Exhibit 99.2

©EQRx 2021 | REMAKING MEDICINE v v v v5 March 23, 2022, 8:00am ET Conference Call and Webcast Q4 & FY 2021 Financial Results, Business Update ©EQRx 2022 | REMAKING MEDICINE

©EQRx 2021 | REMAKING MEDICINE DISCLAIMER This presentation (this “Presentation”) relates to EQRx , Inc. (together with its subsidiaries, “ EQRx ”). EQRx is a new type of pharmaceutical company committed to developing and delivering innovative medicines to patients at radically lo wer prices. Launched in January 2020, EQRx is purpose - built, at scale, with a growing catalog of medicines in development in high - cost drug categories and emerging partne rships with leading payers and providers. Leveraging cutting - edge science and technology and strategic partnerships with stakeholders from across the healthcare system, EQRx aims to provide innovative, patent - protected medicines more efficiently and cost - effectively than ever before. In December 2021 , EQRx completed the business combination among EQRx International, Inc. and CM Life Sciences III and began trading on the Nasdaq Global Market as the combined company under the ti cker symbol EQRX . Forward - Looking Statements. Certain statements in this Presentation may be considered forward - looking statements within the meaning of the provisions of the Private Securities Litigation Reform Act of 1995. Forward - looking statements generally relate to potential future events or EQRx’s potential future financial or operating performance. For example, express or implied statements concerning the following incl ud e forward - looking statements: EQRx’s ability to develop and deliver innovative medicines at radically lower prices, EQRx’s ability to create a new pharma platform that both improves patients’ lives and delivers meaningful savings to payers and heal th systems around the world, EQRx’s plans and timelines for the clinical development and regulatory review of EQRx’s product candidates both in and outside the U.S. including with respect to regulators’ acceptance of clinical data generated b y third parties, the therapeutic potential and clinical benefits and tolerability of EQRx’s product candidates, expectations regarding EQRx’s Global Buyers Club and number of covered lives reached and ability to convert MOUs into binding, definitive agreements, EQRx’s cash runway and estimated cash outflow, as well as other statements regarding plans and market opportunities of EQRx . In some cases, you can identify forward - looking statements by terminology such as (but not limited to) “may”, “should”, “expec t”, “intend”, “will”, “estimate”, “anticipate”, “believe”, “predict”, “potential”, “could”, “project”, “budget”, “forecast”, “ant ici pate”, “plan”, “design” or “continue”, or the negatives of these terms or variations of them or similar terminology. Such forward - looking statements are subject to risks, uncertainties, and other factors that could cause actual results to differ mate rially from those expressed or implied by such forward - looking statements. These forward - looking statements are based upon estimates and assumptions that, while considered reasonable by EQRx and its management, are inherently uncertain. New risks and uncertainties may emerge from time to time, and it is not possibl e to predict all risks and uncertainties. Factors that may cause actual results to differ materially from current expectations include, but are not limi ted to, various factors beyond EQRx’s control, including changes in the competitive and highly regulated industries in which EQRx operates, the timing and outcome of the EQRx’s planned interactions with regulatory authorities, delay of any current and future clinical trials or the development or regul at ory approval of aumolertinib , sugemalimab or EQRx’s other drug candidates, as well as other general economic conditions and other risks, uncertainties and factors set forth in t he section entitled “Risk Factors” and “Cautionary Note Regarding Forward - Looking Statements” in EQRx’s most recent Annual Report on Form 10 - K or Quarterly Report on Form 10 - Q filings, and other documents filed by EQRx from time to time with the SEC, as well as factors associated with companies, such as EQRx , that operate in the biopharma industry, including uncertainty in the timing or results of preclinical studies and clinical trials, product acceptance and/or receipt of regulatory approvals for product candidates, including any delays and other impacts from the ongoing COVID - 19 pandemic. Nothing in this Presentation should be regarded as a representation by any person that the forward - looking statements set forth herein will be achieved or that any of the contemplated results of such forward - looking statements will be achieved. You should not place undue reliance on forward - looking statements in this Presentation, which speak only as of the date they are made and are qualified in their entirety by reference to the cautionary statements herein. EQRx does not undertake or accept any duty to release publicly any updates or revisions to any forward - looking statements to reflect any change in its expectations or any change in events, conditions or circumstances on which any such statement is based. This Presentation does not purport to summarize all of the conditions, risks and other attributes of an investment in EQRx . Industry and Market Data. Certain information contained in this Presentation relates to or is based on studies, publications, surveys and EQRx’s own internal estimates and research. In this Presentation, EQRx relies on and refers to publicly available information and statistics regarding market participants in the sectors in which EQRx competes and other industry data. Any comparison of EQRx to any other entity assumes the reliability of the information available to EQRx . EQRx obtained this information and statistics from third - party sources, including reports by market research firms and company filing s. In addition, all of the market data included in this Presentation involve a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while EQRx believes its internal research is reliable, such research has not been verified by any independent source, and EQRx has not independently verified the information. Trademarks. The EQRx logo and other trademarks or service marks or EQRx appearing in this Presentation are the property of EQRx . This Presentation also contains registered marks, trademarks, and trade names of other companies. All other registered marks, and trade names appearing herein are the property of their respective holders. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this Presentation may be listed without the TM, SM © or ® symbols. 2 ©EQRx 2022 | REMAKING MEDICINE

©EQRx 2021 | REMAKING MEDICINE IMPROVE HEALTH FOR ALL WITH GREAT, INNOVATIVE, AFFORDABLE MEDICINES OUR MISSION

©EQRx 2021 | REMAKING MEDICINE Today’s 3 key takeaways Expanding the Global Buyers Club; goal to have MOUs with payers that cover 350M lives by the end of 2022 $1.7B in cash and cash equivalents*, with expected runway into 2025 Expect first submissions to be ex - US in 2H 2022 for lead programs; continuing to engage with the FDA * As of December 31, 2021 ©EQRx 2022 | REMAKING MEDICINE 4 FINANCIALS CATALOG OF MEDICINES BUYERS CLUB GLOBAL

©EQRx 2021 | REMAKING MEDICINE 5 Aumolertinib | 3rd - generation EGFR TKI Phase 3 trial in patients with 1L EGFR+ NSCLC* demonstrated statistically significant PFS benefit , with the potential to mitigate EGFR wild - type mediated toxicities Completed PK study conducted in the US and New Zealand in an ethnically diverse population By mid - 2022, initiate US - led trial to compare aumolertinib , to aumolertinib + chemo, to osimertinib for the treatment of 1L EGFR+ NSCLC to assess the applicability of the pivotal trial results to current medical practice in a diverse patient population Clinical Evidence ©EQRx 2022 | REMAKING MEDICINE Expect first submissions to be ex - US in 2H 2022 for 1L EGFR+ NSCLC; received Innovation Passport designation pursuant to ILAP pathway in the UK ex - US Regulatory Continuing to engage with the FDA; will provide updates on approach and timelines when we have further clarity US Regulatory Expect first submissions to be ex - US in 2H 2022, continuing to engage with the FDA This is an investigational asset. Safety and efficacy have not been established and there is no guarantee that the outcome of these studies will result in approval by a regulatory authority. NSCLC: non - small cell lung cancer

©EQRx 2021 | REMAKING MEDICINE 6 Sugemalimab | anti - PD - L1 antibody Phase 3 Stage IV NSCLC trial demonstrated statistically significant PFS and OS benefit on pre - specified analyses, regardless of tumor pathologic subtype or PD - L1 expression levels Phase 3 Stage III NSCLC trial demonstrated statistically significant PFS benefit , treating patients with either sequential or concurrent chemoradiotherapy; expect results from pre - specified OS analysis in 2023 In 2H 2022, initiate US - led trial to compare sugemalimab to other approved checkpoint inhibitor(s) to assess the applicability of the pivotal trial results to current medical practice in a diverse patient population Clinical Evidence ©EQRx 2022 | REMAKING MEDICINE Expect first submissions to be ex - US in 2H 2022 for Stage IV NSCLC; received Innovation Passport designation pursuant to ILAP pathway in the UK ex - US Regulatory Breakthrough Designation for ENKTL, with planned submission in 2023 Continuing to engage with the FDA; will provide updates on approach and timelines for NSCLC US Regulatory Expect first submissions to be ex - US in 2H 2022, continuing to engage with the FDA This is an investigational asset. Safety and efficacy have not been established and there is no guarantee that the outcome of these studies will result in approval by a regulatory authority.
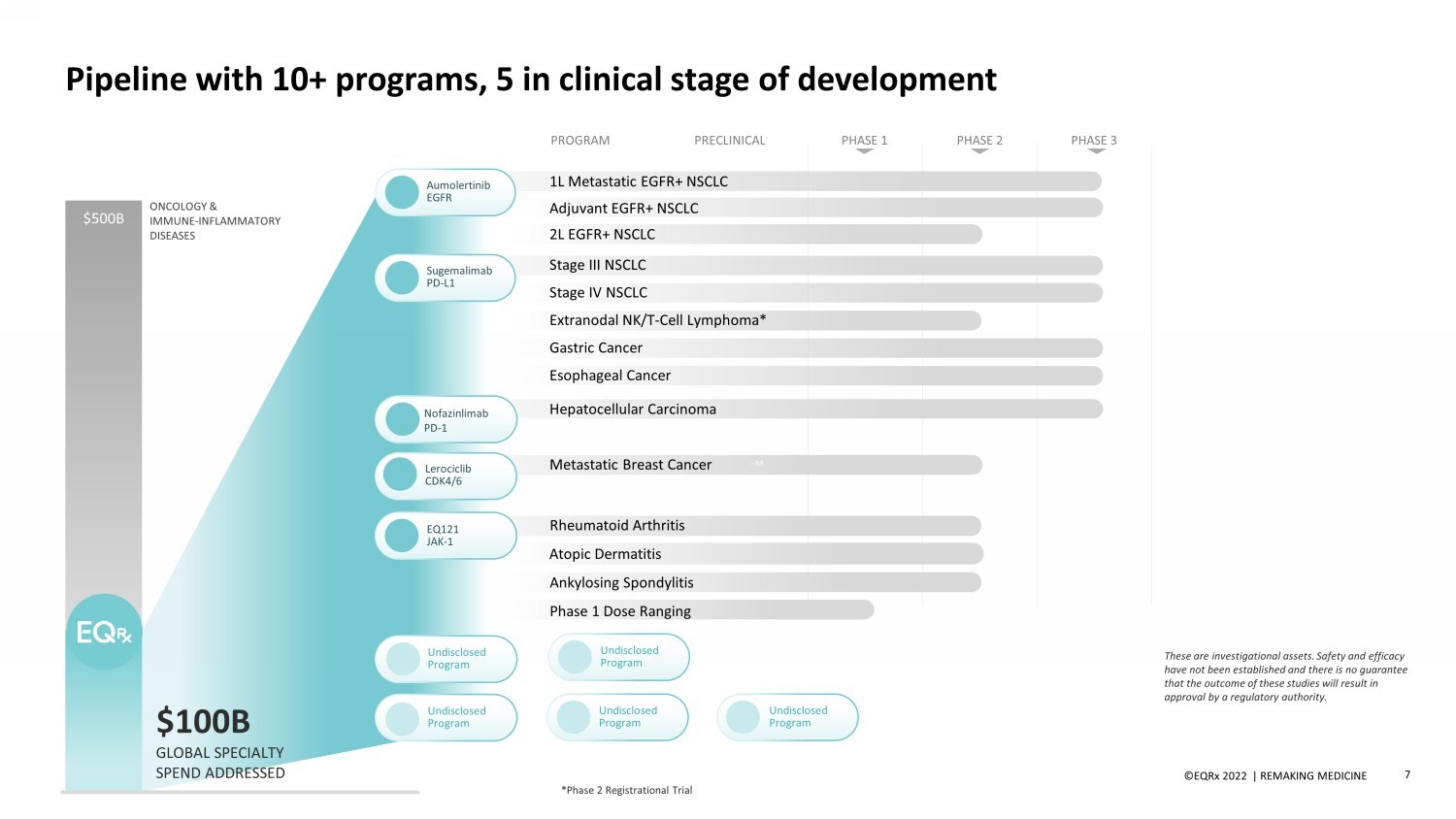
©EQRx 2021 | REMAKING MEDICINE 7 Pipeline with 10+ programs, 5 in clinical stage of development ONCOLOGY & IMMUNE - INFLAMMATORY DISEASES Undisclosed Program Undisclosed Program Undisclosed Program Undisclosed Program Undisclosed Program $500B GLOBAL SPECIALTY SPEND ADDRESSED $100B Nofazinlimab PD - 1 Hepatocellular Carcinoma Sugemalimab PD - L1 Stage III NSCLC Stage IV NSCLC Extranodal NK/T - Cell Lymphoma* Gastric Cancer Esophageal Cancer Adjuvant EGFR+ NSCLC 1L Metastatic EGFR+ NSCLC 2L EGFR+ NSCLC Aumolertinib EGFR PRECLINICAL PROGRAM PHASE 2 PHASE 3 PHASE 1 ©EQRx 2022 | REMAKING MEDICINE *Phase 2 Registrational Trial – Phase 1 Dose Ranging Ankylosing Spondylitis Atopic Dermatitis Rheumatoid Arthritis EQ121 JA K - 1 Lerociclib CDK4/6 Metastatic Breast Cancer – M These are investigational assets. Safety and efficacy have not been established and there is no guarantee that the outcome of these studies will result in approval by a regulatory authority.

©EQRx 2021 | REMAKING MEDICINE 8 Selectively add to our portfolio x Address a drug class of high - cost burden to patients and society and deliver meaningful value to the members of the Global Buyers Club x Equally good or better medicine x Areas where there are known, clear and causal mechanisms of action x Patent - protected, innovative drugs with sufficient patent runway in the class x Opportunity to capture significant share of market 5 + PRECLINICAL / DISCOVERY CLINICAL 5 CATALOG OF MEDICINES Criteria: ©EQRx 2022 | REMAKING MEDICINE
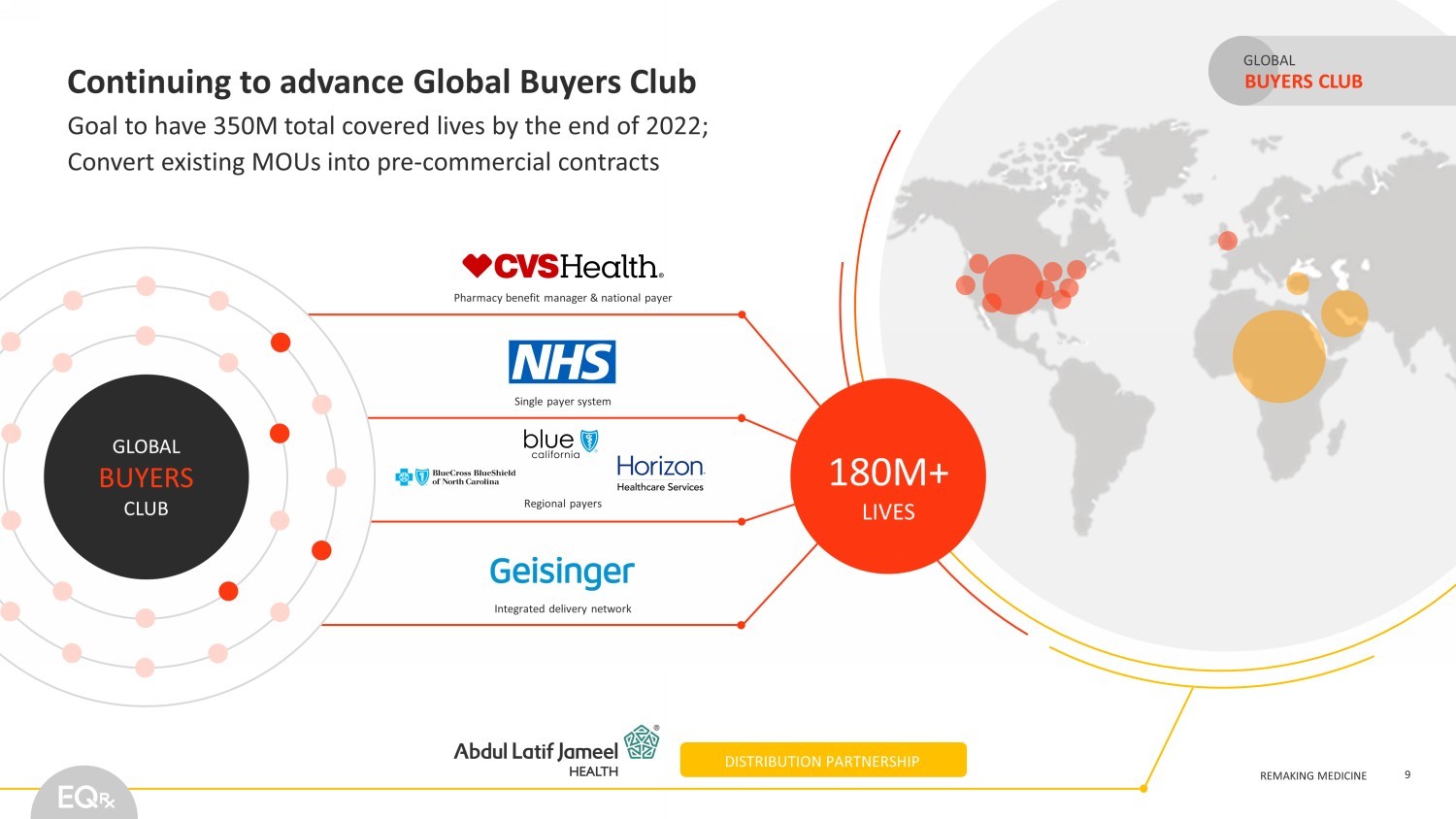
©EQRx 2021 | REMAKING MEDICINE REMAKING MEDICINE DISTRIBUTION PARTNERSHIP Pharmacy benefit manager & national payer Single payer system Regional payers Integrated delivery network GLOBAL BUYERS CLUB 180M+ LIVES Continuing to advance Global Buyers Club Goal to have 350M total covered lives by the end of 2022; Convert existing MOUs into pre - commercial contracts BUYERS CLUB GLOBAL 9
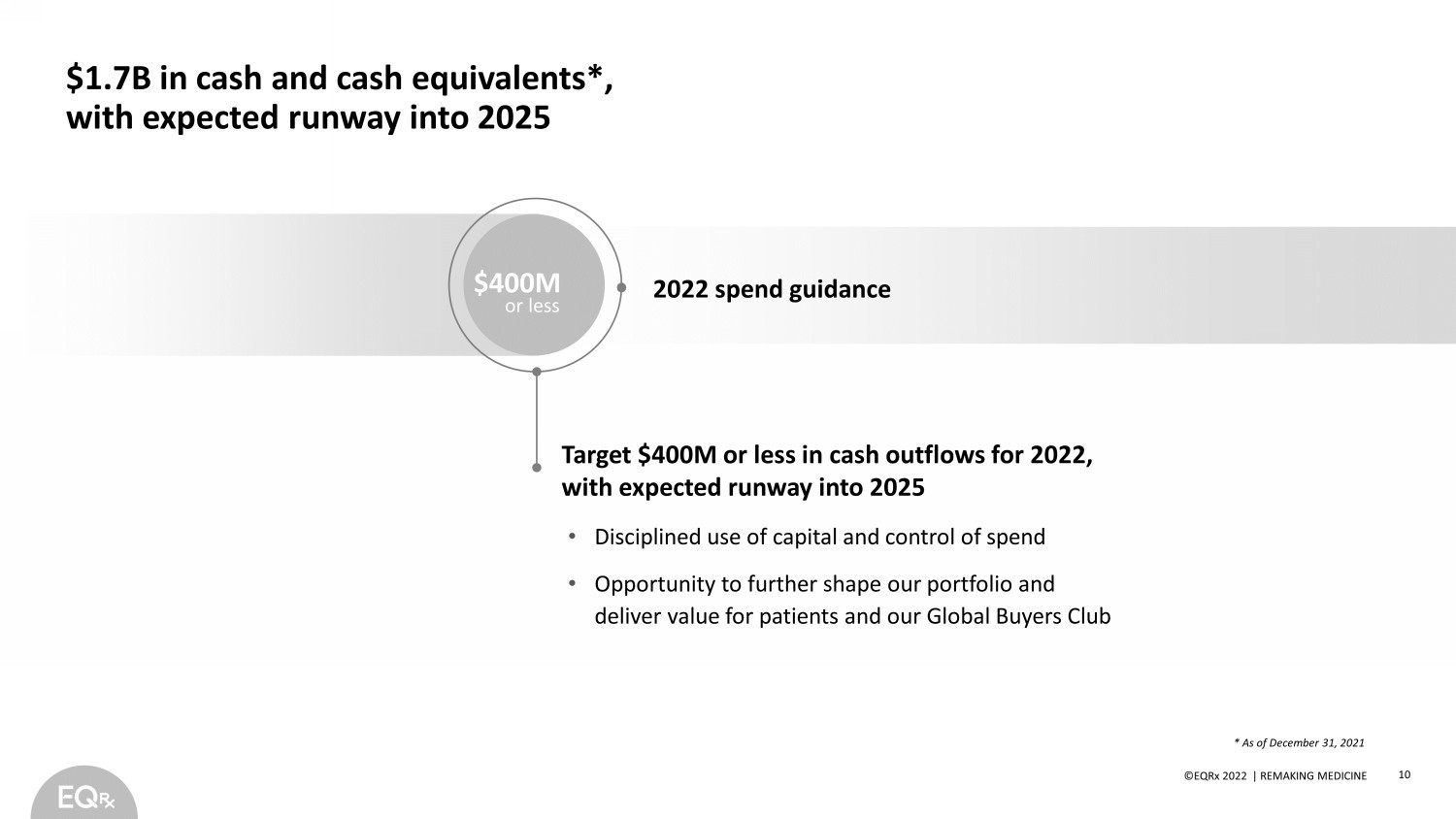
©EQRx 2021 | REMAKING MEDICINE Very selectively add to our portfolio where we can: $1.7B in cash and cash equivalents*, with expected runway into 2025 • Disciplined use of capital and control of spend • Opportunity to further shape our portfolio and deliver value for patients and our Global Buyers Club Target $400M or less in cash outflows for 2022, with expected runway into 2025 2022 spend guidance 10 ©EQRx 2022 | REMAKING MEDICINE $400M or less * As of December 31, 2021
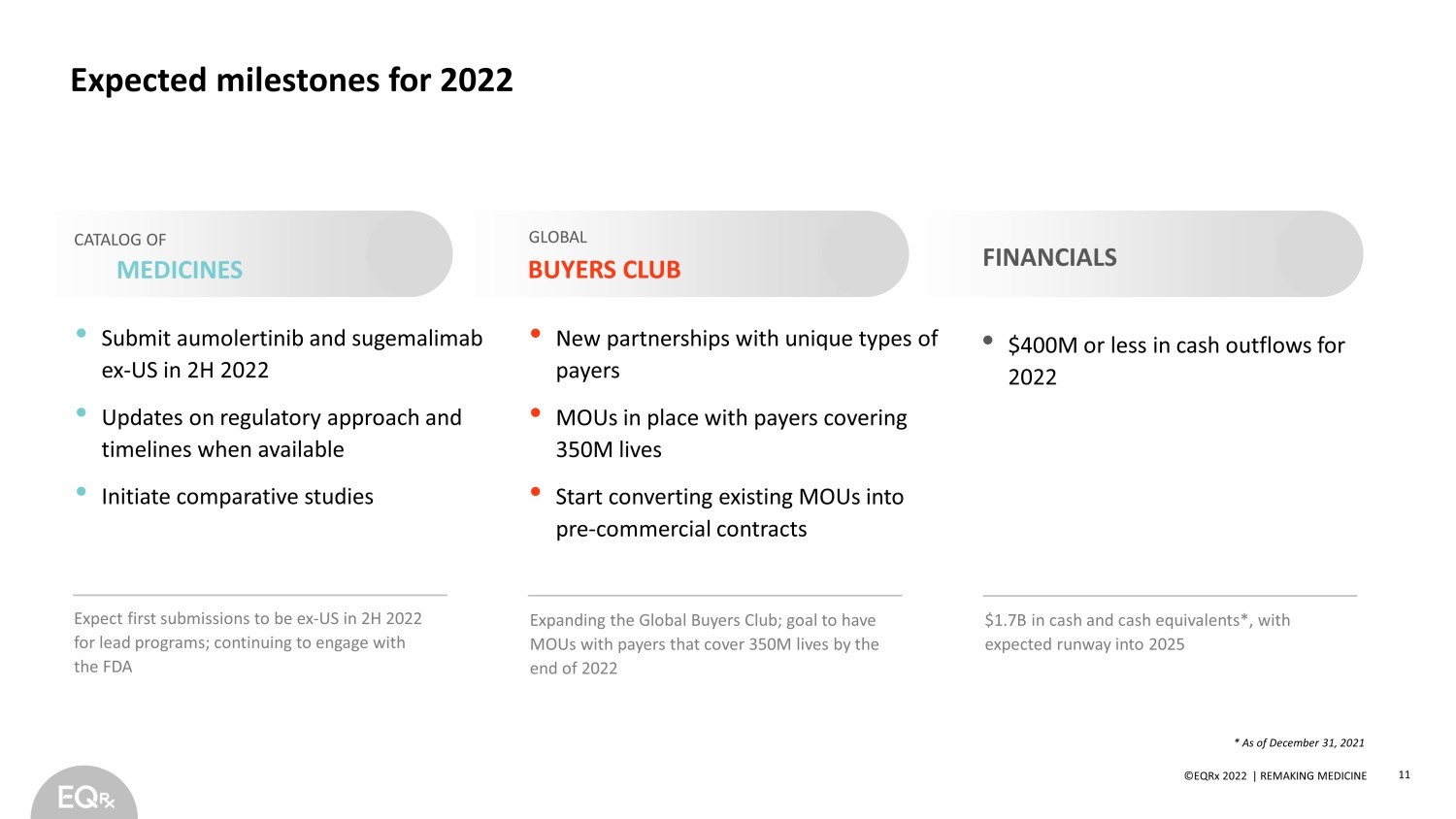
©EQRx 2021 | REMAKING MEDICINE Expected milestones for 2022 • Submit aumolertinib and sugemalimab ex - US in 2H 2022 • Updates on regulatory approach and timelines when available • Initiate comparative studies • $400M or less in cash outflows for 2022 • New partnerships with unique types of payers • MOUs in place with payers covering 350M lives • Start converting existing MOUs into pre - commercial contracts FINANCIALS CATALOG OF MEDICINES BUYERS CLUB GLOBAL * As of December 31, 2021 11 ©EQRx 2022 | REMAKING MEDICINE Expanding the Global Buyers Club; goal to have MOUs with payers that cover 350M lives by the end of 2022 $1.7B in cash and cash equivalents*, with expected runway into 2025 Expect first submissions to be ex - US in 2H 2022 for lead programs; continuing to engage with the FDA

REMAKING MEDICINE This is NEW PHARMA











