Confidential Draft No. 4 as confidentially submitted to the United States Securities and Exchange
Commission pursuant to Section 106(a) of the Jumpstart Our Business Startups Act of 2012 on August 14,
2023 and is not being filed publicly under the Securities Act of 1933, as amended.
Registration No. 333-______________
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
CONFIDENTIAL SUBMISSION
FORM S-1
REGISTRATION STATEMENT UNDER
THE SECURITIES ACT OF 1933
Elevai Labs, Inc.
(Exact name of registrant as specified in its charter)
Not Applicable
(Translation of Registrant’s Name into English)
| Delaware | 5912 | 85-1399981 | ||
| (State or other jurisdiction of | (Primary Standard Industrial | (I.R.S. Employer | ||
| incorporation or organization) | Classification Code Number) | Identification Number) |
120 Newport Center Drive, Ste. 250
Newport Beach, CA 92660
866-794-4940
(Address, including zip code, and telephone number, including area code, of principal executive offices)
Dr. Jordan R. Plews
Chief Executive Officer
120 Newport Center Drive, Ste. 250
Newport Beach, CA 92660
866-794-4940
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
William Rosenstadt, Esq. Mengyi “Jason” Ye, Esq. | Ying Li, Esq. Guillaume de Sampigny, Esq. Hunter Taubman Fischer & Li LLC New York, NY 10022
|
Approximate date of commencement of proposed sale to public: From time to time after the effective date of this registration statement.
If any securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act, check the following box. ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ | |||
| Non-accelerated filer ☒ | Smaller reporting company ☒ | |||
| Emerging growth company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until this registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the U.S. Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities, and it is not soliciting offers to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION DATED AUGUST 14, 2023 |
[●] Shares of Common Stock

Elevai Labs, Inc.
This is a firm commitment initial public offering of $[●] of shares of common stock par value US$0.0001 (“Common Stock”) per share of Elevai Labs, Inc. (the “Company”, “Elevai”, “we”, “us”, “our”), a Delaware corporation, which amount we have assumed to be equal to [●] shares of Common Stock using the midpoint of the price range on the cover page of this prospectus. We anticipate that the initial public offering price of our shares of Common Stock will be between $[●] and $[●] per share.
There is currently no public market for our Common Stock. We have applied to have our shares of our common stock listed on the Nasdaq Capital Market under the symbol “ELAB”, and this offering will not close unless such application has been approved. We cannot guarantee that we will be successful in listing on Nasdaq; however, we will not complete this offering unless we are so listed.
We are an “emerging growth company” under the federal securities laws and have elected to comply with certain reduced public company reporting requirements. See “Prospectus Summary-Implications of Being an Emerging Growth Company.”
Investing in our shares involves a high degree of risk. You should carefully consider the matters described under the caption “Risk Factors” beginning on page 15.
We are a “smaller reporting company” as defined under federal securities laws and, as such, have elected to comply with certain reduced public company disclosure requirements in this prospectus and future filings.
Upon the completion of this offering, our outstanding shares will consist of [●] shares of Common Stock, which includes [●] shares of our common stock issuable upon the conversion of all outstanding shares of our convertible preferred stock simultaneously with the completion of this offering (as described below), and assuming the underwriters do not exercise their over-allotment option to purchase additional shares of Common Stock, or [●] shares of Common Stock, assuming the over-allotment option is exercised in full.
Neither the United States Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriter discounts(1) | $ | $ | ||||||
| Proceeds to us, before expenses(2) | $ | $ | ||||||
| (1) | The underwriter, Univest Securities, LLC, will receive compensation in addition to the discounts. The registration statement, of which this prospectus is a part, also registers for sale [●] warrants to purchase shares of Common Stock to be issued to the underwriter (based on the assumed offering price of $[●] per share, the midpoint of the range set forth on the cover page of this prospectus, assuming no exercise of the underwriter’s over-allotment option). We have agreed to issue the warrants to the underwriter as a portion of the underwriting compensation payable to the underwriter in connection with this offering. See “Underwriting” for a description of compensation payable to the underwriter. |
| (2) | The total estimated expenses related to this offering are set forth in the section entitled “Expenses Relating to this Offering.” |
We have granted a 45-day option to the underwriter to purchase up to additional [●] shares of Common Stock, representing 15% of the shares of Common Stock offered at the initial public offering, to cover over-allotments, if any.
The underwriter expects to deliver the shares of Common Stock on or about [●], 2023.
Univest Securities, LLC

The date of this prospectus is [●], 2023
TABLE OF CONTENTS
You should rely only on the information contained in this prospectus, any amendment or supplement to this prospectus or any free writing prospectus prepared by or on our behalf. Neither we nor the underwriter have authorized any other person to provide you with different or additional information. Neither we nor the underwriter take responsibility for, nor can we assure you as to the reliability of, any other information that others may provide. The underwriter is not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. The information contained in this prospectus is accurate only as of the date of this prospectus or such other date stated in this prospectus, and our business, financial condition, results of operations and/or prospects may have changed since those dates.
Except as otherwise set forth in this prospectus, neither we nor the underwriter have taken any action to permit a public offering of these securities outside the United States or to permit the possession or distribution of this prospectus outside the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to the offering of these securities and the distribution of this prospectus outside the United States.
Unless the context otherwise requires, in this prospectus, the term(s) “we”, “us”, “our”, “Company”, “our company”, “our business” and “Elevai” refer to Elevai Labs, Inc. and, unless the context requires otherwise, its subsidiaries.
i
This prospectus contains forward-looking statements. All statements other than statements of historical facts contained in this prospectus, including, without limitation, statements regarding our future results of operations and financial position, business strategy, transformation, strategic priorities and future progress, are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “project,” “believe,” “estimate” or “predict” “or the negative of these terms or other similar expressions. The forward-looking statements in this prospectus are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition and results of operations. These forward-looking statements speak only as of the date of this prospectus and are subject to a number of important factors that could cause actual results to differ materially from those in the forward-looking statements, including the factors described in the sections entitled “Risk Factors” and in our periodic filings with the SEC. Because forward-looking statements are inherently subject to risks and uncertainties, you should not rely on these forward-looking statements as predictions of future events. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.
ii
This summary highlights selected information appearing elsewhere in this prospectus. Because it is a summary, it may not contain all of the information that may be important to you. To understand this offering fully, you should read this entire prospectus carefully, including the information set forth under the heading “Risk Factors” and our financial statements.
Prospectus Conventions
Except where the context otherwise requires and for purposes of this prospectus only, references to:
| ● | “Exchange Act” are to the United States Securities Exchange Act of 1934, as amended; |
| ● | “FY2021”, “FY2022” are to fiscal year ended December 31, 2021, and December 31, 2022, respectively; |
| ● | “cGMP” are to current good manufacturing practices. |
| ● | “CM” are to conditioned media. |
| ● | “Common Stock” are to our Common Stock with a par value of $0.001 per share |
| ● | “hUMSCs” are to human umbilical mesenchymal stem cells. |
| ● | “KOL” are to key opinion leader. |
| ● | “SEC” or “Securities and Exchange Commission” are to United States Securities and Exchange Commission; |
| ● | “Securities Act” are to the U.S. Securities Act of 1933, as amended; and |
| ● | “Elevai”, “ELEVAI”, “our business”, “our Company”, “Company”, “we”, “us”, “our” and “Group” are to Elevai Labs, Inc., a Delaware corporation and, unless the context requires otherwise, its subsidiaries. |
Prospectus Definitions of Commonly Used Terms
For additional context of commonly used terms within this prospectus the following definitions are applicable when used herein:
| ● | “Conditioned Media” refers to the media or solution within which cells have been grown and have released various types of molecules such as growth factors, cytokines, and extracellular vesicles. This media can then be collected and used to study the effects of these molecules on other cells or tissues; | |
| ● | “Comedogenicity” refers to the potential of a substance or ingredient to clog pores and contribute to the formation of comedones (blackheads and whiteheads), which are a characteristic feature of acne. Comedogenic substances are more likely to cause acne or exacerbate existing acne-prone skin. In the context of skincare and cosmetic products, non-comedogenic formulations are preferred for individuals with acne-prone skin to reduce the likelihood of pore-clogging and acne development; | |
| ● | “Extracellular Vesicles” (EVs) are small membrane-bound particles released by cells into the extracellular environment. These vesicles play a crucial role in cell-to-cell communication by carrying proteins, lipids, and genetic material like ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) from one cell to another; |
1
| ● | “Exosomes” refers to small membrane-bound vesicles that are released by cells that are involved in intercellular communication. They contain various types of biomolecules such as proteins, lipids, and nucleic acids, which can be transferred between cells and may modulate and support these natural cellular processes; | |
| ● | “hUMSC” stands for human umbilical cord-derived mesenchymal stem cells. hUMSC are adult stem cells that can differentiate into various cell types. hUMSCs can be isolated from the Wharton’s Jelly of the umbilical cord, and have shown therapeutic potential in various diseases such as osteoarthritis, myocardial infarction, and neurodegenerative diseases; | |
| ● | “Hyperpigmentation” is characterized by the appearance of the darkening of the skin due to the increased production of melanin, the pigment that gives skin its color. It can be caused by various factors such as exposure to sunlight, hormonal changes, and skin injuries. Hyperpigmentation may be treated with various topical agents such as hydroquinone, retinoids, and corticosteroids, as well as with cosmetic procedures such as chemical peels and laser therapy; | |
| ● | “Mesenchymal stem cells” (MSCs) are a type of multipotent adult stem cell that can differentiate into various cell types, including bone, cartilage, muscle, and fat cells. MSCs are found in various tissues, such as bone marrow, adipose tissue, and umbilical cord blood. They play a critical role in tissue repair and regeneration, as well as modulating immune responses; | |
| ● | “Modulation” refers to the regulation or adjustment of biological processes at various levels, including genes, proteins, cells, and physiological systems; | |
| ● | “Tumorigenic” describes the ability of a substance, cell, or process to induce the formation of tumors or cancerous growths. Tumorigenic substances or agents may cause genetic mutations, disrupt normal cell function, or promote uncontrolled cell growth, ultimately leading to the development of tumors; and | |
| ● | “White label” refers to a product that is manufactured by one company but sold under another company’s brand name. |
We have made rounding adjustments to some of the figures included in this prospectus. Accordingly, numerical figures shown as totals in some tables may not be an arithmetic aggregation of the figures that preceded them.
Unless the context indicates otherwise, all information in this prospectus assumes no exercise by the underwriters of their over-allotment option.
We obtained certain industry, market and competitive position data in this prospectus from our own internal estimates, surveys and research and from publicly available information, including private agency, industry and general publications and research, surveys and studies conducted by third parties, including the sources cited in “Business – Market, Industry and Other Research-Based Data”. None of these industry sources or governmental agencies are affiliated with us, and the information contained in this report has not been reviewed or endorsed by any of them. While we have not independently verified the data and information contained therein and such data and information may have been collected using third-party methodologies, we believe that the data and information, including projections based on a number of assumptions, from these third-party publications and reports used in this prospectus is reliable. However, the industries in which we operate may not grow at the rate projected by market data, or at all. Industry publications, research, surveys, studies, and forecasts generally state that the information they contain has been obtained from sources believed to be reliable but that the accuracy and completeness of such information is not guaranteed. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications and uncertainties as the other forward-looking statements in this prospectus. These forecasts and forward-looking information are subject to uncertainty and risk due to a variety of factors, including those described under “Risk Factors”. These and other factors could cause results to differ materially from those expressed in the forecasts or estimates from independent third parties and us.
2
The Company
We are a physician-dispensed skincare company with a focus on modernizing aesthetic skincare. We conduct research and development to advance innovative and science-driven topical skincare that complements the medical aesthetics industry. Upon our founding in 2020, we initiated our research and development phase for our current product formulations. Since 2022, we have principally employed a business-to-business model in which we produce and commercialize a new generation of topical skincare products that contain our proprietary stem cell-derived Elevai ExosomesTM designed to enhance the appearance of skin.
Our exosome manufacturing process from source to skin is known as ‘Precision Regenerative Exosome Technology™’ or ‘PREx™’. PREx™ utilizes advanced patent-pending stem cell processing technology as part of our cohesive production process involving carefully controlled stem cell culture to produce stem cell derived factors that are featured in our topical exosome products. Specifically, as referenced herein “exosomes” are small membrane-bound vesicles that are released by cells that are involved in intercellular communication. They contain various types of biomolecules such as proteins, lipids, and nucleic acids, which can be transferred between cells and may modulate and support these natural cellular processes.
Our proprietary PREx™ biotechnology process yields exosome lots from human umbilical cord-derived mesenchymal stem cells (“hUMSC”) for our specialty physician-dispensed skincare products. hUMSC are adult stem cells that can differentiate into various cell types. hUMSCs can be isolated from the Wharton’s Jelly portion of the umbilical cord and have shown therapeutic potential in various diseases such as osteoarthritis, myocardial infarction, and neurodegenerative diseases. Our products are comprised of topical cosmetic solutions to enhance the appearance of skin. Our products are not drug products or considered regenerative medicine, nor have any of our products received FDA approval. Our cosmetic products are not intended to prevent, treat or cure diseases or medical conditions. Moreover, our cosmetic products are not intended to be injected or delivered intravenously. Instead, our exosome-infused skincare products are topically applied to the skin to aid in the reduction of the appearance of a range of the most common cosmetic skin conditions, including the appearance of skin firmness, oxidative stress, photodamage, hyperpigmentation, and texture of soft tissue deficits, such as reducing the appearance of fine lines and wrinkles.
Market, Industry and Other Research-Based Data
We currently distribute our cosmetics products through two distinct channels, including a business-to-business sales channel where we sell our products directly within the United States and through our distribution sales channel where we sell our products directly to distributors with international or regional reach under exclusive and non-exclusive territorial agreements. We have employed a combination of both distribution channels via distribution agreements and directed business-to-business channels to optimize our sales reach and strategy.
The term ‘physician-dispensed’ refers to a sales channel where cosmetics products are exclusively sold in physician clinics or medically directed businesses by licensed medical professionals or that have a medical professional on staff, such as medical spas. Our products are only available through a medically-directed business and are geared towards nourishing, protecting and supporting healthy looking skin. Such cosmetics products are highly sought after by consumers making them one of the fastest growing segments of the personal care market.1 Consumers turn to cosmetics to enhance the appearance of dull or aging skin and to brighten the skin by lessening the appearance of a myriad of aesthetics concerns such as unwanted pigmentation, acne, melasma and rosacea. They view these products as alternatives to medications and may try cosmetics products before using medicinal products. Physicians also value well designed topical skincare products formulated and manufactured with our biotechnology for their complementary aesthetic effects in conjunction with medications to improve skin appearance and to enhance the benefits of in-office procedures.
Our business-to-business model channel within the physician-dispensed cosmetics skincare market utilizes both online sales, and our trained direct sales force comprised of employed, and independently contracted aesthetic account managers. This business-to-business sales channel is distinct from our leverage of non-exclusive distribution agreements third-party distributors or resellers, who in turn sell our products to end customers. Under distribution agreements our relationship between the seller and the buyer is more indirect, because our distributors serve as an intermediaries, however we believe scaling our product lines through larger distribution sales channels will lead to faster brand expansion, recognition and market reach.
The skincare segment within the physician-dispensed market is projected to grow by a 9.9% CAGR to reach $12.8 billion by 2027 with the US physician-dispensed cosmetics market valued at $5.9 billion in 2020 alone.2 Outside the United States, the physician-dispensed skincare market varies by country due to cultural differences and regulatory requirements. Cultural desires for skin with lighter and more of an even pigmentation have created large and growing aesthetic skincare demands throughout Asia, particularly in Japan, China, Korea, and India. European and certain South American countries, such as Brazil, also present large skincare markets due to the complementary growth in cosmetic procedures and willingness on the part of their consumers to spend discretionary income on aesthetic enhancements. The global physician-dispensed cosmeceuticals market size was valued at $16.52 billion in 2020 and is projected to reach $35.33 billion by 2028, growing at a CAGR of 9.8% from 2021 to 2028.3
| 1 | U.S. Beauty & Personal Care 2023-2026 | Statista. |
| 2 | Physician-dispensed Cosmeceuticals – Global Market Trajectory & Analytics | Research & Markets |
| 3 | Physician-dispensed Cosmeceuticals Market Size, Share & Forecast | Verified Market Research |
3
Current Products and Products in Development
Our products rely on Elevai ExosomesTM that are derived from, ethically sourced and thoroughly tested, human umbilical mesenchymal stem cells (“hUMSCs”) originating from umbilical cord tissue. We purchase our hUMSCs from third parties that source umbilical tissue from consenting donors and are manufactured under current Good Manufacturing Practices (“cGMP”) conditions. We infuse our product lines with exosomes derived from these hUMSCs which are replete with growth factors. Our cosmetic topical products do not contain any living cells but do include our Elevai ExosomesTM. Our products and their safety are regulated by the FDA, however our products and all cosmetics generally do not require FDA approval before being sold. Nonetheless, the FDA may pursue enforcement action against products on the market that are not in compliance with applicable laws. See “Regulations” for more information.
We have integrated the use of stem cell exosomes into our initial product line: our Elevai Post Treatment E-Series™. The E-Series™ is comprised of two post-skincare procedure care products that target the face and neck, and upper chest regions. Our products include Empower™, and Enfinity™ serums, which are sold exclusively through our business-to-business model channel and via our distribution agreements channel.
Empower™ is our after-treatment topical product that supports the appearance of healthy skin and promotes an even toned complexion. Empower™ serum is a concentrated serum, designed specifically for application post ablative procedures and treatments such as such as energy device treatments, mid-depth chemical peels, micro needling, or injectables. Enfinity™ is our continuing care product that we recommend for daily use. Our Enfinity™ daily serum is a stable serum for at-home daily use that contains a blend of Elevai ExosomesTM combined with complementary stem cell growth factors. This daily product contains complimentary skincare ingredients available to support the appearance of healthy skin including Elevai ExosomesTM, vitamin C, hyaluronic acid, and copper peptides. Our exosome-based products, Enfinity™ are designed to remain shelf stable, are subject to minimal degradation over time when used and stored as directed, and do not require freezing or reconstitution prior to each use.
We further believe that our products have the potential to be used in a number of applications and other procedures beyond their current use. For example, we are in the early stages of evaluating the adjunctive use of our exosomes in the promotion of healthy hair growth cycles and noticeable improvement to hair appearance, fullness and thickness.
Competition
The market for medical aesthetic skincare products is highly competitive, and we expect the intensity of competition to increase in the future. Our principal competitors are large, well-established companies in the fields of pharmaceuticals, cosmetics, medical devices and health care.
Our largest direct competitors in the physician-dispensed cosmetic skincare market, inclusive of both distribution and business-to-business market channels for our medical aesthetics cosmetics products include SkinCeuticals, a division of L’Oréal S.A., Skinbetter Science LLC, a division of L’Oréal S.A., SkinMedica, Inc., a division of Allergan, Inc., ZO Skin Health, 51% owned by BlackStone, PCA Skin, EltaMD, each a division of Colgate-Palmolive, Dermalogica, Murad, each a division of Unilever, and Alastin Skincare, a division of Galderma.
Our competitors strictly in the business-to-business channels for medical aesthetics skincare products include The Beauty Company (Nasdaq:SKIN), Waldencast (Nasdaq:WALD), Inmode (Nasdaq: INMD, Evolus (Nasdaq: EOLS), Revance (Nasdaq: RVNC), and Cynosure.
4
Operational and Competitive Strengths
We face competition from both traditional cosmetics brands, such as retail-focused products, as well as other high-end cosmetics brands in the physician-dispensed cosmetics space. We believe the primary competitive factors in our favor is our Elevai ExosomesTM though our company exhibits the following additional operational and competitive strengths:
Our Next Generation Technology and Early Results:
Elevai ExosomesTM remain our key ingredient and main competitive strength, which is produced under proprietary and cGMP-compliant conditions in our state-of-the-art laboratory. We have a proprietary process to stimulate our ethically sourced cGMP grade hUMSCs to produce stem-cell derived exosomes. This process is designed to ensure that our customers consistently receive a stable, and potent product using strict standard operating procedures under laboratory controlled in-vitro culture conditions. Thereafter, we work closely with our formulation partners so that each batch of product is mixed according to our strict specifications. We believe we are one of the few in the physician-dispensed aesthetics industry to incorporate next generation biotechnology into its product lines. We believe that many of our competitors market products that contain inferior synthetic exosomes, exosomes from inferior sources, or ingredients that can be purchased anywhere. We are conducting ongoing sponsored validation studies involving individuals with noticeable skin pigmentation and redness to determine if there is an improvement in the appearance of skin pigmentation and redness issues when our topical products containing our Elevai ExosomesTM are applied daily. Subjects in one of our validation studies were analyzed by an advanced imaging and analysis device called “VISIA” to determine what percentage of those subjects’ facial skin showed evidence of a change in detected levels of hyper pigmentation after twice-daily application of our Enfinity™ daily serum over the course of approximately 12 weeks. After twelve weeks of twice daily topical application of our Enfinity™ daily serum, follow up VISIA scans showed a six to twenty percent reduction in the area of facial skin recorded with hyper pigmentation as compared to their initial VISIA scans. There we found that after multiple-week application of our products, those hyper pigmented regions appeared less dark, less pronounced or noticeable, and the skin appeared to display a more balanced skin tone and texture. This early positive assessment is based on our comparing quantified values of image data that are taken at multiple time points throughout the validation study in order make our well quantified comparison of skin quality at the timepoints recorded. There, the imaging data showed the intensity of the remaining hyperpigmentation on those subjects’ facial skin was visibly reduced as compared to initial VISIA scans. However, we note that we continue to determine if we can better quantify this reduction in pigmentation intensity as further evidence of performance is analyzed over the course of our validation studies. At this early stage, the continued success of positive results of our products is highly subjective to consumers and we have yet to complete formal clinical validation studies with a large cohort to demonstrate support for the performance claims of our products, such as their ability to aesthetically improve the skin. Furthermore, any statements contained herein regarding our topical cosmetic and exosome-containing serums have not been reviewed or approved by the FDA. Similarly, the United States FDA has relatively limited experience regulating cosmetics derived from stem cells, and as of the date of this prospectus, there are no FDA approved medical products utilizing exosomes.
Our Product Quality, Ongoing Research and Seamless Production Process:
Many of our early-stage competitors employ contract manufacturers and labs to handle all portions their production. Our California-based laboratory and production facility helps us protect our trade secrets by keeping our core processes for exosome production in-house and eliminates our need to rely on contractors that may use damaged products of inferior quality, or dangerous/unstable ingredients solely for the purpose of manufacturing our Elevai ExosomesTM. Our streamlined commercialization process is quality controlled from stem cell acquisition, through exosome production, to specifying our standards to our contractors for formulation and bottling, ensuring continuity across the process to limit damage to our product’s exosomes and actives. Additionally, our aesthetic account managers and senior-level staff are highly supportive of our physician clients who rely on the quality of our product literature and educational material. This literature allows our physician clients to provide the best information to their clients whose experience may be ultimately enhanced by choosing to use our product lines post-procedure.
Although we are an early-stage company, we have integrated the production of our Elevai ExosomesTM with our general production process. We do not outsource any aspect of our exosome production process or license any core technology. We also have the capability to commercialize a variety of products derived from stem cells containing innovative encapsulated stem cell produced factors and quickly introduce new competitive products and existing product enhancements. This capability is harnessed by our ability to produce unique ingredient in our own lab like Elevai ExosomesTM. These natural stem cell factors are a core ingredient, and an ingredient that we believe few others can commercialize or approximate. We maintain the ability and know-how to modulate the way the stem cells are cultured in our laboratory space. Through modulation, we are able to produce different versions of our stem cell exosomes, and tailor them for different purposes, such as potentially supporting and promoting a healthy hair growth cycle. As we continue to grow our production outputs, we expect to multiply our modalities and deliver newly and more narrowly tailored versions of exosomes to the market in the form of our cosmetic products.
Although our ultimate goal is to achieve vertical integration, our current focus is on promoting the manufacture of our top-quality products and reducing our costs to produce next-generation cosmetics for the physician-dispensed cosmetic skincare market at favorable price points while generating healthy margins. We believe our products will remain attractive to most consumers by pricing them at rates that are competitive with existing and emerging post-care and aesthetics cosmetics companies while remaining below a pricing tier reserved for more top-end direct-to-consumer products like those from La Mer Technology, Inc. Similarly, we believe that our pricing strategy is competitive with other competing physician dispensed skincare brands that do not contain exosomes. We believe this price point is still attainable for consumers in the physician-dispensed cosmetic skincare market even though our products employ the integration of topical exosomes that is in a similar class as existing skincare products, but through a newer manufacturing process which we believe allows our brand to market better quality and more purified extracellular vesicles in our products. Thus, we chose to favorably price ourselves at the top of the range that we believe the physician-dispensed cosmetic skincare market will positively respond to.
5
Our Products Ease of Use, Quality Ingredients, and Post-Procedure Benefits:
We believe our products often complement the experience- and improve the results-of most physician in-office or medical spa aesthetic face and body treatments that include laser treatment, microneedling and ablative surgical procedures. We designed our products to provide benefits without any blood draw or needling. Our products may also ease uneven looking or puffy skin texture associated with the post-procedure healing process by including ingredients that assist in soothing and supporting the skin for the appearance of a more even skin tone.
To attain customer satisfaction with our products after aesthetic face and body treatments, we carefully select high-quality active ingredients to aid in the healing process. These includes hydrating hyaluronic acid and ceramides, to support skin health for any skin type. Alongside our Elevai ExosomesTM, our products are packed with bioavailable forms of vitamin C, and skin-restoring copper peptides. Our products are integrated into post-procedure or treatment protocols and have achieved positive results under third-party dermal safety evaluations. Each of our products underwent clinical dermal safety evaluations and there was no skin reactivity observed at any time over the multi-week study.
We culture our hUMSCs under carefully controlled conditions in our lab without the use of animal components or byproducts, such as Fetal Bovine Serum (“FBS”). Aside from our moral compass, there are many reasons to avoid animal components in our production process in particular. While this includes safety to avoid animal borne viruses, there is more consistency and predictability for high quality exosomes when culturing hUMSCs. Although there is much variability in any animal-derived component, they remain the primary way that most scientists around the world grow cells in laboratories. We aim to ensure that our products do not contain any parabens, phthalates, or animal byproducts, and we never test on animals.
We believe the application of our topical products can reduce redness, brighten skin, improve wrinkles and skin texture to promote healthy looking skin and the appearance of rejuvenation. Depending on consumer needs, our skin products are designed to either be directly applied topically after an aesthetics or ablative procedure or applied daily. At this preliminary stage, the continued success of the early positive results of our products is highly subjective to consumers and we have yet to complete clinical validation studies to demonstrate support for any performance claims of our products, such as their ability to aesthetically improve the skin. Furthermore, any statements contained herein regarding our topical cosmetic and exosome-containing serums have not been reviewed or approved by the FDA.
Established Partnerships with Major Industry Players and Our Local Community:
Our position as an early mover in utilizing topical exosome skincare technology in the physician-dispensed markets has attracted various industry leaders to become our non-exclusive or exclusive partners, creating an extensive network for us to leverage. We believe the expertise and market coverage of both our exclusive and non-exclusive distribution agreements with channel partners broaden our executional capability, reduce our execution risk, and provide immediate market access to increase the speed at which our products can reach the market. These partnerships solidify our position as a smaller company with substantial technological expertise. Additionally, our exclusive and non-exclusive distribution partnerships have allowed our products to enter Asian and Canadian international markets via our third-party distributors, in a capital efficient manner. In addition to our white-label distribution agreement, we may plan to pursue strategic co-development opportunities and arrangements that further enhance our product pipeline to create effective synergies to supplement our product offerings in the physician-dispensed market. Our current collaboration with many high-volume distributors provides valuable knowledge that we believe will enhance our early mover advantage.
On April 1, 2023, the Mitacs-Accelerate Grants Program via the Office of Commercialization and Industry Engagement (OCIE) Dalhousie University in Nova Scotia, Canada awarded our team $90,000 Canadian Dollars under a two-year research grant in relation to a project entitled “Multiomic characterization of stem cell derived extracellular vesicles for supporting the skin.” Under this project, we will engage an intern from Dalhousie University’s Department of Process Engineering & Applied Science under the tutelage of Dr. Stansislav Sokolenko who is responsible for completing a report about the project that is reviewed by their faculty supervisor and presented to our team. The primary aim of this research project, called “ELV3000”, is to establish new and novel techniques for characterizing the bioactive ‘payload’ of our Elevai ExosomesTM in order to provide us with a greater understanding of how specific exosome contents may be attributable to positive skincare outcomes. The secondary aim of the research project will be to further optimize our Elevai Exosomes™ production process to eventually improve our products through the eventual ability to exert greater control over exosome payloads. This detailed characterization will be conducted using a combination of traditional and advanced techniques and will build on other work currently being performed by us and our contract research partners.
Additionally, we partner with local California universities through a federally funded program called “CareerCONNECTED” Federal Work Study (“CCFWS”) to maintain roots in the surrounding area. The CareerCONNECTED program provides low-income students an opportunity to learn real world skills they would not traditionally receive in an academic setting. 60% of the interns’ pay is federally funded and we pay the other 40% of their salary. We benefit immensely from these interns and believe it is mutually beneficial to our growth to work with eager, academic minded individuals that can help us with our more time intensive tasks that slow down our general operations. This in turn helps our lab team reduce production time to make our exosome enriched media. Along with the interns assisting the lab team, we in turn teach them essential lab skills that will benefit them going forward in their science careers. We believe the program gives us an advantage in training future scientists to our specifications and potentially selecting future employees from the intern pool that are already received high quality training that can meet our lab specifications. Any future opportunity to hire our trained interns reduces the time and the opportunity costs that we would normally incur with training a newly hired, full time lab tech.
6
We continue to grow through allying with channel partners, local universities, and strategic investors globally and expect these relationships will enhance our credibility, relationship with the surrounding community, generate better leads, and future conversion of customers. These investments will ultimately enable us to be more agile in achieving our goals in the shortest time and leverage further investment into our technological strengths alongside our partners’ connections and relationships.
Our Well Recognized and Award-winning Team and Brand:
We produce our Elevai ExosomesTM using a proprietary process called Precision Regenerative Exosome Technology, or PREx™, which has been developed and perfected by Jordan R. Plews, PhD. Dr. Plews is a published biochemical engineer with expertise in molecular biology and stem cells, which we believe will enable our ability to scale our concepts as we develop other novel product lines. We believe we can efficiently bridge the knowledge-gap between engineering and processing because of our research and aptitude in both fields. Using both vocations, improves our ability to isolate re-agents and stem-cell material to identify novel proof of concepts on a biochemical and molecular level while efficiently harnessing processes to produce and market those concepts at scale.
Our second founder, Dr. Hatem Abou-Sayed (known professionally as “Tim Sayed MD”), is a double board-certified plastic surgeon with nearly two decades of experience in the medical aesthetics market. Dr. Sayed was instrumental in positioning us as an exosome focused aesthetic skincare brand and forming the foundational engagements with our hUMSC suppliers to build on the established scientific background and legacy of our suppliers. In that capacity he helped to develop the application of our cosmetics products to complement device-based medical aesthetic procedures.
Under both founders’ guidance, we have made a number of strategic hires to assemble our management heads who in turn have recruited an experienced sales and marketing team. Together, our team has a demonstrated its ability to identify new business opportunities and to develop our business by growing our global distribution networks. Similarly, we are privileged to include a number of strategic advisors and consultants as members of our team including James R. Headley, NorthStrive Companies Inc., Kevin Green, and Crystal Muilenburg.
To that end, our brand has received a number of awards and accreditations, and we have been featured in exposés in recognition of our products and innovation. Those awards and recognitions include the People’s Choice Award after presenting at the Octane Aesthetics Tech Summit annual event, as part of the small business accelerator called the LaunchPad SBDC (Small Business Development Center). Additionally, we have been featured in the Aesthetic Guide Magazine, New Beauty Magazine, Grazia Magazine and MedEsthetics Magazine, among others.
Strategy
We believe we have the potential to be one of the most disruptive brands in the physician-dispensed cosmetics skincare market. We are in the early stages of new product development and have significant room to grow by attracting more consumers to the brand, making our current products more widely available and offering more innovative products to our consumers. We expect the United States to be the largest source of our growth over the next few years and see ample opportunity to expand in select international markets. We also believe we have an opportunity to improve our margins through greater operating leverage and efficiency once we begin distributing our product more widely.
Our Technology and Research:
We believe we are one of the first to adapt stem cell technology from cGMP grade hUMSCs to produce purified extracellular vesicles, also referred to as exosomes into topical skincare products to capture market share in the high growth physician-dispensed cosmetics skincare market. This strategy is not only based on our understanding of consumers’ interest in the appearance of a quicker post-procedure recovery, but our research into what the physician-dispensed cosmetics skincare market is currently lacking and our belief in our products’ ability—based on early imaging data leveraging quantitative analysis and visual assessments of photographic progress photos. Our imaging study data is gathered utilizing an advanced imaging and analysis device made by Canfield Scientific, called “VISIA”. This complexion analysis system captures high-quality, standardized images that are monitored following a medical aesthetic procedure at regular intervals to assess redness, discomfort, tone, texture, wrinkles, and other measures of skin appearance.
Since 2020, we have invested in the creation of a commercial process that began in 2022 which leverages the use of hUMSCs to produce extracellular vesicles, or exosomes in our products because not only do these factors have the ability to enhance the appearance of the skin, but they can do so without the tumorigenic or ethical concerns associated with the use of embryonic stem cells or induced pluripotent stem cells.24 Because we recognized the potential of utilizing hUMSCs for the skin, it was natural for us to utilize them as the basis for formulating our products. This is founded on our belief that our products can improve the appearance of skin prone to appearing temporarily red and puffy that is normally experienced by consumers while attending to their aesthetics needs in physicians’ offices or medical spas.
| 24 | Gao, F., et al., Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis, 2016. 7: p. e2062. |
7
A Visionary and Experienced Management Team:
We have made significant investments in our business over the past three years by building our own exosome manufacturing lab, hiring top talent to help us build functional and streamlined capabilities in our commercialization process. Our management team comes from leading international skincare companies, with world-class research, marketing, and e-commerce experience to implement growth strategies and drive operational improvements.
Brand and Product Expansion:
We plan to continue to grow our young brand’s reputation. We plan to continue to expand our brand by attending events, presenting at scientific and medical aesthetic and cosmetic skincare conferences, and conducting clinical validation studies to further validate the aesthetic results of our products. We believe what differentiates us from many traditional cosmetics companies is our lean, but aggressive ability to make fast market-driven decisions and execute with quality control standards. We believe we have a major speed-to-market advantage over many other companies because of our size and aptitude in bioprocessing. Similarly, we are highly responsive to market-trends alongside physician and aesthetics consumer needs alike. We will continue to leverage our executional excellence as we combine our aptitude in stem cell research and bioprocessing while seeking to become the preferred partner of our key customers. Additionally, we have a robust product pipeline that we believe is likely to address the many evolving needs of customers, physicians, and clinicians in the aesthetic and cosmetics market ultimately increasing our branding and the number of customers we serve. Should any of our pipeline products over perform, especially those product lines that may focus on aesthetic needs other than skin appearance it may be advantageous for our branding to spin off those product lines and further focus on our core market: skin improvement and appearance.
Channel Expansion, Production Capacity, and International Growth:
While our current focus is on the physician-dispensed market, we intend to expand into other sales channels including e-commerce by growing the information available on our website and making our products available for purchase through our medical-spa, physician and physician group partners’ websites. We believe being featured on a variety of partner websites will strengthen our brand and provide a unique direct-to-consumer e-commerce model via our business-to-business relationships where our e-commerce partners receive a share of product purchase revenue. Ultimately, our business-to-business model will strengthen our relationships with our physician partners, while an eventual e-commerce model broadens our market exposure and drive traffic and conversion to our other social media profiles. Additionally, we expect our current and prospective exclusive and non-exclusive distribution agreements to penetrate global markets and pique consumer interest not typically within our current reach. We believe both our products and white-label products can drive new market demand for our brand in those international markets that our distribution partners sell our products in.
We intend to expand the production capacity of our products and to develop new pipeline products in response to a number of potential growth factors, including: our organic growth, the development of research and development of pipeline products, and the expected increase in our product popularity, expansions of our distributor networks and channels through exclusive and non-exclusive international distributional agreements, and other potential strategic partnerships with industry leaders. Moreover, in addition to and as a result of the foregoing growth factors we expect an increase in orders for our products and a continuous rise in sales volume in the future based on our market research and estimates. To keep pace with this rise in sales volume, we anticipate the need to expand our production capacity by the end of quarter one of 2024. This expansion will enable us to meet our projected demand and we anticipate doubling our production capacity would cost between $1,500,000 and $2,000,000. This additional capital would cover our expenses relating to expanded capacity, including increased rent, additional lab equipment, and an increase to our overall headcount. We expect a production capacity expansion will lower manufacturing costs through economies of scale and improve overall cost-efficiency and profit margins. Ultimately, we can provide our products at a competitive price, especially to cost-sensitive physicians or medical spa owners, and consumers in the skincare aesthetics market, who may be relatively new to the concept of medical aesthetics cosmetic skincare.
We expect to expand our product offerings in response to this estimated growth, which are subject to additional costs. To expand a single pipeline product, we currently estimate capital requirements of approximately $250,000 for equipment to support the initial development of that product. We further estimate an additional $100,000 worth of funds to arrange for the testing protocol, clinical validations and to fully launch any product at scale. We estimate the operational framework to prepare for the launch of a pipeline product such as a topical haircare product, would take six to twelve months of development work with an additional four to six months to fully scale such product before an official product launch. We estimate that this pipeline development and scaling effort would not likely begin until the end of quarter one of 2024 and conclude until quarter two of 2025. This estimate depends on whether we may need to expand our operations and development work of any new pipeline product, which is dependent on the results of the development work, continued research and initial rounds of validation testing.
8
Currently, we directly distribute our products directly and indirectly. We distribute directly in the United States through both our business-to-business sales channel and indirectly through our sales distribution channel via licensing and manufacturing agreements with third-party distributors. Under our distribution agreements, third party distributors include our products in their suite of domestic and international sales. Under this sales distribution channel, we sell our products to a distributor, who resells our products to the physician practice customers after placing their order in a designated territory that is either exclusive or non-exclusive to that distributor. We have broadened our sales channel to include our cosmetic product offerings at medical spa locations. In March 2023, we engaged in negotiations with a privately held aesthetics and dermatology company that operates thirteen medical spa locations throughout the United States, many of which are housed inside boutique and upscale hotels. In March 2023, we entered negotiations to provide our cosmetic products to the spa operator. In May 2023, we began supplying a number of that company’s medical spa locations that provide ablative services with our complementary cosmetic products. We aim to supply products to three of these medical spa locations by July 2023 to their customers who receive micro-needling, and laser treatments. Our principal goal is to expand our relationship with the medical spa company in order to provide our cosmetic product to all thirteen locations by May 2024. To that end, we plan to negotiate additional agreements with the remaining locations throughout 2023.
To bolster our regional sales, we entered into a non-exclusive authorized distribution agreement in August 2022 with Refine USA, LLC (“Refine”), one of the preeminent manufacturers and distributors of innovative aesthetic medical device technologies and clinical grade skincare. Under the agreement, Refine may purchase unlimited quantities of our topical cosmetics subject to minimum order size limits and distribute them throughout the United States to their network of consumers and physicians. As we continue to enhance our own United States sales channels, we are focused on bringing on third-party distributors in key large international markets, such as Canada, Europe, Brazil, Southeast Asia and the Middle East. We also plan to drive the distribution of our products through strategic relationships in specific countries, such as Japan. To continue growing in the physician-dispensed market, we intend to onboard more direct sales representatives that can reach geographic markets we currently do not have a presence in and partner with cosmetic device companies to co-market and sell our products. To this end, in January 2022, we signed a white label non-exclusive authorized global distribution agreement with premier aesthetic device company DermapenWorld Inc. (“DermaPenWorld”), which expanded the reach of our branding and serum integration. Under a ‘white label’ agreement our products or ingredients may be combined with another company’s ingredients or products and sold under that company’s brand name. Essentially, under these arrangements we provide products or ingredients that can be customized with branding and packaging to match the branding of the company that will be selling the product directly. Pursuant to that white label agreement, we provide a shared distributor wholesale quantities of our serums that are formulated together with DermapenWorld’s and products which are marketed globally subject to previously agreed upon key performance indicators. In addition, between February and May 2023, we entered three exclusive distribution agreements with three separate distributors in Canada, Kuwait, and Vietnam whereby those distributors are permitted to promote, market, sell and distribute our products within their designated countries. Under these exclusive distribution agreements, we granted each a nonexclusive, nontransferable, royalty free license to use our branded products and marketing literature. Because we are entering new markets under each contract, we also arranged for each distributor to assist us in obtaining any required registrations, licenses and other applicable governmental approvals necessary to import, sell and distribute our products at our expense. On June 26, 2023, pursuant to the terms of our white label non-exclusive authorized global distribution agreement with DermaPenWorld, we sent a termination letter to DermaPenWorld providing notice that we will not renew the distribution agreement upon its expiration. The termination notice is effective as of the end of business on January 16, 2024.
Once commercially viable, as our manufacturing process becomes more vertically integrated, we may expand our business model to provide mesenchymal stem cells (MSCs) to companies needing MSCs, large-scale stem cell culturing, or those companies developing MSC-derived products. This expanded role would position the company as a contract manufacturer and distributor of MSCs because we may become a dependable and well-regarded source of exosomes enriched cosmetic products which have been designed for topical application to assist in the recovery after the delivery of medical aesthetic services. Nonetheless, we aim to remain focused on developing and marketing topical products that are enhanced by unique stem cell derived additives and possibly expand from there into other high-science aesthetic skincare applications, given our biochemical engineering and bioprocessing prowess and ability to manufacture laboratory-based skincare additives ourselves. Thus, any expansion into our role as a manufacturer and distributor of MSCs would only come to fruition if funding and bandwidth were such that we felt we met all our aesthetic market goals and had remaining capacity, or we identified an overwhelming interest and opportunity to benefit from such a paradigm shift. In expanding under this strategic channel, we would likely partner, license, or outsource any non-aesthetics-based business so as not to complicate or defocus our Elevai product brand.
Research and Development
Our research and development efforts are focused on improving and enhancing our existing products as well as developing new products. We undertake research and development on new product formulations and execute studies on existing products and future products that demonstrate what we believe to be the high-quality design of our formulas and the powerful performance of our products.
While we currently primarily focus on bringing physician-dispensed cosmetic aesthetics products to market and supporting the skin, we are in the process of researching and developing applications for hair, both on the face and head, and have ongoing research into additional, customized applications. Currently, many companies and competitors alike talk about exosomes as though they are one single ingredient with the innate ability to do many different jobs. However, research shows that certain exosomes released by certain cells are directly correlated with the cells they originate from and those particular exosomes’ capabilities and contents vary based on the cell type used and the way those cells have been treated or cultured.5 Thus, this research shows the contents of exosomes vary widely depending on the cell type used to generate them, their culture conditions, their processing and storage conditions, and how they are applied or used. Knowing this, we use highly-trained professionals to isolate and culture our hUMSCs and the resulting secreted exosomes are produced using strict protocols.
After formulation, all pipeline products are tested for integrity, safety, and performance. Prior to launch, our pipeline products undergo several safety tests, including, but not limited to, human repeat insult patch tests, used to help predict the likelihood of induced allergic contact dermatitis, comedogenicity tests, to prevent the product clogging pores, and cumulative irritation tests, to evaluate the skin irritation potential and safety of individual ingredients or cosmetic compounds. Our products and their ingredients are also tested at multiple steps in the process to avoid any microbial contamination.
| 5 | Kugeratski, Fernanda G., and Raghu Kalluri. “Exosomes as mediators of immune regulation and immunotherapy in cancer.” The FEBS journal 288.1 (2021): 10-35; Lobb, Richard J., et al. “Oncogenic transformation of lung cells results in distinct exosome protein profile similar to the cell of origin.” Proteomics 17.23-24 (2017): 1600432; Camussi, Giovanni, et al. “Exosome/microvesicle-mediated epigenetic reprogramming of cells.” American journal of cancer research 1.1 (2011): 98. |
9
We currently work with Radyus Research to utilize a number of advanced analytical techniques that we believe will help us improve our current processes and keep our brand at the forefront of exosome product production. To analyze our exosomes, we and Raydus Research leverage NanoSight, a nanoparticle tracking analysis instrument so we may evaluate the proteomic characteristics (or characterization of the protein makeup) of our exosomes. Through this thorough process we access the make-up of our finalized exosomes while balancing the efficiency of different adjustments to our cell-culturing production process.
We are also in the early stages of evaluating the adjunctive and topical use of our exosomes on promoting healthy hair growth cycle and hair fullness through the use of pipeline serums, shampoos, and conditioners. We hope and expect our studies to show if topically applied, our hUMSC’s exosomes will have a positive impact as it relates to noticeably reducing the appearance of hair shedding. We anticipate this effect will be primarily through hydrating and nourishing the scalp and topically applying nutrients to the scalp.
Manufacturing
We have exclusively developed our manufacturing process through our management team’s experience in formulating robust skin care products, which we believe provides us with a competitive edge. Success in manufacturing our exosomes requires refined processes that are reliable, scalable, and economical. In our lab, we grow our ethically sourced stem cells and trigger them such that they produce exosomes under our proprietary method.
As of the date of this prospectus, we own or have an agreement in principle for the right to purchase the related manufacturing processes, methods, and formulations. Moreover, we also oversee our leased laboratory space in California which operate under Good Laboratory Practices (“GLP”) and adhere to Good Manufacturing Practices (“GMP”) for the production of our cosmetic products. Moreover, our products are formulated by a third-party in an FDA inspected facility that adheres to GMP guidelines because GMP guidelines promote the manufacture of our products at the highest recommended safety and quality standards for cosmetic products.
Our facility contains multiple cell and tissue culture suites containing biosafety cabinets and cell culture incubators. Not only does our facility provide a significant amount of cold storage and processing space that permit large-scale culture of hUMSCs and the ability to mass produce of stem cell-derived exosomes but allows us to perform cryo-preservation, cryo-storage, various forms of microscopy and cell analysis. Some additional key features of our facility include 24/7 security, advanced climate control, increased cold storage, additional cell culture and R&D suites to perform supplemental in-house research.
Intellectual Property
We have developed a comprehensive portfolio of intellectual property, consisting of patent applications, trademarks, domain names, know-how and trade secrets. As of the date of this prospectus, we have 15 registered trademarks inclusive of 12 global trademarks and 3 United States trademarks, and 18 trademark applications pending, 2 registered domain names, 3 non-provisional patent applications filed, 1 provisional patent application and 3 International Patent Corporation Treaty (“PCT”) applications filed.
Our Precision Regenerative Exosome Technology™, or PREx™, process is used to produce Elevai Exosomes™ and the exact process remains a trade secret. We have strategically decided to not pursue a patent around the process.
We believe our intellectual property adequately protects our products and technology and may prevent others from commercializing products or methods substantially similar to ours.
Corporate History and Structure
Elevai Labs, Inc. was incorporated in Delaware in June 2020 under the original name Reactive Medical Labs Inc. In June 2021, we entered into a stock transfer agreement with Reactive Medical Inc., a Canadian company, whereby we purchased substantially all of the assets and liabilities of Reactive Medical Inc. Under the stock transfer agreement, we acquired 100% of the issued and outstanding common shares of Reactive Medical Inc. Immediately before the stock transfer agreement BWL Investments Ltd., a British Columbia Canada corporation owned 100% of the issued and outstanding common shares of Reactive Medical Inc. In consideration of 100% of the issued and outstanding common shares of Reactive Medical Inc., we issued 100 shares of our Common Stock to BWL Investments Ltd. Upon completion of the stock transfer agreement, Reactive Medical Inc. became our wholly owned subsidiary. In September 2022, Reactive Medical Inc. changed its name to Elevai Research Inc. Our principal executive offices are located at 120 Newport Center Dr. #250, Newport Beach, CA 92660. As of the date of this prospectus, we are qualified to do business as a foreign corporation in the state of California. Our telephone number is 866-794-4940. Our website address is https://elevailabs.com. Information contained on our website or connected thereto does not constitute part of, and is not incorporated by reference into, this prospectus or the registration statement of which it forms a part.
10
Elevai has one wholly owned subsidiary, Elevai Research Inc. (FKA Reactive Medical Inc.).
The following diagram sets forth the structure of the Company as of the date of this prospectus an after giving effect to the offering based on a proposed number of [●] shares of Common Stock being offered (assuming no exercise of the over-allotment option by the underwriters) and the automatic conversion of our preferred stock simultaneous with this offering:

Implications of Being an Emerging Growth Company
As a company with less than $1.235 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include:
| - | a requirement to have only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations disclosure; |
| - | an exemption from the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended, or the Sarbanes-Oxley Act, in the assessment of our internal control over financial reporting; |
| - | reduced disclosure about our executive compensation arrangements in our periodic reports, proxy statements and registration statements; and |
| - | exemptions from the requirements of holding non-binding advisory votes on executive compensation and golden parachute arrangements. |
We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of this offering, (b) in which we have total annual revenues of at least $1.235 billion or (c) in which we are deemed to be a large accelerated filer, which means the market value of our Common Stock that is held by non-affiliates exceeds $700.0 million as of the prior December 31, and (2) the date on which we have issued more than $1.235 billion in non-convertible debt during the prior three-year period. We may choose to take advantage of some but not all of these reduced burdens. We have taken advantage of the requirement to have only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations disclosure in this prospectus and we may choose to take advantage of other reduced reporting burdens in future filings. Accordingly, the information contained herein and the information that we provide to our stockholders may differ from the information you might get from other public companies.
Summary Risk Factors
An investment in our shares involves a high degree of risk. If any of the factors below or in the section entitled “Risk Factors” or contained elsewhere in this prospectus occurs, our business, financial condition, liquidity, results of operations and prospects could be materially and adversely affected. These risks are discussed more fully in the section titled “Risk Factors.”
Risks Related to Our Business, Our Brand, Our Products and Our Industry
Risks and uncertainties related to our business include, but are not limited to, the following:
| ● | Our revenues and financial results depend significantly on sales of our Elevai Post Treatment E-Series™. If we are unable to manufacture or sell our Elevai Post Treatment E-Series™, in sufficient quantities and in a timely manner or maintain client acceptance of our Elevai Post Treatment E-Series™, our business will be materially and adversely impacted (see page 15 of this prospectus); |
| ● | Our marketed products and our products under development could be rendered obsolete by technological or other medical advances (see page 17 of this prospectus); |
| ● | We have a limited operating history at our current scale, which may make it difficult to evaluate our business and future prospects (see page 24 of this prospectus); |
| ● | Restrictions on the use of human stem cells, and the ethical, legal and social implications of that research, could prevent us from developing or gaining acceptance for commercially viable products in these areas (see page 29 of this prospectus); |
11
| ● | Our products may be expensive to manufacture, and they may not be profitable if we are unable to control the costs to manufacture them (see page 29 of this prospectus); |
| ● | Our business is based on novel technologies that are inherently expensive, risky and may not be understood by or accepted in the cosmetics marketplace, which could adversely affect our future value (see page 29 of this prospectus); and |
Risks Related to Our Financial Condition
In addition to the risks described above, we are subject to general risks and uncertainties relating our financial condition, including, but not limited to, the following:
| ● | As described in the report of our auditors for the six months ended June 30, 2023, and the years ended December 31, 2021, and 2022 and the notes to our consolidated financial statements, there is substantial doubt about our ability to continue as a going concern, and if we are unable to continue, you may lose your entire investment (see page 30 of this prospectus); |
| ● | We have a history of net losses, and we may not be able to achieve or maintain profitability in the future (see page 30 of this prospectus); |
Risks Related to Our Dependence on Third Parties
Risks and uncertainties related to our dependence on third parties include, but are not limited to, the following:
| ● | We depend on our collaborators to help us develop and test our proposed products, and our ability to develop and commercialize products may be impaired or delayed if collaborations are unsuccessful (see page 32 of this prospectus); |
| ● | If we, or our third-party manufacturers or formulators fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of its business (see page 37 of this prospectus). |
Risks Related to Our Products Legal and Regulatory Risks
Risks and uncertainties related to our legal and regulatory risks include, but are not limited to, the following:
| ● | A recall or suspension of sale of our products, or the discovery of serious safety issues with our products or the incorrect application of such products by medical professionals to which we sell such products, could have a significant negative impact on us (see page 37-38 of this prospectus); |
| ● | Restrictive and extensive government regulation could slow or hinder our production of cosmetics containing a stem-cell byproduct and we may be unsuccessful in our efforts to comply with applicable federal, state and international laws and regulations, which could result in government enforcement actions (see page 40 of this prospectus); |
Risks Related to this Offering and Our Common Stock
In addition to the risks described above, we are subject to general risks and uncertainties relating to this offering, including, but not limited to, the following:
| ● | The price of our Common Stock could be subject to rapid and substantial volatility (see page 43-44 of this prospectus); |
| ● | Our Common Stock has not been publicly traded, and we expect that the price of our Common Stock will fluctuate substantially (see page 44 of this prospectus); |
| ● | We have broad discretion in the use of the net proceeds from this offering and may not use them effectively (see page 44 of this prospectus); |
| ● | You will suffer immediate and substantial dilution (see page 45 of this prospectus); |
| ● | There may not be an active, liquid trading market for our Common Stock (see page 47 of this prospectus); |
| ● | Shares eligible for future sale may adversely affect the market price of our Common Stock, as the future sale of a substantial amount of outstanding Common Stock in the public marketplace could reduce the price of our Common Stock (see page 47 of this prospectus); |
12
Offering Summary
Shares Offered:
| $[●] shares of Common Stock (excluding the underwriter’s over-allotment option), which we have assumed to be [●] shares using the midpoint of the price range on the cover page of this prospectus. | |
| Assumed Public Offering Price: | $[●] per share, the midpoint of the price range on the cover page of this prospectus. | |
| Over-Allotment: | We have granted the underwriter a 45-day option (commencing from the date of this prospectus) to purchase up to an additional fifteen percent of the shares sold in the initial closing of this offering at the public offering price to cover over-allotments, if any. We have assumed this option will be for [●] shares of Common Stock using the midpoint of the price range on the cover page of this prospectus. | |
| Shares Outstanding After the Offering: | [●] shares of Common Stock (or [●] shares of Common Stock if the underwriter exercises the over-allotment option in full). | |
| Lock-up: | We, our directors, executive officers and stockholders holding 5% or more of the issued and outstanding shares of Common Stock, will enter into a lock-up agreement with the Underwriters not to sell, transfer or dispose of any shares of Common Stock for a period of six months from the date of this prospectus. See “Shares Eligible for Future Sale” and “Underwriting.” | |
| Listing: | We plan to list our shares of Common Stock on the Nasdaq Capital Market. This offering will not close unless our application to be listed on Nasdaq has been approved. We cannot guarantee that we will be successful in listing on Nasdaq; however, we will not complete this offering unless we are so listed. | |
| Proposed Nasdaq Capital Market Symbol: | “ELAB” | |
| Transfer Agent: | VStock Transfer, LLC | |
| Use of Proceeds: | We intend to use the net proceeds from this offering to expand our in-house aesthetics sales team, expand our intellectual property portfolio, product lines and formulation and the marketing of those products and general corporate purposes. | |
| Underwriter: | Univest Securities, LLC | |
| Underwriter Warrants: | We have agreed to sell to Univest Securities, LLC, warrants (the “Representative’s Warrants”) to purchase up to a total of [●] shares of Common Stock, or [●] shares of Common Stock in the event the underwriter exercises its over-allotment option (equal to 5% of the aggregate number of shares sold in the offering and in each case assuming the offering price per share is the midpoint of the price range on the cover page of this prospectus) at a price equal to the price of our shares offered hereby. | |
| Dividend policy | We do not intend to pay any dividends on our Common Stock for the foreseeable future. Instead, we anticipate that all of our earnings, if any, will be used for the operation and growth of our business. See “Dividend Policy” for more information. | |
| Risk Factors: | Investing in these securities involves a high degree of risk. As an investor, you should be able to bear a complete loss of your investment. You should carefully consider the information set forth in the “Risk Factors” section of this prospectus before deciding to invest in our Common Stock. |
Shares outstanding after the offering is based on (i) [●] shares of Common Stock that are outstanding as of the date of this prospectus and (ii) [●] shares of Common Stock issuable upon the automatic conversion of our convertible preferred stock simultaneous with this offering, and excludes up to [●] shares of Common Stock underlying options and warrants that we have granted prior to the closing of this offering and [●] shares of Common stock issuable upon exercise of the warrants issued to the underwriter (or [●] shares issuable upon exercise of the warrants issued to the underwriter if the over-allotment option is exercised in full), assuming a per share price in the offering of $[●].
We refer to our series seed preferred stock, series seed 2 preferred stock, and series A preferred stock herein as our “convertible preferred stock.” Except as otherwise indicated, all information in this prospectus assumes the conversion of all outstanding shares of our convertible preferred stock into an aggregate of [●] shares of common stock, which will occur automatically upon the closing of this offering.
13
Summary Financial Data
The following tables summarize our financial data. We derived the summary financial statement data for the years ended December 31, 2022, and December 31, 2021, and for the six months ended June 30, 2023 and 2022 set forth below from our audited financial statements. Our financial statements have been prepared in accordance with U.S. Generally Accepted Accounting Principles. Our historical results are not necessarily indicative of the results that may be expected in the future. You should read the information presented below together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” our financial statements, the notes to those statements and the other financial information contained in this prospectus and the unaudited consolidated pro forma information appearing elsewhere in this prospectus.
Statement of Operations Data:
| For the Years Ended | For the Six Months Ended | |||||||||||||||
| December 31, | June 30, | |||||||||||||||
| 2022 | 2021 | 2023 | 2022 | |||||||||||||
| Revenue | $ | 766,277 | $ | 827 | $ | 459,350 | $ | 195,257 | ||||||||
| Total Cost and Expenses | 2,238,350 | 785,478 | 2,206,456 | 761,289 | ||||||||||||
| Loss from Operations | (1,791,041 | ) | (784,739 | ) | (1,899,719 | ) | (645,084 | ) | ||||||||
| Total Other Income (expense) | (9,227 | ) | - | (460,835 | ) | (3,442 | ) | |||||||||
| Foreign currency translation adjustments | (91 | ) | 1,424 | 375 | 242 | |||||||||||
| Total Comprehensive Loss | $ | 1,800,359 | $ | 783,315 | $ | (2,360,179 | ) | $ | (648,284 | ) | ||||||
| Basic and Diluted Loss per Share of Common Stock | $ | (0.19 | ) | $ | (0.08 | ) | $ | (0.240 | ) | $ | (0.068 | ) | ||||
| Basic and Diluted Weighted Average Shares of Common Stock Outstanding | 9,528,863 | 9,460,664 | 9,838,599 | 9,526,808 | ||||||||||||
Balance Sheet Data:
| As of December 31, | As of June 30, | |||||||||||
| 2022 | 2021 | 2023 | ||||||||||
| Cash | $ | 1,154,901 | $ | 411,858 | $ | 601,265 | ||||||
| Total current assets | 1,551,322 | 636,525 | 1,450,505 | |||||||||
| Total Assets | $ | 1,892,183 | $ | 666,250 | $ | 1,743,954 | ||||||
| Total current liabilities | 588,272 | 215,622 | 1,562,769 | |||||||||
| Long term debt | - | - | - | |||||||||
| Total liabilities | 760,873 | 215,622 | 1,676,666 | |||||||||
| Total stockholders’ deficit | 1,131,310 | 450,628 | 67,288 | |||||||||
| Total Liabilities and stockholders’ deficit | $ | 1,892,183 | $ | 666,250 | $ | 1,743,954 | ||||||
| Working capital (deficit) | $ | 963,050 | $ | 420,903 | $ | (112,264 | ) | |||||
14
Investing in our securities involves risks. Before you make a decision to buy our securities, in addition to the risks and uncertainties discussed above under “Forward-Looking Statements,” you should carefully consider the specific risks set forth herein. If any of these risks actually occur, it may materially harm our business, financial condition, liquidity and results of operations. As a result, the market price of our securities could decline, and you could lose all or part of your investment. Additionally, the risks and uncertainties described in this prospectus are not the only risks and uncertainties that we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may become material and adversely affect our business.
Risks Related to Our Business, Our Brand, Our Products and Our Industry
Our revenues and financial results depend significantly on sales of our Elevai Post Treatment E-Series™. If we are unable to manufacture or sell our Elevai Post Treatment E-Series™ in sufficient quantities and in a timely manner or maintain client acceptance of our Elevai Post Treatment E-Series™, our business will be materially and adversely impacted.
To date, a substantial majority of our revenues have resulted from sales of our principal product line, our Elevai Post Treatment E-Series™. Our Elevai Post Treatment E-Series™ and related products accounted for a substantial majority of our net sales for the years ended December 31, 2022, and 2021. Although we intend to introduce additional products, we expect sales of our Elevai Post Treatment E-Series™ to continue to account for a significant majority of our sales for the foreseeable future. Because our business is highly dependent on our Elevai Post Treatment E-Series™, factors adversely affecting the pricing of, or demand for, these products could have a material and adverse effect on our business. Additionally, our commercial success depends in large part on our ability to sustain market acceptance of our Elevai Post Treatment E-Series™ through physician practices and under both our exclusive and non-exclusive distribution agreements. If existing users of our products determine that our products do not satisfy their requirements, or if our competitors develop a product that is perceived by medical aesthetics consumers, physicians or distributors to better satisfy their respective aesthetics requirements, sales of our Elevai Post Treatment E-Series™, and our total net sales may correspondingly decline which could adversely affect our business, financial condition, results of operations and prospects.
Our results of operations could be harmed if we are unable to accurately forecast demand for our products.
To maintain an adequate inventory supply, we must forecast inventory needs and place orders with our third-party suppliers, formulators and packagers before firm orders are placed by our clients or distribution partners. If we fail to accurately forecast client demand, we may experience excess inventory levels or a shortage of product to deliver to our clients. Factors that could affect our ability to accurately forecast demand for our products include: an unanticipated increase or decrease in demand for our products; our failure to accurately forecast acceptance for our new products; product introductions by competitors; unanticipated changes in general market conditions or other factors, which may result in cancellations of advance orders or a reduction or increase in the rate of reorders or at-once orders placed by clients or distribution partners; the impact on demand due to unseasonable weather conditions; weakening of economic conditions or consumer or client confidence in future economic conditions, which could reduce demand for discretionary items, such as aesthetics services and our complementary cosmetics products; and terrorism or acts of war, or the threat thereof, or political or labor instability or unrest, which could adversely affect consumer or client confidence and spending or interrupt production and distribution of product and raw materials.
15
Inventory levels in excess of client or distribution partner demand may result in inventory write-downs or write-offs and the sale of excess inventory at discounted prices or in less preferred distribution channels, which could impair our brand image and harm our business. In addition, if we underestimate the demand for our products, our third-party suppliers, formulators, and packagers may not be able to facilitate bringing our cosmetics products to market or in time to meet our client or distribution partner requirements, and this could result in delays in the shipment of our products and our ability to recognize revenue, lost sales, as well as damage to our reputation and client and distributor relationships.
The difficulty in forecasting demand also makes it difficult to estimate our future results of operations and financial condition from period to period. A failure to accurately predict the level of demand for our products could adversely affect our business, financial condition, results of operations, and prospects.
We face intense competition, in some cases from companies that have significantly greater resources than we do, which could limit our ability to generate sales.
The market for aesthetic and cosmetic skin health products is highly competitive and we expect the intensity of competition to increase in the future as market acceptance grows and related technology advances. We also expect to encounter increased competition as we enter new markets and as we attempt to penetrate existing markets with new products. We may not be able to compete effectively in these markets, we may face significant pricing pressure from our competitors, and we may lose market share to our competitors. Our principal competitors are large, well-established companies in the fields of pharmaceuticals, medical devices, cosmetics and health care. Our direct competitors include SkinCeuticals, a division of L’Oréal S.A., SkinMedica, Inc., a division of Allergan, Inc., ZO Skin Health, PCA Skin, EltaMD, each a division of Colgate-Palmolive, Dermalogica, Murad and Eminence.
We also face competition from medical device companies offering products used to enhance the skin’s appearance to physicians, such cosmetic device companies that provide complementary microneedling serum treatment, and those companies may offer similar complementary products to physicians, aestheticians, spas, and wellness centers that provide facial treatment services.
We may not be able to successfully expand the use of our current product lines or develop new products.
We are constantly working to improve, extend the stability of and reformulate our existing products. Continued market acceptance of our products will depend on our ability to successfully develop additional applications of our Elevai ExosomesTM. The development of additional applications will require significant commitments of personnel and financial resources and we cannot assure you that they will be successful. If the attempted extensions of our product lines and new applications for our Elevai ExosomesTM are not commercially successful, our business will be adversely affected.
We are researching and working alongside contract research organizations to developing new product lines by applying our Elevai ExosomesTM technology to new agents. We also have applied for intellectual property rights to cover our use of certain patents in relation to additional methods and formulations of our Elevai ExosomesTM. New products, in various stages of development, include skin discoloration, and hair loss products and systems. These development activities, as well as new clinical validation studies to demonstrate aesthetic improvements, which may assist us in the marketing and sale of our cosmetic products. Completion of any clinical validation studies requires significant commitments of our personnel, time, and financial resources. We cannot assure you that we will be able to develop new products or formulations in a timely manner, or at all. Delays in the development or testing processes will cause a corresponding delay in revenue generation from those products. Regardless of whether such new products or formulations are ever released to the market, the expense of such processes, which may be considerable, will have already been incurred and we may not be able to recover such expenses.
We reevaluate our formulation and product development efforts regularly to assess whether our efforts to develop a particular new product or formulation are progressing at a rate that justifies our continued expenditures. On the basis of these reevaluations, we have abandoned development efforts in the past, and may abandon those development efforts in the future. New products that we develop may not be successfully commercialized. If we fail to take a product or technology from the development stage to market on a timely basis, we may incur significant expenses without a near-term financial return or any financial return.
16
Our failure to successfully research and develop additional technologies would impair our ability to grow.
We intend to develop and acquire and market new products and technologies though our own internal research capabilities and through the assistance of CROs. Our business model depends in part on our ability to patents new products and/or technologies. The success of this strategy also depends upon our ability and the ability of our third-party formulators to formulate products under such patents, as well as our ability to manufacture, market and sell such patented products.
We may not be able to internally develop new products or technologies successfully. Moreover, vetting, negotiating and implementing design protocols for new product and formulation development with CROs can be a lengthy and complex process. Other companies, including those with substantially greater financial, research and technology, marketing and sales resources, may compete with us for contracts with CROs and development of these technologies. We may not be able to negotiate with or find acceptable CROs to develop such products on terms that we find acceptable, or at all. As a result, our ability to grow our business or increase our profits could be adversely impacted.
Our marketed products and our products under development could be rendered obsolete by technological or other medical advances.
Our marketed products and our products under development may be rendered obsolete or uneconomical by our competitors’ products or technological advances or those other advances within other markets that may better or more inexpensively address the conditions that our products are designed to address.
All of our products address the condition-, and the enhancement of the appearance-of skin by utilizing our Elevai ExosomesTM. This market is the subject of active research and development by many potential competitors, including major pharmaceutical companies, specialized biotechnology firms, universities, hospitals, clinics and other research institutions. Competitive advances may also include the potential development of new therapies aimed at treating hyperpigmentation and photo-damaged skin that utilize other lab techniques involving stem-cell secretion, lasers or other advanced technologies. While we intend to expand our technological capabilities to remain competitive, research and development by others may render our technology or products obsolete or noncompetitive or result in treatments superior to any therapies we develop, as our competitors may develop and patent products which are better than ours, which could harm our competitive position.
To sustain our continued growth, we will need to increase the size of our organization, and we may encounter difficulties managing our growth, which could adversely affect our results of operations.
If we are able to successfully develop additional products and expand the application of our current products, we may experience growth in the number of our employees and the scope of our operations. To the extent that we acquire and launch additional cosmetics products, the resulting growth and expansion of our sales force will place a significant demand on our financial, managerial and operational resources. Since many of the new cosmetics products or pipeline products we are working on may involve using our technologies under new applications or circumstances, it may require our entering into new markets. We may not be able to accurately forecast the number of employees required, the timing of their hire or the associated cost with our expansion and/or our entrance into new markets. The extent of any expansion we may experience will be driven largely by the success of our new cosmetics products. As a result, management’s ability to project the size of any such expansion and its cost to the company is limited by the following uncertainties: (i) we will not have previously sold any of the new products and applications and the ultimate success of these new products and applications is unknown; (ii) we will be entering new markets; and (iii) the costs associated with any expansion will be partially driven by factors that may not be fully in our control (e.g., timing of hiring, market salary rates, ability to hire new managerial and senior staff). Subject to these uncertainties, we estimate that our current business plan may require us to hire new rounds of employees, specifically for our salesforce within the next 12 months. Due to the uncertainty surrounding the new cosmetics products, this estimate may prove to be incorrect, and our costs could be significantly higher. Our success will also depend on the ability of our executive officers and senior management to continue to implement and improve our operational, information management and financial control systems, particularly in light of our status as a newly public company subject to the reporting requirements of the Securities Exchange Act of 1934, or the Exchange Act, and to expand, train and manage our employee base. Our inability to manage growth effectively could cause our operating costs to grow even faster than we are currently anticipating and adversely affect our results of operations.
17
If we are unable to retain our existing sales force and recruit additional people to join our sales force, our revenue may not increase and may even decline.
Our products are primarily marketed by our sales force to form new accounts with medical practices, and we depend on those medical practices to generate a substantial majority of our revenue. Our current sales force is independently contracted and may terminate their services at any time, and we may experience high turnover among our sales force from year to year. To increase our revenue, we must increase the number of and/or the productivity of our sales force. We must also expand our outreach and outbound efforts to attract, connect and nurture new customers for a wider medical practice base who purchase product and whom we can foster relationships with to promote retention and higher value over the life of the medical practice.
While we take many steps to help train, motivate and retain our sales force, we cannot accurately predict how the number and productivity of our sales force may fluctuate. Our operating results could be harmed if we do not generate sufficient interest in our business and its products to retain and motivate our existing sales force and attract new people to join our sales force.
The number and productivity of our sales force is negatively impacted by several additional factors, including:
| ● | any adverse publicity or negative public perception regarding us, our products or ingredients, our sales distribution channel, or our industry or competitors; |
| ● | lack of interest in, dissatisfaction with, or the technical failure of, existing or new products; |
| ● | lack of compelling products or income opportunities; |
| ● | negative sales force reaction to changes in our sales compensation plans or to our failure to make changes that would be necessary to keep our compensation competitive with the market; |
| ● | interactions with our company, including our actions to enforce our policies and procedures and the quality of our customer service; |
| ● | any regulatory actions or charges against us or others in our industry, as well as regulatory changes that impact product formulations and sales viability; |
| ● | general economic, business and public health conditions, including employment levels, employment trends such as the gig and sharing economies, and pandemics or other conditions that curtail person-to-person interactions; |
| ● | changes in the policies of social media platforms used to prospect or recruit potential consumers and sales force participants; |
| ● | recruiting efforts of our competitors and changes in consumer-loyalty trends; and |
| ● | potential saturation or maturity levels in a given market, which could negatively impact our ability to attract and retain our sales force in such market. |
18
Our growth may suffer if an economic downturn in any of our major markets inhibits consumers from spending their disposable income on aesthetic and skin health products.
Our growth depends significantly on continued economic growth in the markets where we sell our products. Because many treatments in which our products are used are considered cosmetic in nature, they are typically paid directly by consumers out of their disposable income and are not subject to reimbursement by third-party payers such as health insurance organizations. As a result, an economic downturn in any of our major markets could have an adverse effect on the sales and profitability of our products.
Our products may cause undesirable side effects that could limit their use, require their removal from the market or prevent further development.
While there are no known side-effects of our products during any initial or prolonged use, any occurrence of side-effects or appearance that any of the ingredients we use may cause undesirable side-effects could limit consumer purchase and use of our products, particularly if physicians or their medical aesthetics consumers perceive that the risks or discomfort outweigh the benefits or if they perceive that the side effects of competitive products are less significant.
Undesirable side effects that may, in some cases be caused by our products could interrupt, delay or halt our research and development programs, including any clinical validation studies, and could result in adverse regulatory action by the FDA or other regulatory authorities. More severe side effects associated with our products may be observed in the future. Even if we are able to complete the development of a new product and obtain any required regulatory approval, undesirable side effects could prevent us from achieving or maintaining market acceptance of our current or prospective products or could substantially increase the costs and expenses of commercializing any future products. Negative publicity concerning our products, whether accurate or inaccurate, could also reduce market or regulatory acceptance of our products, which could result in decreased product demand, removal from the market or an increased number of product liability claims, whether or not such claims have merit.
Product liability lawsuits could divert our resources, result in substantial liabilities and reduce the commercial potential of our products.
Our business exposes us to the risk of product liability claims that are inherent to the development, clinical validation studies and testing to demonstrate aesthetic improvement and marketing of aesthetic and cosmetic skin products. These lawsuits may divert our management from pursuing our business strategy and may be costly to defend. In addition, if we are held liable in any of these lawsuits, we may incur substantial liabilities and may be forced to limit or forgo further commercialization of those products. Although we maintain general liability insurance in an amount that we believe is reasonably adequate to insulate us from potential claims, this insurance may not fully cover potential liabilities. In addition, our inability to obtain or maintain sufficient insurance coverage at an acceptable cost or to otherwise protect against potential product liability claims could prevent or inhibit the commercial production and sale of our products, which could adversely affect our business.
We are subject to risks associated with doing business internationally.
Our international sales will depend upon the success under both our exclusive and non-exclusive distribution partners and associated marketing efforts of and sales of our third-party distributors via our distribution and license agreements with these partners. Although none of our international distribution or license partners accounted for any of our net sales in 2021 and 2022, our business is subject to certain risks inherent in international business as we continue to expand, many of which are beyond our control. These risks include:
| ● | adverse changes in tariff and trade protection measures; |
| ● | unexpected changes in foreign regulatory requirements; |
| ● | the potential of negative consequences from changes in tax laws; |
19
| ● | the potential of business failure of one or more of our distribution partners; |
| ● | changing economic conditions in countries where our products are sold; unexpected fluctuations in exchange rates; |
| ● | potential political unrest and hostilities; |
| ● | differing degrees of protection for intellectual property; and |
| ● | difficulties in coordinating foreign distribution. |
Any of the foregoing factors could adversely affect our business, financial condition and results of operations. We cannot assure you that we can successfully manage these risks or avoid their effects when doing business internationally.
Potential business combinations could require significant management attention and prove difficult to integrate with our business, which could divert the attention of our management, disrupt our normal course of business, dilute stockholder value and adversely affect our operating results.
If we become aware of potential business combination candidates that are complementary to our business, we may decide to combine with such businesses or acquire their assets in the future. Business combinations generally involve a number of additional difficulties and risks to our business, including:
| ● | failure to integrate management information systems, personnel, research and development and marketing, operations, sales and support; |
| ● | disruption of our ongoing business and diversion of management’s attention from other business matters; |
| ● | potential loss of the acquired company’s customers; |
| ● | failure to further develop or integrate the acquired company’s products or technology successfully; |
| ● | unanticipated costs and liabilities; and |
| ● | other accounting system consequences. |
In addition, we may not realize benefits from any business combination we may undertake in the future. If we fail to successfully integrate such businesses, or the products and technologies associated with such business combinations into our company, the revenue and operating results of the combined company could be adversely affected. Any integration process would require significant time and resources, and we may not be able to manage the process successfully. If our customers are uncertain about our ability to operate on a combined basis, they may delay or cancel orders for our products. We may not successfully evaluate, integrate or utilize the acquired technology and product lines or accurately forecast the financial impact of a combination, including accounting system charges or volatility in the stock price of the combined entity, should it be a publicly traded target. If we fail to successfully integrate other companies with which we may combine in the future, our business could be adversely affected.
20
If we fail to cost-effectively acquire new client accounts or retain our existing clients, our business could be adversely affected. Our sales and profit are dependent upon our ability to expand sales to our existing client relationships and acquire new client accounts.
Our success, and our ability to increase revenue and achieve profitability, depend in part on our ability to cost-effectively acquire new client accounts, retain existing clients and keep existing aesthetics-consumers engaged so that they continue to request and purchase our products. While we intend to continue to invest significantly in sales and marketing to educate medical clients about our brand, our values and our products, there is no assurance that these efforts will generate further demand for our products or expand our client base. Our ability to attract new client accounts and retain our existing accounts will depend on, among other items, the perceived value and quality of our products, consumer demand for thoughtfully designed and innovative cosmetic products at a premium, competitive offerings, our ability to offer new and relevant products and the effectiveness of our marketing efforts. We may also lose loyal clients to our competitors if we are unable to meet client demand for product in a timely manner. If we are unable to cost-effectively acquire new client accounts, retain existing clients and keep existing clients engaged, our business, financial condition, results of operations and prospects could be adversely affected.
Any strategies we employ to pursue this growth are subject to numerous factors outside of our control. Our medical clients continue to be aggressively marketed to for other private label or competitive cosmetic products in the medical aesthetics space, which could reduce demand for our products. The expansion of our business also depends on our ability to increase sales through our distribution agreements and white-label product channels. Any growth within our existing distribution agreement channels may also affect our existing client relationships and present additional challenges, including those related to pricing strategies. Our direct connections to our clients may become more limited as we expand our distribution channels. Additionally, we may need to increase or reallocate spending on marketing and promotional activities, such as temporary price reductions, off-invoice discounts, advertisements, product coupons and other trade activities, and these expenditures are subject to risks, including risks related to our clients’ acceptance of our marketing efforts. Our strategy to grow international sales may also increase our marketing spend. Our failure to obtain new clients, or expand our business with existing clients, could have an adverse effect on our business, financial condition, results of operations and prospects.
We also use paid and non-paid advertising. Our paid advertising may include search engine marketing, display, paid social media and product placement and traditional advertising, such as direct mail, television, radio and magazine advertising. Our non-paid advertising efforts include search engine optimization, non-paid social media and e-mail marketing. We drive a significant amount of traffic to our website via search engines. However, search engines frequently update and change the logic that determines the placement and display of results of a user’s search, such that the purchased or algorithmic placement of links to our website can be negatively affected. Moreover, a search engine could, for competitive or other purposes, alter its search algorithms or results, causing our website to place lower in search query results.
We also drive a significant amount of traffic to our website via social networking or other ecommerce channels used by our current and prospective clients and medical aesthetics cosmetics consumers. As social networking and ecommerce channels continue to rapidly evolve, we may be unable to develop or maintain a presence within these channels. If we are unable to cost-effectively drive traffic to our website, or if the popularity of our social media presence declines, our ability to acquire new clients or interest via consumers could be adversely affected. Additionally, if we fail to increase our revenue per client, generate repeat purchases or maintain high levels of client engagement, our business, financial condition, results of operations and prospects could be adversely affected.
We must expend resources to maintain awareness of our brand, build brand loyalty and generate interest in our products. Our marketing strategies and channels will evolve, and our efforts may or may not be successful.
In order to remain competitive and expand and keep market share for our cosmetics products across our various distribution and business channels, we may need to increase our marketing and advertising spending to maintain and increase brand and client awareness, protect and grow our existing market share or promote new products, which could impact our operating results. Substantial advertising and promotional expenditures may be required to maintain or improve our brand’s market position or to introduce new products to the market, presenting at medical aesthetic conferences, traveling to tradeshows as well as increasingly engaging with non-traditional media. Non-traditional media meets many clients and their cosmetic aesthetics consumers where they are most active which includes outreach through social media and web-based channels, however these channels may not prove successful in building brand awareness. Thus, an increase in our marketing and advertising efforts may not maintain our current reputation or lead to increased market share. Further, social media platforms frequently change the algorithms that determine the ranking and display of results of a user’s search and may make other changes to the way results are displayed, or may increase the costs of such advertising, which can negatively affect the placement of our links and, therefore, reduce the number of visits to our website and social media channels or make such marketing cost-prohibitive. In addition, social media platforms typically require compliance with their policies and procedures, which may be subject to change or new interpretation with limited ability to negotiate, which could negatively impact our marketing capabilities. If we are unable to maintain and promote a favorable perception of our brand and products on a cost-effective basis, our business, financial condition, results of operations and prospects could be adversely affected.
21
Failure to leverage our brand value propositions through our novel exosome technology to compete against well-known cosmetics products, especially during an economic downturn, may adversely affect our ability to achieve or maintain profitability.
In the medical aesthetic cosmetics product category, we compete not only with other widely advertised branded products, but also with well-known branded cosmetics products that have larger market share within the medical aesthetics cosmetics space and may have the capacity to be sold at lower prices. Medical businesses are more likely to purchase our products if they believe that our products provide greater value than less expensive alternatives and if their medical aesthetics consumers demand our products. If the difference in perceived value between our brand and well-known cosmetics products narrows, or if there is a perception of such a narrowing, clients may choose not to buy our products at prices that are profitable for us. We believe that in periods of economic uncertainty, such as the current economic uncertainty surrounding COVID-19, clients may purchase less inventory from us or more from lower-priced brands despite our value proposition. To the extent this occurs, we could experience a reduction in the sales volume of our products or an unfavorable shift in the types of products medical aesthetics consumers demand, which could have an adverse effect on our business, financial condition, results of operations and prospects.
Our brand and reputation may be diminished due to real or perceived quality, safety, aesthetic results or environmental impact issues with our products, which could have an adverse effect on our business, financial condition, results of operations and prospects.
We believe our clients and their medical aesthetics consumers rely on us to provide them with high quality, innovative, well-designed, and effective products. Any loss of confidence on the part of medical aesthetics consumers in our products or the ingredients used in our products, whether related to product contamination or product safety or quality failures, actual or perceived, environmental impacts, or inclusion of prohibited ingredients, or ingredients that are perceived to be “toxic”, could tarnish the image of our brand and could cause consumers to choose other products. Allegations of contamination or other adverse effects on product safety or aesthetic results or suitability for use by a particular consumer or on the environment, even if untrue, may require us to expend significant time and resources responding to such allegations and could, from time to time, result in a recall of a product from any or all of the markets in which the recalled product was distributed. Any such issues or recalls could negatively affect our ability to achieve or maintain profitability and brand image.
We also have no control over our products once purchased by medical aesthetics consumers of our clients. For example, medical aesthetics consumers may store or use our products under conditions and for periods of time inconsistent with approved directions for use or the listed shelf life or required warnings or other governmental guidelines on our labels, which may adversely affect the quality and safety of our products or the perceived quality and safety.
If our products are found to be, or perceived to be, defective or unsafe, or if they otherwise fail to meet our clients and their medical aesthetics consumers’ expectations, our relationships with our client base could suffer, the appeal of our brand could be diminished, we may need to recall some of our products and/or become subject to regulatory action, and we could lose sales or market share or become subject to boycotts or liability claims. In addition, safety or other defects in our competitors’ products or products using our branded name via white-label agreements in other distributed products could reduce overall demand for products with our brand if consumers generally view them to be similar to our products. Any such adverse effect could be exacerbated by our market positioning as a purveyor of high quality, innovative, well-designed, and effective products and may significantly reduce our brand value. Issues regarding the safety, aesthetic results, quality or environmental impact of any of our products, regardless of the cause, may have an adverse effect on our brand, reputation and operating results. Further, the growing use of social and digital media by us, our clients and third parties increases the speed and extent that information or misinformation and opinions can be shared. Negative publicity about us, our brand or our products on social or digital media could seriously damage our brand and reputation. Any loss of confidence on the part of clients and their medical aesthetics consumers in the quality, safety, aesthetic results or environmental suitability of our products would be difficult and costly to overcome, even if such concerns were based on inaccurate or misleading information. If we do not maintain a favorable perception of our brand, and project our positions regarding our product safety effectively, our business, financial condition, results of operations and prospects could be adversely affected.
22
Economic downturns or a change in medical aesthetic consumer preferences, perception and spending habits in the medical aesthetic cosmetic products categories, in particular, could limit aesthetic consumer demand for our cosmetic products and negatively affect our business.
We have positioned our brand to capitalize on growing consumer interest in complementary cosmetics for the medical aesthetics services industry. The medical aesthetics cosmetics product industry is sensitive to national and regional economic conditions and the demand for the products that we distribute may be adversely affected from time to time by economic downturns that impact consumer spending on cosmetics products, including discretionary spending. Future economic conditions such as employment levels, business conditions, housing starts, interest rates, inflation rates, energy and fuel costs, and tax rates could reduce consumer spending or change consumer purchasing habits. Among these changes could be a reduction in the number of medical aesthetics cosmetics products that medical aesthetics consumers purchase when they receive medical aesthetics services, given that many products in this category often have higher retail prices than do their conventional counterparts found in retail stores.
Further, the high-end cosmetics markets in which we operate are subject to changes in consumer preference, trends, new technology, perception and discretionary spending habits. Our performance depends significantly on factors that may affect the level and pattern of medical aesthetics consumer spending in the markets in which we operate. Such factors include medical aesthetics consumer preference, medical aesthetics consumer confidence, medical aesthetics consumer income, medical aesthetics consumer perception of the safety and quality of our products and shifts in the perceived value for our cosmetics products relative to conventional alternatives. The medical aesthetics cosmetics market is also subject to changes in the rate of procedures, which have been increasing in developed countries like the United States. In addition, media coverage regarding the safety or quality of, our products or the biological raw materials, ingredients or bioengineering processes involved in their manufacturing may damage consumer confidence in our cosmetics products.
A general decline in the consumption of our cosmetics products could occur at any time as a result of change in medical aesthetics consumer preference, perception, confidence and spending habits, including an unwillingness to pay a premium or an inability to purchase our products due to financial hardship or increased price sensitivity, which may be exacerbated by the effects of the COVID-19 pandemic or economic downturn. If medical aesthetics consumer preferences shift away from complementary cosmetic products, our business, financial condition and results of operations could be adversely affected.
The success of our products depends on a number of factors Including our ability to accurately anticipate changes in the medical aesthetics cosmetics market demand and medical aesthetics consumer preferences, our ability to differentiate the quality and innovativeness of our cosmetics products from those of our competitors, and the effectiveness of our marketing and advertising campaigns for our cosmetics products. We may not be successful in identifying trends in medical aesthetics preferences and developing cosmetics products that respond to or lead the way in such trends in a timely manner. We also may not be able to effectively promote our cosmetics products and related technologies by our marketing and advertising campaigns and gain market acceptance. If our cosmetics products fail to gain market acceptance, are restricted by regulatory requirements or have quality problems, we may not be able to fully recover costs and expenses incurred in our operation, and our business, financial condition, results of operations and prospects could be adversely affected.
If we cannot maintain our company culture or focus on our purpose as we grow, our success and our business and competitive position may be harmed.
We believe our culture and our mission have been key contributors to our success to date and that the critical nature of the products that we promote a sense of transparency and scientific innovation to our clients. Any failure to preserve our culture or focus on our mission could negatively affect our ability to retain and recruit clients, and personnel, which is critical to our growth and to effectively focus on and pursue our corporate objectives. As we grow and develop the infrastructure of a public company, we may find it difficult to maintain these important values. If we fail to maintain our company culture or focus on our mission our competitive position and business, financial condition, results of operations and prospects could be adversely affected.
If we lose key personnel or are unable to attract and retain other qualified personnel, we may be unable to execute our business plan and our business would be materially adversely affected.
As of May 31, 2023, we had 14 employees. Our success depends on our continued ability to attract, retain and motivate highly qualified management, business development, sales and marketing, product development and other personnel. In the future we may not be able to recruit and retain qualified personnel, particularly for senior sales and marketing, research and product development positions due to intense competition for personnel among businesses like ours, and the failure to do so could have a significant negative impact on our future product sales and business results. Our success depends in large part on the efforts and abilities of Jordan R. Plews, our President and Chief Executive Officer; Graydon Bensler, our Chief Financial Officer; Tim Sayed, our Chief Medical Officer; Brenda Buechler, our Chief Marketing Officer; and Christoph Kraneiss, our Chief Commercial Officer—as well as other members of our senior management and our scientific and technical personnel.
23
Our ability to maintain our competitive position is largely dependent on the services of our senior management and other key personnel, including our co-founder, President and Chief Executive Officer, Jordan R. Plews. The loss of the services of Dr. Plews could have an adverse effect on our business, financial condition, results of operations and prospects. Dr. Plews is a well-recognized bioengineer, doctor and entrepreneur. We believe that the success of our brand depends in part on our ongoing affiliation with Dr. Plews. We have an agreement with Dr. Plews, or the Inventions and Proprietary Information Agreement, which, among other things, includes an assignment to us for any of Dr. Plews inventions, know-how or intellectual property, among other items that are legally protectable that result or relatedly-arise from any work performed in his capacity as our employee and imposes various obligations on us. Should that Inventions and Proprietary Information Agreement terminate and upon twelve months after the termination of the Inventions and Proprietary Information Agreement, we could, among other things, lose our ability to control recruiting by Dr. Plews of any of our employees or consultants. Moreover, upon such termination of the Inventions and Proprietary Information Agreement, we may not be able to limit Dr. Plews use of any of our know-how, processes or reverse engineering of our products and sustain reputational damage. We depend on Dr. Plews appearances at industry functions, conferences and his reach and influence to connect with clients and provide insight on current medical aesthetic cosmetics trends. Thus, the loss of the services of Dr. Plews, or the loss of our ability to use Dr. Plews’s likeness, could have an adverse effect on our business, financial condition, results of operations and prospects.
Although we maintain “key employee” insurance policies on our executive officers that would compensate us for the loss of their services, even if we lose the services of one or more of these individuals, finding a replacement could be difficult, may take an extended period of time and could significantly impede the achievement of our business objectives. Moreover, the “key employee” insurance policy limit may not fully cover interim expenses for the services and expertise we may require in order to maintain relatively uninterrupted operations of our business. This gap in coverage and the time it may take to replace a key employee may have a material adverse effect on our results of operations and financial condition.
We may be unable to accurately forecast revenue and appropriately plan our expenses in the future.
Revenue and results of operations are difficult to forecast because they generally depend on the volume, timing and type of orders we receive across our various distribution channels, all of which are uncertain. Forecasts may be particularly challenging as we intend to expand into new markets and geographies and develop and market new cosmetics products. We base our expense levels and investment plans on our estimates of revenue and gross margin. However, we cannot be sure the same growth rates and trends are meaningful predictors of future growth. If our assumptions prove to be wrong, we may spend more than we anticipate acquiring and retaining our client or may generate lower revenue per client account than anticipated, either of which could have an adverse effect on our business, financial condition, results of operations and prospects.
We have a limited operating history at our current scale, which may make it difficult to evaluate our business and future prospects.
We began commercial operations in 2020 and have a limited history of generating revenue at our current scale. As a result of our relatively short operating history at our current scale, we have limited financial data that can be used to evaluate our business and future prospects. Any evaluation of our business and prospects must be considered in light of our limited operating history, which may not be indicative of future performance. Because of our limited operating history, we face increased risks, uncertainties, expenses, and difficulties, including the risks and uncertainties discussed in this section.
A disruption in our operations could have an adverse effect on our business.
As a company engages in sales domestically and internationally, our operations, including those of our third-party formulators, suppliers, and distribution partners, and other service providers, are subject to the risks inherent in such activities, including industrial accidents, environmental events, strikes and other labor disputes, disruptions in information systems, product quality control, safety, licensing requirements and other regulatory issues, as well as natural disasters, pandemics or other public health emergencies, border disputes, acts of terrorism and other external factors over which we and our third-party manufacturers, suppliers and delivery service providers have no control. The loss of, or damage to, the facilities of our third-party formulators, suppliers and delivery service providers could have an adverse effect on our business, financial condition, results of operations and prospects.
We depend heavily on postal and parcel carriers for the delivery of products sold directly to clients. Interruptions to or failures in these delivery services could prevent the timely or successful delivery of our products. These interruptions or failures may be due to unforeseen events that are beyond our control or the control of our third-party delivery service providers, such as labor unrest or natural disasters Any failure to provide high-quality delivery services to our clients may negatively affect the ordering and likelihood of repeat-purchasing of our clients, damage our reputation and cause us to lose client accounts.
24
The COVID-19 or another pandemic could have an adverse effect on our business, financial condition, results of operations and prospects.
In connection with the COVID-19 pandemic, governments have implemented significant measures, including closures, quarantines, travel restrictions and other social distancing directives, intended to control the spread of the virus. Companies have also taken precautions, such as requiring employees to work remotely, imposing travel restrictions and temporarily closing businesses from time to time. To the extent that these restrictions remain in place, additional prevention and mitigation measures are implemented in the future, or there is uncertainty about the effectiveness of these or any other measures to contain or treat COVID-19 or a variant thereof, there has been and continues to be an adverse impact on global economic conditions and consumer confidence and spending, which could adversely affect our supply chain as well as the demand for our cosmetics products. Although at this time we have not experienced disruptions to our supply chain, and we have not experienced decreases in demand or attributed COVID-19 to any material financial impacts, the fluid nature of the COVID-19 pandemic and uncertainties regarding the related economic impact are likely to result in sustained market uncertainty and turmoil, which could also have an adverse effect on our business, financial condition, results of operations and prospects.
The impact of the COVID-19 pandemic, or any other eventual pandemic on any of our suppliers, formulators, packagers, shippers, distribution partners, medical business clients or transportation or logistics providers may negatively affect the price and availability of our materials and impact our supply chain. If the disruptions caused by the COVID-19 pandemic continue for an extended period of time, our ability to meet the demands of our clients may be materially impacted. In addition, the conditions caused by the COVID-19 pandemic, or another pandemic may negatively impact collections of accounts receivable and cause some of our clients’ businesses to slow, all of which could adversely affect our business, financial condition, results of operations and prospects.
Further, the COVID-19 pandemic may impact medical aesthetic cosmetics consumer demand. Medical aesthetics practices may be impacted if governments continue to implement regional business closures, quarantines, travel restrictions and other social distancing directives to slow the spread of the virus. Further, to the extent our clients’ operations are negatively impacted, their medical aesthetic cosmetics consumers may reduce demand for or spending on our cosmetics products. There may also be significant reductions or volatility in medical aesthetic cosmetics demand for our products due to travel restrictions or social distancing directives, as well as the temporary inability of consumers to purchase our products due to illness, quarantine or financial hardship, shifts in demand away from one or more of our products, decreased consumer confidence and spending or beautification activities, any of which may negatively impact our results, including as a result of an increased difficulty in planning for operations. Additionally, we may be unable to effectively modify our advertising activities to reflect changing medical aesthetic cosmetics interests in beauty, or their general outward appearance, and shopping habits due to event cancellations, reduced in-store visits and travel restrictions, among other things.
It is not currently possible to ascertain the overall impact of the COVID-19 pandemic on our business. However, if the pandemic continues to persist as a severe worldwide health crisis, the disease could have an adverse effect on our business, financial condition, results of operations and prospects, and may also have the effect of heightening many of the other risks described in this “Risk Factors” section.
Our business is at an early stage of product development, and we may not develop additional cosmetics products that can be commercialized or profitably developed and our failure to introduce new products may adversely affect our ability to continue to grow.
Our business is at an early stage of product development. As of the date of this prospectus, we have commercialized two cosmetics products for the medical aesthetics market. We are still in the early stages of identifying and conducting research on potential new cosmetic products.
A key element of our growth strategy depends on our ability to develop and market new products that meet our standards for quality and appeal to our clients and distribution partners. Our pipeline products will require significant research and development, and clinical validation testing to demonstrate aesthetic improvement of any product. We may not be able to successful commercialize or synthetize any of product candidates or commercialize any products at scale that is profitable. Our product candidates may prove to have undesirable and unintended side effects or other characteristics adversely affecting their safety, aesthetic results or cost effectiveness that could prevent or limit their use. Any product using any of our technology may fail to provide the intended aesthetic improvements or achieve aesthetic results or benefits equal to or better than the standard of treatment at the time of testing or after a product may be formulated.
25
The success of our innovation and product development efforts is affected by our ability to anticipate changes in consumer and market preferences within the medical aesthetics cosmetics industry, the technical capability of our laboratory staff, including biochemists and bioengineers, developing and testing product formulas and prototypes, our ability to comply with applicable governmental regulations, and the success of our management and sales and marketing teams in introducing and marketing new products. Our cosmetics product offerings may change over time, which makes it difficult to forecast our future results of operations. There can be no assurance that we will successfully develop and market new products that appeal to clients. For example, product formulas we develop may not contain the product attributes desired by the medical aesthetics consumers that our clients serve. Any such failure may lead to a decrease in our growth, sales and ability to achieve profitability, which could adversely affect our business, financial condition, results of operations and prospects.
Additionally, the development and introduction of new products requires substantial marketing expenditures, which we may be unable to recoup if new products do not gain widespread market acceptance. If we are unsuccessful in meeting our objectives with respect to new or improved products, our business, financial condition, results of operations and prospects could be adversely affected.
We may incur product liability claims that could harm our business.
We sell a variety of topical cosmetics products for topical human use. Our cosmetics are not generally subject to pre-market approval or registration processes so we cannot rely upon a government safety panel to qualify or approve our products for use, and some ingredients may not have long histories of human consumption or use. We rely upon published and unpublished safety information including clinical validation studies on ingredients used in our products and conduct our own clinical validation and safety studies on some key ingredients and products. A product may be safe for the general population when consumed or used as directed but could cause an adverse reaction for some individuals, such as a person who has a health condition or allergies or who is taking a prescription medication. While we include what we believe are adequate instructions and warnings and we have historically had low or no numbers of reported reactions, previously unknown adverse reactions could occur. While we maintain a policy to insure our product liability risks we will continue to periodically evaluate whether any of our products are found to cause any injury or damage and whether we become subject to product liability claims.
As a result of the type of products that we sell, we may be subject to various product liability claims, including that the products fail to meet quality or manufacturing specifications, contain contaminants, include inadequate instructions as to their proper use, include inadequate warnings concerning side effects and interactions with other substances or for persons with health conditions or allergies, or cause adverse reactions or side effects. Consumer protection laws and regulations governing our business continue to expand, and in some states such as California, class-action lawsuits based on increasingly novel theories of liability are expanding. Product liability claims could increase our costs, cause negative publicity, and adversely affect our business and financial results. As we continue to offer an increasing number of new products through large product offerings our product liability risk may increase.
If our sales force or employees provide improper or inappropriate advice regarding our products, their use or safety, we may be subject to additional product liability. If we discover that our products are causing adverse reactions, or if we determine that any of our employees have not properly handled reports of adverse reactions, we could suffer further adverse publicity or government sanctions.
Our employees, independent contractors, consultants, medical professional clients, distributors and vendors may engage in unethical misconduct or other improper sales activities, including noncompliance with regulatory standards and requirements.
We are exposed to the risk that our employees, independent contractors, consultants, medical professional clients, distributors and vendors and other individuals or entities with whom we have arrangements may engage in unethical, fraudulent or illegal activity. Misconduct by these parties could include intentional, reckless and/or negligent conduct or disclosure of unauthorized activities to us that violates: (i) the laws of the FDA, other similar foreign regulatory authorities and foreign governments, including those laws requiring the reporting of true, complete and accurate information to such regulators; (ii) manufacturing standards; or (iii) laws that require the true, complete and accurate reporting of financial information or data. These laws may impact, among other things, future sales, marketing and promotional campaigns.
26
It is not always possible to identify and deter unethical misconduct by our employees, medical professional clients and other third parties, and the precautions we take to detect and prevent these activities may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to be in compliance such laws or regulations. If such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in government investigations, legal proceedings, the imposition of significant fines or other sanctions, including the imposition of monetary penalties, damages, monetary fines, contractual damages, reputational harm, diminished profits and future earnings and curtailment of operations, any of which could adversely affect our ability to operate our business and our results of operations. Whether or not we are successful in defending against such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these claims or investigations, which could have a material adverse effect on our business, financial condition and results of operations.
Moreover, our cosmetics products may be subject to sales and marketing practices subject to business arrangements that may include kickbacks, self-dealing and other abusive practices. Because our medical professional clients receive a markup on each sale of our cosmetics products our medical professional clients may structure their own internal sales and commission programs or promote certain customer incentive programs and other business arrangements that include our cosmetic products and more generally that incentivize monetary gain over effecting positive results for their customers. Such programs may promote perverse incentives relating to the sale, stocking, or purchasing of our cosmetic products. Such programs could negatively impact our marketing capabilities and favorable perception of our brand and products. If we are unable to maintain and promote a favorable perception of our brand and products, our business, financial condition, results of operations and prospects could be adversely affected.
We have limited clinical validation and testing data, and clinical validation testing are subject to extensive regulatory requirements, very expensive, time-consuming and difficult to design and implement. Our products may fail to achieve necessary safety and aesthetic results during clinical validation testing, which may limit our ability to generate revenues from our cosmetics products.
We have not yet invested significantly in wide-ranging clinical validation testing to demonstrate aesthetic improvement and the few studies we have arranged are in early stages as of the date of this prospectus. We cannot assure you that we will be able to continue to invest or develop resources for conducting these tests in the near future. In particular, clinical validation testing can be very expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. The clinical validation trial process is time consuming. Furthermore, failure can occur at any stage of the testing, and we could encounter problems that cause us to abandon or repeat clinical validation testing. The commencement and completion of clinical validation studies may be affected by several factors, including:
| ● | unforeseen safety issues; |
| ● | determination of product applicative issues; |
| ● | inability to demonstrate positive aesthetic results during clinical validation studies; |
| ● | slower than expected rates of participant recruitment; |
| ● | inability to monitor or document participants adequately during or after product application; and |
| ● | developments related to the coronavirus outbreak and impact of it and COVID-19 on the costs and timing associated with the conduct of our clinical validation studies and other related activities. |
Our success depends largely upon consumer satisfaction with the aesthetic results of our products.
In order to generate repeat and referral business from clients, our clients’ medical aesthetics consumers must be satisfied with the aesthetic results of our cosmetics products. Our products are cosmetic in nature and the success of the results are highly subjective. Accordingly, medical aesthetics consumers’ perception of their aesthetic results may greatly vary even if our products and systems associated therewith are shown to be objectively successful. If medical aesthetics consumers are not satisfied with the aesthetic benefits of our products or feel that they are too expensive for the aesthetic results obtained, our reputation and future sales to our clients could suffer.
27
Our products may fail to achieve the broad degree of physician adoption and use or medical aesthetic consumer demand necessary for commercial success.
Our cosmetics products, which as of the date of this prospectus are used solely in a clinical or medical spa setting, may fail to gain sufficient market acceptance by physicians and others in the medical aesthetics community. The commercial success of these products and any future products will depend significantly on the broad adoption and use of the resulting product by physicians for the treatment of aesthetic indications that we may seek to pursue. We are aware that other companies are seeking to develop alternative products and treatments, any of which could impact the demand for our cosmetics products.
The degree and rate of physician adoption of our exosome serums and any future products depend on a number of factors, including the cost, profitability to our clients, medical aesthetic consumer demand, characteristics and aesthetic results of our products. Our success will also depend on our ability to create compelling marketing programs and ability to overcome any biases physicians or consumers may have toward the use, safety and aesthetic results of existing products over ours. Moreover, our competitors may offer more compelling marketing or discounting programs than we are able to offer, including by bundling multiple aesthetic products to provide a more comprehensive offering than we can. We can provide no assurance that health professionals will continue to recommend our products at their current levels, or at all. Additionally, we may be unable to continue to grow our network of health professional clients and therefore may not continue to achieve revenue growth through this channel.
With respect to medical aesthetic consumer demand, use of our cosmetic products is purely elective with a cost that must be borne by the consumer, and costs related to the use of cosmetics is not reimbursable through any third-party payor, such as Medicaid, Medicare or commercial insurance. The decision by a medical aesthetic consumer to purchase our products for aesthetic indications may be influenced by a number of factors, including the cost, aesthetic results, safety, perception, marketing programs for, and physician recommendations of our cosmetics products versus competitive cosmetics products or other procedures provided by the physician.
If our cosmetics products or any future pipeline product fail to achieve the broad degree of physician adoption necessary for commercial success or the requisite medical aesthetic consumer demand, our operating results and financial condition will be adversely affected, which may delay, prevent or limit our ability to generate revenue and continue our business.
The outcome of our clinical and product testing of our products is uncertain, and if we are unable to satisfactorily complete such testing, or if such testing yields unsatisfactory results, we may not achieve the broad degree of physician adoption and use or medical aesthetic consumer demand necessary for commercial success.
We have yet to complete clinical testing to demonstrate our products aesthetic results. The clinical testing of our current products may not demonstrate aesthetic results to the degree we may anticipate or at all. Similarly, this testing may not be completed in a timely manner, if at all, or only after significant increases in costs, program delays or both, all of which could harm our ability to generate revenues. In addition, our products may not prove to be more effective for improving appearance than current cosmetic products on the market. Accordingly, we may have to delay or abandon efforts to research, develop or further market our products.
The failure to adequately demonstrate the aesthetic results could harm our ability to generate revenues and limit a broader degree of physician adoption necessary for commercial success or the requisite medical aesthetic consumer demand. Accordingly, our operating results and financial condition will be adversely affected, which may delay, prevent or limit our ability to generate revenue and continue our business.
Even if we are successful in product testing of our cosmetic exosome-based products, it is unclear whether cosmetic exosome products can serve as the foundation for a commercially viable and profitable business because of other evolving technologies.
Stem cell technology is rapidly developing and could undergo significant change in the future. Such rapid technological development could result in our technologies becoming obsolete. While our cosmetic products appear promising, and even if they achieve positive test results, they may fail to be successfully adopted by physicians for numerous reasons, including, but not limited to, competing cosmetics technologies for the same treatments. There can be no assurance that we will be able to develop a successful market for our cosmetic exosome products based on stem cell technologies.
Moreover, advances in other cosmetic products are rapid and could significantly reduce or entirely eliminate the need for our products. Additionally, keeping up with new technological developments may materially alter the commercial viability of our technology or products and require us to incur significant costs to replace or modify product lines in which we have a substantial investment. We are focused on exosome based cosmetic products, and if this field is substantially unsuccessful, this could jeopardize our success or future results. The occurrence of any of these factors may have a material adverse effect on our business, operating results and financial condition.
28
If we are unable to keep up with rapid technological changes in our field or compete effectively, we will be unable to operate profitably.
We are engaged in activities in the bioengineering and cosmetics field, which is characterized by extensive research efforts and rapid technological progress. If we fail to anticipate or respond adequately to technological developments, our ability to operate profitably could suffer. Research and discoveries by other bioengineering, cosmetics, pharmaceutical or other companies may render our technologies or potential products or services uneconomical or result in products superior to those we develop. Similarly, any technologies, products or services we develop may not be preferred to any existing or newly developed technologies, products or services.
Restrictions on the use of human stem cells, and the ethical, legal and social implications of that research, could prevent us from developing or gaining acceptance for commercially viable products in these areas.
Our stem cells are derived under current Good Manufacturing Practices from Wharton’s Jelly portion of the human umbilical mesenchymal stem cells and captured within twenty-four hours of a full-term healthy birth by consenting donors. Because the use of human umbilical mesenchymal stem cells gives rise to ethical, legal and social issues regarding the appropriate use of these cells, our research related to human umbilical mesenchymal stem cells could become the subject of adverse commentary or publicity and some political and religious groups may still raise opposition to our cosmetics products and practices. In addition, many research institutions, including some of our potential scientific collaborators, have adopted policies regarding the ethical use of human umbilical mesenchymal stem cells, which, if applied to our procedures, may have the effect of limiting the scope of research conducted using our stem cells, thereby impairing our ability to conduct research in this field.
Our products may be expensive to manufacture, and they may not be profitable if we are unable to control the costs to manufacture them.
Our products may be significantly more expensive to manufacture than other traditional cosmetics products currently on the market today. We hope to substantially reduce manufacturing costs through process improvements, development of new methods, increases in manufacturing scale and outsourcing to experienced formulators or manufacturers. If we are not able to make these, or other improvements, and depending on the pricing of our products, our profit margins may be significantly less than that of other cosmetics products on the market today. In addition, we may not be able to charge a high enough price for any cosmetic product we develop, even if they are safe and effective, to make a profit. If we are unable to realize significant profits from our pipeline products, our business would be materially harmed.
Our business is based on novel technologies that are inherently expensive, risky and may not be understood by or accepted in the cosmetics marketplace, which could adversely affect our future value.
The development, commercialization and marketing of cell and tissue-derived cosmetics are at an early-stage, substantially research-oriented, and financially speculative. To date, very few companies have been successful in their efforts to develop and commercialize a stem cell-derived cosmetic products. In general, stem cell products may be susceptible to various risks, including undesirable and unintended side effects, or other characteristics that may prevent or limit their approval or commercial use. Furthermore, the number of people who currently or may use cell or tissue-derived cosmetics is difficult to forecast with accuracy. Our future success is dependent on the establishment of a significant market for cell- and tissue-derived cosmetics and our ability to capture a share of this market with our product lines.
Our development efforts with our cosmetics products are susceptible to the same risks of failure inherent in the development and commercialization of other topical cosmetics products based on new technologies. The novel nature of exosome-based cosmetics creates significant challenges in the areas of product development and optimization, manufacturing, government regulation, and market acceptance. For example, the United States FDA has relatively limited experience regulating cosmetics derived from stem cells, and there are no FDA approved medical products utilizing exosomes.
We may not have sufficient product liability insurance, which may leave us vulnerable to future claims we will be unable to satisfy.
The testing, manufacturing, marketing and sale of stem cell derived products entail an inherent risk of product liability claims. We currently have a limited amount of product liability insurance, which may not be adequate to meet potential product liability claims. In the event we are forced to expend significant funds on defending product liability actions, and in the event those funds come from operating capital, we will be required to reduce our business activities, which could lead to significant losses. Adequate insurance coverage may not be available in the future on acceptable terms, if at all. If available, we may not be able to maintain any such insurance at sufficient levels of coverage and any such insurance may not provide adequate protection against potential liabilities. Whether or not a product liability insurance policy is obtained or maintained in the future, any product liability claim could harm our business or financial condition.
29
Risks Related to Our Financial Condition
As described in the report of our auditors for the six months ended June 30, 2023, and the years ended December 31, 2021, and 2022 and the notes to our consolidated financial statements, there is substantial doubt about our ability to continue as a going concern, and if we are unable to continue, you may lose your entire investment.
The uncertainty about our ability to continue in operation is based on our continuing losses from operation, limited revenue and limited working capital, among other things which existed as of year-end December 31, 2021 and December 31, 2022. As of June 30, 2023 and December 31, 2022, the Company had a net working capital deficit of $112,264 and positive working capital $963,050, respectively, and has an accumulated deficit of $5,082,927 and $2,722,373, respectively. Included in the accumulated deficit are losses of $2,360,554 for the six months ended June 30, 2023, and $1,800,268 for the year ended December 31, 2022. Given all these facts, we are dependent on obtaining funding from operations and the sale of debt or equity to continue as a going concern. The financial statements do not include any adjustments relating to the recoverability of assets and classification of liabilities that might be necessary should we be unable to continue as a going concern.
Our ability to continue as a going concern depends on the success of this offering and receipt of additional funds through debt or equity financing and our operations. In the event we are unable to obtain such funding, we may have to delay, reduce or eliminate certain of our planned operations, including some of our research and development and/or clinical validation studies to demonstrate aesthetic improvement, reduce overall overhead expense, or divest assets. This in turn may have an adverse effect on our ability to realize the value of our assets. If we are unable to continue as a going concern, you may lose all or part of your investment.
We have a history of net losses, and we may not be able to achieve or maintain profitability in the future.
We have incurred net losses each year since our inception, and we may not be able to achieve or maintain profitability in the future. We incurred net losses of $2,360,554 and $648,526, for the six months ended June 30, 2023 and 2022, respectively and $1,800,268 and $784,739 in the years ended December 31, 2022 and 2021, respectively. Our expenses will likely increase in the future as we develop and launch new cosmetics product offerings, expand in existing and new markets, increase our sales and marketing efforts, and continue to invest in our laboratory facility. These efforts may be more costly than we expect and may not result in increased revenue or growth in our business. These offerings may require significant capital investments and recurring costs, maintenance, depreciation, asset life and asset replacement costs, and if we are not able to maintain sufficient levels of utilization of such assets or such offerings are otherwise not successful, our investments may not generate sufficient returns and our financial condition may be adversely affected. Any failure to increase our revenue sufficiently to keep pace with our investments and other expenses could prevent us from achieving or maintaining profitability or positive cash flow on a consistent basis. If we are unable to successfully address these risks and challenges as we encounter them, our business, financial condition, results of operations and prospects could be adversely affected. If we are unable to generate adequate revenue growth and manage our expenses, we may continue to incur significant losses in the future and may not be able to achieve or maintain profitability.
Our current growth may not be indicative of our future growth and, if we begin to grow rapidly, we may not be able to effectively manage our growth or evaluate our future prospects. If we fail to effectively manage our future growth or evaluate our future prospects, our business could be adversely affected.
We have experienced minimal growth since our launch in 2020. For example, our revenue increased from nil in 2020 to $827 in 2021, to $766,277 in 2022, and to $459,350 for the six months ended June 30, 2023. Moreover, the number of our full-time employees increased. This growth has placed significant demands on our management, financial, operational, technological and other resources. The anticipated growth and expansion of our business depends on a number of factors, including our ability to:
| ● | increase awareness of our brand and successfully compete with other companies; |
| ● | price our products effectively so that we are able to attract new consumers and expand sales to our existing clients; |
| ● | expand distribution with new and existing clients; |
| ● | continue to innovate and introduce new products; |
| ● | expand our supplier and fulfillment capacities; |
| ● | maintain quality control over our product offerings; and |
| ● | expand internationally. |
30
Such growth and expansion of our business will place significant demands on our management and operations teams and require significant additional resources, financial and otherwise, to meet our needs, which may not be available in a cost-effective manner, or at all. We expect to continue to expend substantial resources on:
| ● | our sales and marketing efforts to increase brand awareness, further engaging our existing and prospective clients, and driving sales of our products; |
| ● | product innovation and development; |
| ● | general administration, including increased finance, legal and accounting expenses associated with being a public company; and |
| ● | expanding internationally. |
These investments may not result in the growth of our business. Even if these investments do result in the growth of our business, if we do not effectively manage our growth, we may not be able to execute on our business plan, respond to competitive pressures, take advantage of market opportunities, satisfy our client requirements or maintain high-quality product offerings, any of which could adversely affect our business, financial condition, results of operations and prospects. You should not rely on our historical rate of revenue growth as an indication of our future performance or the rate of growth we may experience in any new category or internationally.
In addition, to support continued growth, we must effectively integrate, develop and motivate a large number of new employees while maintaining our corporate culture. We face significant competition for personnel. To attract top talent, we have had to offer, and expect to continue to offer, competitive compensation and benefits packages before we can validate the productivity of new employees. We may also need to increase our employee compensation levels to remain competitive in attracting and retaining talented employees. The risks associated with a rapidly growing workforce will be particularly acute as we choose to expand into new product categories and global markets. Additionally, we may not be able to hire new employees quickly enough to meet our needs. If we fail to effectively manage our hiring needs or successfully integrate new hires, our efficiency, ability to meet forecasts and employee morale, productivity and retention could suffer, which could have an adverse effect on our business, financial condition, results of operations and prospects.
We are also required to manage numerous relationships with various vendors and other third parties. Further growth of our operations, client base, or internal controls and procedures may not be adequate to support our operations. If we are unable to manage the growth of our organization effectively, our business, financial condition, results of operations and prospects may be adversely affected.
We will need additional capital to conduct our operations and develop our products and our ability to obtain the necessary funding is uncertain.
During the six months ended June 30, 2023, and the years ended December 31, 2021, and December 31, 2022 we used a significant amount of cash to finance our continued operations, and we need to obtain significant additional capital resources in order to develop products going forward. We may not be successful in maintaining our normal operating cash flow and the timing of our capital expenditures may not result in cash flows sufficient to sustain our operations through the next twelve months. If financing is not sufficient and additional financing is not available or available only on terms that are detrimental to our long-term survival, it could have a major adverse effect on our ability to pursue our clinical research and product development programs and could ultimately affect our ability to continue to function. The timing and degree of any future capital requirements will depend on many factors, including:
| ● | the accuracy of the assumptions underlying our estimates for capital needs in 2023 and beyond; | |
| ● | scientific progress in our research and development programs; | |
| ● | the magnitude and scope of our research and development programs and our ability to establish, enforce and maintain strategic arrangements for research, development, product testing, manufacturing, third-party agreements and marketing; | |
| ● | the costs involved in preparing, filing, prosecuting, maintaining, defending and enforcing patent claims; | |
| ● | the number and type of pipeline product that we pursue; and | |
| ● | the development of major public health concerns, including the novel coronavirus outbreak or other pandemics arising globally, and the current and future impact of it and COVID-19 on our business operations and funding requirements. |
31
Additional financing through strategic collaborations, public or private equity or debt financings or other financing sources may not be available on acceptable terms, or at all. Additional equity financing could result in significant dilution to our stockholders, and any debt financings will likely involve covenants restricting our business activities. Additional financing may not be available on acceptable terms, or at all. Further, if we obtain additional funds through arrangements with collaborative partners, these arrangements may require us to relinquish rights to some of our technologies, pipeline product or products that we might otherwise seek to develop and commercialize on our own. If sufficient capital is not available, we may be required to delay, reduce the scope of or eliminate one or more of our research or product development initiatives, any of which could have a material adverse effect on our financial condition or business prospects.
Our need for additional capital and ability to raise capital in the future may be limited and our failure to raise capital when needed could prevent us from growing.
We may also encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may increase capital needs or drive spending and depletion of cash resources faster than expected. Accordingly, the Company will need to obtain substantial additional funding in order to continue and maintain its operations. The uncertainties around the Company’s ability to fund operations raise substantial doubt about its ability to continue as a growing concern. Thus, In the future, we may need to raise capital through public or private financing or other arrangements. Such financing may not be available on acceptable terms, or at all, and our failure to raise capital when needed could harm our business. We may sell Common Stock, convertible securities and other equity securities in one or more transactions at prices and in a manner as we may determine from time to time. If we sell any such securities in subsequent transactions, investors in our Common Stock may be materially diluted. New investors in such subsequent transactions could gain rights, preferences and privileges senior to those of holders of our Common Stock. Debt financing, if available, may involve restrictive covenants and could reduce our operational flexibility or ability to achieve or maintain profitability. If we cannot raise funds on acceptable terms, we may be forced to raise funds on undesirable terms, or our business may contract or we may be unable to grow our business or respond to competitive pressures, any of which could have an adverse effect on our business, financial condition, results of operations and prospects.
Risks Related to Our Dependence on Third Parties
We depend on our collaborators to help us develop and test our proposed products, and our ability to develop and commercialize products may be impaired or delayed if collaborations are unsuccessful.
Our strategy for the development, product testing and commercialization of our proposed products may require entering into collaborations with corporate partners, licensors, licensees and others. We may then be dependent upon the subsequent success of these other parties in performing their respective responsibilities and the continued cooperation of our partners. Our potential collaborators may not cooperate with us or perform their obligations under our agreements with them. We cannot control the amount and timing of our collaborators’ resources that will be devoted to our research and development activities related to our collaborative agreements with them. Our collaborators may choose to pursue existing or alternative technologies in preference to those being developed in collaboration with us.
Under agreements with collaborators, we may rely significantly on such collaborators to, among other things:
| ● | design and conduct product testing and studies to demonstrate aesthetic improvement; |
| ● | fund research and development activities with us; |
| ● | pay us fees upon the achievement of milestones; and |
| ● | market with us any commercial products that result from our collaborations. |
Should we collaborate with others in the development and commercialization of potential products, those expected product pipeline timelines may be delayed if collaborators fail to conduct these activities in a timely manner, or at all. In addition, our potential collaborators could terminate their agreements with us, and we may not receive any development or milestone payments. If we do not achieve milestones set forth in the agreements, or if our collaborators breach or terminate their collaborative agreements with us, our business may be materially harmed.
32
Current and future contractual arrangements with licensors or collaborators require or could require that they pay royalties and their failure to do so would adversely affect the level of our future revenues and profits.
Some of our contractual arrangements between us and a licensor, collaborator or other third party in connection with the distribution of our products currently require or may require in the future that those third-parties make royalty or other payments to us. Should those third parties fail to pay those royalties, we would not receive all of the revenue derived from commercial sales of such product.
Our reliance on the activities of our non-employee consultants, third-party vendors, and operational contractors, whose activities are not wholly within our control, may lead to delays in development of our proposed products.
As an early-stage company, we rely extensively upon and have relationships with in-house consultants and with expertise in cosmetics developments strategy or other business matters. These consultants are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. We have limited control over the activities of these consultants and, except as otherwise required by our collaboration and consulting agreements to the extent they exist, can expect only limited amounts of their time to be dedicated to our activities. These consultants may have commitments to other commercial and non-commercial entities. We have limited control over the operations of our consultants and can expect only limited amounts of time to be dedicated to our research, development and business goals.
We currently contract with third-party contractors, and in some cases, a single contractor, for all aspects of the supply, logistics, and formulation of our cosmetics products, and expect to continue to do so to support commercial scale production of our cosmetics products. There are significant risks associated with contracting with third-party suppliers, including their ability to meet the increased need that may result from our increasing any commercialization efforts. This increases the risk that we will not have sufficient quantities of hUMSCs or be able to obtain such quantities at an acceptable cost, which could delay, prevent or impair our development or commercialization efforts.
We currently rely on third-party contract suppliers, packagers, shippers and formulators for all of our required raw materials, bottling and packaging, active ingredients and finished products for our cosmetics products. Because there are a limited number of suppliers for the raw materials that we use to formulate our cosmetics products, we may need to engage alternate suppliers to prevent a possible disruption of the formulations of the materials necessary to produce our cosmetic products. We do not have any control over the availability of hUMSCs that form the basis for our products, raw materials that are formulated along with our exosomes or packaging and bottling supplies that form the basis for our product packaging and bottling. If we or our formulators are unable to purchase these raw materials on acceptable terms, at sufficient quality levels or in adequate quantities, if at all, the development and commercialization of our products or any future products would be delayed, or there would be a shortage in supply, which would impair our ability to meet our development objectives for our pipeline products or generate revenues from the sale of our current line of cosmetics products. We also currently rely on a single supplier and formulator for our hUMSCs, to formulate the final products by adding ingredients to bring finished products to market, and for bottling and packaging our final products. While we believe that alternative sources of commercially viable supply exist for both our hUMSCs and raw materials that we use to formulate our products, there can be no assurance that we will be able to quickly establish additional or replacement sources if needed, and a reduction or interruption in supply could adversely affect our ability to supply our products in a timely or cost-effective manner.
We expect to continue to rely on these formulators, packagers and bottlers or other subcontractors and suppliers to support our commercial requirements in the near future. We plan to continue to rely on third parties for the raw materials, hUMSCs and formulating of our products necessary to produce our products and bring them to market.
Our continuing reliance on third-party contract formulators, and suppliers entails a number of risks, including reliance on the third party for regulatory compliance and quality assurance, the possible breach of the manufacturing or supply agreement by the third party, and the possible termination or nonrenewal of the agreement by the third party at a time that is costly or inconvenient for us. In addition, third-party contract formulators and suppliers may not be able to comply with cGMP requirements, or similar regulatory requirements. If any of these risks transpire, we may be unable to timely retain alternate subcontractors or suppliers on acceptable terms and with sufficient quality standards and production capacity, which may disrupt and delay the commercial sale of our products.
33
Our failure or the failure of our third-party formulators, packagers, shippers, bottlers and suppliers to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products, operating restrictions and criminal prosecutions, any of which could significantly and adversely affect supplies of our products or pipeline products. Any failure or refusal to supply or any interruption in supply of the components for our products could delay, prevent or impair our ability to bring our products to market.
The manufacture and formulation of cosmetics products is complex, and formulators may encounter difficulties in production. If we or any of our third-party formulators encounter any difficulties, our ability to provide our products or any pipeline product candidates commercial sales could be delayed or stopped.
The manufacture and formulation of cosmetics products is complex, and requires significant expertise and capital investment, including the development of advanced manufacturing techniques and process controls. We and our contract manufacturers also comply with cGMP requirements. Formulators of cosmetics products often encounter difficulties in production, particularly in scaling up and validating initial production and contamination controls. These problems include difficulties with production costs and yields, quality control, including stability of the product, quality assurance testing, operator error, shortages of qualified personnel, as well as compliance with strictly enforced federal, state and foreign regulations. Furthermore, if microbial, viral or other contaminations are discovered in our product candidates or in the manufacturing and formulating facilities in which our products are made, such facilities may need to be closed for an extended period of time to investigate and remedy the contamination.
We cannot assure you that any stability or other issues relating to the manufacture of our products or any future pipeline product will not occur in the future. As our formulation and manufacturing processes are scaled up, they may reveal manufacturing challenges or previously unknown impurities that could require resolution in order to proceed with commercial sales of our cosmetics products.
Our reliance on third-party manufacturers and formulators entails risks, including the following:
| ● | the inability to meet our product specifications, including product formulation, and quality requirements consistently; |
| ● | a delay or inability to procure or expand sufficient manufacturing and formulation capacity; |
| ● | manufacturing and product quality issues, including those related to scale-up of manufacturing; |
| ● | costs and validation of new equipment and facilities required for scale-up; |
| ● | a failure to comply with cGMP and similar quality standards; |
| ● | the inability to negotiate or renegotiate formulation and manufacturing agreements with third parties under commercially reasonable terms; |
| ● | termination or nonrenewal of formulation or manufacturing agreements with third parties in a manner or at a time that is costly or damaging to us; |
| ● | the reliance on a limited number of sources, and in some cases, single sources for some of our key materials, such that if we are unable to secure a sufficient supply of these key materials, we will be unable to manufacture and sell our products in a timely fashion, in sufficient quantities or under acceptable terms; |
| ● | the lack of qualified backup suppliers for those materials that are currently or in the future purchased from a sole or single source supplier; |
| ● | operations of our third-party manufacturers, formulators or suppliers could be disrupted by conditions unrelated to our business or operations, including the bankruptcy of the manufacturer, formulator or supplier; |
| ● | resource constraints, including as a result of labor disputes or unstable political environments; |
| ● | carrier disruptions or increased costs that are beyond our control; and |
| ● | the failure to deliver our products under specified storage conditions and in a timely manner. |
34
If we or our third-party formulators or manufacturers were to encounter any of these difficulties, and in particular where we rely on a single formulator and manufacturer, our ability to commercialize our products, would be jeopardized. Any adverse developments affecting commercial formulation or manufacturing of our products or any future pipeline product may result in shipment delays, inventory shortages, lot failures, product withdrawals or recalls, or other interruptions in the supply of our products. We may also have to take inventory write-offs and incur other charges and expenses for products that fail to meet specifications, undertake costly remediation efforts or seek more costly formulation or manufacturing alternatives. Accordingly, failures or difficulties faced at any level of our supply chain could materially adversely affect our business and delay or impede the development and commercialization our products or any future pipeline product and could have a material adverse effect on our business, prospects, financial condition and results of operations.
Because we have limited research and development capabilities, we may become more dependent on third parties to perform research and development for us.
We have limited internal research and development capabilities and currently outsource portions of our product research and development to third-party research companies. In particular, we have relied heavily on services provided by Radyus Research, Inc. partially in the development of new products, and to analyze the proteomic characteristics of our Elevai ExosomesTM. We have received sufficient support from our third-party research partners to help us drive our new product development, and we expect to continue to rely on third parties to assist in our research and develop new products.
There are a limited number of third-party research and development companies that specialize or have the expertise required to assist us in our product development objectives. As a result, it may be difficult for us to engage research and development partners and personnel for our anticipated future needs. If we are unable to arrange for third-party research and development of our products, or to do so on commercially reasonable terms, we may not be able to develop new products or expand the application of our existing products as quickly as we could if we were to only perform research and development of new products internally.
Reliance on third-party research and development partners entails risks to which we would not be subject if we performed the research and development ourselves, including reliance on the third party for maintaining the confidentiality of the proprietary information relating to the product being developed and for maintaining quality assurance, the possibility of breach of the research and development agreement by the third party, and the possibility of termination or non-renewal of the agreement by the third party.
Dependence upon third parties for the research and development of our future products may limit our ability to commercialize and deliver products on a timely and competitive basis.
Certain market opportunity data and forecasts in this prospectus were obtained from third-party sources and were not independently verified by us. We believe the estimates of market opportunity data and forecasts of market growth included in this prospectus are reliable, but may prove to be inaccurate, and even if the markets in which we compete achieve the forecasted growth, our business could fail to grow at similar rates, if at all.
This prospectus contains certain data and information that we obtained from various government and private entity publications and reports. There is no guarantee that any particular number or percentage of market participants covered by our market opportunity estimates will purchase our products at all or generate any particular level of revenue for us. While we have not independently verified the data and information contained therein and such data and information may have been collected using third-party methodologies, we believe that the data and information, including projections based on a number of assumptions, from these third-party publications and reports used in this prospectus is reliable. Any expansion in the skincare or medical aesthetics cosmetics on a number of factors, including the cost and perceived value associated with our product offerings and those of our competitors. Even if the markets in which we compete meet the size estimates and growth forecast in this prospectus, our business could fail to grow at the rate we anticipate, if at all, which could adversely affect our business, financial condition, results of operations and prospects. Our growth is subject to many factors, including our success in implementing our business strategy, which is subject to many risks and uncertainties. Accordingly, the forecasts of market growth included in this prospectus should not be taken as indicative of our future growth. For more information regarding the estimates of market opportunity and forecasts of market growth included in this prospectus, see the section titled “Business— Market, Industry and Other Research-Based Data.”
Because we currently sublease our laboratory to commercialize our products, we will continue to be dependent on third parties for our own manufacturing capabilities for us for some time.
We currently sublease our laboratory space from Stem Express LLC in order to meet our commercial manufacturing needs and do not have a long-term lease. The termination of that lease or any loss of services under that agreement would be difficult for us to replace within a short period of time. We expect to continue to rely on third parties to for laboratory space to continue our commercial production of our exosome products.
35
There are a limited number of third-party laboratories that operate under the FDA’s current Good Manufacturing Practices, or cGMP, regulations and that have the necessary expertise and capacity for us to manufacture our products. As a result, should our current relationship with our landlord change or manufacturing needs change it may be difficult for us to locate laboratories for lease that meet our current or anticipated future needs. If we are unable to arrange for third-party laboratory for us to manufacture of our products in, or to find a lease on commercially reasonable terms, we may not be able to complete development of, market and sell our current or new products.
Reliance on our use of leased laboratories entails risks to which we would not be subject to if we maintained our own laboratory, including reliance on a third-party landlord for regulatory compliance and maintenance of some of the commercial equipment and facilities used in our manufacturing process, and the possibility of early termination or non-renewal of the agreement by the landlord.
As we continue to grow the size of our company, we may need to further invest in the expansion of our leased manufacturing facilities for the potential need to increase our manufacturing capability, product volume and the necessary personnel. However, in order to make that election, we will need to invest substantial additional funds and recruit qualified personnel in order to operate any new or expanded manufacturing laboratory and there can be no assurance that we will successfully recruit enough qualified personnel to staff and manufacture our products. In order to expand we will also rely on an increase in our need for additional raw materials and other laboratory supplies and there can be no assurance that we will be able to make or obtain adequate supplies of our products. If we are not able to recruit or staff sufficient numbers of qualified personnel or acquire enough supplies necessary for our manufacturing process it will be more difficult for us to launch new products and compete effectively.
Dependence upon third parties to lease the facilities to manufacture of our products may reduce our profit margins, or the sale of our products and may limit our ability to develop and deliver products on a timely and competitive basis.
We cannot assure you that we will be able to continue to lease our manufacturing facilities in order to bring commercial quantities of our products to market at acceptable costs. Our inability to do so would adversely affect our operating results and cause our business to suffer.
We or our third-party vendors may experience in the future network or system failures, or service interruptions, including cybersecurity attacks, or other technology risks. Our inability to protect our systems and data against such risks could harm our business and reputation.
Our ability to operate uninterrupted and provide high levels of service depends upon the performance of our internal network, systems and related infrastructure, and those of our third-party vendors. Any significant interruptions in, or degradation of, the quality of the services, including infrastructure storage and support, that these third parties provide to us could severely harm our business and reputation and lead to the loss of customers and revenue. Our internal network, systems, and related infrastructure, in addition to the networks, systems, and related infrastructure of our third-party vendors, may be vulnerable to computer viruses and other malware that infiltrate such systems and networks, as well as physical or electronic security breaches, natural disasters, and similar disruptions. They have been and may continue to be the target of attempts to identify and exploit network and system vulnerabilities, penetrate or bypass security measures in order to interrupt or degrade the quality of the services we receive or provide, or otherwise gain unauthorized access to our networks and systems or those of our third-party vendors. These vulnerabilities or other attempts at access may result from, or be caused by, human error or technology failures, however, they may also be the product of malicious actions by third parties intending to harm our business. The methods that may be used by these third parties to cause interruptions or failures or to obtain unauthorized access to information change frequently, are difficult to detect, evolve rapidly, and are increasingly sophisticated and hard to defend against.
Although we have not experienced any security breaches or attempted security breaches and continue to invest in security measures, we cannot be certain that our defensive measures, and those employed by our third-party vendors, will be sufficient to defend against all such current and future methods.
Any actual or perceived security breach, whether experienced by us or a third-party vendor; the reporting or announcement of such an event, or reports of perceived security vulnerabilities of our systems or the systems of our third-party service providers whether accurate or not; or our failure or perceived failure to respond or remediate an event or make adequate or timely disclosures to the public, regulatory or law enforcement agencies following any such event may be material and lead to harm to our financial condition, business reputation, and prospects of future business due to, among other factors: loss of customer confidence arising from interruptions or outages, delays, failure to meet contractual obligations, and loss of data or public release of confidential data; increase regulatory scrutiny on us; compromise our trade secret and intellectual property; expose us to costly uninsured liabilities such as material fines, penalties, liquidated damages, and overall margin compression due to renegotiation of contracts on less favorable terms or loss of business; liability for claims relating to misuse of personal information in violation of contractual obligations or data privacy laws; and potential theft of our intellectual property.
36
A security breach could occur and persist for an extended period of time without detection. We expect that any investigation of a security breach could take a substantial amount of time, and during such time we may not necessarily know the extent of the harm or how best to remediate it, and certain errors or actions could be repeated or compounded before they are discovered and remediated, all of which could further increase the costs and consequences of such a breach. Further, detecting and remediating such incidents may require specialized expertise and there can be no assurance that we will be able to retain or hire individuals who possess, or otherwise internally develop, such expertise. Our remediation efforts therefore may not be successful. The inability to implement, maintain, and upgrade adequate safeguards could have a material and adverse impact on our business, financial condition and results of operations. Moreover, there could be public announcements regarding any data security-related incidents and any steps we take to respond to or remediate such incidents.
The occurrence of any such failure may also subject us to costly lawsuits, claims for contractual indemnities, as well as divert valuable management, research and development, information technology, and marketing resources toward addressing these issues and delay our ability to achieve our strategic initiatives. In addition, we gather, as permitted by law, non-public, personally-identifiable financial information from customers, such as names, addresses, telephone numbers, bank and credit card account numbers and financial transaction information, and the compromise of such data, which may subject us to fines and other related costs of remediation.
If our third-party suppliers, logistics, and manufacturers do not comply with ethical business practices or with applicable laws and regulations, our reputation, business, financial condition, results of operations and prospects could be harmed.
Our reputation and our clients’ willingness to purchase our products depend in part on our suppliers’, packagers’, manufacturers’, and formulators’ compliance with ethical employment practices, such as with respect to child labor, wages and benefits, forced labor, discrimination, safe and healthy working conditions, and with all legal and regulatory requirements relating to the conduct of their businesses. We do not exercise control over our suppliers, packagers, shippers, manufacturers, and formulators and cannot guarantee their compliance with ethical and lawful business practices. If our suppliers, packagers, shippers, manufacturers, or formulators fail to comply with applicable laws, regulations, safety codes, employment practices, human rights standards, quality standards, environmental standards, production practices, or other obligations, norms, or ethical standards, our reputation and brand image could be harmed, and we could be exposed to litigation, investigations, enforcement actions, monetary liability, and additional costs that would harm our reputation, business, financial condition, results of operations and prospects.
If we, or our third-party manufacturers or formulators fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of its business.
Our research and development activities and our third-party manufacturers’, formulators’ and suppliers’ activities involve the controlled storage, use and disposal of hazardous materials and other hazardous compounds. We and our manufacturers, formulators and suppliers are subject to laws and regulations governing the use, manufacture, storage, handling and disposal of these hazardous materials. In some cases, these hazardous materials and various wastes resulting from their use are stored at our and our manufacturers’ facilities pending their use and disposal. We cannot eliminate the risk of contamination, which could cause an interruption of our commercialization efforts, research and development efforts, business operations and environmental damage resulting in costly clean-up and liabilities under applicable laws and regulations governing the use, storage, handling and disposal of these materials and specified waste products. Although we believe that the safety procedures utilized by our third-party manufacturers for handling and disposing of these materials generally comply with the standards prescribed by these laws and regulations, we cannot guarantee that this is the case or eliminate the risk of accidental contamination or injury from these materials. In such an event, we may be held liable for any resulting damages and such liability could exceed our resources and state or federal or other applicable authorities may curtail our use of certain materials and/or interrupt our business operations. Furthermore, environmental laws and regulations are complex, change frequently and have tended to become more stringent. We cannot predict the impact of such changes and cannot be certain of our future compliance.
Risks Related to Our Products Legal and Regulatory Risks
A recall or suspension of sale of our products, or the discovery of serious safety issues with our products or the incorrect application of such products by medical professionals to which we sell such products, could have a significant negative impact on us.
The FDA and comparable agencies of other countries regulate our cosmetic products. In the United States, FDA regulations govern, among other things, the activities that we perform, including product development, product testing, product labeling, product storage, manufacturing, advertising, promotion, product sales, reporting of certain product adverse events and failures, and distribution.
37
The FDA and equivalent foreign regulatory authorities have the authority to require the recall or suspension, either temporarily or permanently, of commercialized products in the event that a product has a reasonable probability of causing a serious adverse health risk due to adulteration or misbranding. Regulatory authorities have broad discretion to require the recall or suspension of a product or to require that manufacturers alert customers of safety risks. Recalls, suspensions or other notices relating to any products that we distribute would divert managerial and financial resources, and have an adverse effect on our reputation, financial condition and operating results.
In addition, regulatory authorities may require us to, or we may voluntarily, suspend sales of a product if we become aware that the medical professionals to which we sell our products have not followed our instructions for application. For example, when our product is marketed and sold by us to medical professionals throughout the United States and internationally, we include instructions specifying that such product must be applied topically by these medical professionals. Administration outside of those specific directions could result in us running afoul of government rules and regulations.
FDA and FTC may enforce against our cosmetic products if they do not accept our advertising and marketing or if those products are used beyond the intended uses that we authorize.
If our products are marketed outside of their intended use, for example if they are advertised for the treatment, diagnosis, cure, prevention, or mitigation of a disease, then regulatory agencies may issue a warning letter or further investigate our marketing practices to ensure we are complying with advertising and promotional rules that apply to the product category.
Regulations governing our products, including the formulation, registration, marketing and sale of our products, could harm our business.
Our products are subject to extensive government regulation by numerous federal, state and local government agencies and authorities. Many of these laws and regulations involve a high level of subjectivity, are subject to interpretation, and vary significantly from market to market. These laws and regulations can, and often do, have several impacts on our business, including but not limited to:
| ● | delays, or altogether prohibitions, in introducing or selling a product or ingredient in one or more markets; |
| ● | limitations on our ability to import products into a market; |
| ● | limitations on the claims we can make regarding our products; and |
| ● | delays and expenses associated with compliance, such as record keeping, documentation of the properties of certain products, labeling, and scientific substantiation; and |
| ● | product reformulations, or the recall or discontinuation of certain products that cannot be reformulated to comply with new regulations. |
We have observed a general increase in regulatory activity and activism in the United States and across many markets globally where we operate, and the regulatory landscape is becoming more complex with increasingly strict requirements. In particular, the requirements are impacting the ingredients we can include in our products, the accepted quantities of those ingredients and the quality and characterization of the ingredients. Global regulators have in recent years become overall more restrictive on the accepted levels of certain ingredients or sources that we can use in our product, in some cases banning them outright. Further, many of the restrictions regarding ingredient quality are not directly applicable to our products, leaving the possibility that our interpretation of compliance may not match that of the enforcing authorities. Often there is a lack of an equivalent ingredients or source present in the marketplace. In other cases, the removal or reduction of a technical ingredient to stabilize our products, leads to a significant change to the character of the product that may make it no longer desirable or safe to the consumer. If this trend in new regulations continues, we may find it necessary to alter some of the ways we have traditionally marketed our products in order to stay in compliance with a changing regulatory landscape and this could add to the costs of our operations and/or have an adverse impact on our business.
38
Many laws and regulations govern aspects of regulatory oversight of our products although the FDA currently does not have a pre-market approval system for cosmetics. However, cosmetic products may become subject to more extensive regulation in the future and have recently. These events could interrupt the marketing and sale of our products, severely damage our brand reputation and image in the marketplace, increase the cost of our products, cause us to fail to meet customer expectations or cause us to be unable to deliver merchandise in sufficient quantities or of sufficient quality to our stores, any of which could result in lost sales.
Our operations could be harmed if new laws or regulations are enacted that restrict our ability to market or distribute our products or impose additional burdens or requirements on us in order to continue selling our cosmetics products. In addition, the adoption of new regulations or changes in the interpretations and enforcement of existing regulations may result in significant compliance costs or discontinuation of cosmetics products sales and may impair the marketability of our cosmetics products, resulting in significant loss of net sales. We cannot predict the nature of any future laws, regulations, interpretations, or applications, nor can we determine what effect additional governmental regulations or administrative orders, when and if promulgated, would have on our business. If new or existing laws and regulations restrict, inhibit or delay our ability to introduce or market our products or limit the claims we are able to make regarding our cosmetics products, this could have a material adverse effect on our business, financial condition, and operating results. If we fail to comply with the laws and regulations governing our products, we could face enforcement action, and we could be fined or forced to alter or stop selling our cosmetics products.
Government regulations and private party actions relating to the marketing and advertising of our cosmetics products may restrict, inhibit or delay our ability to sell our cosmetics products and harm our business.
Government authorities regulate advertising and product claims regarding the benefits of our cosmetics products. These regulatory authorities may require us to provide an adequate and reasonable basis to substantiate and support any marketing or product benefits claims. What constitutes such reasonable basis to substantiate such claims can vary widely from market to market and there is no assurance that the research and development efforts that we undertake to support our claims will be deemed adequate for any particular product or product marketing claim. If we are unable to show adequate and reliable substantiation for our product claims, or if our marketing materials or the marketing materials of our sales force make claims that exceed the scope of allowed claims for cosmetics that we offer, the United States Food and Drug Administration (the “FDA”), the Federal Trade Commission (the “FTC”) or other regulatory authorities could take enforcement action requiring us to revise our marketing materials, amend our claims or stop selling certain products, which could harm our business.
For example, in recent years, the FDA has issued warning letters to many cosmetic companies alleging improper structure/function claims regarding their cosmetic products, including, for example, product claims regarding gene activity, cellular rejuvenation, repair, anti-aging and rebuilding collagen. There is a degree of subjectivity in determining whether a claim is an improper structure/function claim. Given this subjectivity and our research and development focus on the appearance of skin and the influence of certain stem-cell derived ingredients on skin, there is a risk that we could receive a warning letter, be required to modify our product claims or take other actions to satisfy the FDA if the FDA determines any of our marketing materials include improper structure/function claims for our cosmetic products. In addition, lawyers have filed class action lawsuits against some cosmetics brands after those brands received these FDA warning letters. There can be no assurance that we will not be subject to government actions or class action lawsuits, which could harm our business.
In the United States, the FTC’s Guides Concerning the Use of Endorsements and Testimonials in Advertising (“Guides”) require disclosure of material connections between an endorser and the company they are endorsing, and they generally do not allow marketing using atypical results. Our sales force has historically used testimonials and “before and after” photos to market and sell some of our popular products such as our E-Series™ serums. We intend to continue to use testimonials for our popular products. In highly regulated and scrutinized product categories, such as those that promote healthy hair growth cycles, if we or our sales force fails to comply with the Guides or makes improper product claims, the FTC could bring an enforcement action against us, and we could be fined and/or forced to alter our marketing materials.
39
Our operations could be harmed if we fail to comply with Good Manufacturing Practices.
Across our markets, there are regulations on a diverse range of Good Manufacturing Practices (“GCMPs”) that may eventually apply to us under the recently enacted Modernization of Cosmetic Regulation Act of 2022 (“MoCRA”) which requires the FDA to issue proposed rules relating to GCMPs for cosmetics manufacturers. If we are considered a cosmetic manufacture under MoCRA than we and our vendors may be subject to stringent safety requirements on a variety of topics, including vendor qualifications, ingredient identification, manufacturing controls and record keeping. Ingredient identification requirements, which would require us to confirm the levels, identity and potency of ingredients listed on our product labels within a narrow range, which may be particularly burdensome and difficult for us because our products contain many different ingredients. Additionally, under MoCRA we may be obligated to track and periodically report adverse events to government agencies. Compliance with these increasing regulations may further increase the cost of manufacturing certain of our products as we work with our vendors to assure they are qualified and in compliance. In addition, our operations could be harmed if regulatory authorities determine that we or our vendors are not in compliance with these regulations or if public reporting of adverse events harms our reputation for quality and safety. A finding of noncompliance may result in administrative warnings, penalties or actions impacting our ability to continue selling certain products, including public withdrawals, seizures and recalls. For example, in prior years, our competitors have had product recalls in the United States based on labeling issues. Problems associated with product recalls could be exacerbated due to the global nature of our business because a recall in one jurisdiction could lead to recalls in other jurisdictions.
Restrictive and extensive government regulation could slow or hinder our production of cosmetics containing a stem-cell byproduct and we may be unsuccessful in our efforts to comply with applicable federal, state and international laws and regulations, which could result in government enforcement actions.
Although we seek to conduct our business in compliance with applicable governmental laws and regulations, these laws and regulations are exceedingly complex and often subject to varying interpretations. The cosmetics and stem-cell industry are topics of significant government interest, and thus the laws and regulations applicable to our business are subject to frequent change and/or reinterpretation. As such, there can be no assurance that we will be able, or will have the resources, to maintain compliance with all such laws and regulations. Failure to comply with such laws and regulations, as well as the costs associated with such compliance or with enforcement of such healthcare laws and regulations, may have a material adverse effect on our operations or may require restructuring of our operations or impair our ability to operate profitably.
The research and development of stem cell byproducts is subject to and restricted by extensive regulation by governmental authorities in the United States and other countries. If in the future we become subject to additional FDA and other necessary regulatory approvals, that process may be lengthy, expensive and uncertain which may have a material adverse effect on our operations or may require restructuring of our operations or impair our ability to operate profitably.
New regulations could prohibit physicians from dispensing our cosmetics products directly.
In our primary market, the United States, we market our cosmetics products and systems directly to our physician clients to dispense in their offices. Thereafter, our cosmetics products and systems we sell are dispensed by physicians directly to their medical aesthetics consumers in their offices. In the event state regulations change to limit or prohibit the ability of physicians to dispense our cosmetics products directly to medical aesthetics consumers in their offices, medical aesthetics consumers may be required to purchase our cosmetics products in retail settings or via e-commerce, as opposed to directly from their physicians. If medical aesthetics consumers are unable to purchase our cosmetics products directly from physicians, it could result in medical aesthetics consumers purchasing less of our product than they otherwise would or affect the perception of our cosmetics products are which would harm our business, our operations or impair our ability to operate profitably.
Failure to obtain regulatory approvals in foreign jurisdictions would prevent us from marketing our cosmetics products internationally.
We market our cosmetics products outside of the United States. In order to market our cosmetics products in many non-U.S. jurisdictions we must obtain separate regulatory approvals and comply with numerous and varying regulatory requirements. In others, we do not have to obtain prior regulatory approval but do have to comply with other regulatory restrictions on the manufacturing, marketing and sale of our cosmetics products. We may be unable to file for regulatory approvals and may not receive necessary approvals to commercialize our cosmetics products in any market. The approval procedure varies among countries and can involve additional testing and data review. We may not obtain foreign regulatory approvals on a timely basis, if at all. Moreover, approval by one foreign regulatory authority does not ensure approval by regulatory agencies in other foreign countries or by the FDA. The failure to obtain these approvals could harm our business, our operations or impair our ability to operate profitably.
40
Risks related to Our Intellectual Property
If we fail to protect or enforce our intellectual property or confidential proprietary information relating to our current and any future cosmetics products or cosmetics pipeline product, others could compete against us more directly and we may not be able to compete effectively in our market.
Our success depends in part on our ability to protect our intellectual property rights. We rely on a combination of trademarks, trade secrets, confidential proprietary information, domains, patent rights and other intellectual property rights to protect our intellectual property. In addition, to protect our trade secrets, confidential information and other intellectual property rights, we have entered into confidentiality agreements with third parties, and confidential information and invention assignment agreements with employees, consultants and advisors. There can be no assurances that we will be able to enforce these agreements or alternatively, these agreements may be deemed to be unenforceable. If we cannot adequately protect or enforce our intellectual property rights, we may not be able to adequately compete, and our business and prospects could be adversely affected.
Certain of our technology may not be subject to protection through patents, which leaves us vulnerable to theft of our technology.
Certain parts of our know-how and technology are not patentable or are trade secrets. To protect our proprietary position in such know-how and technology, we have entered and intend to require all employees, consultants, advisors and collaborators to enter into confidentiality and invention ownership agreements with us. These agreements may not provide meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure. Further, in the absence of patent protection, competitors who independently develop substantially equivalent technology may harm our business.
We may not be able to protect our proprietary technology, which could harm our ability to operate profitably.
The molecular biology, stem cells, cosmetics, and bioprocessing industries place considerable importance on obtaining patent and trade secret protection for new technologies, cosmetics products and processes. Our success will depend, to a substantial degree, on our ability to obtain and enforce patent protection for our cosmetics products, preserve any trade secrets and operate without infringing the proprietary rights of others. We cannot assure you that:
| ● | we will succeed in obtaining any patents, obtain them in a timely manner, or that the breadth or degree of protection that any such patents will protect our interests; |
| ● | the use of our technology will not infringe on the proprietary rights of others; |
| ● | patent applications relating to our potential cosmetics products or technologies will result in the issuance of any patents or that, if issued, such patents will afford adequate protection to us or will not be challenged, invalidated or infringed; or |
| ● | patents will not be issued to other parties, which may be infringed by our potential cosmetics products or technologies. |
We are aware of certain patents that have been granted to others and certain patent applications that have been filed by others with respect to other stem cell technologies and the use of exosomes for cosmetic aesthetics purposes. The fields in which we operate have been characterized by significant efforts by competitors to establish dominant or blocking patent rights to gain a competitive advantage, and by considerable differences of opinion as to the value and legal legitimacy of competitors’ purported patent rights and the technologies they actually utilize in their businesses.
Considerable research in the areas of stem cells, molecular biology, cosmetics, and bioprocessing is being performed in countries outside of the United States, and a number of our competitors are located in those countries. The laws protecting intellectual property in some of those countries may not provide adequate protection to prevent our competitors from misappropriating our intellectual property.
Patents held by other persons may result in infringement claims against us that are costly to defend and which may limit our ability to use the disputed technologies and prevent us from pursuing research and development or commercialization of potential cosmetics products.
A number of biotechnology and other companies, universities and research institutions have filed patent applications or have been issued patents relating to exosomes, stem cells, and other technologies potentially relevant to or required by our expected cosmetics products. We cannot predict which, if any, of such applications will issue as patents or the claims that might be allowed. We are aware that a number of companies have filed applications relating to stem cells. We are also aware of a number of patent applications and patents claiming use of exosomes and other modified cells to improve aesthetics.
41
If third party patents or patent applications contain claims infringed by either our licensed technology or other technology required to make and use our potential cosmetics products and such claims are ultimately determined to be valid, we might not be able to obtain licenses to these patents at a reasonable cost, if at all, or be able to develop or obtain alternative technology. If we are unable to obtain such licenses at a reasonable cost, we may not be able to develop some cosmetics products commercially. We may be required to defend ourselves in court against allegations of infringement of third-party patents. Patent litigation is very expensive and could consume substantial resources and create significant uncertainties. An adverse outcome in such a suit could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties, or require us to cease using such technology.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our target markets and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented, declared generic or determined to be infringing on other marks. We may not be able to protect our rights in these trademarks and trade names, which we need in order to build name recognition with potential partners or customers in our target markets. If we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively, and our business may be adversely affected.
If we infringe or are alleged to infringe intellectual property rights of third parties, our business could be harmed.
Our research, development and commercialization activities may infringe or otherwise violate or be alleged to infringe or otherwise violate patents owned or controlled by other parties. Competitors in the field of aesthetics and cosmetics have developed large portfolios of patents and patent applications in fields relating to our business. Additionally, there may also be patent applications that have been filed but not published that, when issued as patents, could be asserted against us. These third parties could bring claims against us that would cause us to incur substantial expenses and, if successful against us, could cause us to pay substantial damages and/or we could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit. Further, if a patent infringement suit were brought against us, during the pendency of the litigation, we could be forced to stop or delay research, development, manufacturing or sales of the product or product candidate that is the subject of the suit.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of our competitors or are in breach of non-competition or non-solicitation agreements with our competitors.
We may employ individuals who were previously employed at universities or pharmaceutical or cosmetics companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, and we are not currently subject to any claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties, we may in the future be subject to such claims. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
We may need to license intellectual property from third parties, and such licenses may not be available or may not be available on commercially reasonable terms.
A third party may hold intellectual property, including patent rights that are important or necessary to the development of our future cosmetics product. It may be necessary for us to use the patented or proprietary technology of third parties to commercialize our prospective cosmetic products, in which case we would be required to obtain a license from these third parties. There can be no assurance that such third parties will grant us the necessary licenses on commercially reasonable terms or at all. Failure to obtain such licenses on commercially reasonable terms could limit or eliminate our ability to develop or commercialize our future product candidates, which would have a negative impact on our business and results of operations.
42
Risks Related to Our Capital Requirements and Finances
If we fail to generate sufficient cash flow from our operations, we will be unable to continue to develop and commercialize new cosmetics products.
We expect capital outlays and operating expenditures to increase over the next several years as we expand our operations, and our commercialization, product validation studies, research and development and manufacturing activities. We believe that our net cash provided by operating activities and existing cash and cash equivalents will be sufficient to fund our operations for at least the next two years. However, our present and future funding requirements will depend on many factors, including, among other things:
| ● | the level of research and development investment required to maintain and improve our competitive position; |
| ● | the success of our product sales and related collections; |
| ● | our need or decision to acquire or license complementary businesses, cosmetics products or technologies or acquire complementary businesses; |
| ● | costs relating to the expansion of the sales force, management and operational support; |
| ● | competing technological and market developments; and |
| ● | costs relating to changes in regulatory policies or laws that affect our operations. |
As a result of these factors, we may need to raise additional funds, and we cannot be certain that such funds will be available to us on acceptable terms when needed, if at all. In addition, if we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish potentially valuable rights to our future cosmetics products or proprietary technologies, or grant licenses on terms that are not favorable to us. If we cannot raise funds on acceptable terms, we may not be able to expand our operations, develop new cosmetics products, take advantage of future opportunities or respond to competitive pressures or unanticipated customer requirements.
We will incur increased costs as a result of being a public company.
As public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. We will incur costs associated with our public company reporting requirements. We also anticipate that we will incur costs associated with recently adopted corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002. We expect these rules and regulations to increase our legal and financial compliance costs and to make some activities more time-consuming and costly. In order to comply with these requirements, we need to address the material weaknesses we have identified. While we have taken some steps to improve our financial accounting organization and processes to date, we still need to make a number of additional changes, including adding staff in the areas of finance, tax, internal controls and internal audit. We also need to adopt and implement additional policies and procedures to strengthen our financial reporting capability and plan to invest in an enterprise resource planning system. However, the process of designing and implementing an effective financial reporting system is a continuous effort that will require us to anticipate and react to changes in our business and the economic and regulatory environments. All of this will also require us to expend significant resources. We also expect these rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. We are currently evaluating these new rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
Risks Related to this Offering and Our Common Stock
The price of our Common Stock could be subject to rapid and substantial volatility.
There have been instances of extreme stock price run-ups followed by rapid price declines and strong stock price volatility with recent initial public offerings, especially among those with relatively smaller public floats. As a relatively small-capitalization company with a relatively small public float, we may experience greater stock price volatility, extreme price run-ups, lower trading volume, and less liquidity than large-capitalization companies. In particular, our Common Stock may be subject to rapid and substantial price volatility, low volumes of trades, and large spreads in bid and ask prices. Such volatility, including any stock run-ups, may be unrelated to our actual or expected operating performance and financial condition or prospects, making it difficult for prospective investors to assess the rapidly changing value of our Common Stock.
43
The market price of our Common Stock may fluctuate significantly in response to numerous factors, many of which are beyond our control, including:
| ● | actual or anticipated fluctuations in our revenue and other operating results; |
| ● | the financial projections we may provide to the public, any changes in these projections or our failure to meet these projections; |
| ● | actions of securities analysts who initiate or maintain coverage of us, changes in financial estimates by any securities analysts who follow our company, or our failure to meet these estimates or the expectations of investors; |
| ● | announcements by us or our competitors of significant services or features, technical innovations, acquisitions, strategic relationships, joint ventures, or capital commitments; |
| ● | price and volume fluctuations in the overall stock market, including as a result of trends in the economy as a whole; |
| ● | lawsuits threatened or filed against us; and |
| ● | other events or factors, including those resulting from war or incidents of terrorism, or responses to these events. |
In addition, if the trading volumes of our Common Stock are low, persons buying or selling in relatively small quantities may easily influence the price of our Common Stock. This low volume of trades could also cause the price of our Common Stock to fluctuate greatly, with large percentage changes in price occurring in any trading day session. Holders of our Common Stock may also not be able to readily liquidate their investment or may be forced to sell at depressed prices due to low volume trading. Broad market fluctuations and general economic and political conditions may also adversely affect the market price of our Common Stock. As a result of this volatility, investors may experience losses on their investment in our Common Stock. A decline in the market price of our Common Stock also could adversely affect our ability to issue additional Common Stock or other of our securities and our ability to obtain additional financing in the future. No assurance can be given that an active market in our Common Stock will develop or be sustained. If an active market does not develop, holders of our Common Stock may be unable to readily sell the shares they hold or may not be able to sell their shares at all.
Our Common Stock has not been publicly traded, and we expect that the price of our Common Stock will fluctuate substantially.
Prior to this offering, there has been no public market for our Common Stock. An active public trading market may not develop after completion of this offering or, if developed, may not be sustained. The initial public offering price for our shares will be determined by negotiations between us and the representatives of the underwriters and may not be indicative of the market price of our Common Stock after this offering. The market price for our Common Stock after this offering will be affected by a number of factors, including:
| ● | changes in earnings estimates, investors’ perceptions, recommendations by securities analysts or our failure to achieve analysts’ earnings estimates; |
| ● | quarterly variations in our or our competitors’ results of operations; |
| ● | the announcement of new products or service enhancements by us or our competitors; |
| ● | announcements related to litigation; |
| ● | developments in our industry; and |
| ● | general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors. |
We have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not necessarily improve our results of operations or enhance the value of our Common Stock. To the extent (i) we raise more money than required for the purposes explained in the section titled “Use of Proceeds” or (ii) we determine that the proposed uses set forth in that section are no longer in the best interests of our Company, we cannot specify with any certainty the particular uses of such net proceeds that we will receive from our public offering. Our management will have broad discretion in the application of such net proceeds, including working capital, possible acquisitions, and other general corporate purposes, and we may spend or invest these proceeds in a way with which our stockholders disagree. The failure by our management to apply these funds effectively could harm our business and financial condition. Pending their use, we may invest the net proceeds from our public offering in a manner that does not produce income or that loses value.
44
Provisions of Delaware law could discourage a takeover you may consider favorable or could cause current management to become entrenched and difficult to replace.
Delaware law could make it more difficult for other companies to acquire us, even if doing so would benefit our stockholders. Delaware law prohibits us from engaging in any business combination with any “interested stockholder,” meaning generally that a stockholder who beneficially owns more than 15% of our stock cannot acquire us for a period of three years from the date this person became an interested stockholder unless various conditions are met, such as approval of the transaction by our board of directors. These restrictions could have the effect of delaying or preventing a change in control.
You will suffer immediate and substantial dilution.
The initial public offering price per share is substantially higher than the net tangible book value per share immediately after the offering. As a result, you will pay a price per share that substantially exceeds the book value of our assets after subtracting our liabilities.
We do not intend to pay cash dividends on our Common Stock in the foreseeable future.
We currently anticipate that we will retain all future earnings, if any, to finance the growth and development of our business and do not anticipate paying cash dividends on our Common Stock in the foreseeable future. Any payment of cash dividends will depend upon our financial condition, capital requirements, earnings and other factors deemed relevant by our board of directors.
Our operating results may fluctuate, which could cause our stock price to decline.
Our operating results may fluctuate for a variety of reasons, many of which are beyond our control, including:
| ● | fluctuations in revenue, including as a result of adverse market conditions due to the COVID-19 pandemic and the closing of medical offices and travel opportunities as the pandemic abates, the seasonality of market transactions and fluctuations in sales through our medical practice and cosmetics product distribution channels; |
| ● | the amount and timing of our operating expenses; |
| ● | our success in attracting new and maintaining relationships with existing client and distribution partners; |
| ● | our success in executing on our strategy and the impact of any changes in our strategy; |
| ● | the timing and success of product launches, including new products that we may introduce; |
| ● | the success of our marketing efforts; |
45
| ● | adverse economic and market conditions, such as those related to the current COVID-19 pandemic, currency fluctuations and other adverse global events; |
| ● | disruptions in our supply chain, the ability of our third-party manufacturers to produce our products, or ability of our distributors to distribute our products; |
| ● | the impact of competitive developments and our response to those developments; |
| ● | fluctuations in inventory and working capital; |
| ● | our ability to manage our business and future growth; and |
| ● | our ability to recruit and retain employees. |
Fluctuations in our operating results may cause those results to fall below our financial guidance or other projections, or the expectations of analysts or investors, which could cause the price of our Common Stock to decline. Fluctuations in our results could also cause other problems, including, for example, analysts or investors changing their models for valuing our Common Stock, particularly post-pandemic. We could experience short-term liquidity issues, our ability to retain or attract key personnel may diminish, and other unanticipated issues may arise.
We believe that our operating results may vary in the future and that period-to-period comparisons of our operating results may not be meaningful. For example, our overall historical growth rate and the impacts of the COVID-19 pandemic may have overshadowed the effect of seasonal variations on our historical operating results since we launched amid the pandemic. Any seasonal effects may change or become more pronounced over time, which could also cause our operating results to fluctuate. You should not rely on the results of or current operating results as an indication of future performance.
Holders of our Common Stock may also not be able to readily liquidate their investment or may be forced to sell at depressed prices due to low volume trading. Broad market fluctuations and general economic and political conditions may also adversely affect the market price of our Common Stock. As a result of this volatility, investors may experience losses on their investment in our Common Stock. Furthermore, the potential extreme volatility may confuse the public investors of the value of our stock, distort the market perception of our stock price and our company’s financial performance and public image, negatively affect the long-term liquidity of our Common Stock, regardless of our actual or expected operating performance. If we encounter such volatility, including any rapid stock price increases and declines seemingly unrelated to our actual or expected operating performance and financial condition or prospects, it will likely make it difficult and confusing for prospective investors to assess the rapidly changing value of our Common Stock and understand the value thereof.
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our Common Stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups Act, or the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, although we could lose that status sooner if our revenues exceed $1.235 billion, if we issue more than $1 billion in non-convertible debt in a three-year period, or if the market value of our Common Stock held by non-affiliates exceeds $700 million as of any March 31 before that time, in which case we would no longer be an emerging growth company as of the following December 31. We cannot predict if investors will find our Common Stock less attractive because we may rely on these exemptions. If some investors find our Common Stock less attractive as a result, there may be a less active trading market for our Common Stock, and our stock price may be more volatile.
46
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until those standards apply to private companies. We have elected to avail our company of this exemption from new or revised accounting standards and, therefore, will be subject to accounting standards that are available to emerging growth companies.
There may not be an active, liquid trading market for our Common Stock.
Prior to this offering, there has been no public market for our Common Stock. An active trading market for our Common Stock may not develop or be sustained following this offering. You may not be able to sell your shares at the market price, if at all, if trading in our shares is not active. The public offering price was determined by negotiations between us and the underwriters based upon a number of factors. The public offering price may not be indicative of prices that will prevail in the trading market.
Shares eligible for future sale may adversely affect the market price of our Common Stock, as the future sale of a substantial amount of outstanding Common Stock in the public marketplace could reduce the price of our Common Stock.
The market price of our shares could decline as a result of sales of substantial amounts of our shares in the public market or the perception that these sales could occur. In addition, these factors could make it more difficult for us to raise funds through future offerings of our Common Stock. Shares will be outstanding immediately after this offering if the firm commitment is completed and the underwriters do not exercise their over-allotment option and shares if exercised in full. All of the shares sold in the offering will be freely transferable without restriction or further registration under the Securities Act. The remaining shares will be “restricted securities” as defined in Rule 144. These shares may be sold in the future without registration under the Securities Act to the extent permitted by Rule 144 or other exemptions under the Securities Act. See “Shares Eligible for Future Sale”.
We will incur additional costs as a result of becoming a public company, which could negatively impact our net income and liquidity.
Upon completion of this offering, we will become a public company in the United States. As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, Sarbanes-Oxley and rules and regulations implemented by the SEC and the Nasdaq Capital Market require significantly heightened corporate governance practices for public companies. We expect that these rules and regulations will increase our legal, accounting and financial compliance costs and will make many corporate activities more time-consuming and costly.
We do not expect to incur materially greater costs as a result of becoming a public company than those incurred by similarly sized U.S. public companies. In the event that we fail to comply with these rules and regulations, we could become the subject of a governmental enforcement action, investors may lose confidence in us, and the market price of our Common Stock could decline.
The obligation to disclose information publicly may put us at a disadvantage to competitors that are private companies.
Upon completion of this offering, we will be a publicly listed company in the United States. As a publicly listed company, we will be required to file annual reports with the Securities and Exchange Commission. In some cases, we will need to disclose material agreements or results of financial operations that we would not be required to disclose if we were a private company. Our competitors may have access to this information, which would otherwise be confidential. This may give them advantages in competing with our company. Similarly, as a U.S.-listed public company, we will be governed by U.S. laws that our competitors, which are mostly private Chinese companies, are not required to follow. To the extent compliance with U.S. laws increases our expenses or decreases our competitiveness against such companies, our public listing could affect our results of operations.
47
Assuming the sale of [●] shares of Common Stock in this offering at a price of $[●] per share of Common stock, the midpoint of the range set forth on the cover page of this prospectus, after deducting the estimated underwriting discounts, non-accountable expense allowance and offering expenses payable by us, we expect to receive net proceeds of approximately $[●] from this offering. If the underwriter exercises its over-allotment option in full, our aggregate net proceeds will be approximately $[●].
We currently expect to use the net proceeds from this offering (assuming no exercise of the underwriter’s over-allotment option) as follows:
| Description of Use | Estimated Amount of Net Proceeds | |||
| General and Administrative Expenses | $ | |||
| Marketing and Market Expansion | $ | |||
| Research and Development | $ | |||
| Working Capital | $ | |||
| Total: | $ | |||
A $1.00 increase or decrease in the assumed public offering price of $[●] per share would increase or decrease the net proceeds from this offering by approximately $[●], assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts, non-accountable expense allowance and offering expenses payable by us. Similarly, each increase or decrease of 100,000 shares offered would increase or decrease our net proceeds by approximately $[●], assuming the assumed public offering price remains the same, and after deducting estimated underwriting discounts, non-accountable expense allowance and estimated offering expenses payable by us.
Pending our use of the net proceeds from this offering, we may invest the net proceeds in a variety of capital preservation investments, including short-term, investment grade, interest bearing instruments and United States government securities. We do not intend to use the proceeds from this offering to make principal payments, scheduled or early, on any of our outstanding debt.
48
Implications of Being an Emerging Growth Company
We qualify as and elect to be an “emerging growth company” as defined in the Jumpstart our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include, but not limited to:
| ● | reduced disclosure about the emerging growth company’s executive compensation arrangements in our periodic reports, proxy statements and registration statements; and |
| ● | an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002. |
An emerging growth company can also take advantage of the extended transition period provided in Section 13(a) of the Exchange Act for complying with new or revised accounting standards. In other words, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have elected to take advantage of this extended transition period and, as a result, our operating results and financial statements may not be comparable to the operating results and financial statements of companies who have adopted the new or revised accounting standards.
We may take advantage of these provisions for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.235 billion in annual revenue, have more than $700 million in market value of our shares of common stock held by non-affiliates or issue more than $1.0 billion of non-convertible debt over a three-year period.
DETERMINATION OF OFFERING PRICE
The public offering price of the shares we are offering was determined by us in consultation with the underwriter based on discussions with potential investors in light of the history and prospects of our Company, the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment of our management, the public stock price for similar companies, general conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
MARKET INFORMATION FOR THE SHARES
Market Information
There is currently no market for our shares. We intend to apply to have our shares listed on the Nasdaq Capital Market under the symbol “ELAB”. The offering that we are conducting with the prospectus will not close unless the Nasdaq Capital Market has approved our shares for listing.
We have not paid any cash dividends on our shares to date. We intend to retain future earnings, if any, for future operations, expansion and debt repayment and have no current plans to pay cash dividends for the foreseeable future. Any decision to declare and pay dividends in the future will be made at the discretion of the Board and will depend on, among other things, our results of operations, financial condition, cash requirements, contractual restrictions, and other factors that the Board may deem relevant. In addition, our ability to pay dividends may be limited by covenants of any existing and future outstanding indebtedness we or our subsidiaries incur.
49
The following table sets forth our capitalization. Such information is set forth on the following basis:
| ● | on an actual basis for June 30, 2023; |
| ● | on a pro forma basis, giving effect to our sale of [●] shares at a per share price of $[●] and the conversion of all outstanding shares of our convertible preferred stock into an aggregate of [●] shares of our common stock as if such conversion had occurred as of June 30, 2023; and |
| ● | on a pro forma, as adjusted basis, giving effect to the sale by us of [●] shares (excluding any sale of shares pursuant to the underwriter’s over-allotment option), at an assumed public offering price of $[●] per share, after deducting underwriting discounts, non-accountable expense allowance and estimated offering expenses. |
The pro forma and pro forma as adjusted information below is illustrative only and our capitalization following the completion of this offering will be adjusted based on the actual public offering price and other terms of this offering determined at pricing. You should read this table together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our audited financial statements and the related notes appearing elsewhere in this prospectus and our and unaudited consolidated pro forma information appearing elsewhere in this prospectus.
| As of June 30, 2023 | ||||||||||||
| Actual | Pro forma | Pro forma As Adjusted | ||||||||||
| Cash | $ | 601,265 | $ | $ | ||||||||
| Long-term Debt | $ | - | $ | $ | ||||||||
| Stockholders’ Deficit | ||||||||||||
| Common stock, $0.0001 par value, 300,000,000 shares authorized; 9,988,836 and 9,568,475 shares issued and outstanding as of June 30, 2023 and December 31, 2022, respectively | 988 | |||||||||||
| Series seed convertible preferred stock, $0.0001 par value, 213,730 shares authorized; 213,730 shares issued and outstanding as of June 30, 2023 and December 31, 2022 | 21 | |||||||||||
| Series seed 2 convertible preferred stock, $0.0001 par value, 3,635,252 shares authorized; 3,635,252 shares issued and outstanding as of June 30, 2023 and December 31, 2022 | 364 | |||||||||||
| Series A convertible preferred stock, $0.0001 par value, 2,982,003 shares authorized; 1,861,799 shares issued and outstanding as of June 30, 2023 and December 31, 2022 | 186 | |||||||||||
| Additional paid-in capital | 5,148,159 | |||||||||||
| Subscription receivable | - | |||||||||||
| Accumulated deficit | (5,082,927 | ) | ||||||||||
| Accumulated other comprehensive income | 486 | |||||||||||
| Total Stockholders’ Equity | 67,288 | |||||||||||
| Total Capitalization | $ | 1,743,954 | $ | $ | ||||||||
A $1.00 increase or decrease in the assumed public offering price per share would increase or decrease our pro forma as adjusted cash, additional paid-in capital, total stockholders’ equity (deficit) and total capitalization by approximately $[●] assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the underwriting discounts, non-accountable expense allowance and estimated offering expenses payable by us.
50
If you invest in our shares, your interest in our shares will be diluted to the extent of the difference between the offering price per share and the pro forma as adjusted net tangible book value per share after the offering. Net tangible book value represents the amount of total tangible assets less total liabilities, and net tangible book value per share represents net tangible book value divided by the total number of shares outstanding. Dilution results from the fact that the per share offering price is substantially in excess of the pro forma as adjusted net tangible book value per share. Our net tangible book value attributable to stockholders as of June 30, 2023, was $[●], or approximately $[●] per share. Net tangible book value per share as of June 30, 2023, represents the amount of total assets less intangible assets and total liabilities, divided by the number of shares outstanding.
After giving effect to the issuance of [●] shares since [●], June 30, 2023, the receipt of $[●] in connection with such issuances (the “Post-Balance Sheet Issuances”), our pro forma net tangible book value as of June 30, 2023, would have been approximately $[●], or approximately $[●] per share, based on [●] shares outstanding on a pro forma basis.
Our pro forma as adjusted net tangible book value of our shares as of [●] gives further effect to (i) the sale of shares at the assumed public offering price of $[●] per share, after deducting the underwriting discounts, non-accountable expense allowance and estimated offering expenses and assuming the underwriter does not exercise its over-allotment option (assuming no exercise of the over-allotment option) and (ii) the automatic conversion of all outstanding shares of our convertible preferred stock into [●] shares of our common stock. It does not take into consideration any other changes since June 30, 2023. We will issue [●] shares upon completion of the offering (assuming no exercise of the over-allotment option). Our pro forma as adjusted net tangible book value upon the completion of this offering will be approximately $[●], or $[●] per share. This would result in dilution to investors in this offering of approximately $[●] per share, or approximately [●]% from the assumed offering price of $[●] per share. Pro forma as adjusted net tangible book value per share would increase to the benefit of present stockholders by $[●] per share attributable to the purchase of the shares by investors in this offering.
If the underwriter exercises its option to purchase additional shares to cover over-allotments in full, the pro forma as adjusted net tangible book value per share after giving effect to our initial public offering would be approximately $[●] per share, and the dilution in pro forma net tangible book value per share to investors in our initial public offering would be approximately $[●] per share.
The following table sets forth the estimated net tangible book value per share after the offering (assuming no exercise of the over-allotment option) and the dilution to persons purchasing shares.
| Assumed offering price per share | $ | |||||||
| Pro forma net tangible book value per share before the offering | $ | $ | ||||||
| Increase per share attributable to this offering | ||||||||
| Pro forma as adjusted net tangible book value after the offering | $ | $ | ||||||
| Pro forma as adjusted dilution per share to new investors in this offering | $ | $ | ||||||
| Dilution in pro forma as adjusted net tangible book value per | $ |
If any shares are issued upon exercise of outstanding warrants or options, you may experience further dilution.
The following table summarizes, as of June 30, 2023, the differences between the number of shares purchased from us, the total cash consideration paid, and the average price per share paid by our existing stockholders and by our new investors purchasing shares in our initial public offering at the assumed initial public offering price of $[●] per share, before deducting estimated underwriting discounts, non-accountable expense allowance and estimated offering expenses payable by us:
| Shares Purchased | Total Consideration | Average Price Per | ||||||||||||||||||
| Number | Percent | Amount | Percent | Share | ||||||||||||||||
| Existing stockholders | % | % | $ | |||||||||||||||||
| New investors | % | % | $ | |||||||||||||||||
| Total | % | $ | % | |||||||||||||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $[●] per share would increase (decrease) total consideration paid by new investors by $[●] (and net proceeds by approximately $[●]), assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting estimated underwriting discounts, non-accountable expense allowance payable by us.
If the underwriter exercises its option to purchase additional shares to cover over-allotments in full, our existing stockholders would own [●]% and our new investors would own [●]% of the total number of shares of our Common Stock outstanding after our initial public offering.
51
Overview
We are a physician-dispensed skincare company with a focus on modernizing aesthetic skincare. We conduct research and development to advance innovative and science-driven topical skincare that complements the medical aesthetics industry. Upon our founding in 2020, we initiated our research and development phase for our current product formulations. Since 2022, we have principally employed a business-to-business model in which we produce and commercialize a new generation of topical skincare products that contain our proprietary stem cell-derived Elevai ExosomesTM designed to enhance the appearance of skin. We have designed our innovative and meticulously produced Elevai ExosomesTM infused cosmetics for the physician-dispensed industry that we believe has lacked significant and recent new biotechnological innovations or advancements. We expect exosomes, nano-sized, natural delivery mechanism that affect important biological factors, to become a focal point that will change the nature of how medical aesthetics cosmetic skincare and related aesthetic products are used and adopted for years to come.
Our team bridges years of cutting-edge molecular biotechnology research with our company’s flexibility to fully scale pipeline products through our refined competencies in biochemical engineering and bioprocessing prowess. We believe this potent combination of know-how is not found elsewhere in the cosmetics brands utilized by the physician-dispensed skincare industry and these proficiencies validate our early-mover advantage. Although the relationship between biochemical engineering, and stem cell research in cosmetics is not widely understood, we have built a team with mastery in both disciplines. Because we strive to refine the advancement of those intersectant technologies, we are well situated in our ambition to develop and market next-generation products that can improve skin aesthetics while maintaining the ability to fully scale our operations at a level that we believe other companies of a similar size cannot.
Our exosome manufacturing process from source to skin is known as ‘Precision Regenerative Exosome Technology™’ or ‘PREx™’. PREx™ utilizes advanced patent-pending stem cell processing technology as part of our cohesive production process involving carefully controlled stem cell culture to produce stem cell derived factors that are featured in our topical exosome products. Our proprietary PREx™ process yields exosome lots from any hUMSC supplier that provide the source for our specialty physician-dispensed skincare product lines. Our products are comprised of topical cosmetic solutions. These products are not drug products or considered regenerative medicine, nor have any of our products received FDA approval. Our cosmetic products are not intended to prevent, treat or cure diseases or medical conditions. Moreover, our cosmetic products are not intended to be injected or delivered intravenously. Instead, our exosome-infused skincare products are topically applied to the skin to aid in the reduction of the appearance of a range of the most common cosmetic skin conditions, including the appearance of skin firmness, oxidative stress, photodamage, hyperpigmentation, and texture of soft tissue deficits, such as reducing the appearance of fine lines and wrinkles. More specifically, our E-Series™ line is marketed as a post-procedure care cosmetic that is applied to the skin after a medical aesthetics procedure such as skin laser therapy, micro needling, rejuvenation peels and Botox injections.
In addition to antiquated technology, we believe the current skincare market is dominated by confusing marketing claims, leaving skincare consumers with little more than subjective analyses or non-quantitative measures to assess a product before purchasing. In a recent skincare survey, 83% of over 1,000 women surveyed did not believe the marketing claims of the products they used, with over one-third reporting feeling that the product did not improve their skin at all.6 Unfortunately, the cosmetic skincare market in which we compete is riddled with vague, embellished marketing ploys that exaggerate the magnitude of product benefits. In response to this often-challenging marketplace, we founded Elevai with the intention of offering cosmetics products built on over fifteen years of innovative stem-cell biotechnology and scientific development by our Co-founders and Chief Executive Officer. With this goal in mind, we developed a proprietary commercialization process for producing and distributing non-invasive topical cosmetics products featuring our Elevai Exosomes™. Our aptitude in biochemical research and bioprocessing development coupled with a mission to provide scientifically backed skincare technology enables our high potential to become one of the early movers in the medical aesthetics cosmetics industry with international reach. We believe the potency and innovation of our product lines will be supported by our early-study data which will facilitate a shift in the physician-dispensed market towards the use of biotechnology to complement services provided within the medical aesthetics industry.
We seek to revolutionize the physician-dispensed cosmetics skincare market by providing unmet consumer needs to aid those individuals receiving services within the medical aesthetics industry through our innovative, but approachable skin-centric technology: our Elevai ExosomesTM. We believe we will be able to show that our exosomes provide aesthetic benefits and in turn can improve the appearance of skin that is prone to temporary inflammation and damage normally experienced by individuals immediately after receiving cosmetic or medical aesthetic procedures in either a physicians’ office, medical spa or by a licensed aesthetician. Through our ongoing rigorous testing and data analysis, we believe our products have the potential to demonstrate that they complement and enhance commonly performed cosmetic procedures such as skin laser therapy, micro needling, rejuvenation peel, and Botox injections. We believe our products have properties that can reduce the appearance of post-inflammatory hyperpigmentation that typically follows cosmetic or medical aesthetics services, such as laser therapy, micro needling and other minor ablative procedures. We further believe that our products have the potential to be used in a number of applications and other procedures beyond their current use. For example, we are in the early stages of evaluating the adjunctive use of our exosomes in the promotion of healthy hair growth cycles and noticeable improvement to hair appearance, fullness, and thickness.
| 6 | https://www.professionalbeauty.com.au/beauty/most-oz-women-don’t-believe-skincare-claims/ |
52
Our current cosmetic product formulations do not contain living cells, are patent pending and utilize our Elevai ExosomesTM. Elevai ExosomesTM are nano-sized extracellular vesicles packed with growth factors that we believe support skin health, including cytokines, peptides and other small molecules involved in the body’s natural healing. Elevai Exosomes™ are the powerhouse ingredient in all current Elevai products that we believe mimics the elegant repair process of the body alongside carefully selected high-quality active ingredients, such as hyaluronic acid and ceramides, to help maintain skin health for any skin type. While our products contain no living cells, they leverage the novel use of ethically sourced and thoroughly tested, human umbilical mesenchymal stem cells (“hUMSC”) that we culture under proprietary in vitro lab conditions and secrete Elevai Exosomes™ from.
Elevai ExosomesTM are produced from hUMSCs that are derived under current Good Manufacturing Practices (“cGMP”) conditions. Our hUMSCs are rigorously tested from consenting, and carefully selected donors. These hUMSCs then undergo cell expansion in vitro under proprietary conditions in our state-of-the-art laboratory operated under cGMP compliance. We specifically isolate hUMSCs from the Wharton’s Jelly portion of umbilical cords because these stem cells’ conditioned media (“CM”) or stem cells’ secretome is capable of resulting in exosomes with a protein profile that uniquely mimics the profile of proteins that these very young cells produce naturally when supporting the body’s healing and repair process. CM is a collective term for the paracrine soluble factors (a form of cellular communication in which a cell produces to signal to induce changes in nearby cells) produced by stem cells and utilized for intercellular communication. The CM of the hUMSCs we source is made up of growth factors, exosomes, lipids, microvesicles, and nutrients. Our hUMSCs are purified through our proprietary method, resulting in Elevai ExosomesTM. Every product we develop originates with a deep understanding of the skin, what healthy skin needs to look healthy, and how our next-generation, stem-cell exosome technology can help maintain the appearance of healthy skin. We specifically designed our Elevai Exosomes™ to be bottled in stable solutions so they can be topically applied.
hUMSCs, when cultured under the right conditions, produce nano-vesicles (~30-150nm) called exosomes, or extracellular vesicles. Biologically, exosomes are roughly spherical and made up of a lipid bilayer produced by the cell they originate from. This lipid bilayer forms a protective “shell” or outer casing, and within the “shell” exists the exosome payload containing molecules deposited there by the cell that generated the exosome. While exosomes are generated using some of the origin cell’s own cellular material, the exosomes do not contain cells, nor are they explicitly cellular material. Instead, exosomes represent a powerful, nano-sized, natural delivery mechanism for protecting important biological factors and this enables them to be directed to where they are needed most. The use of exosomes has been found to penetrate the skin better, absorb more easily, and protect the active ingredients fused into our products, including stem cell derived proteins, peptides, and growth factors.8
Elevai Exosomes™ are produced in-house through our proprietary manufacturing process, called Precision Regenerative Exosome Technology™ or PREx™ Our PREx™ technology is based on over 15 years of stem-cell research, bioprocessing, pharmaceutical- and biotech-product development by CEO and chief scientist Dr. Jordan R. Plews, PhD. Through PREx™, we have produced exosomes that have been developed into topical serums and creams which we believe aesthetically enhance the skin with a more youthful appearance.
To maintain quality control over our Elevai Exosomes™ commercialization and manufacturing process, we only purchase hUMSCs that are derived and banked by a cGMP-compliant third-party manufacturer. We similarly produce our final products in an FDA-inspected, cGMP compliant facility. The hUMSCs are shipped to our laboratory where we similarly operate our lab under strict good laboratory practice (“GLP”) protocols to produce the highest quality of exosomes for our products. Not only are all our ingredients and products subject to multiple quality control tests before bottling, but each lot is also tested, and batch numbered to enhance safety and traceability. Since every stem cell line is carefully derived and closely monitored throughout the process by highly trained personnel, only the highest quality stem cells are used to generate Elevai Exosomes™. If cells show any deviation in morphology or vitality, or any signs of contamination, the cell lines and exosomes from those cells are discarded. Currently, we are reliant on suppliers and manufacturers for both our raw materials and formulations and packaging of our final products, however we may build up additional manufacturing capabilities to become more vertically integrated. To this end, we are currently exploring the possibility of acquiring products, technologies, or companies to assist in our integrative goals.
We believe that we are at the fore of the biotech aesthetic revolution by innovating new cosmetic products at a level of biotech and aesthetics cosmetics research rarely observed in over-the-counter or physician-dispensed cosmetics skincare market. Our patent pending flagship Elevai E-Series™ products include Empower™ and Enfinity™ which are sold exclusively through our business-to-business model channel and via our distribution agreements channel. These products complement those individuals receiving services within the medical aesthetics industry who are in need of cosmetics to improve the appearance of skin in addition to receiving aesthetics treatments. Empower™ was developed to provide immediate post-treatment skin support and Enfinity™ for ongoing daily aftercare. Both products contain our patent-pending Elevai Exosomes™.
| 7 | Cha, Hyeonjin et al. “Stem Cell-Derived Exosomes and Nanovesicles: Promotion of Cell Proliferation, Migration, and Anti-Senescence for Treatment of Wound Damage and Skin Ageing.” Pharmaceutics vol. 12,12 1135. 24 Nov. 2020, doi:10.3390/pharmaceutics12121135 |
| 8 | Banerjee R. Overcoming the stratum corneum barrier: a nano approach. Drug Deliv Transl Res. 2013 Jun;3(3):205-8. Doi: 10.1007/s13346-013-0149-8. PMID: 25788129 |
| 9 | Liu, Shi-Jie et al. “Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Ameliorate HaCaT Cell Photo-Aging.” Rejuvenation research vol. 24,4 (2021): 283-293. Doi:10.1089/rej.2020.2313 |
53
Separately, regenerative medicines, including those that may contain exosomes usually refers to the use of a cell or a gene therapy to actually repair or replace damaged cells, tissues, or organs as opposed to enhancing the appearance of skin through cosmetics. When these regenerative medicines are advertised to repair, or replace damaged cells, or tissues while treating diseases and conditions in humans, they may be regulated as drugs and biological products under the Federal Food Drug and Cosmetic Act (“FD&C Act”). The FDA regulates the interstate manufacture and distribution of certain biological products, and drugs derived from human cells, tissues and cellular and tissue-based products or “HCT/Ps” under the Public Health Service Act (the “PHSA”). Moreover, to lawfully market a drug that is also a biological product, a biologics license must be in effect under the PHSA. As of the date of this prospectus, we are not aware of any FDA approved exosome biological products or drugs for any use. Our products are not considered a regenerative medicine intended to be used to treat any disease or condition. As a result, we do not believe our products qualify as HCT/Ps as it relates to their intended use as defined by the FDA, we expect they will remain cosmetic products. See “Regulations” for more information.
At this time, our topical products are sold nationally under a business-to-business (“B2B”) model. Separately, we have established licensing and manufacturing agreements with third-party distributors to sell our products internationally in Canada, Kuwait and Vietnam, however we have made no direct international sales as of the date of this prospectus. Our products are primarily sold to medical practices overseen by licensed medical professionals through licensing agreements in which those select outlets are required to provide proof of licensure and sign a reseller’s agreement. These arrangements protect our brand and are designed to ensure that our products remain positioned as professional luxury cosmetics products that are only sold via partners with sufficient training and know-how to properly highlight the unique benefits that our topical products offer. We trust these licensed professionals know when the topical application of our products is most beneficial to any affected region of the skin following an in-office medical aesthetic treatment to aid and hasten the recovery process and to ameliorate the effects of inflammation. In turn, licensed skincare professionals value their relationship with us, as it also protects them from unscrupulous non-professional skincare resellers and online outlets that would simply undercut on price and endanger their repeat business. We believe that physicians and their trained staff are the best source of trustworthy skincare information. Their deep understanding of the science, chemistry and structure of skin gives them clinical insight to select the most effective cosmetics products for their clients such as our cosmetics products.
In addition to providing client-based physician practices, medical spas and licensed aestheticians with product, we offer complimentary educational programs and webinars, and make appearances at medical aesthetic conferences to showcase our cosmetics products and latest research. From time to time, our free informational sessions educate physicians on the variety of benefits carrying our cosmetics products will have on their practices and patients, and under what circumstances it may be advantageous for physicians to implement use of our cosmetics products. We also host patient-driven events to help our physicians connect with those patients seeking to utilize our cosmetics products which helps to grow their practices. Often, we will also provide useful information and in-office literature on our cosmetics products for those patients who may be interested in our products during a regular visit. Moreover, to further expand our product reach we have entered into a white-label supply contract and other regional and international supply contracts with select sales distribution channel partners that distribute our Elevai branded products internationally or incorporate our proprietary Elevai ExosomesTM into their own new product lines to reach customers globally.
As our product lines become more pervasive, we anticipate working with additional distribution channel partners that will fuse our Elevai ExosomesTM into diverse product offerings and increase our overall share of the cosmetics market.
54
Corporate History and Structure
Elevai has one wholly owned subsidiary, Elevai Research Inc. (FKA Reactive Medical Inc.).
The following diagram sets forth the structure of the Company as of the date of this prospectus an after giving effect to the offering based on a proposed number of [●] shares of Common Stock being offered (assuming no exercise of the over-allotment option by the underwriters) and the automatic conversion of our preferred stock simultaneous with this offering:

Reactive Medical Labs Inc. (referred to herein as “Reactive Labs”) was incorporated in Delaware on June 9, 2020. On December 3, 2021, Reactive Labs changed its name to Elevai Labs, Inc. (referred to herein as “Elevai”). Reactive Medical Inc. (referred to herein as “Reactive”) was incorporated in British Columbia, Canada on February 5, 2018. On September 7, 2022, Reactive changed its name to Elevai Research Inc. Elevai Research Inc. is a wholly owned subsidiary of Elevai.
In June 2021, we entered into a stock transfer agreement with Reactive, whereby we purchased substantially all of the assets and liabilities of Reactive. Under the stock transfer agreement, we acquired 100% of the issued and outstanding common shares of Reactive. Immediately before the stock transfer agreement BWL Investments Ltd., a British Columbia Canada corporation owned 100% of the issued and outstanding common shares of Reactive. In consideration of 100% of the issued and outstanding common shares of Reactive we issued 100 shares of our Common Stock to BWL Investments Ltd. Upon completion of the stock transfer agreement, Reactive became our wholly owned subsidiary. In September 2022, Reactive changed its name to Elevai Research Inc.
55
Market, Industry and Other Research-Based Data
Our Market and Industry
We currently distribute our cosmetics products through two distinct channels, including a business-to-business sales channel where we sell our products directly within the United States and through our distribution sales channel where we sell our products directly to distributors with international or regional reach under exclusive and non-exclusive territorial agreements. We have employed a combination of both distribution channels via distribution agreements and directed business-to-business channels to optimize our sales reach and strategy.
We specifically place our products with physicians’ offices and medically directed businesses through our business-to-business model channel via our online sales portal, and our trained direct sales force comprised of employed, and independently contracted aesthetic account managers. Consumers are increasingly looking to their physicians for advice on cosmetic product selection because they are overwhelmed by the marketing hype that often creates unrealistic expectations and some degree of consumer confusion. Gradually, we believe consumers are also looking for individualized skin care regimens and want to know from their physicians, what works and what does not. In addition to the business-to-business sales channels, we indirectly distribute our products within the physician-dispensed cosmetics skincare market through both exclusive and non-exclusive distribution agreements.
The term ‘physician-dispensed’ refers to a sales channel where cosmetics products are exclusively sold in physician clinics or medically directed businesses by licensed medical professionals or that have a medical professional on staff. We include medical spas under this category such as standalone, or hospitality-affiliated clinics focused on cosmetic treatments such as injections, micro needling, and some plastic surgery services. Cosmetic products like ours are only available through a medically-directed business and are geared towards nourishing, protecting and supporting healthy looking skin.
Such cosmetic products are highly sought after by consumers making them one of the fastest growing segments of the personal care market.10 Consumers turn to cosmetics to enhance the appearance of dull or aging skin and to brighten the skin by lessening the appearance of a myriad of aesthetics concerns such as unwanted pigmentation, acne, melasma and rosacea. They view these products as alternatives to medications and often try cosmetics products before seeking medicinal solutions. Physicians also value well designed, topical skincare products formulated and manufactured with our biotechnology for their complementary aesthetic effects in conjunction with medications to improve skin appearance and to enhance the benefits of in-office procedures. Most of our product sales are within the physician-dispensed market through business-to-business channels, chiefly to dermatologists, plastic surgeons, and other physicians who are focused on medical aesthetics and therapeutic skincare, including some physicians practicing in medical esthetician practices. Our products complement those medical aesthetics services provided in these professional settings.
Our business-to-business sales channel within the physician-dispensed cosmetics skincare market utilizes both online sales, and our trained direct sales force comprised of employed, and independently contracted aesthetic account managers. This business-to-business sales channel is distinct from our leverage of non-exclusive distribution agreements third-party distributors or resellers, who in turn sell our products to end customers. Under distribution agreements our relationship between the seller and the buyer is more indirect, because our distributors serve as an intermediaries, however we believe scaling our product lines through larger distribution sales channels will lead to faster brand expansion, recognition and market reach.
The objective of the medical aesthetics industry is to offer medical education and care in a setting that includes spa facilities as well as conventional, complementary, and/or alternative aesthetics-focused therapies and cosmetic treatments. These medical aesthetics services include cosmetic procedures such as micro needling, Botox injections, anti-wrinkle and fine line reduction therapies, acne surgery, fillers and facial and massage services performed with highly specialized lasers and instruments.
| 10 | U.S. Beauty & Personal Care 2023-2026 | Statista. |
56
Industry Data
According to a national survey conducted by the American Society of Plastic Surgeons, in 2022 three-fourths of cosmetic-focused plastic surgery clinics saw an increase in business compared to pre-COVID-19-pandemic levels with almost 30 percent saying business had doubled. 11 We believe this trend reflects a growing desire and acceptance among individuals who sought out cosmetic focused procedures to seek assistance from medical professionals to improve their appearance, including the appearance of their skin. In fact, it is estimated that the global skin care products market size was valued at $130.50 billion in 2021 and is expected to expand at a compound annual growth rate (CAGR) of 4.6% from 2022 to 2030 to reach $196.20 billion by 2030.12 The United States skin care market specifically generated approximately $17.5 billion of revenue in 2020 and is expected to increase to roughly $22.7 billion by 2025.13 By contrast, the global professional skin care market has almost doubled in size in 10 years, from $4.5 billion in 2010 to $8.5 billion in 2021 with an estimated CAGR of 9.9% through 2026.14 Notably, the CAGR gain of +10% in 2021 was in part due to the medical dispensed channel, which topped $2.5 billion.14
The medical professionals who purchase our products dispense them in-office directly to their patients in conjunction with medical aesthetics treatments. Medical aesthetics professionals utilize our topical products to enhance their patients’ treatments or to support their patients’ healing process after treatments, ablative procedures, or post-surgery. These procedures generally include chemical peels, micro-needling, laser skin resurfacing, and other surgeries where the skin may be damaged or inflamed post-surgery or -procedure. The global medical esthetician market size was valued at $10.4 billion in 2021 and is expected to register a CAGR of 11.3% from 2022 to 2028 for an estimated value of $22.4 billion with North America leading total sales by volume.15 By comparison, the United States medical esthetician market was valued at $5.6 billion in 2021 and is expected to register a CAGR of 13.6% from 2022-203016 We believe this expected market growth can be attributed to factors, such as increasing consumer awareness about self-care and anti-aging services along with a rapid expansion of the wellness tourism sector.
| 11 | Inaugural Asps Insights and Trends Report: Cosmetic Surgery 2022. https://www.plasticsurgery.org/documents/News/Trends/2022/trends-report-cosmetic-surgery-2022.pdf. |
| 12 | Skin Care Products Market Size Report, 2022-2030. |
| 13 | U.S. Revenue of Skin Care Market 2012-2025 | Statista. |
| 14 | Professional Skin Care Global Series: Market Analysis and Opportunities | Kline & Company, 15 Dec. 2022. |
| 15 | U.S. Revenue of Skin Care Market 2012-2025 | Statista Research. |
| 16 | Medical Aesthetics Market by Size, Share, Forecasts, & Trends Analysis | Meticulous Research® |
57
We believe the following factors are contributing to the growth in aesthetic treatment procedures and medical aesthetics cosmetics sales:
| ● | Aging demographics and increasing patient focus on improving appearance and youthfulness; |
| ● | “Pre-juvenation” trend amongst millennials seeking aesthetic treatments at a younger age, which extends the lifetime value of a patient and their referrals to the practice; |
| ● | Rising wealth and disposable income as well as a growing middle class; |
| ● | Normalization and increased social acceptance of cosmetic procedures, including for men, driven by media, social media influencers and celebrities; |
| ● | Easier access to aesthetic treatments with the rise and growth of aesthetic chain businesses and medical spas globally; |
| ● | Broadening practitioner base seeking to expand menu of elective, private-pay aesthetic procedures they offer; |
| ● | Growing patient interest in non-invasive or minimally-invasive procedures and awareness of energy-based aesthetic treatments; |
| ● | Increasing popularity of combination treatments amongst patients seeking to address a broader range of indications and treatment areas, longer lasting clinical outcomes; and |
| ● | An increasing number of minimally invasive solutions that has seen a reduction in cost that attracts a broader patient base. |
Since an aging population is more prone to getting wrinkles and frown lines on their skin and forehead, we believe the consumer population is more actively taking skincare procedures and therapies to manage and maintain their appearance. According to the World Health Organization (WHO) “Fact Sheet on Ageing and Health” published in October 2022, the share of the population aged 60 years and over will increase to 1.4 billion by the year 2030, and 1 in 6 people will be aged 60 years or over worldwide by 2030.17 We estimate this increasing geriatric population will directly propel the growth of physician-dispensed cosmetics’ demand for better treatment of such aesthetic issues. Therefore, driving the market’s growth.
| 17 | Ageing and Health, 2022 | World Health Organization |
58
The skincare segment within the physician-dispensed market is projected to grow by a 9.9% CAGR to reach $12.8 billion by 2027 with the US physician-dispensed cosmetics market valued at $5.9 billion in 2020 alone.18 Outside the United States, the physician-dispensed skincare market varies by country due to cultural differences and regulatory requirements. Cultural desires for skin with lighter and more of an even pigmentation have created large and growing aesthetic skincare demands throughout Asia, particularly in Japan, China, Korea, and India. European and certain South American countries, such as Brazil, also present large skincare markets due to the complementary growth in cosmetic procedures and willingness on the part of their consumers to spend discretionary income on aesthetic enhancements. The global physician-dispensed cosmeceuticals market size was valued at $16.52 billion in 2020 and projected to reach $35.33 billion by 2028, growing at a CAGR of 9.8% from 2021 to 2028.19

Physician-dispensed Cosmeceuticals Market Size, Share & Forecast | Verified Market Research
The medical spa market is also growing, with the global medical spa end user market projected to reach $29.5 billion in 2030, a 12% compound annual growth rate from 2017, according to Allied Market Research.20 A medical spa is a combination of an aesthetic medical center and a day spa that provides non-surgical aesthetic medical services under the supervision of a licensed treatment provider (as defined by the state in which it operates). Medical spas strive to blend the best of two worlds—a relaxing spa experience with the specialized treatments typically only found at a dermatology or plastic surgery clinic. Some of the more common medical spa offerings include light, laser and energy-based treatments (for skin rejuvenation, aging skin, acne, and hair removal), dermabrasion/infusion, injectables, as well as chemical peels. Ownership of these medical spas typically includes physicians who have pivoted to or have specialized in aesthetic medicine or entrepreneurs who contract with local medical directors and consultants.
There are a growing number of medical spas, and even those with multi-location businesses accounting for a large part of this market. Within high value global markets, such as the United States, Japan, China, Hong Kong, Australia and Western Europe, we estimate there are hundreds of thousands of locations, with many chains planning to expand their presence. For example, according to Allied Market Research, North America is projected to account for a major share of the global medical spa market through 2030.20 U.S. dominated the North America medical spa market owing to huge number of facilities offering such services, rise in disposable income and aesthetic consciousness across the country. Therefore, we believe that medical spas have significant potential play an important role as purchasers of aesthetic devices and that there is significant opportunity for a company that tailors its product offerings to meet the needs of a wide range of customers.
According to Kline’s Professional Skin Care Global Series: Market Analysis and Opportunities Report, some professional skin care outlets like spas and beauty institutes have experienced a tumultuous few years, but the physician-dispensed skincare market has skyrocketed before, during, and after the pandemic. Kline’s Report also claims that aesthetic non-surgical procedures are a good barometer of the market potential and according to The Aesthetic Society’s recently released 2021 procedure survey report, consumer demand for fillers and Botox were up 42% and 33%, respectively, from the prior year.21 Moreover, escalating demand for face creams, sunscreens, and body lotions across the globe is expected to have a positive impact on the market growth over the forecast period.22 Lastly, the flourishing e-commerce sector is anticipated to boost the global skin care products market growth even further.23
| 18 | Physician-dispensed Cosmeceuticals–- Global Market Trajectory & Analytics | Research & Markets |
| 19 | Physician-dispensed Cosmeceuticals Market Size, Share & Forecast | Verified Market Research |
| 20 | Medical Spa Market Size and Share: Growth Prediction- 2030 (https://www.alliedmarketresearch.com/medical-spa-market) |
| 21 | Procedural Statistics | The Aesthetic Society. |
| 22 | Skin Care Products Market Size Report, 2022-2030 (grandviewresearch.com) |
| 23 | Skin Care Products Market Size Report, 2022-2030 (grandviewresearch.com) |
59
Exosome Research Generally
The roles of exosomes across the body remains a highly active area of academic research, but our and others research leads us to believe that there is a likely correlative relationship between the contents of a given exosome and the cell that produced that exosome, such that different cell types may produce distinct exosomes containing unique payloads specific to those cells producing them. For example, our research shows that hUMSCs are known to play a role in sending, receiving, and/or mediating signals tied to the immune system and cell growth or cell turnover, and are responsible for directing and contributing towards healing, repair, and regeneration. This distinctive feature of hUMSCs is precisely the reason why we have sourced hUMSC’s in order to secrete those highly functional exosomes. Further, hUMSCs’ paracrine functions or those functions that stimulate cell growth through the release of numerous soluble factors promote endogenous repair and regenerative mechanisms when applied topically. Thus, hUMSC-based cell therapies possess a huge clinical potential that has translated into very encouraging results in other pre-clinical and clinical studies investigating the safety and efficacy of hUMSCs use for the treatment of different conditions including skin burns, wounds, scars, wrinkles, and disorders including psoriasis vulgaris, and Romberg’s syndrome.
Some hUMSCs’ exosomes when researched have specifically been shown to enhance the condition of cellular function and repair and this is chiefly based on exosomes’ involvement in the stimulation of new blood vessel formation in a process known as angiogenesis, the inhibition of the infiltration of fibroblast cells generally associated with scarring of skin, and the enhancement of cells neuronal survival and neuronal differentiation.24 Similarly research has shown that these same exosomes appear to promote the stimulation of extracellular matrix remodeling—a process involving the promotion of collagen, elastin, and other proteins that support nearby cells, including those that make up skin tissue. Thus, exosomes when topically applied to skin, appear to provide a variety of potent factors which have been shown to have the ability to modulate inflammation and provide useful biomolecules that help guide and instruct other cells to repair skin damage. As exosomes penetrate the skin, they mimic the body’s natural healing responses to ameliorate skin damage and extrinsic aging caused by exposure to daily aggressors like pollution and ultraviolet-A-and-B-waves from sunlight. Similarly, exosomes appear to support the skin by improving blood flow, and increasing collagen and elastin production which, with prolonged application reveals rejuvenated, more youthful looking skin. Exosomes further provide the skin structural stability, cause a reduction in the signs and symptoms associated with damaged cells local inflammation response, and assist in the regulation of immune cells activities which help the body fight infections and other diseases.
Additionally, some research shows that exosomes naturally promote and support the body’s intercellular communication, or the sharing of instructional messages from one cell to another. Those studies suggest exosomes exude the process in which stem cells send ‘messages’ to other cells, the origin of those cell types (and author of the exosome message) matter, otherwise the instructional information may be ineffectual.
Moreover, research suggests those newly expressed proteins stimulated by healthy young stem cells may fulfill that lack of protein maintained at a cellular level to a degree that may already be affected by the individual’s biological or genetic environmental factors. When exosomes are applied topically, we have also observed that the exosomes protect those proteins and instruct them to travel from cell to cell for further repair. Importantly, we have not observed any bodily rejection of these newly stimulated proteins after any topical application, and we do not expect there to be any because stem cells are immune privileged meaning, they have a very low chance of immune reaction from other human stem cells.25
| 24 | Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, et al. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:1–11. |
| 25 | Machado, Cíntia de Vasconcellos, Paloma Dias da Silva Telles, and Ivana Lucia Oliveira Nascimento. “Immunological characteristics of mesenchymal stem cells.” Revista brasileira de hematologia e hemoterapia 35 (2013): 62-67. (https://www.scielo.br/j/rbhh/a/LsXp5tzvbYbcMchKcZzRTBB/abstract/?lang=en) |
60
Current Products and Products in Development
Our products rely on Elevai ExosomesTM that are derived from, ethically sourced and thoroughly tested, human umbilical mesenchymal stem cells (“hUMSCs”) originating from umbilical cord tissue. Our products include Empower™ and Enfinity™, two post-skincare procedure care serums that target the face and neck, and upper chest regions which are sold exclusively through our business-to-business model channel and via our distribution agreements channel.


61
We purchase our hUMSCs for our products from third parties that source umbilical tissue from consenting donors and are manufactured under current Good Manufacturing Practices (“cGMP”) conditions. We infuse our product lines with exosomes derived from these hUMSCs which are replete with growth factors. Our cosmetic topical products do not contain any living cells but do include our Elevai ExosomesTM. That is because Exosomes are not actually cells. Rather, they are cellular byproducts that are tiny, subcellular, membrane-bound vesicles approximately 30-150 nanometers in diameter. These vesicles are released by and can be derived from almost all cell types, including hUMSCs, whereas growth factors are signaling proteins that assist in hydrating and nourishing skin and do not by their nature have any form of protective envelope or encapsulation naturally. In comparison, exosomes are the delivery mechanism or envelope in which proteins (including growth factors), lipids, and other factors are contained within and can be applied topically to skin. Most notably, hUMSCs’ secretome produced by stem cells, also known as their conditioned media (“CM”) contain exosomes, proteins, growth factors, cytokines and other substances. Many competing products have attempted to synthetically use growth factors to recreate the messaging process from hUMSCs’ CMs, including recombinant bacteria, peptides, and/or small molecules. However, we believe many of these synthetic modalities are ineffective due to their lack of protection or encasement, which our Elevai ExosomesTM provide. Without protection, many synthetic modalities may end up undelivered due to their size, charge, shape, which we believe would not result in positive impact on existing skin concerns or bring skin a more youthful and healthy appearance. Instead, we believe a well-constructed exosome can overcome the deficiencies of synthetic versions. Our platform technology intentionally utilizes hUMSCs derived from umbilical cords of healthy full term newborns. These cells are then triggered under laboratory-controlled in vitro culture conditions to produce a reliable and consistent exosome ingredient harnessed by our Elevai ExosomesTM which support skin health. Our statements herein regarding our topical cosmetic and exosome-containing serums have not been reviewed or approved by the FDA. At this early stage, the continued success of the early positive results of our products is highly subjective to consumers and we have yet to complete clinical validation studies to demonstrate support for any performance claims of our products, such as their ability to aesthetically improve the skin.

Rather than try to synthetically design a specific ingredient or synthetic protein or peptide one at a time, we instead rely on hUMSCs’ innate ability to assist in nourishing, protecting and supporting healthy looking skin in the form of their derived exosomes. Under our PREx™ process, we believe our ethically sourced hUMSCs utilize multiple triggering modalities and protocols, enabling us to create multiple types of exosome messages or message profiles, each suited for a different purpose to enhance the texture and appearance of skin. For example, just as an individual would expect their stem cells to produce exosomes to help them smooth skin texture and replace lost moisture, we expect to trigger similar exosomes, isolate and purify them, and then provide them topically in our product lines in order to nourish, protect and support healthy looking skin. We believe our in vitro culture process to create Elevai ExosomesTM leverages this natural support to the moisture recovery and feeling of rejuvenation to skin, as opposed to pulling together a manmade CM which is essentially an educated guess at what that message construction should look like. Many of these synthetic CMs are utilized by our competitors alongside the addition of peptides or growth factors tied to improving skin appearance, which we believe does not make up for their inferior product design.
Our hUMSCs are triggered under proprietary laboratory conditions via our PREx™ process so as to result in the secretion of exosomes that carry a variety of proteins, including growth factors and calming cytokines which we then purify and capture to include in our topical products. Elevai ExosomesTM that we secrete are about 1/100th the diameter of a cell and their lipid bilayer protects their contents while also helps them to be absorbed by the skin and support the skin’s moisture barrier. We believe that our products promote and support healthy support skin rejuvenation of the skin’s moisture barrier that will help skin look and feel healthy by topically providing growth factors tied to maintaining the skin’s moisture barrier and promoting the appearance of a more radiant and even toned complexion that often decreases with age.
62
As the skin ages there is often an increase in the appearance of age-related pigments and fine lines and wrinkles. This appearance may be attributable to diet, genetics, or environmental/lifestyle factors such as the sun or smoking but it generally occurs naturally with age and causes the skin’s repair systems to break down which may result in visual signs of aging or disease. Working together in harmony we believe hUMSC derived growth factors encapsulated in exosomes have the potential to reduce the appearance of aging by supporting the skin’s natural moisture barrier. Our in-house laboratory experience has shown that when we expose the hUMSC-derived growth factors to artificially aged skin, or when we specifically trigger those growth factors using our proprietary PREx™ processes, we are then able to topically deliver growth factors back to skin, so that it appeared to be firmer and youth as if the skin had naturally and abundantly created those growth factors on its own.
In our experience, the process of hUMSC derived growth factors being repeatedly introduced and absorbed by skin over time often results in skin appearing more youthful as if the skin was expressing proteins typical of younger skin.
We have integrated the use of stem cell exosomes into our initial product line: our Elevai Post Treatment E-Series™. The E-Series™ is comprised of two post-skincare procedure care products that target the face and neck, and upper chest regions. Our products include Empower™-, and Enfinity™-serums, which are sold exclusively through our business-to-business model channel and via our distribution agreements channel. We aim to disrupt the post-procedure market with these products by providing a best-in-class system of topicals that work to complement the aesthetic results of in-office procedures, such as laser skin resurfacing or peels, microdermabrasion, or microneedling, among other procedures. Both E-Series™ products contain our Elevai ExosomesTM, those ultra-small, nanoparticle compartments packed with growth factors known to support skin health alongside hyaluronic acid, vitamin C, ceramides, niacinamide, glutathione and a proprietary blend of peptides to promote our formulations support both skin complexion and appearance. Our Empower™ serum retails at $149 per tube and wholesales for $596 for an eight-product pack. Our Enfinity™ serum retails at $299 a bottle and wholesales for $149 a bottle. Our products are sold as complementary to in-office procedures and are provided at the option of our physician client or at the request of that physician’s customer. In most cases the customer is being examined by the physician beforehand to determine the procedure. Often the customer is purchasing our Enfinity™ serum to precondition the skin prior to the procedure. On the day of the procedure a medical professional applies the Empower™ serum post-procedure. Then the customer is recommended to continue using Enfinity™ at home within 24 to 48 hours of the procedure.
Our products are being dispensed in two different models that are complementary to the service provided. In the first model the physician client includes our products in the price of the entire procedure. In the other models the physician client recommends both products and the patients decide whether they would like to add one or both products. In both models the physician client buys the product at the foregoing price points. The physician client retains the difference in between the wholesale price and the suggested retail price.
Our statements herein regarding our Empower™, and Enfinity™ topical cosmetic and exosome-containing serums have not been reviewed or approved by the FDA. For a discussion of certain risks and governmental regulation related to these products, see “Risk Factors” above and “Regulations” below.
63
Empower™ is our after-treatment topical product that supports skin health and promotes an even toned complexion. Empower™ serum is a concentrated serum, designed specifically for a one-time application post ablative procedures and treatments such as such as post mid-depth chemical peels, post micro needling, and post injectables. The ingredient-rich formulation is designed to support the skin’s moisture recovery and improve the appearance of the health of the skin while promoting the appearance of healthy skin texture.


Empower™ is our after-treatment topical product that supports the appearance of healthy skin and promotes an even toned complexion. Empower™ serum is a concentrated serum, designed specifically for application post ablative procedures and treatments such as such as energy device treatments, mid-depth chemical peels, micro needling, or injectables. Enfinity™ is our continuing care product that we recommend for daily use. Our Enfinity™ daily serum is a stable serum for at-home daily use that contains a blend of Elevai ExosomesTM combined with complementary stem cell growth factors. This daily product contains complimentary skincare ingredients available to support the appearance of healthy skin including Elevai ExosomesTM, vitamin C, hyaluronic acid, and copper peptides. Our exosome-based products, Enfinity™ are designed to remain shelf stable, are subject to minimal degradation over time when used and stored as directed, and do not require freezing or reconstitution prior to each use. We believe that our Enfinity™ serum with repeated use catalyzes healthier looking, and more balanced skin tone and texture, offering results that visibly reduces the appearance of age-related pigment and fine lines.
64



We further believe that our products have the potential to be used in a number of applications and other procedures beyond their current use. For example, we are in the early stages of evaluating the adjunctive use of our exosomes in the promotion of healthy hair growth cycles and noticeable improvement to hair appearance, fullness and thickness.
65
In December 2021, we achieved positive results from two multi-week clinical dermal safety evaluations of our Empower™ serum and Enfinity™ daily serum. For the dermal safety evaluations, Essex Testing Clinic, Inc. (“Essex”) conducted two separate single-center, semi-occlusive patch test trials to evaluate the irritation and sensitization potential of our Empower™ serum and Enfinity™ daily serum in 56 healthy adult male and female volunteers. The studies utilized cumulative as well as repeat insult patch designs, which aim to provide a standard assessment of cutaneous tolerability and safety. The studies’ results demonstrated our Empower™ serum and Enfinity™ daily serum were topically well tolerated. There was no irritation or sensitization caused by our Empower™ serum and Enfinity™ daily serum at any time during the course of the evaluations, and no adverse events and no severe or serious adverse events were reported.
In January 2022, we entered into a white label non-exclusive authorized global distribution agreement and trademark license agreement with the premier aesthetic device company DermapenWorld Inc.. DermaPenWorld are makers of the DermaPen micro needling device and have an established customer base across many geographies. When pressed onto skin, DermaPenWorld’s high quality micro needling device encourages collagen production but causes minor levels of skin trauma and redness. Our Elevai Exosomes™ infused products offer synergistic value to DermapenWorld’s device because our products may be applied afterward to support recovery and enable consumers to conclude their treatment session with what we believe to be less visible redness and faster comfort levels. Under the agreement, DermapenWorld agreed to purchase minimum quantities of Elevai Exosomes™ to be included as a key ingredient within DermapenWorld’s “Dp DermaceuticalsTM Meso-Glide EXO-SKINTM” products that will be distributed globally, spanning across Europe, Asia, Africa, the Americas and Australia. DermapenWorld’s Dp DermaceuticalsTM product line was specifically developed to be used when performing microneedling, which are now enhanced by ELEVAI Exosomes™, along with our Enfinity™ daily serum, which will be packaged in DermapenWorld’s Dp DermaceuticalsTM packaging. The DermapenWorld distribution agreement requires a royalty to be paid for sales within the United States or Canada. In FY 2021 the DermapenWorld distribution agreement formed none of our overall revenue and in FY 2022 no sales under the DermapenWorld distribution agreement were recorded within the United States or Canada. In FY 2022 and FY 2023 sales recorded outside of the United States and Canda under the DermapenWorld distribution agreement formed part of our overall revenue. Because of the foregoing, as of the date of this prospectus we have not received any revenue attributable from royalty payments. On June 26, 2023, pursuant to the terms of our white label non-exclusive authorized global distribution agreement and trademark license agreement with DermaPenWorld, we sent a termination letter to DermaPenWorld providing notice that we will not renew the distribution agreement and trademark license agreement upon their expiration. The termination notice is effective as of the end of business on January 16, 2024.
In August 2022, we entered into a non-exclusive authorized distribution agreement with one of the preeminent manufacturers and distributors of innovative aesthetic medical device technologies and clinical grade skincare, Refine USA, LLC (“Refine”). Under the agreement, Refine may purchase unlimited quantities of Elevai Exosomes™ and distribute them throughout the United States to their network of consumers and physicians.
In June 2022, we subcontracted a Contract Research Organization (“CRO”) to commence the regulatory process in the United Kingdom (“UK”) for our clinical development plan to conduct early phase clinical trials (Phase 1-2) to utilize a pipeline product, preliminarily referred to as “Enlighten”. Enlighten is an early pipeline product we believe may be effective in treating Melasma through the use of exosomes—a skin condition causing dark, discolored patches on the skin. The UK maintains an initiative called the Innovative Licensing and Access Pathway or iLAP that aims to accelerate the timeline to marketing drug authorization in the UK and provides medicine developers expert support and guidance throughout the development process. We believe there are potential cost saving avenues available through working with regulators in the United Kingdom and such approval will alleviate any potential path we may gravitate towards in seeking further approval by the FDA in the United States. We are continuing to look closely at whether a strategic path within the UK is viable and have not fully committed to the iLAP pathway in the UK because it presents many new challenges, some of which require resources we have yet to earmark for the scope of work that we believe is involved in receiving regulatory approval. Thus, at the time of this prospectus our clinical development of Enlighten is currently on hold, but our internal development and formulation testing of Enlighten is ongoing. See “Research and Development” for more information.
66
Aside from our testing the regulatory waters in the UK, there are some indications from our strategic advisory team that we may already have the ability to market a product that can benefit those with melasma or other clinical hyperpigmentation disorders without explicitly targeting a clinical indication like melasma via a traditional regulatory approval path. This alternative cosmetic based approach may be advantageous to our operations since regulatory approval would likely lead to our product being explicitly marked for prescription use and subject to insurance reimbursement. Given our stature as an aesthetics-product designer and -manufacturer, it may not serve our brand well or provide us with as much benefit to take a medicinal pathway. For example, many physician-dispensed cosmetics brands have successfully launched ‘brightening’ products that are clearly aimed at the hyperpigmentation market with fewer data points and technological advances related to their products than we currently maintain. Thus, it is our goal to only make investments towards any regulatory pathway that would involve indicative clinical trials to become a prescription product if it makes financial sense for the company moving forward.
In addition to the above, we are actively seeking potential strategic partnerships in the medical aesthetics skincare market that may add value to our product lines and broaden our distribution. Between February and May of 2023, we entered into three high-volume distribution agreements in Canada, Vietnam, and Kuwait. We also plan to continue to invest in our research and development capabilities which will assist in the expansion of manufacturing and marketing new flagship products in addition to our E-Series™ product line. As of the date of this prospectus, we have several pipeline products in the early development stages that we intend to complement our current product offerings.
Competition
The market for medical aesthetic skincare products is highly competitive, and we expect the intensity of competition to increase in the future. Our principal competitors are large, well-established companies in the fields of pharmaceuticals, cosmetics, medical devices and health care.
We face and will continue to face intense competition. Several of our competitors have greater research and development and marketing capabilities, more diverse distribution channels, and greater financial resources than we do. These competitors may have developed, or could in the future develop, new technologies that compete with our products or render our products obsolete. We are also likely to encounter increased competition as we enter new markets and as we attempt to further penetrate existing markets with new products and expand into new markets via new distribution channels.
Our largest direct competitors in the physician-dispensed cosmetic skincare market, inclusive of both distribution and business-to-business market channels for our medical aesthetics cosmetics products include SkinCeuticals, a division of L’Oréal S.A., Skinbetter Science LLC, a division of L’Oréal S.A., SkinMedica, Inc., a division of Allergan, Inc., ZO Skin Health, 51% owned by BlackStone, PCA Skin, EltaMD, each a division of Colgate-Palmolive, Dermalogica, Murad, each a division of Unilever, and Alastin Skincare, a division of Galderma.
Our competitors strictly in the business-to-business channels for medical aesthetics skincare products include The Beauty Company (Nasdaq:SKIN), Waldencast (Nasdaq:WALD), Inmode (Nasdaq: INMD), Evolus (Nasdaq: EOLS), Revance (Nasdaq: RVNC), and Cynosure.
Operational and Competitive Strengths
We face competition from both traditional cosmetics brands, such as retail-focused products, as well as other high-end cosmetics brands in the physician-dispensed cosmetics space. We believe the primary competitive factors in our favor is our Elevai ExosomesTM though our company exhibits the following additional operational and competitive strengths:
Our Next Generation Technology and Early Results:
Elevai ExosomesTM remain our key ingredient and main competitive strength, which is produced under proprietary and cGMP-compliant conditions in our state-of-the-art laboratory. We have a proprietary process to stimulate our ethically sourced cGMP grade hUMSCs to produce stem-cell derived exosomes. This process is designed to ensure that our customers consistently receive a stable, and potent product using strict standard operating procedures under laboratory controlled in-vitro culture conditions. Thereafter, we work closely with our formulation partners so that each batch of product is mixed according to our strict specifications. We believe we are one of the few in the physician-dispensed cosmetics industry to incorporate next generation biotechnology into its product lines. We believe that many of our competitors market products that contain inferior synthetic exosomes, exosomes from inferior sources, or ingredients that can be purchased anywhere. We are conducting ongoing sponsored validation studies involving individuals with noticeable skin pigmentation and redness to determine if there is an improvement in the appearance of skin pigmentation and redness issues when our topical products containing our Elevai ExosomesTM are applied daily. In fact, subjects in one of our validation studies were analyzed by an advanced imaging and analysis device called “VISIA” to determine what percentage of those subjects’ facial skin showed evidence of a change in detected levels of hyper pigmentation after twice-daily application of our Enfinity™ daily serum over the course of approximately 12 weeks. After twelve weeks of twice daily topical application of our Enfinity™ daily serum, follow up VISIA scans showed a six to twenty percent reduction in the area of facial skin recorded with hyper pigmentation as compared to their initial VISIA scans. There we found that after multiple-week application of our products, those hyper pigmented regions appeared less dark, less pronounced or noticeable, and the skin appeared to display a more balanced skin tone and texture. This early positive assessment is based on our comparing quantified values of image data that are taken at multiple time points throughout the validation study in order make our well quantified comparison of skin quality at the timepoints recorded. There, the imaging data showed the intensity of the remaining hyperpigmentation on those subjects’ facial skin was visibly reduced as compared to initial VISIA scans. However, we note that we continue to determine if we can better quantify this reduction in pigmentation intensity as further evidence of performance is analyzed over the course of our validation studies. At this early stage, the continued success of positive results of our products is highly subjective to consumers and we have yet to complete formal clinical validation studies with a large cohort to demonstrate support for the performance claims of our products, such as their ability to aesthetically improve the skin. Furthermore, any statements contained herein regarding our topical cosmetic and exosome-containing serums have not been reviewed or approved by the FDA. Similarly, the United States FDA has relatively limited experience regulating cosmetics derived from stem cells, and as of the date of this prospectus, there are no FDA approved medical products utilizing exosomes.
67
Our Product Quality, Ongoing Research and Seamless Production Process:
Many of our early-stage competitors employ contract manufacturers and labs to handle all portions of their production. Our California-based laboratory and production facility helps us protect our trade secrets by keeping our core processes in-house and eliminates our need to rely on contractors that may use damaged products of inferior quality, or dangerous/unstable ingredients solely for the purpose of manufacturing our Elevai ExosomesTM. Our streamlined commercialization process is quality controlled from stem cell acquisition, through exosome production, to specifying our standards to our contractors for formulation and bottling, ensuring continuity across the process to limit damage to our products’ exosomes and actives. Additionally, our aesthetic account managers and senior-level staff are highly supportive of our physician clients who rely on the quality of our product literature and educational material. This literature allows our physician clients to provide the best information to their clients whose experience may be ultimately enhanced by choosing to use our product lines post-procedure.
Although we are an early-stage company, we have integrated the production of our Elevai ExosomesTM with our general production process. We do not outsource any aspect of our exosome production process or license any core technology. We also have the capability to commercialize a variety of products derived from stem cells containing innovative encapsulated stem cell produced factors and quickly introduce new competitive products and existing product enhancements. This capability is harnessed by our ability to produce unique ingredient in our own lab like Elevai ExosomesTM. These natural stem cell factors are a core ingredient, and an ingredient that we believe few others can commercialize or approximate. We maintain the ability and know-how to modulate the way the stem cells are cultured in our laboratory space. Through modulation, we are able to produce different versions of our stem cell exosomes, and tailor them for different purposes, such as potentially supporting and promoting a healthy hair growth cycle. As we continue to grow our production outputs, we expect to multiply our modalities and deliver newly and more narrowly tailored versions of exosomes to the market in the form of our cosmetic products.
Although our ultimate goal is to achieve vertical integration, our current focus is on promoting the manufacture of our top-quality products and reducing our costs to produce next-generation cosmetics for the physician-dispensed skincare market at favorable price points while generating healthy margins. We believe our products will remain attractive and favorable by most consumers by pricing them at rates that are competitive with existing and emerging post-care and aesthetics cosmetics companies while remaining below a pricing tier reserved for more top-end direct-to-consumer products like those from La Mer Technology, Inc. Similarly, we believe that our pricing strategy is competitive with other competing physician dispensed skincare brands that do not contain exosomes. We believe this price point is still attainable for consumers in the physician-dispensed cosmetic skincare market even though our products employ the integration of topical exosomes that is in a similar class as existing skincare products, but through a newer manufacturing process which we believe allows our brand to market better quality and more purified extracellular vesicles in our products Thus, we chose to favorably price ourselves at the top of the range that we believe the physician-dispensed cosmetic skincare market will positively respond to.
Our Products Ease of Use, Quality Ingredients, and Post-Procedure Benefits:
We believe our products often complement the experience- and improve the results-of most physician in-office or medical spa aesthetic face and body treatments that include laser treatment, microneedling and ablative surgical procedures. We designed our products to provide benefits without any blood draw or needling. Our products may also ease uneven looking or puffy skin texture associated with the post-procedure healing process by including ingredients that assist in soothing and supporting the skin for the appearance of a more even skin tone.
To promote customers satisfaction with our products after an aesthetic face and body treatment, we carefully select high-quality active ingredients to aid in nourishing the skin. These includes hydrating hyaluronic acid and ceramides, to support skin health for any skin type. Alongside our Elevai ExosomesTM, our products are packed with bioavailable forms of vitamin C, and skin-restoring copper peptides. Our products are integrated into post-procedure or treatment protocols and have achieved positive results under third-party dermal safety evaluations. Each of our products underwent clinical dermal safety evaluations and there was no skin reactivity observed at any time over the multi-week study.
We culture our hUMSCs under carefully controlled conditions in our lab without the use of animal components or byproducts, such as Fetal Bovine Serum (“FBS”). Aside from our moral compass, there are many reasons to avoid animal components in our production process in particular. While this includes safety to avoid animal borne viruses, there is more consistency and predictability for high quality exosomes when culturing hUMSCs. Although there is much variability in any animal-derived component, they remain the primary way that most scientists around the world grow cells in laboratories. We aim to ensure that our products do not contain any parabens, phthalates, or animal byproducts, and we never test on animals.
We believe the application of our topical products can reduce redness, brighten skin, improve wrinkles and skin texture to promote healthy looking skin and the appearance of rejuvenation. Depending on consumer needs, our skin products are designed to either be directly applied topically after an aesthetics or ablative procedure or applied daily. At this early stage, the continued success of the early positive results of our products is highly subjective to consumers and we have yet to complete clinical validation studies to demonstrate support for any performance claims of our products, such as their ability to aesthetically improve the skin. Furthermore, any statements contained herein regarding our topical cosmetic and exosome-containing serums have not been reviewed or approved by the FDA.
Established Partnerships with Major Industry Players and Our Local Community:
Our position as an early mover in utilizing topical exosome skincare technology in the physician-dispensed cosmetic skincare market has attracted various industry leaders to become our exclusive and non-exclusive partners, creating an extensive network for us to leverage. We believe the expertise and market coverage of both our exclusive and non-exclusive distribution agreements with channel partners broaden our executional capability, reduce our execution risk, and provide immediate market access to increase the speed at which our products can reach the market. These partnerships solidify our position as a smaller company with substantial technological expertise. Additionally, our exclusive and non-exclusive distribution partnerships have allowed our products to enter Asian and Canadian international markets via our third-party distributors, in a capital efficient manner. In addition to our white-label distribution agreement, we may plan to pursue strategic co-development opportunities and arrangements that further enhance our product pipeline to create effective synergies to supplement our product offerings in the physician-dispensed cosmetic skincare market. Our current collaboration with many high-volume distributors provides valuable knowledge that we believe will enhance our early mover advantage.
68
On April 1, 2023, the Mitacs-Accelerate Grants Program via the Office of Commercialization and Industry Engagement (OCIE) Dalhousie University in Nova Scotia, Canada awarded our team $90,000 Canadian Dollars under a two-year research grant in relation to a project entitled “Multiomic characterization of stem cell derived extracellular vesicles for supporting the skin”. Under this project, we will engage an intern from Dalhousie University’s Department of Process Engineering & Applied Science under the tutelage of Dr. Stansislav Sokolenko who is responsible for completing a report about the project that is reviewed by their faculty supervisor and presented to our team. The primary aim of this research project, called ‘ELV3000’, is to establish new and novel techniques for characterizing the bioactive ‘payload’ of our Elevai ExosomesTM in order to provide us with a greater understanding of how specific exosome contents may be attributable to positive skincare outcomes. The secondary aim of the research project will be to further optimize our Elevai Exosomes™ production process to eventually improve our products through the eventual ability to exert greater control over exosome payloads. This detailed characterization will be conducted using a combination of traditional and advanced techniques and will build on other work currently being performed by us and our contract research partners.
Additionally, we partner with local California universities through a federally funded program called “CareerCONNECTED” Federal Work Study (“CCFWS”) to maintain roots in the surrounding area. The CareerCONNECTED program provides low-income students an opportunity to learn real world skills they would not traditionally receive in an academic setting. 60% of the interns’ pay is federally funded and we pay the other 40% of their salary. We benefit immensely from these interns and believe it is mutually beneficial to our growth to work with eager, academic minded individuals that can help us with our more time intensive tasks that slow down our general operations. This in turn helps our lab team reduce production time to make our exosome enriched media. Along with the interns assisting the lab team, we in turn teach them essential lab skills that will benefit them going forward in their science careers. We believe the program gives us an advantage in training future scientists to our specifications and potentially selecting future employees from the intern pool that are already received high quality training that can meet our lab specifications. Any future opportunity to hire our trained interns reduces the time and the opportunity costs that we would normally incur with training a newly hired, full time lab tech.
We continue to grow through allying with channel partners, local universities, and strategic investors globally and expect these relationships will enhance our credibility, relationship with the surrounding community, generate better leads, and future conversion of customers. These investments will ultimately enable us to be more agile in achieving our goals in the shortest time and leverage further investment into our technological strengths alongside our partners’ connections and relationships.
Our Well Recognized and Award-winning Team and Brand:
We produce our Elevai ExosomesTM using a proprietary process called Precision Regenerative Exosome Technology, or PREx™, which has been developed and perfected by Jordan R. Plews, PhD under whom we have made a number of strategic hires to assemble our management team. To that end, our brand has received a number of awards and accreditations, and we have been featured in exposés in recognition of our products and innovation. As of the date of prospectus, we received the People’s Choice Award after presenting at the Octane Aesthetics Tech Summit annual event, as part of the small business accelerator called the LaunchPad SBDC (Small Business Development Center). Additionally, we have been featured in the Aesthetic Guide Magazine, New Beauty Magazine, Grazia Magazine and MedEsthetics Magazine, among others.
Jordan R. Plews, our President and Chief Executive Officer who is a University College London and Stanford University trained stem cell scientist and biochemical engineer. Dr. Plews is a well-respected and accomplished industry innovator holding notable accolades involving human stem-cell research to derive high quality human stem-cell growth factors and proteins. This initial research ultimately led him to a co-found the biotechnology focused FactorFive Skincare brand which topically utilizes the power of ethically sourced adult adipose derived stem cells’ growth factors. Alongside his product-line, Dr. Plews commissioned numerous products in the medical aesthetics cosmetics market and those that complement services provided under the medical aesthetics market. Dr. Plews founded Elevai after reaching a pinnacle moment in human-stem cell research to focus on the next generation medical aesthetic technology that focuses on stem-cell derived exosomes, which we believe is the future of skincare.
Dr. Plews is a published biochemical engineer with expertise in molecular biology and stem cells, which we believe will enable our ability to scale our concepts as we develop other novel product lines. We believe we can efficiently bridge the knowledge-gap between engineering and processing because of our research and aptitude in both fields. Using both vocations, improves our ability to isolate re-agents and stem-cell material to identify novel proof of concepts on a biochemical and molecular level while efficiently harnessing processes to produce and market those concepts at scale.
Our second founder, Dr. Hatem Abou-Sayed (known professionally as “Tim Sayed MD”), is a double board-certified plastic surgeon with nearly two decades of experience in the medical aesthetics market. He also holds an executive MBA with a strong marketing and finance focus, and an undergraduate engineering degree with a focus on biotechnology. Dr. Sayed was instrumental in positioning us as an exosome focused aesthetic skincare brand and forming the foundational engagements with our hUMSC suppliers to build on the established scientific background and legacy of our suppliers. In that capacity he helped to develop the application of our cosmetics products to complement device-based medical aesthetic procedures. Moreover, Dr. Sayed recruited and established the leadership role and the onboarding of Dr. Plews. Additionally, Dr. Sayed has introduced us to numerous key opinion leaders, strategic organizations within the medical aesthetics and plastic surgery industry and prospective research collaborators. Dr. Sayed is the current President of the San Diego Plastic Surgery Society and has been in active leadership roles with the American Society of Plastic Surgeons, and the Aesthetic Society, including the Emerging Trends, Public Education, Development and Ethics committees. Dr. Sayed is a well-respected source in the medical aesthetics industry, has authored several publications in peer-reviewed medical journals as well as in periodicals for the public. He has contributed a regular column for Modern Aesthetics Magazine, advising other plastic surgeons on how to modernize their medical offices and provide a better patient experience. Notably, he is a co-author of a textbook chapter on digital tools for plastic surgeons in one of the definitive textbooks for plastic surgeons, Neligan’s Plastic Surgery.
69
In addition, we hired Brenda Buechler as our chief marketing officer because of her track record in successful business leadership and leading brand development in the physician-dispensed aesthetics arena. Her extensive and successful career spans over 23 years in both director and leadership roles at notable companies in the physician-dispensed aesthetics market. Her former roles leading sales (B2C, B2B, and online), branding, and strategic marketing in start-ups as well as large established brands such as Alastin Skincare, and SkinMedica Inc. Brenda is highly adept at branding and developing strategic marketing and communications campaigns that have historically exceeded expectations and driven revenue. For example, during Ms. Buechler’s tenure as director of brand marketing and public relations at Alastin Skincare, Inc., she assembled and managed multiple teams, new product launches, and laid the groundwork for the company’s initial eCommerce and blog infrastructure that led to a 240% growth in B2B eCommerce revenue. Additionally, during her time with SkinMedica Inc., Brenda initiated marketing strategies and research programs across both the physician-dispensed and consumer eCommerce sales channels, in both the B2B and B2C markets. This effort ultimately contributed to overall revenue growth of 100% year over year for over two years straight.
We also have an experienced sales and marketing team which over the span of their careers have demonstrated the ability to identify new business opportunities and to develop the business by growing our global distribution networks. The recent recruitment of our Chief Commercial Officer, Christoph Kraneiss adds a dedicated industry veteran to oversee and further innovate our current product lines and future product offerings. Chris is a business strategist with an impressive track record of growing brands and has a deep, nuanced understanding of the needs of the modern consumer within the physician-dispensed aesthetics market. Mr. Kraneiss has a proven track record of building successful physician-dispensed aesthetic businesses within top globally recognized brands such as his tenure as Senior Vice President at SkinBetter Science and ZO Skin Health, founded by renowned dermatologist Zein Obagi, MD. During his six years at ZO Skin Health, he led his department’s growth from $2M in retail sales to over $100M while reaching profitability within less than three years. Moreover, Chris helped to establish the SkinBetter brand in more than 130 countries and roughly 500,000 points of purchase globally.
Prior to joining Elevai, Chris served as Managing Director for Noon Aesthetics, and Vice President of International Business Development for Higher Education Skincare, a B2C company. We believe that because of Chris’s innovative approach to brand expansion coupled with his passion for Elevai’s mission to offer high quality cosmetics products, he will build on his success in continuing to establish, train, and manage international aesthetic sales teams for our associated retailers, physicians, and distributors alike.
In December of 2022, we strategically hired Ms. Lynnel Anderson, our Director of Aesthetic Clinical Education to lead and structure develop client facing product marketing, education initiatives and resources for our growing brand and product lines. Not only is Ms. Anderson an Army reservist, but Lynnel brings a wealth of knowledge and experience to our team from her previous tenures at Takeda Pharmaceuticals, Syneron, ZO Skin Health and Suneva Medical. While Ms. Anderson is predominantly in charge of our educational efforts, she will also play a pivotal role in servicing and performing outreach to potential key opinion leaders. We believe and have noted Ms. Anderson is an exceptional communicator and negotiator and will help our brand and products to break through the crowded cosmetics market.
Similarly, we are privileged to include a number of strategic advisors and consultants as members of our team including James R. Headley, Braeden Lichti of NorthStrive Companies Inc., Kevin Green, and Crystal Muilenburg.
James R. Headly is an experienced pharmaceutical and cosmetic industry executive who brings comprehensive insight to Elevai as a strategic advisor. James previously served as President and CEO of ZO Skin Health and plays an active role in advising our management team to evaluate strategic growth initiatives, product launches, and prospective partnerships. Headley’s beauty industry background boasts an extensive career beginning with positions with F&R Lazarus Department (Federated Department Stores) now Macy’s, the Estee Lauder Companies, and Warner Cosmetics, which eventually became the Ralph Lauren Fragrance Division of L’Oréal. We believe Mr. Headley’s extensive career will add value to our branding, sales, customer service, industry recognition, and overall brand innovation.
During his time as President and CEO of ZO Skin Health, Mr. Headley was credited for establishing the brand’s archetype which led to ZO’s strategic success. There, he conceived and launched ZO’s physician-dispensed aesthetics products that catapulted ZO into the number one position in the physician-dispensed skincare space. During James’s time at ZO Skin Health, annual sales rose from $2 million to over $100 million. Notably, in 2020, 51% of ZO Skin was sold to the American alternative investment management company Blackstone. We believe James will be an enormous support not only to Elevai’s business development, but to our expanding relationships with key opinion leaders in the aesthetic space. James’s expertise is invaluable to the future of our brand and the growth of the medical aesthetics cosmetics skincare industry.
Braeden Lichti, a co-founder has served as our strategic consultant since July 2020 through his firm NorthStrive Companies Inc. (“NorthStrive”). Mr. Lichti is the founder and CEO of NorthStrive since its inception in 2020. NorthStrive is a California corporation that focuses on identifying public markets venture capital investment opportunities in high-growth early-stage companies. NorthStrive is a sector agnostic privately held firm that has identified and invested, through its principal owners, in industries, including biotechnology, medical devices, and medical aesthetics. We believe Mr. Lichti will be a continued asset to the Company due to his network within the healthcare and aesthetic business community, and his experience identifying investment opportunities. Mr. Lichti will be beneficial to the Company as it seeks to identify new business and capital opportunities which led to the conclusion that NorthStrive should continue to be our advisor.
Kevin Green is an experienced healthcare executive with a broad background in life sciences and biotechnology finance and operations. He has over ten years of business development experience in the medical aesthetics field including Allergan Plc, a division of AbbVie Inc., Elsie Biotechnologies, and Bioniz, LLC. Kevin has been involved in multiple acquisitions for companies that remain our competitors, such as SkinMedica Inc. With Kevin’s broad operations and finance expertise, he brings relevant generalist experience to assist in our business development functions. He has been an advisor to us and made several key introductions between our founders and prominent executives and practitioners in the physician-dispensed aesthetic space. We expect Kevin to continue to network on our behalf and provide key input on key business dealings and product development moving forward.
70
Crystal Muilenburg most recently served as Chief Marketing Officer for Evolus where she led the re-launch of “Jeuveau”, a disruptor brand to Botox Cosmetic. Jeuveau is one of the fastest growing neurotoxin in the United States and considered to be one of the top product launches in aesthetics history. While at Evolus from 2019 to 2023, Crystal also led human relations, operations and supply chain management. There, Crystal established Evolvus’s international business operations. Crystal was Head of Global Marketing for Sienna Biopharmaceuticals, a publicly-traded, clinical stage medical dermatology and aesthetics company from 2017 to 2019. Crystal has worked in the aesthetics industry since 2006 starting with Allergan (now AbbVie Inc.) where she held various domestic and international marketing and communications leadership roles from 2006 to 2017. We believe Crystal will be an asset to our consulting team and work to network on our behalf to assist in key introductions and assist in adjusting our internal procedures and human capital operations.
Strategy
We believe we have the potential to be one of the most disruptive brands in the physician-dispensed cosmetics skincare market. We are in the early stages of new product development and have significant room to grow by attracting more consumers to the brand, making our current products more widely available and offering more innovative products to our consumers. We expect the United States to be the largest source of our growth over the next few years and see ample opportunity to expand in select international markets. We also believe we have an opportunity to improve our margins through greater operating leverage and efficiency once we begin distributing our product more widely.
Our Technology and Research:
We believe we are one of the first to adapt stem cell technology from cGMP grade hUMSCs to produce purified extracellular vesicles, also referred to as exosomes into topical skincare products to capture market share in the high growth physician-dispensed cosmetics skincare market. This strategy is not only based on our understanding of consumers’ interest in the appearance of a quicker post-procedure recovery, but our research into what the physician-dispensed cosmetics skincare market is currently lacking and our belief in our products’ ability—based on early imaging data leveraging quantitative analysis and visual assessments of photographic progress photos. Our imaging study data is gathered utilizing an advanced imaging and analysis device made by Canfield Scientific, called “VISIA”. This complexion analysis system captures high-quality, standardized images that are monitored following a medical aesthetic procedure at regular intervals to assess redness, discomfort, tone, texture, wrinkles, and other measures of skin appearance.
Since 2020, we have invested in the creation of a commercial process that began in 2022 which leverages the use of hUMSCs to produce extracellular vesicles, or exosomes in our products because not only do these factors have the ability to enhance the appearance of the skin, but they can do so without the tumorigenic or ethical concerns associated with the use of embryonic stem cells or induced pluripotent stem cells.25 Because we recognized the potential of utilizing hUMSCs for the skin, it was natural for us to utilize them as the basis for formulating our products. This is founded on our belief that our products can improve the appearance of skin prone to appearing temporarily red and puffy that is normally experienced by consumers while attending to their aesthetics needs in physicians’ offices or medical spa.
A Visionary and Experienced Management Team:
We have made significant investments in our business over the past three years by building our own exosome manufacturing lab, hiring top talent to help us build functional and streamlined capabilities in our commercialization process. Our management team comes from leading international skincare companies, with world-class research, marketing, and e-commerce experience to implement growth strategies and drive operational improvements.
Brand and Product Expansion:
We plan to continue to grow our young brand’s reputation. We plan to continue to expand our brand by attending events, presenting at scientific and medical aesthetic and cosmetic skincare conferences, and conducting clinical validation studies to further validate the aesthetic results of our products. We believe what differentiates us from many traditional cosmetics companies is our lean, but aggressive ability to make fast market-driven decisions and execute with quality control standards. We believe we have a major speed-to-market advantage over many other companies because of our size and aptitude in bioprocessing. Similarly, we are highly responsive to market-trends alongside physician and aesthetics consumer needs alike. We will continue to leverage our executional excellence as we combine our aptitude in stem cell research and bioprocessing while seeking to become the preferred partner of our key customers. Additionally, we have a robust product pipeline that we believe is likely to address the many evolving needs of customers, physicians, and clinicians in the aesthetic and cosmetics market ultimately increasing our branding and the number of customers we serve. Should any of our pipeline products over perform, especially those product lines that may focus on aesthetic needs other than skin appearance it may be advantageous for our branding to spin off those product lines and further focus on our core market: skin improvement and appearance.
| 25 | Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, et al. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:1–11. |
71
Channel Expansion, Production Capacity, and International Growth:
While our current focus is on the physician-dispensed cosmetic skincare market, we intend to expand into other sales channels including e-commerce by growing the information available on our website and making our products available for purchase through our medical-spa, physician and physician group partners’ websites. We believe being featured on a variety of partner websites will strengthen our brand and provide a unique direct-to-consumer e-commerce model via our business-to-business relationships where our e-commerce partners receive a share of product purchase revenue. Ultimately, our business-to-business model will strengthen our relationships with our physician partners, while an eventual e-commerce model broadens our market exposure and drive traffic and conversion to our other social media profiles. Additionally, we expect our current and prospective exclusive and non-exclusive distribution agreements to penetrate global markets and pique consumer interest not typically within our current reach. We believe both our products and white-label products can drive new market demand for our brand in those international markets that our distribution partners sell our products in.
We intend to expand the production capacity of our products and to develop new pipeline products in response to a number of potential growth factors, including: our organic growth, the development of research and development of pipeline products, and the expected increase in our product popularity, expansions of our distributor networks and channels through exclusive and non-exclusive international distributional agreements, and other potential strategic partnerships with industry leaders. Moreover, in addition to and as a result of the foregoing growth factors we expect an increase in orders for our products and a continuous rise in sales volume in the future based on our market research and estimates. To keep pace with this rise in sales volume, we anticipate the need to expand our production capacity by the end of quarter one of 2024. This expansion will enable us to meet our projected demand and we anticipate doubling our production capacity would cost between $1,500,000 and $2,000,000. This additional capital would cover our expenses relating to expanded capacity, including increased rent, additional lab equipment, and an increase to our overall headcount. We expect increasing our production capacity will lower manufacturing costs through economies of scale and improve overall cost-efficiency and profit margins. Ultimately, we can provide our products at a competitive price, especially to cost-sensitive physicians or medical spa owners, and consumers in the skincare aesthetics market, who may be relatively new to the concept of medical aesthetics cosmetic skincare.
We expect to expand our product offerings in response to this estimated growth, which are subject to additional costs. To expand a single pipeline product, we currently estimate capital requirements of approximately $250,000 for equipment to support the initial development of that product. We further estimate an additional $100,000 worth of funds to arrange for the testing protocol, clinical validations and to fully launch any product at scale. We estimate the operational framework to prepare for the launch of a pipeline product such as a topical haircare product, would take six to twelve months of development work with an additional four to six months to fully scale such product before an official product launch. We estimate that this pipeline development and scaling effort would not likely begin until the end of quarter one of 2024 and conclude until quarter two of 2025. This estimate depends on whether we may need to expand our operations and development work of any new pipeline product, which is dependent on the results of the development work, continued research and initial rounds of validation testing.
Currently, we directly distribute our products in the United States through both our business-to-business sales channel and licensing and manufacturing agreements with third-party distributors that include our products in their suite of domestic and international sales through our sales distribution channel. Under this sales distribution channel, we sell our products to a distributor, who resells our products to the physician practice customers after placing their order in a designated territory that is either exclusive or non-exclusive to that distributor. We have broadened our sales channel to include our cosmetic product offerings at medical spa locations. In March 2023, we engaged in negotiations with a privately held aesthetics and dermatology company that operates thirteen medical spa locations throughout the United States, many of which are housed inside boutique and upscale hotels. In March 2023, we entered negotiations to provide our cosmetic products to the spa operator. In May 2023, we began supplying a number of that company’s medical spa locations that provide ablative services with our complementary cosmetic products. From July 2023, onwards we aim supply our our Elevai Post Treatment E-Series™ products to four of medical spa locations to their customers who receive micro-needling, and laser treatments. Our principal goal is to expand our relationship with the medical spa company in order to provide our cosmetic product to all thirteen locations by May 2024. To that end, we plan to negotiate additional agreements with the remaining locations throughout 2023.
72
To bolster our regional sales, we entered into a non-exclusive authorized distribution agreement in August 2022 with Refine USA, LLC (“Refine”), one of the preeminent manufacturers and distributors of innovative aesthetic medical device technologies and clinical grade skincare. Under the agreement, Refine may purchase unlimited quantities of our topical cosmetics subject to minimum order size limits and distribute them throughout the United States to their network of consumers and physicians. As we continue to enhance our own United States sales channels, we are focused on bringing on third-party distributors in key large international markets, such as Canada, Europe, Brazil, Southeast Asia and the Middle East. We also plan to drive the distribution of our products through strategic relationships in specific countries, such as Japan. To continue growing in the physician-dispensed market, we intend to onboard more direct sales representatives that can reach geographic markets we currently do not have a presence in and partner with cosmetic device companies to co-market and sell our products. To this end, in January 2022, we signed a white label non-exclusive authorized global distribution agreement with premier aesthetic device company DermapenWorld, which expanded the reach of our branding and serum integration. Under a ‘white label’ agreement our products or ingredients may be combined with another company’s ingredients or products and sold under that company’s brand name. Essentially, under these arrangements we provide products or ingredients that can be customized with branding and packaging to match the branding of the company that will be selling the product directly. Pursuant to that white label agreement, we provide a shared distributor wholesale quantities of our serums that are formulated together with DermapenWorld’s and products which are marketed globally subject to previously agreed upon key performance indicators. In addition, between February and May 2023, we entered three exclusive distribution agreements with three separate distributors in Canada, Kuwait, and Vietnam whereby those distributors are permitted to promote, market, sell and distribute our products within their designated countries. Under these exclusive distribution agreements, we granted each a nonexclusive, nontransferable, royalty free license to use our branded products and marketing literature. Because we are entering new markets under each contract, we also arranged for each distributor to assist us in obtaining any required registrations, licenses and other applicable governmental approvals necessary to import, sell and distribute our products at our expense. On June 26, 2023, pursuant to the terms of our white label non-exclusive authorized global distribution agreement with DermaPenWorld, we sent a termination letter to DermaPenWorld providing notice that we will not renew the distribution agreement upon its expiration. The termination notice is effective as of the end of business on January 16, 2024.
Once commercially viable, as our manufacturing process becomes more vertically integrated, we may expand our business model to provide mesenchymal stem cells (MSCs) to companies needing MSCs, large-scale stem cell culturing, or those companies developing MSC-derived products. This expanded role would position the company as a contract manufacturer and distributor of MSCs because we may become a dependable and well-regarded source of exosomes enriched cosmetic products which have been designed for topical application to assist in the recovery after the delivery of medical aesthetic services. Nonetheless, we aim to remain focused on developing and marketing topical products that are enhanced by unique stem cell derived additives and possibly expand from there into other high-science aesthetic skincare applications, given our biochemical engineering and bioprocessing prowess and ability to manufacture laboratory-based skincare additives ourselves. Thus, any expansion into our role as a manufacturer and distributor of MSCs would only come to fruition if funding and bandwidth were such that we felt we met all our aesthetic market goals and had remaining capacity, or we identified an overwhelming interest and opportunity to benefit from such a paradigm shift. In expanding under this strategic channel, we would likely partner, license, or outsource any non-aesthetics-based business so as not to complicate or defocus our Elevai product brand.
Research and Development
Our research and development efforts are focused on improving and enhancing our existing products as well as developing new products. We undertake research and development on new product formulations and execute studies on existing products and future products that demonstrate what we believe to be the high-quality design of our formulas and the powerful performance of our products. We are constantly in a state of improving our product offerings and are currently in the process of assessing the physical characteristics of our exosomes with a goal of further optimizing and improving our exosome production capabilities. At times, because of our limited internal research and development capacity, we outsource portions of our product research and development and have joined force with Radyus Research, a contract research organization (CRO) under a formal statement of work to analyze the cell population our cultured hUMSCs produce under varied in-vitro culture conditions and the concentration of exosomes contained therein. Any data resulting from our CRO under this agreed upon statement of work is our own.
73
Together with Radyus Research, we are utilizing a number of advanced analytical techniques to help us improve our current processes and keep our brand at the forefront of exosome products. To analyze our exosomes, we utilize NanoSight, a nanoparticle tracking analysis instrument so we may evaluate the proteomic characteristics (or characterization of the protein makeup) of our exosomes. Through this thorough process we access the make-up of our finalized exosomes while balancing the efficiency of different adjustments to our cell-culturing production process. Taking the time to note the variability in our production based on establishing notable and favorable characteristics of the exosomes produced from those variations help keep our final products optimized. Our analysis is dependent on analyzing characteristics such as those exosomes’ nanoscopic scale (or nanoscale) which is often dependent on the unique culture techniques we may subject our hUMSCs to. Through these measurements, we can better understand those processes that lead to optimal production of exosomes from our hUMSCs that exhibit characteristics that are most effectively absorbed and utilized topically. We will further conduct this analysis from time to time to perform quality control on our processes to confirm the size and distribution of our exosomes and so that we are generating the best possible exosome products under the most efficient production conditions. NanoSight is one of a few technologies to detect the size of exosomes and count them and group them or graph out their distribution. This also helps us to identify whether our process results in fully intact exosomes or if under our culturing process, we may have damaged our hUMSCs. Any such damage tends to release apoptotic bodies (or damaged cells), and allows us to modify our purification process, which can remove most apoptotic bodies. Since we are able to identify those processes that produce the best possible quality exosomes, we are able to remain confident that our products continue to work the way we expect them to.
Radyus Research is integral to our research and development to assist in media formulation. With their assistance, we better understand how to trigger the stem cells into producing the right exosomes and in the right quantities. The media we use to grow the stem cells is constantly being fine-tuned with different growth factors to determine what the right ratios should be and which growth factors that are needed to best optimize our production process. Radyus’ analysis allows us to understand how reproducible our production process from batch to batch is, but also gives us quantitative analysis for how we can best culture our hUMSCs and perfect our PREx™ process.
We are also using a variety of modalities under including the measurement of analytes in our exosomes (protein analysis) using Luminex multiplex assays to look at the ribonucleic acid content (RNA) and microRNA content of the exosomes. These RNA sequencing techniques, transcriptome analysis, and various filtration, centrifugation, and affinity column-based strategies identifies all the mRNA sequences it detects and quantifies them. The data will provide us an idea of what the RNA ‘messages’ are inside our specific formula or mix of exosomes for future production catalogues. We also plan to also run some mix of analyses every time we alter or update our production process to compare each technique and may run this analysis occasionally for quality check purposes. Ultimately, we expect to have data that shows that we are producing multitudes of beneficial growth factors. From there we expect when we alter the process significantly, that those factors will change.
While we currently primarily focus on bringing physician-dispensed cosmetic aesthetics products to market and supporting the skin, we are in the process of researching and developing applications for hair, both on the face and head, and have ongoing research into additional, customized applications. Currently, many companies and competitors alike talk about exosomes as though they are one single ingredient with the innate ability to do many different jobs. However, research shows that certain exosomes released by certain cells are directly correlated with the cells they originate from and those particular exosomes’ capabilities and contents vary based on the cell type used and the way those cells have been treated or cultured.26 Thus, this research shows the contents of exosomes vary widely depending on the cell type used to generate them, their culture conditions, their processing and storage conditions, and how they are applied or used. Knowing this, we use highly-trained professionals to isolate and culture our hUMSCs and the resulting secreted exosomes are produced using strict protocols.
After formulation, all pipeline products are tested for integrity, safety, and performance. Prior to launch, our pipeline products undergo several safety tests, including, but not limited to, human repeat insult patch tests, used to help predict the likelihood of induced allergic contact dermatitis, comedogenicity tests, to prevent the product from clogging pores, and cumulative irritation tests, to evaluate the skin irritation potential and safety of individual ingredients or cosmetic compounds. Our products and their ingredients are also tested at multiple steps in the process to avoid microbial contamination.
In May 2022, we engaged a Contract Research Organization (“CRO”) that commenced a multi-step regulatory process in the United Kingdom (“UK”) for our clinical development plan to conduct early phase clinical trials (Phase 1-2) to utilize an updated version of our Enfinity™ product, preliminarily referred to as ‘Enlighten’, for the treatment of Melasma. Melasma is a common skin condition that causes dark, discolored patches on skin, typically on the face that are darker than a person’s normal skin tone. In addition, our CRO has compiled a case series data on client outcomes to date using the current version of our Enfinity™ product. As of the date of this prospectus, our internal development and formulation testing of Enlighten is ongoing, but the case study is on hold. We expect that if it continues, the resulting series data will provide a strong case for the need for further clinical trials and a publication to summarize the data to strengthen the use case of stem cell exosomes for the treatment of Melasma.
| 26 | Gao, F., et al., Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis, 2016. 7: p. e2062. |
74
If the study continues to progress, we intend to show a meaningful clinical benefit to patients. In order to do so, we will need to devise well-designed clinical trials to demonstrate Enlighten is both safe and effective. We believe registration trials will need to be designed as randomized trials in patients with melasma where one group of patients receive Enlighten and another receive the best available care. We will design the protocol once a meeting with the Medicines and Healthcare Products Regulatory Agency (“MHRA”) has taken place, in order to incorporate its advice and seek inclusion into the Innovative Licensing and Access Pathway (ILAP), a collaborative initiative which offers an accelerated path to market for new indications and repurposed medicines. We aim to perform the Phase I trial at 2 clinical trial centers in the United Kingdom under a clinical trials authorization (“CTA”). Because there are no approved therapies similar to Enlighten currently on any market, we plan to take advantage of the regulatory opportunities afforded to therapies that treat small markets with high unmet needs.
In addition to contacting the MHRA and ILAP, we have been in discussion with the United Kingdom National Institute for Health and Care Research (NIHR) to support our clinical development by brokering relationships with key opinion leaders, and potential investigators. We believe these relationships will assist us in selecting the most conducive sites to conduct our trials for Enlighten. As of the date of this prospectus, our discussion with the MHRA, ILAP and NIHR in relation to the clinical studies and related manufacturing process to develop Enlighten is in early stages of negotiation and administration. We cannot guarantee that the negotiations will be successful. If the negotiations are not successful, we will not pursue the commercialization of our Enlighten product in the near future. As such, this product is in the very early stages of development and may not continue as a product within the Company.
Despite our efforts to engage our CRO and collectively conduct research in the UK into the regulatory approval of our early-stage product, there are some indications from our strategic advisory team that we may already have the ability to market a product that can benefit those with melasma or other clinical hyperpigmentation disorders without explicitly targeting a clinical indication like melasma via the traditional regulatory approval path. Thus, it is our goal to only make the large investment towards any regulatory pathway that would involve indicative clinical trials to become a prescription product if it makes financial sense for the company moving forward.
We are also currently in the early stages of evaluating the adjunctive and topical use of our exosomes on promoting healthy hair growth cycle and hair fullness through the use of pipeline serums, shampoos, and conditioners. We hope and expect our studies to show if topically applied, our hUMSC’s exosomes will have a positive impact as it relates to noticeably reducing the appearance of hair shedding. We anticipate this effect will be primarily through hydrating and nourishing the scalp and topically applying nutrients to the scalp.
We believe hUMSC’s exosomes are packed with a variety of elements that will have a positive impact on promoting healthy hair growth cycles including multiple secreted proteins or growth factors. Among other elements, exosomes can include vascular endothelial growth factor (VEGF), which is a natural growth factor, keratinocyte growth factor (KGF or FGF7) which play an important role in protecting and supporting healthy looking skin, and fibroblast growth factor (FGF2 or bFGF) which are small proteins or peptides that visibly makes hair look and feel softer, shinier, and fuller. Collectively we hope to show that these growth factors will contribute towards the promotion of a healthy growth cycle for follicles by noticeably reducing follicle shedding. Many products on the market often results in tiny, short, and blonde vellus hairs that do not appear to be full or feel softer to the extent that they did prior to having unhealthy looking hair. Exosomes with their complementary growth factors show the potential to promote fuller looking hair follicles with a more aesthetically pleasing girth and length that is associated with fuller looking and feeling hair.
In addition to genetic factors, age, gender, and dietary factors, individuals experiencing unhealthy looking hair often have a high level of inflammation caused by dihydrogen testosterone or DHT which similarly contributes to the lack of blood flow to the scalp. As DHT attaches to hair follicles it reduces the blood flow to that follicle, inflames the cells on the scalp and binds to the androgen receptor on both the scalp and hair follicles. This binding over time causes the follicle to fall into the dormant phase of growth or die because of the lack of nutrients it receives. This often causes hair loss in a pattern attributable to the number and amount of androgen receptors on an individual’s head. Prescription medicinal products marketed to males, such as finasteride, aim to inhibit the enzyme ‘5-alpha-reductase’ which is the enzyme responsible for converting the two molecules of testosterone via a hydrogen into a single molecule, dihydrogen testosterone (DHT). Those products tend to regulate hormonal response and are often associated with undesirable side effects that affect an individual’s hormones generally. As discussed, DHT is often targeted because it binds to androgen receptors, causing inflammation on the scalp which causes hair to fall out and grow back thinner than it was before. While hUMSC’s exosomes do not address the hormonal component of hair loss involving the overproduction of testosterone, we hope that if used persistently, our topical pipeline products will hydrate and nourish the scalp to a level that will supplant the need to take drugs that may result in hormonal imbalances and/or sexual side effects. Alternatively, our products may be taken in addition to prescription hair-loss drugs, such as finasteride, to aid in a more natural appearance of hair thickness and fullness. Additionally, research has shown that females tend to be more sensitive to testosterone and DHT, and their hair growth patterns may also be negatively affected by hormone imbalances attributable to menopause, however prescription DHT inhibitors have been deemed unsafe to be taken by the female population. Notably, our pipeline product will not be subject to these limitations as it relates to female hair loss or thinning, and we feel this will provide our product with an advantage in addition to being an early mover in the use of exosomes as it relates to unhealthy hair growth cycles.
75

Manufacturing
We currently lease a lab and office space within a larger facility that contains over 5,000 square feet of clean lab space where we manufacture exosomes and run daily business operations that are staffed by trained biochemical engineers from some of the top US medical colleges. All lab staff participate in a stringent training program and detailed standard operating procedures are followed throughout the entire commercialization process with multiple checks in place throughout to promote the quality of our product lines.
We have exclusively developed our manufacturing process through our management team’s experience in developing and curating the production needed for our robust skin care products, which we believe provides us with a competitive edge. Success in manufacturing our Elevai ExosomesTM requires refined processes that are reliable, scalable, and economical. In our lab, we grow our ethically sourced stem cells and trigger them such that they produce exosomes under our proprietary method. We take the exosome ingredient from our lab to our formulator on a non-exclusive basis who follows our specified parameters to support our exosomes’ ability to maintain our high quality and integrity standards. We maintain manufacturing scalability and flexibility through our in-house exosome process that we closely monitor to promote quality control in the commercialization of all of our proprietary product concepts. As of the date of this prospectus, we own or have an agreement in principle for the right to purchase the related manufacturing processes, and methods with our third-party vendors. We also maintain an agreement with our formulator which provides us with the right to purchase the product formulas. Moreover, we also oversee our leased laboratory space in California which operate under Good Laboratory Practices (“GLP”) and adhere to Good Manufacturing Practices (“GMP”) for the production of our Elevai ExosomesTM. Similarly, our products are formulated by a third-party in an FDA inspected facility that adheres to GMP guidelines because GMP guidelines promotes the manufacture of our products at the highest recommended safety and quality standards for cosmetic products.
In July 2023, we expanded a portion of our laboratory space by an additional 721 square feet in order to store additional materials which in turn enables us to stage larger batches of our Elevai ExosomesTM and increase the amount of Elevai ExosomesTM we can produce per production-run via our Precision Regenerative Exosome TechnologyTM, or PREx™ Technology. With our expanded space, we estimate our batch size has increased about 50%, therefore we can manufacture about 50% more of our Elevai ExosomesTM per production-run. We believe this expansion will increase our efficiency by enhancing our ability to meet a particular target production level during the timeframe in which we set such a target and with less production-runs via PREx™. Expanding the batch size enables more time in between batches for us to plan, scale, and focus on research and development activities.
Our rental facility contains multiple cell and tissue culture suites containing biosafety cabinets and cell culture incubators. Not only does our facility provide a significant amount of cold storage and processing space that permit large-scale culture of hUMSCs and the ability to mass produce of stem cell-derived exosomes but allows us to perform cryo-preservation, cryo-storage, various forms of microscopy and cell analysis. Some additional key features of our rental facility include 24/7 security, advanced climate control, increased cold storage, additional cell culture and R&D suites to perform supplemental in-house research.
Areas of our rental facility operate under strict aseptic conditions following GLP standards which are used for cell culture and production of the company’s proprietary stem cell exosomes for topical applications while other portions of the lab provide access to a current good manufacturing practices (“cGMP”) suite for ultra-pure, medical-grade research and development. Because we maintain the unique capacity to isolate and culture human umbilical mesenchymal stem cells (“hUMSCs”) for use in preclinical and clinical trials and have access to cGMP labs alongside stem cell research and development labs with class II biosafety cabinets and the latest cellular research and production technologies, we are able to not only focus on cosmetic topical products for the physician-dispensed cosmetics skincare market but also address and explore clinical applications of these products in the future.
76
Our manufacturing process begins once we receive ethically sourced and thoroughly tested hUMSCs, from consenting donors who are themselves rigorously questioned and tested for communicable diseases. Elevai purchases hUMSCs from third-party cGMP sources in the US and in the UK where the Wharton’s Jelly portion of the hUMSCs are captured and extracted within twenty-four hours of a full-term healthy birth. Wharton’s Jelly is a mucous connective tissue primarily made up of hyaluronic acid and chondroitin sulfate and the main purpose of Wharton’s jelly is to provide insulation and protection to the umbilical cord. These properties make our use of these cord derived cells ideal for our commercialization process. This is because these stem cells’ conditioned media (“CM”) or stem cells’ secretome is capable of resulting in exosomes with a protein profile that uniquely mimics the profile of proteins that these very young cells naturally produce when supporting healing and repair process that we believe complement the aesthetic results of our serums in improving the appearance of the skin.
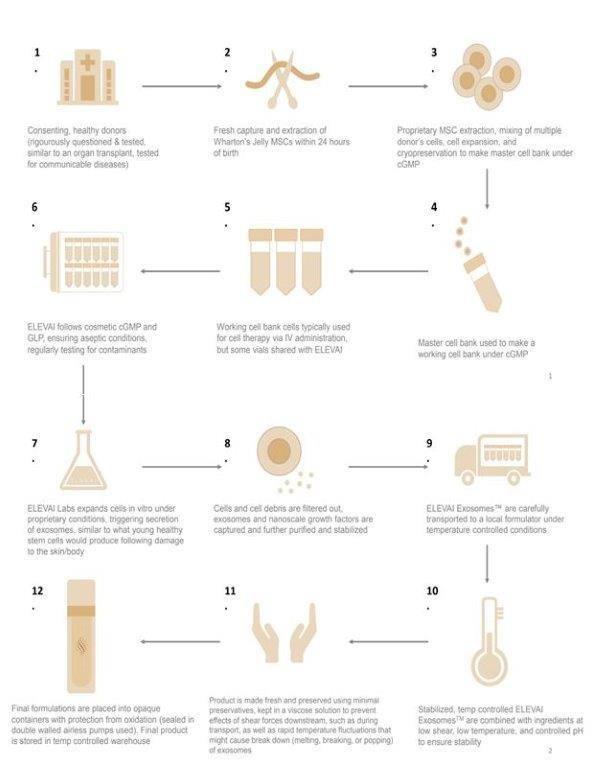
77
We work with multiple suppliers to source our high quality hUMSCs for research and development, which are provided after isolating those hUMSCs from multiple umbilical cords under specific proprietary conditions where cell expansion and cryopreservation is utilized, followed by creation of a master and working cell bank under cGMP. A master cell bank (“MCB”) is a uniform, well-characterized, and carefully preserved set of cells. These cells are derived from a single, well-defined source. The advantage of establishing a MCB is to ensure that set of cells serves as the primary stock of cells from which all subsequent cell banks (e.g., working cell banks) and final products are derived. We believe that establishing a MCB is essential for ensuring the consistency, and quality because there are less variations for characterization and increased quality control of the MCB. The formation of an MCB involves rigorous testing for identity, purity, potency, and stability, as well as screening for the presence of any contaminants, such as bacteria, fungi, viruses, or mycoplasma. Similarly, using multiple cords to source our hUMSCs from a blend of Wharton’s Jelly MSCs from ten or more donor cords ensures that any gene expression issues or donor to donor variability is diluted out, protecting from causing variability in our final product. Using a mixture of donors helps to normalize any issue with any single donor and we believe it to improve our batch consistency and limit the impact of any deficiencies from a single donor.
As of the date of this prospectus, we rely on one supplier to source hUMSCs for our cosmetic products under a non-exclusive supply agreement in principle for the proprietary production of hUMSCs with INmune Bio International Ltd (England) (“INmune UK”). This supply agreement exists in principle at the time of this prospectus, and we are continuing to negotiate its final terms while operating through purchase orders. INmune UK has developed a proprietary know how manufacturing process that reliably produces clinical grade cGMP quality hUMSCs. INmune UK’s hUMSCs are rigorously tested for common sources of contamination (bacterial and fungal contamination) as well as for a wide variety of viruses, including HIV, Hepatitis, Herpes, and current known viruses that are standard to test for when blood or tissue is donated for transplant. These hUMSCs are express shipped to our lab in California on dry ice, where we keep them cryopreserved until processing using PREx™.
These hUMSCs are thereafter subjects in our proprietary production process called Precision Regenerative Exosome TechnologyTM, or PREx™ Technology, which is used to produce Elevai Exosomes™. Cell therapy and biotech companies also currently utilize this same technique of combining multiple, well tested lots of donor cells to promote reliable, consistent production of their cell-based products and this represents the current standard for safety, aesthetic results, and reproducibility for cell-based therapies. Starting from this well tested cell bank of Wharton’s Jelly hUMSCs, we utilize proprietary cell culture techniques to trigger the production of our Elevai ExosomesTM through proprietary controlled in vitro culture conditions. The conditions in which Elevai Exosomes™ are secreted are designed to simulate what young healthy stem cells experience when damage to the skin occurs. Thereafter, we filter out cells and cell debris, and work to isolate exosomes and nanoscale growth factors that are then further purified and stabilized to formulate our products which are rich in Elevai ExosomesTM.
From our lab, we transfer our Elevai ExosomesTM to an FDA inspected cGMP-compliant formulator who specializes in the formulation and manufacture of prescription, over-the-counter pharmaceutical, and cosmetic products. This formulator produces our products pursuant to our specifications under our non-exclusive agreement. The manufacturer is required by law and by our manufacturing standards to comply with current Good Manufacturing Practices. Elevai pre-qualifies its manufacturers and formulators and works only with manufacturer’s that are FDA inspected. This requires our formulators to test both their ingredients and final products for quality, traceability and safety per FDA recommended GMP guidelines for cosmetic products. Additionally, these guidelines include testing each ingredient prior to use in a formulation as well as testing the final product produced for microbial contamination. Once with our formulator, the Elevai ExosomesTM are processed into final formulations and stabilized in a temperature-controlled environment where they are combined with ingredients at a low shear forces and controlled potential of Hydrogen (“pH”) to promote stability. Additionally, the final formulations are preserved using minimal preservatives, kept in viscose solution to prevent effects of shear forces in the downstream processes, such as during transport or rapid temperature fluctuations that may result in the breaking down of exosomes. These final formulations are placed into opaque containers which help protect from oxidation through a sealed and double walled airless pump and final products are stored in a temperature-controlled warehouse prior to being brought to market. We have and will continue to devote significant resources to process development and manufacturing scale-up to optimize process robustness and success rates in developing our Elevai Exosomes TM and other potential product candidates, as well as to reduce per-unit manufacturing costs and enable us to further enhance our scale for regional and global sales.
Once infused with our Elevai ExosomesTM, our products require no additional specialty manufacturing capabilities or unique, sole-source components. This reduces our dependence on any single manufacturer.
78
Intellectual Property
We are committed to developing and protecting our intellectual property and, where appropriate, filing patent applications to protect our technology. Since our establishment, we have focused on building an established brand for our products to achieve brand recognition and to increase our market share. We believe that increased brand awareness will increase sales and sales margins and improve customer loyalty. We have consistently marketed our products under the Elevai brand.
We rely on a combination of patent, copyright, trademark and trade secret laws and other agreements with employees and third parties to establish and protect our proprietary intellectual property rights. We require our officers, employees and consultants to enter into standard agreements containing provisions requiring confidentiality of proprietary information and assignment to us of all inventions made during the course of their employment or consulting relationship. We also enter into nondisclosure agreements with our commercial counterparties and limit access to, and distribution of, our proprietary information. As of the date of this prospectus, we have 15 registered trademarks inclusive of 12 global trademarks and 3 United States trademarks, and 18 trademark applications pending, 2 registered domain names, 3 non-provisional patent applications filed, 1 provisional patent application and 3 International Patent Corporation Treaty (“PCT”) applications filed.
Our Precision Regenerative Exosome Technology™, or PREx™, process is used to produce Elevai Exosomes™ and the exact process remains a trade secret. We have strategically decided to not pursue a patent around the process.
Copyright
As of the date of this prospectus, we have neither registered nor claim authorship over any copyrights.
Trademarks
We own or have filed the following United States trademarks as of May 31, 2023, as noted below:
| No. | Trademark | Owner | Country / Region | Application Number(s) | Application Date | Registration Number(s) | Registration Date |
| 1. | ELEVAI | Elevai Labs, Inc. | United States | 90976081 | 2020-07-19 | 66305839 | 2022-01-25 |
| 2. | EXOSENTIAL | Elevai Labs, Inc. | United States | 88982140 | 2020-02-05 | 6660483 | 2022-03-01 |
| 3. | ELEVAI Logo
| Elevai Labs, Inc. | United States | 90976512 | 2020-11-19 | 6681167 | 2022-03-22 |
| 4. | ELEVAI | Elevai Labs, Inc. | International: | 1657242, 40202209265W | 2022-03-07 | 1657242, 1208477, 2264791, 40202209265W | 2022-03-07 |
| 5. | ELEVAI EXOSOMES | Elevai Labs, Inc. | Australia | 2256931 | 2022-03-17 | 2256931 | 2022-03-17 |
| 6. | ELEVAI EXOSOMES | Elevai Labs, Inc. | International: Designating, the EU | A0122396 | 2022-04-28 | 1663708 | 2022-04-28 |
| 7. | SKINCARE, ELEVATED. | Elevai Labs, Inc. | International: Designating Australia, the EU, the UK | 1676788 | 2022-07-07 | 1676788, 2292774, WO000001676788 | 2022-07-07 |
| 8. | ELEVAI ENLIGHTEN | Elevai Labs, Inc. | International: Designating Australia, New Zealand, Canada, the EU, the UK, China, Japan, Singapore, Vietnam, South Korea, the UAE and Russia | 1732234 | 2023-05-05 | 1732234 | 2023-05-05 |
79
| 9. | EMPOWER | Elevai Labs, Inc. | International: Designating Australia, New Zealand, Canada, the EU, the UK, China, Japan, Singapore, Vietnam, South Korea, the UAE and Russia | 1732226 | 2023-05-05 | 1732226 | 2023-05-05 |
| 10. | ENFINITY | Elevai Labs, Inc. | International: Designating Australia, New Zealand, Canada, the EU, the UK, China, Japan, Singapore, Vietnam, South Korea, the UAE and Russia | 1732116 | 2023-05-05 | 1732116 | 2023-05-05 |
| 11. | ELEVAI | Elevai Labs, Inc. | United States | 90061002 | 2020-07-19 | ||
| 12. |
ELEVAI Logo
| Elevai Labs, Inc. | United States | 90331545 | 2020-11-19 | ||
| 13. | ELEVAI E-SERIES | Elevai Labs, Inc. | United States | 90483829 | 2021-01-22 | ||
| 14. | ELEVAI EXOSOMES | Elevai Labs, Inc. | United States | 97097832 | 2021-10-28 | ||
| 15. | ELEVAI | Elevai Labs, Inc. | Canada | 21806809 | 2022-03-07 | ||
| 16. | ELEVAI | Elevai Labs, Inc. | International: Designating, South Korea, UAE | 1657242 | 2022-03-07 | ||
| 17. | ELEVAI EXOSOMES | Elevai Labs, Inc. | Canada | 1663708 | 2022-04-28 | ||
| 18. | PRECISION REGENERATIVE EXOSOME TECHNOLOGY | Elevai Labs, Inc. | United States | 97641977 | 2022-10-21 | ||
| 19. | PREX | Elevai Labs, Inc. | United States | 97641975 | 2022-10-21 | ||
| 20. | REVERSE ENGINEERING OF NATURE | Elevai Labs, Inc. | United States | 97682790 | 2022-11-17 | ||
| 21. | SKINCARE, ELEVATED. | Elevai Labs, Inc. | Canada | 2203546 | 2022-07-07 | ||
| 22. | EMPOWER | Elevai Labs, Inc. | United States | 97693845 | 2022-11-28 | ||
| 23. | ENFINITY | Elevai Labs, Inc. | United States | 97693841 | 2022-11-28 | ||
| 24. | ELEVAI ENLIGHTEN | Elevai Labs, Inc. | United States | 97622736 | 2022-10-07 | ||
| 25. | ELESOMES | Elevai Labs, Inc. | United States | 97765984 | 2023-01-24 | ||
| 26. | EVOSOMES | Elevai Labs, Inc. | United States | 97765987 | 2023-01-24 | ||
| 27. | REJUVASOMES | Elevai Labs, Inc. | United States | 97815390 | 2023-02-28 |
80
Domain Names
We have the right to use the following domain registration issued in the United States, as noted below:
| Number | Issue Date | Expiration Date | Registration Agency | Domain Name | Owner | |||||
| 1 | 07/10/2020 | 07/10/2023 | GoDaddy Operating Company, LLC. | www.elevaiskincare.com | Reactive Medical Labs Inc. | |||||
| 2 | 11/08/2020 | 11/08/2023 | GoDaddy Operating Company, LLC. | www.elevailabs.com | Reactive Medical Labs Inc. |
Patents
A provisional patent application is a temporary patent application which is not examined by the United States Patent and Trademark Office (“USPTO”). This type of application will not mature into a valid enforceable patent by itself and grants the applicant a 12-month pendency period, the establishment of an official United States patent application filing date for the invention which may be later referenced in a non-provisional application, and the authorized use of a “patent pending” notice for 12 months in connection with the description of the invention under the patent application. Provisional patent applications serve to establish a chain of priority rights for subsequently filed patent applications. Similarly, PCT applications do not mature into valid enforceable patents and serve to establish a chain of priority rights for subsequently filed patent applications. PCT applications are place holder applications to allow the Company access to the Patent Cooperation Treaty rights, specifically related to the ability to enter into the national phase patent prosecution of member nations within thirty months of the earliest priority date. PCT patent applications cover all 152 nations which are signatories of the Patent Cooperation Treaty. Alternatively, a non-provisional patent application is a traditional patent application that requests the USPTO to issue a utility patent. This type of patent protects intellectual property rights for twenty years for any novel, useful, and non-obvious invention.
Below is a table, with footnotes, that includes our United States and International Patent Cooperation Treaty (PCT-Global) patent applications with its referenced property number that are material to our business as of May 4, 2023, as well as our two patent anticipated applications:
| Property No. | Patent title | Application Number and Filling Date | Application Type | Jurisdiction | Ownership Status and Expiration Date | |||||
| 1. | Formulation patent for the exosome formulation based on human umbilical mesenchymal stem cells.(1) | 63/256,593, 10/17/21 | Provisional | USA | Elevai Labs, Inc., 10/17/22 | |||||
| 2. | Formulation patent for the exosome formulation based on human umbilical mesenchymal stem cells. (2) | 17/865,229, 07/14/22 | Non-Provisional | USA | Elevai Labs, Inc., 10/17/41 | |||||
| 3. | Formulation patent for the exosome formulation based on human umbilical mesenchymal stem cells. (3) | PCT/US22/46446, 10/12/22 | Patent Co-operation Treaty | PCT-Global | Elevai Labs, Inc., 04/12/2025 | |||||
| 4. | Method of use of exosome formulation-based product for the regeneration of hair after hair loss. (4) | 18/101,974, 1/26/23 | Non-Provisional | USA | Elevai Labs, Inc., 10/17/41 | |||||
| 5. | Method of use of exosome formulation-based product for the regeneration of hair after hair loss. (5) | PCT/US23/11750, 1/27/23 | Patent Co-operation Treaty | PCT-Global | Elevai Labs, Inc., 07/27/2025 | |||||
| 6. | Method of treating skin pigmentation and formulation for treatment. (6) | 17/977,257, 10/31/22 | Non-Provisional | USA | Elevai Labs, Inc., 10/17/41 | |||||
| 7. | Method of treating skin pigmentation and formulation for treatment. (7) | PCT/US22/54352, 12/30/22 | Patent Co-operation Treaty | PCT-Global | Elevai Labs, Inc., July 30, 2024 |
| 1 | Formulation provisional patent application for the exosome formulation based on human umbilical mesenchymal stem cells. Does not include any methods of use. |
| 2 | Non-provisional patent application based on Property (1). |
| 3 | Patent Co-operation Treaty (PCT) application based on Property (2). |
| 4 | Non-provisional patent application for method of use of exosome formulation-based product of Property (2) for treatment of skin discoloration conditions. Specifically mentions rosacea and melasma. Claims priority to Property (2) and Property (1). |
| 5 | PCT application based on Property (4). |
| 6 | Non-provisional patent application for method of use of exosome formulation-based product of Property (2) for the regeneration of hair after hair loss from androgeneic alopecia, alopecia areta, or telogen effulvium. Claims priority to Property (2) and Property (1). |
| 7 | PCT application based on Property (6). |
81
Below is a table that includes our anticipated United States and International Patent Cooperation Treaty (PCT-Global) patent applications as of May 4med, 2023:
| Patent title | Expected Filling Date | Application Type | Jurisdiction | ||||
| Formulation patent for Exosome-based Product for Treatment of Skin Pigmentation and Method of Use | 07/15/2023 | Non-provisional | USA | N/A | |||
| Formulation patent for Exosome-based Product for Treatment of Skin Pigmentation and Method of Use | 07/28/2023 | Patent Co-operation Treaty | PCT-Global | N/A | |||
If approved, our International PCT patent applications will cover all 152 nations which are signatories of the PCT. However, our IP strategy generally recognizes the United States, United Kingdom, European Union, Canada, Japan, Australia and China as targets for extending patent protection under the PCT. Decisions regarding which countries to extend patent coverage under the PCT is taken on a case-by-case basis, subject to normal business considerations such as value and return on investment.
Sales and Marketing
In the United States, we are primarily a B2B distributor and sell and market our skincare products to top-end dermatologists, plastic surgeons, medical spa owners and other physicians who are focused on aesthetic and therapeutic skincare. As of the date of this prospectus, we primarily sell and market through our team of approximately nine independent contractors or aesthetic account managers (“AAMs”) and direct sales force of four full time AAMs. This team is led by our director of aesthetic clinical education. We intend to replace our nine 1099 AAMs completely with full time AAMs by the end of fiscal year 2024 or sooner. Currently we maintain domestic sales coverage in the Pacific Southwest, Atlantic Northeast including New England, and the Atlantic Southeast regions of the United States and our plan, based on funding, is to add additional coverage in the Pacific Northwest, Midwest and along the Atlantic corridor of the United States. We anticipate that when using the minimum proceeds raised under this offering, it will take us 12 to 18 months to fully transition to our expanded coverage. Once these regional AAMs are in place, those AAMs will receive continuous product and sales education and be groomed to be the regional sales managers in their territories. From there on out we plan to split the territories to have at least one AAM per state. Depending on the growth trajectory we will continue splitting territories until every territory can be serviced by our fully employed AAMs with a white glove concierge service. We intend to implement 30-60-90 day call schedules as well as sales targets per AAM depending on the location of the territory and the size of the territory. Ultimately, our goal is to have fifty full time AAMs in place by the end of 2024.
As of the date of this prospectus, we have nearly 130 accounts across the United States. The medical professionals we sell to dispense our products in-office directly to their patients, a distribution method commonly referred to as the “physician-dispensed” channel. Additionally, we have broadened our sales channel to include our cosmetic product offerings at medical spa locations. In March 2023, we engaged in negotiations with a privately held aesthetics and dermatology company that operates thirteen medical spa locations throughout the United States, many of which are housed inside boutique and upscale hotels. In March 2023, we entered negotiations to provide our cosmetic products to the spa operator. In May 2023, we began supplying a number of that company’s medical spa locations that provide ablative services with our complementary cosmetic products. We aim to supply products to three of these medical spa locations by July 2023 to their customers who receive micro-needling, and laser treatments. Our principal goal is to expand our relationship with the medical spa company in order to provide our cosmetic product to all thirteen locations by May 2024. To that end, we plan to negotiate additional agreements with the remaining locations throughout 2023.
Currently, our professional clients receive a markup on each of our products that they sell to customers of their practices. We believe our professional clients financially and strategically benefit by collecting a margin and competitively adding a new income stream and distinguishing product-line to their practices’ milieu. Additionally, we offer these professional clients the ability to sell our products directly to their customers through our online store where capture a percentage of the sale. We also sell our products nationally on a business-to-business basis through our sales representatives. We believe that our market position in the physician-dispensed channel presents us with the opportunity to increase the market share of our existing products and to launch a range of new products. Moreover, we believe the physician-dispensed distribution model ultimately results in higher patient satisfaction because it is better suited to the provision of system-based skin care than traditional distribution channels. We have built long-term relationships with skin health professionals based on the success of our products during the first few formative years since launching our Elevai Post Treatment E-Series™. We will continue our sales and marketing efforts aimed at helping physicians understand how our products can meet growing patient demand for effective skin care treatments, thereby generating additional sources of revenue for physician practices. Furthermore, we believe that our product offerings, and our experienced sales force, uniquely position us to benefit from growth in the number of physicians who dispense skin care products directly to their patients.
82
Moving forward into 2023, we established three core strategic imperatives to drive our collective marketing and branding campaigns with an overarching goal of opening new physician accounts specifically in the fields of dermatology and plastic surgery. In doing so, we intend to grow our products footprint in order to grow our month over month sales by adding new accounts each month and growing sales in those accounts each month. Our three core strategies include (i) accelerating our branding through product awareness, (ii) focusing on product differentiation and our unique value proposition, and (iii) building well regarded clinical evidence alongside key opinion leader (“KOL”) advocacy for our current and future products.

83
In addition to the foregoing relationships with our core physician practices, we plan to continue accelerating our company, brand, and product awareness through multiple marketing channels. These include driving traffic to our branded website through advertising and paid media. These media partners include but not limited to print and digital advertisements, social media advertising, and email marketing and social media campaigns. Our ongoing email campaigns include keeping our current accounts and leads updated regarding our brand and products, and we plan to expand social media presence on TikTok to reach new audiences. Other valuable marketing includes accelerating our industry-specific advertising and sponsorships at key conferences, ten of which we have identified opportunities for in 2023 alone. Additionally, we attempt to create and build relationships with our physician’s clients through these physicians displaying and marketing our products to their patients. We believe that physicians and their trained staff are the best source of trustworthy skincare information. Their deep understanding of the science, chemistry and structure of skin gives them clinical insight to select the most effective products for patients such as ours. To support this effort, we offer educational programs and webinars on our products and patient events to help these physicians grow their practices. We also offer in-office materials on our products for their patients, as an organic form of direct-to-consumer advertising in addition to public relations programs for patient referrals. Together these paid advertisements, conference sponsorships, public webinars and in-office education represents an important part of our overall marketing and advertising strategy. Overall, we believe educating current and potential consumers will drive consumer inclination to request our products from their physician to drive organic demand and market penetration.
To brand our current and future products, we believe focusing on our differentiation and unique value proposition will set us apart for maximized growth and new and evolving partnerships. We plan to expand unbranded and branded product education for our aesthetic account managers and KOLs in order to position our brand as a leader in the physician-dispensed cosmetics space with both exosome biotechnology and engineering expertise. This cohesion amongst our key representatives will differentiate our brands from competitors at every stage with our source to skin manufacturing and technology. From there, we will propel our brand forward to promote a clear value proposition and business value for Elevai with our team and KOLs in lockstep. Ultimately, we believe this strategy will demonstrate to our current and prospective physician accounts how our brand can bring value to their practice as a business.
Lastly, we intend to build clinical validation study evidence and brand our products through a groundswell of KOL advocacy to establish and expand on core clinic data for our E-Series™ product line and eventual product entrants. As we authenticate and build up quantitative data for new publication opportunities, we plan to validate our product offerings through additional medical aesthetic device pairings. Our ability to form device company collaborations provides additional authenticity and validation amongst larger companies in adjacent cosmetics or aesthetics industries. Our endorsements through branded partnerships will further support KOL development and advocacy through ongoing education and peer-to-peer opportunities including wider endorsements of the Elevai brand to drive physician adoption. A well-developed combination of our core KOLs and co-branding opportunities will present the Elevai brand with the ability to retain a broader reach in the physician-dispensed industry and more of the market share.
Internationally, we have the ability to sell our products through authorized wholesale distributors. As of the date of this prospectus, we have not made any direct international sales. Instead, our Vietnamese and Canadian distributors have sold our products and are two of a handful of distributors with whom we have entered into exclusive and non-exclusive distribution agreements. Under this distribution agreement channel, we sell our products to a distributor, who resells our products to the physician practice customers after placing their order in a designated territory that is either exclusive or non-exclusive to that distributor. In 2022 alone, we signed a white label non-exclusive authorized global distribution agreement with premier aesthetic device company DermapenWorld Inc. and a non-exclusive distribution agreement with medical device technologies manufacturer and clinical grade skincare distributor, Refine USA, LLC. Between February and May 2023, we entered three exclusive distribution agreements with three separate distributors in Canada, Kuwait, and Vietnam whereby those distributors are permitted to promote, market, sell and distribute our products within their designated countries. Under these exclusive distribution agreements, we granted each a nonexclusive, nontransferable, royalty free license to use our branded products and marketing literature. Except for product sales made by our Canadian and Vietnamese distributors, as of the date of this prospectus, our distributors have not made any sales under any of our exclusive distribution agreements.
Our Facilities
Our principal executive office is located at 120 Newport Center Drive, Newport Beach, CA 92660. The office has 500 square feet, and the lease runs from October 2022 to March 2023. The monthly rent is $1,561.
Our medical grade production laboratory spaces are located at 1743 Creekside Dr. Folsom, CA 95630 (the “1743 Lab”), and 1739 Creekside Dr. # 110 Folsom, CA 95630 (the “1739 Lab”). Our 1743 Lab has 1,388 square feet while our 1739 Lab has 1,600 square feet and the lease for both the 1743 Lab and 1739 Lab runs from June 2022 to May 2025. The monthly rent for both the 1743 Lab and 1739 Lab is $13,476.75.
The following table sets forth the leases term and monthly rent:
| Lease Term | Address | Space (square feet) | Average Monthly Rent | |||||||
| May 2023 to September 2023 | 120 Newport Center Drive, Newport Beach, CA 92660 | 500 | $ | 1,561 | ||||||
| June 2022 to May 2025 | 1739 Creekside Dr., Ste. 110 Folsom, CA 95630 | 1,388 | $ | 13,476.75 | (1) | |||||
| June 2022 to May 2025 | 1743 Creekside Dr. Folsom, CA 95630 | 1,600 | ||||||||
| (1) | Our average monthly rental payment of $13,476.75 includes one payment for our laboratory locations at both 1739 and 1743 Creekside Dr. Folsom, CA 95630. |
We use these facilities for administrative purposes, research and development, manufacturing of our products and analysis by our laboratory. We believe that these facilities will satisfy our current manufacturing and research and development needs over the next 12 months. However, if we decide to expand our manufacturing capabilities in order to begin the developing the means produce a new topical haircare product within the next 12 months, we estimate we would require a capital intensive purchase of laboratory new equipment and funds to arrange testing protocols in amounts between $250,000 and $300,000 to establish that type of capability over the course of an estimated seven to eighteen months.
Some members of our management work outside of these premises in office space that we do not rent.
84
Our Employees
As of August 14, 2023, we had 16 full-time employees. The following table sets forth the number of our employees by function:
| Functional Area | Number of Employees(1) | |||
| Operating | 2 | |||
| Research and Development | 4 | |||
| Financial Department | 0 | |||
| Sales | 8 | |||
| Branding/Marketing | 2 | |||
| Total | 16 | |||
| (1) | This figure does not include our approximately nine independently contracted aesthetic account managers, as of the date of this prospectus. |
We provide employee benefits for each employee which include medical, unemployment, and work injury compensation.
Our employees have not formed any employee union or association. We have developed various methods to train our employees adequately for the functions they perform and are aware of the laws and regulations affecting our industry. Our success depends on our ability to attract, retain, and motivate qualified employees. We endeavor to offer employees competitive compensation packages and a positive, dynamic, and creative work environment. We believe that we maintain a good working relationship with our employees and have not experienced any difficulty in recruiting staff for our operations.
Environmental, Health and Safety
We are subject to numerous foreign, federal, provincial, state, municipal and local environmental, health and safety laws and regulations relating to, among other matters, safe working conditions, product stewardship and environmental protection, including those relating to emissions to the air, discharges to land and surface waters, generation, handling, storage, transportation, treatment and disposal of hazardous substances and waste materials, and the registration and evaluation of chemicals. We maintain policies and procedures to monitor and control environmental, health and safety risks, and to monitor compliance with applicable environmental, health and safety requirements. Compliance with such laws and regulations pertaining to the discharge of materials into the environment, or otherwise relating to the protection of the environment, has not had a material effect upon our capital expenditures, earnings or competitive position. However, environmental laws and regulations have tended to become increasingly stringent and, to the extent regulatory changes occur in the future, they could result in, among other things, increased costs to our company. For example, certain states such as California and the U.S. Congress have proposed legislation relating to chemical disclosure and other requirements related to the content of our products that are described in further detail below. See “Regulations”.
Seasonality
We have experienced and expect to continue to experience well documented seasonal fluctuations in the United States in our results of operations. Our revenues tend to decrease as spending tapers off during the summer months because patients tend to receive less treatments that our products are associated with during those summer months.28 More specifically, the cosmetic space tends to experience a dip in revenue at the begging of the first quarter with levels gradually rising and eventually tapering off until the summer months in quarter three. This is often due to the fact many aesthetics doctors and consumers alike are traveling. Not until the end of quarter three or beginning of quarter four does sales revenues tend to pick back up.
Insurance
We maintain certain insurance policies to safeguard us against risks and unexpected events. Although we pride ourselves on our commitment to safety, to mitigate our exposure to liability from any such liability, we maintain a number of policies including workers compensation insurance, directors’ and officers’ insurance, crime insurance, employment practices liability insurance, errors and omissions insurance, cyber first party insurance, cyber third-party insurance, product liability insurance, a commercial umbrella policy, and insurance as required in our leased premises. During the fiscal years 2022 and 2021, we did not make any material insurance claims in relation to our business.
Legal Proceedings
As of the date of this prospectus, there are no active legal proceedings pending or threatened against the Company. However, from time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise.
| 28 | Cutera. “Prepare for Seasonal Trends in Your Aesthetic Business.” Medium, 26 Sept. 2018; Cutera. “Seasonal Trends in Aesthetic Treatments and Demographics” Medium, 18 April. 2018. |
85
Federal, state and local governmental authorities in the United States and other countries regulate, among other things, the testing, production, marketing distribution and sale of prescription and over-the-counter drugs and cosmetics. In the United States, we, our formulators, and are our products are subject to regulation by the Food and Drug Administration (the “FDA”), acting under the Federal Food, Drug, and Cosmetic Act (the “FDCA”), the United States Federal Food, Drug, the Consumer Product Safety Commission (the “CPSC”), acting under the Consumer Product Safety Act, as amended by the Consumer Product Safety Improvement Act of 2008 (the “CPSA”), the Federal Trade Commission (the “FTC”) under the Federal Trade Commission Act, and other federal statutes and federal agency rules. These governmental bodies principally regulate our products primarily on the basis of their intended use, as determined by the labeling claims made for the product and their ingredients, proper labeling, advertising, packaging, marketing, manufacture, safety, shipment, and disposal. While the FDA has the authority to regulate certain exosome medical products deemed to, and stem cell therapies used to, treat medical conditions, we are not aware of any FDA approved exosome medical products for any use.
Both federal and state governmental agencies continue to subject the cosmetic industry to regulatory scrutiny. We believe that we have structured our business operations and relationships with our customers to comply with all applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise.
FDA regulation of cosmetics
The FDCA defines cosmetics as products and their components intended to be rubbed, poured, sprinkled, sprayed on, introduced into, or otherwise applied to the human body or any part thereof for cleansing, beautifying, promoting attractiveness, or altering the appearance. Cosmetic products are not subject to FDA pre-market approval authority, although the FDA can take enforcement action for marketed cosmetic products that are adulterated or misbranded, including violations of product safety requirements, use and quantity of ingredients, labeling and promotion, and methods of manufacture. Although cosmetics are not subject to pre-market approval by the FDA, certain ingredients, such as color additives, must be pre-authorized. If the safety of products or ingredients has not been adequately substantiated, a specific warning label is required. Other warnings may also be mandated pursuant to FDA regulations. As of the date of this prospectus, none of our products maintain a warning label, nor do we anticipate the need for any of our pipeline products to maintain such a warning label.
Additionally, the FDA monitors compliance of the formulation-, mixing-, testing- and bottling-of-cosmetic products through market surveillance and both periodic and random inspections of cosmetic manufacturers and distributors. The FDA may, by regulation, require warning statements on certain cosmetic products for specified hazards associated with such products. As of the date of this prospectus, none of our products maintain a warning statements, nor do we anticipate the need for any of our pipeline products to maintain such a warning statement. FDA regulations also prohibit or otherwise restrict the use of certain types of ingredients in cosmetic products. The labeling of cosmetic products is subject to the requirements of the FDCA, the Fair Packaging and Labeling Act, and other FDA regulations. Similarly, our formulators are FDA compliant through registrations and routine inspections every four years. This is designed to ensure regulated products neither contain false nor misleading labeling and that they are not manufactured under unsanitary conditions. Under the FDCA, it is unlawful to introduce or deliver into interstate commerce a cosmetic that is adulterated or misbranded. A cosmetic may be adulterated if its composition is adversely affected because of its ingredients, handling, packaging, contaminants, or manufacturing. Whereas a cosmetic may be misbranded if its labeling is incorrect, or its packaging is misleading or deceptive. Inspections also may arise from consumer or competitor complaints filed with the FDA. In the event the FDA identifies false or misleading labeling or unsanitary conditions or otherwise a failure to comply with FDA requirements, a cosmetic company like ours may be required by a regulatory authority or that cosmetic company may independently decide to conduct a recall, market withdrawal of a product, make changes to its manufacturing processes or product formulations or labels.
The FDA also evaluates the “intended use” of a product to determine whether it is a drug, cosmetic product, or both. If a product is intended for use in the diagnosis, cure, mitigation, treatment, or prevention of a disease, condition, or is intended to affect the structure or function of the human body, the FDA will regulate the product as a drug. Drug products are subject to applicable requirements under the FDCA. The FDA may also consider labeling claims in determining the intended use of a product. If the FDA considers label claims for a cosmetic company’s products like ours to include claims that affect the structure or function of the human body, or contain an intended use for a disease condition, those products may be regulated as “new” drugs. If such products were regulated as “new” drugs by the FDA, it would be necessary for that company to obtain pre-market approval, which includes, among other things, conducting clinical trials to demonstrate the safety and efficacy of its products in order to continue marketing those products. Whether a drug is intended to be sold over the counter or with a prescription can also determine what type of FDA approval process is necessary. If a drug qualifies for sale over the counter, it need not undergo a premarket approval process if it complies with the requirements of the FDA monograph system.
The FDA may change the regulations in relation to any product category, which may require a change to labeling, product formulation, or analytical testing. In such a case. sufficient resources are required to conduct analytical testing, reformulate the product, or make required label changes, and in certain cases, such regulations may result in a company’s inability to continue or resume marketing these products. Any inquiries or investigations from the FDA, FTC, or other foreign regulatory authorities into the regulatory status of a cosmetic company’s products may cause an interruption in the marketing and sale of those products.
We believe that our products’ intended use, as currently labeled, fall within the FDA definition of cosmetics, and therefore do not and will not require pre-market review and approval. Thus, our cosmetics products may be sold both over the counter and through a physician’s office, subject to state laws governing the commercial practices of physicians.
86
Federal regulation of advertising and promotion
We are subject to regulation by the CPSC under statutes and related regulations under the CPSA that prohibits consumer products that fail to comply with applicable product safety laws, regulations, and standards from the market. The CPSC has the authority to require the recall, repair, replacement or refund of any such banned products or products that otherwise create a substantial risk of injury and may seek penalties for regulatory noncompliance under certain circumstances. The CPSC regulations also require manufacturers of consumer products to report to the CPSC certain types of information regarding products that fail to comply with applicable regulations. Certain state laws also address the safety of consumer products and mandate reporting requirements, and noncompliance may result in penalties or other regulatory action.
The FTC, FDA, and state authorities also regulate the advertising of over-the-counter drugs and cosmetics, as well as exercise general authority to prevent unfair or deceptive trade practices. The advertising regulations specifically relate to any product claims regarding the safety, performance, and benefits of cosmetic products like ours. These regulatory authorities typically require a safety assessment of a given product on a reasonable basis to support any of its marketing claims. What constitutes a reasonable basis for substantiation can vary widely from market to market, and there is no assurance that a cosmetic company like ours may support all claims that will be considered sufficient by the regulatory bodies. The most significant area of risk for such activities relates to improper or unsubstantiated claims about the use and safety of our products. If a company like ours cannot adequately support safety claims, or substantiate product claims, or if a company’s promotional materials make claims that exceed the scope of allowed claims for the classification of the specific product, the FDA, FTC, or other regulatory authority could take enforcement action or impose penalties, such as monetary consumer redress, requiring companies to revise marketing materials, amend claims or stop selling certain products.
We are also subject to a number of United States federal and state and foreign laws and regulations that affect companies conducting business on the internet, including consumer protection regulations that govern retailers and oversee the promotion and sale of merchandise. Many of these laws and regulations are still evolving and being tested in courts and are still subject to wider interpretation. Some of these regulations involve user privacy, data protection, collection, user interface, content, intellectual property, distribution, electronic contracts and other communications, competition, protection of minors, consumer protection, telecommunications, product liability, taxation, economic or other trade prohibitions or sanctions and online payment services. In particular, we are subject to federal, state, and foreign laws regarding privacy and protection of consumer data. Foreign data protection, privacy, and other laws and regulations can often be more restrictive than those protections in the United States. United States federal and state and foreign laws and regulations are constantly evolving and can be subject to significant change. In addition, the application, interpretation, and enforcement of these laws and regulations are often uncertain and may be interpreted and applied inconsistently from country to country and inconsistently with our current policies and practices. There are also a number of legislative proposals pending before the United States Congress, various state legislative bodies, and foreign governments concerning privacy and data protection which could affect us. For example, the European Commission recently published the final draft of new data protection regulations that include operational requirements for companies that receive personal data which are different from those previously in place in the European Union, and also include significant penalties for non-compliance.
Federal regulation of cosmetics manufacturing and Good Manufacturing Practices
The FDA recently promulgated regulations to eventually establish Good Manufacturing Practices (“GCMPs”) regulations consistent with national and international standards for cosmetics. Although portions of the law have yet to be implemented it is worth nothing that President Biden signed into law the Modernization of Cosmetic Regulation Act of 2022 (“MoCRA”) on December 29, 2022 which requires the FDA to issue proposed rules relating to GCMPs within two years after enactment. Additionally, MoCRA requires increased FDA oversight of cosmetics and the ingredients in them by establishing processes similar to those for other FDA-regulated products, that ensures the cosmetic manufacturers provide assurances that the cosmetic products are safe. Specifically, MoCRA creates federal standards for cosmetic products registration, product listing, good manufacturing practice, recordkeeping, recalls, adverse event reporting and safety substantiation. States and local governments are precluded under MoCRA from enacting, implementing, or enforcing requirements for cosmetics that are different from MoCRA’s requirements, with two exceptions: states may prohibit the use of or limit the amount of an ingredient in cosmetic products under state law, and may continue to enforce reporting requirements such as California’s Proposition 65 that were in existence prior to MoCRA’s passage.
MoCRA establishes a definition of an obligations on a “responsible person” including manufacturers, or distributors of a cosmetic and those whose name appears on the products label such as us. However, the law accommodations for smaller sized businesses by defining those responsible persons whose gross annual sales in the United States of cosmetic products for the previous three-year period is less than $1,000,000 as a small business. Under MoCRA, small businesses are not subject to good manufacturing practices, registration, and listing requirements and they’re application under MoCRA are not discussed herein. Pursuant to the statute we fall under the definition of a small business and thus as of the date of this prospectus, the recent adoption of this law is expected to have limited effects on our operations. While we anticipate significant efforts by the FDA to work towards implementing its newly promulgated authority, it may be too early to determine what serious effects if any the law will have on a business of our size. In addition, MoCRA also does not apply to physicians’ offices, health care clinics, entities that provide complimentary cosmetic products, trade shows and others giving free samples or entities that are only doing research. The law also does not apply to distribution facilities including those that prepare labels, relabel, package, repackage, hold, and/or distribute cosmetic products.
Similar to the FDA’s existing authorities for dietary supplements and OTC drugs, under MoCRA a responsible person must (i) maintain records of any health-related adverse events associated with the use of its product for six years (or three years for some small businesses like ours) and, (ii) report to the FDA any serious adverse events no later than 15 days after learning about the issue. A responsible person must also provide any new and material medical information it learns of related to the serious adverse event for one year following the initial submission. MoCRA broadens the scope of what constitutes a serious adverse reportable event to account for particular considerations relevant to the cosmetics sector which includes an infection or “significant disfigurement (including serious and persistent rashes, second- or third-degree burns, significant hair loss, or persistent or significant alteration of appearance), other than as intended, under conditions of use that are customary or usual.”
87
Unlike for food and drug products, the FDA has yet to adopt good manufacturing practice (cGMP) regulations for cosmetics companies like ours as of the date of this prospectus. Under MoCRA the proposed rulemaking for cGMPs for cosmetics products will be published no later than two years after date of enactment (December 29, 2022) with final regulations no later than three years after that date of enactment. However, the FDA does have nonbinding draft guidance document on cosmetic cGMPs that cosmetic manufacturers may follow. Thus, we currently require all of our third-party manufacturers to represent and warrant to us that the products they produce for us are made in accordance with cGMPs, including those in relation to requiring specific documentation, recordkeeping, building and facility design standards, and equipment maintenance, and personnel requirements.
Under the FDA’s guidance recommendations, cosmetic manufacturers should:
| ● | Maintain records describing how their products are manufactured and tested; |
| ● | Determine whether their buildings and facilities are of suitable cleanliness, design, size, and construction; |
| ● | Determine whether their equipment is of appropriate design, size, and workmanship; |
| ● | Ensure that their personnel have the necessary training and expertise to perform their jobs; |
| ● | Handle, use, and store raw materials; |
| ● | Create written standard operating procedures, internal audit procedures, and laboratory controls; and |
| ● | Record and respond to consumer complaints, adverse events, and product recalls. |
Although we are not a cosmetics manufacturer and only manufacture our exosomes as components to be included in our final cosmetic products, we substantially implement the above cGMP practices in order to reduce the risk of manufacturing any adulterated or misbranded cosmetics by following the draft cGMP guidance. Cosmetic companies may also reduce the risk that cosmetics under company control will be tampered with or subject to criminal, terrorist, or other malicious actions by following the FDA’s guidance on security preventive measures for cosmetics processors and transporters.
FDA regulation of regenerative medicine
Although we do not believe that federal regulation of regenerative medicine is applicable to our product offerings, the shifting FDA guidance on human cell and tissue drug, devices and biological products defined under section 351 of the FDCA, is a complex and evolving subject worth citing for further clarification. Until recently, the principal federal statutes regulating medical products include the FDCA and the Public Health Service Act (the “PHSA”), despite both statutes not explicitly addressing these types of biological drugs or products. In the absence of direct Congressional action, the rules regarding human cell and tissue products continues to develop at the administrative level. This complex set of regulations and sub-regulatory guidance documents has been promulgated by the FDA and is regularly updated and published as recently as November 202229.
Regenerative medicines, including those that may contain exosomes, usually refers to the use of a cell or a gene therapy that can actually repair or replace damaged cells, tissues, or organs, as opposed to improving the appearance of skin. When these regenerative medicines are advertised to repair, or replace damaged cells, or tissues while treating diseases and conditions in humans, they may be regulated by the FDA as drugs and biological products under the FDCA. Some of these regenerative medicines may be subject to premarket review and approval requirements under the purview of FDA under the PHSA under which the FDA regulates the interstate manufacture and distribution of certain biological products, and drugs derived from human cells, tissues and cellular and tissue-based products or HCT/Ps. Under 21 CFR 1271.3(d), HCT/Ps are defined as “articles containing or consisting of human cells or tissues that are intended for implantation, transplantation, infusion, or transfer into a human recipient.” HCT/P regulations are promulgated so that those biological products used by practitioners in their treatment of patients are designed to prevent the transmission or spread of communicable diseases. To do so, the FDA created a tiered, risk-based system in which the level of FDA oversight varies depending on the source of the tissue, the degree to which that drug is processed, and the manufacturer’s claims for the final drugs.
Although HCT/Ps include those regenerative medicine therapies that may transplant cells from a donor into a patient to treat medical conditions, as of the date of this prospectus, we are not aware of any FDA approved exosome biological products or drugs for any use. Because our products are neither a regenerative medicine intended to be used to treat any disease or condition and, and we do not believe our products are subject to qualify as HCT/Ps as it relates to their intended use as defined by the FDA and we do not expect them to become subject to the above-mentioned guidance that is regularly published by the FDA.
| 29 | Regulation of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) - Small Entity Compliance Guide | FDA |
88
Other government regulation
In addition to the foregoing laboratory protocols involving the handling of raw materials, we produce minimal non-hazardous bio-waste or discharge in our production of exosomes that is discarded via a contracted third-party hauler that specializes in processing waste in a compliant and controlled manner. To maintain current with our company protocols we maintain policies and procedures to monitor and control environmental, health and safety risks, and to monitor compliance with applicable state and federal environmental, health and safety requirements.
We are also subject to the Occupational Safety and Health Act, import, export and customs regulations as well as the laws and regulations of other countries.
Although our products are free from synthetic or natural fragrances, essential oils, flavors, parabens or phthalates we are further subject to state, and local laws including the California Safe Drinking Water and Toxic Enforcement Act (or Proposition 65) that requires all businesses to notify Californians about products, including cosmetics, that contain chemicals known to the State of California to cause cancer or reproductive harm. The California Safe Cosmetics Act further requires that a cosmetics manufacturer, packer, or distributor named on a cosmetic sold in California provide the California Department of Public Health a list of cosmetic products containing any ingredients known or suspected to cause cancer or reproductive or developmental harm. As of the date of this prospectus, we have not been subject to reporting under the California Safe Cosmetics Act, nor do we expect to be.
In addition, the California’s Cosmetic Fragrance and Flavor Ingredient Right to Know Act of 2020, requires manufacturers of cosmetics sold in California to disclose to the California Department of Public Health (CDPH) Safe Cosmetics Program certain flavor and fragrance ingredients in the cosmetics. Beginning January 1, 2025, California and Maryland have prohibited the manufacture or sale of cosmetics that contain certain intentionally added ingredients. Moreover, New York law sets limits on the amount of 1,4-dioxane that can be in cosmetics and personal care products sold in New York beginning December 31, 2022, with a tighter limit for personal care products beginning December 31, 2023. As of the date of this prospectus, we have not been subject to reporting under the California’s Cosmetic Fragrance and Flavor Ingredient Right to Know Act or New York State regulations regarding personal care products, nor do we expect to be.
89
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read together with our consolidated financial statements and the related notes to those statements included elsewhere in this prospectus. This discussion and analysis and other parts of this prospectus contain forward-looking statements based upon current beliefs, plans and expectations related to future events and our future financial performance that involve risks, uncertainties and assumptions, such as statements regarding our intentions, plans, objectives, expectations, forecasts and projections. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under the section titled “Risk Factors” and elsewhere in this prospectus. You should carefully read the “Risk Factors” to gain an understanding of the important factors that could cause actual results to differ materially from our forward-looking statements. Please also see the section titled “Special Note Regarding Forward-Looking Statements.” All amounts included herein with respect to the fiscal years ended December 31, 2022 and 2021 are derived from our audited consolidated financial statements included elsewhere in this prospectus and for the six-month periods ended June 30, 2023 and 2022 set forth below from our unaudited interim financial statements, each of which we prepared in accordance with U.S. Generally Accepted Accounting Principles or US GAAP.
Organization and Overview of Operations
Elevai Labs, Inc. was incorporated in Delaware in June 2020. We are a physician-dispensed skincare company with a focus on modernizing aesthetic skincare. Elevai conducts research and development to advance innovative and science-driven topical skincare that complements the medical aesthetics industry. We principally employ a business-to-business model in which we produce and commercialize a new generation of topical skincare products that contain our proprietary stem cell-derived Elevai ExosomesTM to enhance the appearance of skin.
In June 2021, we entered into a stock transfer agreement with Reactive Medical Labs, Inc., a Canadian company under common control, whereby we purchased substantially all of the assets and liabilities of Reactive Medical Labs, Inc.
To bring our products to market, we developed a robust process from source to skin (exosome secretion to product bottling) that holds and utilizes advanced patent pending knowledge alongside our cohesive production process. Our specialty product lines are topically applied to the skin to aid in the reduction of the appearance of a range of the most common cosmetic skin conditions, including the appearance of skin firmness, oxidative stress, photodamage, hyperpigmentation, and texture of soft tissue deficits, such as reducing the appearance of fine lines and wrinkles. We primarily sell our products through the physician-dispensed channel.
Outlook
Management’s Plans
Over the next twelve months we intend to focus on:
| ● | Expanding our internal sales force, hiring new employees to accelerate commercialization of our products; |
| ● | Utilizing clinical validation studies to show the aesthetic results of our products; |
| ● | R&D to create new product formulations and bring them to market; |
| ● | Expanding our distribution partnerships internationally; and |
| ● | Expanding our partnerships with complementary device manufacturers. |
90
Results of Operations
Comparison of the six months ended June 30, 2023 and 2022.
The following table provides certain selected financial information of our results of operations for the periods presented:
| Six Months ended June 30, 2023 | Six Months ended June 30, 2022 | Change | ||||||||||
| Revenue | $ | 459,350 | $ | 195,257 | $ | 264,093 | ||||||
| Cost of revenue | $ | 152,613 | $ | 79,052 | $ | 73,561 | ||||||
| Gross profit | $ | 306,737 | $ | 116,205 | $ | 190,532 | ||||||
| Gross profit percentage | 67 | % | $ | 60 | % | 7 | % | |||||
| Depreciation | $ | 5,385 | $ | 1,695 | $ | 3,690 | ||||||
| Marketing and Promotion | $ | 216,727 | $ | 61,489 | $ | 155,238 | ||||||
| Consulting Fees | $ | 233,687 | $ | 138,720 | $ | 94,967 | ||||||
| Office and Administration | $ | 964,009 | $ | 327,417 | $ | 636,592 | ||||||
| Professional Fees | $ | 306,730 | $ | 45,159 | $ | 261,571 | ||||||
| Investor Relations | $ | 75,720 | $ | 13,786 | $ | 61,934 | ||||||
| Research and Development | $ | 217,395 | $ | 78,563 | $ | 138,832 | ||||||
| Foreign exchange (gain) loss | $ | 2,633 | $ | 1,857 | $ | 776 | ||||||
| Travel and entertainment | $ | 184,170 | $ | 92,603 | $ | 91,567 | ||||||
| Total operating expenses | $ | 2,206,456 | $ | 761,289 | $ | 1,445,167 | ||||||
| Loss from operations | $ | (1,899,719 | ) | $ | (645,084 | ) | $ | (1,254,635 | ) | |||
| Other expenses1 | (460,835 | ) | (3,442 | ) | (457,393 | ) | ||||||
| Net loss | $ | (2,360,554 | ) | $ | (648,526 | ) | $ | (1,712,028 | ) | |||
| Total Comprehensive Loss | $ | (2,360,179 | ) | $ | (648,284 | ) | $ | (1,711,895 | ) | |||
| Basic and dilutive loss per common share | $ | (0.240 | ) | $ | (0.068 | ) | $ | (0.172 | ) | |||
| Weighted average number of shares outstanding – basic and diluted | 9,838,599 | 9,526,808 | 311,791 | |||||||||
| 1 | ”Other expenses” relates to interest income, interest expense, loss on sale of equipment and fair value gain/loss on derivative liability. |
Revenue
Revenue for the six months ended June 30, 2023, was $459,350 as compared to $195,257 for the six months ended June 30, 2022, an increase of $264,093.
Our revenue by product category is as follows:
| Six Months ended June 30, 2023 | Six Months ended June 30, 2022 | |||||||
| Enfinity | $ | 257,053 | 78,925 | |||||
| Empower | 202,297 | 20,282 | ||||||
| White label distributor | - | 96,050 | ||||||
| Total Revenue | $ | 459,350 | 195,257 | |||||
During the six months ended June 30, 2022, the Company sold 583 bottles of Enfinity, produced its first production run of Empower and sold 38 single Empower tubes (equivalent to 4.75 eight packs) as well as 37 eight packs of Empower, and sold 1,201 eight packs of Empower to a white label distributor. During the six months ended June 30, 2023, the Company sold 2,081 bottles of Enfinity and sold 486 (eight packs) of Empower tubes. The Company has seen significant growth in sales since its commercialization in Q1 2022.
91
Cost of Revenue
Cost of Revenue for the six months ended June 30, 2023, was $152,613 as compared to $79,052 for the six months ended June 30, 2022.
Our cost of revenue by product category is as follows:
| Six Months ended June 30, 2023 | Six Months ended June 30, 2022 | |||||||
| Enfinity | $ | 98,383 | $ | 28,528 | ||||
| Empower | 54,230 | 12,388 | ||||||
| White label distributor | - | 38,136 | ||||||
| Total Cost of Revenue | $ | 152,613 | $ | 79,052 | ||||
The increase in cost of revenue is directly attributed to the increase in sales during the six months ended June 30, 2023, compared to the six months ended June 30 2022. The following is a breakdown of the components of cost of revenue:
| Six Months ended June 30, 2023 | Six Months ended June 30, 2022 | |||||||
| Cost of inventory | $ | 73,896 | $ | 56,622 | ||||
| Sales commission | 47,417 | 12,622 | ||||||
| Shipping cost | 28,378 | 1,988 | ||||||
| Inventory write down and wastage | 2,922 | 7,820 | ||||||
| Total Cost of Revenue | $ | 152,613 | $ | 79,052 | ||||
Gross Profit
The following is a breakdown of gross profit percentage by product category for the six months ended June 30, 2023, compared to the six months ended June 30, 2022:
| Six Months ended June 30, 2023 | Six Months ended June 30, 2022 | |||||||
| Enfinity | 62 | % | 64 | % | ||||
| Empower | 73 | % | 39 | % | ||||
| White label distributor | - | 60 | % | |||||
| Overall Gross Profit Percentage | 67 | % | 60 | % | ||||
92
Gross profit for the six months ended June 30, 2023, was $306,737 as compared to $116,205 for the six months ended June 30, 2022, an increase of $190,532. This represents an overall gross margin percentage of 67% during the six month period ending June 30, 2023, compared to 60% in the six month period ending June 30, 2022. The overall increase in gross margin percentage is primarily due to the Empower products being sold at a higher margin to increase profit margins compared to six months ended June 30, 2022. Additionally, the six month period ended June 30, 2022 contains white label distributor sales which are sold at a lower gross margin while the six month period ended June 30, 2023 had no white label distributor sales.
The decrease in the gross margin percentage on Enfinity from 64% during the six months ended June 30, 2022 to 62% in the six months ended June 30, 2023, is primarily related to higher sales commissions as the Company hired more sales reps to drive sales. The increase in the gross margin percentage on Empower from 39% during the six months ended June 30, 2022 to 73% in the six months ended June 30, 2023, is primarily related to the write down, during 2022, of Empower tubes that were the wrong size.
Research and Development Expenses (“R&D”)
R&D expenses for the six months ended June 30, 2023, were $217,395 compared to $78,563 for the six months ended June 30, 2022, an increase of $138,832. R&D related to the Company’s Enfinity, Empower and white label distributor products. The increase in R&D is mainly driven by an increase in lab employee headcount from one (1) during the six months ended June 30, 2022, to four (4) in the six months ended June 30, 2023. In addition, the Company was in its old lab location during Q1 2022 compared to the new lab location in Q1 2023 (the Company has been in its new lab since July 2022). The new lab location has a higher production and R&D capacity which brings an increase in rent and utilities. During both the six months ended June 30, 2023 and 2022, the Company's lab staff worked on increasing the efficiency and refining the production process.
Marketing and Promotion
Marketing and promotion expenses for the six months ended June 30, 2023, were $216,727 compared to $61,489 for the six months ended June 30, 2022, an increase of $155,238. The Company increased its marketing and promotion efforts to drive sales, which included giving out product samples with a cost of $64,718 during the six months ended June 30, 2023, compared to only $12,407 during the six months ended June 30, 2022.
Office and Administrative Expenses
Office and administrative expenses for the six months ended June 30, 2023, were $964,009, compared to $327,417 for the six months ended June 30, 2022, an increase of $636,592. The increase is mainly the result of salaries and wages of $608,150 and office rent of $54,138 incurred for the six months ended June 30, 2023, compared to $190,347 and $15,281 in the six months ended June 30, 2022, a combined increase of $456,660. The Company increased its headcount and moved into a larger office location to accommodate the commercialization of its products and growth in operations during the six months ended June 30, 2023. During the six months ended June 30, 2023, office and administrative expenses also include share-based compensation of $178,736, compared to $56,407 in six months ended June 30, 2022, an increase of $122,329. The increase in share-based compensation expense is due to the continued vesting of stock options granted during 2021 and 2022, with additional options issued during 2023. The remaining increase is consistent with the increase in operations in the six months ended June 30, 2023, compared to the six months ended June 30, 2022.
Consulting Fees
Consulting fees for the six months ended June 30, 2023, were $233,687, compared to $138,720 for the six months ended June 30, 2022, an increase of $94,967. During the six months ended June 30, 2023, and 2022, the Company incurred consulting fees in relation to recruitment, strategic introductions, business advisory, international relations, and strategy. In addition, the Company received services from a number of parties (including companies controlled by related parties and CFO) in a consulting capacity. The increase in consulting fees is consistent with the increase in operations.
Professional Fees
Professional fees for the six months ended June 30, 2023, was $306,730, compared to $45,159 for the six months ended June 30, 2022, an increase of $261,571. Professional fees comprise of legal, audit and accounting services. The increase during the six months ended June 30, 2023, is primarily due to an increase in audit, legal and accounting services pursuant to the Company’s goal of filing its preliminary initial registration (S-1 Form) with the SEC and completing an initial public offering (“IPO”).
Travel and Entertainment
Travel and entertainment for the six months ended June 30, 2023, was $184,170, compared to $92,603 for six months ended June 30, 2022, an increase of $91,567. Travel and entertainment expenses are related primarily to costs incurred during the attendance of industry trade shows and conferences. The increase in the six months ended June 30, 2023, compared to 2022 is due to the Company increasing its presence at trade shows and conferences to raise awareness of the Company, its products and to drive business development.
Investor Relations
Investor relations for the six months ended June 30, 2023, was $75,720, compared to $13,786 for the six months ended June 30, 2022. The increase in investor relations spending is consistent with the Company’s growth strategy, which includes promotion to current and potential investors.
93
Liquidity and Capital Resources
The accompanying unaudited condensed interim consolidated financial statements have been prepared on a going concern basis, which implies the Company will continue to realize its assets and discharge its liabilities in the normal course of business. The continuation of the Company as a going concern is dependent upon the continued financial support from its shareholders, the ability of the Company to obtain necessary equity financing to continue operations, and ultimately the attainment of profitable operations.
As of June 30, 2023, and December 31, 2022, the Company had a net working capital deficit of $112,264, and a positive working capital $963,050, respectively, and has an accumulated deficit of $5,082,927 and $2,722,373, respectively. Furthermore, for six months ended June 30, 2023, and 2022, the Company incurred a net loss of $2,360,554 and $648,526, respectively and used $1,654,262 and $489,327 respectively of cash flows for operating activities. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. The accompanying unaudited condensed interim consolidated financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
Our principal liquidity requirements are for working capital, capital expenditure, research and development and inventory production. We fund our liquidity requirements primarily through cash on hand, cash flows from operations, and the issuance of common and preferred stock. As of June 30, 2023, we had cash of $601,265, with $1,154,901 as of December 31, 2022.
The following table provides selected financial data of our financial position as of June 30, 2023, and as of December 31, 2022, respectively.
| June 30, 2023 | December 31, 2022 | Change | ||||||||||
| Current assets | $ | 1,450,505 | $ | 1,551,322 | $ | (100,817 | ) | |||||
| Current liabilities | $ | 1,562,769 | $ | 588,272 | $ | 974,497 | ||||||
| Working capital | $ | (112,264 | ) | $ | 963,050 | $ | (1,075,314 | ) | ||||
The following table summarizes our cash flows from operating, investing, and financing activities for the six months ended June 30, 2023, compared to the six months ended June 30, 2022:
| Six Month Ended June 30, 2023 | Six Month Ended June 30, 2022 | Change | ||||||||||
| Cash used in operating activities | $ | (1,654,262 | ) | $ | (489,327 | ) | $ | (1,164,935 | ) | |||
| Cash used in investing activities | $ | (11,191 | ) | $ | (3,441 | ) | $ | (7,750 | ) | |||
| Cash provided by financing activities | $ | 1,111,089 | $ | 470,912 | $ | 640,177 | ||||||
Cash Used in Operating Activities
For the six months ended June 30, 2023, net cash flows used in operating activities was $1,654,262 compared to $489,327 used during the six months ended June 30, 2022, respectively, primarily due to net loss and timing of settlement of assets and liabilities.
Cash Used in Investing Activities
During the six months ended June 30, 2023, and 2022, we used $11,191 and $3,441, respectively, in investing activities primarily related to the purchase of equipment for our lab space to be used on the production of inventory and research and development, as well as the purchase of equipment for use at conferences and trade shows.
94
Cash Flows from Financing Activities
During the six months ended June 30, 2023, we had cash flows provided by financing activities of $1,111,089 compared to $470,912 financing activities during the six months ended June 30, 2022. During the six months ended June 30, 2023, the Company raised $1,073,589 through the issuance of common stock and common stock purchase warrants, and another $37,500 upon the exercise of stock options in exchange for common stock.
Critical Accounting Policies and Significant Judgments and Estimates
This discussion and analysis of our financial condition and results of operations is based on our condensed interim consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). The preparation of the condensed interim consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to revenue recognition, the collectability of receivables, valuation of inventory, fair value of derivative liabilities and stock options, useful lives and recoverability of long-lived assets, and deferred income tax asset valuation allowances. The Company bases its estimates and assumptions on current facts, historical experience, and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgements about the carrying value of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from those estimates. Estimates and assumptions are reviewed periodically, and the effects of revisions are reflected in the condensed interim consolidated financial statements in the period they are determined.
The Company’s policy for property and equipment requires judgement in determining whether the present value of future expected economic benefits exceeds capitalized costs. The policy requires management to make certain estimates and assumptions about future economic benefits related to its operations. Estimates and assumptions may change if new information becomes available. If information becomes available suggesting that the recovery of capitalized cost is unlikely, the capitalized cost is written off/impaired to the condensed interim consolidated statement of operations.
The assessment of whether the going concern assumption is appropriate requires management to take into account all available information about the future, which is at least, but not limited to, 12 months from the date the financial statements are issued. The Company is aware that material uncertainties related to events or conditions may cast substantial doubt upon the Company’s ability to continue as a going concern.
Revenue Recognition
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers. Since ASU 2014-09 was issued, several additional ASUs have been issued to clarify various elements of the guidance. These standards provide guidance on recognizing revenue, including a five-step model to determine when revenue recognition is appropriate.
The Company recognizes revenue when it satisfies a performance obligation by transferring control over a product to a customer. Revenue is measured based on the consideration the Company expects to receive in exchange for those products. In instances where financial acceptance of the product is specified by the customer, revenue is deferred until all acceptance criteria have been met. Revenues are recognized under ASC 606, “Revenue from Contracts with Customers,” in a manner that reasonably reflects the delivery of its products and services to customers in return for expected consideration.
The Company generates revenue through the sale of skincare products. Revenue from the sale of skincare products are recognized at the point in time when the Company considered revenue realized or realizable and earned, which is typically when all of the five following criteria are met: (1) the contract with the customer is identifiable (i.e. when a sales transaction has been entered into between the Company and the customer), (2) the performance obligation in the contract is identifiable (i.e. the customer has ordered a known quantity of product to be delivered), (3) the transaction price is determinable (i.e. the customer has agreed to the Company’s price for the products ordered), (4) the Company is able to allocate the transaction price to the performance obligations in the contract, and (5) the performance obligations have been satisfied, which is typically upon delivery of the product to the customer.
Transaction prices for performance obligations are explicitly outlined in relevant agreements; therefore, the Company does not believe that significant judgements are required with respect to the determination of the transaction price, including any variable consideration identified.
The Company is responsible for providing the products to customers. As a result, the Company is considered the Principal when providing products to customers. As the Company collects payment at the time of the customer order, its contracts do not have a significant financing component. Customers are entitled to replacement or full refund of any damaged or defective product, after the return of the damaged or defective product to the Company. There were no significant returns or refunds during the six months ended June 30, 2023, and 2022.
95
Foreign Currency Translation
The Company’s functional and reporting currency is the U.S. dollar. The functional currency of the Company’s Canadian subsidiary, Elevai Research Inc. (“Elevai Research”) is the Canadian dollar. Monetary assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the balance sheet date. Non-monetary assets, liabilities, and items recorded in income arising from transactions denominated in foreign currencies are translated at rates of exchange in effect at the date of the transaction. Gains and losses arising on translation or settlement of foreign currency denominated transactions or balances are included in the determination of income.
The accounts of Eleva Research are translated to U.S. dollars using the current rate method. Accordingly, assets and liabilities are translated into U.S. dollars at the period-end exchange rate while revenues and expenses are translated at the average exchange rates during the period. Related exchange gains and losses are included in a separate component of stockholders’ equity as accumulated other comprehensive income (loss).
Inventory
Inventory consists of raw materials, work-in-progress and finished goods and are valued at the lower of cost or net realizable value. The Company’s manufacturing process involves the production of our proprietary stem cell-derived Elevai ExosomesTM. Finished goods consists of a new generation of cosmetic topical products containing our proprietary stem cell-derived Elevai ExosomesTM. Cost is determined using the weighted average cost formula. Net realizable value is determined on the basis of anticipated sales proceeds less the estimated selling expenses. Management compares the cost of inventories with the net realizable value and an allowance is made to write down inventories to net realizable value, if lower.
Stock-Based Compensation
Employees - The Company accounts for share-based compensation under the fair value method which requires all such compensation to employees, including the grant of employee stock options, to be calculated based on its fair value at the measurement date (generally the grant date), and recognized in the condensed interim consolidated statement of operations over the requisite service period.
Nonemployees - During June 2018, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2018-07, Compensation-Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting (“ASU 2018-07”) to simplify the accounting for share-based payments to nonemployees by aligning it with the accounting for share-based payments to employees. Under the requirements of ASU 2018-07, the Company accounts for share-based compensation to non-employees under the fair value method which requires all such compensation to be calculated based on the fair value at the measurement date (generally the grant date) and recognized in the statement of operations over the requisite service period.
During the six months ended June 30, 2023, and 2022, the Company recorded $185,068 and $58,510, respectively, in share-based compensation expense, of which $178,736 and $6,332, and $56,407 and $2,101, respectively is included in office and administration and research and development, respectively.
Determining the appropriate fair value model and the related assumptions requires judgment. During six months ended June 30, 2023, and 2022, the fair value of each option grant was estimated using a Black-Scholes option-pricing model.
The expected volatility represents the historical volatility of comparable publicly traded companies in similar industries, adjusted for variables such as stock price, market capitalization and life cycle. Due to limited historical data, the expected term for options granted is equal to the contractual life. The risk-free interest rate is based on a treasury instrument whose term is consistent with the expected life of stock options. The Company has not paid and does not anticipate paying cash dividends on its shares of common stock; therefore, the expected dividend yield is assumed to be zero.
96
Concentrations
Customers
During the six month period ended June 30, 2023, the Company recorded 16% of its revenue from its largest customers. The Company’s largest customer relates to sales to a wholesaler during the period. During the six months ended June 30, 2022, the Company recorded 49% of its revenue from a single customer. The company's largest customer relates to sales to a wholesaler during the period.
As of June 30, 2023 and December 31, 2022, the Company had $3,549 and $nil receivables due from this customer, respectfully, and $nil and $5,992, respectfully, in customer deposits were received from its largest customer.
The Company expects its dependence on major customers to decrease over time as it enters into additional distributor agreements and builds out its sales team.
Suppliers
During the six month period ended June 30, 2023 and 2022, the Company had 2 key suppliers that represented approximately 57% and 66%, respectively of the cost incurred in the purchase and production of inventory. The table below represents a breakdown of each supplier as a percentage of the cost incurred (Suppliers are shown from largest to smallest and does not necessarily represent the same suppliers period over period):
| Six Months Ended June 30, 2023 | Six Months Ended June 30, 2022 | |||||||
| Supplier 1 | 33 | % | 33 | % | ||||
| Supplier 2 | 24 | % | 33 | % | ||||
| Total | 57 | % | 66 | % | ||||
The Company continually evaluates the performance of its suppliers and the availability of alternatives to substitute or supplement its inventory production supply chain. The Company believes that a breakdown in supply from one of its key suppliers would be overcome in a short amount of time given the availability of alternatives.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditure or capital resources that is material to investors.
Future Related Party Transactions
After completion of this Offering, the Corporate Governance Committee of our Board of Directors (which we will establish and which will consist solely of independent directors) will be required to approve all related party transactions. All related party transactions will be made or entered into on terms that are no less favorable to use than can be obtained from unaffiliated third parties.
97
Impact of Inflation
We do not believe the impact of inflation on our Company is material.
COVID-19 Risk
The COVID-19 pandemic has had, and continues to have, a significant impact around the world, prompting governments and businesses to take unprecedented measures, such as restrictions on travel and business operations, temporary closures of businesses, and quarantine and shelter-in-place orders. The COVID-19 pandemic has at times significantly curtailed global economic activity and caused significant volatility and disruption in global financial markets. The COVID-19 pandemic and the measures taken by many countries in response have affected and could in the future materially impact the Company’s business, results of operations and financial condition.
Certain of our outsourcing partners, component suppliers and logistical service providers have experienced disruptions during the COVID-19 pandemic, resulting in non-material supply shortages. Similar or greater disruptions could occur in the future.
Inflation Risk
We are also exposed to inflation risk. Inflationary factors, such as increases in labor costs, could impair our operating results. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, a high rate of inflation in the future may have an adverse effect on our ability to maintain current levels of gross margin and operating expenses.
Market Risk
Market risk is the risk of loss arising from adverse changes in market rates and prices. Our market risk exposure is generally limited to those risks that arise in the normal course of business, as we do not engage in speculative, non-operating transactions, nor do we utilize financial instruments or derivative instruments for trading purposes.
Comparison of the Years Ended December 31, 2022 and 2021.
The following table provides certain selected financial information of our results of operations for the periods presented:
| Year Ended December 31, 2022 | Year Ended December 31, 2021 | Change | ||||||||||
| Revenue | $ | 766,277 | $ | 827 | $ | 765,450 | ||||||
| Cost of revenue | $ | 318,968 | $ | 88 | $ | 318,880 | ||||||
| Gross profit | $ | 447,309 | $ | 739 | $ | 446,570 | ||||||
| Gross profit percentage | 58 | % | $ | 89 | % | (31 | )% | |||||
| Amortization | $ | 5,034 | $ | 2,385 | $ | 2,649 | ||||||
| Marketing and Promotion | $ | 192,863 | $ | 31,605 | $ | 161,258 | ||||||
| Consulting Fees | $ | 324,395 | $ | 210,402 | $ | 113,993 | ||||||
| Office and Administration | $ | 1,019,708 | $ | 268,175 | $ | 751,533 | ||||||
| Professional Fees | $ | 192,409 | $ | 124,152 | $ | 68,257 | ||||||
| Investor Relations | $ | 74,003 | $ | - | $ | 74,003 | ||||||
| Research and Development | $ | 228,747 | $ | 123,632 | $ | 105,115 | ||||||
| Foreign exchange (gain) loss | $ | 2,749 | $ | (323 | ) | $ | 3,072 | |||||
| Travel and entertainment | $ | 198,442 | $ | 25,450 | $ | 172,992 | ||||||
| Total operating expenses | $ | 2,238,350 | $ | 785,478 | $ | 1,452,872 | ||||||
| Loss from operations | $ | (1,791,041 | ) | $ | (784,739 | ) | $ | (1,006,302 | ) | |||
| Net loss | $ | (1,800,268 | ) | $ | (784,739 | ) | $ | (1,015,529 | ) | |||
| Total Comprehensive Loss | $ | (1,800,359 | ) | $ | (783,315 | ) | $ | (1,017,044 | ) | |||
| Basic and dilutive loss per common share | $ | (0.189 | ) | $ | (0.083 | ) | $ | (0.106 | ) | |||
| Weighted average number of shares outstanding – basic and diluted | 9,528,863 | 9,460,664 | 68,199 | |||||||||
98
Revenue
Revenue for the year ended December 31, 2022 was $766,277 as compared to $827 for the year ended December 31, 2021, an increase of $765,450.
Our revenue by product category is as follows:
| Year Ended December 31, 2022 | Year ended December 31, 2021 | |||||||
| Enfinity | $ | 265,411 | $ | 827 | ||||
| Empower | 156,848 | - | ||||||
| White label distributor | 344,018 | - | ||||||
| Total Revenue | $ | 766,277 | $ | 827 | ||||
During the last quarter of fiscal year 2021, the Company produced its first batch of Enfinity bottles in preparation of commercialization in 2022. The limited number of Enfinity bottles (3) sold during 2021 is due to the timing of production and arrival of the Company’s products at its distribution partner’s warehouse. During 2022, the Company sold 2,114 bottles of Enfinity and produced its first batch and sold 350 (eight packs) of Empower tubes. The Company also entered into a distributor agreement through which it is selling media containing its Elevai ExosomesTM under a white label deal. During 2022, the Company sold approximately 287 liters under this white label distributor agreement.
Cost of Revenue
Cost of Revenue for the year ended December 31, 2022 was $318,968 as compared to $88 for the year ended December 31, 2021.
Our cost of revenue by product category is as follows:
| Year Ended December 31, 2022 | Year ended December 31, 2021 | |||||||
| Enfinity | $ | 101,554 | $ | 88 | ||||
| Empower | 42,554 | - | ||||||
| White label distributor | 174,860 | - | ||||||
| Total Cost of Revenue | $ | 318,968 | $ | 88 | ||||
The increase in cost of revenue is directly attributed to the increase in sales during 2022 compared to 2021. The following is a breakdown of the components of cost of revenue:
| Year Ended December 31, 2022 | Year ended December 31, 2021 | |||||||
| Cost of inventory | $ | 243,285 | $ | 88 | ||||
| Sales commission | 52,508 | - | ||||||
| Shipping cost | 14,880 | - | ||||||
| Inventory write down | 8,295 | - | ||||||
| Total Cost of Revenue | $ | 318,968 | $ | 88 | ||||
Gross Profit
Gross profit for the year ended December 31, 2022 was $447,309 as compared to $739 for the year ended December 31, 2021, an increase of $446,570. This represents an overall gross margin percentage of 58% during 2022, compared to 89% in 2021. The decrease in gross margin is due to a change in the product mix, specifically the lower margin on the Company’s white label distributor sales, and the introduction of sales commission, shipping cost and inventory write down. The inventory write down during 2022 represented Empower tubes that were the wrong size, the Company is not anticipating significant inventory write downs on a go forward basis.
99
The following is a breakdown of gross profit percentage by product category:
| Year Ended December 31, 2022 | Year ended December 31, 2021 | |||||||
| Enfinity | 62 | % | 89 | % | ||||
| Empower | 73 | % | - | |||||
| White label distributor | 49 | % | - | |||||
| Overall Gross Profit Percentage | 58 | % | 89 | % | ||||
Research and Development Expenses
Research and development expenses for the year ended December 31, 2022, were $228,747 compared to $123,632 for the year ended December 31, 2021, an increase of $105,115. Research and Development related to the Company’s Enfinity, Empower and white label distributor products. During 2022, the Company increased its headcount for lab employees from 1 to 4, worked on increasing the efficiency of its production process and moved to a new lab location with increased production capacity. The Company also dealt with supply chain challenges requiring the Company to test and implement changes to its production process.
Marketing and Promotion
Marketing and promotion expenses for the year ended December 31, 2022, were $192,863 compared to $31,605 for the year ended December 31, 2021, an increase of $161,258. During 2022, the Company increased its marketing and promotion efforts to drive sales, which included giving out product samples with a cost of $46,693 (2021 - $7,014).
Office and Administrative Expenses
Office and administrative expenses for the year ended December 31, 2022 were $1,019,708, compared to $268,175 for the year ended December 31, 2021, an increase of $751,533. The increase is mainly the result of salaries and wages of $617,425 and office rent of $73,363 incurred during 2022, compared to $94,335 and $14,870 in 2021, a combined increase of $581,583. The Company increased its headcount and moved into a larger office location to accommodate the commercialization of its products and growth in operations during 2022. During 2022, office and administrative expenses also include share-based compensation of $164,907, compared to $136,486 in 2021, an increase of $28,421. The increase in share-based compensation expense is due to the continued vesting of stock options granted during 2021 and additional options issued during 2022. The remaining increase is consistent with the increase in operations in 2022 compared to 2021.
Consulting Fees
Consulting fees for the year ended December 31, 2022 were $324,395, compared to $210,402 for the year ended December 31, 2021, an increase of $113,993. During 2022 and 2021, the Company incurred consulting fees in relation to recruitment, strategic introductions, business advisory, international relations, and strategy. In addition, the Company received services from a number of parties (including companies controlled by the Company’s Chairman and CFO) in a consulting capacity. The increase in consulting fees is due to an agreement, effective January 2022, between the Company and Northstrive Companies Inc., a company controlled by the Company’s Chairman and former President, which resulted in the Company incurring an additional $120,000 during 2022, compared to $nil in 2021.
Professional Fees
Professional fees for the year ended December 31, 2022 was $192,409, compared to $124,152 for the year ended December 31, 2021, an increase of $68,257. Professional fees comprise of legal, audit and accounting services. The increase during 2022 is primarily due to an increase in audit and accounting services pursuant to the Company’s goal of filing its preliminary initial registration (S-1 Form) with the SEC and completing an initial public offering (“IPO”).
100
Travel and Entertainment
Travel and entertainment for the year ended December 31, 2022 was $198,442, compared to $25,450 for the year ended December 31, 2021, an increase of $172,992. Travel and entertainment expense related primarily to costs incurred during the attendance of industry trade shows and conferences. The increase in 2022 is due to the Company increasing its presence at trade shows and conferences to raise awareness of the Company, its products and to drive business development.
Investor Relations
Investor relations for the year ended December 31, 2022 was $74,003, compared to $nil for the year ended December 31, 2021. The increase in investor relations spending is consistent with the Company’s growth strategy, which includes promotion to current and potential investors.
Liquidity and Capital Resources
The accompanying consolidated financial statements have been prepared on a going concern basis, which implies the Company will continue to realize its assets and discharge its liabilities in the normal course of business. The continuation of the Company as a going concern is dependent upon the continued financial support from its shareholders, the ability of the Company to obtain necessary equity financing to continue operations, and ultimately the attainment of profitable operations.
As of December 31, 2022 and 2021, the Company had a net working capital of $963,050 and $420,903, respectively, and has an accumulated deficit of $2,722,373 and $922,105, respectively. Furthermore, for the years ended December 31, 2022 and 2021, the Company incurred a net loss of $1,800,268 and $784,739, respectively and used $1,585,876 and $660,934, respectively of cash flows for operating activities. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
Our principal liquidity requirements are for working capital, capital expenditure, research and development and inventory production. We fund our liquidity requirements primarily through cash on hand, cash flows from operations, and the issuance of common and preferred stock. As of December 31, 2022 we had cash of $1,154,901, with $411,858 as of December 31, 2021
The following table provides selected financial data as of December 31, 2022 and 2021, respectively.
| December 31, 2022 | December 31, 2021 | Change | ||||||||||
| Current assets | $ | 1,551,322 | $ | 636,525 | $ | 914,797 | ||||||
| Current liabilities | $ | 588,272 | $ | 215,622 | $ | 372,650 | ||||||
| Working capital | $ | 963,050 | $ | 420,903 | $ | 542,147 | ||||||
The following table summarizes our cash flows from operating, investing and financing activities:
| Year Ended December 31, 2022 | Year Ended December 31, 2021 | Change | ||||||||||
| Cash used in operating activities | $ | (1,585,876 | ) | $ | (660,934 | ) | $ | (924,942 | ) | |||
| Cash used in investing activities | $ | (32,027 | ) | $ | (32,482 | ) | $ | 455 | ||||
| Cash provided by financing activities | $ | 2,362,259 | $ | 1,090,577 | $ | 1,271,682 | ||||||
101
Cash Flow from Operating Activities
For the year ended December 31, 2022, net cash flows used in operating activities was $1,585,876 compared to $660,934 used during the year ended December 31, 2021, respectively, primarily due to net loss and timing of settlement of assets and liabilities.
Cash Flows from Investing Activities
During the years ended December 31, 2022 and 2021, we used $32,027 and $32,482, respectively, in investing activities primarily related to the purchase of equipment for our lab space to be used on the production of inventory and research and development. Net cash used in investing activities include proceeds of $3,500 generated on the sale of equipment.
Cash Flows from Financing Activities
During the year ended December 31, 2022, we had cash flow provided by financing activities of $2,362,259 compared to cash flow provided by financing activities of $1,090,577 in 2021, an increase of $1,271,682. During 2022, the Company raised $183,970 through short term convertible notes that were converted into Series A preferred stock and common stock purchase warrants. The Company raised an additional $2,153,289, net of share issuance cost of $33,132, through its Series A preferred stock financing and another $25,000 upon the exercise of stock options in exchange for common shares. In 2021, the Company had a private placement of series seed 2 preferred stock for $1,090,577.
Critical Accounting Policies and Significant Judgments and Estimates
This discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”). The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to revenue recognition, the collectability of receivables, valuation of inventory, fair value of derivative liabilities and stock options, useful lives and recoverability of long-lived assets, and deferred income tax asset valuation allowances. The Company bases its estimates and assumptions on current facts, historical experience and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgements about the carrying value of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from those estimates. Estimates and assumptions are reviewed periodically, and the effects of revisions are reflected in the consolidated financial statements in the period they are determined.
The Company’s policy for property and equipment requires judgement in determining whether the present value of future expected economic benefits exceeds capitalized costs. The policy requires management to make certain estimates and assumptions about future economic benefits related to its operations. Estimates and assumptions may change if new information becomes available. If information becomes available suggesting that the recovery of capitalized cost is unlikely, the capitalized cost is written off/impaired to the consolidated statement of operations.
The assessment of whether the going concern assumption is appropriate requires management to take into account all available information about the future, which is at least, but not limited to, 12 months from the date the financial statements are issued. The Company is aware that material uncertainties related to events or conditions may cast substantial doubt upon the Company’s ability to continue as a going concern.
102
Revenue Recognition
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers. Since ASU 2014-09 was issued, several additional ASUs have been issued to clarify various elements of the guidance. These standards provide guidance on recognizing revenue, including a five-step model to determine when revenue recognition is appropriate.
The Company recognizes revenue when it satisfies a performance obligation by transferring control over a product to a customer. Revenue is measured based on the consideration the Company expects to receive in exchange for those products. In instances where financial acceptance of the product is specified by the customer, revenue is deferred until all acceptance criteria have been met. Revenues are recognized under ASC 606, “Revenue from Contracts with Customers,” in a manner that reasonably reflects the delivery of its products and services to customers in return for expected consideration.
The Company generates revenue through the sale of skincare products. Revenue from the sale of skincare products are recognized at the point in time when the Company considered revenue realized or realizable and earned, which is typically when all of the five following criteria are met: (1) the contract with the customer is identifiable (i.e. when a sales transaction has been entered into between the Company and the customer), (2) the performance obligation in the contract is identifiable (i.e. the customer has ordered a known quantity of product to be delivered), (3) the transaction price is determinable (i.e. the customer has agreed to the Company’s price for the products ordered), (4) the Company is able to allocate the transaction price to the performance obligations in the contract, and (5) the performance obligations have been satisfied, which is typically upon delivery of the product to the customer.
Transaction prices for performance obligations are explicitly outlined in relevant agreements; therefore, the Company does not believe that significant judgements are required with respect to the determination of the transaction price, including any variable consideration identified.
The Company is responsible for providing the products to customers. As a result, the Company is considered the Principal when providing products to customers. As the Company collects payment at the time of the customer order, its contracts do not have a significant financing component. Customers are entitled to replacement or full refund of any damaged or defective product, after the return of the damaged or defective product to the Company. There were no significant returns or refunds during the year ended December 31, 2022 and 2021
Foreign Currency Translation
The Company’s functional and reporting currency is the U.S. dollar. The functional currency of the Company’s Canadian subsidiary, Elevai Research Inc. (“Elevai Reearch”) is the Canadian dollar. Monetary assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the balance sheet date. Non-monetary assets, liabilities, and items recorded in income arising from transactions denominated in foreign currencies are translated at rates of exchange in effect at the date of the transaction. Gains and losses arising on translation or settlement of foreign currency denominated transactions or balances are included in the determination of income.
The accounts of Eleva Research are translated to U.S. dollars using the current rate method. Accordingly, assets and liabilities are translated into U.S. dollars at the period-end exchange rate while revenues and expenses are translated at the average exchange rates during the period. Related exchange gains and losses are included in a separate component of stockholders’ equity as accumulated other comprehensive income (loss).
103
Inventory
Inventory consist of raw materials, work-in-progress and finished goods and are valued at the lower of cost or net realizable value. The Company’s manufacturing process involves the production of our proprietary stem cell-derived Elevai ExosomesTM. Finished goods consists of a new generation of cosmetic topical products containing our proprietary stem cell-derived Elevai ExosomesTM. Cost is determined using the weighted average cost formula. Net realizable value is determined on the basis of anticipated sales proceeds less the estimated selling expenses. Management compares the cost of inventories with the net realizable value and an allowance is made to write down inventories to net realizable value, if lower.
Stock-Based Compensation
Employees - The Company accounts for share-based compensation under the fair value method which requires all such compensation to employees, including the grant of employee stock options, to be calculated based on its fair value at the measurement date (generally the grant date), and recognized in the consolidated statement of operations over the requisite service period.
Nonemployees - During June 2018, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2018-07, Compensation-Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting (“ASU 2018-07”) to simplify the accounting for share-based payments to nonemployees by aligning it with the accounting for share-based payments to employees. Under the requirements of ASU 2018-07, the Company accounts for share-based compensation to non-employees under the fair value method which requires all such compensation to be calculated based on the fair value at the measurement date (generally the grant date), and recognized in the statement of operations over the requisite service period.
During the years ended December 31, 2022 and 2021, the Company recorded $171,869 and $142,355, respectively, in share-based compensation expense, of which $164,907 and $6,962, and $136,486 and $5,869, respectively is included in office and administration and research and development, respectively.
Determining the appropriate fair value model and the related assumptions requires judgment. During the years ended December 31, 2022 and 2021, the fair value of each option grant was estimated using a Black-Scholes option-pricing model.
The expected volatility represents the historical volatility of comparable publicly traded companies in similar industries, adjusted for variables such as stock price, market capitalization and life cycle. Due to limited historical data, the expected term for options granted is equal to the contractual life. The risk-free interest rate is based on a treasury instrument whose term is consistent with the expected life of stock options. The Company has not paid and does not anticipate paying cash dividends on its shares of common stock; therefore, the expected dividend yield is assumed to be zero.
Concentrations
Customers
During the year ended December 31, 2022, the Company recorded 54% of its revenue from its two largest customers, each representing 45% and 9% respectively. The Company’s largest customer, representing $344,018 of revenue, relates to a white label distributor agreement signed during the year. There was no significant concentration of sales during the year ended December 31, 2021.
As of December 31, 2022, the Company had no receivables due from these customers and $5,992 in customer deposits were received from its largest customer.
The Company expects its dependence on these major customers to decrease over time as it enters into additional distributor agreements and builds out its sales team.
104
Suppliers
During the year ended December 31, 2022, the Company had 5 key suppliers that represented approximately 80% (2021 – 73%) of the cost incurred in the purchase and production of inventory. The table below represents a breakdown of each supplier as a percentage of the cost incurred (Suppliers are shown from largest to smallest and does not necessarily represent the same suppliers year over year):
| Year Ended December 31, 2022 | Year ended December 31, 2021 | |||||||
| Supplier 1 | 39 | % | 18 | % | ||||
| Supplier 2 | 14 | % | 17 | % | ||||
| Supplier 3 | 11 | % | 15 | % | ||||
| Supplier 4 | 8 | % | 13 | % | ||||
| Supplier 5 | 8 | % | 10 | % | ||||
| Total | 80 | % | 73 | % | ||||
The Company continually evaluates the performance of its suppliers and the availability of alternatives to substitute or supplement its inventory production supply chain. The Company believes that a breakdown in supply from one of its key suppliers would be overcome in a short amount of time given the availability of alternatives.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditure or capital resources that is material to investors.
Future Related Party Transactions
After completion of this Offering, the Corporate Governance Committee of our Board of Directors (which we will establish and which will consist solely of independent directors) will be required to approve all related party transactions. All related party transactions will be made or entered into on terms that are no less favorable to use than can be obtained from unaffiliated third parties.
Impact of Inflation
We do not believe the impact of inflation on our Company is material.
COVID-19 Risk
The COVID-19 pandemic has had, and continues to have, a significant impact around the world, prompting governments and businesses to take unprecedented measures, such as restrictions on travel and business operations, temporary closures of businesses, and quarantine and shelter-in-place orders. The COVID-19 pandemic has at times significantly curtailed global economic activity and caused significant volatility and disruption in global financial markets. The COVID-19 pandemic and the measures taken by many countries in response have affected and could in the future materially impact the Company’s business, results of operations and financial condition.
Certain of our outsourcing partners, component suppliers and logistical service providers have experienced disruptions during the COVID-19 pandemic, resulting in non-material supply shortages. Similar or greater disruptions could occur in the future.
Inflation Risk
We are also exposed to inflation risk. Inflationary factors, such as increases in labor costs, could impair our operating results. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, a high rate of inflation in the future may have an adverse effect on our ability to maintain current levels of gross margin and operating expenses.
Market Risk
Market risk is the risk of loss arising from adverse changes in market rates and prices. Our market risk exposure is generally limited to those risks that arise in the normal course of business, as we do not engage in speculative, non-operating transactions, nor do we utilize financial instruments or derivative instruments for trading purposes.
105
Our Executive Officers and Directors
The following table sets forth certain information regarding our executive officers and directors as of the date of this prospectus. A biography of each of those officers and directors is further reflected below.
| Name | Age | Position | ||
Jordan R. Plews, PhD | 40 | Chief Executive Officer, President and Director | ||
| Graydon Bensler | 32 | Chief Financial Officer and Director | ||
| Hatem Abou-Sayed, MD | 52 | Chief Medical Officer and Director | ||
| Brenda Buechler | 54 | Chief Marketing Officer | ||
| Christoph Kraneiss | 53 | Chief Commercial Officer | ||
| Jeffrey Parry | 63 | Independent Director and Chair of Nominating Committee | ||
| Crystal Muilenburg | 43 | Independent Director and Chair of Compensation Committee | ||
| Juliana Daley | 35 | Independent Director and Chair of the Audit Committee | ||
| [●] | - | Independent Director(1) |
| (1) | One or more additional independent directors may be appointed to our Board of Directors immediately before or upon effectiveness of the registration statement of which this prospectus forms a part. |
Prior to the consummation of this offering, our Board of Directors will consist of [7] members, Jordan R. Plews, Graydon Bensler, Tim Sayed, Jeffrey Parry, Crystal Muilenburg, Juliana Daley and [●]. No director is currently named as Chair. Each of our directors serves for a term of one year ending on the date of the subsequent annual meeting of stockholders following the annual meeting at which such director was elected. Notwithstanding the foregoing, each director is to serve until his or her successor is elected and qualified or until his death, resignation or removal. Our Board appoints our officers, and each officer is to serve until his or her successor is appointed and qualified or until his or her death, resignation or removal.
Jordan R. Plews PhD, Chief Executive Officer
Dr. Plews is one of our co-founders and has served as our Chief Executive Officer since our inception. Dr. Plews is also serves as President and Director of the Board of the Company. Dr. Plews led the acquisition of Reactive Medical Labs, Inc and the development and design of Elevai ExosomesTM. After completing his undergraduate in biochemical engineering at University College London in the UK, Dr. Plews worked as part of Pfizer’s bioprocess development group before returning to academia for his doctorate in Stem Cell Research and Molecular Biology. He was recruited by Stanford’s School of Medicine and worked under Dr. Joseph Wu, now head of the American Heart Association, and Joe Gold, who was previously Senior Director at Geron Corporation. After completing a certification from Stanford’s Graduate School of Business, Dr. Plews worked as a product and project manager in the biotech field, developing and launching projects to help researchers and clinicians, before eventually transitioning into entrepreneurship and the founding of Elevai.
Prior to cofounding Elevai, Dr. Plews was a senior leader in product management for the oncology division of Natera Inc from 2019-2021, where he led the launch and expansion of Natera Inc.’s first oncology product, Signatera. Signatera is a personalized cancer diagnostic based on next generation sequencing cellular data. Before Natera Inc., from 2015-2019, Dr. Plews served as Chief Scientific Officer of Xytogen Biotech Inc., which produced FACTORFIVE Skincare and a number of white label cosmetic and/or aesthetic products for the physician-dispensed market. Prior to Xytogen Biotech Inc., From 2014-2016 Dr. Plews served as a Global Product Marketing Manager for Becton, Dickinson and Company, focusing on development of products for scientific and regenerative medicine researchers, as well as participating in business development and merger and acquisition activities.
Dr. Plews holds a Bachelor of Engineering and Engineering Doctorate from University College London and completed his post-doctoral research at Stanford University in Stem Cells and Regenerative Medicine.
106
Graydon Bensler, CFA, Chief Financial Officer
Mr. Bensler has served as our Chief Financial Officer since our inception. Mr. Bensler is a financial professional and analyst with over seven years of experience in financial consulting and management for both private businesses and US/Canadian publicly traded companies and is a Chartered Financial Analyst (CFA). In 2017, Mr. Bensler Co-founded an Ed Tech curriculum management and scheduling company that was implanted in academic schools in Canada and the United States. From 2017 to 2019, Mr. Bensler was an account manager at a leading Canadian investor relations firm where he represented publicly traded companies across a wide range of sectors where he worked directly with investment banks, investment brokers and company executives and directors. During his tenure, Mr. Bensler created and conveyed messaging about his clients’ strategic position in the market and successfully guided several companies through multiple financings. From 2019 to 2021, Mr. Bensler was a Senior Associate at Evans & Evans, a Canadian boutique investment banking firm where he led valuations and going public transactions for Canadian and United States companies. In this capacity, Mr. Bensler gained strong knowledge of the capital markets, public company compliance requirements, and regularly interfaced with regulators, auditors, board and executive management. Mr. Bensler has also been a director of publicly traded Health Logic Interactive Inc. (TSXv:CHIP) since 2020. We believe that Mr. Bensler’s past experience as our Chief Financial Officer, his familiarity with both the banking and the financial consulting sectors and his having served as an account manager for similarly situated companies makes him a qualified director for our Company.
Mr. Bensler received his Bachelor of Management and Organizational Studies degree from the University of Western Ontario, with specialization in Finance, and is a CFA Charter holder.
Hatem Abou-Sayed, MD, MBA, FACS, Chief Medical Officer
Dr. Hatem Abou-Sayed (“Dr. Tim Sayed”) is one of our co-founders and has served as our Chief Medical Officer since our inception. Dr. Sayed is a double-board-certified plastic surgeon with two decades of experience and oversees our product development and marketing, and brings academic knowledge and clinical experience in aesthetics, anti-aging, skincare, and health technology. Since 2017, Dr. Sayed has owned and operated his plastic surgery offices in Southern California where he draws patients from around the world and throughout the United States. From 2012-2015, Dr. Sayed served as a medical director and investor at the electronic health records and practice management company: Modernizing Medicine. From 2015-2017, he served as Vice President at Interpreta, Inc., which provides precision medicine clinical interpretation solutions (acquired by Centene Corporation). Since 2017, Dr. Sayed has served as ZamZam Skin, LLC’s (ZamZam) managing member, a Halal skincare product line that is currently in development. Dr. Sayed oversees ZamZam’s marketing strategy and assists the company with administering the financial aspects of ZamZam’s ongoing research and development. Since 2020, Dr. Sayed has served on the advisory board of ‘Yes Doctor’, a patient financing-driven acquisition platform for plastic surgeons. Dr. Sayed’s educational and professional experience in the medical field, his background and connections in the surgical discipline, his having co-founded our business and provided strategic advice makes him a qualified director for our Company.
Dr. Sayed completed medical training at UCSF and surgical residency at Massachusetts General Hospital/Harvard Medical School.
Dr. Sayed holds a B.S. in electrical engineering and computer sciences from University of California, Berkeley, an M.D. from the School of Medicine at University of California, San Francisco, and an M.B.A. from the Kellogg School of Management at Northwestern University.
107
Jeffrey Parry, Independent Director, Chair of the Nominating Committee and member of the of Audit Committee and Compensation Committee
Mr. Parry was appointed as an independent director in June 2023 and is the president of Mystic Marine Advisors LLC, a Connecticut based advisory firm he founded in 1998 focused on emerging and turnaround situations for strategic and financial stakeholders. Jeffrey served as Executive Chairman of TBS Shipping Limited from 2012 to 2018 where he led a successful restructuring and co-founded Valhalla Shipping, Inc with an $167 million equity investment by institutional investors. From July 2008 to October 2009, Mr. Parry was the Chief Executive Officer of Nasdaq-listed Aries Maritime Transport Limited and led a successful turn-around and sale to strategic investors. Mr. Parry was a Managing Director of Poten & Partners, an international energy advisor, from 2001 to 2007 where in 2006 he co-founded Poten Capital Services LLC, a New York based broker-dealer. Earlier in his career, Mr. Parry founded Cool FM and 7X Television in Athens, Greece and served as President of One Fifth Avenue Apartment Corporation. Since 2010, Jeffrey has served as an independent director of Nasdaq listed Globus Maritime Ltd. where he sits on the audit committee. Since 2022, Jeffrey has also become an independent director of Digitrax Entertainment Inc., a Tennessee based music technology start-up. Mr. Parry’s educational and professional experience in business, his background and familiarity in investment banking, his having served as a director of a company listed on Nasdaq makes him a qualified director candidate for our Company.
Jeff holds an MBA in Finance and Accounting from Columbia University and a B.A. in Literature from Brown University.
Crystal Muilenburg, Independent Director, Chair of the Compensation Committee and member of the of Audit Committee and Nominating Committee
Ms. Muilenburg was appointed as an independent director in June 2023 and has more than two decades of experience in marketing, corporate communications and public relations branding for global Fortune 500 companies and public start-ups. Most recently, Crystal served as Chief Marketing Officer for Evolus. While at Evolus from 2019 to 2023, Ms. Muilenburg also led human relations, operations and supply chain management. There, Ms. Muilenburg established Evolvus’s international business operations. Ms. Muilenburg was Head of Global Marketing for Sienna Biopharmaceuticals, a publicly-traded, clinical stage medical dermatology and aesthetics company from 2017 to 2019. Ms. Muilenburg has worked in the aesthetics industry since 2006 starting with Allergan (now AbbVie Inc.) where she held various domestic and international marketing and communications leadership roles from 2006 to 2017. We believe that Ms. Muilenburg’s service as our strategic advisor and her service in executive roles at several companies listed on U.S. exchanges makes her a qualified director candidate for our company.
Ms. Muilenburg is a regular speaker on topics including corporate reputation, product launches, diversity, equity, and inclusion and women’s leadership. She is the Orange County Healthcare Businesswoman’s Association mentor chair and a member of Chief—a private network for businesswomen.
Ms. Muilenburg holds a B.S. degree in Biology from California Polytechnic State University, San Luis Obispo.
Juliana Daley, CPA Independent Director, Chair of the Audit Committee and member of the of Compensation Committee and Nominating Committee
Ms. Daley was appointed as an independent director in June 2023 and holds over eleven years of accounting, controller, and financial reporting experience in the public sector. Ms. Daley has worked a variety of industries in both the United States and Canada. Since July 2021, Ms. Daley has served as Manager of Accounting at Anavex Life Sciences Corp. (NASDAQ: AVXL), a clinical-stage biopharmaceutical company based in New York, NY that is focused on developing treatments for debilitating neurodegenerative and neurodevelopmental diseases. In addition, from August 2021 to July 2022, she served as an independent director and audit committee chair to Vegano Foods (CSE: VAGN) during Vegano Food’s initial public offering in February 2022. From October 2015 to July 2021, Ms. Daley was a Manager of Financial Reporting and Advisory Services to various public companies in the United States and Canada, through her position with the accounting firm, Treewalk (previously ACM Management, Inc.). At Treewalk Ms. Daley assisted clients in meeting their quarterly and annual reporting requirements including the preparation of complete financial reporting packages and managing assurance engagements from start to finish. At Treewalk, she also served as chief financial officer to Makena Resources Inc. (CSE: MKNA) (April 2018 - April 2019) and Naked Brand Group Inc. (NASDAQ: NAKD) (March 2018 - June 2018) until the completion of their prospective mergers in April 2019 and June 2018, respectively. From September 2011 to April 2015, Ms. Daley was employed with Naked Brand Group Inc., where she worked in the accounting department, serving as controller from August 2013 until her departure in April 2015, and where she was also responsible for assisting in various operational functions including EDI implementation, ERP implementation, inventory management, information technology and office administration. From July 2021 to present, Ms. Daley has acted as manager of accounting at Anavex Life Sciences where she assists to in the finalization of all internal reporting, budgeting, and operational matters such as annual SOX audits, quarterly reviews, IT audits, and annual audits. Ms. Daley’s expertise in financial accounting for public companies and her having served as a chief financial officer and controller on companies listed on United States public exchanges makes her a qualified director candidate for our company.
108
In September 2015, Ms. Daley wrote her Common Final Examination (CFE) through the CPA Western School of Business and became designated in May 2016. She also holds a BBA in Accounting and Economics from the University of the Fraser Valley in Abbotsford, British Columbia.
Brenda Buechler, Chief Marketing Officer
Ms. Buechler is the Chief Marketing Officer for Elevai Labs. In this role, she leads the portfolio and brand marketing strategies to help Elevai achieve its commercial targets and support the global vision. Ms. Buechler has a strong track record in leadership in the pharmaceutical and medical aesthetics industries holding sales, marketing, and business development roles over the course of her 24-year career. Prior to her role with Elevai, Ms. Buechler led the Consumer and HCP marketing efforts for Evofem Biosciences from 2019 to 2022 and was responsible for the launch of a first-in-class technology in the women’s health space. Ms. Buechler spent 6 years in marketing and public relations roles with aesthetic industry leaders Alastin Skincare, from 2016 to 2019, and SkinMedica, from 2008 to 2010, where she helped to bring over 15 new, award-winning physician-dispensed skincare products to the market. From 2011 to 2016 Ms. Buechler held various Business Development and Client Services roles within the medical aesthetic and pharmaceutical industries touching over 50 brands throughout her career. Before her tenure in marketing and advertising, Ms. Buechler spent 10 years in pharmaceutical sales from 1997 to 2007 with Merck and Pfizer working across over a dozen disease states and therapeutic categories.
Ms. Buechler is currently an active member of Cosmetic Executive Women (CEW) and the Healthcare Businesswomen’s Association (HBA) where she is currently serving as a group mentor.
Ms. Buechler holds a Bachelor of Arts from San Diego State University.
Christoph Kraneiss, Chief Commercial Officer
Mr. Kraneiss has been our Chief Commercial Officer since August 2022. He holds a proven track record of building successful physician-dispensed aesthetic businesses within top globally recognized brands. Mr. Kraneiss served from 2017-2019 as Senior Vice President of SkinBetter Science, LLC. Similarly, from 2011 -2017, Mr. Kraneiss served as Senior Vice President of ZO Skin Health, Inc., which was founded by renowned dermatologist Zein Obagi, MD. During his 6 years at ZO Skin Health, he led his department from $2M in retail sales to over $100M, establishing the brand in more than 130 countries, and roughly 500,000 points of purchase globally, reaching profitability within less than 3 years. Most recently Mr. Kraneiss served as Managing Director for Noon Aesthetics, Inc. from 2019 - 2021 and as Vice President of International Business Development for Higher Education Skincare, Inc., a B2C company from 2021 – 2022. Mr. Kraneiss has successfully established and managed numerous aesthetic sales teams in different countries and has trained retailers, physicians, and distributors in sales and business strategies, specifically relating to servicing the medical aesthetics industry.
Mr. Kraneiss received both an MBA as well as a bachelor’s degree in marketing and communications from University of Saxony Anhalt and speaks English, German, and Russian fluently.
Director Independence and Financial Experts
Under the rules of the Nasdaq Stock Market, within one year of our initial public offering a majority of our Board members must qualify as independent directors if we are not a “controlled company.” Our proposed Board of Directors will include a majority of independent directors over our non-independent directors. Additional independent directors will be appointed to our Board of Directors immediately before or upon effectiveness of the registration statement of which this prospectus forms a part. At the time of the offering, our independent directors will be Jeffrey Parry, Crystal Muilenburg, Juliana Daley and [●]. The Board has determined the foregoing director are “independent” within the meaning of the corporate governance standards of Nasdaq Rule 5605 and meet the criteria for independence set forth in Rule 10A-3 of the Exchange Act, as such directors do not have a direct or indirect material relationship with our company. A material relationship is a relationship which could, in the view of our Board of Directors, be reasonably expected to interfere with the exercise of a director’s independent judgment. Our independent directors will have regularly scheduled meetings at which only independent directors are present.
Any affiliated transactions will be on terms no less favorable to us than could be obtained from independent parties. Our board of directors will review and approve all affiliated transactions with any interested director abstaining from such review and approval.
Juliana Daley qualifies as an “audit committee financial expert” as defined under the rules and regulations of the SEC.
Board Leadership Structure
The Board believes that it should maintain the flexibility to select the Chairman of the Board and adjust its board leadership structure from time to time. Our Chairman position is currently vacant. The Board determined that having its Chairman position vacant for the time being provides it with optimal flexible opportunity to fill the leadership position when the company is able to recruit additional board members and the vacancy is in its best interests and those of its stockholders. The Board believes that it will locate a Chairman with strategic vision, knowledge of our operations and the industry in which we operate qualify that person to serve as both Chair of the Board
109
Role of the Board in Risk Oversight
One of the key functions of the Board is informed oversight of our risk management process. The Board does not have a standing risk management committee, but rather administers this oversight function directly through the Board as a whole, as well as through various standing committees of the Board that address risks inherent in their respective areas of oversight. In particular, the Board is responsible for monitoring and assessing strategic risk exposure and our audit committee has the responsibility to consider and discuss our major financial risk exposures and the steps our management will take to monitor and control such exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. The audit committee also monitors compliance with legal and regulatory requirements. Our compensation committee also assesses and monitors whether our compensation plans, policies and programs comply with applicable legal and regulatory requirements. Our nominating and corporate governance committee monitors the effectiveness of our governance guidelines.
Board Committees
Prior to the consummation of this offering, the Board intends to establish an audit committee, a compensation committee and a nominating committee, each of which will have the composition and responsibilities described below. Members will serve on these committees until their resignation or until otherwise determined by the Board. Each committee will operate under a charter approved by the Board. Copies of each charter will be posted on the Corporate Governance section of our website at www.elevailabs.com. Our website and the information contained on, or that can be accessed through, our website is not deemed to be incorporated by reference in, and is not considered part of, this prospectus.
Audit Committee
Our audit committee will consist of [●], Jeffrey Parry, Crystal Muilenburg and Juliana Daley will serve as the audit committee chairperson. The Board has determined that [●], be Jeffrey Parry, Crystal Muilenburg and Juliana Daley each meet the requirements for independence and financial literacy under the current Nasdaq listing standards and SEC rules and regulations, including Rule 10A-3. In addition, the Board has determined that Juliana Daley qualifies as an “audit committee financial expert” within the meaning of Item 407(d) of Regulation S-K promulgated under the Securities Act. This designation does not impose any duties, obligations, or liabilities that are greater than are generally imposed on members of the audit committee and the Board. The audit committee will be responsible for, among other things:
| ● | selecting a qualified firm to serve as the independent registered public accounting firm to audit our financial statements; |
| ● | helping to ensure the independence and overseeing the performance of the independent registered public accounting firm; |
| ● | reviewing and discussing the scope and results of the audit with the independent registered public accounting firm and reviewing, with management and that firm, our interim and year-end operating results; |
| ● | reviewing our financial statements and critical accounting policies and estimates; |
| ● | reviewing the adequacy and effectiveness of our internal controls; |
| ● | developing procedures for employees to submit concerns anonymously about questionable accounting, internal accounting controls, or audit matters; |
| ● | overseeing our policies on risk assessment and risk management; |
| ● | overseeing compliance with our code of business conduct and ethics; |
| ● | reviewing related party transactions; and |
| ● | approving or, as permitted, pre-approving all audit and all permissible non-audit services (other than de minimis non-audit services) to be performed by the independent registered public accounting firm. |
The audit committee will operate under a written charter, which satisfies the applicable rules of the SEC and the listing standards of Nasdaq, and which will be available on our website. All audit services to be provided to us and all permissible non-audit services, other than de minimis non-audit services, to be provided to us by our independent registered public accounting firm will be approved in advance by the audit committee.
110
Compensation Committee
Our compensation committee will consist of Jeffrey Parry, Crystal Muilenburg and Juliana Daley and [●]. Crystal Muilenburg will be the chairperson of the compensation committee. The Board has determined that the composition of the compensation committee will meet the requirements for independence under the current Nasdaq listing standards and SEC rules and regulations. Each member of the committee is a non-employee director, as defined in Rule 16b-3 promulgated under the Exchange Act. The compensation committee will be responsible for, among other things:
| ● | reviewing, approving and determining, or making recommendations to the Board regarding, the compensation of our executive officers, including the Chief Executive Officer; |
| ● | making recommendations regarding non-employee director compensation to our full Board of Directors; |
| ● | administering our equity compensation plans and agreements with our executive officers; |
| ● | reviewing, approving and administering incentive compensation and equity compensation plans; and |
| ● | reviewing and approving our overall compensation philosophy. |
The compensation committee will operate under a written charter, which satisfies the applicable rules of the SEC and the listing standards of Nasdaq, and which will be available on our website.
Nominating Committee
Our nominating and corporate governance committee will consist of Jeffrey Parry, Crystal Muilenburg and Juliana Daley and [●]. Jeffery Parry will be the chairperson of the nominating and corporate governance committee. The Board has determined that the composition of the nominating and corporate governance committee will meet the requirements for independence under the current Nasdaq listing standards and SEC rules and regulations. The nominating and corporate governance committee will be responsible for, among other things:
| ● | identifying, evaluating and selecting, or making recommendations to the Board regarding nominees for election to the Board of Directors and its committees; |
| ● | considering and making recommendations to the Board regarding the composition of the Board of Directors and its committees; |
| ● | developing and making recommendations to the Board regarding corporate governance guidelines and matters; |
| ● | overseeing our corporate governance practices; |
| ● | overseeing the evaluation and the performance of the Board and individual directors; and |
| ● | contributing to succession planning. |
The nominating and corporate governance committee will operate under a written charter, which satisfies the applicable rules of the SEC and the Nasdaq listing standards and will be available on our website.
111
Compensation Committee Interlocks and Insider Participation
None of the members of the compensation committee is or has been at any time one of our officers or employees. None of our executive officers currently serves, or in the past fiscal year has served, as a member of the Board or compensation committee (or other Board of Directors committee performing equivalent functions or, in the absence of any such committee, the entire Board of Directors) of any entity that has one or more executive officers serving as a member of the Board or compensation committee.
Code of Ethics
We will adopt a Code of Ethics and Business Conduct that applies to our directors, officers and other employees prior to the consummation of the offering.
Limitation on Liability and Indemnification of Directors and Officers
The Certificate of Incorporation limits our directors’ liability to the fullest extent permitted under the Delaware General Corporation Law (the “DGCL”). The DGCL provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability:
| ● | for any transaction from which the director derives an improper personal benefit; |
| ● | for any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| ● | for any unlawful payment of dividends or redemption of shares; or |
| ● | for any breach of a director’s duty of loyalty to the corporation or its stockholders. |
If the DGCL is amended to authorize corporate action further eliminating or limiting the personal liability of directors, then the liability of our directors will be eliminated or limited to the fullest extent permitted by the DGCL, as so amended.
DGCL law and the Bylaws provide that we will, in certain situations, indemnify our directors and officers and may indemnify other employees and other agents, to the fullest extent permitted by law. Any indemnified person is also entitled, subject to certain limitations, to advancement, direct payment, or reimbursement of reasonable expenses (including attorneys’ fees and disbursements) in advance of the final disposition of the proceeding.
We maintain a directors’ and officers’ insurance policy pursuant to which our directors and officers are insured against liability for actions taken in their capacities as directors and officers. We believe these provisions in the Certificate of Incorporation, Bylaws and these indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, or control persons, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
112
Introduction
We are an emerging growth company, as defined in the JOBS Act. As an emerging growth company, we will be exempt from certain requirements related to executive compensation, including, but not limited to, the requirements to hold a nonbinding advisory vote on executive compensation and to provide information relating to the ratio of total compensation of our Chief Executive Officer to the median of the annual total compensation of all of our employees, each as required by the Investor Protection and Securities Reform Act of 2010, which is part of the Dodd-Frank Wall Street Reform and Consumer Protection Act.
This section provides an overview of our executive compensation programs, including a narrative description of the material factors necessary to understand the information disclosed in the summary compensation table below.
For the year ended 2022, our named executive officers (“Named Executive Officers” or “NEOs”) were:
| ● | Jordan R. Plews, Chief Executive Officer; |
| ● | Brenda Buechler, Chief Marketing Officer; and |
| ● | Christoph Kraneiss, Chief Commercial Officer |
The objective of our compensation program is to provide a total compensation package to each NEO that will enable us to attract, motivate and retain outstanding individuals, align the interests of our executive team with those of our equity holders, encourage individual and collective contributions to the successful execution of our short- and long-term business strategies and reward NEOs for performance.
Compensation of Directors and Named Executive Officers
The following table presents information regarding the total compensation (excluding equity-based compensation reported) awarded to, earned by, and paid to our NEOs for services rendered to us in all capacities for the years indicated.
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Option Awards ($)4 | All Other Compensation ($) | Total ($) | ||||||||||||||||
| Jordan R. Plews1 | 2022 | $ | 200,000 | $ | 10,000 | $ | - | - | $ | 210,000 | ||||||||||||
| CEO, President and Director | 2021 | $ | 50,000 | $ | $ | 50,993 | - | $ | 100,993 | |||||||||||||
| Brenda Buechler2 | 2022 | $ | 79,167 | $ | 5,000 | $ | 143,679 | - | $ | 227,846 | ||||||||||||
| Chief Marketing Officer | 2021 | $ | - | $ | - | $ | - | - | $ | - | ||||||||||||
| Christoph Kraneiss3 | 2022 | $ | 75,000 | $ | - | $ | 121,227 | - | $ | 196,227 | ||||||||||||
| Chief Commercial Officer | 2021 | $ | - | $ | - | $ | - | - | $ | - | ||||||||||||
| 1 | Dr. Jordan R. Plews has served as our Chief Executive Officer since October 1, 2021. |
| 2 | Brenda Buechler has served as our Chief Marketing Officer since August 1, 2022. |
| 3 | Christoph Kraneiss has served as our Chief Commercial Officer since August 8, 2022. |
| 4 | The options granted vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter. As of the date of this prospectus, no options have been exercised by the NEOs. |
113
Employment Arrangements with Named Executive Officers
Jordan R. Plews
In September 2021, we entered into an employment contract with Dr. Jordan R. Plews as the Company’s Chief Executive Officer, effective as of October 1, 2021.
The agreement is at will and subject to termination prior to completion of the services at any time by us, or with 14 days’ prior written notice by Dr. Plews and for any reason not prohibited by law.
Pursuant to the terms and provisions of the agreement: (a) Dr. Plews was appointed as our Chief Executive Officer and undertook and performed the duties and responsibilities normally and reasonably associated with such office; and (b) we agreed to pay Dr. Plews an annual salary of $200,000 in addition to equity compensation in the form of stock options in accordance with our 2020 Equity Incentive Plan, as amended.
Brenda Buechler
In June 2022, we entered into an employment contract with Brenda Buechler as the Company’s Chief Marketing Officer, effective as of August 1, 2022.
The agreement is at will and subject to termination prior to completion of the services at any time by us, or with 14 days’ prior written notice by Ms. Buechler and for any reason not prohibited by law.
Pursuant to the terms and provisions of the agreement: (a) Ms. Buechler was appointed as our Chief Marketing Officer and undertook and performed the duties and responsibilities normally and reasonably associated with such office; and (b) we agreed to pay Ms. Buechler an annual salary of $190,000 in addition to equity compensation in the form of stock options in accordance with our 2020 Equity Incentive Plan, as amended. except that 25% of those stock-options shall not vest and become exercisable until the first anniversary of the grant date and, thereafter, the options shall vest at a rate of 25% per annum and become exercisable with respect to 100% of the shares subject to the option on the fourth anniversary of the grant date.
Christoph Kraneiss
In August 2022, we entered into an employment contract with Christoph Kraneiss as the Company’s Chief Commercial Officer, effective as of August 8, 2022.
The agreement is at will and subject to termination prior to completion of the services at any time by us, or with 14 days’ prior written notice by Mr. Kraneiss and for any reason not prohibited by law.
Pursuant to the terms and provisions of the agreement: (a) Mr. Kraneiss was appointed as our Chief Commercial Officer and undertook and performed the duties and responsibilities normally and reasonably associated with such office; and (b) we agreed to pay Mr. Kraneiss an annual salary of $180,000 in addition to equity compensation in the form of stock options in accordance with our 2020 Equity Incentive Plan, as amended. except that 25% of those stock-options shall not vest and become exercisable until the first anniversary of the grant date and, thereafter, the options shall vest at a rate of 25% per annum and become exercisable with respect to 100% of the shares subject to the option on the fourth anniversary of the grant date.
Outstanding Equity Awards at 2022 Fiscal Year-End for Named Executive Officers of Elevai Labs, Inc.
As of December 31, 2022, the following outstanding equity awards in the form of nonstatutory stock options to our NEOs are as follows:
| NEO | Outstanding | Vested | ||||||
| Jordan R. Plews | 200,000 | - | ||||||
| Brenda Buechler | 110,000 | - | ||||||
| Christoph Kraneiss | 100,000 | - | ||||||
Director Compensation
We did not pay our directors for their services as our directors in our fiscal years 2021 and 2022.
We intend to and have agreed to compensate our independent directors for their service as directors through a mix of cash and stock options. In addition to in-person attendance bonuses, we intend to reimburse our non-employee directors for reasonable travel and out-of-pocket expenses incurred in connection with attending board of director and committee meetings.
114
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Other than employment and other agreements set out elsewhere in this prospectus, the following summarizes those of transactions since December 31, 2020 to which we have been a participant in which the amount involved exceeded or will exceed $25,000, and in which any of our directors, executive officers or beneficial owners of more than 5% of our capital stock or any member of the immediate family of any of the foregoing persons had or will have a direct or indirect material interest.
The Company paid consulting fees of $95,078 and $46,919 to GB Capital Ltd., a company controlled by Graydon Bensler, CFO and Director in 2022 and 2021, respectively.
On June 24, 2020, we issued 8,480,000 shares of Common Stock to the founders of the Company for total consideration of $50,880.
On February 9, 2021, the Company granted 800,000 stock options to four related parties (200,000 stock options each) with a contractual life of ten years and exercise price of $0.60 per share of common stock. These stock options were valued at $203,972 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter. Details of the fair value granted to each individual and the related expense recorded for the year ended December 31, 2022 and 2021 are as follow:
| December 31, 2022 | December 31, 2021 | Fair value of stock options granted | ||||||||||
| Braeden Lichti, Former Chairman and President | $ | 14,181 | $ | 28,170 | $ | 50,993 | ||||||
| Graydon Bensler, CFO and Director | 14,181 | 28,170 | 50,993 | |||||||||
| Jordan R. Plews, CEO, President and Director | 14,181 | 28,170 | 50,993 | |||||||||
| Tim Sayed, Chief Medical Officer | 14,181 | 28,170 | 50,993 | |||||||||
| $ | 56,724 | $ | 112,680 | $ | 203,972 | |||||||
BWL Investments Ltd., a British Columbia Canadian Corporation (“BWL”) owned and managed by Braeden Lichti and Tim Sayed, Chief Medical Officer subscribed to $48,980 and $10,000 in promissory notes, respectively. On July 15,2022, these promissory notes and accrued interest were converted into Series A preferred shares and warrants as follows:
| Series A preferred shares | Warrants | Promissory notes and accrued interest | ||||||||||
| BWL Investments Ltd. | 61,551 | 61,551 | $ | 49,538 | ||||||||
| Tim Sayed, Chief Medical Officer | 12,563 | 12,563 | 10,112 | |||||||||
| 74,114 | 74,114 | $ | 59,650 | |||||||||
On July 20, 2021, the Company granted 200,000 stock options to a former director and related party, Yi Guo, with a contractual life of ten years and exercise price of $0.60 per share of common stock. These stock options were valued at $51,014 using the Black-Scholes Option Pricing Model. The options vested 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter. The share-based compensation expense recorded for the year ended December 31, 2021, relating to these stock options was $14,516. Mr. Guo resigned in fiscal year 2022 and as a result, all unvested stock options at the time of his resignation were forfeited.
Pursuant to an advisory board agreement between us and Jeffery Parry, (an independent director to the Company as of June 1, 2023) dated August 12, 2021, on August 16, 2021, the Company granted Mr. Parry equity compensation in the form of nonstatutory stock options to purchase 41,667 shares of the Company’s Common Stock. Under an amended advisory board agreement between us and Jeffery Parry dated September 30, 2022 additional nonstatutory stock options to purchase 16,000 shares of the Company’s Common Stock were granted to Mr. Parry. The stock options held a contractual life of ten years and exercise price of $0.60 per common stock. These stock options were valued at $10,630 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter. Through unanimous written consent, the Board of the Company amended the vesting schedule for those stock options to accelerate the vesting of such stock options so that those stock options fully vested as of December 3, 2022. On December 16, 2022, Mr. Parry exercised all 41,667 stock options for a total exercise price of $25,000.20. On June 1, 2023, we terminated the advisory board agreement between us and Jeffery Parry.
115
As amended and agreed to on May 1, 2023, and as effective on January 4, 2022, we entered into a consulting agreement (the “CA”) with NorthStrive Companies Inc., a California Corporation (“NorthStrive”) owned and managed by Braeden Lichti. Pursuant to the CA, NorthStrive is to assist us in a variety of business matters, including assistance in our overall investor outreach and communications strategy, and advising us on becoming a “public” company. As of May 31, 2023, the Company had $192,705 (2022 - $120,000, 2021 - $23,520) due to NorthStrive, of which $22,705 (2021 - $23,520) is unsecured, non-interest bearing and are due on demand. $120,000 was due as of December 31, 2022, and the remaining $50,000 was due as of May 31, 2022. The aforementioned fees are due in contemplation for NorthStrive’s advisement under the CA, whereby starting on January 4, 2022, we agreed to compensate NorthStrive $10,000 per month (the “Compensation”). We retain the option, but not the obligation to issue the amount of Compensation due NorthStrive in shares of our Common Stock equal to our series A preferred stock price at $1.34138 per share equal to the value of the Compensation due to NorthStrive for services provided through and up to March 31, 2023 and $3.00 per share equal to the value of the Compensation due to NorthStrive for services provided after March 31, 2023 or via cash payment equal to the amount of Compensation outstanding however, that Compensation due NorthStrive shall accrue interest-free and payment of that Compensation has been deferred until the earlier of either (a) our raising an aggregate of at least US$2,000,000 of equity and/or debt investment from and after October 1, 2022, (b) our becoming listed on any established stock exchange or a national market system, or (c) a determination by our Board that Company has sufficient cash flows to support payment of the Compensation due to NorthStrive at the time of that determination.
On May 1, 2023, as effective on February 1, 2023, we entered into an advisory agreement (the “AA”) with Braeden Litchi which terminates after twenty-two months to strategically assist us in our maintenance of board governance, director recruitment, and direction for our board of directors strategy sessions. The AA was entered into under contemplation of Mr. Litchi’s resignation from our Board effective February 1, 2023, and our desire to maintain Mr. Litchi’s compensation as a valuable advisor to us. Pursuant to the AA, we agreed with Mr. Litchi that in exchange for services under the AA, his options granted on February 9, 2021, to purchase 200,000 shares of our Common Stock under our 2020 Equity Incentive Plan shall continue to vest pursuant to the aforementioned terms under this section.
Prior to our reorganization, BWL Investments Ltd., a British Columbia Canadian Corporation (“BWL”) also owned and managed by Braeden Lichti, owned approximately 29.4% of our issued and outstanding shares of common stock and 100% of the equity interests in Reactive Labs. On June 4, 2021, we issued 100 shares of common stock to BWL in in exchange for substantially all of the assets and liabilities of Reactive Labs.
Braeden Lichti is one of our co-founders and our former chairman and director. He is the current chief executive officer of NorthStrive and BWL, as described herein and may be deemed a “promoter” as defined by Rule 405 of the Securities Act though we elect to refer to him as a “founder” or “organizer” as permitted under Rule 405. There are no other promoters of our company.
In May, and December of 2022, we granted nonstatutory stock options to purchase 250,000 shares of the Company’s common stock to Brenda Buechler, our Chief Marketing Officer, and Christoph Kraneiss, our Chief Commercial Officer. The options maintain a contractual life of ten years and weighted average exercise price of $1.22 per share of common stock. These stock options were valued at $264,906 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter. Details of the fair value granted to each individual and the related expense recorded for the year ended December 31, 2022, are as follow:
| December 31, 2022 | Fair value of stock options granted | |||||||
| Brenda Buechler, Chief Marketing Officer | $ | 43,488 | $ | 143,679 | ||||
| Christoph Kraneiss, Chief Commercial Officer | 28,344 | 121,227 | ||||||
| $ | 71,832 | $ | 264,906 | |||||
On June 1, 2023, we rescinded previously granted but unissued nonstatutory stock options to each of our independent director nominees and instead granted nonstatutory stock options to purchase 240,000 shares of the Company’s common stock to our then independent director nominees and related parties Jeffery Parry, Crystal Muilenburg and Julianna Daley under our 2021 Equity Incentive Plan. The equity compensation grants were directly in relation to the appointment of Mr. Parry, Ms. Daley and Ms. Muilenburg as our independent directors. The options maintain a contractual life of ten years and an exercise price of $5.00 per share of common stock. All options vest at a rate of 25% on the first anniversary of the date of grant and the remaining 75% vest evenly over 36 months thereafter.
116
BENEFICIAL OWNERSHIP OF SHARES
The following tables set forth information regarding the beneficial ownership of our Common Stock and Preferred Stock as of the date of this prospectus on a pre-offering and a post offering basis, for:
| ● | each of our directors and executive officers; |
| ● | all directors and executive officers as a group; and |
| ● | each person who is known to us to own beneficially more than 5% of its Common Stock. |
Beneficial ownership is determined according to the rules of the Commission, which generally provide that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power over that security, including options and warrants that are currently exercisable or exercisable within 60 days. In computing the number of shares of Common Stock beneficially owned by a person and the percentage ownership, we deemed outstanding shares of our Common Stock subject to options and warrants held by that person that are currently exercisable or exercisable within 60 days of the Closing Date. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person.
The percentage ownership of Common Stock reflected below is based on [●] shares of Common Stock outstanding as of the date of this prospectus. The percentage of beneficial ownership prior to this offering in the table below is based on [●]shares of common stock deemed to be outstanding as of July [●], 2023, assuming the conversion of all outstanding shares of our preferred stock into shares of our common stock simultaneously with the completion of this offering (assuming an offering price of $[●] per share, based on the midpoint of the price range on the cover page of this prospectus) and excludes any shares that may be issued if the underwriter exercises its over-allotment option and in each case upon the closing of this offering, and the percentage of beneficial ownership at this offering in the table below is based on [●] shares of Common Stock assumed to be outstanding after the closing of the offering.
Information with respect to beneficial ownership has been furnished by each director, officer or beneficial owner of more than 5% of either Common Stock. Beneficial ownership is determined in accordance with the rules of the SEC and generally requires that such person have voting or investment power with respect to securities. Except as otherwise indicated in the footnotes to the following table, or as required by applicable community property laws, all persons and entities listed have sole voting and investment power for all Common Stock shown as beneficially owned by them. Unless otherwise indicated in the footnotes, the address for each principal stockholder is in the care of our Company at 120 Newport Center Drive, Ste. 250, Newport Beach, CA 92660.
117
As of the date of this prospectus, hereof, we have fifty-nine stockholders of record.
| Number of Shares | Percentage of Shares Outstanding Beneficially Owned | |||||||||||
| Name of Beneficial Owner | Beneficially Owned | Before Offering | After Offering | |||||||||
| 5% or Greater Stockholders | ||||||||||||
| BWL Investments Ltd. (1) | 3,823,965 | 20.24 | % | % | ||||||||
| GB Capital Ltd. (2) | 841,454 | 4.70 | % | % | ||||||||
| JP Bio Consulting LLC(3) | 2,851,454 | 15.92 | % | % | ||||||||
| Hatem Abou-Sayed MD MBA FACS, a Professional Medical Corporation(4) | 1,359,342 | 7.59 | % | |||||||||
| Hongyu Wang | 1,708,406 | 9.54 | % | |||||||||
| Directors, Named Executive Officers and Other Executive Officers: | ||||||||||||
| Jordan R. Plews, Chief Executive Officer and Director | 3,051,454 | 17.04 | % | % | ||||||||
| Graydon Bensler, Chief Financial Officer and Director | 1,041,454 | 5.82 | % | % | ||||||||
| Brenda Buechler, Chief Marketing Officer | 153,727 | * | ||||||||||
| Christoph Kraneiss, Chief Commercial Officer | 100,000 | * | ||||||||||
| Hatem Abou-Sayed, Chief Medical Officer and Director | 1,571,905 | 8.78 | % | % | ||||||||
| Jeffrey Parry, Director | 137,667 | * | ||||||||||
| Crystal Muilenburg, Director | 80,000 | * | ||||||||||
| Juliane Daley, Director | 81,200 | * | ||||||||||
| All executive officers and directors as a group (8 persons) | 5,664,813 | 31.64 | % | % | ||||||||
| * | Denotes less than one (1%) percent |
| † | Unless otherwise indicated, the business address of each of the individuals is our address of c/o Elevai Labs, Inc., 120 Newport Center Drive, Ste. 250, Newport Beach, CA 92660. |
| (1) | Braeden Lichti has sole voting and dispositive power over the shares held by BWL Investments Ltd. |
| (2) | Graydon Bensler has sole voting and dipositive power over the shares held by GB Capital Ltd. |
| (3) | Jordan R. Plews has sole voting and dipositive power over the shares held by JP Bio Consulting LLC. |
| (4) | Hatem Abou-Sayed has sole voting and dipositive power over the shares held by Hatem Abou-Sayed MD MBA FACS, a Professional Medical Corporation |
118
CERTAIN UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS
The following discussion is a summary of certain United States federal income tax considerations generally applicable to the ownership and disposition of our shares. This summary is based upon United States federal income tax law as of the date of this prospectus, which is subject to change or differing interpretations, possibly with retroactive effect. This summary does not discuss all aspects of United States federal income taxation that may be important to particular investors in light of their individual circumstances, including investors subject to special tax rules (e.g., financial institutions, insurance companies, broker-dealers, dealers or traders in securities, tax-exempt organizations (including private foundations), taxpayers that have elected mark-to-market accounting, S corporations, regulated investment companies, real estate investment trusts, passive foreign investment companies, controlled foreign corporations, United States Holders (as defined below) that will hold our shares as part of a straddle, hedge, conversion, or other integrated transaction for United States federal income tax purposes, expatriates or former long-term residents of the United States, or investors that have a functional currency other than the United States dollar), all of whom may be subject to tax rules that differ materially from those summarized below. This summary does not discuss other United States federal tax consequences (e.g., estate or gift tax), any state, local, or non-United States tax considerations or the Medicare tax or alternative minimum tax. In addition, this summary is limited to investors that will hold our securities as “capital assets” (generally, property held for investment) under the Internal Revenue Code of 1986, as amended (the “Code”) and that acquire our shares for cash pursuant to this prospectus. No ruling from the Internal Revenue Service, (the “IRS”) has been or will be sought regarding any matter discussed herein. No assurance can be given that the IRS would not assert, or that a court would not sustain a position contrary to any of the tax aspects set forth below.
For purposes of this summary, a “United States Holder” is a beneficial holder of securities who or that, for United States federal income tax purposes is:
| ● | an individual who is a United States citizen or resident of the United States; |
| ● | a corporation or other entity treated as a corporation for United States federal income tax purposes created in, or organized under the law of, the United States or any state or political subdivision thereof; |
| ● | an estate the income of which is includible in gross income for United States federal income tax purposes regardless of its source; or |
| ● | a trust (A) the administration of which is subject to the primary supervision of a United States court and which has one or more United States persons (within the meaning of the Code) who have the authority to control all substantial decisions of the trust or (B) that has in effect a valid election under applicable Treasury regulations to be treated as a United States person. |
119
A “non-United States Holder” is a beneficial holder of securities who or that is neither a United States Holder nor a partnership for United States federal income tax purposes.
If a partnership (including an entity or arrangement treated as a partnership for United States federal income tax purposes) holds our securities, the tax treatment of a partner, member or other beneficial owner in such partnership will generally depend upon the status of the partner, member or other beneficial owner, the activities of the partnership and certain determinations made at the partner, member or other beneficial owner level. If you are a partner, member or other beneficial owner of a partnership holding our securities, you are urged to consult your tax advisor regarding the tax consequences of the ownership and disposition of our securities.
THIS DISCUSSION OF UNITED STATES FEDERAL INCOME TAX CONSIDERATIONS IS FOR GENERAL INFORMATION PURPOSES ONLY AND IS NOT TAX ADVICE. PROSPECTIVE HOLDERS SHOULD CONSULT THEIR TAX ADVISORS CONCERNING THE UNITED STATES FEDERAL INCOME TAX CONSEQUENCES TO THEM OF OWNING AND DISPOSING OF OUR SECURITIES, AS WELL AS THE APPLICATION OF ANY, STATE, LOCAL AND NON-UNITED STATES. INCOME, ESTATE AND OTHER TAX CONSIDERATIONS.
United States Holders
Taxation of Distributions
If we pay distributions or make constructive distributions (other than certain distributions of our stock or rights to acquire our stock) to United States Holders of our shares, such distributions generally will constitute dividends for United States federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under United States federal income tax principles. Distributions in excess of our current and accumulated earnings and profits will constitute a return of capital that will be applied against and reduce (but not below zero) the United States Holder’s adjusted tax basis in our shares. Any remaining excess will be treated as gain realized on the sale or other disposition of our shares and will be treated as described under “United States Holders—Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Shares” below.
Dividends we pay to a United States Holder that is a taxable corporation will generally qualify for the dividends received deduction if the requisite holding period is satisfied. With certain exceptions (including dividends treated as investment income for purposes of investment interest deduction limitations), and provided certain holding period requirements are met, dividends we pay to a non-corporate United States Holder will generally constitute “qualified dividends” that will be subject to tax at the maximum tax rate accorded to long-term capital gains. If the holding period requirements are not satisfied, a corporation may not be able to qualify for the dividends received deduction and would have taxable income equal to the entire dividend amount, and non-corporate holders may be subject to tax on such dividend at ordinary income tax rates instead of the preferential rates that apply to qualified dividend income.
Gain or Loss on Sale, Taxable Exchange or Other Taxable Disposition of Shares
A United States Holder generally will recognize gain or loss on the sale, taxable exchange or other taxable disposition of our shares. Any such gain or loss will be capital gain or loss and will be long-term capital gain or loss if the United States Holder’s holding period for our shares so disposed of exceeds one year. The amount of gain or loss recognized will generally be equal to the difference between (1) the sum of the amount of cash and the fair market value of any property received in such disposition and (2) the United States Holder’s adjusted tax basis in its shares so disposed of. A United States Holder’s adjusted tax basis in its shares will generally equal the United States Holder’s acquisition cost for such shares (or, in the case of shares received upon exercise of a Warrant, the United States Holder’s initial basis for such shares, as discussed below), less any prior distributions treated as a return of capital. The deductibility of capital losses is subject to limitations. Long-term capital gains recognized by non-corporate United States Holders are generally eligible for reduced rates of tax. If the United States Holder’s holding period for the shares so disposed of is one year or less, any gain on a sale or other taxable disposition of the shares would be subject to short-term capital gain treatment and would be taxed at ordinary income tax rates. The deductibility of capital losses is subject to limitations.
120
Information Reporting and Backup Withholding.
In general, information reporting requirements may apply to dividends paid to a United States Holder and to the proceeds of the sale or other disposition of our shares, unless the United States Holder is an exempt recipient. Backup withholding may apply to such payments if the United States Holder fails to provide a taxpayer identification number, a certification of exempt status or has been notified by the IRS that it is subject to backup withholding (and such notification has not been withdrawn).
Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules will be allowed as a credit against a United States Holder’s United States federal income tax liability and may entitle such holder to a refund, provided the required information is timely furnished to the IRS.
Non-United States Holders
This section applies to you if you are a “Non-United States Holder.” As used herein, the term “Non-United States Holder” means a beneficial owner of our securities who or that is for United States federal income tax purposes:
| ● | a non-resident alien individual (other than certain former citizens and residents of the United States subject to United States tax as expatriates); |
| ● | a foreign corporation; or |
| ● | an estate or trust that is not a United States Holder; |
but generally does not include an individual who is present in the United States for 183 days or more in the taxable year of the disposition. If you are such an individual, you should consult your tax advisor regarding the United States federal income tax consequences of the acquisition, ownership or sale or other disposition of our securities.
Taxation of Distributions.
In general, any distributions we make to a Non-United States Holder of shares, to the extent paid out of our current or accumulated earnings and profits (as determined under United States federal income tax principles), will constitute dividends for United States federal income tax purposes and, provided such dividends are not effectively connected with the Non-United States Holder’s conduct of a trade or business within the United States, we will be required to withhold tax from the gross amount of the dividend at a rate of 30%, unless such Non-United States Holder is eligible for a reduced rate of withholding tax under an applicable income tax treaty and provides proper certification of its eligibility for such reduced rate (usually on an IRS Form W-8BEN or W-8BEN-E). Any distribution not constituting a dividend will be treated first as reducing (but not below zero) the Non-United States Holder’s adjusted tax basis in its shares and, to the extent such distribution exceeds the Non-United States Holder’s adjusted tax basis, as gain realized from the sale or other disposition of our securities, which will be treated as described under “Non-United States Holders - Gain on Sale, Taxable Exchange or Other Taxable Disposition of Shares” below. In addition, if we determine that we are likely to be classified as a “United States real property holding corporation” (see “Non-United States Holders - Gain on Sale, Taxable Exchange or Other Taxable Disposition of Shares” below), we generally will withhold 15% of any distribution that exceeds our current and accumulated earnings and profits.
The withholding tax generally does not apply to dividends paid to a Non-United States Holder who provides a Form W-8ECI, certifying that the dividends are effectively connected with the Non-United States Holder’s conduct of a trade or business within the United States. Instead, the effectively connected dividends will be subject to regular United States federal income tax as if the Non-United States Holder were a United States resident, subject to an applicable income tax treaty providing otherwise. A corporate Non-United States Holder receiving effectively connected dividends may also be subject to an additional “branch profits tax” imposed at a rate of 30% (or a lower applicable treaty rate).
121
Gain on Sale, Taxable Exchange or Other Taxable Disposition of Shares.
A Non-United States Holder generally will not be subject to United States federal income or withholding tax in respect of gain recognized on a sale, taxable exchange or other taxable disposition of our securities unless:
| ● | the gain is effectively connected with the conduct by the Non-United States Holder of a trade or business within the United States (and, under certain income tax treaties, is attributable to a United States permanent establishment or fixed base maintained by the Non-United States Holder); or | |
| ● | we are or have been a “United States real property holding corporation” for United States federal income tax purposes at any time during the shorter of the five-year period ending on the date of disposition or the period that the Non- United States Holder held our securities, and, in the case where our shares are regularly traded on an established securities market, the Non-United States Holder has owned, directly or constructively, more than 5% of our shares at any time within the shorter of the five- year period preceding the disposition or such Non-United States Holder’s holding period for our shares. There can be no assurance that our shares will be treated as regularly traded on an established securities market for this purpose. |
Unless an applicable treaty provides otherwise, gain described in the first bullet point above will be subject to tax at generally applicable United States federal income tax rates as if the Non-United States Holder were a United States resident. Any gains described in the first bullet point above of a Non-United States Holder that is treated as a foreign corporation for United States federal income tax purposes may also be subject to an additional “branch profits tax” imposed at a 30% rate (or lower treaty rate).
If the second bullet point above applies to a Non-United States Holder, gain recognized by such Holder on the sale, exchange or other disposition of our shares will be subject to tax at generally applicable United States federal income tax rates. In addition, a buyer of our shares from such holder may be required to withhold United States federal income tax at a rate of 15% of the amount realized upon such disposition. We will be classified as a United States real property holding corporation if the fair market value of our “United States real property interests” equals or exceeds 50% of the sum of the fair market value of our worldwide real property interests plus our other assets used or held for use in a trade or business, as determined for United States federal income tax purposes. We do not expect to be a United States real property holding corporation immediately after the business combination is completed.
Information Reporting and Backup Withholding.
Information returns will be filed with the IRS in connection with payments of dividends and the proceeds from a sale or other disposition of our shares. A Non-United States Holder may have to comply with certification procedures to establish that it is not a United States person in order to avoid information reporting and backup withholding requirements. The certification procedures required to claim a reduced rate of withholding under a treaty generally will satisfy the certification requirements necessary to avoid the backup withholding as well. Backup withholding is not an additional tax. The amount of any backup withholding from a payment to a Non-United States Holder will be allowed as a credit against such holder’s United States federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
122
Foreign Account Tax Compliance Act Withholding Taxes.
Provisions commonly referred to as “FATCA” generally impose withholding at a rate of 30% on payments of dividends (including constructive dividends) in respect to our securities which are held by or through certain foreign financial institutions” (which is broadly defined for this purpose and in general includes investment vehicles) and certain other non-United States entities unless various United States information reporting and due diligence requirements (generally relating to ownership by United States persons of interests in or accounts with those entities) have been satisfied by, or an exemption applies to, the payee (typically certified as to by the delivery of a properly completed IRS Form W-8BEN-E). Foreign financial institutions located in jurisdictions that have an intergovernmental agreement with the United States governing FATCA may be subject to different rules. Accordingly, the entity through which our shares are held will affect the determination of whether such withholding is required. Similarly, dividends in respect of our shares held by an investor that is a non-financial Non-United States entity that does not qualify under certain exceptions will generally be subject to withholding at a rate of 30% unless such entity either (1) certifies to us or the applicable withholding agent that such entity does not have any “substantial United States owners” or (2) provide certain information regarding the entity’s “substantial United States owners” which will in turn be provided to the United States Department of Treasury. Under certain circumstances, a Non-United States Holder might be eligible for refunds or credits of such withholding taxes, and a Non-United States Holder might be required to file a United States federal income tax return to claim such refunds or credits.
Thirty percent withholding under FATCA was scheduled to apply to payments of gross proceeds from the sale or other disposition of property that produces United States-source interest or dividends beginning on January 1, 2019, but on December 13, 2018, the IRS released proposed regulations that, if finalized in their proposed form, would eliminate the obligation to withhold on gross proceeds. Such proposed regulations also delayed withholding on certain other payments received from other foreign financial institutions that are allocable, as provided for under final Treasury Regulations, to payments of United States-source dividends, and other fixed or determinable annual or periodic income. Although these proposed Treasury Regulations are not final, taxpayers generally may rely on them until final Treasury Regulations are issued. Prospective investors should consult their tax advisors regarding the effects of FATCA on their investment in our securities.
Possible Legislative Tax Changes
The foregoing summary of federal income tax law reflects provisions of recent legislation. However, because, Treasury Regulations and other official interpretations have not been issued with respect to a number of such provisions, their meaning is uncertain. In addition, legislation has been or may be proposed in Congress that might have a substantial and adverse effect on U.S. and Non-United States Holders. United States and Non-United States Holders should consult with their own professional advisers as to all current and possible future proposals with respect to federal, state and local tax legislation and the effect, if any, that such legislation may have on an investment in our common stock. In addition, the United States federal income tax rate (and any other applicable tax rates) may increase during the ownership of the common stock and negatively affect the after-tax returns of the United States and Non-United States Holders. Among other proposed tax changes, the current United States presidential administration has proposed increasing the United States corporate income tax from its current 21% rate.
123
SECURITIES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our Common Stock, and a liquid trading market for our common stock may not develop or be sustained after this offering. Future sales of our common stock, including shares issued upon the exercise of outstanding options or warrants, in the public market after the closing of this offering, or the perception that those sales may occur, could adversely affect the prevailing market price for our common stock from time to time or impair our ability to raise equity capital in the future.
Based on the number of shares of common stock outstanding as immediately prior to the closing of this offering and assuming (i) the automatic conversion of all our outstanding convertibles notes into an aggregate of [●] shares of our common stock (assuming an initial public offering price of $[●] per share, the midpoint of the price range set forth on the cover page of this prospectus) (ii) no exercise of the underwriters’ option to purchase additional shares of common stock and/or warrants, and (v) no exercise of outstanding options to purchase [●] shares of our common stock, upon completion of this offering, we will have an outstanding aggregate of [●] shares of Common Stock. All of the shares sold in this offering will be freely tradable unless purchased by our “affiliates” as such term is defined in Rule 144 under the Securities Act or purchased by existing stockholders and their affiliated entities that are subject to lock-up agreements. All remaining shares of common stock held by existing stockholders simultaneously with the closing of this offering will be “restricted securities,” as such term is defined in Rule 144. These restricted securities were issued and sold in private transactions and are eligible for public sale only if registered under the Securities Act or if they qualify for an exemption from registration under the Securities Act, including the exemptions provided by Rule 144 or Rule 701 of the Securities Act, which rules are summarized below.
As a result of the lock-up agreements referred to below and the provisions of Rule 144 and Rule 701 under the Securities Act, based on the number of shares of our common stock outstanding as of the date of this prospectus, the remaining shares of our common stock will generally become eligible for sale in the public market are as follows:
| Approximate Number of Shares | First Date Available for Sale on the Public Markets | |
| [●] Shares | 181 days after the date of this prospectus, upon expiration of the lock-up agreements referred to below, subject in some cases to applicable volume, manner of sale and other limitations under Rule 144 and Rule 701. |
We may issue shares of common stock from time to time as consideration for future acquisitions, investments or other corporate purposes.
In the event that any such acquisition, investment or other transaction is significant, the number of shares of common stock that we may issue may in turn be significant. We may also grant registration rights covering those shares of common stock issued in connection with any such acquisition and investment.
In addition, the shares of common stock reserved for future issuance under the 2020 Equity Incentive Plan will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, the lock-up agreements, a registration statement under the Securities Act or an exemption from registration, including Rule 144 and Rule 701.
124
Rule 144
Under Rule 144, as currently in effect, once we have been subject to the public company reporting requirements of the Exchange Act for at least 90 days, and we are current in our Exchange Act reporting at the time of sale, a person (or persons whose shares are required to be aggregated) who is not deemed to have been one of our “affiliates” for purposes of Rule 144 at any time during the three months preceding a sale and who has beneficially owned restricted securities within the meaning of Rule 144 for at least six months, including the holding period of any prior owner other than one of our “affiliates,” is entitled to sell those shares in the public market (subject to lock-up agreements, if applicable) without complying with the manner of sale, volume limitations or notice provisions of Rule 144, but subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than “affiliates,” then such person is entitled to sell such shares in the public market without complying with any of the requirements of Rule 144 (subject to any lock-up agreement, if applicable).
In general, under Rule 144, as currently in effect, once we have been subject to the public company reporting requirements of the Exchange Act for at least 90 days, our “affiliates,” as defined in Rule 144, who have beneficially owned the shares proposed to be sold for at least six months, are entitled to sell in the public market, upon expiration of any applicable lock-up agreements and within any three-month period, a number of those shares of our common stock that does not exceed the greater of:
| ● | 1% of the number of shares of common stock then outstanding, which will equal approximately [●] shares of common stock immediately upon the closing of this offering (assuming no exercise of the underwriter’s option to purchase additional shares and no exercise of outstanding options or warrants subsequent to [●], 2023); or |
| ● | the average weekly trading volume of our common stock on during the four calendar weeks preceding the filing of a notice on Form 144 with respect to such sale. |
Such sales under Rule 144 by our “affiliates” or persons selling shares on behalf of our “affiliates” are also subject to certain manner of sale provisions, notice requirements and requirements related to the availability of current public information about us. Notwithstanding the availability of Rule 144, the holders of substantially all of our restricted securities have entered into lock-up agreements as referenced above and their restricted securities will become eligible for sale (subject to the above limitations under Rule 144) upon the expiration of the restrictions set forth in those agreements.
Rule 701
In general, under Rule 701 as currently in effect, any of our employees, directors, officers, consultants or advisors who acquired common stock from us in connection with a written compensatory stock or option plan or other written agreement in compliance with Rule 701 before the effective date of the registration statement of which this prospectus is a part (to the extent such common stock is not subject to a lock-up agreement) and who are not our “affiliates” as defined in Rule 144 during the immediately preceding 90 days, is entitled to rely on Rule 701 to resell such shares beginning 90 days after the date of this prospectus in reliance on Rule 144, but without complying with the notice, manner of sale, public information requirements or volume limitation provisions of Rule 144. Persons who are our “affiliates” may resell those shares beginning 90 days after the date of this prospectus without compliance with minimum holding period requirements under Rule 144 (subject to the terms of the lock-up agreement referred to below, if applicable).
125
Lock-Up Agreements
In connection with this offering, we, our directors, our executive officers and the holders of substantially all of our Common Stock, stock options and other securities convertible into, exercisable or exchangeable for our Common Stock, have agreed, subject to certain exceptions, with the underwriters not to directly or indirectly offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend, or otherwise transfer or dispose of, directly or indirectly, or enter into any hedging, swap or other agreement or transaction that transfers any of the economic consequences of ownership of shares of our Common Stock or any options to purchase shares of our Common Stock, or any securities convertible into or exchangeable for shares of Common Stock, during the period from the date of this prospectus continuing through the date 180 days after the date of this prospectus, except with the prior written consent of the representative of the underwriters, and certain other limited exceptions. These agreements are described in the section titled “Underwriting.”
In addition to the restrictions contained in the lock-up agreements described above, we have entered into agreements with certain security holders, including the amended and restated investors’ rights agreement, our standard form of option agreement, our standard form of restricted stock agreement and our standard form of restricted stock purchase agreement, that contain market stand-off provisions or incorporate market stand-off provisions from our equity incentive plan imposing restrictions on the ability of such security holders to offer, sell or transfer our equity securities for a period of 180 days following the date of this prospectus.
Following the lock-up periods set forth in the agreements described above, and assuming that the representative of the underwriters do not release any parties from these agreements, all of the shares of our Common Stock that are restricted securities or are held by our affiliates as of the date of this prospectus will be eligible for sale in the public market in compliance with Rule 144 under the Securities Act.
Equity Incentive Plans
We intend to file with the SEC a registration statement on Form S-8 under the Securities Act covering the shares of Common Stock reserved for issuance under the 2020 Equity Incentive Plan. Such registration statement is expected to be filed and become effective as soon as practicable after the closing of this offering. Accordingly, shares registered under such registration statement will be available for sale in the open market following its effective date, subject to Rule 144 volume limitations for affiliates and the lock-up agreements described above, if applicable.
126
The following are summaries of the material terms of our amended and restated certificate of incorporation and our amended and restated bylaws, which will be effective upon the closing of this offering. The descriptions of the common stock and preferred stock give effect to changes to our capital structure that will occur immediately prior to the completion of this offering. We refer in this section to our amended and restated certificate of incorporation as our Amended Certificate of Incorporation, and we refer to our amended and restated bylaws as our Amended Bylaws. The material terms outlined herein are not intended to be a complete summary of the rights and preferences of such securities and is qualified by reference to the Certificate of Incorporation, the Bylaws, the Amended Certificate of Incorporation, Amended Bylaws and the warrant-related documents described herein, which are exhibits to the registration statement of which this prospectus is a part. We urge you to read each of the Certificate of Incorporation, the Bylaws, the Amended Certificate of Incorporation, the Amended Bylaws and to refer to the applicable provisions of Delaware law described herein in their entirety for a complete description of the rights and preferences of our securities.
Authorized Common Stock and Preferred Stock
Immediately prior to the completion of this offering, under our Certificate of Incorporation, we are authorized to issue 306,830,985 shares of capital stock, consisting of two classes: 300,000,000 shares of Common Stock, $0.0001 par value per share of which 9,988,836 shares are issued and outstanding as of June 30, 2023 and up to 6,830,985 shares of preferred stock, $0.0001 par value in one or more series from time to time which currently consists of 213,730 shares of series seed convertible preferred stock $0.0001 par value per share, of which 213,730 shares are issued and outstanding, 3,635,252 shares of series seed 2 convertible preferred stock, $0.0001 par value per share, of which 3,635,252 shares and 2,982,003 shares of series A convertible preferred stock, $0.0001 par value per share, of which 1,861,799 shares are issued and outstanding as the date of this prospectus.
Upon completion of this offering, under our Amended Certificate of Incorporation our authorized capital stock will consist of 300,000,000 shares of common stock, par value $0.0001 per share, and [●] shares of preferred stock, par value $0.0001 per share, all of which shares of preferred stock will be undesignated.
Common Stock
Immediately prior to the completion of this offering, there were [●] shares of Common Stock issued and outstanding, held of record by [●] stockholders. Upon the completion of this offering, all our issued preferred stock will automatically convert into common stock. If our offering were to be completed as of the date of this prospectus, our shares of convertible preferred stock would convert into [5,710,781] shares of common stock. In addition, options to purchase [●] shares of Common Stock to purchase [●] shares of Common Stock were also outstanding, and [481,828] warrants to purchase shares of Common Stock were outstanding.
The holders of shares of our Common Stock are entitled to one vote for each share held of record by such holder on all matters submitted to a vote of the stockholders.
The number of authorized shares of Common Stock may be increased or decreased by the affirmative vote of the holders of shares of capital stock of stock of the corporation representing a majority of the votes represented by all outstanding shares of capital stock of the corporation entitled to vote. Our Articles of Incorporation do not provide for cumulative voting. Subject to preferences that may be applicable to any outstanding preferred stock, holders of Common Stock are entitled to receive ratably such dividends as may be declared by the Board of Directors out of funds legally available for that purpose. In the event of our liquidation, dissolution or winding up, the holders of Common Stock are entitled to share ratably in all assets remaining after payment of liabilities, subject to the prior distribution rights of any outstanding preferred stock. The Common Stock has no preemptive or conversion rights or other subscription rights.
Upon completion of this offering all of our issued preferred stock will automatically convert into common stock. And the outstanding shares of Common Stock will be, fully paid and non-assessable. The Company may amend its Articles of Incorporation at any time, by way of a board resolution and without a stockholder meeting consistent with the provisions of the DGCL. Upon liquidation, dissolution or winding-up, the holders of our common stock are entitled to share ratably in all of our assets which are legally available for distribution after the payment of or provision for all liabilities and the liquidation preference of any outstanding preferred stock. The holders of our common stock have no preemptive, subscription, redemption, or conversion rights.
127
Preferred Stock
Immediately prior to the completion of this offering, all outstanding shares of our convertible preferred stock will be converted into shares of our common stock.
Upon the consummation of this offering, under our Amended Certificate of Incorporation our Board of Directors will have the authority, without further action by our stockholders, to issue up to [●] shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms and the number of shares constituting, or the designation of, such series, any or all of which may be greater than the rights of common stock. The issuance of our preferred stock could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon our liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change in control of our Company or other corporate action. Immediately after consummation of this offering, no shares of preferred stock will be outstanding, and we have no present plan to issue any shares of preferred stock.
Immediately prior to the completion of this offering there were 6,830,985 authorized shares of preferred stock, $0.0001 par value, and 5,710,781 shares of preferred stock issued and outstanding in three series as prescribed by our Certificate of Incorporation. Pursuant to our Certificate of Incorporation the conversion ratio of each series of convertible preferred stock is determined by dividing the original issue price of that series of convertible preferred stock by the conversion price, subject to adjustment. Since prior to adjustment the original issue price and conversion price are defined as the same figure, the conversion ratio is 1:1, subject to adjustment. For reference, the original issue price is defined as (a) for our series A preferred stock, $1.34138 per share, which also represents the series A preferred stock conversion price; (b) for our series seed 2 preferred stock $0.30 per share, which also represents the series seed 2 preferred stock conversion price; and (c) for our series seed preferred stock $0.30 per share, which also represents the series seed preferred stock conversion price. The 1:1 conversion of each of the three foregoing series of convertible preferred shares is subject to adjustment in the event of any stock split, stock dividend, combination or other similar recapitalization and other adjustments as set forth in our Certificate of Incorporation. As a result, immediately prior to the completion of this offering, each outstanding share of convertible preferred stock is convertible into shares of common stock on a one-for-one basis.
Consequently immediately prior to the completion of this offering, we had: (i) 213,730 authorized shares of series seed convertible preferred stock, all of which are issued and outstanding and upon completion of this offering, such issued and outstanding shares series seed 1 convertible preferred stock will convert into 213,730 shares of our common stock; (ii) 6,666,667 authorized shares of series seed 2 convertible preferred stock, 3,635,252 of which are issued and outstanding, and upon completion of this offering, such issued and outstanding shares of series seed 2 preferred stock will convert into 3,635,252 shares of our common stock; and (iii) 2,982,003 authorized shares of series a convertible preferred stock, 1,861,799 of which are issued and outstanding, and upon completion of this offering, such issued and outstanding shares of series a preferred stock will convert into 1,861,799 shares of our common stock. Shares of any Preferred Stock that converted will be retired, canceled and will not be reissued.
128
Warrants and Options
On July 15, 2022, we issued 231,828 shares of our series A preferred and 231,828 warrants to purchase shares of our Common stock upon conversion of $186,584 of promissory notes and accrued interest. The warrants have an exercise price of $2.01207 and expire in 5 years.
On March 2, 2023, we issued 250,000 warrants to an investor to purchase shares of our Common stock for total proceeds of $750,000. Each warrant is exercisable at $3.00 per share of Common Stock and expire in 3 years and 180 days, subject to terms prompting our acceleration of their expiration. The warrants are exercisable, in whole or in part at the issue date but such exercisability is subject to a lock-up period that ceases upon the date of our initial public offering and shall continue to be exercisable in whole or in part immediately after the lock-up period.
We have adopted an incentive stock option plan known as our 2020 Equity Incentive Plan (the “Plan”) where our Board or any of its committees can grant issuances of incentives stock options, nonstatutory stock options, and restricted stock to our employees, advisors and directors. The exercise price of incentive stock options and nonqualified stock options will be no less than 100% of the fair value per share of the Company’s Common Stock on the date of grant. If an individual owns common stock representing more than 10% of the voting shares and the grant is an incentive stock option, the price of each share will be at least 110% of the fair value on the date of grant. The aggregate number of shares of Common Stock allocated and made available for issuance pursuant to stock options granted under the Plan will not exceed 1,734,188 shares of Common Stock. As of the date of this prospectus, 1,537,667 options under the Plan were outstanding, and 92,354 were available for future grant. Each option granted under the Plan will carry a term of no more than ten (10) years from the date of grant and the Plan will remain in effect until it is terminated by the Board. The term and vesting periods for options granted under the Plan are determined by the Company’s board of directors.
Lock-Up Restrictions
Certain of our stockholders are subject to certain restrictions on transfer until the termination of applicable lock-up periods. See the section entitled “Share Eligible for Future Sale” for lock-up restrictions on our securities under the lock-up agreements.
Delaware Anti-Takeover Law and Certificate of Incorporation and Bylaw Provisions
Under Section 203 of the DGCL, we will be prohibited from engaging in any Business Combination with any stockholder for a period of three years following the time that such stockholder (the “interested stockholder”) came to own at least 15% of our outstanding voting stock (the “acquisition”), except if:
| ● | the Board approved the acquisition prior to its consummation; |
| ● | the interested stockholder owned at least 85% of the outstanding voting stock upon consummation of the acquisition; or |
| ● | the Business Combination is approved by the Board, and by a 2/3 majority vote of the other stockholders in a meeting. |
129
Generally, a “Business Combination” includes any merger, consolidation, asset or stock sale or certain other transactions resulting in a financial benefit to the interested stockholder. Subject to certain exceptions, an “interested stockholder” is a person who, together with that person’s affiliates and associates, owns, or within the previous three years owned, 15% or more of our voting stock.
Under certain circumstances, declining to opt out of Section 203 of the DGCL will make it more difficult for a person who would be an “interested stockholder” to effect various Business Combinations with the Company for a three-year period. This may encourage companies interested in acquiring the Company to negotiate in advance with the Board because the stockholder approval requirement would be avoided if the Board approves the acquisition which results in the stockholder becoming an interested stockholder. This may also have the effect of preventing changes in the Board and may make it more difficult to accomplish transactions which stockholders may otherwise deem to be in their best interests.
Written Consent by Stockholders
Under the Certificate of Incorporation, subject to the rights of any series of preferred stock then outstanding, any action required or permitted to be taken by our stockholders must be effected at a duly called annual or special meetings of our stockholders and may not be effected by any consent in writing by such stockholders.
Under our Amended Certificate of Incorporation, any action required or permitted to be taken by our stockholders must be effected at a duly called annual or special meetings of our stockholders and may not be effected by any consent in writing by such stockholders.
Special Meeting of Stockholders
Under both our Certificate of Incorporation and Amended Certificate of Incorporation, special meetings of our stockholders may be called only by the chairperson of the Board, our chief executive officer or the Board acting pursuant to a resolution adopted by a majority of the total number of authorized directors whether or not there exist any vacancies in previously authorized directorships and may not be called by any other person or persons. Only such business shall be considered at a special meeting of stockholders as shall have been stated in the notice for such meeting.
Dividends
Under our Certificate of Incorporation, holders of our Common Stock and convertible preferred stock are entitled to dividends pro rata on a pari passu basis according to the number of shares of Common Stock held by such holders. For this purpose, each holder of shares of our convertible preferred stock are treated as if they hold the greatest whole number of shares of Common Stock then issuable upon conversion of all shares of their convertible preferred stock.
Under our Amended Certificate of Incorporation, the holders of our Common Stock are entitled to such dividends or other distribution as may be declared by our board of directors, subject to the DGCL. The directors may from time to time declare dividends (including interim dividends) and other distributions on issued shares of Elevai Labs and authorize payment of the same out of the funds of Elevai Labs lawfully available therefor. No dividend shall be paid otherwise than out of profits or, subject to the restrictions of the DGCL.
Listing
We plan to apply to list our Common Stock on the Nasdaq Capital Market under the symbol “ELAB.” We cannot guarantee that we will be successful in listing on Nasdaq; however, we will not complete this offering unless we receive conditional approval letter.
Transfer Agent and Registrar
We have appointed VStock Transfer, LLC as the transfer agent for our shares. VStock Transfer’s telephone number and address is (212) 828-8436 and 18 Lafayette Place Woodmere, New York 11598.
130
In connection with this offering, we will enter into an underwriting agreement with Univest Securities, LLC, to act as the representative of the underwriters named below (the “Representative”). Subject to the terms and conditions of the underwriting agreement, the underwriters named below have agreed to purchase, and we have agreed to sell to them, the number of shares of Common Stock set forth opposite its name below, at the initial public offering price, less the underwriting discounts, as set forth on the cover page of this prospectus and as indicated below:
| Name of Underwriter | Number of Shares | |||
| Univest Securities, LLC | ||||
| [●] | ||||
The underwriting agreement provides that the obligations of the underwriters to pay for and accept delivery of shares of Common Stock are subject to the approval of certain legal matters by their counsel and to certain other conditions specified in the underwriting agreement. The underwriters are offering the shares of Common Stock when, as and if issued to and accepted by them, subject to prior sale. The underwriters are obligated to take and pay for all of the shares offered by this prospectus if any such shares are taken. However, the underwriters are not required to take or pay for the shares covered by the underwriters’ over-allotment option described below.
Over-Allotment Option
We have granted to the Representative an option, exercisable for 45 days from the closing of this offering, to purchase additional shares of Common Stock equal to fifteen percent (15%) of the total number of shares of Common Stock sold in this offering at the initial public offering price listed on the cover page of this prospectus, less underwriting discounts. The option may be exercised in whole or in part, and may be exercised more than once, during the 45-day option period. The Representative may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with the offering contemplated by this prospectus. To the extent the option is exercised, each underwriter will become obligated, subject to certain conditions, to offer the additional shares of Common Stock on the same terms as those on which the shares are being offered under this prospectus.
Discounts
The underwriters propose to initially offer the shares of Common Stock to the public at the initial public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $[●] per share of Common Stock. If all of the shares of Common Stock offered by us are not sold at the public offering price, the underwriters may change the offering price and other selling terms by means of a supplement to this prospectus. The underwriting discounts are equal to seven percent (7%) of the initial public offering price.
The following table shows the initial public offering price, underwriting discounts, and proceeds before expenses to us. The total amounts are shown assuming either no exercise and full exercise of the over-allotment option we granted to the Representative.
| No Exercise | Full Exercise1 | |||||||
| Public offering price per share | $ | $ | ||||||
| Total | $ | $ | ||||||
| (1) | The fees do not include the Representative’s Warrants or expense reimbursement as described below. |
We have also agreed to pay the Representative by deduction from the net proceeds of the offering contemplated herein, as a non-accountable expense allowance equal to one percent (1%) of the gross proceeds received by us from the sale of the shares of Common Stock at the closing of the offering, including any shares issued pursuant to the exercise of the over-allotment option we granted to the Representative.
131
We have agreed to reimburse the Representative for its reasonable out-of-pocket accountable expenses in connection with this offering, up to a maximum amount of $250,000. The out-of-pocket expenses include but are not limited to road show expenses, fees of the Representative’s legal counsel, and due diligence expenses. As of the date of this prospectus, we have paid $80,000 to the underwriter as an advance against out-of-pocket accountable expenses. Any advance will be returned to us to the extent the representative’s out-of-pocket accountable expenses are not actually incurred in accordance with FINRA Rule 5110(g)(4)(A).
Our total expenses of the offering payable by us, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding the underwriting discounts and non-accountable expense allowance, are approximately $[●].
Right of First Refusal
We have agreed to grant the Representative, for the 18-month period following the closing of this offering, a right of first refusal to provide investment banking services to the Company on an exclusive basis in all matters for which investment banking services are sought by the Company (such right, the “Right of First Refusal”), which right is exercisable in the representative’s sole discretion but is non-assignable. For these purposes, investment banking services shall include, without limitation, (a) acting as lead or joint-lead manager for any underwritten public offering; (b) acting as exclusive lead or joint book-runner or placement agent, initial purchaser in connection with any private offering of securities of the Company; and (c) acting as financial advisor in connection with any sale or other transfer by the Company, directly or indirectly, of a majority or controlling portion of its capital stock or assets to another entity, any purchase or other transfer by another entity, directly or indirectly, of a majority or controlling portion of the capital stock or assets of the Company, and any merger or consolidation of the Company with another entity. In accordance with FINRA Rule 5110(f)(2)(E)(i), such right of first refusal shall not have a duration of more than three years from the date of commencement of sales of the public offering or the termination date of the engagement between us and the underwriter. The Right of First Refusal may be terminated by the Company for cause pursuant to FINRA Rule 5110(g)(5)(B)(i). For the avoidance of doubt, “for cause” termination shall include termination due to any material failure by the Representative to provide the underwriting services contemplated herein.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act and liabilities arising from breaches of representations and warranties contained in the underwriting agreement, or to contribute to payments that the underwriters may be required to make in respect of those liabilities.
Lock-Up Agreements
The Company has agreed in the Underwriting Agreement that, without the prior written consent of the Representative, it will not, for a period of six (6) months from the commencement of the Company’s first day of trading on the Nasdaq (the “Lock-Up Period”), (i) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase or otherwise transfer or dispose of, directly or indirectly, or file with the SEC any registration statement relating to, any shares of capital stock of the Company or any securities convertible into or exercisable or exchangeable for shares of capital stock of the Company; (ii) complete any offering of debt securities of the Company, other than entering into a line of credit with a traditional bank, or (iv) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of capital stock of the Company, whether any such transaction described in clause (i), (ii), or (iii) above is to be settled by delivery of shares of capital stock of the Company or such other securities, in cash or otherwise.
In addition, our officers, directors, and stockholders holding 5% or more of the issued and outstanding Common Stock have agreed, that during the Lock-up Period, without the prior written consent of the Representative, subject to certain exceptions with respect to the Common Stock that they beneficially own, including the issuance of shares upon the exercise of convertible securities and options that may be currently outstanding or which may be issued that they will not directly, or indirectly, (i) offer, pledge, announce the intention to sell, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right to purchase, make any short sale or otherwise transfer or dispose of, directly or indirectly, any shares of Common Stock of the Company or any securities convertible into or exercisable or exchangeable for shares of Common Stock of the Company, whether now owned or hereafter acquired by such person or with respect to which such person has or hereafter acquires the power of disposition; (ii) enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of such securities; (iii) make any demand for or exercise any right with respect to the registration of any such securities; or (iv) publicly disclose the intention to make any offer, sale, pledge or disposition, or to enter into any transaction, swap, hedge or other arrangement relating to any such securities. This means that, for a period of six months following the date of this prospectus, such persons may not offer, sell, pledge or otherwise dispose of these securities without the prior written consent of the Representative or as otherwise agreed.
132
The Representative has no present intention to waive or shorten the lock-up period; however, the terms of the lock-up agreements may be waived at its discretion. In determining whether to waive the terms of the lock-up agreements, the Representative may base its decision on its assessment of the relative strengths of the securities markets and companies similar to ours in general, and the trading pattern of, and demand for, our securities in general.
Representative’s Warrants
We have agreed to issue to the Representative and to register herein warrants to purchase a number of shares equal to five percent (5%) of the total number of shares of Common Stock sold in this offering. The warrants will be exercisable upon issuance, at any time, and from time to time, in whole or in part, commencing from the closing of the offering and expiring five years from the effectiveness of the offering. The warrants are exercisable at a per share price of 100% of the public offering price of the shares offered hereby. The Representative’s Warrant shall not be callable or cancellable.
The Representative’s warrants and the underlying shares are deemed to be compensation by FINRA, and therefore will be subject to a lock-up pursuant to FINRA Rule 5110(c)(1). The Representative’s Warrant may not be sold, transferred, assigned, pledged or hypothecated, or be the subject of any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the securities by any person for a period of 180 days immediately following the commencement of sales of the offering, of which this prospectus forms a part in accordance with FINRA Rule 5110(e)(1), except that they may be assigned, in whole or in part, to any successor, officer, manager, member, or partner of the underwriter, and to members of the syndicate or selling group and their respective officers, managers, members or partners. The Representative’s Warrants may be exercised as to all or a lesser number of shares, will provide for cashless exercise and will contain provisions for immediate “piggyback” registration rights at our expense for a period of seven years from the commencement of sales of the offering and demand registration rights, with one such right being at our expense, for a period of five years from the commencement of sales of the offering. We have registered the Underwriter the shares underlying the Representative’s Warrant in this offering.
Price Stabilization, Short Positions and Penalty Bids
In connection with the offering the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions, penalty bids and passive market making in accordance with Regulation M under the Exchange Act.
| ● | Stabilizing transactions permit the underwriters to make bids or purchases for the purpose of pegging, fixing or maintaining the price of the Common Stock, so long as stabilizing bids do not exceed a specified maximum. |
| ● | Over-allotment involves sales by the underwriters of Common Stock in excess of the number of shares of Common Stock the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares of Common Stock over-allotted by the underwriters is not greater than the number of shares of Common Stock that they may purchase in the over-allotment option. In a naked short position, the number of shares of Common Stock involved is greater than the number of shares of Common Stock in the over-allotment option. The underwriters may close out any covered short position by either exercising their over-allotment option and/or purchasing shares of Common Stock in the open market. |
| ● | Syndicate covering transactions involve purchases of shares of Common Stock in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares of Common Stock to close out the short position, the underwriters will consider, among other things, the price of our shares of Common Stock available for purchase in the open market as compared to the price at which they may purchase shares of Common Stock through the over-allotment option. If the underwriters sell more shares of Common Stock than could be covered by the over-allotment option, a naked short position, the position can only be closed out by buying shares of Common Stock in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares of Common Stock in the open market after pricing that could adversely affect investors who purchase in the offering. |
| ● | Penalty bids permit the underwriters to reclaim a selling concession from a syndicate member when the shares of Common Stock originally sold by the syndicate member are purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
| ● | In passive market making, market makers in the Common Stock who are the underwriters or prospective underwriter may, subject to limitations, make bids for or purchases of our Common Stock until the time, if any, at which a stabilizing bid is made. |
133
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our Common Stock or preventing or retarding a decline in the market price of our Common Stock. As a result, the price of our Common Stock may be higher than the price that might otherwise exist in the open market. These transactions may be effected on the Nasdaq Stock Market or otherwise, and, if commenced, may be discontinued at any time.
Determination of Offering Price
The public offering price of the shares of Common Stock we are offering was determined by us in consultation with the Representative based on discussions with potential investors in light of the history and prospects of our Company, the stage of development of our business, our business plans for the future and the extent to which they have been implemented, an assessment of our management, the public stock price for similar companies, general conditions of the securities markets at the time of the offering and such other factors as were deemed relevant.
Electronic Offer, Sale and Distribution of Securities
This prospectus in electronic format may be made available on websites or through other online services maintained by one or more of the underwriters, or by their affiliates. Other than this prospectus in electronic format, the information on any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter in its capacity as underwriter, and should not be relied upon by investors.
Selling Restrictions
No action has been taken in any jurisdiction (except in the United States) that would permit a public offering of our shares of common stock, or the possession, circulation or distribution of this prospectus or any other material relating to us or our shares of common stock in any jurisdiction where action for that purpose is required. Accordingly, our shares of common stock may not be offered or sold, directly or indirectly, and this prospectus or any other offering material or advertisements in connection with our shares of common stock may be distributed or published, in or from any country or jurisdiction, except in compliance with any applicable rules and regulations of any such country or jurisdiction.
Nasdaq Listing
We will not complete this offering unless our shares have been approved for listing on the Nasdaq Capital Market under the symbol “ELAB”.
134
EXPENSES RELATING TO THIS OFFERING
Set forth below is an itemization of the total expenses, excluding underwriting discounts and the non-accountable expense allowance, expected to be incurred in connection with this offering by us. With the exception of the SEC registration fee, the FINRA filing fee, and the Nasdaq Capital Market listing fee, all amounts are estimates.
| Securities and Exchange Commission Registration Fee | $ | |||
| Nasdaq Capital Market Listing Fee | $ | |||
| FINRA Filing Fee | $ | |||
| Legal Fees and Expenses | $ | |||
| Accounting Fees and Expenses | $ | |||
| Printing Expenses | $ | |||
| Miscellaneous Expenses | $ | |||
| Total Expenses | $ |
These expenses will be borne by us. Underwriting discounts and the non-accountable expense allowance will be borne by us in proportion to the numbers of shares of Common Stock sold in the offering.
The legality and validity of the securities offered from time to time under this prospectus will be passed upon by Ortoli Rosenstadt LLP. The current address of Ortoli Rosenstadt LLP is 366 Madison Avenue, 3rd Floor, New York, NY 10017. Certain legal matters as to the United States federal securities and New York law in connection with this offering will be passed upon for the underwriters by Hunter Taubman Fischer & Li LLC.
Our financial statements as of December 31, 2022 and 2021, and for each of the two years in the period ending December 31, 2022, included in this Prospectus have been audited by TPS Thayer, an independent registered public accounting firm, as stated in their report appearing herein and elsewhere in the Registration Statement (which report expresses an unqualified opinion on the financial statements and includes an explanatory paragraph referring to the entity’s ability to continue as a going concern). TPS Thayer has also performed review of our unaudited interim financial statements as of and for the six months ended June 30, 2023 and 2022. Such financial statements have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing. The current address of TPS Thayer is 1600 Hwy 6 Suite 100, Sugar Land, TX 77478.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities we are offering under this prospectus, we refer you to the registration statement and the exhibits and schedules filed as a part of the registration statement. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the Public Reference Room. The SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC, where our SEC filings are also available. The address of the SEC’s web site is http://www.sec.gov.
135
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
ELEVAI LABS, INC.
Index to Consolidated Financial Statements
For the Years Ended December 31, 2022 and 2021
For the Six Months Ended June 30, 2023 and 2022
F-1
Consolidated Financial Statements of

For the years ended
December 31, 2022 and 2021
(Expressed in United States Dollars)
F-2
Report of Independent Registered Public Accounting Firm
To the Board of Directors and
Shareholders Elevai Labs, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Elevai Labs, Inc. and subsidiaries (collectively, “the Company”) as of December 31, 2022, and 2021, and the related consolidated statements of operations and other comprehensive loss, shareholders’ equity and cash flows for the two year period then ended and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the consolidated financial position of the Company as of December 31, 2022 and 2021, and the consolidated results of its operations and its consolidated cash flows for the two year period ended December 31, 2022 and 2021 in conformity with generally accepted accounting principles in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As described in Note 2 the financial statements, the Company has suffered recurring losses from operations and has stockholders’ deficit that raise substantial doubt about its ability to continue as going concern. Management’s plans regarding these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatements of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provided a reasonable basis for our opinion.

TPS Thayer, LLC
We have served as the Company’s auditor
since 2022 Sugar Land, Texas
June 13, 2023
F-3
Consolidated Balance Sheets
(Expressed in United States dollar)
| As of: | December 31, 2022 | December 31, 2021 | ||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | 1,154,901 | $ | 411,858 | ||||
| Receivables, net | 12,854 | 1,710 | ||||||
| Prepaids and deposits | 153,422 | 62,140 | ||||||
| Inventory, net | 230,145 | 160,817 | ||||||
| Total Current Assets | 1,551,322 | 636,525 | ||||||
| Deposit | 10,773 | |||||||
| Property and equipment, net | 53,535 | 29,725 | ||||||
| Operating lease right-of-use asset | 276,553 | - | ||||||
| TOTAL ASSETS | $ | 1,892,183 | $ | 666,250 | ||||
| LIABILITIES | ||||||||
| Current Liabilities | ||||||||
| Accounts payable and accrued liabilities | $ | 256,325 | $ | 192,102 | ||||
| Customer deposits | 10,172 | - | ||||||
| Due to related parties | 142,704 | 23,520 | ||||||
| Current portion of lease liability | 110,616 | - | ||||||
| Derivative liabilities | 68,455 | - | ||||||
| Total Current Liabilities | 588,272 | 215,622 | ||||||
| Operating lease liability | 172,601 | - | ||||||
| TOTAL LIABILIITES | $ | 760,873 | $ | 215,622 | ||||
| Commitments and Contingencies | ||||||||
| EQUITY | ||||||||
| Series seed 1 preferred stock, $0.0001 par value, 213,730 shares authorized; 213,730 shares issued and outstanding as of December 31, 2022 and 2021 | 21 | 21 | ||||||
| Series seed 2 preferred stock, $0.0001 par value, 3,635,252 shares authorized; 3,635,252 shares issued and outstanding as of December 31, 2022 and 2021, respectively | 364 | 364 | ||||||
| Series A preferred stock, $0.0001 par value, 2,982,003 shares authorized; 1,861,799 and Nil shares issued and outstanding as of December 31, 2022 and 2021 | 186 | - | ||||||
| Common stock, $0.0001 par value, 19,000,000 shares authorized; 9,568,475 and 9,526,808 shares issued and outstanding as of December 31, 2022 and 2021, respectively | 957 | 952 | ||||||
| Additional paid-in capital | 3,852,044 | 1,371,194 | ||||||
| Accumulated other comprehensive income | 111 | 202 | ||||||
| Accumulated deficit | (2,722,373 | ) | (922,105 | ) | ||||
| TOTAL EQUITY | 1,131,310 | 450,628 | ||||||
| TOTAL LIABILITIES AND EQUITY | $ | 1,892,183 | $ | 666,250 | ||||
The accompanying notes are an integral part of these consolidated financial statements
F-4
Consolidated Statements of Operations and Comprehensive Loss
For the years ended December 31, 2022 and 2021
(Expressed in United States dollar)
| December 31, 2022 | December 31, 2021 | |||||||
| Revenue | $ | 766,277 | $ | 827 | ||||
| Cost of sales | 318,968 | 88 | ||||||
| Gross profit | $ | 447,309 | $ | 739 | ||||
| Expenses | ||||||||
| Depreciation | 5,034 | 2,385 | ||||||
| Marketing and promotion | 192,863 | 31,605 | ||||||
| Consulting fees | 324,395 | 210,402 | ||||||
| Office and administrative | 1,019,708 | 268,175 | ||||||
| Professional fees | 192,409 | 124,152 | ||||||
| Investor relations | 74,003 | - | ||||||
| Research and development | 228,747 | 123,632 | ||||||
| Foreign exchange (gain) loss | 2,749 | (323 | ) | |||||
| Travel and entertainment | 198,442 | 25,450 | ||||||
| Total Expenses | $ | 2,238,350 | $ | 785,478 | ||||
| Net loss before other income (expense) | $ | (1,791,041 | ) | $ | (784,739 | ) | ||
| Other income (expense) | ||||||||
| Change in fair value of derivative liabilities | (12,754 | ) | - | |||||
| Interest income | 7,702 | - | ||||||
| Interest expense | (2,629 | ) | - | |||||
| Loss on sale of equipment | (1,546 | ) | - | |||||
| Net loss | $ | (1,800,268 | ) | $ | (784,739 | ) | ||
| Other comprehensive income (loss) | ||||||||
| Currency translation adjustment | (91 | ) | 1,424 | |||||
| Total comprehensive loss | $ | (1,800,359 | ) | $ | (783,315 | ) | ||
| Basic and diluted loss per share | $ | (0.189 | ) | $ | (0.083 | ) | ||
| Weighted average shares outstanding | 9,528,863 | 9,460,664 | ||||||
The accompanying notes are an integral part of these consolidated financial statements
F-5
Consolidated Statements of Changes in Stockholders’ Equity
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
| Series seed 1 preferred stock | Series seed 2 preferred stock | Series A preferred stock | Common Stock | Additional | Accumulated other | |||||||||||||||||||||||||||||||||||||||||||
| Number | Number | Number | Number | paid-in | Accumulated | comprehensive | ||||||||||||||||||||||||||||||||||||||||||
| of shares | Amount | of shares | Amount | of shares | Amount | of shares | Amount | capital | deficit | income | Total | |||||||||||||||||||||||||||||||||||||
| # | $ | # | $ | # | $ | # | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||||||||||||
| Balance, January 1, 2021 | 213,730 | 21 | - | - | - | - | 8,480,000 | 848 | 114,130 | (137,366 | ) | (1,222 | ) | (23,589 | ) | |||||||||||||||||||||||||||||||||
| Private placements | - | - | 3,635,252 | 364 | - | - | - | - | 1,090,213 | - | - | 1,090,577 | ||||||||||||||||||||||||||||||||||||
| Conversion of promissory notes | - | - | - | - | - | - | 1,046,808 | 104 | 24,496 | - | - | 24,600 | ||||||||||||||||||||||||||||||||||||
| Share-based compensation | - | - | - | - | - | - | - | - | 142,355 | - | - | 142,355 | ||||||||||||||||||||||||||||||||||||
| Net loss for the year | - | - | - | - | - | - | - | - | - | (784,739 | ) | - | (784,739 | ) | ||||||||||||||||||||||||||||||||||
| Currency translation adjustment | - | - | - | - | - | - | - | - | - | - | 1,424 | 1,424 | ||||||||||||||||||||||||||||||||||||
| Balance, December 31, 2021 | 213,730 | 21 | 3,635,252 | 364 | - | - | 9,526,808 | 952 | 1,371,194 | (922,105 | ) | 202 | 450,628 | |||||||||||||||||||||||||||||||||||
| Balance, January 1, 2022 | 213,730 | 21 | 3,635,252 | 364 | - | - | 9,526,808 | 952 | 1,371,194 | (922,105 | ) | 202 | 450,628 | |||||||||||||||||||||||||||||||||||
| Private placements | - | - | - | - | 1,629,971 | 163 | - | - | 2,186,258 | - | - | 2,186,421 | ||||||||||||||||||||||||||||||||||||
| Share issuance cost | - | - | - | - | - | - | - | - | (33,132 | ) | - | - | (33,132 | ) | ||||||||||||||||||||||||||||||||||
| Conversion of promissory notes | - | - | - | - | 231,828 | 23 | - | - | 130,860 | - | - | 130,883 | ||||||||||||||||||||||||||||||||||||
| Share-based compensation | - | - | - | - | - | - | - | - | 171,869 | - | - | 171,869 | ||||||||||||||||||||||||||||||||||||
| Exercise of stock options | - | - | - | - | - | - | 41,667 | 5 | 24,995 | - | - | 25,000 | ||||||||||||||||||||||||||||||||||||
| Net loss for the year | - | - | - | - | - | - | - | - | - | (1,800,268 | ) | - | (1,800,268 | ) | ||||||||||||||||||||||||||||||||||
| Currency translation adjustment | - | - | - | - | - | - | - | - | - | - | (91 | ) | (91 | ) | ||||||||||||||||||||||||||||||||||
| Balance, December 31, 2022 | 213,730 | 21 | 3,635,252 | 364 | 1,861,799 | 186 | 9,568,475 | 957 | 3,852,044 | (2,722,373 | ) | 111 | 1,131,310 | |||||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
F-6
Consolidated Statements of Cash Flows
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
| December 31, 2022 | December 31, 2021 | |||||||
| Operating activities | ||||||||
| Net loss | $ | (1,800,268 | ) | $ | (784,739 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 6,512 | 2,757 | ||||||
| Interest expense | 2,614 | 600 | ||||||
| Share-based compensation | 171,869 | 142,355 | ||||||
| Straight-line rent expense | 6,664 | - | ||||||
| Loss on sale of equipment | 1,546 | - | ||||||
| Change in fair value of derivative liabilities | 12,754 | - | ||||||
| Changes in operating assets and liabilities: | ||||||||
| Receivables | (11,547 | ) | (1,355 | ) | ||||
| Prepaid expenses and deposits | (102,055 | ) | (58,840 | ) | ||||
| Inventory | (69,328 | ) | (160,817 | ) | ||||
| Accounts payable and accrued liabilities | 65,191 | 192,086 | ||||||
| Customer deposits | 10,172 | - | ||||||
| Due to related parties | 120,000 | 7,019 | ||||||
| Cash flows used in operating activities | $ | (1,585,876 | ) | $ | (660,934 | ) | ||
| Investing activities | ||||||||
| Purchase of equipment | (35,527 | ) | (32,482 | ) | ||||
| Proceeds on disposal of equipment | 3,500 | |||||||
| Cash flows used in investing activities | $ | (32,027 | ) | $ | (32,482 | ) | ||
| Financing activities | ||||||||
| Exercise of stock options | 25,000 | - | ||||||
| Proceeds from the issuance of series A preferred stock | 2,153,289 | - | ||||||
| Proceeds from the issuance of series seed 2 preferred stock | - | 1,090,577 | ||||||
| Proceeds from notes payable | 183,970 | - | ||||||
| Cash flows provided by financing activities | $ | 2,362,259 | $ | 1,090,577 | ||||
| Effect of exchange rate changes on cash | (1,313 | ) | 1,346 | |||||
| Increase in cash | 743,043 | 398,507 | ||||||
| Cash, beginning of period | 411,858 | 13,351 | ||||||
| Cash, ending of period | $ | 1,154,901 | $ | 411,858 | ||||
| Supplemental cash flow information: | ||||||||
| Cash paid for interest | - | - | ||||||
| Cash paid for taxes | - | - | ||||||
| Non-cash Investing and Financing transactions: | ||||||||
| Settlement of notes payable and accrued interest through issuance of common stock | - | 24,600 | ||||||
| Settlement of notes payable and accrued interest through issuance of series A preferred stock and warrants | 186,584 | - | ||||||
The accompanying notes are an integral part of these consolidated financial statements
F-7
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
| 1. | Organization and nature of operations |
Elevai Labs, Inc. (“Elevai”) was incorporated under the laws of the State of Delaware on June 9, 2020. Elevai and its 100% owned subsidiary, Elevai Research Inc, are collectively referred to in these consolidated financial statements as “the Company”.
The Company is a skincare development company engaged in the design, manufacture, and marketing of skincare products in the skincare industry. The Company’s principal activities are developing and manufacturing skincare products.
Reorganization
A reorganization of the legal structure was completed in June 2021. On June 4, 2021, Elevai acquired 100% of Elevai Research Inc. (formerly Reactive Medical Inc.) (“Elevai Research”).
Prior to the reorganization, BWL Investments Ltd. (“BWL”) owned approximately 29.4% of the issued and outstanding common shares of Elevai and 100% of Elevai Research. The other significant shareholder of Elevai was JP Bio Consulting LLC (“JP”), also holding approximately 29.4% of the issued and outstanding common shares of Elevai. In addition, BWL and JP (the “Controlling Shareholders”) had entered into an agreement to act in concert and vote together in favor of the acquisition of Elevai Research.
Since the Company is effectively controlled by the same Controlling Shareholders before and after the reorganization, it has been accounted for as an acquisition under common control. The assets and liabilities of Elevai Research are consolidated retrospectively from the date of incorporation of Elevai Research on February 5, 2018, as both entities were under common control since inception.
| 2. | Going Concern |
These audited consolidated financial statements have been prepared on a going concern basis, which implies the Company will continue to realize its assets and discharge its liabilities in the normal course of business. The continuation of the Company as a going concern is dependent upon the continued financial support from its shareholders and the ability of the Company to obtain necessary equity financing to continue operations, and ultimately the attainment of profitable operations.
As of December 31, 2022 and 2021, the Company had a net working capital of $963,050 and $420,903, respectively, and has an accumulated deficit of $2,722,373 and $922,105, respectively. Furthermore, for the years ended December 31, 2022 and 2021, the Company incurred a net loss of $1,800,268 and $784,739, respectively and used $1,585,876 and $660,934, respectively of cash flows for operating activities. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. These audited consolidated financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
The assessment of whether the going concern assumption is appropriate requires management to take into account all available information about the future, which is at least, but not limited to, 12 months from the date the financial statements are issued. The Company is aware that material uncertainties related to events or conditions may cast substantial doubt upon the Company’s ability to continue as a going concern.
F-8
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Management’s plans that alleviate substantial doubt about the Company’s ability to continue as a going concern include raising additional debt or equity financing (refer to note 17). In addition, in February 2023, the Company filed its preliminary initial registration (S-1 Form) with the SEC pursuant to its goal of completing an initial public offering (“IPO”). The Company plans to use funds raised in a successful IPO to accelerate new product development, inventory production, increasing its sales force and expanding into new markets.
The outbreak of the coronavirus, also known as “COVID-19”, has spread across the globe and is impacting worldwide economic activity. Conditions surrounding the coronavirus continue to rapidly evolve and government authorities have implemented emergency measures to mitigate the spread of the virus. The outbreak and the related mitigation measures may have an adverse impact on global economic conditions as well as on the Company’s business activities. The extent to which the coronavirus may impact the Company’s business activities will depend on future developments, such as the ultimate geographic spread of the disease, the duration of the outbreak, travel restrictions, business disruptions, and the effectiveness of actions taken in the USA and Canada and other countries to contain and treat the disease. These events are highly uncertain and as such, the Company cannot determine their financial impact at this time. While certain restrictions are presently in the process of being relaxed, it is unclear when the world will return to the previous normal, if ever. This may adversely impact the expected implementation of the Company’s plans moving forward.
| 3. | Summary of Significant Accounting Policies |
Basis of Presentation
The consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States (“U.S. GAAP”) and are expressed in U.S. dollars. These consolidated financial statements include the accounts of the Company and its wholly owned subsidiary. All intercompany accounts and transactions were eliminated upon consolidation.
This summary of significant accounting policies of the Company is presented to assist in understanding the Company’s consolidated financial statements. The consolidated financial statements and notes are representations of the Company’s management who are responsible for their integrity and objectivity. These accounting policies conform to accounting principles generally accepted in the United States of America and have been consistently applied in the preparation of the consolidated financial statements.
Principles of Consolidation
The consolidated financial statements include the account of Elevai, and its 100% owned subsidiary, Elevai Research. All intercompany accounts, transactions and profits were eliminated in the consolidated financial statements.
F-9
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to revenue recognition, the collectability of receivables, valuation of inventory, fair value of derivative liabilities and stock options, useful lives and recoverability of long-lived assets, and deferred income tax asset valuation allowances. The Company bases its estimates and assumptions on current facts, historical experience and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgements about the carrying value of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from those estimates. Estimates and assumptions are reviewed periodically, and the effects of revisions are reflected in the consolidated financial statements in the period they are determined.
Foreign Currency Translation
The Company’s functional and reporting currency is the U.S. dollar. The functional currency of Elevai Research is the Canadian dollar. Monetary assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the balance sheet date. Non-monetary assets, liabilities, and items recorded in income arising from transactions denominated in foreign currencies are translated at rates of exchange in effect at the date of the transaction. Gains and losses arising on translation or settlement of foreign currency denominated transactions or balances are included in the determination of income.
The accounts of Elevai Research are translated to U.S. dollars using the current rate method. Accordingly, assets and liabilities are translated into U.S. dollars at the period-end exchange rate while revenues and expenses are translated at the average exchange rates during the period. Related exchange gains and losses are included in a separate component of stockholders’ equity as accumulated other comprehensive income (loss).
Reportable Segments and Geographic Areas
The Company has one reportable segment. The Company’s activities are interrelated, and each activity is dependent upon and supportive of the other. Accordingly, all significant operating decisions are based on analysis of financial products provided as a single global business.
F-10
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
The majority of the Company’s operations and assets are located in the United States. Elevai Research, the Company’s Canadian subsidiary, is located in Canada and provide limited operational support. The following is a summary of the Company’s operations, assets and liabilities split between the Unites States and Canada:
| United States | Canada | Total | ||||||||||
| Revenue | $ | 766,277 | $ | - | $ | 766,277 | ||||||
| Cost of sales | 318,968 | - | 318,968 | |||||||||
| Gross profit | $ | 447,309 | $ | - | $ | 447,309 | ||||||
| Expenses | 2,084,777 | 153,573 | 2,238,350 | |||||||||
| Other income (expense) | (9,227 | ) | - | (9,227 | ) | |||||||
| Net loss | $ | 1,646,695 | $ | 153,573 | $ | 1,800,268 | ||||||
| Current Assets | $ | 1,487,928 | $ | 63,394 | $ | 1,551,322 | ||||||
| Non-current assets | 338,607 | 2,254 | 340,861 | |||||||||
| Total Assets | $ | 1,826,535 | $ | 65,648 | $ | 1,892,183 | ||||||
| Current liabilities | $ | 558,880 | $ | 29,392 | $ | 588,272 | ||||||
| Non-current liabilities | 172,601 | - | 172,601 | |||||||||
| Total Liabilities | $ | 731,481 | $ | 29,392 | $ | 760,873 | ||||||
| Total Equity | $ | 1,095,054 | $ | 36,256 | $ | 1,131,310 | ||||||
Revenue Recognition
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers. Since ASU 2014-09 was issued, several additional ASUs have been issued to clarify various elements of the guidance. These standards provide guidance on recognizing revenue, including a five-step model to determine when revenue recognition is appropriate.
The Company recognizes revenue when it satisfies a performance obligation by transferring control over a product to a customer. Revenue is measured based on the consideration the Company expects to receive in exchange for those products. In instances where financial acceptance of the product is specified by the customer, revenue is deferred until all acceptance criteria have been met. Revenues are recognized under ASC 606, “Revenue from Contracts with Customers,” in a manner that reasonably reflects the delivery of its products and services to customers in return for expected consideration.
The Company generates revenue through the sale of skincare products. Revenue from the sale of skincare products are recognized at the point in time when the Company considered revenue realized or realizable and earned, which is typically when all of the five following criteria are met: (1) the contract with the customer is identifiable (i.e. when a sales transaction has been entered into between the Company and the customer), (2) the performance obligation in the contract is identifiable (i.e. the customer has ordered a known quantity of product to be delivered), (3) the transaction price is determinable (i.e. the customer has agreed to the Company’s price for the products ordered), (4) the Company is able to allocate the transaction price to the performance obligations in the contract, and (5) the performance obligations have been satisfied, which is typically upon delivery of the product to the customer.
F-11
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Transaction prices for performance obligations are explicitly outlined in relevant agreements; therefore, the Company does not believe that significant judgements are required with respect to the determination of the transaction price, including any variable consideration identified.
The Company is responsible for providing the products to customers. As a result, the Company is considered the Principal when providing products to customers. As the Company collects payment at the time of the customer order, its contracts do not have a significant financing component. Customers are entitled to replacement or full refund of any damaged or defective product, after the return of the damaged or defective product to the Company. There were no significant returns or refunds during the year ended December 31, 2022 and 2021.
Research and development
Research and development costs are expensed as incurred in accordance with ASC 730, Research and Development. The Company incurs research and development costs in the pursuit of new products and improving the formulation of existing products. Examples of research costs include laboratory research, studies, surveys, and other activities aimed at acquiring new knowledge. Development costs include expenses incurred in the process of applying research findings or other knowledge to a plan or design for a new product or process. Examples of development costs include engineering, design, testing, and other activities aimed at developing a product or process for commercial production.
Development costs may be capitalized if the following criteria are met: (1) technological feasibility has been established, (2) the Company intends to complete the product or process. (3) the Company has the ability to use or sell the product or process, (4) the product or process will generate future economic benefits, and (5) the costs can be reliably measured.
As of December 31, 2022 and 2021, the Company has not capitalized any development cost.
Marketing and promotion
Costs associated with marketing and promoting the Company’s products are expensed when incurred. The Company includes the cost of products given out as samples in marketing and promotion expenses.
Leases
The Company accounts for leases in accordance with ASC 842, “Leases”. We determine if an arrangement meets the definition of a lease at inception of the contract. Leases are classified as either operating or capital leases. All of the Company’s leases have been assessed as operating leases. Accounting for operating leases, other than short term leases, results in operating lease right-of-use (“ROU”) assets, operating lease liabilities - current, and operating lease liabilities - noncurrent on the balance sheets.
ROU assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments over the lease term. As our lease do not provide an implicit rate, we use our incremental borrowing rate based on the estimated rate of interest for collateralized borrowing over a similar term of the lease payments at commencement date. The operating lease ROU asset also includes any lease payments made and excludes lease incentives. Our lease terms may include options to extend or terminate the lease when it is reasonably certain that we will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
F-12
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Income Taxes
The Company accounts for income taxes using the asset and liability method in accordance with ASC 740, “Income Taxes”. The asset and liability method provides that deferred income tax assets and liabilities are recognized for the expected future tax consequence of temporary differences between the financial reporting and taxes basis of assets and liabilities, and for operating loss and tax credit carryforwards. Deferred income tax assets and liabilities are measured using the currently enacted tax rates and laws that will be in effect when the differences are expected to reverse. The Company records a valuation allowance to reduce deferred income tax assets to the amount that it believes more likely than not to be realized. In making such a determination, the Company considers all available positive and negative evidence, including future reversals of existing taxable temporary differences, projected future taxable income tax planning, strategies and results of recent operations. If the Company determines that such deferred tax assets will be recognized in the future in excess of the net recorded amount then the deferred tax asset valuation will be adjusted which would reduce the provision for income taxes. Significant judgments and estimates are required in the determination of the consolidated income tax expense. As of December 31, 2022 and 2021, the Company did not have any amounts recorded pertaining to tax assets or liabilities as the Company has incurred losses since inception and has taken a full valuation allowance against its tax loss carry forwards. In addition, the Company did not have any amounts recorded pertaining to tax expense or recovery.
The Company records uncertain tax provisions in accordance with ASC 740 based on a two-step process whereby (1) a determination is made about whether it is more likely than not that the tax positions will be sustained based on the technical merits of the position and (2) for those tax positions that meet the more likely than not recognition threshold, the Company recognizes the largest amount of tax benefit that is more than 50 percent likely to be realized upon ultimate settlement with the related tax authority.
As of December 31, 2022 and 2021, the Company did not have any amounts recorded pertaining to uncertain tax positions. The Company recognizes interest and penalties related to uncertain tax positions in office and administrative expense. The Company did not incur any penalties or interest during the years ended December 31, 2022 and 2021.
Concentration of Credit Risk
Cash, receivables and refundable deposits are the only financial instruments that are potentially subject to credit risk. The Company places its cash in what it believes to be credit-worthy financial institutions. Receivables relate to sales taxes paid that is reimbursable from the Canadian government and timing differences on receiving proceeds from sales transactions processed through customer credit cards. Refundable deposits relate to the Company’s security deposit on lease agreements.
F-13
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
As at December 31, 2022, 92% of the Company’s cash were held with Silicon Valley Bank (“SVB”). On March 10, 2023, SVB failed after a bank run and was shut down by the Federal Deposit Insurance Corp (FDIC). The Company suffered no losses and had full access to funds shortly after SVB was placed under the control of the FDIC. The Company has since taken steps to protect its cash from credit risk by placing its funds with, JPMorgan Chase Bank, the largest bank in the United States and the world by market capitalization.
Risks and Uncertainties
The Company is subject to risks from, among other things, competition associated with the industry in general, other risks associated with financing, liquidity requirements, rapidly changing customer requirements, limited operating history, foreign currency exchange rates and the volatility of public markets.
Contingencies
Certain conditions may exist as of the date the consolidated financial statements are issued, which may result in a loss to the Company, but which will only be resolved when one or more future events occur or fail to occur. The Company’s management and legal counsel assess such contingent liabilities, and such assessment inherently involves judgement. In assessing loss contingencies related to legal proceedings that are pending against the Company or un-asserted claims that may result in such proceedings, the Company’s legal counsel evaluates the perceived merits of any legal proceedings or un-asserted claims as well as the perceived merits of the amount of relief sought or expected to be sought.
If the assessment of a contingency indicates it is probable that a material loss has been incurred and the amount of the liability can be estimated, then the estimated liability would be accrued in the Company’s consolidated financial statements. If the assessment indicates that a potential material loss contingency is not probable but is reasonably possible, or is probable but cannot be estimated, then the nature of the contingent liability, together with an estimate of the range of possible loss if determinable and material would be disclosed. Loss contingencies considered to be remote by management are generally not disclosed unless they involve guarantees, in which case the guarantee would be disclosed.
Cash and Cash Equivalents
Cash includes cash on hand and cash in demand deposits. Cash equivalents include all highly liquid instruments with original maturities of three months or less. As of December 31, 2022 and 2021, the Company did not hold any cash equivalents.
F-14
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Receivables
All receivables under standard terms are due thirty (30) days from the date billed. If the funds are not received within thirty (30) days, the customer is contacted to arrange payment. The Company uses the allowance method to account for uncollectable receivables. As of December 31, 2022 and 2021, there was no allowance for uncollectable receivables recorded.
Inventory
Inventory consist of raw materials, work-in-progress and finished goods and are valued at the lower of cost or net realizable value. The Company’s manufacturing process involves the production of our proprietary stem cell-derived Elevai ExosomesTM. Finished goods consists of a new generation of cosmetic topical products containing our proprietary stem cell-derived Elevai ExosomesTM. Cost is determined using the weighted average cost formula. Net realizable value is determined on the basis of anticipated sales proceeds less the estimated selling expenses. Management compares the cost of inventories with the net realizable value and an allowance is made to write down inventories to net realizable value, if lower.
Property and Equipment
Property and equipment is stated at cost less accumulated depreciation. Renewals and betterments that materially extend the life of assets are capitalized. Expenditures for maintenance and repairs are expensed as incurred. Property and equipment is depreciated using the straight-line method. The estimated useful lives of property and equipment are generally as follows:
| Lab equipment | 7-year straight-line |
| Furniture and fixtures | 7-year straight-line |
| Computers | 5-year straight-line |
Impairment of Long-Lived Assets
The Company reviews long-lived assets such as equipment for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. If the total of the expected undiscounted future cash flows is less than the carrying value of the asset, a loss is recognized for the excess of the carrying amount over the fair value of the asset.
The Company’s policy for property and equipment requires judgement in determining whether the present value of future expected economic benefits exceeds capitalized costs. The policy requires management to make certain estimates and assumptions about future economic benefits related to its operations. Estimates and assumptions may change if new information becomes available. If information becomes available suggesting that the recovery of capitalized cost is unlikely, the capitalized cost is written off/impaired to the consolidated statement of operations.
Derivative Financial Instruments
The Company does not use derivative instruments to hedge exposures to cash flow, market, or foreign currency risks. The Company evaluates all of its financial instruments, including issued stock purchase warrants, to determine if such instruments are derivatives or contain features that qualify as embedded derivatives. For derivative financial instruments that are accounted for as liabilities, the derivative instrument is initially recorded at its fair value and is then re-valued at each reporting date, with changes in the fair value reported in the consolidated statement of operations. The Company uses the Black-Scholes option-pricing model to value the derivative instruments at inception and subsequent valuation dates. The classification of derivative instruments, including whether such instruments should be recorded as liabilities or as equity, is reassessed at the end of each reporting period.
F-15
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Common Stock Warrants
The Company classifies as equity any warrants that (i) require physical settlement or net-share settlement or (ii) provide the Company with a choice of net-cash settlement or settlement in its own shares (physical settlement or net-share settlement). The Company classifies as assets or liabilities any warrants that (i) require net-cash settlement (including a requirement to net cash settle the contract if an event occurs and if that event is outside the Company’s control), (ii) gives the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement) or (iii) that contain reset provisions that do not qualify for the scope exception. The Company assesses classification of its common stock warrants at each reporting date to determine whether a change in classification is required. Warrants classified as liabilities are initially recorded at fair value, with gains and losses arising from changes in fair value recognized in other income (expense) in the consolidated statements of operations at each period end while such instruments remain outstanding.
Financial Instruments and Fair Value Measurements
The Company analyzes all financial instruments with features of both liabilities and equity under ASC 480, “Distinguishing Liabilities from Equity,” and ASC 815 “Derivatives and Hedging”.
ASC 820, “Fair Value Measurements and Disclosures,” requires disclosure of the fair value of financial instruments held by the Company. ASC 825, “Financial Instruments,” defines fair value, and establishes a three-level valuation hierarchy for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The carrying amounts reported in the consolidated balance sheets for receivables and current liabilities each qualify as financial instruments and are a reasonable estimate of their fair values because of the short period of time between the origination of such instruments and their expected realization and their current market rate of interest. The three levels of valuation hierarchy are defined as follows:
Level 1
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the asset or liability such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
F-16
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Level 3
Level 3 applies to asset or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The Company’s financial instruments consist of cash, receivables, accounts payable and accrued liabilities, notes payable, due to related parties and derivative liabilities. Except for cash and derivative liabilities, the Company’s financial instruments’ carrying amounts, excluding unamortized discounts, approximate their fair values due to their short term to maturity. Cash is measured and recognized at fair value based on level 1 inputs for all periods presented. Derivative liabilities are measured and recognized at fair value based on level 3 inputs.
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| December 31, 2022: | ||||||||||||||||
| Cash | $ | 1,154,901 | $ | - | $ | - | $ | 1,154,901 | ||||||||
| Derivative liabilities | - | - | 68,455 | 68,455 | ||||||||||||
| $ | 1,154,901 | $ | - | $ | 68,455 | $ | 1,223,356 | |||||||||
| December 31, 2021: | ||||||||||||||||
| Cash | $ | 411,858 | $ | - | $ | - | $ | 411,858 | ||||||||
Loss per Share
The Company computes net income (loss) per share in accordance with ASC 260, “Earnings per Share”. ASC 260 requires presentation of both basic and diluted earnings per share (“EPS”) on the face of the consolidated statement of operations. Basic EPS is computed by dividing net income (loss) available to common shareholders (numerator) by the weighted average number of shares outstanding (denominator) during the period. Diluted EPS gives effect to all dilutive potential shares of common stock outstanding during the period using the treasury stock method and convertible preferred stock using the if-converted method. In computing diluted EPS, the average stock price for the period is used in determining the number of shares assumed to be purchased from the exercise of stock options or warrants. Diluted EPS excludes all potential shares if their effect is anti-dilutive.
The Company’s preferred stock, stock options and warrants outstanding as of December 31, 2022 and 2021, are considered potential common shares that could dilute earnings per share, but were not included in the diluted loss per share computation because their effect was antidilutive for the periods presented. As a result, there is no difference between the computation of basic and diluted loss per shares for the periods presented.
Share-Based Compensation
Employees - The Company accounts for share-based compensation under the fair value method which requires all such compensation to employees, including the grant of employee stock options, to be calculated based on its fair value at the measurement date (generally the grant date), and recognized in the consolidated statement of operations over the requisite service period.
F-17
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Nonemployees - During June 2018, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) 2018-07, Compensation-Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting (“ASU 2018-07”) to simplify the accounting for share-based payments to nonemployees by aligning it with the accounting for share-based payments to employees. Under the requirements of ASU 2018-07, the Company accounts for share-based compensation to non-employees under the fair value method which requires all such compensation to be calculated based on the fair value at the measurement date (generally the grant date), and recognized in the statement of operations over the requisite service period.
During the years ended December 31, 2022 and 2021, the Company recorded $171,869 and $142,355, respectively, in share-based compensation expense, of which $164,907 and $6,962, and $136,486 and $5,869, respectively is included in office and administration and research and development, respectively.
Determining the appropriate fair value model and the related assumptions requires judgment. During the years ended December 31, 2022 and 2021, the fair value of each option grant was estimated using a Black-Scholes option-pricing model.
The expected volatility represents the historical volatility of comparable publicly traded companies in similar industries, adjusted for variables such as stock price, market capitalization and life cycle. Due to limited historical data, the expected term for options granted is equal to the contractual life. The risk-free interest rate is based on a treasury instrument whose term is consistent with the expected life of stock options. The Company has not paid and does not anticipate paying cash dividends on its shares of common stock; therefore, the expected dividend yield is assumed to be zero.
New Accounting Standards
Recently Adopted Accounting Standards
In August 2020, the FASB issued ASU 2020-06, ASC Subtopic 470-20 “Debt—Debt with Conversion and Other Options” and ASC subtopic 815-40 “Hedging—Contracts in Entity’s Own Equity”. The standard reduced the number of accounting models for convertible debt instruments and convertible preferred stock. Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting; and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. The amendments in this update are effective for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. The adoption of this standard did not have a significant impact on the Company’s consolidated financial statements.
F-18
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Recently Issued Accounting Standards
The Company assesses the adoption impacts of recently issued, but not yet effective, accounting standards by the Financial Accounting Standards Board on the Company’s consolidated financial statements.
In March 2022, the FASB issued ASU 2022-02, ASC Subtopic 326 “Credit Losses”: Troubled Debt Restructurings and Vintage Disclosures. Since the issuance of Accounting Standards Update No. 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments, the Board has provided resources to monitor and assist stakeholders with the implementation of Topic 326. Post-Implementation Review (PIR) activities have included forming a Credit Losses Transition Resource Group, conducting outreach with stakeholders of all types, developing educational materials and staff question-and-answer guidance, conducting educational workshops, and performing an archival review of financial reports. ASU No. 2022-02 is effective for annual and interim periods beginning after December 15, 2022.
The Company does not expect the standard to have a significant impact on its consolidated financial statements.
In June 2022, the FASB issued ASU 2022-03, ASC Subtopic 820 “Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions”. The FASB is issuing this Update (1) to clarify the guidance in Topic 820, Fair Value Measurement, when measuring the fair value of an equity security subject to contractual restrictions that prohibit the sale of an equity security, (2) to amend a related illustrative example, and (3) to introduce new disclosure requirements for equity securities subject to contractual sale restrictions that are measured at fair value in accordance with Topic 820.
Stakeholders asserted that the language in the illustrative example resulted in diversity in practice on whether the effects of a contractual restriction that prohibits the sale of an equity security should be considered in measuring that equity security’s fair value. Some stakeholders apply a discount to the price of an equity security subject to a contractual sale restriction, whereas other stakeholders consider the application of a discount to be inappropriate under the principles of Topic 820.
For public business entities, the amendments in this Update are effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2024, and interim periods within those fiscal years. Early adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance.
The Company does not expect the standard to have a significant impact on its consolidated financial statements.
F-19
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
| 4. | Receivables |
As of December 31, 2022 and 2021, receivables consisted of the following:
| December 31, 2022 | December 31, 2021 | |||||||
| Trade receivable | $ | 4,180 | $ | - | ||||
| Sales taxes receivable | 8,674 | 1,710 | ||||||
| $ | 12,854 | $ | 1,710 | |||||
The Company records sales taxes receivable for recoverable sales taxes paid on eligible purchases in its Canadian subsidiary.
| 5. | Prepaids and Deposits |
As of December 31, 2022 and 2021, prepaid and deposits consisted of the following:
| December 31, 2022 | December 31, 2021 | |||||||
| Prepaid expenses | $ | 89,819 | $ | 31,176 | ||||
| Deposits | 24,376 | 30,964 | ||||||
| Deferred share issuance and listing expense | 50,000 | - | ||||||
| $ | 164,195 | $ | 62,140 | |||||
As of December 31, 2022 and 2021, the security deposit on the Company’s long term lease in the amount of $10,773 and $Nil, respectively, is classified as a non-current deposit on the balance sheet.
| 6. | Inventory |
As of December 31, 2022 and 2021, inventory consisted of the following:
| December 31, 2022 | December 31, 2021 | |||||||
| Raw materials | $ | 81,133 | $ | 84,020 | ||||
| Work in progress | 116,984 | 28,116 | ||||||
| Finished goods | 32,028 | 48,681 | ||||||
| $ | 230,145 | $ | 160,817 | |||||
Cost of inventory recognized as expense in cost of sales for the years ended December 31, 2022 and 2021, totaled $251,580 and $88, respectively.
F-20
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
| 7. | Property and Equipment |
| Equipment | Furniture and Fixtures | Computers | Total | |||||||||||||
| Cost | ||||||||||||||||
| Balance, December 31, 2020 | $ | - | $ | - | $ | - | $ | - | ||||||||
| Additions | 32,482 | - | - | 32,482 | ||||||||||||
| Balance, December 31, 2021 | $ | 32,482 | $ | - | $ | - | $ | 32,482 | ||||||||
| Additions | 24,222 | 8,365 | 2,940 | 35,527 | ||||||||||||
| Disposal | (6,188 | ) | - | - | (6,188 | ) | ||||||||||
| Foreign currency translation | - | - | (181 | ) | (181 | ) | ||||||||||
| Balance, December 31, 2022 | $ | 50,516 | $ | 8,365 | $ | 2,759 | $ | 61,640 | ||||||||
| Accumulated depreciation | ||||||||||||||||
| Balance, December 31, 2020 | $ | - | $ | - | $ | - | $ | - | ||||||||
| Depreciation expense | 2,757 | - | - | 2,757 | ||||||||||||
| Balance, December 31, 2021 | $ | 2,757 | $ | - | $ | - | $ | 2,757 | ||||||||
| Depreciation expense | 5,437 | 548 | 527 | 6,512 | ||||||||||||
| Disposal | (1,142 | ) | - | - | (1,142 | ) | ||||||||||
| Foreign currency translation | - | - | (22 | ) | (22 | ) | ||||||||||
| Balance, December 31, 2022 | $ | 7,052 | $ | 548 | $ | 505 | $ | 8,105 | ||||||||
| Net book value | ||||||||||||||||
| December 31, 2021 | $ | 29,725 | $ | - | $ | - | $ | 29,725 | ||||||||
| December 31, 2022 | $ | 43,464 | $ | 7,817 | $ | 2,254 | $ | 53,535 | ||||||||
During the years ended December 31, 2022 and 2021, the Company capitalized depreciation of $1,478 and $372, respectively as part of the production of inventory.
| 8. | Operating Lease |
During 2022, the Company entered into a noncancelable operating lease that includes two property location, one which is being used as the Company’s office and the other as its lab for research and development and the production of inventory. The lease had a commencement date of June 1, 2022 and expires on May 31, 2025, after which the term will continue on a month-to-month basis.
The Company recognized a total lease cost related to its noncancelable operating lease of $73,802 for the year ended December 31, 2022, compared to a cash outflow related to lease payments of $67,138. The lease cost has been allocated as follows based on the square footage of each property location.
| December 31, 2022 | ||||
| Office space, recorded in office and administration | $ | 51,745 | ||
| Lab space, recorded in research and development | 19,004 | |||
| Lab space, capitalized to production of inventory | 3,053 | |||
| $ | 73,802 | |||
F-21
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
As of December 31, 2022 the Company recorded a security deposit of $10,773 (note 5).
Future minimum lease payments under the Company’s operating lease that has an initial noncancelable lease term in excess of one year at December 31, 2022 are as follows:
| Year ended December 31, | Total | |||
| 2023 | $ | 129,276 | ||
| 2024 | 129,276 | |||
| 2025 | 53,865 | |||
| Thereafter | - | |||
| 312,417 | ||||
| Less: Imputed interest | (29,200 | ) | ||
| Operating lease liability | 283,217 | |||
| Operating lease lability – current | 110,616 | |||
| Operating lease lability – non-current | $ | 172,601 | ||
The Company used a discount rate of 8% as its incremental cost of borrowing and the remaining lease term as of December 31, 2022 is 2.42 years.
| 9. | Accounts Payable and Accrued Liabilities |
As of December 31, 2022 and 2021, accounts payable and accrued liabilities consisted of the following:
| December 31, 2022 | December 31, 2021 | |||||||
| Accounts payable | $ | 222,461 | $ | 98,175 | ||||
| Accrued liabilities | 33,864 | 93,927 | ||||||
| $ | 256,325 | $ | 192,102 | |||||
As of December 31, 2022 and 2021, accounts payable and accrued liabilities include $11,621 and $Nil, respectively that is due to related parties in the ordinary course of business.
| 10. | Notes Payable |
On August 24, 2020, the Company received $24,000 in short-term financing (the “notes payable”) from two unrelated third parties. The notes payable bear interest at 6% per annum and had a maturity date of August 24, 2021. On January 24, 2021, the notes payable and accrued interest of $600 were settled upon conversion into common stock.
In April and May 2022, the Company issued promissory notes to five investors (including two related parties of the Company) for a total amount of $183,970. The promissory notes carried simple interest at a rate of 8% per annum. On July 15 2022, the promissory notes and accrued interest of $2,614, converted into the Series A financing round in accordance with the original terms of the agreements. The conversion price was set at $0.80 (60% of the Series A financing round price) and as a result the noteholders received 231,828 Series A preferred shares. In addition, the conversion terms contained a 100% warrant coverage ratio resulting in the note holders receiving 231,828 common stock purchase warrants with an exercise price of $2.01 (150% of the Series A financing round price).
F-22
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
| 11. | Derivative liabilities |
We analyzed the common stock purchase warrants issued as partial settlement of the promissory notes payable on July 15, 2022, against the requirements of ASC 480, Distinguishing Liabilities from Equity, and determined that the warrants should be classified as financial liabilities since the terms allows for a cashless net share settlement at the option of the holder.
ASC 815, Derivatives and Hedging, requires that the warrants be accounted for as derivative liabilities with initial and subsequent measurement at fair value with changes in fair value recorded as other income (expense).
A continuity of the Company’s common stock purchase derivative liability warrants is as follows:
| Derivative liabilities | ||||
| Balance, January 1, 2021 and December 31, 2021 | $ | - | ||
| Addition of new derivatives recognized as partial settlement of promissory notes | 55,701 | |||
| Change in fair value of derivative liabilities | 12,754 | |||
| Outstanding, December 31, 2022 | $ | 68,455 | ||
We determined our derivative liabilities to be a Level 3 fair value measurement during the year and used the Black-Scholes Option Pricing Model to calculate the fair value as of initial recognition and as of December 31, 2022. The Black-Scholes Option Pricing Model requires six basic data inputs: the exercise or strike price, expected time to expiration or exercise, the risk-free interest rate, the current stock price, the estimated volatility of the stock price in the future, and the dividend rate. Changes to these inputs could produce a significantly higher or lower fair value measurement.
The following assumptions were used in the Black-Scholes option pricing model:
| December 31, 2022 | July 15, 2022 | |||||||
| Risk-free interest rate | 4.73 | % | 3.12 | % | ||||
| Expected life 1 | 0.75 years | 0.6 years | ||||||
| Expected dividend rate | 0.00 | 0.00 | % | |||||
| Expected volatility | 100 | % | 100.00 | % | ||||
F-23
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
As of December 31, 2022, the following warrants were outstanding:
| Outstanding | Expiry date1 | Weighted average exercise price ($) | ||||||
| 75,840 | April 27, 2027 | 2.01 | ||||||
| 63,037 | May 9, 2027 | 2.01 | ||||||
| 80,388 | May 24, 2027 | 2.01 | ||||||
| 12,563 | May 25, 2027 | 2.01 | ||||||
| 231,828 | 2.01 | |||||||
As of December 31, 2022, the weighted average life of warrants outstanding was 4.36 years.
| 1 | Although the warrants had an original term to expiry of 5 years, the Company used the expected time to complete its IPO as the expected life in the Black-Scholes option pricing model as any outstanding warrants would automatically convert to common stock (net share settlement) upon completion of the Company’s IPO. |
| 12. | Equity |
Common Stock
Authorized
As of December 31, 2022 and 2021, the Company had 19,000,000 common stock authorized, each having a par value of $0.0001.
Issued and outstanding
As of December 31, 2022 and 2021, the Company had 9,568,475 and 9,526,808 shares issued and outstanding, respectively
Transactions during the year ended December 31, 2022
On December 13, 2022, the Company issued 41,667 common stock upon the exercise of 41,667 stock options with an exercise price of $0.60 per common stock for $25,000, of which $5 was recognized in common stock and the remaining $24,995 in additional paid-in capital.
Transactions during the year ended December 31, 2021
On January 24, 2021, the Company issued 1,046,808 common stock upon conversion of $24,600 of notes payable and accrued interest, of which $104 was recognized in common stock and the remaining $24,496 in additional paid-in capital.
F-24
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Preferred Stock
Authorized
As of December 31, 2022 and 2021, the Company had 213,730 stock of Series Seed 1 preferred stock authorized, each having a par value of $0.0001 per stock.
As of December 31, 2022 and 2021, the Company had 3,635,252 stock of Series Seed 2 preferred stock authorized, each having a par value of $0.0001 per stock.
As of December 31, 2022, the Company had 2,982,003 (2021 – Nil) stock of Series A preferred stock authorized, each having a par value of $0.0001 per stock.
The holders of Preferred Stock shall have the right to convert their shares of Preferred Stock, at any time, into shares of Common Stock at a conversion price of 1:1.
Issued and outstanding
As of December 31, 2022 and 2021, the Company had 213,730 Series Seed 1 preferred stock issued and outstanding.
As of December 31, 2022 and 2021, the Company had 3,635,252 Series Seed 2 preferred stock issued and outstanding.
As of December 31, 2022 and 2021, the Company had 1,861,799 and Nil Series A preferred stock issued and outstanding, respectively.
Transactions during the year ended December 31, 2022
On July 15, 2022, the Company closed the first tranche of its Series A Financing and issued 1,090,029 Series A preferred shares for gross proceeds of $1,462,146, of which $109 was recognized in preferred stock and the remaining $1,462,037 in additional paid-in capital. In addition, the Company issued 231,828 Series A preferred shares and 231,828 common stock purchase warrants upon conversion of $186,584 of promissory notes and accrued interest, of which $23 was recognized in preferred stock, $55,701 as derivative liabilities at fair value, and the remaining $130,860 in additional paid-in capital.
On July 27, 2022, the Company closed the second tranche of its Series A Financing and issued 349,790 Series A preferred shares for gross proceeds of $469,207, of which $35 was recognized in preferred stock and the remaining $469,172 in additional paid-in capital.
On August 4, 2022, the Company closed the third tranche of its Series A Financing and issued 111,884 Series A preferred shares for gross proceeds of $150,080, of which $11 was recognized in preferred stock and the remaining $150,069 in additional paid-in capital.
On October 10, 2022, the Company closed the fourth tranche of its Series A Financing and issued 78,268 Series A Preferred shares for gross proceeds of $104,988, of which $8 was recognized in preferred stock and the remaining $104,980 in additional paid-in capital.
F-25
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
The Company incurred shared issuance cost of $33,132 in connection with its Series A Financing which has been recorded as a deduction from additional paid-in capital.
Transactions during the year ended December 31, 2021
On August 24, 2021, the Company issued 3,635,252 Series Seed 2 preferred stock for $1,090,577, of which $364 was recognized in preferred stock and the remaining $1,090,213 in additional paid-in capital.
Stock Options
The Company has a stock option plan included in the Company’s 2020 Equity Incentive Plan (the “Plan”) where the Board of Directors or any of its committees can grant Incentive Stock Options, Nonstatutory Stock Options, and Restricted Stock to employees, advisors and directors of the Company. As of December 31, 2022 and 2021, the aggregate number of shares allocated and made available for issuance pursuant to stock options granted under the Plan shall not exceed 1,534,188 shares (note 17). The plan shall remain in effect until it is terminated by the Board of Directors.
Transactions during the year ended December 31, 2022
On April 25, 2022, the Company granted 45,000 stock options with a contractual life of ten years and exercise price of $0.60 per common stock. These stock options were valued at $11,617 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
From June 1, 2022 to November 1, 2022, the Company granted 262,000 stock options with a contractual life of ten years and exercise price of $1.34 per common stock. These stock options were valued at $317,652 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
From September 1, 2022 to December 12, 2022, the Company granted 105,000 stock options with a contractual life of ten years and exercise price of $5.00 per common stock. These stock options were valued at $115,868 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
Transactions during the year ended December 31, 2021
On February 9, 2021, the Company granted 841,667 stock options with a contractual life of ten years and exercise price of $0.60 per common stock. These stock options were valued at $214,595 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
On February 28, 2021, the Company granted 50,000 stock options with a contractual life of ten years and exercise price of $0.60 per common stock. These stock options were valued at $12,775 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
F-26
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
On July 20, 2021, the Company granted 200,000 stock options with a contractual life of ten years and exercise price of $0.60 per common stock. These stock options were valued at $51,014 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
On August 16, 2021, the Company granted 41,667 stock options with a contractual life of ten years and exercise price of $0.60 per common stock. These stock options were valued at $10,630 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
The following assumptions were used in the Black-Scholes option pricing model:
| December 31, 2022 | December 31, 2021 | |||||||
| Risk-free interest rate | 2.81% - 4.07 | % | 1.18% - 1.45 | % | ||||
| Expected life | 10 years | 10 years | ||||||
| Expected dividend rate | 0.00 | % | 0.00 | % | ||||
| Expected volatility | 100 | % | 100.00 | % | ||||
| Forfeiture rate | 0.00 | % | 0.00 | % | ||||
The continuity of stock options for the years ended December 31, 2022 and 2021 is summarized below:
| Number of stock options | Weighted average exercise price | |||||||
| Outstanding, January 1, 2021 | - | $ | - | |||||
| Granted | 1,133,334 | 0.60 | ||||||
| Outstanding, December 31, 2021 | 1,133,334 | $ | 0.60 | |||||
| Granted | 412,000 | 2.19 | ||||||
| Forfeited | (137,500 | ) | 0.60 | |||||
| Exercised | (41,667 | ) | 0.60 | |||||
| Outstanding, December 31, 2022 | 1,366,167 | 1.08 | ||||||
As of December 31, 2022, the following options were outstanding, entitling the holders thereof the right to purchase one common stock for each option held as follows:
| Outstanding | Vested | Expiry date | Weighted average exercise price ($) | |||||||||
| 841,667 | 403,302 | February 8, 2031 | 0.60 | |||||||||
| 50,000 | 23,962 | February 27, 2031 | 0.60 | |||||||||
| 62,500 | 62,500 | July 19, 2031 | 0.60 | |||||||||
| 45,000 | - | April 25, 2032 | 0.60 | |||||||||
| 16,000 | - | June 1, 2032 | 1.34 | |||||||||
| 110,000 | - | July 1, 2032 | 1.34 | |||||||||
| 100,000 | - | August 8, 2032 | 1.34 | |||||||||
| 16,000 | - | September 30, 2032 | 1.34 | |||||||||
| 80,000 | - | September 30, 2032 | 5.00 | |||||||||
| 10,000 | - | October 15, 2032 | 1.34 | |||||||||
| 10,000 | - | November 1, 2032 | 1.34 | |||||||||
| 5,000 | - | November 1, 2032 | 5.00 | |||||||||
| 20,000 | - | December 12, 2032 | 5.00 | |||||||||
| 1,366,167 | 489,764 | 1.08 | ||||||||||
As of December 31, 2022 and 2021, the weighted average life of stock options outstanding was 8.58 years and 9.21 years, respectively.
F-27
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
| 13. | Related Party Transactions |
Related parties consist of the following individuals and corporations:
| ● | Braeden Lichti, former Chairman and President, significant shareholder through BWL Investments Ltd. Resigned as President effective October 11, 2022. | |
| ● | Jordan R. Plews, CEO and Director, significant shareholder through JP Bio Consulting LLC | |
| ● | Graydon Bensler, CFO and Director | |
| ● | Yi Guo, Former Director, resigned effective September 29, 2022 | |
| ● | Tim Sayed, Chief Medical Officer | |
| ● | Brenda Buechler, Chief Marketing Officer | |
| ● | Christoph Kraneiss, Chief Commercial Officer | |
| ● | GB Capital Ltd., controlled by Graydon Bensler | |
| ● | JP Bio Consulting LLC, significant shareholder and controlled by Jordan R. Plews | |
| ● | BWL Investments Ltd., significant shareholder and controlled by Braeden Lichti | |
| ● | Northstrive Companies Inc., controlled by Braeden Lichti |
Key management personnel include those persons having authority and responsibility for planning, directing, and controlling the activities of the Company as a whole. The Company has determined that key management personnel consist of members of the Company’s Board of Directors, corporate officers, and individuals with more than 10% control.
Remuneration attributed to key management personnel are summarized as follows:
| December 31, 2022 | December 31, 2021 | |||||||
| Consulting fees | $ | 215,078 | $ | 46,919 | ||||
| Salaries | 403,180 | 50,000 | ||||||
| Share-based compensation | 129,980 | 127,196 | ||||||
| $ | 748,238 | $ | 224,115 | |||||
The Company incurred consulting fees of $95,078 (2021 - $46,919) to GB Capital Ltd., a company controlled by Graydon Bensler, CFO and Director. In addition, the Company incurred consulting fees of $120,000 (2021 - $nil) to Northstrive Companies Inc., a company controlled by the Company’s former Chairman and President.
F-28
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Jordan R. Plews, CEO and Director, earned a Salary of $222,446 and $50,000, respectively during the years ended December 31, 2022 and 2021 (includes a bonus of $10,000 and employer taxes of $12,446).
Brenda Buechler, Chief Marketing Officer, earned a Salary of $107,937 during the year ended December 31, 2022 (includes a bonus of $5,000 and employer taxes of $7,937).
Christoph Kraneiss, Chief Commercial Officer, earned a Salary of $72,792 during the year ended December 31, 2022 (includes employer taxes of $5,292).
On February 9, 2021, the Company granted 800,000 stock options to four related parties (200,000 stock options each) with a contractual life of ten years and exercise price of $0.60 per share of common stock. These stock options were valued at $203,972 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter. Details of the fair value granted to each individual and the related expense recorded for the years ended December 31, 2022 and 2021 are as follow:
| December 31, 2022 | December 31, 2021 | Fair value of stock options granted | ||||||||||
| Braeden Lichti, former Chairman and President | $ | 14,181 | $ | 28,170 | $ | 50,993 | ||||||
| Graydon Bensler, CFO and Director | 14,181 | 28,170 | 50,993 | |||||||||
| Jordan R. Plews, CEO and Director | 14,181 | 28,170 | 50,993 | |||||||||
| Tim Sayed, Chief Medical Officer | 14,181 | 28,170 | 50,993 | |||||||||
| $ | 56,724 | $ | 112,680 | $ | 203,972 | |||||||
On July 20, 2021, the Company granted 200,000 stock options to a related party, Yi Guo, former Director, with a contractual life of ten years and exercise price of $0.60 per share of common stock. These stock options were valued at $51,014 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter. On October 17, 2022, Yi Guo resigned from the board of directors of the Company and as a result, 137,500 unvested options were forfeited. The remaining 62,500 vested option remain exercisable for 3 months after the resignation. The share-based compensation expense recorded for the years ended December 31, 2022 and 2021 relating to these stock options was $1,424 and $14,516, respectively.
F-29
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
During 2022, the Company granted 250,000 stock options to two related parties (150,000 stock options to Brenda Buechler, Chief Marketing Officer, and 100,000 options to Christoph Kraneiss, Chief Commercial Officer) with a contractual life of ten years and weighted average exercise price of $1.22 per share of common stock. These stock options were valued at $264,906 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter. Details of the fair value granted to each individual and the related expense recorded for the year ended December 31, 2022 is as follow:
| December 31, 2022 | Fair value of stock options granted | |||||||
| Brenda Buechler, Chief Marketing Officer | $ | 43,488 | $ | 143,679 | ||||
| Christoph Kraneiss, Chief Commercial Officer | 28,344 | 121,227 | ||||||
| $ | 71,832 | $ | 264,906 | |||||
BWL Investments Ltd. and Tim Sayed, Chief Medical Officer subscribed to $48,980 and $10,000 in promissory notes (Note 10), respectively. On July 15,2022, these promissory notes and accrued interest were converted into Series A preferred shares and warrants as follows:
| Series A preferred shares | Warrants | Promissory notes and accrued interest | ||||||||||
| BWL Investments Ltd. | 61,551 | 61,551 | $ | 49,538 | ||||||||
| Tim Sayed, Chief Medical Officer | 12,563 | 12,563 | 10,112 | |||||||||
| 74,114 | 74,114 | $ | 59,650 | |||||||||
As of December 31, 2022 and 2021, the Company had $142,705 and $23,520, respectively due to companies controlled by Braeden Lichti, of which $22,705 and $23,520, respectively is unsecured, non-interest bearing and are due on demand. The remaining $120,000 due as of December 31, 2022, is payable to Northstrive Companies Inc. for consulting services rendered by Braeden Lichti (the “Fees”). Payment of the Fees will be deferred until the earlier of either (a) the Company raising an aggregate of at least $2,000,000 of equity and/or debt investment from and after October 1, 2022, (b) the Company becomes listed on any established stock exchange or a national market system including without limitation the New York Stock Exchange, the Nasdaq Capital Market of The Nasdaq Stock Market, or (c) the Board determines that the Company has sufficient cash flows to support payment of the foregoing amounts of Fees due at the time of that determination. The Fees shall be payable in cash payment or in the form of Series A preferred stock priced at $1.34138 per share (the “Original Series A Issue Price”) equal to the value of the Fees then due.
As of December 31, 2022, accounts payable and accrued liabilities include $7,165 in consulting fees payable to Graydon Bensler, CFO and Director, $1,485 to companies controlled by Braeden Lichti, and $2,971 to Jordan R. Plews, CEO and Director, for expenses incurred on behalf of the Company.
| 14. | Income Tax |
During the years ended December 31, 2022 and 2021, there is $Nil and $Nil current and deferred income tax expense, respectively, reflected in the Statement of Operations and Comprehensive Loss.
F-30
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
The following are the components of income before income tax reflected in the Consolidated Statement of Operations and Comprehensive Loss for the years ended December 31, 2022 and 2021:
Component of Loss Before Income Tax
| December 31, 2022 | December 31, 2021 | |||||||
| Net loss before income tax | $ | (1,800,268 | ) | $ | (784,739 | ) | ||
| Effective tax rate | 27.87 | % | 27.87 | % | ||||
| Expected recovery | (501,735 | ) | (218,707 | ) | ||||
| Share-based compensation | 47,906 | 39,678 | ||||||
| Other non-deductible items | 3,555 | - | ||||||
| Foreign exchange | 3,602 | 217 | ||||||
| Tax rate differences | 1,292 | 549 | ||||||
| Change in valuation allowance | 445,380 | 178,263 | ||||||
| Tax expense (recovery) | - | - | ||||||
Deferred income taxes arise from temporary differences between the tax and financial statement recognition of revenue and expense. In evaluating the ability to recover the deferred tax assets within the jurisdiction from which they arise, the Company considered all available positive and negative evidence, including scheduled reversals of deferred tax liabilities, projected future taxable income, tax planning strategies, and recent financial operations. In projecting future taxable income, the Company began with historical results adjusted for changes in accounting policies and incorporates assumptions including the amount of future pretax operating income, the reversal of temporary differences, and the implementation of feasible and prudent tax planning strategies. These assumptions require significant judgement about the forecasts of future taxable income and are consistent with the plans and estimates the Company is using to manage the underlying businesses. In evaluating objective evidence that historical results provide, the Company consider three years of cumulative operating income (loss).
During the year ended December 31, 2022, the Company had aggregate net operating losses for income tax purposes of $1,615,645 (2021 – $642,237) to offset future taxable income in the United States and Canada. As of December 31, 2022, the deferred tax asset related to these loss carry forwards amounted to approximately $662,000 (2021 - $217,000) and were fully reserved. Management believes that it is not yet more likely than not that these assets will be realized in the near future.
| 15. | Commitments and Contingencies |
There were no commitments as of December 31, 2022 and 2021 or during the years then ended.
As of December 31, 2022, the Company had an ongoing dispute with a vendor regarding unpaid invoices. The Company disputed the services claimed to have been rendered by the vendor. In May 2023, the Company and the vendor agreed to settle the matter, resulting in the Company agreeing to pay a final settlement of $9,225, an amount that is significantly less than the unpaid invoices originally claimed by the vendor. The Company included the settlement amount in accrued liabilities as of December 31, 2022. There were no contingencies as of December 31, 2021 or during the year then ended.
F-31
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
| 16. | Concentrations |
Customers
During the year ended December 31, 2022, the Company recorded 54% of its revenue from its two largest customers, each representing 45% and 9% respectively. The Company’s largest customer, representing $344,018 of revenue, relates to a white label distributor agreement signed during the year. There was no significant concentration of sales during the year ended December 31, 2021.
As of December 31, 2022, the Company had no receivables due from these customers and $5,992 in customer deposits were received from its largest customer.
The Company expects its dependence on these major customers to decrease over time as it enters into additional distributor agreements and builds out its sales team.
Suppliers
During the year ended December 31, 2022, the Company had 5 key suppliers that represented approximately 80% (2021 – 73%) of the cost incurred in the purchase and production of inventory. The table below represents a breakdown of each supplier as a percentage of the cost incurred (Suppliers are shown from largest to smallest and does not necessarily represent the same suppliers year over year):
| December 31, 2022 | December 31, 2021 | |||||||
| Supplier 1 | 39 | % | 18 | % | ||||
| Supplier 2 | 14 | % | 17 | % | ||||
| Supplier 3 | 11 | % | 15 | % | ||||
| Supplier 4 | 8 | % | 13 | % | ||||
| Supplier 5 | 8 | % | 10 | % | ||||
| 80 | % | 73 | % | |||||
The Company continually evaluates the performance of its suppliers and the availability of alternatives to substitute or supplement its inventory production supply chain. The Company believes that a breakdown in supply from one of its key suppliers would be overcome in a short amount of time given the availability of alternatives.
| 17. | Subsequent Events |
Management has evaluated events subsequent to the year ended December 31, 2022 up to June 13, 2023, for transactions and other events that may require adjustment of and/or disclosure in the consolidated financial statements.
Authorized Common Stock and Option Pool Increase
The Company increased the number of common stock authorized for issuance from 19,000,000 to 300,000,000. In addition, the Company increased the aggregate number of shares allocated and made available for issuance pursuant to stock options granted under the Plan from 1,534,188 to 1,734,188 shares.
F-32
Elevai Labs, Inc.
Notes to the Consolidated Financial Statements
For the years ended December 31, 2022 and 2021
(Expressed in United States dollars)
Stock Options
In January 2023, 62,500 stock options with an exercise price of $0.60 were exercised in exchange for 62,500 shares of common stock for total proceeds of $37,500.
The Company granted 172,500 stock options (includes 80,000 each to two of its newly appointed independent directors) with a contractual life of ten years and exercise price of $5.00 per common stock.
Common Shares and Warrants
On March 2, 2023, the Company issued 250,000 common stock and 250,000 warrants to purchase common stock for total proceeds of $750,000. Each warrant is exercisable at $3.00 per common stock. The warrants shall be exercisable, in whole or in part at the issue date but such exercisability shall cease upon the date of the Company’s IPO and listing of its common shares on the Nasdaq Capital Market or other Trading Market and shall continue to be exercisable in whole or in part immediately after the Lock-up Period but no later than the Warrant Expiration Date or Accelerated Warrant Expiration Date (the “Exercise Period”). In the event of the Company’s initial public offering and listing of shares of its common stock on a Trading Market, the Company shall notify the holder at least fifteen (15) calendar days prior to the consummation of such IPO. “Trading Market” shall mean a “national securities exchange” that has registered with the SEC under Section 6 of the Securities Exchange Act of 1934. The Expiration Date shall be the earlier of (i) three years and one hundred eighty (180) days from the issue date (the “Warrant Expiration Date”) or (ii) upon the Company’s reasonable judgment and written notice to the purchaser, of the Company’s option to accelerate the Warrant Expiration Date whereby upon purchaser’s receipt of the Company’s written notice of acceleration during the Exercise Period, the Purchaser’s option to exercise any number of warrants shall occur no later than fourteen (14) days following the receipt of the written notice of acceleration (the “Accelerated Warrant Expiration Date”). For the avoidance of doubt, it shall be reasonable for the Company to accelerate the Expiration Date of this warrant to coincide with transactions including, but not limited to (i) a change of control including but not limited to the voluntary or involuntary sale, assignment, transfer or other disposition, or transfer by operation of law, of more than 50% of any direct or indirect equity interest of the Company; or (ii) a subsequent capital financing other than the IPO consisting of but not limited to an offer or proposal for, or indication of interest in, the issuance of debt or the capital stock of the Company.
In April and May 2023, the Company issued an additional 107,861 common stock for total proceeds of $323,589.
F-33
Condensed Consolidated Financial Statements of

For the six months ended June 30, 2023, and 2022
(Expressed in United States Dollars)
(Unaudited)
F-34
Elevai Labs Inc.
Condensed Consolidated Balance Sheets
As of June 30, 2023, and December 31, 2022
(Unaudited - Expressed in United States dollar)
| As of: | June 30, 2023 | December 31, 2022 | ||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | 601,265 | $ | 1,154,901 | ||||
| Receivables, net | 34,437 | 12,854 | ||||||
| Prepaids and deposits | 336,940 | 153,422 | ||||||
| Inventory, net | 477,863 | 230,145 | ||||||
| Total Current Assets | 1,450,505 | 1,551,322 | ||||||
| Deposits | 10,773 | 10,773 | ||||||
| Property and equipment, net | 58,950 | 53,535 | ||||||
| Operating lease right-of-use asset | 223,726 | 276,553 | ||||||
| TOTAL ASSETS | $ | 1,743,954 | $ | 1,892,183 | ||||
| LIABILITIES | ||||||||
| Current Liabilities | ||||||||
| Accounts payable and accrued liabilities | $ | 631,605 | $ | 256,325 | ||||
| Customer deposits | 85,366 | 10,172 | ||||||
| Due to related parties | 202,982 | 142,704 | ||||||
| Derivative liabilities | 527,701 | 68,455 | ||||||
| Current portion of operating lease liability | 115,115 | 110,616 | ||||||
| Total Current Liabilities | 1,562,769 | 588,272 | ||||||
| Operating lease liability | 113,897 | 172,601 | ||||||
| TOTAL LIABILIITES | $ | 1,676,666 | $ | 760,873 | ||||
| EQUITY | ||||||||
| Series seed 1 preferred stock, $0.0001 par value, 213,730 shares authorized; 213,730 shares issued and outstanding as of June 30, 2023 and December 31, 2022 | 21 | 21 | ||||||
| Series seed 2 preferred stock, $0.0001 par value, 3,635,252 shares authorized; 3,635,252 shares issued and outstanding as of June 30, 2023 and December 31, 2022 | 364 | 364 | ||||||
| Series A preferred stock, $0.0001 par value, 2,982,003 shares authorized; 1,861,799 shares issued and outstanding as of June 30, 2023 and December 31, 2022 | 186 | 186 | ||||||
| Common stock, $0.0001 par value, 300,000,000 shares authorized; 9,988,836 and 9,568,475 shares issued and outstanding as of June 30, 2023 and December 31, 2022, respectively | 999 | 957 | ||||||
| Additional paid-in capital | 5,148,159 | 3,852,044 | ||||||
| Accumulated other comprehensive income | 486 | 111 | ||||||
| Accumulated deficit | (5,082,927 | ) | (2,722,373 | ) | ||||
| TOTAL EQUITY | 67,288 | 1,131,310 | ||||||
| TOTAL LIABILITIES AND EQUITY | $ | 1,743,954 | $ | 1,892,183 | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements
F-35
Elevai Labs Inc.
Condensed Consolidated Statements of Operations and Comprehensive Loss
For the three and six months ended June 30, 2023 and 2022
(Unaudited – Expressed in United States dollar)
| Three months ended June 30, 2023 | Three months ended June 30, 2022 | Six months ended June 30, 2023 | Six months ended June 30, 2022 | |||||||||||||
| Revenue | $ | 316,530 | 170,464 | 459,350 | 195,257 | |||||||||||
| Cost of sales | 108,180 | 64,857 | 152,613 | 79,052 | ||||||||||||
| Gross profit | $ | 208,350 | 105,607 | 306,737 | 116,205 | |||||||||||
| Expenses | ||||||||||||||||
| Depreciation | 2,888 | 807 | 5,385 | 1,695 | ||||||||||||
| Marketing and promotion | 114,051 | 20,144 | 216,727 | 61,489 | ||||||||||||
| Consulting fees | 149,723 | 79,898 | 233,687 | 138,720 | ||||||||||||
| Office and administrative | 529,950 | 174,605 | 964,009 | 327,417 | ||||||||||||
| Professional fees | 168,933 | 12,065 | 306,730 | 45,159 | ||||||||||||
| Investor relations | 37,452 | 8,164 | 75,720 | 13,786 | ||||||||||||
| Research and development | 133,654 | 41,622 | 217,395 | 78,563 | ||||||||||||
| Foreign exchange loss (gain) | 2,374 | (206 | ) | 2,633 | 1,857 | |||||||||||
| Travel and entertainment | 122,655 | 53,097 | 184,170 | 92,603 | ||||||||||||
| Total Expenses | $ | 1,261,680 | 390,196 | 2,206,456 | 761,289 | |||||||||||
| Net loss before other income (expense) | $ | (1,053,330 | ) | (284,589 | ) | (1,899,719 | ) | (645,084 | ) | |||||||
| Other income (expense) | ||||||||||||||||
| Loss on sale of equipment | - | (1,546 | ) | - | (1,546 | ) | ||||||||||
| Interest income | 349 | 86 | 5,456 | 86 | ||||||||||||
| Interest expense | (4,409 | ) | (1,982 | ) | (7,045 | ) | (1,982 | ) | ||||||||
| Change in fair value of derivative liabilities | (222,468 | ) | - | (459,246 | ) | - | ||||||||||
| Net loss | $ | (1,279,858 | ) | (288,031 | ) | (2,360,554 | ) | (648,526 | ) | |||||||
| Other comprehensive income (loss) | ||||||||||||||||
| Currency translation adjustment | 262 | 72 | 375 | 242 | ||||||||||||
| Net loss and comprehensive loss | $ | (1,279,596 | ) | (287,959 | ) | (2,360,179 | ) | (648,284 | ) | |||||||
| Basic and diluted loss per share | $ | (0.128 | ) | $ | (0.030 | ) | $ | (0.240 | ) | $ | (0.068 | ) | ||||
| Weighted average shares outstanding | 9,976,725 | 9,526,808 | 9,838,599 | 9,526,808 | ||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements
F-36
Elevai Labs Inc.
Condensed Consolidated Statements of Changes in Equity
For the six months ended June 30, 2023 and 2022
(Unaudited – Expressed in thousands of United States Dollars, except for share amount)
| Series seed 1 preferred stock | Series seed 2 preferred stock | Series A preferred stock | Common Stock | Additional | Accumulated other | |||||||||||||||||||||||||||||||||||||||||||
| Number of shares | Amount | Number of shares | Amount | Number of shares | Amount | Number of shares | Amount | paid-in capital | Accumulated deficit | comprehensive income | Total | |||||||||||||||||||||||||||||||||||||
| # | $ | # | $ | # | $ | # | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||||||||||||
| Balance, January 1, 2022 | 213,730 | 21 | 3,635,252 | 364 | - | - | 9,526,808 | 952 | 1,371,194 | (922,105 | ) | 202 | 450,628 | |||||||||||||||||||||||||||||||||||
| Series A preferred shares subscription | - | - | - | - | - | - | - | - | 284,960 | - | - | 284,960 | ||||||||||||||||||||||||||||||||||||
| Share-based compensation | - | - | - | - | - | - | - | - | 58,510 | - | - | 58,510 | ||||||||||||||||||||||||||||||||||||
| Net loss for the period | - | - | - | - | - | - | - | - | - | (648,526 | ) | - | (648,526 | ) | ||||||||||||||||||||||||||||||||||
| Currency translation adjustment | - | - | - | - | - | - | - | - | - | - | 242 | 242 | ||||||||||||||||||||||||||||||||||||
| Balance, June 30, 2022 | 213,730 | 21 | 3,635,252 | 364 | - | - | 9,526,808 | 952 | 1,714,664 | (1,570,631 | ) | 444 | 145,814 | |||||||||||||||||||||||||||||||||||
| Balance, January 1, 2023 | 213,730 | 21 | 3,635,252 | 364 | 1,861,799 | 186 | 9,568,475 | 957 | 3,852,044 | (2,722,373 | ) | 111 | 1,131,310 | |||||||||||||||||||||||||||||||||||
| Private placement | - | - | - | - | - | - | 357,861 | 36 | 1,073,553 | - | - | 1,073,589 | ||||||||||||||||||||||||||||||||||||
| Exercise of stock options | - | - | - | - | - | - | 62,500 | 6 | 37,494 | - | - | 37,500 | ||||||||||||||||||||||||||||||||||||
| Share-based compensation | - | - | - | - | - | - | - | - | 185,068 | - | - | 185,068 | ||||||||||||||||||||||||||||||||||||
| Net loss for the period | - | - | - | - | - | - | - | - | - | (2,360,554 | ) | - | (2,360,554 | ) | ||||||||||||||||||||||||||||||||||
| Currency translation adjustment | - | - | - | - | - | - | - | - | - | - | 375 | 375 | ||||||||||||||||||||||||||||||||||||
| Balance, June 30, 2023 | 213,730 | 21 | 3,635,252 | 364 | 1,861,799 | 186 | 9,988,836 | 999 | 5,148,159 | (5,082,927 | ) | 486 | 67,288 | |||||||||||||||||||||||||||||||||||
F-37
Elevai Labs Inc.
Condensed Consolidated Statements of Changes in Equity
For the three months ended June 30, 2023 and 2022
(Unaudited – Expressed in thousands of United States Dollars, except for share amount)
| Series seed 1 preferred stock | Series seed 2 preferred stock | Series A preferred stock | Common Stock | Additional | Accumulated other | |||||||||||||||||||||||||||||||||||||||||||
| Number of shares | Amount | Number of shares | Amount | Number of shares | Amount | Number of shares | Amount | paid-in capital | Accumulated deficit | comprehensive income | Total | |||||||||||||||||||||||||||||||||||||
| # | $ | # | $ | # | $ | # | $ | $ | $ | $ | $ | |||||||||||||||||||||||||||||||||||||
| Balance, April 1, 2022 | 213,730 | 21 | 3,635,252 | 364 | - | - | 9,526,808 | 952 | 1,402,238 | (1,282,600 | ) | 372 | 121,347 | |||||||||||||||||||||||||||||||||||
| Series A preferred shares subscription | - | - | - | - | - | - | - | - | 284,960 | - | - | 284,960 | ||||||||||||||||||||||||||||||||||||
| Share-based compensation | - | - | - | - | - | - | - | - | 27,466 | - | - | 27,466 | ||||||||||||||||||||||||||||||||||||
| Net loss for the period | - | - | - | - | - | - | - | - | - | (288,031 | ) | - | (288,031 | ) | ||||||||||||||||||||||||||||||||||
| Currency translation adjustment | - | - | - | - | - | - | - | - | - | - | 72 | 72 | ||||||||||||||||||||||||||||||||||||
| Balance, June 30, 2022 | 213,730 | 21 | 3,635,252 | 364 | - | - | 9,526,808 | 952 | 1,714,664 | (1,570,631 | ) | 444 | 145,814 | |||||||||||||||||||||||||||||||||||
| Balance, April 1, 2023 | 213,730 | 21 | 3,635,252 | 364 | 1,861,799 | 186 | 9,880,975 | 988 | 4,714,674 | -3,803,069 | 224 | 913,388 | ||||||||||||||||||||||||||||||||||||
| Private placement | - | - | - | - | - | - | 107,861 | 11 | 323,578 | - | - | 323,589 | ||||||||||||||||||||||||||||||||||||
| Share-based compensation | - | - | - | - | - | - | - | - | 109,907 | - | - | 109,907 | ||||||||||||||||||||||||||||||||||||
| Net loss for the period | - | - | - | - | - | - | - | - | - | (1,279,858 | ) | - | (1,279,858 | ) | ||||||||||||||||||||||||||||||||||
| Currency translation adjustment | - | - | - | - | - | - | - | - | - | - | 262 | 262 | ||||||||||||||||||||||||||||||||||||
| Balance, June 30, 2023 | 213,730 | 21 | 3,635,252 | 364 | 1,861,799 | 186 | 9,988,836 | 999 | 5,148,159 | (5,082,927 | ) | 486 | 67,288 | |||||||||||||||||||||||||||||||||||
The accompanying notes are an integral part of these condensed consolidated financial statements
F-38
Elevai Labs Inc.
Condensed Consolidated Statements of Cash Flows
For the six months ended June 30, 2023 and 2022
(Unaudited - Expressed in United States dollars)
| June 30, 2023 | June 30, 2022 | |||||||
| Operating activities | ||||||||
| Net loss | $ | (2,360,554 | ) | $ | (648,526 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation | 5,825 | 2,203 | ||||||
| Share-based compensation | 185,068 | 58,510 | ||||||
| Straight-line rent expense | (1,378 | ) | (2,730 | ) | ||||
| Change in fair value of derivative liabilities | 459,246 | - | ||||||
| Loss on sale of equipment | 1,546 | |||||||
| Changes in non-cash working capital: | ||||||||
| Receivables | (21,480 | ) | (2,238 | ) | ||||
| Prepaid expenses and deposits | (183,518 | ) | (4,144 | ) | ||||
| Inventory | (247,718 | ) | (51,442 | ) | ||||
| Accounts payable and accrued liabilities | 375,053 | 18,544 | ||||||
| Customer deposits | 75,194 | 78,950 | ||||||
| Due to related parties | 60,000 | 60,000 | ||||||
| Cash flows used in operating activities | $ | (1,654,262 | ) | $ | (489,327 | ) | ||
| Investing activities | ||||||||
| Purchase of equipment | (11,191 | ) | (6,941 | ) | ||||
| Proceeds from sale of equipment | 3,500 | |||||||
| Cash flows used in investing activities | $ | (11,191 | ) | $ | (3,441 | ) | ||
| Financing activities | ||||||||
| Proceeds from issuance of common stock and warrants | 1,073,589 | - | ||||||
| Exercise of stock options | 37,500 | - | ||||||
| Obligation to issue shares | 284,960 | |||||||
| Proceeds from convertible debenture | 185,952 | |||||||
| Cash flows provided by financing activities | $ | 1,111,089 | $ | 470,912 | ||||
| Effect of exchange rate changes on cash | 728 | (19 | ) | |||||
| Change in cash | (553,636 | ) | (21,875 | ) | ||||
| Cash, beginning of period | 1,154,901 | 411,858 | ||||||
| Cash, ending of period | $ | 601,265 | $ | 389,983 | ||||
| Supplemental cash flow information: | ||||||||
| Cash paid for interest | $ | 4,898 | $ | - | ||||
| Cash paid for taxes | - | - | ||||||
| Non-cash Investing and Financing transactions: | $ | - | $ | - | ||||
The accompanying notes are an integral part of these condensed consolidated financial statements
F-39
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
| 1. | Organization and nature of operations |
Elevai Labs Inc. (“Elevai”) was incorporated under the laws of the State of Delaware on June 9, 2020. Elevai and its 100% owned subsidiary, Elevai Research Inc. ("Elevai Research"), are collectively referred to in these unaudited condensed consolidated financial statements as “the Company”.
The Company is a skincare development company engaged in the design, manufacture, and marketing of skincare products in the skincare industry. The Company’s principal activities are developing and manufacturing skincare products.
| 2. | Going Concern |
These unaudited condensed consolidated financial statements have been prepared on a going concern basis, which implies the Company will continue to realize its assets and discharge its liabilities in the normal course of business. The continuation of the Company as a going concern is dependent upon the continued financial support from its shareholders and the ability of the Company to obtain necessary equity financing to continue operations, and ultimately the attainment of profitable operations.
As of June 30, 2023 and December 31, 2022, the Company had a net working capital deficit of $112,264, and a positive working capital $963,050, respectively, and has an accumulated deficit of $5,082,927 and $2,722,373, respectively. Furthermore, for the six months ended June 30, 2023 and 2022, the Company incurred a net loss of $2,360,554 and $648,526, respectively and used $1,654,262 and $489,327, respectively of cash flows for operating activities. These factors raise substantial doubt regarding the Company’s ability to continue as a going concern. These unaudited condensed consolidated financial statements do not include any adjustments to the recoverability and classification of recorded asset amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
The assessment of whether the going concern assumption is appropriate requires management to take into account all available information about the future, which is at least, but not limited to, 12 months from the date the financial statements are issued. The Company is aware that material uncertainties related to events or conditions may cast substantial doubt upon the Company’s ability to continue as a going concern.
Management’s plans that alleviate substantial doubt about the Company’s ability to continue as a going concern include raising additional debt or equity financing. In addition, in February 2023, the Company filed its preliminary initial registration (S-1 Form) with the SEC pursuant to its goal of completing an initial public offering (“IPO”). The Company plans to use funds raised in a successful IPO to accelerate new product development, inventory production, increasing its sales force and expanding into new markets.
F-40
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
The outbreak of the coronavirus, also known as “COVID-19”, has spread across the globe and is impacting worldwide economic activity. Conditions surrounding the coronavirus continue to rapidly evolve and government authorities have implemented emergency measures to mitigate the spread of the virus. The outbreak and the related mitigation measures may have an adverse impact on global economic conditions as well as on the Company’s business activities. The extent to which the coronavirus may impact the Company’s business activities will depend on future developments, such as the ultimate geographic spread of the disease, the duration of the outbreak, travel restrictions, business disruptions, and the effectiveness of actions taken in the USA and Canada and other countries to contain and treat the disease. These events are highly uncertain and as such, the Company cannot determine their financial impact at this time. While certain restrictions are presently in the process of being relaxed, it is unclear when the world will return to the previous normal, if ever. This may adversely impact the expected implementation of the Company’s plans moving forward.
| 3. | Summary of Significant Accounting Policies |
Basis of Presentation
These unaudited condensed consolidated financial statements have been prepared in accordance with rules and regulations of the Securities and Exchange Commission (“SEC”) and generally accepted accounting principles in the United States (“U.S. GAAP”) for interim financial information and are expressed in United States dollars. Accordingly, the unaudited condensed consolidated financial statements do not include all of the information and footnotes required by generally accepted accounting principles for complete financial statements. In the opinion of management, we have included all adjustments considered necessary for a fair presentation and such adjustments are of a normal recurring nature. These unaudited condensed consolidated financial statements should be read in conjunction with the consolidated financial statements for the years ended December 31, 2022 and 2021. The results of operations for the six months ended June 30, 2023, are not necessarily indicative of the results to be expected for the full fiscal year ending December 31, 2023.
Principles of Consolidation
The unaudited condensed consolidated financial statements include the accounts of Elevai, and its 100% owned subsidiary, Elevai Research. All intercompany accounts, transactions and profits were eliminated in the unaudited condensed consolidated financial statements.
Use of Estimates
The preparation of the unaudited condensed consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company regularly evaluates estimates and assumptions related to revenue recognition, the collectability of receivables, valuation of inventory, fair value of derivative liabilities and stock options, useful lives and recoverability of long-lived assets, and deferred income tax asset valuation allowances. The Company bases its estimates and assumptions on current facts, historical experience and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgements about the carrying value of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from those estimates. Estimates and assumptions are reviewed periodically, and the effects of revisions are reflected in the consolidated financial statements in the period they are determined.
F-41
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
Foreign Currency Translation
The Company’s functional and reporting currency is the U.S. dollar. The functional currency of Elevai Research is the Canadian dollar. Monetary assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the balance sheet date. Non-monetary assets, liabilities, and items recorded in income arising from transactions denominated in foreign currencies are translated at rates of exchange in effect at the date of the transaction. Gains and losses arising on translation or settlement of foreign currency denominated transactions or balances are included in the determination of income.
The accounts of Elevai Research are translated to U.S. dollars using the current rate method. Accordingly, assets and liabilities are translated into U.S. dollars at the period-end exchange rate while revenues and expenses are translated at the average exchange rates during the period. Related exchange gains and losses are included in a separate component of stockholders’ equity as accumulated other comprehensive income (loss).
New Accounting Standards
Recently Adopted Accounting Standards
In August 2020, the FASB issued ASU 2020-06, ASC Subtopic 470-20 “Debt—Debt with Conversion and Other Options” and ASC subtopic 815-40 “Hedging—Contracts in Entity’s Own Equity”. The standard reduced the number of accounting models for convertible debt instruments and convertible preferred stock. Convertible instruments that continue to be subject to separation models are (1) those with embedded conversion features that are not clearly and closely related to the host contract, that meet the definition of a derivative, and that do not qualify for a scope exception from derivative accounting; and (2) convertible debt instruments issued with substantial premiums for which the premiums are recorded as paid-in capital. The amendments in this update are effective for fiscal years beginning after December 15, 2021, including interim periods within those fiscal years. The adoption of this standard did not have a significant impact on the Company’s unaudited condensed consolidated financial statements.
In March 2022, the FASB issued ASU 2022-02, ASC Subtopic 326 “Credit Losses”: Troubled Debt Restructurings and Vintage Disclosures. Since the issuance of Accounting Standards Update No. 2016-13, Financial Instruments—Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments, the Board has provided resources to monitor and assist stakeholders with the implementation of Topic 326. Post-Implementation Review (PIR) activities have included forming a Credit Losses Transition Resource Group, conducting outreach with stakeholders of all types, developing educational materials and staff question-and-answer guidance, conducting educational workshops, and performing an archival review of financial reports. ASU No. 2022-02 is effective for annual and interim periods beginning after December 15, 2022. The adoption of this standard did not have a significant impact on the Company’s unaudited condensed consolidated financial statements.
F-42
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
Recently Issued Accounting Standards
The Company assesses the adoption impacts of recently issued, but not yet effective, accounting standards by the Financial Accounting Standards Board on the Company's unaudited condensed consolidated financial statements.
In June 2022, the FASB issued ASU 2022-03, ASC Subtopic 820 “Fair Value Measurement of Equity Securities Subject to Contractual Sale Restrictions”. The FASB is issuing this Update (1) to clarify the guidance in Topic 820, Fair Value Measurement, when measuring the fair value of an equity security subject to contractual restrictions that prohibit the sale of an equity security, (2) to amend a related illustrative example, and (3) to introduce new disclosure requirements for equity securities subject to contractual sale restrictions that are measured at fair value in accordance with Topic 820.
Stakeholders asserted that the language in the illustrative example resulted in diversity in practice on whether the effects of a contractual restriction that prohibits the sale of an equity security should be considered in measuring that equity security’s fair value. Some stakeholders apply a discount to the price of an equity security subject to a contractual sale restriction, whereas other stakeholders consider the application of a discount to be inappropriate under the principles of Topic 820.
For public business entities, the amendments in this Update are effective for fiscal years beginning after December 15, 2023, and interim periods within those fiscal years. For all other entities, the amendments are effective for fiscal years beginning after December 15, 2024, and interim periods within those fiscal years. Early adoption is permitted for both interim and annual financial statements that have not yet been issued or made available for issuance.
The Company does not expect the standard to have a significant impact on its consolidated financial statements.
| 4. | Receivables |
As of June 30, 2023 and December 31 2022, receivables consisted of the following:
| June 30, 2023 | December 31, 2022 | |||||||
| Trade receivable | $ | 31,179 | $ | 4,180 | ||||
| Sales taxes receivable | 3,258 | 8,674 | ||||||
| $ | 34,437 | $ | 12,854 | |||||
The Company records sales taxes receivable for recoverable sales taxes paid on eligible purchases in its Canadian subsidiary. As at June 30, 2023, and December 31, 2022, the Company recorded a provision for doubtful accounts of $nil and $nil, respectively.
F-43
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
| 5. | Prepaids and Deposits |
As of June 30, 2023 and December 31, 2022, prepaid and deposits consisted of the following:
| June 30, 2023 | December 31, 2022 | |||||||
| Prepaid expenses | $ | 83,002 | $ | 89,819 | ||||
| Deposits | 27,771 | 24,376 | ||||||
| Deferred share issuance and listing expense | 236,940 | 50,000 | ||||||
| $ | 347,713 | $ | 164,195 | |||||
| Prepaids and deposits - current | 336,940 | 153,422 | ||||||
| Deposits- non-current | 10,773 | 10,773 | ||||||
As of June 30, 2023 and December 31, 2022, the security deposit on the Company’s long-term lease in the amount of $10,773 is classified as a non-current deposit on the balance sheet.
| 6. | Inventory |
As of June 30, 2023 and December 31 2022, inventory consisted of the following:
| June 30, 2023 | December 31, 2022 | |||||||
| Raw materials | $ | 250,351 | $ | 81,133 | ||||
| Work in progress | 96,013 | 116,984 | ||||||
| Finished goods | 131,499 | 32,028 | ||||||
| $ | 477,863 | $ | 230,145 | |||||
Cost of inventory recognized as expense in cost of sales for the six months ended June 30, 2023 and 2022, totaled $73,896 and $18,486, respectively. In addition, the cost of inventory relating to samples given out and expensed in marketing and promotion for the six months ended June 30, 2023 and 2022 totaled $64,718 and $12,408, respectively. As at June 30, 2023, and December 31, 2022, the Company recorded an allowance for inventory of $nil and $nil, respectively.
F-44
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
| 7. | Property and equipment |
| Equipment | Furniture and Fixtures | Computers | Total | |||||||||||||
| Cost | ||||||||||||||||
| Balance, December 31, 2021 | $ | 32,482 | $ | - | $ | - | $ | 32,482 | ||||||||
| Additions | 24,222 | 8,365 | 2,940 | 35,527 | ||||||||||||
| Disposal | (6,188 | ) | - | - | (6,188 | ) | ||||||||||
| Foreign currency translation | - | - | (181 | ) | (181 | ) | ||||||||||
| Balance, December 31, 2022 | $ | 50,516 | $ | 8,365 | $ | 2,759 | $ | 61,640 | ||||||||
| Additions | 2,658 | 8,533 | - | 11,191 | ||||||||||||
| Foreign currency translation | - | - | 63 | 63 | ||||||||||||
| Balance, June 30, 2023 | $ | 53,174 | $ | 16,898 | $ | 2,822 | $ | 72,894 | ||||||||
| Accumulated depreciation | ||||||||||||||||
| Balance, December 31, 2021 | $ | 2,757 | $ | - | $ | - | $ | 2,757 | ||||||||
| Depreciation | 5,437 | 548 | 527 | 6,512 | ||||||||||||
| Disposal | (1,142 | ) | - | - | (1,142 | ) | ||||||||||
| Foreign currency translation | - | - | (22 | ) | (22 | ) | ||||||||||
| Balance, December 31, 2022 | $ | 7,052 | $ | 548 | $ | 505 | $ | 8,105 | ||||||||
| Depreciation | 4,340 | 1,207 | 279 | 5,826 | ||||||||||||
| Foreign currency translation | - | - | 13 | 13 | ||||||||||||
| Balance, June 30, 2023 | $ | 11,392 | $ | 1,755 | $ | 797 | $ | 13,944 | ||||||||
| Net book value | ||||||||||||||||
| December 31, 2022 | $ | 43,464 | $ | 7,817 | $ | 2,254 | $ | 53,535 | ||||||||
| June 30, 2023 | $ | 41,782 | $ | 15,143 | $ | 2,025 | $ | 58,950 | ||||||||
During the six months ended June 30, 2023 and 2022, the Company capitalized depreciation of $440 and $508, respectively as part of the production of inventory.
| 8. | Operating lease |
On June 1, 2022, the Company entered into a noncancelable operating lease that includes two property location, one which is being used as the Company’s office and the other as its lab for research and development and the production of inventory. The lease had a commencement date of June 1, 2022 and expires on May 31, 2025, after which the term will continue on a month-to-month basis.
The Company recognized a total lease cost related to its noncancelable operating lease of $63,259 and $10,543, for the six months ended June 30, 2023 and June 30, 2022, respectively. The lease cost has been allocated as follows based on the square footage of each property location.
| June 30, 2023 | June 30, 2022 | |||||||
| Office space, recorded in office and administration | $ | 44,353 | $ | 7,392 | ||||
| Lab space, recorded in research and development | 16,613 | 3,151 | ||||||
| Lab space, capitalized to production of inventory | 2,293 | - | ||||||
| $ | 63,259 | $ | 10,543 | |||||
F-45
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
As of June 30, 2023 and December 31, 2022, the Company recorded a security deposit of $10,773. (Note 5)
Future minimum lease payments under the Company’s operating lease that has an initial noncancelable lease term in excess of one year at June 30, 2023 are as follows:
| As of June 30, 2023 | Total | |||
| 2023 | $ | 64,638 | ||
| 2024 | 129,276 | |||
| 2025 | 53,865 | |||
| Thereafter | - | |||
| 247,779 | ||||
| Less: Imputed interest | (18,767 | ) | ||
| Operating lease liability | 229,012 | |||
| Operating lease lability – current | 115,115 | |||
| Operating lease lability – non-current | $ | 113,897 | ||
The Company used a discount rate of 8% as its incremental cost of borrowing and the remaining lease term as of June 30, 2023, is 1.92 years (December 31, 2022 – 2.42 years).
| 9. | Accounts payable and accrued liabilities |
As of June 30, 2023 and December 31, 2022, accounts payable and accrued liabilities consisted of the following:
| June 30, 2023 | December 31, 2022 | |||||||
| Accounts payable | $ | 537,600 | $ | 222,461 | ||||
| Accrued liabilities | 94,005 | 33,864 | ||||||
| $ | 631,605 | $ | 256,325 | |||||
As of June 30, 2023 and December 31, 2022, accounts payable and accrued liabilities include $15,093 and $11,621, respectively that is due to related parties in the ordinary course of business.
| 10. | Notes payable |
In April and May 2022, the Company issued promissory notes to five investors (including two related parties of the Company) for a total amount of $183,970. The promissory notes carried simple interest at a rate of 8% per annum. On July 15 2022, the promissory notes and accrued interest of $2,614, converted into the Series A financing round in accordance with the original terms of the agreements. The conversion price was set at $0.80 (60% of the Series A preferred shares financing round price) and as a result the noteholders received 231,828 Series A preferred shares. In addition, the conversion terms contained a 100% warrant coverage ratio resulting in the note holders receiving 231,828 common stock purchase warrants with an exercise price of $2.01 (150% of the Series A financing round price).
F-46
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
| 11. | Derivative liabilities |
We analyzed the common stock purchase warrants issued as partial settlement of the promissory notes payable on July 15, 2022 (Note 10), against the requirements of ASC 480, Distinguishing Liabilities from Equity, and determined that the warrants should be classified as financial liabilities since the terms allows for a cashless net share settlement at the option of the holder.
ASC 815, Derivatives and Hedging, requires that the warrants be accounted for as derivative liabilities with initial and subsequent measurement at fair value with changes in fair value recorded as other income (expense).
A continuity of the Company’s common stock purchase derivative liability warrants is as follows:
| Derivative liabilities | ||||
| December 31, 2021 | $ | - | ||
| Addition of new derivatives recognized as partial settlement of promissory notes | 55,701 | |||
| Change in fair value of derivative liabilities | 12,754 | |||
| Outstanding, December 31, 2022 | $ | 68,455 | ||
| Change in fair value of derivative liabilities | 459,246 | |||
| Outstanding, June 30, 2023 | $ | 527,701 | ||
We determined our derivative liabilities to be a Level 3 fair value measurement and used the Black-Scholes Option Pricing Model to calculate the fair value as of initial recognition and subsequent reporting period. The Black-Scholes Option Pricing Model requires six basic data inputs: the exercise or strike price, expected time to expiration or exercise, the risk-free interest rate, the current stock price, the estimated volatility of the stock price in the future, and the dividend rate. Changes to these inputs could produce a significantly higher or lower fair value measurement.
The following assumptions were used in the Black-Scholes option pricing model:
| June 30, 2023 | December 31, 2022 | July 15, 2022 | ||||||||||
| Risk-free interest rate | 4.49 | % | 4.73 | % | 3.12 | % | ||||||
| Expected life1 | 3.83 years | 0.75 years | 0.6 years | |||||||||
| Expected dividend rate | 0.00 | % | 0.00 | % | 0.00 | % | ||||||
| Expected volatility | 100 | % | 100 | % | 100.00 | % | ||||||
| 1 | On April 28, 2023, the Company amended the warrant agreements for the 231,828 derivative liability warrants outstanding. The amendment removed the clause to automatically convert warrants to shares on IPO date and all warrants were given an expiry date of April 27, 2027. This led to an increase in the expected life input in the Black-Scholes model as of June 30, 2023 compared to the December 31, 2022 or March 31, 2022, when the Company used the expected IPO date to calculate the expected life of the warrants. |
F-47
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
As of June 30, 2023, the following derivative liability warrants were outstanding:
| Outstanding | Expiry date1 | Weighted average exercise price ($) | ||||||
| 75,840 | April 27, 2027 | 2.01 | ||||||
| 63,037 | April 27, 2027 | 2.01 | ||||||
| 80,388 | April 27, 2027 | 2.01 | ||||||
| 12,563 | April 27, 2027 | 2.01 | ||||||
| 231,828 | 2.01 | |||||||
As of December 31, 2022, the following derivative liability warrants were outstanding:
| Outstanding | Expiry date1 | Weighted average exercise price ($) | ||||||
| 75,840 | April 27, 2027 | 2.01 | ||||||
| 63,037 | May 9, 2027 | 2.01 | ||||||
| 80,388 | May 24, 2027 | 2.01 | ||||||
| 12,563 | May 25, 2027 | 2.01 | ||||||
| 231,828 | 2.01 | |||||||
As of June 30, 2023 and December 31, 2022, the weighted average life of derivative liability warrants outstanding was 3.83 and 4.36 years, respectively.
| 1 | On April 28, 2023, the Company amended the warrant agreements for the 231,828 derivative liability warrants outstanding. The amendment removed the clause to automatically convert warrants to shares on IPO date and all warrants were given an expiry date of April 27, 2027. This led to an increase in the expected life input in the Black-Scholes model as of June 30, 2023 compared to the December 31, 2022 or March 31, 2022, when the Company used the expected IPO date to calculate the expected life of the warrants. |
| 12. | Equity |
Common Stock
Authorized
As of June 30, 2023 and December 31, 2022, the Company had 300,000,000 and 19,000,000 common stock authorized, respectively, each having a par value of $0.0001.
Issued and outstanding
As of June 30, 2023, and December 31, 2022, the Company had 9,988,836 and 9,568,475 shares issued and outstanding, respectively.
Transactions during the six months ended June 30, 2023
On January 6, 2023, the Company issued 62,500 common stock upon the exercise of 62,500 stock options with an exercise price of $0.60 per common stock for $37,500, of which $6 was recognized in common stock and the remaining $37,494 in additional paid-in capital.
On March 2, 2023, the Company issued 250,000 common stock and 250,000 common stock purchase warrants for $750,000, of which $25 was recognized in common stock and the remaining $749,975 in additional paid-in capital. These warrants are accounted for as equity warrants.
On April 14, 2023, the Company issued 97,681 common stock, of which $10 was recognized in common stock and the remaining $293,579 in additional paid-in capital.
On May 15, 2023, the Company issued 10,000 common stock, of which $1 was recognized in common stock and the remaining $29,999 was recognized in additional paid-in capital.
Transactions during the six months ended June 30, 2022.
There was no common stock transactions during the six months ended June 30, 2022.
F-48
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
Preferred Stock
Authorized
As of June 30, 2023 and December 31, 2022, the Company had 213,730 stock of Series Seed 1 preferred stock authorized, each having a par value of $0.0001 per stock.
As of June 30, 2023 and December 31, 2022, the Company had 3,635,252 stock of Series Seed 2 preferred stock authorized, each having a par value of $0.0001 per stock.
As of June 30, 2023 and December 31, 2022, the Company had 2,982,003 stock of Series A preferred stock authorized, each having a par value of $0.0001 per stock.
Issued and outstanding
As of June 30, 2023 and December 31, 2022, the Company had 213,730 Series Seed 1 preferred stock issued and outstanding.
As of June 30, 2023 and December 31, 2022, the Company had 3,635,252 Series Seed 2 preferred stock issued and outstanding.
As of June 30, 2023 and December 31, 2022, the Company had 1,861,799 Series A preferred stock issued and outstanding.
Transactions during the six-month ended June 30, 2023, and June 30, 2022.
There was no preferred stock transactions during the six months ended June 30, 2023 and 2022.
Equity Warrants
Transactions during the six-month ended June 30, 2023.
On March 2, 2023, the Company issued 250,000 common stock and 250,000 common stock purchase warrants. Each warrant is exercisable at $3.00 per common stock. The warrants shall be exercisable, in whole or in part at the issue date but such exercisability shall cease upon the date of the Company’s IPO and listing of its common shares on the Nasdaq Capital Market or other Trading Market and shall continue to be exercisable in whole or in part immediately after the Lock-up Period but no later than the Warrant Expiration Date or Accelerated Warrant Expiration Date (the “Exercise Period”). In the event of the Company’s initial public offering and listing of shares of its common stock on a Trading Market, the Company shall notify the holder at least fifteen (15) calendar days prior to the consummation of such IPO. “Trading Market” shall mean a "national securities exchange" that has registered with the SEC under Section 6 of the Securities Exchange Act of 1934. The Expiration Date shall be the earlier of (i) three years and one hundred eighty (180) days from the issue date (the “Warrant Expiration Date”) or (ii) upon the Company’s reasonable judgment and written notice to the purchaser, of the Company’s option to accelerate the Warrant Expiration Date whereby upon purchaser’s receipt of the Company’s written notice of acceleration during the Exercise Period, the Purchaser’s option to exercise any number of warrants shall occur no later than fourteen (14) days following the receipt of the written notice of acceleration (the “Accelerated Warrant Expiration Date”). For the avoidance of doubt, it shall be reasonable for the Company to accelerate the Expiration Date of this warrant to coincide with transactions including, but not limited to (i) a change of control including but not limited to the voluntary or involuntary sale, assignment, transfer or other disposition, or transfer by operation of law, of more than 50% of any direct or indirect equity interest of the Company; or (ii) a subsequent capital financing other than the IPO consisting of but not limited to an offer or proposal for, or indication of interest in, the issuance of debt or the capital stock of the Company.
F-49
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
Transactions during the six months ended June 30, 2022.
There was no equity warrant activity during the six months ended June 30, 2022.
As of June 30, 2023, the following equity warrants were outstanding:
| Outstanding | Expiry date | Weighted average exercise price ($) | ||||||
| 250,000 | August 28, 2023 | 3.00 | ||||||
| 250,000 | 3.00 | |||||||
As of December 31, 2022, there were no equity warrants outstanding.
As of June 30, 2023, and December 31, 2022, the weighted average life of equity warrants outstanding was 3.16 and Nil years, respectively.
Stock Options
The Company has a stock option plan included in the Company’s 2020 Equity Incentive Plan (the “Plan”) where the Board of Directors or any of its committees can grant Incentive Stock Options, Nonstatutory Stock Options, and Restricted Stock. The aggregate number of shares allocated and made available for issuance pursuant to stock options granted under the Plan shall not exceed 1,734,188 shares. The plan shall remain in effect until it is terminated by the Board of Directors.
Transactions during the six-month ended June 30, 2023.
On February 1, 2023, the Company granted 10,000 stock options with a contractual life of ten years and an exercise price of $5.00 per common stock. These stock options were valued at $10,767 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
From May 12, 2023 to June 30, 2023, the Company granted 222,500 stock options (includes 80,000 each to two of its newly appointed independent directors) with a contractual life of ten years and an exercise price of $5.00 per common stock. These stock options were valued at $584,787 using the Black-Scholes Option Pricing Model. The options vest 25% on the first vesting date and the remaining 75% vest evenly over 36 months thereafter.
On June 30, 2023, the Company cancelled and reissued 80,000 options previously issued to an advisor of the Company upon their appointment as a director effective June 1, 2023. The cancelled and re-issued options had the same exercise price of $5.00 per common stock and the same vesting terms and expiry date, and as such the cancellation and reissuance had no impact on the Company’s consolidated financial statements.
Transactions during the six months ended June 30, 2022.
On April 25, 2022, the Company granted 45,000 stock options with a contractual life of ten years and an exercise price of $0.60 per common stock. These stock options were valued at $11,617 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
On June 1, 2022, the Company granted 16,000 stock options with a contractual life of ten years and an exercise price of $1.34 per common stock. These stock options were valued at $19,393 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
F-50
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
The following assumptions were used in the Black-Scholes option pricing model during the six months ended June 30, 2023, and year ended December 31, 2022:
| June 30, 2023 | December 31, 2022 | |||||||
| Risk-free interest rate | 3.39-3.65 | % | 2.81% - 4.07 | % | ||||
| Expected life | 10 years | 10 years | ||||||
| Expected dividend rate | 0.00 | % | 0.00 | % | ||||
| Expected volatility | 100 | % | 100 | % | ||||
| Forfeiture rate | 0.00 | % | 0.00 | % | ||||
The continuity of stock options for the period ended June 30, 2023 and year ended December 31, 2022 is summarized below:
| Number of stock options | Weighted average exercise price | |||||||
| Outstanding, December 31, 2021 | 1,133,334 | $ | 0.60 | |||||
| Granted | 412,000 | 2.19 | ||||||
| Forfeited | (137,500 | ) | 0.60 | |||||
| Exercised | (41,667 | ) | 0.60 | |||||
| Outstanding, December 31, 2022 | 1,366,167 | 1.08 | ||||||
| Granted | 232,500 | 5.00 | ||||||
| Exercised | (62,500 | ) | 0.60 | |||||
| Outstanding, June 30, 2023 | 1,536,167 | 1.69 | ||||||
As of June 30, 2023, the following options were outstanding, entitling the holders thereof the right to purchase one common stock for each option held as follows:
| Outstanding | Vested | Expiry date | Weighted average exercise price ($) | |||||||||
| 841,667 | 508,512 | February 8, 2031 | 0.60 | |||||||||
| 50,000 | 30,214 | February 27, 2031 | 0.60 | |||||||||
| 45,000 | 13,125 | April 25, 2032 | 0.60 | |||||||||
| 16,000 | 4,000 | June 1, 2032 | 1.34 | |||||||||
| 110,000 | - | July 1, 2032 | 1.34 | |||||||||
| 100,000 | - | August 8, 2032 | 1.34 | |||||||||
| 16,000 | - | September 30, 2032 | 1.34 | |||||||||
| 80,000 | - | September 30, 2032 | 5.00 | |||||||||
| 10,000 | - | October 15, 2032 | 1.34 | |||||||||
| 10,000 | - | November 1, 2032 | 1.34 | |||||||||
| 5,000 | - | November 1, 2032 | 5.00 | |||||||||
| 20,000 | - | December 12, 2032 | 5.00 | |||||||||
| 10,000 | - | February 1, 2033 | 5.00 | |||||||||
| 50,000 | - | April 16, 2033 | 5.00 | |||||||||
| 80,000 | - | May 1, 2033 | 5.00 | |||||||||
| 80,000 | - | January 25, 2033 | 5.00 | |||||||||
| 10,000 | - | June 27, 2033 | 5.00 | |||||||||
| 2,500 | - | July 10, 2033 | 5.00 | |||||||||
| 1,536,167 | 555,851 | 1.69 | ||||||||||
F-51
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
As of June 30, 2023, the weighted average life of stock options outstanding was 8.34 years (December 31, 2022 – 8.58 years).
During the six months ended June 30, 2023 and 2022, the Company recorded $185,068 and $58,510, respectively, in share-based compensation expense, of which $178,735 and $6,333, and $56,407 and $2,103, respectively is included in office and administration and research and development, respectively.
| 13. | Related Party Transactions |
Related parties consist of the following individuals and corporations:
| ● | Braeden Lichti, Chairman and former President, significant shareholder through BWL Investments Ltd. Resigned as President effective October 11, 2022. | |
| ● | Jordan Plews, CEO and Director, significant shareholder through JP Bio Consulting LLC | |
| ● | Graydon Bensler, CFO and Director | |
| ● | Yi Guo, Former Director, resigned effective September 29, 2022 | |
| ● | Tim Sayed, Chief Medical Officer | |
| ● | Brenda Buechler, Chief Marketing Officer | |
| ● | Christoph Kraneiss, Chief Commercial Officer | |
| ● | Jeffrey Parry, Director (appointed June 1, 2023) | |
| ● | Julie Davey, Director (appointed June 1, 2023) | |
| ● | Crystal Muilenburg, Director (appointed June 1, 2023) | |
| ● | GB Capital Ltd., controlled by Graydon Bensler | |
| ● | JP Bio Consulting LLC, significant shareholder and controlled by Jordan Plews | |
| ● | BWL Investments Ltd., significant shareholder and controlled by Braeden Lichti | |
| ● | Northstrive Companies Inc., controlled by Braeden Lichti |
Key management personnel include those persons having authority and responsibility for planning, directing, and controlling the activities of the Company as a whole. The Company has determined that key management personnel consist of members of the Company’s Board of Directors, corporate officers, and individuals with more than 10% control. The remuneration of directors and key management personnel is as follows:
| Three months ended June 31, 2023 |
Three months | Six months ended June 31, 2023 | Six months ended June 30, 2022 | |||||||||||||
| Consulting fees | $ | 40,288 | $ | 39,963 | $ | 91,538 | $ | 93,422 | ||||||||
| Salaries | 159,814 | 53,825 | 316,253 | 107,937 | ||||||||||||
| Share-based compensation | 75,369 | 23,155 | 122,355 | 50,399 | ||||||||||||
| $ | 275,471 | $ | 116,943 | $ | 530,146 | $ | 251,758 | |||||||||
F-52
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
During the six months ended June 30, 2023, the Company incurred consulting fees of $31,538 (June 30, 2022 - $32,422) to GB Capital Ltd., a company controlled by Graydon Bensler, CFO and Director. In addition, the Company incurred consulting fees of $60,000 (June 30, 2022 - $60,000) to Northstrive Companies Inc., a company controlled by the Company’s Chairman and former President.
Jordan Plews, CEO and Director, earned a Salary of $111,523 and $107,937, respectively during the six months period ended June 30, 2023 and 2022 (includes employer taxes of $11,522 and $7,937, respectively).
Brenda Buechler, Chief Marketing Officer, earned a Salary of $106,123 and $nil, respectively during the six month periods ended June 30, 2023 and 2022 (includes employer taxes of $11,123 and $nil, respectively).
Christoph Kraneiss, Chief Commercial Officer, earned a Salary of $98,608 and $nil, respectively during the six month periods ended June 30, 2023 and 2022 (includes employer taxes of $8,608 and $nil, respectively).
On February 9, 2021, the Company granted 800,000 stock options to four related parties (200,000 stock options each) with a contractual life of ten years and exercise price of $0.60 per share of common stock. These stock options were valued at $203,972 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
On June 1, 2023, the Company granted 160,000 stock options to directors of the company (80,000 stock options each) with a contractual life of ten years and exercise price of $5.00 per share of common stock. These stock options were valued at $420,521 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter.
On June 1, 2023, the Company cancelled and re-issued 80,000 to a director of the company with a contractual life of ten years and exercise price of $5.00 per share of common stock. The cancelled and re-issued options had the same exercise price of $5.00 per common stock and the same vesting terms and expiry date, and as such the cancellation and reissuance is not expected to impact on the Company’s consolidated financial statements. (Note 12).
Details of the fair value of the options granted to each individual and the related expense recorded for the six month periods ended June 30, 2023 and 2022 are as follow:
| Six months ended June 31, 2023 | Six months ended June 30, 2022 | Fair value of stock options granted | ||||||||||
| Braeden Lichti, Former Chairman and President | $ | 3,927 | $ | 4,820 | $ | 50,993 | ||||||
| Graydon Bensler, CFO and Director | 3,927 | 4,820 | 50,993 | |||||||||
| Jordan Plews, CEO and Director | 3,927 | 4,820 | 50,993 | |||||||||
| Tim Sayed, Chief Medical Officer | 3,927 | 4,820 | 50,993 | |||||||||
| Jeffrey Parry, Director | 5,210 | - | 107,669 | |||||||||
| Julie Davey, Director | 13,428 | - | 210,245 | |||||||||
| Crystal Muilenburg, Director | 11,199 | - | 210,245 | |||||||||
| $ | 45,545 | $ | 19,280 | $ | 732,131 | |||||||
F-53
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
On July 20, 2021, the Company granted 200,000 stock options to a related party, Yi Guo, former Director, with a contractual life of ten years and exercise price of $0.60 per share of common stock. These stock options were valued at $51,014 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter. On October 17, 2022, Yi Guo resigned from the board of directors of the Company and as a result, 137,500 unvested options were forfeited. The remaining 62,500 vested option remain exercisable for 3 months after the resignation. On January 6, 2023, Yi Guo exercised the remaining 62,500 options. The share-based compensation expense recorded for the six months ended June 30, 2023 and 2022 relating to these stock options was $Nil and $15,441, respectively.
During the second and third quarter of 2022, the Company granted 250,000 stock options to two related parties (150,000 stock options to Brenda Buechler, Chief Marketing Officer, and 100,000 options to Christoph Kraneiss, Chief Commercial Officer) with a contractual life of ten years and weighted average exercise price of $1.22 per share of common stock. These stock options were valued at $264,906 using the Black-Scholes Option Pricing Model. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter. Details of the fair value granted to each individual and the related expense recorded for the six months ended June 30, 2023 and 2022 is as follow:
| Six months ended June 31, 2023 | Six months ended June 30, 2022 | Fair value of stock options granted | ||||||||||
| Brenda Buechler, Chief Marketing Officer | $ | 41,426 | $ | 1,097 | $ | 143,679 | ||||||
| Christoph Kraneiss, Chief Commercial Officer | 35,384 | - | 121,227 | |||||||||
| $ | 76,810 | $ | 1,097 | $ | 264,906 | |||||||
As of June 30, 2023 and December 31, 2022, the Company had $202,983 and $142,705, respectively due to companies controlled by Braeden Lichti, of which $22,983 and $22,705, respectively is unsecured, non-interest bearing and are due on demand. The remaining $180,000 and $120,000, respectively due as of June 30, 2023 and December 31, 2022, is payable to Northstrive Companies Inc. for consulting services rendered by Braeden Lichti (the “Fees”). Payment of the Fees will be deferred until the earlier of either (a) the Company raising an aggregate of at least $2,000,000 of equity and/or debt investment from and after October 1, 2022, (b) the Company becomes listed on any established stock exchange or a national market system including without limitation the New York Stock Exchange, the Nasdaq Capital Market of The Nasdaq Stock Market, or (c) the Board determines that the Company has sufficient cash flows to support payment of the foregoing amounts of Fees due at the time of that determination. The Fees earned prior to March 31, 2023 shall be payable in cash payment or in the form of Series A preferred stock priced at $1.34138 per share (the “Original Series A Issue Price") equal to the value of the Fees then due. While the Fees earned after April 1, 2023 shall be payable in cash payment or in the form of Series A preferred stock priced at $3 per share.
As of June 30, 2023, accounts payable and accrued liabilities include $7,390 (December 31, 2022 - $7,165) in consulting fees payable to Graydon Bensler, CFO and Director, $5,805 (December 31, 2022 - $1,485) to companies controlled by Braeden Lichti, and $1,898 (December 31, 2022 - $2,971) to Jordan Plews, CEO and Director, for expenses incurred on behalf of the Company.
F-54
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
| 14. | Commitments and Contingencies |
There were no commitments as of June 30, 2023 and December 31, 2022 or during the periods then ended.
The Company had an ongoing dispute with a vendor regarding unpaid invoices. The Company disputed the services claimed to have been rendered by the vendor. In May 2023, the Company and the vendor agreed to settle the matter, resulting in the Company agreeing to pay a final settlement of Cnd$12,500 (approximately $9,225), an amount that is significantly less than the unpaid invoices originally claimed by the vendor. The Company included the settlement amount in accrued liabilities as of December 31, 2022 and the amount was paid over to the vendor during the six months ended June 30, 2023.
| 15. | Concentrations |
Customers
During the six month period ended June 30, 2023, the Company recorded 16% of its revenue from its largest customers. The Company’s largest customer relates to sales to a wholesaler during the period. During the six months ended June 30, 2022, the Company recorded 49% of its revenue from a single customer. The company's largest customer relates to sales to a wholesaler during the period.
As of June 30, 2023 and December 31, 2022, the Company had $3,549 and $nil receivables due from this customer, respectfully, and $nil and $5,992, respectfully, in customer deposits were received from its largest customer.
The Company expects its dependence on major customers to decrease over time as it enters into additional distributor agreements and builds out its sales team.
F-55
Elevai Labs Inc.
Notes to the Condensed Consolidated Financial Statements
(Unaudited - Expressed in United States dollars)
Suppliers
During the six month period ended June 30, 2023 and 2022, the Company had 2 key suppliers that represented approximately 57% and 66%, respectively of the cost incurred in the purchase and production of inventory. The table below represents a breakdown of each supplier as a percentage of the cost incurred (Suppliers are shown from largest to smallest and does not necessarily represent the same suppliers period over period):
| Six Months Ended June 30, 2023 | Six Months Ended June 30, 2022 | |||||||
| Supplier 1 | 33 | % | 33 | % | ||||
| Supplier 2 | 24 | % | 33 | % | ||||
| 57 | % | 66 | % | |||||
The Company continually evaluates the performance of its suppliers and the availability of alternatives to substitute or supplement its inventory production supply chain. The Company believes that a breakdown in supply from one of its key suppliers would be overcome in a short amount of time given the availability of alternatives.
| 16. | Subsequent Events |
Management has evaluated events subsequent to the six months ended June 30, 2023 up to August 14, 2023, for transactions and other events that may require adjustment of and/or disclosure in the consolidated financial statements.
Leases
In July 2023, the Company’s monthly lease payments increased from $10,773 to $13,477 per month, as the Company amended their existing lease to include an additional 721 square feet of office space.
Stock Options
The Company granted 1,500 stock options with a contractual life of ten years and an exercise price of $5.00 per common stock.
F-56
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the estimated expenses to be borne by the registrant in connection with the securities being registered hereby. With the exception of the SEC registration fee, the FINRA filing fee, and the Nasdaq Capital Market listing fee, all amounts are estimates.
| Securities and Exchange Commission Registration Fee | $ | |||
| Nasdaq Capital Market Listing Fee | $ | |||
| FINRA | $ | |||
| Legal Fees and Expenses | $ | |||
| Accounting Fees and Expenses | $ | |||
| Printing Expenses | $ | |||
| Miscellaneous Expenses | $ | |||
| Total Expenses | $ |
These expenses will be borne by us. Underwriting discounts and the non-accountable expense allowance will be borne by us in proportion to the numbers of shares of Common Stock sold in the offering.
Item 14. Indemnification of Directors and Officers
Section 145 of the Delaware General Corporation Law (the “DGCL”) provides that a corporation may indemnify directors and officers as well as other employees and individuals against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with any threatened, pending or completed actions, suits or proceedings in which such person is made a party by reason of such person being or having been a director, officer, employee or agent of the Company. The DGCL provides that Section 145 is not exclusive of other rights to which those seeking indemnification may be entitled under any bylaws, agreement, vote of stockholders or disinterested directors or otherwise. The Company’s Certificate of Incorporation and Bylaws provide for indemnification by the Company of its directors and officers to the fullest extent permitted by the DGCL.
Section 102(b)(7) of the DGCL permits a corporation to provide in its certificate of incorporation that a director of the corporation shall not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except for liability (1) for any breach of the director’s duty of loyalty to the corporation or its stockholders, (2) for acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law, (3) for unlawful payments of dividends or unlawful stock repurchases, redemptions or other distributions or (4) for any transaction from which the director derived an improper personal benefit. The Company’s Certificate of Incorporation provides for such limitation of liability to the fullest extent permitted by the DGCL.
The Company maintains standard policies of insurance under which coverage is provided (1) to its directors and officers against loss arising from claims made by reason of breach of duty or other wrongful act, while acting in their capacity as directors and officers of the Company, and (2) to the Company with respect to payments which may be made by the Company to such officers and directors pursuant to any indemnification provision contained in the Company’s Second Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws or otherwise as a matter of law.
II-1
Item 15. Recent Sales of Unregistered Securities
Since our inception, we have made the following issuances of unregistered equity securities:
| ● | On June 24, 2020, we issued 8,480,000 shares of common stock to the founders of the Company for total consideration of $50,880; |
| ● | On September 30, 2020, we issued 213,730 shares of preferred stock in connection with a series seed preferred stock investment agreement in exchange for gross proceeds of $64,119; |
| ● | On January 24, 2021, we issued 1,046,808 shares of common stock in connection with the conversion of promissory notes in exchange for gross proceeds of $24,600; |
| ● | On June 4, 2021, we issued 100 shares of common stock to BWL Investments Ltd. in exchange for substantially all of the assets and liabilities of Reactive Medical Labs Inc.; |
| ● | On August 24, 2021, we issued 3,635,252 shares of preferred stock in connection with a series seed 2 preferred stock investment agreement for a sales price of $0.30 per share in exchange for gross proceeds of $1,090,577; |
| ● | In April and May 2022, the Company issued promissory notes to 5 investors (including 2 directors of the Company) for a total amount of $183,970. The promissory notes carry simple interest at a rate of eight percent (8%) per annum. The notes also carry 100% warrant coverage. Each lender is entitled to receive a warrant to purchase shares of common stock. Each warrant is exercisable for that number of shares of common stock determined by dividing the warrant coverage amount by the conversion price and shall have a term of five (5) years. The exercise price for the common stock purchasable upon exercise of the warrants shall be equal to 150% of the price per share paid for the conversion shares. The promissory notes and all accrued but unpaid interest automatically converted into the next equity financing, the Series A round. The conversion price was 60% of the price paid per share for equity securities in the next equity financing, the Series A round. On July 15 2022, $183,970 of principal and $2,614 of accrued interest, converted into the series A round for a total of 231,828 shares and 231,828 warrants. The warrants have an exercise price of $2.01207. |
| ● | On July 15, 2022, the Company closed the first tranche of its series A financing and issued 1,090,029 series A preferred shares for gross proceeds of $1,462,146 (excluding the conversion of the promissory notes referred to immediately above). |
| ● | On July 27, 2022, the Company closed the second tranche of its series A financing and issued 349,790 series A preferred shares for gross proceeds of $469,207. |
II-2
| ● | On August 4, 2022, the Company closed the third tranche of its series A financing and issued 111,884 series A preferred shares for gross proceeds of $150,080. |
| ● | On October 10, 2022, the Company closed the fourth tranche of its series A financing and issued 78,268 series A preferred shares for gross proceeds of $104,988. |
| ● | In December 2022, 41,667 stock options with an exercise price of $0.60 were exercised in exchange for 41,667 shares of Common Stock for total proceeds of $25,000. |
| ● | In January 2023, 62,500 stock options with an exercise price of $0.60 were exercised in exchange for 62,500 shares of Common Stock for total proceeds of $37,500. |
| ● | On March 2, 2023, the Company amended its certificate of incorporation to increase the number of common stock authorized for issuance from 19,000,000 to 300,000,000. On the same date, the Company increased the aggregate number of shares allocated and made available for issuance pursuant to stock options granted under the Company’s 2020 equity incentive plan from 1,534,188 to 1,734,188 shares. |
| ● | On March 3, 2023, the Company issued 250,000 shares of common stock for gross proceeds of $750,000 |
| ● | On March 3, 2023, the Company issued 250,000 warrants to purchase 250,000 shares of common stock for gross proceeds of $750,000. Each warrant has a term of three years and 180 days subject to the Company’s ability to accelerate the warrant expiration date. The warrants have an exercise price of $3.00. |
| ● | From March 15, 2023, to May 19, 2023, the Company issued 107,863 shares of common stock for gross proceeds of $ 323,589. |
| ● | On June 1, 2023, the Company granted 240,000 stock options to each of its independent directors with a contractual life of ten years and exercise price of $5.00 per common stock. The options vest 25% on the first anniversary of the grant date and the remaining 75% vest evenly over 36 months thereafter. |
No underwriters were involved in the foregoing sales of securities. These issuances were made pursuant to the exemption from registration contained in Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder.
II-3
16. Exhibits
| (a) | Exhibits. The following exhibits are included herein or incorporated herein by reference: |
| Exhibit No. | Description | |
| 1.1 | Form of Underwriting Agreement* | |
| 3.1 | Certificate of Incorporation of Elevai Labs, Inc.** | |
| 3.2 | Amendment to Second Amended and Restated Certificate of Incorporation of Elevai Labs, Inc.** | |
| 3.3 | Form of Third Amended and Restated Certificate of Incorporation of Elevai Labs, Inc., to be effective upon the closing of this offering* | |
| 3.4 | By-laws* | |
| 3.5 | Form of Amended and Restated Bylaws of Elevai Labs, Inc., to be effective upon the closing of this offering* | |
| 4.1 | Form of Common Stock Share Certificate* | |
| 4.2 | Form of Underwriter’s Warrant (included in Exhibit 1.1)* | |
| 5.1 | Opinion of Ortoli Rosenstadt LLP regarding the validity of the securities being registered* | |
| 10.1 | Stock Transfer Agreement, dated June 4, 2021, between BWL Investments Ltd. And Reactive Medical Labs, Inc.* | |
| 10.2 | 2020 Equity Incentive Plan, as amended, and forms of award agreements thereunder* | |
| 10.3 | Form of Amended and Restated Consulting Agreement between Elevai Labs, Inc. and NorthStrive Companies Inc.* | |
| 10.4 | Form of Advisory Agreement between Elevai Labs, Inc. and Braeden Lichti* | |
| 10.5 | Form of Independent Director Offer Letter* | |
| 10.6 | Authorized Distributor Agreement, dated August 30, 2022, between Elevai Labs, Inc. and Refine USA, LLC* | |
| 10.7 | Authorized Distributor and Trademark License Agreement, dated January 17, 2022, between Elevai Labs, Inc. and Dermapenworld Pty Ltd* | |
| 14.1 | Code of Ethics* | |
| 21.1 | List of subsidiaries* | |
| 23.1 | Consent of Ortoli Rosenstadt LLP (included in Exhibit 5.1)* | |
| 23.2 | ||
| 24.1 | Powers of Attorney (included in the signature page to this registration statement) | |
| 99.1 | Audit Committee Charter* | |
| 99.2 | Compensation Committee Charter* | |
| 99.3 | Nominating Committee Charter* | |
| 107 | Filing Fee Table* |
| * | To be filed by amendment |
| ** | Previously submitted |
Item 17. Undertakings
| (a) | The undersigned registrant hereby undertakes: |
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | to include any prospectus required by Section 10(a)(3) of the Securities Act; |
| (ii) | to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| (iii) | to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
provided, however, that: Paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the SEC by the registrant pursuant to Section 13 or Section 15(d) of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), that are incorporated by reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration statement.
II-4
| (2) | That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| (4) | That, for the purpose of determining liability under the Securities Act to any purchaser: |
| (i) | Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the date the filed prospectus was deemed part of and included in the registration statement; and |
| (ii) | Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5) or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii) or (x) for the purpose of providing the information required by Section 10(a) of the Securities Act shall be deemed to be part of and included in the registration statement as of the earlier of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date. |
| (5) | That, for the purpose of determining liability of the registrant under the Securities Act to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (b) | Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Company pursuant to the foregoing provisions, or otherwise, the Company has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Company of expenses incurred or paid by a director, officer or controlling person of the Company in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Company will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue. |
II-5
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form S-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in New York, NY, on [●], 2023.
| By: | ||
Jordan R. Plews | ||
| Chief Executive Officer (Principal Executive Officer) |
We, the undersigned directors and officers of the Registrant, hereby severally constitute and appoint Jordan R. Plews, as singly, our true and lawful attorney in fact, with full power to him, and to singly, to sign for us and in our names in the capacities indicated below, the registration statement on Form S-1 filed herewith, and any and all pre-effective and post-effective amendments to said registration statement, and any registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, in connection with the registration under the Securities Act of 1933, as amended, of equity securities of the Registrant, and to file or cause to be filed the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney, and to him, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as each of us might or could do in person, and hereby ratifying and confirming all that said attorney, or their substitute or substitutes, shall do or cause to be done by virtue of this Power of Attorney.
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date | ||
| [●] | Chief Executive Officer, President, and Director | [●], 2023 | ||
Jordan R. Plews | (Principal Executive Officer) | |||
| [●] | Chief Financial Officer and Director | [●], 2023 | ||
| Graydon Bensler | (Principal Financial Officer and Principal Accounting Officer) | |||
| [●] | Director | [●], 2023 | ||
| Hatem (Tim) Abou-Sayed | ||||
| [●] | Independent Director | [●], 2023 | ||
| Jeffrey Parry | ||||
| [●] | Independent Director | [●], 2023 | ||
| Crystal Muilenburg | ||||
| [●] | Independent Director | [●], 2023 | ||
| Juliana Daley |
II-6

