Free signup for more
- Track your favorite companies
- Receive email alerts for new filings
- Personalized dashboard of news and more
- Access all data and search results
Filing tables
Filing exhibits
ATAI similar filings
- 6 Feb 24 Departure of Directors or Certain Officers
- 23 Jan 24 Entry into a Material Definitive Agreement
- 9 Jan 24 Other Events
- 4 Jan 24 Regulation FD Disclosure
- 4 Jan 24 Entry into a Material Definitive Agreement
- 14 Nov 23 Results of Operations and Financial Condition
- 13 Oct 23 Departure of Directors or Certain Officers
Filing view
External links
Exhibit 99.1

Healingmental health disorders so that everyone everywhere can live a more fulfilled life. Company Overview Company Overview – January 2024

All references in this presentation to “we”, “us”, “our”, “atai”, or the “Company” refer to ATAI Life Sciences N.V. and its consolidated subsidiaries, unless the context otherwise requires. This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. We intend such forward-looking statements to be covered under by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended.. All statements other than statements of historical facts contained in this presentation, including statements regarding our future results of operations and financial position, industry dynamics, business strategy and plans and our objectives for future operations, are forward-looking statements. These statements represent our opinions, expectations, beliefs, intentions, estimates or strategies regarding the future, which may not be realized. In some cases, you can identify forward-looking statements by terms such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “could,” “intends,” “targets,” “projects,” “contemplates,” “believes,” “estimates,” “predicts,” “potential” or “continue” or the negative of these terms or other similar expressions that are intended to identify forward-looking statements. Forward-looking statements are based largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, short term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including without limitation the important factors described in the section titled “Risk Factors” in our most recent Annual Report on Form 10-K filed with the Securities and Exchange Commission (“SEC”), as updated by our subsequent filings with the SEC, that may cause our actual results, performance or achievements to differ materially and adversely from those expressed or implied by the forward-looking statements. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. We caution you therefore against relying on these forward-looking statements, and we qualify all of our forward-looking statements by these cautionary statements. The forward-looking statements included in this presentation are made only as of the date hereof. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. Moreover, neither we nor our advisors nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements. Neither we nor our advisors undertake any obligation to update any forward-looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, except as may be required by law. You should read this presentation with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect. Unless otherwise indicated, information contained in this presentation concerning our industry, competitive position and the markets in which we operate is based on information from independent industry and research organizations, other third-party sources and management estimates. Management estimates are derived from publicly available information released by independent industry analysts and other third-party sources, as well as data from our internal research, and are based on assumptions made by us upon reviewing such data, and our experience in, and knowledge of, such industry and markets, which we believe to be reasonable. In addition, projections, assumptions and estimates of the future performance of the industry in which we operate or of any individual competitor and our future performance are necessarily subject to uncertainty and risk due to a variety of factors, including those described above. These and other factors could cause results to differ materially from those expressed in the estimates made by independent parties and by us. Industry publications, research, surveys and studies generally state that the information they contain has been obtained from sources believed to be reliable, but that the accuracy and completeness of such information is not guaranteed. Forecasts and other forward-looking information obtained from these sources are subject to the same qualifications and uncertainties as the other forward-looking statements in this presentation. This presentation contains excerpts of testimonials from individuals who have been treated with compounds or derivatives of the compounds underlying our product candidates in the context of third-party studies or otherwise that are solely intended to be illustrative and not representative of the potential for beneficial results of such compounds. Our product candidates are in preclinical or clinical stages of development and none of our product candidates have been approved by the FDA or any other regulatory agency. Any trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the products or services of the Company. Disclaimer 02

1 Mental health disorders are one of the largest global health burdens; in 2019, 1 in every 8 people, or 970 million people, around the world were living with a mental disorder1 3 Eight clinical-stage drug development programs and strategic investments, each with a robust package of prior clinical evidence 2 atai’s objective is to achieve clinically meaningful and sustained behavioral change in mental health patients by developing rapid-acting and durable therapeutics 4 Validated operating model and ability to capture value: IPO of COMPASS Pathways in 2020 and licensing deal between Otsuka and atai subsidiary Perception Neuroscience in 2021 5 Cash, marketable securities, and committed term loan funding are expected to provide runway into 20262 atai Life Sciences: Healing mental health disorders so that everyone everywhere can live a more fulfilled life 03 World Health Organization Committed term loan funding includes $45M of additional capital that can be drawn not subject to milestones under the facility with Hercules Capital; marketable securities includes money market funds, U.S. Treasury securities, commercial paper, corporate notes/bonds, U.S. government agencies securities, and public equities

Our strategy will be delivered through a robust portfolio of psychedelic and non-psychedelic drug development programs and strategic investments 04 PSYCHEDELIC PROGRAMS & STRATEGIC INVESTMENTS Preclin Phase 1 Phase 2 Phase 3 Programs / Investments RL-007 / Pro-cognitive neuromodulator3 GRX-917 / Deuterated etifoxine COMP3601 / Psilocybin VLS-01 / DMT DMX-1002 / Ibogaine EMP-01 / R-MDMA EGX-A & EGX-B / Novel 5-HT2A Receptor Agonists BPL-0032 / 5-MEO-DMT ELE-1012 / Psilocin Primary Indication Cognitive Impairment Associated with Schizophrenia Generalized Anxiety Disorder Treatment-Resistant Depression Treatment-Resistant Depression Opioid Use Disorder Post-Traumatic Stress Disorder & others Undisclosed Treatment-Resistant Depression Major Depressive Disorder NON-PSYCHEDELIC PROGRAMS 2 Strategic Investment in Beckley PsyTech 1 Strategic Investment in Compass Pathways 3 RL-007 compound is (2R, 3S)-2-amino-3-hydroxy-3-pyridin-4-yl-1-pyrrolidin-1-yl-propan-1-one(L)-(+) tartrate salts Strategic Investment
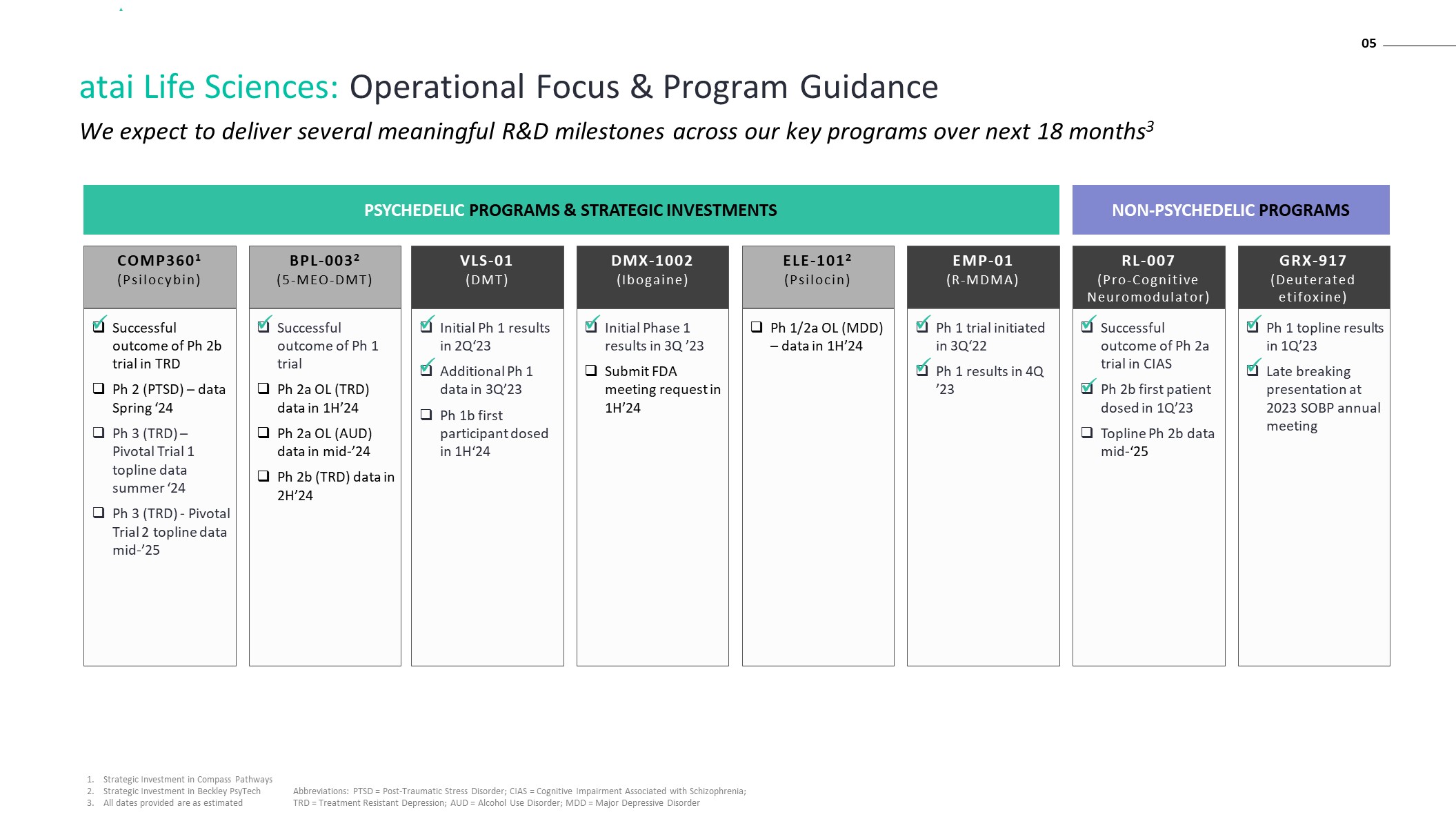
We expect to deliver several meaningful R&D milestones across our key programs over next 18 months3 05 atai Life Sciences: Operational Focus & Program Guidance Initial Phase 1 results in 3Q ’23 Submit FDA meeting request in 1H’24 Initial Ph 1 results in 2Q‘23 Additional Ph 1 data in 3Q’23 Ph 1b first participant dosed in 1H‘24 Ph 1 topline results in 1Q’23 Late breaking presentation at 2023 SOBP annual meeting Successful outcome of Ph 2a trial in CIAS Ph 2b first patient dosed in 1Q’23 Topline Ph 2b data mid-‘25 RL-007 (Pro-Cognitive Neuromodulator) GRX-917(Deuterated etifoxine) VLS-01 (DMT) DMX-1002 (Ibogaine) EMP-01 (R-MDMA) COMP3601 (Psilocybin) Ph 1 trial initiated in 3Q‘22 Ph 1 results in 4Q ’23 Successful outcome of Ph 2b trial in TRD Ph 2 (PTSD) – data Spring ‘24 Ph 3 (TRD) – Pivotal Trial 1 topline data summer ‘24 Ph 3 (TRD) - Pivotal Trial 2 topline data mid-’25 BPL-0032 (5-MEO-DMT) Successful outcome of Ph 1 trial Ph 2a OL (TRD) data in 1H’24 Ph 2a OL (AUD) data in mid-’24 Ph 2b (TRD) data in 2H’24 ELE-1012 (Psilocin) Ph 1/2a OL (MDD) – data in 1H’24 PSYCHEDELIC PROGRAMS & STRATEGIC INVESTMENTS NON-PSYCHEDELIC PROGRAMS Strategic Investment in Compass Pathways Strategic Investment in Beckley PsyTech All dates provided are as estimated Abbreviations: PTSD = Post-Traumatic Stress Disorder; CIAS = Cognitive Impairment Associated with Schizophrenia; TRD = Treatment Resistant Depression; AUD = Alcohol Use Disorder; MDD = Major Depressive Disorder

BPL-003 (5-MeO-DMT) for TRD & Alcohol Use Disorder 06

12mg (n=5) 10mg (n=5) 8mg (n=5) 6mg (n=4) 4mg (n=4) 2.5mg (n=4) 1mg (n=4) Mean plasma concentration levels (ng/ml) Time post-dose (minutes) BPL-003 Phase 1 Pharmacokinetic Profile Key Findings Safety All adverse events (AEs) were mild (89.5%) or moderate (10.5%); no Serious AEs occurred Most common AEs (>10%) : nasal discomfort, nausea, vomiting, and headache Pharmacokinetics (PK) Exposure was dose-proportionate Rapid onset: mean Tmax of 6-17 min Short duration: mean t1/2 of 15-30 min Pharmacodynamics (PD) Subjects were psychedelic naive All subjects on doses ≥6mg achieved intensity scores ≥7 Perceptual effects generally fully resolved within 60 - 90 mins BPL-003 Phase 1 Subjective Intensity Ratings 0 12 30 60 90 12mg (n=5) 10mg (n=5) 8mg (n=5) 6mg (n=4) Time post-dose (minutes) Mean subjective drug intensity (SDI) Results from completed Phase 1 SAD study showed BPL-003 had a favorable safety profile and was well tolerated whilst demonstrating dose proportionate PK/PD profile BPL-003: Intranasal 5-MeO-DMT 0 12 30 60 90 Abbreviations: SAD = Single Ascending Dose; PK = Pharmacokinetic; PD = Pharmacodynamic 7 07

BPL-003 Phase 2a is an open-label monotherapy study in TRD patients BPL-003 Phase 2a Clinical Trial Design Abbreviations: MADRS = Montgomery–Åsberg Depression Rating Scale; CGI-S = Clinical Global Impressions-Severity; PGIC = Patient's Global Impression of Change; EQ-5D = EuroQol-5D 8 Screening 10mg (n=12) Open label Day 2 85 Data expected for Ph 2a (TRD) in 1H24 Core Study (12 weeks) 57 29 8 Key Inclusion Criteria Patients with moderate-severe treatment resistant depression Montgomery-Asberg Depression Rating Scale (MADRS) score ≥24 Willing and able to discontinue current antidepressants Key Objectives: Primary Endpoint: Safety and tolerability of BPL-003 monotherapy Key Secondary Endpoints: MADRS change at Day 2, 8, 29, 57 and 85 CGI-S, PGIC, EQ-5D 08

BPL-003 Phase 2b is a randomized, double-blind, single-dose monotherapy study in moderate to severe TRD patients BPL-003 Phase 2b Clinical Trial Design 1 Patients entering the open-label extension are randomized 1:1 to receive either a single 12mg dose or a biphasic 4mg and 8mg dose approximately 10 minutes apart Abbreviations: MADRS = Montgomery–Åsberg Depression Rating Scale; CGI-S = Clinical Global Impressions-Severity; PGIC = Patient's Global Impression of Change; EQ-5D = EuroQol-5D 9 Randomization (1:1:1; n=225) Day 0 1 28 56 Wk-8 Washout 12 mg1 Core Study (8 weeks) Primary Analysis Day 1 28 56 Data expected for Ph 2b (TRD) in 2H24 (first patient dosed Oct 2023) Open label extension (8 weeks) 0.3 mg (n=75) 8 mg (n=75) 12 mg (n=75) 1st Dose 2nd Dose Key Inclusion Criteria Patients with treatment-resistant depression Hamilton Depression Scale (HAM-D) >= 19 Willing and able to discontinue current antidepressants Key Objectives: Primary Endpoint: MADRS change from baseline at day 28 Key Secondary Endpoints: MADRS change at Day 1, 7 and 56 CGI-S, PGIC, EQ-5D 7 09

VLS-01 (DMT) for TRD 10

World Health Organization Salzer, “National Estimates of Recovery-Remission From Serious Mental Illness”, Psychiatry Online (2018) Health Volunteer Study Designed for a potential rapid, sustained reduction in depressive symptoms from a single dose Product Overview: VLS-01 for Depression Lead indication overview ~300m Global sufferers of depression in 20191 33% Patients who have inadequate response or relapse after current treatments2 Depression is a mood disorder that affects the thoughts and behavior of an individual, leading to psychological, physical, and social problems Treatment resistant depression (TRD) is diagnosed after two failed courses of antidepressants FDA approved depression treatments can be characterized by a slow onset, long-term side effects and inadequate response rate 11 Global disease burden VLS-01 Key Product Features Designed for rapid onset and sustained efficacy after single dose Short duration of psychedelic effect (~30 to 45 minutes) with improved tolerability and convenience from OTF delivery relative to other psychedelics in development for depression DMT (N,N-Dimethyltryptamine) in an oral transmucosal film (OTF) Lead: Treatment Resistant DepressionPotential expansions: Eating Disorders, Substance Use Disorders Final Phase 1 data reported in 3Q ’23 Phase 1b first participant expected in 1H ‘243 PRODUCT INDICATIONS CURRENT STATUS INTELLECTUAL PROPERTY Granted U.S. patent covering OTF administration of DMT, supported by several pending U.S. and PCT patent applications
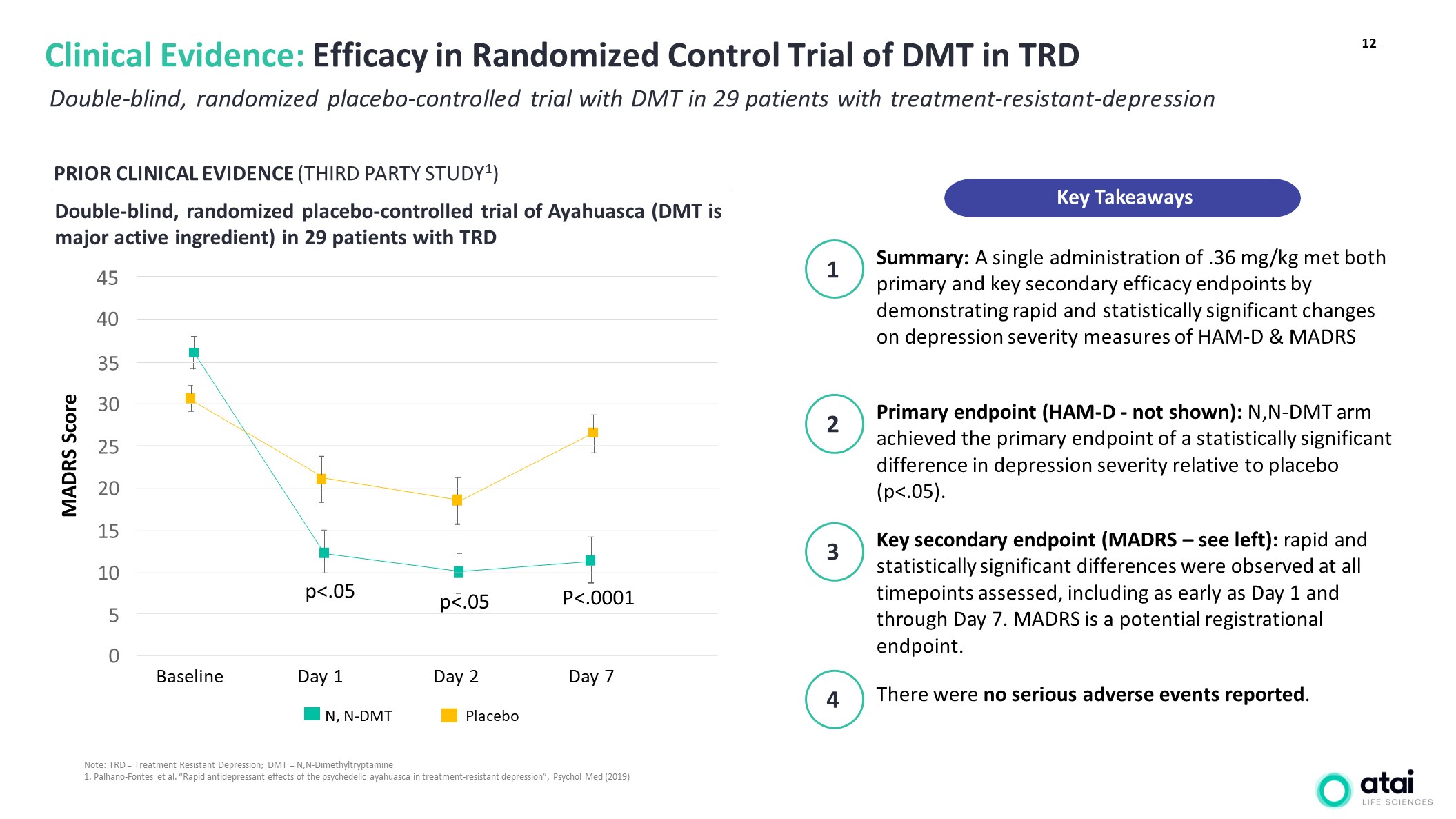
12 Double-blind, randomized placebo-controlled trial with DMT in 29 patients with treatment-resistant-depression Clinical Evidence: Efficacy in Randomized Control Trial of DMT in TRD PRIOR CLINICAL EVIDENCE (THIRD PARTY STUDY1) Double-blind, randomized placebo-controlled trial of Ayahuasca (DMT is major active ingredient) in 29 patients with TRD Placebo N, N-DMT P<.0001 p<.05 p<.05 Baseline Day 1 Day 2 Day 7 45 40 35 30 25 20 15 10 5 0 MADRS Score Note: TRD = Treatment Resistant Depression; DMT = N,N-Dimethyltryptamine 1. Palhano-Fontes et al. “Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression”, Psychol Med (2019) Summary: A single administration of .36 mg/kg met both primary and key secondary efficacy endpoints by demonstrating rapid and statistically significant changes on depression severity measures of HAM-D & MADRS Primary endpoint (HAM-D - not shown): N,N-DMT arm achieved the primary endpoint of a statistically significant difference in depression severity relative to placebo (p<.05). 1 2 There were no serious adverse events reported. 4 Key secondary endpoint (MADRS – see left): rapid and statistically significant differences were observed at all timepoints assessed, including as early as Day 1 and through Day 7. MADRS is a potential registrational endpoint. 3 Key Takeaways
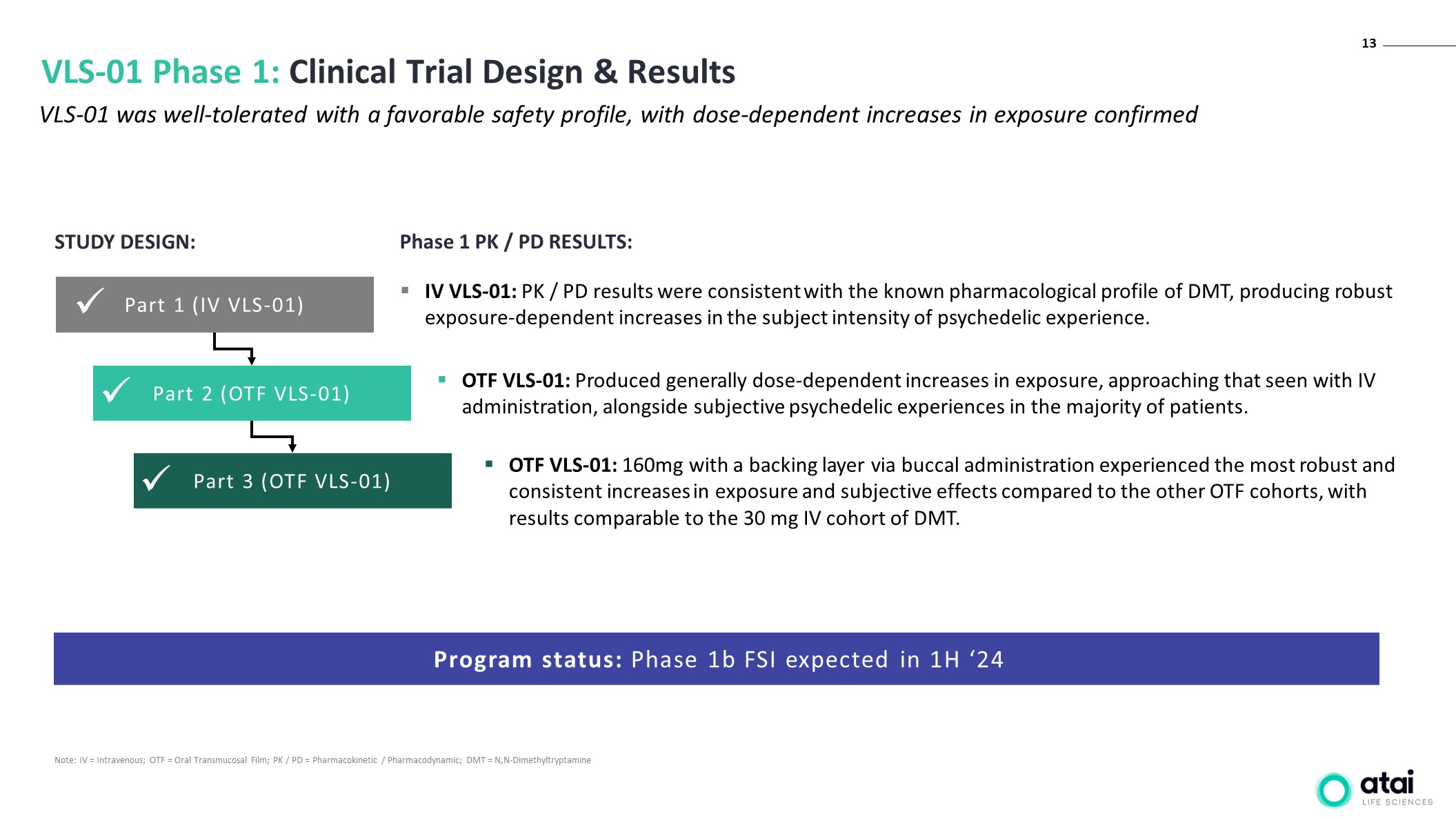
13 VLS-01 Phase 1: Clinical Trial Design & Results VLS-01 was well-tolerated with a favorable safety profile, with dose-dependent increases in exposure confirmed Note: IV = Intravenous; OTF = Oral Transmucosal Film; PK / PD = Pharmacokinetic / Pharmacodynamic; DMT = N,N-Dimethyltryptamine Program status: Phase 1b FSI expected in 1H ‘24 IV VLS-01: PK / PD results were consistent with the known pharmacological profile of DMT, producing robust exposure-dependent increases in the subject intensity of psychedelic experience. Part 3 (OTF VLS-01) Part 2 (OTF VLS-01) Part 1 (IV VLS-01) STUDY DESIGN: OTF VLS-01: Produced generally dose-dependent increases in exposure, approaching that seen with IV administration, alongside subjective psychedelic experiences in the majority of patients. OTF VLS-01: 160mg with a backing layer via buccal administration experienced the most robust and consistent increases in exposure and subjective effects compared to the other OTF cohorts, with results comparable to the 30 mg IV cohort of DMT. Phase 1 PK / PD RESULTS:

COMP 360 (psilocybin) for TRD, PTSD and Anorexia 14

COMP360 Phase 2b trial showed a rapid, sustained reduction in depressive symptoms PRODUCT PHARMA-COLOGY PRODUCT FEATURES INDICATIONS CURRENT STATUS INTELLECTUAL PROPERTY Oral Psilocybin (COMP360) 5-HT2A-R agonist Rapid onset, potential for sustained efficacy after single dose Primary: Treatment Resistant Depression, Anorexia Nervosa, PTSD Potential: Major Depressive Disorder, Autism, Bipolar Disorder, Chronic Cluster Headache Phase 3 pivotal trial 1 data expected summer-24 Phase 3 pivotal trial 2 data expected mid-25 Proprietary formulation of synthetic psilocybin, COMP360 SUMMARY: COMP360 HIGHLIGHT COMP360 demonstrated efficacy in reducing depressive symptom severity with rapid and durable response in Phase 2b study PRIOR EVIDENCE IN HUMANS (COMP360 PHASE 2b) 15.5%1 OWNERSHIP Note: MADRS = Montgomery-Åsberg Depression Rating Scale; COMP360 = a proprietary high-purity, polymorphic crystalline formulation of psilocybin; In COMPASS’s model of psilocybin therapy, COMP360 is administered in conjunction with psychological support from specially trained therapists. Ownership percentage as of Sep 30th, 2023 Post-hoc analysis showed results were also positive at the other time points listed for 25mg dose, however, the nonsignificant finding for the comparison between the 10mg group and the 1mg group terminated significance testing based on the prespecified hierarchical test procedure for all subsequent key secondary efficacy end points. 15 Primary Endpoint

16 COMPASS Pathways is currently conducting a Phase 3 pivotal program, with topline data expected in summer-2024 and mid-2025 Week 6 Primary endpoint1 Week 3 Proceeded by long-term follow up Pivotal study 1 Single dose monotherapy (COMP 005) COMP360 25mg Placebo Randomization = 2:1 Target N2 = 255 Topline data expected: summer-2024 Pivotal study 2 Fixed repeat dose monotherapy (COMP 006) COMP360 25mg COMP360 25mg COMP360 10mg COMP360 10mg COMP360 1mg COMP360 1mg Randomization = 2:1:1 Target N2 = 568 Topline data expected: mid-2025 Week 0 Week 0 Source: Compass Pathways Capital Markets Day presentation as of May 11th, 2023 Primary endpoint = Change from baseline in MADRS total score at week 6 The participant population (TRD definition and core inclusion / exclusion criteria) remains unchanged compared to Phase 2b Proceeded by long-term follow up Pivotal Phase 3 Trial Designs Week 6 Primary endpoint1

ELE-101 (psilocin) for MDD 17

Potential benefits of psilocybin’s active moiety in an optimized delivery and treatment model ELE-01: IV Psilocin 1 Psilocin simulations based on primary data from Brown et al. 2017, Madsen et al . 2019, Hasler et al. 1997, and Carhart-Harris et al. 2011. 2 Holze F. et al (2023). Pharmacokinetics and Pharmacodynamics of Oral Psilocybin Administration in Healthy Participants. Clin Pharmacol Ther. Psilocin pharmacokinetics for IV psilocin (simulated) vs. oral psilocybin1 Typical plasma psilocin concentrations at effective dose of 25mg psilocybin1 Perceptual threshold2 Expected benefits of IV psilocin vs oral psilocybin: Reduced variability Shorter-half life = shorter duration of psychedelic effect, anticipated to be <2 hours 18 18
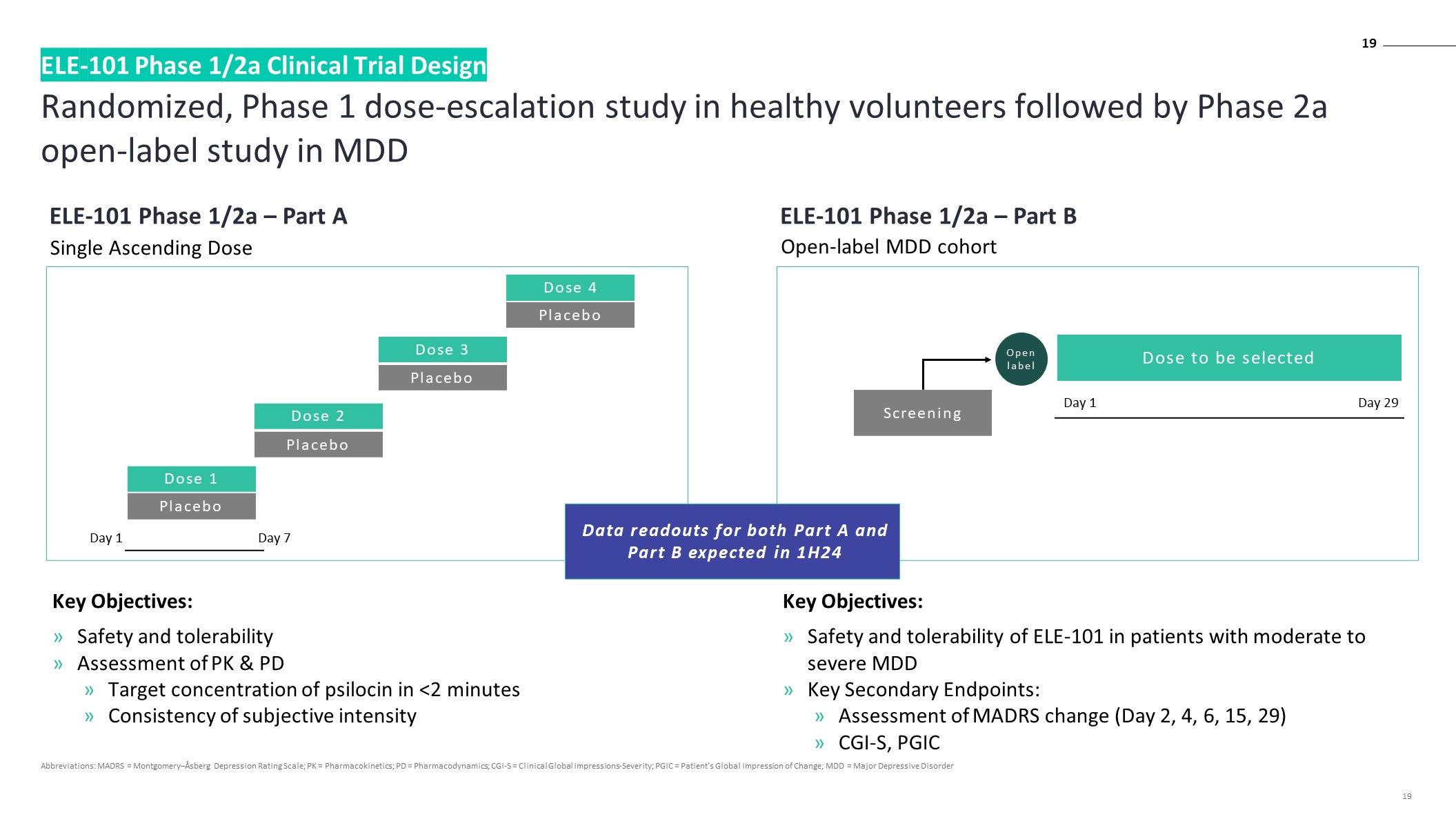
Randomized, Phase 1 dose-escalation study in healthy volunteers followed by Phase 2a open-label study in MDD ELE-101 Phase 1/2a Clinical Trial Design 19 ELE-101 Phase 1/2a – Part A Key Objectives: Safety and tolerability Assessment of PK & PD Target concentration of psilocin in <2 minutes Consistency of subjective intensity Key Objectives: Safety and tolerability of ELE-101 in patients with moderate to severe MDD Key Secondary Endpoints: Assessment of MADRS change (Day 2, 4, 6, 15, 29) CGI-S, PGIC Single Ascending Dose ELE-101 Phase 1/2a – Part B Open-label MDD cohort Data readouts for both Part A and Part B expected in 1H24 Screening Dose to be selected Open label Day 1 Day 29 Dose 1 Placebo Dose 2 Placebo Dose 3 Placebo Dose 4 Placebo Day 1 Day 7 Abbreviations: MADRS = Montgomery–Åsberg Depression Rating Scale; PK = Pharmacokinetics; PD = Pharmacodynamics; CGI-S = Clinical Global Impressions-Severity; PGIC = Patient's Global Impression of Change; MDD = Major Depressive Disorder 19

DMX-1002 (ibogaine) for Substance Use Disorder 20

Post traumatic stress disorder and traumatic brain injury, respectively World Health Organization Salzer, “National Estimates of Recovery-Remission From Serious Mental Illness”, Psychiatry Online (2018) Designed to have a rapid, sustained reduction in depressive symptoms through psychedelic effects Product Overview: DMX-1002 for Opioid Use Disorder Lead indication overview ~3m US OUD Incidence in 20202 >100k Opioid-related deaths in US in 2022 Substance use disorders are highly prevalent and characterized by an inability to control the use of a legal or illegal drugs, such as opioids (including prescription opioids) or alcohol. Current standard of care for OUD primarily consists of psychosocial support and synthetic full and partial opioid receptor agonists (methadone & buprenorphine), where approximately 30% of patients achieve treatment success (defined as >80% illicit opioid free weeks). In addition, long-acting opioid antagonists (naltrexone) lead to a proportion of patients achieving treatment success. 21 Global disease burden DMX-1002 Key Product Features A single dose of ibogaine delivered in a monitored setting may support withdrawal and long-term relapse prevention in Opioid Use Disorder patients Prior clinical evidence: In third-party open label studies, ibogaine was associated with significantly reduced opioid cravings, both at discharge and at one month post treatment, as well as improved mood in patients with OUD In addition, a double-blind, placebo-controlled study in subjects with cocaine use disorder demonstrated a statistically significant benefit on urine confirmed relapse of a single administration of ibogaine compared to placebo Lead: Opioid Use Disorder (“OUD”)Potential expansions: Add’l Substance Use Disorders, PTSD, TBI1 Phase 1 results reported in Q3’23 Expect to submit FDA meeting request in 1H’24 PRODUCT INDICATIONS CURRENT STATUS INTELLECTUAL PROPERTY Issued and pending method of treatment claims for OUD DMX-1002 is an oral formulation of ibogaine, which is an indole alkaloid with potential for clinical benefit through oneirophrenic effects

22 Results from an open-label study of 8-12 mg/kg of ibogaine in patients seeking detoxification from opioids and cocaine Clinical Evidence: Efficacy of ibogaine in Open-Label Safety and Efficacy Study PRIOR CLINICAL EVIDENCE (THIRD PARTY STUDY1) Note: TRD = Treatment Resistant Depression; DMT = N,N-Dimethyltryptamine 1 Mash et al., “Ibogaine Detoxification Transitions Opioid and Cocaine Abusers Between Dependence and Abstinence: Clinical Observations and Treatment Outcomes” (2018) 1 2 Safety: Ibogaine was reported to be well tolerated with no serious adverse events. 4 Key Takeaways HCQ-29 Subscale Pre-dose (N=75) 5 4 3 2 1 0 Discharge (N=74) 1 Month (N=37) Emotionality(negative mood state) Compulsivity(lack of confidence in ability to quit) Purposefulness(desire of intent to use) Expectancy(expected positive benefits of drug use) Self-reported dimensions of craving Efficacy – Post-Acute Withdrawal Syndrome: signs and symptoms at post dose assessments were reduced compared to pre-dose baseline withdrawal severity measures. Objective signs of opioid withdrawal were mild and none were exacerbated at later time points. 3 Efficacy – Relapse Prevention (shown left): Opioid dependent patients had significant reductions in the mean scores of four HCQ-29 domains of craving measured at program discharge and out to 1 month for patients continuing through study completion. Cravings are an important mediator of relapse. Summary: A single-dose of ibogaine showed reductions in self-reported opioid cravings in 74 opioid dependent patients.

Demonstrated safety level and plasma concentrations of DMX-1002 in line with previous trials Phase 1 Study: DMX-1002 Trial Design & Results Summary COMPLETED PHASE 1 TRIAL: SINGLE ASCENDING DOSE No serious adverse events reported Nearly all adverse events were mild-to-moderate (>94%), consistent with prior trials of ibogaine Potential therapeutic plasma levels DMX-1002's 9 mg/kg achieved plasma concentrations in line with those described in previous studies where therapeutic effects were observed Asymptomatic QTc Prolongation One of two participants in cohort 3, asymptomatic QTc prolongation was observed, with no cardiac arrythmias. The QTcF change of 90-94ms resolved without intervention or sequelae SUMMARY OF PHASE 1 RESULTS Cohort 1 N = 12 3 mg/kg Cohort 2 N = 6 6 mg/kg Cohort 3 N = 2 9 mg/kg Day 1: Single oral dose <Day 6: Inpatient Safety Monitoring: Cardiac safety Adverse events PK Population: Healthy male participants Design: Single-blinded, cross-over study. All participants received placebo first, followed by DMX-1002 at a second visit 23

DMX-1002 has the potential to become the first & best in-class treatment for OUD, minimizing risk of relapse SUMMARY DMX-1002 could potentially become a paradigm-shifting therapy for Opioid Use Disorder (OUD) Current standard of care for OUD is medication therapy, requiring opioid substitutes that carry significant side effects Sustained relapse prevention Single dose administered in monitored setting, providing both withdrawal support and oneiric experience driving sustained remission Single Therapeutic Episode Minimal Abuse Potential No Opioid Side Effects High Adherence / Low Risk of Relapse Cholinergic, glutamatergic and monoaminergic receptor modulator Mechanism of Action Ibogaine (DMX-1002) Therapy Medication Assisted Therapy1 Daily therapy given in substitution of opioid in outpatient setting in attempt to wean off from opioid Mu-agonist Partial Mu-agonist Mu-antagonist Methadone Buprenorphine Naltrexone Withdrawal Support2 Therapies given for symptomatic management during supervised withdrawal (detoxification) Alpha-2 agonist Alpha-2 agonist Clonidine Lofexidine Current strategies for withdrawal support have high rates of relapse Note: OUD = Opioid Use Disorder Source: Publicly available information, including company websites and clinicaltrials.gov, GlobalData, Evaluate Pharma (both as of 2022) Current Standard of Care Rarely used given high rates of relapse. Used primarily in institutional or penitentiary settings DemeRx 24

RL-007 for Cognitive Impairment 25

World Health Organization Bora et al, Cognitive Impairment in Schizophrenia and Affective Psychoses: Implications for DSM-V Criteria and Beyond GlobalData (as of 6/1/2023) 4. Schaffer et al., 2013 Demonstrated consistent pro-cognitive effects in prior clinical trials, with a favorable safety profile in >500 subjects Product Overview: RL-007 for Cognitive Impairment Lead indication overview ~24m Global sufferers of Schizophrenia¹ >80% Patients with Schizophrenia experiencing significant cognitive impairment2 Cognitive impairment associated with schizophrenia (CIAS) is characterized by attention, learning, memory, and exec function deficits Such deficits result in cognitive function around 2.5 standard deviations below the mean of the general population4 CIAS is a common and major cause of disability in schizophrenia, with more than 80% of patients showing significant impairment2 No FDA approved treatments3 26 Global disease burden RL-007 Key Potential Product Features Pro-cognitive effects demonstrated across four prior clinical studies, including two Phase 1 and two Phase 2 trials Consistent “inverted-U” dose response across clinical & preclinical studies Demonstrated safety & tolerability with no evidence of sedative side effects across the 10 clinical studies in >500 subjects Oral, pro-cognitive neuromodulator Lead: Cognitive impairment associated with schizophreniaPotential expansions: Cognitive disorders including Alzheimer’s dementia and/or Autism Phase 2a CIAS trial completed in H2’21Phase 2b first patient dosed in 1Q’23 Phase 2b data expected in mid’25 PRODUCT INDICATIONS CURRENT STATUS INTELLECTUAL PROPERTY Issued composition of matter, formulation and method of use IP

27 Third-Party Phase 2 study in DPNP showed statistically significant positive cognitive signals (exploratory endpoints) Note: * = P< 0.05 vs Placebo; N=60 patients/treatment group; dosed TID = 3x/day dosing; randomized, cross-over design Clinical Evidence: Efficacy on Cognitive Endpoints in a Phase 2 Study Phase II, randomized, placebo-controlled, crossover clinical study in subjects with diabetic peripheral neuropathic pain (DPNP) that assessed improvements in verbal learning and memory as an exploratory endpoint 4-week placebo periods were compared to 4-week RL-007 periods “Intermediate-dose escalation” RL-007 40mg (first week) to 80mg (n=60) “High-dose escalation” RL-007 150mg (first week) to 300mg (n=60) Background RL-007 showed statistically significant pro-cognitive effects on learning and memory within the “Intermediate-Dose escalation” 40mg to 80mg arm. 1 2 Key Takeaways Delayed recall 0.4 0.3 0.2 0.1 0 -0.1 -0.2 -0.3 0.35 0.3 0.25 0.2 0.15 0.1 0.05 RL-007 (40 & 80 mg TID) RL-007 (150 & 300 mg TID) RL-007 (40 & 80 mg TID) RL-007 (150 & 300 mg TID) Cohen’s d effect size Diff. vs. Placebo Cohen’s d effect size Diff. vs. Placebo * * Direction of Improvement Direction of Improvement 0 3 The 40 to 80mg arm patients also reported a statistically significant improvement on the Cognitive and Physical Function Questionnaire (p = 0.021) Inverted U-shaped dose response whereby intermediate doses yield greater clinical activity is replicated and consistent with from prior clinical and preclinical studies Verbal learning
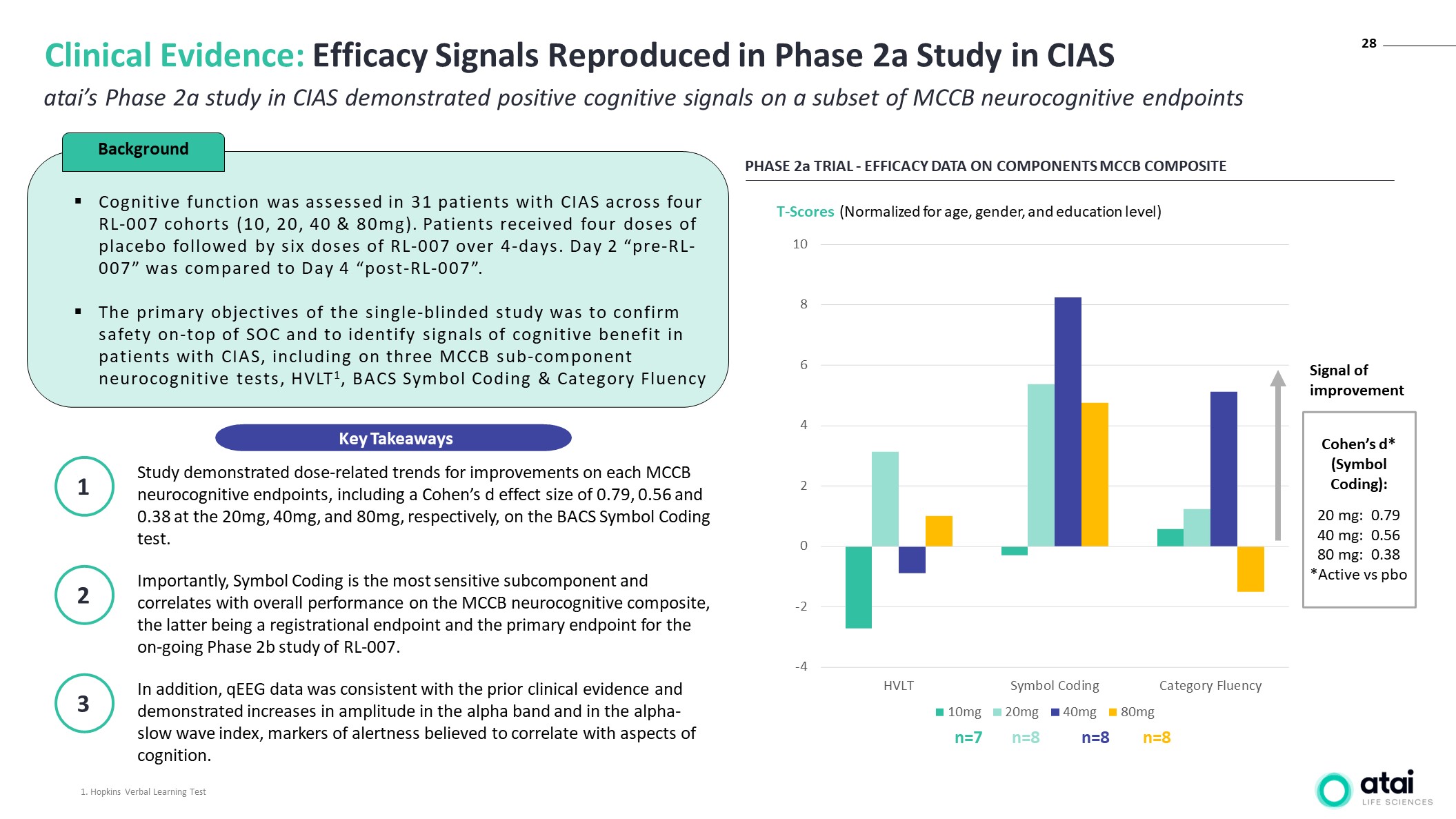
PHASE 2a TRIAL - EFFICACY DATA ON COMPONENTS MCCB COMPOSITE Signal of improvement n=7 n=8 n=8 n=8 Cohen’s d* (Symbol Coding): 20 mg: 0.79 40 mg: 0.56 80 mg: 0.38 *Active vs pbo T-Scores (Normalized for age, gender, and education level) atai’s Phase 2a study in CIAS demonstrated positive cognitive signals on a subset of MCCB neurocognitive endpoints Clinical Evidence: Efficacy Signals Reproduced in Phase 2a Study in CIAS Study demonstrated dose-related trends for improvements on each MCCB neurocognitive endpoints, including a Cohen’s d effect size of 0.79, 0.56 and 0.38 at the 20mg, 40mg, and 80mg, respectively, on the BACS Symbol Coding test. 1 Importantly, Symbol Coding is the most sensitive subcomponent and correlates with overall performance on the MCCB neurocognitive composite, the latter being a registrational endpoint and the primary endpoint for the on-going Phase 2b study of RL-007. 2 In addition, qEEG data was consistent with the prior clinical evidence and demonstrated increases in amplitude in the alpha band and in the alpha-slow wave index, markers of alertness believed to correlate with aspects of cognition. 3 Cognitive function was assessed in 31 patients with CIAS across four RL-007 cohorts (10, 20, 40 & 80mg). Patients received four doses of placebo followed by six doses of RL-007 over 4-days. Day 2 “pre-RL-007” was compared to Day 4 “post-RL-007”. The primary objectives of the single-blinded study was to confirm safety on-top of SOC and to identify signals of cognitive benefit in patients with CIAS, including on three MCCB sub-component neurocognitive tests, HVLT1, BACS Symbol Coding & Category Fluency Background Key Takeaways 1. Hopkins Verbal Learning Test 28

29 Clinical Trial Design: RL-007 Phase 2b Study Note: MCCB = MATRICS Consensus Cognitive Battery; BACS = Brief Assessment of Cognition in Schizophrenia; CIAS = Cognitive Impairment Associated with Schizophrenia; TID = 3x/day dosing Trial status: First patient dosed in 1Q’23, Topline data anticipated mid’25 Randomized, placebo-controlled study of RL-007 in ~234 patients with CIAS RL-007 TID (40mg) RL-007 TID (20mg) Placebo R Randomized 1:1:1 Primary Endpoint: MCCB neurocognitive composite score at Week 6 Key Secondary Endpoints: Select Individual Components of MCCB, including BACS Symbol Coding Clinical Global Impression Score Week 6 Day 1

GRX-917 for Anxiety Disorders 30
World Health Organization Anxiety and Depression Association of America (2021) GlobalData (as of 6/1/2023) - All recent approvals by the FDA have been reformulations of long-standing antidepressant and benzodiazepine options Designed to have rapid onset of anxiolytic activity but without the negative side effects seen with benzodiazepines Product Overview: GRX-917 for Anxiety Disorders Lead indication overview ~300m Anxiety disorder sufferers in 20191 #1 Most common mental health disorder1 Anxiety disorders develop when feelings of apprehension and unease persist over an extended period and potentially worsen over time 50% of US patients go untreated as a result of sub-optimal treatment options2 No FDA approved novel treatments over the past decade3 31 Global disease burden GRX-917 Key Product Features Demonstrated rapid onset activity of anxiolytic activity (non-deuterated etifoxine approved in France) Review of ~14m prescriptions in France underscores the strong safety track record for etifoxine Differentiated tolerability profile, with limited sedative, addictive and/or cognitive impairing properties, unlike benzodiazepines Deuterated etifoxine HCl oral dosage form (GRX-917) Lead: Anxiety Disorders (e.g., GAD, SAD, PTSD, etc.) Phase 1 trial completed in H2’22 Exploring partnership and external funding opportunities PRODUCT INDICATIONS CURRENT STATUS INTELLECTUAL PROPERTY Issued composition of matter on deuterated etifoxine (GRX-917) and corresponding methods of use

32 Note: PK / PD = Pharmacokinetic / Pharmacodynamic; qEEG = Quantitative electroencephalography; BID = Twice daily Demonstrated a rapid and dose-dependent PK/PD effect along with a favourable safety profile Phase 1 Study: GRX-917 Trial Design & Results Summary COMPLETED PHASE 1 TRIAL Part 2: Multiple Ascending Dose Part 1: Single Ascending Dose 42 healthy subjects: 5 cohorts 25mg to 500mg PD Endpoint: qEEG 60 healthy subjects: 5 cohorts 100mg to 300mg BID PD Endpoint: qEEG SAFETY/PK/PD TREATMENT SAFETY/PK/PD TREATMENT Safe & well-tolerated Well-tolerated with no dose limiting toxicities, with adverse effects comparable to that of placebo Target engagement demonstrated Dose-dependent increases in qEEG beta power Sedation comparable to placebo Sedation in-line with placebo, which was consistent with EEG results and which did not show decreases in qEEG alpha power SUMMARY OF PHASE 1 RESULTS
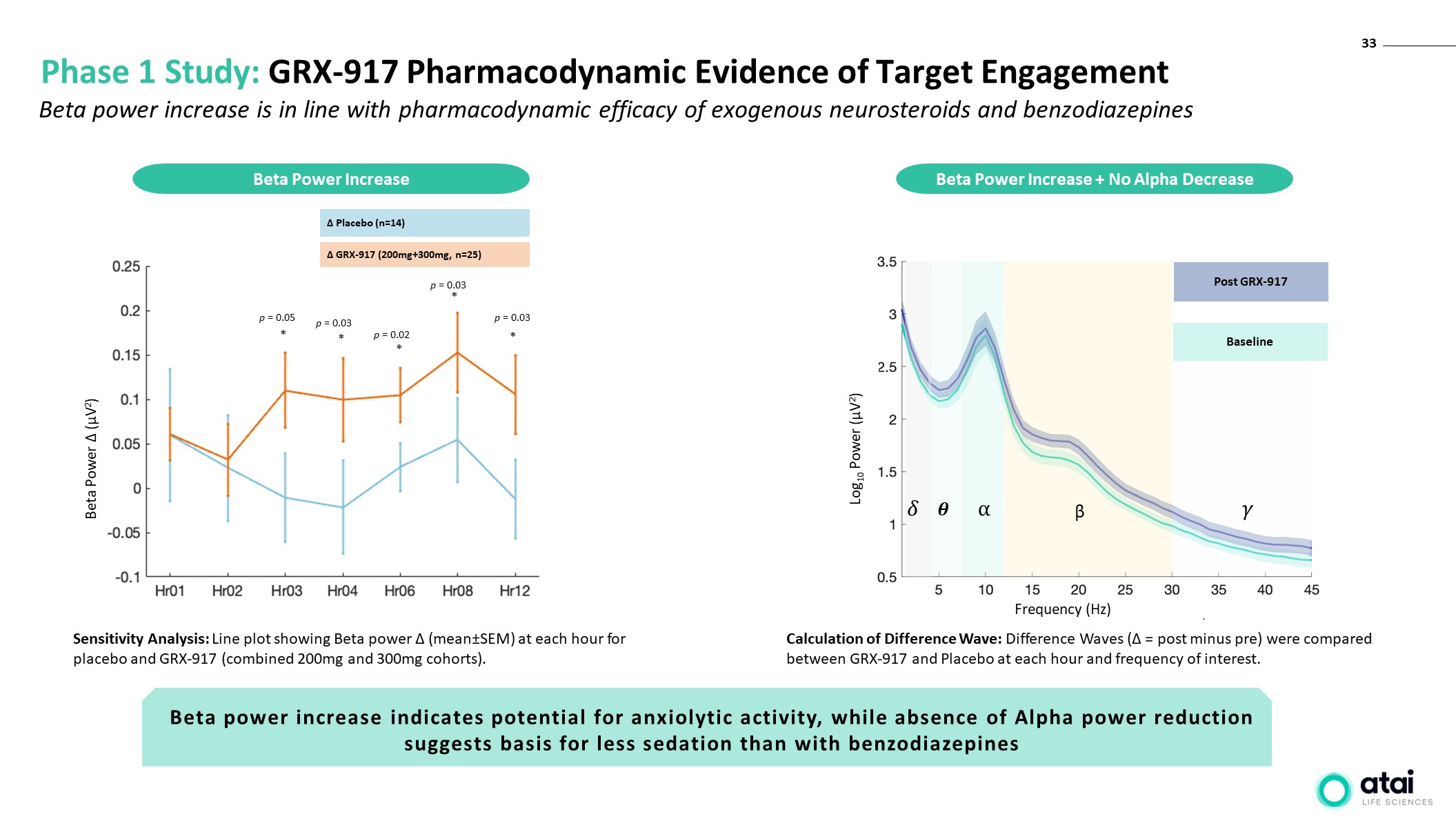
33 Phase 1 Study: GRX-917 Pharmacodynamic Evidence of Target Engagement Beta Power ∆ (µV2) ∆ Placebo (n=14) ∆ GRX-917 (200mg+300mg, n=25) p = 0.05 p = 0.03 p = 0.02 p = 0.03 p = 0.03 * * * * * Sensitivity Analysis: Line plot showing Beta power ∆ (mean±SEM) at each hour for placebo and GRX-917 (combined 200mg and 300mg cohorts). Beta Power Increase Beta Power Increase + No Alpha Decrease ⍺ �� �� β �� Post GRX-917 Baseline Log10 Power (µV2) Frequency (Hz) Beta power increase is in line with pharmacodynamic efficacy of exogenous neurosteroids and benzodiazepines Beta power increase indicates potential for anxiolytic activity, while absence of Alpha power reduction suggests basis for less sedation than with benzodiazepines Calculation of Difference Wave: Difference Waves (∆ = post minus pre) were compared between GRX-917 and Placebo at each hour and frequency of interest.

Nasdaq: ATAI