Exhibit 99.2
Transform Therapies. Reimagine Lives. February 6, 2025 Alumis and ACELYRIN Merger Investor Presentation |

Disclaimer Forward - Looking Statements This communication contains forward - looking statements within the meaning of federal securities laws, including the “safe harbor” provisions of th e Private Securities Litigation Reform Act of 1995. Such statements are based upon current plans, estimates and expectations of management of Alumis Inc. (“Alumis”) and ACELYRIN, Inc. (“ACELYRIN”) in light of historical results and trends, current conditions and potential future de velopments, and are subject to various risks and uncertainties that could cause actual results to differ materially from such st atements. The inclusion of forward - looking statements should not be regarded as a representation that such plans, estimates and expectations will be achieved. Words such as “anticipate,” “e xpect,” “project,” “intend,” “believe,” “may,” “will,” “should,” “plan,” “could,” “continue,” “target,” “contemplate,” “estim ate ,” “forecast,” “guidance,” “predict,” “possible,” “potential,” “pursue,” “likely,” and words and terms of similar substance used in connection with any discussion of future pl ans , actions or events identify forward - looking statements. All statements, other than statements of historical facts, including ex press or implied statements regarding the proposed transaction; the conversion of equity interests contemplated by the agreement and plan of merger, dated as of February 6, 2025, by and among the parties (the “merger agreement”); the issuance of common stock of Alumis contemplated by the merger ag ree ment; the expected filing by Alumis with the Securities and Exchange Commission (the “SEC”) of a registration statement on Form S - 4 (the “registration statement”) and a join t proxy statement/prospectus of Alumis and ACELYRIN to be included therein (the “joint proxy statement/prospectus”); the expe cte d timing of the closing of the proposed transaction; the ability of the parties to complete the proposed transaction considering the various closing conditions; the expected bene fit s of the proposed transaction; the sufficiency of the combined company’s capital resources; the combined company’s cash runwa y; the competitive ability and position of the combined company;the clinical pipeline of the combined company; and any assumptions underlying any of the foregoing, are forward - looking statements. Risks and uncertainties include, among other things, (i) the risk that the proposed transaction may not be completed in a tim ely basis or at all, which may adversely affect Alumis's and ACELYRIN's businesses and the price of their respective securities; (ii) the potential failure to receive, on a timely ba si s or otherwise, the required approvals of the proposed transaction, including stockholder approvals by both Alumis's stockholders and ACELYRIN's stockholders, and the potential failure to satisfy the other conditions to the consummation of th e transaction; (iii) the effect of the announcement, pendency or completion of the proposed transaction on each of Alumis's or ACELYRIN's ability to attract, motivate, retain and hire key personnel and maintain relationships with partners, suppliers a nd others with whom Alumis or ACELYRIN does business, or on Alumis's or ACELYRIN's operating results and business generally; (iv) that the proposed transaction may divert management’s attention from each of Alumis's and ACELYRIN's ongoing business operations; (v) the risk of any legal proceedings related to the proposed transaction or othe rw ise, or the impact of the proposed transaction thereupon, including resulting expense or delay; (vi) that Alumis or ACELYRIN may be adversely affected by other economic, business and/or competitive factors; (vii) t he occurrence of any event, change or other circumstance that could give rise to the termination of the merger agreement, includ ing in circumstances which would require Alumis or ACELYRIN to pay a termination fee; (viii) the risk that restrictions during the pendency of the proposed transaction may impa ct Alumis's or ACELYRIN's ability to pursue certain business opportunities or strategic transactions; (ix) the risk that the anticipated be nefits and synergies of the proposed transaction may not be fully realized or may take longer to realize than expected; (x) the impact of legislative, regulatory, economic, compe tit ive and technological changes; (xi) risks relating to the value of Alumis securities to be issued in the proposed transaction ; ( xii) the risk that integration of the proposed transaction post closing may not occur as anticipated or the combined company may not be able to achieve the growth prospects expected from th e t ransaction; (xiii) the effect of the announcement, pendency or completion of the proposed transaction on the market price of the common stock of each of Alumis and ACELYRIN; (xiv) the implementation of each of Alumis's and ACELYRIN's business model and strategic plans for product candidates and pipeline, and challenges inherent in developing, c ommercializing, manufacturing, launching, marketing and selling potential existing and new products and product candidates; ( xv) the scope, progress, results and costs of developing Alumis's and ACELYRIN's product candidates and any future product candidates, including conducting preclinical studies and clinical tr ia ls, and otherwise related to the research and development of Alumis's and ACELYRIN's pipeline; (xvi) the timing and costs involved in obtaining and maintaining regulatory approval for Alumis's and ACELYRIN's current or future product candidates, and any related restrictions, limitations and/or warnings in the label o f any approved product; (xvii) the market for, adoption (including rate and degree of market acceptance) and pricing and reimbursement of Alumis's and ACELYRIN's product candidates, if approved, and their respective abilities to compete with therapies and procedures that ar e rapidly growing and evolving; (xviii) uncertainties in contractual relationships, including collaborations, partnerships, l ice nsing or other arrangements and the performance of third party suppliers and manufacturers; (xix) the ability of each of Alumis and ACELYRIN to establish an d maintain intellectual property protection for products or avoid or defend claims of infringement; (xx) Alumis’s ability to successfully integrate ACELYRIN’s operations and personnel and; (xxi) potential delays in initiating, enrolling or completing preclinical studies and clinical trials. These risks, as well as other risks related to the proposed transaction, will be described in the registration statement and the joint proxy statement/prospectus that will be filed with the SEC in connection with the proposed transaction. While the list of factors presented here and the list of factors to be presented in the registration statement are considered representative, no such list should be considered to be a complete sta tem ent of all potential risks and uncertainties. For additional information about other factors that could cause actual results to differ materially from those described in the forward looking statements, please refer to Alumis’s and ACELYRIN’s respective periodic reports and other filings with the SEC, including the risk factors identified in Alumis’s and ACELYRIN’s most recent Quarterly Reports on Form 10 - Q and/or Annual Reports on Form 10 - K. The risks and uncertainties descr ibed above and in the SEC filings cited above are not exclusive and further information concerning Alumis and ACELYRIN and their respect ive businesses, including factors that potentially could materially affect their respective businesses, financial conditions or o pe rating results, may emerge from time to time. Readers are urged to consider these factors carefully in evaluating these forward looking statements, and not to place undue reliance on any forward - looking statements, which speak only as of the date hereof. Readers should also carefully review the risk factors descr ibed in other documents Alumis and ACELYRIN file from time to time with the SEC. The forward - looking statements included in this communication are made only as of the date hereof. Alumis assumes no obligation and does not intend to update these forward - looking statements, even if new information becomes available in the future, except as required by law. Additional Information and Where to Find It In connection with the proposed merger, Alumis intends to file with the SEC the registration statement, which will include th e j oint proxy statement/prospectus. After the registration statement has been declared effective by the SEC, the joint proxy sta tem ent/prospectus will be delivered to stockholders of Alumis and ACELYRIN. BEFORE MAKING ANY VOTING OR INVESTMENT DECISION, SECURITY HOLDERS OF Alumis AND ACELYRIN ARE URGED TO RE AD THE JOINT PROXY STATEMENT/PROSPECTUS (INCLUDING ALL AMENDMENTS AND SUPPLEMENTS THERETO) AND OTHER DOCUMENTS RELATING TO THE M ERG ER THAT WILL BE FILED WITH THE SEC WHEN THEY BECOME AVAILABLE BECAUSE THEY WILL CONTAIN IMPORTANT INFORMATION ABOUT THE PROPOSED ME RGER. Investors and security holders will be able to obtain copies of the joint proxy statement/prospectus (when available) a nd other documents filed by Alumis and ACELYRIN with the SEC, without charge, through the website maintained by the SEC at www.sec.gov. Copies of the doc ume nts filed with the SEC by Alumis will be available free of charge under the SEC Filings heading of the Investor Relations section of Alumis’s website at investors.alumis.com. Copies of the documents filed with the SEC by ACELYRIN will be available free of charge under the Financials & Filings heading of th e I nvestor Relations section of ACELYRIN’s website investors.ACELYRIN.com. Participants in the Solicitation Alumis and ACELYRIN and their respective directors and executive officers may be deemed to be participants in the solicitation of pr ox ies in respect of the proposed transaction. Information about Alumis’s directors and executive officers is set forth in Alumis’s registration statement on Form S - 1/A (File No. 333 - 280068), which was filed with the SEC on June 24, 2024. Information about ACELYRIN’s directors and executive officers is set for th in the proxy statement for ACELYRIN’s 2024 Annual Meeting of Stockholders, which was filed with the SEC on April 22, 2024, an d ACELYRIN’s Current Reports on Form 8 - K filed with the SEC on May 28, 2024, August 13, 2024 and December 10, 2024. Stockholders may obtain additional information regarding the int erests of such participants by reading the registration statement and the joint proxy statement/prospectus and other relevant ma terials to be filed with the SEC regarding the proposed merger when they become available. Investors should read the joint proxy statement/prospectus carefully when it beco mes available before making any voting or investment decisions. No Offer or Solicitation This communication shall not constitute an offer to sell or the solicitation of an offer to buy any securities or a solicitation of any vote or ap proval, nor shall there be any sale of securities in any jurisdiction in which such offer, solicitation or sale would be unla wfu l prior to registration or qualification under the securities laws of any such jurisdiction. No offering of securities shall be made except by means of a prospectus meeting the re quirements of Section 10 of the Securities Act of 1933, as amended. 2 © Alumis

3 © Alumis Creating Well Capitalized Company with Multiple Upcoming Expected Development Milestones and Extended Runway into 2027 〉 Creates a late - stage clinical biopharma company dedicated to innovating, developing and commercializing transformative therapies for immune - mediated diseases 〉 Differentiated pipeline with multiple upcoming milestones expected, including: 〉 Topline data from Phase 3 ONWARD trials for Alumis’ ESK - 001 in moderate - to - severe plaque psoriasis on track for readout in 1H 2026 〉 Topline data from Phase 2b LUMUS trial in systemic lupus erythematosus on track for readout in 2026 〉 Phase 2 clinical trial initiation for Alumis ’ A - 005 in MS 〉 Advancing lonigutamab , a subcutaneously delivered anti - IGF - 1R currently being investigated in a Phase 2 clinical trial in thyroid eye disease 〉 Pro forma cash of ~$737 million as of December 31, 2024, provides runway into 2027 beyond multiple expected clinical readouts 〉 Combined company to benefit from world - class leadership team with proven record of operating discipline and capital efficiency
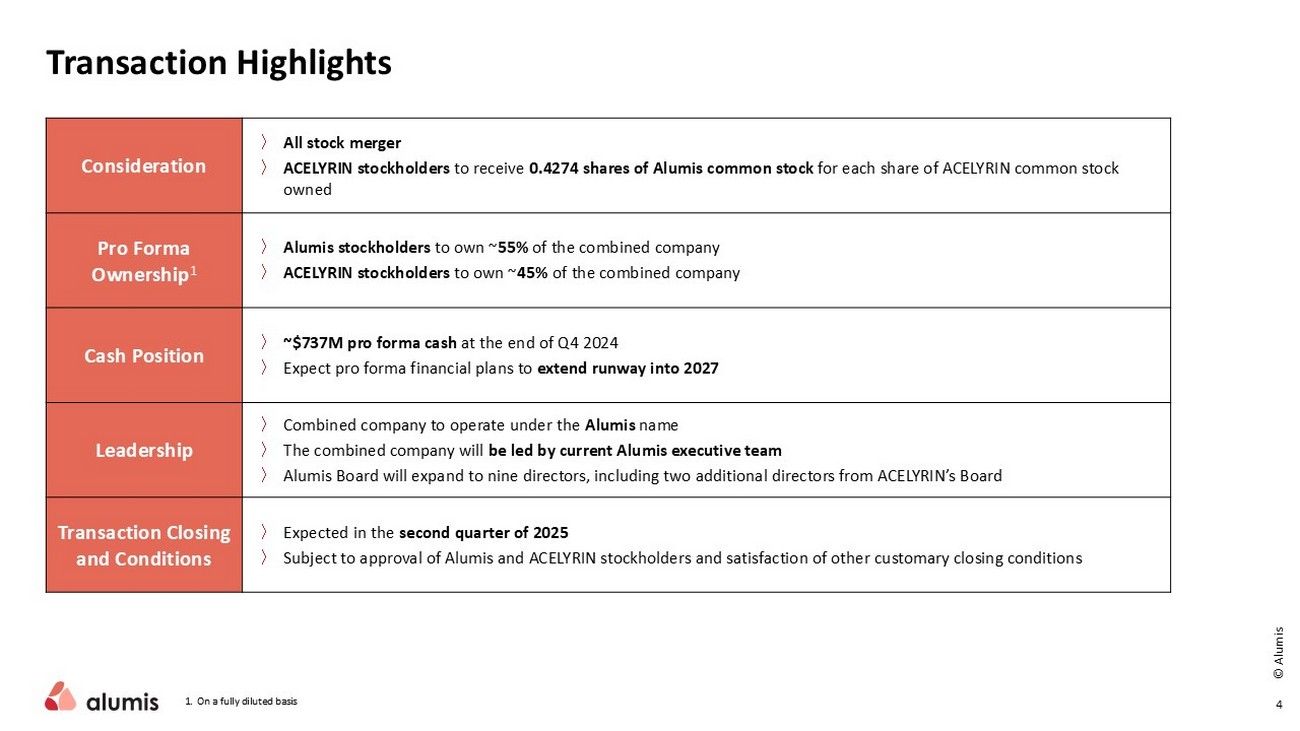
4 © Alumis 〉 All stock merger 〉 ACELYRIN stockholders to receive 0.4274 shares of Alumis common stock for each share of ACELYRIN common stock owned Consideration 〉 Alumis stockholders to own ~ 55% of the combined company 〉 ACELYRIN stockholders to own ~ 45% of the combined company Pro Forma Ownership 1 〉 ~$737M pro forma cash at the end of Q4 2024 〉 Expect pro forma financial plans to extend runway into 2027 Cash Position 〉 Combined company to operate under the Alumis name 〉 The combined company will be led by current Alumis executive team 〉 Alumis Board will expand to nine directors, including two additional directors from ACELYRIN’s Board Leadership 〉 Expected in the second quarter of 2025 〉 Subject to approval of Alumis and ACELYRIN stockholders and satisfaction of other customary closing conditions Transaction Closing and Conditions 1. On a fully diluted basis Transaction Highlights

Late - Stage Pipeline With Multiple Near - Term Catalysts 5 © Alumis 〉 Post transaction close, the company will consider development plan for lonigutamab in the context of the broader combined portfolio to drive long - term value for stockholders ANTICIPATED MILESTONES DEVELOPMENT PHASE 3 PHASE 2 PHASE 1 PRECLINICAL TARGET 1H 2026 : Phase 3 topline data Moderate - to - Severe Plaque Psoriasis ( PsO ) ESK - 001 (TYK2) 2026: Phase 2b topline data Systemic Lupus Erythematosus (SLE) Other suitable development opportunities for ESK - 001 include expanded Psoriasis indication, and/or other immunological indications outside the CNS Add’l indications TBD PsA, IBD, etc. To be determined Thyroid Eye Disease (TED) Lonigutamab (anti - IGF - 1R) 2025: Initiate Phase 2 in MS 2026: Phase 2 topline data Multiple Sclerosis (MS) A - 005 (TYK2) Expanded opportunities for A - 005 include other neuroinflammatory or neurodegenerative indications Add’l indications TBD PAR, AD, etc . YE25: New clinical candidate Undisclosed IRF5, Additional Targets

6 © Alumis Ongoing Phase 2b trial of ESK - 001 in systemic lupus erythematosus Phase 2 trial of A - 005 in multiple sclerosis A - 005 Ongoing Phase 3 trials of ESK - 001 in m oderate - to - severe plaque psoriasis Topline data readout expected in 1H 2026 Topline data readout expected in 2026 Initiation expected in 2025 To pline data expected in 2026 Alumis Pipeline: Development Plan is on Track
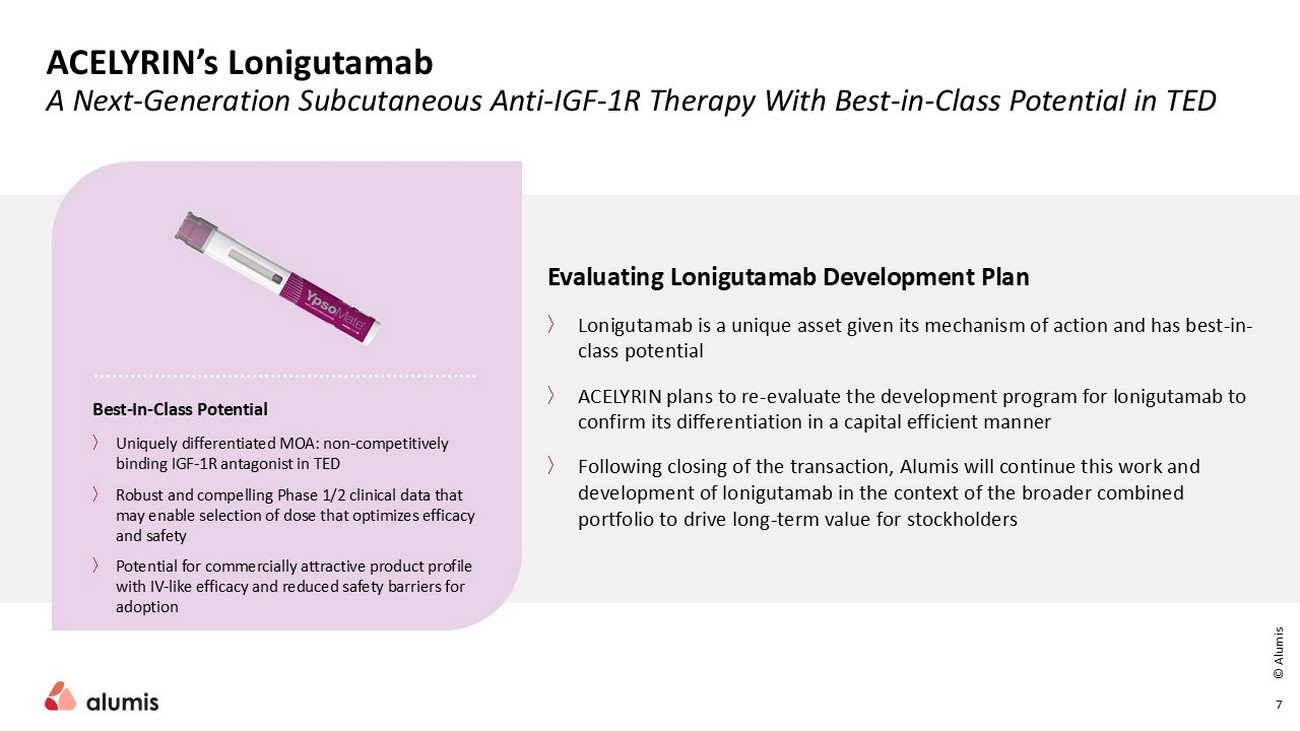
ACELYRIN’s Lonigutamab A Next - Generation Subcutaneous Anti - IGF - 1R Therapy With Best - in - Class Potential in TED 7 Evaluating Lonigutamab Development Plan 〉 Lonigutamab is a unique asset given its mechanism of action and has best - in - class potential 〉 ACELYRIN plans to re - evaluate the development program for lonigutamab to confirm its differentiation in a capital efficient manner 〉 Following closing of the transaction, Alumis will continue this work and development of lonigutamab in the context of the broader combined portfolio to drive long - term value for stockholders Best - In - Class Potential 〉 Uniquely differentiated MOA: non - competitively binding IGF - 1R antagonist in TED 〉 Robust and compelling Phase 1/2 clinical data that may enable selection of dose that optimizes efficacy and safety 〉 Potential for commercially attractive product profile with IV - like efficacy and reduced safety barriers for adoption © Alumis

8 © Alumis F lexibility and Runway to Advance L ate - stage T herapies 〉 Standalone: Alumis and ACELYRIN had cash, cash equivalents and marketable securities of ~$289 million and ~$448 million, respectively, on a preliminary basis, as of December 31, 2024 〉 Combined Company: Pro forma cash of ~$737 million as of December 31, 2024 〉 Strong Financial Position: Provides runway into 2027 to advance expanded pipeline through multiple planned key data readouts across several clinical trials
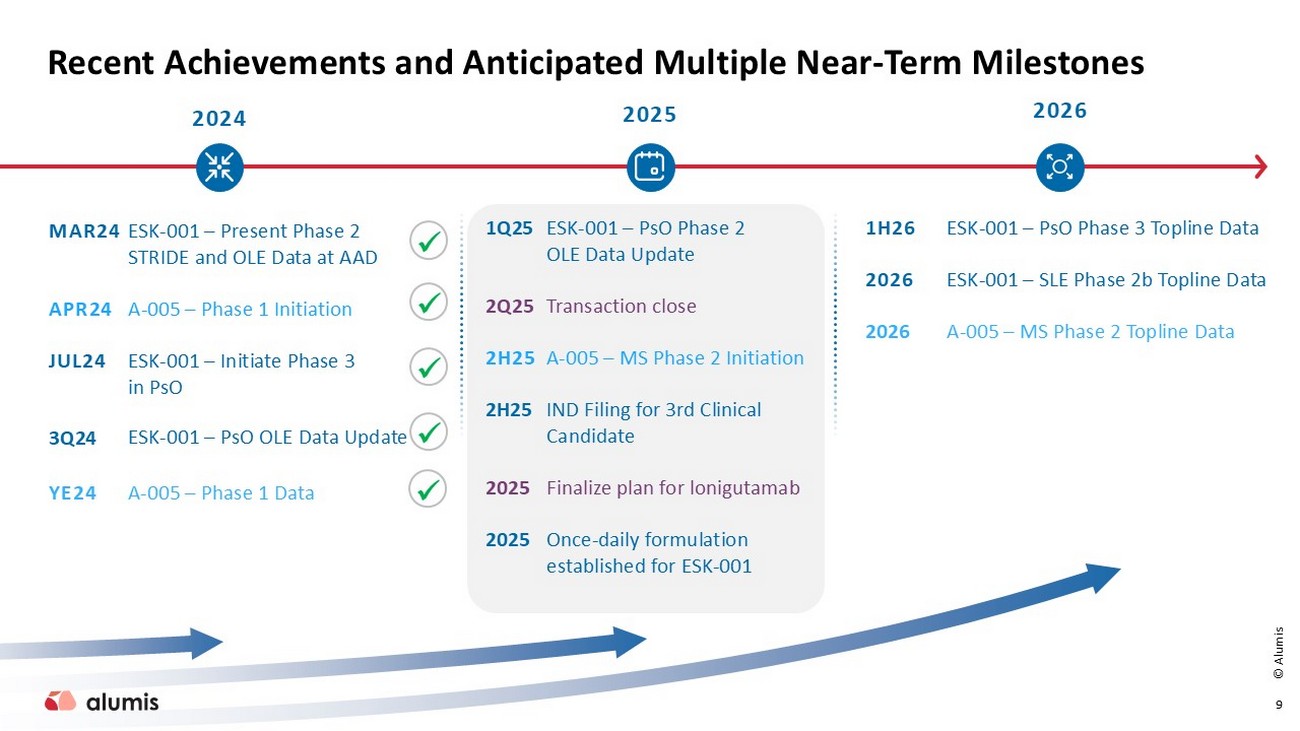
x x Recent Achievements and Anticipated Multiple Near - Term Milestones 2024 2025 2026 MAR24 APR24 JUL24 3Q24 YE24 ESK - 001 – Present Phase 2 STRIDE and OLE Data at AAD A - 005 – Phase 1 Initiation ESK - 001 – Initiate Phase 3 in PsO ESK - 001 – PsO OLE Data Update A - 005 – Phase 1 Data 1H26 2026 2026 ESK - 001 – PsO Phase 3 Topline Data ESK - 001 – SLE Phase 2b Topline Data A - 005 – MS Phase 2 Topline Data ESK - 001 – PsO Phase 2 OLE Data Update Transaction close A - 005 – MS Phase 2 Initiation IND Filing for 3rd Clinical Candidate Finalize plan for lonigutamab Once - daily formulation established for ESK - 001 1Q25 2 Q25 2H25 2H25 2025 2025 x 9 x x © Alumis

Alumis /ACELYRIN Combination © Alumis 10 Late - Stage Clinical Biopharma Company Dedicated to Innovating, Developing and Commercializing Transformative Therapies for Immune - mediated Diseases Strong Portfolio, Large Opportunity 〉 Three potential best - in - class molecules: two advanced clinical TYK2 inhibitors and an IGF - 1R inhibitor provide strategic opportunities across a broad range of immune - mediated diseases 〉 ESK - 001: Near term pivotal data with two major value inflection points expected in large indications, high efficacy oral molecul es provide significant market maker potential 〉 A - 005: Potentially first - in - class fully CNS - penetrant TYK2 inhibitor expands breadth of addressable indications, including those within the CNS 〉 Lonigutamab : Potentially differentiated profile with favorable efficacy and safety data in TED Well capitalized and proven leadership to execute 〉 Pro forma combined cash of ~$737 million as of December 31, 2024 provides runway into 2027 beyond multiple expected clinical readouts 〉 Experienced team with strong track record of operating discipline, capital efficiency and value creation
Thank You!
Appendix
13 Alumis: A Well Characterized Late - Stage Portfolio with Significant Opportunity Genomic, proteomic and clinical insights validate and drive our Research and Development strategy and increase the probability of technical success (PTS) of our programs Experienced team with strong track record of operating discipline, capital efficiency and value creation Proven Leadership High efficacy oral molecules provide significant market maker potential High PTS Near Term Pivotal Data Large Opportunity Two highly differentiated advanced clinical TYK2 inhibitors provide strategic opportunities across a broad range of immune mediated diseases ESK - 001: Two major value inflection points in large indications A - 005: First - in - class fully CNS - penetrant TYK2 inhibitor expands breadth of addressable indications, including those within the CNS First - in - class Market Maker © Alumis
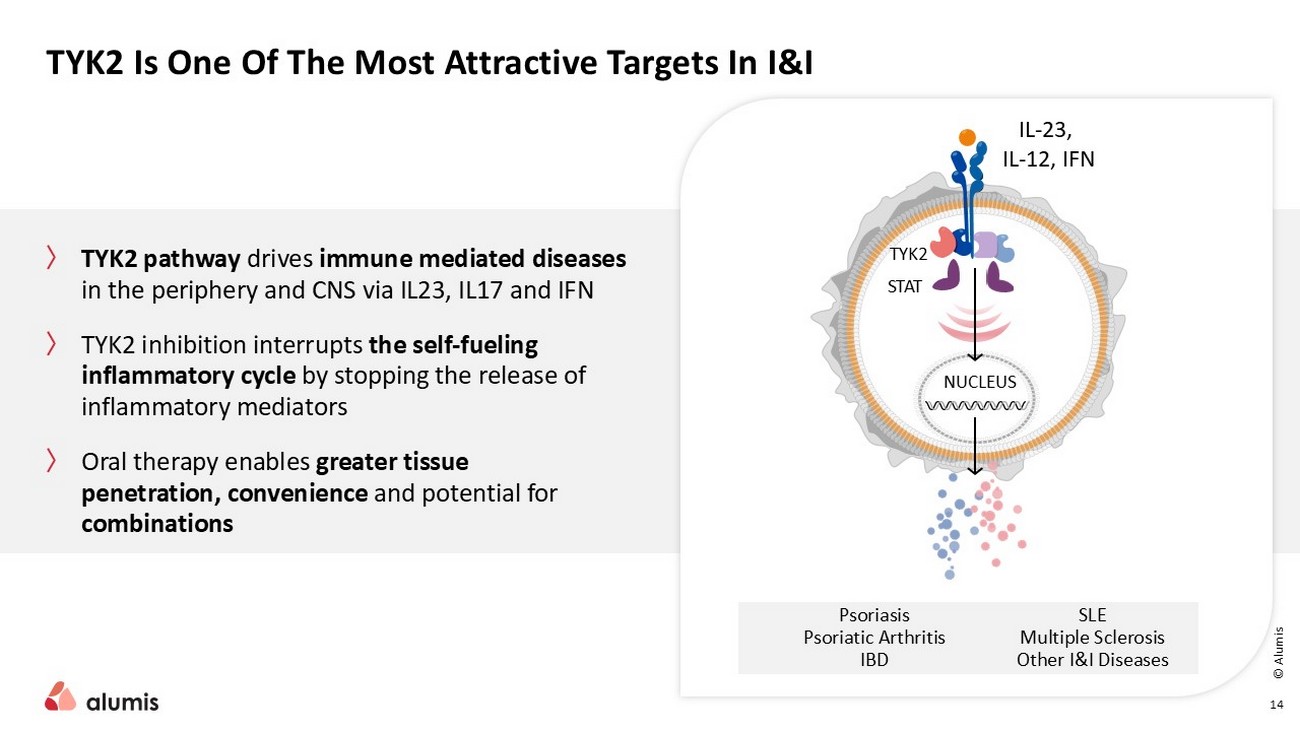
〉 TYK2 pathway drives immune mediated diseases in the periphery and CNS via IL23, IL17 and IFN 〉 TYK2 inhibition interrupts the self - fueling inflammatory cycle by stopping the release of inflammatory mediators 〉 Oral therapy enables greater tissue penetration, convenience and potential for combinations TYK2 Is One Of The Most Attractive Targets In I&I 14 … TYK2 STAT NUCLEUS IL - 23, IL - 12, IFN Psoriasis Psoriatic Arthritis IBD SLE Multiple Sclerosis Other I&I Diseases © Alumis

15 ESK - 001 and A - 005: A Highly Differentiated TYK2i Portfolio Phase 3 dose fully inhibits TYK2 by every measure in skin and blood* 〉 Maximal Target Inhibition Long term PASI and sPGA response rates competitive with high efficacy biologics 〉 High Response Oral No major safety signals to date with up to two years of active therapy 〉 Long Term Safety Significant and prolonged cerebral spinal fluid (CSF) exposure 〉 Full CNS Penetration High peripheral and CSF exposure achieves maximal target inhibition 〉 Maximal Target Inhibition Chronic tox ongoing, CMC and clinical pharmacology support Phase 2 〉 Phase 2 Ready ESK - 001: next - generation TY2 inhibitor for immune - mediated diseases A - 005: first - in - class CNS - penetrant TYK2 inhibitor for neuroinflammatory diseases © Alumis *in vitro HWB, ex vivo Ph1 PD assays in HV, and RNAseq of Ph2 PsO patients
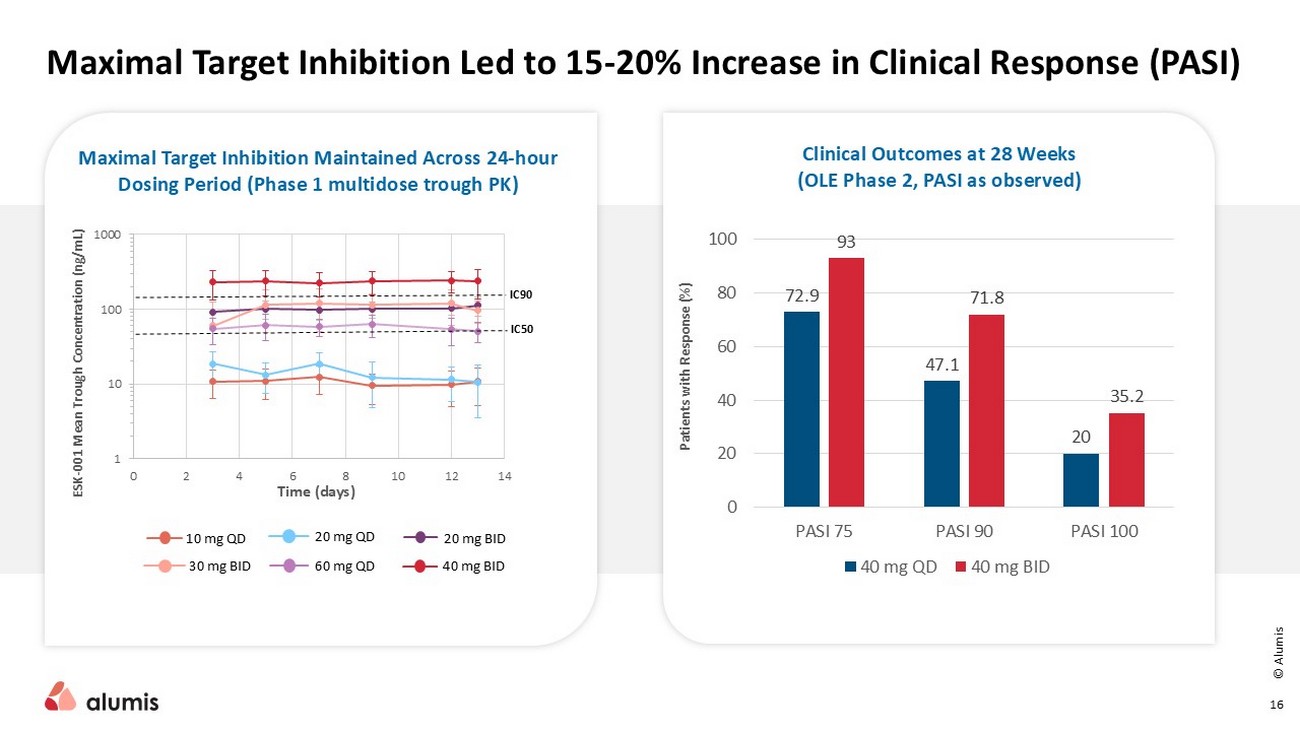
© Alumis 16 Maximal Target Inhibition Led to 15 - 20% Increase in Clinical Response (PASI) Maximal Target Inhibition Maintained Across 24 - hour Dosing Period ( Phase 1 multidose trough PK) Clinical Outcomes at 28 Weeks (OLE Phase 2, PASI as observed) 1 10 100 1000 0 2 4 6 8 10 12 14 ESK - 001 Mean Trough Concentration (ng/mL) Time (days) IC90 IC50 10 mg QD 20 mg QD 20 mg BID 30 mg BID 40 mg BID 60 mg QD Patients with Response (%) 72.9 47.1 20 93 71.8 35.2 0 20 40 60 80 100 PASI 75 PASI 90 PASI 100 40 mg QD 40 mg BID
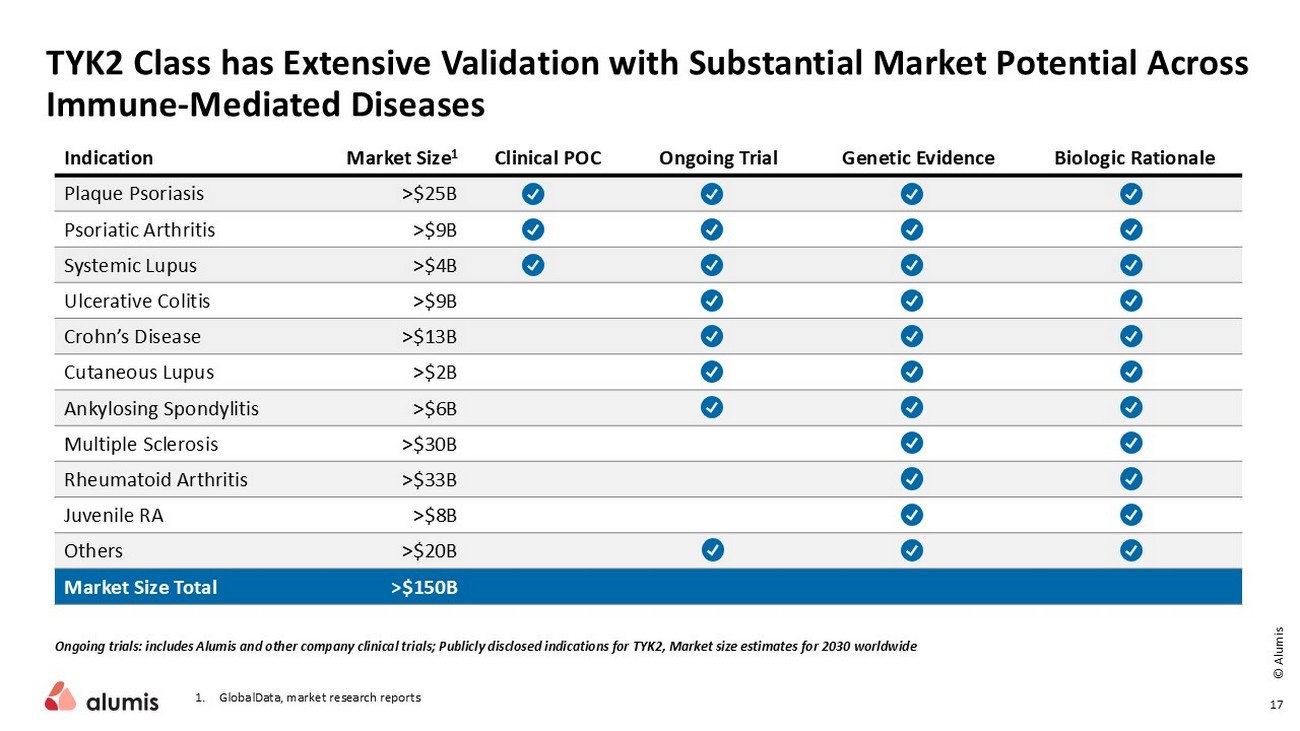
TYK2 Class has Extensive Validation with Substantial Market Potential Across Immune - Mediated Diseases Ongoing trials: includes Alumis and other company clinical trials; Publicly disclosed indications for TYK2, Market size estimates for 2030 worldwide Biologic Rationale Genetic Evidence Ongoing Trial Clinical POC Market Size 1 Indication >$25B Plaque Psoriasis >$9B Psoriatic Arthritis >$4B Systemic Lupus >$9B Ulcerative Colitis >$13B Crohn’s Disease >$2B Cutaneous Lupus >$6B Ankylosing Spondylitis >$30B Multiple Sclerosis >$33B Rheumatoid Arthritis >$8B Juvenile RA >$20B Others >$150B Market Size Total 1. GlobalData , market research reports 17 © Alumis
© Alumis 18 Lonigutamab – A Unique Mechanism for TED Patients 〉 Robust subcutaneous efficacy in TED Patients comparable to SoC and investigational anti - IGF - 1R IV agents 〉 First clinical subcutaneous data in TED patients informs clinical development 〉 Optimized safety profile : potential for lower risks of hearing impairment, menstrual disorders and hyperglycemia 〉 Ability to be used chronically and flexibly to tailor to patient treatment goals 〉 Smallest, lowest volume autoinjector for an anti - IGF - 1R agent

















