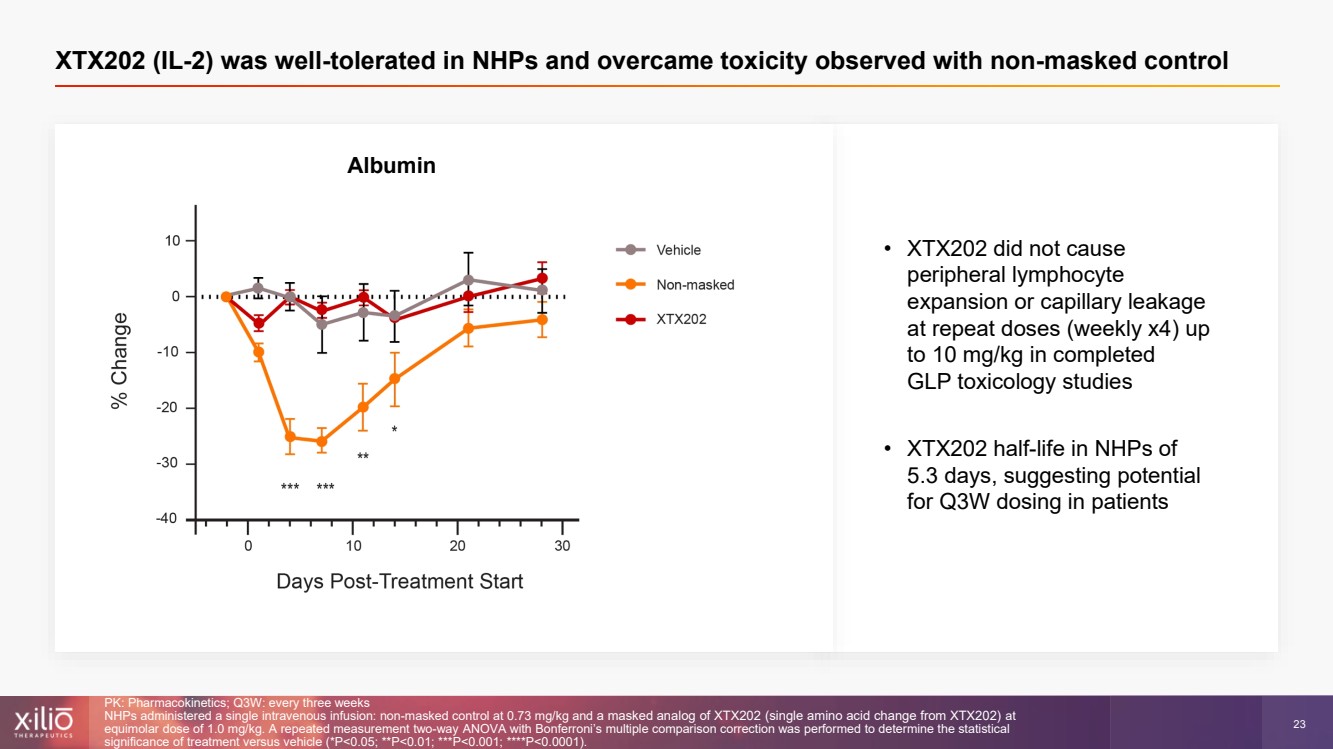
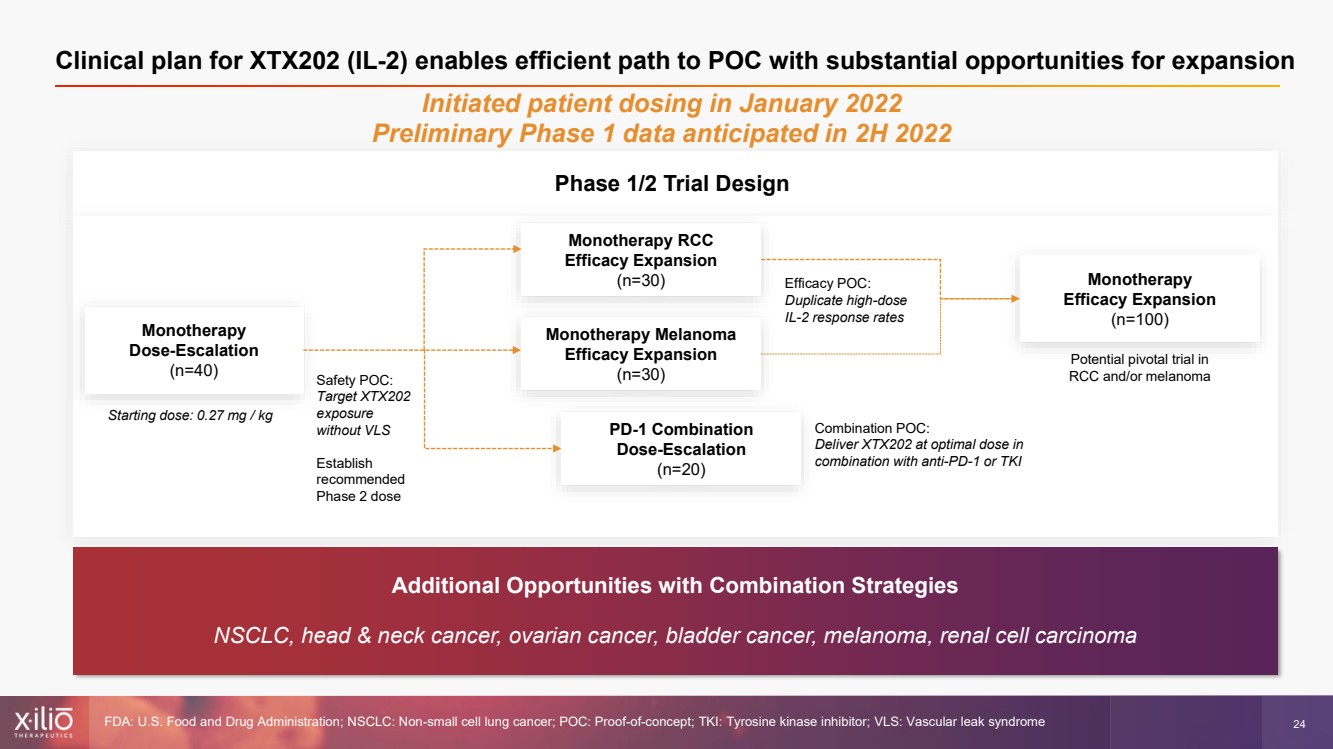
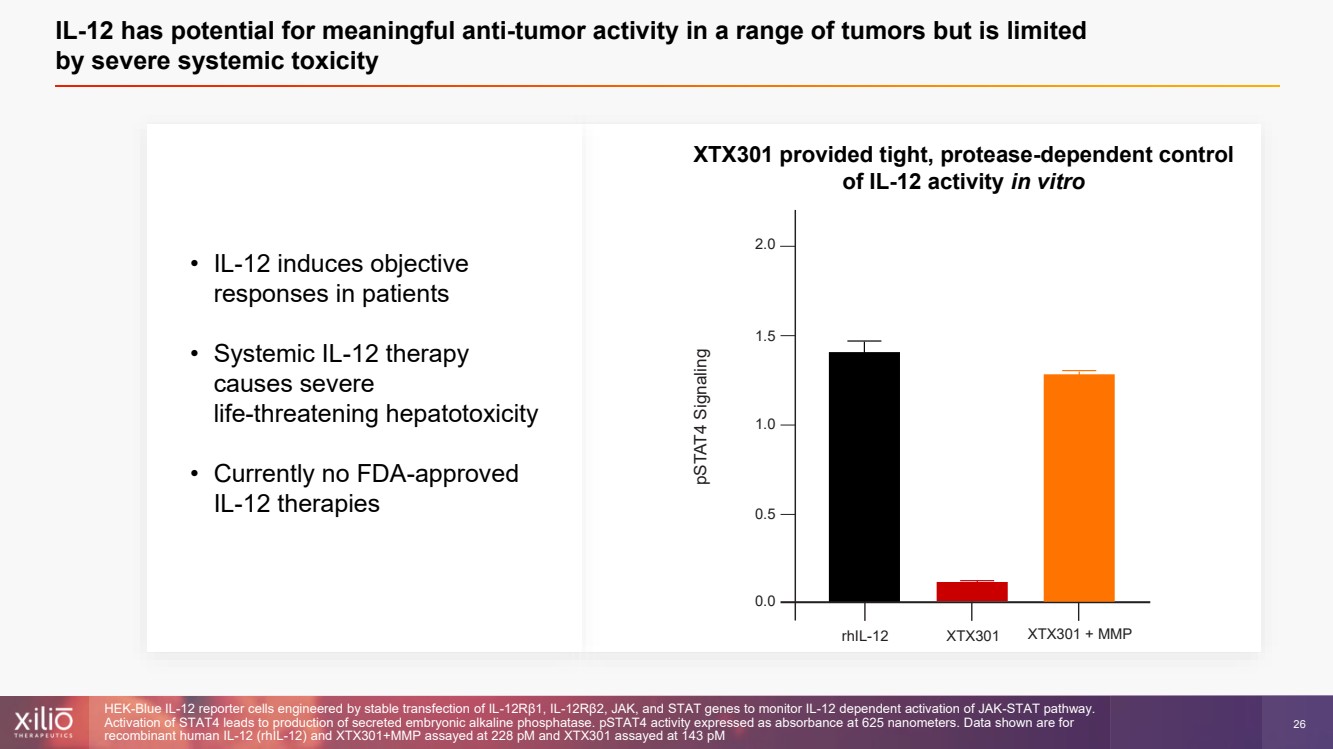
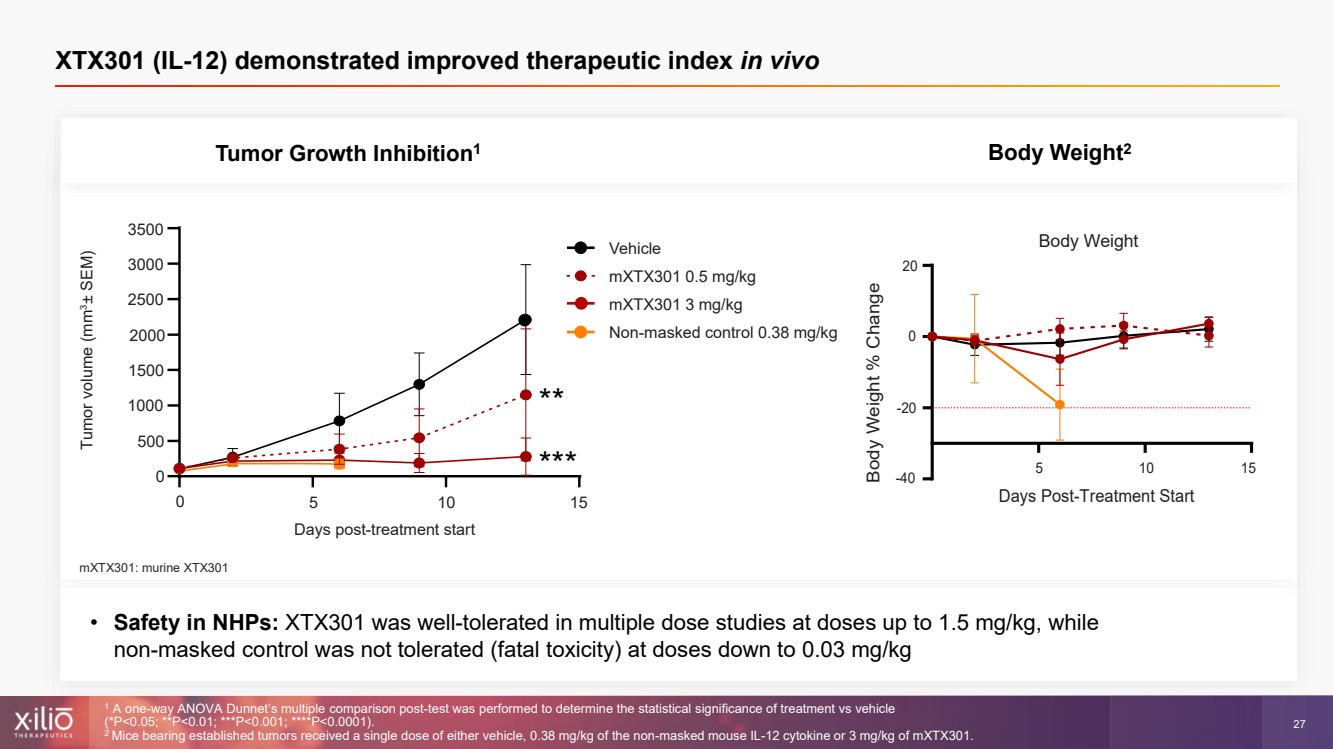
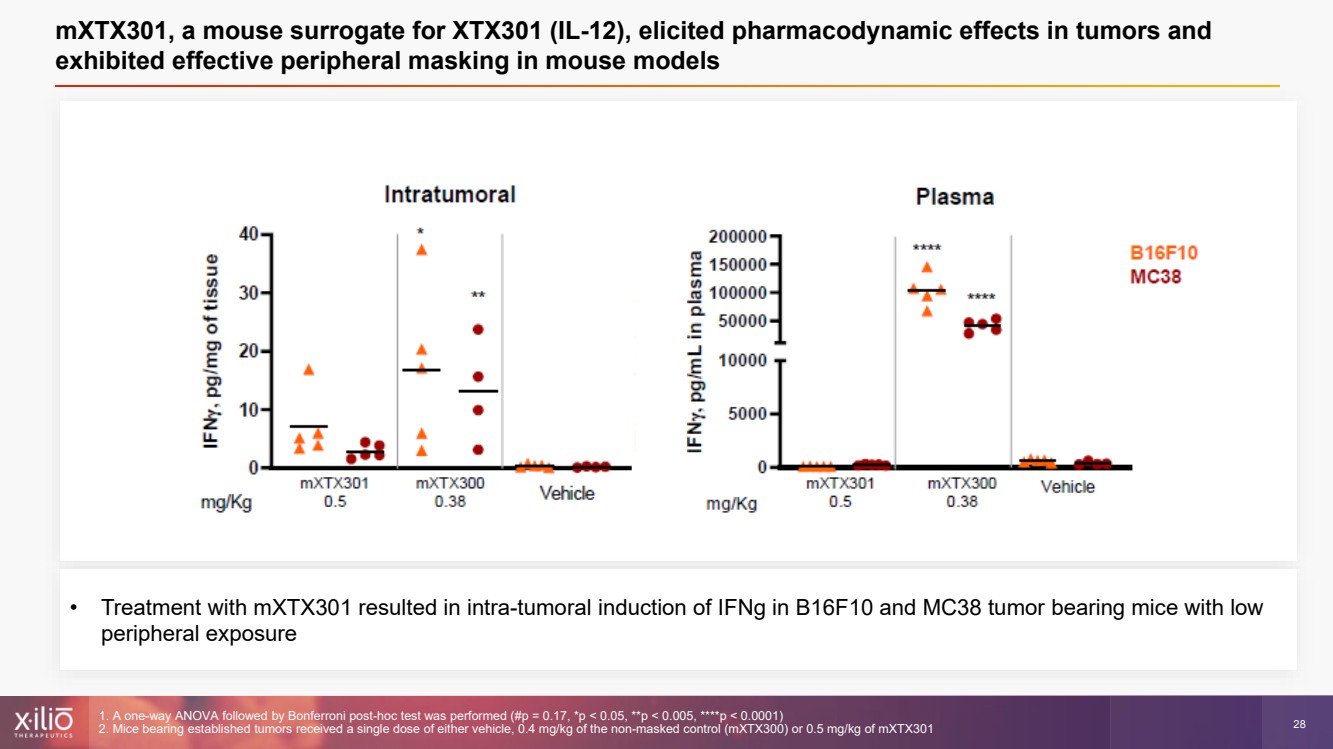
| 2 This presentation contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 , as amended, including, without limitation, statements regarding plans, strategies, timelines and expectations for Xilio’s current or future approved product ca ndidates, including without limitation, plans and timing related the presentation of preliminary clinical data for XTX101 and XTX202 and the submission of an IND for XT X301; the potential benefits of any of Xilio’s current or future product candidates in treating patients; Xilio’s ability to fund its operating expenses and cap ital expenditure requirements with its cash and cash equivalents; and Xilio’s strategy, goals and anticipated financial performance, milestones, business plans and focus .. The words “aim,” “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “pr edict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward - looking statements, although not all forward - looking statement s contain these identifying words. Any forward - looking statements in this presentation are based on management’s current expectations and beliefs and are subject to a number of risks, uncertainties and important factors that may cause actual events or results to differ materially from those expressed or implied by any forward - lo oking statements contained in this presentation, including, without limitation, risks and uncertainties related to ongoing and planned research and development act ivities, including initiating, conducting or completing preclinical studies and clinical trials and the timing and results of such preclinical studies or cl ini cal trials; the delay of any current or planned preclinical studies or clinical trials or the development of Xilio’s current or future product candidates; Xilio’s ability to obtain and maintain sufficient preclinical and clinical supply of current or future product candidates; Xilio’s advancement of multiple early - stage programs; X ilio’s ability to successfully demonstrate the safety and efficacy of its product candidates and gain approval of its product candidates on a timely basis, if at all; results from preclinical studies or clinical trials for Xilio’s product candidates, which may not support further development of such product candidates; acti ons of regulatory agencies, which may affect the initiation, timing and progress of current or future clinical trials; Xilio’s ability to obtain, maintain and enfo rce patent and other intellectual property protection for current or future product candidates; the impact of international trade policies on Xilio’s business, including U.S. and China trade policies; Xilio’s ability to obtain and maintain sufficient cash resources to fund current or future operating expenses and capital expenditure require men ts; and the impact of the COVID - 19 pandemic on Xilio’s business, operations, strategy, goals and anticipated milestones. These and other risks and uncertainties are described in greater detail in the section entitled “Risk Factors” in Xilio’s fil ing s with the U.S. Securities and Exchange Commission (SEC), including Xilio’s most recently annual report on Form 10 - K and any other filings that Xilio has made or may ma ke with the SEC in the future. Any forward - looking statements contained in this presentation represent Xilio’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Except as required by law, Xilio explicitly disclaims any obligation to update any forward - look ing statements. Forward - looking statements |