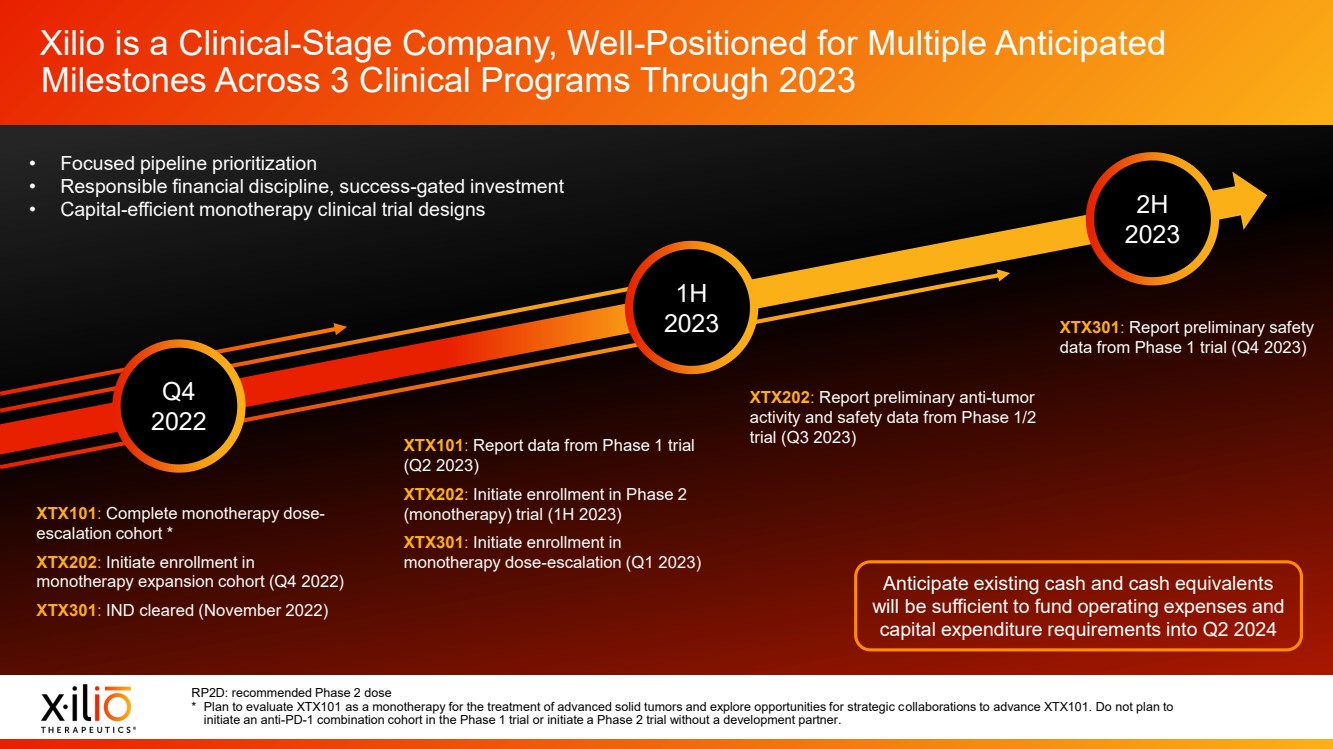
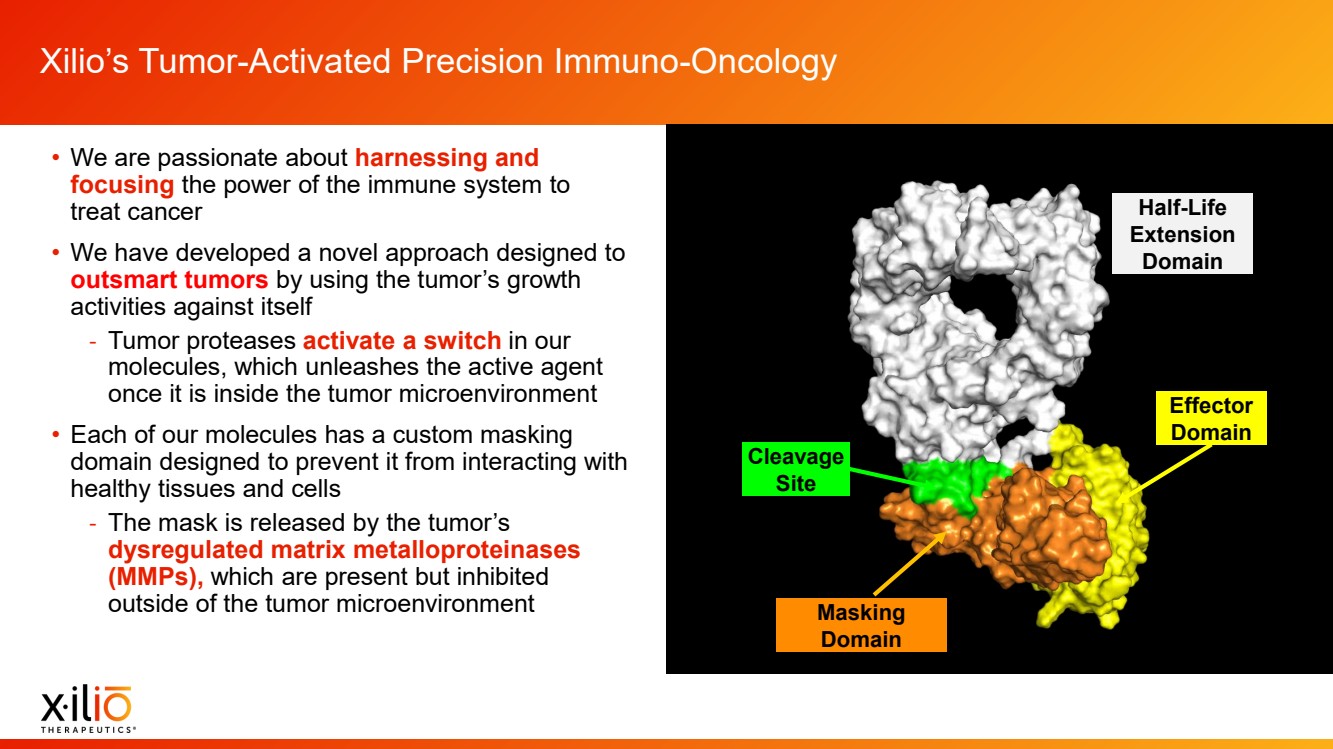
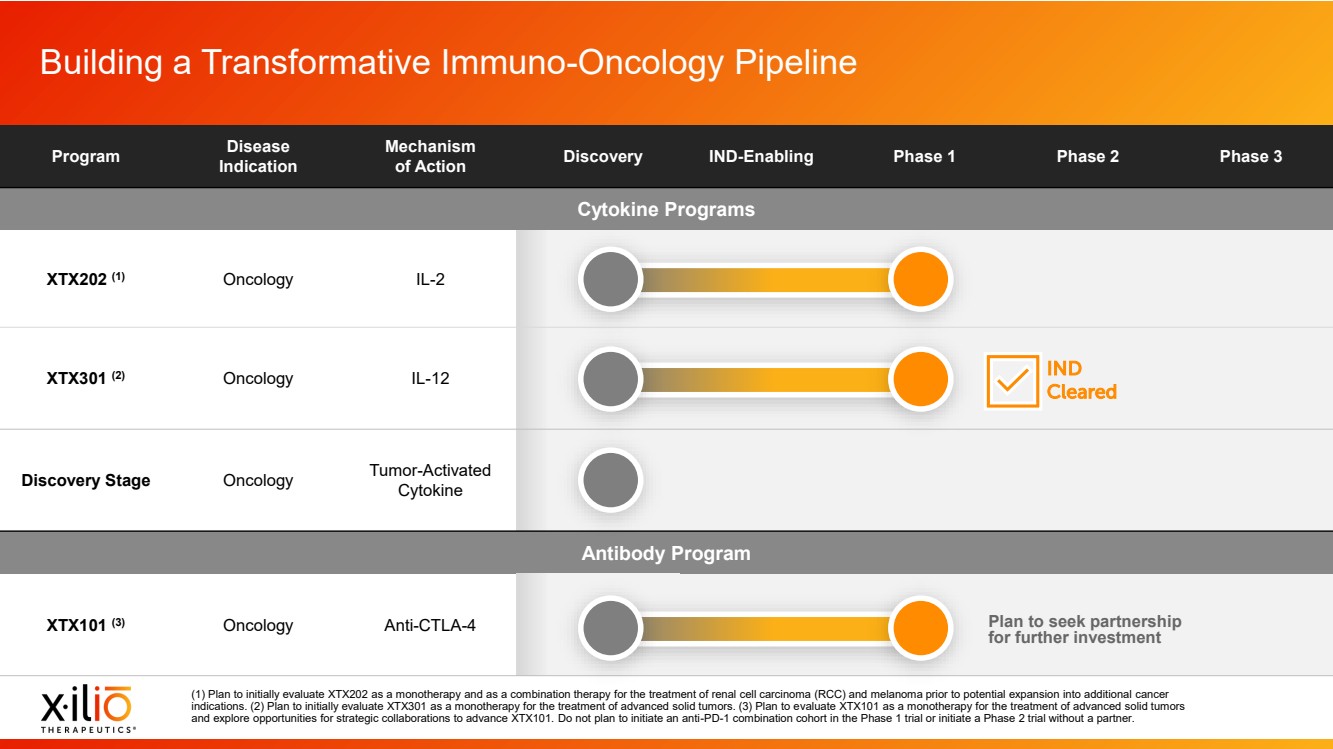
| 2 Placeholder area for citations and references Forward - Looking Statements This presentation contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 , as amended, including, without limitation, statements regarding plans, timing and expectations related to the initiation of patient enrollment in a monotherapy expansion cohort for the Phase 1 clinical trial fo r XTX202, the initiation of patient enrollment in a Phase 2 clinical trial for XTX202 and reporting data from the Phase 1/2 clinical trial for XTX202; plans, timing and expectations related to the initiation of pati ent enrollment in the planned Phase 1 clinical trial for XTX301 and reporting data from the Phase 1 clinical trial for XTX301; plans, timing and expectations related to completing monotherapy dose - escalation for the Phas e 1 clinical trial for XTX101 and reporting data from the Phase 1 clinical trial for XTX101; plans, timing and expectations related to potential collaborations to advance XTX101; plans, timing and expectations rel ated to progressing its next research - stage program; the potential benefits of any of Xilio’s current or future product candidates in treating patients; Xilio’s ability to fund its operating expenses and capital expenditure requirements with its existing cash and cash equivalents; and Xilio’s strategy, goals and anticipated financial performance, milestones, business plans and focus. The words “aim,” “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “pr edict,” “project,” “potential,” “continue,” “seek,” “target” and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words. Any forward - lo oking statements in this presentation are based on management’s current expectations and beliefs and are subject to a number of important risks, uncertainties and other factors that may cause actual events or r esu lts to differ materially from those expressed or implied by any forward - looking statements contained in this presentation, including, without limitation, risks and uncertainties related to ongoing and plan ned research and development activities, including initiating, conducting or completing preclinical studies and clinical trials and the timing and results of such preclinical studies or clinical trials; the delay of any current or planned preclinical studies or clinical trials or the development of Xilio’s current or future product candidates; Xilio’s ability to obtain and maintain sufficient preclinical and clinical supply of current or future product candidates; Xilio’s advancement of multiple early - stage programs; there can be no assurance that interim or preliminary preclinical or clinical data or results will be predictive of future preclinical or cli nic al data or results, including, without limitation, the preliminary intra - tumoral pharmacodynamic data reported for a single patient treated with XTX202 who had an optional on - treatment tumor biopsy and was the first patient f or whom a tumor biopsy analysis was available as of the date hereof; Xilio’s ability to successfully demonstrate the safety and efficacy of its product candidates and gain approval of its product candidates on a t ime ly basis, if at all; results from preclinical studies or clinical trials for Xilio’s product candidates, which may not support further development of such product candidates; actions of regulatory agencies, which may a ffe ct the initiation, timing and progress of current or future clinical trials; Xilio’s ability to obtain, maintain and enforce patent and other intellectual property protection for current or future product candidates; Xilio’s ability to obtain and maintain sufficient cash resources to fund current or future operating expenses and capital expenditure requirements; the impact of international trade policies on Xilio’s business, including U.S. and China trade policies; and Xilio’s ability to seek, establish and maintain a collaboration or partnership to develop XTX101 with a collaborator or partner. These and other risks and uncertainties are described in greater detail in the section entitled “Risk Factors” in Xilio’s filings with the U.S. Securities and Exchange Commission (SEC), including Xilio’s most recently filed annual report on Form 10 - K and quarterly report on Form 10 - Q, as well as any other filings that Xilio has made or may make with the SEC in the future. Any forward - looking statements contained in this presentation represent Xilio’s views only as of the date hereof and should not be relied upon as representing its views as of any subsequent date. Except as r equired by law, Xilio explicitly disclaims any obligation to update any forward - looking statements. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data ob tai ned from third - party sources and Xilio’s own internal estimates and research. While Xilio believes these third - party studies, publications, surveys and other data to be reliable as of the date of this presentation, Xilio has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources. In addition, no independent source has evaluated the reasonableness or accuracy of our internal estimates or research and no reliance should be made on any information or statements made in this presentation relating to or based on such internal esti mat es and research. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their resp ect ive owners. |