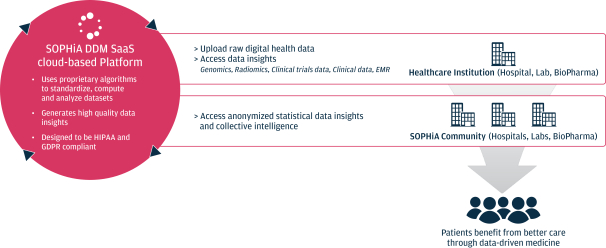
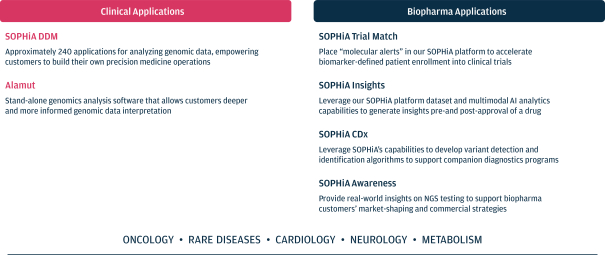
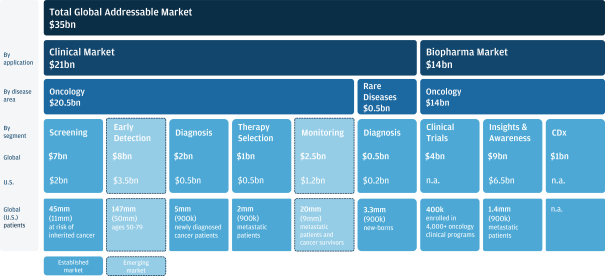
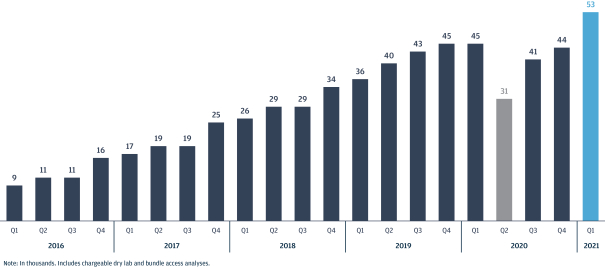
Risk factors
Investing in our ordinary shares involves a high degree of risk. You should carefully consider the risks and uncertainties described below together with all of the other information contained in this prospectus, including our consolidated financial statements, including the notes thereto, included elsewhere in this prospectus, before deciding to invest in our ordinary shares. If any of the events or developments described below were to occur, our business, results of operations, financial condition and prospects could suffer materially, the trading price of our ordinary shares could decline and you could lose all or part of your investment. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently believe to be immaterial may also adversely affect our business. If any of the following risks occur, our business, results of operations, financial condition and prospects could be materially and adversely affected.
Risks Related to the Development of Our SOPHiA Platform and Related Solutions, Products and Services
We may not be successful in expanding features, applications and data modalities of our SOPHiA platform and related solutions, products and services.
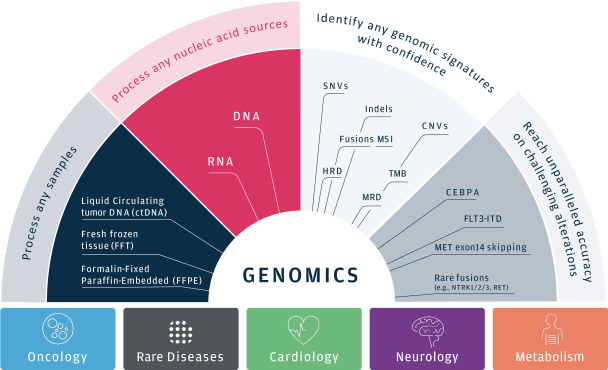
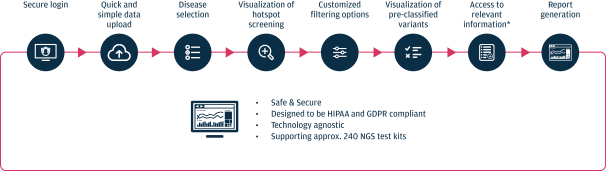
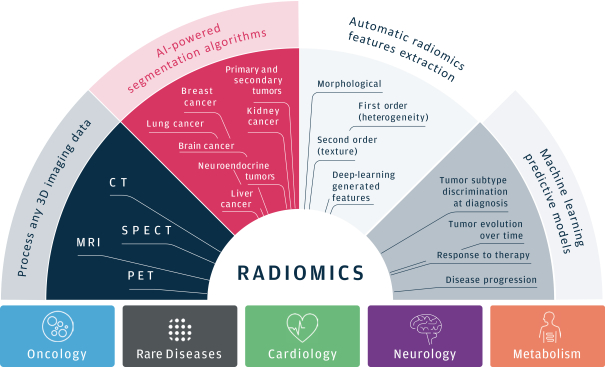
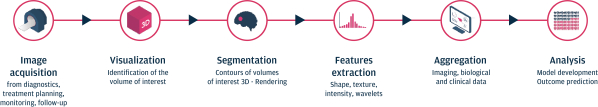
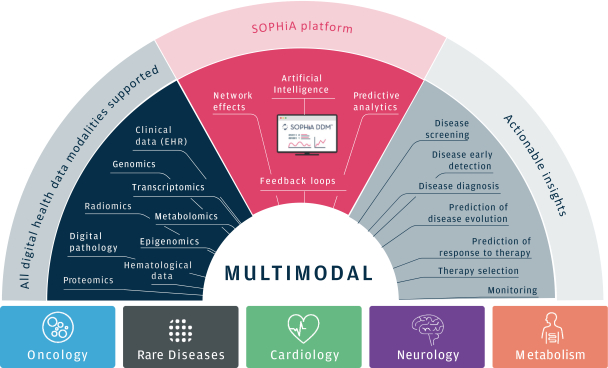
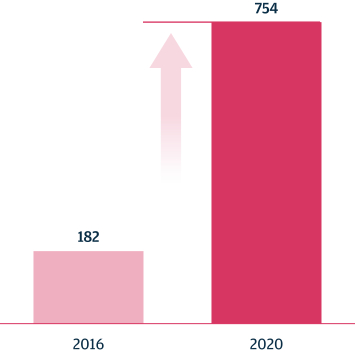
As of March 31, 2021, our SOPHiA platform offered approximately 240 genomics applications across oncology, rare diseases, infectious diseases, cardiology, neurology, metabolism and other disease areas. A major part of our long-term strategy is bringing new high-impact content to our customers through updates to our platform, which may include expanding our platform with additional features, applications and data modalities and related solutions, products and services. We expect to make significant investments to advance these efforts.
Enhancing our platform and developing new related solutions, products and services is a speculative and risky endeavor. Features, applications, data modalities and services that initially show promise may fail to achieve the desired results or may not achieve acceptable levels of analytical accuracy or utility. We may need to alter our platform, products or services in development and repeat studies before we identify a potentially successful feature, application, data modality, product or service. Platform, service and product development is expensive, may take years to complete and can have uncertain outcomes. Failure can occur at any stage of the development. Even if we confirm that our platform can be successfully employed for additional features, applications and data modalities, those features, applications and data modalities may be limited in scope to only some diseases, disease segments, patient markets or geographies. If, after development, a new feature, application, data modality, service or product appears successful, we or our collaborators may, depending on the nature of the feature, application, data modality, service or product, need to obtain FDA’s, European Medicines Agency’s (the “EMA”) and other regulatory clearances, authorizations or approvals before we can market the feature, application, data modality, service or product. The FDA’s and EMA’s clearance, authorization or approval pathways are likely to require significant time and expenditures. The FDA, EMA or other applicable regulatory authority may not clear, authorize or approve any feature, application, data modality, service or product we develop. Even if we develop a feature, application, data modality, service or product that receives regulatory clearance, authorization or approval, we or our collaborators would need to commit substantial resources to commercialize, sell and market the feature, application, data modality, service or product and the feature, application, data modality, service or product may never achieve significant market acceptance among various stakeholders and be commercially successful. Furthermore, we purposefully built our SOPHiA platform in a decentralized manner and strategically positioned it as a “universal operating-system” for multiomics and multimodal data analytics in order to provide for a broad range of product and service expansion opportunities. However, certain jurisdictions, such as the Netherlands, implemented centralized services architectures for EHR where all patient data passes through a single, often government-run, entity rather than being shared directly between the healthcare providers. Such centralized
16