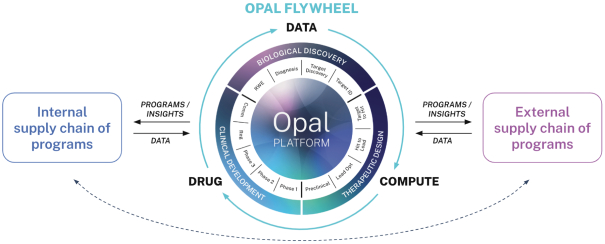
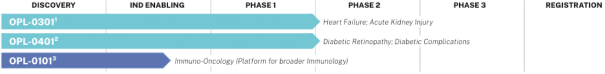
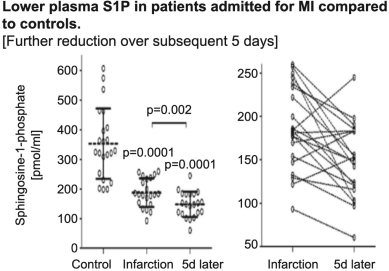
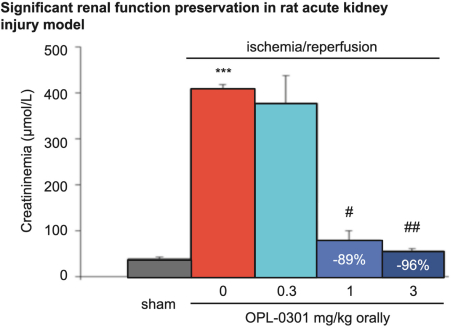
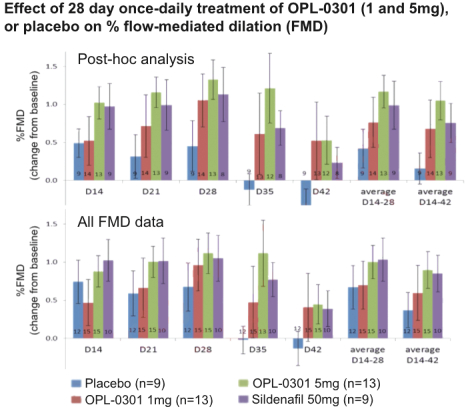
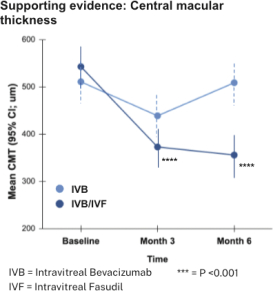
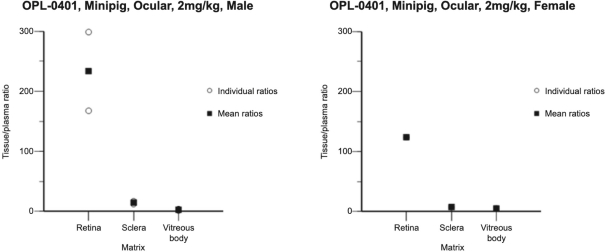
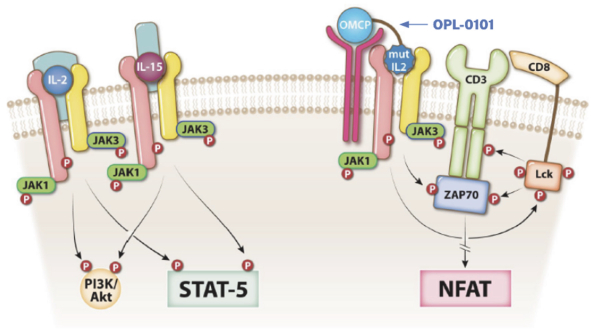
“Financial Statements” has the meaning specified in Section 4.8(a).
“Foreign Benefit Plan” means each Company Benefit Plan that is governed by the laws of any jurisdiction outside of the United States or provides compensation or benefits to any current or former director, officer, individual consultant, worker or employee of a Company Party or any of the Company Parties’ Subsidiaries (or any dependent thereof) who resides outside of the United States.
“Forward Purchase Agreement” means the Forward Purchase Agreement entered into as of March 3, 2021, between Acquiror and Sponsor, as further amended, restated, modified or supplemented from time to time.
“GAAP” means generally accepted accounting principles in the United States as in effect from time to time.
“Goodwin” has the meaning specified in Section 11.18(b).
“Goodwin Privileged Communications” has the meaning specified in Section 11.18(b).
“Governing Documents” means the legal document(s) by which any Person (other than an individual) establishes its legal existence or which govern its internal affairs. For example, the “Governing Documents” of a corporation are its certificate of incorporation and bylaws, the “Governing Documents” of a limited partnership are its limited partnership agreement and certificate of limited partnership, the “Governing Documents” of a limited liability company are its operating agreement and certificate of formation and the “Governing Documents” of an exempted company are its memorandum and articles of association.
“Governmental Authority” means any federal, state, provincial, municipal, local or foreign government, governmental authority, regulatory or administrative agency, governmental commission, department, board, bureau, agency or instrumentality, court or tribunal.
“Governmental Authorization” has the meaning specified in Section 4.5.
“Governmental Order” means any order, judgment, injunction, decree, writ, stipulation, determination or award, in each case, entered by or with any Governmental Authority.
“Hazardous Material” means any (i) pollutant, contaminant, chemical, (ii) industrial, solid, liquid or gaseous toxic or hazardous substance, material or waste, (iii) petroleum or any fraction or product thereof, (iv) asbestos or asbestos-containing material, (v) polychlorinated biphenyl, (vi) chlorofluorocarbons, and (vii) other substance, material or waste, in each case, which are regulated under any Environmental Law or as to which liability may be imposed pursuant to Environmental Law.
“Healthcare Laws” means all applicable Laws relating to patient care or human health and safety, including, as amended from time to time, any such Law pertaining to the research (including preclinical, nonclinical, and clinical research or studies), development, testing, production, manufacture, analysis, transfer, storing, distribution, importation, exportation, use, handling, quality, packaging, approval, labeling, marketing, promotion, pricing, third-party reimbursement or sale of drugs, biological products or medical devices, including (i) the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 301 et seq.), and the United States Public Health Service Act (42 U.S.C. § 201 et seq.); (ii) all applicable Laws relating to any federal health care program (as such term is defined in 42 U.S.C. § 1320a-7b(f)), including the federal Anti-Kickback Statute (42 U.S.C. § 1320a-7b(b)), the Stark Anti-Self-Referral Law (42 U.S.C. § 1395nn), the civil False Claims Act (31 U.S.C. § 3729 et seq.), the administrative False Claims Law (42 U.S.C. § 1320a-7b(a)), Sections 1320a-7, 1320a-7a, and 1320a-7b of Title 42 of the United States Code, any criminal laws relating to health care fraud and abuse, including but not limited to 18 U.S.C. Sections 286, 287, 1347 and 1349 and the health care fraud criminal provisions under the Health Insurance Portability and Accountability Act of 1996, 42 U.S.C. §§ 1320d et seq., and any comparable
A-10