Luxembourg, Malta, Republic of Moldova, Monaco, Montenegro, Morocco, the Netherlands, North Macedonia, Norway, Poland, Portugal, Romania, San Marino, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey), seven issued patents in other foreign jurisdictions (India, Japan, Mexico, South Africa (two patents), Taiwan, Ukraine) and 18 pending foreign patent applications, including one pending European patent application, with claims covering the composition of matter of OCS-01. Patents (including any patents that issue from such patent applications) in this family will expire in 2037, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
As of October 31, 2022, we also owned a patent family that consisted of one U.S. non-provisional patent application and 20 additional foreign patent applications in other jurisdictions, including one European patent application, directed to specific formulations of OCS-01 and methods for stabilizing the composition for use as an eye drop. Patents, if issued from patent applications in this family, will expire in 2040, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
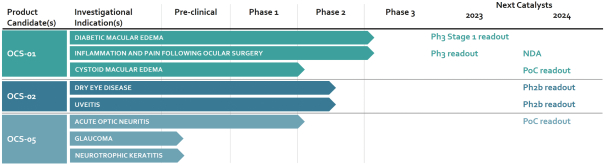
OCS-02
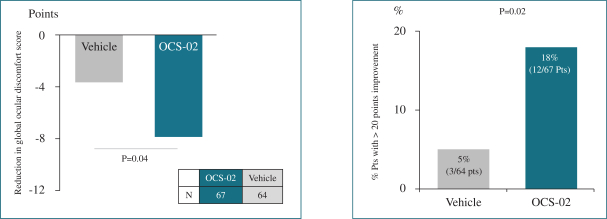
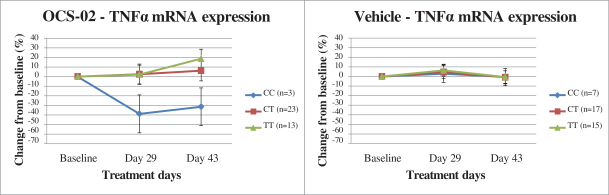
Regarding our OCS-02 product candidate, as of October 31, 2022, we exclusively licensed from Novartis under the Novartis Agreement, in the licensed field as defined in the Novartis Agreement, one patent family that consisted of three issued U.S. patents and two granted European patents (respectively one European patent validated in 36 jurisdictions (Albania, Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Great Britain, Greece, Hungary, Iceland, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Monaco, the Netherlands, North Macedonia, Norway, Poland, Portugal, Romania, Serbia, Slovakia, Slovenia, Spain, Sweden, Switzerland, Turkey) and another European patent validated in six jurisdictions (France, Germany, Great Britain, Italy, Spain, Switzerland), 25 issued patents in other foreign jurisdictions (Australia (three patents), Brazil, Canada, Chile (two patents), China, India, Hong-Kong (2 patents), Japan, Republic of Korea (two patents), Mexico (three patents), Philippines, Russia, South Africa, Taiwan (three patents), Ukraine, Uruguay) and eight patent applications pending in other foreign jurisdictions, with claims covering composition of matter of OCS-02 or methods of use. Patents (including any patents that issue from such patent applications) will expire in 2031, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
In addition, as of October 31, 2022, we exclusively licensed from Novartis under the Novartis Agreement, in the licensed field as defined in the Novartis Agreement, six additional patent families covering composition of matter of OCS-02 or methods of use, including a biomarker for patient selection, which patents (including any patents that issue from patent applications in these families) will expire between 2023 and 2037, without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees. Under the terms of the Novartis Agreement, Novartis is responsible for the prosecution and maintenance of these six patent families.
OCS-03
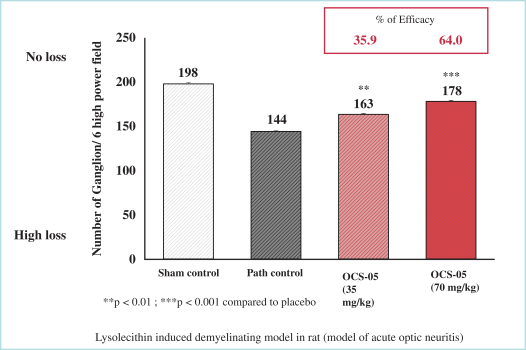
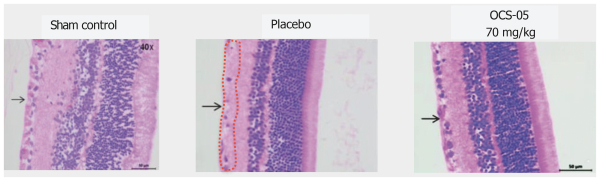
As of October 31, 2022, we also owned a pending PCT application with claims covering composition of matter of OCS-3 and its use. In order for any future patent applications to claim the benefit of such PCT application, they must be filed not later than 12 months after the filing date of such PCT application. Patents, if issued from national phases of such PCT application, will expire in 2041, assuming national phases filings within the 30-month entering to national phase period, and without giving effect to any potential patent term extensions and patent term adjustments and assuming payment of all appropriate maintenance, renewal, annuity or other governmental fees.
222