UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-Q
(Mark One)
| ☒ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended June 30, 2021
or
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission File Number: 001-40493
ATAI Life Sciences N.V.
(Exact name of registrant as specified in its charter)
| The Netherlands | Not Applicable | |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) | |
ATAI Life Sciences N.V. c/o Mindspace Krausenstraße 9-10 Berlin, Germany | Not Applicable | |
| (Address of principal executive offices) | (Zip Code) | |
+49 89 2153 9035
(Registrant’s telephone number, including area code)
N/A
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Trading Symbol(s) | Name of each exchange on which registered | ||
| Common shares, par value €0.10 per share | ATAI | The Nasdaq Stock Market LLC (Nasdaq Global Market) |
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☐ No ☒
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ | |||
| Emerging growth company | ☒ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of August 11, 2021, the registrant had 154,819,776 common shares, par value €0.10 per share, outstanding.
FORM 10-Q
Table of Contents
| Page | ||||||
| 1 | ||||||
PART I. FINANCIAL INFORMATION | 2 | |||||
Item 1. | Financial Statements (Unaudited) | 2 | ||||
Condensed Consolidated Balance Sheets as of June 30, 2021 and December 31, 2020 | 2 | |||||
| 3 | ||||||
| 4 | ||||||
| 5 | ||||||
Condensed Consolidated Statements of Cash Flows for the Six Months Ended June 30, 2021 and 2020 | 6 | |||||
| Notes to Condensed Consolidated Financial Statements | 7 | |||||
Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations | 51 | ||||
Item 3. | Quantitative and Qualitative Disclosures About Market Risk | 77 | ||||
Item 4. | Controls and Procedures | 78 | ||||
PART II. OTHER INFORMATION | ||||||
Item 1. | Legal Proceedings | 80 | ||||
Item 1A. | Risk Factors | 81 | ||||
Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds | 82 | ||||
Item 3. | Defaults Upon Senior Securities | 83 | ||||
Item 4. | Mine Safety Disclosures | 83 | ||||
Item 5. | Other Information | 83 | ||||
Item 6. | Exhibits | 83 | ||||
| Signatures | 85 | |||||
i
CAUTIONARY NOTE REGARDING FORWARD LOOKING STATEMENTS
This Quarterly Report on Form 10-Q (this “Quarterly Report”) contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All statements contained in this Quarterly Report other than statements of historical fact, including statements regarding our future operating results and financial position, the success, cost and timing of development of our product candidates, including the progress of preclinical and clinical trials, the commercialization of our current product candidates and any other product candidates we may identify and pursue, if approved, including our ability to successfully build a specialty sales force and commercial infrastructure to market our current product candidates and any other product candidates we may identify and pursue, the timing of and our ability to obtain and maintain regulatory approvals, our business strategy and plans, potential acquisitions, and the plans and objectives of management for future operations and capital expenditures, are forward-looking statements. The words “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “could,” “would,” “project,” “plan,” “potentially,” “preliminary,” “likely,” and similar expressions are intended to identify forward-looking statements.
We have based these forward-looking statements largely on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, business strategy, short-term and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties, and assumptions, including without limitation: we are a clinical-stage biopharmaceutical company and have incurred significant losses since our inception, and we anticipate that we will continue to incur significant losses for the foreseeable future; we will require substantial additional funding to achieve our business goals, and if we are unable to obtain this funding when needed and on acceptable terms, we could be forced to delay, limit or terminate our product development efforts; our limited operating history may make it difficult to evaluate the success of our business and to assess our future viability; we have never generated revenue and may never be profitable; our product candidates contain controlled substances, the use of which may generate public controversy; clinical and preclinical development is uncertain, and our preclinical programs may experience delays or may never advance to clinical trials; we currently rely on qualified therapists working at third-party clinical trial sites to administer certain of our product candidates in our clinical trials and we expect this to continue upon approval, if any, of our current or future product candidate, and if third-party sites fail to recruit and retain a sufficient number of therapists or effectively manage their therapists, our business, financial condition and results of operations would be materially harmed; we cannot give any assurance that any of our product candidates will receive regulatory approval, which is necessary before they can be commercialized; research and development of drugs targeting the central nervous system, or CNS, is particularly difficult, and it can be difficult to predict and understand why a drug has a positive effect on some patients but not others; we face significant competition in an environment of rapid technological and scientific change; third parties may claim that we are infringing, misappropriating or otherwise violating their intellectual property rights, the outcome of which would be uncertain and may prevent or delay our development and commercialization efforts; a change in our effective place of management may increase our aggregate tax burden; we identified material weaknesses in connection with our internal control over financial reporting; and a pandemic, epidemic, or outbreak of an infectious disease, such as the COVID-19 pandemic, may materially and adversely affect our business, including our preclinical studies, clinical trials, third parties on whom we rely, our supply chain, our ability to raise capital, our ability to conduct regular business and our financial results. Other risk factors include the important factors described in the section titled “Risk Factors” in our final prospectus dated June 17, 2021, filed with the Securities and Exchange Commission (“SEC”) pursuant to Rule 424(b) under the Securities Act, that may cause our actual results, performance or achievements to differ materially and adversely from those expressed or implied by the forward-looking statements.
Any forward-looking statements made herein speak only as of the date of this Quarterly Report, and you should not rely on forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, performance, or achievements reflected in the forward-looking statements will be achieved or will occur. Except as required by applicable law, we undertake no obligation to update any of these forward-looking statements for any reason after the date of this Quarterly Report on Form 10-Q or to conform these statements to actual results or revised expectations.
GENERAL
Unless the context otherwise requires, all references in this Quarterly Report to “we,” “us,” “our,” “ATAI” or the “Company” refer to ATAI Life Sciences N.V. and its consolidated subsidiaries.
All reports we file with the SEC are available for download free of charge via the Electronic Data Gathering Analysis and Retrieval (EDGAR) System on the SEC’s website at www.sec.gov. We also make electronic copies of our reports available for download, free of charge, through our investor relations website at ir.atai.life as soon as reasonably practicable after filing such material with the SEC.
We may announce material business and financial information to our investors using our investor relations website at ir.atai.life. We therefore encourage investors and others interested in ATAI to review the information that we make available on our website, in addition to following our filings with the SEC, webcasts, press releases and conference calls. Information contained on our website is not part of this Quarterly Report.
1
PART I – FINANCIAL INFORMATION
| Item 1. | Financial Statements (Unaudited) |
CONDENSED CONSOLIDATED BALANCE SHEETS
(Amounts in thousands, except share and per share amounts)
| June 30, | December 31, | |||||||
| 2021 | 2020 | |||||||
| (unaudited) | ||||||||
Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 453,622 | $ | 97,246 | ||||
Prepaid expenses and other current assets | 3,964 | 2,076 | ||||||
Short term notes receivable - related party | — | 226 | ||||||
|
|
|
| |||||
Total current assets | 457,586 | 99,548 | ||||||
Property and equipment, net | 331 | 71 | ||||||
Deferred offering costs | — | 1,575 | ||||||
Equity method investments | 19,780 | — | ||||||
Other investments held at fair value | 6,886 | — | ||||||
Other investments | 16,107 | 8,044 | ||||||
Long term notes receivable | 1,388 | 911 | ||||||
Long term notes receivable - related parties | 3,194 | 1,060 | ||||||
Other assets | 689 | 339 | ||||||
|
|
|
| |||||
Total assets | $ | 505,961 | $ | 111,548 | ||||
|
|
|
| |||||
Liabilities and Stockholders’ Equity | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 6,202 | $ | 3,083 | ||||
Accrued liabilities | 7,824 | 9,215 | ||||||
Deferred revenue | 120 | — | ||||||
Short-term notes payable | 39 | — | ||||||
|
|
|
| |||||
Total current liabilities | 14,185 | 12,298 | ||||||
Contingent consideration liability - related parties | 2,466 | 1,705 | ||||||
Convertible promissory notes - related parties, net of discounts and deferred issuance costs | 1,176 | 1,199 | ||||||
Convertible promissory notes and derivative liability (including a related party convertible promissory note and derivative liability of $0 million and $0.3 million at June 30, 2021 and December 31, 2020, respectively) | — | 978 | ||||||
Other liabilities | 3,239 | — | ||||||
|
|
|
| |||||
Total liabilities | 21,066 | 16,180 | ||||||
|
|
|
| |||||
Commitments and contingencies (Note 15) | ||||||||
Stockholders’ equity: | ||||||||
Common stock, €0.10 par value ($0.12 par value at June 30, 2021 and December 31, 2020, respectively); 750,000,000 and 173,116,704 shares authorized at June 30, 2021 and December 31, 2020, respectively; 154,819,776 and 114,735,712 shares issued and outstanding at June 30, 2021 and December 31, 2020, respectively | 17,299 | 13,372 | ||||||
Additional paid-in capital | 691,382 | 261,626 | ||||||
Accumulated other comprehensive income (loss) | 3,937 | 5,819 | ||||||
Accumulated deficit | (237,768 | ) | (189,995 | ) | ||||
|
|
|
| |||||
Total stockholders’ equity attributable to ATAI Life Sciences N.V. stockholders | 474,850 | 90,822 | ||||||
Noncontrolling interests | 10,045 | 4,546 | ||||||
|
|
|
| |||||
Total stockholders’ equity | 484,895 | 95,368 | ||||||
|
|
|
| |||||
Total liabilities and stockholders’ equity | $ | 505,961 | $ | 111,548 | ||||
|
|
|
| |||||
See accompanying notes to the unaudited condensed consolidated financial statements
2
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(Amounts in thousands, except share and per share amounts)
(unaudited)
| Three Months Ended | Six Months Ended | |||||||||||||||
| June 30, | June 30, | |||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
License revenue | $ | — | $ | — | $ | 19,880 | $ | — | ||||||||
Operating expenses: | ||||||||||||||||
Research and development | 16,026 | 2,854 | 21,611 | 4,998 | ||||||||||||
Acquisition of in-process research and development | 7,962 | 120 | 8,934 | 120 | ||||||||||||
General and administrative | 37,331 | 2,851 | 46,604 | 4,421 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating expenses | 61,319 | 5,825 | 77,149 | 9,539 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Loss from operations | (61,319 | ) | (5,825 | ) | (57,269 | ) | (9,539 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Other income (expense), net: | ||||||||||||||||
Interest income | 35 | 18 | 72 | 38 | ||||||||||||
Change in fair value of contingent consideration liability - related parties | (911 | ) | (42 | ) | (660 | ) | (66 | ) | ||||||||
Change in fair value of short term notes receivable - related party | — | — | — | 718 | ||||||||||||
Change in fair value of convertible promissory notes | — | (1,260 | ) | — | (133 | ) | ||||||||||
Change in fair value of derivative liability | — | — | 41 | — | ||||||||||||
Unrealized loss on other investments held at fair value | (5,460 | ) | — | (5,460 | ) | — | ||||||||||
Unrealized gain on other investments | — | — | — | 19,856 | ||||||||||||
Loss on conversion of convertible promissory notes | (513 | ) | — | (513 | ) | — | ||||||||||
Gain on consolidation of a variable interest entity | 3,543 | — | 3,543 | — | ||||||||||||
Other income (expense), net | (2,676 | ) | (37 | ) | (1,302 | ) | (119 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Total other income (expense), net | (5,982 | ) | (1,321 | ) | (4,279 | ) | 20,294 | |||||||||
|
|
|
|
|
|
|
| |||||||||
Net income (loss) before income taxes | (67,301 | ) | (7,146 | ) | (61,548 | ) | 10,755 | |||||||||
Provision for income taxes | (58 | ) | — | (64 | ) | — | ||||||||||
Gain on dilution of equity method investment | 16,923 | — | 16,923 | — | ||||||||||||
Losses from investments in equity method investees, net of tax | (2,937 | ) | (9,811 | ) | (4,640 | ) | (11,831 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Net loss | (53,373 | ) | (16,957 | ) | (49,329 | ) | (1,076 | ) | ||||||||
Net loss attributable to redeemable noncontrolling interests and noncontrolling interests | (4,912 | ) | (600 | ) | (1,556 | ) | (1,022 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Net loss attributable to ATAI Life Sciences N.V. stockholders | $ | (48,461 | ) | $ | (16,357 | ) | $ | (47,773 | ) | $ | (54 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
Net loss per share attributable to ATAI Life Sciences N.V. stockholders — basic and diluted | $ | (0.37 | ) | $ | (0.18 | ) | $ | (0.38 | ) | $ | (0.00 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
Weighted average common shares outstanding attributable to ATAI Life Sciences N.V. stockholders — basic and diluted | 132,265,075 | 90,709,312 | 125,797,732 | 90,709,312 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
See accompanying notes to the unaudited condensed consolidated financial statements
3
CONDENSED CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
(Amounts in thousands)
(unaudited)
| Three Months Ended | Six Months Ended | |||||||||||||||
| June 30, | June 30, | |||||||||||||||
| 2021 | 2020 | 2021 | 2020 | |||||||||||||
Net loss | $ | (53,373 | ) | $ | (16,957 | ) | $ | (49,329 | ) | $ | (1,076 | ) | ||||
Other comprehensive loss: | ||||||||||||||||
Foreign currency translation adjustments, net of tax | 2,110 | 784 | (1,916 | ) | (106 | ) | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Comprehensive income (loss) | $ | (51,263 | ) | $ | (16,173 | ) | $ | (51,245 | ) | $ | (1,182 | ) | ||||
Comprehensive income (loss) attributable to redeemable noncontrolling interests and noncontrolling interests | (4,912 | ) | (600 | ) | (1,556 | ) | (1,022 | ) | ||||||||
Foreign currency translation adjustments, net of tax attributable to noncontrolling interests | 150 | (20 | ) | (34 | ) | (7 | ) | |||||||||
|
|
|
|
|
|
|
| |||||||||
Comprehensive loss attributable to redeemable noncontrolling interests and noncontrolling interests | (4,762 | ) | (620 | ) | (1,590 | ) | (1,029 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Comprehensive income (loss) attributable to ATAI Life Sciences N.V. stockholders | $ | (46,501 | ) | $ | (15,553 | ) | $ | (49,655 | ) | $ | (153) | |||||
|
|
|
|
|
|
|
| |||||||||
See accompanying notes to the unaudited condensed consolidated financial statements
4
CONDENSED CONSOLIDATED STATEMENTS OF REDEEMABLE NONCONTROLLING
INTERESTS AND STOCKHOLDERS’ EQUITY
(Amounts in thousands, except share and per share amounts)
(unaudited)
| Accumulated | Total | |||||||||||||||||||||||||||||||||||||||
| Other | Stockholders’ | |||||||||||||||||||||||||||||||||||||||
| Redeemable | Additional | Share | Comprehensive | Equity Attributable to | Total | |||||||||||||||||||||||||||||||||||
| Noncontrolling | Common Stock | Paid-In | Subscriptions | Income | Accumulated | ATAI Life Sciences N.V. | Noncontrolling | Stockholders’ | ||||||||||||||||||||||||||||||||
| Interests | Shares | Amount | Capital | Receivable | (Loss) | Deficit | Stockholders | Interests | Equity | |||||||||||||||||||||||||||||||
Balances at December 31, 2020 | $ | — | 114,735,712 | $ | 13,372 | $ | 261,626 | $ | — | $ | 5,819 | $ | (189,995 | ) | $ | 90,822 | $ | 4,546 | $ | 95,368 | ||||||||||||||||||||
Issuance of common shares, net of issuance costs of $4.9 million | — | 15,552,688 | 1,881 | 162,497 | (140,868 | ) | — | — | 23,510 | — | 23,510 | |||||||||||||||||||||||||||||
Issuance of common shares under the Hurdle Share Option Plan (see Note 12) | — | 7,281,376 | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||
Issuance of noncontrolling interest | — | — | — | — | — | — | — | — | 885 | 885 | ||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | 212 | — | — | — | 212 | — | 212 | ||||||||||||||||||||||||||||||
Foreign currency translation adjustment, net of tax | — | — | — | — | — | (3,842 | ) | — | (3,842 | ) | (184 | ) | (4,026 | ) | ||||||||||||||||||||||||||
Net income | — | — | — | — | — | — | 688 | 688 | 3,356 | 4,044 | ||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
Balances as of March 31, 2021 | $ | — | 137,569,776 | $ | 15,253 | $ | 424,335 | $ | (140,868 | ) | $ | 1,977 | $ | (189,307 | ) | $ | 111,390 | $ | 8,603 | $ | 119,993 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
Settlement of issuance of common shares, net of issuance costs of $4.9 million | — | — | — | — | 140,868 | — | — | 140,868 | — |
| 140,868 |
| ||||||||||||||||||||||||||||
Issuance of common shares, net of issuance costs of $9.0 million | — | 17,250,000 | 2,046 | 229,535 | — | — | — | 231,581 | — | 231,581 | ||||||||||||||||||||||||||||||
Issuance of noncontrolling interest | 2,555 | — | — | — | — | — | — | — | 3,649 | 3,649 | ||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | 37,512 | — | — | — | 37,512 | — | 37,512 | ||||||||||||||||||||||||||||||
Foreign currency translation adjustment, net of tax | — | — | — | — | — | 1,960 | — | 1,960 | 150 | 2,110 | ||||||||||||||||||||||||||||||
Net income | (2,555 | ) | — | — | — | — | — | (48,461 | ) | (48,461 | ) | (2,357 | ) | (50,818 | ) | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
Balances as of June 30, 2021 | $ | — | 154,819,776 | $ | 17,299 | $ | 691,382 | $ | — | $ | 3,937 | $ | (237,768 | ) | $ | 474,850 | $ | 10,045 | $ | 497,032 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||
| Accumulated | Total | |||||||||||||||||||||||||||||||||||
| Other | Stockholders’ | |||||||||||||||||||||||||||||||||||
| Redeemable | Additional | Comprehensive | Equity Attributable | Total | ||||||||||||||||||||||||||||||||
| Noncontrolling | Common Stock | Paid-In | Income | Accumulated | to ATAI Life Sciences N.V. | Noncontrolling | Stockholders’ | |||||||||||||||||||||||||||||
| Interests | Shares | Amount | Capital | (Loss) | Deficit | Stockholders | Interests | Equity | ||||||||||||||||||||||||||||
Balances at December 31, 2019 | $ | 142 | 90,709,312 | $ | 10,510 | $ | 69,819 | $ | (1,426 | ) | $ | (20,152 | ) | $ | 58,751 | $ | 887 | $ | 59,638 | |||||||||||||||||
Stock-based compensation expense | — | — | — | 41 | — | — | 41 | — | 41 | |||||||||||||||||||||||||||
Foreign currency translation adjustment, net of tax | — | — | — | — | (903 | ) | — | (903 | ) | 13 | (890 | ) | ||||||||||||||||||||||||
Net income (loss) | (33 | ) | — | — | — | — | 16,302 | 16,302 | (389 | ) | 15,913 | |||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
Balances as of March 31, 2020 | $ | 109 | 90,709,312 | $ | 10,510 | $ | 69,860 | $ | (2,329 | ) | $ | (3,850 | ) | $ | 74,191 | $ | 511 | $ | 74,702 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
Stock-based compensation expense | — | — | — | 41 | — | — | 41 | — | 41 | |||||||||||||||||||||||||||
Issuance of subsidiary shares in connection with the Columbia stock purchase agreement (Note 16) | — | — | — | 120 | — | — | 120 | — | 120 | |||||||||||||||||||||||||||
Foreign currency translation adjustment, net of tax | — | — | — | — | 804 | 804 | (20 | ) | 777 | |||||||||||||||||||||||||||
Net income (loss) | (109 | ) | — | — | — | — | (16,357 | ) | (16,357 | ) | (491 | ) | (16,848 | ) | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
Balances as of June 30, 2020 | $ | — | $ | 90,709,312 | $ | 10,510 | $ | 70,021 | $ | (1,525 | ) | $ | (20,207 | ) | $ | 58,799 | $ | — | $ | 58,800 | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
See accompanying notes to the unaudited condensed consolidated financial statements
5
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts in thousands)
(unaudited)
| Six Months Ended | ||||||||
| June 30, | ||||||||
| 2021 | 2020 | |||||||
Cash flows from operating activities | ||||||||
Net loss | $ | (49,329 | ) | $ | (1,076 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
Depreciation and amortization expense | 25 | 5 | ||||||
Amortization of debt discount | 191 | 15 | ||||||
Change in fair value of contingent consideration liability- related parties | 660 | 66 | ||||||
Change in fair value of short term notes receivable - related parties | — | (718 | ) | |||||
Change in fair value of convertible promissory notes | — | 133 | ||||||
Change in fair value of derivative liability | (41 | ) | 12 | |||||
Change in fair value of warrant liability | 40 | — | ||||||
Unrealized loss on other investments held at fair value | 5,460 | — | ||||||
Unrealized gains on other investments | — | (19,856 | ) | |||||
Gain on dilution of equity method investment | (16,923 | ) | — | |||||
Loss on conversion of convertible notes | 513 | — | ||||||
Gain on consolidation of a variable interest entity | (3,543 | ) | — | |||||
Losses from investments in equity method investees | 4,641 | 11,831 | ||||||
In-process research and development expense | 8,934 | 120 | ||||||
Stock-based compensation expense | 37,724 | 82 | ||||||
Unrealized foreign exchange gains | — | (155 | ) | |||||
Other | 41 | (57 | ) | |||||
Changes in operating assets and liabilities: | ||||||||
Prepaid expenses and other current assets | (1,674 | ) | (513 | ) | ||||
Accounts payable | 2,380 | 252 | ||||||
Accrued liabilities | (3,846 | ) | 752 | |||||
Deferred revenue | 120 | — | ||||||
|
|
|
| |||||
Net cash used in operating activities | (14,627 | ) | (9,107 | ) | ||||
|
|
|
| |||||
Cash flows from investing activities | ||||||||
Purchases of property and equipment | (298 | ) | (8 | ) | ||||
Capitalized internal-use software development costs | (155 | ) | — | |||||
Cash acquired in asset acquisitions, net | 47 | — | ||||||
Cash paid for investments in equity method investees | (5,359 | ) | — | |||||
Cash paid for other investments | (23,445 | ) | (17,823 | ) | ||||
Purchases of long term notes receivable - related party | — | (1,198 | ) | |||||
Loans to related parties | (2,624 | ) | — | |||||
Cash paid for other assets | (195 | ) | — | |||||
|
|
|
| |||||
Net cash used in investing activities | (32,029 | ) | (19,029 | ) | ||||
|
|
|
| |||||
Cash flows from financing activities | ||||||||
Proceeds from issuance of common stock | 409,884 | — | ||||||
Cash paid for common stock issuance costs | (10,161 | ) | — | |||||
Proceeds from issuance of share option awards | 534 | — | ||||||
Proceeds from sale of investment | 2,417 | — | ||||||
Proceeds from issuance of convertible promissory notes | 1,588 | 13,011 | ||||||
|
|
|
| |||||
Net cash provided by financing activities | 404,262 | 13,011 | ||||||
|
|
|
| |||||
Effect of foreign exchange rate changes on cash | (1,230 | ) | (204 | ) | ||||
|
|
|
| |||||
Net increase (decrease) in cash and cash equivalents | 356,376 | (15,329 | ) | |||||
|
|
|
| |||||
Cash and cash equivalents – beginning of the period | 97,246 | 30,062 | ||||||
|
|
|
| |||||
Cash and cash equivalents – end of the period | $ | 453,622 | 14,733 | |||||
|
|
|
| |||||
Supplemental disclosures of non-cash investing and financing information: | ||||||||
Fair value of noncontrolling interests issued in connection with asset acquisitions | $ | 885 | $ | — | ||||
Fair value of noncontrolling interests issued in connection with consolidation of a VIE | $ | 392 | $ | — | ||||
Fair value redeemable noncontrolling interests issued in connection with consolidation of a VIE | $ | 2,555 | $ | — | ||||
Issuance of subsidiary shares in connection with the conversion of convertible notes | $ | 3,257 | $ | — | ||||
Common stock issuance costs in accounts payable | $ | 230 | $ | — | ||||
Common stock issuance costs in accrued liabilities | $ | 1,958 | $ | — | ||||
Conversion of short term notes receivable for other investments | $ | — | $ | 9,003 | ||||
Issuance of subsidiary shares in connection with a stock purchase agreement | $ | — | $ | 120 | ||||
Issuance of derivative instrument related to convertible promissory notes | $ | 646 | $ | 203 | ||||
See accompanying notes to the unaudited condensed consolidated financial statements
6
1. Organization and Description of Business
ATAI Life Sciences N.V. (“ATAI”) is the parent company of ATAI Life Sciences AG and, along with its subsidiaries, is a clinical-stage biopharmaceutical company aiming to transform the treatment of mental health disorders. ATAI was founded to address the significant unmet need and lack of innovation in the mental health treatment landscape as well as the emergence of therapies that previously may have been overlooked or underused, including psychedelic compounds and digital therapies.
Since inception, ATAI has either created wholly owned subsidiaries or has made investments in certain controlled entities, including variable interest entities (“VIEs”) for which ATAI is the primary beneficiary under the VIE model (collectively, the “Company”). ATAI is headquartered in Berlin, Germany.
Corporate Reorganization and Initial Public Offering
ATAI was incorporated pursuant to the laws of the Netherlands as a Dutch private company with limited liability on September 10, 2020 for the purposes of becoming a holding company for ATAI Life Sciences AG and for the purposes of consummating the corporate reorganization described below. ATAI has not conducted any operations prior to the corporate reorganization other than activities incidental to its formation. ATAI Life Sciences AG was formed as a separate company on February 7, 2018.
In contemplation of the consummation of ATAI’s initial public offering (“IPO”) of common shares, ATAI undertook a corporate reorganization (the “Corporate Reorganization”). The Corporate Reorganization consisted of several steps as described below:
| • | Exchange of ATAI Life Sciences AG Securities for ATAI Life Sciences B.V. Common Shares and Share Split: In April 2021, the existing shareholders of ATAI Life Sciences AG each became a party to a separate notarial deed of issue under Dutch law and (i) subscribed for new common shares in ATAI Life Sciences B.V. and (ii) transferred their respective shares in ATAI Life Sciences AG, on a 1 to 10 basis (the “Exchange Ratio”), to ATAI Life Sciences B.V. as a contribution in kind on the common shares in ATAI Life Sciences B.V. As a result of the issuance of common shares in ATAI Life Sciences B.V. to the shareholders of ATAI Life Sciences AG and the contribution and transfer of their respective shares in ATAI Life Sciences AG to ATAI Life Sciences B.V., ATAI Life Sciences AG became a wholly owned subsidiary of ATAI Life Sciences B.V. No shareholder rights or preferences changed as a result of the share for share exchange. In connection with such exchange, the common share in ATAI Life Sciences B.V. held by Apeiron was cancelled. On June 7, 2021, shares of ATAI Life Sciences B.V. were split applying a ratio of 1.6 to one, and the nominal value of the shares was reduced to €0.10, pursuant to a shareholders’ resolution and amendment to the articles of association. |
| • | Conversion of ATAI Life Sciences B.V. into ATAI Life Sciences N.V.: Immediately preceding the Company’s IPO, the legal form of ATAI Life Sciences B.V. was converted from a Dutch private company with limited liability to a Dutch public company, and the articles of association of ATAI Life Sciences N.V., became effective. Following the Corporate Reorganization, ATAI Life Sciences N.V. became the holding company of ATAI Life Sciences AG. |
The Corporate Reorganization, as described above, is considered a continuation of ATAI Life Sciences AG resulting in no change in the carrying values of assets or liabilities. As a result, the financial statements for periods prior to the Corporate Reorganization are the financial statements of ATAI Life Sciences AG as the predecessor to ATAI for accounting and reporting purposes. All share, per-share and related information presented in these condensed consolidated financial statements and corresponding disclosure notes have been retrospectively adjusted, where applicable, to reflect the impact of the share exchange and share split resulting from the Corporate Reorganization. In connection with the Corporate Reorganization, outstanding share awards and option grants of ATAI Life Sciences AG were exchanged for share awards and option grants of ATAI Life Sciences B.V. with identical restrictions.
On June 22, 2021, ATAI closed the IPO of its common stock on Nasdaq. As part of the IPO, the Company issued and sold 17,250,000 shares of its common stock, which included 2,250,000 shares sold pursuant to the exercise of the
7
underwriters’ over-allotment option, at a public offering price of $15.00 per share. The Company received net proceeds of approximately $231.6 million from the IPO, after deducting underwriters’ discounts and commissions of $18.1 million and offering costs of $9.0 million.
Impact of COVID-19 Pandemic
In December 2019, a novel strain of coronavirus, severe acute respiratory syndrome coronavirus 2, or SARS-CoV-2, was identified in Wuhan, China. On March 11, 2020, the World Health Organization designated the outbreak of COVID-19, the disease associated with SARS-CoV-2, as a global pandemic. Governments and businesses around the world have taken unprecedented actions to mitigate the spread of COVID-19, including, but not limited to, shelter-in-place orders, quarantines, significant restrictions on travel, as well as restrictions that prohibit many employees from going to work.
The Company has been actively monitoring the impact of the COVID-19 pandemic, including variants, on its employees and business. Although some research and development timelines have been impacted by delays related to the COVID-19 pandemic, the Company has not experienced material financial impacts on its business and operations as a result of the COVID-19 pandemic. The Company has undertaken a number of business continuity measures to mitigate potential disruption to its operations and in order to preserve the integrity of its research and development programs. The extent of the impact of COVID-19 on the Company’s future operational and financial performance will depend on certain developments, including the duration and spread of the pandemic, including its variants, the rate and success of vaccination roll-out efforts, impact on employees and vendors all of which are uncertain and cannot be predicted. At this point, the extent to which COVID-19 may impact the Company’s future financial condition or results of operations is uncertain.
Liquidity and Going Concern
The Company has incurred significant losses and negative cash flows from operations since its inception. As of June 30, 2021, the Company had cash and cash equivalents of $453.6 million and its accumulated deficit was $237.8 million. The Company has historically financed its operations through the sale of equity securities, sale of convertible notes and revenue generated from licensing and collaboration arrangements. The Company has not generated any revenues to date from the sale of its product candidates and does not anticipate generating any revenues from the sale of its product candidates unless and until it successfully completes development and obtains regulatory approval to market its product candidates.
The Company currently expects that its existing cash as of June 30, 2021 will be sufficient to fund its operating expenses and capital expenditure requirements for at least the next 12 months from the date the unaudited condensed consolidated financial statements are issued.
2. Basis of Presentation and Summary of Significant Accounting Policies
Basis of Presentation
The accompanying condensed consolidated financial statements, which include the accounts of ATAI, its wholly owned subsidiaries and controlled entities, are presented in accordance with generally accepted accounting principles in the United States of America (“GAAP”) and applicable rules and regulations of SEC regarding interim financial reporting. Certain information and footnote disclosures normally included in the financial statements prepared in accordance with U.S. GAAP have been condensed or omitted in accordance with such rules and regulations. All intercompany transactions and accounts have been eliminated in consolidation.
8
The unaudited interim condensed consolidated financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary for a fair statement of the Company’s financial position, its results of operations and comprehensive loss, and its cash flows for the periods presented. The results of operations for the three and six months ended June 30, 2021 are not necessarily indicative of the results to be expected for the year ending December 31, 2021 or for any other future annual or interim period.
These condensed consolidated financial statements should be read in conjunction with the Company’s audited consolidated financial statements included in the prospectus dated June 17, 2021 (“Prospectus”) that forms a part of the Company’s Registration Statements on Form S-1 (File Nos. 333-255383 and 333-257184), as filed with the SEC pursuant to Rule 424(b)(4) promulgated under the Securities Act of 1933, as amended.
Significant Accounting Policies
During the six months ended June 30, 2021, there were no significant changes to the Company’s significant accounting policies as described in the Company’s audited consolidated financial statement as of and for the year ended December 31, 2020 except as described below.
Use of Estimates
The preparation of condensed consolidated financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements, and the reported amounts of expenses during the reporting period. Significant estimates and assumptions made in the accompanying condensed consolidated financial statements include, but are not limited to the fair value of the Company’s short term notes receivable—related party with COMPASS Pathways plc, convertible promissory notes issued in connection with the 2020 convertible note agreement (the “2020 Convertible Notes”), contingent consideration liability—related parties, derivative liability associated with the Perception convertible promissory notes, redeemable noncontrolling interests, and IPR&D assets and noncontrolling interests recognized in acquisitions, the valuations of common shares and share-based awards, and accruals for research and development costs.
The Company bases its estimates on historical experience and on various other assumptions that are believed to be reasonable. Actual results may differ from those estimates or assumptions.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with original maturities of three months or less from the purchase date to be cash equivalents. As of June 30, 2021 and December 31, 2020, cash and cash equivalents consisted of cash on deposit and cash held in high-yield savings accounts and money market funds.
Fair Value Measurements
Assets and liabilities recorded at fair value on a recurring basis in the consolidated balance sheets are categorized based upon the level of judgment associated with the inputs used to measure their fair values. Fair value is defined as the exchange price that would be received for an asset or an exit price that would be paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. Valuation techniques used to measure fair value must maximize the use of observable inputs and minimize the
9
use of unobservable inputs. The authoritative guidance on fair value measurements establishes a three-tier fair value hierarchy for disclosure of fair value measurements as follows:
Level 1—Observable inputs such as unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date;
Level 2—Inputs (other than quoted prices included in Level 1) are either directly or indirectly observable for the asset or liability. These include quoted prices for similar assets or liabilities in active markets and quoted prices for identical or similar assets or liabilities in markets that are not active; and
Level 3—Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
To the extent that the valuation is based on models or inputs that are less observable or unobservable in the market, the determination of fair value requires more judgment. Accordingly, the degree of judgment exercised by the Company in determining fair value is greatest for instruments categorized in Level 3. A financial instrument’s level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement.
The Company’s contingent consideration liability—related parties, the 2020 Convertible Notes, derivative liability associated with the Perception convertible promissory notes, investment in Intelgenx Technologies Corp. Initial Warrants and Additional Units Warrant and warrant liability with Neuronasal Inc. are carried at fair value, determined according to Level 3 inputs in the fair value hierarchy described above (See Note 7). The carrying amount reflected in the accompanying consolidated balance sheets for cash, prepaid expenses and other current assets, accounts payable and accrued expenses approximate their fair values, due to their short-term nature.
The carrying amounts of the Company’s convertible promissory notes—related parties issued in 2018 and 2020 (collectively, the “2018 Convertible Notes”) do not approximate fair value because the fair value is driven by the underlying value of the Company’s common stock to which the notes are able to be converted. As of June 30, 2021, the carrying amount and fair value amount of the convertible promissory notes issued in 2018 was $0.2 million and $44.3 million, respectively. As of June 30, 2021, the carrying amount and fair value amount of the convertible promissory notes issued in 2020 was $1.0 million and $232.3 million, respectively. As of December 31, 2020, the carrying amount and fair value amount for convertible promissory note issued in 2018 was $0.2 million and $12.3 million, respectively. As of December 31, 2020, the carrying amount and fair value amount for convertible promissory note issued in 2020 was $1.0 million and $64.4 million, respectively.
The carrying amounts of the Perception convertible promissory notes issued during 2020, do not approximate fair value because carrying amounts are net of unamortized debt discounts. The fair value of the Perception convertible promissory notes was determined based on the changes in expectation and increase in probability of occurrence of certain conversion events, including a qualified equity financing and a licensing transaction, that would have beneficial conversion terms for the note holders. In June 2021, Perception convertible promissory notes converted into shares of Series A preferred stock of Perception pursuant to their original terms. As of June 30, 2021, there were no Perception convertible promissory notes outstanding. As of December 31, 2020, the carrying amount and fair value amount for Perception convertible promissory notes was $0.8 million and $4.6 million, respectively. See Note 10 for additional discussion.
Fair Value Option
As permitted under Accounting Standards Codification 825, Financial Instruments, or ASC 825, the Company has elected the fair value option to account for its investment in IntelGenx Technologies Corp. (“IntelGenx”) common stock which otherwise would be subject to ASC 323. In accordance with ASC 825, the Company records these common stock investments at fair value with changes in fair value recorded as a component of other income (expense), net in the consolidated statement of operations and comprehensive loss.
10
Convertible Promissory Notes and Derivative Instruments
The Company does not use derivative instruments to hedge exposures to interest rate, market, or foreign currency risks. The Company evaluates all of its financial instruments, including convertible promissory notes, to determine if such instruments contain features that meet the definition of embedded derivatives. Embedded derivatives must be separately measured from the host contract if all the requirements for bifurcation are met. The assessment of the conditions surrounding the bifurcation of embedded derivatives depends on the nature of the host contract. Bifurcated embedded derivatives are recognized at fair value, with changes in fair value recognized in the consolidated statements of operations at each reporting period. Bifurcated embedded derivatives are classified with the related host contract in the Company’s consolidated balance sheets.
On March 16, 2020, Perception entered into a convertible promissory note agreement with the Company and other investors, including related parties, which provided for the issuance of convertible notes of $3.3 million to the Company and $0.6 million to other investors. On December 1, 2020, Perception entered into an additional convertible promissory note agreement with the Company and other investors, including related parties, which provided for the issuance of convertible notes of up to $12.0 million to the Company in aggregate of which (i) $6.2 million and $0.8 million were issued in December 2020 and January 2021, respectively, under the First Tranche Funding and (ii) $5.0 million was issued under the Second Tranche Funding (See Note 10). The Perception convertible promissory notes issued to the Company represent intercompany debt and are eliminated upon consolidation.
In addition, the Perception convertible promissory notes contain certain embedded features, which are redemption features and meet the definition of derivative instruments. The Company classifies these instruments as a liability on its consolidated balance sheets as the redemption features involve substantial discounts, provide for the accelerated repayment of the notes upon the occurrence of specified events, and are not clearly and closely related to its host instrument. The derivative liability was initially recorded at fair value upon issuance of the convertible promissory notes and is subsequently remeasured to fair value at each reporting date. Both the Perception convertible promissory notes and the derivative liability have been classified as long-term and presented as convertible promissory notes and derivative liability in the Company’s consolidated balance sheets.
Changes in the fair value of the derivative asset and liability are recognized as a component of other income (expense), net in the consolidated statements of operations. Changes in the fair value of the derivative asset and liability will continue to be recognized until the warrants and convertible promissory notes are no longer outstanding.
Warrant Liability
The Company accounts for the warrants in accordance with the guidance contained in ASC 815-40 under which the warrants do not meet the criteria for equity treatment and must be recorded as liabilities and is included other liabilities in the consolidated balance sheet. Accordingly, we classify the warrants as liabilities at their fair value and adjust the warrants to fair value at each reporting period. This liability is subject to re-measurement at each balance sheet date until exercised, and any change in fair value is recognized in our statement of operations. The fair value of the warrant liability is measured using a Black Scholes pricing model. Assumptions and estimates are made in determining an appropriate risk-free interest rate, volatility, term, dividend yield, discount due to exercise restrictions, and the fair value of common stock. Any significant adjustments to the unobservable inputs would have a direct impact on the fair value of the warrant liability.
Licenses of Intellectual Property
The Company may enter into collaboration and licensing arrangements for research and development, manufacturing, and commercialization activities with counterparties for the development and commercialization of its product candidates. The agreements may have units of account within the scope of ASC 606 where the counterparties meet the definition of a customer as well as units of account within the scope of ASC 808 where both parties are determined to be active participants.
The arrangements may contain multiple components, which may include (i) licenses, or options to obtain licenses to the Company’s intellectual property or sale of the Company’s license, (ii) research and development
11
activities, (iii) participation on joint steering committees, and (iv) the manufacturing of commercial, clinical or preclinical material. Payments pursuant to these arrangements may include non-refundable, upfront payments, milestone payments upon the achievement of significant development events, research and development reimbursements, sales milestones, and royalties on product sales. The amount of variable consideration is constrained until it is probable that the revenue is not at a significant risk of reversal in a future period. The contracts into which the Company enters generally do not include significant financing components.
In determining the appropriate amount of revenue to be recognized as it fulfills its obligations under each of its collaboration and license agreements, the Company performs the following steps: (i) identification of the promised goods or services in the contract within the scope of ASC 606; (ii) determination of whether the promised goods or services are performance obligations including whether they are capable of being distinct and distinct in the context of the contract; (iii) measurement of the transaction price, including the constraint on variable consideration; (iv) allocation of the transaction price to the performance obligations; and (v) recognition of revenue when (or as) the Company satisfies each performance obligation. As part of the accounting for these arrangements, the Company must use significant judgment to determine: a) the number of performance obligations based on the determination under step (ii) above; b) the transaction price under step (iii) above; c) the stand-alone selling price for each performance obligation identified in the contract for the allocation of transaction price in step (iv) above; and d) the measure of progress in step (v) above. The Company uses judgment to determine whether milestones or other variable consideration, except for sales-based milestones and royalties on license arrangements, should be included in the transaction price as described further below.
If a license to the Company’s intellectual property is determined to be distinct from the other promises or performance obligations identified in the arrangement, the Company recognizes revenue from consideration allocated to the license when the license is transferred to the customer and the customer is able to use and benefit from the license. In assessing whether a promise or performance obligation is distinct from the other elements, the Company considers factors such as the research, development, manufacturing and commercialization capabilities of the counterparties and the availability of its associated expertise in the general marketplace. In addition, the Company considers whether the counterparties can benefit from a promise for its intended purpose without the receipt of the remaining elements, whether the value of the promise is dependent on the unsatisfied promise, whether there are other vendors that could provide the remaining promise, and whether it is separately identifiable from the remaining promise. For licenses that are combined with other promises, the Company utilizes judgment to assess the nature of the combined performance obligation to determine whether the combined performance obligation is satisfied over time or at a point in time and, if over time, the appropriate method of measuring progress for purposes of recognizing revenue. The Company evaluates the measure of progress as of each reporting period and, if necessary, adjusts the measure of performance and related revenue recognition. The measure of progress, and thereby periods over which revenue should be recognized, is subject to estimates by management and may change over the course of the arrangement. Such a change could have a material impact on the amount of revenue the Company records in future periods.
Customer Options: If an arrangement is determined to contain customer options that allow the customer to acquire additional goods or services such as research and development services or manufacturing services, the goods and services underlying the customer options are not considered to be performance obligations at the inception of the arrangement unless a material right is provided to the customer. If the customer option does not represent a material right, the obligation to provide such goods and services is contingent on exercise of the option, and the associated consideration is not included in the transaction price. If a customer option is determined to include a significant and incremental discount and, therefore, represents a material right, the material right is recognized as a separate performance obligation at the outset of the arrangement. The Company allocates the transaction price to material rights based on the relative standalone selling price.
Milestone Payments: At the inception of each arrangement that includes milestone payments, the Company evaluates whether the milestones are considered probable of being achieved and estimates the amount to be included in the transaction price using the most-likely amount method. If it is probable that a significant revenue reversal would not occur, the associated milestone value is included in the transaction price. Milestone payments that are not within the control of the Company or the licensee, such as regulatory approvals, are not considered probable of being achieved until those approvals are received. The Company evaluates factors such as the scientific, clinical, regulatory, commercial, and other risks that must be overcome to achieve the respective milestone in making this assessment. There is considerable judgment involved in determining whether it is probable that a significant revenue reversal would not occur. At the end of each subsequent reporting period, the Company reevaluates the probability
12
of achievement of all milestones subject to constraint and, if necessary, adjusts its estimate of the overall transaction price. Any such adjustments are recorded on a cumulative catch-up basis, which would affect revenues and earnings in the period of adjustment.
Royalties: For license arrangements that include sales-based royalties, including milestone payments based on a level of sales, and the license is deemed to be the predominant item to which the royalties relate, the Company recognizes revenue at the later of (i) when the related sales occur, or (ii) when the performance obligation to which some or all of the royalty has been allocated has been satisfied (or partially satisfied). To date, the Company has not recognized any royalty revenue resulting from any of its licensing arrangements.
Stock-Based Compensation
The Company accounts for all stock-based payment awards granted to employees, directors and non-employees as stock-based compensation expense based on their grant date fair value. The Company grants equity awards under its stock-based compensation programs, which may include stock options and restricted common stock. The measurement date for employee awards is the date of grant, and stock-based compensation costs are recognized as expense over the requisite service period, which is the vesting period, on a straight-line basis. Since the adoption of ASU 2018-07, the measurement date for non-employee awards is the date of grant, and stock-based compensation costs are recognized in the same period and in the same manner as if the entity had paid cash for the goods or services. Stock-based compensation expense is classified in the accompanying condensed consolidated statements of operations based on the function to which the related services are provided. The Company has elected to recognize forfeitures of stock-based compensation awards as they occur.
The Company recognizes the compensation cost of awards subject to service-based and performance-based vesting conditions using the accelerated attribution method over the requisite service period if the performance- based vesting conditions are probable of being met. Recognition of compensation cost relating to awards that vest on a “Liquidity Event” (as defined in the award or Partnership agreements) will be deferred until the consummation of such transaction.
The Company calculates the fair value of stock options granted using the Black-Scholes option-pricing model with the following assumptions:
Expected Volatility—The Company estimated volatility for option grants by evaluating the average historical volatility of a peer group of companies for the period immediately preceding the option grant for a term that is approximately equal to the options’ expected life.
Expected Term—The expected term of the Company’s options represents the period that the stock-based awards are expected to be outstanding. The Company has generally elected to use the “simplified method” by analogy for estimating the expected term of options, whereby the expected term equals the arithmetic average of the vesting term and the original contractual term of the option.
Risk-Free Interest Rate—The risk-free interest rate is based on the implied yield with an equivalent expected term at the grant date.
Dividend Yield—The Company has not declared or paid dividends to date and does not anticipate declaring dividends. As such, the dividend yield has been estimated to be zero.
As part of the valuation of stock-based compensation under the Black-Scholes option pricing model, it is necessary for the Company to estimate the fair value of its common stock. Prior to the closing of the IPO, the fair value of the Company’s common stock was estimated on each grant date. Given the absence of a public trading market, and in accordance with the American Institute of Certified Public Accountants’ Practice Guide, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, the Company exercised reasonable judgment and considered numerous objective and subjective factors to determine its best estimate of the fair value of its common stock. The estimation of the fair value of the common stock considered factors including the following: the estimated present value of the Company’s future cash flows; the Company’s business, financial condition and results of operations; the Company’s forecasted operating performance; the illiquid nature of the Company’s common stock; industry information such as market size and growth; market capitalization of comparable companies and the estimated value of transactions such companies have engaged in; and macroeconomic conditions.
After the closing of the IPO, the Company’s board of directors determined the fair value of each share of common stock underlying stock-based awards based on the closing price of the Company’s common stock as reported by Nasdaq on the date of grant.
13
Net Income (Loss) per Share Attributable to Common Stockholders
The Company computed basic net income (loss) per share attributable to common stockholders by dividing net income (loss) attributable to common stockholders by the weighted-average number of common stock outstanding for the period, without consideration for potentially dilutive securities. The Company computes diluted net income (loss) per common share after giving consideration to all potentially dilutive common stock, including convertible notes and stock options, outstanding during the period determined using the if-converted and treasury-stock methods, respectively, except where the effect of including such securities would be antidilutive.
Recently Adopted Accounting Pronouncements
In August 2020, the FASB issued ASU 2020-06, “Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815—40)” (“ASU 2020-06”). ASU 2020-06 simplifies the accounting for certain financial instruments with characteristics of liabilities and equity, including convertible instruments and contracts on an entity’s own equity. The ASU is part of the FASB’s simplification initiative, which aims to reduce unnecessary complexity in U.S. GAAP. The ASU’s amendments are effective for the Company for fiscal years beginning after December 15, 2023 and interim periods within those fiscal years, with early adoption permitted. The Company early adopted this standard on January 1, 2021 applying the modified retrospective transition approach. Upon adoption of ASU 2020-06, the embedded conversion option related to the 2018 Convertible Notes is no longer separated from the host contract and recognized within additional paid-in-capital and is instead accounted for as a single liability measured at amortized cost within convertible promissory notes—related parties in the condensed consolidated balance sheets. Therefore, the unamortized debt discount of $8,000 was eliminated.
3. Acquisitions
2021 Acquisitions
PsyProtix, Inc.
In February 2021, the Company jointly formed PsyProtix with Chymia, LLC (“Chymia”). PsyProtix was created for the purpose of exploring and developing a metabolomics-based precision psychiatry approach, initially targeting the stratification and treatment of Treatment Resistant Depression (“TRD”) patients. In February 2021, pursuant to a Series A Preferred Stock Purchase Agreement (the “PsyProtix Purchase Agreement”), the Company acquired shares of PsyProtix’s Series A preferred stock in exchange for an initial payment of $0.1 million in cash. In addition, pursuant to the PsyProtix Purchase Agreement, the Company agreed to make aggregate payments to PsyProtix of up to $4.9 million upon the achievement of specified clinical milestones to complete the purchase of the shares and provide additional funding to PsyProtix. The PsyProtix Purchase Agreement resulted in the Company holding a 75.0% voting interest and Chymia holding a 25.0% voting interest in PsyProtix. In connection with the Company’s agreement for additional funding, PsyProtix issued the corresponding Series A preferred shares to the Company provided that the shares are held in an escrow account (the “PsyProtix Escrow Shares”). The PsyProtix Escrow Shares will be released, from time to time, to the Company upon PsyProtix achieving certain milestones as defined in the PsyProtix Purchase Agreement with cash payments to be made by the Company. In addition, the Company has the right, but not the obligation, to make payment for the certain PsyProtix Escrow Shares at any time, regardless of the achievement of any milestones. The PsyProtix Escrow Shares have voting and all other rights until an event of default occurs where the Company fails to make a payment within 10 days following the written notice
14
of the achievement of the relevant milestone. In the event of default, a pro rata portion of the PsyProtix Escrow Shares will automatically be surrendered and be deemed forfeited and canceled, and could result in the Company losing control of PsyProtix’s board of directors and its controlling financial interest in PsyProtix. In addition, prior to the occurrence of the earlier of a certain milestone event or reaching of the Company’s capital contribution threshold of $5.0 million, PsyProtix will issue additional shares of common stock to Chymia to maintain Chymia’s current ownership percentage. This anti-dilution right was concluded to be embedded in the common shares held by Chymia.
Immediately following the closing of the PsyProtix Purchase Agreement, PsyProtix loaned $0.1 million to Chymia in exchange for a duly executed promissory note (the “Chymia Note”). The Chymia Note shall accrue interest at a 5% rate per annum until payment in full. The aggregate principal amount of $0.1 million, together with all accrued and unpaid interest and all other amounts payable are due to be paid on the date that is the earlier of (i) five years from the promissory note agreement date or (ii) the occurrence of a liquidation event or a deemed liquidation event (as defined in the PsyProtix’s certificate of incorporation). As of June 30, 2021, the Chymia Note was $0.1 million and included as a component of long-term notes receivable—related parties on the condensed consolidated balance sheets.
The PsyProtix Purchase Agreement provided the Company unilateral rights to control all decisions related to the significant activities of PsyProtix. The Company concluded that PsyProtix was not considered a business based on its assessment under ASC 805 and accounted for the Company’s acquisition in PsyProtix as an initial consolidation of a VIE that is not a business under ASC 810 (See Note 4). The assets acquired, liabilities assumed, and noncontrolling interest in the transaction were measured based on their fair values. The Company did not recognize a gain or a loss in connection with the consolidation of PsyProtix as the fair value of the consideration paid of $0.1 million was equivalent to the fair value of the identifiable assets acquired of $0.1 million.
Psyber, Inc.
Psyber is a globally based startup focused on the development of brain-computer interface-enabled digital therapeutics for treating mental health issues. Psyber was created as a joint venture between the Company and the founders of Psyber. In February 2021, pursuant to a Series A Preferred Stock Purchase Agreement (the “Psyber Purchase Agreement”), the Company acquired shares of Psyber’s Series A preferred stock in exchange for an initial payment of $0.2 million in cash. In addition, pursuant to the Psyber Purchase Agreement, the Company agreed to make aggregate payments to Psyber of up to $1.8 million upon the achievement of specified clinical milestones to complete the purchase of the shares and provide additional funding to Psyber. The Psyber Purchase Agreement resulted in the Company holding a 75.0% voting interest and the founders of Psyber jointly holding a 25.0% voting interest in Psyber. In connection with the Company’s agreement for additional funding, Psyber issued the corresponding Series A preferred shares to the Company provided that the shares are held in an escrow account (the “Psyber Escrow Shares”). The Psyber Escrow Shares will be released, from time to time, to the Company upon Psyber achieving certain milestones as defined in the Psyber Purchase Agreement with cash payments to be made by the Company. In addition, the Company has the right, but not the obligation, to make payment for the certain Psyber Escrow Shares at any time, regardless of the achievement of any milestones. The Psyber Escrow Shares have voting and all other rights until an event of default occurs where the Company fails to make a payment within 10 days following the written notice of the achievement of the relevant milestone. In the event of default, a pro rata portion of the Psyber Escrow Shares will automatically be surrendered and be deemed forfeited and canceled, and could result in the Company losing control of Psyber’s board of directors and its controlling financial interest in Psyber. In addition, prior to the occurrence of the earlier of a certain milestone event or reaching of the Company’s capital contribution threshold of $2.0 million, Psyber will issue additional shares of common stock to the founders of Psyber to maintain the founders’ current ownership percentage. This anti-dilution right was concluded to be embedded in the common shares held by the founders of Psyber.
The Psyber Purchase Agreement provided the Company unilateral rights to control all decisions related to the significant activities of Psyber. The Company concluded that Psyber was not considered a business based on its assessment under ASC 805 and accounted for the Company’s acquisition in Psyber as an initial consolidation of a VIE that is not a business under ASC 810 (See Note 4). The assets acquired, liabilities assumed, and noncontrolling interest in the transaction were measured based on their fair values. The Company recognized a gain on consolidation of $2,000. The gain was calculated as the sum of the consideration paid of $0.2 million, less the fair value of identifiable net assets acquired of $0.2 million.
15
InnarisBio, Inc.
In February 2021, the Company jointly formed InnarisBio with UniQuest Pty Ltd (“UniQuest”) for the purpose of adding a solgel-based direct-to-brain intranasal drug delivery technology to the Company’s platform. In March 2021, pursuant to a Series A Preferred Stock Purchase Agreement (the “InnarisBio Purchase Agreement”), the Company acquired shares of InnarisBio’s Series A preferred stock in exchange for an initial payment of $1.1 million in cash. In addition, pursuant to the InnarisBio Purchase Agreement, the Company agreed to make aggregate payments to InnarisBio of up to $3.9 million upon the achievement of specified clinical milestones to complete the purchase of the shares and provide additional funding to InnarisBio. The InnarisBio Purchase Agreement resulted in the Company holding an 82.0% voting interest and UniQuest holding a 18.0% voting interest in InnarisBio. In connection with the Company’s agreement for additional funding, InnarisBio issued the corresponding Series A preferred shares to the Company provided that the shares are held in an escrow account (the “InnarisBio Escrow Shares”). The InnarisBio Escrow Shares will be released, from time to time, to the Company upon InnarisBio achieving certain milestones as defined in the InnarisBio Purchase Agreement with cash payments to be made by the Company. In addition, the Company has the right, but not the obligation, to make payment for the certain InnarisBio Escrow Shares at any time, regardless of the achievement of any milestones. The InnarisBio Escrow Shares have voting and all other rights until an event of default occurs where the Company fails to make a payment within 10 days following the written notice of the achievement of the relevant milestone. In the event of default, a pro rata portion of the InnarisBio Escrow Shares will automatically be surrendered and be deemed forfeited and cancelled and could result in the Company losing control of InnarisBio’s board of directors and its controlling financial interest in InnarisBio.
The InnarisBio Purchase Agreement provided the Company unilateral rights to control all decisions related to the significant activities of InnarisBio. The Company concluded that InnarisBio was not considered a business based on its assessment under ASC 805 and accounted for the Company’s acquisition in InnarisBio as an initial consolidation of a VIE that is not a business under ASC 810 (See Note 4). The assets acquired, liabilities assumed, and noncontrolling interest in the transaction were measured based on their fair values. The Company recognized a loss on consolidation of $7,000 for the six months ended June 30, 2021. The loss was calculated as the sum of the consideration paid of $1.1 million, the fair value of the noncontrolling interest issued of $0.9 million, less the fair value of identifiable net assets acquired of $2.0 million. The fair value of the contingent milestone payments of $0.1 million was included in the total purchase consideration for the noncontrolling interest and recognized as a liability by InnarisBio at the date of acquisition. The fair value of the IPR&D acquired of $1.0 million was reflected as acquired in-process research and development expense on the condensed consolidated statements of operations as it had no alternative future use at the time of the acquisition.
Neuronasal, Inc.
Neuronasal, Inc. (“Neuronasal”) is developing a novel intranasal formulation of N-acetylcysteine for acute mild traumatic brain injury. The Company first acquired investments in Neuronasal in December 2019 pursuant to a Preferred Stock Purchase Agreement (the “Neuronasal PSPA”). In December 2019, in connection with the original purchase of the preferred shares, Neuronasal and the Company entered into the Secondary Sale and Put Right Agreement (the “Neuronasal Secondary Sale Agreement”), whereby upon the achievement of certain contingent development milestones, existing common shareholders have the right to sell and the Company has the option but not the obligation to purchase additional shares of common stock at a price determined based on the fair market value per share on the date of exercise. These options are contingent upon the exercise of the options by Neuronasal’s common shareholders to sell shares to the Company. On March 10, 2021, pursuant to the Neuronasal PSPA, the Company purchased additional Series A preferred shares for approximately $0.8 million based on the achievement of certain development milestones. Also, pursuant to the Neuronasal Secondary Sale Agreement, the Company purchased additional common shares for approximately $0.3 million. On May 17, 2021, pursuant to the Neuronasal PSPA the Company exercised its option to purchase additional shares of Series A preferred stock of Neuronasal for an aggregate cost of $1.0 million. The additional purchase on May 17, 2021 resulted in the Company obtaining an aggregate 55.99% ownership interest in Neuronasal, including the Company’s previously acquired investments in Neuronasal’s common and preferred stock, and provided the Company with control of Neuronasal’s board of directors and the unilateral rights to control all decisions related to the significant activities of Neuronasal. Prior to May 17, 2021, the Company accounted for its investments in Neuronasal’s common stock under the equity method and Neuronasal’s preferred stock under the measurement alternative (See Note 5). Following the closing of this acquisition on May 17, 2021, the results of Neuronasal have been consolidated in the Company’s consolidated financial statements.
16
The Company concluded that Neuronasal was not considered a business based on its assessment under ASC 805 and accounted for the Company’s acquisition in Neuronasal as an initial consolidation of a variable interest entity (“VIE”) that is not a business under ASC 810 (See Note 4). The assets acquired, liabilities assumed, and noncontrolling interest in the transaction were measured based on their fair values. The Company recognized a gain of $3.5 million. The gain was calculated as the sum of the consideration paid of $1.0 million, the fair value of the noncontrolling interest issued of $3.0 million, the carrying value of the Company’s investments in Neuronasal’s common stock and preferred stock prior to May 17, 2021 of $0.8 million, less the fair value of identifiable net assets acquired of $8.3 million. The fair value of the IPR&D acquired of $8.0 million was charged to research and development expense as it had no alternative future use at the time of the acquisition.
All acquisitions discussed above were considered as asset acquisitions and no goodwill was recognized upon consolidation.
4. Variable Interest Entities and a Voting Interest Entity
Consolidated VIEs
At each reporting period, the Company reassesses whether it remains the primary beneficiary for Variable Interest Entities (“VIEs”) consolidated under the VIE model. For the acquisitions further described in Note 3, the Company determined that PsyProtix, Inc., Psyber, Inc., InnarisBio, Inc., and Neuronasal, Inc. are VIEs as each entity does not have sufficient equity at risk to carry out its principal activities without additional subordinated financial support.
As of June 30, 2021 and December 31, 2020, the Company has accounted for the following investments as VIEs, excluding the wholly owned subsidiaries:
Consolidated Entities | Relationship as of June 30, 2021 | Relationship as of December 31, 2020 | Date Control Obtained | Ownership % June 30, 2021 | Ownership % December 31, 2020 | |||||||||
Perception Neuroscience Holdings, Inc. | Controlled VIE | Controlled VIE | November 2018 | 58.9 | % | 50.1 | % | |||||||
Kures, Inc. | Controlled VIE | Controlled VIE | August 2019 | 54.1 | % | 54.1 | % | |||||||
EntheogeniX Biosciences, Inc. | Controlled VIE | Controlled VIE | November 2019 | 80.0 | % | 80.0 | % | |||||||
DemeRx IB, Inc. | Controlled VIE | Controlled VIE | December 2019 | 59.5 | % | 59.5 | % | |||||||
Recognify Life Sciences, Inc. | Controlled VIE | Controlled VIE | November 2020 | 51.9 | % | 51.9 | % | |||||||
PsyProtix, Inc. | Controlled VIE | — | February 2021 | 75.0 | % | — | ||||||||
Psyber, Inc. | Controlled VIE | — | February 2021 | 75.0 | % | — | ||||||||
InnarisBio, Inc. | Controlled VIE | — | March 2021 | 82.0 | % | — | ||||||||
Neuronasal, Inc. | Controlled VIE | Investment | May 2021 | 56.0 | % | 37.2 | % | |||||||
The entities consolidated by the Company are comprised of wholly and partially owned entities for which the Company is the primary beneficiary under the VIE model as the Company has (i) the power to direct the activities that most significantly impact the VIE’s economic performance and (ii) the obligation to absorb losses that could potentially be significant to the VIE, or the right to receive benefits from the VIE that could potentially be significant to the VIE. The results of operations of the consolidated entities are included within the Company’s condensed consolidated financial statements from the date of acquisition to June 30, 2021.
As of June 30, 2021 and December 31, 2020, the assets of the consolidated VIEs can only be used to settle the obligations of the respective VIEs. The liabilities of the consolidated VIEs are obligations of the respective VIEs and their creditors have no recourse to the general credit or assets of ATAI.
17
The following table presents the assets and liabilities (excluding intercompany balances that were eliminated in consolidation) for all VIEs as of June 30, 2021 (in thousands):
| Perception | Kures | EntheogeniX | DemeRx IB | Recognify | PsyProtix | Psyber | InnarisBio | Neuronasal | ||||||||||||||||||||||||||||
Assets: | ||||||||||||||||||||||||||||||||||||
Current assets: | ||||||||||||||||||||||||||||||||||||
Cash | $ | 26,741 | $ | 762 | $ | 401 | $ | 5,351 | $ | 1,493 | $ | 91 | $ | 174 | $ | 816 | $ | 691 | ||||||||||||||||||
Unbilled receivable | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Prepaid expenses and other current assets | 1,638 | 84 | — | 431 | 15 | — | — | — | 560 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
Total current assets | 28,379 | 846 | 401 | 5,782 | 1,508 | 91 | 174 | 816 | 1,251 | |||||||||||||||||||||||||||
Property and equipment, net | 2 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Goodwill | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Long term notes receivable | — | — | — | 1,075 | — | 102 | — | — | — | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
Total assets | $ | 28,381 | $ | 846 | $ | 401 | $ | 6,857 | $ | 1,508 | $ | 193 | $ | 174 | $ | 816 | $ | 1,251 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
Liabilities: | ||||||||||||||||||||||||||||||||||||
Current liabilities: | ||||||||||||||||||||||||||||||||||||
Accounts payable | $ | 743 | $ | 210 | $ | 26 | $ | 495 | $ | 19 | $ | — | $ | 1 | $ | — | $ | 253 | ||||||||||||||||||
Accrued liabilities | 947 | 356 | 31 | 154 | 264 | 9 | 69 | — | 384 | |||||||||||||||||||||||||||
Deferred revenue | 120 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Convertible promissory notes and derivative liability - current portion | — | — | — | — | — | — | — | — | 38 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
Total current liabilities | 1,810 | 566 | 57 | 649 | 283 | 9 | 70 | — | 675 | |||||||||||||||||||||||||||
Convertible promissory notes and derivative liability | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
Contingent consideration liability | 2,363 | — | — | — | — | — | — | 103 | — | |||||||||||||||||||||||||||
Other non-current liabilities | — | — | — | — | — | — | — | — | 289 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
Total liabilities | $ | 4,173 | $ | 566 | $ | 57 | $ | 649 | $ | 283 | $ | 9 | $ | 70 | $ | 103 | $ | 964 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||
The following table presents the assets and liabilities (excluding intercompany balances that were eliminated in consolidation) for all consolidated VIEs as of December 31, 2020 (in thousands):
| Perception | Kures | EntheogeniX | DemeRx IB | Recognify | ||||||||||||||||
Assets: | ||||||||||||||||||||
Current assets: | ||||||||||||||||||||
Cash | $ | 6,527 | $ | 1,264 | $ | 652 | $ | 7,252 | $ | 1,895 | ||||||||||
Prepaid expenses and other current assets | 768 | 124 | — | 193 | 44 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total current assets | 7,295 | 1,388 | 652 | 7,445 | 1,939 | |||||||||||||||
Property and equipment, net | 4 | — | — | — | — | |||||||||||||||
Long term notes receivable | — | — | — | 1,060 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total assets | $ | 7,299 | $ | 1,388 | $ | 652 | $ | 8,505 | $ | 1,939 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Liabilities: | ||||||||||||||||||||
Current liabilities: | ||||||||||||||||||||
Accounts payable | $ | 564 | $ | 220 | $ | 35 | $ | 230 | $ | 64 | ||||||||||
Accrued liabilities | 297 | 229 | 11 | 92 | 66 | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total current liabilities | 861 | 449 | 46 | 322 | 130 | |||||||||||||||
Convertible promissory notes and derivative liability | 978 | — | — | — | — | |||||||||||||||
Contingent consideration liability | 1,705 | — | — | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total liabilities | $ | 3,544 | $ | 449 | $ | 46 | $ | 322 | $ | 130 | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
18
Noncontrolling Interests
The Company recognizes noncontrolling interests related to its consolidated VIEs and provides a rollforward of the noncontrolling interests balance, as follows (in thousands):
| Perception | Recognify | Psyber | InnarisBio | Neuronasal | Total | |||||||||||||||||||
Balance as of December 31, 2020 | $ | — | $ | 4,546 | $ | — | $ | — | $ | — | $ | 4,546 | ||||||||||||
Issuance of noncontrolling interests | — | — | 8 | 877 | — | 885 | ||||||||||||||||||
Net income (loss) attributable to noncontrolling interests - common | 1,755 | — | (8 | ) | (877 | ) | — | 870 | ||||||||||||||||
Net income (loss) attributable to noncontrolling interests - preferred | 2,608 | (122 | ) | — | — | — | 2,486 | |||||||||||||||||
Comprehensive loss attributable to noncontrolling interests | (184 | ) | — | — | — | — | (184 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balance as of March 31, 2021 | $ | 4,179 | $ | 4,424 | $ | — | $ | — | $ | — | $ | 8,603 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Issuance of noncontrolling interests | 3,257 | — | — | — | 392 | 3,649 | ||||||||||||||||||
Net income (loss) attributable to noncontrolling interests - common | (1,755 | ) | (217 | ) | — | — | (392 | ) | (2,364 | ) | ||||||||||||||
Net income (loss) attributable to noncontrolling interests - preferred | 7 | — | — | — | — | 7 | ||||||||||||||||||
Comprehensive loss attributable to noncontrolling interests | 150 | — | — | — | — | 150 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Balance as of June 30, 2021 | $ | 5,838 | $ | 4,207 | $ | — | $ | — | $ | — | $ | 10,045 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
| Perception | Kures | Total | ||||||||||
Balance as of December 31, 2019 | $ | 487 | $ | 400 | $ | 887 | ||||||
Issuance of noncontrolling interests | — | — | — | |||||||||
Repurchase of noncontrolling interest | — | — | — | |||||||||
Net loss attributable to noncontrolling interests - common | — | — | — | |||||||||
Net loss attributable to noncontrolling interests - preferred | (297 | ) | (92 | ) | (389 | ) | ||||||
Comprehensive loss attributable to noncontrolling interests | 13 | | — | | 13 | |||||||
|
|
|
|
|
| |||||||
Balance as of March 31, 2020 | $ | 203 | $ | 308 | $ | 511 | ||||||
|
|
|
|
|
| |||||||
Issuance of noncontrolling interests | — | — | — | |||||||||
Repurchase of noncontrolling interest | — | — | — | |||||||||
Net loss attributable to noncontrolling interests - common | — | — | — | |||||||||
Net loss attributable to noncontrolling interests - preferred | (183 | ) | (308 | ) | (491 | ) | ||||||
Comprehensive loss attributable to noncontrolling interests | (20 | ) | — | (20 | ) | |||||||
|
|
|
|
|
| |||||||
Balance as of June 30, 2020 | $ | — | $ | — | $ | — | ||||||
|
|
|
|
|
| |||||||
Redeemable Noncontrolling Interests
In connection with the consolidation of Kures, the Company recognized the shares of Kures common stock and Series A-1 preferred stock held by the founders of Kures as redeemable noncontrolling interests as they contain embedded put options that are exercisable by the founders following a successful completion of a future event, which is not solely within the control of the Company. The redeemable noncontrolling interests were initially measured at fair value upon issuance and are redeemable at fair value at the holder’s option upon the successful completion or occurrence of future events. As of June 30, 2021 and December 31, 2020, the Company did not adjust the carrying value of the redeemable noncontrolling interests based on their estimated redemption values since it was not probable that the events that would allow the shares to become redeemable would occur. Subsequent adjustments to increase or decrease the carrying values of the redeemable noncontrolling interests to their estimated redemption values will be made if and when it becomes probable that such events will occur.
In connection with the consolidation of DemeRx IB, the Company recognized common stock held by DemeRx as redeemable noncontrolling interests as they are redeemable upon the occurrence of events that are not solely within the control of the Company. The redeemable noncontrolling interests were initially measured at fair value upon issuance and are redeemable at fair value at the holder’s option upon the successful completion of future events. As of June 30, 2021 and December 31, 2020, the Company did not adjust the carrying value of the redeemable noncontrolling interests based on their estimated redemption values since it was not probable that the events that would allow the shares to become redeemable would occur. Subsequent adjustments to increase or decrease the carrying values of the redeemable noncontrolling interests to their estimated redemption values will be made if and when it becomes probable that such events will occur.
19
In connection with the consolidation of Neuronasal, the Company recognized the shares of Neuronasal common stock held by the founders of Neuronasal as redeemable noncontrolling interests as they contain embedded put options that are exercisable by the founders following a successful completion of a future event, which is not solely within the control of the Company. The redeemable noncontrolling interests were initially measured at fair value upon issuance and are redeemable at fair value at the holder’s option upon the successful completion or occurrence of future events. As of June 30, 2021, the Company did not adjust the carrying value of the redeemable noncontrolling interests based on their estimated redemption values since it was not probable that the events that would allow the shares to become redeemable would occur. Subsequent adjustments to increase or decrease the carrying values of the redeemable noncontrolling interests to their estimated redemption values will be made if and when it becomes probable that such events will occur.
Redeemable noncontrolling interests are classified in temporary equity as they are redeemable based on events that are not solely within the control of the Company. As of June 30, 2021 and December 31, 2020, the balance of redeemable noncontrolling interests in temporary equity on the condensed consolidated balance sheets was zero. The amount of net loss attributable to redeemable noncontrolling interests of $2.6 million and $0.1 million are included in consolidated net loss on the face of the condensed consolidated statements of operations for the three months ended June 30, 2021 and 2020, respectively.
The following table provides a rollforward of the redeemable noncontrolling interests balance (in thousands):
| Kures | Neuronasal | Total | ||||||||||
Balance as of December 31, 2020 | $ | — | $ | — | $ | — | ||||||
Issuance of redeemable noncontrolling interests | — | — | — | |||||||||
Net loss attributable to redeemable noncontrolling interests - common | — | — | — | |||||||||
|
|
|
|
|
| |||||||
Balance as of March 31, 2021 | $ | — | $ | — | $ | — | ||||||
|
|
|
|
|
| |||||||
Issuance of redeemable noncontrolling interests | — | 2,555 | 2,555 | |||||||||
Net loss attributable to redeemable noncontrolling interests - common | — | (2,555 | ) | (2,555 | ) | |||||||
|
|
|
|
|
| |||||||
Balance as of June 30, 2021 | $ | — | $ | — | $ | — | ||||||
|
|
|
|
|
| |||||||
| Kures | Total | |||||||
Balance as of December 31, 2019 | $ | 142 | $ | 142 | ||||
Net loss attributable to redeemable noncontrolling interests - preferred | (33 | ) | (33 | ) | ||||
|
|
|
| |||||
Balance as of March 31, 2020 | $ | 109 | $ | 109 | ||||
|
|
|
| |||||
Net loss attributable to redeemable noncontrolling interests - preferred | (109 | ) | (109 | ) | ||||
|
|
|
| |||||
Balance as of June 30, 2020 | $ | — | $ | — | ||||
|
|
|
| |||||
Non-consolidated VIEs and a VOE
The Company evaluated the nature of its investments in Innoplexus AG (“Innoplexus”), DemeRx NB, Inc. (“DemeRx NB”) and IntelGenx and determined that the investments are VIEs as of the date of the Company’s initial investment through June 30, 2021. The Company is not the primary beneficiary as it did not have the power to direct the activities that most significantly impact the investments’ economic performance and therefore concluded that it did not have a controlling financial interest that would require consolidation as of June 30, 2021 and December 31, 2020.
The Company will reevaluate if the investments meet the definition of a VIE upon the occurrence of specific reconsideration events. The Company accounted for these investments under either the equity method or the measurement alternative included within ASC 321 (See Note 5). As of June 30, 2021, the Company’s maximum exposure for its non-consolidated VIEs was $23.0 million relating to the carrying values in other investments and other investments held at fair value and $3.2 million relating to the carrying value in long term notes receivable – related party. As of December 31, 2020, the Company’s maximum exposure for its non-consolidated VIEs was $8.0 million relating to the carrying values in its other investments and $0.2 million relating to the carrying value in short term notes receivable—related party.
The Company evaluated the nature of its investment in Gaba Therapeutics, Inc. (“Gaba”) and determined that Gaba was a VIE through May 21, 2021 when the Company exercised its option to purchase additional shares or Series A Preferred stock for an aggregate purchase price of $5.0 million (see Note 5). Prior to the option exercise, the Company was not the primary beneficiary as it did not have the power to direct the activities that most significantly impact the investment’s economic performance and therefore concluded that it did not have a controlling financial interest that would require consolidation through May 21, 2021. The completion of the Series A Preferred stock purchase in May 2021 was deemed to be a reconsideration event at which point Gaba was no longer deemed a VIE as Gaba now had sufficient equity at risk to finance its activities through the initial development period without additional subordinated financial support. Entities that do not qualify as a VIE are assessed for consolidation under the voting interest model (“VOE model”). Under the VOE model, the Company consolidates the entity if it determines that it, directly or indirectly, has greater than 50% of the voting shares and that other equity holders do not have substantive voting, participating or liquidation rights. While the Company holds greater than 50% of the outstanding equity interest of Gaba, the Company does not have the power to control the entity. Concurrent with the exercise of the option, the Company executed a side letter with the other equity holders of Gaba agreeing to forego the rights to additional seats on the Board of Directors, resulting in the Company lacking the ability to control the investee. The Company concluded that it does not have a controlling financial interest that would require consolidation under the VOE model and accounted for the investments in Gaba preferred stock under the measurement alternative per ASC 323 (See Note 5).
20
As disclosed in Note 5, as of June 30, 2021, the Company is obligated to purchase additional shares of Series A preferred stock of GABA for up to $1.5 million upon the achievement of certain specified contingent clinical development milestones. This amount has not been included in the Company’s determination of the maximum exposure of loss presented for its non-consolidated VIEs.
The Company had an investment in COMPASS Pathways plc (formerly known as Compass Pathfinder Holding Limited) (“COMPASS”) which was determined to be an investment in a VIE as of December 31, 2019 and through the date prior to its initial public offering in September 2020 (“COMPASS IPO”); however, the Company was not the primary beneficiary as it did not have the power to direct the activities that most significantly impact the investment’s economic performance and therefore concluded that it did not have a controlling financial interest that would require consolidation during this period as of December 31, 2019 and through September 2020. The completion of the COMPASS IPO in September 2020 was deemed to be a reconsideration event. Upon the completion of the COMPASS IPO, the Company’s investment in COMPASS was no longer deemed an investment in a VIE as COMPASS now had sufficient equity at risk to finance its activities without additional subordinated financial support. Entities that do not qualify as a VIE are assessed for consolidation under the voting interest model (“VOE model”). Under the VOE model, the Company consolidates the entity if it determines that it, directly or indirectly, has greater than 50% of the voting shares and that other equity holders do not have substantive voting, participating or liquidation rights. From the date of the COMPASS IPO through December 31, 2020, the Company’s voting interest was 26.3% which included the voting rights provided under the voting agreements as further described in Note 5 below. In April 2021, the voting agreements were terminated. On May 4, 2021, the Company purchased additional equity investments in COMPASS common stock. From the date of the additional investment through June 30, 2021, the Company’s voting interest was 19.4%. The Company concluded that it did not have a controlling financial interest that would require consolidation under the VOE model and accounted for the investments in COMPASS common stock under the equity method (See Note 5).
5. Equity Method Investments and Other Investments
Equity Method Investments
As of June 30, 2021 and December 31, 2020, the Company accounted for the following investments in the investee’s common stock under the equity method (amounts in thousands):
| As of June 30, 2021 | As of December 31, 2020 | |||||||||||||||||||
| Date First | Common Stock | Carrying | Common Stock | Carrying | ||||||||||||||||
Investee | Acquired | Ownership % | Value | Ownership % | Value | |||||||||||||||
Innoplexus A.G. | August 2018 | 35.0 | % | $ | — | 35.0 | % | $ | — | |||||||||||
COMPASS Pathways plc(2) | December 2018 | 19.4 | % | 19,780 | 22.1 | % | — | |||||||||||||
GABA Therapeutics, Inc | November 2020 | 7.5 | %(1) | — | 7.5 | %(1) | — | |||||||||||||
Neuronasal, Inc | October 2020 | n/a | (3) | — | 9.8 | %(1) | — | |||||||||||||
|
|
|
| |||||||||||||||||
Total | $ | 19,780 | $ | — | ||||||||||||||||
|
|
|
| |||||||||||||||||
| (1) | The Company is deemed to have significant influence over this entity through its total ownership interest in the entity’s equity, including the Company’s investment in the respective entity’s preferred stock, described below in Other Investments. |
| (2) | Prior to the consummation of the COMPASS IPO in September 2020, COMPASS undertook a corporate reorganization. As part of the corporate reorganization, COMPASS became a wholly owned subsidiary of COMPASS Rx Limited. COMPASS Rx Limited was re-registered as a public limited company and renamed COMPASS Pathways plc. |
| (3) | Neuronasal common stock was accounted for under the equity method until the entity was consolidated on May 17, 2021 (See Note 3). |
21
Other Investments
The Company has accounted for its other investments that do not have a readily determinable fair value under the measurement alternative. As of June 30, 2021 and December 31, 2020, the carrying values of other investments, which consisted of investments in the investee’s preferred stock and common stock not in the scope of ASC 323 were as follows (in thousands):
| June 30, | December 31, | |||||||
| 2021 | 2020 | |||||||
GABA Therapeutics, Inc. | $ | 14,682 | $ | 5,519 | ||||
DemeRx NB, Inc. | 1,067 | 1,096 | ||||||
Juvenescence Limited | 358 | 368 | ||||||
Neuronasal, Inc. | — | 1,061 | ||||||
|
|
|
| |||||
Total | $ | 16,107 | $ | 8,044 | ||||
|
|
|
| |||||
The Company’s investments in the preferred stock of COMPASS (through September 2020), Neuronasal (through May 2021), Innoplexus, GABA, and DemeRx NB are not considered as in-substance common stock due to the existence of substantial liquidation preferences and therefore did not have subordination characteristics that were substantially similar to the common stock. Although the Company’s investment in Juvenescence Limited (Juvenescence) is in common stock, it is not able to exercise significant influence over the operating and financial decisions of Juvenescence. The Company concluded that its ownership interests in above Other Investments do not have a readily determinable available fair value and are accounted for under the measurement alternative. Under the measurement alternative, the Company measured its other investments at cost, less any impairment, plus or minus, if any, observable price changes in orderly transactions for an identical or similar investment of the same issuer.
During the three and six months ended June 30, 2021 and 2020 there were no observable changes in price recorded related to the Company’s Other Investments.
During the three and six months ended June 30, 2021 and 2020, the Company evaluated all of its other investments to determine if certain events or changes in circumstance during these time periods in 2021 and 2020 had a significant adverse effect on the fair value of any of its investments in non-consolidated entities. Based on this analysis, the Company did not note any impairment indicators existed associated with the Company’s Other Investments.
Innoplexus AG
Innoplexus AG is a technology company that provides “Data as a Service” and “Continuous Analytics as a Service” solutions that aims to help healthcare organizations leverage their technologies and expedite the drug development process across all stages—preclinical, clinical, regulatory and commercial. The Company first acquired investments in Innoplexus in August 2018.
As of December 31, 2020, the Company owned 35.0% of the common stock issued by Innoplexus. The Company has significant influence over Innoplexus through its noncontrolling representation on the investee’s supervisory board. Accordingly, the Company’s investment in Innoplexus’ common stock was accounted for in accordance with the equity method. The Company’s investment in Innoplexus’ preferred stock did not meet the criteria for in-substance common stock. As such, the investment in Innoplexus’ preferred stock was accounted for under the measurement alternative as discussed below.
In February 2021, the Company entered into a Share Purchase and Assignment Agreement (the “Innoplexus SPA”) to sell its shares of common and preferred stock held in Innoplexus to a current investor of Innoplexus (the “Purchaser”) in exchange for an initial purchase price of approximately $2.4 million. In addition, the Company is entitled to receive contingent payments based on the occurrence of subsequent equity transactions or liquidity events at Innoplexus as determined under the Innoplexus SPA.
Pursuant to the Innoplexus SPA, the Purchaser is required to hold a minimum number of shares equivalent to the number of shares purchased from the Company through December 31, 2026. In the event that the Purchaser is in breach of this requirement, the purchaser is required to pay the Company an additional purchase price of approximately $9.6 million. The transaction was accounted for as a secured financing as it did not qualify for sale accounting under ASC Topic 860, Transfers and Servicing (ASC 860), due to the provision under the Innoplexus SPA which constrained the Purchaser from its right to pledge or exchange the underlying shares and provided more than a trivial benefit to the Company. The initial proceeds from the transaction were reflected as a secured borrowing liability of $2.4 million as of June 30, 2021, which is included in Other liabilities in the Company’s condensed consolidated balance sheet. The Company will continue to account for its investment in Innoplexus’ common stock under the equity method of accounting and its investment in Innoplexus’ preferred shares under the measurement alternative.
22
In addition, the Innoplexus SPA also provides the rights for the Company to receive additional consideration with a maximum payment outcome of $22.3 million should the equity value of Innoplexus exceed certain thresholds upon the occurrence of certain events. The Company concluded that this feature met the definition of a derivative which required bifurcation. As the probability of the occurrence of certain events defined in the Innoplexus SPA was less than remote, the Company concluded that the fair value of the embedded derivative ascribed to this feature was de minimis as of June 30, 2021.
The carrying value of the Company’s investment in Innoplexus was zero as of June 30, 2021 and December 31, 2020.
COMPASS Pathways plc
COMPASS Pathways plc is a mental health care company dedicated to pioneering the development of a new model of psilocybin therapy with its product COMP360. The Company first acquired investments in COMPASS in December 2018.
Common Stock Investment
During the first quarter of 2020, the Company’s investment in COMPASS common stock, which was accounted for under the equity method, was reduced to zero after the Company recognized its proportionate share of COMPASS’ net loss from investments in equity method investees. Immediately prior to the completion of the COMPASS IPO, the different classes of issued share capital of COMPASS Pathways plc were reorganized into a single class of ordinary shares through a reverse share split. Upon the consummation of the COMPASS IPO, all of the Company’s outstanding shares of COMPASS, including 7,052,003 shares of COMPASS preferred stock were converted into 7,935,663 new ordinary shares of COMPASS Pathways plc. Upon the COMPASS Preferred Stock Conversion, the Company accounted for the transaction under the equity method and recorded the carrying value of the Company’s investment in COMPASS’ preferred shares of $53.1 million in equity method investments in the condensed consolidated balance sheets.
The carrying value of the investment in COMPASS common stock was reduced to zero as of December 31,2020 due to IPR&D charge with no alternative future use. Since the Company has no obligation to provide financing support to COMPASS, the Company is not required to record further losses exceeding the carrying value of the investment. As of December 31, 2020, the Company owned 22.1% of COMPASS ordinary shares. Based on quoted market prices, the market value of the Company’s ownership in COMPASS was $378.1 million as of December 31, 2020.
On May 4, 2021, COMPASS completed an additional round of equity financing through the offering of 4,000,000 American Depositary Shares (ADS). The COMPASS ADS have identical rights including voting rights as the ordinary shares issued and outstanding. The Company participated in this financing round but did not purchase enough shares to maintain its ownership percentage. The Company acquired 140,000 ADS at an aggregate price of $5.0 million which resulted in a decrease in the Company’s equity ownership percentage in COMPASS and a gain on dilution of $16.9 million. The additional shares purchased was not made to fund prior period losses. As of June 30, 2021, the Company owned 19.4% of the COMPASS ordinary shares. Based on quoted market prices, the market value of the Company’s ownership in COMPASS was $308.1 million as of June 30, 2021.
From the original acquisition of COMPASS common shares in December 2018 through the COMPASS IPO, the Company is deemed to have significant influence over COMPASS through its ownership interest in COMPASS’ equity, including the Company’s investment in COMPASS preferred stock, described below in Other Investments, and the Company’s noncontrolling representation on the COMPASS’ board of directors. Accordingly, the Company’s investment in COMPASS’ common stock was accounted for in accordance with the equity method. The Company’s investment in COMPASS’ preferred stock did not meet the criteria for in-substance common stock. As such, the investment in COMPASS’ preferred stock was accounted for under the measurement alternative as discussed below. Upon the completion of the COMPASS IPO through June 30, 2021, the Company is deemed to continue to have significant influence over COMPASS primarily through its ownership interest in COMPASS’ equity. Accordingly, the Company’s investment in COMPASS’ common stock was accounted for in accordance with the equity method through June 30, 2021.
In December 2020, the Company entered into two voting agreements with COMPASS registered shareholders. The voting agreements provided the Company the voting rights attached to the COMPASS ordinary shares held by such COMPASS shareholders. As of December 31, 2020, the Company held 26.3%
23
voting interest in COMPASS, which included the voting rights provided under the voting agreements. The voting agreements did not provide the Company control over COMPASS nor additional board seats and therefore had no impact on the Company’s investment in COMPASS under the equity method. In April 2021, both voting agreements were terminated.
During the three months ended June 30, 2021 and 2020, the Company recognized its proportionate share of COMPASS’ net loss of $2.1 million and $ 9.8 million, respectively, as losses from investments in equity method investees, net of tax on the condensed consolidated statements of operations. During the six months ended June 30, 2021 and 2020, the Company recognized its proportionate share of COMPASS’ net loss of $2.1 million and $11.8 million, respectively, as losses from investments in equity method investees, net of tax on the condensed consolidated statements of operations. During the three and six months ended June 30, 2020, the Company’s proportionate share of COMPASS’ net loss was more than the Company’s proportionate share using the common stock ownership percentage described above because the aggregate net losses attributable to the Company’s investment in COMPASS common stock reduced the carrying amount to zero in the first quarter of 2020. Accordingly, the remaining COMPASS’ net losses attributable to the Company was determined based on the Company’s ownership percentage of each class of preferred stock in COMPASS and recorded to the Company’s investments in COMPASS preferred stock discussed below.
Preferred Stock Investment
The Company’s preferred stock ownership in COMPASS is included in Other Investments and obtained through a series of related party transactions since 2018. In connection with COMPASS’ secondary Series A preferred stock offering in March 2020, the Company’s investment in COMPASS’ Series A preferred shares were remeasured to fair value due to the observable price change, resulting an aggregate gain of $19.9 million in unrealized gains on other investments in the condensed consolidated statements of operations during the six months ended June 30, 2020.
In March 2020, the Company purchased additional shares of COMPASS Series A preferred stock for £16.1 million or approximately $17.8 million under the secondary Series A preferred stock purchase. In April 2020, COMPASS entered into the Series B preferred stock subscription agreement with other investors for issuance of its Series B preferred stock, which resulted in an automatic conversion of the Company’s COMPASS convertible notes receivable, totaling £6.2 million or $7.6 million on the date of conversion, into shares of COMPASS Series B preferred stock at a conversion price per share representing a 15% discount to the price per share paid by the investors in the COMPASS Series B preferred stock issuance (the “COMPASS Notes Conversion”) (See Note 6). In addition, in April 2020, the Company purchased additional shares of COMPASS Series B preferred stock for $5.3 million and the purchase was completed in August 2020. In September 2020, in connection with the COMPASS IPO, all of the Company’s outstanding shares of 7,052,003 COMPASS preferred stock were converted into new ordinary shares of COMPASS Pathways plc as discussed above (the “COMPASS Preferred Stock Conversion”). Upon the COMPASS Preferred Stock Conversion, the Company accounted for the transaction under the equity method and recorded the carrying value of the Company’s investment in COMPASS’ preferred shares of $53.1 million in equity method investments in the condensed consolidated balance sheets. As of December 31, 2020, the COMPASS Other Investment balance was zero as the Company had no outstanding shares of preferred stock in COMPASS.
GABA Therapeutics, Inc.
GABA is a California based biotechnology company focused on developing its GRX-917 for anxiety, depression and a broad range of neurological disorders. The Company is deemed to have significant influence over GABA through its total ownership interest in GABA’ equity, including the Company’s investment in GABA’s preferred stock, and the Company’s noncontrolling representation on GABA’s board of directors.
Common Stock Investment
The Company’s investment in GABA’s common stock was accounted for in accordance with the equity method. The Company’s investment in GABA’s preferred stock did not meet the criteria for in-substance common stock. As such, the investment in GABA’s preferred stock is accounted for under the measurement alternative as discussed below.
The carrying value of the investment in GABA common stock was reduced to zero as of December 31, 2020 due to IPR&D charge with no alternative future use and remained zero as of June 30, 2021. Accordingly, GABA’s net losses attributable to the Company were determined based on
24
the Company’s ownership percentage of preferred stock in GABA and recorded to the Company’s investments in GABA preferred stock discussed below. During the three and six months ended June 30, 2021, the Company recognized its proportionate share of GABA’s net loss of $0.4 million and $1.1 million, respectively as losses from investments in equity method investees, net of tax on the condensed consolidated statements of operations.
Preferred Stock Investment
In August 2019, GABA and the Company entered into the Preferred Stock Purchase Agreement (the “GABA PSPA”), whereby GABA issued shares of its Series A preferred stock to the Company at a price of approximately $5.5 million. At closing, the Company had over 20% of overall ownership interest in GABA and a noncontrolling representation on the board. On May 15, 2021, GABA and the Company entered into an Amendment to Preferred Stock Purchase Agreement (the Amended GABA PSPA”) under which the GABA PSPA was amended. Pursuant to the Amended PSPA, GABA issued additional shares of its Series A preferred stock to the Company at a price of approximately $0.6 million. As of June 30, 2021 and December 31, 2020, the investment in GABA’s preferred stock was recorded in Other Investments on the condensed consolidated balance sheets under the measurement alternative under ASC 321.
Pursuant to the GABA PSPA, the Company is obligated to purchase additional shares of Series A preferred stock for up to $10.0 million with the same price per share as its initial investment, upon the achievement of specified contingent clinical development milestones. On April 13, 2021, pursuant to the GABA PSPA, the Company purchased additional shares of Series A preferred stock of GABA, for an aggregate cost of $5.0 million based on the achievement of certain development milestones. On May 21, 2021, the Company exercised its option to purchase additional shares of Series A preferred stock prior to the achievement of certain development milestone for an aggregate cost of $5.0 million. As of June 30, 2021, the Company completed the purchase of the additional shares of Series A preferred stock for $10.0 million pursuant to the GABA PSPA. Pursuant to the Amended GABA PSPA, the Company is obligated to purchase additional shares of Series A preferred stock from GABA for up to $1.5 million with the same price per share as its initial investment upon the achievement of specified contingent clinical development milestones. The obligation to purchase additional shares of Series A preferred stock from GABA was $1.5 million as of June 30, 2021.
In accordance with the amended GABA PSPA, the Company also has the option but not the obligation to purchase the aforementioned additional shares of Series A preferred stock at any time prior to the achievement of any milestone at the same price per share as its initial investment. In August 2019, pursuant to the Right of First Refusal and Co-Sale Agreement, the Company has the option but not the obligation to purchase additional shares of common stock for up to $2.0 million from the existing common shareholders.
The Company has evaluated the contingent obligation (forward) and option and concluded that they both: (i) represent freestanding financial instruments as they are legally detachable and separately exercisable from the underlying shares; and (ii) are equity securities under ASC Topic 321, Investments—Equity Securities (ASC 321). The Company accounted for the contingent obligation based on the measurement alternative under ASC 321 which is included in Other Investments as of June 30, 2021 and December 31, 2020. In November 2020 the Company exercised its option to purchase additional shares of common stock of GABA at a price of approximately $1.8 million pursuant to an Omnibus Amendment Agreement under which the Right of First Refusal and Co-Sale Agreement was amended.
Neuronasal, Inc.
Neuronasal is developing a novel intranasal formulation of N-acetylcysteine (“NAC”) for acute mild traumatic brain injury.
Common Stock Investment
In October 2020, upon the achievement of certain development milestones, the Company made a cash contribution of $0.3 million in exchange for 9.8% of the outstanding common stock of Neuronasal.
On March 10, 2021, upon the achievement of certain development milestones, the Company made another cash contribution of $0.5 million in exchange for 10.8% of the outstanding common stock of Neuronasal. The Company recorded its investment in Neuronasal common stock at the carrying cost basis of $0.5 million. At the date of the investment, a basis difference was identified as the cost basis of the Company’s investment in Neuronasal exceeded the Company’s proportionate share of the underlying net assets in Neuronasal. The Company concluded that the basis
25
differences were primarily attributable to Neuronasal’s IPR&D associated with Neuronasal’s novel intranasal formulation of NAC. As the Company’s investments in Neuronasal did not meet the definition of a business due to substantially all of the estimated fair value of the gross assets was concentrated in NAC, the basis differences were attributable to the IPR&D with no alternative future use, were immediately expensed on the dates of investments. The Company’s proportionate share of the basis difference exceeded its carrying value of the equity method investment in Neuronasal and as a result, the March 2021 equity investment balance of $0.5 million was reduced to zero. For the six months ended June 30, 2021, the Company recognized losses from investments in equity method investees, net of tax of $0.5 million in association with the basis difference charge in the Company’s condensed consolidated statements of operations.
The Company is deemed to have significant influence over Neuronasal through its total ownership interest in Neuronasal’s equity through the acquisition date of May 17, 2021 (see Note 3), including the Company’s investment in Neuronasal’s preferred stock, and the Company’s noncontrolling representation on Neuronasal’s board of directors. Accordingly, the Company’s investment in Neuronasal’s common stock was accounted for in accordance with the equity method. The Company’s investment in Neuronasal’s preferred stock did not meet the criteria for in-substance common stock. As such, the investment in Neuronasal’s preferred stock is accounted for under the measurement alternative as discussed below.
The carrying value of the investment in Neuronasal common stock was reduced to zero as of December 31,2020 due to IPR&D charges with no alternative future use. Accordingly, Neuronasal’s net losses attributable to the Company was determined based on the Company’s ownership percentage of preferred stock in Neuronasal and recorded to the Company’s investments in Neuronasal preferred stock discussed below. During the three and six months ended June 30, 2021, immediately prior to the acquisition, the Company recognized its proportionate share of Neuronasal’s net loss of $0.4 million and $1.0 million, respectively as losses from investments in equity method investees, net of tax on the condensed consolidated statements of operations.
Preferred Stock Investment
In December 2019, Neuronasal and the Company entered into the Neuronasal PSPA and the Neuronasal Secondary Sale Agreement, whereby Neuronasal issued shares of its Series A preferred stock to the Company at a price of approximately $0.5 million. At closing, the Company has a less than 20% of ownership interest in Neuronasal and a noncontrolling representation on the board. In October 2020, pursuant to the Neuronasal PSPA, the Company purchased additional Series A preferred shares at a price of approximately $0.8 million. The investment in Neuronasal preferred shares was recorded in Other Investments on the condensed consolidated balance sheets under the measurement alternative under ASC 321as of June 30, 2021 and December 31, 2020.
In October 2020, pursuant to the Neuronasal PSPA, the Company purchased additional Series A preferred shares at a price of approximately $0.8 million upon the achievement of a specified contingent clinical development milestone. On March 10, 2021, pursuant to the Neuronasal PSPA, the Company purchased additional Series A preferred shares for approximately $0.8 million based on the achievement of certain development milestones. Also, pursuant to the Neuronasal Secondary Sale Agreement, the Company purchased additional common shares for approximately $0.3 million. The obligation to purchase additional shares of Series A preferred stock from Neuronasal, and shares of common stock from the existing common shareholders was $1.5 million as of June 30, 2021.
On May 17, 2021, pursuant to the Neuronasal PSPA and the Neuronasal Secondary Sale Agreement, the Company, at its sole option, purchased additional shares of Series A preferred stock of Neuronasal for an aggregate cost of $1.0 million. Upon the closing of the purchase occurred on May 17, 2021, the Company obtained a controlling financial interest in Neuronasal. The Company derecognized its other investments in Neuronasal and began to consolidate the operations of Neuronasal into its financial statements. Please see Note 3, “Acquisitions” for further discussion.
26
DemeRx NB
In December 2019, the Company jointly formed DemeRx NB with DemeRx. DemeRx and DemeRx NB entered into a Contribution Agreement whereby DemeRx assigned all of its rights, title, and interests in and to all of its assets relating to DMX-1002, Noribogaine, in exchange for shares of common stock of DemeRx NB. DemeRx NB will use the contributed intellectual property to develop Noribogaine. Noribogaine is an active metabolite of ibogaine designed to have a longer plasma half-life and potentially reduced hallucinogenic effects compared to ibogaine.
In connection with the Contribution Agreement, the parties entered into a Series A Preferred Stock Purchase Agreement (the “DemeRx NB PSPA”) pursuant to which the Company purchased shares of Series A preferred stock of DemeRx NB at a purchase price of $1.0 million. At closing, the Company has less than 20% of ownership interest in DemeRx NB and a noncontrolling representation on the board. The investment in DemeRx NB was recorded in Other Investments on the condensed consolidated balance sheets under the measurement alternative under ASC 321.
In accordance with the DemeRx NB PSPA, the Company also has the option but not the obligation to purchase additional shares of Series A preferred stock at a purchase price of up to $19.0 million with the same price per share as its initial investment. As of June 30, 2021, the Company has not exercised its option to purchase any shares of Series A preferred stock of DemeRx NB. The Company has evaluated the option and concluded that it: (i) represents a freestanding financial instrument as it is legally detachable and separately exercisable from the underlying shares; and (ii) is an equity security under ASC 321. The Company accounted for the option based on the measurement alternative under ASC 321, which is included in Other Investments as of June 30, 2021 and December 31, 2020.
Other Investments Held at Fair Value
IntelGenx Technologies Corp.
IntelGenx is a novel drug delivery company focused on the development and manufacturing of novel oral thin film products for the pharmaceutical market. In March 2021, IntelGenx and the Company entered into the Strategic Development Agreement and Purchaser Rights Agreement (“PPA”). On May 14, 2021, IntelGenx and the Company executed a Securities Purchase Agreement (the “IntelGenx SPA”) after obtaining IntelGenx shareholder approval, whereby IntelGenx issued shares of its common stock and warrants to the Company at a price of approximately $12.3 million. Each warrant (“the Initial Warrants”) entitles the Company to purchase one share at a price of $0.35 for a period of three years from the closing of the initial investment. Pursuant to the IntelGenx SPA, the Company has the right to purchase (in cash, or in certain circumstances, the Company’s equity) additional units for a period of three years from the closing of the initial investment (the “Additional Unit Warrants”). Each Additional Unit Warrant will be comprised of (i) one share of common stock and (ii) one half of one warrant (the “Additional Warrants”). The price for the Additional Unit Warrants will be (i) until the date which is 12 months following the closing and the purchase does not result in the Company owning more than 74,600,000 common shares of IntelGenx, $0.331 (subject to certain exceptions), and (ii) until the date which is 12 months following the closing and the purchase results in the Company owning more than 74,600,000 common shares of IntelGenx or following the date which is 12 months following the closing regardless of the number of shares held by the Company, the lower of (A) a 20% premium to the volume weighted average price of the common share for the thirty trading days immediately preceding the news release of the additional closing, and (B) $0.50 if purchased in the second year following closing or $0.75 if purchased in third year following closing. Each Additional Warrant will entitle the Company, for a period of three years from the date of issuance, to purchase one share at the lesser of either (i) a 20% premium to the price of the corresponding additional share, or (ii) the price per share under which shares of IntelGenx are issued under convertible instruments that were outstanding on February 16, 2021, provided that the Company may not exercise Additional Warrants to purchase more than the lesser of (x) 44,000,000 common shares of IntelGenx, and (y) the number of common shares issued by IntelGenx under outstanding convertibles held by other investors as of February 16, 2021. Following the initial closing, the Company held a 25% voting interest in IntelGenx. Pursuant to the PPA, the Company is entitled to designate a number of directors to the IntelGenx’s board of directors in the same proportion as the shares of common stock held by the Company to the outstanding of IntelGenx common shares.
Pursuant to the Strategic Development Agreement, the Company engages IntelGenx to conduct research and development projects (“Development Project”) using IntelGenx’s proprietary oral thin film technology. Under the terms of the Strategic Development Agreement, the Company can select four (4) program products. As of the effective date of the Strategic Development Agreement, the Company nominated two (2) program products - DMT and Salvinorin A. 20% of any funds that IntelGenx received or will receive through the Company’s equity investment under the IntelGenx SPA will be available to be credited towards research and development services that IntelGenx conducts for the Company under the Development Projects. No material research and development services have been performed as of June 30, 2021.
27
The Company has significant influence over IntelGenx through ownership interest in IntelGenx’s equity and the Company’s noncontrolling representation on IntelGenx’s board of directors. The Company qualified for and elected to account for its investment in the IntelGenx common stock under the fair value option. The Company believes that the fair value option better reflects the underlying economics of the IntelGenx common stock investment. The Initial Warrants and Additional Units Warrant are accounted for at fair value under ASC 321. The Company determined that the initial aggregate fair value equals to the transaction price and recorded the common shares $3.0 million, the Initial Warrants at $1.2 million and the Additional Unit Warrants at $8.2 million on a relative fair value basis resulting in no initial gain or loss recognized in the consolidated statements of operations. Subsequently, changes in fair value of the common shares and the Warrants are recorded as a component of other income (expense), net in the consolidated statement of operations and comprehensive loss. As of June 30, 2021, the common shares and the Warrants are recorded at $6.9 million within other investments held at fair value in the condensed consolidated balance sheets.
During the three and six months ended June 30, 2021, the Company recognized the change in fair value of the investment in IntelGenx’s common stock and Warrants of $5.5 million loss in the condensed consolidated statements of operations.
Summarized Financial Information
The following is a summary of financial data for investments accounted for under the equity method of accounting (in thousands):
Balance Sheets
| June 30, 2021 | ||||||||||||
| Compass | Neuronasal(1) | GABA | ||||||||||
Current assets | $ | 334,035 | $ | — | $ | 10,954 | ||||||
Non-current assets | 1,126 | — | — | |||||||||
|
|
|
|
|
| |||||||
Total assets | $ | 335,161 | $ | — | $ | 10,954 | ||||||
|
|
|
|
|
| |||||||
Current liabilities | $ | 7,801 | $ | — | $ | 162 | ||||||
Non-current liabilities | — | — | — | |||||||||
|
|
|
|
|
| |||||||
Total liabilities | $ | 7,801 | $ | — | $ | 162 | ||||||
|
|
|
|
|
| |||||||
| December 31, 2020 | ||||||||||||
| Compass | Neuronasal(1) | GABA | ||||||||||
Current assets | $ | 202,404 | $ | 351 | $ | 3,302 | ||||||
Non-current assets | 1,052 | 10 | — | |||||||||
|
|
|
|
|
| |||||||
Total assets | $ | 203,456 | $ | 361 | $ | 3,302 | ||||||
|
|
|
|
|
| |||||||
Current liabilities | $ | 6,895 | $ | 686 | $ | 430 | ||||||
Non-current liabilities | — | 48 | — | |||||||||
|
|
|
|
|
| |||||||
Total liabilities | $ | 6,895 | $ | 734 | $ | 430 | ||||||
|
|
|
|
|
| |||||||
28
Statements of operations
| Three Months Ended | ||||||||||||
| June 30, 2021 | ||||||||||||
| Compass | Neuronasal(1) | GABA | ||||||||||
Revenue | $ | — | $ | — | $ | — | ||||||
Loss from continuing operations | $ | (19,528 | ) | $ | (409 | ) | $ | (387 | ) | |||
Net loss | $ | (19,528 | ) | $ | (409 | ) | $ | (387 | ) | |||
| Three Months Ended | ||||||||||||
| June 30, 2020 | ||||||||||||
| Compass | Neuronasal(1) | GABA | ||||||||||
Revenue | $ | — | $ | — | $ | — | ||||||
Loss from continuing operations | $ | (17,687 | ) | $ | (382 | ) | $ | (929 | ) | |||
Net loss | $ | (17,687 | ) | $ | (382 | ) | $ | (929 | ) | |||
| Six Months Ended | ||||||||||||
| June 30, 2021 | ||||||||||||
| Compass | Neuronasal(1) | GABA | ||||||||||
Revenue | $ | — | $ | — | $ | — | ||||||
Loss from continuing operations | $ | (33,130 | ) | $ | (985 | ) | $ | (1,046 | ) | |||
Net loss | $ | (33,130 | ) | $ | (985 | ) | $ | (1,046 | ) | |||
| Six Months Ended | ||||||||||||
| June 30, 2020 | ||||||||||||
| Compass | Neuronasal(1) | GABA | ||||||||||
Revenue | $ | — | $ | — | $ | — | ||||||
Loss from continuing operations | $ | (26,392 | ) | $ | (514 | ) | $ | (1,956 | ) | |||
Net loss | $ | (26,392 | ) | $ | (514 | ) | $ | (1,956 | ) | |||
| (1) | Results from operations for Neuronasal are through May 17, 2021 at which point the entity is consolidated. |
6. Notes Receivable
Long Term Notes Receivable – related party
Loan to IntelGenx Corp.
On March 8, 2021, the Company and IntelGenx entered into a loan agreement under which the Company provided the aggregate principal amount of $2.0 million (the “March Term Loan”). Pursuant to the loan agreement, IntelGenx may, by written notice, request an advance up to an additional $0.5 million as an additional term loan if no event of default has occurred as defined in the loan agreement. On May 11, 2021, the Company paid an additional advance of $0.5 million as an additional term loan (the “May Term Loan”, and together with the March Term Loan the “Term Loans”). The Term Loans were originally due to mature 120 days following the special shareholder meeting of IntelGenx Tech Corp. to approve additional investment in IntelGenx Tech Corp. by the Company. On May 14, 2021, the Company entered into an amendment agreement to the loan agreement under which the Maturity Date will be the first business day following the first closing of a subscription for additional units if the proceeds from such subscription amount to at least $3.0 million. The loan bears an annualized interest rate of 8% and such interest is accrued daily. The principal amount of the Term Loans plus any accrued interest shall become due and payable on the Maturity Date.
Pursuant to the terms of the Term Loans, upon the occurrence of an event of default, the Company may accelerate the Term Loans and declare the principal and any accrued and unpaid interests of the Term Loans to be immediately due and payable. In addition, IntelGenx may prepay the Term Loans in whole or in part at any time without premium or penalty. Any prepayment of the principal shall be accompanied by a payment of interest accrued to date thereon. The Company concluded that these embedded features do not meet the criteria to be bifurcated and separately accounted for as derivatives.
29
The Company recorded the Term Loans at cost which included the principal balance of the note and accrued interest, net of any payments received, in Long term notes receivables – related parties on its condensed consolidated balance sheets. As of June 30, 2021, the Term Loans have an outstanding balance of $2.5 million. During the three and six months ended June 30, 2021, the recognized interest income associated with the Term Loans was immaterial.
7. Fair Value Measurement
The following table presents information about the Company’s financial assets and liabilities that are measured at fair value on a recurring basis and indicates the fair value hierarchy of the valuation (in thousands):
| Fair Value Measurements as of | ||||||||||||||||
| June 30, 2021 Using: | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
Assets: | ||||||||||||||||
Cash equivalents: | ||||||||||||||||
Money market funds | $ | 398,528 | $ | — | $ | — | $ | 398,528 | ||||||||
Other investment held at fair value | — | 2,248 | 4,638 | 6,886 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
| $ | 398,528 | $ | 2,248 | $ | 4,638 | $ | 405,416 | |||||||||
|
|
|
|
|
|
|
| |||||||||
Liabilities: | ||||||||||||||||
Contingent consideration liability - related parties | $ | — | $ | — | $ | 2,466 | $ | 2,466 | ||||||||
Warrant Liability | $ | — | $ | — | $ | 289 | $ | 289 | ||||||||
|
|
|
|
|
|
|
| |||||||||
| $ | — | $ | — | $ | 2,755 | $ | 2,755 | |||||||||
|
|
|
|
|
|
|
| |||||||||
| Fair Value Measurements as of | ||||||||||||||||
| December 31, 2020 Using: | ||||||||||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
Assets: | $ | — | ||||||||||||||
Liabilities: | ||||||||||||||||
Contingent consideration liability - related parties | $ | — | $ | — | $ | 1,705 | $ | 1,705 | ||||||||
Derivative liability | — | — | 214 | 214 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
| $ | — | $ | — | $ | 1,919 | $ | 1,919 | |||||||||
|
|
|
|
|
|
|
| |||||||||
During the three and six months ended June 30, 2021 and 2020, there were no transfers between Level 1, Level 2 or Level 3.
Valuation of COMPASS Note Receivable-Related Party
The fair value of the COMPASS Notes at issuance and financial reporting dates was estimated based on significant inputs not observable in the market, which represent Level 3 measurements within the fair value hierarchy. The Company estimated the fair value of the COMPASS Notes during the first quarter of 2020 and immediately prior to the conversion of the notes in April 2020 using the fair value of the Series B preferred stock of COMPASS. The fair value of the Notes was estimated to be $9.0 million immediately prior to the conversion of the notes. Once the notes were converted, the acquired shares were recorded at a price per share equal to the fair value of the Series B shares of £1,350 or $1,654. The change in fair value in the COMPASS Notes from December 31, 2019 to its conversion to Series B preferred stock in April 2020, were $0.7 million and included in change in fair value of short term notes receivable—related party in the condensed consolidated statements of operations.
Contingent Consideration Liability—Related Parties—Perception and Innaris Bio
The contingent consideration liability—related parties in the table above relates to milestone and royalty payments in connection with the acquisition of Perception and InnarisBio. The fair value of the contingent consideration liability—related parties
30
was determined based on significant inputs not observable in the market, which represent Level 3 measurements within the fair value hierarchy. The fair value of the contingent milestone and royalty liabilities was estimated based on the discounted cash flow valuation technique. The technique considered the following unobservable inputs:
| • | the probability and timing of achieving the specified milestones and royalties as of each valuation date, |
| • | the probability of executing the license agreement, |
| • | the expected first year of revenue, and |
| • | market-based discount rates |
The fair value of the contingent milestone and royalty liabilities for InnarisBio was estimated to be $0.1 million as of June 30, 2021.
The fair value of the Perception contingent milestone and royalty liabilities could change in future periods depending on prospects for the outcome of R-Ketamine milestone meetings with the FDA or other regulatory authorities, and whether the Company realizes a significant increase or decrease in sales upon commercialization. The most significant assumptions in the discounted cash flow valuation technique that impacts the fair value of the milestone contingent consideration are the projected milestone timing and the probability of the milestone being met. Further, significant assumptions in the discounted cash flow that impacts the fair value of the royalty contingent consideration are the projected revenue over ten years, the timing of royalties on commercial revenue, and the probability of success rate for a commercial R-Ketamine product. As of the fourth quarter of 2020, Perception negotiated a license transaction with a third-party pharmaceutical company that closed in March 2021. The Company used a scenario-based model (“SBM”) to consider the Company’s estimate of 80 percent probability that the transaction would happen and the 20 percent probability that it would fail to close. The valuation used inputs that were unobservable inputs with the most significant being the discount rates for royalties on projected clinical milestones and commercial revenue, probability of the transaction closing, and probability of success estimates over the following ten years.
As of June 30, 2021, the license transaction had closed and the scenario-based method was no longer used (See Note 16). The valuation used inputs that were unobservable with the most significant being the discount rates for royalties on projected clinical milestones and commercial revenue and the probability of success estimates over the following ten years.
The fair value of the contingent milestone and royalty liabilities for Perception was estimated to be $2.4 million and $1.7 million as of June 30, 2021 and December 31, 2020, respectively.
The fair value of the contingent milestone and royalty liabilities could change in future periods depending on the prospects for the first patient dosing and the outcome of obtaining approval from FDA or regulatory authorities for potential drug product using the solgel-based direct-to-brain intranasal drug delivery technology, and whether the Company realizes a significant increase or decrease in sales upon commercialization. The most significant assumptions in the income approach valuation technique used to estimate the contingent liabilities are the probability of each milestone being met, the probability of number of drug products being developed, projected milestone timing and discount rate.
31
The following table summarizes significant unobservable inputs that are included in the valuation of contingent consideration lability – related for Perception parties as of June 30, 2021 and December 31, 2020:
| June 30, 2021 | December 31, 2020 | |||||||||||||||||
| Weighted | Weighted | |||||||||||||||||
Valuation Technique | Significant Unobservable Inputs | Input Range | Average | Input Range | Average | |||||||||||||
Discounted cash flow | Milestone contingent consideration: | |||||||||||||||||
Discount rate | 5.9% | 5.9% | 8.4% to 14.1% | 9.4% | ||||||||||||||
Projected milestone timing | 3.5 years | 3.5 years | 4.0 to 4.3 years | 4.1 years | ||||||||||||||
Probability of the milestone | 51.9% | 51.9% | 10.5% to 48.7% | 34.8% | ||||||||||||||
Discounted cash flow with SBM | Royalty contingent consideration: | |||||||||||||||||
Discount rate for royalties | 13.0% | 13.0% | 12.0% to 13.0% | 12.5% | ||||||||||||||
Discount rate for royalties on milestones | 5.9% | 5.9% | 8.4% | 8.4% | ||||||||||||||
Projected commercial revenue | | $270.3 to $2,031 million | | N/A | | $77.5 to $3,542 million | | N/A | ||||||||||
Projected clinical milestone revenue | | $6.0 to $30.0 million | | N/A | | $6.0 to $30.0 million | | N/A | ||||||||||
Timing of royalties on commercial revenue | 7.8 years | 7.8 years | 7.8 to 8.5 years | 8.1 years | ||||||||||||||
Timing of royalties on clinical milestone revenue | 0.8 year | 0.8 year | 1.3 years | 1.3 years | ||||||||||||||
Probability of success rate | 26.5% to 100.0% | 29.9% | 3.95% to 100.0% | 12.6% | ||||||||||||||
Probability of the close of the license transaction (1) | N/A | N/A | 80.0% | 80.0% | ||||||||||||||
| (1) | This input was used in fourth quarter of 2020 in relation to a potential license transaction that Perception has with a third-party pharmaceutical company. |
Valuation of 2020 Convertible Notes Payable
The fair value of the 2020 Convertible Notes at issuance and at each reporting period was estimated based on significant inputs not observable in the market, which represents a Level 3 measurement within the fair value hierarchy. The Company used a SBM to incorporate estimates and assumptions concerning company prospects and market indications into a model to estimate the value of the notes. The most significant estimates and assumptions used as inputs in the SBM valuation technique impacting the fair value of the 2020 Convertible Notes are those concerning type, timing and probability of specific scenario outcomes. At the issuance dates of the 2020 Convertible Notes, a qualified financing was assumed to occur within the year following issuance. Specifically, the Company discounted the cash flows for fixed payments by using annualized discount rates that were applied across valuation dates from issuance dates of the 2020 Convertible Notes until conversion in November 2020. The discount rates were based on certain considerations including: time to payment, an assessment of the credit position of ATAI, market yields of companies with similar credit risk at the date of valuation estimation, and calibrated rates based on the fair value relative to the original issue price from the 2020 Convertible Notes.
Payments that are sensitive to the total equity value of the Company at the date of payment were valued at each valuation date using an option pricing model (“OPM”). Key assumptions used in the OPM included risk free rate, volatility across the period of the valuation dates, dividend yield, and a period of estimation commensurate with time until payment. The inputs to the option pricing model were determined based on assessment of the Company’s most recent financing transaction, assessed and adjusted for the market value of a group of publicly traded peer guideline companies and relevant equity indices as of each valuation date from issuance to conversion.
32
The following table summarizes significant unobservable inputs by valuation technique that are included in the valuation of the 2020 Convertible Notes from the issuance date of the notes in January 2020 to June 30, 2020:
| June 30, 2020 | ||||||
| Weighted | ||||||
Valuation Technique | Significant Unobservable Inputs | Input Range | Average | |||
SBM | Discount rate | 0.6% to 7.2% | 1.6% | |||
| Expected term | 0.5 to 1.0 years | 0.8 years | ||||
| Probability scenarios: | ||||||
Conversion upon a financing event | 50.0% to 60.0% | 52.0% | ||||
OPM | Risk free rate | -0.6% to -0.7% | -0.6% | |||
| Volatility | 70.0% to 80.0% | 75.0% | ||||
| Dividend yield | 0% | 0% | ||||
Valuation of Derivative Liability—Perception Convertible Notes
The derivative liability in the table above relates to the embedded conversion features in connection with the Perception Convertible Notes issued in 2020 and 2021 discussed in Note 10. The Perception March 2020 Notes contained a derivative, which is related to embedded conversion feature upon a qualified financing transaction. The Perception December 2020 Notes contained a derivative, which is related to embedded conversion features upon a qualified financing transaction and a licensing transaction. The fair value of the embedded conversion features at issuance of the Perception Convertible Notes and subsequent financial reporting dates was estimated based on significant inputs not observable in the market, which represent Level 3 measurements within the fair value hierarchy. The Company used a SBM to incorporate estimates and assumptions concerning company prospects and market indications into a model to estimate the value of the derivative liability. An SBM considers a range of various potential scenario outcomes assumed to occur with associated probabilities. Cash flow outcomes are then discounted to present value to estimate fair value. The SBM procedure is as follows: (i) estimate future cash flows that arise from scenario outcomes, (ii) discount the cash flows to present value using a market-based discount rate and (iii) probability weight the present values to form a probability weighted, expected return analysis that estimates fair value at the subject valuation date. The most significant estimates and assumptions used as inputs in the SBM valuation technique impacting the fair value of the embedded conversion features are those concerning the scenario outcomes’ type, timing and probability.
At the issuance dates of the Perception Convertible Notes and at December 31, 2020, a qualified financing and a licensing transaction were assumed to occur within the year following issuance which the Company estimated 20 percent and 80 percent probability of occurrence of a qualified financing and a licensing transaction, respectively.
As the derivative liability associated with the Perception March 2020 Notes was related to the embedded conversion feature upon a qualified financing transaction the fair value of the derivative liability associated with the Perception March 2020 Notes was reduced to zero because of a zero percent probability of the occurrence of a qualified financing transaction as of June 30, 2021.
The Company calculated the payment due to the holders of Perception Convertible Notes with and without the embedded conversion feature and discounted to present value. The Company discounted the cash flows using a discount rate of 17.0 percent annualized at the issuance dates, and at December 31, 2020 based on an assessment of the credit position of Perception and market yields of companies with similar credit risk at the date of valuation estimation.
On May 31, 2021, the Company issued convertible notes under the Second Tranche Funding (see Note 10). In connection with the issuance of these notes, the Company determined the fair value of the derivative liability related to the embedded conversion option by calculating the payment due to the holders of these notes with and without the conversion feature. The Company discounted the cash flows using a discount rate of 18.0 percent annualized at the issuance date, based on an assessment of the credit position of Perception and market yields of companies with similar credit risk at the date of valuation estimation.
33
On June 10, 2021, the Perception Convertible Notes converted into shares of Series A preferred stock of Perception pursuant to their original terms. The Company remeasured the embedded derivatives related to the Perception Convertible Notes at fair value immediately prior to conversion on June 10, 2021. The Company calculated the payments due to the holders of Perception Convertible Notes with and without the conversion feature. The Company discounted the cash flows using a discount rate of 18.0 percent at June 10, 2021, based on an assessment of the credit position of Perception and market yields of companies with similar credit risk at the date of valuation estimation.
The fair value of the embedded conversion features, including the embedded conversion features associated with the notes issued under the Second Tranche Funding was determined to be $0.8 million immediately before the conversion of the Perception Convertible Notes on June 10, 2021 and reduced to zero upon conversion of the notes. The fair value of the embedded conversion features was determined to be $0.2 million as of December 31, 2020.
The significant unobservable inputs that are included in the valuation of the derivative liability as of December 31, 2020 include:
| December 31, 2020 | ||||
| Weighted | ||||
Significant Unobservable Inputs | Input Range | Average | ||
Discount rate | 17.0% | 17.0% | ||
Expected term | 1 year | 1 year | ||
Probability scenarios: | ||||
Qualified financing transaction | 20% | 20% | ||
Licensing transaction | 80% | 80% | ||
Warrant Liability
The warrant liability in the table above relates to issued and outstanding warrants to purchase shares of Neuronasal’s common stock acquired in connection with the acquisition of Neuronasal. The warrants was classified as other liability in the accompanying condensed consolidated balance sheet as the underlying common stock was determined to be contingently, but not currently, redeemable. The warrant liability was recorded at fair value utilizing the Black-Scholes option pricing model which represent Level 3 measurements within the fair value hierarchy. The Black Scholes option pricing model is based on the estimated market value of the underlying common stock at the valuation measurement date, the remaining contractual term of the warrant, risk-free interest rates, expected dividends, and expected volatility of the price of the underlying common stock. The Company adjusted the carrying value of the warrant to its estimated fair value at each reporting date, with any related increase or decrease in the fair value recorded as an increase or decrease to other income (expense) in the statements of operations.
The fair value of the warrant liability was estimated to be $0.2 million as of June 30, 2021.
The following table summarizes significant unobservable inputs that are included in the valuation of the warrant lability as of June 30, 2021:
| June 30, 2021 | ||||
Exercise Price | $ | 0.01 | ||
Stock Price | $ | 36.12 | ||
Dividend Yield | 0.00 | % | ||
Expected Term (in Years) | 3.25 | |||
Risk-Free Interest Rate | 0.51 | % | ||
Expected Volatility | 98 | % | ||
34
IntelGenx Common Stock, Initial Warrants and Additional Units Warrant
The Company’s investment in IntelGenx consist of Common Stock Initial Warrants and Additional Units Warrant (collectively the “Warrants”). The Company determined Warrants do not meet the definition of derivative instrument per ASC 815. The Company determined that the initial aggregate fair value equals to the transaction price and recorded the common shares $3.0 million, the Initial Warrants at $1.2 million and the Additional Units Warrant at $8.2 million on a relative fair value basis resulting in no initial gain or loss recognized in the consolidated statements of operations.
The fair value of Common Shares is estimated by applying discount for lack of marketability (DLOM) of 13.7% as of May 14, 2021 and 10.7% as of June 30, 2021.
The Initial Warrant asset was recorded at fair value utilizing the Black-Scholes option pricing model. The Black Scholes option pricing model is based on the estimated market value of the underlying common stock at the valuation measurement date, the remaining contractual term of the warrant, risk-free interest rates, expected dividends, and expected volatility of the price of the underlying common stock. The expected volatility is based on a peer group volatility which is a Level 3 input within the fair value hierarchy.
The following table summarizes significant inputs that are included in the valuation of the Initial Warrants as of June 30, 2021:
| June 30, 2021 | ||||
Value of Underlying | $ | 0.46 | ||
Exercise Price | $ | 0.35 | ||
Risk Free Rate | 0.43 | % | ||
Expected Term (in Years) | 2.9 | |||
Expected Volatility | 100 | % | ||
Dividend Yield | 0.00 | % | ||
The fair value of the Additional Units is estimated using a Binomial Lattice in a risk-neutral framework (a special case of the Income Approach). Specifically, the future stock price of the IntelGenX is modeled assuming a Geometric Brownian Motion (GBM) in a risk-neutral framework. For each modeled future price, the Additional Unit is calculated based on the contractual terms (incorporating any optimal early exercise), and then discounted at the term-matched risk-free rate. Finally, the value of the Additional Units is calculated as the probability-weighted present value over all future modeled payoffs.
The following table summarizes significant unobservable inputs that are included in the valuation of the Additional Units Warrant as of June 30, 2021:
| June 30, 2021 | ||||
Tranche 1 Number Units | 14,920,000 | |||
Tranche 2 Number Units | 115,080,000 | |||
Additional Warrants Term (in years) | 3.00 | |||
Additional Units Term (in Years) | 2.87 | |||
Maximum Term (in Years) | 5.87 | |||
Stock Price | $ | 0.460 | ||
Expected Volatility | 100 | % | ||
Warrant Strike | $ | 0.556 | ||
Unit Purchase Price 1st Year | $ | 0.331 | ||
Unit Purchase Price 2nd Year | $ | 0.500 | ||
Unit Purchase Price 3rd Year | $ | 0.750 | ||
Wfraction | 0.68 | |||
Risk-Free Rate | 1.02 | % | ||
Dividend Yield | 0.00 | % | ||
Number of time-steps | 500 | |||
The following table provides a roll forward of the aggregate fair values of the Company’s financial instruments described above, for which fair value is determined using Level 3 inputs (in thousands):
| Other Investments Held at Fair Value | Contingent Consideration liability - related parties | Derivative Liability | Warrant Liability | |||||||||||||
Balance as of December 31, 2020 | $ | — | $ | 1,705 | $ | 214 | $ | — | ||||||||
Initial fair value of instrument | — | 101 | 304 | — | ||||||||||||
Change in fair value | — | (251 | ) | (41 | ) | — | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Balance as of March 31, 2021 | $ | — | $ | 1,555 | $ | 477 | $ | — | ||||||||
|
|
|
|
|
|
|
| |||||||||
Initial fair value of instrument | 9,358 | — | 343 | 249 | ||||||||||||
Change in fair value | (4,720 | ) | 911 | — | 40 | |||||||||||
Extinguishment of liability | — | — | (820 | ) | — | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Balance as of June 30, 2021 | $ | 4,638 | $ | 2,466 | $ | — | $ | 289 | ||||||||
|
|
|
|
|
|
|
| |||||||||
| Compass Notes Receivable - related party | Contingent Consideration liability - related parties | 2020 Convertible Notes Payable | Derivative Liability | |||||||||||||
Balance as of December 31, 2019 | $ | 8,244 | $ | 572 | $ | — | $ | — | ||||||||
Initial fair value of instrument | — | — | — | 31 | ||||||||||||
Issuance of notes payable | — | — | 9,707 | — | ||||||||||||
Change in fair value | 718 | 24 | (1,127 | ) | — | |||||||||||
Foreign currency transaction adjustments | 41 | — | (38 | ) | — | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Balance as of March 31, 2020 | $ | 9,003 | $ | 596 | $ | 8,542 | $ | 31 | ||||||||
|
|
|
|
|
|
|
| |||||||||
Initial fair value of instrument | — | — | — | 184 | ||||||||||||
Issuance of notes payable | — | — | 2,668 | — | ||||||||||||
Conversion of notes receivable | (9,003 | ) | — | — | — | |||||||||||
Change in fair value | — | 42 | 1,260 | — | ||||||||||||
Foreign currency transaction adjustments | — | — | 212 | — | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Balance as of June 30, 2020 | $ | — | $ | 638 | $ | 12,682 | $ | 215 | ||||||||
|
|
|
|
|
|
|
| |||||||||
8. Prepaid Expenses and Other Current Assets
Prepaid expenses consist of the following (in thousands):
| June 30, 2021 | December 31, 2020 | |||||||
Prepaid research and development related expenses | $ | 2,408 | $ | 313 | ||||
Research and development tax credit | 1,029 | 556 | ||||||
Sales tax receivables | 315 | 509 | ||||||
Prepaid insurance | 101 | 144 | ||||||
Other | 111 | 554 | ||||||
|
|
|
| |||||
Total | $ | 3,964 | $ | 2,076 | ||||
|
|
|
| |||||
9. Accrued Liabilities
Accrued liabilities consist of the following (in thousands):
| June 30, 2021 | December 31, 2020 | |||||||
Accrued accounting, legal, and other professional fees | $ | 3,324 | $ | 2,858 | ||||
Taxes payable | 1,323 | 997 | ||||||
Accrued external research and development expenses | 1,138 | 347 | ||||||
Accrued payroll | 1,103 | 1,098 | ||||||
Accrued advisory fees | — | 3,819 | ||||||
Other liabilities | 936 | 96 | ||||||
|
|
|
| |||||
Total | $ | 7,824 | $ | 9,215 | ||||
|
|
|
| |||||
35
10. Convertible Promissory Notes
2018 Convertible Promissory Notes—Related Parties
Convertible promissory notes—related parties, net of discounts and deferred issuance costs, consisted of the following (in thousands):
| June 30, 2021 | December 31, 2020 | |||||||
Convertible notes issued in November 2018 | $ | 190 | $ | 195 | ||||
Convertible notes issued in October 2020 | 995 | 1,022 | ||||||
Unamortized discount and deferred issuance costs | (9 | ) | (18 | ) | ||||
|
|
|
| |||||
Total | $ | 1,176 | $ | 1,199 | ||||
|
|
|
| |||||
During November 2018, the Company executed a terms and conditions agreement (the “Convertible Note Agreement”) under which it would issue up to €1.0 million or $1.2 million in convertible promissory notes to investors. An investor would become a party to the Convertible Note Agreement and would be issued a convertible promissory note by executing and delivering a subscription form. In November 2018, certain investors subscribed to the Convertible Note Agreement and the Company issued convertible promissory notes in the aggregate principal amount of €0.2 million or $0.2 million.
In October 2020, certain investors subscribed to the Convertible Note Agreement and the Company issued the remainder of the 2018 Convertible Notes in the aggregate principal amount of €0.8 million or $1.0 million (collectively, the “2018 Convertible Notes”). The total aggregate principal amount of the 2018 Convertible Notes is $1.2 million as of December 31, 2020. The 2018 Convertible Notes are non-interest-bearing, unsecured and are due and payable on September 30, 2025, unless previously redeemed, converted, purchased or cancelled (the “Maturity Date”). Each 2018 Convertible Note has a face value of €1 and is convertible into one share of ATAI Life Sciences AG upon the payment of €17.00. Conversion rights may be exercised by a noteholder at any time prior to maturity, except during certain periods subsequent to the consummation of the IPO. The 2018 Convertible Notes may be declared for early redemption by the noteholders upon occurrence of specified events of default, including payment default, insolvency and a material adverse change in the Company’s business, operations or financial or other condition. Upon early redemption, the conversion right with respect to the 2018 Convertible Notes may no longer be exercised.
In connection with the Convertible Note Agreement, the Company issued convertible notes in the principal amounts of €0.1 million or $0.1 million to the founders of Perception, who are also related parties of the Company in November 2018 (See Note 18). Perception is a biotech firm acquired by the Company on November 5, 2018. Upon the purchase of certain assets of Perception in November 2018, Perception was deemed to have been a VIE, of which the Company is the primary beneficiary (See Note 4).
In addition, in connection with the Convertible Note Agreement, the Company issued convertible notes in the principal amounts of €0.5 million or $0.6 million to Apeiron, the family office of the Company’s founder, and €0.3 million or $0.4 million to one other shareholder of the Company and the founder of COMPASS in October 2020 (See Note 18).
The Company concluded that both the embedded conversion feature, which is exercisable by the investor at any time during the maturity, and the contingent put option, which would trigger upon the occurrence of an event of default of the 2018 Convertible Notes, do not meet the criteria to be bifurcated and separately accounted for as derivatives and were recorded net of discount and issuance costs, or a reduction to the carrying value of the notes issued in November 2018, with a corresponding adjustment to additional paid in capital. The discount is being amortized using the effective interest method over the period from the respective date of issuance to the Maturity Date.
The Company determined that the October 2020 notes were issued in exchange for services previously provided by the Company’s founders and other shareholders and were fully vested and non-forfeitable upon issuance. These instruments were therefore considered share based compensation awards to non-employees, and the instruments were initially measured and recorded at their grant date fair value based on a Black-Scholes option-pricing model.
The fair value of the October 2020 notes exceeded the principal amount that will be due at maturity. Therefore, at initial recognition, the October 2020 notes were accounted for as convertible debt issued at a substantial premium, such that the face value of the note is recorded as a liability premium was recorded as paid-in capital.
36
2020 Convertible Promissory Notes
In January 2020, the Company executed a terms and conditions agreement (the “2020 Convertible Note Agreement”) under which it would issue up to €30.0 million, or $33.5 million, in convertible promissory notes to various investors. The total aggregate principal amount of the 2020 Convertible Notes was $12.4 million as of June 30, 2020.
For the three and six months ended June 30, 2020, the interest expense and change in fair value in the 2020 Convertible Notes from its various issuance dates to the conversion date totaled $1.2 million and $0.1 million, respectively and is included in change in fair value of convertible promissory notes in the condensed consolidated statements of operations.
Perception Convertible Promissory Notes
On March 16, 2020, Perception entered into a convertible promissory note agreement with the Company and other investors, including related parties, which provided for the issuance of convertible notes of $3.9 million (the “Perception Note Purchase Agreement”).
The notes bear interest at an annual rate of 5% and are due and payable on June 30, 2022, unless earlier converted (the “Perception March 2020 Notes”).
On December 1, 2020, Perception entered into an additional convertible promissory note agreement (the “Perception December 2020 Convertible Note Agreement”) with the Company and other investors, including related parties, which provided for the issuance of convertible notes of up to $12.0 million. Pursuant to the Perception December 2020 Convertible Note Agreement, the convertible notes are issued in two tranches: (i) up to $7.0 million under the first tranche funding (the “First Tranche Funding”), with $6.2 million and $0.8 million issued in December 2020 and January 2021, respectively, and (ii) up to an additional $5.0 million under the second tranche funding (the “Second Tranche Funding”), was issued in May 2021.
Under the Second Tranche Funding, Perception issued $4.2 million to the Company, $0.2 million to Apeiron, and $0.3 million to Sonia Weiss Pick and Family, and $0.4 million to other investors.
The notes bear interest at an annual rate of 5% and are due and payable on February 28, 2022, unless earlier converted (the “Perception December 2020 Notes” and together with the Perception March 2020 Notes, the “Perception Convertible Notes”).
In the event of a qualified sale of preferred stock resulting in gross proceeds to Perception of at least $5.0 million, all the principal and accrued and unpaid interest under the Perception Convertible Notes will automatically convert, into the same equity securities issued by Perception at a 25% discount from the lowest price of the security issued. In the event that Perception receives upfront proceeds of $5.0 million or more in a licensing transaction, all the principal and accrued and unpaid interest under the Perception convertible notes will automatically convert, into shares of Series A Preferred Stock of Perception at a price per share of $0.75 for the Perception March 2020 Notes and 75% of the fair market value of the Series A Preferred Stock of Perception for the Perception December 2020 Notes. Upon a change in control of Perception, all the principal and accrued and unpaid interest under the Perception Convertible Notes will automatically convert into shares of Series A Preferred Stock of Perception at a price per share of $0.75. The Perception Convertible Notes issued to the Company represent intercompany debt and are eliminated upon consolidation.
The Perception March 2020 Notes contained an embedded conversion features in the event of a qualified financing whereas the Perception December 2020 Notes contained both embedded conversion features in the event of a qualified financing and upon the occurrence of a licensing transaction. The Company concluded that both the embedded conversion features met the definition of embedded derivatives that were required to be bifurcated and accounted for as a separate unit of accounting.
As of December 31, 2020, the Company recorded the fair value of the derivative liabilities of $0.4 million as a liability with the offset being recorded as a debt discount on the issuance dates of the Perception Convertible Notes.
37
Both the liability and the offsetting debt discount are presented together in convertible promissory notes and derivative liability on the consolidated balance sheets. The resulting debt discount is being amortized to interest expense using the effective interest method over the terms of the Perception Convertible Notes. This interest expense is recorded in other income (expense), net in the consolidated statements of operations. The derivative liabilities are subsequently remeasured to fair value at each reporting date with changes in fair value recognized as a component of other income (expense), net in the consolidated statements of operations.
Upon issuance of the notes under the Second Tranche Funding, the Company recorded the fair value of the derivative liabilities of $0.3 million as a liability with an offset being recorded as a debt discount.
On June 10, 2021, Perception received proceeds of $20.0 million pursuant to the licensing and collaboration arrangement between Perception and Otsuka Pharmaceutical Co., LTD (“Otsuka”) (See Note 16). Upon receipt of the proceeds, the Perception Convertible Notes automatically converted into 6,456,595 shares of Series A preferred stock of Perception pursuant to their original terms. The Company, Sonia Weiss Pick and Family, Apeiron, and other investors received 5,403,791 shares, 440,415 shares, 27,809 shares and 584,580 shares of Perception Series A preferred stock, respectively, upon conversion of the Perception Convertible Notes. The amounts associated with the shares of Perception Series A preferred stock issued to the Company represent intercompany transactions and are eliminated upon consolidation.
The Company remeasured the derivative liability immediately prior to the conversion of the Perception Notes and recorded an immaterial net gain for the three months ended June 30, 2021. The Company recorded a net gain of $41,000 resulting from the change in fair value of the derivative liability for the six months ended June 30, 2021. The conversion of the Perception December 2020 Notes was accounted for as an extinguishment as the notes were converted pursuant to an embedded conversion feature upon a licensing transaction, which was determined to be a redemption feature. Accordingly, the Company recorded a loss on extinguishment of notes of $0.5 million in the condensed consolidated statements of operations for the three and six months ended June 30, 2021. The loss on extinguishment of notes represents the difference between (i) carrying value including derivative liability of the Perception December 2020 Notes of $2.2 million and (ii) the fair value of Perception Series A preferred stock into which the notes converted of $2.7 million. The conversion of the Perception March 2020 Notes was accounted for as a conversion as the notes converted pursuant to a conversion feature. Accordingly, the Company derecognized the carrying amount of the Perception March 2020 notes issued to Sonia Weiss and Family and other investors in the aggregate amount of $0.6 million with an offset to Series A preferred stock, and no gain or loss was recognized. The shares issued upon conversion of the Perception March 2020 and December 2020 Notes issued to the Company represent an intercompany transaction and, therefore, eliminate in consolidation.
As of December 31, 2020, the fair value of the derivative liability was $0.2 million, including an immaterial amount of derivative liability relating to Sonia Weiss Pick and Family. As of June 30, 2020, the fair value of the derivative liability was $0.2 million, including $0.1 million of derivative liability relating to Sonia Weiss Pick and Family and Apeiron. The Company recorded a net loss of $12,000 resulting from the change in the fair value of derivative for the three months ended June 30, 2020. The Company recorded a net gain $44,000 resulting from the change in fair value of the derivative liability for the six months ended June 30, 2020.
The Company recognized interest expense of $0.1 million, including amortization of debt discount of $93,000 during the three months ended June 30, 2021. The Company recognized interest expense of $0.2 million, including amortization of debt discount of $0.2 million during the six months ended June 30, 2021. As of December 31, 2020, the unamortized debt discount on the Perception Convertible Notes was $0.3 million. As of June 30, 2021, there was no unamortized debt discount due to the conversion of the Perception Convertible Notes into Series A convertible preferred stock of Perception on June 10, 2021. The debt issuance costs associated with the Perception Convertible Notes were not material.
11. Common Stock
In January 2021, pursuant to an additional closing from the common stock issuance in November and December 2020, the Company issued and sold 2,133,328 shares of common stock to Apeiron at the same issuance price, for cash proceeds of $12.2 million. In March 2021, the Company issued and sold 13,419,360 shares of common stock to new and existing investors, including related parties, at a price of €9.69 or $11.71 per share, for cash proceeds of $152.2 million, net of issuance costs of $4.9 million.
38
On June 22, 2021, ATAI closed the IPO of its common stock on Nasdaq. As part of the IPO, the Company issued and sold 17,250,000 shares of its common stock, which included 2,250,000 shares sold pursuant to the exercise of the underwriters’ over-allotment option, at a public offering price of $15.00 per share. The Company received net proceeds of $231.6 million from the IPO, after deducting underwriters’ discounts and commissions of $18.1 million and offering costs of $9.0 million.
All common shareholders have identical rights. Each share of common stock entitles the holder to one vote on all matters submitted to the stockholders for a vote.
All holders of common stock are entitled to receive dividends, as may be declared by the Company’s board of directors. Upon liquidation, common stockholders will receive distribution on a pro rata basis. As of June 30, 2021 and December 31, 2020, no cash dividends have been declared or paid.
12. Stock-Based Compensation
Atai Life Sciences 2020 Equity Incentive Plan
Effective August 21, 2020, the Company adopted an equity-based compensation plan, the 2020 Equity Incentive Plan (as amended from time to time, “2020 Incentive Plan”). The 2020 Incentive Plan is administered by the Company’s Board. The plan is intended to encourage ownership of shares by employees, directors and certain consultants to the Company in order to attract and retain such individuals, to induce them to work for the benefit of the Company and to provide additional incentive for them to promote the success of the Company. The 2020 Incentive Plan enables the Company to grant incentive stock options or nonqualified stock options, restricted stock awards and other stock-based awards to executive officers, directors and employees and consultants of the Company.
The Company has reserved up to 22,658,192 shares of common stock, excluding any shares issued under its Hurdle Share Option Program described below, for issuance to executive officers, directors, other employees and consultants of the Company pursuant to the 2020 Incentive Plan. Shares that are expired, terminated, surrendered, or canceled without having been fully exercised will be available for future awards. As of June 30, 2021, 0 shares were available for future grants under the 2020 Incentive Plan and all subsequent grants are subject to the Atai Life Sciences 2021 Incentive Award Plan discussed below.
Atai Life Sciences 2021 Incentive Award Plan
Effective April 23, 2021, the Company adopted and our shareholders approved the 2021 Incentive Award Plan (“2021 Incentive Plan”). The 2021 Incentive Plan is administered by the Company’s Board. The plan is intended to encourage ownership of shares by employees, directors, and certain consultants to the Company in order to attract and retain such individuals, to induce them to work for the benefit of the Company or of an affiliate and to provide additional incentive for them to promote the success of the Company. The 2021 Incentive Plan enables the Company to grant incentive stock options or nonqualified stock options, restricted stock awards and other stock-based awards to executive officers, directors and other employees and consultants of the Company.
The Company has reserved up to 16,000,000 shares of common stock, for issuance to executive officers, directors and employees and consultants of the Company pursuant to the 2021 Incentive Plan. Shares that are expired, terminated, surrendered, or canceled without having been fully exercised will be available for future awards. As of June 30, 2021, 14,573,575 shares were available for future grants under the 2021 Incentive Plan.
Stock Options
The stock options outstanding noted below consist primarily of both service and performance-based options to purchase Common Stock. These stock options have a five-year contractual term. These awards are subject to the risk of forfeiture until vested by virtue of continued employment or service to the Company.
The December 31, 2020 stock options outstanding balance noted below includes 3,176,976 stock options that will vest over a four-year service period, only if and when a “Liquidity Event” (as defined in the 2020 Incentive Plan) occurs within five years of the date of grant. During the six months ended June 30, 2021, the Company modified the vesting terms of 2,464,720 of these options held by 12 employees such that, if the Company achieves an Initial Public Offering (“IPO”) (as defined in the awards) by June 30, 2021 or December 31, 2021, an additional 25% or 12.5%, respectively, will accelerate and vest upon the occurrence of the transaction. In each case provided, however, no option shall become vested before the first anniversary of the respective vesting start date. The Company applied modification accounting under ASC 718, which resulted in a new measurement of compensation cost, and the original grant-date fair value of the award is no longer used to measure compensation cost for the award. The weighted average fair value on the new measurement date amounted to $4.97. In June of 2021, the Company achieved a Liquidity Event and therefore began recognizing expense during the period.
39
In addition, during the six months ended June 30, 2021, the Company cancelled 1,152,192 stock options held by 3 employees and concurrently granted 4,543,936 stock options under the HSOP Plan (as defined and described below) (“Exchange Options”). The Company applied modification accounting under ASC 718, which resulted in a new measurement of compensation cost, and the original grant-date fair value of the award is no longer used to measure compensation cost for the award. The weighted average fair value on the new measurement date amounted to $4.20. Refer to the Atai Life Sciences Hurdle Share Option Plan for more information on these stock options.
The following is a summary of stock option activity for from December 31, 2020 to June 30, 2021:
| Number of Options | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
Outstanding as of December 31, 2020 | 11,331,232 | $ | 1.54 | 4.64 | $ | 47,735 | ||||||||||
Granted | 14,051,289 | (1) | 8.69 | — | — | |||||||||||
Exercised | — | — | — | — | ||||||||||||
Cancelled or forfeited | (1,584,528 | )(2) | 2.37 | — | — | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Outstanding as of June 30, 2021 | 23,797,993 | (3) | $ | 5.71 | 4.49 | $ | 304,470 | |||||||||
|
|
|
|
|
|
|
| |||||||||
Options exercisable as of June 30, 2021 | 5,512,278 | $ | 0.85 | 4.14 | $ | 97,291 | ||||||||||
|
|
|
|
|
|
|
| |||||||||
| (1) | Includes (a) 5,391,184 stock options that will vest over a two to four-year service period, only if and when a “Liquidity Event” (as defined in the awards) occurs within five years of the date of grant. If the Company achieves an IPO (as defined in the awards) by June 30, 2021 or December 31, 2021, an additional 25% or 12.5%, respectively, the stock options will accelerate and vest upon the occurrence of the transaction, (b) 5,241,785 stock options that will vest over a one to four-year service period, only if and when a “Liquidity Event” (as defined in the awards) occurs within five years of the date of grant (c) 1,460,784 stock options that will vest at the end of a four-year service period and upon the satisfaction of specified performance-based vesting conditions, only if and when a “Liquidity Event” (as defined in the awards) occurs within five years of the date of grant, (d) 624,000 stock options that will vest over a three-year service period, only if and when a “Liquidity Event” (as defined in the awards) occurs within five years of the date of grant, (e) 400,688 stock options that will vest over a four-year service period and upon the satisfaction of specified performance-based vesting conditions including liquidity-based conditions, only if and when a “Liquidity Event” (as defined in the awards) occurs within five years of the date of grant. If the Company achieves an IPO (as defined in the awards) by June 30, 2021 or December 31, 2021, an additional 25% or 12.5%, respectively, will accelerate and satisfy the service-based vesting condition upon the occurrence of the transaction, (f) 400,000 stock options that will vest over a two-year service period and upon the satisfaction of specified market-based conditions tied to price of the Company’s publicly traded shares, only if and when a “Liquidity Event” (as defined in the awards) occurs within five years of the date of grant, (g) 338,112 stock options that will vest over a four-year service period and upon the satisfaction of specified performance-based vesting conditions including liquidity-based conditions, only if and when a “Liquidity Event” (as defined in the awards) occurs within five years of the date of grant, (h) 100,640 stock options that will vest over a four-year service period and upon the satisfaction of specified performance-based vesting conditions, only if and when a “Liquidity Event” (as defined in the awards) occurs within five years of the date of grant, and (i) 94,096 stock options that will vest only if and when a “Liquidity Event” (as defined in the awards) occurs within five years of the date of grant. |
| (2) | Includes 1,152,192 Exchange Shares |
| (3) | With the satisfaction of the Liquidity Event (as defined in the awards) during the three months ended June 30, 2021, the outstanding options include (a) 14,051,289 stock options as described in footnote (1) less 392,336 stock options forfeited, (b) 4,566,848 vested stock options yet to be exercised as of June 30, 2021, (c) 3,027,408 stock options that will vest at the end of a four-year service period and upon the satisfaction of specified performance-based vesting conditions, and (d) 2,544,784 stock options that will vest over a two to four-year service period. |
The weighted-average grant-date fair value of options granted during the six months ended June 30, 2021, was $6.39.
The Company estimates the fair values of stock options using the Black-Scholes option-pricing model on the date of grant. During the six months ended June 30, 2021, the assumptions used in the Black-Scholes option pricing model were as follows:
| June 30, 2021 | ||
Weighted average expected term in years | 3.64 | |
Weighted average expected stock price volatility | 81.2% | |
Risk-free interest rate | (0.76)%-1.27% | |
Expected dividend yield | 0% |
40
For the three months ended June 30, 2021 and 2020, the Company recorded stock-based compensation expense of $20.6 million and $0.0 million, respectively. For the six months ended June 30, 2021 and 2020, the Company recorded stock-based compensation expense of $20.6 million and $0.0 million, respectively.
As of June 30, 2021, total unrecognized compensation cost related to the unvested stock-based awards was $85.4 million, which is expected to be recognized over a weighted average period of 2.41 years.
Atai Life Sciences Hurdle Share Option Plan
In August 21, 2020, the Partnership (as defined below) approved and implemented an employee stock option plan for selected executives, employees, and consultants of the Partnership (so-called Hurdle Share Options Program or “HSOP Plan”), which became effective on January 2, 2021, the date the first grants under the HSOP were made (“HSOP Options”). This plan is primarily aimed at German-based executives, employees, and consultants of the Company (collectively as “HSOP Participants”). The purpose of the HSOP Plan is to permit these individuals to indirectly participate in the appreciation in value of the Company through a German law private partnership, ATAI Life Sciences HSOP GbR (the “Partnership”). The HSOP Plan was established under the Partnership Agreement of the Partnership. The HSOP Plan requires the exercise price to be equal to the fair value of the shares on the date of grant.
The Partnership has reserved up to 8,000,000 shares (“HSOP Shares”) pursuant to the HSOP Plan. The Partnership is authorized to subscribe for the additional shares under HSOP Plan. Each HSOP Option contains both service and performance-based vesting conditions, including a liquidity-based condition, and gives the holder the option to purchase HSOP Shares. As of June 30, 2021, 718,624 shares were available for future grants under the HSOP Plan.
The HSOP Plan mimics the economics of a typical stock option plan, however, HSOP Options result in HSOP Shares being issued to the Partnership at the grant date. The grantee is required to pay a nominal value (€0.06 per share) for the shares upon grant (“Nominal Upfront Payment”). The nominal amount paid at the grant date is refundable if the HSOP Options do not vest or are forfeited. Otherwise, the nominal amount is refundable until the later of the occurrence of a Liquidity Event (as defined in the “HSOP Plan”) or the exercise date.
The HSOP Shares issued under the HSOP plan to the Partnership are indirectly owned by HSOP Options holders via their interest in the Partnership. However, each HSOP Option holder signed a nonrevocable power of attorney ceding virtually all rights and decisions, including their rights as shareholders to the Managing Partner (as defined in the Partnership agreement) of the Partnership. HSOP Option holders have a forfeitable right to distributions until the HSOP Options vest, at which time the right becomes nonforfeitable. Accordingly, the HSOP Shares issued to the Partnership and allocated to the HSOP Options holders are not considered outstanding for accounting purposes. Therefore, the Company accounted for the Nominal Upfront Payment as an in-substance early exercise provision under ASC 718 as the nominal amount is deducted from the exercise price upon exercise. As of June 30, 2021, the $0.5 million Nominal Upfront Payment was recorded as an other liability on the condensed consolidation balance sheets. The HSOP Options include a provision that requires the HSOP Options holders pay compensation equal to 2% per annum interest on the unpaid exercise price less the €0.06 nominal amount paid upon grant (“Non-recourse Loan”) upon qualifying events (as defined in the Partnership agreement), which occurred on April 23, 2021 currently with the corporate reorganization discussed in Note 1.
The 2% per annum interest rate is fixed and not linked to something other than a service, performance, or market condition, therefore, the Company accounted for the fixed rate interest charge as an in-substance non-recourse loan in a stock compensation arrangement under ASC 718. In such cases, the rights and obligations embodied in a transfer of equity shares to an employee for a note that provides no recourse to other assets or the employee (other than the correlating shares) are substantially the same as those embodied in a grant of share options. The 2% per annum interest was considered in the valuation of the HSOP Options.
HSOP Options
The HSOP Options outstanding noted below consist of service and performance-based options to purchase HSOP Shares. These HSOP Options have a fifteen-year contractual term. These HSOP Options vest over a three to four-year service period, only if and when a “Liquidity Event” (as defined in the Partnership agreement) occurs within fifteen years of the date of grant. If a Change in Control (as defined in the Partnership agreement) or in the event the holder’s service with the Partnership is terminated due to his death or disability by June 30, 2021 or December 31, 2021, an additional 25% or 12.5%, respectively, HSOP options will accelerate and vest upon the occurrence of the transaction. These awards are subject to the risk of forfeiture until vested by virtue of continued employment or service to the Company. In June of 2021, the Company achieved a Liquidity Event and therefore began recognizing expense during the period.
41
The following is a summary of stock option activity for from December 31, 2020 to June 30, 2021:
| Number of Options | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
Outstanding as of December 31, 2020 | — | — | — | — | ||||||||||||
Granted | 7,281,376 | (1) | 6.64 | — | — | |||||||||||
Exercised | — | — | — | — | ||||||||||||
Cancelled or forfeited | — | — | — | — | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Outstanding as of June 30, 2021 | 7,281,376 | $ | 6.64 | 14.51 | $ | 86,346 | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Options exercisable as of June 30, 2021 | 2,736,372 | $ | 6.63 | 14.51 | $ | 32,483 | ||||||||||
|
|
|
|
|
|
|
| |||||||||
| (1) | Includes 4,543,936 Exchange Shares |
The weighted-average grant-date fair value of options granted during the six months ended June 30, 2021, was $4.37.
The Company estimates the fair values of stock options using the Black-Scholes option-pricing model on the date of grant. During the six months ended June 30, 2021, the assumptions used in the Black-Scholes option pricing model were as follows:
| June 30, 2021 | ||
Weighted average expected term in years | 8.00 | |
Weighted average expected stock price volatility | 70.0% | |
Risk-free interest rate | (0.70)%-(0.65)% | |
Expected dividend yield | 0% |
For the three months ended June 30, 2021 and 2020, the Company recorded stock-based compensation expense of $16.7 million and $0.0 million, respectively. For the six months ended June 30, 2021 and 2020, the Company recorded stock-based compensation expense of $16.7 million and $0.0 million, respectively.
As of June 30, 2021, total unrecognized compensation cost related to the unvested stock-based awards was $14.3 million which is expected to be recognized over a weighted average period of 1.53 years.
Kures 2019 Stock Option and Grant Plan
Effective August 27, 2019, Kures adopted an equity-based compensation plan. The Kures 2019 Stock Option and Grant Plan provides for Kures to grant incentive stock options or nonqualified stock options, restricted stock awards and other stock-based awards to employees, directors, consultants of Kures.
Kures has reserved up to 954,315 shares of common stock for issuance to directors of Kures pursuant to the Kures 2019 Stock Option and Grant Plan. At June 30, 2021, there was 600,000 stock option issued and outstanding and 354,315 shares were available for future grants under the Kures 2019 Stock Option and Grant Plan.
The Kures 2019 Stock Option and Grant Plan is administered by Kures’ board of directors. Shares that are expired, terminated, surrendered, or canceled without having been fully exercised will be available for future awards.
42
Stock Options
The stock options outstanding noted below consist primarily of service-based options to purchase Common Stock, the majority of which vest over a four-year period and have a ten-year contractual term. These awards are subject to the risk of forfeiture until vested by virtue of continued employment or service to the Company. The following is a summary of stock option from December 31, 2020 to June 30, 2021:
| Number of Options | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Term (Years) | Aggregate Intrinsic Value | |||||||||||||
Outstanding as of December 31, 2020 | 600,000 | $ | 0.10 | 9.58 | $ | — | ||||||||||
Granted | — | — | — | — | ||||||||||||
Exercised | — | — | — | — | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Cancelled or forfeited | — | — | — | — | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Outstanding as of June 30, 2021 | 600,000 | $ | 0.10 | 9.08 | $ | — | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Options exercisable as of June 30, 2021 | 237,500 | $ | 0.10 | 9.08 | $ | — | ||||||||||
|
|
|
|
|
|
|
| |||||||||
For the three months ended June 30, 2021 and 2020, the Company recorded stock-based compensation expense of $2,618 and $0.0, respectively. For the six months ended June 30, 2021 and 2020, the Company recorded stock-based compensation expense of $5,207 and $0.0, respectively. As of June 30, 2021, total unrecognized compensation cost related to the unvested stock-based awards was $0.1 million, which is expected to be recognized over a weighted average period of 2.16 years.
Kures Restricted Common Stock Awards
Immediately following the acquisition detailed in Note 3, the Board of Directors of Kures issued 4,937,530 unvested restricted common shares to directors of Kures. The restricted common stock vest over a two to three-year period, subject to the risk of forfeiture until vested by virtue of continued employment or service to the Company.
The Company measures all non-cash share-based awards using the fair value on the date of grant and recognizes compensation expense for those awards on a straight-line basis over the requisite service period, which is generally the period from the grant date to the end of the vesting period.
The Company reflects restricted stock awards as issued and outstanding shares of common stock when vested and the shares have been delivered to the individual. The following table summarizes Kures’ restricted common stock awards activity from December 31, 2020 to June 30, 2021:
| RSA | Weighted Average Grant Date Fair Value | |||||||
Unvested balance as of December 31, 2020 | 2,743,066 | $ | 0.10 | |||||
Granted | — | — | ||||||
Vested | (822,924 | ) | 0.10 | |||||
Forfeited | — | — | ||||||
|
|
|
| |||||
Unvested balance as of June 30, 2021 | 1,920,142 | $ | 0.10 | |||||
|
|
|
| |||||
For the three months ended June 30, 2021 and 2020, the Company recorded stock-based compensation expense associated with restricted stock awards of $42,761 and $41,033, respectively. For the six months ended June 30, 2021 and 2020, the Company recorded stock-based compensation expense associated with restricted stock awards of $83,343 and $82,067, respectively.
The fair value of restricted stock that vested during the six months ended June 30, 2021 was $0.1 million. As of June 30, 2021, total unrecognized compensation cost related to the unvested stock-based awards was $0.2 million, which is expected to be recognized over a weighted average period of 1.16 years.
43
Recognify Restricted Common Stock Awards
Immediately following the acquisition detailed in Note 3, the Board of Directors of Recognify issued 1,017,917 unvested restricted common shares to directors and consultants of Recognify. The restricted common stock typically vest over a two to four-year period, subject to the risk of forfeiture until vested by virtue of continued employment or service to the Company.
The Company reflects restricted stock awards as issued and outstanding shares of common stock when vested and the shares have been delivered to the individual. The following table summarizes Recognify’ restricted common stock awards activity from December 31, 2020 to June 30, 2021:
| RSA | Weighted Average Grant Date Fair Value | |||||||
Unvested balance as of December 31, 2020 | 952,387 | $ | 1.71 | |||||
Granted | ||||||||
Vested | (198,684 | ) | 1.71 | |||||
Forfeited | — | — | ||||||
|
|
|
| |||||
Unvested balance as of June 30, 2021 | 753,703 | $ | 1.71 | |||||
|
|
|
| |||||
The Company acquired Recognify in November 2020. The Company determined Recognify is a VIE and consolidated its result of operations within the Company’s consolidated financial statements.
For the three months ended June 30, 2021, the Company recorded stock-based compensation expense of $0.2 million. For the six months ended June 30, 2021, the Company recorded stock-based compensation expense of $0.3 million.
The total fair value of shares vested during the six months ended June 30, 2021, was $0.3 million. As of June 30, 2021, total unrecognized compensation cost related to the unvested stock-based awards was $1.3 million, which is expected to be recognized over a weighted average period of 2.18 years.
Stock-Based Compensation
Stock-based compensation expense is allocated to either research and development or general and administrative expense on the consolidated statements of operations based on the cost center to which the option holder belongs.
For the three months ended June, 2020, the Company recorded total stock-based compensation expense associated with Kures’ restricted stock awards of $41,033 within research and development expense on the condensed consolidated statements of operations. For the six months ended June, 2020, the Company recorded total stock-based compensation expense associated with Kures’ restricted stock awards of $82,067 within research and development expense on the condensed consolidated statements of operations.
44
The following table summarizes the total stock-based compensation expense by function for the three months ended June 30, 2021, which includes expense related to stock options and restricted stock awards (in thousands):
| Three Months Ended June 30, 2021 | ||||||||||||||||||||||||
| Atai ESOP | Atai HSOP | Kures Stock Options | Kures RSA | Recognify RSA | Total | |||||||||||||||||||
Research and development | $ | 8,698 | $ | — | $ | 3 | $ | 43 | $ | 115 | $ | 8,859 | ||||||||||||
General and administrative | 11,940 | 16,650 | — | — | 63 | $ | 28,653 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total share based compensation expense | $ | 20,638 | $ | 16,650 | $ | 3 | $ | 43 | $ | 178 | $ | 37,512 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
The following table summarizes the total stock-based compensation expense by function for the six months ended June 30, 2021, which includes expense related to stock options and restricted stock awards (in thousands):
| Six Months Ended June 30, 2021 | ||||||||||||||||||||||||
| Atai ESOP | Atai HSOP | Kures Stock Options | Kures RSA | Recognify RSA | Total | |||||||||||||||||||
Research and development | $ | 8,698 | $ | — | $ | 5 | $ | 83 | $ | 222 | $ | 9,008 | ||||||||||||
General and administrative | 11,940 | 16,650 | — | — | 125 | $ | 28,715 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
Total share based compensation expense | $ | 20,638 | $ | 16,650 | $ | 5 | $ | 83 | $ | 347 | $ | 37,723 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||
13. Income Taxes
The Company records its quarterly income tax expense by utilizing an estimated annual effective tax rate applied to its period to date earnings as adjusted for any discrete items arising during the quarter. The tax effect for discrete items are recorded in the period in which they occur. The Company recorded $58,000 and $0 income tax expense for the three months ended June 30, 2021 and 2020. The Company recorded $64,000 and $0 income tax expense for the six months ended June 30, 2021 and 2020. The Company continues to maintain a full valuation allowance against its deferred tax assets consistent with prior periods.
45
14. Net Loss Per Share
Basic and diluted net loss per share attributable to ATAI stockholders were calculated as follows (in thousands, except share and per share data):
| Three Months Ended | Six Months Ended | |||||||||||||||
| June 30, 2021 | June 30, 2020 | June 30, 2021 | June 30, 2020 | |||||||||||||
Numerator: | ||||||||||||||||
Net income | $ | (53,373 | ) | $ | (16,957 | ) | $ | (49,329 | ) | $ | (1,076 | ) | ||||
Net income (loss) attributable to redeemable noncontrolling interests and noncontrolling interests | (4,912 | ) | (600 | ) | (1,556 | ) | (1,022 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Net income attributable to ATAI Life Sciences N.V. shareholders - basic and diluted | $ | (48,461 | ) | $ | (16,357 | ) | $ | (47,773 | ) | $ | (54 | ) | ||||
Denominator: | ||||||||||||||||
Weighted average common shares outstanding attributable to ATAI Life Sciences N.V. stockholders - basic and diluted | 132,265,075 | 90,709,312 | 125,797,732 | 90,709,312 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Net income per share attributable to ATAI Life Sciences N.V. shareholders - basic and diluted | $ | (0.37 | ) | $ | (0.18 | ) | $ | (0.38 | ) | $ | (0.00 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
HSOP Shares issued to the Partnership and allocated to the HSOP Options holders are not considered outstanding for accounting purposes and not included in the calculation of basic weighted average common shares outstanding in the table above because the HSOP Option holders have a forfeitable right to distributions until the HSOP Options vest, at which time the right becomes nonforfeitable.
The following also represents maximum amount of outstanding shares of potentially dilutive securities were excluded from the computation of diluted net income (loss) per share attributable to common shareholders for the periods presented because including them would have been antidilutive:
Potentially dilutive securities to the Company’s common shares:
| As of June 30, | ||||||||
| 2021 | 2020 | |||||||
Options to purchase common stock | 23,797,993 | — | ||||||
HSOP options to purchase common stock | 7,281,376 | — | ||||||
2020 Convertible Promissory Notes (Note 11) | — | 3,679,485 | ||||||
2018 Convertible Promissory Notes - Related Parties (Note 11) | 16,000,000 | 2,560,000 | ||||||
|
|
|
| |||||
| 47,079,369 | 6,239,485 | |||||||
|
|
|
| |||||
The 2018 Convertible Promissory Notes – related party that would be issuable upon the exercise of conversion rights of convertible note holders for 1,000,000 and 160,000 shares of common stock of ATAI Life Sciences AG, respectively. The 2018 Convertible Promissory Notes – related party remained outstanding following completion of the share exchange and ATAI Life Sciences AG became the wholly owned subsidiary of ATAI Life Sciences N.V after the Corporate Reorganization described in Note 1 through June 30, 2021. Upon conversion, it is expected the shares would be exchanged on a one-for-sixteen basis for shares of ATAI Life Sciences N.V. which is reflected in the table above.
The 2020 Convertible Notes converted into 8,773,056 of shares of the Company’s common stock in November 2020 in connection with a qualified financing transaction, and therefore these shares were not included as of June 30, 2021 in the table above.
46
15. Commitments and Contingencies
Research and Development Agreements
The Company may also enter into contracts in the normal course of business with clinical research organizations for clinical trials, with contract manufacturing organizations for clinical supplies and with other vendors for preclinical studies, supplies and other services and products for operating purposes.
Indemnification
In the ordinary course of business, the Company may provide indemnifications of varying scope and terms to vendors, lessors, business partners, board members, officers and other parties with respect to certain matters, including, but not limited to, losses arising out of breach of such agreements, services to be provided by the Company, negligence or willful misconduct of the Company, violations of law by the Company, or intellectual property infringement claims made by third parties. In addition, the Company has entered into indemnification agreements with directors and certain officers and employees that will require the Company, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service as directors, officers or employees. No demands have been made upon the Company to provide indemnification under such agreements, and thus, there are no claims that the Company is aware of that could have a material effect on the Company’s consolidated financial statements.
The Company also maintains director and officer insurance, which may cover certain liabilities arising from its obligation to indemnify the Company’s directors. To date, the Company has not incurred any material costs and has not accrued any liabilities in the consolidated financial statements as a result of these provisions.
Contingencies
From time to time, the Company may become involved in legal proceedings arising in the ordinary course of business. The Company is unable to predict the outcome of these matters or the ultimate legal and financial liability, and at this time cannot reasonably estimate the possible loss or range of loss and accordingly has not accrued a related liability. At each reporting date, the Company evaluates whether or not a potential loss amount or a potential range of loss is probable and reasonably estimable under the provisions of the authoritative guidance that addresses accounting for contingencies. The Company accrues a liability when a loss is considered probable and the amount can be reasonably estimated. When a material loss contingency is reasonably possible but not probable, the Company does not record a liability, but instead discloses the nature and the amount of the claim, and an estimate of the loss or range of loss, if such an estimate can be made. Legal fees are expensed as incurred. The Company currently believes that the outcome of these legal proceedings, either individually or in the aggregate, will not have a material effect on its consolidated financial position, results of operations or cash flows.
16. License Agreements
Otsuka License and Collaboration Agreement
On March 11, 2021, the Company entered into a license and collaboration agreement (the “Otsuka Agreement”) with Otsuka Pharmaceutical Co., LTD (“Otsuka”) under which the Company granted exclusive rights to Otsuka to develop and commercialize products containing arketamine, known as PCN-101, in Japan for the treatment of any depression, including treatment-resistant depression, or major depressive disorder or any of their related symptoms or conditions. Under the terms of the Otsuka Agreement, Otsuka received an exclusive right to develop and commercialize products containing PCN-101 in Japan at its own cost and expense. The Company retained all rights to PCN-101 outside of Japan.
47
Otsuka owed the Company an upfront, non-refundable payment of $20.0 million as of the execution of the Otsuka Agreement. The Company is also entitled to receive aggregate payments of up to $35.0 million if certain development and regulatory milestones are achieved for the current or a new intravenous formulation of a product and up to $66.0 million in commercial milestones upon the achievement of certain commercial sales thresholds. Otsuka is obligated to pay the Company a tiered, double-digit royalties on net sales of products containing PCN-101 in Japan, subject to reduction in certain circumstances.
The Otsuka Agreement will expire upon the fulfillment of Otsuka’s royalty obligations on a product-by-product basis. Otsuka shall have the right to terminate this agreement in its entirety for convenience at any time (a) on ninety (90) days’ prior written notice to Perception if such notice is given before the first regulatory approval of the first licensed product in the Otsuka territory, or (b) on one hundred and eighty(180) days’ prior written notice to Perception if such notice is given on or after the first regulatory approval of the first licensed product in the Otsuka territory. The Otsuka Agreement may be terminated in its entirety at any time during the term upon written notice by either party if the other party is in material breach of its obligations and has not cured such breach within thirty (30) days in the case of a payment breach, or within ninety (90) days in the case of all other breaches.
The Company first assessed the Otsuka Agreement under ASC 808 to determine whether the Otsuka Agreement or units of accounts within the Otsuka Agreement represent a collaborative arrangement based on the risks and rewards and activities of the parties.
The Company concluded that Otsuka is a customer in the context of the Otsuka Agreement and the units of account are within the scope of ASC 606. The Company determined that the combined promise of the exclusive license to PCN-101 and non-exclusive license to conduct clinical trials in Asia are a single performance obligation. The Company determined that the option rights for CMC study data, additional research services and development supply do not represent material rights to Otsuka as these options were issued at standalone selling prices. As such, they are not performance obligations at the outset of the arrangement.
Based on this assessment, the Company concluded three performance obligations exists at the outset of the Otsuka Agreement: (i) the exclusive license to PCN-101 and exclusive license to conduct clinical trials in Japan, (ii) Global Requested Ongoing Clinical Studies and (iii) Global Ongoing Clinical Studies. The Company determined that the upfront payment of $20.0 million constitutes the transaction price at the outset of the Otsuka Agreement. Future potential milestone payments were fully constrained as the risk of significant revenue reversal related to these amounts has not yet been resolved. The achievement of the future potential milestones is not within the Company’s control and is subject to certain research and development success or regulatory approvals and therefore carry significant uncertainty. The Company will reevaluate the likelihood of achieving future milestones at the end of each reporting period. As all performance obligations have been satisfied if the risk of significant revenue reversal is resolved, any future milestone revenue from the arrangement will be added to the transaction price (and thereby recognized as revenue) in the period the risk is resolved.
For the three and six months ended June 30, 2021, there have been no milestones achieved under the Otsuka Agreement and the Company did not recognize any revenue associated with the Otsuka Agreement based on performance completed during that period. The Company satisfied the performance obligation related to the license upon delivery of the license and recognized the amount of $19.7 million allocated to the license as license revenue during the six months ended June 30, 2021. Additionally, the Company recognized revenues of $0.2 million related to certain research and development services during the six months ended June 30, 2021. As of June 30, 2021, the Company had current deferred revenue of $0.1 million due to certain research and development services under the Otsuka Agreement which will be recognized over time as the respective study results are delivered.
Accelerate License Agreement
On April 27, 2021, Psyber entered into a license arrangement with Accelerate Technologies Pte. Ltd. (“Accelerate”), whereby Accelerate grants Psyber non-exclusive rights to license and use the technology to commercialize of Psyber’s BCI-enabled companion digital therapeutics in United States of America, Singapore,
48
Member Countries of the European Union, Canada, Australia and New Zealand as a potential treatment for mental health and behavior change, such as substance use disorders including opioid use disorder, mood and anxiety disorders including post-traumatic stress disorder, and treatment-resistant depression. Psyber will pay Accelerate an upfront payment of $0.1 million, up to $0.3 million upon the achievement of certain clinical and sale milestones, and low to mid single digit royalty payments based on net sales.
Columbia Stock Purchase and License Agreement
In June 2020, Kures entered into a license agreement with Columbia, pursuant to which, Kures obtained an exclusive license under certain patents and technical information to discover, develop, manufacture, use and commercialize such patents or other products in all uses and applications (“Columbia IP”). In addition, in consideration for the rights to the Columbia IP, Kures entered into a Stock Purchase Agreement (the “SPA”) with Columbia in contemplation of the license agreement. Pursuant to the SPA, Kures issued to Columbia certain shares of the Kures’ capital stock, representing 5% of Kures common stock on a fully diluted basis, in accordance with the terms and conditions of the SPA. Kures can, from time to time, issue to Columbia additional shares of Kures’ common stock, at a per share price equal to the then fair market value of each such share. The antidilution protection provision shall be maintained up to and through the achievement of certain milestone events. At the acquisition date, the Company recorded the fair value of the shares of Kures common stock issued to Columbia of $0.1 million to Company’s additional-paid-in-capital and a debit to research and development expense for the corresponding acquired in-process research and development as it had no alternative future use at the time of the acquisition. In addition, Kures is obligated to pay tiered royalties ranging in the low to mid-single-digit percentage based on net sales of products licensed under the agreement. If Kures receives revenue from sublicensing any of its rights under the agreement, Kures is also obligated to pay a portion of that revenue to Columbia. Starting from the fourth anniversary of the effective date of the Kures License Agreement, Kures is obligated to pay Columbia annual license fees ranging from $10,000 to $0.1 million, creditable against royalties. Kures is also obligated to make milestone payments aggregating up to $15.5 million upon the achievement of certain clinical or regulatory and sales-based milestones for the first indication for each of the licensed product and up to $7.3 million for each subsequent indication for each of such products. In addition, Kures is obligated to pay Columbia a portion of the non-royalty sublicense payments it receives from a third party receiving a sublicense to practice the rights licensed to Kures under the license agreement, ranging from a low teen to low double-digit percentage. Kures has the right to terminate the Columbia agreement for any reason upon a 90-day notice and if Columbia materially breaches the agreement and fails to remedy any such default. Columbia has the right to terminate the Columbia agreement if Kures declares bankruptcy, becomes insolvent or otherwise materially breaches the agreement and fails to remedy any such default within specified cure periods. Such termination does not preclude Columbia’s rights to any milestone payments, royalties, and other payments described above. The Columbia agreement will remain in effect until terminated by the parties according to their rights. During the three and six months ended June 30, 2020, the Company made no material payments in connection with the Columbia agreement.
17. Related Party Transactions
ATAI Formation
In connection with the formation of ATAI in 2018, the Company entered into a series of transactions with its shareholders, Apeiron, Galaxy Group Investments LLC. (“Galaxy”) and HCS Beteiligungsgesellschaft mbH (“HCS”) whereby these shareholders contributed their investments in COMPASS, Innoplexus and Juvenescence to the Company in exchange for ATAI’s common stock of equivalent value. Apeiron is the family office of the Company’s founder who owns 21.0% and 21.7% of the outstanding common stock in the Company as of June 30, 2021 and December 31, 2020, respectively. Galaxy is a NYC-based multi-strategy investment firm that owns 7% and 8% of the outstanding common stock in the Company as of June 30, 2021 and December 31, 2020, respectively. HCS is a German venture capital firm that owns 4% and 6% of the outstanding common stock in the Company as of June 30, 2021 and December 31, 2020, respectively.
Convertible Note Agreements with Perception
In March 2020, Perception entered into the Perception Note Purchase Agreement with the Company and other investors, including related parties, which provided for the issuance of convertible notes of up to $3.9 million, among which Perception issued convertible notes in the aggregate principal amount of $3.3 million to the Company and $0.3 million to Sonia Weiss Pick and Family, and $0.3 million to other investors. In addition, in December 2020, Perception entered into the Perception December 2020 Convertible Note Agreement with the Company and other investors, including related parties, which provided for the issuance of convertible notes of up to $12.0 million in two tranches. Under the First Tranche Funding of $7.0 million, Perception issued an aggregate principal amount of $5.8 million to the Company and $0.4 million to other investors as of December 31, 2020 and $0.2 million to Apeiron, $0.5 million to Sonia Weiss Pick and Family, and $0.1 million to other investors in January 2021. Under the Second Tranche Funding of $5.0 million, Perception issued an aggregate of $4.2 million to the Company, $0.2 million to Apeiron, $0.3 million to Sonia Weiss Pick and Family, and $0.4 million to other investors.
On June 10, 2021, the Company received $20.0 million pursuant to the licensing and collaboration arrangement with Otsuka. Upon receipt of the proceeds, the Perception Convertible Notes automatically converted into Series A preferred stock pursuant to their original terms. Sonia Weiss Pick and Family and Aperion received 440,415 shares and 27,809 shares of Perception Series A preferred stock, respectively, upon conversion of the Perception Convertible Notes. The conversion of the Perception December 2020 Notes was accounted for an extinguishment. The March 2020 Notes were accounted for as a conversion. These transactions are further described in Note 10.
Common Stock
In January 2021, pursuant to an additional closing from the common stock issuance in November and December 2020, the Company issued and sold 2,133,328 shares of common stock to Apeiron at the same issuance price, for cash proceeds of $12.2 million. In March 2021, in connection with the Company’s issuance of 13,419,360 shares of common stock, at a price of €9.69 or $11.71 per share, the Company issued common shares to Apeiron for a total purchase price of $14.5 million, and issued common shares to Presight II, L.P. for a total purchase price of $13.9 million (See Note 11). Apeiron is the co-managing member of the general partner of Presight II, L.P.
Directed Share Program
In connection with ATAI’s initial public offering, the underwriters reserved 27% of the common shares for sale at the initial offering price to the Company’s managing directors, supervisory directors and certain other parties. Apeiron participated in the program and purchased $10.5 million of common stock.
49
Consulting Agreement with Mr. Angermayer
In January 2021, the Company entered into a consulting agreement, (the “Consulting Agreement”), with Mr. Angermayer, one of the Company’s co-founders and supervisory director. Apeiron is the family office and merchant banking business of Mr. Angermayer. Pursuant to the Consulting Agreement, Mr. Angermayer agreed to render services to the Company on business and financing strategies in exchange for 624,000 shares under the 2020 Incentive Plan upon achievement of certain performance targets. The Consulting Agreement expires on March 31, 2024. As a result of this agreement, for the three and six months ended June 30, 2021, the Company recorded $0.3 million of stock-based compensation included in general and administrative expense in its condensed consolidated statement of operations.
Related Party Receivable
In February 2021, the Company advanced $0.8 million to a member of the management team to cover the personal payroll and income taxes on their taxable income from the exercise of stock options. This receivable was repaid in May 2021.
18. Defined Contribution Plan
The Company has a defined contribution retirement savings plan under Section 401(k) of the Internal Revenue Code. This plan allows eligible employees to defer a portion of their annual compensation The Company made an immaterial amount of 401(k) contributions for the three months and six months ended June 30, 2021 and 2020, respectively.
19. Subsequent Events
Purchase of Psyber Preferred Stock
In July 2021, pursuant to the Psyber Purchase Agreement, the Company purchased additional Series A preferred shares for an aggregate cost of approximately $0.7 million based on the achievement of certain development milestones.
Termination of Attersee Credit Line
In July 2021, the Company terminated its credit line with Attersee, which remained unused as of the date of cancellation.
50
| Item 2. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
You should read the following discussion and analysis of our financial condition and results of operations together with our condensed financial statements and related notes included in this Quarterly Report and our audited consolidated financial statements and related notes thereto for the year ended December 31, 2020, included in our prospectus dated June 17, 2021 (the “Prospectus”), as filed with the Securities and Exchange Commission (the “SEC”), pursuant to Rule 424(b)(4) under the Securities Act of 1933, as amended, (the “Securities Act”), relating to our Registration Statements on Form S-1 (File No. 333-255383).
This discussion contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. We intend such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). In some cases, you can identify these statements by forward-looking words such as “may,” “will,” “expect,” “believe,” “anticipate,” “intend,” “could,” “should,” “estimate,” or “continue,” and similar expressions or variations. Such forward-looking statements are subject to risks, uncertainties and other factors that could cause actual results and the timing of certain events to differ materially from future results expressed or implied by such forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those identified below, and those discussed in the section titled “Risk Factors” included in the Prospectus. The forward-looking statements in this Quarterly Report represent our views as of the date of this Quarterly Report. Except as may be required by law, we assume no obligation to update these forward-looking statements or the reasons that results could differ from these forward-looking statements. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this Quarterly Report. Additionally, our historical results are not necessarily indicative of the results that may be expected for any period in the future. All references to years, unless otherwise noted, refer to our fiscal years, which end on December 31. Unless the context otherwise requires, all references in this subsection to “we,” “us,” “our,” “ATAI” or the “Company” refer to ATAI and its consolidated subsidiaries.
Business Overview
We are a clinical-stage biopharmaceutical company aiming to transform the treatment of mental health disorders. We founded atai Life Sciences in 2018 as a response to the significant unmet need and lack of innovation in the mental health treatment landscape, as well as the emergence of therapies that previously may have been overlooked or underused, including psychedelic compounds and digital therapeutics. We have built a pipeline of 11 development programs and six enabling technologies, each led by focused teams with deep expertise in their respective fields and supported by our internal development and operational infrastructure. We believe that several of our therapeutic programs’ target indications have potential market opportunities of at least $1 billion in annual sales, if approved. One of our ATAI companies, Recognify Life Sciences, has initiated a Phase 2a trial in the United States. In addition, we plan to initiate Perception’s Phase 2 trial for TRD and DemeRx’s Phase 1/2 OUD trial in Q3 2021. Additionally, we plan to initiate three Phase 2 trials and also expect to initiate four Phase 1 trials in 2022.
Our Emerging Clinical and Preclinical Programs
The table below summarizes the status of our product candidate portfolio as of the filing date of this Quarterly Report. Our pipeline currently consists of therapeutic candidates across multiple neuropsychiatric indications including depression, cognitive impairment associated with schizophrenia, or CIAS, SUD, anxiety, mTBI and PTSD. We currently hold at least a majority interest, or have options to obtain a majority interest, in each of these atai companies.
51
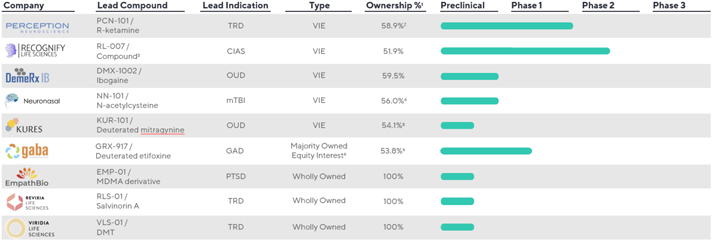
Majority Owned Companies
Note: TRD = Treatment-resistant depression; CIAS = Cognitive impairment associated with schizophrenia; OUD = Opioid use disorder;
GAD = Generalized anxiety disorder; mTBI = Mild traumatic brain injury; DMT = N,N-dimethyltryptamine;
MDMA = 3,4-Methylenedioxymethamphetamine; PTSD = Post-traumatic stress disorder, VIE = Variable interest entity.
| (1) | Unless otherwise indicated herein, ownership percentage based on ownership of securities with voting rights as of June 30, 2021. |
| (2) | Perception does not give effect to the shares of common stock issuable after giving full effect to the anti-dilution feature of the Stock Purchase Agreement, which would not impact our majority position in Perception. |
| (3) | RL-007 compound is (2R, 3S)-2-amino-3-hydroxy-3-pyridin-4-yl-1-pyrrolidin-1-yl-propan-1-one(L)-(+) tartrate salt. |
| (4) | Neuronasal ownership does not give effect to the obligation to acquire further shares upon the achievement of specified development milestones which may increase the ownership to up to 64.5%. |
| (5) | Kures ownership does not give effect to the obligation to acquire further shares upon the achievement of specified development milestones which may increase the ownership to up to 67.9%. |
| (6) | Operational involvement through MSA model, including Srinivas Rao serving as GABA CMO; GABA ownership does not give effect to the obligation to acquire further shares upon the achievement of specified development milestones which may increase the ownership to up to 54.2%. |
Perception Neuroscience: PCN-101 for TRD
| • | Product concept: PCN-101 is a parenteral formulation of R-ketamine, a glutamatergic modulator that is a component of ketamine and being developed as a rapid-acting antidepressant, with the potential to be an at-home non-dissociative alternative to S-ketamine (marketed as SPRAVATO). |
| • | Prior evidence in humans: In a third-party clinical trial, another formulation of R-ketamine was observed to produce a rapid and durable response with limited dissociative side effects in patients with TRD. In September 2020, Perception Neuroscience completed a Phase 1 trial of PCN-101 supporting the advancement of PCN-101 into a Phase 2 trial. |
| • | Upcoming milestones: We expect to initiate a Phase 2 randomized, double blind, placebo-controlled trial in patients with treatment-resistant depression in the third quarter and anticipate the trial to run through late 2022. The trial will assess the efficacy and safety, dose response and duration of action in patients with TRD. |
Recognify Life Sciences: RL-007 for CIAS
| • | Product concept: RL-007, a cholinergic, glutamatergic and GABA-B receptor modulator, is an orally available compound that is thought to alter the excitatory/inhibitory balance in the brain to produce pro-cognitive effects. We are developing this compound for the treatment of cognitive impairments associated with schizophrenia. |
| • | Prior evidence in humans: In third-party studies, other formulations of this compound have been shown to effect a significant improvement in aspects of cognitive function in both experimental paradigms involving healthy subjects as well as in a Phase 2 trial in patients suffering from diabetic peripheral neuropathic pain. |
| • | Recent advancements: In April 2021, Recognify initiated a Phase 2a study for RL-007, after receiving IND clearance from the U.S. Food and Drug Administration to commence clinical trials for the treatment of Cognitive Impairment Associated with Schizophrenia (CIAS). The study is designed to evaluate the effects of RL-007 on safety, tolerability, electroencephalogram-based biomarkers and cognition. |
| • | Upcoming milestones: We expect topline results from the Phase 2a single-arm, multiple dose trial in patients with CIAS in late 2021. |
DemeRx IB: DMX-1002 for OUD
| • | Product concept: DMX-1002 is an oral formulation of ibogaine, a cholinergic, glutamatergic and monoaminergic receptor modulator that is a naturally occurring psychedelic product isolated from a West African shrub, that we are developing for the treatment of OUD. |
| • | Prior evidence in humans: In third-party studies evaluating other formulations of ibogaine, significant reductions in opioid cravings were observed, both at discharge and at one month post treatment, and were associated with improved mood in patients with OUD. |
| • | Upcoming milestones: We expect to initiate the Phase 1 component of Phase 1/2 trial of DMX-1002 in recreational drug users and healthy volunteers to be initiated in Q3 and to read out safety data in early 2022. The trial is designed to assess safety, tolerability, pharmacokinetics, and efficacy, and the results will inform future studies in patients with opioid use disorder. |
GABA: GRX-917 for GAD
| • | Product concept: GRX-917 is an oral formulation of a deuterated version of etifoxine, a compound that has a long history of prescription use in France for treating anxiety disorders. GRX-917 is designed to provide rapid anxiolytic activity with improved tolerability to current treatments for anxiety in the United States. |
| • | Prior evidence in humans: Etifoxine has been observed to have the rapid onset of anxiolytic activity of benzodiazepines without their sedating or addicting properties. Furthermore, etifoxine is not associated with abuse, dependence or respiratory depression and has been observed to have no significant impact on motor skills or cognition. |
| • | Recent advancements: In June 2021, GABA initiated a randomized, double blind, placebo-controlled Phase 1 trial. The study will evaluate safety, tolerability, pharmacokinetics, as well as pharmacodynamics using qEEG. |
| • | Upcoming milestones: We expect topline results from the Phase 1 single ascending dose/multiple ascending dose program in early 2022. |
Neuronasal: NN-101 for mTBI
| • | Product concept: NN-101 is a novel intranasal formulation of NAC. NAC is believed to stimulate the synthesis of GSH, an endogenous antioxidant that plays a protective role in the pathogenesis of mTBI. |
| • | Prior evidence in humans: An orally administered formulation of NAC was shown to increase the probability of mTBI symptom resolution at seven days in a third-party study conducted by the U.S. Army. Neuronasal has also completed a pilot study of NN-101 in nine healthy volunteers. In this pilot study, NN-101 was observed to be approximately 20 times and 100 times more brain-penetrant compared to IV and oral NAC, respectively, and was well tolerated. |
Viridia Life Sciences: VLS-01 for TRD
| • | Product concept: VLS-01 is a formulation of DMT, the active moiety of the traditional, hallucinogenic drink ayahuasca. DMT is characterized by an intrinsically short duration of psychedelic effect with a serum half-life estimated at less than 10 minutes. VLS-01 is formulated to provide a psychedelic experience lasting 30 to 45 minutes, thus potentially allowing for a shorter clinic visit compared to many other psychedelic compounds that may require a patient to be monitored for four or more hours. |
| • | Prior evidence in humans: Ayahuasca has shown significant antidepressant effects compared with placebo at one, two and seven days after dosing in a double-blind, randomized, placebo-controlled third-party clinical trial in patients with TRD. |
EmpathBio: EMP-01 for PTSD
| • | Product concept: EMP-01 is an oral formulation of an MDMA derivative being developed for the treatment of PTSD. We are developing EMP-01 for the potential to have an improved therapeutic index compared to MDMA. |
| • | Prior evidence in humans: In a meta-analysis of 21 third-party trials of other formulations of MDMA-combined with psychotherapy for the treatment of PTSD, the benefits of such treatment were statistically significant versus placebo or active placebo-assisted therapy alone. In addition, a recent third-party randomized, double-blind, placebo-controlled phase 3 study with 90 patients with severe PTSD, showed statistically significant reduction in PTSD symptoms in the MDMA-assisted psychotherapy group versus placebo. |
Revixia Life Sciences: RLS-01 for TRD
| • | Product concept: RLS-01 is a formulation of SalA, a naturally occurring psychedelic compound with pharmacology differentiated from that of psilocybin or DMT, being developed for the treatment of TRD and other indications. |
| • | Prior evidence in humans: In a third-party study of another formulation of SalA, the effects of the compound were observed to be similar to those of psilocybin based upon functional brain imaging. We believe these data combined with anecdotal usage reports suggest that SalA may possess rapid-acting antidepressant properties. |
Kures: KUR-101 for OUD
| • | Product concept: KUR-101 is an oral formulation of deuterated mitragynine being developed for the treatment of OUD. Mitragynine is a component of the leaves of kratom (Mitragnyna speciosa). |
| • | Prior evidence in humans: Kratom has a long history of traditional medicine use as an analgesic in parts of Southeast Asia, and its use in the United States has increased in recent years, particularly amongst individuals seeking to reduce prescription opioid consumption or manage opioid withdrawal symptoms. Published third-party human data involving isolated mitragynine are limited, but recent mechanistic insights suggest that this compound may be well-suited for the medically assisted therapy of OUD. |
DemeRx NB: DMX-1001 for OUD
| • | Product concept: DMX-1001 is an oral formulation of noribogaine being developed for the treatment of OUD. Noribogaine is an active metabolite of ibogaine designed to have a longer plasma half-life and potentially reduced hallucinogenic effects compared with ibogaine. |
| • | Prior evidence in humans: Three third-party clinical trials have been conducted, testing various doses of another formulation of noribogaine in both healthy subjects and opioid dependent subjects undergoing detoxification. We believe the results from these trials support further development. |
Our Ownership Position in COMPASS
In addition to our emerging clinical and preclinical programs and enabling technologies, we led the Series A financing round in 2018 for COMPASS, co-led their Series B financing round in 2020 and continue to hold a significant equity ownership position in COMPASS. COMPASS is developing its investigational COMP360 psilocybin therapy, which comprises administration of COMP360 with psychological support from specially trained therapists, with an initial focus on TRD. The therapeutic potential of psilocybin administered in conjunction with psychological support has been shown in multiple academic-sponsored studies, which did not involve COMP360, specifically exhibiting rapid reductions in depression symptoms after a single high dose with no SAEs. COMPASS evaluated COMP360 in conjunction with psychological support in a Phase 2b trial that concluded in June 2021 and expects to report data from this trial in late 2021. The randomized, double-blind, dose-ranging study investigated the safety and efficacy of psilocybin therapy in 233 patients, the largest clinical trial with psilocybin to date. As of June 30, 2021, we beneficially owned 8,075,663 shares representing 19.4 % equity interest in COMPASS. Certain of our founding investors were also seed investors and founders of COMPASS. Our interest in the product candidates of COMPASS is limited to the potential appreciation of our equity interest.
Recent Developments
Purchase of GABA Shares
In April 2021, pursuant to the GABA Preferred Stock Purchase Agreement, we purchased additional shares of Series A preferred stock of GABA for an aggregate cost of $5.0 million based on the achievement of certain development milestones. In May 2021, we exercised our option to purchase additional shares of Series A preferred stock prior to the achievement of certain development milestone for an aggregate cost of $5.0 million. The purchase of additional shares of Series A preferred stock resulted in us holding an 53.8% equity interest in the outstanding common stock and Series A preferred stock of GABA.
Purchase of COMPASS Ordinary Shares
In May 2021, we purchased additional ordinary shares of COMPASS (represented by American Depositary Shares) common stock for an aggregate cost of $5.0 million. Following the close of the additional purchase, we held a 19.4% equity interest in COMPASS ordinary shares.
Purchase of IntelGenx Shares
In May 2021, we entered into the IntelGenx Share Purchase Agreement, (“SPA”), whereby IntelGenx issued shares of its common stock and warrants to us at an aggregate price of approximately $12.3 million. Pursuant to the IntelGenx SPA, we have the right to purchase additional shares of common stock at a price determined in the IntelGenx SPA. Following the initial close of the transaction, we held a 25% voting interest in IntelGenx.
52
Consolidation of Neuronasal
In May 2021, pursuant to the Neuronasal Preferred Share Purchase Agreement, we exercised our option to purchase additional shares of Series A preferred stock of Neuronasal for an aggregate cost of $1.0 million. The purchase of additional shares of Series A preferred stock resulted in us holding an 56.0% equity interest in the outstanding common stock and Series A preferred stock of Neuronasal as of the date of purchase. Following the closing of this share purchase, the results of Neuronasal have been consolidated in our condensed consolidated financial statements.
Financial Overview
Since our inception in 2018, we have focused substantially all of our efforts and financial resources on acquiring and developing product and technology rights, establishing our platform, building our intellectual property portfolio and conducting research and development activities for our product candidates within our ATAI companies that we consolidate based on our controlling financial interest of such entities. We operate a decentralized model to enable scalable drug or technological development at our ATAI companies. Our ATAI companies drive development of our programs and enabling technologies that we have either acquired a controlling or significant interest in or created de novo. We believe that this model provides our development teams the support and incentives to rapidly advance their therapeutic candidates or technologies in a cost-efficient manner. We look to optimize deployment of our capital in order to maximize value for our stakeholders.
Wholly owned subsidiaries and variable interest entities with greater than 50% ownership and deemed control are consolidated in our financial statements, and our net income (loss) is reduced for the non-controlling interest of the VIE’s share, resulting in net income(loss) attributable to ATAI stockholders.
Investments, where we have ownership in the underlying company’s equity greater than 20% and less than 50%, or where we have significant influence, are recorded under the equity method. We then record income(loss) in equity method investments for our proportionate share of the underlying company’s net results until the investment balance is adjusted to zero. If we make subsequent additional investments in that same company, we may record additional gains(losses) based on changes to our investment basis and also may record additional income(loss) in equity method investments.
We do not have any products approved for sale and have not generated any revenue from product sales. We have funded our operations to date primarily with proceeds from the sale of our common stock and from issuances of convertible notes.
We were incorporated pursuant to the laws of the Netherlands on September 10, 2020. As more fully described in the Prospectus in the section titled “Corporate Reorganization,” and in the Notes to Condensed Consolidated Financial Statements appearing elsewhere in this Quarterly Report, we undertook a corporate reorganization, or the Corporate Reorganization on April 23, 2021. In April 2021, all of the outstanding shares in ATAI Life Sciences AG were contributed and transferred to ATAI Life Sciences N.V. in a capital increase in exchange for newly issued common shares of ATAI Life Sciences N.V. on a 1 to 10 basis, and, as a result, ATAI Life Sciences AG became a wholly owned subsidiary of ATAI Life Sciences N.V. Furthermore, on June 7, 2021, shares of ATAI Life Sciences N.V. were split applying a ratio of 1.6 to one. The Corporate Reorganization is considered a continuation of ATAI Life Sciences AG resulting in no change in the carrying values of assets or liabilities. As a result, the financial statements for periods prior to the Corporate Reorganization are the financial statements of ATAI Life Sciences AG as the predecessor to ATAI Life Sciences N.V. for accounting and reporting purposes. All share, per-share and financial information presented and corresponding disclosures have been retrospectively adjusted, where applicable, to reflect the impact of the share exchange and share split resulting from the Corporate Reorganization. In connection with the Corporate Reorganization, outstanding share awards and option grants of ATAI Life Sciences AG were exchanged for share awards and option grants of ATAI Life Sciences N.V. with identical restrictions.
53
On June 22, 2021, we completed an IPO on Nasdaq, in which we issued and sold 17,250,000 shares of our common stock at a public offering price of $15.00 per share, including 2,500,000 shares of common stock sold pursuant to the underwriters’ exercise of their option to purchase additional shares of common stock, for aggregate net proceeds of $231.6 million, after deducting underwriting discounts and commissions of $18.1 million and offering costs of $9.0 million. Prior to the IPO, we received gross cash proceeds of $361.5 million from sales of our common stock and convertible notes.
We have incurred significant operating losses since our inception. Our net loss attributable to ATAI Life Sciences N.V. stockholders was $48.5 million and $16.4 million for the three months ended June 30, 2021 and 2020, respectively, and $47.8 million and $0.05 million for the six months ended June 30, 2021 and 2020, respectively. As of June 30, 2021 and December 31, 2020, our accumulated deficit was $237.8 million and $190.0 million, respectively. Our ability to generate product revenue sufficient to achieve profitability will depend substantially on the successful development and eventual commercialization of product candidates at our ATAI companies and at our ATAI companies that we consolidate based on our controlling financial interest of such entities as determined under the variable interest entity model, or VIEs. We expect to continue to incur significant expenses and increasing operating losses for at least the next several years.
Our historical losses resulted principally from costs incurred in connection with research and development activities and general and administrative costs associated with our operations. In the future, we intend to continue to conduct research and development, preclinical testing, clinical trials, regulatory compliance, market access, commercialization and business development activities that, together with anticipated general and administrative expenses, will result in incurring further significant losses for at least the next several years. Our operating losses stem primarily from development of our mental health research programs. Furthermore, we expect to incur additional costs associated with operating as a public company, including audit, legal, regulatory, and tax-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance premiums, and investor relations costs. As a result, we will need substantial additional funding to support our continuing operations and pursue our growth strategy. Until such time as we can generate significant revenue from sales of our product candidates, if ever, we expect to finance our operations through a combination of equity offerings, debt financings, strategic collaborations and alliances or licensing arrangements. Our inability to raise capital as and when needed could have a negative impact on our financial condition and ability to pursue our business strategies. There can be no assurances, however, that our current operating plan will be achieved or that additional funding will be available on terms acceptable to us, or at all.
As of June 30, 2021, we had cash and cash equivalents of $453.6 million We believe that our existing cash will be sufficient for us to fund our operating expenses and capital expenditure requirements for at least the next 12 months. We have based this estimate on assumptions that may prove to be wrong, and we could exhaust our available capital resources sooner than we expect. See “Liquidity and Capital Resources—Liquidity Risk” below.
Factors Affecting our Results
We believe that the most significant factors affecting our results of operations include:
Acquisitions/Investments
To continue to grow our business and to aid in the development of our various product candidates, we are continually acquiring and investing in companies that share our common goal towards advancing transformative treatments, including psychedelic compounds and digital therapeutics, for patients that suffer from mental health disorders. During the three months ended June 30, 2021, we spent $28.8 million on investments in GABA, COMPASS, IntelGenx and Neuronasal.
54
Research and Development Expenses
Our ability to successfully develop innovative product candidates through our programs will be the primary factor affecting our future growth. Our approach to the discovery and development of our product candidates is still being demonstrated. As such, we do not know whether we will be able to successfully develop any products. Developing novel product candidates requires a significant investment of resources over a prolonged period of time, and a core part of our strategy is to continue making sustained investments in this area. We have chosen to leverage our platform to initially focus on advancing our product candidates in the area of mental health.
All of our product candidates are still in development stages, and we have incurred and will continue to incur significant research and development costs for their preclinical studies and clinical trials. We expect that our research and development expenses will constitute the most substantial part of our expenses in future periods in line with the advancement and expansion of the development of our product candidates.
Acquisition of In-Process Research and Development Expenses
In an asset acquisition, including the initial consolidation of a VIE that is not a business, acquired in-process research and development, or IPR&D, with no alternative future is charged to the condensed consolidated statements of operations as a component of operating expenses at the acquisition date.
Since inception, we have grown primarily by continually acquiring and investing in other companies. Our IPR&D expenses were $8.0 million and $9.0 million, representing 13.0% and 11.6% of our total operating expenses for the three and six months ended June 30, 2021, respectively. Our IPR&D expenses for the three and six months ended June 30, 2020 were $0.1 million. As we continue to acquire and invest in companies, we expect our IPR&D expenses to increase in absolute amounts and continue to represent a significant percentage of our total operating expenses.
Stock-Based Compensation
In August 2020, we adopted the 2020 Equity Incentive Plan and the Hurdle Share Option Plan, which allowed us to grant stock-based awards to executive officers, directors, employees and consultants. Prior to our IPO, we issued stock options that vest over a two to four-year service period, only if and when a “Liquidity Event” (as defined in the plans) occurs, with accelerated vesting if a Liquidity Event occurred by specified dates. Upon the closing of our IPO, the stock-based award vesting contingent upon a Liquidity Event was no longer deferred. For the three and six months ended June 30, 2021, stock-based compensation of $37.5 million and $37.7 million, respectively.
Impact of COVID-19
In December 2019, a novel strain of coronavirus, severe acute respiratory syndrome coronavirus 2, or SARS-CoV-2, was identified in Wuhan, China. On March 11, 2020, the World Health Organization designated the outbreak of COVID-19, the disease associated with SARS-CoV-2, as a global pandemic. Governments and businesses around the world have taken unprecedented actions to mitigate the spread of COVID-19, including, but not limited to, shelter- in-place orders, quarantines, significant restrictions on travel, as well as restrictions that prohibit many employees from going to work.
We have been actively monitoring the impact of the COVID-19 pandemic, including variants, on our employees and our business. Although some of our research and development timelines have been impacted by delays related to the COVID-19 pandemic, we have not experienced material financial impacts on our business and operations as a result of the COVID-19 pandemic. We have undertaken a number of business continuity measures to mitigate potential disruption to our operations and in order to preserve the integrity of our research and development programs. However, the impact on our future results will largely depend on future developments related to COVID-19, which are highly uncertain and cannot be predicted with confidence, such as the emergence of new variants, the rate and success of vaccination roll-out efforts, the ultimate duration and spread of the outbreak, the continuing impact of the COVID-19 pandemic on financial markets and the global economy, travel restrictions, social distancing and other mitigation measures in the United States and other countries, business closures or business disruptions and the effectiveness of actions taken in the United States and other countries to contain, treat, and prevent the disease, including the availability and effectiveness of vaccines.
55
Basis of Presentation and Consolidation
Since our inception, we have created wholly-owned subsidiaries or made investments in certain controlled entities, including partially-owned subsidiaries for which we have majority voting interest under the VOE model or for which we are the primary beneficiary under the VIE model, which we refer to collectively as our consolidated entities. Ownership interests in entities over which we have significant influence, but not a controlling financial interest, are accounted for as cost and equity method investments. Ownership interests in consolidated entities that are held by entities other than us are reported as redeemable convertible noncontrolling interests and noncontrolling interests in our condensed consolidated balance sheets. Losses attributed to redeemable convertible noncontrolling interests and noncontrolling interests are reported separately in our condensed consolidated statements of operations.
Components of Our Results of Operations
Revenue
On March 11, 2021, we entered into a license and collaboration agreement, or the Otsuka Agreement, with Otsuka Pharmaceutical Co., LTD, or Otsuka, under which we granted exclusive rights to Otsuka to develop and commercialize certain products containing arketamine in Japan for the treatment of depression and other select indications. We received an upfront, non-refundable payment of $20.0 million in June 2021 and we are also eligible to receive up to $35.0 million if certain development and regulatory milestones are achieved and up to $66.0 million in commercial milestones upon the achievement of certain commercial sales thresholds. We are eligible to receive a tiered, double-digit royalties on net sales of licensed products subject to reduction in certain circumstances.
In March 2021, we satisfied the performance obligation related to the license upon delivery of the license and recognized the amount of $19.7 million allocated to the license as license revenue. Additionally, we recognized revenues of $0.2 million related to certain research and development services. As of June 30, 2021, we had current deferred revenue of $0.1 million due to certain research and development services under the Otsuka Agreement which will be recognized over time as the respective study results are delivered. To date, there have been no milestones achieved under the Otsuka Agreement. License revenue of $0 and $19.9 million was recorded for the three and six months ended June 30, 2021, respectively.
For the foreseeable future, we may generate revenue from reimbursements of services under the Otsuka Agreement, as well as milestone payments under our current and/or future collaboration agreements. We do not expect to generate any revenue from the sale of products unless and until such time that our product candidates have advanced through clinical development and regulatory approval, if ever. We expect that any revenue we generate, if at all, will fluctuate from quarter-to-quarter as a result of the timing and amount of payments relating to such services and milestones and the extent to which any of our products are approved and successfully commercialized. If we fail to complete preclinical and clinical development of product candidates or obtain regulatory approval for them, our ability to generate future revenues and our results of operations and financial position would be adversely affected.
Operating Expenses
Research and Development Expenses
Research and development expenses consist primarily of costs incurred for our research activities, including our discovery efforts and the development of our product candidates, which include:
| • | employee-related expenses, including salaries, related benefits and stock-based compensation, for employees engaged in research and development functions; |
| • | expenses incurred in connection with the preclinical and clinical development of our product candidates, including our agreements with third parties, such as consultants and CROs; |
| • | expenses incurred under agreements with consultants who supplement our internal capabilities; |
| • | the cost of lab supplies and acquiring, developing and manufacturing preclinical study materials and clinical trial materials; |
| • | costs related to compliance with regulatory requirements; |
56
| • | facilities, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities, insurance and other operating costs; and |
| • | payments made in connection with third-party licensing agreements. |
Research and development costs, including costs reimbursed under our collaboration with Otsuka, are expensed as incurred, with reimbursements of such amounts being recognized as revenue. We account for nonrefundable advance payments for goods and services that will be used in future research and development activities as expenses when the service has been performed or when the goods have been received.
Our direct research and development expenses are tracked on a program-by-program basis for our product candidates and consist primarily of external costs, such as fees paid to outside consultants, CROs, CMOs and research laboratories in connection with our preclinical development, process development, manufacturing and clinical development activities. Our direct research and development expenses by program also include fees incurred under third-party license agreements.
We do not allocate internal research and development expenses consisting of employee and contractor-related costs, to specific product candidate programs because these costs are deployed across multiple product candidate programs under research and development and, as such, are separately classified.
Research and development activities are central to our business model. Product candidates in later stages of clinical development generally have higher development costs than those in earlier stages of clinical development, primarily due to the increased size and duration of later-stage clinical trials. We expect that our research and development expenses will continue to increase for the foreseeable future in connection with our planned preclinical and clinical development activities in the near term and in the future.
The successful development of our product candidates is highly uncertain. As such, at this time, we cannot reasonably estimate or know the nature, timing and estimated costs of the efforts that will be necessary to complete the remainder of the development of these product candidates. We are also unable to predict when, if ever, material net cash inflows will commence from our product candidates. This is due to the numerous risks and uncertainties associated with developing products, including the uncertainty of whether (i) any clinical trials will be conducted or progress as planned or completed on schedule, if at all, (ii) we obtain regulatory approval for our product candidates and (iii) we successfully commercialize product candidates.
Acquisition of In-Process Research and Development Expenses
Acquisition of in-process research and development expenses consist of acquired in-process research and development with no future alternative use based on the probability of clinical success. We expect our acquisition of IPR&D expenses to increase as we continue to grow and expand.
General and Administrative Expenses
General and administrative expenses consist primarily of salaries and other related costs, including stock-based compensation, for personnel in our executive, finance, corporate and business development and administrative functions, professional fees for legal, patent, accounting, auditing, tax and consulting services, travel expenses and facility-related expenses, which include allocated expenses for rent and maintenance of facilities, advertising, and information technology-related expenses.
We expect that our general and administrative expenses will increase in the future as we increase our general and administrative headcount to support our continued research and development and potential commercialization of our product candidates. We also expect to incur increased expenses associated with being a public company, including increased costs of accounting, audit, legal, regulatory and tax-related services associated with maintaining compliance with exchange listing and SEC requirements, director and officer insurance costs, and investor and public relations costs.
57
Other Income (Expense), Net
Interest Income
Interest income consists of interest earned on cash balances held in interest-bearing accounts and interest earned on notes receivable. We expect that our interest income will fluctuate based on the timing and ability to raise additional funds as well as the amount of expenditures for our research and development of our product candidates and ongoing business operations.
Change in Fair Value of Contingent Consideration Liability—Related Parties
Changes in fair value of contingent consideration liability—related parties, consists of subsequent remeasurement of our contingent consideration liability—related parties with Perception and InnarisBio for which we have elected the fair value option. See “—Liquidity and Capital Resources—Indebtedness” below for further discussion of our contingent consideration liability—related parties.
Change in Fair Value of Short Term Notes Receivable—Related Party
Changes in fair value of short term notes receivable—related party, including interest, consists of subsequent remeasurement of our short term notes receivable-related party with COMPASS for which we have elected the fair value option. The COMPASS notes were converted during 2020. See “—Liquidity and Capital Resources—Indebtedness” below for further discussion of our short term notes receivable – related party.
Change in Fair Value of Convertible Promissory Notes
Changes in fair value of convertible promissory notes consists of subsequent remeasurement of our convertible promissory notes for which we have elected the fair value option. See “—Liquidity and Capital Resources—Indebtedness” below for further discussion of our convertible promissory notes.
Change in Fair Value of Derivative Liability
Changes in fair value of derivative liability consists of subsequent remeasurement of our derivative liability relating to certain embedded features contained in the Perception convertible promissory notes for which we have elected the fair value option. The Perception convertible promissory notes were converted during June 2021. See “—Liquidity and Capital Resources—Indebtedness” below for further discussion the Perception convertible promissory notes.
Unrealized Loss on Other Investments Held at Fair Value
In May 2021, we received IntelGenx common stock, warrants and additional unit warrants for a price of approximately $12.3 million. We determined that the initial aggregate fair value is equal to the transaction price and recorded the common shares at $3.0 million, the warrants at $1.2 million and the additional unit warrants at $8.2 million on a relative fair value basis resulting in no initial gain or loss recognized in the condensed consolidated statements of operations. Subsequently, changes in fair value of the common shares, the warrants and additional unit warrants are recorded as a component of other income (expense), net in the condensed consolidated statement of operations.
Unrealized Gain on Other Investments
In March 2020, we entered into a series of transactions including the purchase of additional shares of COMPASS Series A and Series B preferred stock under the secondary Series A preferred stock purchase agreement and the Series B preferred stock subscription agreement, respectively. In April 2020, COMPASS entered into a Series B preferred stock subscription agreement with other investors for issuance of its Series B preferred stock, which resulted in the automatic conversion of our COMPASS convertible notes receivable into shares of COMPASS Series B preferred stock. We remeasured our investment in COMPASS’ Series A preferred shares to fair value due to the observable price change in connection with COMPASS’ secondary Series A preferred stock purchase in March 2020 and recognized unrealized gains on other investments in the condensed consolidated statements of operations in association with the transaction.
58
Other Income (Expense), net
Other income (expense), net consists principally of interest expense, foreign currency transactions gains and losses, impairment related to our other investments and credits related to our research and development tax credits which are claimed from the Australian tax authority, in respect to qualifying research and development costs incurred.
Income Tax
For our consolidated entities, deferred income taxes are provided for the effects of temporary differences between the amounts of assets and liabilities recognized for financial reporting purposes and the amounts recognized for income tax purposes. Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
We regularly assess the need to record a valuation allowance against net deferred tax assets if, based upon the available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. Accordingly we continue to maintain a full valuation allowance against our deferred tax assets as of June 30, 2021, consistent with prior periods, which primarily relate to our German and international tax loss carryforwards. In assessing the realizability of deferred tax assets, we consider whether it is more likely than not that some or all of the deferred tax assets will not be realized. The future realization of deferred tax assets is subject to the existence of sufficient taxable income of the appropriate character (e.g., ordinary income or capital gain) as provided under the carryforward provisions of local tax law. We consider the scheduled reversal of deferred tax liabilities (including the effect in available carryback and carryforward periods), future projected taxable income, including the character and jurisdiction of such income, and tax-planning strategies in making this assessment.
Unrecognized tax benefits arise when the estimated benefit recorded in the financial statements differs from the amounts taken or expected to be taken in a tax return because of the considerations described above. As of June 30, 2021 and December 31, 2020, we had no unrecognized tax benefits.
Losses from Investments in Equity Method Investees, Net of Tax
Losses from investments in equity method investees, net of tax consists of our share of equity method investees losses on the basis of our equity ownership percentage, IPR&D charges resulting from basis differences and impairment related to our equity method investments.
Net Loss Attributable to Redeemable Noncontrolling Interests and Noncontrolling Interests
Net loss attributable to redeemable noncontrolling interests and noncontrolling interests in our condensed consolidated statements of operations is a result of our investments in certain of our consolidated VIEs, and consists of the portion of the net loss of these consolidated entities that is not allocated to us. Net losses in consolidated VIEs are attributed to redeemable noncontrolling interests and noncontrolling interests considering the liquidation preferences of the different classes of equity held by the shareholders in the VIE and their respective interests in the net assets of the consolidated VIE in the event of liquidation, and their pro rata ownership. Changes in the amount of net loss attributable to redeemable noncontrolling interests and noncontrolling interests are directly impacted by changes in the net loss of our VIEs and our ownership percentage changes.
59
Results of Operations
Comparison of the Three Months Ended June 30, 2021 and 2020 (unaudited)
| Three months ended June 30, | ||||||||||||||||
| 2021 | 2020 | $ Change | % Change | |||||||||||||
| (in thousands, except percentages) | ||||||||||||||||
Operating expenses: | ||||||||||||||||
Research and development | $ | 16,026 | $ | 2,854 | $ | 13,172 | 461.5 | % | ||||||||
Acquisition of in-process research and development | 7,962 | 120 | 7,842 | 6535.0 | % | |||||||||||
General and administrative | 37,331 | 2,851 | 34,480 | 1209.4 | % | |||||||||||
|
|
|
|
|
| |||||||||||
Total operating expenses | 61,319 | 5,825 | 55,494 | 952.7 | % | |||||||||||
|
|
|
|
|
| |||||||||||
Loss from operations | (61,319 | ) | (5,825 | ) | (55,494 | ) | 952.7 | % | ||||||||
|
|
|
|
|
| |||||||||||
Other income (expense), net: | ||||||||||||||||
Interest income | 35 | 18 | 17 | 94.4 | % | |||||||||||
Change in fair value of contingent consideration liability - related parties | (911 | ) | (42 | ) | (869 | ) | 2069.0 | % | ||||||||
Change in fair value of convertible promissory notes | — | (1,260 | ) | 1,260 | (100.0 | %) | ||||||||||
Unrealized loss on other investments held at fair value | (5,460 | ) | — | (5,460 | ) | 100.0 | % | |||||||||
Loss on conversion of convertible promissory notes | (513 | ) | — | (513 | ) | 100.0 | % | |||||||||
Gain on consolidation of a variable interest entity | 3,543 | — | 3,543 | 100.0 | % | |||||||||||
Other income (expense), net | (2,676 | ) | (37 | ) | (2,639 | ) | 7132.4 | % | ||||||||
|
|
|
|
|
| |||||||||||
Total other income (expense), net | (5,982 | ) | (1,321 | ) | (4,661 | ) | 352.8 | % | ||||||||
|
|
|
|
|
| |||||||||||
Net loss before income taxes | (67,301 | ) | (7,146 | ) | (60,155 | ) | 841.8 | % | ||||||||
Provision for income taxes | (58 | ) | — | (58 | ) | 100.0 | % | |||||||||
Gain on dilution of equity method investment | 16,923 | — | 16,923 | 100.0 | % | |||||||||||
Losses from investments in equity method investees, net of tax | (2,937 | ) | (9,811 | ) | 6,874 | (70.1 | %) | |||||||||
|
|
|
|
|
| |||||||||||
Net loss | (53,373 | ) | (16,957 | ) | (36,416 | ) | 214.8 | % | ||||||||
Net loss attributable to redeemable noncontrolling interests and noncontrolling interests | (4,912 | ) | (600 | ) | (4,312 | ) | 718.7 | % | ||||||||
|
|
|
|
|
| |||||||||||
Net loss attributable to ATAI Life Sciences AG stockholders | $ | (48,461 | ) | $ | (16,357 | ) | $ | (32,104 | ) | 196.3 | % | |||||
|
|
|
|
|
| |||||||||||
License Revenue
No license revenue was recognized for the three months ended June 30, 2021 or June 30, 2020.
60
Research and Development Expenses
The table and discussion below present research and development expenses for the three months ended June 30, 2021 and 2020:
| Three months ended June 30, | ||||||||||||||||
| 2021 | 2020 | Change | % Change | |||||||||||||
| (in thousands, except percentages) | ||||||||||||||||
Direct research and development expenses by program: | ||||||||||||||||
PCN-101 (Perception) | $ | 2,373 | $ | 1,086 | $ | 1,286 | 118.4 | % | ||||||||
DMX-1002 (DemeRx IB) | 949 | 152 | 797 | 524.2 | % | |||||||||||
RL-007 (Recognify) | 676 | — | 676 | 100.0 | % | |||||||||||
VLS-01 (Viridia) | 646 | 2 | 644 | 28618.0 | % | |||||||||||
KUR-101 (Kures) | 376 | 920 | (544 | ) | (59.1 | %) | ||||||||||
EMP-01 (EmpathBio) | 249 | — | 249 | 100.0 | % | |||||||||||
Novel drug delivery (InnarisBio) | 200 | — | 200 | 100.0 | % | |||||||||||
RLS-01 (Revixia) | 188 | — | 188 | 100.0 | % | |||||||||||
Novel compounds (EntheogeniX) | 133 | 248 | (114 | ) | (46.2 | %) | ||||||||||
NN-01 (Neuronasal) | 122 | — | 122 | 100.0 | % | |||||||||||
Other (Introspect, Psyber, Psyprotix) | 89 | — | 89 | 100.0 | % | |||||||||||
Unallocated research and development expenses: | ||||||||||||||||
Personnel expenses | 9,851 | 365 | 9,486 | 2596.2 | % | |||||||||||
Professional and consulting services | 118 | 65 | 53 | 82.0 | % | |||||||||||
Other | 56 | 16 | 40 | 257.9 | % | |||||||||||
|
|
|
|
|
| |||||||||||
Total research and development expenses | $ | 16,026 | $ | 2,854 | $ | 13,172 | 461.6 | % | ||||||||
|
|
|
|
|
| |||||||||||
Research and development expenses were $16.0 million for the three months ended June 30, 2021, compared to $2.9 million for the three months ended June 30, 2020. The increase of $13.1 million was primarily attributable to $9.5 million of personnel costs, which included $8.7 million of stock-based compensation and an increase of $3.6 million of direct costs at the platform companies as discussed below.
The $1.2 million increase in direct costs for PCN-101 was primarily due to an increase of $0.9 million in clinical development costs, and $0.2 million in consulting and personnel related costs.
The $0.8 million increase in direct costs for DMX-1002 program was primarily due to an increase of $0.3 million preclinical activities, $0.2 million in manufacturing and $0.1 million in clinical development costs.
The direct costs of $0.7 million for RL-007 program were $0.5 million in clinical development costs and $0.2 million of personnel related costs, which included $0.1 million of stock-based compensation expense.
The direct costs for VLS-01 program were $0.6 million of manufacturing and control processes and other preclinical activities.
The decrease of $0.5 million in direct costs for KUR-101 was primarily due to a $0.5 million decrease in preclinical activities.
The direct costs for EMP-001 were $0.2 million of manufacturing and control processes costs and other preclinical activities.
The direct costs for InnarisBio were $0.2 million of preclinical activities.
The direct costs for RLS-01 were $0.2 million of manufacturing and control processes costs and other preclinical activities.
The $0.1 million decrease in direct costs for EntheogeniX was primarily due to a $0.2 million decrease in data and analytical support costs, partially offset by a $1.0 million increase in manufacturing and control processes costs and other preclinical activities.
The direct costs for NN-01, which are from the date of acquisition in May 2021 were $0.1 million in clinical development costs.
61
During the three months ended June 30, 2021, we did not incur any significant direct costs in association with IntroSpect, Psyber, or Psyprotix; direct costs associated with these programs were related to the ramp up of preclinical development and initial clinical-stage activities.
Acquisition of In-Process Research and Development Expense
| Three Months Ended June 30, | ||||||||||||||||
| 2021 | 2020 | Change | % Change | |||||||||||||
| (in thousands, except percentages) | ||||||||||||||||
Acquisition of in-process research and development expense by program: | ||||||||||||||||
Neuronasal | $ | 7,962 | $ | — | $ | 7,962 | 100.0 | % | ||||||||
KUR-101 (Kures) | — | 120 | (120 | ) | 100.0 | % | ||||||||||
|
|
|
|
|
| |||||||||||
Total acquisition of in-process research and development expense | $ | 7,962 | $ | 120 | $ | 7,842 | 6535.0 | % | ||||||||
|
|
|
|
|
| |||||||||||
Acquisition of in-process research and development expenses was $8.0 million for the three months ended June 30, 2021, which was IPR&D acquired from Neuronasal in May 2021. Acquisition of in-process research and development expenses was $0.1 million for the three months ended June 30, 2020, which was IPR&D acquired from Kures. The acquired IPR&D were all considered to have no future alternative use.
General and Administrative Expenses
General and administrative expenses were $37.3 million for the three months ended June 30, 2021 compared to $2.9 million for the three months ended June 30, 2020. The increase of $34.5 million, was attributable to $30.2 million of personnel costs, which included $28.7 million of stock-based compensation, $3.4 million of professional fees, and $0.9 million other costs related to support of our platform growth and public company requirements.
Interest Income
Interest income for the three months ended June 30, 2021 and 2020 primarily consisted of interest earned on our cash balances and notes receivable during these periods. We had interest income for the three months ended June 30, 2021 and 2020 of $35,000 and $18,000, respectively.
Change in Fair Value of Contingent Consideration Liability—Related Parties
The milestone and royalty payments in relation to the acquisition of Perception Neuroscience were recorded at the acquisition date or at the exercise date related to the call option, and is subsequently remeasured to fair value as of June 30, 2021, resulting in an expense of $0.9 million and $0.04 million being recognized for the three months ended June 30, 2021 and 2020, respectively. The increase of $0.86 million was primarily attributable to Perception’s completion of its Phase 1 clinical trial in September 2020, which increased the probability of the milestone event occurring, and a potential license agreement with a third-party pharmaceutical company, which would include an upfront payment and additional milestone payments. As the license agreement had not been executed as of December 31, 2020, we used a probability weighted approach for the royalty payments, where 80% was applied to the license scenario and 20% was applied to the no-license scenario. At March 31, 2021, the license transaction had closed and the scenario-based method with 80%/20% probability was no longer used.
The milestone and royalty payments in relation to the acquisition of InnarisBio were recorded at the acquisition date and is subsequently remeasured to fair value as of June 30, 2021, resulting in an immaterial expense being recognized for the three months ended June 30, 2021 because there were no material changes to any of the significant assumptions used that impacts the fair value of the contingent liability.
Change in Fair Value of Convertible Promissory Notes
Change in fair value of convertible promissory notes for the three months ended June 30, 2020 was $1.3 million, which was primarily associated with the change in fair value of our 2020 convertible notes, or the 2020 Notes. The change in fair value of the 2020 Notes was primarily attributable to an increase in the fair value of the underlying common stock in 2020 leading up to the conversion of the convertible promissory notes into our common shares in November 2020. We did not recognize a change in fair value of convertible promissory notes for the three months ended June 30, 2021.
62
Unrealized Loss on Other Investments Held at Fair Value
In May 2021, we received IntelGenx common stock, warrants and additional unit warrants for a price of approximately $12.3 million. We determined that the initial aggregate fair value is equal to the transaction price and recorded the common shares at $3.0 million, the warrants at $1.2 million and the additional unit warrants at $8.2 million on a relative fair value basis resulting in no initial gain or loss recognized in the condensed consolidated statements of operations. Subsequently, changes in fair value of the common shares, the warrants and additional unit warrants are recorded as a component of other income (expense), net in the condensed consolidated statement of operations. During the three months ended June 30, 2021, we recognized $5.5 million of unrealized loss on other investments held at fair value.
Loss on Conversion of Convertible Promissory Notes
Loss on conversion of convertible promissory notes for the three months ended June 30, 2021 was $0.5 million. In June 2021, upon the funding of the Otsuka license and collaborative agreement, the Perception convertible promissory notes were converted into Perception Series A preferred stock. The loss represents the difference between (i) carrying value including derivative liability of the Perception December 2020 Notes of $2.2 million and (ii) the fair value of Perception Series A preferred stock into which the notes converted of $2.7 million. There was no loss on conversion of convertible promissory notes recorded in the three months ended June 30, 2020.
Gain on Consolidation of a Variable Interest Entity
Gain on consolidation of a variable interest entity for the three months ended June 30, 2021 was $3.5 million. We purchased additional shares of Neuronasal in May 2021 and recognized a gain of $3.5 million. The gain was calculated as the sum of the consideration paid of $1.0 million, the fair value of the noncontrolling interest issued of $3.0 million, the carrying value of our investments in Neuronasal’s common stock and preferred stock prior to May 2021 of $0.8 million, less the fair value of identifiable net assets acquired of $8.3 million. The fair value of the IPR&D acquired of $8.3 million was charged to research and development expense as it had no alternative future use at the time of the acquisition. There was no gain on asset acquisition of a variable interest entity recorded in the three months ended June 30, 2020.
Other Income (Expense), Net
Other expense, net for the three months ended June 30, 2021 was $2.7 million, compared to $.04 million for the three months ended June 30, 2020. The increase of $2.7 million was primarily related to foreign currency expense.
Income Tax
We incurred income tax expense of $58,000 for the three months ended June 30, 2021. The income tax expense relates to book profits and thus taxable profits generated in one of our United States subsidiaries. Given our early stage development and lack of prior earnings history, we have a full valuation allowance primarily related to German and overseas tax loss carryforwards that we do not consider more likely than not to be realized. We did not incur income tax expense for the three months ended June 30, 2020
Losses from Investments in Equity Method Investees
Losses from investment in equity method investees for the three months ended June 30, 2021 and 2020 were $2.9 million and $9.8 million, respectively. Loss from investment in equity method investees represents our share of equity method investee losses on the basis of our equity ownership percentages or based on our proportionate share of the respective class of securities in our other investments in the event that the carrying amount of our equity method investments was zero.
63
Comparison of the Six Months Ended June 30, 2021 and 2020 (unaudited)
| Six months ended June 30, | ||||||||||||||||
| 2021 | 2020 | $ Change | % Change | |||||||||||||
| (in thousands, except percentages) | ||||||||||||||||
License revenue | $ | 19,880 | $ | — | 19,880 | 100.0 | % | |||||||||
Operating expenses: | ||||||||||||||||
Research and development | 21,611 | 4,998 | 16,613 | 332.4 | % | |||||||||||
Acquisition of in-process research and development | 8,934 | 120 | 8,814 | 7345.0 | % | |||||||||||
General and administrative | 46,604 | 4,421 | 42,183 | 954.2 | % | |||||||||||
|
|
|
|
|
| |||||||||||
Total operating expenses | 77,149 | 9,539 | 67,610 | 708.8 | % | |||||||||||
|
|
|
|
|
| |||||||||||
Loss from operations | (57,269 | ) | (9,539 | ) | (47,730 | ) | 500.4 | % | ||||||||
|
|
|
|
|
| |||||||||||
Other income (expense), net: | ||||||||||||||||
Interest income | 72 | 38 | 34 | 89.5 | % | |||||||||||
Change in fair value of contingent consideration liability - related parties | (660 | ) | (66 | ) | (594 | ) | 100.0 | % | ||||||||
Change in fair value of short term notes receivable - related party | — | 718 | (718 | ) | (100 | %) | ||||||||||
Change in fair value of convertible promissory notes | — | (133 | ) | 133 | (100 | %) | ||||||||||
Change in fair value of derivative liability | 41 | — | 41 | 100.0 | % | |||||||||||
Unrealized loss on other investments held at fair value | (5,460 | ) | — | (5,460 | ) | 100.0 | % | |||||||||
Unrealized gain on other investments | — | 19,856 | (19,856 | ) | (100 | %) | ||||||||||
Loss on conversion of convertible promissory notes | (513 | ) | — | (513 | ) | 100.0 | % | |||||||||
Gain on consolidation of a variable interest entity | 3,543 | — | 3,543 | 100.0 | % | |||||||||||
Other income (expense), net | (1,302 | ) | (119 | ) | (1,183 | ) | 994.1 | % | ||||||||
|
|
|
|
|
| |||||||||||
Total other income (expense), net | (4,279 | ) | 20,294 | (24,573 | ) | (121 | %) | |||||||||
|
|
|
|
|
| |||||||||||
Net income (loss) before income taxes | (61,548 | ) | 10,755 | (72,303 | ) | (672 | %) | |||||||||
Provision for income taxes | (64 | ) | — | (64 | ) | 100.0 | % | |||||||||
Gain on dilution of equity method investment | 16,923 | — | 16,923 | 100.0 | % | |||||||||||
Losses from investments in equity method investees, net of tax | (4,640 | ) | (11,831 | ) | 7,191 | (61 | %) | |||||||||
|
|
|
|
|
| |||||||||||
Net loss | (49,329 | ) | (1,076 | ) | (48,253 | ) | 4484.5 | % | ||||||||
Net loss attributable to redeemable noncontrolling interests and noncontrolling interests | (1,556 | ) | (1,022 | ) | (534 | ) | 52.3 | % | ||||||||
|
|
|
|
|
| |||||||||||
Net loss attributable to ATAI Life Sciences AG stockholders | $ | (47,773 | ) | $ | (54 | ) | $ | (47,719 | ) | 88368.5 | % | |||||
|
|
|
|
|
| |||||||||||
License Revenue
License revenue was $19.9 million for the six months ended June 30, 2021, which related to a license and collaboration agreement entered into with Otsuka Pharmaceutical Co., LTD, or Otsuka, whereby Otsuka was granted an exclusive right to develop and commercialize products containing PCN-101 in Japan at its own cost and expense. The license revenue was recognized upon delivery of the license to Otsuka during the period.
64
Research and Development Expenses
The table and discussion below present research and development expenses for the six months ended June 30, 2021 and 2020:
| Six months ended June 30, | ||||||||||||||||
| 2021 | 2020 | Change | % Change | |||||||||||||
| (in thousands, except percentages) | ||||||||||||||||
Direct research and development expenses by program: | ||||||||||||||||
PCN-101 (Perception Neuroscience) | $ | 4,072 | $ | 1,787 | $ | 2,286 | 127.9 | % | ||||||||
DMX-1002 (DemeRx IB) | 1,835 | 361 | 1,474 | 408.4 | % | |||||||||||
RL-007 (Recognify) | 1,076 | — | 1,076 | 100.0 | % | |||||||||||
VLS-01 (Viridia) | 1,067 | 2 | 1,065 | 47316.2 | % | |||||||||||
KUR-101 (Kures) | 688 | 1,594 | (906 | ) | (56.8 | %) | ||||||||||
EMP-01 (EmpathBio) | 331 | — | 331 | 100.0 | % | |||||||||||
RLS-01 (Revixia) | 280 | — | 280 | 100.0 | % | |||||||||||
Novel compounds (EntheogeniX) | 245 | 363 | (118 | ) | (32.5 | %) | ||||||||||
Novel drug delivery (InnarisBio) | 224 | — | 224 | 100.0 | % | |||||||||||
NN-01 (Neuronasal) | 122 | — | 122 | 100.0 | % | |||||||||||
Other (Introspect, Psyber, Psyprotix) | 65 | 2 | 64 | 3394.2 | % | |||||||||||
Unallocated research and development expenses: | ||||||||||||||||
Personnel expenses | 11,119 | 680 | 10,439 | 1534.9 | % | |||||||||||
Professional and consulting services | 303 | 104 | 200 | 192.4 | % | |||||||||||
Other | 182 | 105 | 76 | 72.5 | % | |||||||||||
|
|
|
|
|
| |||||||||||
Total research and development expenses | $ | 21,611 | $ | 4,998 | $ | 16,613 | 326.9 | % | ||||||||
|
|
|
|
|
| |||||||||||
Research and development expenses were $21.6 million for the six months ended June 30, 2021, compared to $5.0 million for the six months ended June 30, 2020. The increase of $16.6 million was primarily attributable to $10.4 million of personnel costs, which included $8.9 million in stock-based compensation and an increase of $5.9 million of direct costs at the platform companies as discussed below.
The $2.3 million increase in direct costs for PCN-101 was primarily due to an increase of $1.0 million in clinical development costs, $0.6 million in drug manufacturing costs, $0.3 million in preclinical activities, and $0.4 million in consulting and personnel related costs.
The $1.5 million increase in direct costs for DMX-1002 program was primarily due to an increase of $0.7 million in clinical development cost, $0.4 million in preclinical activities, $0.3 million in manufacturing, and $0.1 million increase in personnel related costs.
The direct costs of $1.1 million for RL-007 were $0.6 million of clinical development costs, $0.4 million of personnel related costs, which included $0.2 million of stock-based compensation expense and $0.1 million in manufacturing and control processes costs.
The direct costs for VLS-01 program were $1.1 million of manufacturing and control processes and other preclinical activities.
The $0.9 million decrease in direct costs for KUR-101 was primarily due to a $0.8 million reduction in preclinical activities, and a $0.2 million decrease in manufacturing and control processes costs, offset by a $0.1 million increase in clinical development costs.
The direct costs for EMP-001 were $0.3 million of manufacturing and control processes costs and other preclinical activities.
The direct costs for RLS-01 were $0.3 million of manufacturing and control processes costs and other preclinical activities.
The decrease of $0.1 million in direct costs for EntheogeniX was primarily due to a $0.2 million decrease in data and analytical support costs, partially offset by a $0.1 million increase in manufacturing and control processes costs.
The direct costs for NN-01, which are from the date of acquisition in May 2021, were $0.1 million of clinical development costs.
65
During the six months ended June 30, 2021, we did not incur any significant direct costs in association with IntroSpect, Psyber, or Psyprotix; direct costs associated with these programs were related to the ramp up of preclinical development and initial clinical-stage activities.
Acquisition of In-Process Research and Development Expense
| Six Months Ended June 30, | ||||||||||||||||
| 2021 | 2020 | Change | % Change | |||||||||||||
| (in thousands, except percentages) | ||||||||||||||||
Acquisition of in-process research and development expense by program: | ||||||||||||||||
Neuronasal | $ | 7,962 | $ | — | $ | 7,962 | 100.0 | % | ||||||||
InnarisBio | 972 | 972 | 100.0 | % | ||||||||||||
KUR-101 (Kures) | 120 | (120 | ) | 100.0 | % | |||||||||||
|
|
|
|
|
| |||||||||||
Total acquisition of in-process research and development expense | $ | 8,934 | $ | 120 | $ | 8,814 | 7361.7 | % | ||||||||
|
|
|
|
|
| |||||||||||
Acquisition of in-process research and development expenses was $9.0 million for the six months ended June 30, 2021, which was IPR&D acquired from Neuronasal in May 2021 and InnarisBio in March 2021. Acquisition of in-process research and development expenses was $0.1 million for the six months ended June 30, 2020, which was IPR&D acquired from Kures. The acquired IPR&D were all considered to have no future alternative use.
General and Administrative Expenses
General and administrative expenses were $46.6 million for the six months ended June 30, 2021 compared to $4.4 million for the six months ended June 30, 2020. The increase of $42.2 million, was attributable to $32.7 million of personnel costs, which included $28.7 million of stock-based compensation, $8.2 million of professional fees, and $1.3 million other costs related to support of our platform growth and public company requirements.
Interest Income
Interest income for the six months ended June 30, 2021 and 2020 primarily consisted of interest earned on our cash balances and notes receivable during these periods. We had interest income for the six months ended June 30, 2021 and 2020 of $72,000 and $38,000, respectively.
Change in Fair Value of Contingent Consideration Liability—Related Parties
The milestone and royalty payments in relation to the acquisition of Perception Neuroscience were recorded at the acquisition date or at the exercise date related to the call option, and is subsequently remeasured to fair value as of June 30, 2021, resulting in an expense of $0.7 million and $0.07 million being recognized for the six months ended June 30, 2021 and 2020, respectively. The increase of $0.6 million was primarily attributable to Perception’s completion of its Phase 1 clinical trial in September 2020, which increased the probability of the milestone event occurring, and a potential license agreement with a third-party pharmaceutical company, which would include an upfront payment and additional milestone payments. As the license agreement had not been executed as of December 31, 2020, we used a probability weighted approach for the royalty payments, where 80% was applied to the license scenario and 20% was applied to the no-license scenario. At March 31, 2021, the license transaction had closed and the scenario-based method with 80%/20% probability was no longer used.
The milestone and royalty payments in relation to the acquisition of InnarisBio were recorded at the acquisition date and is subsequently remeasured to fair value as of June 30, 2021, resulting in an immaterial expense being recognized for the six months ended June 30, 2021 because there were no material changes to any of the significant assumptions used that impacts the fair value of the contingent liability.
66
Change in Fair Value of Short Term Notes Receivable—Related Party
Change in fair value of short term notes receivable with COMPASS for the six months ended June 30, 2020 was $0.7 million. The COMPASS notes were converted during 2020. No change in fair value of short term notes receivable of related parties was recognized for the six months ended June 30, 2021.
Change in Fair Value of Convertible Promissory Notes
Change in fair value of convertible promissory notes for the six months ended June 30, 2020 was $0.1 million, which was primarily associated with the change in fair value of our 2020 convertible notes, or the 2020 Notes. The change in fair value of the 2020 Notes was primarily attributable to an increase in the fair value of the underlying common stock in 2020 leading up to the conversion of the convertible promissory notes into our common shares in November 2020. No changes in fair value of convertible promissory notes were recognized for the six months ended June 30, 2021 as the 2020 Notes were converted in November 2020.
Change in Fair Value of Derivative Liability
Change in fair value of derivative liability was $0.04 million for the six months ended June 30, 2021, which was primarily due to the additional issuance of convertible promissory notes in January 2021 and the increased probability of a potential licensing transaction with a third-party pharmaceutical company and a decrease in the probability of a potential preferred equity financing round. We did not recognize a change in fair value of derivative liability for the six months ended June 30, 2020.
Unrealized Loss on Other Investments Held at Fair Value
In May 2021, we received IntelGenx common stock, warrants and additional unit warrants for a price of approximately $12.3 million. We determined that the initial aggregate fair value is equal to the transaction price and recorded the common shares at $3.0 million, the warrants at $1.2 million and the additional unit warrants at $8.2 million on a relative fair value basis resulting in no initial gain or loss recognized in the condensed consolidated statements of operations. Subsequently, changes in fair value of the common shares, the warrants and additional unit warrants are recorded as a component of other income (expense), net in the condensed consolidated statement of operations. During the six months ended June 30, 2021, we recognized $5.5 million of unrealized loss on other investments held at fair value.
Unrealized Gain on Other Investments
Unrealized gain on other investments for the six months ended June 30, 2021 was zero compared to $19.9 million for the six months ended June 30, 2020. The $19.9 million gain in 2020 mainly related to our remeasurement of our investment in COMPASS’ Series A preferred shares to fair value due to the observable price change in connection with COMPASS’ secondary Series A preferred stock purchase in March 2020.
Loss on Conversion of Convertible Promissory Notes
Loss on conversion of convertible promissory notes for the six months ended June 30, 2021 was $0.5 million. In June 2021, upon the funding of the Otsuka license and collaborative agreement, the Perception convertible promissory notes were converted into Perception Series A preferred stock. The loss represents the difference between (i) carrying value including derivative liability of the Perception December 2020 Notes of $2.2 million and (ii) the fair value of Perception Series A preferred stock into which the notes converted of $2.7 million. There was no loss on conversion of convertible promissory notes recorded in the six months ended June 30, 2020.
Gain on Consolidation of a Variable Interest Entity
Gain on consolidation of a variable interest entity was $3.5 million for the six months ended June 30, 2021. We purchased additional shares of Neuronasal in May 2021 and recognized a gain of $3.5 million. The gain was calculated as the sum of the consideration paid of $1.0 million, the fair value of the noncontrolling interest issued of $3.0 million, the carrying value of our investments in Neuronasal’s common stock and preferred stock prior to May 2021 of $0.8 million, less the fair value of identifiable net assets acquired of $8.3 million. The fair value of the IPR&D acquired of $8.3 million was charged to research and development expense as it had no alternative future use at the time of the acquisition. There was no gain on consolidation of a variable interest entity recorded in the six months ended June 30, 2020.
Other Income (Expense), Net
Other expense, net for the six months ended June 30, 2021 was $1.3 million, compared to $0.1 million for the six months ended June 30, 2020. The increase of $1.2 million was primarily related to foreign currency expense.
Income Tax
We incurred income tax expense for $64,000 for the six months ended June 30, 2021. The income tax expense relates to book profits and thus taxable profits generated in one of our United States subsidiaries. Given our early stage development and lack of prior earnings history, we have a full valuation allowance primarily related to German and overseas tax loss carryforwards that we do not consider more likely than not to be realized. We did not incur income tax expense for the six months ended June 30, 2020.
Losses from Investments in Equity Method Investees
Losses from investment in equity method investees for the six months ended June 30, 2021 and 2020 were $4.6 million and $11.8 million, respectively. Loss from investment in equity method investees represents our share of equity method investee losses on the basis of our equity ownership percentages or based on our proportionate share of the respective class of securities in our other investments in the event that the carrying amount of our equity method investments was zero.
67
Liquidity and Capital Resources
Sources of Liquidity
In June 2021, we completed our IPO of 17,250,000 shares of our common stock at a price to the public of $15.00 per share, including the exercise in full by the underwriters of their option to purchase 2,250,000 additional shares of our common stock. We received aggregate net proceeds of $231.6 million, after underwriting discounts and commissions of $18.1 million and offering costs of $9.0 million. Since our inception through June 30, 2021, sources of capital raised to fund our operations were comprised of aggregate gross proceeds of $630.0 million from sales of our common stock and convertible notes. As of June 30, 2021, we had cash and cash equivalents of $453.6 million.
Convertible Promissory Notes
In November 2018, we issued an aggregate principal amount of $0.2 million of convertible notes, or the 2018 Convertible Notes. The 2018 Convertible Notes are non-interest-bearing and have a maturity date of September 30, 2025, unless previously redeemed, converted, purchased or cancelled. In October 2020, we issued an additional principal amount of $1.0 million of 2018 Convertible Notes. Each note has a face value of €1 and is convertible into one ordinary share of ATAI Life Sciences AG upon the payment of €17.00. We expect each of the 2018 Convertible Notes to be amended to allow for conversion into sixteen ordinary shares of ATAI Life Sciences N.V. As of June 30, 2021 an aggregate principal amount of $1.2 million of the 2018 Convertible Notes remained outstanding.
Investments
While a significant potential source of liquidity resides in our investment in COMPASS ordinary shares, we do not expect that our investment in COMPASS will be a material source of liquidity in the near term. Based on quoted market prices, the market value of our ownership in COMPASS was $308.1 million as of June 30, 2021. As of June 30, 2021, the carrying value of our investment in COMPASS was $19.8 million under the equity method. As a result of additional ordinary shares issued by COMPASS in May 2021, including additional shares purchased by us for an aggregate cost of $5.0 million, our ownership interest in COMPASS was reduced to 19.4%.
Liquidity Risks
As of June 30, 2021, we had cash and cash equivalents of $453.6 million. We believe that our cash and cash equivalents will be sufficient to fund our projected operating expenses and capital expenditures through at least the next 12 months.
We expect to incur substantial additional expenditures in the near term to support our ongoing activities. Additionally, we expect to incur additional costs as a result of operating as a public company. We expect to continue to incur net losses for the foreseeable future. Our ability to fund our product development and clinical operations as well as commercialization of our product candidates, will depend on the amount and timing of cash received from planned financings.
Our future capital requirements will depend on many factors, including:
| • | the time and cost necessary to complete ongoing and planned clinical trials; |
| • | the outcome, timing and cost of meeting regulatory requirements established by the FDA, the EMA and other comparable foreign regulatory authorities; |
| • | the progress, timing, scope and costs of our preclinical studies, clinical trials and other related activities for our ongoing and planned clinical trials, and potential future clinical trials; |
| • | the costs of commercialization activities for any of our product candidates that receive marketing approval, including the costs and timing of establishing product sales, marketing, distribution and manufacturing capabilities, or entering into strategic collaborations with third parties to leverage or access these capabilities; |
| • | the amount and timing of sales and other revenues from our product candidates, if approved, including the sales price and the availability of coverage and adequate third party reimbursement; |
68
| • | the cash requirements in purchasing additional equity from certain of our ATAI companies upon the achievement of specified development milestone events; |
| • | the cash requirements of developing our programs and our ability and willingness to finance their continued development; |
| • | the cash requirements of any future acquisitions or discovery of product candidates; and |
| • | the time and cost necessary to respond to technological and market developments, including other products that may compete with one or more of our product candidates. |
A change in the outcome of any of these or other variables with respect to the development of any of our product candidates could significantly change the costs and timing associated with the development of that product candidate. Further, our operating plans may change in the future, and we may need additional funds to meet operational needs and capital requirements associated with such operating plans. If we are unable to obtain this funding when needed and on acceptable terms, we could be forced to delay, limit or terminate our product development efforts.”
Until such time, if ever, as we can generate substantial product revenue, we expect to finance our operations through a combination of equity financings, debt financings, collaborations with other companies or other strategic transactions. We do not currently have any committed external source of funds. Debt financing and preferred equity financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making acquisitions or capital expenditures or declaring dividends. If we are unable to raise additional funds through equity or debt financings or other arrangements when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Further, our operating plans may change, and we may need additional funds to meet operational needs and capital requirements for clinical trials and other research and development activities. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated product development programs.
Cash Flows
The following table summarizes our cash flows for six months ended June 30, 2021 and 2020:
| June 30, | ||||||||
| 2021 | 2020 | |||||||
| (in thousands) | ||||||||
Net cash used in operating activities | $ | (14,627 | ) | $ | (9,107 | ) | ||
Net cash used in investing activities | (32,029 | ) | (19,029 | ) | ||||
Net cash provided by financing activities | 404,262 | 13,011 | ||||||
Effect of foreign exchange rate changes on cash | (1,230 | ) | (204 | ) | ||||
|
|
|
| |||||
Net increase (decrease) in cash | 356,376 | $ | (15,329 | ) | ||||
|
|
|
| |||||
Net Cash Used in Operating Activities
Net cash used in operating activities was $14.6 million for the six months ended June 30, 2021, which consisted of a net loss of $49.3 million, adjusted by non-cash charges of $37.7 million and net cash outflows from the change in operating assets and liabilities of $3.0 million. The non-cash charges primarily consisted of $37.7 million of stock-based compensation, $8.9 million of IPR&D considered to have no future alternative use, $5.5 million of unrealized loss on other investments held at fair value and $4.6 million of losses from our equity method investments partially offset by $16.9 million of gain on investment dilution. The net cash outflows from the change in operating assets and liabilities were primarily due to a $3.8 million decrease in accrued liabilities and a $1.7 million increase in prepaid expenses offset by a $2.4 million increase in accounts payable and $0.1 million increase in deferred revenue.
69
Net cash used in operating activities was $9.1 million in the six months ended June 30, 2020, which consisted of a net loss of $1.1 million, adjusted by non-cash adjustments of $8.5 million and net cash inflows from the change in operating assets and liabilities of $0.5 million. The non-cash charges primarily consisted of $19.9 million of unrealized gains on other investments associated with COMPASS, $0.7 million related to the change in the fair value of short term note receivable with a related party, offset by $11.8 million of losses from investments in equity method investees, $0.1 million related to the change in the fair value of convertible promissory notes, and $0.1 million of in process research and development expense. The net cash inflows from the change in operating assets and liabilities were primarily due to a $1.0 million increase in accounts payable and accrued liabilities, offset by a $0.5 million increase in prepaid expenses.
Net Cash Used in Investing Activities
Net cash used in investing activities was $32.0 million for the six months ended June 30, 2021, primarily driven by additional investments of $23.4 million in our other investments, $5.4 million additional investments into equity-method investees, $0.3 million of purchases of property, plant and equipment, $0.2 million of capitalized internal-use software development costs, $2.6 million of loans to related parties and $0.2 million of purchase of other assets.
Net cash used in investing activities was $19.0 million in the six months ended June 30, 2020, primarily driven by additional investments of $17.8 million in our other investments and $1.2 million of long term notes receivable additional investments.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $404.3 million for the six months ended June 30, 2021, primarily due to $400.3 million of net proceeds from the issuance of our common stock, $2.4 million of proceeds from our sale of Innoplexus investments treated as a secured financing, and $1.6 million of proceeds from the issuance of convertible promissory notes.
Net cash provided by financing activities was $13.0 million in the six months ended June 30, 2020, primarily due to $13.0 million from the issuance of convertible promissory notes.
Indebtedness
Convertible Notes
Between November 2018 and June 2021, we issued an aggregate of $34.3 million of convertible notes.
In November 2018, we issued an aggregate principal amount of $0.2 million of convertible notes, or the 2018 Convertible Notes. The 2018 Convertible Notes are non-interest-bearing and have a maturity date of September 30, 2025, unless previously redeemed, converted, purchased or cancelled. In October 2020, we issued an additional principal amount of $1.0 million of 2018 Convertible Notes. Each note has a face value of €1 and is convertible into one ordinary share of ATAI Life Sciences AG upon the payment of €17.00. Conversion rights may be exercised by a noteholder at any time prior to maturity, except during certain periods subsequent to the consummation of the IPO. As of June 30, 2021 and December 31, 2020, an aggregate principal amount of $1.2 million of the 2018 Convertible Notes remained outstanding.
During the year ended December 31, 2020, we issued an aggregate of $30.4 million of the 2020 Notes. The 2020 Notes accrue interest at a rate of 5% per annum and have a maturity date of January 31, 2022, unless previously redeemed, converted, purchased or cancelled. The 2020 Notes are convertible upon mandatory conversion events into shares of ATAI Life Sciences N.V., subject to certain dilution adjustments. In November 2020, all of the outstanding principal and accrued interest under the 2020 Notes was automatically converted into shares of common stock.
70
In March 2020, we received proceeds of $0.6 million from the issuance of Perception Notes, as defined below, to third party investors. In December 2020, January 2021, and May 2021 we received $0.4 million, $0.8 million, and $0.8 million respectively, in proceeds from the issuance of additional Perception Notes. The Perception Notes are convertible upon mandatory conversion events into shares of Perception. As of June 30, 2021 and December 31, 2020, $0 million and $1.0 million, respectively, of the Perception Notes remained outstanding.
Promissory Note
In December 2019, we executed a promissory note payable to DemeRx IB whereby we agreed, under a contribution agreement and a Series A Preferred Stock Purchase Agreement, or the DemeRx IB SPA, to make aggregate payments to DemeRx IB of up to $17.0 million upon the achievement of specified clinical and regulatory milestones. As of June 30, 2021, we had made aggregate payments of $10.0 million pursuant to the DemeRx IB SPA.
Investment in Convertible Promissory Notes—Related Party
On May 15, 2019, we purchased convertible promissory notes from Kures, or the Kures Notes, in an aggregate principal amount of $0.1 million that earned interest at an annual rate of 5% and matured on December 31, 2019. We qualified for and elected the fair value option. All principal and interest accrued under the Kures Notes was converted into shares of Series A-1 preferred stock in connection with Kures’ sale of Series A-1 preferred stock in August 2019.
On September 27, 2019, we purchased convertible promissory notes from COMPASS for a total principal amount of $4.0 million, and on November 6, 2019, we purchased an additional convertible promissory note for $4.2 million, together, the COMPASS Notes. The COMPASS Notes bear interest at an annual rate of 3%, which was considered contingent in nature and therefore no earned interest was recorded. We qualified for and elected the fair value option. All principal amounts under the COMPASS Notes were converted into shares of Series B preferred stock in connection with COMPASS’ sale of Series B preferred stock in April 2020.
On March 16, 2020, Perception Neuroscience entered into a convertible promissory note agreement with us and certain other unrelated investors, or the Perception Note Purchase Agreement, pursuant to which Perception Neuroscience issued $3.9 million in principal amount of convertible notes in aggregate. Under the Perception Note Purchase Agreement, Perception Neuroscience issued convertible notes, or the Perception Notes, in the aggregate principal amount of $3.3 million to us and $0.6 million to other investors, including related parties. The Perception Notes bear interest at an annual rate of 5% and are due and payable on June 30, 2022 unless earlier converted. In December 2020, Perception Neuroscience issued additional convertible notes to us, certain related parties and third party investors in the aggregate principal amount of $7.0 million, of which $5.8 million was issued to us and $1.2 million was issued to other investors, including related parties. In May 2021, Perception Neuroscience issued additional convertible notes to us, certain related parties and third party investors in the aggregate principal amount of $5.0 million, of which $4.2 million was issued to us and $0.8 million was issued to other investors, including related parties, as part of its second tranche funding. The notes bear interest at an annual rate of 5% and are due and payable on February 28, 2022, unless earlier converted. Perception Neuroscience may not prepay in whole or in part without our consent. In June 2021, the convertible promissory notes were converted.
In January 2021, pursuant to the Perception Note Purchase Agreement, Perception issued an aggregate principal amount of $0.8 million to other investors, including related parties, as part of its first tranche funding.
Contractual Obligations and Commitments
We have entered into other contracts in the normal course of business with certain CROs, CMOs and other third parties for preclinical research studies and testing, clinical trials and manufacturing services. These contracts do not contain any minimum purchase commitments and are cancelable by us upon written notice. Payments due upon cancellation consist only of payments for services provided and expenses incurred, including noncancelable obligations of our service providers, up to the date of cancellation. The amounts and timing of such payments are not known.
71
In addition, under various licensing and related agreements to which we are a party, we are obligated to pay annual license maintenance fees and may be required to make milestone payments and to pay royalties and other amounts to third parties. The payment obligations under these agreements are contingent upon future events, such as our achievement of specified milestones or generating product sales, and the amount, timing and likelihood of such payments are not known. Such contingent payment obligations are described below. For additional information regarding our license agreements described below, see Note 17 to our condensed consolidated financial statements included elsewhere in this Quarterly Report.
Columbia Stock Purchase Agreement
In June 2020, Kures and Columbia entered into a stock purchase agreement, or the Kures SPA. Pursuant to the Kures SPA, Kures can, from time to time, issue to Columbia additional shares of Kures’ common stock, at a per share price equal to the then fair market value of each such share, and shall be deemed to have been paid in partial consideration for the execution, delivery and performance by Columbia of the Kures License Agreement. If Kures proposes to sell any equity securities or securities convertible into equity securities, Columbia will have the right to purchase up to 5% of such securities. These rights shall terminate upon the occurrence of an IPO, if Kures becomes subject to periodic reporting requirements under Section 12(g) or 15(d) of the Exchange Act or certain liquidation events. Columbia also has certain co-sale rights. At the acquisition date, we recorded the fair value of the shares of Kures common stock issued to Columbia of $0.1 million to our additional-paid-in-capital and a debit to research and development expense.
GABA Preferred Stock Purchase Agreement
We entered into the Preferred Stock Purchase Agreement, or the GABA PSPA, in August 2019 with GABA Therapeutics LLC, and purchased shares of Series A preferred stock of GABA at a price of approximately $5.5 million. In addition, pursuant to the GABA PSPA, we are obligated to purchase additional shares of Series A preferred stock, at the same price as the original transaction, for up to $10.0 million, upon the achievement of specified contingent development milestones.
In October 2020, we entered into an Omnibus Amendment Agreement, or the GABA Omnibus Amendment Agreement, with GABA and GABA Therapeutics LLC under which the Right of First Refusal and Co-Sale Agreement was amended. Pursuant to the GABA Omnibus Amendment, GABA Therapeutics LLC granted us the right to purchase additional shares of common stock of GABA held by GABA Therapeutics LLC at the call option purchase price of $1.8 million. In November 2020, we exercised the call option and made a cash contribution of $1.8 million in exchange for additional shares of common stock of GABA.
In April 2021, pursuant to the GABA PSPA, we purchased additional shares of Series A preferred stock of GABA for an aggregate cost of $5.0 million based on the achievement of certain development milestones.
In May 2021, we purchased additional shares of Series A preferred stock prior to the achievement of certain development milestones for an aggregate cost of $5.0 million. The GABA PSPA terminates upon the occurrence of certain liquidation events.
In May 2021, we, GABA and GABA Therapeutics LLC entered into an Amendment Agreement under which the GABA PSPA was amended. Pursuant to the Amendment Agreement, we purchased additional shares of GABA Series A preferred stock at a price of approximately $0.6 million. We are obligated to purchase additional shares of GABA Series A preferred stock for up to $1.5 million with the same price per share as our initial investment and additional shares of GABA common stock for up to $1.0 million upon the achievement of specified contingent development milestones.
Further in accordance with the GABA PSPA, we have the option but not the obligation to purchase the aforementioned additional shares of Series A preferred stock at any time prior to the achievement of any of the specified milestones. Additionally, we have the Right of First Refusal and Co-Sale Agreement with GABA Therapeutics LLC, under which we have the option but not the obligation to purchase shares of common stock for up to $2.0 million from the existing common shareholders.
72
As of June 30, 2021, we had made aggregate payments of $15.5 million pursuant to the GABA PSPA, $1.8 million pursuant to the GABA Omnibus Amendment Agreement and $0.6 million pursuant to the Amendment Agreement.
Neuronasal Preferred Stock Purchase Agreement
Under our Preferred Stock Purchase Agreement, or the Neuronasal PSPA, and the Secondary Sale and Put Right Agreement, or the Neuronasal Secondary Sale Agreement, entered with Neuronasal in December 2019, we are obligated to purchase additional shares of Series A preferred stock from Neuronasal, and shares of common stock from the existing common shareholders, at the same price as the original transaction, at a purchase price of approximately $3.8 million, upon the achievement of specified contingent clinical development milestones.
In October 2020, pursuant to the Neuronasal PSPA, we purchased additional Series A preferred shares at a price of approximately $0.8 million upon the achievement of a specified contingent clinical development milestone.
In March 2021, pursuant to the Neuronasal PSPA and the Neuronasal Secondary Sale Agreement, we purchased additional Series A preferred shares and additional common shares for an aggregate of approximately $1.1 million based on the achievement of certain development milestones.
In May 2021, pursuant to the Neuronasal PSPA and the Neuronasal Secondary Sale Agreement, we exercised our option to purchase additional Series A preferred shares for an aggregate of approximately $1.0 million.
Under the Neuronasal PSPA, we have the option but not the obligation to purchase additional shares of Series A preferred stock, at the same price as the original transaction, at a purchase price of up to approximately $1.0 million upon achievement of certain contingent clinical development milestones by a specified date. Additionally, pursuant to the Neuronasal Secondary Sale Agreement, upon the achievement of certain development milestones, existing common shareholders have the right to sell and we have the option but not the obligation to purchase additional shares of common stock at a price determined based on the fair market value per share. These options are contingent only upon the exercise of the options of the common shareholders.
Additionally, under the Neuronasal PSPA, we have a right of first offer, which requires Neuronasal to first offer us new securities it proposes to sell. The Neuronasal PSPA terminates upon the occurrence of certain liquidation events. The Neuronasal Secondary Sale Agreement terminates when shares of Neuronasal are no longer held by us or our affiliates, Neuronasal consummates a sale of its securities pursuant to a registration statement or the consummation of certain mergers or consolidations.
As of June 30, 2021, we had made aggregate payments of $3.7 million pursuant to this agreement.
Kures Preferred Stock Purchase Agreement
We entered into the Preferred Stock Purchase Agreement, or the Kures PSPA, in August 2019 with Kures, where we purchased shares of Series A-1 preferred stock of Kures for an aggregate purchase price of $3.5 million. The Kures PSPA provided us with control of Kures’ board of directors, resulting in us having unilateral rights to control all decisions related to the significant activities of Kures. In connection with the Kures PSPA, we are required to purchase up to approximately $5.5 million of Series A-2 preferred stock upon the achievement of specified clinical milestones. The Kures PSPA also contains a call option, such that we have the right, but not the obligation, to purchase up to a certain number of shares of Series B preferred stock upon the achievement of specified clinical milestones. As of June 30, 2021, we have not exercised our option to purchase any shares of Series B preferred stock of Kures.
As of June 30, 2021, we had made aggregate payments of $3.5 million pursuant to the Kures PSPA.
Perception Preferred Stock Purchase Agreement
We formed ATAI US 2, Inc., or ATAI US 2, an entity formed for the sole purpose of effecting the acquisition and a wholly owned subsidiary of Perception, entered into a series of transactions to acquire 100% of the equity of Perception Neuroscience, a pre-clinical stage biotech company. In connection with the Perception SPA and the Rollover Agreement between us, Perception and Perception Neuroscience, Perception acquired the outstanding
73
common shares of Perception Neuroscience, or the Rollover Shares, in exchange for aggregate consideration which consisted of (i) a $4.0 million cash payment by Perception at closing ($4.6 million purchase price, less transaction costs of Perception Neuroscience assumed by Perception of $0.6 million), (ii) contingent consideration payable to a founder of Perception Neuroscience of $2.4 million based on the achievement of certain development milestones and royalties on future revenues and (iii) issuance of Class B common shares of Perception to the founders of Perception Neuroscience, representing a 100% interest in the common equity of Perception. In connection with the Perception SPA, we are required to make milestone payments and sub-single-digit royalty payments to a founder of Perception Neuroscience upon the achievement of certain development milestones and royalties on future revenues. Also, in connection with the Perception SPA, Perception entered into a call option agreement with one of the founders of Perception Neuroscience, whereby Perception was granted an option to repurchase 2,350,000 shares of its Class B common stock from the founder. Upon the exercise of the call option, the other founder was entitled to receive a contingent consideration payment.
In connection with the acquisition of Perception Neuroscience by Perception and, ultimately, ATAI US 2, and pursuant to the Perception Preferred Stock Purchase Agreement or Perception PSPA, we purchased shares of Perception’s Series A preferred stock for approximately $9.5 million. The Perception PSPA provided us with control of Perception’s board of directors, resulting in us having unilateral rights to control all decisions related to the significant activities of Perception. Pursuant to a Secondary Perception Preferred Stock Purchase Agreement, we sold shares of Series A preferred stock to secondary investors for approximately $1.6 million in November and December of 2018 under the same terms and conditions of the original purchase. In addition, under the Perception PSPA, Perception was granted the option to sell, and we had the obligation to purchase additional shares of Perception Series A preferred stock at a price equal to Perception PSPA purchase price upon the exercise of the call option. In April 2019, Perception exercised the call option with the founder resulting in the redemption and cancellation of Perception Class B common shares. The exercise of the call option and the related purchase of the noncontrolling interest resulted in a cash payment of $1.0 million.
As of June 30, 2021, we had made aggregate payments of $4.0 million pursuant to the Perception SPA and $10.5 million pursuant to the Perception PSPA.
DemeRx NB Options
We entered into a Series A Preferred Stock Purchase Agreement, or the DemeRx NB PSPA, pursuant to which we purchased shares of Series A Preferred Stock of DemeRx NB at a purchase price of $1.0 million. In accordance with the DemeRx NB PSPA, we also have the option but not the obligation to purchase additional shares of Series A preferred stock at a purchase price of up to $19.0 million. As of June 30, 2021, we have not exercised our option to purchase any shares of Series A preferred stock of DemeRx NB. The DemeRx NB PSPA can be terminated with the written consent of all parties.
As of June 30, 2021, we had made aggregate payments of $1.0 million pursuant to the DemeRx NB PSPA.
DemeRx IB Preferred Stock Purchase Agreement
In December 2019, we entered into the DemeRx IB SPA, pursuant to which we purchased shares of Series A Preferred Stock of DemeRx IB in exchange for an initial payment of $5.0 million in cash and a promissory note issued by us payable to DemeRx IB. Under the promissory note, we agreed to make aggregate payments to DemeRx IB of up to $17.0 million upon the achievement of specified clinical and regulatory milestones. As of June 30, 2021, we had made aggregate payments of $0 million pursuant to the DemeRx IB SPA.
Further, in connection with the promissory note issued, we pledged and assigned to DemeRx IB a portion of shares of our Series A preferred stock of DemeRx IB, or the Pledged Shares, as security under the promissory note. The Pledged Shares have voting and all other rights until an event of default occurs where we fail to make a payment when due. In the event of default, a pro rata portion of the Pledged Shares will automatically be surrendered and be deemed forfeited and canceled.
Recognify Preferred Stock Purchase Agreement
We entered into the Preferred Stock Purchase Agreement, or the Recognify PSPA, in November 2020 with Recognify, where we purchased shares of Series A preferred stock of Recognify at a purchase price of $2.0 million. In addition, pursuant to the Recognify PSPA, we agreed to make aggregate payments to Recognify of up to
74
$18.0 million upon the achievement of specified clinical and regulatory milestones to complete the purchase of the shares and provide additional funding to Recognify. In connection with the Recognify PSPA for additional funding, Recognify issued the corresponding Series A preferred shares to the us provided that the shares, or the Escrow Shares, were held in an escrow account. The Escrow Shares will be released, from time to time, to us upon Recognify achieving certain milestones as defined in the Recognify PSPA with cash payments to be made by us.
In addition, we have the right, but not the obligation, to make payment for the certain Escrow Shares at any time, regardless of the achievement of any milestones. The Escrow Shares have voting and all other rights until an event of default occurs where we fail to make a payment within 10 days following the written notice of the achievement of the relevant milestone. In the event of default, a pro rata portion of the Escrow Shares will automatically be surrendered and be deemed forfeited and canceled, and could result in us losing control of Recognify’s board of directors and our controlling financial interest in Recognify.
In May 2021, pursuant to the Recognify PSPA, we purchased additional shares of Series A preferred stock prior to the achievement of certain development milestone for an aggregate cost of $0.5 million.
As of June 30, 2021, we had made aggregate payments of $2.5 million pursuant to the Recognify PSPA.
EntheogeniX License Agreement
In November 2019, EntheogeniX entered into a license agreement with Cyclica relating to EntheogeniX’s drug discovery and development initiatives. Pursuant to the agreement, EntheogeniX obtained a limited, non-transferable, and non-exclusive right, solely for the term of the agreement, to access and use Cyclica’s hosted and cloud-based software platforms, solely for the purposes of screening certain compounds generated by Cyclica pursuant to the license agreement. Upon execution of the agreement, EntheogeniX paid Cyclica an upfront service fee of $0.1 million. In addition, EntheogeniX is obligated to make aggregate milestone payments to Cyclica of up to $0.3 million upon the achievement of specified development milestones. The term of the license agreement will continue for the life of EntheogeniX and may only be terminated by either party following a non-curable material breach of the shareholders agreement between Cyclica and EntheogeniX.
PsyProtix Purchase Agreement
In February 2021, we jointly formed PsyProtix with Chymia, LLC, or Chymia. PsyProtix was created for the purpose of exploring and developing a metabolomics-based precision psychiatry approach, initially targeting the stratification and treatment of TRD patients. In February 2021, pursuant to a Series A Preferred Stock Purchase Agreement, or the PsyProtix Purchase Agreement, we acquired shares of PsyProtix’s Series A preferred stock in exchange for an initial payment of $0.1 million in cash. In addition, pursuant to the PsyProtix Purchase Agreement, we agreed to make aggregate payments to PsyProtix of up to $4.9 million upon the achievement of specified clinical milestones to complete the purchase of the shares and provide additional funding to PsyProtix.
Psyber Purchase Agreement
In February 2021, pursuant to a Series A Preferred Stock Purchase Agreement, or the Psyber Purchase Agreement, we acquired shares of Psyber’s Series A preferred stock in exchange for an initial payment of $0.2 million in cash. In addition, pursuant to the Psyber Purchase Agreement, we agreed to make aggregate payments to Psyber of up to $1.8 million upon the achievement of specified clinical milestones to complete the purchase of the shares and provide additional funding to Psyber.
InnarisBio Preferred Stock Purchase Agreement
In February 2021, we jointly formed InnarisBio with UniQuest Pty Ltd, or UniQuest, for the purpose of adding a solgel-based direct-to-brain intranasal drug delivery technology to our platform. In March 2021, pursuant to a Series A Preferred Stock Purchase Agreement, or the InnarisBio Purchase Agreement, we acquired shares of InnarisBio’s Series A preferred stock in exchange for an initial payment of $1.1 million in cash. In addition, pursuant to the InnarisBio Purchase Agreement, we agreed to make aggregate payments to InnarisBio of up to $3.9 million upon the achievement of specified clinical milestones to complete the purchase of the shares and provide additional funding to InnarisBio.
75
For additional information regarding our contingent commitments and future put rights or options associated with our investments, see Note 5 to our consolidated financial statements included elsewhere in this Quarterly Report.
Off-Balance Sheet Arrangements
As of June 30, 2021, we did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K. While we have investments classified as VIEs, their purpose is not to provide off-balance sheet financing.
Recent Accounting Pronouncements
See Note 2, “Summary of Significant Accounting Policies—Recently Adopted Accounting Pronouncements” to our unaudited condensed consolidated financial statements appearing under Part 1, Item 1 for more information.
Critical Accounting Policies and Estimates
There have been no significant changes to our critical accounting policies from our disclosure reported in “Critical Accounting Policies and Estimates” in the section titled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in our Prospectus.
JOBS Act
We are an emerging growth company, as defined in the JOBS Act. We intend to rely on certain of the exemptions and reduced reporting requirements provided by the JOBS Act. As an emerging growth company, we are not required to, among other things, (i) provide an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act, and (ii) comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis). We have elected to use the extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that (i) we are no longer an emerging growth company or (ii) we affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, our consolidated financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
As described in Note 2 to our condensed consolidated financial statements included elsewhere in this Quarterly Report, we have early adopted accounting standards, as the JOBS Act does not preclude an emerging growth company from adopting a new or revised accounting standard earlier than the time that such standard applies to private companies. We expect to use the extended transition period for any other new or revised accounting standards during the period in which we remain an emerging growth company.
We will remain an emerging growth company until the earliest to occur of: (1) the last day of the fiscal year (a) following the fifth anniversary of the completion of our initial public offering, or December 31, 2026, (b) in which we have total annual gross revenues of $1.07 billion or more, or (c) in which we are deemed to be a large accelerated filer under the rules of the SEC, which means the market value of our outstanding common stock held by non-affiliates exceeds $700 million as of last business day of our most recently completed second fiscal quarter, and (2) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years.
Further, even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company,” which would allow us to take advantage of many of the same exemptions from disclosure requirements, including reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. We cannot predict if investors will find our common shares less attractive because we may rely on these exemptions. If some investors find our common shares less attractive as a result, there may be a less active trading market for our common shares and our share price may be more volatile.
76
| Item 3. | Quantitative and Qualitative Disclosures about Market Risk |
We are exposed to market risks in the ordinary course of our business. Market risk represents the risk of loss that may impact our financial position due to adverse changes in financial market prices and rates. Our market risk exposure is primarily the result of fluctuations in interest rates and foreign currency exchange rates. In addition, our portfolio of notes receivables is exposed to credit risk in the form of non-payment or non-performance. In mitigating our credit risk, we consider multiple factors, including the duration and terms of the note and the nature of and our relationship with the counterparty.
Interest Rate Sensitivity
Our primary exposure to market risk is interest rate sensitivity, which is affected by changes in the general level of U.S. interest rates. As of June 30, 2021, we had cash and cash equivalents of $453.6 million. We generally hold our cash in interest-bearing demand deposit accounts. Due to the nature of our cash, a hypothetical 100 basis point change in interest rates would not have a material effect on the fair value of our cash. Our cash is held for working capital purposes. We do not enter into investments for trading or speculative purposes.
As of June 30, 2021, we had $1.2 million in convertible promissory notes – related parties, net, which was comprised of non-interest-bearing borrowings under the 2018 Convertible Notes. Based on the principal amounts of the convertible promissory notes and the interest rate assigned to the convertible promissory notes, an immediate 10% change in interest rates would not have a material impact on our convertible promissory notes, financial position or results of operations.
As of June 30, 2021, the carrying amount of our short and long-term notes receivables was an aggregate amount of $4.6 million. Based on the principal amounts of the notes receivable and the interest rates assigned to each note receivable as per their respective contracts, an immediate 10% change in the interest rates would not have a material impact on our notes receivables, financial position or results of operations.
Foreign Currency Exchange Risk
Our reporting and functional currency is the U.S. dollar, and the functional currency of our foreign subsidiaries is generally the respective local currency. The assets and liabilities of each of our foreign subsidiaries are translated into U.S. dollars at exchange rates in effect at each balance sheet date. Adjustments resulting from translating foreign functional currency financial statements into U.S. dollars are recorded as a separate component on the condensed consolidated statements of comprehensive loss. Equity transactions are translated using historical exchange rates. Expenses are translated using the average exchange rate during the previous month. Gains or losses due to transactions in foreign currencies are included in interest and other income, net in our condensed consolidated statements of operations.
The volatility of exchange rates depends on many factors that we cannot forecast with reliable accuracy. We have experienced and will continue to experience fluctuations in foreign exchange gains and losses related to changes in foreign currency exchange rates. In the event our foreign currency denominated assets, liabilities, revenue, or expenses increase, our results of operations may be more greatly affected by fluctuations in the exchange rates of the currencies in which we do business. We have not engaged in the hedging of foreign currency transactions to date, although we may choose to do so in the future.
A hypothetical 10% change in the relative value of the U.S. dollar to other currencies during any of the periods presented would not have had a material effect on our consolidated financial statements.
77
| Item 4. | Controls and Procedures |
Limitations on Effectiveness of Controls and Procedures
In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives. In addition, the design of disclosure controls and procedures must reflect the fact that there are resource constraints and that management is required to apply judgment in evaluating the benefits of possible controls and procedures relative to their costs.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated, as of the end of the period covered by this Quarterly Report, the effectiveness of our disclosure controls and procedures (as that term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based on that evaluation, our principal executive officer and principal financial officer concluded that our disclosure controls and procedures were not effective at reasonable assurance level as of June 30, 2021 as a result of the material weaknesses described in our Prospectus and below.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. In connection with the preparation of our consolidated financial statements for the years ended December 31, 2019 and 2020, we identified material weaknesses in our internal control over financial reporting. The material weaknesses that were identified were related to the design of internal controls as follows: (1) the lack of a sufficient number of trained professionals with the expertise to design, implement and execute a formal risk assessment process and formal accounting policies, procedures and controls over accounting and financial reporting to ensure the timely recording, review, and reconciliation of financial transactions while maintaining a segregation of duties; (2) the lack of formal processes and controls specific to the identification and recording of expense transactions, including stock-based compensation, completely and accurately, and in the appropriate period; and (3) there were not a sufficient number of trained professionals with the appropriate U.S. GAAP technical expertise to identify, evaluate and account for complex transactions and review valuation reports prepared by external specialists. As a result, we did not design and maintain formal accounting policies, processes and controls related to complex transactions necessary for an effective financial reporting process. These deficiencies constitute material weaknesses in the design of our internal controls over financial reporting. As a result of the material weaknesses, we have relied, in part, on the assistance of outside advisors with expertise in these matters to assist us in the preparation of our consolidated financial statements and in our compliance with SEC reporting obligations and expect to continue to do so while we remediate these material weaknesses.
Management’s Remediation Efforts
As disclosed in our Prospectus, we have identified and begun to implement several steps, as further described below, designed to remediate the material weaknesses described in this Item 4 and to enhance our overall control environment. Although we intend to complete the remediation process as promptly as possible, we cannot at this time estimate how long it will take to remediate these material weaknesses, and our remediation plan may not prove to be successful. We will not consider the material weaknesses remediated until our enhanced controls are operational for a sufficient period of time and tested, enabling management to conclude that the enhanced controls are operating effectively. As of June 30, 2021, the material weaknesses had not been remediated.
Our remediation plan includes, but is not limited to, the following measures:
| • | Formalizing our processes and internal control documentation and strengthening supervisory reviews by our financial management. |
78
| • | Hiring additional qualified accounting and finance personnel and engaging financial consultants to enable the implementation of internal control over financial reporting and segregating duties amongst accounting and finance personnel. |
| • | Planning to implement certain accounting systems to automate manual processes. |
| • | We will also continue to engage third parties as required to assist with technical accounting, application of new accounting standards, tax matters, and valuations of our equity instruments, contingent consideration, notes receivable and acquired in-process research and development. |
While the foregoing measures are intended to effectively remediate the material weaknesses described in this Item 4, it is possible that additional remediation steps will be necessary. As such, as we continue to evaluate and implement our plan to remediate the material weaknesses, our management may decide to take additional measures to address the material weaknesses or modify the remediation steps described above. Until these material weaknesses are remediated, we plan to continue to perform additional analyses and other procedures to help ensure that our consolidated financial statements are prepared in accordance with GAAP.
Changes in Internal Control over Financial Reporting
We are taking actions to remediate the material weaknesses relating to our internal controls over financial reporting, as described above. Except as discussed above, there were no changes in our internal control over financial reporting (as that term is defined in Rules 13a-15(d) or 15d-15(d) of the Exchange Act) identified in management’s evaluation pursuant to during the quarter ended June 30, 2021 that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
79
| Item 1. | Legal Proceedings |
See Note 15 “Commitments and Contingencies” to our condensed consolidated financial statements in Item 1, Part I of this Quarterly Report for information regarding certain legal proceedings in which we are involved, which is incorporated by reference into this Part II, Item 1.
80
| Item 1A. | Risk Factors |
Investing in our common shares involves a high degree of risk. In addition to the other information set forth in this Quarterly Report, you should carefully consider the factors described in the section titled “Risk Factors” in our Prospectus. There have been no material changes to the risk factors described in the Prospectus. If any of the risk factors described in the Prospectus actually materializes, our business, financial condition and results of operations could be materially adversely affected. In such an event, the market price of our common shares could decline and you may lose all or part of your investment. Additional risks that we currently do not know about or that we currently believe to be immaterial may also impair our business. We may disclose changes to such risk factors or disclose additional risk factors from time to time in our future filings with the SEC.
81
| Item 2. | Unregistered Sales of Equity Securities and Use of Proceeds. |
Unregistered Sales of Equity Securities
Set forth below is information regarding unregistered securities issued by us during the three months ended June 30, 2021. Also included is the consideration received by us for such unregistered securities and information relating to the section of the Securities Act, or rule of the SEC, under which exemption from registration was claimed.
| (a) | Issuance of Common Shares. |
In April 2021, in connection with the corporate reorganization, we issued an aggregate of 137,569,776 common shares of ATAI Life Sciences N.V. to the shareholders of ATAI Life Sciences AG, which included accredited investors, director nominees and employees.
| (b) | Equity Awards. |
From April 1, 2021 to June 30, 2021, we granted our executive officers, directors and employees and consultants options to purchase an aggregate of 4,270,208 common shares, at a weighted average exercise price of $9.64 per share under our 2020 Equity Incentive Plan. As of June 30, 2021, 4,270,208 of such options remain outstanding.
Unless otherwise stated, the issuances of the above securities were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act or Regulation D promulgated thereunder, Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving any public offering or pursuant to benefit plans and contracts relating to compensation as provided under Rule 701, or Regulation S. No underwriter or underwriting discount or commission was involved in any of the transactions set forth above.
Use of Proceeds
On June 17, 2021, the SEC declared effective our registration statement on Form S-1 (File No. 333-255383), as amended, filed in connection with our initial public offering, and a registration statement on Form S-1 MEF (File No. 333-257184) was effective on filing on June 17, 2021 (collectively, the “Registration Statement”). Pursuant to the Registration Statement, we registered the offer and sale of 17,250,000 of our common shares with a proposed maximum aggregate offering price of approximately $258.8 million. Credit Suisse Securities (USA) LLC, Citigroup Global Markets Inc., Cowen and Company, LLC and Berenberg Capital Markets LLC acted as representatives of the underwriters for the offering. On June 22, 2021, we issued and sold 17,250,000 of our common shares (including 2,250,000 common shares in connection with the full exercise of the underwriters’ option to purchase additional shares) at a price to the public of $15.00 per share. Upon completion of the initial public offering on June 22, 2021, we received net proceeds of approximately $231.6 million, after deducting underwriting discounts and commissions of $18.1 million and offering expenses of $9.0 million. No payments for such expenses were made directly or indirectly to (i) any of our officers or directors or their associates, (ii) any persons owning 10% or more of any class of our equity securities or (iii) any of our affiliates.
The offering terminated after the sale of all securities registered pursuant to the Registration Statement. As of June 30, 2021, net proceeds of approximately $231.6 million from our initial public offering have been invested in a variety of capital preservation investments, including term deposits, and short-term, investment-grade and interest-bearing instruments. There has been no material change in the expected use of the net proceeds from our initial public offering as described in the Prospectus relating to our Registration Statement.
82
| Item 3. | Defaults Upon Senior Securities. |
None.
| Item 4. | Mine Safety Disclosures. |
Not applicable.
| Item 5. | Other Information. |
None
| Item 6. | Exhibits. |
83
| 10.9 | Amendment to Preferred Stock Purchase Agreement, dated as of May 15, 2021 by and among ATAI Life Sciences AG, GABA Therapeutics, LLC and GABA Therapeutics, Inc. | S-1/A | 333- 255383 | 10.26 | 6/4/2021 | |||||||
| 31.1 | Certification of Principal Executive Officer pursuant to Exchange Act Rule 13a-14(a). | * | ||||||||||
| 31.2 | Certification of Principal Financial Officer pursuant to Exchange Act Rule 13a-14(a). | * | ||||||||||
| 32.1 | Certification of Principal Executive Officer pursuant to 18 U.S.C. Section 1350. | ** | ||||||||||
| 32.2 | Certification of Principal Financial Officer pursuant to 18 U.S.C. Section 1350. | ** | ||||||||||
| 101.INS | Inline XBRL Instance Document - the Instance Document does not appear in the interactive data file because its XBRL tags are embedded within the Inline XBRL document | *** | ||||||||||
| 101.SCH | Inline XBRL Taxonomy Extension Schema Document | *** | ||||||||||
| 101.CAL | Inline XBRL Taxonomy Extension Calculation Linkbase Document | *** | ||||||||||
| 101.DEF | Inline XBRL Taxonomy Extension Definition Linkbase Document | *** | ||||||||||
| 101.LAB | Inline XBRL Taxonomy Extension Label Linkbase Document | *** | ||||||||||
| 101.PRE | Inline XBRL Taxonomy Extension Presentation Linkbase Document | *** | ||||||||||
| 104 | Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) | *** | ||||||||||
| * | Filed herewith. |
| ** | Furnished herewith. |
| *** | To be filed by amendment. |
84
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| ATAI LIFE SCIENCES N.V. | ||||||
| Date: August 16, 2021 | By: | /s/ Florian Brand | ||||
| Florian Brand | ||||||
Chief Executive Officer and Managing Director (Principal Executive Officer) | ||||||
| Date: August 16, 2021 | By: | /s/ Greg Weaver | ||||
| Greg Weaver | ||||||
Chief Financial Officer and Managing Director (Principal Financial Officer and Principal Accounting Officer) | ||||||
85