Exhibit 99.1
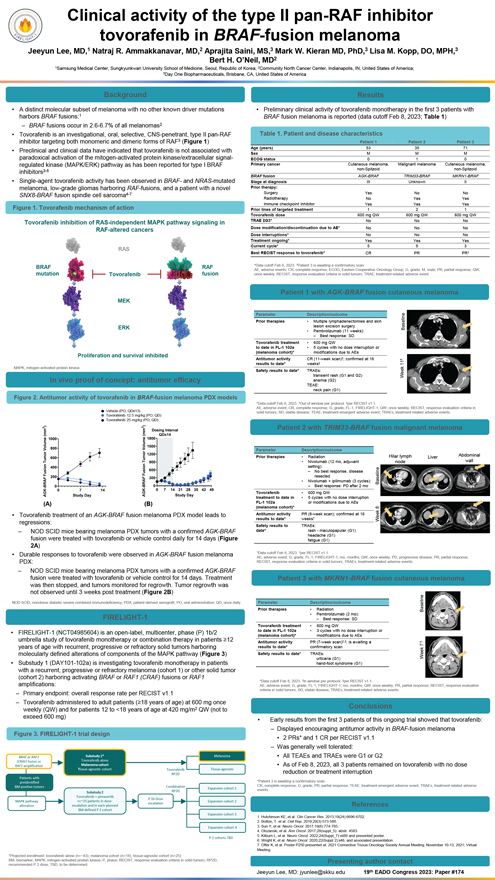
Clinical activity of the type II pan-RAF inhibitor tovorafenib in BRAF-fusion melanoma Jeeyun Lee, MD,1 Natraj R. Ammakkanavar, MD,2 Aprajita Saini, MS,3 Mark W. Kieran MD, PhD,3 Lisa M. Kopp, DO, MPH,3 Bert H. O’Neil, MD2 1Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea; 2Community North Cancer Center, Indianapolis, IN, United States of America; 3Day One Biopharmaceuticals, Brisbane, CA, United States of America Background • A distinct molecular subset of melanoma with no other known driver mutations harbors BRAF fusions:1 – BRAF fusions occur in 2.6-6.7% of all melanomas2 • Tovorafenib is an investigational, oral, selective, CNS-penetrant, type II pan-RAF inhibitor targeting both monomeric and dimeric forms of RAF3 (Figure 1) • Preclinical and clinical data have indicated that tovorafenib is not associated with paradoxical activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) pathway as has been reported for type I BRAF inhibitors3-5 • Single-agent tovorafenib activity has been observed in BRAF- and NRAS-mutated melanoma, low-grade gliomas harboring RAF-fusions, and a patient with a novel SNX8-BRAF fusion spindle cell sarcoma4-7 Figure 1. Tovorafenib mechanism of action Tovorafenib inhibition of RAS-independent MAPK pathway signaling in RAF-altered cancers RAS BRAF RAF mutation Tovorafenib fusion MEK ERK feration and survival inhibite MAPK, mitogen-activated protein kinase. In vivo proof of concept: antitumor efficacy Figure 2. Antitumor activity of tovorafenib in BRAF-fusion melanoma PDX models Vehicle (PO, QDx13) Tovorafenib 12.5 mg/kg (PO, QD) Tovorafenib 25 mg/kg (PO, QD) (A) (B) • Tovorafenib treatment of an AGK-BRAF fusion melanoma PDX model leads to regressions: – NOD SCID mice bearing melanoma PDX tumors with a confirmed AGK-BRAF fusion were treated with tovorafenib or vehicle control daily for 14 days (Figure 2A) • Durable responses to tovorafenib were observed in AGK-BRAF fusion melanoma PDX: – NOD SCID mice bearing melanoma PDX tumors with a confirmed AGK-BRAF fusion were treated with tovorafenib or vehicle control for 14 days. Treatment was then stopped, and tumors monitored for regrowth. Tumor regrowth was not observed until 3 weeks post treatment (Figure 2B) NOD SCID, nonobese diabetic severe combined immunodeficiency; PDX, patient-derived xenograft; PO, oral administration; QD, once daily. FIRELIGHT-1 • FIRELIGHT-1 (NCT04985604) is an open-label, multicenter, phase (P) 1b/2 umbrella study of tovorafenib monotherapy or combination therapy in patients 12 years of age with recurrent, progressive or refractory solid tumors harboring molecularly defined alterations of components of the MAPK pathway (Figure 3) • Substudy 1 (DAY101-102a) is investigating tovorafenib monotherapy in patients with a recurrent, progressive or refractory melanoma (cohort 1) or other solid tumor (cohort 2) harboring activating BRAF or RAF1 (CRAF) fusions or RAF1 amplifications: – Primary endpoint: overall response rate per RECIST v1.1 – Tovorafenib administered to adult patients (18 years of age) at 600 mg once weekly (QW) and for patients 12 to <18 years of age at 420 mg/m2 QW (not to exceed 600 mg) Figure 3. FIRELIGHT-1 trial design BRAF or RAF1 Substudy 1* Melanoma (CRAF) fusion or Tovorafenib alone RAF1 amplification Melanoma cohort Tissue-agnostic cohort Tovorafenib Tissue-agnostic Patients with RP2D preidentified Combination BM-positive tumors RP2D Expansion cohort 1 Substudy 2 Tovorafenib + pimasertib P 1b Dose MAPK pathway n=~25 patients in dose- Expansion cohort 2 escalation alteration escalation and in each planned BM-defined P 2 cohort Expansion cohort 3 Expansion cohort 4 P 2 cohorts TBD *Projected enrollment: tovorafenib alone (n=~43); melanoma cohort (n=18); tissue-agnostic cohort (n=25). BM, biomarker; MAPK, mitogen-activated protein kinase; P, phase; RECIST, response evaluation criteria in solid tumors; RP2D, recommended P 2 dose; TBD, to be determined. Results • Preliminary clinical activity of tovorafenib monotherapy in the first 3 patients with BRAF fusion melanoma is reported (data cutoff Feb 8, 2023; Table 1) Table 1. Patient and disease characteristics Patient 1 Patient 2 Patient 3 Age (years) 53 35 71 Sex M M M ECOG status 0 1 0 Primary cancer Cutaneous melanoma, Malignant melanoma Cutaneous melanoma, non-Spitzoid non-Spitzoid BRAF fusion AGK-BRAF TRIM33-BRAF MKRN1-BRAF Stage at diagnosis III Unknown II Prior therapy: Surgery Yes No No Radiotherapy No Yes Yes Immune checkpoint inhibitor Yes Yes Yes Prior lines of targeted treatment 1 2 1 Tovorafenib dose 600 mg QW 600 mg QW 600 mg QW TRAE G3* No No No Dose modification/discontinuation due to AE* No No No Dose interruptions* No No No Treatment ongoing* Yes Yes Yes Current cycle* 5 5 3 Best RECIST response to tovorafenib* CR PR PR *Data cutoff Feb 8, 2023. Patient 3 is awaiting a confirmatory scan. AE, adverse events; CR, complete response; ECOG, Eastern Cooperative Oncology Group; G, grade; M, male; PR, partial response; QW, once weekly; RECIST, response evaluation criteria in solid tumors; TRAE, treatment-related adverse event. Patient 1 with AGK-BRAF fusion cutaneous melanoma Parameter Description/outcome Prior therapies • Multiple lymphadenectomies and skin lesion excision surgery Baseline • Pembrolizumab (11 weeks): – Best response: SD Tovorafenib treatment • 600 mg QW to date in FL-1 102a • 5 cycles with no dose interruption or (melanoma cohort)* modifications due to AEs Antitumor activity CR (11-week scan); confirmed at 16 results to date* weeks 11 Safety results to date* TRAEs: transient rash (G1 and G2) Week anemia (G2) TEAE: neck pain (G1) *Data cutoff Feb 8, 2023. Out of window per protocol. per RECIST v1.1. AE, adverse event; CR, complete response; G, grade; FL-1, FIRELIGHT-1; QW, once weekly; RECIST, response evaluation criteria in solid tumors; SD, stable disease; TEAE, treatment-emergent adverse event; TRAEs, treatment-related adverse events. Patient 2 with TRIM33-BRAF fusion malignant melanoma Parameter Description/outcome Prior therapies • Radiation Hilar lymph Liver Abdominal • Nivolumab (12 mo, adjuvant node wall setting): – No best response, disease resected • Nivolumab + ipilimumab (3 cycles): Baseline – Best response: PD after 2 mo Tovorafenib • 600 mg QW treatment to date in • 5 cycles with no dose interruption FL-1 102a or modifications due to AEs (melanoma cohort)* 8 Antitumor activity PR (8-week scan); confirmed at 16 results to date* weeks Week Safety results to TRAEs: date* rash - maculopapular (G1) headache (G1) fatigue (G1) *Data cutoff Feb 8, 2023. per RECIST v1.1. AE, adverse event; G, grade; FL-1, FIRELIGHT-1; mo, months; QW, once weekly; PD, progressive disease; PR, partial response; RECIST, response evaluation criteria in solid tumors; TRAEs, treatment-related adverse events. Patient 3 with MKRN1-BRAF fusion cutaneous melanoma Parameter Description/outcome Prior therapies • Radiation Baseline • Pembrolizumab (2 mo): – Best response: SD Tovorafenib treatment • 600 mg QW to date in FL-1 102a • 3 cycles with no dose interruption or (melanoma cohort)* modifications due to AEs Antitumor activity PR (7-week scan),; is awaiting a results to date* confirmatory scan 7 Safety results to date* TRAEs: urticaria (G1) Week hand-foot syndrome (G1) *Data cutoff Feb 8, 2023. In window per protocol. per RECIST v1.1. AE, adverse event; G, grade; FL-1, FIRELIGHT-1; mo, months; QW, once weekly; PR, partial response; RECIST, response evaluation criteria in solid tumors; SD, stable disease; TRAEs, treatment-related adverse events. Conclusions • Early results from the first 3 patients of this ongoing trial showed that tovorafenib: – Displayed encouraging antitumor activity in BRAF-fusion melanoma • 2 PRs* and 1 CR per RECIST v1.1 – Was generally well tolerated: • All TEAEs and TRAEs were G1 or G2 • As of Feb 8, 2023, all 3 patients remained on tovorafenib with no dose reduction or treatment interruption *Patient 3 is awaiting a confirmatory scan. events CR, complete . response; G, grade; PR, partial response; TEAE, treatment-emergent adverse event; TRAEs, treatment-related adverse References 1. Hutchinson KE, et al. Clin Cancer Res. 2013;19(24):6696-6702. 2. Botton, T. et al. Cell Rep. 2019;29(3):573-588. 3. Sun Y, et al. Neuro Oncol. 2017;19(6):774-785. 4. Olszanski, et al. Ann Oncol. 2017;28(suppl_5): abstr. 4583. 5. Kilburn L, et al. Neuro Oncol. 2022;24(Suppl_7):vii89 and presented poster. 6. Wright K, et al. Neuro Oncol. 2020;22(Suppl 2):ii46, and associated presentation. 7. Offer K, et al. Poster P250 presented at: 2021 Connective Tissue Oncology Society Annual Meeting; November 10-13, 2021; Virtual Meeting. Presenting author contact Jeeyun Lee, MD: jyunlee@skku.edu 19th EADO Congress 2023: Paper #174
