
May 2024 Corporate Presentation Advancing Medicines for Solid Tumors

Important Notice and Disclaimers Except for statements of historical fact, any information contained in this presentation may be a forward-looking statement that reflects the Company’s current views about future events and is subject to risks, uncertainties, assumptions and changes in circumstances that may cause events or the Company’s actual activities or results to differ significantly from those expressed in any forward-looking statement. In some cases, you can identify forward- looking statements by terminology such as “may”, “will”, “should”, “plan”, “predict”, “expect”, “estimate”, “anticipate”, “intend”, “goal”, “strategy”, “believe”, “could”, “would”, “potential”, “project”, “continue” and similar expressions and variations thereof. Forward-looking statements may include statements regarding the Company’s business strategy, cash flows and funding status, potential growth opportunities, clinical development activities, the timing and results of preclinical research, clinical trials and potential regulatory approval and commercialization of product candidates. Although the Company believes that the expectations reflected in such forward- looking statements are reasonable, the Company cannot guarantee future events, results, actions, levels of activity, performance or achievements. Any forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described under the heading “Risk Factors” in documents the Company has filed with the SEC. Any forward-looking statements speak only as of the date of this presentation and the Company undertakes no obligation to revise or update any forward-looking statements to reflect events or circumstances after the date hereof. 2 Context Therapeutics Inc. - May 2024 Certain information contained in this Presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company's own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this Presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. This presentation discusses product candidates that are under preclinical and clinical study, and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these product candidates for the use for which such product candidates are being studied. While the Company believes its internal research is reliable, such research has not been verified by any independent source. All the scientific, preclinical and clinical data presented within this presentation are – by definition prior to completion of the clinical trial and a clinical study report – preliminary in nature and subject to further quality checks including customary source data verification. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. Forward Looking Statement Executive Summary
Lead Program: CTIM-76, a Claudin 6 (CLDN6) x CD3 Bispecific Antibody Executive Summary Context Therapeutics Inc. - May 20243 Lead Asset CTIM-76 is a potentially best-in-class CLDN6 T cell engaging (TCE) bispecific antibody • CLDN6 is enriched in a wide range of cancers, but absent or expressed at low levels in normal adult tissue • CTIM-76 is highly selective for CLDN6, exhibits excellent preclinical efficacy and tolerability • IND cleared, targeting first patient enrolled in mid-2024 Phase 1 Trial Phase 1 trial to focus on CLDN6-positive gynecologic and testicular cancers • Clinical proof of concept achieved with BNT211 CART, highlighting the potential for TCE in reproductive cancers1,2 • Reproductive cancer focus creates clinical efficiencies for CTIM-76 program • Potential to expand clinical footprint once competitors establish proof of concept in other tumor types (e.g., NSCLC) TCE Gaining Momentum Recent TCE clinical data demonstrates promising efficacy and safety in solid tumors • Clinical activity across a broad range of targets, including DLL3, PSMA, and STEAP1 • Clinical activity across multiple tumor types, including SCLC, mCRPC, and neuroendocrine • Low rates Grade ≥ 3 cytokine release syndrome (CRS) Well Capitalized Supported by high-quality investor syndicate with expected cash runway into 2028 • Recently announced private placement funds estimated duration of Phase 1 dose escalation/expansion trial with runway post data readout 1 Reproductive cancers include endometrial, ovarian, and testicular ; 2 Mackensen, ESMO 2023 SCLC = small cell lung cancer ; NSCLC = non- small cell lung cancer; mCRPC = metastatic castrate resistant prostate cancer
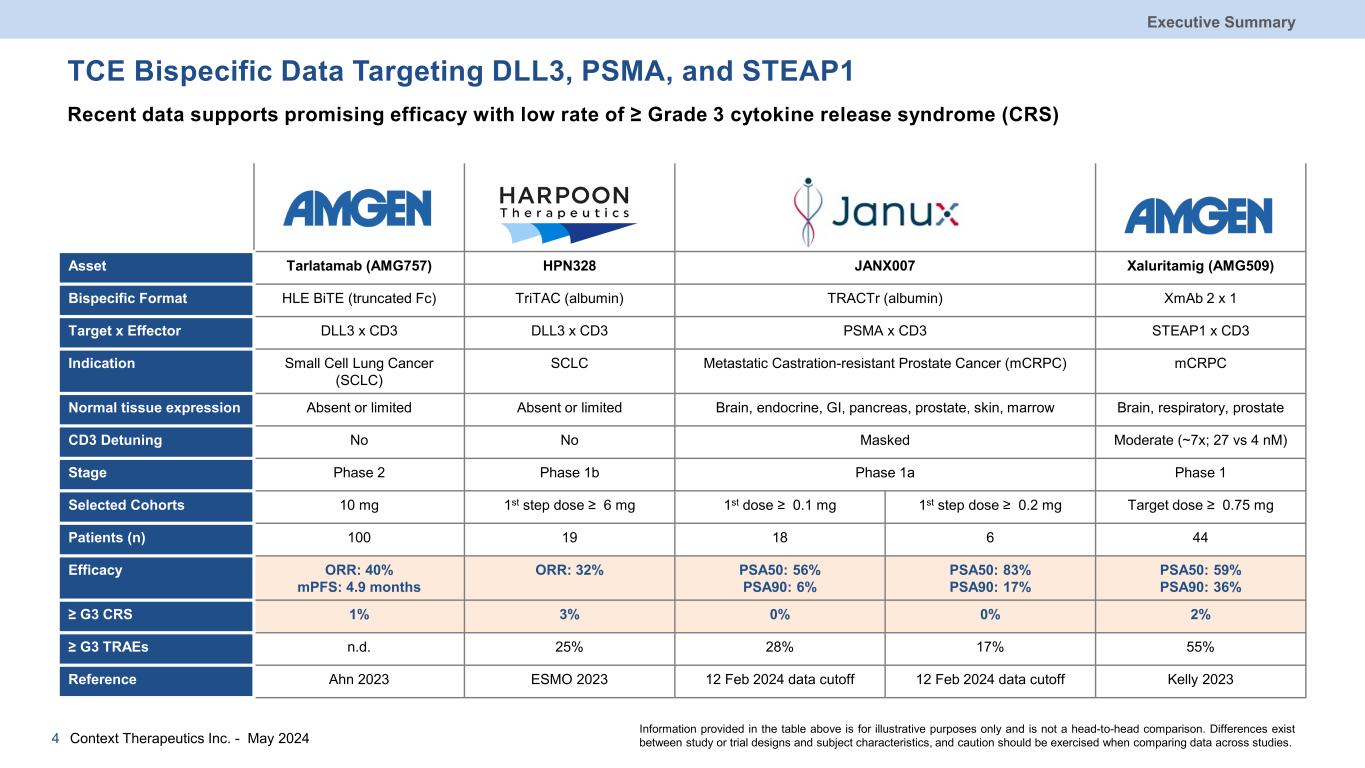
TCE Bispecific Data Targeting DLL3, PSMA, and STEAP1 Recent data supports promising efficacy with low rate of ≥ Grade 3 cytokine release syndrome (CRS) Asset Tarlatamab (AMG757) HPN328 JANX007 Xaluritamig (AMG509) Bispecific Format HLE BiTE (truncated Fc) TriTAC (albumin) TRACTr (albumin) XmAb 2 x 1 Target x Effector DLL3 x CD3 DLL3 x CD3 PSMA x CD3 STEAP1 x CD3 Indication Small Cell Lung Cancer (SCLC) SCLC Metastatic Castration-resistant Prostate Cancer (mCRPC) mCRPC Normal tissue expression Absent or limited Absent or limited Brain, endocrine, GI, pancreas, prostate, skin, marrow Brain, respiratory, prostate CD3 Detuning No No Masked Moderate (~7x; 27 vs 4 nM) Stage Phase 2 Phase 1b Phase 1a Phase 1 Selected Cohorts 10 mg 1st step dose ≥ 6 mg 1st dose ≥ 0.1 mg 1st step dose ≥ 0.2 mg Target dose ≥ 0.75 mg Patients (n) 100 19 18 6 44 Efficacy ORR: 40% mPFS: 4.9 months ORR: 32% PSA50: 56% PSA90: 6% PSA50: 83% PSA90: 17% PSA50: 59% PSA90: 36% ≥ G3 CRS 1% 3% 0% 0% 2% ≥ G3 TRAEs n.d. 25% 28% 17% 55% Reference Ahn 2023 ESMO 2023 12 Feb 2024 data cutoff 12 Feb 2024 data cutoff Kelly 2023 Executive Summary Context Therapeutics Inc. - May 20244 Information provided in the table above is for illustrative purposes only and is not a head-to-head comparison. Differences exist between study or trial designs and subject characteristics, and caution should be exercised when comparing data across studies.

CLDN6 Therapies Have the Potential to Reach a Large Patient Population >50,000 patients per year in the US only in Relapse/Refractory (R/R) Setting Executive Summary 5 Context Therapeutics Inc. - May 2024 Initial indications of interest based on: • CLDN6 prevalence • Patient population size • Observed clinical responses • Potential accelerated pathway Selected Cancer indications Incidence R/R Incidence CLDN6 Positive Patient Population Based on R/R Incidence Endometrial 65,900 14,000 51%1 7,140 Ovarian 19,900 12,800 44%1 5,632 Testicular 9,910 400 94%1 376 Non-Small Cell Lung 201,229 110,653 26%1 28,769 Breast 290,600 43,800 2-41%2,8,9 9,417 Gastric 26,380 11,090 13-55%6,7 3,771 Sarcoma 17,100 12,390 20%11 2,478 Glioma 19,000 10,000 21%6 2,100 Bladder 81,180 17,100 2-8%2,10 855 Small Cell Lung 35,511 19,527 2%2 391 Malignant Rhabdoid 50 500 29-44%2,3-5 183 1 Context internal data; 2 Reinhard, Science, 2020; 3 Wang, Diagn Pathol., 2013; 4 Micke, Intl J Cancer, 2014; 5 Soini, Pol J Path, 2022; 6 Antonelli, Brain Pathol., 2011; 7 Sullivan, Am J Surg Pathol., 2012; 8 Jia, Intl J Clin Exp Pathol., 2019; 9 Yafang, J Breast Cancer, 2011; 10 Ushiku, Histopath., 2012; 11 Mackensen, Nature Medicine, 2023. Incidences based on public estimates; Relapsed/refractory (R/R) or last-line patient population approximated by annual mortality; CLDN6 target prevalence is based on IHC or RNAseq from published reports. Patient population derived from midpoint of CLDN6 positive population multiplied by R/R incident population.
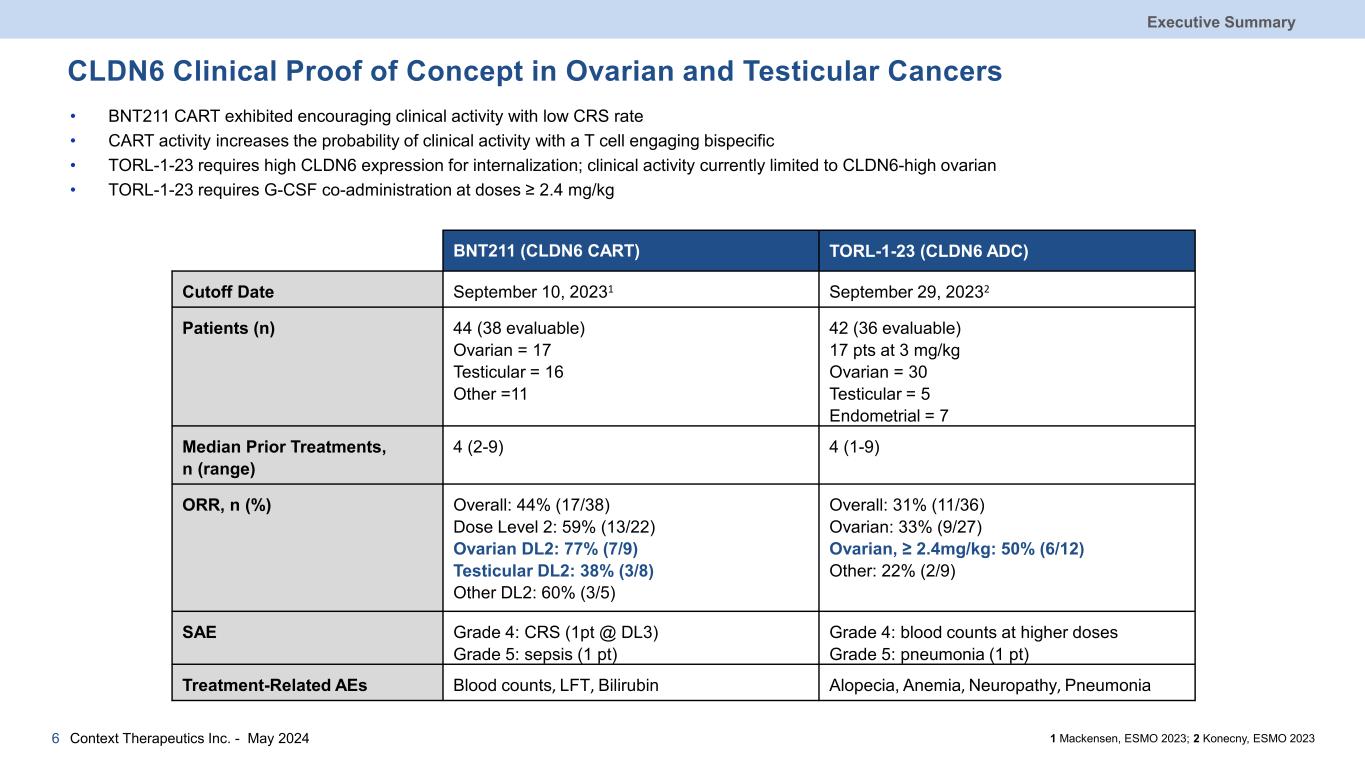
CLDN6 Clinical Proof of Concept in Ovarian and Testicular Cancers Executive Summary Context Therapeutics Inc. - May 20246 BNT211 (CLDN6 CART) TORL-1-23 (CLDN6 ADC) Cutoff Date September 10, 20231 September 29, 20232 Patients (n) 44 (38 evaluable) Ovarian = 17 Testicular = 16 Other =11 42 (36 evaluable) 17 pts at 3 mg/kg Ovarian = 30 Testicular = 5 Endometrial = 7 Median Prior Treatments, n (range) 4 (2-9) 4 (1-9) ORR, n (%) Overall: 44% (17/38) Dose Level 2: 59% (13/22) Ovarian DL2: 77% (7/9) Testicular DL2: 38% (3/8) Other DL2: 60% (3/5) Overall: 31% (11/36) Ovarian: 33% (9/27) Ovarian, ≥ 2.4mg/kg: 50% (6/12) Other: 22% (2/9) SAE Grade 4: CRS (1pt @ DL3) Grade 5: sepsis (1 pt) Grade 4: blood counts at higher doses Grade 5: pneumonia (1 pt) Treatment-Related AEs Blood counts, LFT, Bilirubin Alopecia, Anemia, Neuropathy, Pneumonia 1 Mackensen, ESMO 2023; 2 Konecny, ESMO 2023 • BNT211 CART exhibited encouraging clinical activity with low CRS rate • CART activity increases the probability of clinical activity with a T cell engaging bispecific • TORL-1-23 requires high CLDN6 expression for internalization; clinical activity currently limited to CLDN6-high ovarian • TORL-1-23 requires G-CSF co-administration at doses ≥ 2.4 mg/kg

Claudin 6 (CLDN6) Target biology and therapeutic rationale Context Therapeutics Inc. - May 20247
CLDN6 is an Oncofetal Protein Oncofetal proteins are considered favorable candidates for immunotherapy • Normally present at higher levels during embryonic development • Turned off or have low levels of expression in adult tissues • Increased expression across many solid tumors CLDN6 Target Biology Context Therapeutics Inc. - May 20248 Oncofetal Characteristics of CLDN6 Huan, Mol Med Reports, 2021
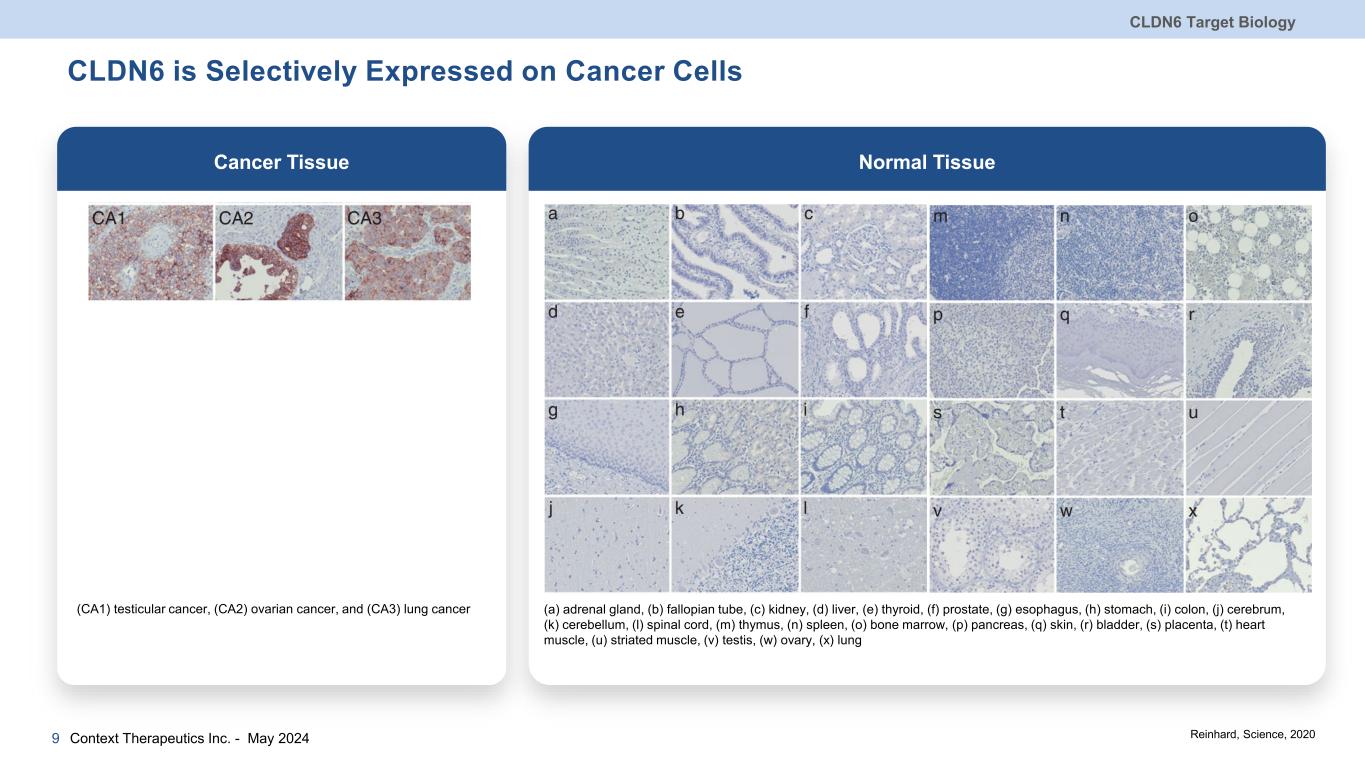
CLDN6 is Selectively Expressed on Cancer Cells CLDN6 Target Biology Context Therapeutics Inc. - May 20249 (a) adrenal gland, (b) fallopian tube, (c) kidney, (d) liver, (e) thyroid, (f) prostate, (g) esophagus, (h) stomach, (i) colon, (j) cerebrum, (k) cerebellum, (l) spinal cord, (m) thymus, (n) spleen, (o) bone marrow, (p) pancreas, (q) skin, (r) bladder, (s) placenta, (t) heart muscle, (u) striated muscle, (v) testis, (w) ovary, (x) lung (CA1) testicular cancer, (CA2) ovarian cancer, and (CA3) lung cancer Reinhard, Science, 2020 Normal TissueCancer Tissue
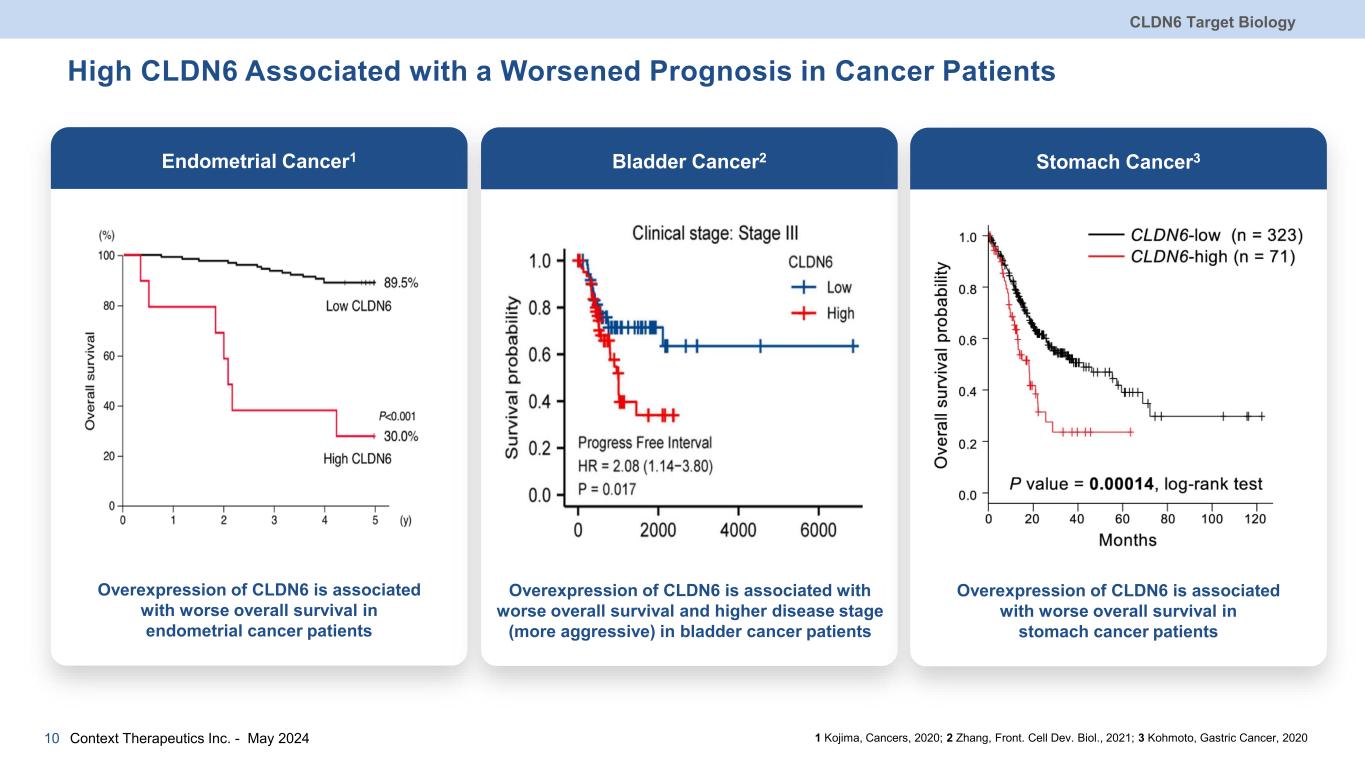
High CLDN6 Associated with a Worsened Prognosis in Cancer Patients CLDN6 Target Biology Context Therapeutics Inc. - May 202410 Endometrial Cancer1 Overexpression of CLDN6 is associated with worse overall survival in endometrial cancer patients 1 Kojima, Cancers, 2020; 2 Zhang, Front. Cell Dev. Biol., 2021; 3 Kohmoto, Gastric Cancer, 2020 Bladder Cancer2 Overexpression of CLDN6 is associated with worse overall survival and higher disease stage (more aggressive) in bladder cancer patients Stomach Cancer3 Overexpression of CLDN6 is associated with worse overall survival in stomach cancer patients

CLDN6 Has Limited Overlap with Competing Targets for Female Reproductive Cancers CLDN6 Target Biology Context Therapeutics Inc. - May 202411 Bishop, AnalyzeR [Data Set] Accessed March 1, 2024 Female Reproductive Cancer Correlation between CLDN6 and other targets via RNAseq biopsy data CLDN6 vs. FRαCLDN6 vs. MesothelinCLDN6 vs. CDH6 CLDN6 vs. B7H3

CTIM-76 Claudin 6 x CD3 Development Candidate Context Therapeutics Inc. - May 202412

Developing a Highly Selective CLDN6 Antibody is Challenging CTIM-76 Program Context Therapeutics Inc. - May 202413 CLDN9 CLDN6 CLDN4 CLDN3 CLDN5 CLDN8 CLDN17 CLDN2 CLDN14 CLDN20 CLDN7 CLDN1 CLDN19 CLDN34 CLDN12 CLDN23 CLDN16 CLDN24 CLDN22 CLDN25 CLDN18 CLDN11 CLDN15 CLDN10 • CLDN6 antigen is conformationally dependent, which limits access to antibody-antigen binding • Antigen binding region is highly conserved with CLDN3, CLDN4, and CLDN9, making CLDN6- selective binding a challenge1 • CLDN6 selectivity is required to avoid off-target liabilities identified in murine knockout and knockdown studies with CLDN3 (intestine)2, CLDN4 (liver, pancreas)3, and CLDN9 (liver, ear)4 1 Screnci, Cancer Res, 2022; 2 Tanaka, J Hepatol, 2018; 3 Cordat, Physiology, 2019; Li, FEBS Open Bio, 2020; 4 Nakano, PLoS Genet, 2009 Human CLDN Family Tree
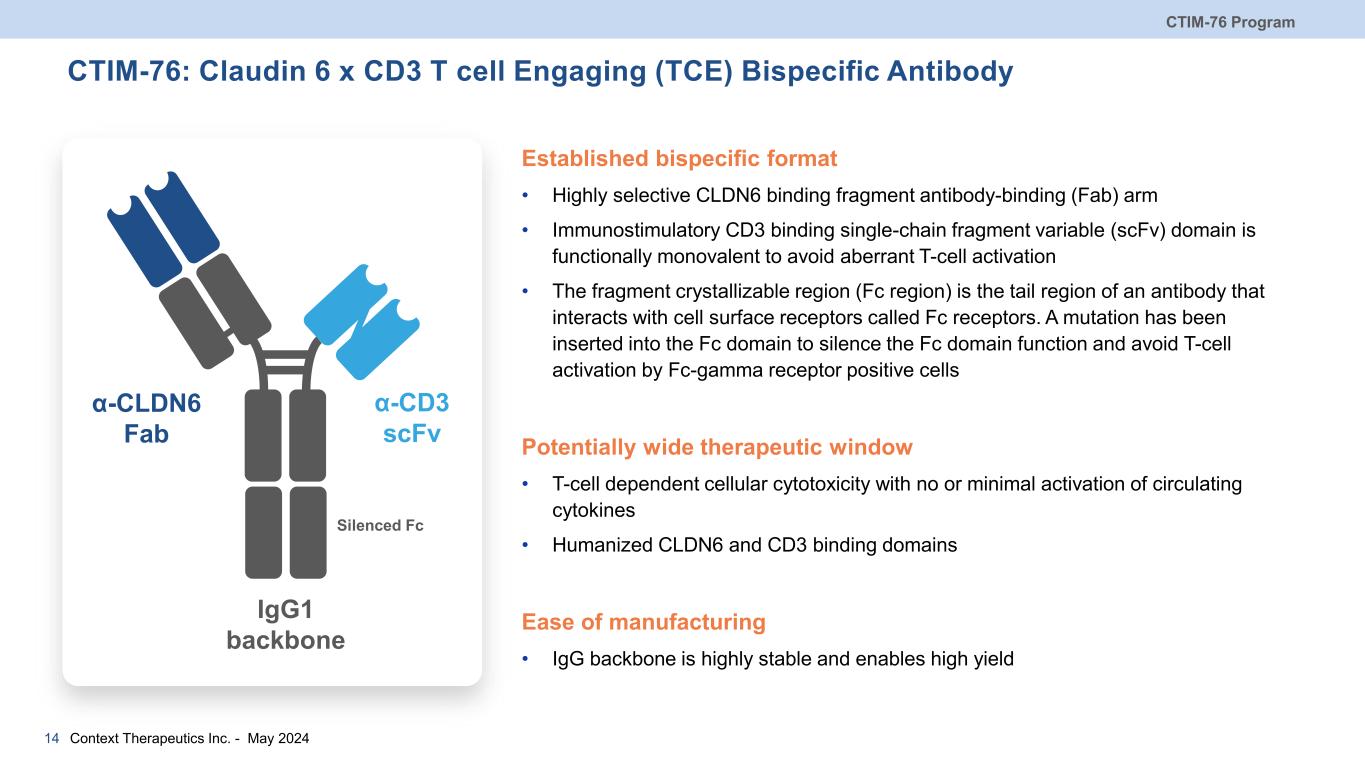
CTIM-76: Claudin 6 x CD3 T cell Engaging (TCE) Bispecific Antibody Established bispecific format • Highly selective CLDN6 binding fragment antibody-binding (Fab) arm • Immunostimulatory CD3 binding single-chain fragment variable (scFv) domain is functionally monovalent to avoid aberrant T-cell activation • The fragment crystallizable region (Fc region) is the tail region of an antibody that interacts with cell surface receptors called Fc receptors. A mutation has been inserted into the Fc domain to silence the Fc domain function and avoid T-cell activation by Fc-gamma receptor positive cells Potentially wide therapeutic window • T-cell dependent cellular cytotoxicity with no or minimal activation of circulating cytokines • Humanized CLDN6 and CD3 binding domains Ease of manufacturing • IgG backbone is highly stable and enables high yield CTIM-76 Program Context Therapeutics Inc. - May 202414 α-CLDN6 Fab α-CD3 scFv IgG1 backbone Silenced Fc

CTIM-76 Program CTIM-76: T cell engaging (TCE) CLDN6 x CD3 Bispecific Antibody Context Therapeutics Inc. - May 202415 Selectivity Potency In Vivo Efficacy • CTIM-76 CLDN6 EC50 of 3.41 nM (binding) • CTIM-76 preferentially binds to CLDN6 over CLDN3/4/9 • CLDN3/4/6/9 were transiently transfected in HEK-293F cells (4:1 Target:GFP) >10,000x • Potency assay provides a better assessment for a TCE bispecific than binding assays for off-target liabilities associated with CLDN3, CLDN4, or CLDN9 • CTIM-76 CLDN6 EC50 of 0.0004 nM (cytotoxicity) • CTIM-76 preferentially targets CLDN6, with minimal binding and cytotoxicity against CLDN9-expressing cells >500x 0 150 300 450 600 0 7 14 21 Tu m or V ol um e (m m 3) Day Vehicle 0.01mg/kg 0.1mg/kg 1.0mg/kg Tumor Regression • CTIM-76 effectively engaged systemically administered human PBMC cells to promote significant tumor regression and complete responses in OVCAR3 (~96,000 CLDN6 copies per cell) ovarian xenograft models in mice • CTIM-76 was well tolerated in OVCAR3 xenograft study • NSG-b2m knockout mice (n=14/arm) engrafted with human PBMCs and bearing advanced subcutaneous OVCAR3 tumor xenografts were treated twice per week
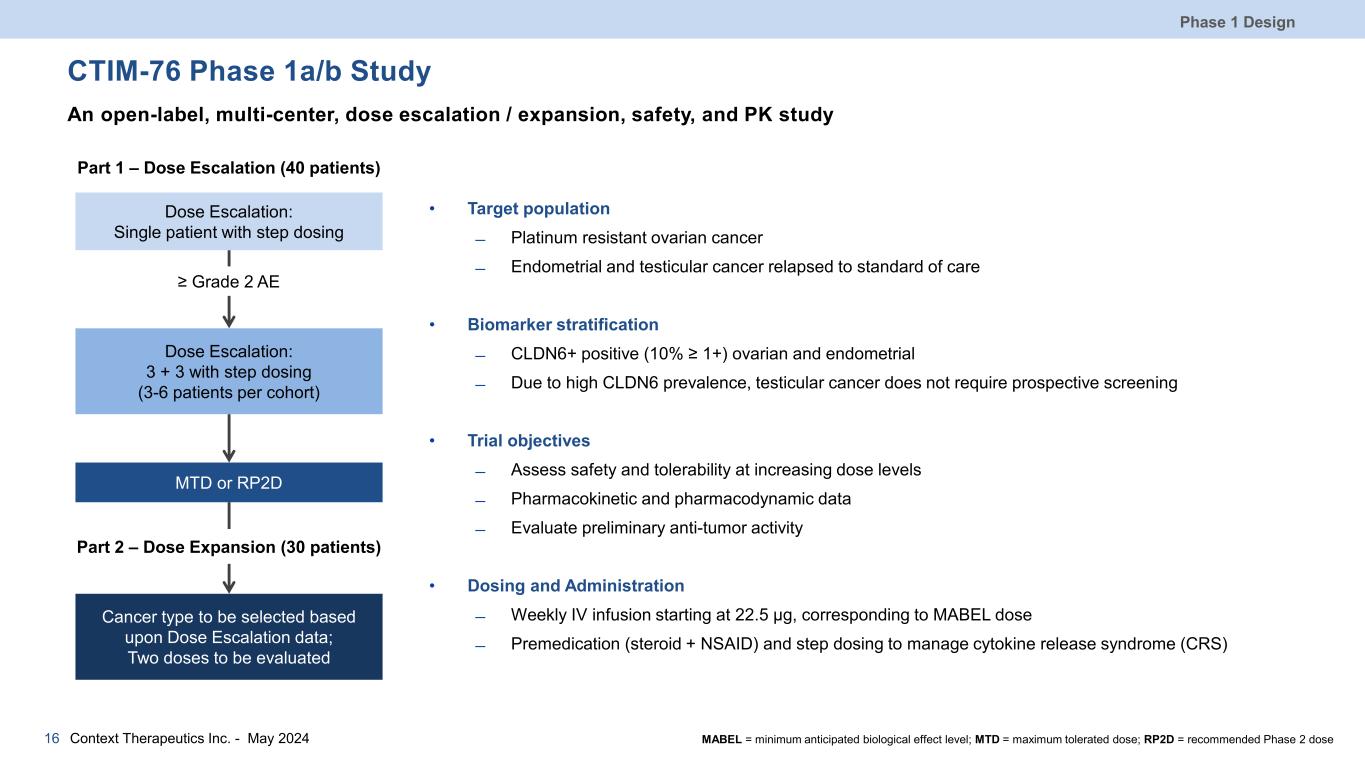
CTIM-76 Phase 1a/b Study An open-label, multi-center, dose escalation / expansion, safety, and PK study • Target population ̶ Platinum resistant ovarian cancer ̶ Endometrial and testicular cancer relapsed to standard of care • Biomarker stratification ̶ CLDN6+ positive (10% ≥ 1+) ovarian and endometrial ̶ Due to high CLDN6 prevalence, testicular cancer does not require prospective screening • Trial objectives ̶ Assess safety and tolerability at increasing dose levels ̶ Pharmacokinetic and pharmacodynamic data ̶ Evaluate preliminary anti-tumor activity • Dosing and Administration ̶ Weekly IV infusion starting at 22.5 µg, corresponding to MABEL dose ̶ Premedication (steroid + NSAID) and step dosing to manage cytokine release syndrome (CRS) Phase 1 Design 16 Dose Escalation: Single patient with step dosing Dose Escalation: 3 + 3 with step dosing (3-6 patients per cohort) MTD or RP2D Cancer type to be selected based upon Dose Escalation data; Two doses to be evaluated ≥ Grade 2 AE Part 2 – Dose Expansion (30 patients) Part 1 – Dose Escalation (40 patients) Context Therapeutics Inc. - May 2024 MABEL = minimum anticipated biological effect level; MTD = maximum tolerated dose; RP2D = recommended Phase 2 dose

Competitive Landscape 17 Context Therapeutics Inc. - May 2024
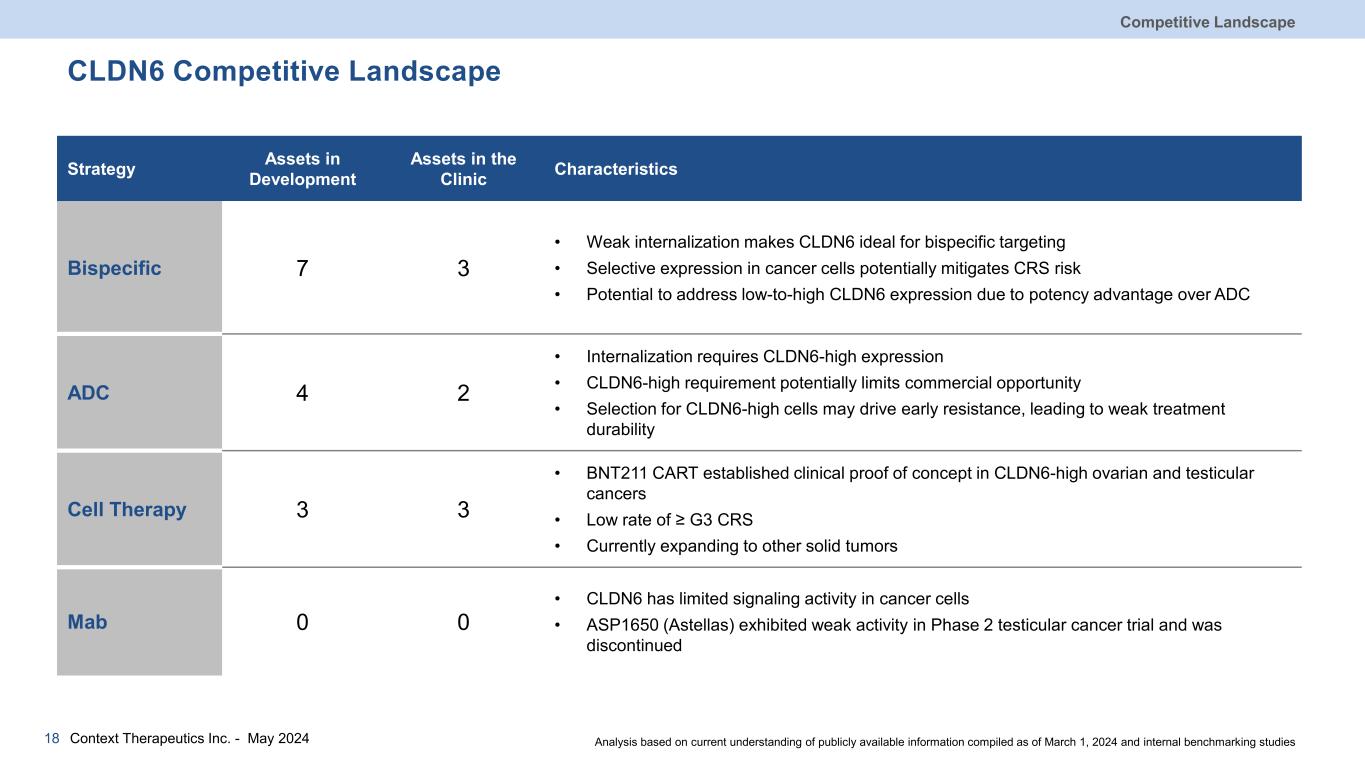
CLDN6 Competitive Landscape Strategy Assets in Development Assets in the Clinic Characteristics Bispecific 7 3 • Weak internalization makes CLDN6 ideal for bispecific targeting • Selective expression in cancer cells potentially mitigates CRS risk • Potential to address low-to-high CLDN6 expression due to potency advantage over ADC ADC 4 2 • Internalization requires CLDN6-high expression • CLDN6-high requirement potentially limits commercial opportunity • Selection for CLDN6-high cells may drive early resistance, leading to weak treatment durability Cell Therapy 3 3 • BNT211 CART established clinical proof of concept in CLDN6-high ovarian and testicular cancers • Low rate of ≥ G3 CRS • Currently expanding to other solid tumors Mab 0 0 • CLDN6 has limited signaling activity in cancer cells • ASP1650 (Astellas) exhibited weak activity in Phase 2 testicular cancer trial and was discontinued Competitive Landscape Context Therapeutics Inc. - May 202418 Analysis based on current understanding of publicly available information compiled as of March 1, 2024 and internal benchmarking studies

CLDN6 Competitive Landscape Context Therapeutics Inc. - May 202419 Scalable Manufacturing Process Complex Manufacturing Process Potential / Disclosed Safety Liabilities Selectivity for CLDN6 vs CLDN3,4,9 Limited Information on Asset Limited Selectivity Deprioritized CLDN6-CAR-NK CAR-NK + IL7 FPI Q2 22 TJ-C64B2 2+2 bsAb CLDN6x4IBB TORL-1-23 CLDN6 + MMAE FPI Q4 21 SAIL66 bsAb CLDN6xCD3 FPI Q1 23 Analysis based on current understanding of publicly available information compiled as of May 1, 2024 and internal benchmarking studies; 1 Anticipated first patient enrolled 2 TJ-C64B deprioritization per Q2 2023 earnings guidance; 3 Pham et al, AMG 794, a Claudin 6-targeted half-life extended (HLE) bispecific T cell engager (BITE®), AACR 2022; 4 Hamilton, First-in-human study of SC-004, AACR 2020; FPI = First Patient In Phase 1 trial; DLT = Dose Limiting Toxicity CTIM-761 bsAb CLDN6xCD3 FPI mid-2024 Competitive Landscape Undisclosed CAR-NK IND 2H 23 Undisclosed bsAb CLDN6xCD3 IND Q4 23 BNT211 CAR-T + CARVac FPI Q3 20 AMG-7943 BiTE CLDN6xCD3 FPI Q1 23 BNT142 mRNA BsAb CLDN6xCD3 FPI Q1 22 XmAb541 2+1 bsAb CLDN6xCD3 IND Q4 23 DS-9606a CLDN6/CLDN9 + 2nd gen toxin FPI Q2 22 GB-7008-01 CLDN6/CLDN9 + MMAE Status Unknown Selective, Potent, Scalable SC0044 CLDN6/CLDN9 + PBD Ph 1 DLT NBL-028 bsAb CLDN6 x CD137 FPI Q2 24
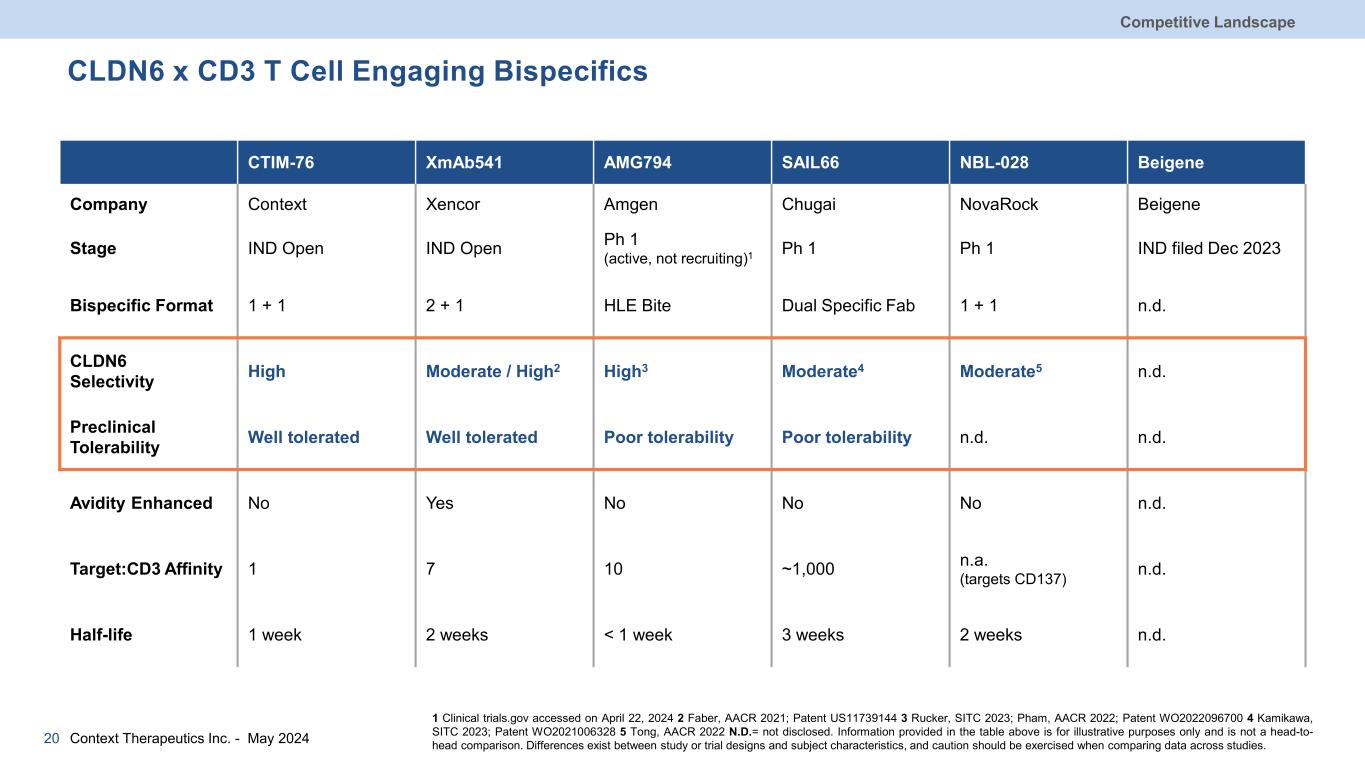
CLDN6 x CD3 T Cell Engaging Bispecifics Competitive Landscape 20 CTIM-76 XmAb541 AMG794 SAIL66 NBL-028 Beigene Company Context Xencor Amgen Chugai NovaRock Beigene Stage IND Open IND Open Ph 1 (active, not recruiting)1 Ph 1 Ph 1 IND filed Dec 2023 Bispecific Format 1 + 1 2 + 1 HLE Bite Dual Specific Fab 1 + 1 n.d. CLDN6 Selectivity High Moderate / High2 High3 Moderate4 Moderate5 n.d. Preclinical Tolerability Well tolerated Well tolerated Poor tolerability Poor tolerability n.d. n.d. Avidity Enhanced No Yes No No No n.d. Target:CD3 Affinity 1 7 10 ~1,000 n.a. (targets CD137) n.d. Half-life 1 week 2 weeks < 1 week 3 weeks 2 weeks n.d. 1 Clinical trials.gov accessed on April 22, 2024 2 Faber, AACR 2021; Patent US11739144 3 Rucker, SITC 2023; Pham, AACR 2022; Patent WO2022096700 4 Kamikawa, SITC 2023; Patent WO2021006328 5 Tong, AACR 2022 N.D.= not disclosed. Information provided in the table above is for illustrative purposes only and is not a head-to- head comparison. Differences exist between study or trial designs and subject characteristics, and caution should be exercised when comparing data across studies.Context Therapeutics Inc. - May 2024

Corporate 21 Context Therapeutics Inc. - May 2024

Corporate Experienced Leadership Team • Experienced team • Our management team is supported by a Board with strong public company operating and governance experience Focus on Execution Martin Lehr CEO and Director Alex Levit, Esq Chief Legal Officer Jennifer Minai, CPA Chief Financial Officer Chris Beck, MBA SVP Operations Context Therapeutics Inc. - May 202422

Corporate Investment Highlights (Nasdaq: CNTX) Solid Tumors Large Unmet Need Claudin 6 High-Value Target IND cleared April 2024 On track for first patient enrolled mid-2024 Milestones Oncology experience Strong Team Expected cash runway into 2028 Cash Runway Context Therapeutics Inc. - May 202423

Advancing Medicines for Solid Tumors © Context Therapeutics 2024























